Abstract
The cloning and expression of a family of five modular-type mannuronan C-5-epimerase genes from Azotobacter vinelandii (algE1 to -5) has previously been reported. The corresponding proteins catalyze the Ca2+-dependent polymer-level epimerization of β-d-mannuronic acid to α-l-guluronic acid (G) in the commercially important polysaccharide alginate. Here we report the identification of three additional structurally similar genes, designated algE6, algE7, and algY. All three genes were sequenced and expressed in Escherichia coli. AlgE6 introduced contiguous stretches of G residues into its substrate (G blocks), while AlgE7 acted as both an epimerase and a lyase. The epimerase activity of AlgE7 leads to formation of alginates with both single G residues and G blocks. AlgY did not display epimerase activity, but a hybrid gene in which the 5′-terminal part was exchanged with the corresponding region in algE4 expressed an active epimerase. Southern blot analysis of genomic A. vinelandii DNA, using the 5′ part of algE2 as a probe, indicated that all hybridization signals originated from algE1 to -5 or the three new genes reported here.
Alginate is a linear copolymer composed of β-d-mannuronic acid (M) and its C-5 epimer, α-l-guluronic acid (G). The M and G residues are organized in blocks of consecutive M residues (M blocks), consecutive G residues (G blocks), or alternating M and G (MG blocks), and the lengths and distributions of the different block types vary among alginates isolated from brown algae or from different bacteria belonging to the genera Azotobacter and Pseudomonas (36, 37). Alginates are the most abundant polysaccharides in brown algae (comprising up to 40% of the dry matter), and their functions are to supply strength and flexibility to the algal tissues (38). The bacterium Azotobacter vinelandii produces alginate both as a vegetative state capsule and as an integrated part of a particular resting stage form (cyst) of this organism (31). The opportunistic pathogen Pseudomonas aeruginosa produces alginate as a capsule-like exopolysaccharide during infection of the lungs of cystic fibrosis patients (12, 23). Alginates from brown algae and A. vinelandii have M, G, and MG blocks (29, 36, 37), while alginates from P. aeruginosa and other Pseudomonas species do not contain G blocks (34, 36). In contrast to the alginates produced by brown algae, bacterial alginates are partially O-acetylated at O-2 and/or O-3 on mannuronic acid residues (36).
The relative amount and distribution of G residues determine most of the physicochemical properties of the polymer. Alginates with G blocks can form gels by reversible cross-linking with divalent cations such as Ca2+, Ba2+, and Sr2+ (41), and the gelling and viscosifying properties of alginate are utilized in pharmaceutical, food, textile, and paper industries (26). In addition, alginate has a very interesting potential in a variety of biotechnological applications and in biomedicine. Alginate rich in M blocks stimulates cytokine production (27) and has a much higher antitumor activity than alginates with a high fraction of G blocks (14). G-rich alginates can be used for encapsulation of cells and enzymes (35), and Langerhans islets immobilized in alginates rich in G have been evaluated as a potential treatment for type 1 diabetes (39, 40).
Both in brown algae and in alginate-producing bacteria, the polymer is first synthesized as mannuronan, and the enzyme mannuronan C-5-epimerase catalyzes the epimerization of M to G at the polymer level (7, 12, 21, 22). Ertesvåg et al. (7) have previously reported the cloning and expression of five genes encoding a family of Ca2+-dependent epimerases in A. vinelandii (algE1 to -5). The deduced AlgE protein sequences consist of two types of structural modules, designated A (385 amino acids each; one or two copies) and R (155 amino acids each; one to seven copies), and each R module contains four to six nine-amino-acid-long repeated sequences corresponding to putative Ca2+-binding motifs. The molecular masses of AlgE1 to -5 vary from 57.7 (AlgE4) to 191 kDa (AlgE3), depending on the number of A and R modules in the proteins. Four of the epimerase genes are clustered in the chromosome (algE1 to -4), while algE5 is located in another part of the A. vinelandii genome. Nuclear magnetic resonance (NMR) spectroscopy analyses demonstrate that the reaction products at least of AlgE2 and AlgE4 differ with respect to sequence distributions of M and G residues. AlgE2 leads to formation of mainly G blocks, while AlgE4 forms predominantly alginates with MG blocks.
The A. vinelandii chromosome also encodes a Ca2+-independent mannuronan C-5-epimerase, designated AlgG (30). Sequence alignments demonstrate that algG does not belong to the algE gene family but shares 66% sequence identity to a mannuronan C-5-epimerase gene (also designated algG) from P. aeruginosa (12). The algG gene in P. aeruginosa is localized in a cluster of alg genes encoding enzymes involved in alginate biosynthesis, and sequence analysis of genomic DNA flanking algG in A. vinelandii suggests that this gene also is part of an alg gene cluster organized as in P. aeruginosa (30).
Southern blot analysis of genomic A. vinelandii DNA using the 5′-terminal 800 bp in the A sequence of algE2 as the probe (A probe) demonstrated that the chromosome probably encodes more A-like sequences than are present in algE1 to -5 (7). In this report, we show that the A. vinelandii genome encodes two additional mannuronan C-5-epimerase genes, designated algE6 and algE7, and also a third highly related gene apparently not encoding an active epimerase.
MATERIALS AND METHODS
Bacterial strains, phages, and plasmids.
Strains, phages, and plasmids are listed in Table 1.
TABLE 1.
Bacterial strains, phages, and plasmids used
| Strain, phage, or plasmid | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) | 45 |
| SURE | sbcC recB recJΔ(mcrCB-hsdSMR-mrr) endA1 gyrA96 Tcr Kmr | 18 |
| NM538 | supF hsdR trpR lacY | 13 |
| A. vinelandii E | 21 | |
| P. aeruginosa 8830 | 3 | |
| Phages | ||
| EPn | Recombinant EMBL3 phages isolated from an A. vinelandii gene library; identified by hybridization against the A probe | This study |
| Plasmids | ||
| pLITMUS28 | Apr, ColE1 replicon; cloning vector | New England Biolabs |
| pLITMUS29 | Apr, ColE1 replicon; cloning vector | New England Biolabs |
| pTrc99A | Apr, ColE1 replicon; expression vector | 1 |
| pSelect-1 | Tcr, mutagenesis vector | Promega |
| pBG2 | Derivative of pLITMUS28 in which a 6.0-kb BglII-HindIII DNA fragment from EP1 was subcloned into the corresponding sites of pLITMUS28 | This study |
| pBG5 | Derivative of pLITMUS28 in which a 3.0-kb BglII DNA fragment (one site originate from the λ vector) from EP35 was subcloned into the corresponding site of pLITMUS28 | This study |
| pBG6 | Derivative of pLITMUS28 in which a 2.9-kb KpnI DNA fragment from EP35 was subcloned into the corresponding site of pLITMUS28 | This study |
| pBG18 | Derivative of pLITMUS29 in which a 7.8-kb BglII-EcoRI DNA fragment from EP35 was subcloned into the corresponding sites of pLITMUS29 | This study |
| pBG19 | Derivative of pLITMUS28 in which a 3.5-kb NcoI DNA fragment from pBG2 was subcloned into the corresponding site of pLITMUS28 | This study |
| pBG21 | Derivative of pLITMUS29 in which a 3.5-kb XhoI-AvrII (restriction sites in the polylinker) DNA fragment from pBG19 was subcloned into the corresponding sites of pLITMUS29 | This study |
| pBG23 | Derivative of pTrc99A in which a 2.6-kb NcoI-XmaI DNA fragment from EP35 was subcloned into the corresponding sites of pTrc99A | This study |
| pBG24 | Derivative of pTrc99A in which a 1.0-kb NcoI-XmaI DNA fragment from pBG18 was subcloned into the corresponding sites of pTrc99A. The NcoI site was generated by using PCR on pBG18 | This study |
| pBG25 | Derivative of pTrc99A in which a 1.9-kb NcoI-XmaI DNA fragment from pBG23 was subcloned into the corresponding sites of pTrc99A. The NcoI site was generated by using PCR on pBG23 | This study |
| pBG27 | Derivative of pBG25 in which a 0.9-kb XmaI-HindIII DNA fragment from pBG18 was subcloned into the corresponding sites of pBG25. The HindIII site was generated by using PCR on pBG18 | This study |
| pBG28 | Derivative of pLITMUS29 in which a 2.7-kb KpnI-BsaW1 DNA fragment from pBG6 was subcloned into pLITMUS29 cleaved by KpnI and AgeI | This study |
| pBG29 | Derivative of pBG24 in which a 1.8-kb XmaI (restriction site in algE6)-HindIII (restriction site in the vector) DNA fragment from pBG28 was subcloned into the corresponding sites of pBG24 | This study |
| pBG31 | Derivative of pSelect-1 in which a 3.5-kb XbaI-HindIII DNA fragment from pBG21 was subcloned into the corresponding sites of pSelect-1 | This study |
| pBG32 | Derivative of pBG31 in which an NcoI site was introduced in the insert by site-specific mutagenesis | This study |
| pBG33 | Derivative of pTrc99A in which a 2.1-kb NcoI-SalI DNA fragment from pBG32 was subcloned into the corresponding sites of Trc99A | This study |
| pHH4 | Derivative of pTrc99A in which a 1.8-kb NcoI-XmaI DNA fragment encoding AlgE4 was subcloned into the corresponding sites of pTrc99A | 7 |
| pBG36 | A 1.5-kb BstEII DNA fragment (one site originate from the vector) from pHH4 ligated to a 4.8-kb BstEII DNA fragment from pBG33 | This study |
| pBG41 | A 1.5-kb BstEII DNA fragment (one site originate from the vector) from pBG33 ligated to a 4.5-kb BstEII DNA fragment from pHH4 | This study |
Growth of bacteria and phages.
A. vinelandii was grown at 30°C with shaking in nitrogen-free medium (9.8 mM K2HPO4-KH2PO4, 0.8 mM MgSO4 · 7H2O, 3.4 mM NaCl, 8.7 μM Na2MoO4 · 2H2O, 54 μM FeSO4 · 7H2O, 1% sucrose). Escherichia coli cells were for most purposes grown in L broth or on L agar at 37°C (32). For all activity measurements, the cells were grown in threefold-concentrated L broth. When relevant, the E. coli media were supplemented with 0.2 mg of ampicillin per ml (unless otherwise stated). When the cells were to be used for growth of phages, the L broth was supplemented with 10 mM MgSO4 and 0.2% maltose.
Standard recombinant DNA technology.
Restriction endonuclease digestions, ligations, and agarose gel electrophoresis were performed in accordance with standard protocols (32). Transformations were performed as described by Chung et al. (2). Genomic DNA from A. vinelandii was isolated by using a genomic DNA kit from Qiagen. Plasmid isolations were performed by the use of a plasmid midi kit from Qiagen (for sequencing) or a Wizard miniprep kit from Promega. DNA sequencing was performed by using cycle sequencing with an Amply Taq kit on an Applied Biosystems model 373 automatic sequencer. The genes were sequenced on both strands by the Biotechnology Centre, University of Oslo, and at Medigene, Martinsried, Germany.
Screening of an A. vinelandii gene library and Southern blot analyses.
An EMBL3 gene library prepared from A. vinelandii DNA (5) was plated on E. coli NM538 on L agar (32). The overlaying agar contained 0.7% agarose, 0.2% maltose, and 10 mM MgSO4. A total of 6,000 phages were examined in the screening procedure. The Dig system (Biochemica, Boehringer Mannheim) was used to label the A probe (random-primed DNA labeling), and gene library screening and Southern blot analyses were performed as specified by the manufacturer.
Protein sequence comparisons and relationships.
Protein alignments were done with the MACAW program (33), and phylogenetic analyses were done with the PHYLIP program package, version 3.5c (9). Protein distance matrices were calculated with the Protdist program, using a Dayhoff PAM matrix (4). The Fitch program rooted with AlgE7A was used to create the unrooted tree for the A modules, while the Neighbour program rooted with AlgE7R2 was used to create the unrooted tree for the R modules (11). The Drawgram program was used to plot the phenograms.
Cloning of algE6, algE7, algY, and the hybrid genes algE4-algY and algY-algE4 into expression vectors.
The open reading frame encoding AlgE6 was cloned into pTrc99A by a two-step protocol. In step 1, the 1.0-kb 5′ end of algE6 was PCR amplified from pBG18 (Fig. 1A). PCR primer 1 (5′ GAAGCGGAGCCATGGATTACAACG 3′) was constructed to change two of the bases proximal and upstream of the ATG start codon from AT to CC (underlined), introducing a new NcoI site. Primer 2 was a vector primer for pLITMUS28 (5′ CGCCAGGGTTTTCCCAGTCACGAC 3′ (New England Biolabs). The PCR fragment was then ligated into pTrc99A, generating pBG24. In step 2, a 1.8-kb DNA fragment from pBG28 containing the 3′ part of algE6 was ligated into pBG24, generating pBG29.
FIG. 1.
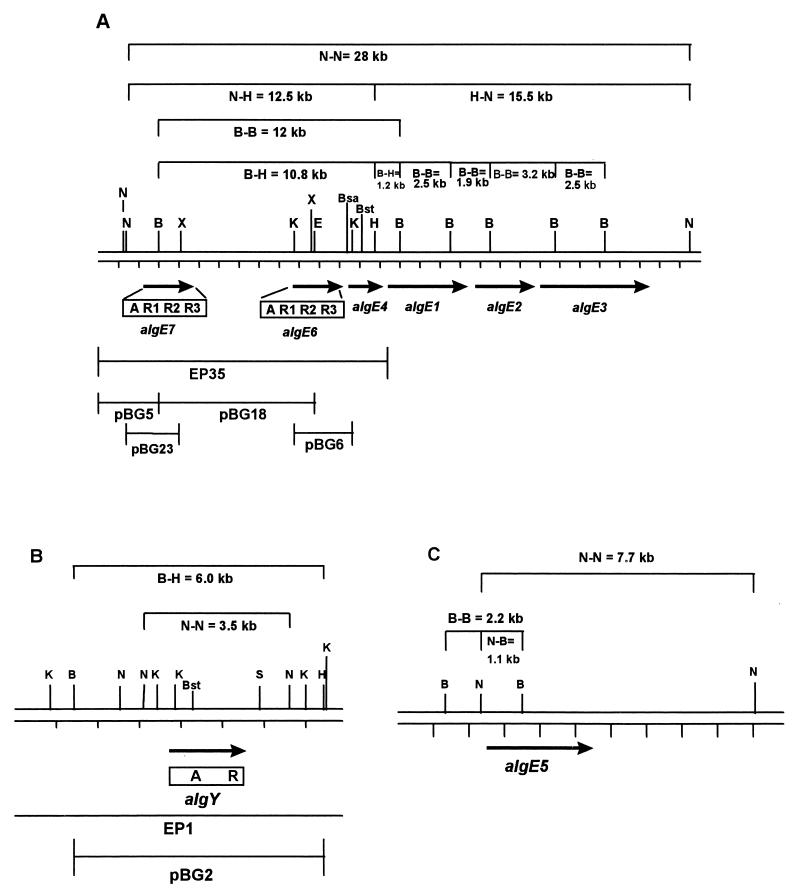
Physical map of genomic A. vinelandii DNA showing the chromosomal locations of algE1 to -4, algE6 and algE7 (A), algY (B), and algE5 (C). The genes and their direction of transcription are indicated by filled arrows. The modular structure of the deduced AlgE6, AlgE7, and AlgY proteins are illustrated below the arrows. The inserts in the different plasmids and phages are presented as lines. Restriction sites: BglII (B), BsaWI (Bsa), BstEII (Bst), EcoRI (E), HindIII (H), KpnI (K), NcoI (N), SalI (S), and XmaI (X). For BsaWI, EcoRI, KpnI, XmaI (A), SalI (B), and BstEII (A and B), only the restriction sites used in the different cloning steps are shown (see Materials and Methods and Table 1). Note that only part of the insert in EP1 is shown.
The open reading frame encoding AlgE7 was cloned into pTrc99A by a two-step PCR protocol. In step 1, primers 3 and 4 were used on pBG23 (Fig. 1A) to PCR amplify the 5′ 1.9-kb part of algE7. Primer 3 served to change two of the bases proximal and upstream of the ATG start codon from AG to CC (underlined) in order to generate a new NcoI site (5′ AGCGAAGCCCATGGAATACAACG 3′). Primer 4 (5′ CACACTACCATCGGCGCT ACG 3′) is a vector primer for pTrc99A. The resulting PCR product was ligated into pTrc99A, generating pBG25. In step 2, PCR was used on pBG18 (Fig. 1A) to amplify the 3′ 0.9 kb of algE7 by using the vector primer 5′ AGCGGATAACAATTTCACACAGGA 3′ (New England Biolabs) for pLITMUS28 and primer 5 (5′ TCCGAAGCTTGCCCGAATGAAACGATCC 3′; the HindIII site is underlined), which generates a HindIII site downstream of the 3′ end in algE7. This PCR product was ligated into pBG25, generating pBG27.
The open reading frame encoding AlgY was cloned into pTrc99A as a 2.1-kb DNA fragment from pBG32 (generating pBG33) by using site-specific mutagenesis on pBG31 to change two of the bases proximal and upstream of the ATG start codon from AA to CC (introducing a new NcoI site). The site-directed in vitro Altered Sites mutagenesis system was obtained from Promega and used according to the manufacturer’s instructions. The oligonucleotide used to introduce an NcoI site overlapping the start codon was 5′ AAGCGGATCCATGGATTTCAACGT 3′ (the new bases after mutagenesis are underlined).
Two new plasmids, designated pBG36 and pBG41, were constructed by using a common restriction site (BstEII) in algY and algE4 (bp 598 to 604 in algY and bp 599 to 605 in algE4). algE4 was previously cloned into pTrc99A, generating pHH4 (7).
Expression of AlgE6, AlgE7, AlgY, AlgE4-AlgY (encoded by pBG36) and AlgY-AlgE4 (encoded by pBG41) and preparation of crude extracts.
One hundred milliliters of culture medium was inoculated to 1% from an overnight culture of strains SURE(pBG27), SURE(pBG29), SURE(pBG33), JM109(pBG36), and JM109(pBG41) in the same medium. The JM109(pBG36) medium was supplemented with 0.5 mg of ampicillin per ml due to some plasmid loss problems. After 3 h of incubation with shaking, the production of the corresponding enzymes was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) at a concentration of 0.5 mM. The cells were harvested 3 to 4 h after induction, and the optical density at 600 nm was measured. The harvested cells were resuspended in 10 ml of MC buffer [20 mM 3-(N-morpholino)propanesulfonic acid (pH 6.9), 2.2 mM CaCl2] and then disrupted by ultrasonication. The broken cells were centrifuged at 27,000 × g for 30 min, and the supernatants were filtered through a 0.2-μm-pore-size Millipore filter prior to activity measurements and partial purification by fast protein liquid chromatography (FPLC).
Measurements of epimerase activity by radioisotope assays.
5-3H-labeled alginate (specific activity, 144,330 dpm/mg of alginate, 95% mannuronic acid) was prepared by growing P. aeruginosa in a medium containing 5-3H-labeled glucose and used as substrate for the crude cell extracts in the radioisotope assays (30). Epimerase activities were measured in reaction mixtures (total volume of 0.6 ml) containing 0.1 mg of tritiated M-enriched alginate, 10 μl of cell extract, and MC buffer. Incubations were performed at 37°C for 1 h, and the reactions were terminated by adding 15 μl of 5 M NaCl and 0.8 ml of isopropanol. The alginate was precipitated at −80°C for 30 min and then centrifuged at 27,000 × g for 30 min. One milliliter of supernatant was used for determination of released 3H in a scintillation counter. All assays were performed in duplicate.
Partial purification of AlgE6, AlgE7, and AlgE4-AlgY by FPLC and measurements of epimerase activity by NMR spectroscopy.
The filtered crude cell extracts were loaded onto a HiTrap Q Sepharose HP column (Pharmacia) equilibrated with MC buffer. The three enzymes were eluted with a 0 to 1 M NaCl gradient (in MC buffer). AlgE6 was eluted between 0.3 and 0.35 M NaCl, AlgE7 was eluted between 0.35 and 0.45 M NaCl, and AlgE4-AlgY was eluted between 0.37 and 0.42 M NaCl. The purification factors for AlgE6, AlgE7, and AlgE4-AlgY were 4.2, 9.2, and 14.5, respectively. The fractions with epimerase activity were used directly as a source of enzyme for incubation with alginate.
The partially purified proteins were incubated with 1 ml M-enriched unlabeled alginate from P. aeruginosa (7.5-mg/ml stock solution, 95% mannuronic acid; prepared as described for the labeled alginate) in MC buffer, and the reaction mixtures (total volume of 6 ml) were incubated at 37°C for 5, 8, and 18 h (AlgE6), 10 h (AlgE7), and 8 h (AlgE4-AlgY hybrid). The reactions were terminated by chelation of Ca2+ with addition of Na2EDTA (0.5 M, pH 8.0) to 10 mM, and the solutions were dialyzed extensively against distilled water before preparation for NMR spectroscopy (17).
Nucleotide sequence accession numbers.
The algE6, algE7, and algY nucleotide sequence data were deposited in the GenBank database under accession no. AF099799, AF099800, and AF099801, respectively.
RESULTS
Screening of an A. vinelandii gene library and Southern blot analyses of phage DNA.
The A probe was used to screen a phage EMBL3 gene library prepared from A. vinelandii DNA. Forty reproducibly hybridizing plaques were identified, and DNA was prepared from each of the corresponding clones. The DNA preparations were digested with a series of restriction endonucleases selected on the basis of the known sequences of the regions containing algE1 to -5). Southern blot analyses of these digests (using the same probe as described above) indicated that 10 of the 40 clones contained A-hybridizing sequences not belonging to algE1 to -5. Further analyses of the 10 new clones showed that they originated from two separate regions of the A. vinelandii chromosome. One of these regions, represented by six phage clones, were found to be closely linked to the region from algE1 to -4. Further analyses of the DNA in these phages showed that one of them (EP35) covered all of the A-hybridizing signals represented by the group, and the insert in this phage was in addition found to cover algE4. The remaining four phages all overlapped and were found to contain only one A-homologous sequence. Phage EP1 was chosen for further studies of this group.
DNA sequencing and identification of three new putative epimerase genes.
DNA fragments from the insert of phage EP35 were subcloned into the plasmid vector pLITMUS28, and the resulting recombinant plasmids were designated pBG5, pBG6, and pBG18 (Fig. 1A). DNA sequencing of parts of the inserts in these plasmids led to the identification of two new putative epimerase genes, designated algE6 and algE7. The 3′ end of algE6 was found to lie only 276 bp upstream of algE4, while the 3′ end of algE7 was found to lie about 5 kb upstream of the start of algE6.
In phage EP1, only one region hybridized to the A probe, and this region was also subcloned into pLITMUS28, generating plasmid pBG2 (Fig. 1B). Sequencing of the relevant part of the subcloned region led to the identification of yet another putative epimerase gene. For reasons described below, this putative gene was assigned another type of designation, algY.
Amino acid sequence analyses of the deduced proteins encoded by algE6, algE7, and algY.
Inspection of the deduced amino acid sequences of AlgE6 and AlgE7 showed that the A module represented the amino-terminal part of the putative proteins and that the sequences also contained three repeats each of the sequences homologous to the R modules found in AlgE1 to -5 (Fig. 1A). This structure is thus new, since none of the proteins AlgE1 to -5 contain three R modules. A similar analysis of AlgY revealed that the modular structure of this putative protein is AR, as in AlgE4 (Fig. 1B). Alignment of the sequences of the A modules of the three putative proteins (Fig. 2) showed that the percentages of homology were 64 (AlgE6-AlgE7), 65 (AlgE6-AlgY), and 63 (AlgE7-AlgY). Based on this alignment, we constructed a consensus sequence, ConA*, and aligned it against the previously reported consensus sequence (ConA) of the A modules in AlgE1 to -5. The percentage of homology between the two sequences is 72, suggesting that all A sequences have a common evolutionary origin. However, closer inspection of the alignments showed that some local regions were not very similar to those previously reported. This point is most clearly seen for the 11 amino acids double underlined in Fig. 2 (amino acids 117 to 127 in the ConA sequence). All of these residues are 100% conserved in the A modules of AlgE1 to -5 (7). The AlgE6 sequence deviates by only one residue (isoleucine to alanine) from this sequence, but in AlgE7 and AlgY five and seven, respectively, of the residues are different. Three of these nonconserved amino acids are identical in AlgE7 and AlgY (residues 121 to 123 in the ConA sequence). Similarly, amino acids 92 to 102 in ConA are all identical in AlgE1 to -5, and two of these residues are different in AlgE6 (single underlining in Fig. 2). In AlgE7 and AlgY, on the other hand, 6 of the 11 residues are different. Two of these nonconserved amino acids (residues 98 and 99) are identical in AlgE7A and AlgYA, where a serine and an asparagine are replaced with a histidine and an aspartic acid, respectively.
FIG. 2.
Alignment of the A modules in AlgE6, AlgE7, and AlgY. The ConA* sequence represents the consensus sequence (more than 50% identity) derived from AlgE6A, AlgE7A, and AlgYA; the ConA sequence represents the consensus sequence (more than 50% identity) derived from the A modules in AlgE1 to -5. Open spaces indicate no identity, and dashes indicate gaps. Single dots indicate that the corresponding amino acid is the same as in ConA*, while double dots represent amino acid identity between ConA* and ConA. Each number to the right indicates the position (relative to the N-terminal end of the deduced protein) of the terminal residue in that line.
The R modules of AlgE6, AlgE7, and AlgY were also aligned (Fig. 3); as previously reported for AlgE1 to -5, these sequences deviate much more from each other than do the A modules. However, the degree of similarity is clearly sufficient to conclude that the R sequences of the three new genes are closely related to those in AlgE1 to -5. The putative Ca2+-binding motifs found to be repeated four to six times N terminally in the R modules of AlgE1 to -5 are also found in the modules reported here. Interestingly, such a motif is repeated seven times in the middle R module of AlgE7. The deduced consensus sequence (ConR*) of the R modules of the three new genes shows, as expected, very significant homology to that previously reported for AlgE1 to -5 (ConR). The terminal amino acids of all deduced enzymes were given the specific designation S in AlgE1 to -5 because they seemed to represent extra residues relative to all the other R modules (7), and the same appears to be true for the new sequences reported here. Moreover, the deduced R modules of AlgE6 are separated by short (10 residues) amino acid sequences, six of which are the same (Fig. 3). Note also that three of these six residues are prolines.
FIG. 3.
Alignment of the R modules in AlgE6, AlgE7, and AlgY. Notation is as in Fig. 2 except that open spaces indicate 50% identity or less. The vertical lines at the top indicate the start of each nonameric putative Ca2+-binding motif, while the broken vertical lines near the termini represent the end of the R modules (left) and the start of the S motifs (right). The S motif refers to the extra terminal amino acids in AlgE6, AlgE7, and AlgY, which are not a formal part of the R modules in the proteins (7). The ConS* sequence represents the consensus sequence (50% identity or more) derived from the S motifs in AlgE6A, AlgE7A and AlgYA; the ConS sequence represents the consensus sequence (more than 50% identity) derived from AlgE1S to -E5S.
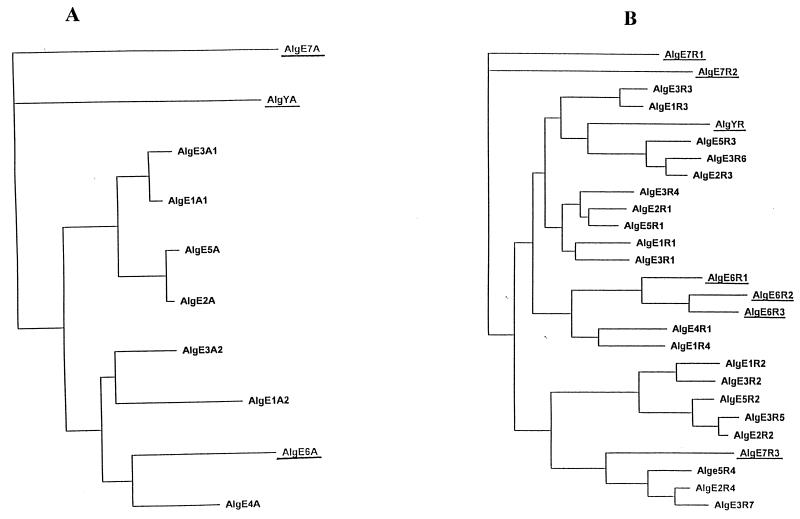
Based on all available sequence data, we constructed an evolutionary tree of both the A (Fig. 4A) and R (Fig. 4B) modules. According to this analysis, the A modules in AlgE7 and AlgY are the two sequences that are most diverse relative to all the other A sequences. The AlgE6 A module, on the other hand, groups together with several others and appears to be most closely related to the A module of AlgE4. This analysis therefore correlates well with the observations of sequence deviations in local regions.
FIG. 4.
Phenograms constructed on the basis of alignments of the A modules in AlgE1 to -7 and AlgY (A) and the R modules in AlgE1 to -7 and AlgY (B). The A and R modules in AlgE6, AlgE7, and AlgY are underlined.
The relationships among the R modules are more complex than for the A modules, but in general, strongly related R modules tend to be in the same position relative to the A modules, as previously reported (7). However, the three R modules in AlgE6 form a separate subgroup, and two of the R modules in AlgE7 (R1 and R2) are much less related to all other modules according to this analysis.
The sequences upstream of algE1 to -5 (M1 to M5) show significant sequence similarity but do not contain any obvious promoter sites of the E. coli ς70 type (7). Some of the conserved regions in M1 to M5 are also present upstream of algE6 and algE7 (M6 and M7), while the upstream region of algY (MY) was very different from all of the other corresponding regions. No sequences putatively involved in binding of prokaryotic sigma factors could be identified in M6, M7, and MY.
Expression analyses and measurements of epimerase activities.
Some of the bases 5′ to the putative start ATG of algE6, algE7, and algY were substituted in order to generate NcoI restriction endonuclease sites facilitating insertion of the genes at the ATG start site of the expression vector pTrc99A. The corresponding plasmids, designated pBG29 (algE6), pBG27 (algE7), and pBG33 (algY), were then transformed into E. coli SURE, and cultures of the corresponding transformants were used for analyses of epimerase expression from the trc promoter. The results of these experiments showed that IPTG-induced cells containing pBG29 and pBG27 expressed activities consistent with the expression of mannuronan C-5-epimerases. However, no activity was detected in extracts prepared from cells containing pBG33 (Table 2). This latter result was quite surprising since it is the only example among eight of a gene containing A and R modules that do not express epimerase activity in E. coli.
TABLE 2.
Activities of AlgE6, AlgE7, AlgY, AlgE4-AlgY, and AlgY-AlgE4 expressed in E. coli
| Enzyme | 3H dpm released/OD600 unit of cell culturea |
|---|---|
| AlgE6 | 20,200 |
| AlgE7 | 30,600 |
| AlgY | 0 |
| AlgE4-AlgY | 17,300 |
| AlgY-AlgE4 | 0 |
Observed blank values (no enzyme added) were in the range 40 to 60 dpm and have been subtracted. OD, optical density at 600 nm.
As described above (Fig. 2), local stretches of amino acid sequences in the N-terminal half of AlgY (and AlgE7) are quite different from the corresponding sequences in AlgE1 to -6. One possible interpretation of this is that AlgY throughout evolution has developed some function other than epimerization, explaining the observed lack of epimerase activity after expression in E. coli. To analyze this hypothesis further, we substituted the 5′ part (encoding the first 200 amino acids) of algY with the corresponding part of algE4, generating plasmid pBG36 (encoding the hybrid protein AlgE4-AlgY). This experiment was facilitated by the presence of a common BstEII site in the same position (according to the alignments) in the two genes. We also constructed the reciprocal plasmid, pBG41 (encoding the hybrid protein AlgY-AlgE4), in which the corresponding 3′-terminal part of algY (encoding the terminal 337 amino acids) was substituted with that of algE4. Plasmids pBG36 and pBG41 were transformed into E. coli JM109, and the corresponding cell extracts were analyzed with respect to epimerase activity after IPTG induction (Table 2). These experiments showed that pBG36 expressed strong epimerase activity, while no activity was detected from pBG41. We therefore conclude that one or more amino acid residues encoded by the sequence upstream of the BstEII restriction site in algY is responsible for the lack of epimerase activity.
Analysis of reaction products by NMR spectroscopy.
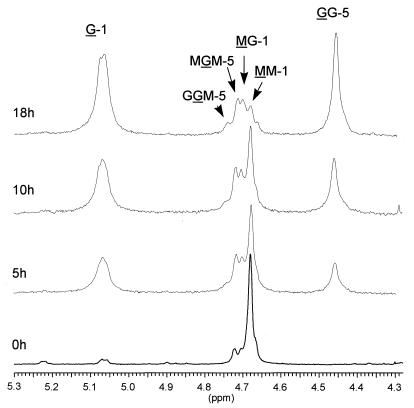
The recombinant proteins expressed by plasmids pBG29 (AlgE6), pBG27 (AlgE7), and pBG36 (active AlgE4-AlgY hybrid) were partially purified by FPLC and incubated with M-rich alginate, and the reaction products were finally analyzed by NMR spectroscopy. The data confirmed that AlgE6 is an epimerase and that it introduces G blocks into its substrate (Fig. 5 and Table 3). This enzyme is therefore functionally related to AlgE2 (7). The NMR data show that within 18 h the M-rich substrate, initially devoid of G blocks, is converted to a polymer with an average number of G residues in the G blocks, NG > 1 ≈ 15. This finding signifies a polymer with high cooperative binding capacity for calcium ions, and with a predicted strong gel-forming capacity.
FIG. 5.
1H NMR spectroscopy monitoring the action of AlgE6 on an alginate with molar fractions of 0.95 and 0 for M and GG, respectively. The samples were incubated for 5, 10, and 18 h, and the spectra were recorded at 90°C on a Bruker Avance DPX 300 MHz instrument. The samples contained 10 mg of alginate per ml in D2O at pH 6.8.
TABLE 3.
Chemical composition and sequential parameters of alginate epimerized with AlgE6 and AlgE4-AlgY
| Sample | Molar fraction
|
NG > 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| G | M | GG | MG/GM | MM | MGG/GGM | MGM | GGG | ||
| Substrate | 0.05 | 0.95 | <0.05 | 0.05 | 0.90 | <0.05 | 0.05 | <0.05 | 0 |
| AlgE6, 18 h | 0.78 | 0.22 | 0.57 | 0.21 | 0.005 | 0.04 | 0.17 | 0.53 | 15 |
| AlgE4-AlgY, 8 h | 0.36 | 0.64 | <0.05 | 0.35 | 0.29 | <0.05 | 0.35 | <0.05 | 0 |
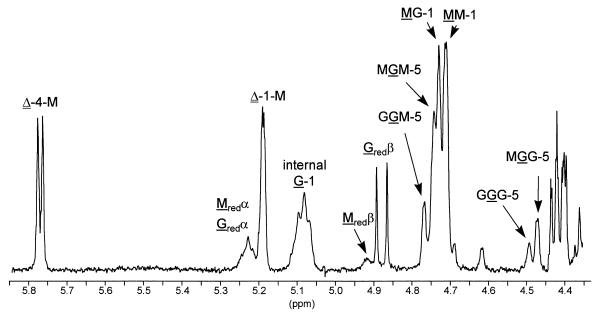
The NMR spectrum of alginate incubated with AlgE7 also confirmed that this protein is an epimerase (Fig. 6). Furthermore, the spectrum shows that the epimerization pattern is different from that of AlgE6, in that AlgE7 introduces both single G residues and G blocks. Even more interesting, however, are the high intensities of the resonance signals from the reducing ends as well as two additional peaks at 5.77 and 5.19 ppm arising from unsaturated protons. These latter protons are due to the formation of unsaturated 4-deoxy-l-erythro-hex-4-enepyranosyluronate residues (Δ) by a β-elimination reaction (19). AlgE7 thus exhibits both epimerase and lyase activities, a property not previously reported for any other enzyme. From the intensities of the resonance signals of the end groups, the average size of the unsaturated oligouronide was estimated (6) to represent a pentasaccharide containing approximately 40% guluronic acid residues, of which 50% are internal G and the other half represent the G residues at the reducing ends. The lyase activity is apparently highly specific since the G residues predominates at the reducing end, while the unsaturated nonreducing end exclusively is Δ-M-.
FIG. 6.
1H NMR spectrum of alginate epimerized by AlgE7 (same alginate substrate as in Fig. 5). In the spectrum, G, M, Gred, Mred, and Δ denote internal G residues, internal M residues, reducing G and M residues and 4-deoxy-l-erythro-hex-4-enepyranosyluronate residues, respectively; each numbers refer to the position of the proton in the pyranosyl ring, and the nonunderlined M and G refer to the neighbor residues. The spectrum was recorded as explained in the legend to Fig. 5.
The NMR spectrum of alginate epimerized by the active AlgE4-AlgY hybrid shows that this enzyme is an epimerase that predominantly produces alginate with MG blocks, similar to AlgE4 (Fig. 7 and Table 3). Whether this epimerization pattern is a direct result of the presence of the N-terminal part of the AlgE4 A module is not known.
FIG. 7.
1H NMR spectrum of alginate epimerized by the AlgE4-AlgY hybrid (same alginate substrate as in Fig. 5). The spectrum was recorded as explained in the legend to Fig. 5.
Southern blot analyses of genomic A. vinelandii DNA.
Since all known algE-type epimerase genes have now been sequenced, we were able to carry out a Southern blot analysis to see if all hybridizing signals could be assigned to algE1 to -7 or algY. Genomic A. vinelandii DNA was digested with BglII, NcoI, and HindIII (or combinations thereof) and subjected to Southern blot analysis using the A fragment as a probe (Fig. 8). All of the hybridization signals obtained in this analysis could be explained by the predicted hybridizing fragments shown in Fig. 1. These results therefore indicate that all algE-type genes present in the A. vinelandii genome have now been identified (see also Discussion).
FIG. 8.
Southern blot analysis of genomic A. vinelandii DNA hybridized against the A probe. The DNA was digested with BglII (lane 1), BglII-NcoI (lane 2), NcoI (lane 3), BglII-HindII (lane 4), and NcoI-HindIII (lane 5). Numbers on the left represent a λ-HindIII standard (in kilobases). Sizes of the hybridizing DNA fragments should be compared to those predicted in Fig. 1. Note that the signal intensities are strongly influenced by the number of A modules in a given fragment and by the extent of homology to the probe.
DISCUSSION
The A. vinelandii algE gene family represents an unusually complex system with respect to both gene structure and functional role, at least for a prokaryotic organism. Multicopy genes (20, 24, 42, 44) and proteins with repeated homologous sequences (10, 16, 25, 43) have previously been reported for some bacteria, but a chromosomal arrangement combining these elements as in the epimerase gene family appears to be rather unique. The reason why A. vinelandii has evolved at least seven structurally and functionally related genes to control the structure of its alginates is not fully understood. However, it seems very likely that the existence of these genes is strongly related to the ability of A. vinelandii to form metabolically dormant cysts, in which alginates of different structures presumably play a crucial role. In the cyst stage, the modified vegetative cells are surrounded by a capsule consisting of a thin outer layer (exine) and a thicker inner layer (intine). Alginates account for 40 and 72% of the exine and intine carbohydrates, respectively, and while the intine material resembles the alginates in the vegetative cell capsule (mostly MM and MG blocks; M/G ratio = 1.8), the alginates in the exine layer are richer in polyguluronic acid (M/G ratio = 0.45), and 42% of the diads are GG (28). If the predicted role of the epimerase system in cyst formation is correct, it also implies that the epimerase gene family can probably be seen as important components in a microbial differentiation process, analogous to the extensively studied spore formation in other bacteria.
If the above hypothesis is correct, it seems probable that each epimerase catalyzes the formation of alginates with different physical properties, as was previously shown for AlgE2 (G-block formation) and AlgE4 (MG-block formation). The NMR analyses of the AlgE6 product indicate that this enzyme is functionally related to AlgE2, but this does not necessarily mean that their reaction products are structurally or functionally equivalent. It could, for instance, be that the lengths of the G blocks and the spacing between them are different, and such differences might affect the properties of the corresponding alginates significantly. In fact, currently available data indicate that AlgE6 is capable of forming alginates with G blocks longer than those formed by AlgE2. It is well established in the literature that the alginate gel strength increases with increasing G-block length (38, 41), and the observations described above therefore also may be significant with respect to the use of in vitro-epimerized alginates in industry and biotechnology.
Interestingly, AlgE7 displays both epimerase and lyase activities. Gacesa (15) has proposed that the reaction mechanisms of these two types of enzymes are very similar. Both enzyme activities includes removal of the proton at C-5 in the uronic acid, but instead of replacing the C-5 proton in the final reaction step (epimerization), the lyase activity catalyzes a β elimination of the 4-O-glycosidic bond, producing unsaturated sugar derivatives at the nonreducing end. According to this proposal, it seems probable that the same active site is involved for both reactions with AlgE7. Whether the lyase reaction can be viewed as an abortive epimerization or as an epimerase-independent reaction is not clear. If the former is true, the apparent specificity for the G-↓-G-M or G-↓-M-M glycosidic linkage provides information about the direction of the epimerase action. A more thorough study of epimerase-lyase coupling is under way in our laboratory. The biological significance, if any, of the AlgE7 lyase activity is not known. It is possible, however, that cyst formation needs a fraction of shorter alginate oligomers and that AlgE7 contributes to this. A role in cyst germination also cannot be excluded.
The inability of algY to encode an active epimerase in E. coli was surprising, as all other enzymes belonging to this family are active after expression in this host. A Western blot analysis (antibody kindly provided by Hilde Kristin Høidal) showing that AlgY is produced in IPTG-induced E. coli cells excluded the possibility that the lack of activity is caused by some expression problems. The significance of this is unknown, but it could be that the enzyme is synthesized in an inactive form in E. coli, that it needs an unknown cofactor, or that it displays some activity other than epimerization. It could, for instance, be that it is a lyase with a substrate specificity not detected in our analyses. Concerning the latter hypothesis, it should be emphasized that some of the nonconserved amino acids are similar in AlgE7A and AlgYA. These particular residues are located in the N-terminal part of the enzyme, which when exchanged with the corresponding part from AlgE4 leads to an active epimerase.
The Southern blot analysis of genomic A. vinelandii DNA indicated that the chromosome does not contain any other genes hybridizing to the A sequence. Recent experiments have shown that the A module is responsible for the epimerization reaction (8), and it is therefore likely that all active AlgE proteins encoded by A. vinelandii have now been identified. The epimerase genes appear to be localized in three separate physical locations, in which algE5 and algY are localized separately. Moreover, algE7 is separated by approximately 5 kb from the main block containing algE1 to -4 and algE6. All of the enzymes therefore clearly do not originate from the same transcript. An operon organization in the block of six genes seems also unlikely, since the length of the region (25 kb) and the spacing between the genes are larger than expected for an operon structure (Fig. 1A and reference 7). By combining transcriptional and Western blot analyses of A. vinelandii cell cultures prepared during vegetative growth and at different stages in the encystment process, it should be possible to approach these problems in the near future.
ACKNOWLEDGMENTS
We thank Wenche Iren Strand for performing the NMR analyses.
This work was supported by grants from the Norwegian Research Council and Pronova Biopolymers AS.
REFERENCES
- 1.Amann E, Ochs B, Abel K-J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 2.Chung C T, Niemala S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darzins A, Chakrabarty A M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984;159:9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dayhoff M O. Atlas of protein sequence and structure. Vol. 5 1979. , Suppl. 3. National Biomedical Research Foundation, Washington, D.C. [Google Scholar]
- 5.Ertesvåg H, Doseth B, Larsen B, Skjåk-Bræk G, Valla S. Cloning and expression of an Azotobacter vinelandii mannuronan C-5-epimerase gene. J Bacteriol. 1994;176:2846–2853. doi: 10.1128/jb.176.10.2846-2853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ertesvåg H, Erlien F, Skjåk-Bræk G, Rehm B, Valla S. Biochemical properties and substrate specificities of a recombinantly produced Azotobacter vinelandii alginate lyase. J Bacteriol. 1998;180:3779–3784. doi: 10.1128/jb.180.15.3779-3784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ertesvåg H, Høidal H K, Hals I K, Rian A, Doseth B, Valla S. A family of modular type mannuronan C-5-epimerase genes controls alginate structure in Azotobacter vinelandii. Mol Microbiol. 1995;16:719–731. doi: 10.1111/j.1365-2958.1995.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 8.Ertesvåg, H., and S. Valla. Unpublished data.
- 9.Felsenstein J. Distance methods for inferring phylogenies: a justification. Evolution. 1984;38:16–24. doi: 10.1111/j.1558-5646.1984.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 10.Finnie C, Zorreguieta A, Hartley N M, Downie J A. Characterization of Rhizobium leguminosarum exopolysaccharide glycanases that are secreted via a type 1 exporter and have a novel heptapeptide repeat motif. J Bacteriol. 1998;180:1691–1699. doi: 10.1128/jb.180.7.1691-1699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitch W M, Margoliash E. Construction of phylogenetic trees. Science. 1967;155:279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- 12.Franklin M J, Chitnis C E, Gacesa P, Sonesson A, White D C, Ohman D E. Pseudomonas aeruginosa AlgG is a polymer level alginate C-5-mannuronan epimerase. J Bacteriol. 1994;176:1821–1830. doi: 10.1128/jb.176.7.1821-1830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frischauf A, Lehrach H, Poustka A, Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983;170:827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- 14.Fujihara M, Nagumo T. The effect of the content of d-mannuronic acid and l-guluronic acid blocks in alginates on antitumor activity. Carbohydr Res. 1992;224:343–347. doi: 10.1016/0008-6215(92)84123-a. [DOI] [PubMed] [Google Scholar]
- 15.Gacesa P. Alginate modifying enzymes. A proposed unified mechanism of action for the lyases and epimerases. FEBS Lett. 1987;212:199–202. [Google Scholar]
- 16.Gerngross U T, Romaniec M P M, Kobayashi T, Huskisson N S, Demain A L. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal SL-protein reveals an unusual degree of homology. Mol Microbiol. 1993;8:325–334. doi: 10.1111/j.1365-2958.1993.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 17.Grasdalen H. High-field, 1H-n.m.r. spectroscopy of alginate: sequential structure and linkage conformations. Carbohydr Res. 1983;118:255–260. [Google Scholar]
- 18.Greener A. E. coli SURE™ strain. Clone ‘uncloneable’ DNA. Strategies (Stratagene) 1990;3:5–6. [Google Scholar]
- 19.Heyrand A, Gey C, Leonard C, Rochas C, Girond S, Kloareg B. NMR spectroscopy analysis of oligoguluronates and oligomannuronates prepared by acid or enzymatic hydrolysis of homopolymeric blocks of alginic acid. Application to the determination of the substrate specificity of Haliotis tuberculata alginate lyase. Carbohydr Res. 1996;289:11–23. doi: 10.1016/0008-6215(96)00060-2. [DOI] [PubMed] [Google Scholar]
- 20.Hinton S M, Slaughter C, Eisner W, Fisher T. The molybden-pterin binding protein is encoded by a multigene family in Clostridium pasteurianum. Gene. 1987;54:211–219. doi: 10.1016/0378-1119(87)90489-6. [DOI] [PubMed] [Google Scholar]
- 21.Larsen B, Haug A. Biosynthesis of alginate. Part 1. Composition and structure of alginate produced by Azotobacter vinelandii. Carbohydr Res. 1971;17:287–296. doi: 10.1016/s0008-6215(00)82536-7. [DOI] [PubMed] [Google Scholar]
- 22.Magdwick J, Haug A, Larsen B. Polymannuronic acid 5-epimerase from the marine alga Pelvetia canaliculata. Acta Chem Scand. 1973;27:3592–3594. doi: 10.3891/acta.chem.scand.27-3592. [DOI] [PubMed] [Google Scholar]
- 23.May T B, Chakrabarty A M. Pseudomonas aeruginosa: genes and enzymes of alginate synthesis. Trends Microbiol. 1994;2:151–157. doi: 10.1016/0966-842x(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 24.McTavish H, Laquier F, Arciero D, Logan M, Mundfrom G, Fuchs J A, Hooper A B. Multiple copies of genes coding for electron transport proteins in the bacterium Nitrosomonas europaea. J Bacteriol. 1993;175:2445–2447. doi: 10.1128/jb.175.8.2445-2447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montamedi H, Cai S J, Elliston K O. Structural organization of a multifunctional polyketide synthase involved in the biosynthesis of the macrolide immunosuppressant FK506. Eur J Biochem. 1997;244:74–80. doi: 10.1111/j.1432-1033.1997.00074.x. [DOI] [PubMed] [Google Scholar]
- 26.Onsøyen E. Commercial applications of alginates. Carbohydr Eur. 1996;14:26–31. [Google Scholar]
- 27.Otterlei M, Østgaard K, Skjåk-Bræk G, Smidsrød O, Soon-Shiong P, Espevik T. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother. 1991;10:286–291. doi: 10.1097/00002371-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Page W J, Sadoff H L. Relationship between calcium and uronic acids in the encystment of Azotobacter vinelandii. J Bacteriol. 1975;122:145–151. doi: 10.1128/jb.122.1.145-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panikkar R, Brasch D J. Composition and block structure of alginates from New Zealand brown seaweeds. Carbohydr Res. 1996;293:119–132. [Google Scholar]
- 30.Rehm B H, Ertesvåg H, Valla S. A new Azotobacter vinelandii mannuronan C-5-epimerase gene (algG) is part of an alg gene cluster physically organized in a manner similar to that in Pseudomonas aeruginosa. J Bacteriol. 1996;178:5884–5889. doi: 10.1128/jb.178.20.5884-5889.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadoff H L. Encystment and germination of Azotobacter vinelandii. Bacteriol Rev. 1975;39:516–539. doi: 10.1128/br.39.4.516-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schuler G D, Altshul S F, Lipman D J. A workbench for multiple alignment construction and analysis. Protein Struct Funct Genet. 1991;9:180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- 34.Sherbrock-Cox V, Russell N J, Gacesa P. The purification and chemical characterisation of the alginate present in extracellular material produced by mucoid strains of Pseudomonas aeruginosa. Carbohydr Res. 1984;135:147–154. doi: 10.1016/0008-6215(84)85012-0. [DOI] [PubMed] [Google Scholar]
- 35.Skjåk-Bræk G, Espevik T. Application of alginate gels in biotechnology and biomedicine. Carbohydr Eur. 1996;14:19–25. [Google Scholar]
- 36.Skjåk-Bræk G, Grasdalen H, Larsen B. Monomer sequence and acetylation pattern in some bacterial alginates. Carbohydr Res. 1986;154:239–250. doi: 10.1016/s0008-6215(00)90036-3. [DOI] [PubMed] [Google Scholar]
- 37.Skjåk-Bræk G, Martinsen A. Applications of some algal polysaccharides in biotechnology. In: Guiry M D, Blunden G, editors. Seaweed resources in Europe: uses and potential. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 219–257. [Google Scholar]
- 38.Smidsrød O, Draget K I. Chemistry and physical properties of alginates. Carbohydr Eur. 1996;14:6–13. [Google Scholar]
- 39.Soon-Shiong P, Feldman E, Nelson R, Heintz R, Yao Q, Yao Z, Zheng T, Merideth N, Skjåk-Bræk G, Espevik T, Smidsrød O, Sandford P. Long-term reversal of diabetes by the injection of immunoprotected islets. Proc Natl Acad Sci USA. 1993;90:5843–5847. doi: 10.1073/pnas.90.12.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soon-Shiong P, Heintz R E, Merideth N, Yao Q X, Yao Z, Zheng T, Murphy M, Moloney M K, Schmehl M, Harris M, Mendez R, Sandford P A. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 41.Stokke B T, Smidsrød O, Bruheim P, Skjåk-Bræk G. Distribution of uronate residues in alginate chains in relation to alginate gelling properties. Macromolecules. 1991;24:4637–4645. [Google Scholar]
- 42.Tamaki S J, Gold S, Robeson M, Manulis S, Keen N T. Structure and organization of the pel genes from Erwinia chrysanthemi EC16. J Bacteriol. 1988;170:3468–3478. doi: 10.1128/jb.170.8.3468-3478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson S A, Wang L L, Sparling P F. Cloning and nucleotide sequence of frpC, a second gene from Neisseria meningitidis encoding a protein similar to RTX cytotoxins. Mol Microbiol. 1993;9:85–96. doi: 10.1111/j.1365-2958.1993.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang S-Z, Chen J-S, Johnson J L. The presence of five nifH-like sequences in Clostridium pasteurianum: sequence divergence and transcription properties. Nucleic Acids Res. 1988;16:439–454. doi: 10.1093/nar/16.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]