Abstract
BACKGROUND:
Prone positioning and neuromuscular blocking agents (NMBAs) are frequently used to treat severe respiratory failure from COVID-19 pneumonia. Prone positioning has shown to improve mortality, whereas NMBAs are used to prevent ventilator asynchrony and reduce patient self-inflicted lung injury. However, despite the use of lung-protective strategies, high death rates in this patient population have been reported.
METHODS:
We retrospectively examined the factors affecting prolonged mechanical ventilation in subjects receiving prone positioning plus muscle relaxants. The medical records of 170 patients were reviewed. Subjects were divided into 2 groups according to ventilator-free days (VFDs) at day 28. Whereas subjects with VFDs < 18 d were defined as prolonged mechanical ventilation, subjects with VFDs ≥18 d were defined as short-term mechanical ventilation. Subjects’ baseline status, status at ICU admission, therapy before ICU admission, and treatment in the ICU were studied.
RESULTS:
Under the proning protocol for COVID-19, the mortality rate in our facility was 11.2%. The prognosis may be improved by avoiding lung injury in the early stages of mechanical ventilation. According to multifactorial logistic regression analysis, persistent SARS-CoV-2 viral shedding in blood (P = .03), higher daily corticosteroid use before ICU admission (P = .007), delayed recovery of lymphocyte count (P < .001), and higher maximal fibrinogen degradation products (P = .039) were associated with prolonged mechanical ventilation. A significant relationship was found between daily corticosteroid use before admission and VFDs by squared regression analysis (y = −0.00008522x2 + 0.01338x + 12.8; x: daily corticosteroids dosage before admission [prednisolone mg/d]; y: VFDs/28 d, R2 = 0.047, P = .02). The peak point of the regression curve was 13.4 d at 78.5 mg/d of the equivalent prednisolone dose, which corresponded to the longest VFDs.
CONCLUSIONS:
Persistent SARS-CoV-2 viral shedding in blood, high corticosteroid dose from the onset of symptoms to ICU admission, slow recovery of lymphocyte counts, and high levels of fibrinogen degradation products after admission were associated with prolonged mechanical ventilation in subjects with severe COVID-19 pneumonia.
Keywords: COVID-19, invasive mechanical ventilation, ventilator-free days, corticosteroid use, viral shedding, prone therapy, muscle relaxants
Introduction
Severe COVID-19 pneumonia requiring invasive mechanical ventilation results in high mortality.1-3 It is crucial to preserve the strategy of low tidal volume (VT) ventilation and limited inspiratory airway pressure as lung-protective ventilation in patients with severe COVID-19 pneumonia. In addition, corticosteroid use and prone positioning have been reported to improve the prognosis.4-7 We treated subjects with severe COVID-19 pneumonia with a prone positioning protocol, including continuous use of neuromuscular blocking agents (NMBAs), from the early phase of mechanical ventilation to reduce lung injury due to COVID-19–induced increases in inspiratory effort and ventilator-induced lung injury (VILI) in ICU at Osaka University Hospital.
Osaka University Hospital is an exclusive center for patients with severe COVID-19 pneumonia. This is among one of the hospitals selected by the Osaka Prefectural Government for referring patients with severe pneumonia requiring invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) in the Osaka prefectural area. Whereas most patients were transferred from other hospitals, a few were brought from home by ambulance and admitted to the ICU within a few hours of their arrival. Most patients were intubated at the referring hospital or the emergency department of this hospital. Indications for ECMO were also determined by physicians of the previous hospitals, the emergency department of Osaka University Hospital, or the ICU, according to the patients’ status. If patients had symptoms of circulatory deterioration, physicians added extracorporeal cardiac support to the patients’ treatment.
Prone positioning is recommended in cases of moderate to severe ARDS,4,8,9 including patients with severe COVID-19 pneumonia receiving invasive mechanical ventilation.10-13 Awake prone positioning may also be helpful in mild COVID-19 pneumonia.14-16 Moreover, it could also be effective in patients with severe COVID-19 pneumonia requiring invasive mechanical ventilation. It has also been suggested that high respiratory drive secondary to gas exchange and respiratory mechanics impairment may lead to lung injury in patients with ARDS, known as patient self-inflicted lung injury, and that mechanical ventilation may worsen the prognosis.17,18 In such cases, continuous muscle relaxant administration may help control the progression of lung injury.19 We made a prone positioning protocol with muscle relaxants to treat severe COVID-19 pneumonia. We applied it to all the intubated subjects with severe COVID-19 unless the subjects had contraindications to the protocol. The effect of prone positioning added to other recommended therapeutic interventions for the treatment of COVID-19–related ARDS is unknown, although it might be more effective in comparison to Botta’s1 report in the Netherland and meta-analysis by Lim et al.2 It is well known in non–COVID-related ARDS that mortality rates are often high despite the use of lung-protective ventilation. In this study, we retrospectively examined factors affecting the prognosis of severe COVID-19 pneumonia in subjects receiving the prone positioning protocol with NMBAs.
QUICK LOOK.
Current knowledge
Prone positioning and corticosteroid use has been effective in patients with severe COVID-19 pneumonia. Factors influencing prolonged mechanical ventilation under prone positioning protocol with neuromuscular blocking agents (NMBAs) are unknown.
What this paper contributes to our knowledge
A prolonged SARS-CoV-2 viral shedding of blood, high corticosteroid dose from the onset of symptoms to ICU admission, slow recovery of lymphocyte counts, and high levels of fibrinogen degradation products after ICU admission were factors associated with prolonged mechanical ventilation in subjects with severe COVID-19 pneumonia treated with the prone positioning protocol with NMBAs. An equivalent prednisolone dose of 78.5 mg/d before ICU admission increased the number of ventilator-free days.
Methods
Study Population
The medical records of 170 consecutive patients admitted to the special ICU for patients with severe COVID-19 pneumonia at the Osaka University Hospital from July 2020–September 2021 were reviewed retrospectively. Data on clinical characteristics from ICU admission to hospital discharge were collected from subject records. This study was approved by the institutional review board of Osaka University Hospital (number 20545).
Prone Positioning Protocol With Muscle Relaxants
The treatment protocol for subjects with severe COVID-19 pneumonia was as follows: Neuromuscular blockade with rocuronium was continuously administered 48 h after admission to the ICU to prevent subject self-inflicted lung injury. Attending physicians adjusted the administration rate of the drug at 20–40 mg/h to achieve the disappearance of spontaneous inspiratory efforts and cough reflex. Sedatives, including propofol, dexmedetomidine, midazolam and isoflurane, were also used to avoid awareness with paralysis. Fentanyl and morphine were used as analgesics. Attending physicians determined selection and administration rates of the drugs according to the subject’s condition. Prone positioning was started on admission day to minimize VILI.15 Postural change from supine to prone was performed in the late afternoon and then from prone to supine the following morning. Subjects were initially planned for 3 sessions of prone positioning, with each session lasting for 16 h or more. The attending ICU physicians made the final decision of the application of prone positioning and continuous administration of muscle relaxants according to the status of each subject. Pressure-targeted ventilation was chosen to maintain better synchrony between the subject’s inspiratory effort and the ventilator. Generally, target VT was in the range of 6–8 mL/kg predicted body weight. However, individual target VT values were determined by the attending physician according to the status of each subject.
Arterial blood gases were evaluated regularly every 6 h by the attending nurse or physician and more frequently if needed. To achieve 40–50 s of activated partial thromboplastin time, we routinely administered unfractionated heparin. Intravenous dexamethasone 6 mg/d was routinely started at ICU admission5 and was scheduled for 10 d. Some subjects who were transferred to our facility from other hospitals received corticosteroids before transfer. Such subjects received a shorter corticosteroid duration. Dexamethasone administration was prolonged if the attending physician deemed it necessary. Dexamethasone was gradually tapered if needed and subsequently discontinued. In subjects who did not improve with dexamethasone administration, high-dose methylprednisolone was administered according to the attending physician’s decision.
SARS-CoV-2 reverse transcription polymerase chain reaction (PCR) using blood samples was performed twice a week after March 2020 using a specific reagent (LightMix Modular SARS-CoV E-gene, TIB Molbiol Syntheselabor GmbH, Berlin, Germany). The cycle threshold of blood PCR (blood PCR-Ct) was collected. More than 38 of the blood PCR-Ct samples were negative in this assay.
Data Collection
Subjects’ baseline status, status at ICU admission, therapy before ICU admission, and treatment in the ICU were studied. Data on subjects’ status and treatment in previous hospitals were collected from referral letters from the previous hospitals. In most cases, because subjects were already intubated and their families were in isolation, it was difficult to obtain previous medical complications of subjects before ICU admission. Subject data, including mechanical ventilation settings, ventilatory data, and arterial blood gas measurements at ICU admission and during prone positioning, were obtained from the subject’s clinical records. Subjects were divided into 2 groups according to the number of ventilator-free days (VFDs) at day 28 following ICU admission. Subjects with VFDs < 18 d were categorized as prolonged mechanical ventilation, whereas those with VFDs ≥ 18 d were categorized as short mechanical ventilation. The following data of each group were compared. If a subject was discharged from the ICU on invasive mechanical ventilation, we investigated the respiratory status on the 90th d of ICU admission by asking the next hospital.
Data at ICU admission including age, sex, body mass index, interval between symptom onset and ICU admission, interval between symptom onset and intubation, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, PaO2/FIO2, and dynamic respiratory system compliance (CRS) were collected. Daily laboratory data, including C-reactive protein, fibrinogen degradation products, white blood cell counts, and lymphocyte counts, were collected until day 14 after ICU admission. Vaccination, use of antiviral drugs, and total corticosteroid dosages were recorded for evaluation of therapy before ICU admission. The amounts of corticosteroids were expressed as equivalent doses of prednisolone according to the following equations: methylprednisolone (mg) × 1.25 = prednisolone (mg), dexamethasone (mg) × 6.67 = prednisolone (mg).20 Daily corticosteroid dose was used to adjust the differences in clinical time courses among subjects. Daily corticosteroid dose was calculated as total corticosteroid dosage divided by the intervals between onset of symptoms and ICU admission.
Data on therapy in the ICU including the numbers of sessions of prone positioning; intervals between intubation and prone positioning; indication of ECMO; duration of ECMO; and use of other immunomodulating agents, antiviral drugs, and high-dose methylprednisolone, defined as > 0.5 g/d, were collected. Death, re-intubation, tracheotomy, liberation from mechanical ventilation, discharge with full-time mechanical ventilation, discharge with intermittent mechanical ventilation, duration of mechanical ventilation, and ICU days were collected to compare the prognosis of each group.
Statistical Analysis
Continuous variables were compared using analysis of variance (ANOVA). Categorical variables were expressed as numbers with percentages, and values were compared using Pearson chi-square test. Univariate logistic regression analyses of data set characteristics were conducted to evaluate factors associated with the risk of prolonged mechanical ventilation. In addition, multivariate logistic regression analyses of data set characteristics were performed with the selected factors according to the risk of prolonged mechanical ventilation. One or 2 factors were chosen from each category: subjects baseline status, status at ICU admission, therapy before ICU admission, and treatment in ICU.
In subjects who had > 2 measuring points of SARS-CoV-2 blood PCR-Ct, the regression line of increasing blood PCR-Ct values was calculated in each subject. The slope and y-intercept of the regression line of the blood PCR-Ct were determined. The y-intercept of the regression line indicated the amounts of SARS-CoV-2 virus in blood at ICU admission. The slope of the regression line indicated the speed of decrease of SARS-CoV-2 virus in blood after ICU admission. Many subjects presented decreasing lymphocyte counts at ICU admission and increasing lymphocyte counts after ICU admission. The regression line of increasing lymphocyte counts was calculated in each subject. The slope and y-intercept of the regression line of lymphocyte counts were collected.
Time courses of blood PCR-Ct until the achievement of a negative PCR test during ICU were compared in prolonged group and short group. Time courses of C-reactive protein, fibrinogen degradation products, and lymphocyte counts were compared in the prolonged and short groups within 10 d after ICU admission. Two-way ANOVA was used to compare changes in blood PCR-Ct, C-reactive protein, fibrinogen degradation products, and lymphocyte counts between the 2 groups. Daily corticosteroid dosage before ICU admission was recorded to evaluate the effects of treatment before ICU admission on VFDs within 28 d after ICU admission. We described the possible non-linear associations between daily corticosteroid dosage before ICU admission and VFDs using a quadratic regression curve in the regression model with the least squares method.
For all analyses, P < .05 was considered statistically significant. All data were analyzed with the JMP Pro statistical software version 15.2.1 (JMP Statistical Discovery, Cary, North Carolina).
Results
The characteristics of subjects at ICU admission are shown in Table 1. PaO2/FIO2, CRS, and lymphocyte counts of the prolonged group were less than those of the short group. Age, body mass index, and APACHE II score of the prolonged group were greater than those of the short group. C-reactive protein, fibrinogen degradation products, and white blood cell were not different between the 2 groups. Therapy before ICU admission is also shown in Table 1. No subject had a history of vaccination in either group. The percentages of subjects who received corticosteroids were not different between the 2 groups. The amounts of daily corticosteroids before ICU admission in the prolonged group were greater than those in the short group. The use of antiviral drugs was not different between the 2 groups.
Table 1.
Characteristics of Subjects at ICU Admission
The treatment characteristics of subjects in the ICU are shown in Table 2. The proportion of subjects who received prone positioning with NMBA did not differ between the 2 groups. The frequency of prone positioning in the prolonged group was greater than in the short group. Intervals between intubation and prone positioning of the prolonged group were longer than those of the short group. ECMO was used in more subjects in the prolonged group than in the short group. Two subjects in the prolonged group received venous-arterial ECMO for cardiopulmonary resuscitation. The other subjects received venous-venous ECMO due to hypoxemia. Whereas no subject received high-dose methylprednisolone in the short group, 9 subjects were received high-dose methylprednisolone in the prolonged group. Only one subject received high-dose methylprednisolone at ICU admission day, whereas the other subjects received high-dose methylprednisolone after the tenth day of ICU admission.
Table 2.
Treatment Characteristics of Subjects in the ICU
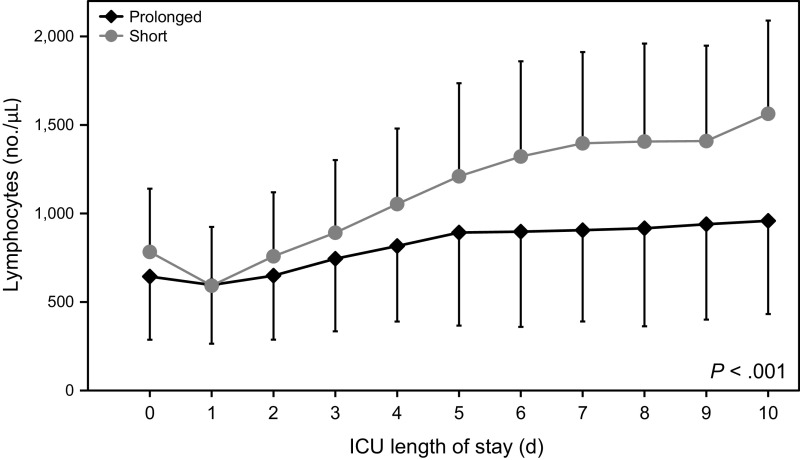
The characteristics of subjects in the ICU are shown in Table 3. Lymphocyte counts and slope of the regression line of lymphocyte counts in the prolonged group were less than those in the short group, indicating that the lymphocyte counts and speed of increase of lymphocyte counts in the prolonged group were less than those in the short group. The time course of lymphocyte counts during 10 d after ICU admission in each group in shown in Figure 1. Lymphocyte counts in the prolonged group were significantly less than those in the short group. Maximal fibrinogen degradation products and maximal C-reactive protein during 2 weeks after ICU admission in the prolonged group were greater than those in the short group. Time courses of fibrinogen degradation products and C-reactive protein during 10 d after ICU admission in each group are shown in Figures 2 and 3, respectively. Fibrinogen degradation products and C-reactive protein of the prolonged group were significantly greater than those of the short group.
Table 3.
Characteristics of Subjects in the ICU
Fig. 1.
Time course of lymphocyte counts within 10 d after ICU admission in the prolonged and short mechanical ventilation groups.
Fig. 2.
Time course of fibrinogen degradation products within 10 d after ICU admission in the prolonged and short mechanical ventilation groups.
Fig. 3.
Time course of C-reactive protein within 10 d after ICU admission in the prolonged and short mechanical ventilation groups.
Driving pressures and PEEP during the prone positioning protocol were not different between the prolonged and short groups (Supplemental Figs A and B, see related supplementary materials at http://www.rcjournal.com). The averages of driving pressures and PEEP were < 14 cm H2O and > 9.8 cm H2O, respectively. VT/predicted body weight, CRS, pH, PaCO2, and PaO2/FIO2 during the prone positioning protocol were different between the prolonged and short groups (Supplemental Figs C, D, E, F, and G, see related supplementary materials at http://www.rcjournal.com). Average VT/predicted body weight of both groups was achieved between 6–8 mL/kg predicted body weight except for the values of the short group on the ICU admission day.
Blood PCR-Ct was measured in 118 of 170 subjects. Time course of SARS-CoV-2 blood PCR-Ct during ICU in the prolonged and short groups is shown in Figure 4. The characteristics of blood PCR-Ct in the ICU are shown in Table 4. The proportion of subjects who were negative for blood PCR-Ct at the first test after ICU admission was similar between the 2 groups. The slope and y-intercept of the regression line of SARS-CoV-2 blood PCR-Ct in the prolonged group were less than those in the short group. The time course of SARS-CoV-2 blood PCR-Ct in each group is shown in Figure 1, revealing that the amount of SARS-CoV-2 in the blood of the prolonged group was greater than that of the short group. Prognostic characteristics are shown in Table 5. In 28 subjects in the prolonged group with full-time mechanical ventilation at ICU discharge, 20 were liberated from mechanical ventilation; the outcome was unknown in 5, and 3 were ventilator dependent at the 90th d of ICU admission. In 4 subjects in the prolonged group with intermittent mechanical ventilation at ICU discharge, 3 subjects were liberated from mechanical ventilation at the 90th day of ICU admission.
Fig. 4.
Time course of SARS-CoV-2 blood polymerase chain reaction (PCR) cycle threshold during ICU in the prolonged and short mechanical ventilation groups. PCR = polymerase chain reaction.
Table 4.
Cycle Threshold of Blood Polymerase Chain Reaction of Subjects in the ICU
Table 5.
Prognostic Characteristics of Subjects
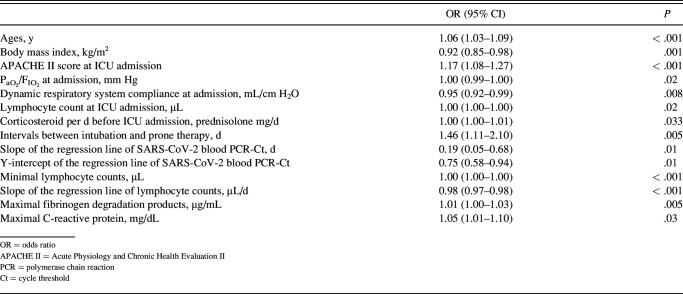
The results of the univariate logistic regression analysis for associated factors of prolonged mechanical ventilation are shown in Table 6, whereas the results of the multivariate logistic regression analysis are shown in Table 7. Age and body mass index were selected as factors of subjects’ baseline status. APACHE II score and y-intercept of the regression line of SARS-CoV-2 blood PCR-Ct were selected as factors of status at ICU admission, daily corticosteroid dosage before ICU admission as a factor of therapy before ICU admission, the interval between intubation and prone therapy as a factor of treatment in the ICU, and slope of the regression line of lymphocyte counts and maximal fibrinogen degradation products as factors of characteristics during ICU. According to the multifactorial logistic regression analysis for associated factors of prolonged mechanical ventilation, lower y-intercept of the regression line of SARS-CoV-2 blood PCR-Ct, higher daily corticosteroid dosage before ICU admission, the smaller slope of the regression line of lymphocyte count, and higher maximal fibrinogen degradation products were associated with prolonged mechanical ventilation in subjects with severe COVID-19 pneumonia.
Table 6.
Univariate Logistic Regression Analysis for Factors Associated With Prolonged Mechanical Ventilation
Table 7.
Multifactorial Logistic Regression Analysis for Factors Associated With Prolonged Mechanical Ventilation
Figure 5 shows a significant relationship between daily corticosteroid dosage before ICU admission and VFDs within 28 d after ICU admission. The peak point of the regression curve was 13.4 d at 78.5 mg/d of the equivalent prednisolone dose.
Fig. 5.
Relationship between daily corticosteroid dosage before ICU admission and ventilator-free days (VFDs) within 28 d after ICU admission. A significant relationship was shown by squared regression analysis between daily corticosteroid dosage before ICU admission and VFDs within 28 d. Corticosteroid doses were converted to the equivalent dose of prednisolone. The peak point of the regression curve was 13.4 d at 78.5 mg/d of prednisolone.
Discussion
A slow rate of decrease in blood virus level, high corticosteroid dose from the onset of the symptoms to ICU admission, slow recovery of lymphocyte counts, and high levels of fibrinogen degradation products were factors associated with prolonged mechanical ventilation in subjects with severe COVID-19 pneumonia. A prolonged duration of mechanical ventilation was also seen in older subjects and those with low body mass index, high APACHE II scores, and delayed prone positioning after intubation.
We determined the threshold of prolonged mechanical ventilation was 10 d after ICU admission. Previous studies indicated that the timing of tracheostomy for subjects who received invasive mechanical ventilation was 10 d post intubation and initiation of mechanical ventilation.21,22 Subsequently, subjects were divided into 2 groups according to 18 VFDs in 28 d after ICU admission. Whereas Botta et al1 reported that the average and median of VFDs at day 28 were 6.8 d and 0 d, respectively, those of the present study were 12.4 d and 17 d. The threshold of the present study is suitable for examining the data as it divides the subjects into 50% approximately.
Although there are many reports on risk factors for mechanical ventilation in ARDS secondary to COVID-19 pneumonia, few direct studies on prognostic factors associated with prolonged mechanical ventilation have been conducted. In this study, many differences were found between the prolonged and short groups. One or 2 representative factors were found to be significant from each aspect: subjects’ baseline status, status at ICU admission, therapy before ICU admission, and treatment in the ICU. According to the results of the multifactorial logistic regression analysis, several factors were associated with prolonged mechanical ventilation.
The lower y-intercept of the regression line of SARS-CoV-2 blood PCR-Ct, which indicated persistent viral shedding in the blood, was associated with prolonged mechanical ventilation. Persistent viral shedding, especially through nasopharyngeal route, is associated with the severity of COVID-19 pneumonia as well as poor outcomes.23,24 The present study indicated that low values in blood PCR-Ct were likely associated with prolonged mechanical ventilation. Viral clearance of blood samples was reported to be faster than that of nasopharyngeal samples.25 In this study, only 10.2% of the subjects had a negative in blood PCR-Ct at ICU admission. Blood PCR-Ct was often not negative at the start of mechanical ventilation in severely ill subjects with COVID-19 pneumonia. The result of this study suggests that persistent viral shedding in blood samples was associated with the prognosis of the disease.
Lymphocytopenia has been reported to occur in many subjects with COVID-19 pneumonia.26,27 Low lymphocyte count–to-white blood cell ratios have been reported to reduce survival.28,29 This study also showed that a slower recovery rate of lymphocyte counts was associated with prolonged mechanical ventilation. Previous reports suggest a link between severity and coagulopathy in COVID-19 pneumonia.29-32 In the present study, high levels of fibrinogen degradation products were associated with prolonged mechanical ventilation, consistent with previous reports showing a significant relationship of coagulopathy with poor outcomes.
After ICU admission, 6 mg/d of dexamethasone was administered daily for 10 d, according to a previous report, and the administration period was extended at the attending physician’s discretion.5 Prior to admission at our facility, subjects were treated with varying dosages of corticosteroids by previous physicians, ranging from nil to high. As a treatment before ICU admission, corticosteroid doses before ICU admission were associated with prolonged mechanical ventilation. Corticosteroids administered at a dosage 11.8 mg/d of dexamethasone or an equivalent prednisolone dose of 78.5 mg/d were seen to achieve the longest VFDs. A comparative report of dexamethasone doses at 6 mg/d and 12 mg/d suggests that 12 mg/d may be more effective than 6 mg/d, although the difference in efficacy is not significant.33,34 It has been reported that a medium dose of prednisolone at 200–250 mg/d is also effective.7,35 Since some subjects received higher doses prior to ICU admission, in this study, the effect of corticosteroid administration itself could not be examined. However, this study can conclude that administering large doses of corticosteroids prior to the subject becoming severely ill was associated with prolonged mechanical ventilation. The study suggests that the safe upper limit of corticosteroids is approximately 80 mg/d of prednisolone or its equivalent. Increasing the dose of a corticosteroid may not improve its efficacy while at the same time leading to increased complications.36 Immunosuppression has been reported as a factor associated with persistent viral shedding.22 Li et al37 reported that taking high or medium doses of corticosteroids, which indicated an equivalent dose of > 80 mg/d of prednisolone, delayed viral clearance. In the present study, higher daily corticosteroid dosage was also found to affect lymphocytopenia and viral shedding of blood.
A common indication of prone positioning and NMBAs is severe ARDS. COVID-19 pneumonia had a very high mortality rate and no established treatments. Therefore, we applied the early prone positioning protocol with NMBAs. In this protocol, prone positioning, considered effective in reducing lung injury, was performed for 3 consecutive d after ICU admission.4,8-12 Continuous infusion of 20–40 mg/h of rocuronium, which helps to prevent subject self-inflicted lung injury and patient-ventilator asynchrony, was given for 2 consecutive d immediately after ICU admission.17,18,38 According to the global data report, the mortality of patients with COVID-19 pneumonia requiring invasive mechanical ventilation was > 30%.1-3 The mortality rate of patients in our facility was 11.2%, which was considerably less than those of these reports. The prognosis of COVID-19 may be improved by actively reducing VILI in the early stages of invasive mechanical ventilation. However, there was no comparator group, and this effect requires additional research on the effectiveness of our strategy.
Viral load and other laboratory data might only indicate the time course and severity of COVID-19 infection. There may be little knowledge of appropriate times and amounts for drugs, such as corticosteroids, in the treatment strategy for COVID-19. The prognosis may be improved if immunosuppressive drugs can be adjusted according to the degree of inflammation. Furthermore, lung injury may be reduced if we can adjust the protocol of prone positioning and muscle relaxant administration from an early disease stage. Further research is needed to examine these matters.
This study has several limitations. First, this study was a retrospective and single-center study, which used the same treatment protocol. It is necessary to consider different treatment methods to study the effectiveness of a particular therapy. Second, the effects of other treatments could not be confirmed in this study. Several new therapies have been developed, but their effects could not be examined. The vaccine for SARS-CoV-2 was not given to all subjects, and its effects could also not be evaluated. Third, since many subjects had already been intubated at ICU admission, and their families were often unable to visit the hospital, it was difficult to accurately assess most subjects’ baseline status. A wide range of basic factors could not be examined in this study. Therefore, it was not possible to investigate the broad effects of fundamental subject factors. Fourth, since a few subjects had been intubated at previous hospitals, the precise VFDs could not be calculated. However, since most subjects were brought to this ICU within 24 h of intubation, this time gap could be considered to be minimal.
Conclusions
In summary, a slow rate of decrease in blood virus level, high corticosteroid dose from the onset to ICU admission, slow recovery of lymphocyte counts, and high level of fibrinogen degradation products after ICU admission were associated with prolonged mechanical ventilation in subjects with severe COVID-19 pneumonia. Furthermore, the severity of pneumonia and the subjects’ characteristics also influenced outcome.
Footnotes
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
REFERENCES
- 1.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, et al. ; PRoVENT-COVID Collaborative Group. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multi-center, observational cohort study. Lancet Respir Med 2021;9(2):139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim ZJ, Subramaniam A, Ponnapa RM, Blecher G, Kadam U, Afroz A, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med 2021;203(1):54-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greco M, De Corte T, Ercole A, Antonelli M, Azoulay E, Citerio G, et al. ; ESICM UNITE-COVID investigators. Clinical and organizational factors associated with mortality during the peak of first COVID-19 wave: the global UNITE-COVID study. Intensive Care Med 2022;48(6):690-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. ; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368(23):2159-2168. [DOI] [PubMed] [Google Scholar]
- 5.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384(8):693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno G, Carbonell R, Martin-Loeches I, Solé-Violán J, Correig I, Fraga E, et al. ; COVID-19 SEMICYUC Working Group. Corticosteroid treatment and mortality in mechanically ventilated COVID-19–associated acute respiratory distress syndrome (ARDS) patients: a multi-center cohort study. Ann Intensive Care 2021;11(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano EJ, Fonseca Fuentes X, Corsini Campioli C, O’Horo JC, Abu Saleh O, Odeyemi Y, et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest 2021;159(3):1019-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guérin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, et al. Prone position in ARDS patients: why, when, how, and for whom. Intensive Care Med 2020;46(12):2385-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010;36(4):585-599. [DOI] [PubMed] [Google Scholar]
- 10.Bell J, William PC, Kreisel C, Sonti R, Cobb N. Predicting impact of prone position on oxygenation in mechanically ventilated patients with COVID-19. J Intensive Care Med 2022;37(7):883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathews KS, Soh H, Shaefi S, Wang W, Bose S, Coca S, et al. Prone positioning and survival in mechanically ventilated patients with coronavirus disease 2019–related respiratory failure. Crit Care Med 2021;49(7):1026-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharat A, Simon M, Guérin C. Prone position in COVID-19–associated acute respiratory failure. Curr Opin Crit Care 2022;28(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelhamer MC, Wesson PD, Solari IL, Jensen DL, Steele WA, Dimitrov VG, et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J Intensive Care Med 2021;36(2):241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlov I, He H, McNicholas B, Perez Y, Tavernier E, Trump MW, et al. Awake prone positioning in non-intubated patients with acute hypoxemic respiratory failure due to COVID-19. Respir Care 2022;67(1):102-114. [DOI] [PubMed] [Google Scholar]
- 15.Kaur R, Vines DL, Mirza S, Elshafei A, Jackson JA, Harnois LJ, et al. Early versus late awake prone positioning in non-intubated patients with COVID-19. Crit Care 2021;25(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esperatti M, Busico M, Fuentes NA, Gallardo A, Osatnik J, Vitali A, et al. ; Argentine Collaborative Group on High Flow and Prone Positioning. Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19–related acute respiratory failure treated with high-flow nasal oxygen: a multi-center cohort study. Crit Care 2022;26(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carteaux G, Parfait M, Combet M, Haudebourg AF, Tuffet S, Mekontso DA. Patient self-inflicted lung injury: a practical review. JCM 2021;10(12):2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida T, Grieco DL, Brochard L, Fujino Y. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr Opin Crit Care 2020;26(1):59-65. [DOI] [PubMed] [Google Scholar]
- 19.Torbic H, Krishnan S, Harnegie MP, Duggal A. Neuromuscular blocking agents for ARDS: a systematic review and meta-analysis. Respir Care 2021;66(1):120-128. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013;9(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng H, Fang Q, Chen K, Zhang X. Early versus late tracheotomy in ICU patients: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2021;100(3):e24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plummer AL, Gracey DR. Consensus conference on artificial airways in patients receiving mechanical ventilation. Chest 1989;96(1):178-180. [DOI] [PubMed] [Google Scholar]
- 23.Batra A, Clark JR, Kang AK, Ali S, Patel TR, Shlobin NA, et al. Persistent viral RNA shedding of SARS-CoV-2 is associated with delirium incidence and six-month mortality in hospitalized COVID-19 patients. Geroscience 2022;44(3):1241-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vena A, Taramasso L, Di Biagio A, Mikulska M, Dentone C, De Maria A, et al. ; GECOVID study group. Prevalence and clinical significance of persistent viral shedding in hospitalized adult patients with SARS-CoV-2 infection: a prospective observational study. Infect Dis Ther 2021;10(1):387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joukar F, Kalurazi TY, Khoshsorour M, Taramian S, Mahfoozi L, Balou HA, et al. Persistence of SARS-CoV-2 RNA in the nasopharyngeal, blood, urine, and stool samples of patients with COVID-19: a hospital-based longitudinal study. Virol J 2021;18(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peñuelas O, Del Campo-Albendea L, de Aledo ALG, Añón JM, Rodríguez-Solís C, Mancebo J, et al. Long-term survival of mechanically ventilated patients with severe COVID-19: an observational cohort study. Ann Intensive Care 2021;11(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, et al. Hematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol 2020;7(9):e671-e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180(7):934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395(10239):1763-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, Wahlin RR, et al. ; COVID STEROID 2 Trial Group. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA 2021;326(18):1807-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granholm A, Munch MW, Myatra SN, Vijayaraghavan BKT, Cronhjort M, Wahlin RR, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxemia: a preplanned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med 2022;48(1):45-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papamanoli A, Yoo J, Grewal P, Predun W, Hotelling J, Jacob R, et al. High-dose methylprednisolone in non-intubated patients with severe COVID-19 pneumonia. Eur J Clin Invest 2021;51(2):e13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres A, Motos A, Cillóniz C, Ceccato A, Fernández-Barat L, Gabarrús A, et al. ; CIBERESUCICOVID Project Investigators. Major candidate variables to guide personalized treatment with steroids in critically ill patients with COVID-19: CIBERESUCICOVID study. Intensive Care Med 2022;48(7):850-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Liao X, Zhou Y, Wang L, Yang H, Zhang W, et al. Association between glucocorticoids treatment and viral clearance delay in patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis 2021;21(1):1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver L, Das A, Saffaran S, Yehya N, Scott TE, Chikhani M, et al. High risk of patient self-inflicted lung injury in COVID-19 with frequently encountered spontaneous breathing patterns: a computational modelling study. Ann Intensive Care 2021;11(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]