Abstract
With the rapid success in the development of mRNA vaccines against COVID-19 and with a number of mRNA-based drugs ahead in the pipelines, mRNA has catapulted to the forefront of drug research, demonstrating its substantial effectiveness against a broad range of diseases. As the recent global pandemic gradually fades, we cannot stop thinking about what the world has gained: the realization and validation of the power of mRNA in modern medicine. A significant amount of research has now been concentrated on developing mRNA drugs and vaccine platforms against infectious and immune diseases, cancer, and other debilitating diseases and has demonstrated encouraging results. Here, based on the CAS Content Collection, we provide a landscape view of the current state, outline trends in the research and development of mRNA therapeutics and vaccines, and highlight some notable patents focusing on mRNA therapeutics, vaccines, and delivery systems. Analysis of diseases disclosed in patents also reveals highly investigated diseases for treatments with these medicines. Finally, we provide information about mRNA therapeutics and vaccines in clinical trials. We hope this Review will be useful for understanding the current knowledge in the field of mRNA medicines and will assist in efforts to solve its remaining challenges and revolutionize the treatment of human diseases.
Keywords: mRNA, vaccine, therapeutic, COVID-19, infectious disease, cancer
The COVID-19 mRNA vaccines were developed and approved at unprecedented speed and have demonstrated significant effectiveness against infections and acute COVID in the real world. Although the idea of using mRNA as a simple and promising way to deliver vaccines or therapeutic drugs had been around for decades before the onset of the recent global pandemic, the success of mRNA vaccines against COVID has created huge enthusiasm around this concept and significantly boosted development and applications of this class of medicines in other areas. As the pandemic gradually fades, we cannot stop thinking about what the world has gained in this chaos: the realization and validation of the power of mRNA in modern medicine.
Messenger RNA (mRNA) is the molecule that carries genetic information from DNA in the cell nucleus to the cytosol for synthesizing proteins by ribosomes. While most of conventional therapies work by binding and inhibiting hyperactive disease-causing proteins, mRNA therapies can restore protein activities for treating diseases caused by the loss of certain protein functions. Moreover, mRNA therapy is explicit, as defined by the nucleic acid sequence, and very unlikely to have an off-target effect. Compared to antibody or cell therapies, mRNA is also much easier to synthesize and purify on large scales. Another advantage is that mRNA is transient and does not enter the cell nucleus; therefore, it is very unlikely to cause any genetic mutations in cells.
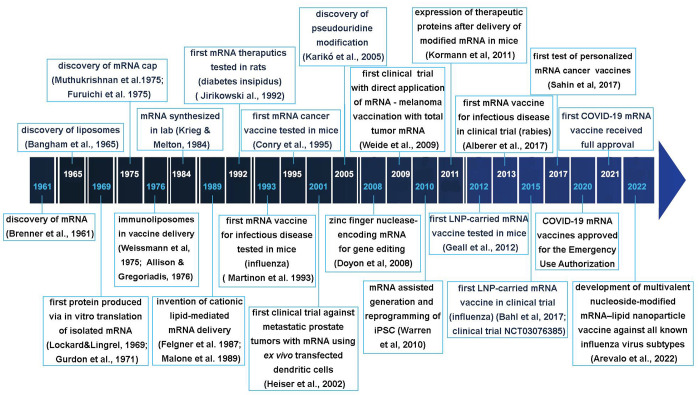
Many key research findings have contributed to the advancement of mRNA’s medical applications. Early research on mRNA’s stability and translational activity provided the foundation for developing mRNA-based vaccines and drugs. Comprehensive exploration of nucleic acids in the 1950s and 1960s brought the discovery of mRNA.1−3 Since then, mRNA has been the subject of systematic basic and applied research aimed at various diseases (Figure 1).
Figure 1.
A timeline of the milestone discoveries and key technologies leading to the successful development of mRNA therapeutics and vaccines; work from references (1, 4, 6, 9−13, 20−22, 24, 25, 28−45).
In the first decades after its discovery, the research was mainly focused on understanding the structural and functional aspects of mRNA and its metabolism in eukaryotic cells, in parallel with developing tools for mRNA recombinant engineering. Later, the 5′-cap on mRNA was discovered.4,5 In the 1980s, in vitro transcription from engineered DNA templates by means of a bacteriophage SP6 promoter and RNA polymerase led to the production of mRNA in cell-free systems.6 Although attempts in using liposomes to deliver mRNA into cells to induce protein expression date from the 1970s,7,8 the invention of cationic lipids9 was the decisive step in enabling nucleic acid transport into cells which resulted in the first cationic lipid-assisted mRNA delivery.9,10
In the 1990s, preclinical evaluation of in vitro mRNA transcription began for applications such as protein substitution and cancer and infectious diseases vaccinations.11−19 In 1992, a team of scientists working at Scripps Research Institute used mRNA to transiently reverse diabetes insipidus in rats.11 Albeit the concept of mRNA vaccines sounds relatively new, it was actually first suggested in 1995, for encoding cancer antigens.13 The accrued expertise was valuable in solving some of the obstacles associated with mRNA pharmaceuticals such as its short half-life and unfavorable immunogenicity.
In 2005, a solution was found on how to prevent activation of the immune response against the injected mRNA per se by inserting a naturally occurring modified nucleoside: pseudouridine.20 The invention of the pseudouridine modification and further exploration on mRNA led to the first human trial of a mRNA vaccine against melanoma in 2008.21 In the following years, numerous preclinical and clinical trials on mRNA-based vaccines against infectious diseases and cancer were completed.22,23 In 2009, the first trial on cancer immunotherapy using mRNA-based vaccines in human subjects with metastatic melanoma was conducted.21 In 2010, it was shown that pseudouridine-modified mRNA might be applied as a safe approach for effectively reprogramming cells to pluripotency.24 The first clinical trial of personalized mRNA-based cancer vaccine was performed in 2017.25 In 2021, a successful use of lipid nanoparticles (LNPs) comprising Streptococcus pyogenes Cas9 mRNA and a CRISPR guide RNA in patients with transthyretin amyloidosis with polyneuropathy was reported.26
Two human mRNA vaccines against COVID-19 received Emergency Use Authorization in 2020 and were finally approved in 2021.27−29 This was only brought about by decades of research on mRNA-based therapeutics. The lessons learned during the COVID-19 mRNA vaccines development were recently applied in formulating a multivalent nucleoside-modified mRNA flu vaccine.30
As it became clear that mRNA vaccines provide a promising alternative to conventional vaccine approaches due to their high efficiency, potential for rapid development, low-cost manufacture, and capacity for scale-up, as well as safe administration, significant efforts have been concentrated on developing mRNA drug and vaccine platforms against infectious diseases, cancer, and other debilitating diseases and have demonstrated encouraging results.
This Review provides a detailed overview of mRNA drugs and vaccines and considers future directions and challenges in advancing this promising platform to widespread therapeutic use. We examine data from the CAS Content Collection,46 the largest human-curated collection of published scientific information and analyze the publication landscape of recent research in order to reveal the research trends in published documents and to provide insights into the scientific advances in the area. We also discuss the evolution of the key concepts in the field, the major technologies, and their development pipelines with company research focuses, disease categories, development stages, and publication trends. We hope that this report can serve as a useful resource for understanding the current state of knowledge in the field of mRNA medicines and the remaining challenges to fulfill the potential of this new class of medicines.
Landscape of Scientific Publications Related to mRNA Therapeutics and Vaccines
Trend of mRNA Publications over Time
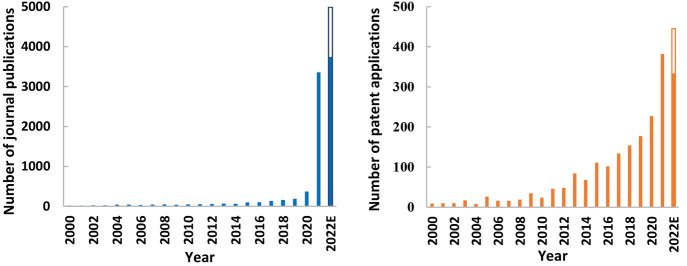
Based on the analysis of CAS document collection, a total of 9,322 research papers have been published in the field of mRNA therapeutics and vaccines. Due to the small number of published papers before 2000, the analysis of the development trend focused on those papers published since 2000. As shown in the left panel of Figure 2, the number of published papers in this area showed a slow growth prior to 2020 followed by a significant increase each year afterward. From 2000 to 2019, the annual number of publications in the global mRNA field was less than 200, and the growth in publication was relatively slow. Due to the impact of the novel coronavirus outbreak at the end of 2019, mRNA technology has attracted wide attention from researchers. After 2020, the number of published papers in this area has shown a rapid growth trend, with the number of published papers increasing to 3,361 in 2021 and nearly 5,000 in 2022.
Figure 2.
Annual number of published journal articles (left) and patents (right) on mRNA therapeutics and vaccines. The data for 2022 include extrapolated numbers for October to December 2022.
A total of 2,089 patents related to mRNA therapeutics and vaccines have been published worldwide, according to CAS document collection. Due to the small number of patents before 2000, the trend analysis also focused on the analysis of patents since 2000. As shown in the right panel of Figure 2, the number of patents each year grew slowly from 2000 to 2010 with some fluctuations, and the annual publications were all below 30. Between 2011 and 2019, the number of mRNA-related patents worldwide increased from 46 to 177 each year. Stimulated by the COVID-19 pandemic, the annual number of patents increased dramatically after 2020, increasing to 382 in 2021 and likely to nearly 450 in 2022. We also performed trend analyses for patents on mRNA therapeutic, mRNA vaccines and delivery systems separately. The results for those are described in the Supporting Information.
Distribution of Research Topics
Distribution of Topics in Journal Publications
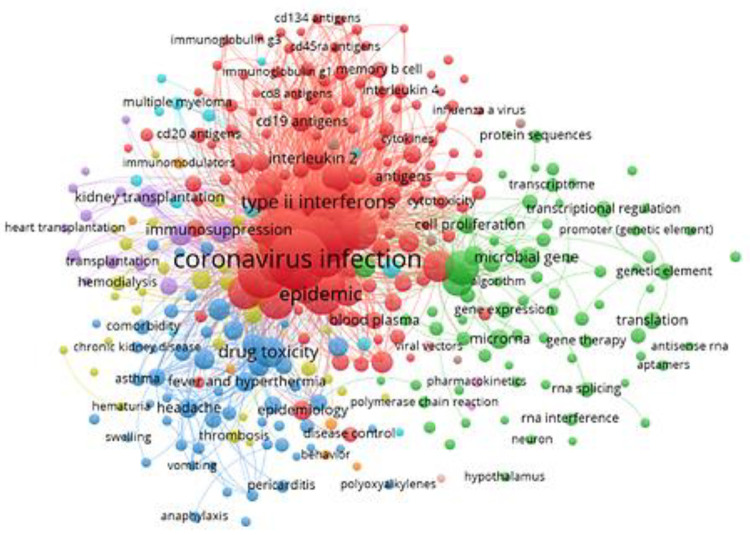
This report then examined CAS-indexed concepts in mRNA therapeutic and vaccine journal articles in order to reveal emerging trends or the more specific focus of research and development in this field (Figure 3). From the distribution of research topics based on the index concepts, it seems that the research has thus far focused on research in immunology, mechanisms of action, and disease indications. Among the immune research (red dots), key concepts include immunoglobulin G, viral spike glycoproteins, neutralizing antibodies, immunogenicity, etc. In terms of mechanism of action studies (green dots), key concepts include signal transduction, transcriptional regulation, genetic elements, RNA splicing, etc. In the treatment of diseases (blue dots), key concepts include diabetes, hypertension, myocarditis, and cardiovascular disease. In terms of indicator studies (yellow dots), key concepts include c-reactive protein, leukocyte, blood platelet, etc. Conceivably, due to the outbreak of the COVID pandemic and the development of mRNA vaccines against this disease, the cluster surrounding coronavirus infection accounts for a very large portion of journal publications.
Figure 3.
Concepts and their co-occurrence in journal articles related to mRNA therapeutics or vaccines.
Distribution of Topics in Patents
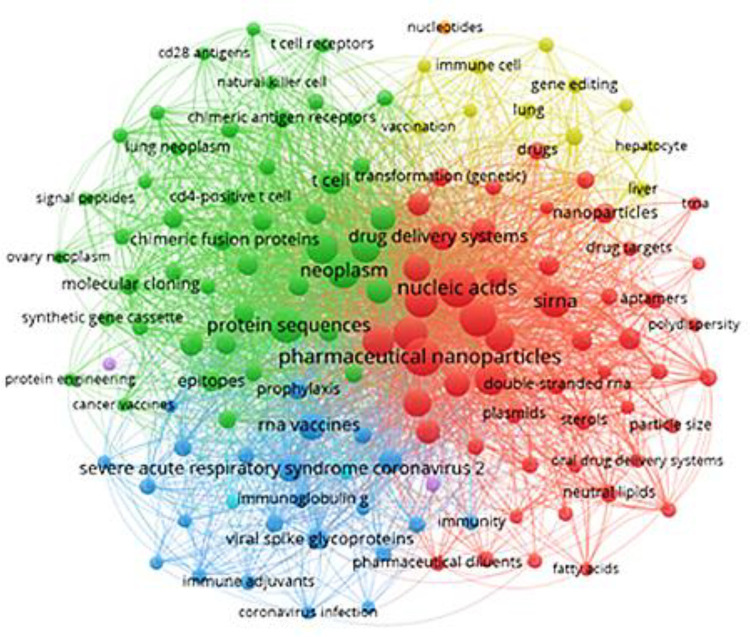
Key concepts in the field of mRNA therapeutics and vaccines were also examined with a concept cluster analysis for patents within the CAS Content Collection (Figure 4). Unlike journals, a significant portion of the patents focused on drug delivery such as nanoparticles (red dots) and immunotherapy in addition to SARS-CoV-2-related studies, etc. In terms of immunotherapy (green dots), key concepts include chimeric fusion proteins, chimeric antigen receptors, cancer immunotherapy, T cell receptors, etc. In other treatment aspects (blue dots), key concepts include RNA vaccines, immune adjuvants, etc. The high occurrence of all these terms reflects the emphasis of most R&D activities in this area.
Figure 4.
Concepts and their co-occurrence in patents related to mRNA therapeutics or vaccines.
Major Countries Contributing to the R&D of mRNA Therapeutics and Vaccines
Top Countries/Regions
Among the top 20 countries and regions in terms of scientific research output (Figure 5), the United States has the largest scientific research output in both journal articles and patents, with 29.8% and 45.9% of the world’s total output in these two types of publications, respectively, and significantly outnumbers those by other countries/regions. Germany ranks distantly the second in total scientific output, accounting for 7.4% of journal articles and 16.5% of patents. China ranks the third in scientific research output, with 6.8% of journal articles and 13.4% of patents. Italy, Japan, and the United Kingdom ranked fourth, fifth, and sixth, respectively, in total output.
Figure 5.
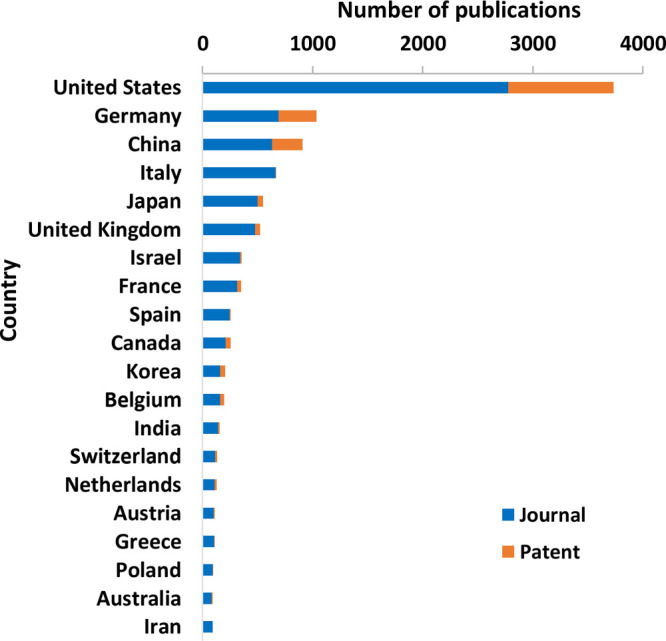
Major countries/regions with journal articles and patents related to mRNA therapeutics or vaccines.
Patent Output Trend over Time for Top Countries
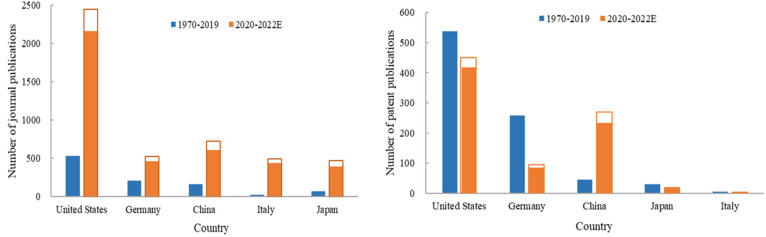
Figure 6 shows the contributions by the top five countries for the periods of 1970–2019 and 2020–2022 (the period following the outbreak of the COVID-19 pandemic). The U.S. is in the lead position in both periods for both journal and patent publications. The number of journal articles published by China from 1970 to 2019 was slightly lower than that of Germany, but increased rapidly during the period of 2020–2022, exceeding Germany during this period. The numbers of journal articles published by Italy and Japan were also small (<80) during 1970–2019 but increased significantly (>390) during 2020–2022 (left panel).
Figure 6.
Journal publications (left) and patent output (right) from the top five countries during 1970–2019 vs 2020–2022.
The numbers of patent applications by organizations in the U.S. and Germany during 1970–2019 were higher than those in 2020–2022, indicating the presence of sufficient R&D activities in these two countries before the onset of COVID-19 (Figure 6, right panel). While the numbers of patents from these two countries decreased during the three-year period of 2020–2022, the numbers were still higher than those of other countries, reflecting their leading roles. The trend of patent output from China appears to be different from that of other countries as indicated by the low activity during 1970–2019 and 5-fold increase during the period of 2020–2022, indicating that China is rapidly increasing its technological development in the mRNA therapeutics and vaccines due to the impact of the COVID-19 pandemic.
Patent Filing Strategies Revealed by Analysis of Patent Application Flows among Major Countries/Regions
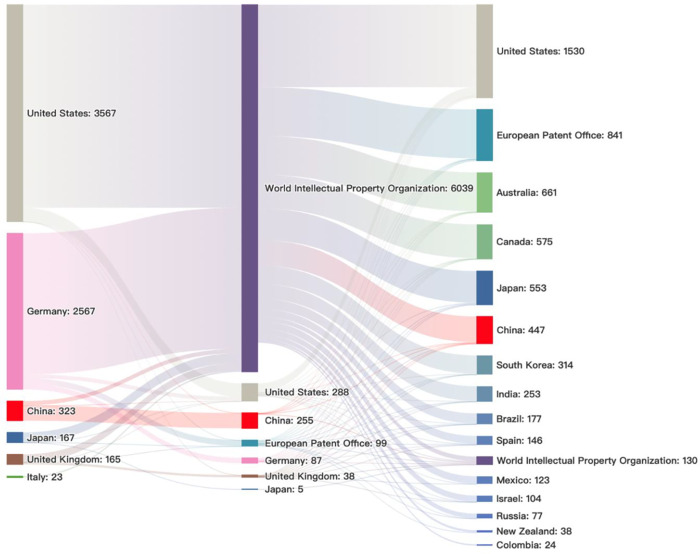
This report then examined the pattern of patent application flow related to technology development of mRNA therapeutics and vaccines. Our studies show that the U.S., Germany, the U.K., Japan, and Italy all have applied for technology protection in some overseas markets (Figure 7). In contrast, the majority of patents initiated by organizations in China were filed domestically to the Chinese Patent Office with some to the World Intellectual Property Organization, indicating that those Chinese organizations have placed less emphasis on seeking overseas protection of their intellectual properties. The U.S., Germany, the U.K., Japan, and Italy mainly distributed patents to other countries through the World Intellectual Property Organization. Among them, the number of patents distributed through the World Intellectual Property Organization in the U.S., Germany, Japan, and Italy accounted for more than 90%, and the number of patents distributed through the World Intellectual Property Organization in the U.K. accounted for more than 75.0%. The U.S. ranks first in the volume of patents distributed through the World Intellectual Property Organization, followed by Australia, Canada, Japan, and China. Countries such as the U.S., Australia, Canada, Japan, and China are also preferred when distributing patents through the European Patent Office.
Figure 7.
Flow of patent applications from the top six countries to patent offices around the world. Note: The column on the left is for home countries from which the patent assignees are located. The middle column represents the patent offices around the world that the priority patents in the patent families were filed to. The right column represents countries/regions where the intellectual properties are protected when those patents were applied through WIPO.
Major Organizations Contributing to the R&D of mRNA Therapeutics and Vaccines
Organizations in Journal Publications
Among the top 15 organizations in the world publishing mRNA journal articles in this area (Table 1), 12 are from the U.S., indicating that the U.S. has a predominant role in basic research. Israel, the U.K., and China each has one organization ranking among the top 15. In terms of the nature of the top 15 organizations, 11 are universities, 3 scientific research institutions, and 1 company. The top three are Harvard University, the University of California, and the University of Pennsylvania, with 108, 84, and 84 journal articles, respectively. Tel Aviv University in Israel ranks fourth globally, with 74 papers. Johns Hopkins University, Moderna, Washington University, the U.S. Centers for Disease Control and Prevention, and the U.S. National Institutes of Health all ranked in the top 10. The University of Oxford in the U.K. ranks eighth in the world, with 57 articles. It may be worth mentioning that the U.S. Centers for Disease Control and Prevention and Mount Sinai Hospital account for more than 94% of the published journal articles in 2020–2022, indicating the high level of dedication of these two organizations to this area in recent years, probably related to the effort on fighting the COVID-19.
Table 1. Top 15 Organizations Publishing Journal Articles on RNA Therapeutics or Vaccinesa.
| Ranking | Organization | No. of journal publications | Country | Organization type | No. of journal publications in the past 3 years (% of total) |
|---|---|---|---|---|---|
| 1 | Harvard University | 108 | U.S. | University | 91 (84.3%) |
| 2 | University of California | 84 | U.S. | University | 60 (71.4%) |
| 2 | University of Pennsylvania | 84 | U.S. | University | 52 (61.9%) |
| 4 | Tel Aviv University | 74 | Israel | University | 67 (90.5%) |
| 5 | Johns Hopkins University | 71 | U.S. | University | 58 (81.7%) |
| 6 | Moderna | 62 | U.S. | Company | 35 (56.5%) |
| 7 | Washington University | 58 | U.S. | University | 39 (67.2%) |
| 8 | University of Oxford | 57 | U.K. | University | 50 (87.7%) |
| 9 | Centers for Disease Control and Prevention | 55 | U.S. | Scientific research institution | 52 (94.5%) |
| 9 | National Institutes of Health | 55 | U.S. | Scientific research institution | 48 (87.3%) |
| 9 | Yale University | 55 | U.S. | University | 50 (90.9%) |
| 12 | The University of Hong Kong | 53 | China | University | 46 (86.8%) |
| 13 | Cornell University | 51 | U.S. | University | 45 (88.2%) |
| 13 | Mount Sinai Hospital | 51 | U.S. | Scientific research institution | 48 (94.1%) |
| 13 | Stanford University | 51 | U.S. | University | 35 (68.6%) |
Data from the CAS Content Collection.
Organizations in Patent Publications
Among the top 15 organizations with a high number of patents in this area (Table 2), the dominant organizations are mainly located in the U.S. (8 out of 15) followed by Germany (4 out of 15). Among these top patent applicants, companies are the main source of patents (11 out of 15). Thus, as expected, the major R&D effort of commercial companies has been focusing on the development of patentable technologies in this area. Moderna of the U.S. has produced most patents, followed by CureVac and BioNTech of Germany. The Chinese Academy of Science had the highest percentage of patents in the past three years, accounting for 70.6%, which may be indicative of an emphasis of R&D in this area by this organization in more recent years.
Table 2. Top 15 Organizations with Patent Applications on RNA Therapeutics or Vaccinesa.
| Ranking | Organizations | No. of patent applications | Country | Organization type | No. of patent applications in the past 3 years (% of total) |
|---|---|---|---|---|---|
| 1 | Moderna | 207 | U.S. | Company | 61 (29.5%) |
| 2 | CureVac | 150 | Germany | Company | 15 (10.0%) |
| 3 | BioNTech | 135 | Germany | Company | 59 (43.7%) |
| 4 | Translate Bio | 78 | U.S. | Company | 48 (61.5%) |
| 5 | Tron | 53 | Germany | Company | 11 (20.8%) |
| 6 | Alnylam Pharmaceuticals | 30 | U.S. | Company | 2 (6.7%) |
| 7 | Shire Human Genetic Therapies | 28 | U.S. | Company | 0 (0.0%) |
| 8 | University of Pennsylvania | 27 | U.S. | University | 13 (48.1%) |
| 9 | Arcturus Therapeutics | 23 | U.S. | Company | 8 (34.8%) |
| 10 | Acuitas Therapeutics | 21 | Canada | Company | 5 (23.8%) |
| 11 | Chinese Academy of Sciences | 17 | China | Scientific research institution | 12 (70.6%) |
| 12 | Massachusetts Institute of Technology | 16 | U.S. | University | 4 (25.0%) |
| 12 | University of California | 16 | U.S. | University | 6 (37.5%) |
| 14 | Ethris | 15 | Germany | Company | 2 (13.3%) |
| 15 | Evox Therapeutics | 14 | U.K. | Company | 4 (28.6%) |
Data from the CAS Content Collection.
Patent Distribution among Key Technologies
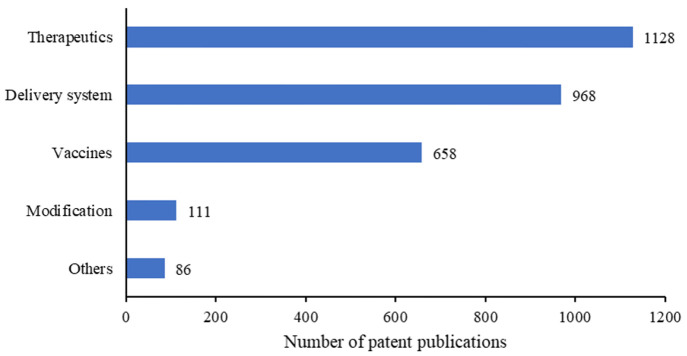
From the perspective of technology classification, mRNA patents mainly include therapeutic technology, delivery technology, vaccine technology, and mRNA modification technology (Figure 8). Out of 2,089 patents, 1,129 patents are related to the development of mRNA therapeutics for disease treatment, 977 related to development of delivery technology, and 659 related to vaccines. The total number is larger than 2,089 because some patents covered more than one specific technology area. The number of patent applications in these three types of technology account for 93.3% of the total patents, whereas the number of patents about mRNA modification technology is relatively small, though this technology is crucial to the success of mRNA vaccines and therapeutics.
Figure 8.
Distribution of patents among key classes of mRNA therapeutics and vaccines.
Analysis of Patents Related to mRNA Therapeutics
Top Countries/Regions with Published Patents Related to mRNA Therapeutics
Figure 9 shows the top 10 countries/regions where patent applicants in the field of mRNA therapeutics are located. The U.S. and Germany are the top two countries with 550 and 214 patents, respectively. The patents from these two countries account for about 67.7% of the total global patents in this area, reflecting the predominant role of these two countries in this area. China has filed 89 patents in this field, ranking distantly third.
Figure 9.
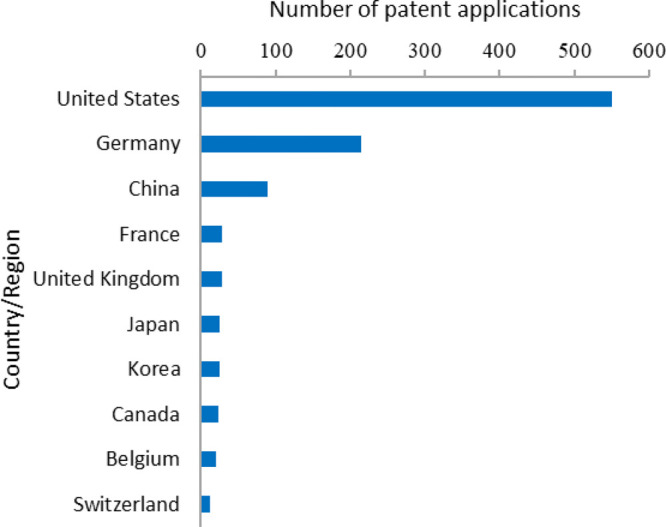
Distribution of the top 10 countries/regions in the global field of mRNA therapeutic patents.
Top Organizations with Published Patents Related to mRNA Therapeutics
Table 3 lists the top 11 organizations with patents related to mRNA therapeutics. As can be seen from the table, most of these organizations are in the U.S. (six) and Germany (four) with one in the U.K. Most of these patent applicants are commercial companies (nine) and only two are universities, indicating a heavy role of commercial companies in leading the R&D effort on mRNA therapeutics. The top five companies are Moderna, BioNTech, CureVac, Translate Bio, and Tron, with Moderna holding the largest number of patents (121).
Table 3. Top Patent Applicants for the Development of mRNA Therapeuticsa.
| Ranking | Organizations | No. of patent applications | Country | Organization type |
|---|---|---|---|---|
| 1 | Moderna | 121 | U.S. | Company |
| 2 | BioNTech | 92 | Germany | Company |
| 3 | CureVac | 79 | Germany | Company |
| 4 | Translate Bio | 36 | U.S. | Company |
| 5 | Tron | 34 | Germany | Company |
| 6 | Shire Human Genetic Therapies | 22 | U.S. | Company |
| 7 | University of Pennsylvania | 16 | U.S. | University |
| 8 | The Ohio State University | 15 | U.S. | University |
| 9 | Arcturus Therapeutics | 13 | U.S. | Company |
| 10 | Ethris | 10 | Germany | Company |
| 10 | Evox Therapeutics | 10 | U.K. | Company |
| 10 | Massachusetts Institute of Technology | 10 | U.S. | University |
Data from the CAS Content Collection.
Notable Patents Related to R&D of mRNA Therapeutics
Table 4 highlights several of the most notable patents focused on the development of mRNA therapeutics.
Table 4. Notable Patents on mRNA Therapeutics.
| Patent number | Organization | Patent title |
|---|---|---|
| WO2013096709 | Moderna, USA | Increasing the viability or longevity of an organ or organ explant using modified mRNAs for proteins essential for organ survival |
| WO2015058069 | Moderna, USA | Polynucleotides for tolerizing cellular systems |
| WO2016201377 | Moderna, USA | Preparation of targeted adaptive vaccines for treatment of inflammatory disease, autoimmune disease and cancers |
| WO2017214175 | Moderna, USA | Modified RNA encoding VEGF-A in formulations for treatment of heart failure and other diseases |
| WO2018160540 | Sanofi, France; BioNTech, Germany | Therapeutic RNA and uses in treating solid tumor cancers |
| WO2018222890 | Arcturus Therapeutics, USA | Synthesis and structure of high potency RNA therapeutics |
| WO2019178006 | SQZ Biotechnologies Co., USA | Immunogenic epitope and adjuvant-modified T cells for intracellular delivery of tumor or exogenous antigen to enhance immune response against cancer and infection |
| WO2020056147 | Moderna, USA | Polynucleotides encoding glucose-6-phosphatase for the treatment of glycogen storage disease |
| WO2020097409 | Moderna, USA | Use of mRNA encoding OX40L in combination with immune checkpoint inhibitor to treat cancer in human patients |
| WO202011811 | Arcturus Therapeutics, USA | Compositions and methods for treating ornithine transcarbamylase deficiency |
| WO2020154189 | Sanofi, France | Therapeutic RNA for treatment of advanced stage solid tumor |
| WO2020227615 | Moderna, USA | Polynucleotides encoding methylmalonyl-CoA mutase for the treatment of methylmalonic acidemia |
| WO2020260685 | eTheRNA Immunotherapies, Belgium | Antitumor therapy comprising mRNA molecules encoding tumor-associated antigens and checkpoint inhibitors |
| WO2021021988 | Translate Bio, USA | Treatment of cystic fibrosis by delivery of nebulized mRNA encoding Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) |
| WO2021058472 | BioNTech and TRON, Germany | Combination treatment using therapeutic antibody and interleukin 2 (IL-2) |
| WO2021198157 | BioNTech, Germany | mRNA compositions (RiboMab) expressing claudin-18.2-targeting antibody and anticancer uses thereof |
| WO202120771 | Verve Therapeutics, USA | Base editing of ANGPTL3 and methods of using same for treatment of cardiovascular disease |
| WO2021214204 | BioNTech, Germany | RNA constructs and uses thereof |
| WO2022136266 | BioNTech, Germany | Therapeutic RNA for treating cancer |
Patent application WO2020097409 by Moderna, USA features methods for treating ovarian cancer, as well as other cancers such as solid tumors, lymphomas, and epithelial origin cancers, by administering mRNA encoding an OX40L polypeptide, also known as CD252. The disclosure also presents pharmaceutical composition for intratumoral administration comprising lipid nanoparticles with a mRNA encoding a human OX40L. The disclosure also features combination therapies, such as the use of mRNA encoding an OX40L polypeptide in combination with a checkpoint inhibitor, such as an anti-PD-L1 antibody.
Patent application WO2021198157 by BioNTech, Germany, provides RNA technologies for targeting Claudin-18.2 (CLDN-18.2) polypeptides. Such RNA technologies can be useful for the treatment of Claudin-18.2 pos. cancer, including biliary cancers, ovarian cancers, gastric cancers, gastroesophageal cancers, and pancreatic cancers. Noteworthy is a mRNA formulation encoding monoclonal IgG1, such as Zolbetuximab (Claudiximab).
Patent application WO2018160540 by Sanofi, France, and BioNTech, Germany, relates to the field of therapeutic mRNAs for the treatment of solid tumors, including medical preparation comprising mRNA encoding an IL-12sc protein, an IL-15 sushi protein, an IFNα protein, and a GM-CSF protein. The disclosed pharmaceutical formulations are for use in a method of preventing cancer metastasis.
Patent application WO2020260685 by eTheRNA Immunotherapies, Belgium, relates to combinations of mRNAs encoding CD40, caTLR4, and CD70 with mRNAs encoding tumor-associated antigens for use as therapeutic vaccine in the treatment of metastatic cancer patients, primarily with malignant melanoma disease, but also to other cancer types. The disclosed therapies may further encompass the administration of checkpoint inhibitors. The invention provides administration schemes focusing on administration of the therapeutic into lymph nodes, the so-called intranodal therapy.
Analysis of Patents Related to mRNA Vaccines
Top Countries/Regions with Published Patents Related to mRNA Vaccines
The patent applicants in the global mRNA vaccine field are mainly from the U.S., China, Germany, and countries and regions shown in Figure 10. The U.S., China, and Germany have 220, 144, and 134 patent applications respectively, making them the three countries with the strongest strength in this technology field. It needs to point out that China does not yet have a mRNA vaccine on market probably due to its late involvement in this area and thus the patent data here does not necessarily reconcile with the market data and correlate with the impact. Countries such as Belgium, the U.K., and France have less than 30 patent applications in this field.
Figure 10.
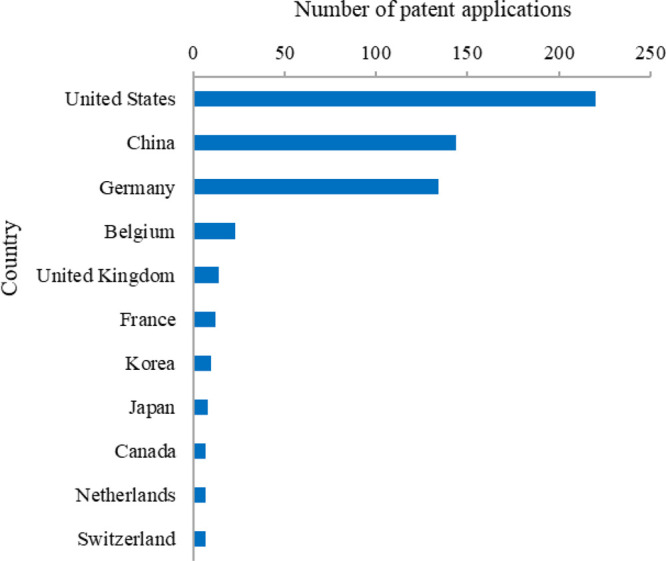
Distribution of mRNA vaccine patents among countries/regions with the largest numbers of patents.
Distribution of mRNA Vaccine Patents among Top Organizations
Table 5 lists the top 12 organizations in terms of mRNA vaccine patent output. Among them, five are from the U.S., three from Germany, two from China, and one each from the U.K. and Belgium. Like other technical fields, companies are the main contributors of mRNA vaccine patents (8 out of 12). The top 3 companies are CureVac (92), Moderna (59), and BioNTech (38).
Table 5. Global Patent Applications by Major Institutions in the Field of mRNA Vaccinesa.
| Order | Organization | No. of patent applications | Country | Organization type |
|---|---|---|---|---|
| 1 | CureVac AG | 92 | Germany | Company |
| 2 | Moderna | 59 | U.S. | Company |
| 3 | BioNTech | 38 | Germany | Company |
| 4 | TRON GmbH | 21 | Germany | Company |
| 5 | Chinese Academy of Sciences | 10 | China | Scientific research institution |
| 6 | GlaxoSmithKline Biologicals S.A. | 10 | U.K. | Company |
| 7 | Translate Bio | 7 | U.S. | Company |
| 8 | CanSino Biologics | 6 | China | Company |
| 8 | eTheRNA Immunotherapies | 6 | Belgium | Company |
| 8 | University of California | 6 | U.S. | University |
| 8 | University of Florida | 6 | U.S. | University |
| 8 | University of Pennsylvania | 6 | U.S. | University |
Data from the CAS Content Collection.
Most Notable Patents Related to mRNA Vaccine R&D
Over 650 patents related to mRNA vaccine development with their associated information were identified in this study. Tables 6 and 7 list several intriguing patents focused on the mRNA vaccines for infectious diseases and cancers, respectively, the two major disease classes for which mRNA vaccines are being developed.
Table 6. Notable Patents Focused on the Development of mRNA Vaccines for Infectious Diseases.
| Patent number | Organization | Patent title |
|---|---|---|
| WO2021213924 | BioNTech, Germany | Coronavirus RNA vaccine encoding SARS-CoV-2 spike protein for preventing COVID-19 |
| WO2020190750 | Moderna, U.S. Dept. of Health and Human Services, USA | Preparation of HIV Env- and lentivirus Gag protein-encoding mRNA VLP vaccine to induce broad-spectrum neutralizing antibodies for treating HIV infection |
| WO2018151816 | Moderna, USA | Immunogenic compositions for Zika virus including cationic lipid nanoparticles encapsulating mRNA having an open reading frame encoding a viral, bacterial or parasitic antigen, a pan HLA DR-binding epitope (PADRE) and a 5′ terminal cap modified to increase mRNA translation efficiency |
| WO2021155243 | Moderna, USA | Respiratory virus vaccine compositions |
| WO2013055905 | Novartis, Switzerland | Recombinant self-replicating polycistronic RNA molecules expressing multiple herpes virus proteins and their use in vaccines for inducing neutralizing antibodies |
| WO2021159040 | Moderna, USA | Engineering SARS CoV-2 mRNA vaccines expressing key neutralizing domains of spike protein, individually or in combination, for inducing protective immunity and immunotherapy |
| WO2021251453 | Daiichi Sankyo, University of Tokyo, Japan | Nucleic acid lipid particle vaccine encapsulated with severe acute respiratory syndrome coronavirus 2 messenger ribonucleic acid |
| WO2021159130 | Moderna, USA; U.S. Dept. of Health and Human Services, USA | Preparation of SARS CoV-2 mRNA vaccines encoding full-length spike protein variant, stabilized into a prefusion conformation, encapsulated in a lipid nanoparticle formulation |
| WO2021255270 | Ziphius Vaccines, Belgium; Universiteit Gent, Belgium | Self-amplifying COVID-19 RNA vaccine encoding SARS-CoV-2 Spike and Nucleocapsid protein antigen and alphavirus Nonstructural protein |
| WO2017070613 | Moderna, USA | Human cytomegalovirus RNA vaccines |
| WO2021204179 | Suzhou Abogen Biosciences, China | Nucleic acid vaccines for coronavirus |
| WO2021226436 | Translate Bio, USA; Sanofi, France | Optimized nucleotide sequences encoding SARS-COV-2 antigens |
| WO2017070623 | Moderna, USA | Herpes simplex virus RNA vaccine |
| WO2021160346 | Institut Pasteur, France | Nucleic acid vaccine against severe acute respiratory syndrome coronavirus SARS-CoV-2 |
| WO2022171182 | Stemirna Therapeutics, China | Vaccine reagent for treating or preventing coronavirus mutant strain |
| CA3132188 | Providence Therapeutics Holdings, Canada | Compositions and methods for the prevention and/or treatment of COVID-19 |
| WO2021183563 | Arcturus Therapeutics, USA | Coronavirus vaccine compositions and methods |
| WO2022150717 | Moderna, USA | Seasonal RNA influenza virus vaccines |
| WO2022129918 | Imperial College, UK Innovations Ltd, U.K. | Engineering a thermally stabilized self-amplifying RNA vaccine based on Venezuelan Equine Encephalitis virus backbone encoding SARS-CoV-2 spike glycoprotein encapsulated in lipid nanoparticle formulation for preventing and/or treatment of COVID-19 |
| WO2022178196 | Sanofi Pasteur, USA | Meningococcal B recombinant vaccine |
| WO2022137133 | CureVac, Germany; GlaxoSmithKline Biologicals SA, U.K. | RNA vaccine against SARS-CoV-2 variants |
| WO2022116528 | Suzhou CureMed Biomedical Technology, China | Circular RNA vaccine containing circular RNA and kit for detecting novel coronavirus neutralizing antibody |
| US20220325255 | University of Texas, USA | Compositions and methods for treating viral infections targeting TRIM7 |
Table 7. Notable Patents Focused on the Development of mRNA Vaccines for Cancer.
| Patent number | Organization | Patent title |
|---|---|---|
| WO2021155149 | Genentech, USA; BioNTech SE, Germany; F. Hoffmann-La Roche, Switzerland | Methods of inducing neoepitope-specific T cells with a PD-1 axis binding antagonist and an RNA vaccine |
| WO2015024664 | CureVac, Germany | Composition comprising mRNA encoding a combination of tumor antigens as vaccine for treating prostate cancer |
| WO2012019168 | ModernaTX, USA | Use of modified mRNA encoding melanocyte stimulating hormone, insulin and granulocyte colony-stimulating factor in prevention or treatment of disorders |
| WO2020097291 | ModernaTX, USA | Cancer vaccines comprising mRNA(s) encoding peptide epitopes (neoepitopes) and formulated as lipid nanoparticles |
| WO2020141212 | eTheRNA Immunotherapies NV, Belgium | mRNA vaccine |
| WO2022008519 | BioNTech SE, Germany; TRON – Translationale Onkologie Mainz, Germany | Therapeutic RNA for HPV-positive cancer |
| WO2015024666 | CureVac, Germany | RNA vaccine for treating lung cancer |
| WO2012159643 | BioNTech AG, Germany; TRON – Translationale Onkologie Mainz, Germany | Individualized vaccines for cancer |
| WO2015014869 | BioNTech AG, Germany; TRON – Translationale Onkologie Mainz, Germany | Determination of expression pattern of a set of tumor antigens including Cxorf61, CAGE1, PRAME and others to select cancer therapy regimen |
| WO2022009052 | Janssen Biotech, USA | Prostate neoantigens and their uses |
| WO2022081764 | RNAimmune, USA | Pan-ras mRNA cancer vaccines |
| WO2014082729 | BioNTech AG, Germany; Mainz Gemeinnuetzige GmbH, Germany | Individualized vaccines for cancer |
| WO2016180467 | BioNtech Cell & Gene Therapies, Germany; TRON – Translationale Onkologie Mainz, Germany | Enhancing the effect of car-engineered T cells by means of nucleic acid vaccination |
Patent application WO2021255270 (Ziphius Vaccines, Belg.; Universiteit Gent) discloses a self-amplifying COVID-19 vaccine comprising a mRNA encoding SARS-CoV-2 spike protein, nucleocapsid protein, and alphavirus nonstructural proteins (nsp1–4) in thermally stabilizing RNA vaccine formulation. Patent application WO2022129918 (Imperial College Innovations Limited, U.K.) discloses novel uses and methods for thermally stabilizing RNA vaccine formulations, including self-amplifying RNA replicons derived from Venezuelan Equine Encephalitis virus encoding SARS-CoV-2 spike protein encapsulated in lipid nanoparticles. The higher and prolonged in vivo translation improves the efficacy of self-amplifying RNA vaccines even in low dose.
Patent application WO2022137133 (CureVac AG and GlaxoSmithKline Biologicals) discloses that the mRNA vaccine encoding variants of highly immunogenic SARS-CoV-2 spike protein in lipid nanoparticle formulation induces neutralizing antibodies and immune cell responses against SARS-CoV-2.
Patent application WO2021155243 (Moderna) discloses a vaccine comprising a codon optimized human respiratory syncytial virus (hRSV) nucleic acid encoding a stabilized prefusion form of an hRSV F glycoprotein variant formulated in the lipid nanoparticles. In vivo study was conducted to evaluate the immunogenicity, efficacy, and safety of the mRNA vaccine in mice and the RSV cotton rat model.
Patent application WO2022116528 (Suzhou CureMed Biomedical Technology) discloses a circular RNA (circRNA) vaccine comprising a specific internal ribosome entry site (IRES) element and receptor domain of SARS-CoV-2 spike protein without 5′ or 3′ ends. The covalently closed structure of circRNA prevents the degradation by exonucleases and improves its biostability. They exemplified the further application for making circRNAs encoding erythropoietin, anti-PD1 antibody, interleukin 15, prostate cancer specific antigen PAP, and CD16 CAR receptor for protein expression in 293T cells.
Patent application US20220325255 (University of Texas) discloses antiviral compositions including mRNA encoding for a TRIM7 protein encapsulated into a lipid nanoparticle (LNP), as well as methods for impairing enterovirus replication for treating viral infections. The disclosure provides for the first time E3 ligase targeting an enterovirus protein and the first demonstration that a viral membrane remodeling protein is subject to degradation as a host antiviral strategy.
Patent application WO2021155149 by Genentech, BioNTech, and Hoffmann-La Roche AG discloses mRNA vaccines composed of mRNAs encoding up to 20 neoepitopes (two decatopes) deriving from cancer-specific mutations in patients formulated in cationic liposomes (Table 7). The first-in-human phase Ia and Ib studies of a mRNA vaccine as a monotherapy and in combination with atezolizumab were conducted in patients with advanced or metastatic solid tumors. They showed innate and neoantigen-specific immune responses induced by the mRNA vaccine alone and combined with atezolizumab.
Patent application WO2015024664 by CureVac discloses development of a mRNA-based personalized cancer vaccine encoding prostate cancer-associated antigens, prostate-specific antigen), PSMA (prostate-specific membrane antigen), PSCA (prostate stem cell antigen), STEAP (six transmembrane epithelial antigen of the prostate), MUC1 (mucin 1) and PAP (prostatic acid phosphatase) for treating prostate cancer.
Patent application WO2016180467 by BioNtech and TRON Translationale Onkologie Mainz discloses administering to the mammal T cells genetically modified to express a chimeric antigen receptor (CAR) targeted to antigen. Antigen is selected from claudin 18.2, claudin 6, CD19, CD20, CD22, CD33, CD123, mesothelin, CEA, c-Met, PSMA, GD-2, or NY-ESO-1. CAR-transgenic human CD8+ T cells targeting claudin-6 proliferated in response to CLDN6-transfected autologous dendritic cells. Murine CLDN6-CAR T cells were able to proliferate strongly in response to murine BMDCs expressing human CLDN6 antigen after RNA transfer.
Patent application WO2020097291 by Moderna discloses mRNA cancer vaccines composed of mRNAs encoding 3–50 neoepitopes formulated in cationic lipid nanoparticles. A phase I study was undertaken to assess the safety, tolerability, and immunogenicity of mRNA vaccine monotherapy in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. A randomized phase II clinical study was conducted in patients with resected cutaneous melanoma.
Analysis of Patents Related to mRNA Delivery Systems
Top Countries/Regions with Published Patents Related to mRNA Delivery Systems
Patent applications in the field of mRNA delivery systems are mainly from the U.S., China, Germany, and other countries, as shown in Figure 11. The U.S. has the largest number of patents (480), which equals to almost the combined number of patents from other countries.
Figure 11.
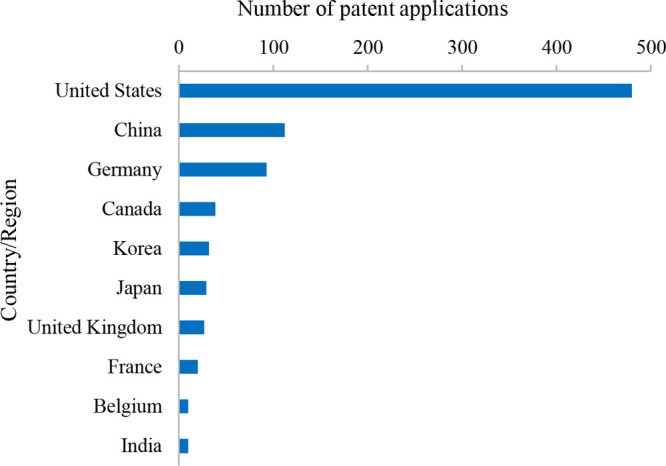
Distribution of patents among the top 10 countries/regions in the field of mRNA delivery systems.
Top Organizations with Published Patents Related to mRNA Delivery Systems
As shown in Table 8, like that in the field of mRNA therapeutics, global patent applicants in the field of mRNA delivery systems are primarily located in the U.S. (6 of top 10), Germany (2 out of 10)), Canada (1 out of 10), and the U.K. (1 out of 10. In terms of the nature of organizations, companies are the main source of these patents (9 out of 10), The top 5 patent applicants are Modena, Translate Bio, CureVac, Alnylam Pharmaceuticals, and BioNTech with Modena having the largest number of patents (64).
Table 8. Top Patent Applicants in the Field of mRNA Delivery Systemsa.
| Number | Organizations | No. of patent applications | Country | Organization type |
|---|---|---|---|---|
| 1 | Moderna | 64 | U.S. | Company |
| 2 | Translate Bio | 45 | U.S. | Company |
| 3 | CureVac AG | 30 | Germany | Company |
| 4 | Alnylam Pharmaceuticals | 24 | U.S. | Company |
| 5 | BioNTech | 22 | Germany | Company |
| 6 | Acuitas Therapeutics | 19 | Canada | Company |
| 7 | Shire Human Genetic Therapies | 15 | U.S. | Company |
| 8 | Massachusetts Institute of Technology | 13 | U.S. | University |
| 9 | Arcturus Therapeutics | 12 | U.S. | Company |
| 9 | Evox Therapeutics Ltd. | 12 | U.K. | Company |
Data from the CAS Content Collection.
Key Technology Layout in the Field of mRNA Modification
From the annual trend, the number of global patent applications in the field of mRNA modification fluctuates, with a significant increase and peak in 2013, followed by a decline (Supplemental Figure S3). The trend of patent applications was relatively stable from 2015 to 2018, and after a brief decline in 2019, the number of patent applications showed a slow growth trend from 2020.
From the distribution of patent research topics, the global field of mRNA modification focuses on drug delivery types, carrier materials, nucleic acid modification, mechanism research, and other aspects (Supplemental Figure S4). Among them, in terms of the type of administration, key concepts include intraperitoneal injections, pharmaceutical intravenous injections, subcutaneous injections, etc. With regard to carrier materials, nanoparticles are infused with cationic lipids, pharmaceutical nanoparticles, pharmaceutical liposomes, etc. Nucleic acid modifications include oligonucleotide analogs, nucleotide analogs, peptide nucleic acids, etc. In terms of mechanism research, key concepts include transcription, growth factors, membrane proteins, etc.
From the perspective of patent origin countries, the patentees in the global field of mRNA modification are mainly from the U.S., Germany, China, Japan, and France (Supplemental Figure S5). Among them, the U.S. filed the largest number of patents in the field, Germany and China are in second and third places, with Japan and France last filing a relatively small numbers of patents.
From the perspective of patent application institutions, global patent application institutions in the field of mRNA modification are mainly concentrated in the U.S. and Germany (Supplemental Table S1). Among the top 10 patent-filing organizations, six are from the U.S. and four are from Germany. From the perspective of institutional nature, companies are still the main body of patent application, accounting for about 70% of all applications. The top five patent filers were Modena, CureVac, BioNTech, Alnylam Pharmaceuticals, and Translate Bio, which also ranked among the top 5 in the field of mRNA modification.
Notable Patents on mRNA Delivery and Modification
RNAs, which are hydrophilic and negatively charged, cannot diffuse across cell membranes; thus, they require delivery vectors and/or chemical modification to reach their targets. mRNAs may also be quickly hydrolyzed by circulating RNases. As such, when administered systemically, RNA delivery systems need to protect the RNA against serum nucleases, bypass the undesirable immune reaction against mRNA per se, avoid nonspecific interactions with serum proteins, and block renal clearance.47 Thus, delivery vehicles and chemical modifications are of the utmost importance for the success of the mRNA therapeutics. Table 9 summarizes some notable patents disclosing essential advances in these areas.
Table 9. Notable Patents Focused on mRNA Delivery and Modification.
| Patent number | Organization | Patent title | Disclosure highlight |
|---|---|---|---|
| WO2013086373 | Alnylam Pharmaceuticals, USA | Lipids for the delivery of nucleic acids | LNP components for RNA delivery |
| WO2014093924 | Moderna, USA | Preparation, cytotoxicity, apoptosis, and transcription of modified nucleic acid molecules and uses thereof | Modification of mRNA |
| WO2016070166 | Arcturus Therapeutics, USA | Translatable messenger RNA analogs containing unlocked nucleomonomers and with prolonged in vivo half-lives for therapeutic uses | mUNA or mRNA analogs with unlocked nucleomonomers |
| US10808242 | BioNTech, Germany | Method for reducing immunogenicity of RNA by constructing A-rich and U-poor mRNA for use in therapy | mRNA modifications for decreasing nonspecific immunogenicity by mRNA itself |
| WO2017117528 | Acuitas Therapeutics, Canada | Preparation of lipids and lipid nanoparticle formulations for delivery of nucleic acids | LNP components and formulation for nucleic acid delivery |
| WO2017176974 | The Ohio State University, USA | Biodegradable amino-ester nanomaterials for nucleic acid delivery | LNPs for delivery of RNAs including siRNA, miRNA, and mRNA |
| WO2017212009 | Curevac AG, Germany | Hybrid carriers comprising cationic peptide or polymer and lipidoid | A nucleic acid delivery system comprised of a cationic peptide or polymer and a lipidoid compound |
| US20180125989 | Translate Bio, USA | Imidazole cholesterol ester (ICE)-based lipid nanoparticle formulation for delivery of mRNA | Methods of formulating nucleic acid containing LNP |
| WO2018009838 | Rubius Therapeutics, USA | Compositions and methods related to therapeutic erythroid cell systems expressing exogenous RNA encoding a protein | Therapeutic erythroid cell systems expressing exogenous RNA encoding a protein |
| WO2018013525 | Translate Bio, USA | Nucleic acid conjugates and uses thereof | Conjugates comprising sugars, folates and cell-penetrating peptides for delivering mRNA |
| WO2019092145 | Evox Therapeutics, U.K. | Exosomes comprising RNA therapeutics | Methods for using extracellular vesicles to encapsulating nucleic acid-based therapeutics such as mRNA, circular RNA, miRNA, etc. |
| WO2020070040 | Johannes Gutenberg-University Mainz and BioNTech, Germany | RNA particles comprising polysarcosine | LNPs for delivering mRNAs |
| WO2020061367 | Moderna, USA | Preparation of compounds and lipid nanoparticle compositions for intracellular delivery of therapeutic agents | LNPs for drug delivery |
| WO2020097540 | Arbutus Biopharma Corp., Canada | Methods and lipid nanoparticles for delivering mRNA and siRNA in treatment of diseases | LNPs for mRNA delivery |
| WO2020263883 | Moderna, USA | Endonuclease-resistant messenger RNA and uses thereof | Chemically modified mRNA that increases mRNA stability |
| CN110747214 | Shenzhen Zhenzhi Medical Technology, China | Preparation of mRNA-antibody fusion molecule and its use for drug delivery | Preparation of antibody–mRNA fusion/conjugate with puromycin as the linker for targeted delivery of mRNA therapeutics. |
| WO2020160397 | Moderna, USA | Methods of preparing lipid nanoparticles | LNP formulation |
| WO2021001417 | BioNTech, Germany | RNA formulations suitable for therapy | Self-amplifying RNA formulated in various polymers |
| WO2021231854 | Moderna, USA | Lipid nanoparticle compositions comprising an mRNA therapeutic and an effector molecule | System that features a tethered molecule to further increase the level and/or activity of mRNA therapeutics formulated in LNP |
| WO2021257262 | Yale University, USA | Poly(amine-co-ester) polymers with modified end groups and enhanced pulmonary delivery | PEGlyated poly(amine-co-ester) polymers with modified end groups for enhanced delivery of mRNA to the lung by inhalation |
| WO2022032154 | Moderna, USA | Compositions for the delivery of payload molecules to airway | LNPs comprising payload molecules such as mRNA therapeutics to be delivered to airway cells |
| WO2016176330 | University of Pennsylvania, USA; Acuitas Therapeutics, Canada | Nucleoside-modified mRNAs encoding antigens for inducing an adaptive | Modified antigen mRNA delivered in LNP induced adaptive immune response without inducing innate immunity |
| WO2020191103 | Arcturus Therapeutics, USA | Method of making lipid-encapsulated RNA nanoparticles | Detailed method for making RNA-encapsulating LNP |
| WO2021250263 | eTheRNA Immunotherapie, Belgium | Lipid nanoparticles comprising ionizable lipid, phospholipid, sterol, PEG lipid and mRNA | LNP components for RNA delivery |
| WO2022175815 | Pfizer, USA | Methods of protecting RNA | Methods of protecting RNA against degradation and components comprising free amino acids for this purpose |
| WO2019246203 | University of Texas, USA | Lipid nanoparticle compositions for delivery of mRNA and long nucleic acids | Compositions for delivery of long nucleic acids (>80 nucleotides), such as mRNAs, including cationic ionizable lipid, phospholipid, PEGylated lipid, and a steroid |
| WO2022236093 | Carnegie Mellon University, USA | Lipid nanoparticle-mediated mRNA delivery to the pancreas | LNP composition for mRNA delivery to the pancreas containing: cationic helper lipid, cholesterol analog, PEG-based compound, ionizable lipidoid, and mRNA |
| US20230043677 | Oregon State University, USA | Inhalable therapeutics | Nanoparticles for mRNA delivery suitable for nebulization and/or delivering mRNA by inhalation |
| WO2022155598 | Tufts College and Brigham and Women’s Hospital, USA | Lipid nanoparticles for targeted delivery of mRNA | LNP composition for specific delivery of CRISPR-Cas9 mRNA to the lung or liver |
Patent application WO2013086373 by Alnylam Pharmaceuticals, USA, relates to novel cationic lipids that can be used in combination with other lipid components such as a neutral lipid, a sterol such as cholesterol, and a PEG-lipid conjugate capable of reducing aggregation, to form lipid nanoparticles with oligonucleotides, to facilitate the cellular uptake and endosomal escape, and to knockdown target mRNA both in vitro and in vivo. Exemplary LNP composition comprised 50% cationic lipid, 10% DSPC, 38.5% cholesterol, and 1.5% PEG-DMG (average PEG molecular weight of 2000).
Patent application WO2020160397 by Moderna, USA, provides methods of producing LNP formulations and the produced LNP formulations thereof. It reflects the recent efforts toward “bedside” and/or “point-of-care” formulations, whereby mRNA may be encapsulated within preformed vesicles that were prepared at an earlier date. This mode of production offers advantages in the context of clinical supply, as empty LNP vesicles may be produced and stored separately prior to recombination with mRNA in a clinical compound setting. Specifically, bedside formulations may promote increased stability, since mRNA and empty raw materials can be stored in separately optimized conditions. Process complexity and cost of goods may be reduced since the LNP preparation occurs independent of cargo, enabling a platform approach for multiple mRNA or active agent constructs. The empty LNP plus mRNA modality may be referred to as “post-hoc”. The concept of post hoc loading as described in the present invention may enable control and/or optimization of each step separately. Further, the post hoc loading may enable mRNA addition at timescales that enable point-of-care formulation, e.g., months or years following empty LNP production.
Patent application WO2017176974 by The Ohio State University, USA, relates to biodegradable amino-ester lipid nanoparticles for efficient delivery of RNAs including siRNA, miRNA, and mRNA. Provided are also compositions including an amino-ester lipid compound of the invention, a noncationic lipid, a PEG-lipid conjugate, a sterol, and an active agent such as mRNA, which can be used to correct a mutation in a genome. For example, mRNAs can be delivered to correct mutations that cause hemophilia due to mutations in the genes encoding Factor VIII (hemophilia A) or Factor IX (hemophilia B).
Patent application WO2019092145 by Evox Therapeutics, UK, pertains to extracellular vesicles (Evs), specifically exosomes, as delivery vehicles for nucleic acid-based therapeutics. The distinctive properties of the extracellular vesicles (Evs), and specifically their nanosized subgroup, the exosomes—their innate stability, low immunogenicity, biocompatibility, and good biomembrane penetration capacity—allow them to function as superior natural nanocarriers for efficient drug delivery and are currently viewed as the rising star in drug delivery.48 The nucleic acid therapeutics of the present invention are loaded into Evs using inventive engineering protein and nucleic acid engineering strategies to enhance loading into Evs and to facilitate release of the nucleic acid cargo molecules inside target cells.
Patent application US10808242 by BioNTech, Germany, is focused on decreasing immunogenicity of RNA. The provided methods for decreasing immunogenicity of RNA comprise modifying the nucleotide sequence of the RNA by reducing the uridine (U) content, by elimination of U nucleosides from the nucleotide sequence of the RNA and/or a substitution of U nucleosides by nucleosides other than U in the nucleotide sequence of the RNA. Using RNA having decreased immunogenicity allows administration of RNA as a drug to a subject, e.g., in order to obtain expression of a pharmaceutically active peptide or protein, without eliciting an immune response which would interfere with therapeutic effectiveness of the RNA or induce adverse effects in the subject.
Patent application WO2020069718 by Johannes Gutenberg-University Mainz and BioNTech, Germany, relates to RNA particles for delivery of RNA to target tissues after parenteral administration and compositions comprising such RNA particles. Specifically, polysarcosine–lipid conjugates are featured as suitable components for the assembly of RNA nanoparticles. By now, PEG has been the most widely used and gold standard “stealth” polymer in drug delivery. However, PEG has been found to exhibit some undesired effects such as lowering transfection efficiency, accelerated blood clearance induced by anti-PEG antibodies, and/or complement activation, as well as inducing a specific immune response. The present invention shows that polysarcosine-lipid conjugates avoid the disadvantages accompanied by the use of PEG. Polysarcosine–lipid conjugates enable manufacturing of RNA nanoparticles with different techniques, resulting in defined surface properties and controlled size ranges. Manufacturing can be done by robust processes that are compliant with the requirements for pharmaceutical manufacturing. The particles can be end-group functionalized with different moieties to modulate charge or to introduce specific molecular moieties like ligands.
Patent application US20230043677 by the Oregon State University, USA, relates to nanoparticle composition for encapsulating a therapeutic agent, such as a mRNA, suitable for nebulization and/or delivery of the nebulized formulation to the lungs by inhalation. The nanoparticles comprise an ionizable lipid, a cholesterol derivative, a structural lipid, and a PEG lipid.
Patent application WO2022155598 by Tufts College and Brigham and Women’s Hospital, USA, discloses a highly potent nonviral LNP-mediated CRISPR-Cas9 delivery system for liver or lung delivery of Cas9 mRNA, and demonstrates its efficacy by targeting the Angptl3 gene. The system is composed of a leading tail-branched bioreducible lipidoid (306-012B) co-formulated with an optimized mixture of excipient lipid molecules, and it successfully co-delivers SpCas9 mRNA and a single guide RNA targeting Angptl3 via a single administration.
The success of mRNA-based COVID-19 vaccines have demonstrated the effectiveness of two key strategies for developing RNA medications: the chemical modifications of mRNA uridine to pseudouridine and 5′-capping, as well as the lipid nanoparticle delivery vectors, paving the way for further advancement of mRNA therapeutics and vaccines.
Application of mRNA Therapeutics and Vaccines in Disease Treatment and Prevention
Analysis of Diseases Claimed in Patents Related to mRNA Therapeutics and Vaccines among Diseases
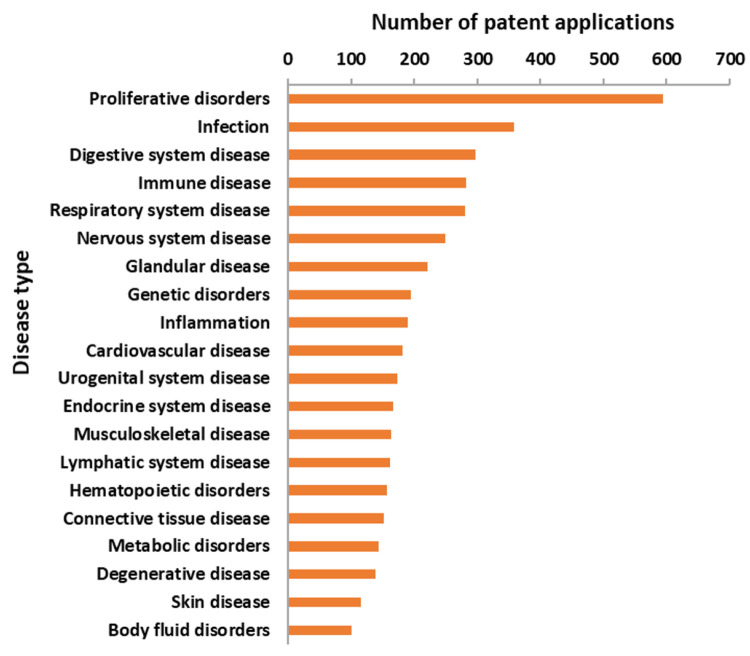
Diseases covered in mRNA patents include 69 primary disease classes, and the top 20 disease classes are shown in Figure 12. Proliferative disorders such as neoplasm, are claimed in the largest number of patents (600) followed by infectious diseases (358), indicating strong focus on application of mRNA medicines in these two areas. From the anatomical perspective, digestive system diseases, immune diseases, respiratory system diseases, nervous system diseases, and glandular diseases have been studies with relative high frequencies in patents. Disease classes such as genetic disorders and others are involved in fewer than 200 patent applications.
Figure 12.
Distribution of patents for the top 20 primary diseases.
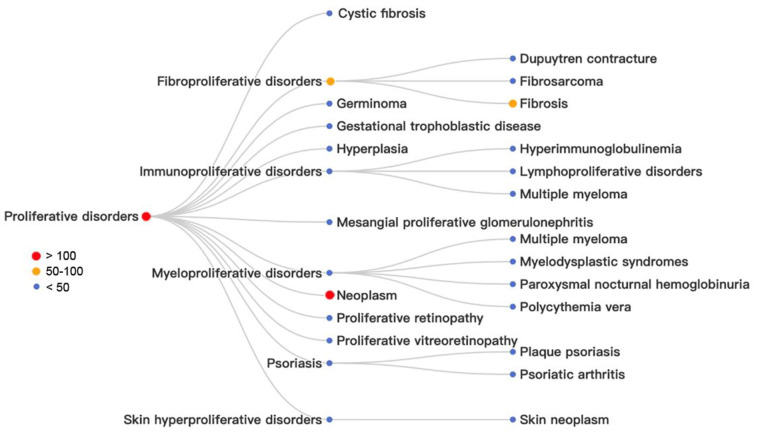
Analysis of Proliferative Diseases Disclosed in Patents Related to mRNA Therapeutics and Vaccines
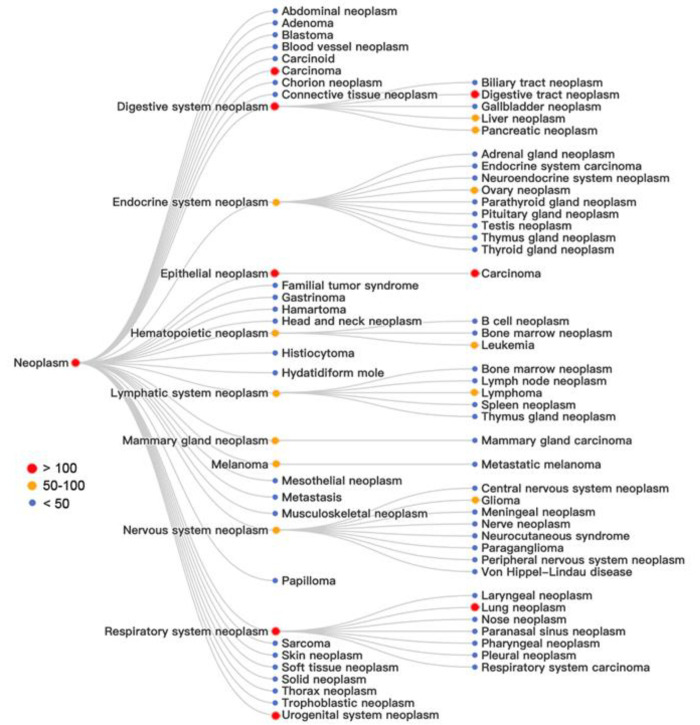
Figure 13 shows classes of diseases claimed by patents related to mRNA therapeutics and vaccine development. The diseases are arranged hierarchically with the number range of patents involved indicated by dots in different colors. As shown in the figure, proliferative diseases such as neoplasm are the most investigated diseases in the R&D of mRNA therapeutics and vaccines. Within this broader class of diseases, neoplasm is the most explored for mRNA therapeutics and vaccines and appeared in 528 patents, indicating a strong interest in applying this new class of medicines to cancer prevention and treatment. As shown in Figures 13 and 14, among the more specific diseases, digestive system neoplasm, lung neoplasm, urogenital system neoplasm, and various forms of carcinoma (i.e., epithelial tissue-derived neoplasm) attracted the most attention, with more than 100 patents involved in each case.
Figure 13.
Analysis of proliferative diseases claimed by patents related to mRNA therapeutics and vaccines: red, more than 100 patents; orange, 50–100 patents; blue, less than 50 patents.
Figure 14.
Analysis of key concepts in patents related to neoplasm: red, more than 100 patents; orange, 50–100 patents; blue, less than 50 patents.
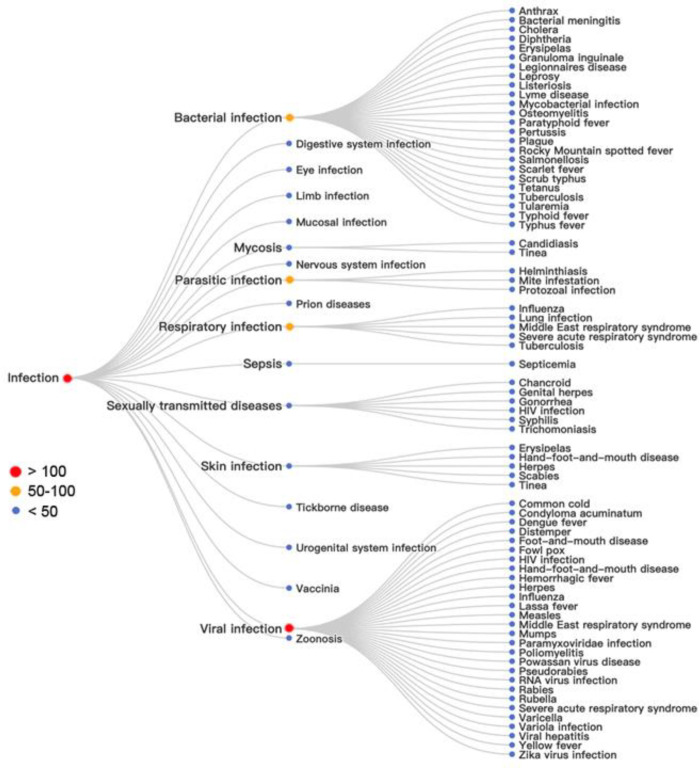
Patent Distribution among Infectious Diseases
As shown along the hierarchical tree of infectious diseases in Figure 15, there are 16 classes and over 60 specific diseases explored by patents on mRNA therapeutics or vaccines. Among those classes of diseases, viral infection has been most examined. Bacterial infections and parasitic infections were also of high concern. From the anatomical system perspective, respiratory system infection received more attention than other systems. Most of these patents are related to vaccine development against infectious diseases.
Figure 15.
Analysis of infectious diseases claimed by patents related to mRNA therapeutics and vaccines: red, more than 100 patents; orange, 50–100 patents; blue, less than 50 patents.
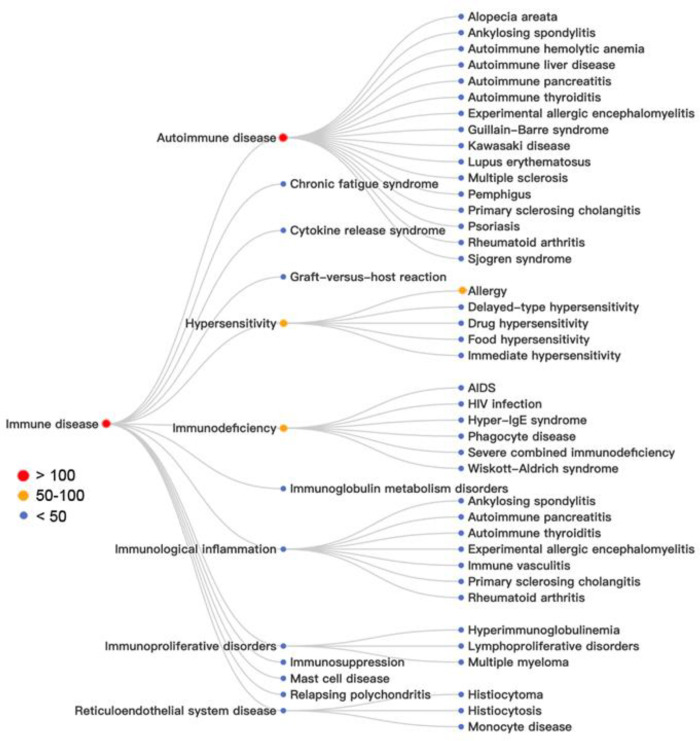
Analysis of Immune Diseases Disclosed by Patents Related to mRNA Therapeutics and Vaccines
Among various immune diseases examined by the mRNA therapeutic and vaccine patents, autoimmune diseases, followed by hypersensitivity (exaggerated response or over reaction to an antigen such as in the case of allergy) and immunodeficiency diseases, received the most attention as shown in Figure 16.
Figure 16.
Analysis of key concepts in patents related to immune diseases: red, more than 100 patents; orange, 50–100 patents; blue, less than 50 patents.
mRNA Vaccines and Therapeutics in Clinical Trials
Action Mechanism of mRNA Vaccines and Therapeutics
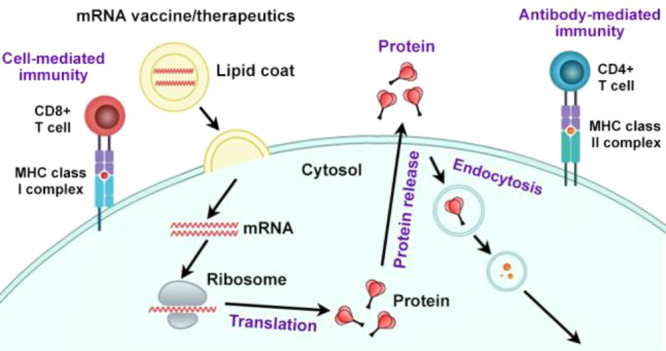
When the lipid nanoparticle (LNP) encapsulating mRNAs encoding targeted protein (antigenic protein) are administered in the body, LNP-mRNAs are engulfed by endocytosis and mRNAs are released to cytosol through endosomal escaping mechanism in antigen-presenting cells (no shown in the figure).49 Inside the ribosomes, a cellular machinery, proteins are translated based on the mRNAs. mRNA-encoded protein therapeutics use synthetic mRNAs that produce the desired proteins, such as antibodies, cytokines, and enzymes inside the human body. For vaccines, mimicking the viral infection process, intracellular produced antigens mainly elicit cell-mediated and antibody-mediated immunities. (Figure 17) First, the proteasome degrades antigenic proteins into peptide epitopes, which are transported into the endoplasmic reticulum and loaded onto major histocompatibility complex class I molecules (MHC I). The MHC I-peptide epitope complexes are presented on the surface of cells that bind to the T cell receptor to activate CD8+ T cells and kill infected or cancer cells (cell-mediated immunity). The antigenic proteins are transported via the Golgi apparatus and released to the outside of the cells. The secreted proteins are endocytosed by antigen-presenting cells, degraded, and loaded onto the MHC II peptide inside endosomes. The MHC II-peptide epitope complexes are presented on the surface of cells, which is recognized by CD4+ T cells facilitating B cells to make antigen-specific antibodies (antibody-mediated immunity).50
Figure 17.
Mechanism of action of mRNAs vaccines and therapeutics (adapted and modified from ref (50)).
mRNA Vaccines in Clinical Trials
Top companies for mRNA vaccine research include Moderna, BioNTech, Pfizer, and CureVac. Examination of diseases investigated by mRNA vaccines in clinical trials revealed that the vast majority (80%) of mRNA vaccines were designed for infectious diseases such as coronavirus infection, influenza, human immunodeficiency virus (HIV), rabies, and respiratory syncytial virus (RSV), among others. Other mRNA vaccines in the pipeline are being researched for various forms of cancers.
Table 10 lists mRNA vaccine candidates for infectious diseases currently in at least phase II trials. All mRNA vaccine candidates are developed for COVID-19 and encode the spike protein of SARS-CoV-2 or its receptor-binding domain. mRNA-1283 is a potential refrigerator-stable COVID-19 vaccine comprising mRNA encoding a SARS-CoV-2 spike protein N-terminal fragment and receptor-binding domain formulated in lipid nanoparticles. It is considered as the “next generation” vaccine candidate aiming for a pan-human coronavirus domain vaccine.
Table 10. mRNA Vaccine Candidates for Infectious Diseases in Phase 2 or More Advanced Clinical Trialsa.
| Vaccine name | CAS Registry Number | Disease indication | Antigen | Company |
|---|---|---|---|---|
| BNT-162b2 (B.1.1.7 + B.1.617.2) | 2883464-25-1 | COVID-19 | Prefusion stabilized S protein of SARS-CoV-2 B.1.1.7 and B.1.1.617.2 variants | BioNTech, Pfizer |
| BNT162b2 (B.1.351) | Pending | COVID-19 | Prefusion stabilized S protein of SARS-CoV-2 B.1.351 variant | BioNTech,Pfizer |
| BNT 162b2 (B.1.1.529) | Pending | COVID-19 | Prefusion stabilized S protein of SARS-CoV-2 B.1.1.529 variant | BioNTech, Pfizer |
| BNT-162b2 (WT/OMI BA.1) | Pending | COVID-19 | Prefusion stabilized S protein of SARS-CoV-2 WT and BA.1 variant | BioNTech, Pfizer |
| BNT-162b5 (WT/OMI BA.2) | Pending | COVID-19 | Prefusion stabilized S protein of SARS-CoV-2 WT and BA.2 variant | BioNTech, Pfizer |
| mRNA 1273 | Pending | COVID-19 | The full-length prefusion stabilized S protein | Moderna |
| mRNA 1273.211 | 2805221-47-8 | COVID-19 | Prefusion stabilized S protein of the SARS-CoV-2 and B.1.351 variant | Moderna |
| mRNA 1273.214 | Pending | COVID-19 | bivalent of SARS-CoV-2 spike protein from Beta and Delta variants | Moderna |
| mRNA 1273.351 | 2642373-67-7 | COVID-19 | the full-length prefusion stabilized S protein of the SARS-CoV-2 B.1.351 variant | Moderna |
| mRNA 1273.529 | 2763208-92-8 | COVID-19 | Prefusion stabilized S protein of the SARS-CoV-2 B.1.1.529 variant | Moderna |
| mRNA 1273.617 | 2882950-03-8 | COVID-19 | Prefusion stabilized S protein of the SARS-CoV-2 B.1.1.617.2 variant | Moderna |
| mRNA 1283 | 2696398-77-1 | COVID-19 | SARS-CoV-2 spike protein receptor-binding domain and N-terminal fragment | Moderna |
| mRNA 1283.529 | Pending | COVID-19 | Prefusion stabilized S protein of the SARS-CoV-2 B.1.1.529 variant | Moderna |
| mRNA 1283.211 | 2882951-80-4 | COVID-19 | Prefusion stabilized S protein of the SARS-CoV-2 B.1.351 variant | Moderna |
| LVRNA 009 | Pending | COVID-19 | SARS-CoV-2 spike protein | AIM Vaccine |
| ARCT 165 | 2714576-70-0 | COVID-19 | Self-Transcribing and Replicating mRNA encoding SARS-CoV-2 spike protein variants | Arcturus Therapeutics |
| ARCT 154 | 2698334-90-4 | COVID-19 | Self-Transcribing and Replicating mRNA encoding SARS-CoV-2 spike protein | Arcturus Therapeutics |
| ARCT 021 | 2541451-24-3 | COVID-19 | Self-Transcribing and Replicating mRNA encoding SARS-CoV-2 spike protein variants | Arcturus Therapeutics |
| BCD 250b | 2756425-11-1 | COVID-19 | The receptor-binding domain of SARS-CoV-2 spike protein | Biocad |
| COVID-19 mRNA vaccine | Pending | COVID-19 | SARS-CoV-2 spike protein | CanSino Biologics |
| SYS 6006 | Pending | COVID-19 | SARS-CoV-2 spike protein | CSPC Pharmaceutical |
| DS 5670 | 2749556-96-3 | COVID-19 | SARS-CoV-2 spike protein | Daiichi Sankyo |
| HDT 301 | 2437182-02-8 | COVID-19 | Self-amplifying RNA encoding SARS-CoV-2 spike protein | Emcure Pharmaceuticals |
| EG-COVID | Pending | COVID-19 | SARS-CoV-2 spike protein | EyeGene |
| PTX-COVID19-B | 2726459-47-6 | COVID-19 | SARS-CoV-2 spike protein | Providence Therapeutics |
| SW-BIC-213 | 2699076-70-3 | COVID-19 | The full-length SARS-CoV-2 spike protein | Stemirna Therapeutics |
| ABO1009-DP | Pending | COVID-19 | SARS-CoV-2 omicron variant spike protein | Suzhou Abogen Biosciences |
| ARCoV | 2543878-98-2 | COVID-19 | The receptor-binding domain of SARS-CoV-2 spike protein | Suzhou Abogen Biosciences |
| Awcorna | Pending | COVID-19 | SARS-CoV-2 spike protein receptor-binding domain | Walvax Biotechnology |
| Comirnaty | 2417899-77-3 | COVID-19 | The full-length prefusion stabilized S protein | BioNTech |
| Spikevax | 2430046-03-8 | COVID-19 | The full-length prefusion stabilized S protein | Moderna |
| mRNA 1073 | 2760527-92-0 | COVID-19 + influenza | Prefusion stabilized S protein of SARS-CoV-2 and hemagglutinin | Moderna |
| mRNA 1345 | 2766353-31-3 | Respiratory syncytial virus infection | RSV prefusion stabilized F glycoprotein | Moderna |
| BNT 161 | 2760529-48-2 | Influenza | Hemagglutinin from H1N1 and B/Yamagata influenza strains | BioNTech |
| mRNA 1010 | 2760527-87-3 | Influenza | Hemagglutinin from four seasonal influenza strains | Moderna |
| mRNA 1020 | 2760527-90-8 | Influenza | Hemagglutinin and neuraminidase antigens | Moderna |
| mRNA 1030 | 2760527-91-9 | Influenza | Hemagglutinin and neuraminidase antigens | Moderna |
| MRT 5407 | 2900351-99-5 | Influenza | Quadrivalent influenza vaccine | Sanofi |
| mRNA 1893 | 2882947-97-7 | Zika virus infection | Structural proteins of Zika virus | Moderna |
| mRNA 1325 | 2882946-55-4 | Zika virus infection | Structural proteins of Zika virus | Moderna |
| mRNA 1647 | 2882946-59-8 | CMV infection | Six mRNAs coding for pentamer viral antigen and glycoprotein B of CMV | Moderna |
Data from the CAS Content Collection, Clinicaltrials.gov, and Pharmaprojects.
Vaccine no longer in development.
In addition to mRNA vaccines for SARS-CoV-2 infection, several mRNA vaccine candidates for infection by other viruses such as RSV, influenza virus, Zika virus, and cytomegalovirus (CMV) have entered clinical trials. In January 2023, Moderna announced that RSV vaccine, mRNA-1345, demonstrated vaccine efficacy of 83.7% at preventing symptoms in older adults in randomized phase III trial.51 Moderna plans to submit mRNA-1345 for regulatory approval in the first half of 2023.52
Two research groups examined levels of neutralizing antibodies and differences in CD4+ or CD8+ T cell responses induced by monovalent and bivalent COVID-19 booster vaccines for protecting against omicron variants. Neither group observed superior immune responses to bivalent booster vaccines compared to monovalent vaccines. Most of neutralizing antibodies elicited by vaccines targeting newer variants still recognize only the original virus because of “immune imprinting” in which the body repeats its immune response to the first variant encountered.53,54 However, fine-tuning the dosage of booster vaccines might increase their efficacy of protection again immune-escape COVID-19 variants.55
The success of COVID-19 mRNA vaccine has revealed the application potential of mRNA platform not only for expansion to other infectious diseases but also for cancers (Table 11), especially as therapeutic vaccines The clinical study of mRNA vaccines has shown good efficacy in the treatment of melanoma, non-small-cell lung cancer (NSCLC), and prostate cancer. Ref. Autogene cevumeran (RO 7198457, BNT122), jointly developed by BioNTech and Genentech, is a mRNA-based individualized neoantigen specific immunotherapy (iNeST). It encodes up to 20 neoepitopes defined by the patient’s tumor-specific mutations delivered in an RNA-lipoplex formulation. A combination of intravenously administered autogene cevumeran combined with anti-PD-L1 immune checkpoint inhibitor atezolizumab is conducted in patients with locally advanced or metastatic solid tumors has entered a first-in-human phase I study. It has induced strong CD4+ and CD8+ T cell immunity against neoantigens. Randomized phase II studies of autogene cevumeran for patients with melanoma in combination with pembrolizumab, for individuals with non-small-cell lung cancer (NSCLC) in combination with atezolizumab, and for individuals with colorectal cancer (CRC) are currently ongoing.
Table 11. mRNA Vaccines in Phase 2 or More Advanced Clinical Trials for Cancersa.
| Vaccine name | CAS Registry Number | Disease indication | Antigen | Company |
|---|---|---|---|---|
| Autogene cevumeran | 2365453-34-3 | Melanoma; colorectal cancer | Patient-specific neoantigens | BioNTech |
| mRNA 4157 | 2741858-84-2 | Melanoma | Up to 34 neoantigens | Moderna |
| mRNA 4359 | 2900354-08-5 | Melanoma; non-small-cell lung carcinoma | IDO and PD-L1 | Moderna |
| BNT 111 | 2755828-88-5 | Melanoma | Mix of four melanoma-associated antigens | BioNTech |
| BNT 112 | 2900354-09-6 | Prostate cancer | Mix of five prostate cancer-specific antigens | BioNTech |
| BNT 113 | 2882951-85-9 | PV16+ head-and-neck squamous carcinoma | HPV16-derived tumor antigens (oncoprotein E6 and E7) | BioNTech |
| CV 9202 | 1665299-76-2 | Non-small-cell lung cancer | NY-ESO-1, MAGE C1, MAGE C2, TPBG (5T4), survivin, MUC1 | CureVac |
| CV 9103 | 2882951-83-7 | Prostate cancer | Mix of four prostate cancer-associated antigens | CureVac |
| SW 1115C3 | 2882951-82-6 | Non-small-cell lung cancer; esophageal cancer | Patient-specific neoantigens | Stemirna Therapeutics |
| Rocapuldencel T; AGS 003 | 2396421-01-3 | Non-small-cell lung cancer; lung cancer; bladder and renal cancer | Autologous tumor antigen and CD40L-loaded dendritic cell immunotherapy | Argos Therapeutics |
Data from the CAS Content Collection, Clinicaltrials.gov, and Pharmaprojects.
BNT111 developed with the BioNTech’s FixVac platform encodes four tumor-associated antigens (TAAs), the cancer-testis antigen NYESO-1, the human melanoma-associated antigen A3 (MAGE-A3), tyrosinase, and putative tyrosine-protein phosphatase (TPTE) and is encapsulated in an RNA-lipoplex formulation. It has entered phase II clinical trials to treat advanced melanoma and has gained FDA fast track designation in 2021. A report from a phase II trial showed that the use of BNT111 alone or in combination with PD-1 antibody can induce tumor antigen-specific CD4+ and CD8+ T cell immune responses.
CV9201, developed by CureVac, is an RNA-based therapeutic vaccine encoding five NSCLC antigens. The first-in-human, multicenter, phase I/IIa study was conducted in 7 patients with locally advanced NSCLC and 39 patients with metastatic NSCLC. The result demonstrated that specific immune responses against at least one antigen were detected in 63% of patients after treatment and the frequency of activated IgD+ CD38hi B cells increased by more than 2-fold in 60% of evaluated patients.56
Moderna’s personalized mRNA cancer vaccine, mRNA-4157, encodes 34 unique neoantigen genes that may stimulate specific T cell responses. Phase I trials showed that this vaccine is safe and tolerable in monotherapy or in combination with pembrolizumab.57 In December 2022, Moderna and Merck announced that mRNA-4157 in combination with anti-PD-1 antibody, pembrolizumab reduced the risk of recurrence or death by 44% in patients with stage III/IV melanoma compared with pembrolizumab monotherapy based on their randomized phase IIb trial.58
mRNA Therapeutics in Clinical Trials
Top companies for mRNA therapeutic research include BioNTech, Moderna, Arcturus Therapeutics, AstraZeneca, and Sanofi. mRNA therapeutics have a broad range of targeted diseases. Consistent with the patent-disease analysis data above, mRNA therapeutics are being developed largely for cancers, followed by metabolic, cardiovascular, infectious, immunological, and respiratory diseases.
mRNA therapeutic products currently in clinical trials are examined in Table 12 to reveal a landscape view of the current progress in mRNA therapeutics in the clinical development pipeline. A select few are also examined in further detail below to showcase the variety of mRNA therapeutics, their mechanism of actions, and their targeted disease indications.
Table 12. mRNA Therapeutic Products in Clinical Trials62a.
| mRNA drug name | CAS Registry Number | Disease indications | Description | Company |
|---|---|---|---|---|
| A-001; TriMix-MEL; ECL-006; E011-MEL | 2877674-59-2 | Melanoma | A mixture of three mRNAs encoding constitutively activated CLT4, CD40L, and TLR4 plus mRNAs for five melanoma-associated antigens (tyrosinase, gp100, MAGE-A3, MAGE-C2, and PRAME), which activate key immune cells against cancer | eTheRNA Immunotherapies |
| ARCT-810; LUNAR-OTC | 2877704-48-6 | Ornithine trans-carbamylase deficiency | mRNA encoding ornithine transcarbamylase formulated in a lipid nanoparticle to correct the enzyme deficiency | Arcturus Therapeutics |
| AZD-8601 | 2603440-18-0 | Heart failure and ischemic cardiovascular diseases | mRNA encoding vascular endothelial growth factor A to stimulate new vascular blood vessel formation and repair as well as regenerate heart cells | AstraZeneca |
| BD-111 | 2901016-63-3 | Herpetic viral keratitis | Viral-like particle drug delivery system used to transduce cas9 mRNA that directly targets and cuts the viral genome of herpes simplex virus 1 to effectively remove the virus | BD Gene |
| BNT-141 | 2877707-22-5 | Solid tumors | mRNA encoding a monoclonal antibody targeting claudin 18, a protein commonly expressed in multiple cancers | BioNTech |
| BNT-142 | 2877707-34-9 | Solid tumors | mRNA encoding a bispecific antibody targeting CD3, a protein involved in activation of certain types of T cells, and claudin 6 (CLDN6), a protein highly expressed in certain cancers | BioNTech |
| BNT-151 | 2877709-82-3 | Solid tumors | A nucleoside-modified, cationic lipoplexes-loaded mRNA encoding an interleukin-2 (IL-2) variant to stimulate anti-cancer T cells | BioNTech |
| BNT-152 | 2877709-92-5 | Solid tumors | A nucleoside-modified mRNA encoding interleukin-7 to stimulate anti-cancer T cells | BioNTech |
| BNT-153 | 2877709-93-6 | Solid tumors | A nucleoside-modified mRNA encoding interleukin 2 (IL-2) to stimulate anti-cancer T cells | BioNTech |
| LioCynx-M004; Lion TCR | 2901015-92-5 | Hepatitis B virus-related hepatocellular carcinoma | A genetically modified autologous cell therapy derived from T cells transfected with mRNA encoding to express a T-cell receptor that recognizes the hepatitis B surface antigen on the surface of HBV-related cancer cells | Lion TCR |
| MEDI-1191 | 2877712-03-1 | Solid tumors | LNP-encapsulated mRNA encoding IL-12 to increase intratumor production of IL-2 via intratumoral injection | Moderna |
| mRNA-2752 | 2878461-50-6 | Solid tumors | Lipid nanoparticle-encapsulated mRNAs encoding human T cell co-stimulator, OX40L, and proinflammatory cytokines, IL-23 and IL36γ, for intratumoral injection | Moderna |
| mRNA-3705 | 2878470-78-9 | Methylmalonic acidemia | Lipid nanoparticle-encapsulated mRNA encoding the mitochondrial enzyme methyl-CoA mutase that is deficient in methylmalonic acidemia | Moderna |
| mRNA-3745 | 2878574-58-2 | Glycogen storage disease type 1a | Lipid nanoparticle-encapsulated mRNA encoding glucose 6-phosphatase to restore the deficient enzyme responsible for converting glycogen into glucose for treatment of type 1a glycogen storage disease | Moderna |
| mRNA-3927 | 2878577-32-1 | Propionic acidemia | Lipid nanoparticle-encapsulated mRNAs encoding propionyl-CoA carboxylase α subunit and β subunit to restore the deficient enzyme and reduce toxic buildup of some substances in propionic acidemia | Moderna |
| mRNA-6231b | 2878577-39-8 | Autoimmune diseases | Lipid nanoparticle-encapsulated modified mRNA encoding a mutated form of human IL-2 fused to human serum albumin to increase its half-life to restore IL-2- and T-cell-mediated immune homeostasis | Moderna |
| MRT-5005b | 2328142-67-0 | Cystic fibrosis | Inhalable form of engineered mRNA variant encoding fully functional cystic fibrosis transmembrane conductance regulator protein for restoring lung function in cystic fibrosis; Phase I/II clinical trial (NCT03375047) showed no improvement in lung function but did discover that repeated inhaled doses of mRNA are safe63 | Translate Bio (acquired by Sanofi) |
| SAR-441000 | 2879301-17-2 | Solid tumors | Mixture of four mRNAs encoding IL-2 single chain, IL-15 fused to the sushi domain of IL-15Rα, GM-CSF, and interferon α2b, which have been reported as mediators of tumor regression | Sanofi, BioNTech |
| SQZ-eAPC-HPV | 2879306-51-9 | HPV and solid tumors | mRNA-based cell therapeutic agent that delivers five mRNAs for HPV16 protein antigens and immune-stimulating proteins, including CD86 and membrane bound IL-2 and IL-12, into four different types of engineered immune cells (monocytes, T-cells, B-cells, and NK cells) of cancer patients in a single step | SQZ Biotechnologies |
| UX053; LUNAR-GSDIII | 2901003-30-1 | Glycogen storage disease type III | Lipid nanoparticle-encapsulated mRNA therapeutic encoding the glycogen debranching enzyme; replacing the defective AGL gene product allows cells to break down glycogen using normal pathways | Ultragenyx, Arcturus Therapeutics |
| Verve-101 | 2894841-30-4 | Heterozygous familial hypercholesterolemia | mRNA-based lipid nanoparticle therapeutic that targets the liver and base-editing in the PCSK9 gene to disrupt PCSK9 protein production to lower LDL cholesterol and treat cardiovascular disease | Verve Therapeutics |
Data from the CAS Content Collection, Clinicaltrials.gov, and Pharmaprojects.
Drug no longer in development.
Arcturus Therapeutics developed and is currently evaluating a mRNA therapeutic for the treatment of ornithine transcarbamylase (OTC) deficiency that currently has no FDA-approved treatments. The urea cycle enzyme OTC helps remove ammonia from liver cells, and a deficiency leads to high ammonia levels. Utilizing their lipid-mediated nucleic acid delivery system (LUNAR), Arcturus is currently researching LUNAR-OTC in a phase II clinical trial (NCT05526066) to evaluate its safety and tolerability in participants with OTC deficiency.59
AZD8601 is a mRNA therapeutic developed through a collaboration between AstraZeneca and Moderna. It encodes for VEGF-A, a paracrine factor important for new blood vessel formation and progenitor cell division that contributes to repair and regeneration of the heart. A phase II clinical trial (NCT03370887) examined the safety, tolerability, and exploratory efficacy of an intra-myocardium AZD8601 injection in patients with moderately impaired systolic function undergoing coronary artery bypass grafting surgery. Trial results revealed no serious side effects and a rising trend in efficacy for the treatment group.60
SQZ Biotechnologies, in collaboration with Roche, has developed a mRNA-based cell therapeutic called SQZ-eAPC-HPV. SQZ-eAPC-HPV delivers five mRNAs for HPV16 protein antigens and immune-stimulating proteins, including CD86 and membrane-bound IL-2 and IL-12, into four different types of engineered immune cells (monocytes, T-cells, B-cells, and NK cells). A phase I/II clinical trial (NCT05357898) is currently recruiting to assess safety and tolerability, antitumor activity, and immunogenic and pharmacodynamic effects of SQZ-eAPC-HPV as monotherapy and in combination with pembrolizumab in patients with recurrent, locally advanced, or metastatic HPV16+ solid tumors.
BioNTech developed BNT141, a mRNA that encodes secreted IgG antibodies targeting claudin 18.2, a protein commonly expressed on certain solid tumors. It is being researched in phase I/II clinical trial number NCT04683939, looking at its safety and pharmacokinetics in patients with Claudin 18.2-positive solid tumors.61
Methods
This study used the CAS Content Collection as the primary data source, which aggregates and connects key scientific details disclosed in more than 50,000 journals and patents from 64 issuing authorities, as well as many other sources to cover the scope of science from chemistry and life sciences to engineering, materials science, and agriculture. A search query that searched for words or phrases in titles, abstracts, keywords, CAS index terms, and substances was developed to extract all the scientific journal articles and patents related to mRNA vaccines and therapeutics. The resulting patents were then intellectually reviewed to 1) remove false drops and 2) classify each document into one or more of the following classes: a) vaccines, b) therapeutics, and/or c) delivery system as well as mRNA modification methods. During this review process, the likely intriguing/notable/highly influential patents were also tagged for further analyses.
Once such reviews were done, the answer set containing over 2,000 patents and more than 9,000 journal articles was further analyzed for patents and journal articles separately for the categories of mRNA vaccines and mRNA therapeutics. Specific analyses included the trends over time; landscape analyses including country distribution, patent office distribution, flow of patents from applicant home countries to patent offices, and most influential organizations; and topic cluster analyses. Most notable patents, diseases involved, and specific mRNA therapeutics and vaccines in clinical trials were also examined based on both CAS-licensed data and publicly available information. The analysis tools include Thomson Data Analyze (DDA), VOSviewer, OmniGraphSketcher, and ECharts.
At the document level, this report focused more on patents than journal articles. A group of patent applications covering the same or similar technical content is called a patent family. Patents for the same technological invention may be filed in multiple countries or regions, and the CAS database maintains these related applications as one record for indexing; this record is referred to as the basic patent for most analyses in this report. Individual patent applications from the same patent family may be applied for in different countries and/or patent offices. This properly represents the distribution of patent applications filed by applicants’ countries (i.e., applicants from different countries or organizations); individual patent applications from the same family are counted separately for geographic distribution and patent flow analysis. The country of origin of a patented technology is determined based on the country location of the patent assignee (or applicant/inventor). The country analysis in this report is based on the country location of the patent applicant for determination of the country of origin of the technology or the actual country owning the technology.
Outlook and Perspectives
The remarkable success of mRNA vaccines against COVID-19 has strongly motivated interest in mRNA as a way of delivering therapeutic proteins. However, a variety of challenges remain to be resolved before mRNA can be verified as a common therapeutic modality with wide-ranging relevance to both rare and common diseases. The challenges in developing better mRNA formulations are as follows:
• Enhancing specific protein expression: Elaboration of the mRNA cargo to augment the time and amount of protein production in vivo, including advances in the design of the primary chemical structure of the mRNA, novel forms of circular and self-amplifying mRNA, and improved purification strategies. The most critical advances in mRNA vaccines and therapeutics are due to the discovery that the insertion of chemically modified nucleosides, specifically in uridine moieties, can significantly increase protein expression. So far, over 130 chemical modifications of RNA have been described, and the properties and effects of multiple other RNA chemical modifications remain to be explored.20,64 All parts of the mRNA—the cap, 5′ and 3′ regions, open reading frame, and polyadenylated tail—can be optimized to augment protein expression.65 In addition to the amount of protein expression, a crucial hurdle of mRNA therapeutics for chronic diseases is the relatively short time period of protein production, which requires repetitive administrations. Self-amplifying mRNAs (saRNAs) make use of the self-replication of an RNA alphavirus, which can amplify RNA transcripts in the cytoplasm.66,67 Circular mRNAs (circRNAs) provide another approach for extending the duration of protein production. The circular structure shields circRNA from exonuclease attacks, which prolongs the RNA lifespan, thus raising the total protein yield.68
• Improving mRNA packaging and delivery systems, including ionizable LNPs, peptide-based nanoparticles, cells, and cell-based extracellular vesicles. The rapid clinical implementation of mRNA-based vaccines and therapies has become possible due to the development of efficient delivery vehicles to protect and transport the highly unstable mRNAs. Various smart delivery systems have been invented to improve key features such as circulation time in blood stream, biodistribution, cargo loading, and release.
Lipid-based nanoparticles are currently the most widely studied and clinically advanced vehicles for mRNA delivery.47,69−71 The most extensively used are cationic and ionizable LNPs.72 Although cationic lipid-based nanoparticles have shown promise in certain therapies, their possible cytotoxicity73 and relatively short circulation time74 have somehow impeded their clinical application. To deal with these issues, a variety of novel lipid–PEG conjugates and ionizable lipids have been synthesized and tested. Ionizable lipids remain neutral at physiological pH, which reduces their toxicity and increases to some degree their circulation half-life.75 Furthermore, the protonation of ionizable lipids at acidic pH not only allows successful condensation and encapsulation of mRNAs, but also allows the escape of mRNAs from the acidic endosomes.70,76 The PEG shell set up by PEGylated lipids considerably prolongs the circulation half-life of the LNPs and reduces their aggregation; it also cut back unfavorable interactions with serum proteins.77
Another promising approach uses extracellular vesicles and especially their smallest version, the exosomes, as a delivery vehicle.48 Exosomes are nanosized vesicles enclosed by a lipid bilayer membrane. They are produced by most eukaryotic cells and subsequently released in the extracellular space, providing means of efficient intercellular communication and signaling, including transport of bioactive molecules such as proteins, nucleic acids, and metabolites between cells and across biological barriers.78,79 As natural delivery vehicles, exosomes possess multiple benefits, such as biocompatibility and low immunogenicity. It has been reported that exosomes originated from various cell types do not provoke toxicity and are well-tolerated after repetitive dosing.80 Thus, they can be viewed as promising mRNA delivery systems in cases in which repeated dosing is required.
Peptides represent other versatile, biocompatible, and targetable RNA carriers. From this class, cell-penetrating peptides (CPPs) that can penetrate the cellular membrane and transfer to the cytoplasm are used most frequently for RNA delivery by forming a variety of nanocomplexes depending on the formulation settings and the properties of the used CPPs and RNAs.81,82
A further novel biological alternative to lipid nanoparticle carriers is the use of cell-based delivery systems, exploiting the cellular paracrine communication ability to directly deliver proteins synthesized by mRNA brought into cells ex vivo. It provides certain advantages, such as biocompatibility, lack of toxicity, and extended half-life in circulation. Various modifications are feasible by introducing mRNA into cells such as immune cells, blood cells, and others.83−85 Recently, a bacteria-mediated vehicle for oral mRNA vaccine delivery has been also developed. This multiple-target vaccine successfully explored engineered Salmonella-based vector to deliver mRNA vaccine against Delta and Omicron variants of COVID-19.86,87
• Targeting mRNA therapeutics to certain tissues and engineering of delivery systems with tissue-specific affinity. Fulfilling the potential of mRNA therapeutics requires proper targeting. Liver is the largest internal organ in human body, performing vital roles in various physiological activities, thus large amount of disease targets potentially receptive to mRNA therapies reside within the liver hepatocytes. A promising liver-targeted N-acetylgalactosamine (GalNAc) ligand has been assessed in various trials for enhancing the cellular uptake and tissue specific delivery of mRNAs.88 GalNAc-based delivery relies on the ability of the hepatocytes to express the asialoglycoprotein receptor, a high-capacity, rapidly internalizing receptor that binds glycoproteins via receptor-mediated endocytosis. In recent years, encouraging progress has been made in the field of GalNAc conjugates, underscoring the value of targeting moieties.89,90 The conjugation of GalNAc moieties to oligonucleotides represents a promising and safe approach for liver-targeted delivery of nucleic acid therapeutics.
• Selective delivery of mRNA therapeutics to organs other than liver requires specifically developed packaging and delivery systems with appropriate affinity. The recent efforts on developing better delivery systems and/or administration routes for this purpose have produced some exciting results. For example, Daniel Siegwart’s team has recently developed a method of Selective ORgan Targeting (SORT) that uses various engineered lipid nanoparticles to selectively deliver mRNA to extrahepatic organs.91 Qiaobing Xu’s team has identified an endogenously lymph node-directing lipid nanoparticle system.92 Kathryn A. Whitehead’s team devised a strategy of delivering cationic helper lipid-containing lipid nanoparticles by intraperitoneal administration to deliver mRNA to pancreas, especially the insulin-producing cells.93 Gaurav Sahay’s lab has developed a LNP system with increased polyethylene glycol (PEG) and inclusion of a cholesterol analog, β-sitolsterol to optimize the delivery of the nebulized, LNP-based mRNA for fibrosis transmembrane conductance regulator (CFTR) protein as potential inhalable LNP-based mRNA therapies.94
• Strategies for allowing repeated dosing for the treatment of chronic conditions. The development of mRNA therapeutics raises further challenges as compared to those with mRNA vaccines. While immunization requires only a small amount of protein production, as the immune system will amplify the antigenic signal, mRNA therapeutics require much higher level of protein to reach a therapeutic level. The tissue bioavailability, half-life in circulation, and carrier efficiency to deliver to the targeted tissue remain challenging. Another major difficulty is the repeated dosing, which is typically required in the treatment of chronic diseases and which activates innate immunity in the long run, with a resultant reduction of therapeutic protein expression.65 Regardless of these hurdles, however, a variety of emerging technologies are currently under development with the effort to deal with them.26,95−97
Despite these challenges, impressive progress has been made in the research and development of mRNA therapeutics and vaccines. Most recently, Luis A. Rojas et al. have reported the promising result of a Phase 1 clinical trial in which personalized uridine mRNA neoantigen vaccines induced substantial T cell activity in postsurgery patients with pancreatic ductal adenocarcinoma.98 The remarkable success of the mRNA vaccines for COVID-19, along with recent expansion of this technology into treatment of most lethal cancers, has validated the great promise of this new class of medications and significantly enhanced the chance to witness their extensive applications in the near future.
Acknowledgments
The authors sincerely appreciate the CAS Data, Analytics & Insights team for their assistance in data extraction. The authors want to thank Caroline Ma and Sunny Yu for their effort in facilitating collaboration and Dharmini Patel for project management. The authors are grateful to the leadership team from their two organizations: Xiwen Liu, Manuel Guzman, Gilles Georges, Michael Dennis, Craig Stephens, and Dawn Riedel. We are also grateful to the consulting experts for their guidance during the research process. The Chinese National Science Library (NSL) work was funded by the Youth Innovation Promotion Association for Chinese Academy of Science (No. 2023182), NSTL “Research on Key Technologies of mRNA Vaccine R&D Pipeline” (E11Z0416), and Chinese Academy of Sciences Literature and Information Capacity Building Project in 2022 (E2290436).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.3c00047.
SI-1, trend over time for patents related to mRNA therapeutics; SI-2, trend over time for patents related to mRNA vaccines; SI-3, trend over time for patents on mRNA delivery systems; SI-4, trend over time for patents related to mRNA modification; SI-5, analysis of research topics in mRNA therapeutics patents; SI-6, analysis of research topics in mRNA vaccine patents; SI-7, analysis of research topics in patents related to mRNA modification; and SI-8, top countries and organizations in the field of mRNA modification (PDF)
Author Contributions
† D.L. and C.L. contributed equally to this paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Brenner S.; Meselson M.; Jacob F. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 1961, 190, 576–581. 10.1038/190576a0. [DOI] [PubMed] [Google Scholar]
- Jacob F.; Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 1961, 3, 318–356. 10.1016/S0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Gros F.; Hiatt H.; Gilbert W.; Kurland C. G.; Risebrough R. W.; Watson J. D. Unstable Ribonucleic Acid Revealed by Pulse Labelling of Escherichia Coli. Nature 1961, 190, 581–585. 10.1038/190581a0. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S.; Both G. W.; Furuichi Y.; Shatkin A. J. 5′-Terminal 7-methylguanosine in eukaryotic messenger-RNA is required for translation. Nature 1975, 255, 33–37. 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y.; Miura K. I. Blocked structure at 5′ terminus of messenger-RNA from cytoplasmic polyhedrosis-virus. Nature 1975, 253, 374–375. 10.1038/253374a0. [DOI] [PubMed] [Google Scholar]
- Krieg P. A.; Melton D. A. Functional messenger-RNAs are produced by SP6 invitro transcription of cloned cDNAs. Nucleic Acids Res. 1984, 12, 7057–7070. 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro M. J.; Giacomoni D.; Lavelle D. O. N.; Paxton W.; Dray S. Evidence for translation of rabbit globin mRNA after liposomemediated insertion into a human cell line. Nature 1978, 274, 921–923. 10.1038/274921a0. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature 1978, 274, 923–924. 10.1038/274923a0. [DOI] [PubMed] [Google Scholar]
- Felgner P. L.; Gadek T. R.; Holm M.; Roman R.; Chan H. W.; Wenz M.; Northrop J. P.; Ringold G. M.; Danielsen M. Lipofection - A Highly Efficient, Lipid-Mediated DNA-Transfection Procedure. Proc. Natl. Acad. Sci. U. S. A. 1987, 84, 7413–7417. 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. W.; Felgner P. L.; Verma I. M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. U. S. A 1989, 86, 6077–6081. 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirikowski G. F.; Sanna P. P.; Maciejewskilenoir D.; Bloom F. E. Reversal of diabetes-insipidus in Brattleboro rats - intrahypothalamic injection of vasopressin messenger-RNA. Science 1992, 255, 996–998. 10.1126/science.1546298. [DOI] [PubMed] [Google Scholar]
- Martinon F.; Krishnan S.; Lenzen G.; Magne R.; Gomard E.; Guillet J. G.; Levy J. P.; Meulien P. Induction of Virus-Specific Cytotoxic T-Lymphocytes in-Vivo by Liposome-Entrapped Messenger-RNA. Eur. J. Immunol. 1993, 23, 1719–1722. 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- Conry R. M.; Lobuglio A. F.; Wright M.; Sumerel L.; Pike M. J.; Feng J. N.; Benjamin R.; Lu D.; Curiel D. T. Characterization of a messenger-RNA polynucleotide vaccine vector. Cancer Res. 1995, 55, 1397–1400. [PubMed] [Google Scholar]
- Boczkowski D.; Nair S. K.; Snyder D.; Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996, 184, 465–472. 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W. Z.; Hoon D. S.; Huang S. K.; Fujii S.; Hashimoto K.; Morishita R.; Kaneda Y. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum. Gene Ther. 1999, 10, 2719–2724. 10.1089/10430349950016762. [DOI] [PubMed] [Google Scholar]
- Mandl C. W.; Aberle J. H.; Aberle S. W.; Holzmann H.; Allison S. L.; Heinz F. X. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat. Med. 1998, 4, 1438–1440. 10.1038/4031. [DOI] [PubMed] [Google Scholar]
- Hoerr I.; Obst R.; Rammensee H. G.; Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur. J. Immunol. 2000, 30, 1–7. . [DOI] [PubMed] [Google Scholar]
- Koido S.; Kashiwaba M.; Chen D.; Gendler S.; Kufe D.; Gong J. Induction of Antitumor Immunity by Vaccination of Dendritic Cells Transfected with MUC1 RNA1. J. Immunol. 2000, 165, 5713–5719. 10.4049/jimmunol.165.10.5713. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V; Forg P; Dalemans W; Chlichlia K; Zeng Y; Fournier P; von Hoegen P Intra-pinna anti-tumor vaccination with self-replicating infectious RNA or with DNA encoding a model tumor antigen and a cytokine. Gene Ther. 2000, 7, 1137–1147. 10.1038/sj.gt.3301220. [DOI] [PubMed] [Google Scholar]
- Kariko K.; Buckstein M.; Ni H. P.; Weissman D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Weide B.; Pascolo S.; Scheel B.; Derhovanessian E.; Pflugfelder A.; Eigentler T. K.; Pawelec G.; Hoerr I.; Rammensee H.-G.; Garbe C. Direct Injection of Protamine-protected mRNA: Results of a Phase 1/2 Vaccination Trial in Metastatic Melanoma Patients. J. Immunother. 2009, 32, 498–507. 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- Sahin U.; Karikó K.; Türeci Ö. mRNA-based therapeutics — developing a new class of drugs. Nat. Rev. Drug Discovery 2014, 13, 759–780. 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- Weissman D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. 10.1586/14760584.2015.973859. [DOI] [PubMed] [Google Scholar]
- Warren L.; Manos P. D.; Ahfeldt T.; Loh Y. H.; Li H.; Lau F.; Ebina W.; Mandal P. K.; Smith Z. D.; Meissner A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U.; Derhovanessian E.; Miller M.; Kloke B. P.; Simon P.; Löwer M.; Bukur V.; Tadmor A. D.; Luxemburger U.; Schrörs B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- Gillmore J. D.; Gane E.; Taubel J.; Kao J.; Fontana M.; Maitland M. L.; Seitzer J.; O’Connell D.; Walsh K. R.; Wood K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. 10.1056/NEJMoa2107454. [DOI] [PubMed] [Google Scholar]
- Vaccines and Related Biological Products Advisory Committee Meeting. Moderna COVID-19 Vaccine. FDA Briefing Document. https://www.fda.gov/media/144434/download (accessed Dec 22, 2020).
- Emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) in individuals 16 years of age and older. https://www.fda.gov/media/144414/download (accessed Dec 22, 2020).
- FDA Approves First COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed Dec 27, 2021).
- Arevalo C. P.; Bolton M. J.; Le Sage V.; Ye N.; Furey C.; Muramatsu H.; Alameh M.-G.; Pardi N.; Drapeau E. M.; Parkhouse K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. 10.1126/science.abm0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A. D.; Standish M. M.; Watkins J. C. Diffusion of Univalent Ions Across Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252. 10.1016/S0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Lockard R. E.; Lingrel J. B. Synthesis of mouse hemoglobin beta-chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem. Biophys. Res. Commun. 1969, 37, 204–212. 10.1016/0006-291X(69)90720-7. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B.; Lane C. D.; Woodland H. R.; Marbaix G. Use of frog eggs and oocytes for study of messenger RNA and its translation in living cells. Nature 1971, 233, 177–182. 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G.; Bloomgarden D.; Kaplan R.; Cohen C.; Hoffstein S.; Collins T.; Gotlieb A.; Nagle D. General Method for Introduction of Enzymes by Means of Immunoglobulin-Coated Liposomes, into Lysosomes of Deficient Cells. Proc. Natl. Acad. Sci. U. S. A. 1975, 72, 88–92. 10.1073/pnas.72.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C.; Gregoriadis G.. Liposomes as Immunological Adjuvants. In Lymphocytes, Macrophages, and Cancerl Mathé G., Florentin I., Simmler M.-C., Eds.; Springer: Berlin, Heidelberg, 1976; pp 58–64. [DOI] [PubMed] [Google Scholar]
- Geall A. J.; Verma A.; Otten G. R.; Shaw C. A.; Hekele A.; Banerjee K.; Cu Y.; Beard C. W.; Brito L. A.; Krucker T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 14604–14609. 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberer M.; Gnad-Vogt U.; Hong H. S.; Mehr K. T.; Backert L.; Finak G.; Gottardo R.; Bica M. A.; Garofano A.; Koch S. D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- Safety, Tolerability, and Immunogenicity of VAL-506440 in Healthy Adult Subjects; ClinicalTrials.gov, 2017. [Google Scholar]
- Bahl K.; Senn J. J.; Yuzhakov O.; Bulychev A.; Brito L. A.; Hassett K. J.; Laska M. E.; Smith M.; Almarsson Ö.; Thompson J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther.: J. Am. Soc. Gene Therapy 2017, 25, 1316–1327. 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) in individuals 18 years of age and older. https://www.fda.gov/media/144638/download (accessed Dec 22, 2020).
- Federici S.; Kredo-Russo S.; Valdés-Mas R.; Kviatcovsky D.; Weinstock E.; Matiuhin Y.; Silberberg Y.; Atarashi K.; Furuichi M.; Oka A.; et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 2022, 185, 2879–2898.e24. 10.1016/j.cell.2022.07.003. [DOI] [PubMed] [Google Scholar]
- Felgner J. H.; Kumar R.; Sridhar C. N.; Wheeler C. J.; Tsai Y. J.; Border R.; Ramsey P.; Martin M.; Felgner P. L. Enhanced Gene Delivery and Mechanism Studies with A Novel Series of Cationic Lipid Formulations. J. Biol. Chem. 1994, 269, 2550–2561. 10.1016/S0021-9258(17)41980-6. [DOI] [PubMed] [Google Scholar]
- Heiser A.; Coleman D.; Dannull J.; Yancey D.; Maurice M. A.; Lallas C. D.; Dahm P.; Niedzwiecki D.; Gilboa E.; Vieweg J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Invest. 2002, 109, 409–417. 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon Y.; McCammon J. M.; Miller J. C.; Faraji F.; Ngo C.; Katibah G. E.; Amora R.; Hocking T. D.; Zhang L.; Rebar E. J.; et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 702–708. 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormann M. S. D.; Hasenpusch G.; Aneja M. K.; Nica G.; Flemmer A. W.; Herber-Jonat S.; Huppmann M.; Mays L. E.; Illenyi M.; Schams A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- CAS Content Collection. https://www.cas.org/about/cas-content (accessed Jan 18, 2023).
- Sasso J. M.; Ambrose B. J. B.; Tenchov R.; Datta R. S.; Basel M. T.; DeLong R. K.; Zhou Q. A. The Progress and Promise of RNA Medicine—An Arsenal of Targeted Treatments. J. Med. Chem. 2022, 65, 6975–7015. 10.1021/acs.jmedchem.2c00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov R.; Sasso J. M.; Wang X.; Liaw W.-S.; Chen C.-A.; Zhou Q. A. Exosomes—Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. 10.1021/acsnano.2c08774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugeri M.; Nawaz M.; Papadimitriou A.; Angerfors A.; Camponeschi A.; Na M.; Hölttä M.; Skantze P.; Johansson S.; Sundqvist M.; et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nature Communications 2019, 10, 4333. 10.1038/s41467-019-12275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meet the mRNA vaccine rookies aiming to take down COVID-19. http://biotech-spain.com/en/articles/meet-the-mrna-vaccine-rookies-aiming-to-take-down-covid-19/ (accessed Feb 21, 2023).
- Carvalho T., mRNA vaccine effective against RSV respiratory disease. Nat. Med. 2023, DOI: 29755. 10.1038/d41591-023-00017-7 [DOI] [PubMed] [Google Scholar]
- Moderna Announces mRNA-1345, an Investigational Respiratory Syncytial Virus (RSV) Vaccine, Has Met Primary Efficacy Endpoints in Phase 3 Trial in Older Adults. https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx (accessed Jan 30, 2023).
- Collier A.-r. Y.; Miller J.; Hachmann N. P.; McMahan K.; Liu J.; Bondzie E. A.; Gallup L.; Rowe M.; Schonberg E.; Thai S. Immunogenicity of the BA.5 Bivalent mRNA Vaccine Boosters. bioRxiv Preprint 2022, 2022.2010.2024.513619. 10.1101/2022.10.24.513619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Bowen A.; Valdez R.; Gherasim C.; Gordon A.; Liu L.; Ho D. D. Antibody responses to Omicron BA.4/BA.5 bivalent mRNA vaccine booster shot. bioRxiv Preprint 2022, 2022.2010.2022.513349. 10.1101/2022.10.22.513349. [DOI] [Google Scholar]
- Offit P. A. Bivalent Covid-19 Vaccines — A Cautionary Tale. N. Engl. J. Med. 2023, 388, 481. 10.1056/NEJMp2215780. [DOI] [PubMed] [Google Scholar]
- Sebastian M.; Schröder A.; Scheel B.; Hong H. S.; Muth A.; von Boehmer L.; Zippelius A.; Mayer F.; Reck M.; Atanackovic D.; et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. 2019, 68, 799–812. 10.1007/s00262-019-02315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman J.; Burris H.; Clarke J.; Patel M.; Cho D.; Gutierrez M.; Julian R.; Scott A.; Cohen P.; Frederick J.; et al. 798 Safety, tolerability, and immunogenicity of mRNA-4157 in combination with pembrolizumab in subjects with unresectable solid tumors (KEYNOTE-603): an update. J. ImmunoTherapy .Cancer 2020, 8, A477–A477. 10.1136/jitc-2020-SITC2020.0798. [DOI] [Google Scholar]
- Moderna and Merck Announce mRNA-4157/V940, an Investigational Personalized mRNA Cancer Vaccine, in Combination with Keytruda(R) (Pembrolizumab), Met Primary Efficacy Endpoint in Phase 2B Keynote-942 Trial. https://investors.modernatx.com/news/news-details/2022/Moderna-and-Merck-Announce-mRNA-4157V940-an-Investigational-Personalized-mRNA-Cancer-Vaccine-in-Combination-with-KEYTRUDAR-pembrolizumab-Met-Primary-Efficacy-Endpoint-in-Phase-2b-KEYNOTE-942-Trial/default.aspx (accessed Jan 30, 2023).
- Pipeline of Arcturus mRNA Therapeutic and Vaccines. https://arcturusrx.com/mrna-medicines-pipeline/#lunarOTC (accessed Jan 26, 2023).
- AZD8601 EPICCURE Phase II trial demonstrated safety and tolerability in patients with heart failure. https://www.astrazeneca.com/media-centre/press-releases/2021/azd8601-epiccure-phase-ii-trial-demonstrated-safety-and-tolerability-in-patients-with-heart-failure.html (accessed Jan 26, 2023).
- Breakthrough technologies across four different drug classes to revolutionize medicine. https://www.biontech.com/int/en/home/pipeline-and-products/pipeline.html#bnt141-solid-tumors-1 (accessed Jan 26, 2023).
- Clinical Trials. https://www.clinicaltrials.gov/ (accessed Apr 20, 2023).
- Taylor N. P.BIOTECH: Translate Bio’s mRNA fails to improve lung function in cystic fibrosis patients. https://www.fiercebiotech.com/biotech/translate-bio-s-mrna-fails-to-improve-lung-function-cystic-fibrosis-patients (accessed Jan 26, 2023).
- Schaefer M.; Kapoor U.; Jantsch M. F. Understanding RNA modifications: the promises and technological bottlenecks of the ‘epitranscriptome’. Open Biol. 2017, 7, 170077. 10.1098/rsob.170077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner E.; Yang R.; Foo K. S.; Goedel A.; Chien K. R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 2022, 40, 1586–1600. 10.1038/s41587-022-01491-z. [DOI] [PubMed] [Google Scholar]
- Kim D. Y.; Atasheva S.; McAuley A. J.; Plante J. A.; Frolova E. I.; Beasley D. W. C.; Frolov I. Enhancement of protein expression by alphavirus replicons by designing self-replicating subgenomic RNAs. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 10708–10713. 10.1073/pnas.1408677111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay P. F.; Hu K.; Blakney A. K.; Samnuan K.; Brown J. C.; Penn R.; Zhou J.; Bouton C. R.; Rogers P.; Polra K.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523. 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselhoeft R. A.; Kowalski P. S.; Anderson D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018, 9, 2629. 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunovska K.; Loughrey D.; Dahlman J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov R.; Bird R.; Curtze A. E.; Zhou Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- Hou X.; Zaks T.; Langer R.; Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Sun C.; Wang C.; Jankovic K. E.; Dong Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S.; Wang Y.; Gong Y.; Lin X.; Zhao Y.; Zhi D.; Zhou Q.; Zhang S. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol. Res. (Camb.) 2018, 7, 473–479. 10.1039/C8TX00005K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; Tian J.; Chen X. Effect of surface properties on liposomal siRNA delivery. Biomaterials 2016, 79, 56–68. 10.1016/j.biomaterials.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R.; Hope M. J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N.; Weissman D.; Whitehead K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discovery 2021, 20, 817–838. 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R.; Minamitake Y.; Peracchia M. T.; Trubetskoy V.; Torchilin V.; Langer R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- Su S.-A.; Xie Y.; Fu Z.; Wang Y.; Wang J.-A.; Xiang M. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget 2017, 8, 25700. 10.18632/oncotarget.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D.; Zickler A. M.; El Andaloussi S. Dosing extracellular vesicles. Adv. Drug Del. Rev. 2021, 178, 113961. 10.1016/j.addr.2021.113961. [DOI] [PubMed] [Google Scholar]
- Boisguérin P.; Konate K.; Josse E.; Vivès E.; Deshayes S. Peptide-Based Nanoparticles for Therapeutic Nucleic Acid Delivery. Biomedicines 2021, 9, 583. 10.3390/biomedicines9050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.; Li X.; Zhao R.; Wu E.; Du Q.; Guo J.; Wang L.; Zhang F. Creating de novo peptide-based bioactivities: from assembly to origami. RSC Adv. 2022, 12, 25955–25961. 10.1039/D2RA03135C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Alarcón C. N.; Liu B.; Watson F.; Searles S.; Lee C. K.; Keys J.; Pi W.; Allen D.; Lammerding J.; et al. Genetically engineered and enucleated human mesenchymal stromal cells for the targeted delivery of therapeutics to diseased tissue. Nat. Biomed. Eng. 2022, 6, 882–897. 10.1038/s41551-021-00815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B.; Zhao B.; Wang K.; Guo Y.; Lu Q.; Zheng L.; Li A.; Qiao J. Neutrophils mediated multistage nanoparticle delivery for prompting tumor photothermal therapy. J. Nanobiotechnol. 2020, 18, 138. 10.1186/s12951-020-00682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman P. M.; Hood E. D.; Ferguson L. T.; Zhao Z.; Siegel D. L.; Mitragotri S.; Brenner J. S.; Muzykantov V. R. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv. Drug Deliv. Rev. 2021, 178, 113992. 10.1016/j.addr.2021.113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloren K. K. S.; Jawalagatti V.; Hewawaduge C.; Chandran S.; Park J.-Y.; Lee J. H. Salmonella-mediated oral delivery of multiple-target vaccine constructs with conserved and variable regions of SARS-CoV-2 protect against the Delta and Omicron variants in hamster. Microbes Infect. 2023, 25, 105101. 10.1016/j.micinf.2023.105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland K. L.; Brenneman K. E. Salmonella as a vaccine delivery vehicle. Expert Rev. Vaccines 2013, 12, 1033–1045. 10.1586/14760584.2013.825454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H.; Zhu X.; Li S.; Wang P.; Fang J. Liver-Targeted Delivery of Oligonucleotides with N-Acetylgalactosamine Conjugation. ACS Omega 2021, 6, 16259–16265. 10.1021/acsomega.1c01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacker A. J.; Voutila J.; Catley M.; Blakey D.; Habib N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 2020, 28, 1759–1771. 10.1016/j.ymthe.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan J.; Muñoz-Muñoz J.; Turnbull G.; Sim N.; Penny S.; Moschos S. Beyond GalNAc! Drug delivery systems comprising complex oligosaccharides for targeted use of nucleic acid therapeutics. RSC Adv. 2022, 12, 20432–20446. 10.1039/D2RA01999J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q.; Wei T.; Farbiak L.; Johnson L. T.; Dilliard S. A.; Siegwart D. J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Ye Z.; Huang C.; Qiu M.; Song D.; Li Y.; Xu Q. Lipid nanoparticle-mediated lymph node—targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, e2207841119. 10.1073/pnas.2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed J. R.; Yerneni S. S.; Arral M. L.; LoPresti S. T.; Chaudhary N.; Sehrawat A.; Muramatsu H.; Alameh M.-G.; Pardi N.; Weissman D.; et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic cells via macrophage-mediated gene transfer. Sci. Adv. 2023, 9, eade1444. 10.1126/sciadv.ade1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Jozic A.; Lin Y.; Eygeris Y.; Bloom E.; Tan X.; Acosta C.; MacDonald K. D.; Welsher K. D.; Sahay G. Engineering Lipid Nanoparticles for Enhanced Intracellular Delivery of mRNA through Inhalation. ACS Nano 2022, 16, 14792–14806. 10.1021/acsnano.2c05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B.; Isaacs C.; Cheng L.; Asrani K. H.; Subramanian R. R. SERPINA1 mRNA as a Treatment for Alpha-1 Antitrypsin Deficiency. J. Nucleic Acids 2018, 2018, 8247935. 10.1155/2018/8247935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakova Y.; Kowalski P. S.; Huang Y.; Gonzalez J. T.; Heartlein M. W.; DeRosa F.; Delcassian D.; Anderson D. G. mRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019, 27, 1415–1423. 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienke C.; Kolb L.; Diken E.; Streuber M.; Kirchhoff S.; Bukur T.; Akilli-Öztürk Ö.; Kranz L. M.; Berger H.; Petschenka J.; et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153. 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- Rojas L. A.; Sethna Z.; Soares K. C.; Olcese C.; Pang N.; Patterson E.; Lihm J.; Ceglia N.; Guasp P.; Chu A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. 10.1038/s41586-023-06063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.