Abstract
Post-traumatic epilepsy (PTE) occurs in some patients after moderate/severe traumatic brain injury (TBI). Although there are no approved therapies to prevent epileptogenesis, levetiracetam (LEV) is commonly given for seizure prophylaxis due to its good safety profile. This led us to study LEV as part of the Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx) Project. The objective of this work is to characterize the pharmacokinetics (PK) and brain uptake of LEV in naïve control rats and in the lateral fluid percussion injury (LFPI) rat model of TBI after either single intraperitoneal doses or a loading dose followed by a 7-day subcutaneous infusion. Sprague-Dawley rats were used as controls and for the LFPI model induced at the left parietal region using injury parameters optimized for moderate/severe TBI. Naïve and LFPI rats received either a bolus injection (intraperitoneal) or a bolus injection followed by subcutaneous infusion over 7 days. Blood and parietal cortical samples were collected at specified time points throughout the study. LEV concentrations in plasma and brain were measured using validated high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) methods. Noncompartmental analysis and a naive-pooled compartmental PK modeling approach were used. Brain-to-plasma ratios ranged from 0.54 to 1.4 to 1. LEV concentrations were well fit by one-compartment, first-order absorption PK models with a clearance of 112 ml/h per kg and volume of distribution of 293 ml/kg. The single-dose pharmacokinetic data were used to guide dose selection for the longer-term studies, and target drug exposures were confirmed. Obtaining LEV PK information early in the screening phase allowed us to guide optimal treatment protocols in EpiBioS4Rx.
SIGNIFICANCE STATEMENT
The characterization of levetiracetam pharmacokinetics and brain uptake in an animal model of post-traumatic epilepsy is essential to identify target concentrations and guide optimal treatment for future studies.
Introduction
The risk of epilepsy after traumatic brain injury (TBI) has been reported to be ∼3%–4% (Bazarian et al., 2009; Burke et al., 2021). However, the post-traumatic epilepsy (PTE) risk increases with more severe injury and occurs in up to 25% of patients after moderate/severe traumatic brain injury (TBI) (Bazarian et al., 2009). In PTE, unprovoked seizures occur over the course of weeks, months, or years after TBI. Although there are numerous studies of PTE development (Ali et al., 2019; Gorter et al., 2019; Saletti et al., 2019; Mukherjee et al., 2020), the pathophysiological mechanisms of PTE that govern which patients are more likely to develop PTE are not well understood (Brady et al., 2019). Further, there are no approved therapies to prevent epileptogenesis after TBI.
The Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx) Project is a multidisciplinary program aimed at identifying biomarkers of epileptogenesis and therapies to prevent PTE after TBI (Engel, 2019). One of the EpiBioS4Rx projects is a multicenter preclinical therapy screening platform using the lateral fluid percussion injury (LFPI) rat model of TBI. Here our team has implemented pharmacokinetic (PK) studies early in preclinical research to screen drug candidates and optimize doses and dosing regimens for the longer-term studies. A key aspect of this project is the use of multiple sites around the world to increase reproducibility and also to consider site-specific factors that could contribute to the variability of results.
Levetiracetam (LEV) is commonly given for early post-TBI seizure prophylaxis due to its good safety profile (Chaari et al., 2017). LEV modulates synaptic neurotransmitter release through binding to the synaptic vesicle protein SV2A in the brain. It is approved by the US Food and Drug Administration (FDA) as both oral and injectable formulations for the treatment of certain seizure disorders. Although some animal studies have suggested that LEV may possess antiepileptogenic properties, confirmatory studies are needed (Klitgaard and Pitkänen, 2003; Brady et al., 2019; Casillas-Espinosa et al., 2019; Klein et al., 2020). For these reasons, EpiBios4Rx selected it as an early drug candidate and to use as a reference for comparison with potential other targeted drug therapies.
The objective of this single-center study was to characterize the PK and brain uptake of LEV in naïve and injured animals in two studies: 1) after a single intraperitoneal dose and 2) after (Löscher et al., 1998) an intraperitoneal loading dose followed by a 7-day subcutaneous infusion (i.e., the time period used for early post-TBI seizure prophylaxis). A sparse sampling population PK model approach was to minimize the number of animals used in the study. The single-dose PK data were used for screening purposes as well as to guide dose selection for the longer-term studies. Osmotic minipumps were used to deliver LEV subcutaneously so as to maintain target concentrations over 7 days.
Materials and Methods
Animal Study
Animals and Injury Induction
Approval was obtained through the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine prior to study initiation (IACUC #20170107). Male ∼11- week-old Sprague-Dawley rats (Taconic Farms, Rensselaer, NY) were used as naïve controls or after LFPI induction. LFPI animals were submitted to a 5-mm-diameter circular left parietal craniotomy (coordinates of the craniotomy center: −4.5 mm posterior to the bregma, 3 mm left of the sagittal suture) (McIntosh et al., 1989). The craniotomy was performed, keeping the dura matter intact, and a female open end of a Luer lock of an 18G needle trimmed to 4-mm diameter was placed on it as an adaptor and fixed with 3M Vetbond (3M Animal Care Products, St Paul, MN) and dental cement. After withdrawal of the anesthesia and when the first toe pinch response was noted, the rat was connected with the Luer lock adaptor to the fluid percussion injury (FPI) device (AmScien Fluid Percussion Device model FP 302; Richmond, VA). A pressure pulse was delivered by the FPI device at injury parameters optimized to induce moderate/severe TBI with a mortality of ∼30%: 3.26 ± 0.1 atmospheres (atm) (Kharatishvili et al., 2006).
Levetiracetam Formulation and Administration
LEV was purchased from MedChemExpress (HY-B0106; NJ). For the bolus injection, a 200-mg/ml solution diluted in 0.9% sterile saline was prepared at the day of experiment. For the osmotic minipump (ALZET 2ML1; Durect, Cupertino, CA) preparation, the concentration of the solution was calculated according to manufacturer instructions based on the body weight of the animal to deliver 200 mg/kg per day. The minipump was prepared on the previous day of the experiment and kept overnight at 37°C for priming.
Single-Dose Intraperitoneal PK Study
A single intraperitoneal bolus injection of 200 mg/kg LEV was administered in naïve rats (n = 6). Blood samples were collected from the lateral tail vein prior to dosing and 0.5, 1, 2, 4, or 6 hours after the administration under isoflurane anesthesia. A 6-hour collection window was considered adequate to capture the elimination phase, assuming a half-life of 1.5–2 hours. Three blood samples were collected from each rat at the designated time points to reduce the number of animals used for this study. Brain samples were collected at 2 hours or 6 hours after LEV bolus injection. Rats were anesthetized with isoflurane 5% in 100% oxygen prior to harvesting brain tissue. The results were collected and analyzed according to a preset plan with three blood samples per time point collected. This provided an adequate number of data points to characterize the PK while minimizing the number of animals used in the study.
Loading Dose and 7-Day Continuous Infusion PK Study
A single intraperitoneal bolus injection of 200 mg/kg LEV was administered in naïve rats or immediately after LFPI (n = 9). Subcutaneous (s.c.) ALZET 2ML1 minipump placement was done 1 hour later under isoflurane (Henry Schein, Melville, NY) anesthesia (5% induction, 2% maintenance). LEV was given as a bolus of 200 mg/kg i.p. followed by 200 mg/kg per day s.c. via minipump for 7 days, at which time the minipump was removed. Blood samples (three per group per time point) were collected at baseline and 1, 2, 73, 121, or 169 hours (i.e., prior to minipump removal) after the bolus administration. Additional blood samples were collected 0.5, 2, or 4 hours after the minipump removal on day 7. Right and left parietal cortical samples were collected at 2 or 121 hours after the bolus LEV administration or 4 hours after minipump removal on day 7. Rats were anesthetized with isoflurane 5% in 100% oxygen prior to harvesting brain tissue. The results were collected and analyzed according to a preset plan with three blood samples per time point collected to provide an adequate number of data points to characterize the PK while minimizing the number of animals used in the study.
Dose Selection Rational
A 200-mg/kg dose converted to human equivalent dose using the US Food and Drug Administration (FDA) guidance is equivalent to 32 mg/kg, which is within the range of doses used for status epilepticus, which range from 20 to 60 mg/kg (Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017; Nene et al., 2019; Sathe et al., 2021). The human equivalent daily dose of 32 mg/kg per day is on the high end of doses that have been used for prophylaxis after traumatic brain injury, which range from <1000 to >3000 mg per day (Patanwala et al., 2016; Ohman et al., 2023).
Blood Collection
Blood was collected from isoflurane-anesthetized rats via lateral tail vein puncture into K2-EDTA microtainer tubes (#365975, BD Microtainer; BD Biosciences). Blood was centrifuged at 3000 g for 10 minutes at 4°C immediately after collection, and the supernatant plasma was collected and stored at −20°C until analysis. Aliquots were kept in Eppendorf LoBind Tubes (#022431064; Fisher Scientific) at −20°C until use.
Brain Samples Collection
Parietal cortical samples were harvested after removal of meninges and superficial vasculature in phosphate-buffered saline on ice. The left (LCCX) and right cerebral cortices (RCCX) were dissected, flash frozen, and stored at −80°C. Brain and plasma samples were analyzed for LEV concentrations as described below.
Measurement of LEV in Brain and Plasma Samples
LEV was extracted from plasma and brain samples using acetonitrile. The rat brain tissue was homogenized in PBS using a bullet blender and zirconium beads and sonicated for 3 minutes. Two milliliters of acetonitrile was added to 0.1 ml of plasma or homogenate and vortexed for ∼5 seconds. All samples were centrifuged for 10 minutes at 2000 g, and the supernatant was then collected in clean glass tubes and evaporated at 35°C using nitrogen. The samples were reconstituted in 0.15 ml of mobile phase and filtered using 13-mm Pall Acrodisc (Pall Corporation, MI) syringe filters (0.2 micron) for liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis. Deuterated levetiracetam (Levetiracetam-D6; Sigma-Aldrich) was used as the internal standard.
Quality control samples were prepared at three concentrations: low (10 μg/ml), mid (100 μg/ml), and high (500 μg/ml) for brain and plasma samples. The quality control samples were mixed well, aliquoted into labeled microcentrifuge tubes, and stored at −80°C. Ten calibration standards were prepared using serial dilutions of a standard, resulting in concentrations ranging from 1 to 1000 μg/ml of LEV. Stock solutions for quality control and calibration standards were prepared separately.
The samples were analyzed using a TSQ Quantum Access triple quad mass spectrometer (Thermo Scientific, CA) with an electrospray and a Dionex Ultimate 3000 HPLC system (CA) that included an autosampler and pump. Reverse phase chromatographic separation was performed using an InfinityLab Poroshell 120 EC-C18 (Agilent, CA) column (2.1 × 100 mm, 2.7 μm) (Supplemental Fig. 1). The analytes were separated using isocratic mobile phase with a composition of 25% 10 mM ammonium acetate and 75% acetonitrile at a flow rate of 0.15 ml/min and run time of 5 minutes. Excalibur software (Thermo Scientific) was used for data acquisition and analysis. The conditions for LC-MS/MS included heated electrospray ionization (HESI) with a vaporizing temperature of 300°C and capillary temperature of 350°C. The spray voltage was set at 3500 V and collision gas pressure of 1.5 millitorrs (mTorr) for the multiple reaction monitor (MRM). The detection of LEV was done using positive polarity. The mass-to-charge (m/z) ratio for parent and product ions used for the multiple reaction monitoring method was 171 and 126.
Quality control samples were evaluated for precision and accuracy. Precision was determined using the percent coefficient of variation (%CV) and accuracy by the percentage of measured concentration relative to the nominal concentration. The limit of detection (LOD) was determined using a signal-to-noise ratio of 3.0 using a neat standard. The lower limit of quantitation (LLOQ) was determined to be the lowest concentration at which an analyte in the sample matrix was measured within accuracy (±20%) and precision (<20%) limits. The standard concentrations were linear from 1 to 1000 mg/l. Relative standard deviation for replicate measurements in plasma samples was less than 5%. Drug concentrations were calculated using the linear equation determined by the absorbance areas of the calibration standards.
Pharmacokinetic Analysis
Using the LEV plasma concentration-time data after a single intraperitoneal loading dose, PK parameters were calculated using noncompartmental analysis (Phoenix, V 8.1; Certara, NC) with pooled naïve averaging. Maximum concentration (Cmax), area under the concentration-time curve (AUC), terminal phase half-life (t1/2), total body clearance (CL/F), and volume of distribution (Vd/F), were calculated. AUC was calculated using the linear-log trapezoidal method. Brain-to-plasma ratios were calculated by dividing the plasma concentration by the brain concentration at any given time point.
One-, two-, and three compartment PK models were also evaluated using a naïve-pooled population approach with Phoenix, NLME (V 8.1; Certara, NC) (Supplemental Fig. 2). The best fit model was determined by the log-likelihood ratio, Akaike’s information, Schwarz criterion, visual observation of goodness-of-fit plots (including residual plots), and precision of model parameters. A first-order conditional estimation method was used. The final structural model was one compartment with a proportional error model. The best fit model was determined by successful minimization, the objective function value, visual observation of goodness-of-fit plots (including residual plots), and precision of model parameters. The compartmental PK model was used to simulate concentration-time profiles (n = 100) for a single intraperitoneal loading dose followed by a subcutaneous constant infusion (assuming the same bioavailability for both routes of administration). The bioavailability was assumed to be similar for both routes of administration given that levetiracetam is completely absorbed with very little first-pass metabolism (F > 90%), similar to humans (Benedetti et al., 2004).
A paired Student’s t test was used to compare LEV concentrations in LCCX and RCCX. A P value less than 0.05 was considered statistically significant.
Results
Single Intraperitoneal Loading Dose PK
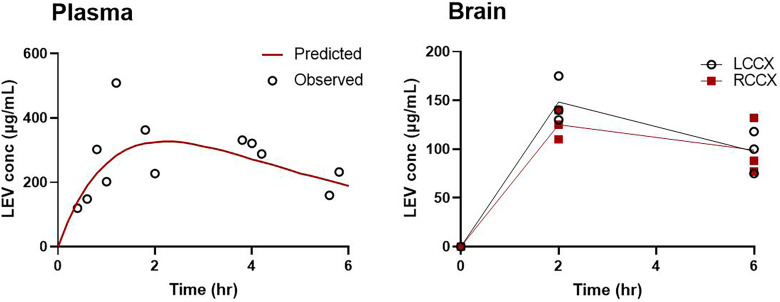
Figure 1 shows the LEV concentration-time profiles in plasma and brain in naïve rats after a single 200-mg/kg intraperitoneal bolus dose. After dosing, maximum LEV concentrations in the plasma occurred at 1.5 hours and then declined in a monoexponential manner. The brain concentrations generally followed those in plasma, which had a time to reach maximum concentration (Tmax) of 2 hours. Brain concentrations at 2 hours approached those in the plasma with mean brain-to-plasma ratios of 0.79 and 0.61 in the LCCX and RCCX, respectively. At 6 hours, the mean brain-to-plasma ratios were 0.87 and 0.70 in the LCCX and RCCX, respectively. Although LCCX mean concentrations were greater than for the RCCX, these differences are not statistically significant.
Fig. 1.
Levetiracetam plasma and brain (LCCX and RCCX) concentrations after a single intraperitoneal dose (200 mg/kg) in naïve rats.
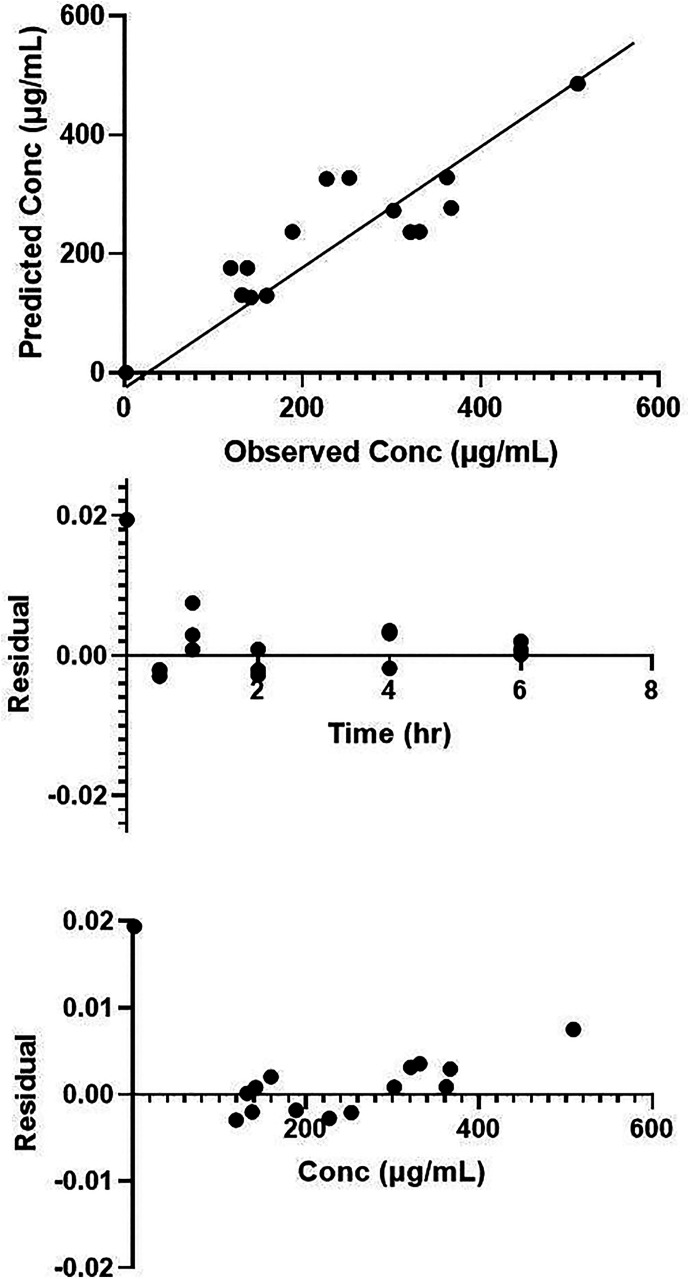
Noncompartmental analysis resulted in a Tmax of 1 hour, Cmax of 326 (S.E. = 142) μg/ml, and AUC of 1200 (S.E. = 210) μg*h/ml. One-, two-, and three-compartment PK models were also evaluated using a naïve-pooled approach. LEV concentrations were best fit by a first-order absorption, one-compartment PK model (Fig. 1; Supplemental Model Text). The typical value estimates of the PK parameters are provided in Table 1. Although the precision of the model parameters was adequate, the percent relative standard error of the parameter estimate (%RSE) for the absorption rate constant, Ka, was high (68%). This is likely due to the limited number of data points used to characterize the absorption phase. The proportional error model best described the residual unexplained variability of 30%. Goodness-of-fit plots (predicted vs. observed plasma concentrations and residual plots) are shown in Fig. 2. The primary PK parameters correspond with an elimination half-life of 2 hours, which agrees with previously published literature (Löscher et al., 1998).
TABLE 1.
Levetiracetam pharmacokinetic parameter estimates using a first-order, one-compartment PK model
| Parameter | Estimatea | %RSE |
|---|---|---|
| Ka (1/h) | 0.46 | 66 |
| V/F (ml) | 73.3 | 41 |
| CL/F (ml/h) | 28.0 | 18 |
| RUV (%CV) | 29 | 29 |
| t1/2 (h) | 1.8 |
CL, clearance; %CV: percent coefficient of variation; F, bioavailability; Ka, absorption rate constant; %RSE: percent relative standard error of the parameter estimate; RUV, residual unexplained variability; t1/2, plasma elimination half-life; V, volume of distribution.
aEstimates are for typical rat (250 g).
Fig. 2.
Goodness-of-fit plots for first order, one-compartment PK model. Panel (A): predicted concentration versus observed concentration; panel (B): residual versus time; panel (C): residual versus concentration.
Intraperitoneal Loading Dose and 7-Day Subcutaneous Continuous Infusion PK
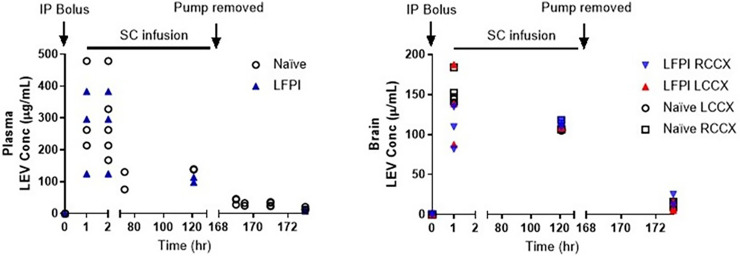
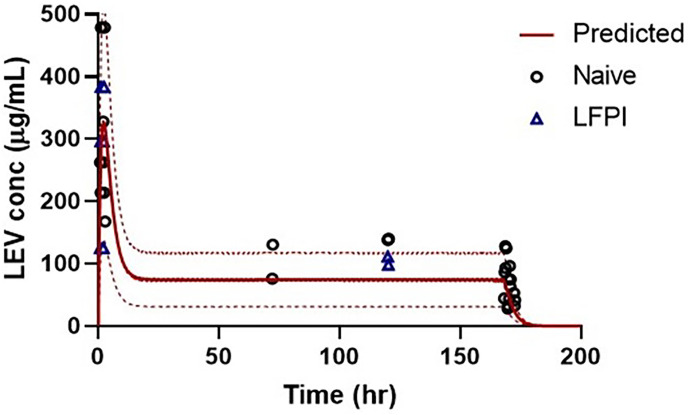
LEV concentrations in brain and plasma are shown in Fig. 3. LEV concentrations after the loading dose were similar to those obtained in the single-dose study, with maximum concentrations ranging from ∼100 to 400 μg/ml at 2 hours postdosing. Further, LEV plasma concentrations were maintained at ∼100 μg/ml during the subcutaneous minipump dosing. Brain-to-plasma ratios were higher in LFPI rat brains, especially in the left cortex (site of injury) (Table 2). Although mean LEV concentrations were consistently higher in the left than in the right cortex of LFPI rats, these differences were only statistically significant at the 7-day collection period 4 hours after minipump removal (P = 0.02). LEV concentrations in the LCCX of the LFPI rats were also statistically greater than in the naïve rats 4 hours after minipump removal (P = 0.02). Similar to brain concentrations, LEV brain-to-plasma ratios are generally greater in LFPI versus control rats, suggesting greater uptake into and possibly slower clearance from the injured brain. Figure 4 shows the LEV concentrations predicted using the PK model and the actual concentrations. The observed values were similar to the predictions, and the means were within the predicted 90% confidence interval.
Fig. 3.
Levetiracetam plasma and brain concentrations after a single intraperitoneal (i.p.) dose of 200 mg/kg LEV administered in naïve rats or immediately after LFPI followed by a subcutaneous (s.c.) continuous infusion of 200 mg/kg per day LEV for 7 days.
TABLE 2.
Mean (S.D.) levetiracetam brain concentrations and corresponding mean brain-to-plasma ratios in naïve and LFPI rats with continuous 7-day LEV subcutaneous treatment (n = 3 per group per time point)
| Naïve | LFPI | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | RCCX | LCCX | RCCX | LCCX | ||||
| Conc (μg/ml) | Brain: Plasma | Conc (μg/ml) | Brain: Plasma | Conc (μg/ml) | Brain: Plasma | Conc (μg/ml) | Brain: Plasma | |
| 1 | 161 (20.1) | 0.54 | 131 (13.2) | 0.63 | 109 (26.4) | 0.72 | 138 (49.9) | 0.90 |
| 120 | 110 (16.7) | 0.76 | 106 (11.3) | 0.79 | 109 (15.4) | 1.06 | 124 (13.0) | 1.2 |
| 172 | 12.0 (1.48) | 0.72 | 9.91 (3.93)b | 0.95 | 9.55 (5.51)a | 0.85 | 18.5 (6.61)a,b | 1.4 |
aStatistically significant difference between RCCX and LCCX of LFPI (P = 0.02, Student’s t test).
bStatistically significant difference in LCCX concentrations between naïve and LFPI rats (P = 0.02, Student’s t test).
Fig. 4.
Comparison of predicted and observed plasma LEV concentrations after a single i.p. dose (200 mg/kg) LEV administered in naïve rats or immediately after LFPI followed by s.c. continuous infusion of 200 mg/kg per day LEV for 7 days. Predicted concentration shown with line, 95% upper and lower confidence intervals shown with dotted lines, and observed concentrations shown with symbols.
LEV Safety and Tolerability
Animals did not have any alterations in motor activity and coordination after LEV administration other than those typically observed after brain trauma. Although in our study the loading dose was 200 mg/kg followed by 200 mg/kg per day, LEV did not cause ataxia or sedation or any other adverse effects as previously observed (Glien et al., 2002).
Discussion
Administration of LEV using a subcutaneously implanted minipump was well tolerated and resulted in maintained LEV plasma concentrations of ∼100 μg/ml over the course of the 7-day study. Brain levels closely followed those in the plasma with brain-to-plasma ratios of 0.8–1. A PK model developed using data from a single intraperitoneal dose adequately predicted the drug concentrations attained after a single loading dose followed by 7-day subcutaneous infusion and can be used to simulate various doses/dosage regimens.
LEV PK reported here are consistent with findings by Löscher et al. (1998). In that study, single 54-mg/kg i.p. doses given to kindled and age-matched nonkindled rats resulted in maximum concentration of 54.8–60.3 μg/ml. Assuming linear pharmacokinetics, the dose-normalized concentrations would be in general agreement with our reported maximum concentration of ∼300 μg/ml for single 200-mg/kg i.p. doses in naïve rats. Elimination half-lives of 1.8 and 2.6 hours were also similar for the two studies (Löscher et al., 1998). In contrast, Glien et al. (2002) reported that a mean dose of 389 mg/kg per day via subcutaneous infusion resulted in a mean plasma concentration of 43.6 μg/ml. Our daily dose of 200 mg/mg per day results in plasma concentrations of ∼100 μg/ml. This discrepancy might be explained by differences in the two animal models, drug delivery methodology, and/or analytical assays. Further, Glien et al. (2002) used female Wistar rats, whereas our study was in male Sprague-Dawley rats. Sex differences in both metabolic and absorption pathways in rodents have been reported (Waxman and Holloway, 2009; Afonso-Pereira et al., 2018; Kutsukake et al., 2019). Nonetheless, our PK model developed using single-dose data was validated using the concentration-time data from the longer-term study showing that similar concentrations were attained across the two studies. This observation also supports that LEV PK is not substantially affected by TBI. Since LEV is currently available for both oral administration and injection for human use, there is substantial clinical information regarding drug exposure and safety.
There is substantial clinical information regarding drug exposure and safety. The peak concentrations measured in this study (108–470 μg/ml after a loading dose and 67–140 μg/ml during maintenance dosing) were similar to those that are attained in humans after 60 mg/kg LEV administered over 10-minute infusion, a dosing regimen that was safely and effectively used in an established status epilepticus (Sathe et al., 2021). In that study, concentrations measured ∼60 minutes after the start of infusion ranged from 80 to 160 μg/ml. Similarly, LEV concentrations of 60–189 μg/ml have also been reported in adults and children with epilepsy after mean i.v. doses of 34 and 47 mg/kg (Wheless et al., 2009). In contrast, a study of LEV for prevention of PTE reported mean maximum concentrations of 60.2 μg/ml after 55 mg/kg per day dose administered orally, nasogastrically, or intravenously (Klein et al., 2012). In this study, intravenous dosing resulted in mean Cmax concentrations of 78.4 μg/ml, generally lower than our concentrations (67–140 μg/ml) measured during maintenance dosing. Nonetheless, based on these reports, doses that attain these concentrations in humans have been shown to be generally safe, suggesting the ability to translate our preclinical research. A key property for CNS-acting drugs is reaching the site of action at a therapeutic concentration. The dosing regimen used in this study resulted in good brain uptake with drug concentrations that closely followed plasma. Accumulation in the brain was observed as the brain-to-plasma ratios increased over the course of the 7-day study. Tong and Patsalos (2001) have also shown prolonged efflux from the brain, consistent with our observations, which may extend its duration of action. Although uptake is not instantaneous, as demonstrated by a delay in achieving maximal brain concentrations, distribution into the brain remains rapid and appears sufficient for use after TBI. For example, even though LEV is less lipophilic and enters brain more slowly than the antiseizure medications fosphenytoin and valproic acid, it was found to be equally as effective in the treatment of established status epilepticus (Chamberlain et al., 2020). We also observed greater LEV concentrations in the left cerebral cortex, likely a result of increased leakiness of the blood-brain barrier in the ipsilateral cortex. Although it is possible that multidrug transporters are altered in the blood-brain barrier of LFPI rats, LEV is not considered a substrate for these transporters, suggesting that passive transport is increased upon injury. Notably, these differences were rather modest and may not be a consideration for local toxicity. It does present an avenue for more targeted drug delivery to the injured area.
Obtaining PK information early in the drug research and screening phase allows for PK considerations to play a role in drug selection and dose optimization early in drug development. We were able to 1) confirm that the dose used in our animal model produced clinically relevant plasma concentrations, 2) characterize the brain permeability, and 3) verify that the dosing regimen resulted in drug concentrations that were well tolerated in both naïve and LFPI animals. Further, a PK model was built using early PK data and was used to design a study to attain target steady-state concentrations for the longer-term studies. These data will subsequently be used to confirm that target exposures are attained at multiple sites. Further, the lack of differences in the pharmacokinetics between naïve and LFPI rats will allow us to use naïve animals to answer PK-related questions, reducing costs and study times. These models will be validated in future studies utilizing LEV and used to inform design of human clinical studies. Specifically, the PK model developed here will be to verify drug exposures from the longer-term efficacy studies, refine the PK model, and link with PD models relating drug concentration with response. This will allow for the prediction of response for various doses and dosing regimens. This approach may reduce development time, help to reach go/no-go decisions faster, and ensure harmonization of protocol across study sites.
Limitations include the relatively small number of doses and animals studied, which hindered the ability to provide an accurate estimate for the absorption rate constant. Further, given destructive sampling, the pharmacokinetic model did not include estimates of interindividual variability. Despite these limitations, these models do provide insight into drug exposures at doses that have shown biologic promise. These models will be validated in future antiepileptogenic studies and may ultimately be used to inform design of human clinical studies.
Abbreviations
- AUC
area under the concentration-time curve
- LCCX
left cerebral cortex
- LEV
levetiracetam
- LFPI
lateral fluid percussion injury
- PK
pharmacokinetic
- PTE
post-traumatic epilepsy
- RCCX
right cerebral cortex
- TBI
traumatic brain injury
Authorship Contributions
Participated in research design: Coles, Saletti, Lisgaras, Casillas-Espinosa, O’Brien, Moshé, Galanopoulou.
Conducted experiments: Saletti, Lisgaras, Casillas-Espinosa, Liu, Li, Jones, Shultz, Ali, Brady, Yamakawa, Hudson, Silva, Braine.
Contributed new reagents or analytic tools: Mishra.
Performed data analysis: Coles, Saletti, Lisgaras, Casillas-Espinosa, Galanopoulou.
Wrote or contributed to the writing of the manuscript: Coles, Saletti, Casillas-Espinosa, Cloyd, Galanopoulou.
Footnotes
The study was supported by National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grant U54-NS100064] (to A.S.G. and S.L.M.), [Grant R01-NS091170] (to A.S.G.), [Grant U54-NS43209] (to S.L.M.), and [Grant RFA-NS-16-012] (to T.J.O.); National Institutes of Health National Institute of Child Health and Human Development [Grant U54-HD090260] (to A.S.G.); US Department of Defense [Grant W81XWH-18-1-0612] (to A.S.G. and S.L.M.); NHMRC Early Career Fellowship [APP1087172] (to. P.J.C.-E.); NHMRC Program [APP1091593] (to T.J.O.) and Investigator [APP1176426] (to T.J.O.); American Epilepsy Society seed grant (to A.S.G.); and research funding from the Heffer Family Foundation, the Segal Family Foundation, and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families (to A.S.G. and S.L.M.).
The authors have declared a conflict of interest. A.S.G. is Editor in Chief of Epilepsia Open and has received royalties for publications from MedLink, Elsevier, and Morgan and Claypool publishers. S.L.M. is serving as Associate Editor of Neurobiology of Disease and is on the editorial board of Brain and Development, Pediatric Neurology, and Physiological Research. He receives from Elsevier an annual compensation for his work as Associate Editor of Neurobiology of Disease and royalties from two books that he coedited. He has received consultant’s fees from UCB and Pfizer. P.J.C.-E. acknowledges that his institution has received funding from Eisai, Supernus, KaosKey, and Praxis Pharmaceuticals. T.J.O. acknowledges that his institution has received consultancy and research funding from UCB Pharma, Eisai, ES Therapeutics, Zynerba, Praxis Pharmaceuticals, and Biogen.
Primary laboratory of origin: Center for Orphan Drug Research, Experimental and Clinical Pharmacology (University of Minnesota College of Pharmacy, Minneapolis, MN).
This work was previously presented as an abstract as follows: Coles L, Lisgaras CP, Liu W, Saletti PG, Casillas-Espinosa P, Jones N, Shultz S, Ali I, Brady R, Cloyd J, et al. (2019) The pharmacokinetics and brain distribution of potential antiepileptogenic therapies in a rat model of post-traumatic epilepsy. American Epilepsy Society Annual Meeting; 2019 Dec 6–10; Baltimore, MD. American Epilepsy Society, Chicago, IL.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Afonso-Pereira F, Dou L, Trenfield SJ, Madla CM, Murdan S, Sousa J, Veiga F, Basit AW (2018) Sex differences in the gastrointestinal tract of rats and the implications for oral drug delivery. Eur J Pharm Sci 115:339–344. [DOI] [PubMed] [Google Scholar]
- Ali I, Silva JC, Liu S, Shultz SR, Kwan P, Jones NC, O’Brien TJ (2019) Targeting neurodegeneration to prevent post-traumatic epilepsy. Neurobiol Dis 123:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N (2009) Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil 24:439–451. [DOI] [PubMed] [Google Scholar]
- Benedetti MS, Coupez R, Whomsley R, Nicolas JM, Collart P, Baltes E (2004) Comparative pharmacokinetics and metabolism of levetiracetam, a new anti-epileptic agent, in mouse, rat, rabbit and dog. Xenobiotica 34:281–300. [DOI] [PubMed] [Google Scholar]
- Brady RD, Casillas-Espinosa PM, Agoston DV, Bertram EH, Kamnaksh A, Semple BD, Shultz SR (2019) Modelling traumatic brain injury and posttraumatic epilepsy in rodents. Neurobiol Dis 123:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J, Gugger J, Ding K, Kim JA, Foreman B, Yue JK, Rabinowitz M, et al. (2021) Association of Posttraumatic Epilepsy With 1-Year Outcomes After Traumatic Brain Injury. JAMA Netw Open 4:e2140191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi S, Goyal MK, Modi M, Bhalla A, Singh P (2015) Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci 22:959–963. [DOI] [PubMed] [Google Scholar]
- Chamberlain JMKapur JShinnar SElm JHolsti MBabcock LRogers ABarsan WCloyd JLowenstein D, et al. ; Neurological Emergencies Treatment Trials; Pediatric Emergency Care Applied Research Network investigators (2020) Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet 395:1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J (2019) Epileptogenesis, traumatic brain injury, and biomarkers. Neurobiol Dis 123:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Löscher W (2002) Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia 43:350–357. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Aronica E, van Vliet EA (2019) The roof is leaking and a storm is raging: repairing the blood–brain barrier in the fight against epilepsy. Epilepsy Curr 19:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujjar AR, Nandhagopal R, Jacob PC, Al-Hashim A, Al-Amrani K, Ganguly SS, Al-Asmi A (2017) Intravenous levetiracetam vs phenytoin for status epilepticus and cluster seizures: a prospective, randomized study. Seizure 49:8–12. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A (2006) A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140:685–697. [DOI] [PubMed] [Google Scholar]
- Klein PFriedman AHameed MQKaminski RMBar-Klein GKlitgaard HKoepp MJozwiak SPrince DARotenberg A, et al. (2020) Repurposed molecules for antiepileptogenesis: missing an opportunity to prevent epilepsy? Epilepsia 61:359–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PHerr DPearl PLNatale JLevine ZNogay CSandoval FTrzcinsky SAtabaki SMTsuchida T, et al. (2012) Results of phase II pharmacokinetic study of levetiracetam for prevention of post-traumatic epilepsy. Epilepsy Behav 24:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard H, Pitkänen A (2003) Antiepileptogenesis, neuroprotection, and disease modification in the treatment of epilepsy: focus on levetiracetam. Epileptic Disord 5 (Suppl 1):S9–S16. [PubMed] [Google Scholar]
- Kutsukake T, Furukawa Y, Ondo K, Gotoh S, Fukami T, Nakajima M (2019) Quantitative analysis of UDP-glucuronosyltransferase UGT1A and UGT2B mRNA expression in the rat liver and small intestine: sex and strain differences. Drug Metab Dispos 47:38–44. [DOI] [PubMed] [Google Scholar]
- Löscher W, Hönack D, Rundfeldt C (1998) Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther 284:474–479. [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL (1989) Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28:233–244. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Arisi GM, Mims K, Hollingsworth G, O’Neil K, Shapiro LA (2020) Neuroinflammatory mechanisms of post-traumatic epilepsy. J Neuroinflammation 17:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundlamuri RC, Sinha S, Subbakrishna DK, Prathyusha PV, Nagappa M, Bindu PS, Taly AB, Umamaheswara Rao GS, and Satishchandra P (2015) Management of generalised convulsive status epilepticus (SE): A prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam--Pilot study. Epilepsy Res 114:52– 58. [DOI] [PubMed] [Google Scholar]
- Nene DMundlamuri RCSatishchandra PPrathyusha PVNagappa MBindu PSRaghavendra KSaini JBharath RDThennarasu K, et al. (2019) Comparing the efficacy of sodium valproate and levetiracetam following initial lorazepam in elderly patients with generalized convulsive status epilepticus (GCSE): a prospective randomized controlled pilot study. Seizure 65:111–117. [DOI] [PubMed] [Google Scholar]
- Ohman K, Kram B, Schultheis J, Sigmon J, Kaleem S, Yang Z, Lee HJ, Vatsaas C, Komisarow J (2023) Evaluation of levetiracetam dosing strategies for seizure prophylaxis following traumatic brain injury. Neurocrit Care 38:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanwala AE, Kurita A, Truong E (2016) Low-dose levetiracetam for seizure prophylaxis after traumatic brain injury. Brain Inj 30:156–158. [DOI] [PubMed] [Google Scholar]
- Saletti PG, Ali I, Casillas-Espinosa PM, Semple BD, Lisgaras CP, Moshé SL, Galanopoulou AS (2019) In search of antiepileptogenic treatments for post-traumatic epilepsy. Neurobiol Dis 123:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe AGMishra UIvaturi VBrundage RCCloyd JCElm JJChamberlain JMSilbergleit RKapur JLowenstein DH, et al. (2021) Early exposure of fosphenytoin, levetiracetam, and valproic acid after high-dose intravenous administration in young children with benzodiazepine-refractory status epilepticus. J Clin Pharmacol 61:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Patsalos PN (2001) A microdialysis study of the novel antiepileptic drug levetiracetam: extracellular pharmacokinetics and effect on taurine in rat brain. Br J Pharmacol 133:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Holloway MG (2009) Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheless JW, Clarke D, Hovinga CA, Ellis M, Durmeier M, McGregor A, Perkins F (2009) Rapid infusion of a loading dose of intravenous levetiracetam with minimal dilution: a safety study. J Child Neurol 24:946–951. [DOI] [PubMed] [Google Scholar]