Abstract
Background
Gastric anisakiasis typically causes severe abdominal symptoms; however, we incidentally detected asymptomatic gastric anisakiasis cases during esophagogastroduodenoscopy. The factors associated with developing acute abdominal symptoms induced by gastric anisakiasis remain unclear. Therefore, this study aimed to investigate the clinical factors associated with abdominal symptoms of gastric anisakiasis by comparing symptomatic and asymptomatic cases.
Methods
This was a retrospective cohort study involving 264 patients diagnosed with gastric anisakiasis at nine hospitals in Japan between October 2015 and October 2021. We analyzed patients’ medical records and endoscopic images and compared the clinical factors between the symptomatic and asymptomatic groups.
Results
One hundred sixty-five patients (77.8%) were diagnosed with abdominal symptoms, whereas 47 (22.2%) were asymptomatic. Older age, male sex, diabetes mellitus, gastric mucosal atrophy, and gastric mucosal atrophy of the Anisakis penetrating area were significantly more common in the asymptomatic group than in the symptomatic group. Multivariate analysis revealed that age (p = 0.007), sex (p = 0.017), and presence or absence of mucosal atrophy (p = 0.033) were independent factors for the occurrence of acute abdominal symptoms. In addition, cases that were Helicobacter pylori naïve, with an elevation of white blood cells, or without an elevation of eosinophils were more common in the symptomatic group than in the asymptomatic group.
Conclusions
Age, sex, and presence or absence of gastric mucosal atrophy were the clinical factors associated with the occurrence of acute abdominal symptoms. Older and male patients and those with gastric mucosal atrophy were less likely to show abdominal symptoms. The mechanisms of the occurrence of symptoms induced by gastric anisakiasis remain unclear; however, our results will help clarify this issue in the future.
Keywords: Gastric anisakiasis, Abdominal pain, Asymptomatic
Introduction
Gastrointestinal anisakiasis is a zoonotic parasitic infection caused by ingesting raw or uncooked seafood infected with nematodes of the genus Anisakis. Anisakis simplex is the most common cause of the infection [1, 2]. Most cases of anisakiasis are reported from Japan, with approximately 20,000 cases occurring yearly [3], possibly due to the Japanese culture of ingesting raw fish. In recent years, Japanese foods (sushi and sashimi) have become popular worldwide and are expected to cause an increased incidence of gastrointestinal anisakiasis [4]. There have been reports of gastrointestinal anisakiasis in various countries and regions [5, 6], and this disease has been recognized as a public health concern. Anisakis larvae may parasitize the esophagus, stomach, small bowel, and colon; however, most gastrointestinal anisakiasis cases are gastric anisakiasis, representing approximately 95% of cases [7]. Gastrointestinal anisakiasis is characterized by an acute abdomen, and the typical symptom of gastric anisakiasis is acute severe epigastric pain with a few hours after ingesting infected seafood. Other symptoms may include nausea and vomiting. Symptoms usually develop within 48 h (peaking within 6 h) [8].
Gastric anisakiasis is generally thought to cause severe abdominal symptoms; however, we incidentally detected asymptomatic gastric anisakiasis cases during esophagogastroduodenoscopy (EGD) for screening or medical checkups. To date, only a few case reports of asymptomatic gastric anisakiasis exist [9, 10]; however, such cases are sometimes encountered in actual clinical practice. Factors associated with developing abdominal symptoms induced by gastric anisakiasis remain unclear. A previous study showed that Anisakis simplex tended to penetrate non-atrophic mucosa more than atrophic mucosa, and patients with normal mucosal infections were more likely to exhibit clinical symptoms [11]. Gastric mucosal atrophy is yellowish-pale and unsmooth mucosa mainly associated with Helicobacter pylori (H. pylori) infection. Another group reported an association between clinical manifestations and H. pylori infection [12]. However, these studies were conducted at a single center with a small sample size and predominantly symptomatic cases; no study analyzing the clinical factors between symptomatic and asymptomatic cases in a multicenter setting with a large sample size exists. Therefore, we conducted a multicenter retrospective cohort study to investigate factors associated with acute abdominal symptoms induced by gastric anisakiasis.
Materials and methods
Study design
This was a multicenter, retrospective cohort study conducted at nine hospitals in Japan, in compliance with the principles of the Declaration of Helsinki of 1964 and revised versions. The study protocol was approved by the institutional review board of Tonan Hospital (approval number 547) and all participating institutions. Written informed consent for EGD was obtained from all the patients. All participants were given opportunities to decline participation in this study using the opt-out method on each participating hospital’s website. This study was registered on the University Hospital Medical Information Network (Registration number UMIN 000046411).
Patients
This study included consecutive patients diagnosed with gastric anisakiasis at nine hospitals in Japan between October 2015 and October 2021. Patients who were reinfected with Anisakis within the period were also included. The inclusion criteria were cases aged ≥ 20 years, diagnosed with gastric anisakiasis using EGD, and wherein Anisakis larvae were removed with forceps. Gastric anisakiasis was defined as Anisakis larvae penetrating into the gastric wall. The exclusion criteria were clinical symptoms or endoscopic findings that could not be evaluated, and non-provision of clinical information. Patients of multiple Anisakis larvae infection (two or more Anisakis larvae found concurrently) were also excluded because it was impossible to determine which larvae were causing symptoms.
Outcome measures
We analyzed patients’ medical records and endoscopic images, including background, comorbidities, regular use of non-steroidal anti-inflammatory drugs (NSAIDs) or acid secretion inhibitors, degree of gastric mucosal atrophy, location of Anisakis larvae, gastric mucosal atrophy of Anisakis larvae’s penetrating area, surrounding edema, erythema or erosion, and complications associated with the endoscopic procedure. If available, we also analyzed the H. pylori infection status and laboratory data, including white blood cell (WBC) counts (cut off < 8600/µL), the percentage of eosinophils (cut off < 6%), and C-reactive protein (CRP) level (cut off < 0.14 mg/dL). The degree of gastric mucosal atrophy was assessed using the Kimura-Takemoto classification [13]. This classification categorized the extent of atrophy into closed-type (C1, C2, and C3) and open-type (O1, O2, and O3). C0 indicated no atrophic mucosa, whereas C1 to O3 indicated atrophic mucosa. In this study, we analyzed whether there was a difference in the occurrence of symptoms depending on the degree of mucosal atrophy. The presence or absence of mucosal atrophy of Anisakis larvae penetrating area was defined as follows: an endoscope that showed homogeneously reddish and smooth mucosa with regular arrangement of collecting venules (RAC) indicated no atrophy, whereas yellowish-pale mucosa and unsmooth mucosa without RAC indicated the presence of atrophy [14]. Gastric mucosal atrophy, mucosal edema, erythema, or erosion were evaluated by endoscopic images. H. pylori infection status was divided into three groups. If any of the tests (urea breath, rapid urease, stool antigen, or serum H. pylori immunoglobulin G antibody test) were positive, the patient was considered to have H. pylori infection. If the tests were negative and the patient had a positive history of eradication, or if any of the tests were negative and endoscopic findings showed mucosal atrophy, the patient was considered to have H. pylori eradication. If one or more of these tests were negative and endoscopic findings showed C0, the patient was considered H. pylori naïve. We categorized patients into two groups according to their clinical symptoms: (a) symptomatic group: patients with acute abdominal pain with or without nausea, or vomiting; and (b) asymptomatic group: patients without abdominal symptoms, such as those incidentally detected during EGD for screening or medical checkups. We compared the clinical findings between the two groups to investigate the clinical factors associated with the occurrence of acute abdominal symptoms.
Statistical analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria) [15]. Quantitative variables were expressed as medians, whereas categorical variables were presented as total numbers and percentages. Pearson’s chi-squared and the Mann-Whitney U tests were used as appropriate. Multivariate analysis was performed using a logistic regression analysis. Statistical significance was set at p < 0.05.
Results
Patients’ characteristics
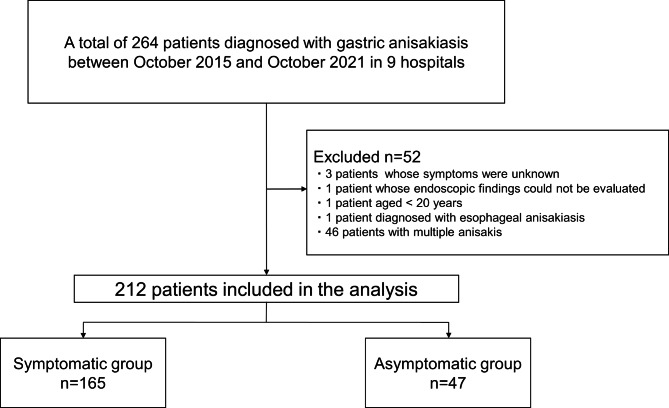
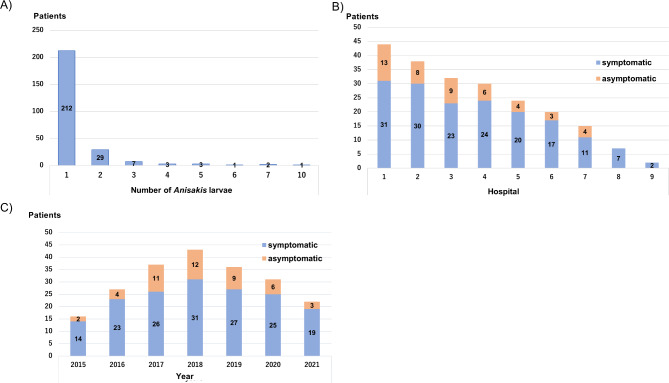
A total of 264 patients diagnosed with gastric anisakiasis at nine hospitals between October 2015 and October 2021 were enrolled in this study. Of the 264 patients, 52 were excluded for the following reasons: unknown clinical symptoms (n = 3), inability to evaluate endoscopic findings (n = 1), age < 20 years (n = 1), esophageal anisakiasis (n = 1), and multiple Anisakis larvae infections (n = 46). Therefore, 212 patients were finally analyzed (Fig. 1). Patients’ baseline characteristics are presented in Table 1. There were 116 male and 96 female patients, with a median patient age of 53 years. Of all the patients, 7.1% had diabetes mellitus. Furthermore, 3.3% and 17.5% of patients took NSAIDs and acid secretion inhibitors, respectively. One hundred sixty-five patients (77.8%) were diagnosed with abdominal symptoms, whereas 47 (22.2%) were asymptomatic. According to the classification of background gastric mucosal atrophy, 145 patients (68.4%) were classified as C0, 46 (21.7%) as C1–C3, and 21 (9.9%) as O1–O3. The locations of Anisakis larvae were as follows: the upper third (n = 74, 34.9%), middle third (n = 96, 45.3%), and lower third (n = 42, 19.8%) of the stomach. According to the gastric circumference, 30 larvae were detected in the lesser curvature (14.2%), 123 in the greater curvature (58.0%), 25 in the anterior wall (11.8%), and 34 in the posterior wall (16.0%). The presence of atrophy in the Anisakis larvae’s penetrating area was positive in 43 (20.3%) patients. Surrounding edema, erythema, or erosion was positive in 164 (77.4%) lesions. No complications were associated with the endoscopic procedures. In this study, no cases reinfected with Anisakis were observed within the period. Detailed data about the number of Anisakis larvae, the number of patients per hospital, and the number of patients per year are shown in Fig. 2.
Fig. 1.
Flow chart outlining the selection of patients
Table 1.
Patient characteristics
| n = 212 (%) | |
|---|---|
| Age (years) | |
| Median (range) | 53 (21–89) |
| Sex | |
| Male | 116 (54.7) |
| Female | 96 (45.3) |
| Abdominal pain | |
| Present | 165 (77.8) |
| Absent | 47 (22.2) |
| Comorbidities | |
| Ischemic heart disease | 10 (4.7) |
| Liver cirrhosis | 2 (0.9) |
| Diabetes mellitus | 15 (7.1) |
| CKD with dialysis | 1 (0.5) |
| NSAIDs | 7 (3.3) |
| Acid secretion inhibitor | 37 (17.5) |
| Degree of gastric mucosal atrophy | |
| No atrophy | 145 (68.4) |
| Closed type | 46 (21.7) |
| Open type | 21 (9.9) |
| Location | |
| Upper third | 74 (34.9) |
| Middle third | 96 (45.3) |
| Lower third | 42 (19.8) |
| Circumference | |
| Anterior wall | 25 (11.8) |
| Posterior wall | 34 (16.0) |
| Greater curvature | 123 (58.0) |
| Lesser curvature | 30 (14.2) |
| Mucosa of penetrating area | |
| Atrophy | 43 (20.3) |
| No atrophy | 169 (79.7) |
| Edema, erythema, erosion | |
| Positive | 164 (77.4) |
| Negative | 48 (22.6) |
CKD; chronic kidney disease, NSAIDs; non-steroidal anti-inflammatory drugs
Fig. 2.
A) Number of patients and number of Anisakis larvae. B) Number of patients per hospital. C) Number of patients per year
Comparison of clinical findings between symptomatic and asymptomatic groups
A comparison of the clinical findings between the symptomatic and asymptomatic groups is shown in Table 2. The median age was 49 years in the symptomatic group and 64 years in the asymptomatic group, indicating that the patients in the asymptomatic group were significantly older (p < 0.001). The number of female patients in the symptomatic group was significantly higher than that in the asymptomatic group (p = 0.046). Furthermore, there were significant differences in the presence of liver cirrhosis (p = 0.048) and diabetes mellitus (p = 0.026) between the two groups. In contrast, the administration of NSAIDs or acid secretion inhibitors was not significantly different between the two groups. Regarding the degree of mucosal atrophy, 75.2% and 44.7% of patients were classified as having no atrophic mucosa in the symptomatic and asymptomatic groups, respectively, with a significant difference (p = 0.001). However, there was no significant difference in the penetrating location or circumference of Anisakis, and the larvae were more common in the greater curvature of the upper or middle stomach. The presence of atrophy in the Anisakis larvae penetrating area was positive in 17.0% of patients in the symptomatic group and 31.9% of those in the asymptomatic group, with a significant difference (p = 0.038). There was no significant difference in the presence of surrounding erosion, edema, or erythema between the two groups.
Table 2.
Comparison of clinical findings between symptomatic and asymptomatic groups
| Symptomatic group | Asymptomatic group |
||
|---|---|---|---|
| n = 165 (%) | n = 47 (%) | p value | |
| Age (years) | |||
| Median (range) | 49 (21–82) | 64 (38–89) | < 0.001 |
| Sex | 0.046 | ||
| Male | 84 (50.9) | 32 (68.1) | |
| Female | 81 (49.1) | 15 (31.9) | |
| Comorbidities | |||
| Ischemic heart disease | 7 (4.2) | 3 (6.4) | 0.700 |
| Liver cirrhosis | 0 (0.0) | 2 (4.3) | 0.048 |
| Diabetes mellitus | 8 (3.8) | 7 (14.9) | 0.026 |
| CKD with dialysis | 0 (0.0) | 1 (2.1) | 0.220 |
| NSAIDs | 6 (3.6) | 1 (2.1) | 0.874 |
| Acid secretion inhibitor | 25 (15.2) | 12 (25.5) | 0.126 |
| Gastric mucosa | 0.001 | ||
| No atrophy | 124 (75.2) | 21 (44.7) | |
| Atrophy | 41 (24.8) | 26 (55.3) | |
| Location | 0.386 | ||
| Upper third | 57 (34.5) | 17 (36.2) | |
| Middle third | 72 (43.6) | 24 (51.1) | |
| Lower third | 36 (21.8) | 6 (12.8) | |
| Circumference | 0.163 | ||
| Anterior wall | 15 (9.1) | 10 (21.3) | |
| Posterior wall | 28 (17.0) | 6 (12.8) | |
| Greater curvature | 97 (58.8) | 26 (55.3) | |
| Lesser curvature | 25 (15.2) | 5 (10.6) | |
| Mucosa of penetrating area | 0.038 | ||
| Atrophy | 28 (17.0) | 15 (31.9) | |
| No atrophy | 137 (83.0) | 32 (68.1) | |
| Edema, erythema, erosion | 0.429 | ||
| Positive | 130 (78.8) | 34 (72.3) | |
| Negative | 35 (21.2) | 13 (27.7) | |
CKD; chronic kidney disease, NSAIDs; non-steroidal anti-inflammatory drugs
Pearson’s chi-squared test, Mann-Whitney U test
We performed a multivariate analysis of factors associated with acute abdominal symptoms, including age, sex, diabetes mellitus, presence or absence of mucosal atrophy, and mucosal atrophy of the penetrating area (Table 3). Age (p = 0.007), sex (p = 0.017), and the presence or absence of mucosal atrophy (p = 0.033) were independent clinical factors for the occurrence of acute abdominal symptoms.
Table 3.
Multivariate analysis of factors associated with acute abdominal symptoms
| odds ratio | 95% CI | p value | |
|---|---|---|---|
| Age | 0.965 | 0.940–0.990 | 0.007 |
| Sex | 0.406 | 0.193–0.852 | 0.017 |
| Diabetes mellitus | 0.592 | 0.176–1.990 | 0.397 |
| Mucosal atrophy | 0.393 | 0.166–0.927 | 0.033 |
| Mucosal atrophy of penetrating area | 0.774 | 0.193–0.852 | 0.602 |
Logistic regression analysis
We analyzed atrophic mucosal cases to determine the relationship between the degree of mucosal atrophy and the occurrence of acute abdominal symptoms (Table 4). First, we divided atrophic mucosal cases into closed- and open- type and found that the degree of mucosal atrophy was significantly associated with the occurrence of symptoms (p = 0.037).
Table 4.
Relationship between the degree of mucosal atrophy and the occurrence of abdominal symptoms
| Degree of mucosal atrophy | |||
|---|---|---|---|
|
Closed type n = 46 (%) |
Open type n = 21 (%) |
p value | |
| Symptomatic | 32 (69.6) | 9 (42.9) | 0.037 |
| Asymptomatic | 14 (30.4) | 12 (57.1) | |
Pearson’s chi-squared test
H. pyloristatus and laboratory data.
H. pylori infection status, WBC counts, the percentage of eosinophils, and CRP levels were examined in 98 (46.2%), 123 (58.0%), 92 (43.4%), and 106 (50.0%) patients, respectively (Table 5). Regarding the H. pylori status, 70 patients (42.4%) in the symptomatic group and 28 (59.6%) in the asymptomatic group were examined. There was a significantly higher proportion of H. pylori-naïve patients in the symptomatic group than in the asymptomatic group (p < 0.01). WBC counts were examined in 98 (59.4%) and 25 (53.2%) patients in the symptomatic and asymptomatic groups, respectively. In the symptomatic group, 50 patients (51.0%) had WBC counts above the cut-off level, whereas all patients in the asymptomatic group had WBC counts within the normal limit. There was a significant difference between the groups (p < 0.001). Regarding the percentage of eosinophils, 83 (50.3%) and 9 (19.1%) patients were examined in the symptomatic and asymptomatic groups, respectively. The results show that 95.2% and 66.6% of the patients in the symptomatic and asymptomatic groups, respectively, exhibited a normal percentage of eosinophils (p = 0.002). CRP levels were examined in 92 (55.8%) and 14 (29.8%) patients in the symptomatic and asymptomatic groups, respectively, and no significant difference was observed (p = 0.200).
Table 5.
Comparison of Helicobacter pylori infection status and laboratory data between symptomatic and asymptomatic groups
| Symptomatic group |
Asymptomatic group | p value | |
|---|---|---|---|
| Hp status (n = 98) | 0.007 | ||
| Infected | 7 (10.0%) | 3 (10.7%) | |
| Eradicated | 22 (31.4%) | 18 (64.3%) | |
| Naïve | 41 (58.6%) | 7 (25.0%) | |
| WBC (n = 123) | |||
| Median (range) (/µL) | 8600 (1400–21,570) | 5300 (2900–7800) | |
| W.N.L / over | 48 / 50 | 25 / 0 | < 0.001 |
| Eosinophils (n = 92) | |||
| Median (range) (%) | 1.0 (0-16.5) | 2.0 (0.4-8) | |
| W.N.L / over | 79 / 4 | 6 / 3 | 0.002 |
| CRP (n = 106) | |||
| Median (range) (mg/dL) | 0.2 (0-7.8) | 0.0 (0-7.4) | |
| W.N.L / over | 42 / 50 | 9 / 5 | 0.200 |
Hp; Helicobacter pylori, WBC; White blood cell, W.N.L; Within the normal limit, CRP; C-reactive protein
Pearson’s chi-squared test
Discussion
Anisakis infection can cause different types of disease: gastrointestinal anisakiasis, ectopic anisakiasis, gastro-allergic anisakiasis, and specific IgE-positive asymptomatic type [16]. Gastric anisakiasis causes acute abdominal pain a few hours after ingesting infected seafood, and the mechanism of the occurrence of abdominal symptoms remains unclear. Physical irritation from Anisakis larval penetration or type I and/or type III allergic reactions were considered one of the causes [17]. In recent years, animal studies using rats have reported that Anisakis larvae causes hemorrhages in gastric tissue and mixed inflammatory cell infiltration in neutrophils and macrophages [18, 19]. In addition, proinflammatory cytokines and miRNAs have been investigated [18]; however, their mechanisms remain unknown, which is an issue for the future.
In this study, a relatively large number of asymptomatic cases (22.2%) were found, and there may be more undiagnosed asymptomatic Anisakis cases in clinical practice than we think. Moneo et al. indicated that the high number of specific IgE-positive individuals suggests that many asymptomatic patients remain underdiagnosed in endemic countries and that there may actually be more Anisakis infections [16]. Although the location of Anisakis was not significantly associated with symptoms in previous reports [20, 21], the greater curvature of the upper or middle third stomach was the most common site of penetration, and careful observation of this area is essential for diagnosing gastric anisakiasis.
Univariate analysis revealed significant differences in age, sex, liver cirrhosis, diabetes mellitus, presence or absence of mucosal atrophy, and mucosal atrophy of the penetrating area. Liver cirrhosis showed a subtle result because of the minimal number of patients with positive results. Diabetes mellitus was more common in the asymptomatic group. Elevated pain thresholds have been reported in patients with diabetic neuropathy [22]. Although their severity has not been investigated, patients with diabetes mellitus may be less likely to experience symptoms. Diabetes mellitus was not a significant factor in the multivariate analysis, and this may be associated with the age difference between the two groups. In general, the prevalence of diabetes mellitus increases in older adults [23], and age may have been a confounding factor in our analysis. Multivariate analysis revealed that age, sex, and presence or absence of mucosal atrophy were independent factors for the occurrence of symptoms. The elderly and male patients were significantly more common in the asymptomatic group. This may be because pain thresholds may differ by age and sex. Although there are various opinions about pain thresholds, it has been reported that pain thresholds increase with age, and males may have higher pain thresholds than females depending on the type of pain [24, 25]. Gastric mucosal atrophy was also significantly associated with abdominal symptoms; however, mucosal atrophy of the penetrating area was not an independent risk factor. These results suggest that the presence or absence of gastric mucosal atrophy, rather than mucosal atrophy of the penetrating area, is associated with the occurrence of symptoms. Furthermore, this study found that advanced mucosal atrophy was less likely to cause symptoms. Gastric mucosal atrophy is associated with intragastric pH, and mucosal atrophy results in elevated pH [26]. Anisakis simplex is more active at lower pH values and its penetration rate into agar gel increases with decreasing pH [11, 27]. It has been shown that symptoms disappear as Anisakis activity decreases [28], suggesting that differences in intragastric pH may have led to differences in Anisakis activity and contributed to the differences in symptom occurrence, although the detailed mechanism of abdominal pain remains unclear.
Although not all cases could be examined, there were significant differences in H. pylori status, WBC count, and percentage of eosinophils between the two groups. H. pylori infection leads to gastric mucosal atrophy. Because gastric mucosal atrophy was significantly associated with symptoms in this study, it seems consistent that the H. pylori infection status is indirectly associated with the occurrence of symptoms. In the symptomatic group, the WBC count was above the normal limit in approximately half of the patients. In contrast, all patients in the asymptomatic group had WBC counts within the normal limit. Although leukocytosis was infrequently observed in a previous report [4], our results suggest that the symptoms are accompanied by inflammation. However, in the asymptomatic group, the timing of infection by Anisakis was unknown, and the possibility that the WBC count was elevated immediately after infection cannot be excluded. The CRP level may not have elevated because of the short duration from the occurrence of symptoms. Eosinophilia has been reported to be infrequent in gastric anisakiasis cases [4], consistent with the present study, wherein eosinophilia was less frequent in the symptomatic group. Meanwhile, the percentage of eosinophils was frequently elevated in the asymptomatic group; however, the number of patients was too small, making the interpretation of these results difficult.
This study had several limitations. First, this study was a retrospective design, and we could not investigate the H. pylori infection status or laboratory data in all cases. In addition, it was impossible to search in detail whether there were any symptoms in asymptomatic cases (for example, patients may have experienced mild symptoms some time ago). In addition, we did not investigate whether abdominal symptoms improved after the removal of Anisakis larvae in patients in the symptomatic group. Second, there was selection bias. These results may differ when comparing many cases because there may be more undiagnosed asymptomatic cases. Third, histological examination and molecular analysis of the removed Anisakis larvae were not performed. In Japan, Anisakis simplex is the major etiological agent of human anisakiasis [29], although different species of Anisakis are causative depending on the region. However, a previous study suggested that survival rates in gastric juice or the larval penetrating activity varies among species and that they express genes involved in pathogenicity in a different manner [30–32]. The lack of a species molecular identification is an important limitation, and future analyses with species considerations are needed.
In conclusion, this is the first study to compare the risk factors for acute abdominal symptoms induced by gastric anisakiasis. Age, sex, and presence or absence of mucosal atrophy were the clinical factors associated with the occurrence of symptoms. Older and male patients or those with gastric mucosal atrophy were less likely to show acute symptoms, suggesting that some cases might not have been diagnosed with gastric anisakiasis. However, the mechanisms of symptom occurrence remain unclear and we believe that this study’s results will help clarify this issue.
Acknowledgements
None.
List of abbreviations
- EGD
Esophagogastroduodenoscopy
- H. pylori
Helicobacter pylori
- NSAIDs
non-steroidal anti-inflammatory drugs
- WBC
white blood cell
- CRP
C-reactive protein
- RAC
regular arrangement of collecting venules
Author contributions
Conception and design: Y.O., Y.T., T.S. Material preparation and data collection: T.I., H.S., F.T., Y.A., Y.K., A.S., S.O., T.J., Y.T. Writing-original draft: Y.O. Writing-review and editing: Y.O., T.S., T.I., H.S., F.T., Y.A., Y.K., A.S., S.O., T.J., Y.T., S.M., H.H., H.M., S.K., M.M., and H.K. All authors have read and approved the final manuscript.
Funding
None.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. In the future we may consider asking our patients for their permission to share clinical data for research purposes.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This retrospective cohort study was conducted at nine hospitals in Japan, in compliance with the principles of the Declaration of Helsinki of 1964 and later versions. The study protocol was approved by the institutional review board of Tonan Hospital (approval number 547) and all participating institutions. This study was registered on the University Hospital Medical Information Network (Registration number UMIN 000046411). Written informed consent for EGD was obtained from all the patients. All participants were given opportunities to decline participation in this study using the opt-out method on each participating hospital’s website.
Consent for publication
Not applicable
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guardone L, Armani A, Nucera D, Costanzo F, Mattiucci S, Bruschi F. Human anisakiasis in Italy: a retrospective epidemiological study over two decades. Parasite. 2018;25:41. doi: 10.1051/parasite/2018034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattiucci S, Fazii P, De Rosa A, Paoletti M, Megna AS, Glielmo A, et al. Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection, Italy. Emerg Infect Dis. 2013;19:496–9. doi: 10.3201/eid1903.121017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiyama H, Shiroyama M, Yamamoto I, Ishikawa T, Morishima Y. Anisakiasis annual incidence and causative species, Japan, 2018–2019. Emerg Infect Dis. 2022;28:2105–8. doi: 10.3201/eid2810.220627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim T, Song HJ, Jeong SU, Choi EK, Cho YK, Kim HU, et al. Comparison of the clinical characteristics of patients with small bowel and gastric anisakiasis in jeju island. Gut Liver. 2013;7:23–9. doi: 10.5009/gnl.2013.7.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kołodziejczyk L, Szostakowska B, Sobecka E, Szczucki K, Stankiewicz K. First case of human anisakiasis in Poland. Parasitol Int. 2020;76:102073. doi: 10.1016/j.parint.2020.102073. [DOI] [PubMed] [Google Scholar]
- 6.Patiño JA, Olivera MJ. Gastro-allergic anisakiasis: the first case reported in Colombia and a literature review. Biomedica. 2019;39:241–6. doi: 10.7705/biomedica.v39i2.3936. [DOI] [PubMed] [Google Scholar]
- 7.Shimamura Y, Muwanwella N, Chandran S, Kandel G, Marcon N. Common symptoms from an uncommon infection: gastrointestinal anisakiasis. Can J Gastroenterol Hepatol. 2016;2016:5176502. doi: 10.1155/2016/5176502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takabayashi T, Mochizuki T, Otani N, Nishiyama K, Ishimatsu S. Anisakiasis presenting to the ED: clinical manifestations, time course, hematologic tests, computed tomographic findings, and treatment. Am J Emerg Med. 2014;32:1485–9. doi: 10.1016/j.ajem.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Nakaji K, Kumamoto M, Wada Y, Nakae Y. Asymptomatic gastric and colonic anisakiasis detected simultaneously. Intern Med. 2019;58:2263–4. doi: 10.2169/internalmedicine.2657-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada T, Ohwada S. Case of gastric anisakiasis with no symptoms. Clin Case Rep. 2020;8:1833–4. doi: 10.1002/ccr3.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai T, Akao N, Seki T, Kumagai T, Ishikawa H, Ohta N, et al. Molecular genotyping of anisakis larvae in Middle Eastern Japan and endoscopic evidence for preferential penetration of normal over atrophic mucosa. PLoS ONE. 2014;9:e89188. doi: 10.1371/journal.pone.0089188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimamura Y, Honda H, Fukuda K. Is there a link between clinical manifestation of gastric anisakiasis and Helicobacter pylori infection? Clin Endosc. 2017;50:510. doi: 10.5946/ce.2017.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh K, Kimura K, Taniguchi Y, Yoshida Y, Kihira K, Takimoto T, et al. Distribution of inflammation and atrophy in the stomach of Helicobacter pylori-positive and -negative patients with chronic gastritis. Am J Gastroenterol. 1996;91:963–9. [PubMed] [Google Scholar]
- 14.Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376–81. doi: 10.1055/s-2002-25281. [DOI] [PubMed] [Google Scholar]
- 15.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moneo I, Carballeda-Sangiao N, González-Muñoz M. New Perspectives on the diagnosis of Allergy to Anisakis spp. Curr Allergy Asthma Rep. 2017;17:27. doi: 10.1007/s11882-017-0698-x. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Iida M. Gastrointestinal anisakidosis. Stomach Intestine. 2002;37:429–36. [Google Scholar]
- 18.Hrabar J, Trumbić Ž, Bočina I, Bušelić I, Vrbatović A, Mladineo I. Interplay between proinflammatory cytokines, miRNA, and tissue lesions in Anisakis-infected Sprague-Dawley rats. PLoS Negl Trop Dis. 2019;13:e0007397. doi: 10.1371/journal.pntd.0007397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bušelić I, Trumbić Ž, Hrabar J, Vrbatović A, Bočina I, Mladineo I. Molecular and Cellular Response to Experimental Anisakis pegreffii (Nematoda, Anisakidae) third-stage larval infection in rats. Front Immunol. 2018;9:2055. doi: 10.3389/fimmu.2018.02055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakizoe S, Kakizoe H, Kakizoe K, Kakizoe Y, Maruta M, Kakizoe T, et al. Endoscopic findings and clinical manifestation of gastric anisakiasis. Am J Gastroenterol. 1995;90:761–3. [PubMed] [Google Scholar]
- 21.Shimamura Y, Ishii N, Ego M, Nakano K, Ikeya T, Nakamura K, et al. Multiple acute infection by Anisakis: a case series. Intern Med. 2016;55:907–10. doi: 10.2169/internalmedicine.55.5628. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki C, Kon T, Funamizu Y, Ueno T, Haga R, Nishijima H, et al. Elevated pain threshold in patients with asymptomatic diabetic neuropathy: an intraepidermal electrical stimulation study. Muscle Nerve. 2016;54:146–9. doi: 10.1002/mus.25158. [DOI] [PubMed] [Google Scholar]
- 23.Charvat H, Goto A, Goto M, Inoue M, Heianza Y, Arase Y, et al. Impact of population aging on trends in diabetes prevalence: a meta-regression analysis of 160,000 japanese adults. J Diabetes Investig. 2015;6:533–42. doi: 10.1111/jdi.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104–13. doi: 10.1016/j.neubiorev.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012;153:602–18. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Marotta F, Hayakawa K, Mikami Y, Morello P, Sugai M, Morita T. Relationship between gastrin cell number, serum, antral mucosa and luminal gastrin concentration and gastric acidity in antral atrophic gastritis. Gut. 1990;31:279–81. doi: 10.1136/gut.31.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima F, Ueda K, Fujimoto S. Evaluating method of the penetration capacity of Anisakis larvae with an agar. Clin Parasitol. 2012;23:64–6. [Google Scholar]
- 28.Sekimoto M, Nagano H, Fujiwara Y, Watanabe T, Katsu K, Doki Y, et al. Two cases of gastric anisakiasis for which oral administration of a medicine containing wood creosote (Seirogan) was effective. Hepatogastroenterology. 2011;58:1252–4. doi: 10.5754/hge11052. [DOI] [PubMed] [Google Scholar]
- 29.Umehara A, Kawakami Y, Araki J, Uchida A. Molecular identification of the etiological agent of the human anisakiasis in Japan. Parasitol Int. 2007;56:211–5. doi: 10.1016/j.parint.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki J, Murata R, Hosaka M, Araki J. Risk factors for human Anisakis infection and association between the geographic origins of Scomber japonicus and anisakid nematodes. Int J Food Microbiol. 2010;137:88–93. doi: 10.1016/j.ijfoodmicro.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Arizono N, Yamada M, Tegoshi T, Yoshikawa M. Anisakis simplex sensu stricto and Anisakis pegreffii: biological characteristics and pathogenetic potential in human anisakiasis. Foodborne Pathog Dis. 2012;9:517–21. doi: 10.1089/fpd.2011.1076. [DOI] [PubMed] [Google Scholar]
- 32.Cavallero S, Lombardo F, Su X, Salvemini M, Cantacessi C, D’Amelio S. Tissue-specific transcriptomes of Anisakis simplex (sensu stricto) and Anisakis pegreffii reveal potential molecular mechanisms involved in pathogenicity. Parasit Vectors. 2018;11:31. doi: 10.1186/s13071-017-2585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. In the future we may consider asking our patients for their permission to share clinical data for research purposes.