Abstract
Background
A limited number of studies have directly examined the effect of whole eggs on body weight and composition in adults, and they have led to inconsistent results. This study aimed to summarize the evidence on the effect of whole egg consumption on body weight and body composition in adults from clinical trials.
Methods
Online databases were searched from inception to April 2023 for clinical trials that directly or indirectly assessed the effect of whole eggs consumption on anthropometric measures including body weight, body mass index (BMI), waist circumference (WC), and fat-free mass (FFM) in adults. A random effects model was used for meta-analysis.
Results
In total, 32 controlled clinical trials were included in the systematic review. The analyses revealed that whole egg consumption has no significant effect on body weight (n = 22), BMI (n = 13), WC (n = 10), and FFM (n = 4, P > 0.05). The subgroup analyses showed that whole egg consumption has an increasing effect on body weight and BMI in studies that lasted more than 12 weeks and in unhealthy participants (P < 0.05). A significant increasing effect on BMI was found in studies that the control group did not receive any egg (P < 0.05). Moreover, in studies that there was no significant difference in energy intake between the intervention and control groups, weight, and WC were significantly increased (P < 0.05). Additionally, in studies that participants in the control group received another food or supplement, studies with calorie restriction, and studies on healthy subjects, whole egg intake significantly decreased BMI (P < 0.05).
Conclusions
Although whole egg consumption had no adverse effect on body composition and body weight, in overall, it might increase body weight in long term. Egg consumption beneficially affects BMI in healthy people and during weight loss diet.
Systematic review registration
This systematic review and meta-analysis is registered in the International Prospective Register of Systematic Reviews (PROSPERO, Registration number: CRD42022308045).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-023-02277-3.
Keywords: Systematic review, Meta-analysis, Egg, Body weight, Body composition, Body mass index, Waist circumference
Background
Obesity as an important global health issue is associated with the risk of several chronic diseases, such as high blood pressure, cardiovascular disease (CVD)s [1], type 2 diabetes (T2D) [2], and osteoarthritis [3]. The worldwide prevalence of overweight and obesity has dramatically increased over the past decades and now approximately one-third of adults are overweight or obese throughout the world [4]. Several factors including genetic and environmental (unhealthy diet, physical inactivity, and air pollution) features are associated with overweight and obesity [5]. Although factors such as education and schooling, changes in technology, and urbanization are effective in the prevalence of obesity [6], but they can almost be prevented by lifestyle changes such as a well-balanced diet and regular physical activity [7]. A Western dietary pattern, which mainly consists of red and processed meat, refined grains, and eggs is associated with increased obesity and overweight [8]. A cohort study suggested that animal protein intake may be positively associated with overweight and obesity, while plant-based protein intake has an inverse association [9]. In addition, studies have shown that snacks, fast foods [10], and sweetened beverages [11] consumption are associated with overweight and obesity. Moreover, high fat intake can lead to greater food intake and weight gain [12]. One of the foods that is high in fat and cholesterol is egg [13].
Eggs are rich in minerals, vitamins, and bioactive compounds and important dietary sources of high-biological value protein, as well [13]. Each large egg is 50 g and provides 78 kcal, 6.29 g protein, and 5.3 g fat, 186 mg of which is cholesterol [14]. Bioactive compounds of egg can have antimicrobial, antioxidant, and anticancer effects [13]. Additionally, researchers have suggested that egg-rich diets can have protective effects against metabolic syndrome (MetS) by increasing HDL levels and reducing inflammation [13, 15]. Moreover, it has been observed that eggs may help weight management due to their high protein content [16]. On the other hand, the high levels of cholesterol content in eggs [13], however, plays a key role in maintaining the structure and function of the brain [17], and it might adversely affect the lipid profile [18]. The association between egg consumption and CVDs has been widely investigated. A meta-analysis found a dose–response positive relation between egg consumption and CVDs [19]. However, Sangah Shin et al. [20] suggested that higher egg consumption may reduce the odds for MetS and all its components. A population-based study performed on Chinese adults showed that egg consumption may improve body fat distribution and it might be beneficial for weight management [21].
Although, few studies [21] have directly examined the association of egg consumption with weight and body distribution, many studies have reported the effect of egg intake on weight and body composition as their secondary outcomes. The results of some studies showed a reducing effect of egg consumption on body weight and composition [22]; however, other studies showed no significant effect on body weight [23].
To the best of our knowledge, to date, limited evidence of reviews conducted on the effect of whole egg consumption on body weight and body composition and the evidence on this issue is conflicting. Therefore, this systematic review and meta-analysis study aimed to summarize the evidence on the effect of whole egg consumption on body weight and body composition.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline was followed for conducting the current systematic review and meta-analysis [24]. The study protocol was also registered in the international prospective register of systematic reviews (PROSPERO) database (http://www.crd.york.ac.uk/PROSPERO, registration number: CRD42022308045).
Search strategy
A systematic search was conducted from inception to the 23rd of April 2023 to find related articles on online databases including Scopus, PubMed, and ISI (Web of Science). There was no language or any other restriction. The terms used are presented in Additional file 1. Two authors independently screened the titles and abstracts of the articles inception to the 23th of April 2023 (AE and FZ). Finally, references of the selected articles were checked to detect additional related articles.
Eligibility criteria
Studies from inception to the 23rd of April 2023 were included in the current systematic review, if (a) examined the effect of whole egg consumption on anthropometric measurements including body weight, body mass index (BMI), waist circumference (WC), fat mass, and fat-free mass (FFM), compared to the control group; (b) were controlled clinical trials in design; and (c) performed in adults (≥ 18 years). Studies were excluded if (a) they did not report outcomes of interest, (b) the intervention period was less than 3 weeks, and (c) conducted on pregnant or lactating women.
Data extraction
Data extraction was conducted by two researchers independently (AE and FZ), and any disagreement was resolved in consultation with third investigator (ASA). The following data was extracted for each included study: publication details (authors, publication year, geographic region), the characteristics of participants (age, sex, number of subjects in intervention and control groups, the health condition of participants), and study characteristics [design (parallel, randomized, cross-over, or factorial intervention), duration, number of study arms, and the amount of whole egg and comparison food used)]. We extracted the mean and standard deviation (SD) for baseline, change, and post-intervention values for outcome markers.
Risk of bias assessment
Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials was used to evaluate the quality of eligible studies [25]. The assessment was conducted by one author and a second author double-checked the risk assessment. The following domains were evaluated for each study: (1) randomization process, (2) deviations from the intended interventions (effect of assignment to intervention), (3) deviations from the intended interventions (effect of adhering to intervention), (4) missing outcome data, and (5) measurement of the outcome. Studies were categorized as low risk if all domains were rated as low risk, and some concern if at least one domain was assessed to have some concern and high risk if one or more domains were categorized as high risk. The risk of bias in non-randomized clinical trials was assessed at the outcome level by using the risk of bias in the non-randomized studies of interventions (ROBINS-I) tool [26]. Two authors (AE and SB) performed the quality assessments, and disagreements were resolved by a third author (ASA).
Statistical analysis
Mean difference and its corresponding standard error (SE) in the change from baseline for body weight, BMI, WC, and FFM between intervention and control groups were calculated and used as effect size for meta-analysis. If the SD for change values were not provided, they were calculated by assuming 0.5 as the correlation coefficient between baseline and end-of-treatment values. In addition, all analyzes were performed with correlation coefficients of 0.2 and 0.8 to make sure that our study was not sensitive to the selected correlation coefficient. Mean values with confidence interval (CI) and also medians, and their interquartile range (IQR) were also converted to mean ± SD using suggested formulas [27].
The overall weighted mean differences and corresponding 95% CIs were calculated by using a random-effects model [28] which takes the between-study heterogeneity into account. Cochrane Q test and I2 statistic were used to assess the between studies heterogeneity [29]. To investigate possible sources of heterogeneity, several subgroup analyses based on participants’ health status (healthy, unhealthy with chronic diseases), duration of the intervention (between 3 and 12 weeks/ ≥ 12 weeks), geographic region (USA/other countries), study design (parallel/cross-over), dose of the intervention (< 12 whole eggs per week/ ≥ 12 whole eggs per week), difference in energy intake between the two groups (yes/no/unclear), calorie restriction along with whole egg intervention (yes/no), and the type of food in the control group (no egg/foods except egg) were conducted. To assess the robustness of the overall result, a sensitivity analysis was performed by removing studies from the analyses one by one [30]. Publication bias was checked using Begg’s funnel plots and asymmetry tests (Begg’s test and Egger’s test) [31]. All statistical analyses were performed using STATA version 11.2 (Stata Corp, College Station, TX), and P values less than 0.05 were considered as statistically significant.
Results
Study selection
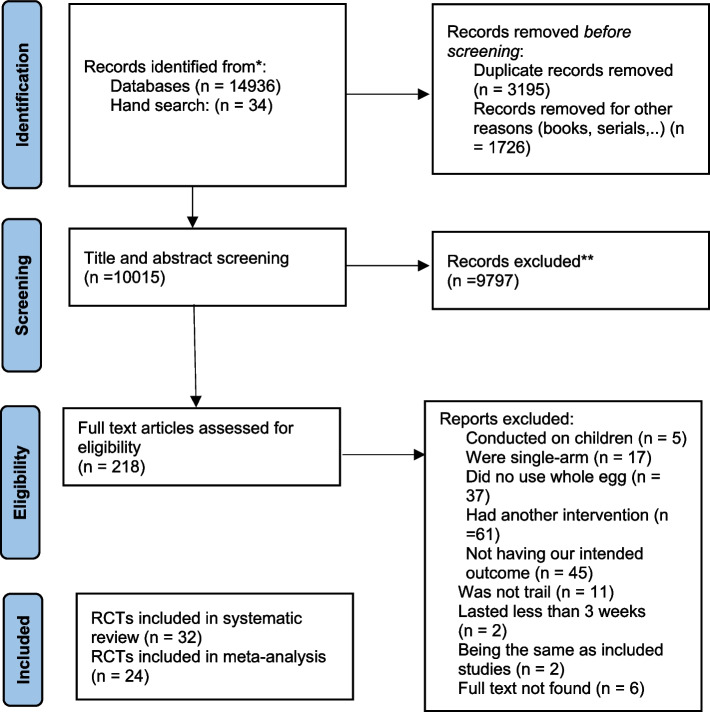
Our systematic search led to 14,970 studies from PubMed, Scopus, Web of Science, and hand search. After removing duplicates and non-article documents (books, series, …), 10,015 studies were screened through reading titles/abstracts. A total of 9797 irrelevant studies were excluded and finally, after reading the full text of 218 potentially relevant studies, 186 studies were excluded because of the following reasons: 5 studies were conducted on children, 17 studies were single-arm, 37 studies did not use the whole egg, 61 studies had another intervention or used enriched eggs for intervention, 11 studies were not the clinical trial in design, 45 studies did not have our intended outcomes, 2 study lasted less than 3 weeks, 2 studies had the same population and protocol as already included studies, and the full text of 6 studies were not available. Therefore, 32 eligible studies were included in our systematic review and meta-analysis [22, 23, 32–61]. The study selection process is provided in Fig. 1.
Fig. 1.
Flow diagram for study selection process
Study characteristics
Characteristics of the included studies are shown in Table 1. Eligible studies were published between 1981 and 2022. More than half of the studies were conducted in the USA [22, 23, 32, 34–36, 39, 42, 43, 46, 47, 49, 51, 53, 58, 59, 61]. Five studies were conducted in Australia [37, 38, 40, 44, 60], two in Thailand [45, 52], two in the UK [50, 57], two in India [54, 55], and one in Denmark [56], Pakistan [48], Finland [41], and Mexico [33]. Nineteen studies had a cross-over design [22, 23, 33, 35, 36, 39, 42, 43, 45, 47, 48, 51–57, 61] and 13 were parallel clinical trials [32, 34, 37, 38, 40, 41, 44, 46, 49, 50, 58–60]. All studies were performed on both sexes except two studies one of which was done in females [52], and the other one was conducted in males [59]. Participants in 20 studies were healthy individuals [32, 34, 36, 39–41, 43, 46, 47, 49–51, 53–60]; in five studies, the participants were with T2D or prediabetes [22, 33, 37, 38, 44]; in four studies, they were with MetS [35, 42, 48, 61]; two studies included participants with hyperlipidemia [45, 57]; and participants in a study by Katz et al. [23] were affected by established coronary artery disease (CAD). One of the included studies had two intervention and control groups, as in the intervention groups, one group received two whole eggs per day and the other received two whole eggs per day + 1000 kcal energy deficit diet; therefore, we considered this study as two studies [58]. There were three types of control groups among the studies: most studies excluded whole eggs from the diet of controls [22, 32, 36, 41, 44–50, 52–55, 59, 61] or the control group received fewer whole eggs than intervention group [37, 38, 56, 57, 60]. In eight studies, control groups received a high carbohydrate breakfast without whole egg [23, 33, 34, 39, 40, 42, 43, 58], and one study provided them walnut [51]; and in one study, the control group received choline supplements [35]. Putadechakum et al. [45] reported the values of FFM as a percentage, and in another study, only the overall effect was reported and the difference by diet allocation was not mentioned [44]. So their studies were not included in the meta-analysis for FFM. In the study conducted by Harman et al. [50], only geometric averages were reported, and seven studies not provided data needed for meta-analysis [51–57]; therefore, these studies were only reported in the systematic review.
Table 1.
Characteristics of clinical trials that were included in systematic review
| Authors, publication year | Country | Design | Gender | Duration (weeks) | Health status of participants | Type and amount of intervention | Type and amount of control | Reported data and result |
|---|---|---|---|---|---|---|---|---|
| Daly et al. 2022 [60] | Australia | Parallel | Male and female | 12 | Healthy |
Intervention 1: 7 whole eggs/week Intervention 2: 12 whole eggs/week |
2 eggs/week | Weight ↔ |
| Njike et al. 2021 [61] | USA | Cross-over | Male and female | 6 | MetS | 2 eggs/week | No egg |
Weight ↔ BMI ↔ WC ↔ |
| Keogh et al. 2020 [40] | Australia | Parallel | Male and female | 24 | Healthy | 10 whole eggs/week | breakfast cereal | Weight ↔ |
| Maki et al. 2020 [42] | USA | Cross-over | Male and female | 4 | MetS/prediabetes | 12 whole eggs/week | energy-matched breakfast meals without eggs contained higher-CHO foods | Weight ↔ |
| Marissa DiBella et al. 2020 [35] | USA | Cross-over | Male and female | 4 | MetS | 21 whole eggs/week | choline supplements (choline bitartrate, CB) |
Weight ↔ BMI ↔ WC ↔ |
| Shakoor et al. 2020 [48] | Pakistan | Cross-over | Male and female | 5 | MetS | 14 whole eggs/week | No egg | BMI ↔ |
| Aljohi et al. 2019 [32] | USA | Parallel | Male and female | 48 | Healthy | no more than 2 whole eggs per day, 12 eggs per week and not to go > 2 days without eating eggs | No egg | BMI ↔ |
| Fuller et al. 2018 [37] | Australia | Parallel | Male and female | 24 | Prediabetes/T2D | 12 whole eggs/week | < 2 eggs/week |
WC ↔ FFM ↔ |
| Missimer et al. 2017 [43] | USA | Cross-over | Male and female | 4 | Healthy | 12 whole eggs/week | one packet of oatmeal per day |
Weight ↔ BMI ↔ WC ↔ |
| DiMarco et al. 2017 [36] | USA | Cross-over | Male and female | 4 | Healthy |
Intervention 1: 7 whole eggs/week Intervention 2: 14 whole eggs/week Intervention 3: 21 whole eggs/week |
No egg |
BMI ↔ WC ↔ |
| Fuller et al. 2016 [38] | Australia | Parallel | Male and female | 12 | Prediabetes/T2D | 12 whole eggs/week | less than 2 eggs/wk |
Weight ↔ WC ↔ FFM ↔ |
| Njike et al. 2016 [22] | USA | Cross-over | Male and female | 12 | T2D | 10–14 whole eggs/week | No egg |
Weight ↑ BMI ↑ WC ↔ |
| Clayton et al. 2015 [34] | USA | Parallel | Male and female | 12 | Healthy | egg-based breakfasts (14 whole eggs/week) | bagel-based breakfasts (9-cm-diameter bagel and the selected options) |
Weight ↔ FFM ↔ |
| Katz et al. 2015 [23] | USA | Cross-over | Male and female | 6 | Established CAD |
breakfast with 2 eggs daily (14 whole eggs/week) |
a high-carbohydrate breakfast daily |
Weight ↔ BMI ↔ |
| Ballesteros et al., 2015 [33] | Mexico | Cross-over | Male and female | 5 | T2D | 7 whole eggs/week | 40 g of oatmeal with 2 cups (472 mL) of lactose-free milk/day |
Weight ↔ BMI ↔ |
| Bonny Burns-Whitmore et al. 2014 [51] | USA | Cross-over | Male and female | 8 | Healthy | 6 whole eggs/week | walnuts (28.4 g, 6x/week) | Weight ↔ |
| Khosla et al., 2013 [46] | USA | Parallel | Male and female | 14 | Healthy | At least 10 whole eggs/week | No egg | Weight ↔ |
| Putadechakum et al. 2013 [45] | Thailand | Cross-over | Male and female | 4 | Hyperlipidemic |
Intervention 1: 7 whole eggs/week Intervention 2: 21 whole eggs/week |
No egg |
Weight ↔ BMI ↔ WC ↔ FFM ↔ |
| Taweesak Techakriengkrai et al. 2012 [52] | Thailand | Cross-over | Female | 4 | Hypercholesterolemic | 21 whole eggs/week | 1 egg/day |
Weight ↔ BMI ↔ |
| Pearce et al. 2011 [44] | Australia | Parallel | Male and female | 12 | T2D | high-protein high-cholesterol (14 whole eggs/week) | High-protein low-cholesterol (no eggs with 100 g of lean protein, meat, chicken or fish) | Weight ↔ |
| Vislocky et al. 2009 [49] | USA | Parallel | Male and female | 8 | Healthy | 12 whole eggs/week | No egg |
Weight ↔ FFM ↔ |
| Harman et al. 2008 [50] | UK | Parallel | Male and female | 12 | Healthy | 14 whole eggs/week | No egg | Weight ↔ |
| Vander Wal et al. 2008 [58] | USA | Parallel | Male and female | 8 | Healthy | 14 whole eggs/week | Bagel breakfast |
BMI ↓ Weight ↓ WC ↔ |
| 14 whole eggs/week + 1000 kcal energy deficit low fat weight loss diet | bagel breakfast + 1000 ca energy deficit low fat weight loss diet |
BMI ↓ Weight ↓ WC ↓ |
||||||
| Katz et al. 2005 [39] | USA | Cross-over | Male and female | 6 | Healthy | 14 whole eggs/week | 60 g uncooked whole oats | BMI ↔ |
| Tannock et al. 2005 [53] | USA | Cross-over | Male and female | 4 | Healthy |
Intervention 1: 14 whole eggs/week Intervention 2: 28 whole eggs/week |
No eggs | Weight ↔ |
| Chakrabarty et al. 2004 [54] | India | Cross-over | Male and female | 8 | Healthy | 7 whole eggs/week | No egg |
Weight ↔ BMI ↔ |
| Chakrabarty et al. 2002 [55] | India | Cross-over | Male and female | 8 | Healthy | 7 whole eggs/week | No egg |
Weight ↔ BMI ↔ |
| Schnohr et al. 1994 [56] | Denmark | Cross-over | Male and female | 6 | Healthy | 14 whole eggs/week | Usual diet | Weight ↔ |
| Lehtimaki et al. 1992 [41] | Finland | Parallel | Male and female | 3 | Healthy | 21 whole eggs/week | No egg | Weight ↔ |
| Edington et al. 1987 [57] | UK | Cross-over | Male and female | 8 |
Group1: healthy Group 2: hyperlipidemia |
7 whole eggs/week | 2 eggs/ week | Weight ↔ |
| Sacks et al. 1984 [47] | USA | Cross-over | Male and female | 3 | Healthy | 7 whole eggs/week | No egg | Weight ↔ |
| Flaim et al. 1981 [59] | USA | Parallel | Male | 5 | Healthy | 28 whole eggs/week | No egg | Weight ↑ |
BMI Body mass index, FFM Fat-free mass, WC Waist circumference, MetS Metabolic syndrome, T2D Type 2 diabetes, CAD Cardiovascular disease
↑Significant increasing effect, ↓significant decreasing effect, ↔non-significant effect
Risk of bias assessment
The results of the risk of bias assessment of included randomized studies are shown in Table 2. Only two studies reported allocation concealment [38, 61]. In one study, whole eggs were given in the form of muffins, so the participants and investigators were blind to the intervention and control groups [47]; however, in other studies, blinding was not possible. The result of the risk of bias assessment for 6 studies was rated as “with some concern” [33, 36, 39, 43, 47, 61] mainly because they did not have any information about their allocation method, 23 studies were high risk [22, 23, 32, 34, 35, 37, 40, 42, 44–46, 48–60] mainly due to lack of information about the blinding of outcome assessors, and one study [38] was rated as low risk of bias. One included non-RCT assessed using the ROBINS-I tool [41]. It was scored as the critical risk of bias (Table 3).
Table 2.
Study quality and risk of bias assessment using Cochrane risk of bias tool for randomized trials
| Author, publication year | Randomization process | Deviations from the intended interventions (effect of assignment to intervention) | Deviations from the intended interventions (effect adhering to intervention) | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall |
|---|---|---|---|---|---|---|---|
| Daly et al. 2022 [60] | Some concern | Some concern | low | Low | high | Some concern | high |
| Njike et al. 2021 [61] | low | Some concern | low | low | low | low | Some concern |
| Keogh et al. (2020) [40] | Some concern | Some concern | low | low | high | Some concern | High |
| Maki et al. (2020) [42] | Some concern | Some concern | low | Low | high | Some concern | High |
| Shakoor et al. (2020) [48] | Some concern | Some concern | low | low | high | Some concern | High |
| DiBella et al (2020) [35] | Some concern | Some concern | low | low | high | Some concern | High |
| Aljohi et al. (2019) [32] | Some concern | Some concern | low | low | high | Some concern | High |
| Fuller et al. (2018) [37] | Some concern | low | low | low | high | Some concern | High |
| Missimer et al. (2017) [43] | Some concern | Some concern | low | low | low | Some concern | Some concern |
| Dimarco et al. (2017) [36] | Some concern | Some concern | low | Low | high | Some concern | Some concern |
| Fuller et al. (2016) [38] | low | low | low | low | low | low | Low |
| Njike et al. (2016) [22] | Some concern | low | low | low | high | Some concern | High |
| Clayton et al. (2015) [34] | Some concern | Some concern | low | low | high | low | High |
| Katz et al. (2015) [23] | Some concern | low | low | low | high | low | High |
| Ballesteros et al. (2015) [33] | Some concern | Some concern | low | low | low | Some concern | Some concern |
| Burns-Whitmore et al. (2014) [51] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Rueda & Pramod Khosla (2013) [46] | Some concern | Some concern | low | low | high | Some concern | High |
| Putadechakum et al. (2013) [45] | Some concern | Some concern | Low | low | high | Some concern | High |
| Techakriengkrai et al. (2012) [52] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Pearce et al. (2011) [44] | Some concern | Some concern | low | Low | high | Some concern | High |
| Vislocky et al. (2009) [49] | Some concern | Some concern | Low | low | high | Some concern | High |
| Harman et al. (2008) [50] | Some concern | Some concern | low | low | high | Some concern | High |
| Vander Wal et al. (2008) [58] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Katz et al. (2005) [39] | Some concern | Some concern | low | low | low | Some concern | Some concern |
| Tannock et al. (2005) [53] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Chakrabarty et al. (2004) [54] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Chakrabarty et al. (2002) [55] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Schnohr et al. (1994) [56] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Edington et al. (1987) [57] | Some concern | Some concern | Low | Low | High | Some concern | High |
| Sacks et al. (1984) [47] | Some concern | low | low | low | low | low | Some concern |
| Flaim et al. (1981) [59] | Some concern | Some concern | Low | Low | High | Some concern | High |
Table 3.
Study quality and risk of bias assessment by using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool
| Author, publication year | Risk of confounding | Risk of selection bias | Risk of misclassification of interventions | Risk of deviation from intended interventions | Risk of missing data | Risk of misclassification of outcomes | Risk of reporting bias | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Lehtimaki et al. 1992 [41] | Critical | Low | Low | Low | Low | No information | Low | Critical |
Findings from the meta-analyses
The effect of whole egg consumption on body weight
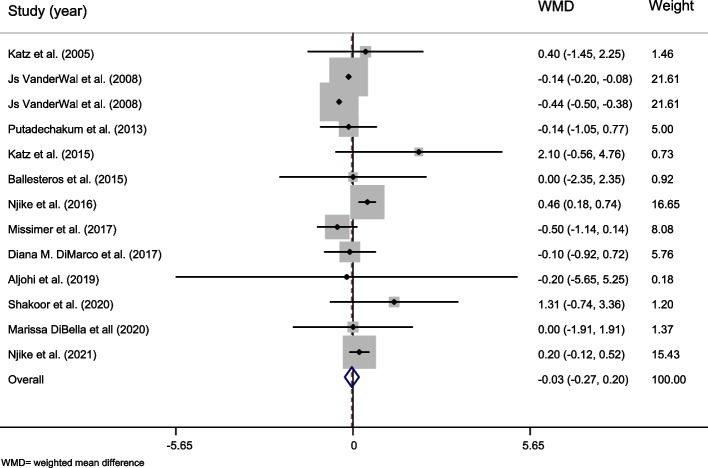
In total, 21 articles reported data on weight related to whole egg consumption [22, 23, 32–35, 37, 38, 40–47, 49, 58–61]. A total of 1117 subjects participated in these studies. The overall results did not show a significant effect of whole egg consumption on body weight [weighted mean difference (WMD) = 0.234, 95% CI = − 0.207–0.675, P = 0.299) (Table 4 and Fig. 2). A high level of heterogeneity was observed between studies (Cochran Q test, P < 0.001, I2 = 84.7%). Based on subgroup analyses, results showed a significant positive effect on body weight in studies that lasted 12 weeks or more (WMD = 0.517, 95% CI 0.013–1.021, P = 0.044) and studies on unhealthy participants (WMD = 0.452, 95% CI 0.059–0.846, P = 0.024. In addition, whole egg consumption had a significant increasing effect on weight in studies that there was no significant difference in energy intakes between the controls and intervention group (WMD = 0.589, 95% CI 0.031–1.147, P = 0.039, Table 4).
Table 4.
The overall effect of whole egg consumption on body weight and by subgroups, using random-effects model
| Study group | Number of studies | Number of participants | Meta-analysis | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| WMD | 95% CI | P effect | Q statistic | p within group | I2 (%) | P between group | |||
| Country | |||||||||
| USA | 14 | 544 | 0.197 | − 0.351, 0.746 | 0.480 | 119.24 | 0.000 | 89.2% | 0.001> |
| Other countries | 8 | 573 | 0.371 | − 0.077, 0.820 | 0.105 | 2.32 | 0.940 | 0.0% | |
| Study design | |||||||||
| Parallel | 13 | 802 | 0.121 | − 0.421, 0.663 | 0.662 | 98.36 | 0.000 | 87.8% | 0.001> |
| Cross-over | 9 | 315 | 0.409 | − 0.140, 0.958 | 0.145 | 12.65 | 0.124 | 36.8% | |
| Control group | |||||||||
| Nothing | 11 | 616 | 0.333 | − 0.096, 0.763 | 0.128 | 14.50 | 0.151 | 31.0% | 0.001> |
| Other foods or supplements | 11 | 501 | 0.130 | − 0.494, 0.753 | 0.684 | 92.80 | 0.000 | 89.2% | |
| Duration | |||||||||
| Less than 12 weeks | 13 | 505 | − 0.007 | − 0.556, 0.542 | 0.979 | 92.11 | 0.000 | 87% | 0.001> |
| 12 weeks and more | 9 | 612 | 0.517 | 0.013, 1.021 | 0.044 | 11.55 | 0.172 | 30.7% | |
| Health status | |||||||||
| Healthy | 12 | 534 | − 0.137 | − 0.763, 0.489 | 0.668 | 80.13 | 0.000 | 86.3% | 0.001> |
| Unhealthy | 10 | 583 | 0.452 | 0.059, 0.846 | 0.024 | 13.80 | 0.130 | 34.8% | |
| Dose of intervention | |||||||||
| Less than 12 eggs per week | 4 | 145 | − 0.216 | − 1.491, 1.058 | 0.739 | 0.04 | 0.998 | 0.0% | 0.683 |
| 12 eggs per week and more | 20 | 1071 | 0.256 | − 0.193, 0.705 | 0.264 | 137.45 | 0.000 | 86.2% | |
| Energy intake differences | |||||||||
| No | 17 | 823 | 0.589 | 0.031, 1.147 | 0.039 | 33.76 | 0.006 | 52.6% | 0.001> |
| Yes | 1 | 30 | 0.300 | − 0.287, 0.887 | 0.316 | 0.00 | |||
| Unclear | 4 | 264 | − 0.667 | − 1.370, 0.037 | 0.063 | 52.56 | 0.000 | 94.3% | |
| Calorie restriction | |||||||||
| No | 18 | 769 | 0.367 | − 0.107, 0.841 | 0.129 | 49.48 | 0.000 | 65.6% | 0.001> |
| Yes | 4 | 348 | − 0.099 | − 1.305, 1.106 | 0.871 | 18.44 | 0.000 | 83.7% | |
| Overall | 22 | 1117 | 0.234 | − 0.207, 0.675 | 0.299 | 136.85 | 0.000 | 84.7% | - |
WMD Weighted mean difference, CI Confidence interval
Fig. 2.
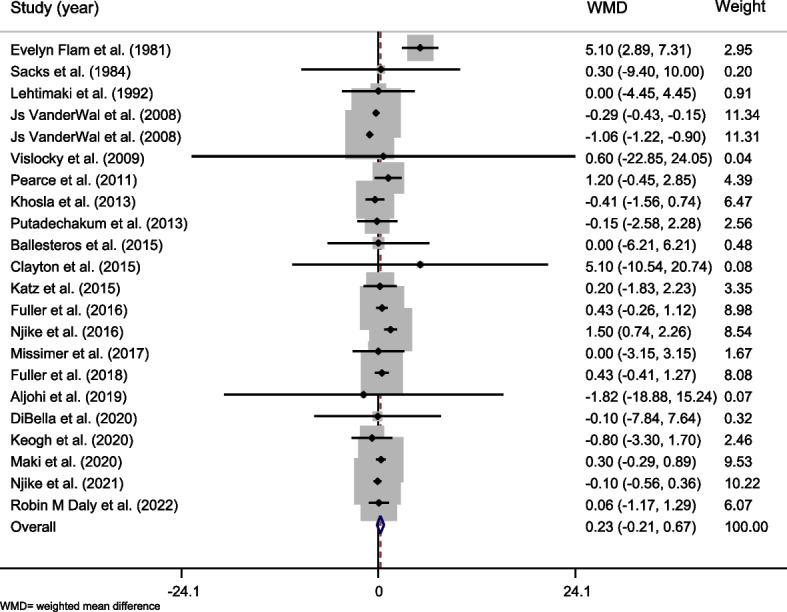
Forest plot representing the effect of egg consumption on body weight using a random-effects model
The effect of whole egg consumption on BMI
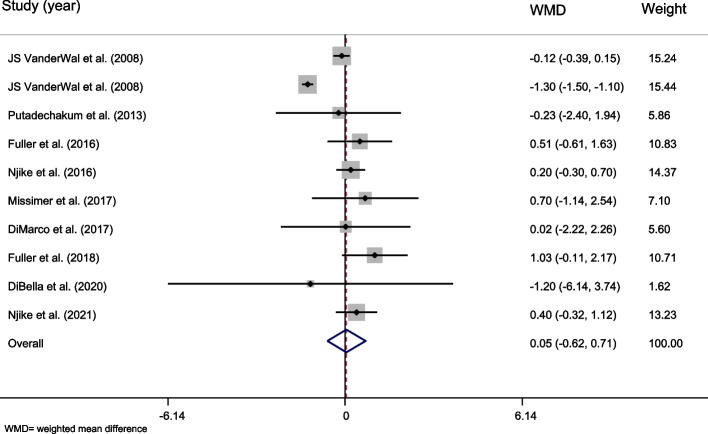
In total, 12 studies with 13 effect sizes and 570 participants were included in the meta-analysis [22, 23, 32, 33, 35, 36, 39, 43, 45, 48, 58, 61]. The overall effect of whole egg consumption on BMI was not significant (WMD = − 0.035, 95% CI = − 0.265–0.196, P = 0.769, Table 5 and Fig. 3). The between-study heterogeneity was significant (Cochran Q test, P = 0.000, I2 = 87%). Subgroup analysis revealed that whole egg consumption significantly increased BMI in studies that excluded whole egg in the diet of controls (WMD = 0.308, 95% CI 0.111–0.505, P = 0.002), in studies that lasted 12 weeks and more (WMD = 0.458, 95% CI 0.181–0.735, P = 0.001), and in unhealthy participants (WMD = 0.337, 95% CI:0.137 – 0.538, P = 0.001). In contrast, subgroup analysis showed significant decreasing effect of whole egg consumption on BMI in trials that gave controls another food or supplement (WMD − 0.270, 95% CI − 0.532 – − 0.008, P = 0.043), in trials with calorie restriction (WMD = − 0.440, 95% CI − 0.499 – − 0.381, P = 0.000) and in healthy individuals (WMD = − 0.286, 95% CI − 0.538 – − 0.035, P = 0.026, Table 5).
Table 5.
The overall effect of whole egg consumption on body mass index (BMI) and by subgroups, using random-effects model
| Study group | Number of studies | Number of participants | Meta-analysis | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| WMD | 95% CI | P effect | Q statistic | P within group | I2% | P between group | |||
| Country | |||||||||
| USA | 10 | 450 | − 0.046 | − 0.287, 0.194 | 0.707 | 90.08 | 0.000 | 90.0% | 0.378 |
| Other countries | 3 | 120 | 0.087 | − 0.694, 0.868 | 0.828 | 1.62 | 0.445 | 0.0% | |
| Study design | |||||||||
| Parallel | 3 | 197 | − 0.290 | − 0.580, 0.001 | 0.051 | 50.00 | 0.000 | 96.0% | 0.001> |
| Cross-over | 10 | 373 | 0.177 | − 0.096, 0.449 | 0.203 | 11.92 | 0.218 | 24.5% | |
| Control group | |||||||||
| Nothing | 6 | 239 | 0.308 | 0.111, 0.505 | 0.002 | 4.43 | 0.490 | 0.0% | 0.001> |
| Other foods or supplement | 7 | 331 | − 0.270 | − 0.532, − 0.008 | 0.044 | 54.19 | 0.000 | 88.9% | |
| Duration | |||||||||
| Less than 12 weeks | 11 | 493 | − 0.148 | − 0.374, 0.079 | 0.201 | 65.67 | 0.000 | 84.8% | 0.001> |
| 12 weeks and more | 2 | 77 | 0.458 | 0.181, 0.735 | 0.001 | 0.06 | 0.813 | 0.0% | |
| Health status | |||||||||
| Healthy | 6 | 330 | − 0.286 | − 0.538, − 0.035 | 0.026 | 51.15 | 0.000 | 90.2% | 0.001> |
| Unhealthy | 7 | 240 | 0.337 | 0.137, 0.538 | 0.001 | 5.28 | 0.508 | 0.0% | |
| Dose of intervention | |||||||||
| Less than 12 eggs per week | 3 | 136 | − 0.047 | − 0.637, 0.544 | 0.877 | 0.03 | 0.984 | 0.0% | 0.471 |
| 12 eggs per week and more | 12 | 541 | − 0.036 | − 0.269, 0.197 | 0.762 | 92.40 | 0.000 | 88.1% | |
| Energy intake differences | |||||||||
| No | 9 | 349 | 0.150 | − 0.128, 0.428 | 0.290 | 10.87 | 0.209 | 26.4% | 0.001> |
| Yes | 0 | - | - | - | - | - | - | - | |
| Unclear | 4 | 221 | − 0.243 | − 0.533, 0.047 | 0.100 | 52.88 | 0.000 | 94.3% | |
| Calorie restriction | |||||||||
| No | 12 | 491 | 0.079 | − 0.199, 0.356 | 0.579 | 27.06 | 0.005 | 59.3% | 0.001> |
| Yes | 1 | 79 | − 0.440 | − 0.499, − 0.381 | 0.000 | 0.00 | |||
| Overall | 13 | 570 | − 0.035 | − 0.265, 0.196 | 0.769 | 92.48 | 0.000 | 87.0% | - |
WMD Weighted mean difference, CI Confidence interval
Fig. 3.
Forest plot representing the effect of egg consumption on body mass index (BMI) using a random-effects model
The effect of whole egg consumption on waist circumference
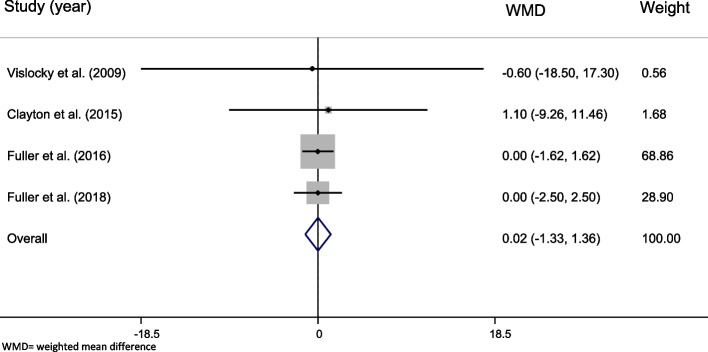
Ten effect sizes from 9 studies [22, 35–38, 43, 45, 58, 61] (n = 665) reported the effect of whole egg consumption on WC (Table 6). The overall effect was not significant (WMD = 0.046, 95% CI = − 0.616–0.709, P = 0.891, Fig. 4). The overall heterogeneity was high (Cochran Q test, P = 0.000, I2 = 90.1%). In subgroup analysis, a significant effect of egg consumption on WC was observed in trials with no significant difference in energy intake between the intervention and control groups (WMD = 0.350, 95% CI = 0.003–0.698, P = 0.048, Table 6).
Table 6.
The overall effect of whole egg consumption on waist circumference (WC) and by subgroups, using random-effects model
| Study group | Number of studies | Number of participants | Meta-analysis | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| WMD | 95% CI | P effect | Q statistic | P within group | I2% | P between group | |||
| Country | |||||||||
| USA | 7 | 326 | − 0.149 | − 0.910, 0.612 | 0.702 | 77.11 | 0.000 | 92.2% | 0.001> |
| Other countries | 3 | 339 | 0.647 | − 0.101, 1.395 | 0.090 | 1.12 | 0.570 | 0.0% | |
| Study design | |||||||||
| Parallel | 4 | 420 | − 0.094 | − 1.043, 0.855 | 0.846 | 63.61 | 0.000 | 95.3% | 0.001> |
| Cross-over | 6 | 245 | 0.253 | − 0.134, 0.639 | 0.200 | 1.00 | 0.963 | 0.0% | |
| Control group | |||||||||
| Nothing | 6 | 442 | 0.345 | − 0.010, 0.700 | 0.057 | 2.18 | 0.824 | 0.0% | 0.001> |
| Other food or supplement | 4 | 223 | − 0.486 | − 1.507, 0.535 | 0.351 | 49.95 | 0.000 | 94.0% | |
| Duration | |||||||||
| Less than 12 weeks | 7 | 365 | − 0.236 | − 1.043, 0.571 | 0.567 | 62.64 | 0.000 | 90.4% | 0.001> |
| 12 weeks and more | 3 | 300 | 0.360 | − 0.064, 0.783 | 0.096 | 1.80 | 0.407 | 0.0% | |
| Health status | |||||||||
| Healthy | 4 | 236 | − 0.398 | − 1.372, 0.576 | 0.423 | 50.56 | 0.000 | 94.1% | 0.001> |
| Unhealthy | 6 | 429 | 0.345 | − 0.013, 0.704 | 0.059 | 2.47 | 0.781 | 0.0% | |
| Dose of intervention | |||||||||
| Less than 12 eggs per week | 2 | 107 | − 0.077 | − 1.624, 1.471 | 0.923 | 0.01 | 0.924 | 0.0% | 0.444 |
| 12 eggs per week and more | 10 | 665 | 0.049 | − 0.614, 0.712 | 0.885 | 90.97 | 0.000 | 90.1% | |
| Energy intake differences | |||||||||
| No | 8 | 513 | 0.350 | 0.003, 0.698 | 0.048 | 2.69 | 0.912 | 0.0% | 0.001> |
| Yes | - | - | - | - | - | - | - | - | |
| Unclear | 2 | 152 | − 0.714 | − 1.870, 0.442 | 0.226 | 47.04 | 0.000 | 97.9% | |
| Calorie restriction | |||||||||
| No | 8 | 458 | 0.024 | − 0.195, 0.244 | 0.827 | 4.13 | 0.765 | 0.0% | 0.001> |
| Yes | 2 | 207 | − 0.205 | − 2.484, 2.074 | 0.860 | 15.67 | 0.000 | 93.6% | |
| Overall | 10 | 665 | 0.046 | − 0.616, 0.709 | 0.891 | 90.87 | 0.000 | 90.1% | - |
WMD Weighted mean difference, CI Confidence interval
Fig. 4.
Forest plot representing the effect of egg consumption on waist circumference (WC) using a random-effects model
The effect of whole egg consumption on fat-free mass
The effect sizes obtained from four studies [34, 37, 38, 49] with 304 participants did not show any significant effect of whole egg consumption on FFM (WMD = 0.015, 95% CI = − 1.328–1.358, P = 0.982, Fig. 5). No significant effect was found in subgroup analyses (Table 7).
Fig. 5.
Forest plot representing the effect of egg consumption on body fat-free mass (FFM) using a random-effects model
Table 7.
The overall effect of whole egg consumption on fat free mass (FFM) and by subgroups, using random-effects model
| Study group | Number of studies | Number of participants | Meta-analysis | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| WMD | 95% CI | P effect | Q statistic | P within group | I2% | P between group | |||
| Country | |||||||||
| USA | 2 | 36 | 0.673 | − 8.292, 9.639 | 0.883 | 0.03 | 0.872 | 0.0% | 0.884 |
| Other countries | 2 | 268 | 0.000 | − 1.359, 1.359 | 1.000 | 0.00 | 1.000 | 0.0% | |
| Duration | |||||||||
| Less than 12 weeks | 1 | 11 | − 0.600 | − 18.498, 17.298 | 0.948 | 0.00 | 0.884 | ||
| 12 weeks and more | 3 | 293 | 0.019 | − 1.329, 1.366 | 0.978 | 0.04 | 0.979 | 0.0% | |
| Health status | |||||||||
| Healthy | 2 | 36 | 0.673 | − 8.292, 9.639 | 0.883 | 0.03 | 0.872 | 0.0% | 0.946 |
| Unhealthy | 2 | 268 | 0.000 | − 1.359, 1.359 | 1.000 | 0.00 | 1.000 | 0.0% | |
| Overall | 4 | 304 | 0.015 | − 1.328, 1.358 | 0.982 | 0.05 | 0.997 | 0.0% | - |
WMD Weighted mean difference, CI Confidence interval
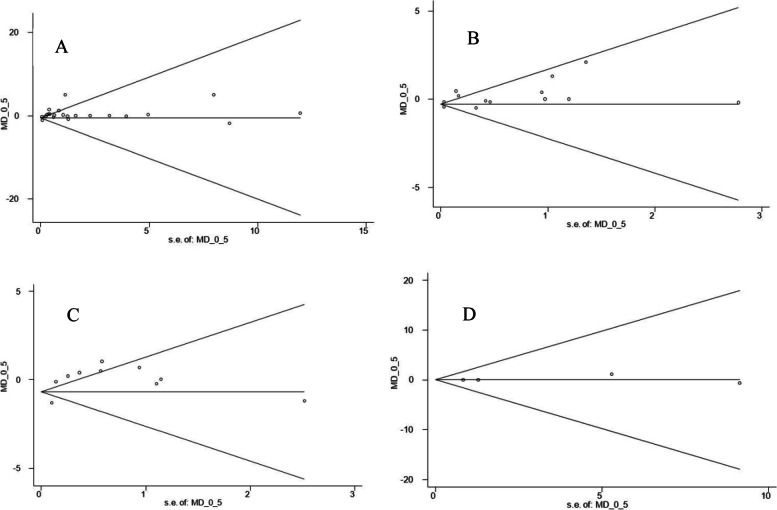
Publication bias and sensitivity analysis
Sensitivity analysis did not show any tangible changes after removing the studies one by one from the meta-analyses. Also, no publication bias was observed for the meta-analysis of BMI (Begg’s test, P = 0.463; Egger’s test, P = 0.222), WC (Begg’s test, P = 0.05; Egger’s test, P = 0.1) and FFM (Begg’s test, P = 1.000; Egger’s test, P = 0.599). However, Begg’s test for meta-analysis of body weight was shown to be significant (Begg’s test, P = 0.006; Egger’s test, P = 0.046) using statistical asymmetry tests (Fig. 6). Therefore, a trim and fill analysis was used to see if correcting the asymmetry by imputing studies changes the overall effects. The analysis could not add studies and the overall effect was not changed (WMD = 0.234, 95% CI = − 0.207–0.675, P = 0.299).
Fig. 6.
Begg’s funnel plots presenting the effect sizes versus their standard errors (SEs) for controlled trials that assessed the effect of whole egg consumption on weight (A), body mass index (BMI) (B), waist circumference (WC) (C), and fat free mass (FFM) (D)
Discussion
The current systematic review and meta-analysis of clinical trials were conducted on the effect of whole egg consumption on body weight and body composition in adults. We did not find a significant effect of whole egg consumption on body weight, BMI, WC, and FFM in adults. However, subgroup analyses revealed that whole egg consumption might significantly increase body weight and BMI in studies longer than 12 weeks and in unhealthy subjects. In addition, an increasing effect of whole egg consumption on BMI was observed in studies that the control group did not receive any food as a replacement for egg. In trials with no significant difference in energy intake between the intervention and control groups, a significant increasing effect of egg consumption on weight and WC was found. Furthermore, in trials that the control groups received another food or supplement, in studies with calorie restriction and in healthy participants, a significant decrease was observed in BMI.
Most studies have considered eggs as an important source of cholesterol and have linked them to chronic diseases, especially CVDs and diabetes [62, 63]. But eggs also are rich in high-quality protein, phospholipids, and antioxidants [13] that can have beneficial effects.
A limited number of human studies have directly examined the effect of whole egg consumption on body weight. A study on rats demonstrated that whole egg-based diet may reduce body weight gain and visceral fat in rats [64]. A randomized clinical trial that investigated the effect of egg intake on appetite found that eating an egg-based breakfast could reduce appetite and short-term energy intake [65]. Evidence suggests that eggs may control appetite and increase satiety by inhibiting or stimulating certain hormones like anorexigenic hormones, such as peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) [66]. Contrary to these results, the present systematic review and meta-analysis showed that whole egg intake does not lead to weight loss but also has no effect on weight gain. The reason for this inconsistency may be the short duration of the previous studies, because our systematic review in subgroup analysis revealed that when whole egg is consumed for 12 weeks or more, it can increase body weight and BMI. And also our meta-analysis showed that when there is no difference in energy intake, egg consumption can significantly increase weight. Therefore, in the case of routine and long-term consumption of egg, it is better to take its calorie content into account in the diet. Additionally, we found that whole egg intake has an increasing effect on weight and BMI in unhealthy individuals. However, there is no clear mechanism for this increasing effect, it seems people with diseases such as T2D and CVDs should take whole eggs with caution.
This meta-analysis shows a decreasing effect of whole egg consumption on BMI when there was calorie restriction. The study findings also revealed that BMI increased significantly in studies that excluded eggs from the diet of controls. Previous studies have examined the relationship between egg intake and BMI. Guangzhou Biobank Cohort Study and meta-analysis, a prospective study in 2019, has investigated the association between egg consumption, CVD mortality, and a meta-analysis has reported a negative association between egg consumption and BMI after adjusting by some confounder variables [67]. As mentioned, evidence has shown that egg consumption can increase short-term satiety and fullness and possibly also be effective in controlling weight and reducing BMI. In addition, a clinical trial that examined the effect of an egg breakfast compared to a bagel breakfast on weight loss found that eggs consumed for breakfast can lead to weight loss and decreasing BMI when it is combined with an energy-restricted diet [58] which supports our findings. Although the mechanism involved is not well understood, the extra protein content of egg may contribute to this effect [58].
In our study, egg consumption had no significant effect on WC. Similar to our results, in previous cohort studies and meta-analyses, no association was found between egg consumption and WC [67]. However, in the subgroup analysis, we found an increasing effect of egg intake on WC in the studies that had no difference in energy intake between the intervention and control groups.
In the results of our study, no significant effect was also found on FFM. A recent study that examined the relationship between egg consumption, serum cholesterol levels, and body composition distribution in Korean adult, found no significant association between egg consumption and FFM which support our results [68].
Our study is the first systematic review and meta-analysis that examined the effect of whole egg intake on body weight and composition. It can also be mentioned that a complete and unrestricted search inception to the 23rd of April 2023 was performed for this study. The present study had also some limitations that should be noted. First, heterogeneity between studies were significant. Second, although the magnitude was not high a significant publication bias was observed for the meta-analysis of body weight. Third, only one study had low risks of bias and the majority of included studies had some concerns due to the lack of information about outcome assessors blinding and method of allocation. Moreover, in most included studies, the method of cooking eggs was not specified. As different cooking methods can lead to different effects of food on weight and body composition, it is recommended that future studies consider this issue. It is also important to note that weight and anthropometry measurements were not the primary outcome of most of the included studies, and these studies may not be accurate enough for reporting these secondary outcomes. The studies included in the present meta-analyses were conducted on adults, so the results of this systematic review and meta-analyses cannot be generalized to other age groups. In addition, a number of studies did not provide the needed data for calculating effect sizes [50–57]. The results of these studies were in line with the current findings. Therefore, the overall findings might not change if they were included in the meta-analyses.
Conclusion
In conclusion, the result of the present systematic review and meta-analysis indicates that whole egg intake might not significantly affect body weight and body composition. However, egg consumption might have adverse effects on body weight and BMI if highly consumed over a long-term period and if consumed by adults with chronic diseases. This review findings also revealed that whole egg might result in better weight reduction if consumed in the context of an energy-restricted diet and if consumed by healthy individuals. Studies that have directly investigated the effect of whole egg consumption on body weight and composition are still lacking, and the mechanisms of this effect have not yet been properly elucidated. Therefore, strong clinical trial studies are needed to measure the effect of whole egg consumption, especially the long-term effect, on weight and anthropometric indices.
Supplementary Information
Additional file 1: Supplementary table 1. Search strategy used for each online database.
Acknowledgements
The authors would like to thank the research council of Nutrition and Food Security Research Center of Shahid Sadoughi University of Medical Sciences for their great cooperation.
Abbreviations
- CVD
Cardiovascular disease
- T2D
Type 2 diabetes
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- BMI
Body mass index
- WC
Waist circumference
- FFM
Fat free mass
- SD
Standard deviation
- SE
Standard error
- CAD
Coronary artery disease
- WMD
Weighted mean difference
- CI
Confidence interval
- IQR
Interquartile range
- PYY
Peptide YY
- GLP-1
Glucagon-like peptide-1
Authors’ contributions
A.SA. and S.B. conceived the study. A.SA. and S.B. designed the search strategy. A.E., F.Z., and S.B. conducted the search, study selection, and data extraction. S.B. and A.SA. conducted the statistical analyses. A.E. wrote the first draft of the manuscript. S.B. and A.SA. critically revised the manuscript. The authors read and approved the final version of the manuscript.
Funding
The current study was funded by Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arezoo Sadat Emrani and Sara Beigrezaei contributed equally to this work.
References
- 1.Higgins M, Kannel W, Garrison R, Pinsky J, STOKES III JJAMS. Hazards of obesity-the Framingham experience. Acta Med Scand. 1987;222(S723):23–36. doi: 10.1111/j.0954-6820.1987.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119(7_Part_2):655–60. doi: 10.7326/0003-4819-119-7_Part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 4.Chooi YC, Ding C, Magkos FJM. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Cardel M, Donahoo WTJE. Social and environmental factors influencing obesity. Endotext [Internet]. 2019.
- 6.Rosin O. The economic causes of obesity: a survey. J Econ Surveys. 2008;22(4):617–647. doi: 10.1111/j.1467-6419.2007.00544.x. [DOI] [Google Scholar]
- 7.Botchlett R, Wu CJJodm, Syndrome M. Diet composition for the management of obesity and obesity-related disorders. J Diabetes Mellitus Metabolic Syndrome. 2018;3:10. doi: 10.28967/jdmms.2018.01.18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang X, Li Y, Liu A, Zhang Q, Hu X, Du S, et al. Dietary pattern and its association with the prevalence of obesity and related cardiometabolic risk factors among Chinese children. PLOS ONE. 2012;7(8):e43183. doi: 10.1371/journal.pone.0043183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bujnowski D, Xun P, Daviglus ML, Van Horn L, He K, Stamler J. Longitudinal association between animal and vegetable protein intake and obesity among men in the United States: the Chicago Western Electric Study. J Am Diet Assoc. 2011;111(8):1150–5.e1. doi: 10.1016/j.jada.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Onge MP, Keller KL, Heymsfield SB. Changes in childhood food consumption patterns: a cause for concern in light of increasing body weights. Am J Clin Nutr. 2003;78(6):1068–1073. doi: 10.1093/ajcn/78.6.1068. [DOI] [PubMed] [Google Scholar]
- 11.Sanigorski AM, Bell AC, Swinburn BA. Association of key foods and beverages with obesity in Australian schoolchildren. Public Health Nutr. 2007;10(2):152–157. doi: 10.1017/S1368980007246634. [DOI] [PubMed] [Google Scholar]
- 12.Golay A. Bobbioni EJIjoo, Obesity rmdjotIAftSo. The role of dietary fat in obesity. 1997;21:S2–11. [PubMed] [Google Scholar]
- 13.Andersen CJ. Bioactive egg components and inflammation. Nutrients. 2015;7(9):7889–7913. doi: 10.3390/nu7095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuang H, Yang F, Zhang Y, Wang T, Chen G. The impact of egg nutrient composition and its consumption on cholesterol homeostasis. Cholesterol. 2018;2018:6303810. doi: 10.1155/2018/6303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkan FJAMJ. Metabolik sendrom. Ankara Med J. 2013;13(2):85–90. [Google Scholar]
- 16.Son M, Wang XF, Wu JP. Egg consumption for appetite control and body weight regulation. In: Wu J, editor. Eggs as functional foods and nutraceuticals for human health. Food Chemistry Function and Analysis. 2019;142019:40–59.
- 17.Hussain G, Wang J, Rasul A, Anwar H, Imran A, Qasim M, et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019;18(1):26. doi: 10.1186/s12944-019-0965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weggemans RM, Zock PL, Katan MB. Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: a meta-analysis. Am J Clin Nutr. 2001;73(5):885–891. doi: 10.1093/ajcn/73.5.885. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhou C, Zhou X, Li L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis. 2013;229(2):524–530. doi: 10.1016/j.atherosclerosis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Shin S, Lee H-W, Kim CE, Lim J, Lee J-K, Lee S-A, et al. Egg consumption and risk of metabolic syndrome in Korean adults: results from the health examinees study. Nutrients. 2017;9(7):687. doi: 10.3390/nu9070687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Zhao Y, Li Q, Dang S, Yan H. Body fat mass, fat distribution and egg consumption: a population-based study in chinese adults. J Am Coll Nutr. 2020;39(6):528–536. doi: 10.1080/07315724.2019.1700200. [DOI] [PubMed] [Google Scholar]
- 22.Njike VY, Ayettey RG, Rajebi H, Treu JA, Katz DL. Egg ingestion in adults with type 2 diabetes: effects on glycemic control, anthropometry, and diet quality—a randomized, controlled, crossover trial. BMJ Open Diabetes Res Care. 2016;4(1):e000281. doi: 10.1136/bmjdrc-2016-000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz DL, Gnanaraj J, Treu JA, Ma YY, Kavak Y, Njike VY. Effects of egg ingestion on endothelial function in adults with coronary artery disease: a randomized, controlled, crossover trial. Am Heart J. 2015;169(1):162–169. doi: 10.1016/j.ahj.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JAJChfsroi. Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions. 2019:205–28.
- 26.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical research ed) 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hozo SP, Djulbegovic B, Hozo IJBmrm. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP. Thompson SGJSim. Quantifying heterogeneity in a meta-analysis. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Smith GD, Altman D. Systematic reviews in health care: meta-analysis in context: Wiley; 2008.
- 31.Egger M, Smith GD, Schneider M, Minder CJB. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aljohi H, Dopler-Nelson M, Cifuentes M, Wilson TA. The consumption of 12 Eggs per week for 1 year does not alter fasting serum markers of cardiovascular disease in older adults with early macular degeneration. J Nutr Intermed Metab. 2019;15:35–41. doi: 10.1016/j.jnim.2018.11.004. [DOI] [Google Scholar]
- 33.Ballesteros MN, Valenzuela F, Robles AE, Artalejo E, Aguilar D, Andersen CJ, et al. One egg per day improves inflammation when compared to an oatmeal-based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Nutrients. 2015;7(5):3449–3463. doi: 10.3390/nu7053449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton ZS, Scholar KR, Shelechi M, Hernandez LM, Barber AM, Petrisko YJ, et al. Influence of resistance training combined with daily consumption of an egg-based or bagel-based breakfast on risk factors for chronic diseases in healthy untrained individuals. J Am Coll Nutr. 2015;34(2):113–119. doi: 10.1080/07315724.2014.946622. [DOI] [PubMed] [Google Scholar]
- 35.DiBella M, Thomas MS, Alyousef H, Millar C, Blesso C, Malysheva O, et al. Choline intake as supplement or as a component of eggs increases plasma choline and reduces interleukin-6 without modifying plasma cholesterol in participants with metabolic syndrome. Nutrients. 2020;12(10):3120. doi: 10.3390/nu12103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiMarco DM, Missimer A, Murillo AG, Lemos BS, Malysheva OV, Caudill MA, et al. Intake of up to 3 Eggs/day increases HDL cholesterol and plasma choline while plasma trimethylamine-N-oxide is unchanged in a healthy population. Lipids. 2017;52(3):255–263. doi: 10.1007/s11745-017-4230-9. [DOI] [PubMed] [Google Scholar]
- 37.Fuller NR, Sainsbury A, Caterson ID, Denyer G, Fong M, Gerofi J, et al. Effect of a high-egg diet on cardiometabolic risk factors in people with type 2 diabetes: the diabetes and egg (DIABEGG) study-randomized weight-loss and follow-up phase. Am J Clin Nutr. 2018;107(6):921–931. doi: 10.1093/ajcn/nqy048. [DOI] [PubMed] [Google Scholar]
- 38.Gatternig K, Widhalm K. Für Sie gelesen: The effect of a high-egg diet on cardiovascular risk factors in people with typ 2 diabetes: the diabetes and egg (DIABEGG) study - a 3-mo randomized controlled trial. J fur Ernahrungsmedizin. 2016;18(1):10. [Google Scholar]
- 39.Katz DL, Evans MA, Nawaz H, Njike VY, Chan W, Comerford BP, et al. Egg consumption and endothelial function: a randomized controlled crossover trial. Int J Cardiol. 2005;99(1):65–70. doi: 10.1016/j.ijcard.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Keogh JB, Clifton PM. No difference in weight loss, glucose, lipids and vitamin D of eggs for breakfast compared with cereal for breakfast during energy restriction. Int J Environ Res Public Health. 2020;17(23):8827. doi: 10.3390/ijerph17238827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehtimäki T, Moilanen T, Solakivi T, Laippala P, Ehnholm C. Cholesterol-rich diet induced changes in plasma lipids in relation to apolipoprotein E phenotype in healthy students. Ann Med. 1992;24(1):61–66. doi: 10.3109/07853899209164146. [DOI] [PubMed] [Google Scholar]
- 42.Maki KC, Palacios OM, Kramer MW, Trivedi R, Dicklin MR, Wilcox ML, et al. Effects of substituting eggs for high-carbohydrate breakfast foods on the cardiometabolic risk-factor profile in adults at risk for type 2 diabetes mellitus. Eur J Clin Nutr. 2020;74(5):784–795. doi: 10.1038/s41430-020-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Missimer A, DiMarco DM, Andersen CJ, Murillo AG, Vergara-Jimenez M, Fernandez ML. Consuming two eggs per day, as compared to an oatmeal breakfast, decreases plasma ghrelin while maintaining the LDL/HDL ratio. Nutrients. 2017;9(2):89. doi: 10.3390/nu9020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearce KL, Clifton PM, Noakes M. Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Br J Nutr. 2011;105(4):584–592. doi: 10.1017/S0007114510003983. [DOI] [PubMed] [Google Scholar]
- 45.Putadechakum S, Phanachet P, Pakpeankitwattana V, Klangjareonchai T, Roongpisuthipong C. Effect of daily egg ingestion with thai food on serum lipids in hyperlipidemic adults. ISRN nutrition. 2013;2013:580213. doi: 10.5402/2013/580213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rueda JM, Khosla P. Impact of breakfasts (with or without eggs) on body weight regulation and blood lipids in university students over a 14-week semester. Nutrients. 2013;5(12):5097–5113. doi: 10.3390/nu5125097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacks FM, Marais GE, Handysides G, Salazar J, Miller L, Foster JM, et al. Lack of an effect of dietary saturated fat and cholesterol on blood pressure in normotensives. Hypertension (Dallas, Tex : 1979) 1984;6(2 Pt 1):193–8. doi: 10.1161/01.HYP.6.2.193. [DOI] [PubMed] [Google Scholar]
- 48.Shakoor H, Khan MI, Sahar A, Khan MKI, Faiz F, Basheer AH. Development of omega-3 rich eggs through dietary flaxseed and bio-evaluation in metabolic syndrome. Food Sci Nutr. 2020;8(6):2619–2626. doi: 10.1002/fsn3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vislocky LM, Pikosky MA, Rubin KH, Vega-López S, Gaine PC, Martin WF, et al. Habitual consumption of eggs does not alter the beneficial effects of endurance training on plasma lipids and lipoprotein metabolism in untrained men and women. J Nutr Biochem. 2009;20(1):26–34. doi: 10.1016/j.jnutbio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Harman NL, Leeds AR, Griffin BA. Increased dietary cholesterol does not increase plasma low density lipoprotein when accompanied by an energy-restricted diet and weight loss. Eur J Nutr. 2008;47(6):287–293. doi: 10.1007/s00394-008-0730-y. [DOI] [PubMed] [Google Scholar]
- 51.Burns-Whitmore B, Haddad E, Sabaté J, Rajaram S. Effects of supplementing n-3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free-living lacto-ovo- vegetarians: a randomized, crossover, free-living intervention study. Nutr J. 2014;13(1):1–9. doi: 10.1186/1475-2891-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Techakriengkra T, Klangjareonchai T, Pakpeankitwattana V, Sritara P, Roongpisuthipong C. The effect of ingestion of egg and low density lipoprotein (LDL) oxidation on serum lipid profiles in hypercholesterolemic women. Songklanakarin J Sci Technol. 2012;34(2):173–178. [Google Scholar]
- 53.Tannock LR, O’Brien KD, Knopp RH, Retzlaff B, Fish B, Wener MH, et al. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation. 2005;111(23):3058–3062. doi: 10.1161/CIRCULATIONAHA.104.506188. [DOI] [PubMed] [Google Scholar]
- 54.Chakrabarty G, Manjunatha S, Bijlani RL, Ray RB, Mahapatra SC, Mehta N, et al. The effect of ingestion of egg on the serum lipid profile of healthy young Indians. Indian J Physiol Pharmacol. 2004;48(3):286–292. [PubMed] [Google Scholar]
- 55.Chakrabarty G, Bijlani RL, Mahapatra SC, Mehta N, Lakshmy R, Vashisht S, et al. The effect of ingestion of egg on serum lipid profile in healthy young free-living subjects. Indian J Physiol Pharmacol. 2002;46(4):492–498. [PubMed] [Google Scholar]
- 56.Schnohr P, Thomsen OO, Riis Hansen P, Boberg-Ans G, Lawaetz H, Weeke T. Egg consumption and high-density-lipoprotein cholesterol. J Intern Med. 1994;235(3):249–251. doi: 10.1111/j.1365-2796.1994.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 57.Edington J, Geekie M, Carter R, Benfield L, Fisher K, Ball M, et al. Effect of dietary cholesterol on plasma cholesterol concentration in subjects following reduced fat, high fibre diet. Br Med J (Clin Res Ed) 1987;294(6568):333–336. doi: 10.1136/bmj.294.6568.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wal JSV, Gupta A, Khosla P, Dhurandhar NV. Egg breakfast enhances weight loss. Int J Obes. 2008;32(10):1545–1551. doi: 10.1038/ijo.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flaim E, Ferreri LF, Thye FW, Hill JE, Ritchey SJ. Plasma lipid and lipoprotein cholesterol concentrations in adult males consuming normal and high cholesterol diets under controlled conditions. Am J Clin Nutr. 1981;34(6):1103–1108. doi: 10.1093/ajcn/34.6.1103. [DOI] [PubMed] [Google Scholar]
- 60.Daly RM, De Ross B, Gianoudis J, Tan SY. Dose-response effect of consuming commercially available eggs on wintertime serum 25-hydroxyvitamin D concentrations in young australian adults: a 12-week randomized controlled trial. J Nutr. 2022;152(7):1702–1710. doi: 10.1093/jn/nxac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Njike VY, Treu JA, Kela GCM, Ayettey RG, Comerford BP, Siddiqui WT. Egg consumption in the context of plant-based diets and cardiometabolic risk factors in adults at risk of type 2 diabetes. J Nutr. 2021;151(12):3651–3660. doi: 10.1093/jn/nxab283. [DOI] [PubMed] [Google Scholar]
- 62.Geiker NRW, Larsen ML, Dyerberg J, Stender S, Astrup A. Egg consumption, cardiovascular diseases and type 2 diabetes. Eur J Clin Nutr. 2018;72(1):44–56. doi: 10.1038/ejcn.2017.153. [DOI] [PubMed] [Google Scholar]
- 63.Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(1):146–159. doi: 10.3945/ajcn.112.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saande CJ, Bries AE, Pritchard SK, Nass CA, Reed CH, Rowling MJ, et al. Whole egg consumption decreases cumulative weight gain in diet-induced obese rats. J Nutr. 2020;150(7):1818–1823. doi: 10.1093/jn/nxaa114. [DOI] [PubMed] [Google Scholar]
- 65.Vander Wal JS, Marth JM, Khosla P, Jen KL, Dhurandhar NV. Short-term effect of eggs on satiety in overweight and obese subjects. J Am Coll Nutr. 2005;24(6):510–515. doi: 10.1080/07315724.2005.10719497. [DOI] [PubMed] [Google Scholar]
- 66.Nilsson M, Holst JJ, Björck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr. 2007;85(4):996–1004. doi: 10.1093/ajcn/85.4.996. [DOI] [PubMed] [Google Scholar]
- 67.Xu L, Lam TH, Jiang CQ, Zhang WS, Zhu F, Jin YL, et al. Egg consumption and the risk of cardiovascular disease and all-cause mortality: Guangzhou Biobank Cohort Study and meta-analyses. Eur J Nutr. 2019;58(2):785–796. doi: 10.1007/s00394-018-1692-3. [DOI] [PubMed] [Google Scholar]
- 68.Shim JE, Seo YG. Relationship between egg consumption and body composition as well as serum cholesterol level: Korea National Health and Nutrition Examination Survey 2008–2011. J Clin Med. 2021;10(24):5918. doi: 10.3390/jcm10245918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary table 1. Search strategy used for each online database.
Data Availability Statement
Not applicable.