Abstract
Anakinra is a recombinant human interleukin-1 receptor antagonist approved for the treatment of inflammatory diseases. Kineret is available as a solution prepared in a borosilicate glass syringe. For implementing a placebo-controlled double-blind randomized clinical trial, anakinra is commonly transferred into plastic syringes. However, there is limited data on anakinra’s stability in polycarbonate syringes. We described the results of our previous studies on the use of anakinra in glass (VCUART3) versus plastic syringes (VCUART2) compared with placebo. These studies were conducted in patients with ST-segment elevation myocardial infarction (STEMI), and we assessed the anti-inflammatory effects of anakinra versus placebo by comparing the area under the curve for high-sensitivity cardiac reactive protein (AUC-CRP) levels during the first 14 days of STEMI, its clinical effects on heart failure (HF) hospitalization, cardiovascular death, or new diagnosis of HF as well as adverse events profile between groups. The levels of AUC-CRP were 75 (50–255 mg·day/l) for anakinra in plastic syringes versus 255 (116–592 mg·day/l) in placebo and 60 (24–139 mg·day/l) and 86 (43–123 mg·day/l) for anakinra once and twice daily in glass syringes, respectively, compared with placebo 214 (131–394 mg·day/l). The rate of adverse events was also comparable between groups. There were no differences in the rate of HF hospitalization or cardiovascular death in patients who received anakinra in plastic or glass syringes. Fewer cases of new-onset heart failure occurred in patients receiving anakinra in plastic or glass syringes compared with placebo. Anakinra stored in plastic (polycarbonate) syringes provides comparable biologic and clinical effect to glass (borosilicate) syringes.
SIGNIFICANCE STATEMENT
Anakinra (Kineret) 100 mg administered subcutaneously in patients with ST-segment elevation myocardial infarction (STEMI) for a duration of up to 14 days appears to have comparable safety and biological efficacy signals when delivered in prefilled glass or transferred into plastic polycarbonate syringes. This may have important implications for the feasibility of designing clinical trials in STEMI and other clinical conditions.
Introduction
Anakinra (Kineret; Swedish Orphan Biovitrum, Stockholm, Sweden) is a nonglycosylated recombinant human interleukin-1 (IL-1) receptor antagonist approved by the US Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis and other autoinflammatory conditions. Anakinra blocks the IL-1 receptor and prevents IL-1α and IL-1β from transducing a proinflammatory signal. (Dinarello, 2019; Abbate et al., 2020a) Originally approved for the treatment of rheumatoid arthritis, anakinra showed potential benefits in diseases not only limited to the joints (Furst, 2004) but also skin (Berk and Bayliss 2008; Tzanetakou et al., 2016), lung and respiratory tract (Rogliani et al., 2015; Calverley et al., 2017), heart and pericardium (Brucato et al., 2016; Correia et al., 2020; Imazio et al., 2020), blood vessels (Johnston et al., 2014), and multisystem complicated disorders such as COVID-19 (Huet et al., 2020; Kyriazopoulou et al., 2021), Behcet’s syndrome, (Fabiani et al., 2017), Still’s disease (Vastert et al., 2019), and Sjogren Syndrome. (Norheim et al., 2012) Anakinra is available in a single formulation as 100 mg in a 0.67-ml solution prepared in a glass syringe to be administered through the subcutaneous route.
During the past two decades, since the initial FDA approval in 2001, anakinra has been used in more than 150 clinical trials testing whether inhibition of IL-1 would favorably affect outcomes in a variety of diseases. In some instances, anakinra was administered through a different route (i.e., intravenous), in which case the content of the anakinra syringe was diluted in an infusion bag (Isambert et al., 2018; Monteagudo et al., 2020; Charlesworth et al., 2021). The conduct of clinical trials commonly requires the use of placebo. Obtaining matching anakinra and placebo syringes from the manufacturer is, however, often not possible, and therefore the investigators need to create matching syringes by transferring the content of the anakinra (Kineret) solution into different syringes, and syringes in plastic polycarbonate tend to be more widely available than glass syringes. There is, however, limited data on anakinra’s stability in plastic polycarbonate syringes. Given the widespread use of anakinra in clinical investigations in patients with acute and chronic inflammatory conditions, comparing the safety and efficacy of anakinra dispensed in glass versus plastic syringes may help the design of future studies in terms of potential cost reductions.
The aim of the current analysis was to compare whether administering anakinra from the content of the Kineret glass syringes after transfer and storage in plastic polycarbonate syringes provides the same biologic activity as administering directly from the Kineret glass syringes.
Materials and Methods
Clinical Trial Identifiers
We retrieved individual patient data from two clinical trials: VCUART2 (Abbate et al., 2013) and VCUART3 (Abbate et al., 2020b) (https://www.clinicaltrials.gov NCT00175018 and NCT01950299, respectively).
Inclusion and Exclusion Criteria
Both VCUART2 (Abbate et al., 2013) and VCUART3 (Abbate et al., 2020b) studies were purposefully designed with overlapping inclusion and exclusion criteria of patients with ST-segment elevation myocardial infarction (STEMI), defined as chest pain (or equivalent) with an onset within 12 hours and ST-segment elevation (>1 mm) in two or more anatomically contiguous leads on the electrocardiogram that is new or presumably new, adult age, presenting within 12 hours of pain onset, and enrolled within 12 hours of reperfusion. The exclusion criteria shared between the two studies included cardiac arrest, unsuccessful percutaneous coronary intervention, hemodynamic instability, preexisting severe congestive heart and/or severe left ventricular dysfunction [left ventricular ejection fraction (LVEF) <20%], severe aortic or mitral valve disease, pregnancy, chronic infections, autoinflammatory or autoimmune disease, or cancer. As the VCUART2 study incorporated cardiovascular magnetic resonance studies, individuals with contraindications to magnetic resonance imaging were excluded from VCUART2, whereas VCUART3 did not include magnetic resonance imaging.
Enrollment Period
Patients were enrolled at Virginia Commonwealth University (VCU) Health (Richmond, VA) for VCUART2 (September 2010 to May 2012) and at three clinical sites for VCUART3 (July 2014 to December 2017), including VCU Health, Virginia Cardiovascular Specialists (Richmond, VA), and Medstar Washington Hospital Center (Washington, DC).
Investigational Treatment
Patients in VCUART2 were randomly assigned in a 1:1 ratio to anakinra (Kineret) 100 mg per day in 0.67 ml or matching NaCl (0.9%) placebo injected subcutaneously for 14 days. The content of the Kineret syringe was transferred to a plastic polycarbonate syringe to create a matching colorless placebo NaCl 0.9% solution. Individual syringes were aseptically prepared according to USP 797 standards by the Investigational Pharmacy and dispensed daily during the inpatient phase, and the patient received the remaining syringes at discharge to complete the 14 days of treatment. The polycarbonate syringe was drawn back to a volume of 0.67 ml with no syringe attached, and the anakinra solution was expelled from the needle tip of the glass prefilled syringe into the open tip of the polycarbonate syringe. A sterile plastic needle cover was then placed on the polycarbonate syringe.
Patients in VCUART3 were randomized 1:1:1 to anakinra 100 mg twice daily, anakinra 100 mg once daily, alternating with placebo once daily or placebo twice daily for 14 days. The syringes containing anakinra, Kineret, or matching placebo were purchased from Swedish Orphan Biovitrum, so no transfer in plastic syringes was necessary.
Inflammatory Response after STEMI
High-sensitivity C-reactive protein (hsCRP) was measured at baseline, 72 hours, and 14 days in both studies (LabCorp). The area under the curve of CRP (CRP-AUC) after 14 days was estimated using the linear trapezoidal method for each subject and compared between the anakinra group and placebo group using three time points: one for baseline (before treatment), one for 72 hours (discharge), and one for outpatient follow-up at 14 days, allowing for variance in time to sampling but assuming a fix time for data calculation. To calculate the high-sensitivity C-reactive protein area under the curve (hsCRP-AUC), we used the following formula: (A + B)*1.5 + (B + C)*5.5, where A is the baseline hsCRP, B is the hsCRP level at 72 hours, and C is the hsCRP level at the end of treatment (14 days).
Efficacy and Safety Outcomes
Patients had in-person clinical follow-up at 14 days and 12 weeks in both studies and at 6 and 12 months for VCUART3. Events included assessing adverse events related to treatment and the incidence of death and heart failure (HF) events, including HF hospitalization, new HF, and cardiovascular death.
Statistical Analysis
Continuous variables are reported as median and interquartile range (IQR) and were compared between groups using a Mann-Whitney U test. Categorical data are reported as number and percentage and were compared using the χ2 test or Fisher’s exact test as appropriate. The analyses were completed using SPSS version 27.0 (SPSS, Chicago, IL).
Results
Patients’ Characteristics
The individual characteristics of the patients are presented in the initial reports for VCUART2 (Abbate et al., 2013) and VCUART3 (Abbate et al., 2020b). VCUART2 included 30 patients randomly assigned 1:1 to anakinra or placebo, with a median age of 57 years and 22 (73%) males. VCUART3 included 99 patients randomly assigned 1:1:1 to anakinra twice daily, anakinra once daily, or placebo, with a median age of 55 years and 80 (80%) males.
Area under the Curve for High-Sensitivity C-Reactive Protein
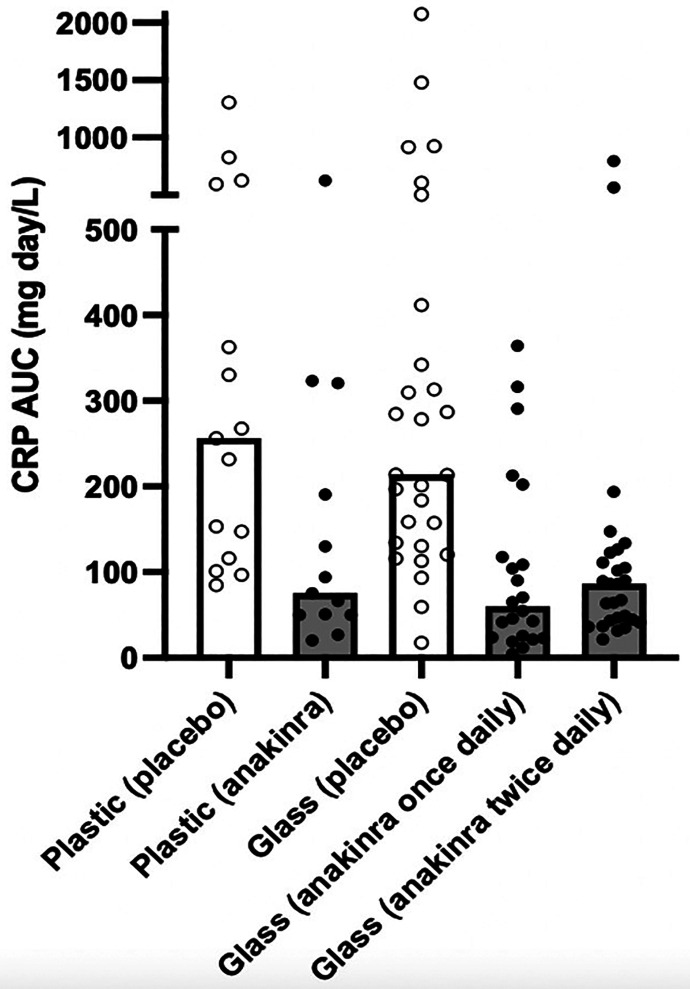
Treatment with anakinra led to a significant reduction in AUC-CRP in both VCUART2 and VCUART3 and independent of whether plastic or glass syringes were used (Fig. 1; Table 1). The levels of AUC-CRP were 75 (50–255 mg•day/l) for anakinra in plastic syringes versus 255 (116–592 mg•day/l) in placebo in VCUART2 and 60 (24–139 mg•day/l) and 86 (43–123 mg•day/l) for anakinra once and twice daily in glass syringes, respectively, compared with placebo 214 (131–394 mg•day/l) in VCUART3.
Fig. 1.
Comparison of the anakinra effects on AUC-CRP at 14 days between VCUART2 and VCUART3 studies.
TABLE 1.
Effects of anakinra on hsCRP-AUC at 14 days during STEMI
| VCUART2 (Plastic Syringes) | VCUART3 (Glass Syringes) | ||||
|---|---|---|---|---|---|
| hsCRP-AUC (mg•day/l) | Placebo | Anakinra | Placebo | Anakinra once daily | Anakinra twice daily |
| Median | 256 | 75 | 214 | 60 | 86 |
| Interquartile Range | 116–592 | 50–255 | 131–394 | 24–139 | 43–123 |
Efficacy Outcomes
There was one (7%) HF hospitalization and four (27%) HF events in the placebo group and no hospitalizations for HF and one event (7%) in the anakinra group using plastic syringes in VCUART2 (Table 2). In VCUART3, using glass syringes, there were four (11%) hospitalizations for HF in the placebo group and none in either anakinra groups; nine (26%) had HF events in the placebo compared with three (9%) and three (10%) in the anakinra once daily and twice daily, respectively (Table 2).
TABLE 2.
Clinical events in VCUART2 and VCUART3 trials
Data are listed as number (%).
| Clinical Events | VCUART2 (Plastic Syringes) | VCUART3 (Glass Syringes) | |||
|---|---|---|---|---|---|
| Placebo | Anakinra | Placebo | Anakinra once daily | Anakinra twice daily | |
| Safety Outcomes | |||||
| Injection site reactions | 2 (13%) | 3 (20%) | 1 (3%) | 6 (18%) | 8 (26%) |
| Drug discontinuation | 0 | 3 (20%) | 1 (3%) | 3 (9%) | 2 (6%) |
| Serious infection | 2 (13%) | 2 (13%) | 5 (14%) | 3 (9%) | 6 (19%) |
| Efficacy Outcomes | |||||
| HF hospitalization or CV death | 1 (7%) | 0 | 4 (11%) | 0 | 0 |
| Death | 1 (7%) | 0 | 1 (3%) | 0 | 0 |
| New onset HF (all forms) | 4 (27%) | 1 (7%) | 9 (26%) | 3 (9%) | 3 (10%) |
CV, cardiovascular.
Safety Outcomes
There were two (13%) injection site reactions and two (13%) serious infections in the placebo group and three (20%) injection site reactions and two (13%) serious infections in the anakinra group using plastic syringes in VCUART2 (Table 2). In VCUART3, using glass syringes, there was one (3%) injection site reaction in the placebo group and six (18%) and eight (26%) in anakinra once daily and twice daily groups, respectively; five (14%) had serious infections in the placebo compared with three (9%) and six (19%) in the anakinra once daily and twice daily, respectively (Table 2).
Discussion
We herein compare for the first time the biologic effects of anakinra provided in Kineret borosilicate glass syringes compared with plastic polycarbonate syringes by comparing the effects of treatment on AUC-CRP and HF events versus placebo in VCUART2 and VCUART3 studies. These studies evaluated the role of anakinra in reducing inflammation in patients with STEMI. Using polycarbonate instead of borosilicate syringes did not significantly impact the 14-day anti-inflammatory response (assessed by AUC-CRP) nor the clinical effects of anakinra in this population. This finding suggests that anakinra is stable in plastic polycarbonate syringes and has implications for designing future randomized clinical trials, including a placebo arm, using biologic agents such as anakinra.
There is a significant inflammatory response even after successful revascularization in patients with STEMI, which predicts adverse cardiac remodeling and overall risk of new onset HF (Seropian et al., 2014; Seropian et al., 2016; Toldo and Abbate 2018; Bellis et al., 2021; Frantz et al., 2022). The result of targeted anti-inflammatory treatment with IL-blockade has shown great promise. (Abbate et al., 2010; Abbate et al., 2013; Abbate et al., 2015; Ridker et al., 2017; Van Tassell et al., 2018; Everett et al., 2019; Abbate et al., 2020b). The benefits of IL-1 blockade is not limited to patients with STEMI, as anakinra also improves cardiac function and cardiorespiratory fitness in patients with HF (Van Tassell et al., 2012; Van Tassell et al., 2017; Buckley et al., 2018; Trankle et al., 2018; Abbate et al., 2020a). Considering the potential benefit of IL-1 blockade in patients with STEMI and HF, assessing the stability/compatibility of anakinra in glass versus plastic syringes would be helpful for designing future randomized clinical trials.
Anakinra is available as a solution prepared in a borosilicate glass syringe. For implementing a placebo-controlled double-blind randomized clinical trial, anakinra is commonly transferred into plastic syringes. Similarly, in VCUART2 study, plastic syringes of anakinra were prepared daily for inpatient use and in batch at the day of discharge, dispensed for up to a 9-day use. An unanswered question has been regarding the stability of anakinra in a nonglass (plastic, polycarbonate) container in a situation where the original containers would not feasible or expensive for use in clinical trials. No significant change was observed for the visual appearance of any syringes while kept in the refrigerator and protected from light. Proteins are labile and their long-term preservation in solution can be challenging, as they may lose their activity because of proteolysis and aggregation (Hovorka and Schöneich 2001; Berkowitz et al., 2012; Lopalco and Stella, 2016). Therefore, the extent of storage shelf life can vary from a few days to years. Kineret supplied as prefilled glass syringes contains recombinant IL-1Ra (100 mg) in a 0.67-ml solution (pH 6.5) containing disodium EDTA (0.12 mg), sodium chloride (5.48 mg), anhydrous citric acid (1.29 mg), and polysorbate 80 (0.70 mg) in water. This formulation has shown stability in prefilled syringes for up to 10 years in a recent in vitro biophysical and cellular characterizations assessment (Peng and Wang, 2018). The available in vivo data on anakinra characteristics are limited. Nevertheless, there are few published studies on the short-term intravenous infusions of anakinra (4–6 hours) in intravenous bags (Wohlfarth et al., 2019; Mehta et al., 2020; Phadke et al., 2021; Pontali et al., 2021). Although it is questionable whether the quantitative analysis of protein substances in samples taken from the solution admixture is needed, biologic response can aid in finding the answer to the stability concern for longer durations of use in nonglass syringes. In the VCUART2 study, transferring anakinra to plastic syringes did not affect the biologic response compared with placebo measured as the reduction in AUC-CRP or clinical outcomes, including cardiovascular death, HF hospitalization, or new onset HF. Furthermore, the reduction in the AUC-CRP was comparable to when used in the prefilled glass syringes in the VCUART3 study. These data suggest that anakinra maintains its anti-inflammatory and clinical activity when used as described, including being stored in polycarbonate syringes for up to 9 days. Although the results of this study should be translated to other biologic compounds with caution, it may support the use of this and other agents for the design of future clinical trials, obviating the need for glass syringes in placebo preparations. This may allow cost reductions associated with research on these widely studied agents in patients with acute and chronic inflammatory conditions as well as cardiovascular diseases.
Limitations
In this study, we compared the clinical findings of VCUART2 and VCUART3 studies to assess the difference between the application of glass versus plastic syringes in clinical practice. Although this approach appears sufficient to show comparable AUC-CRP values between groups and clinical events, it may have some limitations. Firstly, different populations of patients were studied in these two trials, and patients were not randomized between groups. Secondly, we did not measure the concentration of anakinra levels in the plastic versus glass syringes nor its pharmacokinetic levels in the patients. There is a lack of formal stability and compatibility studies for anakinra, and our data are limited to comparing its clinical effects in VCUART2 and VCUART3 clinical trials. Finally, we only compare the AUC of CRP values as the only pharmacodynamic parameter between groups that, although a strong and independent predictor of outcomes in STEMI, is influenced by multiple variables such as the initial CRP value, the extent of the infarct, the time of peak measurement, the value of the peak, the time of follow-up assessments, and its value and may therefore be affected by variables other than the anakinra concentration in the syringes.
Conclusions
Anakinra 100 mg administered subcutaneously in patients with STEMI for a duration of up to 14 days with up to 9 days self-administered as an outpatient appears to have a comparable safety and efficacy when stored in the prefilled borosilicate glass or transferred into plastic polycarbonate syringes. Although we cannot exclude minor differences between using glass versus plastic syringes, these results may be important implications for the feasibility of designing clinical trials in STEMI and other clinical conditions.
Acknowledgments
Swedish Orphan Biovitrum LLC (Stockholm, Sweden) has provided study medication (anakinra) and matching placebo free of cost for VCUART3 study but had no role in the study design, conduct, analysis, or reporting.
Abbreviations
- AUC
area under the curve
- CRP
C-reactive protein
- HF
heart failure
- hsCRP
high-sensitivity C-reactive protein
- hsCRP-AUC
high-sensitivity C-reactive protein area under the curve
- IL-1
interleukin-1
- STEMI
ST-segment elevation myocardial infarction
Authorship Contributions
Participated in research design: Sculthorpe, Pak, Van Tassell, Abbate.
Conducted experiments: Sculthorpe, Pak, Lipinski, Roberts, Markley, Trankle, Canada, Wohlford, Dixon, Van Tassell, Abbate.
Performed data analysis: Talasaz, Golino, Van Tassell, Abbate.
Wrote or contributed to the writing of the manuscript: Talasaz, Sculthorpe, Pak, Lipinski, Roberts, Markley, Trankle, Canada, Wohlford, Golino, Dixon, Van Tassell, Abbate.
Footnotes
The VCUART2 study was funded by Scientist Development [Grant 10SDG 3030051] from the American Heart Association (Dallas, TX), the Presidential Research Incentive Program of Virginia Commonwealth University (VCU) (to A.A.), and by the internal funds of VCU Pauley Heart Center and Victoria Johnson Research Laboratories. The VCUART3 study is supported by a grant from National Institutes of Health National Heart, Lung, and Blood Institute [Grant 1R34-HL121402-01] (to B.W.V.T. and A.A.).
No author has an actual or perceived conflict of interest with the contents of this article.
1Dr. Van Tassell and Dr. Abbate contributed equally to this manuscript.
References
- Abbate AKontos MCAbouzaki NAMelchior RDThomas CVan Tassell BWOddi CCarbone STrankle CRRoberts CS, et al. (2015) Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol 115:288–292. [DOI] [PubMed] [Google Scholar]
- Abbate AKontos MCGrizzard JDBiondi-Zoccai GGVan Tassell BWRobati RRoach LMArena RARoberts CSVarma A, et al. ; VCU-ART Investigators (2010) Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] pilot study). Am J Cardiol 105:1371–1377.e1. [DOI] [PubMed] [Google Scholar]
- Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA (2020a) Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res 126:1260–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbate ATrankle CRBuckley LFLipinski MJAppleton DKadariya DCanada JMCarbone SRoberts CSAbouzaki N, et al. (2020b) Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc 9:e014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbate AVan Tassell BWBiondi-Zoccai GKontos MCGrizzard JDSpillman DWOddi CRoberts CSMelchior RDMueller GH, et al. (2013) Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol 111:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis A, Di Gioia G, Mauro C, Mancusi C, Barbato E, Izzo R, Trimarco B, Morisco C (2021) Reducing cardiac injury during ST-elevation myocardial infarction: a reasoned approach to a multitarget therapeutic strategy. J Clin Med 10:2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk DR, Bayliss SJ (2008) Neutrophilic dermatoses in children. Pediatr Dermatol 25:509–519. [DOI] [PubMed] [Google Scholar]
- Berkowitz SA, Engen JR, Mazzeo JR, Jones GB (2012) Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov 11:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucato AImazio MGattorno MLazaros GMaestroni SCarraro MFinetti MCumetti DCarobbio ARuperto N, et al. (2016) Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 316:1906–1912. [DOI] [PubMed] [Google Scholar]
- Buckley LFCarbone STrankle CRCanada JMErdle CORegan JAViscusi MMKadariya DBillingsley HArena R, et al. (2018) Effect of interleukin-1 blockade on left ventricular systolic performance and work: a post hoc pooled analysis of 2 clinical trials. J Cardiovasc Pharmacol 72:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PMA, Sethi S, Dawson M, Ward CK, Finch DK, Penney M, Newbold P, van der Merwe R (2017) A randomised, placebo-controlled trial of anti-interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary disease. Respir Res 18:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth JEGWilson SQureshi ABlanco EMitchell ASegal SKelly DWeitz JO’Shea DBailey K, et al. (2021) Continuous intravenous anakinra for treating severe secondary haemophagocytic lymphohistiocytosis/macrophage activation syndrome in critically ill children. Pediatr Blood Cancer 68:e29102. [DOI] [PubMed] [Google Scholar]
- Correia ETO, Dos Santos Barbetta LM, de Almeida JPCL, Mesquita ET (2020) Anakinra in recurrent pericarditis: current evidence on clinical use, effectiveness, and safety. J Cardiovasc Pharmacol 76:42–49. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2019) The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol 15:612–632. [DOI] [PubMed] [Google Scholar]
- Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM (2019) Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139:1289–1299. [DOI] [PubMed] [Google Scholar]
- Fabiani C, Sota J, Tosi GM, Franceschini R, Frediani B, Galeazzi M, Rigante D, Cantarini L (2017) The emerging role of interleukin (IL)-1 in the pathogenesis and treatment of inflammatory and degenerative eye diseases. Clin Rheumatol 36:2307–2318. [DOI] [PubMed] [Google Scholar]
- Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J (2022) Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J 43:2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst DE (2004) Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clin Ther 26:1960–1975. [DOI] [PubMed] [Google Scholar]
- Hovorka S, Schöneich C (2001) Oxidative degradation of pharmaceuticals: theory, mechanisms and inhibition. J Pharm Sci 90:253–269. [DOI] [PubMed] [Google Scholar]
- Huet TBeaussier HVoisin OJouveshomme SDauriat GLazareth ISacco ENaccache JMBézie YLaplanche S, et al. (2020) Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2:e393–e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazio MAndreis ADe Ferrari GMCremer PCMardigyan VMaestroni SLuis SALopalco GEmmi GLotan D, et al. (2020) Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol 27:956–964. [DOI] [PubMed] [Google Scholar]
- Isambert NHervieu ARébé CHennequin ABorg CZanetta SChevriaux ARichard CDerangère VLimagne E, et al. (2018) Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): a single-arm phase 2 study. OncoImmunology 7:e1474319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, Lu G, Owens GK, Upchurch GR Jr, Ailawadi G (2014) Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 130(11, Suppl 1)S51–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazopoulou EPoulakou GMilionis HMetallidis SAdamis GTsiakos KFragkou ARapti ADamoulari CFantoni M, et al. (2021) Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 27:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopalco A, Stella VJ (2016) Effect of molecular structure on the relative hydrogen peroxide scavenging ability of some α-keto carboxylic acids. J Pharm Sci 105:2879–2885. [DOI] [PubMed] [Google Scholar]
- Mehta P, Cron RQ, Hartwell J, Manson JJ, Tattersall RS (2020) Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol 2:e358–e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo LA, Boothby A, Gertner E (2020) Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol 2:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norheim KB, Harboe E, Gøransson LG, Omdal R (2012) Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome--a double blind, randomised clinical trial. PLoS One 7:e30123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Wang X (2018) Kineret protein solution survives ten years. J Pharm Biomed Anal 160:383–385. [DOI] [PubMed] [Google Scholar]
- Phadke O, Rouster-Stevens K, Giannopoulos H, Chandrakasan S, Prahalad S (2021) Intravenous administration of anakinra in children with macrophage activation syndrome. Pediatr Rheumatol Online J 19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontali EVolpi SSignori AAntonucci GCastellaneta MBuzzi DMontale ABustaffa MAngelelli ACaorsi R, et al. (2021) Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J Allergy Clin Immunol 147:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PMEverett BMThuren TMacFadyen JGChang WHBallantyne CFonseca FNicolau JKoenig WAnker SD, et al. ; CANTOS Trial Group (2017) Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131. [DOI] [PubMed] [Google Scholar]
- Rogliani P, Calzetta L, Ora J, Matera MG (2015) Canakinumab for the treatment of chronic obstructive pulmonary disease. Pulm Pharmacol Ther 31:15–27. [DOI] [PubMed] [Google Scholar]
- Seropian IM, Sonnino C, Van Tassell BW, Biasucci LM, Abbate A (2016) Inflammatory markers in ST-elevation acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 5:382–395. [DOI] [PubMed] [Google Scholar]
- Seropian IM, Toldo S, Van Tassell BW, Abbate A (2014) Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 63:1593–1603. [DOI] [PubMed] [Google Scholar]
- Toldo S, Abbate A (2018) The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 15:203–214. [DOI] [PubMed] [Google Scholar]
- Trankle CRCanada JMCei LAbouzaki NOddi-Erdle CKadariya DChristopher SViscusi MDel Buono MKontos MC, et al. (2018) Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol 122:1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanetakou V, Kanni T, Giatrakou S, Katoulis A, Papadavid E, Netea MG, Dinarello CA, van der Meer JWM, Rigopoulos D, Giamarellos-Bourboulis EJ (2016) Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol 152:52–59. [DOI] [PubMed] [Google Scholar]
- Van Tassell BWArena RAToldo SMezzaroma EAzam TSeropian IMShah KCanada JVoelkel NFDinarello CA, et al. (2012) Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One 7:e33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell BWCanada JCarbone STrankle CBuckley LOddi Erdle CAbouzaki NADixon DKadariya DChristopher S, et al. (2017) Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail 10:e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell BWLipinski MJAppleton DRoberts CSKontos MCAbouzaki NMelchior RMueller GGarnett JCanada J, et al. (2018) Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): a randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol 41:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastert SJ, Jamilloux Y, Quartier P, Ohlman S, Osterling Koskinen L, Kullenberg T, Franck-Larsson K, Fautrel B, de Benedetti F (2019) Anakinra in children and adults with Still’s disease. Rheumatology (Oxford) 58 (Suppl 6):vi9–vi22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth P, Agis H, Gualdoni GA, Weber J, Staudinger T, Schellongowski P, Robak O (2019) Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med 34:723–731. [DOI] [PubMed] [Google Scholar]