ABSTRACT
The emergence of TMexCD1-TOprJ1, a novel transferable resistance-nodulation-division (RND)-type efflux pump conferring resistance to tigecycline, is now a serious public health issue in the world. Here, we found that melatonin synergistically enhanced the antibacterial efficacy of tigecycline against tmexCD1-toprJ1-positive Klebsiella pneumoniae by disrupting the proton driving force and efflux function to promote the accumulation of tigecycline into cells, damaging cell membrane integrity and causing the leakage of cell contents. The synergistic effect was further validated by a murine thigh infection model. The results revealed that the melatonin/tigecycline combination is a potential therapy to combat resistant bacteria carrying the tmexCD1-toprJ1 gene.
KEYWORDS: tigecycline resistance, melatonin/tigecycline combination, synergistic effect, melatonin/tigecycline hybrid
INTRODUCTION
Antimicrobial resistance (AMR) is rapidly becoming a global crisis in public health (1). In particular, the emergence of carbapenem-resistant Enterobacteriaceae (CRE) poses a great threat to human and animal health (2–5). As one of the members of the Enterobacteriaceae, Klebsiella pneumoniae can cause a variety of infectious diseases such as urinary tract infections, bacteremia, pneumonia, and liver abscesses (6, 7). Currently, carbapenem-resistant and hypervirulent K. pneumoniae (hvKP) strains are continually reported, and tigecycline, a semisynthetic derivative of minocycline with a broad spectrum of antimicrobial activity, is particularly important in the defense against infections caused by the above-described strains (8, 9). However, a novel plasmid-harbored resistance-nodulation-division (RND) efflux pump gene cluster, tmexCD1-toprJ1, which confers multidrug resistance (MDR) including that to tigecycline, was identified among Klebsiella pneumoniae isolates from chickens and patients in China recently. It leads to difficulties in treatment options for Klebsiella infections and poses a threat to public health (10).
The development of antimicrobials has been a constant challenge, and only a small number of new antibiotics are currently being introduced to the clinic (11). Therefore, feasible approaches are urgently needed to increase the number of therapeutic options. It is becoming a new consensus strategy that repurposing approved drugs as potential antibiotic adjuvants to greatly enhance the efficacy of antibiotics already in use offers a timely and economical means to combat AMR. Based on this idea, screening efflux pump inhibitors (EPIs) administered along with antibiotics is a substantial and promising approach to fight efflux-mediated resistance strains. Despite ongoing efforts (12, 13), there are no equivalent EPIs approved because of pharmacological and safety reasons.
Melatonin (N-acetyl-5-methoxytryptamine) can transmit the sensation of “darkness” and contribute to circadian oscillation synchronization (14). It is also endowed with other physiological effects such as blood pressure regulation, suppression of oncogenesis, and immunity (15–18). In addition to the pineal gland, melatonin is also produced by possibly all extrapineal organs like skin, retina, kidney, etc., as well as plants and other phototrophic organisms (14, 19, 20). Previous work demonstrated that melatonin was well tolerated and had low toxicity. In spite of multiple beneficial effects of melatonin, its potential application in treating pathogenic bacteria remains to be explored. In 2020, Liu et al. (21) reported that melatonin effectively reverses MCR-mediated colistin resistance both in vivo and in vitro through multiple modes of action. However, it was unknown whether melatonin could enhance the activity of tigecycline. Here, we focused on the potency of melatonin as an antibiotic adjuvant to restore the activity of tigecycline against tmexCD1-toprJ1-bearing bacterial strains. We found that melatonin effectively reverses tmexCD1-toprJ1-mediated tigecycline resistance both in vitro and in the mouse infection model. Our data suggest that the nonantibiotic melatonin has promise as a potent and safe antibiotic adjuvant inhibiting tmexCD1-toprJ1 efflux and restoring the clinical efficacy of tigecycline.
RESULTS
The melatonin/tigecycline combination shows synergistic activity against tmexCD1-toprJ1-carrying bacteria.
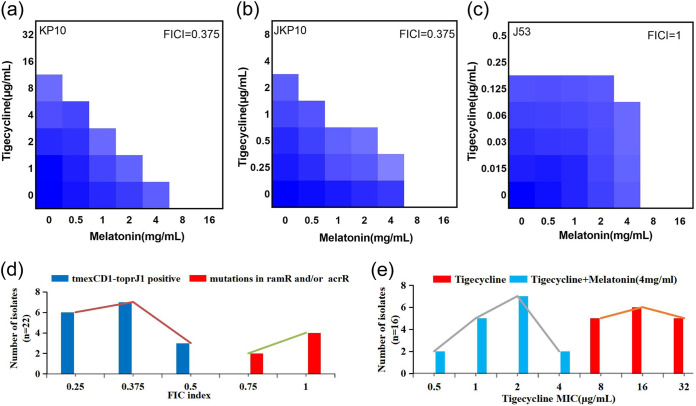
We first initiated checkerboard assays to evaluate the effect of melatonin combined with tigecycline on tmexCD1-toprJ1-carrying K. pneumoniae KP10, its transconjugant JKP10, and the tmexCD1-toprJ1-negative strain Escherichia coli J53. The melatonin/tigecycline combination exhibited a good synergistic effect on tmexCD1-toprJ1-carrying strains KP10 and JKP10 with a fractional inhibitory concentration index (FICI) value of 0.375 (Fig. 1a and b) but, however, a greater-than-additive effect on E. coli J53 without the tmexCD1-toprJ1 gene cluster (FICI = 1) (Fig. 1c), indicating that the combination is specific for tmexCD1-toprJ1 gene-mediated tigecycline resistance. We further verified our hypothesis using 16 clinical virulent K. pneumoniae isolates harboring tmexCD1-toprJ1 and 8 tigecycline-resistant K. pneumoniae isolates with mutations in ramR and acrR. As expected, the FICI values of the 16 tmexCD1-toprJ1-carrying isolates were in the range of 0.25 to 0.5 (Fig. 1d), showing synergistic activity. The MIC50 and MIC90 values decreased from 16 and 32 μg/mL to 2 and 4 μg/mL, respectively, when tigecycline was combined with melatonin (Fig. 1e), while the FICI values ranged from 0.75 to 1 for ramR and/or acrR mutant isolates (Fig. 1d). These results illustrated the selectivity of the synergistic effect of melatonin and tigecycline on tmexCD1-toprJ1-bearing strains.
FIG 1.
Synergistic activity of tigecycline and melatonin against tmexCD1-toprJ1-bearing K. pneumoniae. (a to c) Checkerboard broth microdilution assays between melatonin and tigecycline against KP10, JKP10, and J53. Dark blue regions represent higher cell density and lower inhibition rate of combinational treatment. (d) Distribution of FIC indexes for strains carrying tmexCD1-toprJ and tigecycline-resistant K. pneumoniae strains with mutations of ramR/acrR. The FIC index of tmexCD1-toprJ1-negative bacteria ranged from 0.25 to 0.5, while the FIC index of ramR/acrR mutant strains ranged from 0.75 to 1. Synergy is defined as an FIC index of ≤0.5. (e) Distribution of tigecycline MICs for tmexCD1-toprJ1-bearing strains in the presence/absence of 4 mg/mL melatonin. The MIC50 and MIC90 values were 16 and 32 μg/mL, respectively, when using tigecycline alone; however, both values dropped to 2 μg/mL when tigecycline was combined with 4 mg/mL melatonin.
Melatonin promotes the intracellular accumulation of tigecycline.
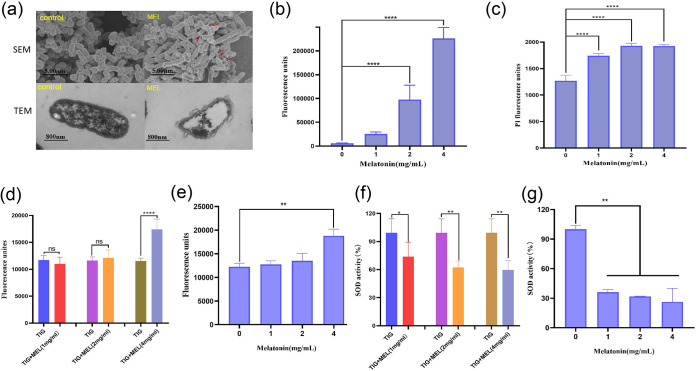
Having shown that melatonin potentiates tigecycline killing of resistant pathogens, we then elucidated the potential mechanisms. First, we determined the effect of melatonin on the proton motive force (PMF), which is pivotal for providing the energy for RND superfamily efflux pumps, by monitoring the fluorescence changes of 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5)]. As shown in Fig. 2a, unlike tigecycline, melatonin significantly (P < 0.01) increased fluorescence in a dose-dependent manner immediately, suggesting that melatonin selectively disrupted the Δψ component of the bacterial PMF. The rhodamine-based assay showed melatonin obviously (P < 0.001) reducing the efflux of rhodamine (Fig. 2b). We further found that the intracellular concentrations of tigecycline detected by high-performance liquid chromatography (HPLC) were markedly increased by nearly 2- to 4-fold when cells were treated with melatonin at 2 mg/mL (1/4 MIC) and 4 mg/mL (1/2 MIC) (Fig. 2c). Considering the finding from a previous report that melatonin could inhibit MCR-1 expression (21), we determined TMexCD1-TOprJ1 expression in the presence of melatonin and tigecycline separately or their combination. Compared with tigecycline and melatonin alone, the expression of tmexC1, tmexD1, and tmprJ1 in KP10 was significantly inhibited by tigecycline (4 μg/mL) combined with melatonin (8 mg/mL) (Fig. 2d). These results showed that melatonin could disrupt the Δψ component of the bacterial PMF; thus, the energy source of RND efflux pumps is blocked, resulting in the accumulation of tigecycline in cells. Additionally, the combination could inhibit the expression of tmexC1, tmexD1, and toprJ1.
FIG 2.
Tigecycline-melatonin combination exerts synergy by dissipating the bacterial proton motive force and inhibiting the expression of TMexCD1-TOprJ1. (a) Membrane potential changes of KP10 upon exposure to melatonin, probed by potentiometric fluorophore DiSC3(5). (b) Function of efflux pump of KP10 carrying tmexCD1-toprJ1 after exposure to various concentrations of melatonin, measured with the fluorescence dye rhodamine. (c) Intracellular accumulation of tigecycline in cells treated with melatonin, determined by HPLC analysis. (d) Relative expression of tmexC1, tmexD1, and toprJ1 in the presence of tigecycline (4 μg/mL), melatonin (2 mg/mL, 4 mg/mL, and 8 mg/mL), and their combinations. All data are expressed as mean ± standard deviation (SD) from three biological replicates, and P values were determined by nonparametric one-way analysis of variance (ANOVA) (*, P < 0.5; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). TIG, tigecycline; MEL, melatonin.
Melatonin disrupts cell membrane permeability and inhibits the oxidative stress and SOS response.
We investigated the morphological changes of cells treated with the sub-MIC of melatonin by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis. The cell outer membrane (OM) was significantly damaged after treatment with melatonin. Compared to the control group, it could be observed that some cells had vacuoles due to the overflow of their contents (Fig. 3a). To further confirm this, we determined the OM and inner membrane permeability of the KP10 strain by use of 1-N-phenyl-naphthylamine (NPN) and propidium iodide (PI), respectively. We found that melatonin (2 and 4 mg/mL) could significantly increase the OM and inner membrane permeability in a dose-dependent manner (Fig. 3b and c). We further determined the reactive oxygen species (ROS) level and superoxide dismutase (SOD) activity in the KP10 strain to validate whether the tigecycline/melatonin combination could cause oxidative damage. The results showed that 4 mg/mL melatonin combined with 4 μg/mL tigecycline could drastically promote the generation of total ROS and decrease SOD activity compared with tigecycline and melatonin separately (P < 0.0001 for the ROS group and P < 0.01 for the SOD group) (Fig. 3d and f), and this oxidative damage was mainly due to melatonin (Fig. 3e and g). The above-described results indicate that melatonin could disrupt cell membrane permeability and inhibit the oxidative stress and SOS response.
FIG 3.
Melatonin disrupts cell membrane permeability and integrity. (a) Morphological changes of KP10 treated with 1/2 MIC (4 mg/mL) of melatonin visualized with SEM and TEM. The destroyed outer membrane is marked with red arrows. (b) That melatonin disrupts the outer membrane of KP10 was found by measuring the fluorescence intensity of 1-N-phenylnaphthylamine (NPN) after exposure to increasing concentrations of melatonin for 1 h. (c) Treatment with different concentrations of melatonin ranging from 0 to 4 mg/mL, determined by propidium iodide (PI) probe. (d and e) Supplementation with 4 mg/mL melatonin significantly increases the production of ROS induced by tigecycline. (f and g) Melatonin supplementation impairs the bacterial oxidative defenses when it is used in combination with tigecycline. SOD activity in cells was measured by biochemical assay. One-way ANOVA among multiple groups was used to calculate P values (ns, not significant; *, P < 0.5; **, P < 0.01; ****, P < 0.0001).
The melatonin/tigecycline combination inhibits bacterial growth and formation of biofilms in vitro.
To further investigate the synergistic bactericidal activity of the melatonin/tigecycline combination, time-dependent killing curves were determined after treatments with melatonin, tigecycline, and a combination of the two. We found that tigecycline (1/4 MIC, 4 μg/mL) had weak bactericidal activities, while melatonin (1/4 MIC, 2 mg/mL) alone showed no bactericidal activities. In contrast, the combination of tigecycline and melatonin displayed obvious bactericidal activities against bacteria, leading to a reduction of bacterial load approximately more than 3 log orders after 24 h of incubation (P < 0.01), compared with the control group (Fig. 4).
FIG 4.
Time-dependent killing and inhibition of biofilm formation by pathogens by the combination of tigecycline and melatonin. KP10 was grown to early exponential phase in LB broth and then treated with PBS, tigecycline (1/4 MIC, 4 μg/mL), or melatonin (1/4 MIC, 2 mg/mL) alone or in combination. The bacterial CFU per milliliter at different time points during 24 h was determined. One-way ANOVA among multiple groups was used to calculate P values (****, P < 0.0001).
Melatonin restores tigecycline activity in vivo.
Given that the combination of tigecycline and melatonin displayed excellent synergistic bactericidal activity against active and dormant pathogens in vitro, we speculated that this combination would reverse TMexCD1-TOprJ1-mediated tigecycline resistance in vivo and thereby restore its clinical efficacy. To test this, we further evaluated the effect of tigecycline or melatonin monotherapy or tigecycline/melatonin combination therapy using a mouse thigh infection model. Encouragingly, the combination therapy displayed more than a 2.5-log-order reduction in CFU compared with tigecycline monotherapy (Fig. 5). These data strongly confirmed that the combination therapy could dramatically rescue infections caused by tmexCD1-toprJ1-positive pathogenic bacteria in vivo.
FIG 5.
Melatonin effectively improves tigecycline efficacy in the mouse thigh infection model. (a) Scheme of the experimental protocols for the mouse infection model. Female mice (n = 8 per group) were infected with a single dose of 1.0 × 106 CFU/mL of bacterial suspension and then treated with PBS, tigecycline (20 mg/kg), melatonin (50 mg/kg), or their combination. After 72 h postinfection, the CFU of KP10 in thighs was counted. (b) Compared with the control and single-drug groups, the combination group showed a marked decrease of the bacterial load in mouse thighs. P values were determined by the Mann-Whitney U test (***, P < 0.001).
DISCUSSION
It has become a consensus that antibiotic adjuvants are considered a promising complement to antibiotics through nonantimicrobial action to synergistically enhance the potency of existing antibiotics against resistant variants. Accumulating studies have shown that an antibiotic-adjuvant combination can positively improve the treatment outcome of multidrug-resistant (MDR) pathogens, including Escherichia coli, K. pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa (22–25). Among these, several are already in clinical use; for example, clavulanic acid, the inhibitor of β-lactamases, has been widely used clinically for decades, effectively enhancing the antibacterial activity of penicillin and cephalosporin antibiotics (26). More combinations are still under study, like a fungal natural product, aspergillomarasmine A (AMA), which recovers meropenem activity by suppressing metallo-β-lactamase (MBL) activity (27). The combination of statins and penicillin was reportedly more effective in controlling methicillin-resistant S. aureus than penicillin monotherapy, as statins can help penicillin penetrate cells to disable PBP2a oligomerization by disrupting the structure of the bacterial membrane (28). An antiprotozoal drug, pentamidine, acting as a membrane disrupter, can potentiate hydrophobic antibiotic activity and overcome acquired colistin resistance (29). It was previously indicated that the hypoglycemic drug metformin and the nonsteroidal anti-inflammatory drug benzydamine restored the activity of tetracycline against pathogens harboring tet(A) and tmexCD1-toprJ1, respectively (30). Melatonin also synergistically increased the activity of colistin against the resistant strains mediated by MCR (21). Thus, antibiotic-nonantibiotic agent combinations represent a promising means to address the widespread emergence of antibiotic-resistant pathogens.
Tigecycline has been considered a last-resort antibiotic to fight multidrug-resistant strains; however, the emergence and rapid global dissemination of plasmid-mediated tigecycline resistance are a great concern. Previously, we characterized efflux tmexCD1-toprJ1-mediated tigecycline resistance in K. pneumoniae (31). Here, we indicate that the neurohormone drug melatonin reversed tmexCD1-toprJ1-mediated tigecycline resistance and remarkably potentiated the antibacterial activity of tigecycline against drug-resistant K. pneumoniae. Mechanistic analysis shows that melatonin can promote the intracellular accumulation of tigecycline by dissipating the ΔΨ component of the bacterial PMF essential for energy synthesis and material transportation. Previous studies found that metformin and benzydamine also damaged the functions of the efflux pump tet(A) and tmexCD1-toprJ1, respectively, by targeting the bacterial PMF (30, 32), indicating that the bacterial PMF may serve as a promising target for the identification of novel antibiotic adjuvants. In this study, we found the combination has no effect on ramR and acrR mutant strains with high-level tigecycline resistance, presumably because the high tigecycline resistance levels are beyond the threshold of the combined action of tigecycline and melatonin.
Our findings suggested that the disruption of the bacterial OM and inner membrane by melatonin was crucial for its potentiation of tigecycline in K. pneumoniae. Collectively, previous studies and our findings showed that melatonin could increase the total ROS level in colistin-resistant E. coli and tigecycline-resistant K. pneumoniae (21), which indicated that ROS damage is involved in their synergistic activity. Importantly, the synergistic effect of this combination has been verified in the mouse thigh infection model. In conclusion, this is the first study to certify the coapplication of melatonin and tigecycline to treat infectious diseases caused by tmexCD1-toprJ1-positive pathogens both in vitro and in animal models. The data of this study and previous findings (21) showed that melatonin exhibits a potent synergistic activity with several antibiotics, in particular, colistin and tigecycline, against resistant pathogens both in vitro and in vivo. Melatonin has been used for a long time with satisfactory safety; however, more studies on the pharmacokinetics of coadministration with tigecycline are warranted to comprehensively explore the potential druggability of melatonin as a novel antibiotic adjuvant, which would greatly benefit treatment of infectious diseases caused by resistant pathogens. In addition, studies have shown that melatonin may have limitations in chronic diseases (33); therefore, further investigation of the efficacy of melatonin-antibiotic combinations in vivo is warranted.
MATERIALS AND METHODS
Bacteria and checkerboard assays.
K. pneumoniae strains used in this study were isolated from chicken cecum, and their characteristics are listed in Table S1 in the supplemental material. Synergistic activity of tigecycline and melatonin was evaluated by checkerboard assays with 2-fold serial dilutions of drugs (8 × 8 matrix). The FIC index (FICI) was calculated according to the following formula: FIC index = FICa + FICb = MICab/MICa + MICba/MICb, where FICa is the FIC index of compound A, FICb is the FIC index of compound B, MICa is the MIC of compound A alone, MICab is the MIC of compound A in combination with compound B, MICb is the MIC of compound B alone, and MICba is the MIC of compound B in combination with compound A. The FICI was interpreted as follows: FICI ≤ 0.5, synergy; FICI < 1, greater than additive; FICI = 1.0, additive; 1 < FICI < 2, less than additive; FICI = 2.0, indifference; and FICI > 2.0, antagonism (34).
Proton motive force assay.
The proton motive force of K. pneumoniae KP10 treated with melatonin was measured with fluorescence probe 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5); 0.5 μM]. Bacteria grown to stationary phase were inoculated into 5 mL LB broth at 37°C with 200-rpm shaking for 8 h. Cultures were washed three times with phosphate-buffered saline (PBS), and then DiSC3(5) was added at a final concentration of 2 μM. The bacterial suspension was incubated with 200-rpm shaking at 37°C for 30 min, and then 180-μL samples were transferred in a black-walled plate and fluorescence was measured immediately in a Synergy H1 microplate reader (BioTek) with an excitation wavelength of 622 nm and emission wavelength of 670 nm. A DiSC3(5) measurement was obtained every 10 min for 120 min, and the melatonin and tigecycline were added at the 10th minute. This assay was performed as previously reported (32).
Efflux pump assay.
The fluorescence dye rhodamine B was used to evaluate the effect of melatonin on the function of tmexCD1-toprJ1-mediated efflux pumps. Stationary-phase K. pneumoniae KP10 was washed three times with PBS, and a final concentration of rhodamine B (20 μM) was added. Next, cultures were placed in an incubator with 200-rpm shaking at 37°C for 30 min. After washing with PBS three times, the bacterial suspension was centrifuged (4,000 × g, 5 min) and resuspended in PBS containing 1% glucose. A different concentration of the melatonin solution was then added and incubated at 37°C for 30 min. Finally, the bacteria were centrifuged at 4,000 × g for 10 min, and then 200 μL was taken to determine the fluorescence intensity using a Synergy H1 microplate reader (excitation at 540 nm, emission at 625 nm).
Tigecycline accumulation analysis.
The accumulation of tigecycline in K. pneumoniae KP10 was assessed as described previously with several modifications (10). Overnight cultures of bacteria were diluted 100-fold in fresh LB broth and grown at 37°C under continuous shaking at 200 rpm to late logarithmic phase. Cells were then harvested by centrifugation at 4°C, washed twice in 50 mM phosphate-buffered saline (PBS, pH 7.0), and resuspended in the same buffer to an optical density at 600 nm (OD600) of 1.0. The cell suspension was incubated for 15 min at 37°C, and then tigecycline was added to the suspension at a final concentration of 100 μg/mL and incubation continued for 30 min. The suspension was divided into four parts, to three of which were added different concentrations of melatonin (10 mg/mL and 20 mg/mL) and 1-(1-Naphthylmethyl)-Piperazine [NMP] (100 μg/mL), and the remaining one was used as a control. Three 500-μL samples were collected at various time points (10, 20, and 30 min), with an equivalent volume of cells also collected to measure the bacterial dry weight at each time point. Each sample was immediately diluted with 500 μL of ice-cold PBS and centrifuged at 6,000 rpm for 10 min at 4°C. Bacterial cell pellets were washed once with 1 mL of ice-cold PBS and then resuspended in 1 mL of 0.1 M glycine hydrochloride (pH 3.0) and shaken at room temperature for 3 h. Samples were then centrifuged at 12,000 rpm for 10 min, and the resulting supernatants were filtered through 0.2-μm-pore-diameter filters. The concentration of tigecycline in the supernatant was determined using a high-performance liquid chromatography apparatus (Thermo Ultimate 3000) equipped with a diode array detector at a detection wavelength of 248 nm according to the United States Pharmacopoeia (USP). Data are shown as micrograms of tigecycline accumulated per gram of bacterial dry weight.
RT-PCR analysis.
K. pneumoniae KP10 was grown to early exponential phase and incubated with tigecycline (4 μg/mL) alone or in combination with melatonin (2 μg/mL, 4 μg/mL, and 8 μg/mL) for 4 h. Then, total RNA was isolated using a Qiagen RNeasy minikit and quantified by measuring the ODs at 260 nm and 280 nm. Reverse transcription of 1 μg RNA was performed using the PrimeScript reverse transcription-PCR (RT-PCR) kit (TaKaRa) following the manufacturer’s protocol. RT-PCR analysis was performed with a 7500 Fast real-time PCR system (Applied Biosystems, CA, USA) using the TB Green quantitative PCR (qPCR) kit (TaKaRa) with primers (Table S2). The relative quantitative method was applied to calculate the fold changes of mRNA expression relative to the reference genes (16S rRNA) in E. coli.
SEM and TEM analysis.
A single clone of KP10 was grown in 5 mL LB broth at 37°C with 200-rpm shaking for 4 h, and then the final concentration of melatonin (4 mg/mL) was injected. After culture at 37°C for 8 h, the bacteria were pelleted by centrifugation at 3,000 rpm for 3 min, and the pellet was washed twice with PBS. Finally, the sample was fixed in 1 mL 2.5% glutaraldehyde at 4°C overnight. After the samples were fixed with glutaraldehyde, they were dried by gradient dehydration with ethanol. After sputter coating with gold, the samples were photographed and observed with scanning electron microscopy (SEM). For transmission electron microscopy (TEM) samples, after fixation with glutaraldehyde, they were fixed with osmic acid for 2 h. After fixation, serial dehydration was carried out, and the samples were soaked for 2 to 4 h. After staining with uranyl acetate and lead citrate for 15 min, the bacterial changes were observed under a TEM and photographs were taken.
Outer membrane permeability and cell membrane integrity.
The fluorescent probe 1-N-phenylnaphthylamine (NPN) (10 μM) with the excitation wavelength of 350 nm and emission wavelength of 420 nm was used to evaluate the outer membrane permeability of KP10 treated with melatonin. The protocols were as follows: overnight KP10 cultures were washed, suspended in PBS with an OD600 of 0.5, and incubated with NPN for 30 min, and then, probed cells were mixed with different concentrations of the melatonin solution and incubated at 37°C for 1 h. Then, 200-μL samples were placed in a 96-well black microplate, and the fluorescence intensity was measured using a Synergy H1 microplate reader. The fluorescence intensity of 10 nM propidium iodide (PI)-labeled cells in the presence of melatonin (0, 1, 2, and 4 mg/mL, respectively) was measured with an excitation wavelength of 535 nm and emission wavelength of 615 nm.
Measurement of ROS level.
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA, 10 μM) was applied to monitor levels of ROS in K. pneumoniae KP10, following the manufacturer’s instructions (Beyotime). Fluorescence intensity was measured with an excitation wavelength of 488 nm and emission wavelength of 525 nm. The intracellular superoxide dismutase (SOD) activity of K. pneumoniae KP10 treated with melatonin, tigecycline, or their combination was measured using the total superoxide dismutase assay kit with WST-8 (S0101; Beyotime, China).
Time-dependent killing studies.
Time-dependent killing was assessed as described previously (21). An overnight culture of K. pneumoniae KP10 was diluted 1:1,000 in LB and incubated for 3 h at 37°C under 200-rpm shaking. K. pneumoniae KP10 was then treated with melatonin (2 mg/mL) and tigecycline (4 μg/mL) separately or their combination for 24 h. At the time points 0, 4, 8, 12, and 24 h, 100-μL cultures were removed, centrifuged, and resuspended in 100 μL sterile PBS (0.01 M, pH 7.4). Subsequently, 10-fold serially diluted suspensions were plated on LB plates and incubated overnight at 37°C. Bacterial colonies were counted, and the primary CFU per milliliter was calculated. Log10 CFU per milliliter was taken as the ordinate and time was taken as the abscissa to make a line graph. Synergy, additive effect, antagonism, and indifference were defined as >2-log kill, <2- but >1-log kill, >1-log growth, and ±1-log kill, respectively. Bactericidal activity (killing) was defined as a ≥103-fold decrease in the number of CFU from input CFU at 4 or 24 h (35, 36). Experiments were performed with three biological replicates.
Mouse thigh infection model.
Animal experiments were carried out at the Laboratory Animal Center of Nanjing Agricultural University, according to the guidelines of Experimental Animal Management Measures of Jiangsu Province, and were approved by the Laboratory Animal Monitoring Committee of Jiangsu Province, China [permit number SYXK (Su) 2017-0007]. The synergy between tigecycline and melatonin was evaluated in the mouse thigh infection model. The dosage of melatonin was determined as previously reported (32). Female BALB/c mice were randomly divided into four groups (n = 8 per group). All mice were first rendered neutropenic by cyclophosphamide (two consecutive doses of 150 and 100 mg/kg of body weight delivered at 4 days and 1 day before infection). Then, 100 μL of KP10 suspension (1.0 × 106 CFU per mouse) was injected into the right thighs of each mouse. After 2 h postinfection, infected mice were treated with PBS, tigecycline (20 mg/kg), melatonin (50 mg/kg), or the combination of tigecycline with melatonin (20 plus 50 mg/kg) by intraperitoneal injections once a day for 3 consecutive days. At 72 h postinfection, mice were euthanized by cervical dislocation. The right thighs were aseptically removed, homogenized, serially diluted, and plated on Mueller-Hinton agar (MHA) containing tigecycline (4 μg/mL) to count bacterial numbers after incubation at 37°C for 24 h.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key Research and Development Program of China (2022YFD1800400 and 2021YFD1800900), the National Natural Science Foundation of China (32002330), and the Natural Science Foundation of Jiangsu Province (BK20200535).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Collignon PJ, McEwen SA. 2019. One Health-its importance in helping to better control antimicrobial resistance. Trop Med Infect Dis 4:22. doi: 10.3390/tropicalmed4010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, Xie L, Yang C, Ma X, Li H, Li W, Zhang X, Liao K, Man S, Wang S, Wen H, Li B, Guo Z, Tian J, Pei F, Liu L, Zhang L, Zou C, Hu T, Cai J, Yang H, Huang J, Jia X, Huang W, Cao B, Wang H. 2018. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother 62:e01882-17. doi: 10.1128/AAC.01882-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He T, Wang R, Liu D, Walsh TR, Zhang R, Lv Y, Ke Y, Ji Q, Wei R, Liu Z, Shen Y, Wang G, Sun L, Lei L, Lv Z, Li Y, Pang M, Wang L, Sun Q, Fu Y, Song H, Hao Y, Shen Z, Wang S, Chen G, Wu C, Shen J, Wang Y. 2019. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat Microbiol 4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Chen C, Cui CY, Zhang Y, Liu X, Cui ZH, Ma XY, Feng Y, Fang LX, Lian XL, Zhang RM, Tang YZ, Zhang KX, Liu HM, Zhuang ZH, Zhou SD, Lv JN, Du H, Huang B, Yu FY, Mathema B, Kreiswirth BN, Liao XP, Chen L, Liu YH. 2019. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat Microbiol 4:1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun S, Gao H, Liu Y, Jin L, Wang R, Wang X, Wang Q, Yin Y, Zhang Y, Wang H. 2020. Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae. Emerg Microbes Infect 9:1102–1113. doi: 10.1080/22221751.2020.1768805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyres KL, Holt KE. 2018. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol 45:131–139. doi: 10.1016/j.mib.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Wyres KL, Lam M, Holt KE. 2020. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol 18:344–359. doi: 10.1038/s41579-019-0315-1. [DOI] [PubMed] [Google Scholar]
- 8.Jo J, Ko KS. 2021. Tigecycline heteroresistance and resistance mechanism in clinical isolates of Acinetobacter baumannii. Microbiol Spectr 9:e101021. doi: 10.1128/Spectrum.01010-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaghoubi S, Zekiy AO, Krutova M, Gholami M, Kouhsari E, Sholeh M, Ghafouri Z, Maleki F. 2022. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis 41:1003–1022. doi: 10.1007/s10096-020-04121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv L, Wan M, Wang C, Gao X, Yang Q, Partridge SR, Wang Y, Zong Z, Doi Y, Shen J, Jia P, Song Q, Zhang Q, Yang J, Huang X, Wang M, Liu JH. 2020. Emergence of a plasmid-encoded resistance-nodulation-division efflux pump conferring resistance to multiple drugs, including tigecycline, in Klebsiella pneumoniae. mBio 11:e02930-19. doi: 10.1128/mBio.02930-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song M, Liu Y, Huang X, Ding S, Wang Y, Shen J, Zhu K. 2020. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat Microbiol 5:1040–1050. doi: 10.1038/s41564-020-0723-z. [DOI] [PubMed] [Google Scholar]
- 12.Rathi E, Kumar A, Kini SG. 2020. Computational approaches in efflux pump inhibitors: current status and prospects. Drug Discov Today 25:1883–1890. doi: 10.1016/j.drudis.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Li X-Z, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaral FGD, Cipolla-Neto J. 2018. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab 62:472–479. doi: 10.20945/2359-3997000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolensky MH, Hermida RC, Portaluppi F. 2017. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med Rev 33:4–16. doi: 10.1016/j.smrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Lok R, van Koningsveld MJ, Gordijn M, Beersma D, Hut RA. 2019. Daytime melatonin and light independently affect human alertness and body temperature. J Pineal Res 67:e12583. doi: 10.1111/jpi.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y. 2017. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63:e12413. doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ. 1995. The influence of season, photoperiod, and pineal melatonin on immune function. J Pineal Res 19:149–165. doi: 10.1111/j.1600-079x.1995.tb00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claustrat B, Leston J. 2015. Melatonin: physiological effects in humans. Neurochirurgie 61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Cipolla-Neto J, Amaral F. 2018. Melatonin as a hormone: new physiological and clinical insights. Endocr Rev 39:990–1028. doi: 10.1210/er.2018-00084. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Jia Y, Yang K, Tong Z, Shi J, Li R, Xiao X, Ren W, Hardeland R, Reiter RJ, Wang Z. 2020. Melatonin overcomes MCR-mediated colistin resistance in Gram-negative pathogens. Theranostics 10:10697–10711. doi: 10.7150/thno.45951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesan N, Perumal G, Doble M. 2015. Bacterial resistance in biofilm-associated bacteria. Future Microbiol 10:1743–1750. doi: 10.2217/fmb.15.69. [DOI] [PubMed] [Google Scholar]
- 23.Douafer H, Andrieu V, Phanstiel OT, Brunel JM. 2019. Antibiotic adjuvants: make antibiotics great again! J Med Chem 62:8665–8681. doi: 10.1021/acs.jmedchem.8b01781. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Li R, Xiao X, Wang Z. 2019. Antibiotic adjuvants: an alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit Rev Microbiol 45:301–314. doi: 10.1080/1040841X.2019.1599813. [DOI] [PubMed] [Google Scholar]
- 25.Tyers M, Wright GD. 2019. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat Rev Microbiol 17:141–155. doi: 10.1038/s41579-018-0141-x. [DOI] [PubMed] [Google Scholar]
- 26.Reading C, Cole M. 1977. Clavulanic acid: a beta-lactamase-inhibiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother 11:852–857. doi: 10.1128/AAC.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, Walsh TR, Coombes BK, Wright GD. 2014. Aspergillomarasmine A overcomes metallo-beta-lactamase antibiotic resistance. Nature 510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster TJ. 2019. Can beta-lactam antibiotics be resurrected to combat MRSA? Trends Microbiol 27:26–38. doi: 10.1016/j.tim.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Stokes JM, MacNair CR, Ilyas B, French S, Cote JP, Bouwman C, Farha MA, Sieron AO, Whitfield C, Coombes BK, Brown ED. 2017. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat Microbiol 2:17028. doi: 10.1038/nmicrobiol.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong Z, Xu T, Deng T, Shi J, Wang Z, Liu Y. 2021. Benzydamine reverses TMexCD-TOprJ-mediated high-level tigecycline resistance in Gram-negative bacteria. Pharmaceuticals (Basel) 14:907. doi: 10.3390/ph14090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Sun N, Liu X, Li F, Sun J, Huang J, Li R, Wang L. 2022. Small clone dissemination of tmexCD1-toprJ1-carrying Klebsiella pneumoniae isolates in a chicken farm. J Glob Antimicrob Resist 29:105–112. doi: 10.1016/j.jgar.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Jia Y, Yang K, Li R, Xiao X, Zhu K, Wang Z. 2020. Metformin restores tetracyclines susceptibility against multidrug resistant bacteria. Adv Sci (Weinh) 7:1902227. doi: 10.1002/advs.201902227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minich DM, Henning M, Darley C, Fahoum M, Schuler CB, Frame J. 2022. Is melatonin the “next vitamin D”?: a review of emerging science, clinical uses, safety, and dietary supplements. Nutrients 14:3934. doi: 10.3390/nu14193934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei WJ, Yang HF. 2017. Synergy against extensively drug-resistant Acinetobacter baumannii in vitro by two old antibiotics: colistin and chloramphenicol. Int J Antimicrob Agents 49:321–326. doi: 10.1016/j.ijantimicag.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ. 2008. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J Antimicrob Chemother 61:365–370. doi: 10.1093/jac/dkm457. [DOI] [PubMed] [Google Scholar]
- 36.Rand KH, Houck HJ. 2004. Synergy of daptomycin with oxacillin and other beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 48:2871–2875. doi: 10.1128/AAC.48.8.2871-2875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00047-23-s0001.doc, DOC file, 0.05 MB (52KB, doc)