Abstract
A nectrioid fungus forming a pinkish colony with mainly solitary phialides producing ellipsoid, aseptate conidia in mucoid packets was isolated from Dirinaria applanata. Our taxonomic study based on morphology and phylogenetic analysis using ITS rDNA sequences revealed that the isolates represented a member of the genus Cylindromonium. Based on further morphological examination, nucleotide sequence comparison, and phylogenetic analysis based on LSU rDNA, tef1, and rpb2 in addition to the phylogenetic analysis using the ITS rDNA sequences, the fungus from Dirinaria represents a new species, which is described here as Cylindromonium dirinariae sp. nov. Furthermore, inoculation experiments revealed that this species can also produce perithecia when inoculated on the host lichen in laboratory environments.
Citation: Ohmaki A, Okane I, Crous PW, Verkley GJM (2023). Cylindromonium dirinariae sp. nov. (Ascomycota, Hypocreales), a new nectrioid lichenicolous species on Dirinaria applanata in Japan. Fungal Systematics and Evolution 11: 1–10. doi: 10.3114/fuse.2023.11.01
Keywords: culture, inoculation, lichenicolous fungi, Nectriaceae, new taxon, phylogeny
INTRODUCTION
Lichenicolous fungi is a term used to circumscribe fungi that grow on lichens. They can interact with their lichen hosts as saprophytes, parasites and commensalistic parasymbionts. Lichenicolous fungi usually establish a symbiotic relationship with a single species or genus of lichens, while some species have a wide host range (Diederich et al. 2018). Approximately 2 300 species of lichenicolous fungi have been described on lichens globally and they are classified into ca. 400 genera, ca. 100 families, 55 orders and 10 classes. Ninety-six percent of the total number of lichenicolous fungi are ascomycetes and four percent of the fungi are basidiomycetes (Diederich et al. 2018). About 166 lichenicolous fungi have been reported from Japan to date (Frisch et al. 2018, Ohmura & Kashiwadani 2018, Tadome et al. 2018, Zhurbenko & Ohmura 2018a, b, Zhurbenko et al. 2018, Zhurbenko & Ohmura 2019, 2020, Frisch et al. 2020, Tadome & Ohmura 2021, 2022, Tadome et al. 2022). In spite of these reports, there has been relatively little research conducted on this fungal group in Japan. Therefore, many species remain to be discovered and described.
The genus Cylindromonium, with the type species C. eugeniicola, was segregated from Acremonium based on analyses of ITS and LSU rDNA sequence data (Summerbell et al. 2011, Crous et al. 2019b). Cylindromonium species are known to be lichenicolous, mycophilic, or saprophytic (Gams 1971, Crous et al. 2019b, 2020, 2021). Cylindromonium was established as a genus to accommodate acremonium-like taxa with unbranched, hyaline, phialidic conidiophores, and cylindrical 1-septate conidia (Crous et al. 2019b). A total of five asexual species have been assigned to Cylindromonium, for which no sexual morph has thus far been reported (Crous et al. 2019b, 2020, 2021). During our research on the diversity of lichenicolous fungi in Japan, a fungus colonising the Physciaceae lichen Dirinaria applanata was found. The purpose of this study is to describe the morphological, physiological, and ecological features of this species, clarify the link to its sexual morph, and discuss its taxonomic placement.
MATERIALS AND METHODS
Collection materials
Field investigations were performed from September 2020 to March 2021 in Tsukuba city, Ibaraki prefecture, Japan. Specimens of the fungus growing on the lichen host D. applanata were found on the bark of Zelkova serrata. A voucher specimen was deposited in the National Museum of Nature and Science (TNS), Tsukuba, Japan. A living ex-type culture was deposited in the Biological Resource Center of the National Institute for Technology and Evaluation (NBRC). Cylindromonium lichenicola strains (CBS 188.70 and CBS 415.70A) were also examined for comparison purposes.
Morphological observations
Samples were observed using a dissecting microscope [M165 C (Leica, Wetzlar, Germany)] and a differential interference contrast compound microscope [BX53 (Olympus, Tokyo, Japan)]. Anatomical examination was performed using hand-cut sections mounted in a drop of water or clear lactophenol. Photographs were taken using a microscope digital camera [Flexacam C3 (Leica, Wetzlar, Germany) or DP23 (Olympus, Tokyo, Japan)]. Dimensions of ascospores, conidia, conidial mass, phialide and hyphal width are given as (minimum–) range of mean ± standard deviation (–maximum) (n = number of measurements). Chemical reactions of the perithecia were observed by using 10 % KOH. To determine if there is a significant difference between each dimension of the present fungus and C. lichenicola, the t-test was performed using Microsoft Excel.
Isolation of fungal cultures
Fungal cultures were isolated from freshly collected material. Mycelium or single ascospores were picked up using a flamed needle and plated on 1 % malt extract agar (MEA). To confirm differences in colony characteristics on each agar medium, mycelial plugs were subcultured on 1 % MEA, potato dextrose agar (PDA) (Nissui Pharmaceutical, Tokyo, Japan), oatmeal agar (OA) (Becton Dickinson and Co, New Jersey, USA), Sabouraud maltose agar (SMA) (Thermo Fisher Scientific, Massachusetts, USA), malt yeast extract agar (MYA) (Ahmadjian 1961) and Sabouraud glucose agar (SGA) (Stocker-Wörgötter 2002), confirming the recipes of these media according to Crous et al. (2019a). Colour of colonies were determined based on Kornerup & Wanscher (1978).
DNA extraction, PCR amplification and sequencing
Perithecia were sampled from specimen TNS-L-131533, mycelium from specimen TNS-L-131534, and mycelia from a culture derived from specimen TNS-L-131535. For DNA extraction, fungal tissues were suspended in 20 μL of DNA extraction buffer [10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 0.01 % sodium dodecyl sulfate (SDS), 0.01 % Proteinase K], incubated at 37 °C for 60 min, and denatured 90 °C for 10 min; 30 μL of sterile distilled water (SDW) added to the tubes, and stored in a freezer at -20 °C.
Partial sequences of the nuc rDNA ITS1-5.8S-ITS2 (ITS), large subunit (LSU) nuc rDNA regions, elongation factor 1-alpha (tef1), and RNA polymerase II second largest subunit (rpb2) were amplified as these regions are frequently used for phylogenetic analyses of Nectriaceae (Lombard et al. 2015, Crous et al. 2019b, 2020, 2021). The ITS region was amplified using the primers ITS5 and ITS4 (White et al. 1990), LSU rDNA using primers LIC24 (Miadlikowska & Lutzoni 2000) and LR7 (Vilgalys & Hester 1990) or LR0R (Rehner & Samuels 1994) and LR6 (Vilgalys & Hester 1990), tef1 using EF1-983F and EF1-1567R (Rehner & Buckley 2005), and rpb2 using RPB2-5F2 and RPB2-7cR (O’Donnell et al. 2007). PCR was performed in a 15 μL reaction volume containing 1 μL DNA template, 7.5 μL GenRED PCR Mix Plus (Nippon Gene, Tokyo, Japan), 1.5 μL each primer (2 pmol/μL), and 3.5 μL distilled water. The PCR was performed in a TaKaRa PCR Thermal Cycler Dice® Touch (TaKaRa, Shiga, Japan) as follows for the ITS region; 5 min at 95 °C, followed by 40 cycles of 30 s at 94 °C, 30 s at 53 °C, 1 min at 72 °C, and a final step of 8 min at 72 °C. PCR conditions for LSU, tef1 and rpb2 were set according to Frisch et al. (2020), Rehner & Buckley (2005) and O’Donnell et al. (2007), respectively.
PCR products were checked by electrophoresis on a 1.5 % agarose gel stained with Midori Green Direct DNA Stain (Nippon Genetics, Tokyo, Japan) and visualised using WSE-5200 Printgraph 2 M (ATTO, Tokyo, Japan). The PCR products were purified using a FastGeneTM Gel/PCR extraction kit (Nippon Genetics, Tokyo, Japan) and ExoSAP-ITTM (Thermo Fisher Scientific, Massachusetts, USA) following the manufacturer’s instructions.
Sequences were obtained via a DNA sequencing service using Applied Biosystems 3730xl DNA analyzer (Thermo Fisher Scientific, Massachusetts, USA) (Eurofins Genomics, Tokyo, Japan). Data and accession number of the voucher specimen, and the obtained sequences from the International Nucleotide Sequence Database (INSD) are shown in Table 1.
Table 1.
Sources of DNA sequence data used in phylogenetic analyses and comparison of sequence data.
| Species | Strain numbers | Host/Substrate | Collection sites | ITS Accession No. | LSU Accession No. | tef1 Accession No. | rpb2 Accession No. | References |
|---|---|---|---|---|---|---|---|---|
| Calostilbe striispora | CBS 133491 | Erythrina glauca | Trinidad and Tobago | KM231789 | – | – | – | Lombard et al. (2015) |
| Ciliciopodium brevipes | CBS 691.83 | Fagus sylvatica | Netherlands | KM231856 | – | – | – | Lombard et al. (2015) |
| Ciliciopodium hyalinum | CBS 106.13 | Soil | Switzerland | KM231857 | – | – | – | Lombard et al. (2015) |
| Cylindrium aeruginosum | CBS 693.83 | Fagus sylvatica | Netherlands | KM231854 | – | – | – | Lombard et al. (2015) |
| Cylindrium elongatum | CBS 685.83A | Fagus sp. | Netherlands | KM231852 | – | – | – | Lombard et al. (2015) |
| Cylindromonium alloxyli | CPC 38159 | Meliola on leaves of Alloxylon pinnatum | Austria | MW175339 | MW175379 | – | MW173114 | Crous et al. (2020) |
| Cylindromonium eugeniicola | CPC 37170 | Leaf litter of Eugenia capensis | South Africa | NR166338 | – | – | – | Crous et al. (2019b) |
| Cylindromonium everniae | CBS 148255 | Evernia prunasti | Netherlands | NR175231 | OK664736 | – | NR175231 | Crous et al. (2021) |
| Cylindromonium dirinariae sp. nov. | TNS–L–131533 | Dirinaria applanata | Japan | LC731273 | LC731274 | LC731275 | LC744391 | This study |
| TNS–L–131534 | Dirinaria applanata | Japan | LC731276 | LC744402 | LC744396 | LC744390 | This study | |
| TNS–L–131535 | Dirinaria applanata | Japan | LC731277 | LC744401 | LC744395 | LC744392 | This study | |
| Cylindromonium lichenicola | CBS 415.70A | Aerial algae | Netherlands | MH859774 | LC744400 | LC744397 | LC744394 | Vu et al. (2019); This study |
| CBS 303.70 | Alnus sp. | Germany | MH859675 | – | – | – | Vu et al. (2019) | |
| CBS 188.70 | Apothecia of lichen | Germany | MH859549 | LC744399 | LC744398 | LC744393 | Vu et al. (2019); This study | |
| Cylindromonium rhabdosporum | CBS 438.66 | Cladonia furcata | Austria | MH858850 | – | – | – | Vu et al. (2019) |
| Falcocladium multivesiculatum | CBS 120386 | Leaf litter | Brazil | JF831936 | – | – | – | Rungjindamai et al. (unpublished) |
| Falcocladium sphaeropedunculatum | CBS 111292 | Leaf litter | Brazil | JF831938 | – | – | – | Rungjindamai et al. (unpublished) |
| Falcocladium thailandicum | CBS 121717 | Eucalyptus camaldulensis | Thailand | JF831939 | – | – | – | Rungjindamai et al. (unpublished) |
| Hyaloseta nolinae | CBS 109837 | Nolina micrantha | USA | KM231846 | – | – | – | Lombard et al. (2015) |
| Lectera colletotrichoides | CBS 109728 | Medicago sativa | Turkey | KM231851 | – | – | – | Lombard et al. (2015) |
| Nectria balansae | CBS 123351 | Coronila sp. | France | HM484552 | – | – | – | Hirooka et al. (2011) |
| Nectria cinnabarina | CBS 125165 | Aesculus sp. | France | HM484548 | – | – | – | Hirooka et al. (2011) |
| Nectria dacryocarpa | CBS 121.87 | Tree fern | Sulawesi | KM231850 | – | – | – | Lombard et al. (2015) |
| Nectria mariae | CBS 125294 | Buxus sempervirens | France | JF832629 | – | – | – | Hirooka et al. (2012) |
| Nectria nigrescens | CBS 125148 | Wood | USA | HM484707 | – | – | – | Hirooka et al. (2011) |
| Phialoseptomonium eucalypti | CBS 145542 | Leaves of Eucalyptus | Australia | MK876402 | – | – | – | Crous et al. (2020) |
| Pochonia sp. | CBS 634.75 | Arcyria sp. | Netherlands | KM231845 | – | – | – | Lombard et al. (2015) |
| CBS 892.7 | Myxomycete | Netherlands | KM231844 | – | – | – | Lombard et al. (2015) | |
| CBS 401.70 | Myxomycete | Netherlands | KM231843 | – | – | – | Lombard et al. (2015) | |
| Rodentomyces reticulatus | CBS 128675 | Rodent dung | Italy | JF832659 | – | – | – | Hirooka et al. (2012) |
| Sarocladium kiliense | CBS 400.52 | Ficus carica | UK | KM231849 | – | – | – | Lombard et al. (2015) |
| Septofusidium berolinense | CBS 731.70 | – | Germany | KM231841 | – | – | – | Lombard et al. (2015) |
| Stachybotrys chartarum | CBS 129.13 | – | Unknown | KM231858 | MH866145 | – | KM232434 | Lombard et al. (2015) |
| Thyronectria lamyi | CBS 417.89 | Berberis vulgaris | Germany | KM231837 | – | – | – | Lombard et al. (2015) |
| Thyronectria pyrrhochlora | CBS 125131 | Acer campestre | Austria | HM484545 | – | – | – | Hirooka et al. (2011) |
| Thyronectria quercicola | CBS 128976 | Quercus ilex | Spain | JF832624 | – | – | – | Hirooka et al. (2012) |
| Thyronectria sinopica | CBS 462.83 | Hedera helix | Netherlands | HM484542 | – | – | – | Hirooka et al. (2011) |
| Tilachlidium brachiatum | CBS 505.67 | Hypholoma fasciculare | Poland | KM231839 | – | – | – | Lombard et al. (2015) |
| Trichonectria rectipila | CBS 132.87 | – | USA | MH862058 | – | – | – | Vu et al. (2019) |
| Trichonectria setadpressa | J.E.20–13 | Lobariella pallida | France | MT153969 | – | – | – | Flakus et al. (2019) |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands; CPC: Culture collection of Pedro Crous, housed at the Westerdijk Fungal Biodiversity Institute; J.E.: The private herbarium of Javier Etayo, Pamplona.
Phylogenetic analysis and comparison of sequence data
Both newly generated ITS sequences and reference sequences were used in the phylogenetic analysis. Sequence data of loci other than ITS do not exist for some isolates of the genus Cylindromonium. Therefore, only ITS, LSU and rpb2 of four Cylindromonium species were used for the phylogenetic analysis (Table 1). ITS sequence data of species with relatively high identity (> 85 %) in the BLAST search and other hypocrealean fungi in the Nectriaceae were included in the analysis to infer the taxonomic position of the targeted fungi (Table 1). In these analyses, Stachybotrys chartarum (KM231858) was chosen as the outgroup (Lombard et al. 2015). Sequences of each locus (ITS, LSU, tef1 and rpb2) were compared with those of C. lichenicola. All sequences analysed in this study were deposited in the DNA Data Bank of Japan (DDBJ), a member of International Nucleotide Sequence Database Collaboration (INSDC).
Assembling forward and reverse strands of the sequenced loci were carried out with MUSCLE v. 3.6 (Edgar 2004) in MEGA v. 7 (Kumar et al. 2016) to obtain consensus sequences. DNA sequences were aligned using the online version MAFFT v. 7 (Katoh et al. 2019) (https://mafft.cbrc.jp/alignment/server/) with default settings. MEGA v. 7 (Kumar et al. 2016) was used to truncate sequences up to the determined edge of the dataset.
Phylogenetic analyses were performed with Maximum likelihood (ML) using an online version W-IQ-Tree v. 1.6.12 (Trifinopoulos et al. 2016) (http://iqtree.cibiv.univie.ac.at/). All characters were equally weighted, and gaps were treated as missing data. The ML analysis for the ITS region alignment using the TIM2+F+I+G4 model and for a combined alignment of the three loci, ITS, LSU, rpb2 using the TN93+G (for ITS and LSU) and TN93+I (for rpb2) were performed with 1 000 bootstrap replicates. FigTree v. 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) and MEGA X were used for plotting the phylogenetic trees. Sequence alignments were deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S30024).
Inoculation experiments
Symptomless thalli of D. applanata, the host lichen of C. dirinariae, and those of the non-host lichen Parmotrema tinctorum were collected in Tsukuba city, Ibaraki prefecture, Japan and confirmed as non-infected via microscopy. Following this step, the lichen surface was cleaned using an ultrasonic cleaner with 0.005 % Aerosol® OT (a surface-active agent) for 1 min. This step was repeated five times. After this process, samples were rinsed with distilled water. Agar pieces including hyphae of isolates of C. dirinariae and C. lichenicola were inoculated onto the lichen thalli and maintained in 90-mm-diam glass Petri dishes with a sheet of wet filter paper to retain a high humidity at room temperature. Lichens inoculated were misted from above and moistened when they began to dry, every 2 to 3 d. In addition, some lichens were not inoculated and maintained under the above conditions as a control.
RESULTS
Taxonomy
Classification: Nectriaceae, Hypocreales, Sordariomycetes.
Cylindromonium dirinariae Ohmaki & Okane, sp. nov. MycoBank MB 846061. Fig. 1.
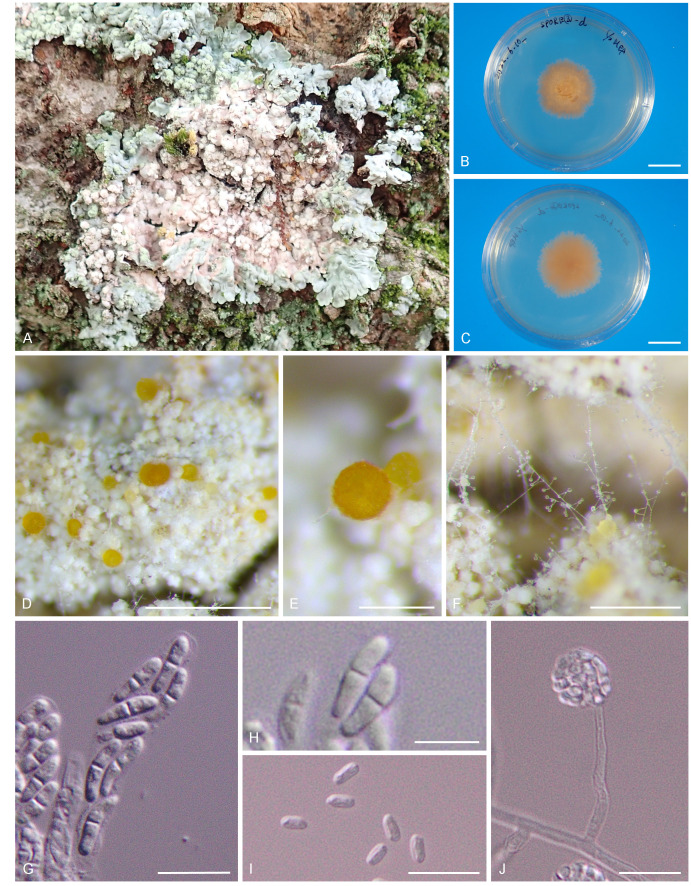
Fig. 1.
Cylindromonium dirinariae. A. C. dirinariae colonizing on Dirinaria applanata. B, C. Colonies on MEA. B. Surface. C. Reverse. D. Growth habit on Dirinaria applanata. E. Perithecium. F. Conidiogenous cells and conidia. G. Asci. H. Ascospores. I. Conidia. J. Phialide and conidial mass. Scale bars: B, C = 3 cm; D = 0.5 mm; E, F = 0.25 mm; G, I, J = 10 μm; H = 5 μm.
Etymology: Name refers to the host genus Dirinaria from which it was isolated.
Diagnosis: Ascomata perithecial, globose, orange; phialides short; conidia aggregated in mucoid packets in the apex of phialides, ellipsoid, aseptate; the species differs from all other Cylindromonium species by its characteristic DNA sequences (ITS, LSU, tef1, rpb2) and from its closest relative C. lichenicola also by its shorter phialides.
Description: Ascomata occur on the upper surface or soralia of the host lichen thallus; perithecial, scattered, globose, 80–100 μm diam, poculiform when dry, pale orange when young but later becoming dark, KOH negative, ascomatal wall layers of textura globosa, 14–18 μm thick. Asci broadly cylindrical to clavate, non-stipitate, (21.2–)24.5 ± 2.3(–29.1) × (3.9–)5.0 ± 0.8(–6.5) μm (n = 12), unitunicate, apex simple, 8-spored. Ascospores biseriate, ellipsoid, hyaline, smooth, medially 1-septate, (5.0–)7.8 ± 1.1(–10.1) × (1.8–)2.6 ± 0.4(–3.7) μm, length/breadth (l/b) = (2.1–)2.6 ± 0.4(–3.9) (n = 40). Mycelium consisting of hyaline, smooth, septate, branched, 2 μm diam hyphae. Conidiogenous cells arising directly from aerial hyphae, hyaline, smooth, subcylindrical, 20–35 μm tall, 2 μm wide at the base, tapering to 1 μm at the apex, phialidic, with non-flared collarette. Conidia solitary, adhering in a slimy mass, hyaline, smooth, aseptate, ellipsoid with obtuse ends, (4.1–)5.9 ± 0.9(–10.3) × (1.5–)2.4 ± 0.4(–3.5) μm, l/b = (1.5–)2.55 ± 0.5(–3.9) (n = 75).
Culture characteristics: Colonies flat, circular or irregular, with moderate aerial mycelium and smooth, lobate margin, reaching 20 mm diam on MEA, PDA, OA, SMA and MYA, 30 mm diam on SGA after 3 wk at 23 °C in darkness. On MEA, PDA, SMA, SGA and MYA surface and reverse strong brownish orange; on OA surface grayish orange.
Host: Dirinaria applanata.
Distribution: Japan.
Typus: Japan, Ibaraki, Tsukuba, Tennodai, Univ. of Tsukuba, 36°06’08”N, 140°06’24”E, lichenicolous on Dirinaria applanata on bark of Zelkova serrata, 1 Aug. 2020, A. Ohmaki, I. Okane, K. Ohmachi, K. Miyazawa & K. Gibu, FAO 005 (holotype TNS-L-131533, culture ex-type NBRC 115852); DDBJ: ITS = LC731273; LSU = LC731274; tef1 = LC731275; rpb2 = LC744391.
Additional materials examined: Japan, Ibaraki, Tsukuba, Tennodai, Univ. of Tsukuba, 36°06’08”N, 140°06’24”E, from Dirinaria applanata on the bark of Zelkova serrata, 1 Aug. 2020, A. Ohmaki, I. Okane, K. Ohmachi, K. Miyazawa & K. Gibu, NBRC 115851 = TNS-L-131534 (FAO 004), DDBJ: ITS = LC731276; LSU = LC744402; tef1 =LC744396; rpb2 =LC744390; 6 Nov. 2020, A. Ohmaki, TNS-L-131536 (FAO 090), in an inoculation experiment with isolates on D. applanata (Ibaraki: Tsukuba, 1 Aug. 2020, A. Ohmaki, I. Okane, K. Ohmachi, K. Miyazawa & K. Gibu, TNS-L-131533); Tsukuba, Tennodai, Univ. of Tsukuba, 36°06’38”N, 140°06’16”E, from Dirinaria applanata on the bark of Zelkova serrata, 15 Mar. 2021, A. Ohmaki, NBRC 115853 = TNS-L-131535 (FAO 006), DDBJ: ITS = LC731277; LSU = LC744401; tef1 = LC744395; rpb2 = LC744392.
Notes: The ex-type strain of C. lichenicola (CBS 425.66) was not available for study, so we conducted morphological observations on other available strains of C. lichenicola (CBS 188.70 and CBS 415.70A) that are similar in terms of their collection sites and hosts. In addition, these strains were also studied when the genus Cylindromonium was established in Crous et al. (2019b).
Morphological features of the C. lichenicola strains (CBS 188.70 and CBS 415.70A) shown in Table 2 correlated well with the original description provided by Gams (1971) [conidia size 5.5–9.8 × 1.5–2.5 μm (l/b = 3.0–4.4), phialide length 30–60 μm, phialide width base 2.0–3.0 μm, apex 0.7–1.5 μm].
Table 2.
Comparison of dimensions (μm) between Cylindromonium dirinariae and C. lichenicola.
| Conidia* | Phialide length* | Phialide width (Base)* | Phialide width (Apex)* | Hyphae width | |
|---|---|---|---|---|---|
| C. dirinariae | (4.1–)5.9 ± 0.9(–10.3) × (1.5–)2.4 ± 0.4(–3.5) | (14.7–)27.4 ± 8.1(–56.7) | (1.7–)2.3 ± 0.3(–3.1) | (1.0–)1.4 ± 0.2(–2.0) | (1.2–)2.0 ± 0.3(–2.6) |
| C. lichenicola | (4.1–)6.8 ± 1.1(–10.0) × (1.5–)2.4 ± 0.5(–3.3) | (33.9–)51.7 ± 7.6(–69.9) | (2.0–)3.2 ± 0.5(–4.0) | (1.1–)1.6 ± 0.3(–2.5) | (1.5–)2.3 ± 0.5(–3.3) |
*Significantly different.
As a result of the t-test, there were significant differences between C. dirinariae and C. lichenicola in conidia, phialide length and width (Table 2). As for phialide length, phialides of C. dirinariae were about half as long as those of C. lichenicola.
Materials examined of Cylindromonium lichenicola: Germany, Probsteierhagen, Schüttbrehm, from unnamed apothecia of lichen on tree bark, Oct. 1996 (living strain CBS 188.70), GenBank: LSU = LC744399; tef1 = LC744398; rpb2 = LC744393. Netherlands, Utrecht, Amelisweerd, from aerial algae on tree bark, Oct. 1968 (living strain CBS 415.70A), GenBank: LSU = LC744400; tef1 = LC744397; rpb2 = LC744394.
Phylogeny
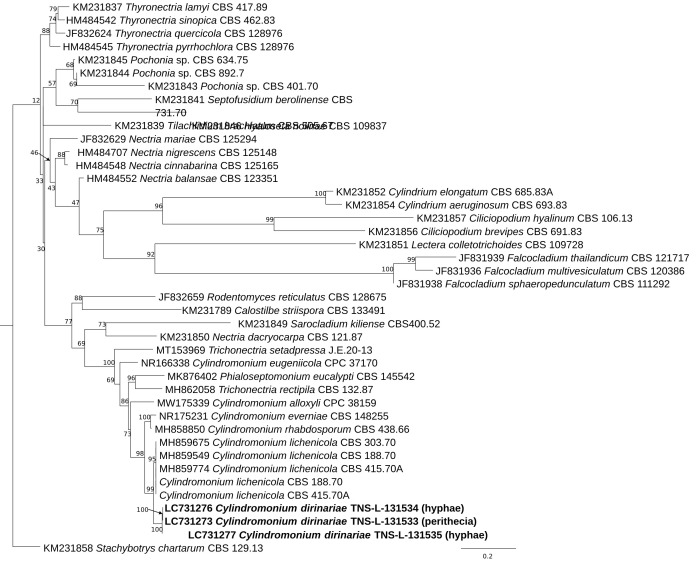
The ITS sequences derived from DNA extracted from a perithecium (specimen TNS-L-131533) and that from mycelia from another specimen (TNS-L-131534) of C. dirinariae were identical. The Phylogenetic analysis based on the ITS region revealed that all three sequences, adding that from mycelia (TNS-L-131535) to the above two, were grouped in a fully supported clade (Fig. 2). The clade was sister to C. lichenicola and linked with five Cylindromonium species including the type species of the genus Cylindromonium, C. everniae. The clade including the Cylindromonium species also included species of Trichonectria (Bionectriaceae) and Phialoseptomonium (Nectriaceae).
Fig. 2.
A phylogenetic tree of Cylindromonium dirinariae and other Nectriaceae and hypocrealean fungi constructed from a maximum-likelihood (ML) analysis based on the ITS sequences. The outgroup is Stachybotrys chartarum. Number at the nodes represents the bootstrap value.
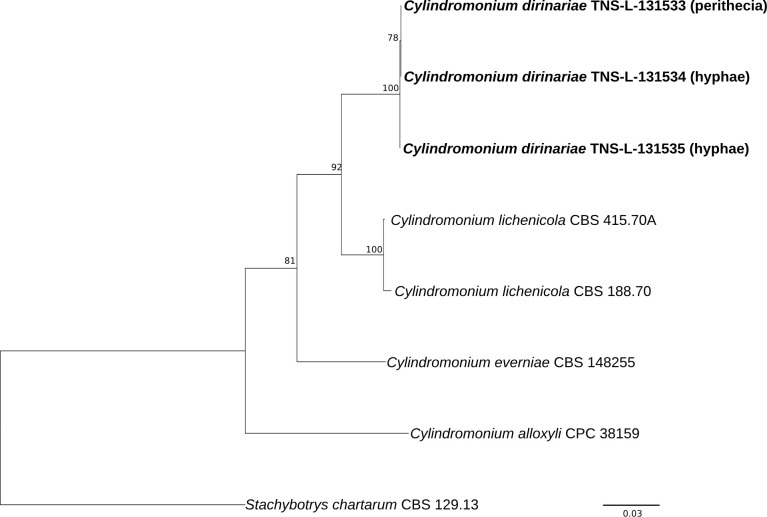
Sequences of LSU, tef1, and rpb2 derived from specimen TNS-L-131533, TNS-L-131534 and TNS-L-131535 of C. dirinariae were identical. The ITS sequence from TNS-L-131535 differed from the other two sequences from TNS-L-131533 and TNS-L-131534 in one site. While the sequences of ITS, LSU, and tef1 of C. lichenicola strains CBS 188.70 and CBS 415.70A were identical, those of rpb2 of the two stains were different in 18 sites. Comparison of C. dirinariae with C. lichenicola (CBS 188.70 and CBS 415.70A) showed that there were 19 gap sites (96.7 % in nucleotide identity) in ITS, 11 gap sites including 2 bp deletions (98.8 %) in LSU, 26 gap sites in tef1 (93.7 %), 153 gap sites (CBS 415.70A vs. C. dirinariae) or 155 gap sites (CBS 188.70 vs C. dirinariae) including 44 bp insertions in rpb2 (84.9–85.1 %). Phylogenetic analysis based on the concatenated sequences of ITS, LSU and rpb2 showed that three sequences of C. dirinariae grouped together with full bootstrap support and clearly segregated from C. lichenicola and other species (Fig. 3).
Fig. 3.
A phylogenetic tree for Cylindromonium dirinariae and other Cylindromonium spp. constructed from a maximum-likelihood (ML) analysis based on concatenated sequence dataset of ITS, LSU rDNA and rpb2. The outgroup is Stachybotrys chartarum. Number above a branch represents the bootstrap value.
Inoculation experiments
The two Cylindromonium spp. studied were able to colonise the inoculated lichens, except for failures due to contamination, extreme dryness or moisture (Fig. 4; Table 3).
Fig. 4.
Lesions on the lichen thalli after inoculation. A. Colony of C. dirinariae on D. applanata. B. Colony of C. dirinariae on P. tinctorum C. Colony of C. lichenicola on D. applanata. D. Colony of C. lichenicola on P. tinctorum. Scale bars = 5 mm.
Table 3.
Colonization rate in inoculation experiments.
| Lichen species inoculated |
Inoculum
|
||
|---|---|---|---|
| Cylindromonium dirinariae | Cylindromonium lichenicola | Control | |
| Dirinaria applanata | 16/20* (80 %)** | 13/15 (87 %) | 3/12 (25 %) |
| Parmotrema tinctorum | 4/4 (100 %) | 2/2 (100 %) | 0/5 (0 %) |
*Number of colonised lichenicolous fungi/the number of inoculated lichens.
**Perithecia produced.
Cylindromonium dirinariae colonised and produced perithecia on the thalli of D. applanata. Colonies reached 5 mm diam about 1 wk post inoculation. Pinkish discolouration was observed in the part of lichen’s thalli covered with hyphae of C. dirinariae. The asexual morph also developed on inoculated thalli. Perithecia developed 2 wk post inoculation. Morphological features of C. dirinariae on the inoculated lichens coincided well with those of C. dirinariae observed in the field.
On the other hand, although C. lichenicola also colonised and produced the asexual morph on D. applanata, no perithecia were produced.
Cylindromonium dirinariae and C. lichenicola colonised and sporulated asexually, but no perithecia were produced on P. tinctorum. The lichen’s thalli were covered with hyphae of the lichenicolous fungi, and discoloured brownish around the point of inoculation. Colonies reached 5 mm diam after 1 wk and 1.5–2 cm diam after 2 wk post inoculation. Lichens used for controls remained healthy. In addition, three single ascospore cultures were obtained of C. dirinariae, and each culture was inoculated onto thalli of D. applanata. As a result, they colonised the lichen and produced perithecia.
DISCUSSION
In the molecular phylogenetic analysis based on ITS sequence data and concatenated sequences of three loci, the three sequences of C. dirinariae clustered in a single clade and positioned as sister to C. lichenicola, which is the most closely related species in morphology and DNA phylogeny (identity = 96.7 %), supported by high bootstrap values. Furthermore, C. dirinariae was related to the group consisting of C. everniae and C. rhabdosporum, supported by high bootstrap values (99 %) (Fig. 2). Comparison of other loci sequences also showed that similarity between C. dirinariae and C. lichenicola were 96.7 % in ITS, 98.8 % in LSU, 93.7 % in tef1, 84.9–85.1 % in rpb2. In addition to phylogeny, C. dirinariae is also morphologically distinct. In our morphological observations and the inoculation experiments, C. dirinariae had 20–35 μm tall phialides and produced perithecia on D. applanata, while C. lichenicola had longer phialides (45–60 μm tall), and failed to produce perithecia on D. applanata and P. tinctorum. Hence, we concluded that C. dirinariae represents a new species. In addition, this is the first report of the sexual morph for the genus Cylindromonium.
The genus Cylindromonium has been reported from Belgium (Gams 1971, Diederich 1989), Germany (Gams 1971, Brackel 2010), Great Britain (Hitch 1995), France (Roux 2012), Luxembourg (Diederich 1989), the Netherlands (Brand et al. 2013, Crous et al. 2021), Czech Republic (Kocourková 2009), India (Joshi et al. 2016), Ukraine (Khodosovtsev et al. 2018), and Australia (Gams 1971, Crous et al. 2020). This is the first report of the genus Cylindromonium from Japan.
Inoculation experiments using single ascospore cultures revealed that this fungus is homothallic. The newly described C. dirinariae was isolated from D. applanata, while the hosts of C. lichenicola are diverse, including the lichens Cladonia, Hypogymnia, Parmelia saxatilis, Tremella cladoniae, lichens overgrowing Stereum species, fungi; Bulgaria inquinans, algal-covered bark, Alnus bark and Betula litter (Gams 1971, Diederich 1989, Hitch 1995, Brackel 2010, Roux 2012, Brand et al. 2013, Tsurykau et al. 2016). Results of our inoculation experiments are suggestive of host specificity; i.e., C. dirinariae was able to form perithecia on its original host lichen, D. applanata, but not on P. tinctorum. Cylindromonium dirinariae could therefore be considered host specific.
Glenn et al. (1997) found that Cylindromonium rhabdosporum (as Acremonium rhabdosporum), occurred on healthy-looking thalli in the field, and perithecia of Nectriopsis rubefaciens (as Nectria rubefaciens) appeared on the same thalli in the closed plates. Although they mentioned the relationship between C. rhabdosporum and N. rubefaciens, they did not confirm that they belong to the same holomorph. Further study is therefore needed to determine the homogeneity between C. rhabdosporum and N. rubefaciens.
Presently there are only a few reports of inoculation experiments of lichenicolous fungi, and they were conducted in the field (Fatma et al. 2019). Glenn et al. (1997) found that continuously moist conditions probably play a pivotal role for lichenicolous fungi to produce perithecia. Inoculation experiments in the moist condition in Petri dishes using axenic cultures of lichenicolous fungi may therefore be a useful technique for studying morphological, physiological and ecological features of lichenicolous fungi. This work has demonstrated the potential of inoculation experiments to investigate the morphological feature of perithecia and host specificity.
Presently the ecology of C. dirinariae remains unclear. Other species of Cylindromonium were reported to be mycophilic or saprophytic (Crous et al. 2019b, 2020). We expect that inoculation experiments will reveal the interaction between lichenicolous fungi and their host lichens which could help us to better understand the ecological role of lichenicolous fungi.
The ascomycete family Nectriaceae includes numerous important plant and human pathogens as well as several facultatively fungicolous or insecticolous species (Rossman 1996, Lombard et al. 2015). Members of Nectriaceae are characterised by uniloculate ascomata that are white, yellow, orange-red or purple, unitunicate asci and phialidic asexual morphs (Rossman et al. 1999, Lombard et al. 2015). In many cases ascomata show a change of colour when mounted in KOH (Lombard et al. 2015). In our study, perithecia of the C. dirinariae were orange in colour and did not react in KOH.
About 400 genera and 2 300 species of lichenicolous fungi are known from lichens, but the actual number of lichenicolous fungal species could be much higher (Diederich et al. 2018). Diederich et al. (2018) estimated 3 000 – 5 000 lichenicolous fungal species will eventually be described based on Hawksworth’s global estimates of fungal diversity (Hawksworth 1991, 2001) and the total number of lichen species (Lücking et al. 2017a, 2017b). Lichenicolous fungi are assumed to be an important source of new species in many groups of fungi, including Nectriaceae.
Acknowledgments
This research was partially financially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 21K19286 (IO).
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
REFERENCES
- Ahmadjian V. (1961). Studies on lichenized fungi. The Bryologist 64: 168–179. [Google Scholar]
- Brackel WV. (2010). Weitere Funde von flechtenbewohnenden Pilzen in Bayern. Beitrag zu einer Checkliste V. Berichte der Bayerischen Botanischen Gesellschaft 80: 5–32. [Google Scholar]
- Brand AM, Sparrius LB, Aptroot A. (2013). 31 nieuwe soorten korstmossen en lichenicole fungi voor Nederland. Buxbaumiella 97: 17–22. [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. (eds) (2019a). Fungal Biodiversity. [Westerdijk Laboratory Manual Series No. 1.]. Utrecht: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ, Lombard L, et al. (2019b). Fungal Planet description sheets: 951–1041. Persoonia 43: 223–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Cowan DA, Maggs-Kölling G, et al. (2020). Fungal Planet description sheets: 1112–1181. Persoonia 45: 251–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Osieck ER, Jurjevi Ž, et al. (2021). Fungal Planet description sheets: 1284–1382. Persoonia 47: 178–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich P. (1989). Les lichens épiphytiques et leurs champignons lichénicoles (macrolichens exceptés) du Luxembourg. Travaux Scientifiques du Musée National D’Histoire Naturelle de Luxembourg 14: 1–268. [Google Scholar]
- Diederich P, Lawrey JD, Ertz D. (2018). The 2018 classification and checklist of lichenicolous fungi, with 2000 nonlichenized, obligately lichenicolous taxa. The Bryologist 121: 340–425. [Google Scholar]
- Edgar RC. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatma MAEW, Abdou MMM, Gehad MEH, et al. (2019). Efficiency of lichenicolous fungi in controlling citrus lichen Xanthoria parietina. Annals of Agricultural Science Moshtohor 57: 507–516. [Google Scholar]
- Flakus A, Etayo J, Miadlikowska J, et al. (2019). Biodiversity assessment of ascomycetes inhabiting Lobariella lichens in Andean cloud forests led to one new family, three new genera and 13 new species of lichenicolous fungi. Plant and Fungal Systematics 64: 283–344. [Google Scholar]
- Frisch A, Grube M, Kashiwadani H, et al. (2018). Arthoniaceae with reddish, K+ purple ascomata in Japan. Phytotaxa 356: 19–33. [Google Scholar]
- Frisch A, Tadome K, Moon KH, et al. (2020). Phylogenetic status of Arthonia phaeophysciae (Arthoniaceae, Ascomycota), a species new to Japan. The Journal of Japanese Botany 95: 133–140. [Google Scholar]
- Gams W. (1971). Cephalosporium-artige Schimmelpilze (Hyphomycetes). Gustav Fischer Verlag, Stuttgart. [Google Scholar]
- Glenn MG, Gomez-Bolea A, Orsi EV. (1997). Effects of thallus damage on interactions of lichens with non-lichenized fungi under natural and laboratory conditions. The Lichenologist 29: 51–65. [Google Scholar]
- Hawksworth DL. (1991). The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycological Research 95: 641–655. [Google Scholar]
- Hawksworth DL. (2001). The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycological Research 105: 1422–1432. [Google Scholar]
- Hirooka Y, Rossman AY, Chaverri P. (2011). A morphological and phylogenetic revision of the Nectria cinnabarina species complex. Studies in Mycology 68: 35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y, Rossman AY, Samuels GJ, et al. (2012). A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Studies in Mycology 71: 1–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitch C. (1995). New, rare and interesting British lichen records. British Lichen Society Bulletin 76: 47–59. [Google Scholar]
- Joshi Y, Falswal A, Tripathi M, et al. (2016). One hundred and five species of lichenicolous biota from India: An updated checklist for the country. Mycosphere 7: 268–294. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodosovtsev A, Darmostuk V, Suija A, et al. (2018). Didymocyrtis trassii sp. nov. and other lichenicolous fungi on Cetraria aculeata. The Lichenologist 50: 529–540. [Google Scholar]
- Kocourková J. (2009). Observations on the genus Neolamya, with the description of the new species N. xanthoparmeliae (Ascomycota, genera incertae sedis). Opuscula Philolichenum 6: 137–148. [Google Scholar]
- Kornerup A, Wanscher JH. (1978). Methuen handbook of colour, 3rd edition. 1–252. Eyre Methuen, London. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 70 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Van der Merwe NA, Groenewald JZ, et al. (2015). Generic concepts in Nectriaceae. Studies in Mycology 80: 189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücking R, Hodkinson BP, Leavitt SD. (2017a). The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota – Approaching one thousand genera. The Bryologist 119: 361–416. [Google Scholar]
- Lücking R, Hodkinson BP, Leavitt SD. (2017b). Corrections and amendments to the 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota. The Bryologist 120: 58–69. [Google Scholar]
- Miadlikowska J, Lutzoni F. (2000). Phylogenetic revision of the genus Peltigera (lichen-forming Ascomycota) based on morphological, chemical, and large subunit nuclear ribosomal DNA data. International Journal of Plant Sciences 161: 925–958. [Google Scholar]
- O’Donnell K, Sarver BA, Brandt M, et al. (2007). Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens-associated US keratitis outbreaks of 2005 and 2006. Journal of Clinical Microbiology 45: 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Kashiwadani H. (2018). Checklist of lichens and allied fungi of Japan. National Museum of Nature and Science Tokyo, Tokyo. [Google Scholar]
- Rambaut A. (2018). FigTree v1.4.4 [accessed 25 October 2020] http://tree.bio.ed.ac.uk/software/figtree/ [Google Scholar]
- Rehner SA, Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98(6): 625–634. [Google Scholar]
- Roux C. (2012). Liste des lichens et champignons lichénicoles de France. Bulletin de la Société Linnéenne de Provence 16: 1–220. [Google Scholar]
- Rossman AY. (1996). Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 88: 1–19. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, et al. (1999). Genera of Bionectriaceae Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Stocker-Wörgötter E. (2002). Resynthesis of photosymbiodemes. In: Protocols in lichenology (Kranner I, Beckett RP, Varma AK. eds). Springer, Berlin: 47-60. [Google Scholar]
- Summerbell RC, Gueidan C, Schroers HJ, et al. (2011). Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Studies in Mycology 68: 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadome K, Ohmura Y. (2021). Two lichenicolous fungi, Illosporium carneum and Ovicuculispora parmeliae (Bionectriaceae, Ascomycota), new to Japan. Bulletin of the National Museum of Nature and Science, Series B (Botany) 47: 21–25. [Google Scholar]
- Tadome K, Ohmura Y. (2022). Six species of montane and subalpine lichenicolous fungi new to Japan. The Journal of Japanese Botany 97: 23–32. [Google Scholar]
- Tadome K, Ohmura Y, Frisch A. (2018). Arthonia molendoi (Arthoniaceae, Ascomycota), a lichenicolous fungus, new to Japan. The Journal of Japanese Botany 93: 407–409. [Google Scholar]
- Tadome K, Ohmura Y, Chaki M. (2022). Spirographa pyramidalis (Spirographaceae, Ascomycota), a lichenicolous fungus, new to Japan. The Journal of Japanese Botany 97: 212–215. [Google Scholar]
- Trifinopoulos J, Nguyen LT, von Haeseler A, et al. (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44: W232–W235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurykau A, Suija A, Heuchert B, et al. (2016). New or otherwise interesting records of lichens and lichenicolous fungi from Belarus II. Herzogia 29: 164–175. [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, De Vries M, et al. (2018). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 91: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego (California): 315–322. [Google Scholar]
- Zhurbenko MP, Ohmura Y. (2018a). Contributions to the knowledge of lichenicolous fungi on Thamnolia. Opuscula Philolichenum 17: 368–373. [Google Scholar]
- Zhurbenko MP, Ohmura Y. (2018b). Perigrapha cetrariae, a new lichenicolous ascomycete on Cetraria from Japan. Folia Cryptogamica Estonica 55: 17–19. [Google Scholar]
- Zhurbenko MP, Ohmura Y. (2019). New and interesting records of lichenicolous fungi from the TNS herbarium: Part I. Opuscula Philolichenum 18: 74–89. [Google Scholar]
- Zhurbenko MP, Ohmura Y. (2020). Contributions to the knowledge of lichenicolous fungi growing on baeomycetoid lichens and Icmadophila, with a key to the species. Lichenologist 52: 437–453. [Google Scholar]
- Zhurbenko MP, Tadome K, Ohmura Y. (2018). Pronectria japonica sp. nov. and a key to the lichenicolous fungi and lichens growing on Ochrolechia. Herzogia 31: 494–504. [Google Scholar]