Abstract

Triple-negative breast cancer (TNBC) remains a disease with a paucity of targeted treatment opportunities. The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that is involved in a wide range of physiological processes, including the sensing of xenobiotics, immune function, development, and differentiation. Different small-molecule AhR ligands drive strikingly varied cellular and organismal responses. In certain cancers, AhR activation by select small molecules induces cell cycle arrest or apoptosis via activation of tumor-suppressive transcriptional programs. AhR is expressed in triple-negative breast cancers, presenting a tractable therapeutic opportunity. Here, we identify a novel ligand of the aryl hydrocarbon receptor that potently and selectively induces cell death in triple-negative breast cancer cells and TNBC stem cells via the AhR. Importantly, we found that this compound, Analog 523, exhibits minimal cytotoxicity against multiple normal human primary cells. Analog 523 represents a high-affinity AhR ligand with potential for future clinical translation as an anticancer agent.
Keywords: aryl hydrocarbon receptor, apoptosis, triple-negative breast cancer, benzimidazoisoquinoline, breast cancer stem cells
Triple-negative breast cancer (TNBC) represents a group of breast cancers defined by the absence of estrogen receptor (ER) and progesterone receptor (PR) expression and the lack of human epidermal growth factor receptor 2 (HER2) amplification or overexpression. TNBCs are frequently aggressive, highly metastatic, and are recalcitrant to the targeted therapies employed in hormone-positive and HER2-amplified breast cancers.1 Despite being superficially classified by the absence of these important immunohistochemical markers, TNBC is highly heterogeneous, and different subtypes exhibit wide variabilities in their molecular features and response to therapies.2 Except immunotherapies,3 PARP inhibitors,4 and antibody–drug conjugates,5 targeted treatments for TNBC are still lacking. There thus exists a clinical need to identify novel therapeutic vulnerabilities for this subtype of cancer.1 Here, we identify a potent small-molecule agonist of the aryl hydrocarbon receptor (AhR) that exhibits highly selective antiproliferative and proapoptotic effects against triple-negative breast cancer cells.
AhR belongs to a superfamily of proteins involved in sensing environmental signals, with family members defined by the presence of both basic helix–loop–helix (bHLH) and PER-ARNT-SIM (PAS) domains. The receptor has a conserved role in sensing and responding to environmental and internal stimuli, binding to both endogenous and xenobiotic small molecules and coordinating adaptive transcriptional responses to maintain organismal homeostasis.6 The transcriptionally inactive AhR is sequestered in the cytoplasm by multiple chaperone proteins and complex members. Upon small-molecule binding to the AhR, the receptor is translocated to the nucleus where it can then interact with the heterodimeric partner aryl hydrocarbon receptor nuclear translocator (ARNT) and other transcriptional coregulators. The AhR–ARNT heterodimer complex binds to regulatory regions of DNA containing the consensus motif (5′-TNGCGTG-3′) or the xenobiotic response element (XRE) and modulates gene transcription. AhR can also modulate gene expression through non-XRE elements7,8 and interactions with other transcription factors, including c-MAF,9 KLF6,10 RelA, and other NF-κB complex members.11,12 The transcriptional programs and resulting biological phenotypes downstream of AhR activation are highly varied, depending on tissue, species, and ligand-specific factors that together influence the outcome.13
While initially studied primarily in the context of xenobiotic metabolism, AhR has prominent functions in controlling the cell cycle, cell fate, and differentiation in multiple cancers. AhR activation by select ligands can induce cell cycle arrest and/or apoptosis through the induction of cell cycle regulatory proteins such as p27Kip114 and p21Cip115 and cell death regulators such as the Fas ligand16 and Bax.17 In certain contexts, AhR activation can enforce differentiation in both stem cells18−20 and cancer cells.21,22 Independent of exogenous ligands, AhR can exert tumor suppression, supported by in vivo studies showing that the loss of AhR drives increased tumor development and cancer hallmarks in colon,23,24 liver,25 and prostate cancer models.26,27 In addition, AhR has been shown to restrict metastasis in lung cancer models.28−30 In hormone-receptor-positive and HER2-overexpressing breast cancers, AhR can inhibit cancer growth via multiple mechanisms, including the induction of cell cycle arrest,31,32 differentiation,33−35 and apoptosis.36
Benzimidazoisoquinolines (BBQs) represent a novel class of high-affinity AhR ligands that are rapidly metabolized and lack overt toxicity in vivo.(37,38) We recently showed that the AhR ligand 11-chloro-7H-benzimidazo[2,1-a]benzo[de]isoquinolin-7-one (11-Cl-BBQ) can inhibit the growth of nonsmall-cell lung cancer cells via AhR activation and the induction of p53, p27Kip1, and p21Cip1.39 11-Cl-BBQ was found to induce an irreversible cell cycle arrest in lung cancer cells but did not induce apoptosis.39 Given the favorable activity and selectivity of 11-Cl-BBQ, we sought to identify novel analogs of 11-Cl-BBQ with improved potency. Here, we identify the dechlorinated derivative of 11-Cl-BBQ, named Analog 523, as a potent AhR-dependent ligand that induces apoptosis in triple-negative breast cancer cells, with the greatest potency in the basal A subtype.
Results
Functional Screening of 11-Cl-BBQ Analogs for Anticancer Activity in TNBC
In order to identify chemical analogs of 11-Cl-BBQ with AhR-dependent growth inhibitory action against triple-negative breast cancer cells, we generated cell lines lacking AhR expression using CRISPR-Cas9 gene editing. AhR knockout (KO) cell lines were generated using the CRISPR-Cas9 lentiCRISPRv2 plasmid, with single guide RNAs (gRNAs) against AhR. In parallel, matched control cell lines were generated with a lentiCRISPRv2 plasmid (CR-V2). Screening for the loss of AhR expression by immunoblotting led to the identification of AhR KO clones (Figure 1A). In order to identify novel analogs of 11-Cl-BBQ, more than 300 chemical analogs of 11-Cl-BBQ were synthesized. Analogs were screened, in a structurally agnostic fashion, using a three-point dose response (0.1, 1, and 10 μM) against vector control (AhR WT) and AhR KO MDA-MB-468 cells, with a relative impact on cell survival measured by a cell survival assay at 48 and 72 h post-treatment. Of the analogs screened, Analog 523 was identified as a top hit based on its potent (>50% inhibition at 0.1 μM) and selective activity against AhR-expressing cells versus AhR KO cells (>70% difference between WT and KO cells at 10 μM) (Figure 1B,C). As a positive control for AhR-dependent growth inhibition, we used CGS-15943, an AhR agonist that we previously identified with proapoptotic effects.16 While multiple other hits from the screen were validated, Analog 523 displayed the highest degree of potency and selectivity and thus was chosen to move forward with further studies. Surprisingly, Analog 523 is structurally very similar to 10-Cl-BBQ and 11-Cl-BBQ and differs only by the loss of the chlorine atom on the benzimidazole ring, resulting in 7H-benzimidazo[2,1-a]benzo[de]isoquinolin-7-one (BBQ) (Figure 1D). To determine whether Analog 523 could activate AhR-dependent transcription, we treated Hepa-1 cells expressing a xenobiotic response element driven reporter with Analog 523 for 16 h (Figure 1E). We observed receptor activation at concentrations as low as 100 pM, and maximal reporter induction was achieved at approximately 100 μM (12–13-fold). This confirmed that Analog 523 activates AhR-mediated transcription and functions as an AhR ligand.
Figure 1.
Identification of Analog 523 as an AhR-dependent anti-cancer compound in triple-negative breast cancer. A) Western blot of AhR expression in vector control (AhR WT) and cr-AhR (AhR KO) MDA-MB-468 cells. B) Plot of 11-Cl-BBQ analogs screened in MDA-MB-468 cells with or without AhR expression, with bars representing AhR-dependent inhibition of cell viability following 72-hour exposure at 10 μM. C) Cell viability of MDA-MB-468 AhR-expressing and AhR KO cells treated with various concentrations of Analog 523 for 72 hours. D) Structures of 11-Cl-BBQ and BBQ (Analog 523). E) Induction of xenobiotic-response element reporter in Hepa-1 cells after 16 hours of treatment with Analog 523. Data represents mean ± standard deviation of three replicates, and results are representative of three independent experiments.
Characterization of AhR-Dependent Anticancer Effects Driven by Analog 523
Given the potent AhR-dependent activity of Analog 523, we chose to further characterize its anticancer effects on the MDA-MB-468 cell line. Short-term (24 h) treatment with 100 nM 523 induced an AhR-dependent S + G2/M phase arrest (Figure 2A), and long-term exposure to 10 nM 523 in a colony forming assay was sufficient to completely inhibit the clonogenicity of AhR-expressing cells while exhibiting a minimal impact on AhR-deficient cells (Figure 2B). Similarly, in the soft-agar clonogenicity assay, an in vitro measure of the metastatic potential, we observed that Analog 523 potently suppressed 3D colony formation at 10 nM (Figure 2C) in an AhR-dependent manner.
Figure 2.
AhR activation by Analog 523 induces cell cycle arrest and inhibits clonogenicity. A) Relative cell cycle distribution of MDA-MB-468 cells (AhR WT and KO) treated with 100 nM of Analog 523 for 24 hours. Cell cycle histogram is representative of at least three independent experiments, with mean +/- the standard error of the mean for three independent experiments plotted on the right. B) Two-dimensional colony forming assay in AhR-expressing and KO MDA-MB-468 cells treated with 10 nM of Analog 523. Right panel represents quantification of colony number. C) Three-dimensional soft-agar assay in AhR-expressing and AhR KO MDA-MB-468 cells treated with 10 nM of Analog 523. Left panel shows images acquired at 20X magnification of clones. Right panel shows quantification of number of clones per field relative to Vehicle control. Colony data represents mean ± standard deviation of three replicates, and results are representative of three independent experiments.
Analog 523 Induces AhR-Dependent Apoptosis and Growth Inhibition in Cancerous but Not Normal Cells
To determine the effectiveness of Analog 523 in three-dimensional cultures, a more physiologically relevant model of solid tumors, we formed spheroid cultures of MDA-MB-468 cells. We exposed spheroids to Analog 523 for 72 h and observed strong, AhR-dependent growth inhibition as measured by viability assays. A comparison of the dose–response curves from two-dimensional (Figure 1C) and spheroid viability assays (Figure 3A) revealed some loss of efficacy in spheroid culture at 0.3 μM, while treatment with 1 μM achieved equivalent inhibitory effects. We next sought to determine whether Analog 523 could induce apoptosis by staining of phosphatidylserine (PS) eversion via fluorophore-conjugated Annexin-V and subsequent quantification by flow cytometry. Annexin-V staining of AhR-expressing and AhR KO cells exposed to Analog 523 revealed potent AhR-dependent induction of apoptosis (Figure 3B). To determine whether AhR activation in normal mammary epithelial cells would induce any undesired cytotoxicity, we utilized both primary human mammary epithelial cells (HMECs) and the nonmalignant mammary epithelial line MCF10A to evaluate if Analog 523 inhibited cell viability. Treatment with concentrations between 0.01 and 10 μM did not induce any cytotoxicity, while treatment with 10 μM 523 minorly inhibited cell viability (∼20%) (Figure 3C). MCF-10A cells express significant levels of AhR protein but exhibited reduced expression compared with the MDA-MB-468 cell line (Figure 3D). We also evaluated the effect of Analog 523 on HEK293T and normal primary human fibroblast viability, representing a nontumorigenic transformed cell line and noncancerous cells from a tissue distinct from mammary epithelial cells, respectively. Analog 523 treatment did not significantly inhibit growth of either cell type (Figure 3D). Together, these data supported Analog 523 acting as an anticancer AhR agonist, with minimal effects on normal breast epithelial cells.
Figure 3.
Analog 523 drives AhR-dependent apoptosis in cancerous, but not normal breast epithelial cells or non-tumorigenic cells. A) Left panel shows quantification of MDA-MB-468 spheroid cell viability (AhR WT and AhR KO) following exposure to Analog 523 for 72 hours. Right panel shows images of spheroids following exposure to Analog 523 for 72 hours. B) Quantification of apoptosis (Annexin-V staining positivity) induced by Analog 523 in AhR-expressing and AhR KO cells following 48-hour exposure. C) Quantification of cell viability in primary human mammary epithelial cells (HMEC) and non-malignant mammary epithelial cells (MCF10A) following 72-hour exposure to Analog 523. D) Western Blot of AhR expression in non-malignant MCF10A versus MDA-MB-468 cells. E) Quantification of cell viability in non-tumorigenic HEK293T cells, and primary normal human fibroblasts following 72-hour exposure to Analog 523. Annexin-V staining and non-tumorigenic cell viability experiments represent mean ± standard error of three experiments. All other data represents mean ± standard deviation of three replicates, and results are representative of three independent experiments.
Analog 523 Inhibits the Growth of Multiple TNBC Cell Lines
We next profiled the ability of Analog 523 to inhibit a panel of triple-negative breast cancer cell lines, in addition to ER-positive (MCF-7) and HER2+ (SKBR-3) breast cancer cells by the cell viability assay (Figure 4A). We found that the MDA-MB-468 cell line (basal A subtype), HCC1187 cells (basal A; immunomodulatory), and HCC70 cells (basal A) were highly sensitive (>50% inhibition at <0.5 μM) to 523 treatment. In addition, the HER2+ SKBR-3 cell line was also sensitive to Analog 523 at concentrations of >1 μM. The remaining cell lines profiled exhibited moderate or minimal sensitivity to Analog 523, with an inhibition of <50% at the concentrations tested. Treatment of HCC70 and HCC1187 cells with 1 μM Analog 523 for 48 h strongly induced apoptosis (Figure 4B,C), consistent with the sensitivity that we observed by viability assays.
Figure 4.
Profiling of Analog 523 in triple-negative breast cancer cell lines. A) Quantification of cell viability from a panel of triple-negative breast cancer cells exposed to Analog 523 for 72 hours. B) Measurement of apoptosis (Annexin-V positivity) induced by Analog 523 in HCC70 TNBC cells (Basal A subtype) after 48 hours of treatment. C) Measurement of apoptosis (Annexin-V positivity) induced by Analog 523 in HCC1187 TNBC cells (Basal A subtype) after 48 hours of treatment. Annexin-V data represents mean +/- standard error of three independent replicates. Viability data represents mean ± standard deviation of three replicates and is representative of three independent experiments.
Analog 523 Suppresses TNBC Stem Cells via AhR
Cancer stem cells represent sources of proliferation, drug resistance, and metastasis in triple-negative breast cancers.40−43 We sought to determine the effects of Analog 523 on cancer stem cells (CSCs) isolated from triple-negative patient-derived breast cancer xenografts.44 In these cells, designated ST1 and ST2, we first determined the levels of AhR expression in these populations and found that both expressed high levels of AhR, with the ST2 clone expressing much greater levels than the ST1 clone (Figure 5A). We next determined whether the AhR pathway was functional in these cells. Treatment with the prototypical AhR ligand TCDD induced nuclear translocation of AhR, as determined by immunocytochemical staining of AhR in ST2 cells treated with a vehicle or 1 nM TCDD (Figure 5B). We confirmed that AhR was transcriptionally active in these cells by measurement of AhR target genes CYP1A1, CYP1B1, and NQO1, following treatment with 1 nM TCDD (Figure 5C).
Figure 5.
Analog 523 inhibits TNBC stem cell growth via AhR. A) Western Blot of AhR expression in two isolated populations of TNBC stem cells (ST1/ST2). B) Immunocytochemical staining of AhR in ST2 cells treated with the AhR ligand TCDD to induce receptor nuclear translocation. C) RT-qPCR of AhR target genes in ST1 and ST2 cells shows functional AhR transcriptional activity. D) Cell viability of ST1 and ST2 cells treated with Analog 523 for 48 hours. E) Western Blot of AhR expression in ST2 cells transfected with scramble siRNA or siRNA against AhR transcript. F) Cell viability of ST2 cells treated with Analog 523 for 24 hours after siRNA-mediated knockdown of AhR. Knockdown is representative of at least two experiments (See Supplemental Figure S1). All other data represents mean ± standard deviation of three replicates.
We next determined whether Analog 523 inhibited the growth of these cells and found that while both the ST1 and ST2 cells were sensitive to Analog 523, ST2 cells were far more sensitive, with 100 nM Analog 523 producing >50% inhibition of viability (Figure 5D). The greater sensitivity of the ST2 cells to Analog 523 is consistent with these cells expressing much higher levels of AhR relative to the ST1 counterparts. We next sought to determine whether the inhibitory effects observed were dependent on AhR. Small-interfering RNA (siRNA) was used to knockdown AhR expression (Figure 5E and Figure S1). Transfection with scrambled siRNA or siRNAs against AhR both negatively affected cell health and protein levels relative to untransfected cells (Figure 5E). We found that ST2 cells transfected with scrambled siRNA controls were more sensitive to treatment with 0.1 or 1 μM 523 relative to those with AhR expression knocked down (siAhR) (Figure 5F), demonstrating that Analog 523 acts via AhR in these cells.
Transcriptional Profiling of Analog 523 in TNBC Cells
To determine the transcriptional programs downstream of AhR following activation by Analog 523, we treated MDA-MB-468 AhR-expressing and MDA-MB-468 AhR KO cells with 250 nM 523 and collected samples at 4 and 12 h post-treatment for global gene expression profiling. We chose 4 h as a time point that would capture a significant fraction of the early, direct AhR-dependent transcriptional targets induced by Analog 523. Most of the fraction of cytosolic AhR has translocated to the nucleus following 2–3 h of ligand treatment with Analog 523 (Figure 5B). We chose 12 h post-treatment as a secondary time point to capture both primary and early secondary responses. AhR-expressing cells treated with Analog 523 for 4 and 12 h shared a core set of 107 differentially expressed, upregulated genes at both 4 and 12 h (overlap p-value = 1 × 10–156) (adjusted p-value < 0.05, log2(FC) > 1). At 4 and 12 h post-treatment, 148 and 157 genes were upregulated, respectively (Figure 6A). Profiling of pathways enriched 4 h after Analog 523 treatment identified networks related to nuclear receptor signaling, Wnt signaling, oxidative stress, and AhR-driven phase I metabolism. Enrichment of these pathways is consistent with previously reported functions of AhR signaling. For instance, disruption of Wnt signaling by AhR expression or activation has been previously reported22,45−47 as well as AhR-dependent induction NRF2 signaling.48,49 At 12 h post-treatment with Analog 523, enriched pathways included NRF2 signaling, Wnt signaling, and oxidation by cytochrome p450, consistent with the induced gene programs at 4 h post-treatment. In addition, we observed induction of gene targets regulated by p53 expression (Figure 6B).
Figure 6.
Transcriptomic profiling of up-regulated targets of Analog 523. A) Venn-Diagram of upregulated genes following Analog 523 treatment for 4 hours and 12 hours in MDA-MB-468 AhR-expressing but not AhR KO cells. Represented genes exhibited fold changes greater than 2 and had adjusted p-values <0.05. B) Bubble plot showing enriched Hallmark Gene Sets from the Molecular Signatures Database (MolSigDB) after 4- and 12 hours treatment with Analog 523. DEGS analyzed exhibited fold changes greater than 1.5 and adjusted p-values <0.05. C) Bubble plot displaying enriched pathways (WikiPathways) following 4- and 12 hours treatment with Analog 523. DEGS analyzed exhibited fold changes greater than 1.5 and adjusted p-values <0.05. D) Enriched Apoptosis Hallmark genes at 4 hours post-treatment with Analog 523. DEGS analyzed exhibited fold changes greater than 1.5 and adjusted p-values <0.05 E) Enriched p53 signaling Hallmark genes common to both 4- and 12 hours post-treatment with Analog 523. DEGS analyzed exhibited fold changes greater than 1.5 and adjusted p-values <0.05.
Hallmark gene sets derived from upregulated targets identified enriched signatures involved in apoptosis at 4 h post-treatment and the p53 signaling pathway (Figure 6C). Inspection of these targets identified proapoptotic mediators including BH3-only proteins such as BMF (4–5-fold), BIK (∼1.5-fold), and NOXA (∼1.5-fold) and mediators related to extrinsic apoptosis signaling such as TNFRSF12A (TWEAKR), IRF, and IL6 (Figure 6D). Also induced were stress-response genes such as GADD45α,50HMOX1,51 and SMAD7,52 which has antiproliferative effects in breast cancer. Induction of p53 transcriptional targets at 4 and 12 h revealed a common set of genes activated at both time points: IER3, PLK3, OSGIN1, LIF, FUCA1, SLC3A2, and HBEGF (Figure 6E). Immediate early response gene 3 (IER3) and oxidative stress-induced growth inhibitor 1 (OSGIN1) exhibited the highest inductions among these targets with greater than 6-fold and 4-fold increases at 12 h, respectively.
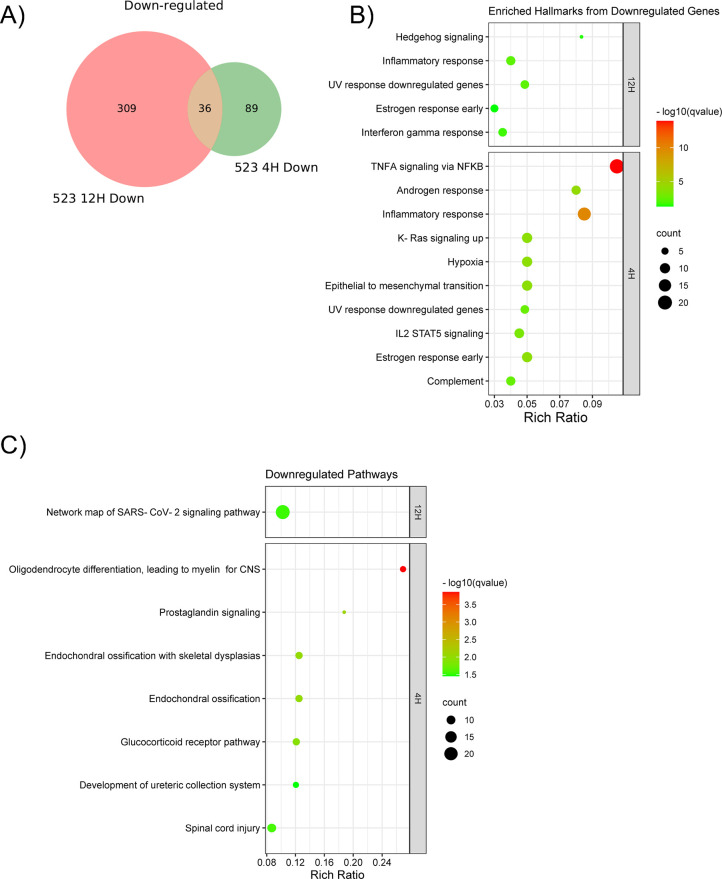
Activation of AhR by Analog 523 resulted in the downregulation of 89 genes at 4 h and 309 genes at 12 h, with a core set of 36 genes common to both time points (overlap p-value = 3.6 × 10–39) (adjusted p-value < 0.05, log2(FC) > 1) (Figure 7A). Gene networks downregulated 4 h post-treatment with Analog 523 included a diverse range of biological pathways involved in processes such as oligodendrocyte myelination, ossification, and glucocorticoid receptor signaling (Figure 7B,C). AhR-dependent control of genes related to these processes has been previously reported.53,54
Figure 7.
Transcriptomic profiling of down-regulated targets of Analog 523. A) Venn-Diagram of downregulated genes following Analog 523 treatment for 4 hours and 12 hours in MDA-MB-468 AhR-expressing but not AhR KO cells. Represented genes exhibited fold changes greater than 2 and had adjusted p-values <0.05. B) Bubble plot showing enriched Hallmark Gene Sets from the Molecular Signatures Database (MolSigDB) after 4- and 12 hours treatment with Analog 523. DEGS analyzed exhibited fold changes greater than 1.5 and adjusted p-values <0.05. C) Bubble plot displaying enriched pathways (WikiPathways) following 4- and 12 hours treatment with Analog 523. DEGS analyzed exhibited absolute fold changes greater than 1.5 and adjusted p-values <0.05.
Discussion
The aryl hydrocarbon receptor (AhR) can inhibit both the development of cancer24,55 and can be therapeutically targeted by select ligands that elicit tumor-suppressive gene programs.56,57 Tumor-suppressive signaling downstream of AhR has been observed in breast cancer31,34 and multiple other cancer types, including lung cancer,28 colon cancer,23,24 glioblastoma,58 medulloblastoma,59 hepatocellular carcinoma,60 melanoma,61,62 and leukemia.63,64 AhR expression and activation by exogenous ligands can suppress cancer growth through a variety of mechanisms, including the induction of cell cycle and cell death regulators, and the restriction of stemness programs.
We originally identified benzimidazoisoquinolines as high-affinity AhR ligands that are rapidly metabolized and do not exert toxicity in vivo.(37,38,65) In this context, these ligands as a class were shown to induce regulatory T-cells via AhR activation and prevent murine graft-versus-host disease.38,65 Analog 523 in these studies was found to be well-tolerated and retained bioactivity in vivo.(38) Building on our previous investigation of 11-Cl-BBQ as an AhR ligand with antiproliferative effects in lung cancer, in this study, we described the identification and characterization of the 11-Cl-BBQ derivative, Analog 523, as a proapoptotic ligand in TNBC.
Transcriptional profiling of the AhR-dependent response to Analog 523 in TNBC cells revealed the early induction of BH3-only proapoptotic Bcl-2 family members and different stress-response factors that can be regulated by the p53 signaling pathway (Figure 6D,E). We recently found that the loss of AhR in p53-deficient or p53-heterozygous backgrounds resulted in an increased tumor incidence and tumor spectrum when compared with AhR wild-type counterparts,55 suggesting a crosstalk between AhR and the p53 signaling pathway. This also indicates a role for AhR in suppressing oncogenic signaling upon p53 deletion. Previous studies indicate that AhR activation by nongenotoxic ligands can increase p53 expression,66 and ARNT, the heterodimer partner of AhR can modulate p53 signaling,67 indicating further crosstalk between these two pathways.
OSGIN1 represents a gene transcriptionally regulated by both NRF2 and p53 (Figure 6E) and is a stress-inducible target gene,68 which can functionally interact with p53.69 Similarly, PLK3 and GADD45α are two stress-inducible targets upregulated by Analog 523 treatment that are involved in the G2/M checkpoint70 and may contribute to the antiproliferative and proapoptotic effects that we observe in response to Analog 523 (Figure 2B). PLK3 can phosphorylate and activate p53 in response to various stress signals such as reactive oxygen species and hypoxia.71−73 MDA-MB-468 cells express only the mutant p53 with an R273H substitution, representing a gain-of-function mutation that supports their survival.74 The induction of wild-type p53 targets such as BMF and ATF3 by Analog 523 in these cells suggests that AhR can transcriptionally activate stress-response genes that are otherwise suppressed directly or indirectly in p53 mutant cells. For instance, in MDA-MB-468 cells, the R273H p53 mutant can indirectly repress BMF expression, and knockdown of mutant p53 drives BMF-dependent apoptosis.74 Similarly, different p53 mutants can repress ATF3 transcription, and notably, ATF3 can restore R273H p53 wild-type activity.75 The induction of p53 tumor suppressor gene targets via activating different stress-responsive transcription factors such as ATF3 and ATF4 is an area of active investigation,76,77 and it is possible that AhR-mediated growth inhibition operates via a similar mode of regulation. Notably, cancer cells expressing mutant R273H exhibit increased sensitivity to oxidative stress and ferroptosis, due in part to p53-dependent inhibition of system xc- transporter expression, which functions to facilitate glutathione production.90,91
We previously showed that BBQs including Analog 523 are well-tolerated and are biologically active in vivo at clinically achievable concentrations (10 mg/kg).38 A previous toxicity study determined a relatively high LD50 value of 660 mg/kg in rats,78 supporting a large therapeutic window. Apart from our studies, relatively little investigation has been done on BBQs as a chemical class regarding their anticancer activity. 10-Cl-BBQ and an analog NAP-6 were shown to possess anticancer activity in triple-negative breast cancer cells, and some of the important structural features governing activity were determined.79 In this respect, Baker et al. found that the naphthylene ring and ortho-disposed substituents on the N-phenyl ring were important for AhR functional activity.79
We previously identified the aryl hydrocarbon receptor as a novel anticancer target in multiple cancers including hepatocellular carcinoma,15,83 triple-negative breast cancer,16,60,93 melanoma,62 and nonsmall-cell lung cancer.39 In triple-negative breast cancer, we have previously identified three AhR ligands with proapoptotic effects including the clinically approved drug raloxifene,60 a novel raloxifene analog,93 and CGS-15943, a preclinical stage molecule originally designed as an adenosine receptor antagonist.16
Transient activation of AhR signaling by many ligands is not inherently toxic, and moreover, AhR represents an attractive drug target for many diseases.37,80 AhR agonists are currently being tested in clinical trials in the context of autoimmune diseases such as multiple sclerosis.81 The recent approval of the AhR ligand Tapinarof for the topical treatment of psoriasis supports the feasibility of translating AhR active compounds for cancer therapy.82 Furthermore, many approved drugs are in fact AhR agonists and hold repurposing potential as anticancer agents.60,62,83−85
Although an in-depth mechanistic understanding of the essential molecular targets downstream of AhR activation by Analog 523 remains to be determined, we demonstrate that Analog 523 acts as a potent AhR agonist with cancer cell-selective antiproliferative and proapoptotic activity. Analog 523 activates transcription of both intrinsic and extrinsic cell death mediators and induces p53 signaling in triple-negative breast cancer cells and TNBC stem cells. We propose Analog 523 as a candidate with high potential for clinical translation as an anticancer agent.
Methods
Luciferase Reporter Gene Assay Reporter gene assays were performed as previously described.87 Hepa1 cells were seeded in 96-well plates (100 uL media) and allowed to attach overnight. The following day, cells were treated with the indicated concentrations of Analog 523, Vehicle (0.1% DMSO) and positive controls (1 nM TCDD and 1 nM 11-Cl-BBQ) by adding 10 uL of each compound at 11X concentration. After 16 hours, cells were lysed by addition of 100 uL of Biotium Lysis Buffer (Fremont, CA, USA). 30 uL of lysate was removed and reporter activity was measured according to the manufacturer’s instructions using a Tropix TR717 microplate luminometer.
Viability Assay
Viability assays were performed by seeding cells at a density of 2000 cells per well in black 96-well plates and the following day (20 h post-treatment) treating with compounds for 48 or 72 h as indicated. Compounds were prepared as stock solutions at concentrations of 11X and 10 μL of the 11X compound added to 100 μL in wells. Viability was determined by the ATP-based CellTiter-Glo viability assay (Promega, Madison WI) according to the manufacturer’s instructions. Luminescence was measured using a Tropix TR717 microplate luminometer. The viability of treated cells was determined by relativizing to vehicle (0.1–0.3% DMSO)-treated wells. For profiling the activity of Analog 523 against the panel of triple-negative breast cancer cell lines, 72 h treatments are represented.
Annexin-V Staining
Cells were seeded in 6-well plates at a density of 300,000 cells/well, and the following day (20 h post-seeding), media was replaced with fresh media containing Analog 523 at indicated concentrations. Annexin-V staining was performed 48 h post-treatment by harvesting cells (floating and attached) with trypsin for 2 min followed by neutralization by addition of serum-containing media, spinning down cells at 2000 rpm, and two washes with ice-cold PBS. Cells were stained with Annexin-V-PerCP conjugates (eBioscience, Waltham, MA, USA) for 15 min on ice before washing out the residual stain (5 mL of cold PBS) and resuspending in 300 μL of 1X binding buffer (eBioscience, Waltham, MA, USA). Data were acquired with a CytoFLEX S flow cytometer (Beckman Coulter, Brea, CA) and analyzed using CytExpert software (Beckman Coulter). Gating to remove debris was performed, and Annexin-V positivity was determined by setting a gate where a distinct population was distinguishable with clearly increased fluorescence by comparison of the vehicle and positive controls. The percentage of cells Annexin-V positive within these gated regions was considered to indicate cells undergoing early and late-stage apoptosis. Ten thousand events were collected per sample, and at least three biological replicates were performed.92,93
Generation of CRISPR-Cas9 Knockout Cell Lines
One vector CRISPR/Cas9 pLentiCRISPR v2 expressing both Cas9, respective CRISPR guide RNAs targeting AhR, and puromycin resistance were purchased from GeneScript (Piscataway, NJ, USA). Lentiviral packaging was described previously.39 The three guide RNA sequences used in this study were as follows: AhR-1, 5′-AAGTCGGTCTCTATGCCGCT-3′; AhR-2, 5′-TTGCTGCTCTACAGTTATCC-3′; AhR-3, 5′-AATTTCAGCGTCAGCTACAC-3′. MDA-MB-468 paired vector control and AhR-deficient cells were generated by lipofectamine-based transfection with either pLentiCRISPR v2 or gRNAs targeting AhR followed by selection for 10 days with 0.5 μg/mL puromycin, before isolating clones and confirming the loss of AhR expression by Western blotting.
Compound Screening
Analogs of 11-Cl-BBQ were synthesized by Praxis Biotech. Screening was performed using MDA-MB-468 AhR-expressing and AhR KO cells previously described. Cells were seeded in 10% serum or 2% serum containing media with 1% pen/strep at a density of 2000 cells/well in 96-well plates. Twenty hours postseeding, cells were treated with 0.1, 1, or 10 μM of each analog (final DMSO concentration of 0.1–0.2%). After 48 and 72 h, cells were harvested. An ATP-based CellTiter-Glo assay kit (Promega, Madison, WI) was used for measuring the cell viability. To calculate the AhR dependence of compound-induced growth inhibition, the % inhibition observed in AhR KO cells was subtracted from the observed % inhibition for AhR WT cells.
Colony Assays
Cells were seeded at a density of 500 cells/well in triplicate in 6-well plates, and the following day (20 h post-treatment), cells were treated with compounds (addition of the 4X compound to existing media to minimize cell disturbance) and allowed to incubate for 2–3 weeks before fixation with 50% methanol/water solution containing 0.1% methylene blue overnight. Colonies were counted manually or with OpenCFU colony counting software. Images were acquired on a ChemiDoc imaging instrument.
Cell Cycle Analysis
Cells were seeded in media containing 10% FBS and 1% P/S at a density of 250,000 cells/well in 6-well plates. The following day (20 h post-treatment), cells were treated with 100 nM 523 for 20 h before harvesting cells by trypsinization, washing with PBS, fixation with 70% ice-cold ethanol while vortexing to minimize clumping, washing out of ethanol followed by permeabilization with 0.1% Triton in PBS, and staining with the Hoechst 33258 dye (1 μg/mL) (Invitrogen, Carlsbad, CA) for 20 min at room temperature. Following staining, the dye was washed out with PBS (5 mL) before resuspension in PBS and acquisition on a flow cytometer. Ten thousand events were captured per sample, and analysis of the singlet population was used for cell cycle distribution analysis. Manual gating was used to delineate the DNA content distribution.
Soft-Agar Assay
Agar solution (3%) was prewarmed (37 °C) until it was fully melted. Warm media (1 mL) was mixed with 0.5 mL of melted 3% agar solution and added into a 6-well plate. The 1% bottom agar layer was incubated at room temperature for 30 min to solidify. Cells were detached using trypsin and resuspended in media. The 0.3% top agar was prepared by mixing 0.45 mL of 1% agar medium with 1.05 mL of the cell suspension. The top layer was added on top of the bottom layer after it solidified. Cells were seeded at the density of 8 × 103 cells/well. After placing the top agar, plates were left at room temperature for 30 min for agar to solidify and then moved into an incubator. The treatments were added 24 h postseeding. The cells were incubated for 30 days. Medium with treatment was added every week, once a week. After 30 days of culture, colonies were stained with 0.02% methylene blue dissolved in 50% MeOH. After one day of incubation at 4 degrees Celsius, the stained colonies were imaged. Images were obtained at 20× and 40× magnification. The number of colonies in a 20× field was counted and used for quantification. Representative individual colonies were imaged at 40× for comparing the size and effect.
Cell Lines
All cell lines, except for the ST1 and ST2 cells, were purchased from ATCC and maintained in 5% CO2, at 37 degrees Celsius, in DMEM or RPMI media containing 10% FBS and 1% penicillin/streptomycin. All HCC cell lines were maintained in RPMI. All MDA cell lines were maintained in DMEM. ZR-75I cells were maintained in RPMI. SKBR3 cells were maintained in DMEM. MCF10A cells were maintained in DMEM/F12 medium with the following supplements added: 5% horse serum, 20 ng/mL epidermal growth factor, 0.5 mg/mL hydrocortisone, 100 ng/mL cholera toxin, 10 μg/mL insulin, and 1% pen/strep. Primary human mammary epithelial cells (HMECs) were obtained from Lonza Biosciences (Basel, Switzerland) and were maintained according to the specified culture protocols in Clonetics mammary epithelial basal medium (Lonza). Mouse Hepa-1 cells were kindly gifted by Dr. Michael Denison at the University of California, Davis, and were maintained in DMEM.
ST1 and ST2 cells were cultured in mammary epithelial growth medium (BioWhittaker) (Lonza), with the following supplements: epidermal growth factor (20 ng/mL) (Sigma), basic fibroblast growth factor (20 ng/mL) (BD Biosciences), heparin (4 μg/mL) (Sigma), B27 supplement (Invitrogen), penicillin/streptomycin (Omega), and gentamycin (35 μg/mL) (Sigma). Cells were gently passaged with Accutase solution. The isolation and characterization of the ST1 and ST2 lines have been previously described.86
Western Blotting
Lysates were prepared in ice-cold RIPA buffer containing protease inhibitors (Pierce protease inhibitor, cat. no. A32963) before addition of Laemmli buffer and boiling. Lysates were resolved on 10% SDS PAGE gels, transferred to PVDF membranes (25 volts for 25 min, semidry transfer), blocked for 1 h with 5% milk in Tris-buffered saline with 0.1% Tween-20, and incubated with primary antibodies at the indicated concentrations in blocking solution overnight at 4 degrees Celsius. Species-specific secondary antibodies conjugated to horseradish peroxidase were used to detect bound primary antibodies and were incubated for 1 h at room temperature (1:2000 in block). The following antibodies were used: anti-AhR (Enzo Life Sciences, 1:1000) and anti-GAPDH (sc-36502, 1:1000, Santa Cruz Biotechnologies) as a loading control.
Spheroid Viability Assay
Cells were trypsinized and resuspended with 10 μL of nanoshuttle per 100,000 cells. Cells were spun at 100g for 5 min then resuspended and spun again at the same speed. This was repeated twice. Cells were then seeded in a clear 96-well plate and placed on a magnetic printer for 24 h before treatment. Treatments were added at an 11X concentration to 100 μL volumes in the plate and allowed to incubate for 72 h before adding a CellTiter-Glo reagent to cells and performing the viability assay according to the manufacturer’s instructions.
RT-qPCR
Total RNA samples were isolated using an E.Z.N.A. total RNA kit (Omega Bio-Tek, Norcross, GA) following the manufacturer’s protocol. Total RNA (1 μg) was used to make cDNA using a transcriptor first strand cDNA synthesis kit (Roche, Basel, Switzerland) in a total volume of 20 μL. cDNA (0.5 μL) was used for each qPCR reaction with a FastStart Universal SYBR Green Master (Rox) (Roche) using standard thermal cycling conditions on a 7500 Fast real-time PCR system (ABI Applied Biosystems). Primers for CYP1A1, CYP1B1, and NQO1 were previously reported.87
Transcriptional Profiling by RNA-Seq
MDA-MB-468 AhR-expressing and AhR KO cells were seeded in 10 cm plates (3.5 million cells/plate) and allowed to attach overnight. The following day, media was replaced with fresh media containing 250 nM Analog 523 or vehicle (0.1% DMSO). Treatments were performed in triplicate. At 4 and 12 h post-treatment, samples were harvested by addition of 700 μL of TRK-lysis buffer and RNA isolation performed according to the manufacturer’s instructions (E.Z.N.A. total RNA kit, Omega Bio-Tek, Norcross, GA). Samples were quantified, and 260/280 and 260/230 values were inspected by NanoDrop. Samples were then sent for quality control, library preparation (poly-A enrichment), and sequencing at Novogene. Sequencing was performed with 150 bp paired-end reads to a depth of 20 million reads per cell. A docker RNA-seq pipeline (cplaisier/star_2_7_1a_grch38_p21; DOI: 10.5281/zenodo.5519663; PMID 34494550) was employed to align reads from FASTQ files to the genome using STAR V2.7.1a (PMID 23104886) and GENCODE genome build GRCh38 release 31 (PMID 30357393), and counts were tabulated using htseq-count (PMID 25260700). DESeq2 (PMID 25516281) was used for subsequent differential gene expression analysis.
Gene ontology (GO TERM) analysis and gene set enrichment analysis were used to determine the AhR-dependent biological processes and pathways downstream of Analog 523. Functional enrichment analysis using the g:Profiler analysis platform88 and gene set enrichment analysis by annotation with The Molecular Signatures Database (MSigDB v7.5.1)89 were used to determine pathways (WikiPathways) activated or repressed downstream of Analog 523 treatment (Figure 6B). GSEA with The Molecular Signatures Database (v7.4 MSigDB) was used to identify transcriptional signatures upregulated at 4 and 12 h post-treatment with Analog 523. Bubble plots were generated with SRPlot.
Immunocytochemistry
ST2 cells were seeded in on a coverslip in 24-well plate at a density of 60,000 cells/well (Thermo Fisher Scientific, Waltham, MA, USA) and after three days of incubation treated with the vehicle or 1 nM TCDD for 2 h before fixation (3.7% paraformaldehyde in PBS), permeabilization (0.1% Triton in PBS), and staining with an anti-AhR antibody (Enzo Life Sciences, Farmingdale, New York, USA) (1:400 in 1% bovine serum albumin/PBS). Following washing, an FITC-conjugated goat-antirabbit antibody (1:600) was incubated for 1 h before washing and mounting with ProLong Gold Antifade with DAPI (Invitrogen). Images were acquired by a cooled CCD camera connected to an Axiovert fluorescence microscope at 20× magnification.
siRNA Knockdown of AhR
Knockdown of AhR expression with siRNA was performed as previously described.16 Briefly, ST2 cells were plated in a gelatin-coated 96-well plate at a density of 2500 cells/well. The following day, cells were transfected with siRNAs (25 nM) against AhR or with scrambled controls for 48 h with a DharmaFect-1 reagent (Horizon Discovery, Waterbeach, United Kingdom). Cells were treated with Analog 523 for 24 h. Confirmation of reduction in AhR protein expression was performed by Western blotting, and the viability was assessed by the CellTiter-Glo viability assay (Promega, Madison, WI, USA).
Statistical Analyses
All data analyses were performed using GraphPad Prism software (San Diego, CA, USA). Bar graphs represent means ± standard deviation, as indicated. For siRNA knockdown experiments and experiments comparing treatments between AhR-expressing and AhR KO cells, two-way ANOVA was used for analysis followed by Tukey’s or Dunnett’s post hoc test. IC50 values were derived using curve fitting in GraphPad Prism software. The cell cycle plot represents the average of three independent experiments as indicated by each individual point. For comparison between Annexin-V positivity in HCC70 and HCC1187 cells following vehicle or Analog 523 treatment, statistical significance was evaluated using Student’s t-test followed by the Holm–Sidak test with a 95% confidence interval (α = 0.05). ns = not significant, P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Acknowledgments
We would like to thank all past and present members of the Kolluri laboratory for their contributions and Brenna L. Stevens, Rhand S. Wood, Kyla M. Guertin, and Jacob Kreitzer for providing excellent laboratory support and technical assistance. Sincere thanks to Drs. Joseph G. Christison, Nancy I. Kerkvliet, Craig B. Marcus, David E. Williams, Andrew B. Buermeyer, and Gonzalo Ureta for helpful conversations. Daniel J. Elson and Bach D. Nguyen were supported in part by the Harvey H. and Donna Morre Basic Cancer Research Fellowship Awards from the Linus Pauling Institute and ARCS Foundation Scholarship (DJE). Daniel J. Elson was supported by the National Institute of Environmental Health Sciences (NIEHS) Training Grant T32ES007060. This research was supported by the American Cancer Society (RSG-13-132-01-CDD), Centro Científico y Tecnológico Ciencia & Vida, FB210008, Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia de ANID (to SB), NIEHS grant number P30ES030287, The U.S. Department of Agriculture’s (USDA) National Institute of Food and Agriculture (NIFA) Multistate project ORE00108E, and the Oregon State University Accelerator Innovation and Development. The sponsors have no role in study design, conclusions, or submission of this manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00253.
(Figure S1) AhR-dependent effects of benzimidazoisoquinoline in TNBC stem cells (PDF)
Author Contributions
Screening and identification of Analog 523 was performed by D.J.E. and B.N. Reporter gene assays, viability assays, cell cycle, 2-day colony assays, apoptosis staining, HMEC culture, TNBC cell line screens, RNA-Seq sample treatment and isolation, and bubble plot visualization were performed by D.J.E. RNA-Sequencing data processing (analysis pipeline), functional enrichment, and generation of PCA, volcano plots, and Venn diagrams were performed by S.F.W. and C.L.P. H.S.J. and D.J.C. cultured and performed experiments with ST1 and ST2 cell lines generated in the laboratory of R.G.O. Analog 523 and 11-Cl-BBQ analogs were synthesized at Praxis Biotech (S.B. and S.C.). N.A.K. conducted experiments on spheroids, MCF10A cell culture, and TNBC cell screening. Y.Z. performed the soft-agar colony forming assay, and D.F. conducted experiments and analyzed data in noncancerous cells. S.K.K. was responsible for study design, supervision, project administration and funding acquisition.
The authors declare no competing financial interest.
Supplementary Material
References
- Bianchini G.; de Angelis C.; Licata L.; Gianni L. Treatment landscape of triple-negative breast cancer — expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. 10.1038/s41571-021-00565-2. [DOI] [PubMed] [Google Scholar]
- Yin L.; Duan J.-J.; Bian X.-W.; Yu S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P.; Cortes J.; Pusztai L.; McArthur H.; Kümmel S.; Bergh J.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- Eikesdal H. P.; Yndestad S.; Elzawahry A.; Llop-Guevara A.; Gilje B.; Blix E. S.; et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann. Oncol. 2021, 32, 240–249. 10.1016/j.annonc.2020.11.009. [DOI] [PubMed] [Google Scholar]
- Bardia A.; Mayer I. A.; Diamond J. R.; Moroose R. L.; Isakoff S. J.; Starodub A. N.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients with Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148. 10.1200/JCO.2016.70.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V.; Quintana F. J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- Tanos R.; Patel R. D.; Murray I. A.; Smith P. B.; Patterson A. D.; Perdew G. H. Aryl hydrocarbon receptor regulates the cholesterol biosynthetic pathway in a dioxin response element-independent manner. Hepatology. 2012, 55, 1994–2004. 10.1002/hep.25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. P.; Li H.; Mitchell K. A.; Joshi A. D.; Elferink C. J. Ah Receptor–Mediated Suppression of Liver Regeneration through NC-XRE–Driven p21Cip1 Expression. Mol. Pharmacol. 2014, 85, 533–541. 10.1124/mol.113.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L.; Quintana F. J.; Pot C.; Joller N.; Xiao S.; Kumar D.; et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010, 11, 854–861. 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. R.; Joshi A. D.; Elferink C. J. The Tumor Suppressor Kruppel-Like Factor 6 Is a Novel Aryl Hydrocarbon Receptor DNA Binding Partner. J. Pharmacol. Exp. Ther. 2013, 345, 419–429. 10.1124/jpet.113.203786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBerry C.; Gonzalez R. M. S.; Shryock N.; Dias A.; Aliberti J. SOCS2-Induced Proteasome-Dependent TRAF6 Degradation: A Common Anti-Inflammatory Pathway for Control of Innate Immune Responses. PLoS One 2012, 7, e38384 10.1371/journal.pone.0038384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C. F. A.; Khan E. M.; Leung P. S. C.; Gershwin M. E.; Chang W. L. W.; Wu D.; Haarmann-Stemmann T.; Hoffmann A.; Denison M. S. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-κB. J. Biol. Chem. 2014, 289, 1866–1875. 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S.; Soshilov A. A.; He G.; Degroot D. E.; Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011, 124, 1–22. 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri S. K.; Weiss C.; Koff A.; Göttlicher M. p27Kip1 induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999, 13, 1742–1753. 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell E. F. 3rd; Jang H. S.; Pearce M.; Kerkvliet N. I.; Kolluri S. K. The aryl hydrocarbon receptor is required for induction of p21Cip1/waf1 expression and growth inhibition by SU5416 in hepatoma cells. Oncotarget. 2017, 8 (15), 25211–25225. 10.18632/oncotarget.16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell E. F.; Jang H. S.; Liefwalker D. F.; Kerkvliet N. I.; Kolluri S. K. Discovery and Mechanistic Characterization of a Select Modulator of AhR-regulated Transcription (SMAhRT) with Anti-cancer Effects. Apoptosis. 2021, 26 (5-6), 307–322. 10.1007/s10495-021-01666-0. [DOI] [PubMed] [Google Scholar]
- Matikainen T.; Perez G. I.; Jurisicova A.; Pru J. K.; Schlezinger J. J.; Ryu H. Y.; et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 2001, 28, 355–360. 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Shah K.; Rao Maradana M.; Delàs M. J.; Metidji A.; Graelmann F.; Llorian M.; et al. Cell-intrinsic Aryl Hydrocarbon Receptor signalling is required for the resolution of injury-induced colonic stem cells. Nat. Commun. 2022, 13, 1827. 10.1038/s41467-022-29098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarino-Palma A.; González-Rico F. J.; Rejano-Gordillo C. M.; Ordiales-Talavero A.; Merino J. M.; Fernández-Salguero P. M. The aryl hydrocarbon receptor promotes differentiation during mouse preimplantational embryo development. Stem Cell Rep. 2021, 16, 2351–2363. 10.1016/j.stemcr.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. I.; Fan Y.; de Gannes M.; Wang Q.; Xia Y.; Puga A. Repression of the Aryl Hydrocarbon Receptor Is Required to Maintain Mitotic Progression and Prevent Loss of Pluripotency of Embryonic Stem Cells. Stem Cells 2016, 34, 2825–2839. 10.1002/stem.2456. [DOI] [PubMed] [Google Scholar]
- Romine K. A.; Nechiporuk T.; Bottomly D.; Jeng S.; Mcweeney S. K.; Kaempf A.; et al. Monocytic Differentiation and AHR Signaling as Primary Nodes of BET Inhibitor Response in Acute Myeloid Leukemia. Blood Cancer Discovery 2021, 2, 518–531. 10.1158/2643-3230.BCD-21-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Marín N.; Merino J. M.; Alvarez-Barrientos A.; Patel D. P.; Takahashi S.; González-Sancho J. M.; Gandolfo P.; Rios R. M.; Muñoz A.; Gonzalez F. J.; Fernández-Salguero P. M. Aryl Hydrocarbon Receptor Promotes Liver Polyploidization and Inhibits PI3K, ERK, and Wnt/β-Catenin Signaling. iScience. 2018, 4, 44–63. 10.1016/j.isci.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H.; Davidson L. A.; Fan Y.-Y.; Goldsby J. S.; Yoon G.; Jin U. H.; Wright G. A.; Landrock K. K.; Weeks B. R.; Wright R. C.; Allred C. D.; Jayaraman A.; Ivanov I.; Roper J.; Safe S. H.; Chapkin R. S. Loss of aryl hydrocarbon receptor potentiates FoxM1 signaling to enhance self-renewal of colonic stem and progenitor cells. EMBO J. 2020, 39, e104319 10.15252/embj.2019104319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H.; Davidson L. A.; Hensel M.; Yoon G.; Landrock K.; Allred C.; Jayaraman A.; Ivanov I.; Safe S. H.; Chapkin R. S. Loss of aryl hydrocarbon receptor promotes colon tumorigenesis in ApcS580/+; KrasG12D/+ mice. Mol. Cancer Res. 2021, 19, 771–783. 10.1158/1541-7786.MCR-20-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.; Boivin G. P.; Knudsen E. S.; Nebert D. W.; Xia Y.; Puga A. the Aryl Hydrocarbon Receptor Functions as a Tumor Suppressor of Liver Carcinogenesis. Cancer Res. 2010, 70, 212–220. 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz W. A.; Lin T. M.; Peterson R. E. The aryl hydrocarbon receptor (AhR) inhibits vanadate-induced vascular endothelial growth factor (VEGF) production in TRAMP prostates. Carcinogenesis 2008, 29, 1077–1082. 10.1093/carcin/bgn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz W. A.; Lin T. M.; Cardiff R. D.; Peterson R. E. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis 2006, 28, 497–505. 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- Nothdurft S.; Thumser-Henner C.; Breitenbücher F.; Okimoto R. A.; Dorsch M.; Opitz C. A.; et al. Functional screening identifies aryl hydrocarbon receptor as suppressor of lung cancer metastasis. Oncogenesis. 2020, 9, 102. 10.1038/s41389-020-00286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.-H.; Li C.-H.; Cheng Y.-W.; Lee C.-C.; Liao P.-L.; Lin C.-H.; et al. The inhibition of lung cancer cell migration by AhR-regulated autophagy. Sci. Rep. 2017, 7, 41927. 10.1038/srep41927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C.; Yang W. H.; Li C. H.; Cheng Y. W.; Tsai C. H.; Kang J. J. Ligand independent aryl hydrocarbon receptor inhibits lung cancer cell invasion by degradation of Smad4. Cancer Lett. 2016, 376, 211–217. 10.1016/j.canlet.2016.03.052. [DOI] [PubMed] [Google Scholar]
- Ahmed S.; Al-Saigh S.; Matthews J. FOXA1 Is Essential for Aryl Hydrocarbon Receptor–Dependent Regulation of Cyclin G2. Mol. Cancer Res. 2012, 10, 636–648. 10.1158/1541-7786.MCR-11-0502. [DOI] [PubMed] [Google Scholar]
- Marlowe J. L.; Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J. Cell. Biochem. 2005, 96, 1174–1184. 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- Ko C. I.; Puga A. Does the aryl hydrocarbon receptor regulate pluripotency?. Curr. Opin. Toxicol. 2017, 2, 1–7. 10.1016/j.cotox.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley E.; Callero M. A.; Berardi D. E.; Campbell P.; Rowland L.; Zylstra D.; et al. AhR ligand Aminoflavone inhibits α6-integrin expression and breast cancer sphere-initiating capacity. Cancer Lett. 2016, 376, 53–61. 10.1016/j.canlet.2016.03.025. [DOI] [PubMed] [Google Scholar]
- Hall J. M.; Barhoover M. A.; Kazmin D.; Mcdonnell D. P.; Greenlee W. F.; Thomas R. S. Activation of the Aryl-Hydrocarbon Receptor Inhibits Invasive and Metastatic Features of Human Breast Cancer Cells and Promotes Breast Cancer Cell Differentiation. Mol. Endocrinol. 2010, 24, 359–369. 10.1210/me.2009-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J.; de Iuliis G. N.; Tarleton M.; McCluskey A.; Sakoff J. A. (Z)-2-(3,4-dichlorophenyl)-3-(1H-pyrrol-2-yl)acrylonitrile exhibits selective antitumor activity in breast cancer cell lines via the aryl hydrocarbon receptor pathway. Mol. Pharmacol. 2018, 93, 168–177. 10.1124/mol.117.109827. [DOI] [PubMed] [Google Scholar]
- Ehrlich A. K.; Kerkvliet N. I. Is chronic AhR activation by rapidly metabolized ligands safe for the treatment of immune-mediated diseases?. Curr. Opin. Toxicol. 2017, 2, 72–78. 10.1016/j.cotox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punj S.; Kopparapu P.; Jang H. S.; Phillips J. L.; Pennington J.; Rohlman D.; O’Donnell E.; Iversen P. L.; Kolluri S. K.; Kerkvliet N. I. Benzimidazoisoquinolines: A new class of rapidly metabolized Aryl hydrocarbon Receptor (AhR) ligands that induce AhR-dependent tregs and prevent murine graft-versus-host disease. PLoS One 2014, 9 (2), e88726 10.1371/journal.pone.0088726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B. D.; Stevens B. L.; Elson D. J.; Finlay D.; Gamble J. T.; Kopparapu P. R.; Tanguay R. L.; Buermeyer A. B.; Kerkvliet N. I.; Kolluri S. K. 11-Cl-BBQ is a Select Modulator of AhR-regulated Transcription that Suppresses Lung Cancer Cell Growth via p53 and p27Kip1. The FEBS Journal 2023, 290, 2064–2084. 10.1111/febs.16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. A.; Bhakta N. R.; Kessenbrock K.; Prummel K. D.; Yu Y.; Takai K.; et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526, 131–135. 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeth G.; Bendahl P. O.; Ringnér M.; Saal L. H.; Gruvberger-Saal S. K.; Lövgren K.; Grabau D.; Fernö M.; Borg A.; Hegardt C. The CD44 + /CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008, 10, R53. 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.; Lee H. J.; Saha S.; Ruan D.; Guo H.; Chan C. H. Inhibition of USP2 eliminates cancer stem cells and enhances TNBC responsiveness to chemotherapy. Cell Death Dis. 2019, 10 (4), 285. 10.1038/s41419-019-1512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurani H.; Razavipour S. F.; Harikumar K. B.; Dunworth M.; Ewald A. J.; Nasir A.; et al. DOT1L Is a Novel Cancer Stem Cell Target for Triple-Negative Breast Cancer. Clin. Cancer Res. 2022, 28, 1948–1965. 10.1158/1078-0432.CCR-21-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro D. J.; Maurer J.; Hebbard L.; Oshima R. G. ROCK1 Inhibition Promotes the Self-Renewal of a Novel Mouse Mammary Cancer Stem Cell. Stem Cells 2013, 31, 12–22. 10.1002/stem.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew L. K.; Sengupta S. S.; Ladu J.; Andreasen E. A.; Tanguay R. L. Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. FASEB J. 2008, 22, 3087–3096. 10.1096/fj.08-109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew L. K.; Simonich M. T.; Tanguay R. L. AHR-dependent misregulation of Wnt signaling disrupts tissue regeneration. Biochem. Pharmacol. 2009, 77, 498–507. 10.1016/j.bcp.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metidji A.; Omenetti S.; Crotta S.; Li Y.; Nye E.; Ross E.; Li V.; Maradana M. R.; Schiering C.; Stockinger B. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity 2018, 49, 353–362.e5. 10.1016/j.immuni.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T. P.; Puga A.; Shertzer H. G. Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chem.-Biol. Interact. 2002, 141, 77–95. 10.1016/s0009-2797(02)00067-4. [DOI] [PubMed] [Google Scholar]
- van den Bogaard E. H.; Bergboer J. G. M.; Vonk-Bergers M.; van Vlijmen-Willems I. M. J. J.; Hato S.; van der Valk P. G. M.; Schröder J. M.; Joosten I.; Zeeuwen P. L. J. M.; Schalkwijk J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Invest. 2013, 123, 917–927. 10.1172/JCI65642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L.; Chen I. T.; Zhan Q.; Bae I.; Chen C. Y.; Gilmer T. M.; et al. Interaction of the p53-Regulated Protein Gadd45 with Proliferating Cell Nuclear Antigen. Science 1994, 266, 1376–1380. 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Meiller A.; Alvarez S.; Drane P.; Lallemand C.; Blanchard B.; Tovey M.; May E. p53-dependent stimulation of redox-related genes in the lymphoid organs of c-irradiated mice-identification of Haeme-oxygenase 1 as a direct p53 target gene. Nucleic Acids Res. 2007, 35, 6924–6934. 10.1093/nar/gkm824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt B. L.; Cao Y.; Redfern A. D.; Chi L. H.; Burrows A. D.; Roslan S.; et al. Activation of canonical BMP4-Smad7 signaling suppresses breast cancer metastasis. Cancer Res. 2020, 80, 1304–1315. 10.1158/0008-5472.CAN-19-0743. [DOI] [PubMed] [Google Scholar]
- Fernández M.; Paradisi M.; D’Intino G.; Del Vecchio G.; Sivilia S.; Giardino L.; Calza L. A single prenatal exposure to the endocrine disruptor 2,3,7,8-tetrachlorodibenzo-p-dioxin alters developmental myelination and remyelination potential in the rat brain. J. Neurochem. 2010, 115, 897–909. 10.1111/j.1471-4159.2010.06974.x. [DOI] [PubMed] [Google Scholar]
- Juricek L.; Carcaud J.; Pelhaitre A.; et al. AhR-deficiency as a cause of demyelinating disease and inflammation. Sci. Rep. 2017, 7, 9794. 10.1038/s41598-017-09621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. L.; Löhr C. V.; Nguyen B. D.; Buermeyer A. B.; Kolluri S. K. Loss of the aryl hydrocarbon receptor increases tumorigenesis in p53-deficient mice. Toxicol. Appl. Pharmacol. 2022, 454, 116191 10.1016/j.taap.2022.116191. [DOI] [PubMed] [Google Scholar]
- Kolluri S. K.; Jin U. H.; Safe S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513. 10.1007/s00204-017-1981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S.; Jin U. H.; Park H.; Chapkin R. S.; Jayaraman A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int. J. Mol. Sci. 2020, 21, 6654. 10.3390/ijms21186654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin U. H.; Karki K.; Cheng Y.; Michelhaugh S. K.; Mittal S.; Safe S. The aryl hydrocarbon receptor is a tumor suppressor–like gene in glioblastoma. J. Biol. Chem. 2019, 294, 11342–11353. 10.1074/jbc.RA119.008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarić N.; Selby M.; Ramaswamy V.; Kool M.; Stockinger B.; Hogstrand C.; Williamson D.; Marino S.; Taylor M. D.; Clifford S. C.; Basson M. A. The AHR pathway represses TGFβ-SMAD3 signalling and has a potent tumour suppressive role in SHH medulloblastoma. Sci. Rep. 2020, 10, 1–16. 10.1038/s41598-019-56876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell E. F.; Koch D. C.; Bisson W. H.; Jang H. S.; Kolluri S. K. The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells. Cell Death Dis. 2014, 5, e1038. 10.1038/cddis.2013.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contador-Troca M.; Alvarez-Barrientos A.; Barrasa E.; Rico-Leo E. M.; Catalina-Fernández I.; Menacho-Márquez M.; et al. The dioxin receptor has tumor suppressor activity in melanoma growth and metastasis. Carcinogenesis 2013, 34, 2683–2693. 10.1093/carcin/bgt248. [DOI] [PubMed] [Google Scholar]
- O’Donnell E. F.; Kopparapu P. R.; Koch D. C.; Jang H. S.; Phillips J. L.; Tanguay R. L.; Kerkvliet N. I.; Kolluri S. K. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS One 2012, 7 (7), e40926 10.1371/journal.pone.0040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunaciu R. P.; Yen A. Activation of the aryl hydrocarbon receptor ahr promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 2011, 71, 2371–2380. 10.1158/0008-5472.CAN-10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunaciu R. P.; Jensen H. A.; MacDonald R. J.; LaTocha D. H.; Varner J. D.; Yen A. 6-Formylindolo(3,2-b)Carbazole (FICZ) Modulates the Signalsome Responsible for RA-Induced Differentiation of HL-60 Myeloblastic Leukemia. Cell 2015, 10, e0135668 10.1371/journal.pone.0135668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich A. K.; Pennington J. M.; Wang X.; Rohlman D.; Punj S.; Löhr C. V.; Newman M. T.; Kolluri S. K.; Kerkvliet N. I. Activation of the Aryl Hydrocarbon Receptor by 10-Cl-BBQ Prevents Insulitis and Effector T Cell Development Independently of Foxp3+ Regulatory T Cells in Nonobese Diabetic Mice. J. Immunol. 2016, 196, 264–273. 10.4049/jimmunol.1501789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M.; Hankinson O. 2,3,7,8-tetrachlorodibenzo-p-dioxin suppresses the growth of human colorectal cancer cells in vitro: Implication of the aryl hydrocarbon receptor signaling. Int. J. Oncol. 2019, 54, 1422–1432. 10.3892/ijo.2019.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella K. A.; Muro I.; Fang G.; Sarkar K.; Mendez O.; Wright C. W. Aryl hydrocarbon receptor nuclear translocator (ARNT) isoforms control lymphoid cancer cell proliferation through differentially regulating tumor suppressor p53 activity. Oncotarget 2016, 7 (10), 10710–10722. 10.18632/oncotarget.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. S.; Matos M. F.; Richter K. E.; Li B.; Scannevin R. H. The NRF2 transcriptional target, OSGIN1, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes. Sci. Rep. 2017, 7, 42054. 10.1038/srep42054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Yao H.; Gan F.; Tokarski A.; Wang Y. Interaction of OKL38 and p53 in Regulating Mitochondrial Structure and Function. PLoS One 2012, 7, e43362 10.1371/journal.pone.0043362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahassi E. M.; Myer D. L.; McKenney R. J.; Hennigan R. F.; Stambrook P. J. Priming phosphorylation of Chk2 by polo-like kinase 3 (Plk3) mediates its full activation by ATM and a downstream checkpoint in response to DNA damage. Mutat. Res. 2006, 596, 166–176. 10.1016/j.mrfmmm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Xie S.; Wang Q.; Wu H.; Cogswell J.; Lu L.; Jhanwar-Uniyal M.; et al. Reactive Oxygen Species-induced Phosphorylation of p53 on Serine 20 Is Mediated in Part by Polo-like Kinase-3. J. Biol. Chem. 2001, 276, 36194–36199. 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- Xie S.; Wu H.; Wang Q.; Cogswell J. P.; Husain I.; Conn C.; et al. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 2001, 276, 43305–43312. 10.1074/jbc.M106050200. [DOI] [PubMed] [Google Scholar]
- Li Z.; Niu J.; Uwagawa T.; Peng B.; Chiao P. J. Function of polo-like kinase 3 in NF-κB-mediated proapoptotic response. J. Biol. Chem. 2005, 280, 16843–16850. 10.1074/jbc.M410119200. [DOI] [PubMed] [Google Scholar]
- Lim L. Y.; Vidnovic N.; Ellisen L. W.; Leong C. O. Mutant p53 mediates survival of breast cancer cells. Br. J. Cancer 2009, 101, 1606. 10.1038/sj.bjc.6605335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.; Wang H.; Lu C.; Malmut S.; Zhang J.; Ren S.; et al. The Activating Transcription Factor 3 Protein Suppresses the Oncogenic Function of Mutant p53 Proteins. J. Biol. Chem. 2014, 289, 8947–8959. 10.1074/jbc.M113.503755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K.; Vu T. T.; Cook W.; Naseri M.; Zhan K.; Nakajima W.; et al. p53-independent Noxa induction by cisplatin is regulated by ATF3/ATF4 in head and neck squamous cell carcinoma cells. Mol. Oncol. 2018, 12, 788–798. 10.1002/1878-0261.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X.; Ahsan N.; Lulla A.; Lev A.; Abbosh P.; Dicker D. T.; et al. P53-independent partial restoration of the p53 pathway in tumors with mutated p53 through ATF4 transcriptional modulation by ERK1/2 and CDK9. Neoplasia. 2021, 23, 304–325. 10.1016/j.neo.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygiene and Sanitation (Gigiena i Sanitariya) (USSR). 1986, Vol. 51 ( (11), ), pp 89; https://pubchem.ncbi.nlm.nih.gov/compound/7H-Benzimidazo_2_1-a_benz_de_isoquinolin-7-one#section=Toxicity.
- Baker J. R.; Pollard B. L.; Lin A. J. S.; Gilbert J.; Paula S.; Zhu X.; et al. Modelling and Phenotypic Screening of NAP-6 and 10-Cl-BBQ, AhR Ligands Displaying Selective Breast Cancer Cytotoxicity in Vitro. ChemMedChem 2021, 16, 1499–1512. 10.1002/cmdc.202000721. [DOI] [PubMed] [Google Scholar]
- Chen J.; Haller C. A.; Jernigan F. E.; Koerner S. K.; Wong D. J.; Wang Y.; Cheong J. E.; Kosaraju R.; Kwan J.; Park D. D.; Thomas B. Modulation of lymphocyte-mediated tissue repair by rational design of heterocyclic aryl hydrocarbon receptor agonists. Sci. Adv. 2020, 6 (3), eaay8230 10.1126/sciadv.aay8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi G.; Dadon Y.; Sasson N.; Steinerman J. R.; Knappertz V.; Vollmer T. L.; et al. CONCERTO: A randomized, placebo-controlled trial of oral laquinimod in relapsing-remitting multiple sclerosis. Mult. Scler. 2022, 28, 608–619. 10.1177/13524585211032803. [DOI] [PubMed] [Google Scholar]
- Bissonnette R.; Gold L. S.; Rubenstein D. S.; Tallman A. M.; Armstrong A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. 10.1016/j.jaad.2020.10.085. [DOI] [PubMed] [Google Scholar]
- Koch D. C.; Jang H. S.; O’Donnell E. F.; Punj S.; Kopparapu P. R.; Bisson W. H.; Kerkvliet N. I.; Kolluri S. K. Anti-androgen flutamide suppresses hepatocellular carcinoma cell proliferation via the aryl hydrocarbon receptor mediated induction of transforming growth factor-β1. Oncogene 2015, 34, 6092–6104. 10.1038/onc.2015.55. [DOI] [PubMed] [Google Scholar]
- Jin U. H.; Lee S.-O.; Pfent C.; Safe S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer 2014, 14, 1–14. 10.1186/1471-2407-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin U. H.; Lee S. O.; Safe S. Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J. Pharmacol. Exp. Ther. 2012, 343, 333–341. 10.1124/jpet.112.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strietz J.; Stepputtis S. S.; Follo M.; Bronsert P.; Stickeler E.; Maurer J. Human Primary Breast Cancer Stem Cells Are Characterized by Epithelial-Mesenchymal Plasticity. Int. J. Mol. Sci. 2021, 22, 1808. 10.3390/ijms22041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell E. F.; Saili K. S.; Koch D. C.; Kopparapu P. R.; Farrer D.; Bisson W. H.; Mathew L. K.; Sengupta S.; Kerkvliet N. I.; Tanguay R. L.; Kolluri S. K. The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PLoS One 2010, 5 (10), e13128 10.1371/journal.pone.0013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudvere U.; Kolberg L.; Kuzmin I.; Arak T.; Adler P.; Peterson H.; et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon A.; Birger C.; Thorvaldsdóttir H.; Ghandi M.; Mesirov J. P.; Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. S.; Duong C. P.; Haupt S.; Montgomery K. G.; House C. M.; Azar W. J.; Pearson H. B.; Fisher O. M.; Read M.; Guerra G. R.; Haupt Y.; Cullinane C.; Wiman K. G.; Abrahmsen L.; Phillips W. A.; Clemons N. J. Inhibiting the system xC–/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat. Commun. 2017, 8, 14844. 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. R.; Oliveira T. G.; Hermann E. R.; Chowanadisai W.; Clarke S. L.; Montgomery M. R. Distinct TP53 Mutation Types Exhibit Increased Sensitivity to Ferroptosis Independently of Changes in Iron Regulatory Protein Activity. Int. J. Mol. Sci. 2020, 21, 6751. 10.3390/ijms21186751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce M. C.; Gamble J. T.; Kopparapu P. R.; O'Donnell E. F.; Mueller M. J.; Jang H. S.; Greenwood J. A.; Satterthwait A. C.; Tanguay R. L.; Zhang X.-K.; Kolluri S. K. Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells. Oncotarget 2018, 9 (40), 26072–26085. 10.18632/oncotarget.25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H.; Pearce M.; O’Donnell E.; Nguyen B.; Truong L.; Mueller M.; Bisson W.; Kerkvliet N.; Tanguay R.; Kolluri S. Identification of a Raloxifene Analog That Promotes AhR-Mediated Apoptosis in Cancer Cells. Biology 2017, 6 (4), 41. 10.3390/biology6040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.