Abstract
The placenta is a fetal organ that performs critical functions to maintain pregnancy and support fetal development, including metabolism and transport of xenobiotics and steroids between the maternal-fetal unit. In vitro placenta models are used to study xenobiotic and steroid disposition, but how well these models recapitulate the human placenta is not well understood. We first characterized the abundance of proteins involved in xenobiotic and steroid disposition in human placental tissue. In pooled human placenta, the following xenobiotic and steroid disposition proteins were detected (highest to lowest), 1) enzymes: glutathione S-transferase P, carbonyl reductase 1, aldo-keto reductase 1B1, hydroxysteroid dehydrogenases (HSD3B1 and HSD11B1), aromatase, epoxide hydrolase 1 (EPHX1) and steryl-sulfatase, and 2) transporters: monocarboxylate transporters (MCT1 and 4), organic anion transporting polypeptide 2B1, organic anion transporter 4, and breast cancer resistance protein (BCRP). Then, the tissue proteomics data were compared with four placental cell lines (BeWo, JEG-3, JAR, and HTR-8/SVneo). The differential global proteomics analysis revealed that the tissue and cell lines shared 1420 cytosolic and 1186 membrane proteins. Although extravillous trophoblast and cytotrophoblast marker proteins were detected in all cell lines, only BeWo and JEG-3 cells expressed the syncytiotrophoblast marker, chorionic somatomammotropin hormone 1. BeWo and JEG-3 cells expressed most target proteins including aromatase, HSDs, EPHX1, MCT1, and BCRP. JEG-3 cells treated with commonly detected phthalates in human biofluids showed dysregulation of steroid pathways. The data presented here show that BeWo and JEG-3 cells are closer to the placental tissue for studying xenobiotic and steroid disposition.
SIGNIFICANCE STATEMENT
This is the first study to compare proteomics data of human placental tissue and cell lines (BeWo, JAR, JEG-3, and HTR-8/SVneo). The placental cell line and tissue proteomes are vastly different, but BeWo and JEG-3 cells showed greater resemblance to the tissue in the expression of xenobiotic and steroid disposition proteins. These data will assist researchers to select an optimum cell model for mechanistic investigations on xenobiotic and steroid disposition in the placenta.
Introduction
The placenta is a vital fetal endocrine organ that establishes the maternal-fetal unit to assist in the pregnancy maintenance and development of the fetus. Alterations in endocrine disposition and metabolism within the placenta have been associated with miscarriage, neurodevelopmental alterations, and physical deformation in the fetus (Gingrich et al., 2020; Vacher et al., 2021). Xenobiotics cannot only disrupt the endocrine functions of the placenta, but they can also cross the placenta and cause fetal toxicity (Wells and Winn, 1996). The placental xenobiotic metabolism and transport pathways are critical for metabolizing xenobiotics and eliminating them back to the maternal side (Unadkat et al., 2004). Xenobiotics can enter the placenta through passive diffusion, facilitated diffusion, or active transport. Once xenobiotics cross into the placenta, they are either transported back to the maternal side unchanged or metabolized in the placenta. Metabolizing enzymes found in the placenta include cytochrome P450s (CYPs), glutathione S-transferases (GSTs), epoxide hydrolase, and sulfotransferases (Syme et al., 2004). Transporters in the placenta, such as breast cancer resistance protein (BCRP), can efflux xenobiotics from the placenta to maternal side, thus preventing xenobiotics from entering the fetal side. Similarly, the placenta also regulates uptake, efflux, and metabolism of steroids that are critical for fetal development. Aromatase (CYP19A1) is the major steroid metabolizing enzyme that converts androgens to estrogens within the placenta (Mendelson and Kamat, 2007), whereas organic anion transporters (OATs) are responsible for the uptake of steroid precursors from maternal blood (Grube et al., 2007; Ugele et al., 2008). Therefore, xenobiotic and steroid disposition is a critical function of the placenta.
Primary cells derived from human tissue are considered a “gold standard” for in vitro investigation of cellular physiology, including xenobiotic and steroid disposition pathways (LeCluyse et al., 2005). However, primary cells are logistically challenging to harvest, obtain, and maintain. Primary placental cells have been used to investigate placental physiology (Colson et al., 2021), but these cells are heterogenous and cannot be used in a high-throughput manner. In vivo animal models present an alternative to investigate placental function (Saeidnia et al., 2015), but interspecies differences in placental development including the xenobiotic and steroid disposition pathways makes translating animal data to humans difficult (Schmidt et al., 2021). Therefore, trophoblastic cell lines are the most commonly used models to study placental physiology in a high throughput manner (Weber et al., 2021).
The common placental cell lines are either choriocarcinoma cell lines (BeWo, JAR, and JEG-3) or immortalized primary cells (HTR-8/SVneo), which have been used to study a variety of placental functions. For example, BeWo cells have been used to study syncytial fusion of placenta cells through differentiation with forskolin treatment (Orendi et al., 2010) as well as xenobiotic transport mediated by BCRP (Crowe and Keelan, 2012). JAR cells have been used to study serotonin transporter reuptake (Decker and Blough, 2018) and the effects of xenobiotics on endocrine pathways (Chen et al., 2010; Hiromori et al., 2016). Similarly, JEG-3 cells have been used to study xenobiotic toxicology (Olivier et al., 2021), specifically the effects of xenobiotics on aromatase (Chen et al., 2017; Xu et al., 2019). HTR-8/SVneo cells are a model for epithelial to mesenchymal transition (Msheik et al., 2020) and have been used to investigate the effects of environmental toxins such as phthalates (Meruvu et al., 2016; Lapehn et al., 2023) and bisphenol A (Lan et al., 2017; Profita et al., 2021). Despite these applications, in vitro models are commonly derived from tumor cells (i.e., choriocarcinomas) and can experience genotypic and phenotypic differences compared with non-pathologic human tissue (Pan et al., 2009; Rothbauer et al., 2017). Moreover, how well these cell lines represent human placental xenobiotic and steroid disposition pathways at the protein level is not well known. In this study, we compared the global proteomes of placental cell lines (BeWo, JAR, JEG-3, and HTR-8/SVneo) to human placental tissue (a pool of 98 individual donors) with an emphasis on the xenobiotic and steroid disposition pathways. Furthermore, the effect of the following commonly used phthalates [di(2-ethylhexyl) phthalate (DEHP), di-n-butyl phthalate (DBP), diisononyl phthalate (DINP), and their metabolites, i.e., monoethylhexyl phthalate (MEHP), monobutyl phthalate (MBP), and monoisononyl phthalate (MINP)] on the JEG-3 proteome and metabolome were evaluated. The data presented in this study have the potential to assist researchers in selecting optimum cell models and interpreting data generated using placental cell lines.
Materials and Methods
Materials
DEHP, DBP, DINP, MEHP, MBP, and MINP were all obtained from AccuStandard (New Haven, CT). DMSO was acquired from Thermo Scientific (Waltham, MA). Dulbecco’s modified Eagle’s medium:Ham’s F-12 (50:50) phenol red free medium (DMEM:F-12) and poly-D-lysine (BioCoat) coated 24-well plates were procured from Corning (Corning, NY). FBS was purchased from GelLifeSciences (Marlborough, MA). Penicillin/streptomycin and PBS were purchased from Gibco (Amarillo, TX). RPMI 1640 with L-glutamate media was purchased from Lonza (Walkersville, MD). Poly-D-lysine coated 6-well plates, T25 flasks, and T75 flasks were from Greiner Bio-one (Monroe, NC). HEPES buffer (1 M) was from VWR Chemicals (Solon, OH) and sodium pyruvate was from MP BioMedicals (Solon, OH). Trypsin, iodoacetamide, and the bicinchoninic acid (BCA) protein assay kit were purchased from Thermo Scientific. MemPer Plus Kit containing the cell wash, solubilization, and permeabilization buffers was procured from Thermo (Rockford, IL). The protease inhibitor cocktail was from Pierce (Waltham, MA). SDS and bovine serum albumin were purchased from Sigma Life Science (Burlington, MA). Liquid chromatography–mass spectrometry (LC-MS) grade formic acid and methanol were purchased from Fisher Chemical (Fair Lawn, NJ). Dithiothreitol was obtained from Fisher Bioreagents (Pittsburgh, PA). Acetone and LC-MS grade acetonitrile were purchased from Sigma Aldrich (St. Louis, MO) and VWR BDH Chemical (Radnor, PA), respectively. Ammonium bicarbonate (ABC, 98% purity) was obtained from Acros Organics (Geel, Belgium).
Tissue Processing
Human placental tissues (n = 98) representing a random sampling with proportion for each race and fetal sex group were procured from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood study (Sontag-Padilla et al., 2016; Paquette et al., 2021). Conditions Affecting Neurocognitive Development and Learning in Early Childhood placentas were collected in Shelby County, Tennessee between 2006 and 2011 with an exclusion criterion of confirmed clinical chorioamnionitis, oligohydramnios, infarction, or previa fetal chromosomal abnormalities (Lewinn et al., 2022). Within 15 minutes of delivery, a piece of placental villous tissue was dissected in the shape of a rectangular prism with approximate dimensions of 2 × 0.5 × 0.5 cm from the placental parenchyma and cut into four ∼0.5-cm cubes. The tissue specimens were placed in a 50-mL centrifuge tube with 20 mL of RNAlater at 4°C for 8–24 hours prior to transferring to an individual 1.8-mL cryovial containing fresh RNAlater. The cryovials were stored at −80°C, and the fetal villous tissue was manually dissected and cleared of maternal decidua. Following dissection, the fetal samples were placed into RNAlater and stored at −80°C. To remove the RNAlater, placenta tissue (18–390 mg) was washed with the cell wash buffer (1 mL). The wash buffer was removed by centrifugation at 300 × g for 5 minutes.
Cell Culture and Harvesting
BeWo, JAR, JEG-3, and HTR-8/SVneo cell lines were obtained from American Type Culture Collection (Manassas, VA). BeWo, JAR, and JEG-3 cells were maintained at 37°C and 5% CO2 in DMEM:F-12 with 10% FBS and 1% penicillin/streptomycin. HTR-8/SVneo cells were maintained in RPMI 1640 with L-glutamate containing 10% FBS, 1% sodium pyruvate (100 mM), 1% penicillin/streptomycin, and 1% HEPES buffer. The cell lines were washed with PBS and harvested via cell scraper in 1 mL PBS. Cell suspension was collected into a 1.5-mL microcentrifuge tube and centrifuged at 500 × g for 2 minutes. The cell pellets were stored in a − 20°C freezer until homogenization.
Isolation of Cytosolic and Membrane-Enriched Fractions of Placenta Tissue and Cell Lines
Placenta tissues were homogenized in the permeabilization buffer containing 0.5% protease inhibitor (PI) (20% w/v) via a handheld homogenizer. Similarly, cell pellets were homogenized in permeabilization buffer (600 µL) containing 0.5% PI by pipetting 10 times to break open the cells. The tissue and cell homogenates were fractionated into cytosolic and membrane enriched fractions following a previously established protocol (Xu et al., 2018). Briefly, the homogenates were incubated at 4°C for 30 minutes with gentle shaking at 350 rpm. The cytosolic fraction was isolated by centrifugation at 16,000 × g for 15 minute at 4°C. The pellets were resuspended in the solubilization buffer: 4% SDS (1:1, final concentration of SDS is 2%) with 0.5% PI and sonicated for 30 seconds prior to incubation at 18°C for 60 minutes with gentle shaking at 350 rpm. The membrane fraction was isolated by centrifugation at 16,000 × g for 15 minutes at 18°C. The total protein content of the cytosolic and membrane fractions was determined utilizing a BCA assay following the manufacturer’s instructions (Thermo Scientific). The cytosolic and membrane fractions were diluted with 100 mM ABC to a protein concentration of 1 mg/mL. Cytosolic and membrane placental tissue fractions were pooled individually prior to digestion.
Protein Digestion
Placenta tissue and cell lines were digested following a previously optimized procedure (Kruger et al., 2022). Briefly, 80 µg of protein sample suspended in 180 µL of 100 mM ABC was mixed with a solution containing 20 µL bovine serum albumin (0.02 mg/mL), 10 µL dithiothreitol (250 mM), and 30 µL of ABC (100 mM). Proteins were denatured and reduced by incubating at 95°C for 10 minutes with gentle shaking at 350 rpm. After cooling for 10 minutes at room temperature, samples were alkylated by incubating with 10 µL of iodoacetamide (500 mM in 100 mM ABC) for 30 minutes in the dark. Proteins were precipitated by adding 1 mL of cold acetone followed by freezing at –80°C for 60 minutes. Acetone was removed after centrifugation at 16,000 × g for 5 minutes at 4°C and samples were dried for 10 minutes. The dried samples were washed by addition of 500 µL of cold methanol followed by centrifugation at 16,000 ×g for 5 minute at 4°C. Methanol was removed and samples were dried for 30 minutes before resuspending the pellet in 80 µL of trypsin solution (3:1 mix of 50 mM ABC: trypsin at 0.2 mg/mL). The reaction was incubated at 37°C for 16 hours at 350 rpm before quenching with 5 µL of 5% formic acid.
Proteomic Data Acquisition
A previously described LC-MS/MS method was used for proteomics data acquisition (Kruger et al., 2022). Briefly, the digested proteins were loaded onto an Easy Spray 1200 series nanoLC coupled Q-Exactive HF MS (Thermo Fisher Scientific) using a Thermo Scientific Acclaim PepMap 100 C18 HPLC column (75 µm × 250 mm) at 1 µg/µL with the column temperature of 35°C and the mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in 80% acetonitrile (B). Data were acquired in data-dependent acquisition mode with a spray voltage of 1.9 kV and capillary temperature of 275°C at an LC gradient (%B) of 2% (0–5 min), 5–30% (5–105 min), 30–40% (105–125 min), 40–100% (125–126 min), and 100% (126–131 min) with a flow rate of 300 nL/min. The automatic gain control (AGC) was set to 1e6, and the full mass spectrometry scanning was a range of m/z 375–1500. The 10 most intense ions were selected to undergo higher energy collisional disassociation with an AGC of 1e5. The survey scans were set to a resolution of 120,000 at and m/z of 200 with a maximum ion injection time of 60 milliseconds and isolation of precursors set to a window of 2.0 m/z. The higher energy C trap dissociation spectra was set to maximum ion injection time of 60 milliseconds and resolution of 30,000 at m/z 200 with a normalized collision energy of 27 eV. Single or unassigned charge states of precursor ions were excluded from the fragmentation selection.
Effect of Phthalates on Steroidal Proteome and Steroid Metabolite Generation in JEG-3 Cells
Cell Culture and Collection
JEG-3 cells were maintained in DMEM:F-12 containing 10% FBS and 1% penicillin/streptomycin at 37°C and 5% CO2. The cells were seeded at 400,000 cells per well in 6-well plates and allowed to adhere overnight. The cells were then treated with 100 µM concentrations of six different phthalates/phthalate metabolites in triplicate, DEHP, MEHP, DBP, MBP, DINP, and MINP. This concentration was selected based on previously reported experiments utilizing phthalates (Lapehn et al., 2023) and represents the higher exposure scenario because urinary phthalate concentrations across the world vary from 0–2540 µg/L depending on lifestyle (Wang et al., 2019). The DMSO concentration in the final incubation was below 0.1% for the phthalate treatments and vehicle control. The media was changed every 12 hours with phthalate/phthalate metabolite containing media. After 72 hours, the cells were collected by removing the media, washing with 1× PBS (1 mL), adding 1× PBS (1 mL), and then scraping the plates. Cell suspension was collected into a 1.5 mL microcentrifuge tube and centrifuged at 500 × g for 2 minutes. The cell pellets were stored in a −20°C freezer until homogenization. The cell pellet was homogenized and digested using trypsin as described above. The proteomics analysis was performed as described above.
To investigate the effect of phthalates on steroid metabolism, a separate set of cells (100,000 cells per well) were seeded in 24-well plates and allowed to adhere overnight. The cells were treated with six phthalates as described above. After 72 hours, the cells were treated with testosterone (1 µM, 200 µL) for 2 hours in PBS to observe the effect of the parent and metabolite phthalates on the metabolism. The reaction was stopped by adding cold acetonitrile (800 µL), and the suspension was collected in 1.5 mL microcentrifuge tubes. The suspension was centrifuged at 16,000 × g for 10 minutes at 4°C, and 900 µL of the supernatant was collected. The sample was dried followed by resuspension in 50 µL of 50:50 water:acetonitrile with 0.2% formic acid. Samples were vortexed, centrifuged at 16,000 × g for 10 minute at 4°C, and 20 µL was transferred to the LC-MS vials for analysis. The sample was loaded onto an Easy Spray 1200 series nanoLC coupled Q-Exactive HF MS (Thermo Fisher Scientific) using a Thermo Scientific Acclaim PepMap 100 C18 HPLC column (75 µm × 250 mm) with the column temperature of 35°C and the mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in 80% acetonitrile (B). Targeted steroid analysis data were acquired in data-independent acquisition (DIA) mode with a spray voltage of 1.9 kV and capillary temperature of 275°C at an LC gradient (%B) of 10% (0–5 min), 10–45% (5–20 min), 45–60% (20–40 min), 60–80% (40–50 min), 80% (50–55 min), 80–100% (55–56 min), and 100% (56–76 min) with a flow rate of 300 nL/min.
The full MS scanning was a range of m/z 70–1000 with the AGC was set to 3e6, a resolution of 120,000, and a maximum IT of 150 milliseconds. Three DIA were run with a resolution of 30,000, AGC of 1e6, and isolation windows of 10, 20, and 50 m/z. The collision energy of each run was a step of 20, 30, and 40 eV. The extracted MS1 chromatograms of steroidal metabolites were analyzed.
Data Visualization and Statistical Analysis
Data-dependent acquisition data were analyzed using MaxQuant (Tyanova et al., 2016a) as previously described (Kruger et al., 2022), except the false discovery rate was set to 1% for both peptides and proteins. MaxQuant used the Andromeda search engine to search the human proteome database curated from Uniprot against the high-resolution MS/MS spectra. The protein identification was done by selecting trypsin as the proteolytic enzyme with two missed cleavages maximum, variable modifications as N-terminal acetylation and methionine oxidation, a fixed modification of carbamidomethylation of cysteine, and unique peptides were used for the label-free quantification analysis. Perseus (Tyanova et al., 2016b) was used to remove 1) potential contaminants, 2) reverse sequence proteins, and 3) proteins only identified by site. Perseus was also used to create the volcano plots with software inbuilt parameters of S0 of 2 and the false discovery rate of 0.05.
The subcellular localization of xenobiotic and steroid disposition proteins were confirmed with Uniprot database, and the proteins were analyzed in their respective fractions (Wiśniewski, 2017). Proteins were normalized utilizing eq. 1 below and the absolute protein levels were determined using eq. 2 via the total protein approach (TPA) (Wiśniewski, 2017).
 |
The difference in the abundance of individual drug metabolizing enzymes and transporter proteins highlighted below was further compared between the cell lines and placental tissue via Student’s t tests. The effects of phthalates were compared with the DMSO control samples via Student’s t tests as well.
DIA metabolomics data were analyzed using XCMS software and individual metabolites were compared utilizing Student’s t tests.
Results
Global Proteomic Differences between Human Placental Tissue and Cell Lines
Our analysis identified 2089, 2748, 2213, 2749, and 2203 cytosolic proteins in placental tissues BeWo, JAR, JEG-3, and HTR-8/SVneo cells, respectively (Fig. 1). A total of 1186 cytosolic proteins were shared among the tissue and the cell lines, whereas 299, 175, 97, 195, and 205 were unique to the placental tissue BeWo, JAR, JEG-3, and HTR-8/SVneo cells, respectively (Fig. 1A). Similarly, we identified 2814, 3056, 2426, 3333, and 2945 membrane proteins in placental tissues BeWo, JAR, JEG-3, and HTR-8/SVneo cells, respectively. A total of 1420 membrane proteins were shared among the tissue and the cell lines, whereas 386, 88, 74, 225, and 362 were unique to the placental tissues BeWo, JAR, JEG-3, and HTR-8/SVneo cells, respectively (Fig. 1B).
Fig. 1.
Number of proteins detected in human placenta and different placental cell lines in the cytosolic (A) and membrane (B) fractions. Total number of proteins detected in the cell lines and placenta (C).
The placenta also shared 58, 16, 35, and 47 unique cytosolic proteins, respectively, with BeWo, JAR, JEG-3, and HTR-8/SVneo cells individually. The placenta also shared 66, 14, 81, and 93 unique membrane proteins, respectively, with BeWo, JAR, JEG-3, and HTR-8/SVneo cells individually. The hierarchical clustering (Fig. 2, A and B) and principal component analysis (PCA) (Fig. 2, C and D) showed unique proteomic profiles of the placental tissue and individual cell lines. In particular, the tissue proteome was distinct from all of the cell lines whereas among the cell lines, JAR and JEG-3 cells clustered together. In general, proteins were detected at significantly higher levels in the placental cell lines compared with the tissue (Fig. 2, E and F). For example, BeWo, JAR, JEG-3, and HTR-8/SVneo cells showed 653, 668, 684, and 658 proteins in cytosolic fraction that were statistical higher, whereas BeWo and JEG-3 had 147 and 101 proteins that were significantly lower in abundance than placenta tissue (2-fold difference between cells vs. tissues and P value <0.05; t test). Similarly, 632, 516, 525, and 519 proteins in the membrane fraction were significantly higher, whereas 192, 209, 256, and 276 were significantly lower in the cell lines compared with the placenta. The fold differences between the shared placental proteins and cell lines are provided in Supplemental Table 1.
Fig. 2.
Quantitative comparison of protein abundance data in human placenta and different placental cell lines represented as hierarchical clustering of the cytosolic (A) and membrane (B) fractions. Principal component analysis (C and D) and Volcano plots with a false discovery rate of 0.05 and fold change of 2 (E and F) of the cytosolic and membrane fractions, respectively.
Subcellular Fraction and Cell Marker Differences
The quality of cytosolic and membrane fractions across the placental cell lines versus tissue were assessed by quantifying subcellular markers (glyceraldehyde-3-phosphate dehydrogenase [GAPDH] and sodium potassium ATPase, respectively). The purity of the cytosolic fraction estimated by GAPDH as a marker was ∼80% or more across all samples (Fig. 3A), and the purity of the membrane fraction estimated by sodium potassium ATPase as a marker was over 99% across all the samples (Fig. 3B). Further, tissue-specific cell-type markers were compared in the cell lines to determine their resemblance to specific placental cells Fig. 3C). The markers of decidual cells (PAEP) and fibroblasts (PECA1) were not detected in any of the cell lines, whereas the extravillous (COL4A1) and cytotrophoblast (IF2B3) markers were expressed in all the cell lines. BeWo and JEG-3 were the only cell lines where the syncytiotrophoblast (CSH1) marker was detected but these levels were significantly lower by >99% than the placenta. Only the choriocarcinoma cell lines (BeWo, JAR, and JEG-3) expressed the Hofbauer cell marker (LAMA2). Placental alkaline phosphatase (PPB1) expression was ∼20-fold higher in the placenta than BeWo and JAR, whereas, JEG-3 cells showed ∼4 order of magnitude fold lower expression of PPB1 than the placenta.
Fig. 3.
Relative expression of cytosolic marker (GAPDH, A) and membrane marker (sodium potassium ATPase, B) in cytosolic and membrane fractions. Relative protein expression of cell type markers in BeWo, JAR, JEG-3, and HTR-8/SVneo cells as compared with human placenta (C). *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.00005. $ indicates proteins that were not detected in at least three replicates. The y-axis for all graphs was converted into log10 scale.
Xenobiotic and Steroid Metabolizing Enzymes and Transporters in the Human Placenta
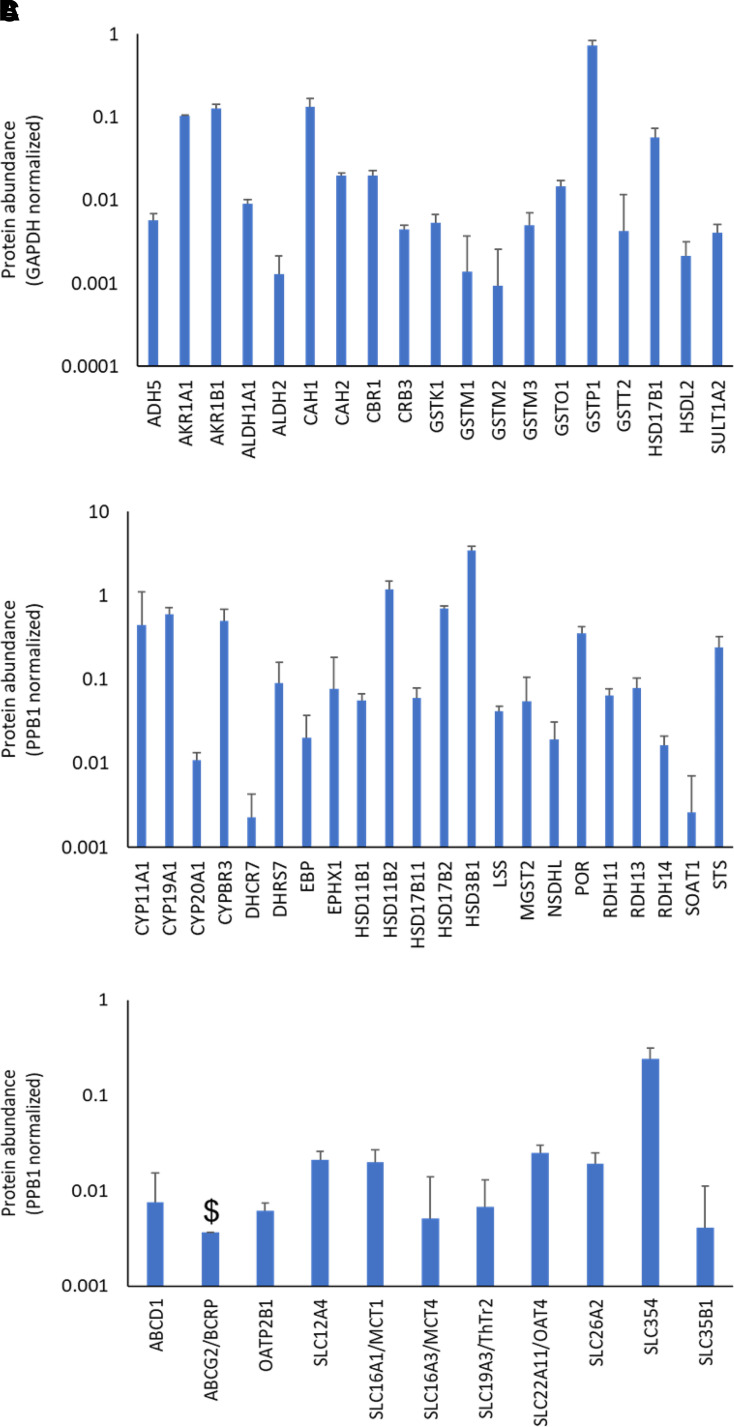
Glutathione S-transferases (GSTs), carbonyl reductase (CAHs), aldo-keto reductases (AKRs), hydroxysteroid dehydrogenases (HSDs), aromatase (CYP19A1), and steryl-sulfatase (STS) were the major enzymes, whereas monocarboxylate transporters (MCTs), organic anion transporting polypeptide 2B1 (OATP2B1), organic anion transporter 4 (OAT4), and breast cancer resistance protein (BCRP) were the major transporter proteins detected in the placenta tissue. GSTP1, AKR1B1, CAH1, HSD17B1, AKR1A1, and CBR1 were the major xenobiotic and steroid metabolizing enzymes in cytosolic fraction with protein levels, 178.1, 57.1, 37.1, 23.5, 18.6, and 9.8 pmol/mg, respectively (Fig. 4A). HSD3B1, HSD11B2, CYP19A1 (aromatase), CYP11A1, HSD17B2, and STS were the major xenobiotic and steroid metabolizing enzymes in the membrane fraction with protein abundance, 108.6, 89.7, 67.6, 35.6, 24.7, and 16.5 pmol/mg, respectively (Fig. 4B). The levels of common xenobiotic and steroid transporter proteins, OAT4, MCT1, OATP2B1, and BCRP in the membrane fraction were low but detectable, 0.94, 0.52, 0.09, and 0.06 pmol/mg, respectively (Fig. 4C). BCRP was only detected in one of the replicates and P-glycoprotein was not detected in any of the samples. The potential reasons for the poor signal of BCRP and P-gp are 1) rigorous protein identification criteria (low false discovery rate) used in the study, and 2) the use of term-placenta where the expression of these proteins is significantly decreased as compared with the first and second trimester placentas (Anoshchenko et al., 2020).
Fig. 4.
Placental protein levels of xenobiotic and steroid disposition related proteins in human placental tissue, cytosolic enzymes (A), membrane associated enzymes (B), and transporters (C). $ indicates proteins that were not detected in all three replicates.
Steroid and Xenobiotic Metabolizing Enzymes and Transporters in Cell Lines Versus Tissue
The levels of xenobiotic and steroid disposition proteins were generally lower in the cell lines as compared with the placenta (Fig. 5). In particular, the metabolizing enzymes in the cytosolic fraction were all lower compared with the placenta. GSTP1 was 10.6-, 2.3-, 2.9-, and 1.9-fold higher in the placenta than in BeWo, JAR, JEG-3, and HTR-8/SVneo cells, respectively. AKR1B1 relative expression in the placenta was 15-fold higher than the HTR-8/SVneo cells, 4.2- and 2.3-fold higher than the BeWo and JAR, and comparable to JEG-3 cells. CAH1 was not detected in the cell lines whereas HSD17B1 was not detected in JAR or HTR-8/SVneo cells and was lower in BeWo and JEG-3 cells by 477.5- and 209.5-fold, respectively. AKR1A1 was 12.3-, 4.2-, 2.7-, and 4.2-fold higher in the placenta than BeWo, JAR, JEG-3, and HTR-8/SVneo cells, respectively. CBR1 was only detected in HTR-8/SVneo cells at the same level as the placental tissue. Interestingly, carboxylesterase 1 (CES1) was not detected in the placenta but was present in BeWo cells.
Fig. 5.
Bubble plot showing the protein levels (y-axis, log10 scale) in BeWo, JAR, JEG-3, and HTR-8/SVneo cells, −log10(P value) (bubble size) and cell types (color) that are significantly different than the placental levels.
Membrane-associated enzymes were either not detected or lower than the placenta. HSD3B1 was not detected in HTR-8/SVneo cells, and the placental expression of HSD3B1 was 570-, 1343-, and 162-fold higher than in BeWo, JAR, and JEG-3 cells, respectively. HSD11B2 was 323-, 17,535-, and 91-fold higher in the placenta than in the BeWo, JAR, and JEG-3 cells and was not detected in HTR-8/SVneo cells. CYP19A1 and CYP11A1 were both lower in the cell lines compared with the placenta. CYP19A1 was 101.6-, 7149.4-, and 41.3-fold lower in BeWo, JAR, and JEG-3 cells but was not detected in HTR-8/SVneo. CYP11A1 was not detected in HTR-8/SVneo cells and was 7.2-, 143.6-, and 14.9-fold lower in BeWo, JAR, and JEG-3 cells, respectively. HSD17B2 was not detected in any of the cell lines. STS was not detected in JAR or HTR-8/SVneo cells but its levels in the placenta were 31.5- and 16.4-fold higher than in the BeWo and JEG-3 cells, respectively.
In terms of transporters, compared with the placenta, OAT4 and OAT2B1 were below the limit of detection across all cell lines. MCT1 was higher in BeWo, JAR, and JEG-3 (2.1-, 1.5-, and 2.4-fold respectively) but was lower in HTR-8/SVneo (52%). BCRP was higher in BeWo (6.4-fold) and JEG-3 cells (1.4-fold) but was not detected in JAR or HTR-8/SVneo cells.
Effect of Phthalates on Steroidal Proteome and Steroid Metabolite Generation in JEG-3 Cells
Because JEG-3 cells showed the most resemblance with the placental xenobiotic and steroid pathways, these cells were treated with phthalates to determine the effects of common phthalates on the placental pathways. At the proteomic level, the following proteins were affected by phthalate treatment in JEG-3 cells, albeit the effect was moderate: CAH2, GTSP1, CBR1, HSD3B1, AKR1A1, AKR1B1, CYP11A1, CYP19A1, HSD17B1, STS, BCRP, and MCT1 (Fig. 6A). Cytosolic enzymes were altered including CAH2 that was 10%–20% significantly higher in DEHP-, MEHP-, MBP-, and MINP-treated cells compared with the vehicle control. Similarly, GTSP1 was significantly higher by 10%–30% in DEHP-, MEHP-, MBP-, and MINP-treated cells. CBR1 was significantly higher (>2-fold, P value = 0.04), whereas HSD3B1 was not detectable after treatment with MEHP. AKR1A1 was 20%–30% significantly higher in the DBP-, MEHP-, and MINP-treated samples versus the vehicle control. AKR1B1 was 20%–40% significantly higher in DEHP-, DBP-, MEHP-, and MINP-treated samples than the vehicle control.
Fig. 6.
Relative protein abundance of androgen and xenobiotic related proteins in JEG-3 cells treated (72 hours) with parent phthalates and their primary metabolites in the cytosolic (A) and membrane transporters (B) and enzymes (C). Metabolite levels of progesterone (D) and testosterone (E) after 72 hours of phthalate treatment that were significantly altered, P = 0.036 and 0.046, respectively. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.00005. $ indicates proteins that were not detected in at least three replicates.
In terms of membrane associated enzymes, CYP19A1 and CYP11A1 were both significantly higher in the MINP treatment (30% and 40% higher, respectively), but only CYP11A1 was significantly higher in the DBP and MEHP treatments, 50% and 20%, respectively, compared with the vehicle control. Similarly, HSD17B1 was significantly higher in the MEHP and MINP treatments (1.5- and 2.3-fold) but was lower in the DINP treatment (0.5-fold). STS was significantly lower in both DBP and MEHP treatments by 0.7-fold compared with the vehicle control. In terms of transporters, BCRP and MCT1 were significantly higher after treatment with MEHP by 20% and 30% than the vehicle control, respectively. BCRP was also 2-fold higher after treatment with DBP but was not detected in all three replicates.
The exploratory targeted steroid metabolite generation analysis revealed that only MINP treatment significantly altered the formation of testosterone and progesterone, which correlate with the increase expression of proteins involved in these pathways (i.e., HSD17B1 and CYP11A1, respectively) (Chatuphonprasert et al., 2018). Progesterone was significantly higher in the MINP treatment by 3.5-fold (Fig. 6B), whereas testosterone was 1.7-fold higher in the MINP treatment compared with the vehicle control of DMSO (Fig. 6C).
Discussion
Studying the effects of xenobiotics on the placenta endocrine pathways is important to determining the potential exposure risks during pregnancy on fetal development. Similarly, how the placenta metabolizes and transports these xenobiotics is also critical to determine their detoxification pathways. In vitro models provide a simple high throughput method to observe the potential placental toxicity and metabolism of xenobiotics. However, cell lines do not represent the placental cellular heterogeneity, so selecting the most comparable model for the specific research question is crucial.
Placental cell lines have been evaluated at the transcriptomics level for their utility to test specific placental functions such as creating the maternal–fetal barrier through cellular fusion (Msheik et al., 2019). However, transcriptomics data often show poor correlation with protein levels and function due to factors such as post-translational modifications and mRNA stability (Christopher et al., 2022). Proteomics data correlate better to the protein activity (e.g., metabolism and transport activity) and provide functional insight into biologic pathways (Monti et al., 2019). In this study, we first showed that the type and abundance of proteins detected in choriocarcinoma cell lines (BeWo, JAR, and JEG-3) and an immortalized cell line (HTR-8/SVneo) were different compared with placental tissue. Then, with respect to xenobiotic and steroid disposition proteins, our data showed that BeWo and JEG-3 cells recapitulated the placental functions better than JAR and HTR-8/SVneo cells.
Our conclusions corroborate with reported functional investigations. For example, BeWo cells have been used to study syncytial fusion (Orendi et al., 2010), antidepressant toxicity (Nabekura et al., 2022), and the uptake of antiepileptic drugs in relation to gabapentin transport (Koishikawa et al., 2022). Both BeWo and JEG-3 cells were used to study the effects of ambient polycyclic aromatic hydrocarbons mixtures on their steroidogenic properties (Drwal et al., 2019) as well as apelin and apelin receptors in the human placenta (Mlyczyńska et al., 2020). BeWo cells have been used to study ABCG2 (BCRP) efflux (Crowe and Keelan, 2012), and JEG-3 cells have been used to study the functions of CYP19A1 and HSDs (Liu et al., 2016). Similarly, JEG-3 cells have recently been used in multiple studies focused on endocrine disruption due to xenobiotic exposure that includes exposures to alkylphenols (Pérez-Albaladejo et al., 2019), phthalates (Zhang et al., 2020), bisphenol A (Xu et al., 2019), acetaminophen (Addo et al., 2021), and cyclosporin A (He et al., 2020). Further, JEG-3 cells have been used in several studies and are regarded as a promising tool in placental toxicological studies (Olivier et al., 2021).
When selecting an in vitro placental model, both the qualitative presence and abundance of individual proteins should be compared with the placenta tissue. For example, in this study, we observed that BeWo, JEG-3, and JAR cells all expressed aromatase whereas HTR-8/SVneo cells did not. Even though both JAR and JEG-3 cells have been used to study the effects of xenobiotics on aromatase (Letcher et al., 1999; Mor et al., 2001; Zhang et al., 2011), our previous study showed that JEG-3 cells express CYP19A1 more consistently than JAR cells (Kruger et al., 2022). This means that results utilizing JAR cells to study CYP19A1 may fluctuate due to the inconsistent levels expressed within the cells.
ABCG2 (BCRP), which effluxes xenobiotics to the maternal side and plays an important role in detoxification, is expressed at 6.4-fold higher levels in BeWo compared with the placenta. Consistent with our previous study (Kruger et al., 2022), JEG-3 cells also expressed BCRP albeit at lower levels than BeWo (1.4-fold higher than placenta). However, unlike our previous finding, the level of BCRP was below the limit of quantification in JAR cells that likely indicates inconsistent expression in this cell line. If the xenobiotic is a substrate of BCRP, BeWo would be expected to have the highest transport rate, followed by JEG-3, due to their protein expression compared with the placenta. HTR-8/SVneo cells did not have detectable levels of BCRP and would most likely not transport the substrate out of the cells efficiently. These variations in protein level must be considered when translating the data to an in vivo setting.
Another consideration while using in vitro cell lines to investigate placental biology is that some cell lines express proteins that are not present in the placental tissue. For example, CES1 is important in metabolizing ester containing pro-drugs and xenobiotics such as phthalates but was not detected in the placenta or other cell lines investigated here and was uniquely detected in BeWo cells. CES1 hydrolyzes aromatic and aliphatic esters but has no catalytic activity toward amides or fatty acyl-CoA esters (Her and Zhu, 2020). Thus, results of studies done with BeWo cells with CES1 metabolized xenobiotics will not potentially translate to human placental tissue. JEG-3 cells have been regarded as a promising tool and our data are consistent with the literature. JEG-3 cells are simpler models that do not undergo fusion like BeWo cells (Al-Nasiry et al., 2006). Our results showed that JEG-3 cells had the most steroidogenic proteins and thus were used to study the endocrine disruption caused by chronic phthalate exposure. Phthalate treatment had varying effects on the proteomic profile and steroid metabolite generation of JEG-3 cells. Interestingly, although the selected phthalates were three parent compounds (DEHP, DINP, and DBP) and their primary metabolites (MEHP, MINP, and MBP), the parent compounds did not have the same results as their respective metabolites. For example, only MINP treatment significantly raised the level of CYP19A1 and was the only treatment to have significantly higher levels of testosterone and progesterone, whereas DINP did not have the same effects. Although these are merely observational data and the mechanisms of moderate change in the protein and metabolite levels warrants further investigations, phthalates and their metabolites have been shown to have varying effects on testosterone, progesterone, and estradiol levels in both pre- and postnatal exposure (Hlisníková et al., 2020). The differential cellular uptake of parent phthalates versus metabolites could be a potential reason for this discrepancy.
Although this study highlighted steroid and xenobiotic proteins, the global proteomics data in Supplemental Table 2 can be used to determine the best in vitro model to represent the placental pathways of interest. This study was limited to detectable proteins, and because the placenta is highly vascularized, blood contamination in the placenta could obscure lower abundance placental proteins. Therefore, some placental proteins may not be detected and a comparison with the cell lines could not be made. Another limitation is that only placentas that were at the end of gestation were used (Lee et al., 2013; Anoshchenko et al., 2020). Thus, the cell lines might have comparable levels to earlier stages of the placenta that are not identified here. The placenta is also a sanctum made up of primarily multi-nucleated fused syncytiotrophoblast cells that has been shown to be difficult to recapitulate in vitro, although there has been some success with BeWo cells. Thus, if studying the fused function is the goal, placental explants are the best method. Despite these limitations, our study is the first to provide and compare the global, steroid, and xenobiotic proteomes of BeWo, JEG-3, JAR, and HTR-8/SVneo cell lines to placental tissue. Future studies on the proteomic differences between undifferentiated and forskolin synctialized BeWo cells and the proteomic shifts through passages of cell lines like HTR-8/SVneo cells that are a mixed population (Abou-Kheir et al., 2017) would be interesting to observe. Nevertheless, the data provided in this manuscript will assist researchers in selecting an optimum cell model for mechanistic investigations on xenobiotic and steroid disposition in the placenta. The data can also be used for interpreting results of the in vitro studies on these cells. Quantitative proteomics differences between placenta tissue and cell lines highlight the need for developing better cellular models that mimic tissue level complexity in proteomics. This research highlights the need for developing and validating the emerging primary human placental models including complex co-culture and organ-on-a-chip models for investigating placenta function.
Acknowledgments
The authors acknowledge Guihua Yue and Matthew Karasu for their technical support.
Data Availability
The authors declare that all the data supporting the findings of this study are contained within the paper.
Abbreviations
- ABC
ammonium bicarbonate
- AGC
automatic gain control
- AKR
aldo-keto reductase
- BCRP
breast cancer resistance protein
- CAH
carbonyl reductase
- CES1
carboxylesterase 1
- CYP
cytochrome P450
- DBP
di-n-butyl phthalate
- DEHP
di(2-ethylhexyl)
- DINP
diisononyl
- DMEM:F-12
Dulbecco’s modified Eagle’s medium:Ham’s F-12 (50:50) phenol red free medium
- EPHX1
epoxide hydrolase 1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GST
glutathione S-transferase
- HSD
hydroxysteroid dehydrogenases
- IAA
iodoacetamide
- LC-MS
liquid chromatography–mass spectrometry
- MBP
monobutyl phthalate
- MCT
monocarboxylate transporter
- MEHP
monoethylhexyl phthalate
- MINP
monoisononyl phthalate
- MS
mass spectrometry
- OAT
organic anion transporter
- PI
protease inhibitor
- STS
steryl-sulfatase
Authorship Contributions
Participated in research design: Kruger, Paquette, Sathyanarayana, Prasad.
Conducted experiments: Kruger, Singh.
Performed data analysis: Kruger, MacDonald, Bammler, Prasad.
Wrote or contributed to the writing of the manuscript: Kruger, Lapehn, Paquette, Singh, MacDonald, Bammler, Enquobahrie, Zhao, Mozhui, Sathyanarayana, Prasad.
Footnotes
This work was supported by the National Institutes of Health NIH Office of the Director [Grant UG3/UH3-OD023271] and National Institute of Environmental Health Sciences [Grant P30-ES007033] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant K99/R00HD096112] (to A.G.P.). Placental tissue samples were procured from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood study that was funded by the Urban Child Institute and National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL1009977].
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This manuscript has been reviewed by PATHWAYS for scientific content and consistency of data interpretation with previous PATHWAYS publications.
No author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at dmd.aspetjournals.org
This article has supplemental material available at dmd.aspetjournals.org
References
- Abou-Kheir W, Barrak J, Hadadeh O, Daoud G (2017) HTR-8/SVneo cell line contains a mixed population of cells. Placenta 50:1–7. [DOI] [PubMed] [Google Scholar]
- Addo KA, Palakodety N, Fry RC (2021) Acetaminophen modulates the expression of steroidogenesis-associated genes and estradiol levels in human placental JEG-3 cells. Toxicol Sci 179:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nasiry S, Spitz B, Hanssens M, Luyten C, Pijnenborg R (2006) Differential effects of inducers of syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells. Hum Reprod 21:193–201. [DOI] [PubMed] [Google Scholar]
- Anoshchenko O, Prasad B, Neradugomma NK, Wang J, Mao Q, Unadkat JD (2020) Gestational age-dependent abundance of human placental transporters as determined by quantitative targeted proteomics. Drug Metab Dispos 48:735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatuphonprasert W, Jarukamjorn K, Ellinger I (2018) Physiology and pathophysiology of steroid biosynthesis, transport and metabolism in the human placenta. Front Pharmacol 9:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen X, Chen X, Hu Z, Li X, Su Y, Li X, Ge R-S (2017) Ziram inhibits aromatase activity in human placenta and JEG-3 cell line. Steroids 128:114–119. [DOI] [PubMed] [Google Scholar]
- Chen S-C, Liao T-L, Wei Y-H, Tzeng C-R, Kao S-H (2010) Endocrine disruptor, dioxin (TCDD)-induced mitochondrial dysfunction and apoptosis in human trophoblast-like JAR cells. Mol Hum Reprod 16:361–372. [DOI] [PubMed] [Google Scholar]
- Christopher JA, Geladaki A, Dawson CS, Vennard OL, Lilley KS (2022) Subcellular transcriptomics and proteomics: a comparative methods review. Mol Cell Proteomics 21:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson A, Depoix CL, Hubinont C, Debiève F (2021) Isolation of primary cytotrophoblasts from human placenta at term. Bio Protoc 11:e4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A, Keelan JA (2012) Development of a model for functional studies of ABCG2 (breast cancer resistance protein) efflux employing a standard BeWo clone (B24). Assay Drug Dev Technol 10:476–484. [DOI] [PubMed] [Google Scholar]
- Decker AM, Blough BE (2018) Development of serotonin transporter reuptake inhibition assays using JAR cells. J Pharmacol Toxicol Methods 92:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drwal E, Rak A, Gregoraszczuk EL (2019) Differential effects of ambient PAH mixtures on cellular and steroidogenic properties of placental JEG-3 and BeWo cells. Reprod Toxicol 86:14–22. [DOI] [PubMed] [Google Scholar]
- Gingrich J, Ticiani E, Veiga-Lopez A (2020) Placenta disrupted: endocrine disrupting chemicals and pregnancy. Trends Endocrinol Metab 31:508–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Reuther S, Meyer Zu Schwabedissen H, Köck K, Draber K, Ritter CA, Fusch C, Jedlitschky G, Kroemer HK (2007) Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos 35:30–35 Drug Metab Dispos. [DOI] [PubMed] [Google Scholar]
- He B, Li QY, Wu YY, Ruan JL, Teng XM, Li DJ, Tang CL (2020) Cyclosporin A protects JEG-3 cells against oxidative stress-induced apoptosis by inhibiting the p53 and JNK/p38 signaling pathways. Reprod Biol Endocrinol 18:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her L, Zhu HJ (2020) Carboxylesterase 1 and Precision Pharmacotherapy: Pharmacogenetics and Nongenetic Regulators. Drug Metab Dispos 48:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromori Y, Yui H, Nishikawa J, Nagase H, Nakanishi T (2016) Organotin compounds cause structure-dependent induction of progesterone in human choriocarcinoma Jar cells. J Steroid Biochem Mol Biol 155 (Pt B):190–198. [DOI] [PubMed] [Google Scholar]
- Hlisníková H, Petrovičová I, Kolena B, Šidlovská M, Sirotkin A (2020) Effects and mechanisms of phthalates’ action on reproductive processes and reproductive health: a literature review. Int J Environ Res Public Health 17:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koishikawa M, Furugen A, Ohyama N, Narumi K, Ishikawa S, Kobayashi M (2022) Uptake of antiepileptic drugs in forskolin-induced differentiated BeWo cells: alteration of gabapentin transport. Xenobiotica 52:405–412. [DOI] [PubMed] [Google Scholar]
- Kruger L, Yue G, Mettu VS, Paquette A, Sathyanarayana S, Prasad B (2022) Differential proteomics analysis of JEG-3 and JAR placental cell models and the effect of androgen treatment. J Steroid Biochem Mol Biol 222:106138. [DOI] [PubMed] [Google Scholar]
- Lan XFu L-JZhang JLiu X-QZhang H-JZhang XMa M-FChen X-MHe J-LLi L-B, et al. (2017) Bisphenol A exposure promotes HTR-8/SVneo cell migration and impairs mouse placentation involving upregulation of integrin-β1 and MMP-9 and stimulation of MAPK and PI3K signaling pathways. Oncotarget 8:51507–51521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapehn S, Houghtaling S, Ahuna K, Kadam L, MacDonald JW, Bammler TK, LeWinn KZ, Myatt L, Sathyanarayana S, Paquette AG (2023) Mono(2-ethylhexyl) phthalate induces transcriptomic changes in placental cells based on concentration, fetal sex, and trophoblast cell type. Arch Toxicol 97:831–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L (2005) Isolation and culture of primary human hepatocytes. Methods Mol Biol 290:207–229. [DOI] [PubMed] [Google Scholar]
- Lee N, Hebert MF, Prasad B, Easterling TR, Kelly EJ, Unadkat JD, Wang J (2013) Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab Dispos 41:2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher RJ, van Holsteijn I, Drenth HJ, Norstrom RJ, Bergman A, Safe S, Pieters R, van den Berg M (1999) Cytotoxicity and aromatase (CYP19) activity modulation by organochlorines in human placental JEG-3 and JAR choriocarcinoma cells. Toxicol Appl Pharmacol 160:10–20. [DOI] [PubMed] [Google Scholar]
- LeWinn KZKarr CJHazlehurst MCarroll KLoftus CNguyen RBarrett ESwan SHSzpiro AAPaquette A, et al. (2022) Cohort profile: the ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS). BMJ Open 12:e064288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mao B, Bai Y, Liu J, Li H, Li X, Lian Q, Ge R-S (2016) Effects of methoxychlor and its metabolite hydroxychlor on human placental 3β-hydroxysteroid dehydrogenase 1 and aromatase in JEG-3 cells. Pharmacology 97:126–133. [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Kamat A (2007) Mechanisms in the regulation of aromatase in developing ovary and placenta. J Steroid Biochem Mol Biol 106:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruvu S, Zhang J, Bedi YS, Choudhury M (2016) Mono-(2-ethylhexyl) phthalate induces apoptosis through miR-16 in human first trimester placental cell line HTR-8/SVneo. Toxicol In Vitro 31:35–42. [DOI] [PubMed] [Google Scholar]
- Mlyczyńska E, Kurowska P, Drwal E, Opydo-Chanek M, Tworzydło W, Kotula-Balak M, Rak A (2020) Apelin and apelin receptor in human placenta: expression, signalling pathway and regulation of trophoblast JEG-3 and BeWo cells proliferation and cell cycle. Int J Mol Med 45:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti C, Zilocchi M, Colugnat I, Alberio T (2019) Proteomics turns functional. J Proteomics 198:36–44. [DOI] [PubMed] [Google Scholar]
- Mor G, Eliza M, Song J, Wiita B, Chen S, Naftolin F (2001) 17alpha-methyl testosterone is a competitive inhibitor of aromatase activity in Jar choriocarcinoma cells and macrophage-like THP-1 cells in culture. J Steroid Biochem Mol Biol 79:239–246. [DOI] [PubMed] [Google Scholar]
- Msheik H, Azar J, El Sabeh M, Abou-Kheir W, Daoud G (2020) HTR-8/SVneo: a model for epithelial to mesenchymal transition in the human placenta. Placenta 90:90–97. [DOI] [PubMed] [Google Scholar]
- Msheik H, El Hayek S, Bari MF, Azar J, Abou-Kheir W, Kobeissy F, Vatish M, Daoud G (2019) Transcriptomic profiling of trophoblast fusion using BeWo and JEG-3 cell lines. Mol Hum Reprod 25:811–824. [DOI] [PubMed] [Google Scholar]
- Nabekura T, Ishikawa S, Tanase M, Okumura T, Kawasaki T (2022) Antidepressants induce toxicity in human placental BeWo cells. Curr Res Toxicol 3:100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier E, Wakx A, Fouyet S, Dutot M, Rat P (2021) JEG-3 placental cells in toxicology studies: a promising tool to reveal pregnancy disorders. Anat Cell Biol 54:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendi K, Gauster M, Moser G, Meiri H, Huppertz B (2010) The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction 140:759–766. [DOI] [PubMed] [Google Scholar]
- Pan C, Kumar C, Bohl S, Klingmueller U, Mann M (2009) Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol Cell Proteomics 8:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AGMacDonald JLapehn SBammler TKruger LDay DBPrice NDLoftus CKannan KMarsit C, et al. (2021) A comprehensive assessment of associations between prenatal phthalate exposure and the placental transcriptomic landscape. Environ Health Perspect 129:97003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Albaladejo E, Lacorte S, Porte C (2019) Differential toxicity of alkylphenols in JEG-3 human placental cells: alteration of P450 aromatase and cell lipid composition. Toxicol Sci 167:336–346. [DOI] [PubMed] [Google Scholar]
- Profita M, Fabbri E, Spisni E, Valbonesi P (2021) Comparing effects and action mechanisms of BPA and BPS on HTR-8/SVneo placental cells†. Biol Reprod 105:1355–1364. [DOI] [PubMed] [Google Scholar]
- Rothbauer M, Patel N, Gondola H, Siwetz M, Huppertz B, Ertl P (2017) A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci Rep 7:5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidnia S, Manayi A, Abdollahi M (2015) From in vitro experiments to in vivo and clinical studies; pros and cons. Curr Drug Discov Technol 12:218–224. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Schmidt A, Markert UR (2021) The road (not) taken - placental transfer and interspecies differences. Placenta 115:70–77. [DOI] [PubMed] [Google Scholar]
- Sontag-Padilla LM, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, Tylavsky F (2016) The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description. RAND Corporation. [Google Scholar]
- Syme MR, Paxton JW, Keelan JA (2004) Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 43:487–514. [DOI] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Cox J (2016a) The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 11:2301–2319. [DOI] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J (2016b) The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 13:731–740. [DOI] [PubMed] [Google Scholar]
- Ugele B, Bahn A, Rex-Haffner M (2008) Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J Steroid Biochem Mol Biol 111:1–6. [DOI] [PubMed] [Google Scholar]
- Unadkat JD, Dahlin A, Vijay S (2004) Placental drug transporters. Curr Drug Metab 5:125–131. [DOI] [PubMed] [Google Scholar]
- Vacher CMLacaille HO’Reilly JJSalzbank JBakalar DSebaoui SLiere PClarkson-Paredes CSasaki TSathyanesan A, et al. (2021) Placental endocrine function shapes cerebellar development and social behavior. Nat Neurosci 24:1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu H, Kannan K (2019) A review of biomonitoring of phthalate exposures. Toxics 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Weise A, Vasheghani F, Göhner C, Fitzgerald JS, Liehr T, Markert UR (2021) Cytogenomics of six human trophoblastic cell lines. Placenta 103:72–75. [DOI] [PubMed] [Google Scholar]
- Wells PG, Winn LM (1996) Biochemical toxicology of chemical teratogenesis. Crit Rev Biochem Mol Biol 31:1–40. [DOI] [PubMed] [Google Scholar]
- Wiśniewski JR (2017) Label-free and standard-free absolute quantitative proteomics using the “Total Protein” and “Proteomic Ruler” approaches. Methods Enzymol 585:49–60. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang X, Ye Y, Li X (2019) Bisphenol A affects estradiol metabolism by targeting CYP1A1 and CYP19A1 in human placental JEG-3 cells. Toxicol In Vitro 61:104615. [DOI] [PubMed] [Google Scholar]
- Xu M, Saxena N, Vrana M, Zhang H, Kumar V, Billington S, Khojasteh C, Heyward S, Unadkat JD, Prasad B (2018) Targeted LC-MS/MS proteomics-based strategy to characterize in vitro models used in drug metabolism and transport studies. Anal Chem 90:11873–11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jiang Z, Zhang L (2011) Effect of triptolide on aromatase activity in human placental microsomes and human placental JEG-3 cells. Arzneimittelforschung 61:727–733. [DOI] [PubMed] [Google Scholar]
- Zhang SSun CZhao SWang BWang HZhang JWang YCheng HZhu LShen R, et al. (2020) Exposure to DEHP or its metabolite MEHP promotes progesterone secretion and inhibits proliferation in mouse placenta or JEG-3 cells. Environ Pollut 257:113593. [DOI] [PubMed] [Google Scholar]