Abstract
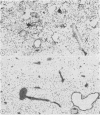
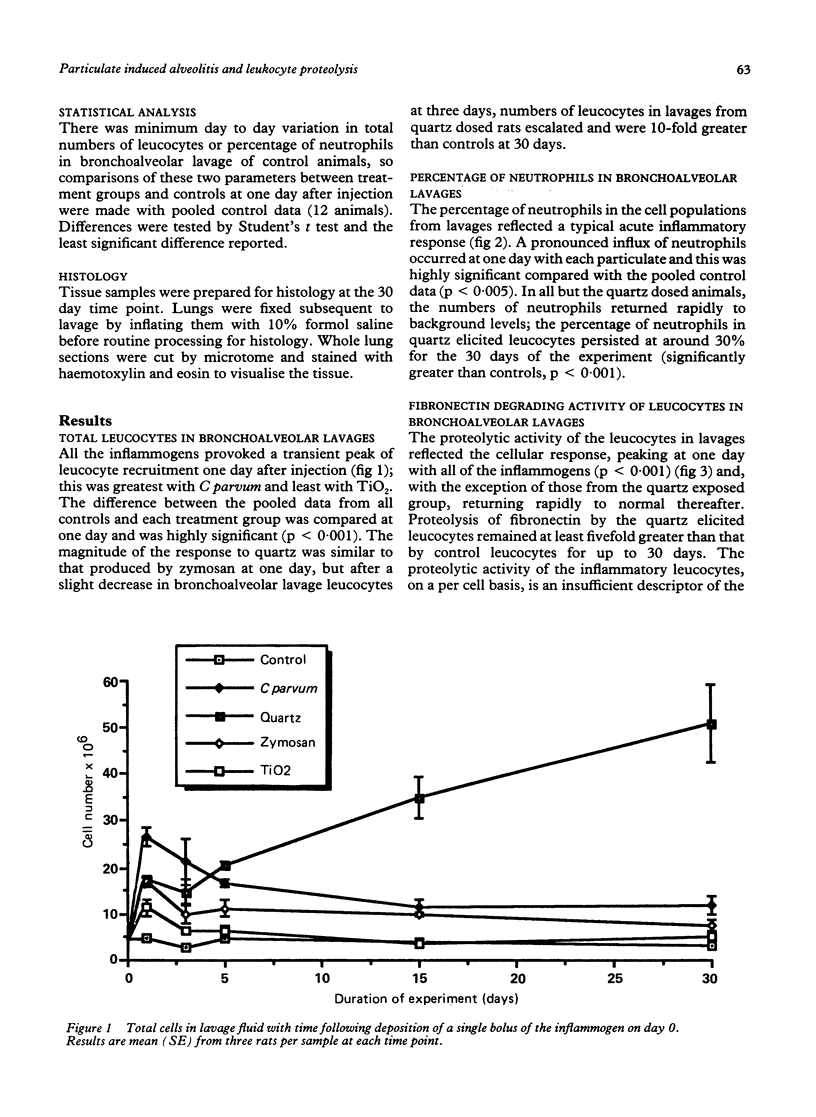
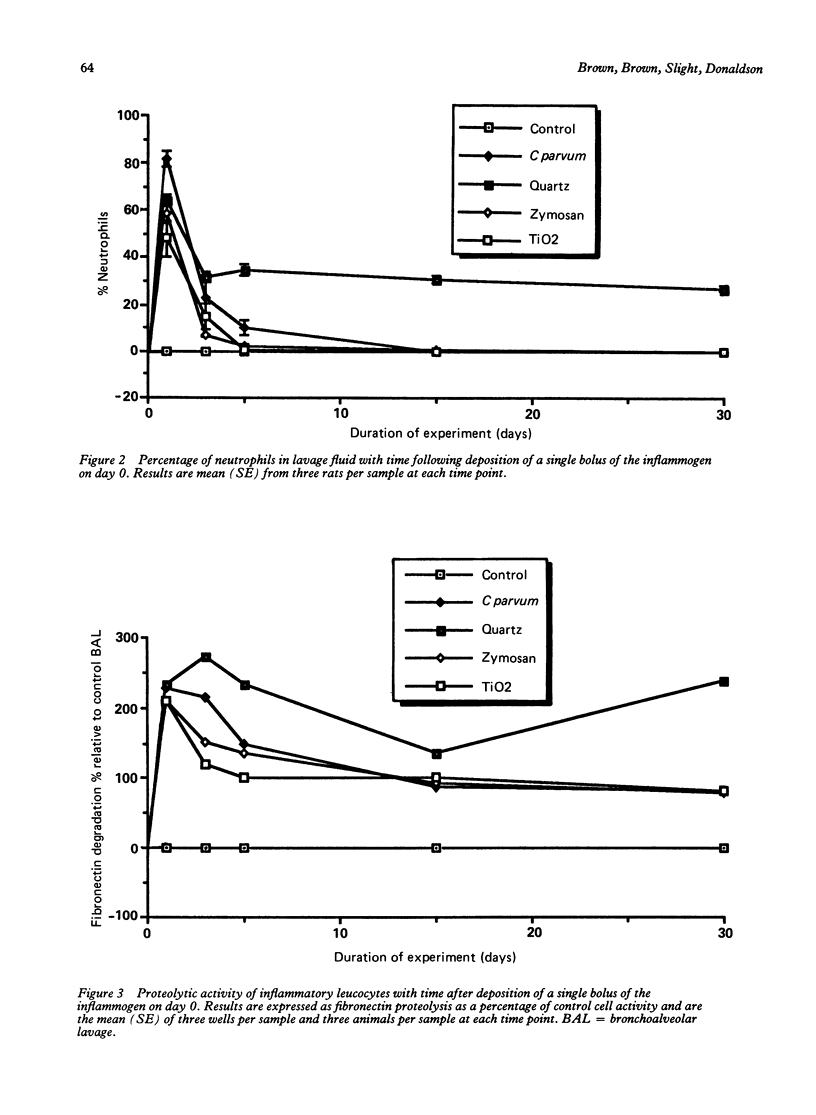
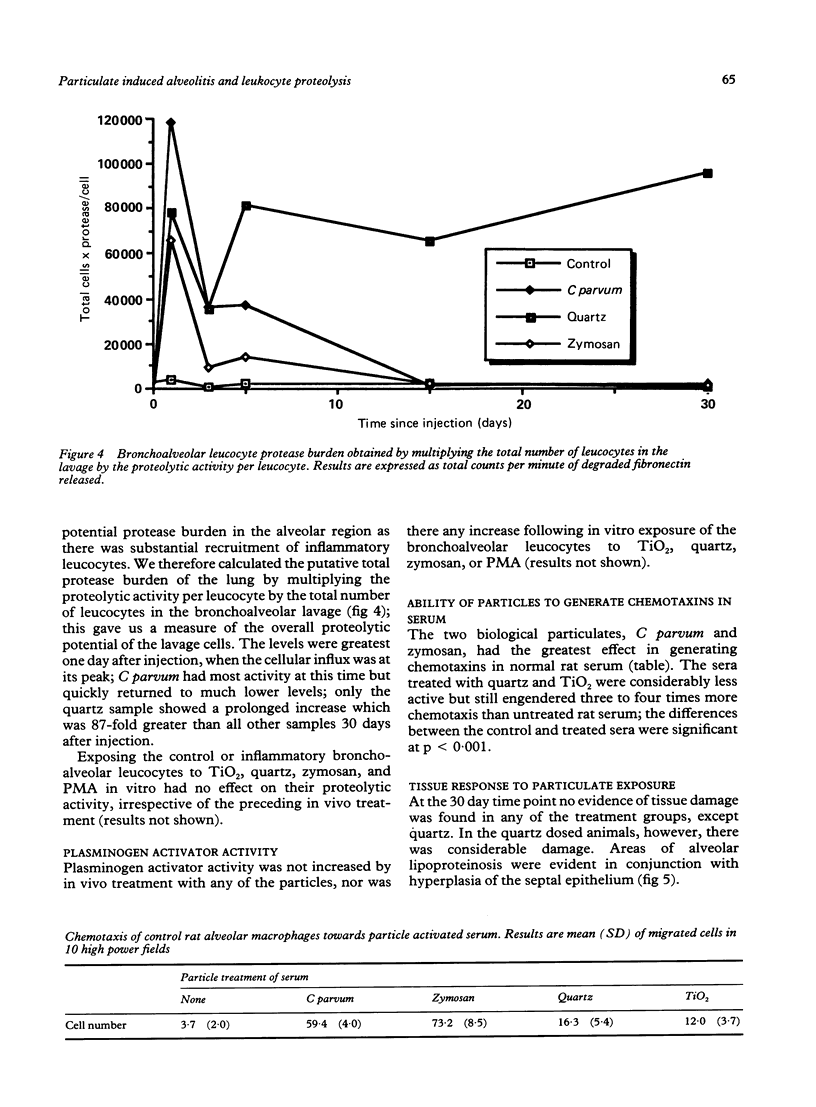
This study assessed the potential harmfulness of particles in the lung by measuring their ability to elicit and maintain an inflammatory response and to damage lung tissue. It compared the inflammogenicity of two nondurable, biological particulates (Corynebacterium parvum and zymosan) with a pathogenic mineral dust (quartz) and a nonpathogenic dust (titanium dioxide) by dosing rats via the intratracheal route and measuring the consequent alveolitis. The magnitude and duration of the inflammatory response were assessed by measuring the total number of leucocytes and the percentage of neutrophils obtained by bronchoalveolar lavage. Two key functional parameters of the lavaged leucocytes--ability to degrade fibronectin and production of plasminogen activator--were also measured. A marked inflammatory response had occurred by one day after instillation, characterised by increases in total leucocyte numbers and percentage of neutrophils in the bronchoalveolar lavages, with all four test materials. In all but the quartz exposed animals, the inflammation subsided rapidly thereafter, approaching control levels by 15 days after injection; in the quartz exposed animals the alveolitis persisted for up to 30 days. All of the inflammogens generated chemotaxins in rat serum in vitro and so, by analogy, might also be expected to generate chemotactic activity in alveolar lining fluid which could contribute to the generation of an inflammatory response. The cellular inflammatory response was accompanied by a concomitant increase in the proteolytic activity of the bronchoalveolar lavage leucocytes but production of plasminogen activator remained unchanged. In vitro exposure to the inflammogens had no effect on the proteolytic activity against fibronectin or on the plasminogen activator activity of bronchoalveolar leucocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Borm P. J., Palmen N., Engelen J. J., Buurman W. A. Spontaneous and stimulated release of tumor necrosis factor-alpha (TNF) from blood monocytes of miners with coal workers' pneumoconiosis. Am Rev Respir Dis. 1988 Dec;138(6):1589–1594. doi: 10.1164/ajrccm/138.6.1589. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The role of cell injury and the continuing inflammatory response in the generation of silicotic pulmonary fibrosis. J Pathol. 1984 Nov;144(3):149–161. doi: 10.1002/path.1711440302. [DOI] [PubMed] [Google Scholar]
- Brown G. M., Donaldson K., Brown D. M. Bronchoalveolar leukocyte response in experimental silicosis: modulation by a soluble aluminum compound. Toxicol Appl Pharmacol. 1989 Oct;101(1):95–105. doi: 10.1016/0041-008x(89)90215-9. [DOI] [PubMed] [Google Scholar]
- Brown G. M., Donaldson K. Degradation of connective tissue components by lung derived leucocytes in vitro: role of proteases and oxidants. Thorax. 1988 Feb;43(2):132–139. doi: 10.1136/thx.43.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Fayerweather W. E. Epidemiologic study of workers exposed to titanium dioxide. J Occup Med. 1988 Dec;30(12):937–942. doi: 10.1097/00043764-198812000-00011. [DOI] [PubMed] [Google Scholar]
- Christner P., Fein A., Goldberg S., Lippmann M., Abrams W., Weinbaum G. Collagenase in the lower respiratory tract of patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985 May;131(5):690–695. doi: 10.1164/arrd.1985.131.5.690. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Beckett S. T., Bolton R. E., Collings P., Middleton A. P. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br J Cancer. 1978 May;37(5):673–688. doi: 10.1038/bjc.1978.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K., Bolton R. E., Jones A., Brown G. M., Robertson M. D., Slight J., Cowie H., Davis J. M. Kinetics of the bronchoalveolar leucocyte response in rats during exposure to equal airborne mass concentrations of quartz, chrysotile asbestos, or titanium dioxide. Thorax. 1988 Jul;43(7):525–533. doi: 10.1136/thx.43.7.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K., Brown G. M. Assessment of mineral dust cytotoxicity toward rat alveolar macrophages using a 51Cr release assay. Fundam Appl Toxicol. 1988 Feb;10(2):365–366. doi: 10.1016/0272-0590(88)90322-3. [DOI] [PubMed] [Google Scholar]
- Donaldson K., Slight J., Bolton R. E. In vitro fibrinolytic activity and viability of rat alveolar macrophages treated with inflammation generating mineral dusts. Agents Actions. 1987 Feb;20(1-2):87–92. doi: 10.1007/BF01965629. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A. Macrophage stimulation and the inflammatory response to asbestos. Environ Health Perspect. 1980 Feb;34:69–74. doi: 10.1289/ehp.803469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Garrett K. C., Richerson H. B., Fantone J. C., Ward P. A., Rennard S. I., Bitterman P. B., Crystal R. G. Pathogenesis of the granulomatous lung diseases. Am Rev Respir Dis. 1984 Sep;130(3):476–496. doi: 10.1164/arrd.1984.130.3.476. [DOI] [PubMed] [Google Scholar]
- Kusaka Y., Donaldson K., Cullen R. T. Lymphocyte modulation by inflammatory bronchoalveolar leukocytes. FEMS Microbiol Immunol. 1990 May;2(1):9–10. doi: 10.1111/j.1574-6968.1990.tb03463.x. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Daniele R. P. Silica stimulation of chemotactic factor release by guinea pig alveolar macrophages. J Reticuloendothel Soc. 1981 Nov;30(5):381–390. [PubMed] [Google Scholar]
- Martin T. R., Chi E. Y., Covert D. S., Hodson W. A., Kessler D. E., Moore W. E., Altman L. C., Butler J. Comparative effects of inhaled volcanic ash and quartz in rats. Am Rev Respir Dis. 1983 Jul;128(1):144–152. doi: 10.1164/arrd.1983.128.1.144. [DOI] [PubMed] [Google Scholar]
- Nolan R. P., Langer A. M., Harington J. S., Oster G., Selikoff I. J. Quartz hemolysis as related to its surface functionalities. Environ Res. 1981 Dec;26(2):503–520. doi: 10.1016/0013-9351(81)90226-7. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablonniere B., Scharfman A., Lafitte J. J., Laine A., Aerts C., Hayem A. Enzymatic activities of bronchoalveolar lavages in coal workers pneumoconiosis. Lung. 1983;161(4):219–228. doi: 10.1007/BF02713867. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille Y., Lwebuga-Mukasa J. S., Polomski L., Merrill W. W., Ingbar D. H., Gee J. B. An in vitro model for polymorphonuclear-leukocyte-induced injury to an extracellular matrix. Relative contribution of oxidants and elastase to fibronectin release from amnionic membranes. Am Rev Respir Dis. 1986 Jul;134(1):134–140. doi: 10.1164/arrd.1986.134.1.134. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Lucey E. C., Stone P. J. Animal models of emphysema. Am Rev Respir Dis. 1986 Jan;133(1):149–169. doi: 10.1164/arrd.1986.133.1.149. [DOI] [PubMed] [Google Scholar]
- Vincent J. H., Jones A. D., Johnston A. M., McMillan C., Bolton R. E., Cowie H. Accumulation of inhaled mineral dust in the lung and associated lymph nodes: implications for exposure and dose in occupational lung disease. Ann Occup Hyg. 1987;31(3):375–393. doi: 10.1093/annhyg/31.3.375. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Warheit D. B., Overby L. H., George G., Brody A. R. Pulmonary macrophages are attracted to inhaled particles through complement activation. Exp Lung Res. 1988;14(1):51–66. doi: 10.3109/01902148809062850. [DOI] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]