ABSTRACT
Cefepime has been reported to cause concentration-related neurotoxicity, especially in critically ill patients with renal failure. This evaluation aimed to identify a dosing regimen providing a sufficient probability of target attainment (PTA) and the lowest justifiable risk of neurotoxicity in critically ill patients. A population pharmacokinetic model was developed based on plasma concentrations over four consecutive days obtained from 14 intensive care unit (ICU) patients. The patients received a median dose of 2,000 mg cefepime by 30-min intravenous infusions with dosing intervals of every 8 h (q8h) to q24h. A time that the free drug concentration exceeds the MIC over the dosing interval (fT>MIC) of 65% and an fT>2×MIC of 100% were defined as treatment targets. Monte Carlo simulations were carried out to identify a dosing regimen for a PTA of 90% and a probability of neurotoxicity not exceeding 20%. A two-compartment model with linear elimination best described the data. Estimated creatinine clearance was significantly related to the clearance of cefepime in nondialysis patients. Interoccasion variability on clearance improved the model, reflecting dynamic clearance changes. The evaluations suggested combining thrice-daily administration as an appropriate choice. In patients with normal renal function (creatinine clearance, 120 mL/min), for the pharmacodynamics target of 100% fT>2×MIC and a PTA of 90%, a dose of 1,333 mg q8h was found to be related to a probability of neurotoxicity of ≤20% and to cover MICs up to 2 mg/L. Continuous infusion appears to be superior to other dosing regimens by providing higher efficacy and a low risk of neurotoxicity. The model makes it possible to improve the predicted balance between cefepime efficacy and neurotoxicity in critically ill patients. (This study has been registered at ClinicalTrials.gov under registration no. NCT01793012).
KEYWORDS: cefepime, population pharmacokinetics, probability of target attainment, neurotoxicity
INTRODUCTION
Cefepime is a fourth-generation cephalosporin antibiotic covering both Gram-positive and Gram-negative bacteria (1). It was first approved in Europe in 1993 and in 1996 by the U.S. Food and Drug Administration (FDA) for the treatment of moderate to severe pneumonia, complicated and uncomplicated urinary tract infections, uncomplicated skin and skin structure infections, intra-abdominal infections, and, later, febrile neutropenia (2, 3). Through its property as a zwitterion, cefepime has an enhanced ability to penetrate the outer cell membrane porins of Gram-negative bacteria more rapidly than third-generation cephalosporins (4). In addition, it is relatively stable to chromosomally encoded AmpC beta-lactamase, representing the treatment of choice in AmpC producers that do not harbor extended-spectrum beta-lactamase enzymes (5). Cefepime has activity comparable to that of ceftazidime against Pseudomonas aeruginosa (4). This broad-spectrum activity of cefepime and its relative beta-lactamase stability make this drug suitable as an empirical treatment for patients in intensive care units (ICUs) (6). The recommended standard dose of cefepime according to EUCAST (European Committee on Antimicrobial Susceptibility Testing) is 1 g every 8 h (q8h) or 2 g q12h (7). The manufacturer’s recommended dose of cefepime for adults with mild to moderate infections and normal kidney function is 0.5 g to 1 g q12h and for those with moderate to severe infections is 1 g to 2 g q12h (8). This dosing regimen has been effective against the vast majority of streptococci, members of the Enterobacterales, and Staphylococcus aureus (9). For patients with febrile neutropenia, the recommended dose is 2 g q8h, all with a 30-min infusion (8). Cefepime doses of 2 g q8h in patients with normal renal function are required to achieve adequate exposure and increase antibacterial effect against susceptible P. aeruginosa infections (10). To account for the mainly renal elimination of cefepime, in patients with an estimated creatinine clearance below 60 mL/min, dose adjustment is required. The manufacturer’s dosing recommendations for the maintenance dose for adults weighing more than 40 kg with renal impairment for severe infection are as follows: 2 g q24h, 1 g every day, and 0.5 g every day for creatinine clearance estimated by the Cockcroft-Gault equation (eCLCR) of 30 to 60 mL/min, 11 to 29 mL/min, and ≤10 mL/min, respectively (8).
Pathophysiological changes in severely ill patients may cause high pharmacokinetic variability, which may result in under- and overexposure to the drug. It is important to understand that the interindividual variability (IIV) of pharmacokinetic (PK) parameters, specifically clearance and volume of distribution, determines the probability of target attainment (PTA) for optimal bacterial cell killing by β-lactams after the administration of a fixed dose (9).
Cefepime is administered parenterally. It exhibits linear pharmacokinetic behavior and low plasma protein binding, which ranges from 16% to 19%, and is widely distributed in body tissues and fluids. It is mainly eliminated by the kidneys through glomerular filtration, with more than 80% of the drug being unchanged in subjects with normal kidney function. The elimination half-life is about 2 to 2.5 h (11). The percentage of time that the free drug concentration exceeds the MIC over the dosing interval (fT>MIC) is the PK/pharmacodynamic (PD) index that best describes its efficacy (12). In line with other beta-lactam antibiotics, the efficacy of cefepime is optimal when the free-drug concentration exceeds the MIC for at least 50% of the dosing interval (13). For Gram-negative bacteria, an fT>MIC of 60 to 70% is generally required to achieve sufficient killing (14). Nicasio et al. reported a PD target of >50% fT>MIC for cefepime in patients with ventilator-associated pneumonia (13), while Roos et al. used a 65% fT>MIC as a target (9). Treatment of severe infections may require the administration of doses that keep free serum concentrations at least 1- to 2-fold higher than the MIC throughout the dosing interval. Accordingly, Aitken et al. used a PD target of 100% fT>2×MIC, or 100% fCmin>2×MIC (free minimum concentration to MIC) ratio of ≥ 2.1 in patients with Gram-negative bacterial pneumonia (15). Al-Shaer et al. suggested PD targets of 100% fT>MIC and 100% fT>4×MIC (16).
A comprehensive review and meta-analysis published in 2006 revealed that the use of cefepime in febrile neutropenic patients was associated with higher mortality (17). The same study team expanded their meta-analysis in 2007 to include all cefepime-treated patients, and they still discovered higher mortality rates than in individuals receiving other broad-spectrum β-lactam antibiotics (18). It has been reported that 15% of all ICU patients will experience one symptom of cefepime neurotoxicity or another (19), but recognition of these symptoms is difficult, as they reflect common conditions in critically ill patients. Symptoms of neurotoxicity include confusion, encephalopathy, abnormal electroencephalography (EEG), nonconvulsive status epilepticus, and severe toxicity leading to coma (20). In general, the neurotoxic symptoms, including encephalopathy, are reversible after discontinuation of cefepime and/or after hemodialysis. The mechanism of the neurotoxic effect is not yet known, but it has been related to the inhibition of γ-aminobutyric acid A (GABA-A) receptors (21). Researchers have been attempting to link the neurotoxicity to plasma cefepime exposure, but the available data are limited by the availability of trough-only concentrations, retrospective study designs, total or calculated unbound concentrations, difficulties in defining the events, and lack of control for covariates affecting these events, which are frequent in intensive care units (16).
To assess the risk for neurotoxicity in this study, the logistic regression model reported by Boschung-Pasquier et al. was used (22). According to the model, at cefepime plasma trough concentrations less than 7.7 mg/L, no patients showed signs of potential neurotoxicity. The risk of neurotoxicity was 25% when plasma trough concentrations were ≥12 mg/L. In patients with trough concentrations of ≥16 mg/L, this risk increased to 50% when plasma trough concentrations were ≥16 mg/L. At plasma trough concentrations of ≥38.1 mg/L, all patients experienced neurotoxicity. On the other hand, Lamoth et al. reported a 50% probability of neurotoxicity at a trough concentration of ≥22 mg/L (23), while Huwyler et al. recommended avoiding a trough concentration of >20 mg/L in patients receiving intermittent dosing and a steady-state concentration of >35 mg/L in patients receiving a continuous infusion (CI) (24) (Table 1).
TABLE 1.
Cefepime-induced neurotoxicity based on plasma trough concentrations reported in various studies
The aims of this study were (i) to quantify PK variability and explore predictors of cefepime clearance and (ii) to identify a dosing regimen of cefepime in critically ill patients providing a sufficient PTA and an acceptably low risk of neurotoxicity.
RESULTS
Fourteen medical-surgical ICU patients were included in the study. Four of the patients were on continuous venovenous hemodialysis (CVVHD), and all but one patient had sepsis. Sepsis was defined according to the European Society of Intensive Care Medicine/Society of Critical Care Medicine Consensus Conference Committee (25). The median numbers of available blood samples per patient were 11.5, 6, 6.5, and 6 on days 1 to 4 of sampling, respectively. A total of 344 plasma concentrations were used for the development of the model. Four very high plasma concentrations ranging between 447.17 and 848.36 mg/L were considered implausible and removed from the evaluation. The patients’ characteristics are shown in Table 2. The median age and body weight of the patients were 62 (range, 22 to 94) years and 70 (45 to 120) kg, respectively.
TABLE 2.
Summary of demographic and clinical characteristics of the patients
| Characteristica | Median (range) or no. of patients |
|---|---|
| eCLCR (mL/min) | 60.8 (16.7–217) |
| mCLCR (mL/min) | 56 (0–219) |
| Body wt (kg) | 70 (45–120) |
| Age (yrs) | 62 (22–94) |
| Sex (male/female) | 6/8 |
| Body ht (m) | 1.68 (1.5–1.83) |
| BMI (kg/m2) | 25 (20–37) |
| Serum albumin concn (mg/dL) | 2.5 (1.8–3.6) |
| Serum creatinine concn (mg/dL) | 0.8 (0.3–4.4) |
| Serum urea concn (mg/dL) | 53 (12–196) |
| Lung transplantation (yes/no) | 4/10 |
| Liver transplantation (yes/no) | 1/13 |
| Dialysis (yes/no) | 4/10 |
| Sepsis (yes/no) | 13/1 |
eCLCR, Cockcroft-Gault creatinine clearance; mCLCR, measured creatinine clearance based on 24-h urinary excretion of creatinine and corresponding plasma concentration of creatinine in patients without hemodialysis; BMI, body mass index.
Population pharmacokinetic model.
A two-compartment model with linear elimination best described cefepime concentrations. The base model was parametrized in terms of elimination clearance for nondialysis patients (CLND), clearance of CVVHD patients (CLD), central volume of distribution (V1), the peripheral volume of distribution (V2), and intercompartmental clearance (Q). IIV was found to be significant on clearance and volume of distribution. Interoccasional variability (IOV) estimated on clearance of both dialysis and nondialysis patients was significant and decreased objective value function (OVF) by 45 points. IOV was tested on the volume of distribution and decreased OVF (by 16.9 points) but was not properly identifiable. Parameter estimates of the final model and 95% confidence interval for the parameter estimates are given in Table 3.
TABLE 3.
Parameter estimates of cefepime obtained from the final modela
| Parameter | NONMEM |
Bootstrap analysis |
||||
|---|---|---|---|---|---|---|
| Estimate | RSE (%) | Shr (%) | Median | RSE (%) | 95% CI | |
| CLD (L/h) | 2.74 | 13 | 2.71 | 9.26 | 2.29–3.19 | |
| CLND (L/h) | 2.94 | 10 | 2.96 | 12.3 | 2.36–3.78 | |
| V1 (L) | 8.88 | 38 | 9.06 | 17.9 | 5.70–12.2 | |
| Q (L/h) | 27.4 | 54 | 24.7 | 22.4 | 16.8–41.7 | |
| V2 (L) | 18.7 | 22 | 18.0 | 12.4 | 14.2–23.5 | |
| eCLCR effect on CLND | 0.896 | 17 | 0.829 | 13.5 | 0.637–1.04 | |
| IIV CLD (CV %) | 22.7 | 15 | 51 | 20.6 | 37.0 | 12.5–27.9 |
| IIV CLND (CV %) | 31.3 | 20 | 21 | 28.8 | 51.4 | 9.80–40.8 |
| IIV V1 (CV %) | 47.3 | 25 | 14 | 44.2 | 42.3 | 26.7–65.0 |
| IIV V2 (CV %) | 19.3 | 26 | 34 | 19.8 | 51.8 | 5.8–29.7 |
| IOV CLND (CV %) | 22.4 | 23 | 8 | 22.3 | 47.6 | 14.1–35.5 |
| Proportional residual error (SD) | 0.278 | 11 | 0.276 | 14.4 | 0.217–0.324 | |
RSE, relative standard error; CI, confidence interval; CLD, elimination clearance in dialysis patients; CLND, elimination in nondialysis patients; V1, central volume of distribution; Q, intercompartmental clearance; V2, peripheral volume of distribution; eCLCR, creatinine clearance estimated by the Cockcroft-Gault equation; IIV, interindividual variability; IOV, interoccasional variability; CV, coefficient of variation; shr, shrinkage; SD, standard deviation.
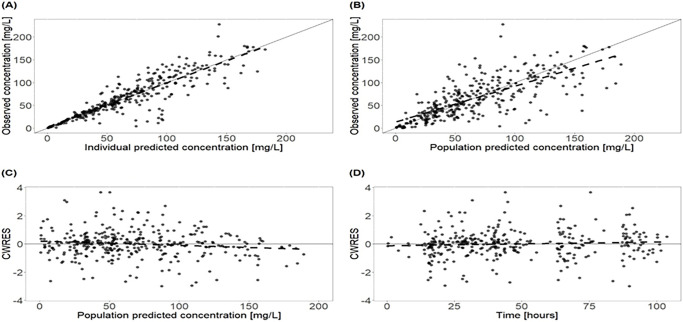
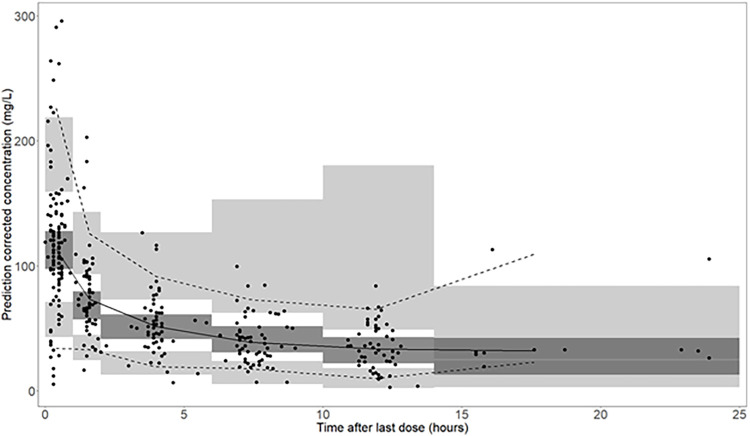
Observations were uniformly distributed along the line of identity; no systematic over- or underprediction was evident from plots of residuals (Fig. 1). Relative standard errors of all parameters in the final model were less than 30% except for V1 and Q, which suggests that parameters were estimated with sufficient precision (Table 3). Figure 2 shows prediction-corrected visual predictive checks (pcVPC), confirming that the developed model was able to capture the central trend and variability in the data except for the last few observations.
FIG 1.
Goodness-of-fits plots for the final covariate cefepime population pharmacokinetic model. (A) Plot of observed concentrations versus individual predictions. (B) Plot of observed concentrations versus population predictions. (C) Plot of conditional weighted residuals (CWRES) versus population predictions. (D) Plot of CWRES versus time after the first dose. The dashed line represents the best linear fit. Black dots represent observed concentrations. The solid line represents the line of identity or unity. The total number of ICU patients is 14, including four patients with continuous venovenous hemodialysis. The patients received a median daily dose of 2,000 mg cefepime by 30-min intravenous infusions.
FIG 2.
Prediction-corrected visual predictive check (n = 1,000). Solid dots represent observed concentrations. The black solid line represents the median, and the lower and upper dashed lines are the 5th and 95th percentiles of observed concentrations. Shaded areas are the respective model-predicted 95% confidence intervals. Dark-shaded areas represent the median prediction interval, and light-shaded areas show the 2.5th and 97.5th percentiles of the simulated data.
In the covariate analysis, eCLCR was found to significantly decrease OVF by 14 points. Serum creatinine was also found to be a significant covariate on clearance but to a lesser extent than eCLCR, decreasing OVF by 7 points. Other covariates, such as measured creatinine clearance (mCLCR), weight, sex, and age, tested on PK parameters did not significantly improve the model.
Monte Carlo simulations.
Monte Carlo simulations were used to assess the combined effect of cefepime dose, dosing interval, and CLCR on both PTA and the risk of neurotoxicity. While the published relationship between cefepime exposure and neurotoxicity was based on trough concentrations, we additionally used the (higher) average concentrations in the logistic regression equation provided by Boschung-Pasquier et al. (22) to increase the safety margin for patients.
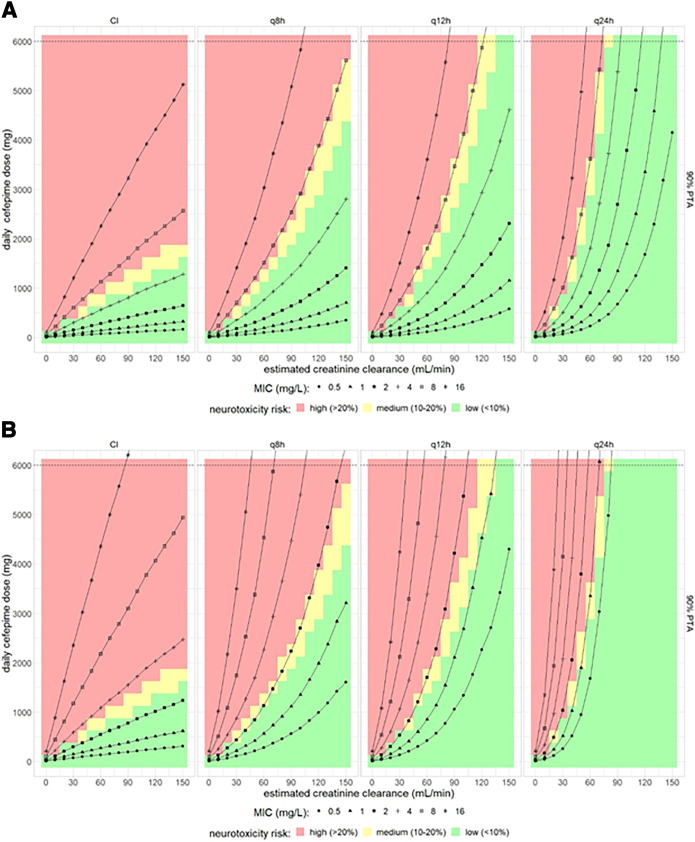
Figure 3A and B show neurotoxicity estimates based on trough concentrations with the pharmacodynamics target of 65% T>MIC and 100% T>2×MIC, respectively. For a PD target of 65% T>MIC and a PTA target of 90%, doses of 500 mg q8h and 2,000 mg q8h predicted around 20% neurotoxicity for patients with eCLCR of 60 mL/min and 150 mL/min, respectively, covering MICs of up to 8 mg/L (Fig. 3A). Increasing the PD target to 100% T>2×MIC, doses of 400 mg q8h and 2,250 mg q8h predicted ≤20% neurotoxicity for patients with eCLCR of 60 mL/min and 150 mL/min, respectively, covering MICs of 2 mg/L (Fig. 3B). With CI, daily doses of 500 mg and 1,250 mg predicted <10% risk of neurotoxicity in patients with eCLCR of 60 mL/min and 150 mL/min, respectively, for PD targets of 65% T>MIC, covering MICs of up to 4 mg/L. For a similar risk of neurotoxicity, doses and renal function increasing the PD target to 100% T>2×MIC reduced MIC coverage to 2 mg/L with CI. Prolonging dosing intervals while maintaining daily doses was predicted to result in a decrease in efficacy (i.e., requiring higher daily doses to cover the same MICs) but even more so in a decrease in the risk of neurotoxicity, while overall any improvement was only marginal when balancing efficacy and neurotoxicity.
FIG 3.
Combined effect of cefepime dose, dosing interval, and estimated creatinine clearance on both PTA and on the probability of neurotoxicity for an efficacy target of (A) 65% T>MIC and (B) 100% T>2×MIC based on plasma trough cefepime concentrations. The color gradient in the background indicates the estimated risk of neurotoxicity. The black lines represent 90% PTA for assumed MICs ranging from 0.5 to 16 mg/L.
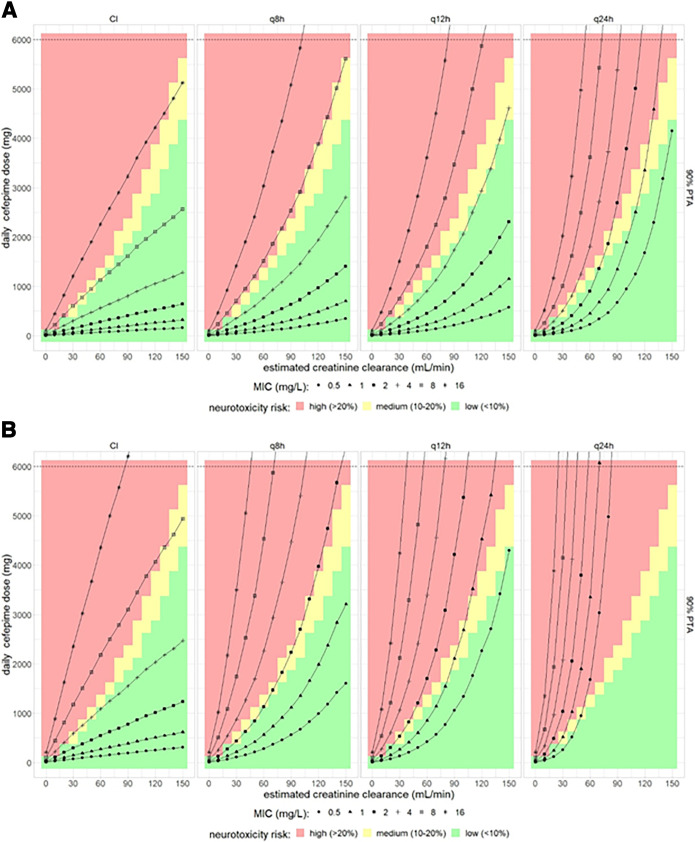
When the risk of neurotoxicity was estimated based on average plasma concentrations, this risk would depend only on daily dose and not on dosing intervals; accordingly, a prolonged dosing interval would deteriorate the efficacy-versus-neurotoxicity risk relationship in this evaluation (Fig. 4A and B).
FIG 4.
Combined effect of cefepime dose, dosing interval, and estimated creatinine clearance on both PTA and on the probability of neurotoxicity for an efficacy target of (A) 65% T>MIC and (B) 100% T>2×MIC based on plasma average cefepime concentrations. The color gradient in the background indicates the estimated risk of neurotoxicity. The black lines represent 90% PTA for assumed MICs ranging from 0.5 to 16 mg/L.
For the pharmacodynamics targets of 100% T>2×MIC and PTA target of 90%, doses of 200 mg q8h and 1,000 mg q8h for a patient with a creatinine clearance of 60 mL/min and 150 mL/min, respectively, was predicted to be related to a probability of neurotoxicity of less than 10% (based on average cefepime concentrations) and providing coverage for MICs up to 1 mg/L. For the same PD target, a daily dose of 1,000 mg and 1,500 mg for CI predicted low neurotoxicity risk for eCLCR values of 60 mL/min and 90 mL/min, respectively, and provided coverage for MICs up to 4 mg/L (Fig. 4B). Doses to achieve 100% T>2×MIC and to cover MICs above 4 mg/L would in all cases be related to a high risk of neurotoxicity. Dosing intervals of q12h and q24h would require higher doses to achieve the pharmacodynamics target and thus would again deteriorate the balance of efficacy versus neurotoxicity risk as assessed based on average cefepime concentrations (Fig. 4B). In summary, it is essential to take renal function into account to select the appropriate daily cefepime dose, and a q8h dosing should be preferred over reduced dosing frequencies, as it provides higher pharmacodynamics target attainment, while a possible benefit regarding neurotoxicity risk because of lower trough values is negligible (Fig. 3A and 4A). Table 4 shows typically suitable doses with an acceptable risk of neurotoxicity for a MIC of 2 mg/L depending on the desired PTA.
TABLE 4.
Suitable predicted cefepime doses administered q8h with an acceptable risk for neurotoxicity (i.e., 20% or lower, based on average concentration) for selected creatinine clearance valuesa
| CLCR (mL/min) | Dose (mg) required for 90% PTA |
|---|---|
| 30 | 440 |
| 60 | 1,100 |
| 90 | 2,300 |
| 120 | 4,000 |
| 150 | 6,500 |
The MIC target is up to 2 mg/L, and the PK/PD target used is 100% T>2×MIC.
While we did not carry out Monte Carlo simulations for CVVHD patients because of the very limited data available, clearance point estimates for patients with CVVHD (CLD, 2.74 L/h) and without CVVHD (CLND, 2.94 L/h) in our model were very similar; thus, the doses predicted for patients with normal kidney function might also be suitable for CVVHD patients in ICUs; however, therapeutic drug monitoring (TDM) in these patients would be desirable to ensure appropriate exposure.
DISCUSSION
A population pharmacokinetic model of cefepime was developed to describe its pharmacokinetics in critically ill patients. Our model enabled stochastic simulations for proposing a dosing regimen balancing antibacterial effects and the risk of neurotoxicity for various degrees of renal impairment.
Balancing efficacy versus risk of neurotoxicity, continuous infusion provided the most favorable results, followed by the shortest dosing interval tested for intermittent infusions, i.e., 8 h. To reach the pharmacodynamics target of 100% T>2×MIC in 90% of patients with an eCLCR of 120 mL/min, a q8h dose of 1,333 mg was related to a ≤20% neurotoxicity risk and covered bacteria with MICs up to 2 mg/L.
A two-compartment model with eCLCR as a covariate on clearance best described the cefepime data, which is in line with previous studies (13, 26, 27). In published evaluations, some authors also suggested a three-compartment model (9); however, the data in our study did not support adding a third compartment. Several studies have described cefepime pharmacokinetics in hospitalized patients (26–29). The typical value of clearance in our study for CLND was 2.96 L/h (mean eCLCR, 87.1 mL/min). This is close to the clearance value previously reported in the literature: Cheng et al. reported a clearance of 2.43 L/h (mean eCLCR, 75.6 mL/min) for five critically ill patients who required extracorporeal membrane oxygenation (ECMO) (27). However, the typical values of clearance reported by other studies (5.99 and 6.33 L/h) were notably higher (26, 29). The difference observed in the studies of Rhodes et al. (29) and Kois et al. (26) may be explained by the fact that in both studies, the mean eCLCR of the cohort was higher, i.e., 126 and 149 mL/min, respectively, than the value of 87.1 mL/min in our study. This also is in agreement with the high dosing regimens suggested for cefepime in both studies.
Renal dysfunction is the major risk factor for cefepime overexposure and thus for cefepime-related toxicity. Among those who experienced neurotoxicity in intensive care units, 80% had renal dysfunction (30), suggesting that a proper dose adjustment may avoid this adverse event. Unfortunately, the assessment of renal function in ICU patients is a challenge (31). Despite the major limitations discussed below, estimated creatinine clearance (eCLCR) is one of the most widely used markers of renal function. In this study, creatinine clearance estimated by the Cockcroft-Gault equation (eClCR) was found to be significantly related to cefepime clearance. In the present study, eCLCR was better than serum creatinine concentration to predict cefepime clearance. Measured creatinine clearance did not improve the model in terms of OVF and goodness of fit. This finding is in line with the conclusions reported by Jonckheere et al. (32). Although determining measured creatinine clearance is considered the method of choice for renal function measurement in critically ill patients (33), it is poorly related to the clearance of drugs such as cefepime (32) and meropenem (34). This might be due to the error-prone nature of urine collection. As eCLCR was a predictor of cefepime clearance, the patients with low eCLCR are expected to reach high plasma averages and trough concentrations if cefepime doses are not reduced accordingly. However, the eCLCR equation was created based on non-critically ill patients and the creatinine measurements were at a steady state (35). In addition, eCLCR is not considered reliable for estimating renal function in obese individuals and is not recommended in subjects older than 65 years (36). These limitations of eCLCR must be considered when the model is applied to patients with highly dynamic renal function.
An important outcome of the model development was that an IIV (coefficient of variation [CV]) of 89% was much higher than IOV on clearance, which reached 21.4% in the base model. This is in line with the high IIV reported by Roos et al. (9) for cefepime in ICU patients and supports the individualized-dose concept for the drug, which can be achieved by taking estimated creatinine clearance as a covariate into account and/or by taking blood samples during treatment and using an intervention approach based on a target concentration, as suggested for aminoglycosides (37). On the other hand, accounting for IOV on clearance of nondialysis patients significantly improved the model in terms of OVF and goodness of fit. IOV describes the changes in cefepime clearance over time which might be due to pathophysiological conditions, concomitant medication, excessive fluid resuscitation, changes in renal function, and other disease treatment-related interventions. Changes in cefepime pharmacokinetics between occasions are difficult to predict; to take them into account, dense TDM would probably be indispensable. More than 90% of patients in our data had sepsis. Cefepime clearance in sepsis patients can be quite variable, making appropriate dosing difficult (2). To reduce the risk of neurotoxicity or suboptimal efficacy in these patients, both plasma concentrations and renal function should be carefully monitored. In this model, variability in kidney function is reflected by estimating IOV on clearance, which can describe unexplained variation of clearance over time, and by covariate modeling, i.e., by incorporating eCLCR as a covariate on clearance of nondialysis patients in the model.
A recently published study involving patients with hospital-acquired/ventilator-associated pneumonia with >50% T>MIC as a treatment target and trough concentration of <20 mg/L as a safety target recommended a dosing regimen of 2,000 mg via infusion every 12 h administered over 4 h in patients with normal renal function (CLCR, 90 to 120 mL/min) for a MIC target of 4 mg/L (38) (Table 5). However, no information on the risk of neurotoxicity was provided. In our study, with a PD target of 65% T>MIC, doses of 500 mg q8h and 1,000 mg q8h with 30 min infusion for eCLCR values of 60 mL/min and 90 mL/min, respectively, appear to be optimal for MICs up to 4 mg/L and 1 mg/L, respectively, with <10% neurotoxicity (Fig. 4A and B). The dosing of 1,000 mg q8h is identical to the EUCAST recommendation for cefepime standard dosing (7), while in a study with patients infected with Pseudomonas aeruginosa, a higher dosage of 2,000 mg q8h was required to provide attainment of similar targets (10), but neurotoxicity was not addressed. Taking a trough concentration of ≥20 mg/L as the toxicity target and 100% T>MIC (with a MIC of 8 mg/L) as the efficacy target, Cheng et al. reported that for patients with a CLCR of 30 mL/min and 65 mL/min, 1,000 mg q12h will achieve 84 to 92% and 46 to 53% probability of efficacy and 8 to 44% and 1 to 8% probability of toxicity, respectively (Table 5). For patients with a CLCR of 120 mL/min, the authors suggest that 1,000 mg q8h will achieve a 40 to 44% probability of efficacy and 1 to 6% probability of toxicity (27). With a PD target of 100% T>2×MIC and neurotoxicity target of <10%, for eCLCR of 60 mL/min and of 120 mL/min, our study found doses of 366 mg q8h and 1,333 mg q8h, respectively, for a PTA of 90% for MICs up to 2 mg/L (Table 4). In this evaluation, we determined optimized dosing regimens irrespective of the vial sizes currently available on the market. Therefore, combinations and divisions of vials of 500 or 1,000 mg might be required to provide doses close to the optimal doses we identified. Several clinical trials have reported high efficacy for continuous infusion (39, 40). Simulations for continuous infusion predicted high efficacy with lower daily doses than those used with q8h, q12h, and q24h administration (Fig. 3 and 4). The predicted neurotoxicity risk was <10%. Continuous infusion also has a pharmacoeconomic advantage over intermittent dosing by achieving the same effect with a lower daily dose of medication (41).
TABLE 5.
Published cefepime dosing recommendations based on trough concentrations and renal functiona
| eCLCR (mL/min) | Dose regimen | Patient population | Trough concn (mg/L) | Time of infusion | MIC (mg/L) | PKPD target (% T>MIC) | Probability of efficacy and neurotoxicity (%)a | Reference |
|---|---|---|---|---|---|---|---|---|
| 90–130 | 2,000 mg q12h | Hospital-acquired-pneumonia patients | <20 | 4 h | 4 | >50 | NA | 38 |
| 30 | 1,000 mg q12h | ICU patients | ≥20 | 30 min | 8 | 100 | E, 84–92; N, 8–44 | 27 |
| 65 | 1,000 mg q12h | ICU patients | ≥20 | 30 min | 8 | 100 | E, 46–53; N, 1–8 | 27 |
| 120 | 1,000 mg q8h | ICU patients | ≥20 | 30 min | 8 | 100 | E, 40– 44; N, 1–6 | 27 |
E, efficacy; N, neurotoxicity; NA, not available.
To date, mainly trough concentrations have been used for the evaluation of cefepime-induced neurotoxicity. We added respective simulations using the same equation based on average concentrations, because the basis for using trough concentrations only was that no other information was available in these studies. However, we expect that the exposure during the rest of the dosing interval is not meaningless, and therefore, we tried to increase the safety margin for patients by using average concentrations. The risk of neurotoxicity but also efficacy were found to be decreased for daily doses with prolonged dosing intervals, i.e., q12h and q24h, when trough concentrations were used, while predicted efficacy was decreased by prolonging dosing intervals; however, when average plasma concentrations were used, the risk for neurotoxicity remained unchanged. Thus, irrespective of the concentrations used to predict neurotoxicity, the shorter dosing interval of 8 h provides the best results in balancing the relationship between efficacy and risk of neurotoxicity.
Beyond modeling, clinical data confirm that patients with estimated glomerular filtration rates (eGFR) of 30 mL/min/1.73 m2 or less have the highest proportion of suspected neurotoxicity (22). TDM in these patients seems to be another useful tool to account for the slower elimination of cefepime. Suttels et al. suggested TDM of cefepime in non-critically ill patients to monitor and prevent adverse events (42). In another study of patients who had CLCR less than 30 mL/min and who were on renal replacement therapy, TDM or an alternative antimicrobial agent was suggested (27). When neurological symptoms appear or worsen, the possibility of a cefepime overdose must be considered, particularly in elderly patients (30). Renal function should also be carefully monitored when cefepime is combined with potentially nephrotoxic antibiotics (such as aminoglycosides) or potent diuretics (43).
This study had several limitations. The small sample size provided limited statistical power for the analysis. Individual PD data in our patients for exploration of a direct link between cefepime concentrations and neurotoxicity were not available. Another issue is heterogeneity in the patient population studied, with some of them being on dialysis. Moreover, little information on the relationship between exposure of cefepime and neurotoxicity was available in the literature.
Conclusion.
In conclusion, the model makes it possible to balance the predicted efficacy and neurotoxicity risk of cefepime in critically ill patients, with emphasis on renal function as the main covariate for exposure. Overall, a daily administration frequency of q8h was found to have advantages over q12h and q24h. For the pharmacodynamics target of 100% T>2×MIC and PTA target of 90%, a dose of 1,333 mg q8h was related to a medium to low neurotoxicity risk and coverage of bacteria with MICs up to 2 mg/L in patients with an eCLCR of 120 mL/min. For MIC targets above 2 mg/L, higher doses are required, leading to a high risk of neurotoxicity. Continuous infusion improves the balance between efficacy and neurotoxicity risk and provides higher efficacy with lower daily doses than q8h, q12h, and q24h administration.
Further evaluations are needed, including studies with larger groups and studies that include experimental pharmacodynamics assessment, to further explore optimal dosing of cefepime in critically ill patients.
MATERIALS AND METHODS
Study design.
This monocentric, prospective observational study was conducted at the Department of Anesthesiology, University Hospital, Ludwig-Maximilians-Universität (LMU) München, Munich, Germany. The study protocol (ClinicalTrials.gov; NCT01793012) was approved by the Ethics Committee of the Medical Faculty of the LMU (registration number 428-12) and conducted following the principles of the Declaration of Helsinki. Written informed consent from patients or their legal representatives was obtained. Patients over the age of 18 who were treated with cefepime and had an ICU stay were included. Following local guidelines, patients received a median dose of 2 g with dosing intervals of q8h, q12h, or q24h as 30-min intravenous infusions. Blood samples were taken on four consecutive days. If feasible/appropriate, the samples were collected prior to and 0.25, 0.5, 1.5, 4, 7.25 or 8, and 12 or 16 h after administration of a cefepime dose and immediately before (trough concentration) the next administration. Demographic information and laboratory data (including serum creatinine) were also collected along with patient-specific clinical information. Creatinine clearance was calculated using the Cockcroft-Gault equation based on daily serum creatinine results (35).
Blood samples were immediately centrifuged and stored at −80°C. Total plasma concentrations of cefepime were determined using a validated liquid chromatography-mass spectrometry method (44). Cefepime-D3,13C1 was used as an internal standard. Validation showed good analytical performance, with a CV for intra- and interassay (n = 5) imprecision of ≤8.6%, and the relative error for inaccuracy was between −8% and −1%.
Population pharmacokinetic analysis.
A population pharmacokinetic model was developed by nonlinear mixed-effects modeling using NONMEM 7.4.3 software (45). Estimation was based on first-order conditional estimation with interaction (FOCE+I). One-, two-, and three-compartment models for plasma concentrations were explored. IIV and IOV were evaluated on clearance and central volume of distribution assuming log-normal distributions of the parameters. IOV was modeled over clearance, assuming that each administration was a separate occasion. For patients on CVVHD, a separate clearance was estimated.
The goodness of fit (GOF) was evaluated by visual assessment of predicted versus observed concentrations and scatterplots of the residuals. For the evaluation of uncertainty around PK parameter estimates, a nonparametric bootstrap (n = 1,000) was performed, and a 95% confidence interval for the parameters was obtained (37). To assess the predictive performance of the final model, pcVPC were performed, comparing observed data with model-based simulated data to assess the adequacy of the predictive ability of the model.
For covariate modeling, covariates were first screened based on physiological plausibility, and later, covariates were plotted against individual empirical Bayes estimates to explore relationships. mCLCR and eCLCR (not weight adjusted) were evaluated, as they are markers of renal function. Demographic characteristics such as total body weight, age, and sex were tested along with serum creatinine, since they are part of typical renal function and the eCLCR equation. Body weight as a covariate on clearance and volume of distribution was also tested by simple allometric scaling with coefficients fixed at 0.75 and 1, respectively. The evaluation was guided by changes in OVF, with a change of 3.84 being considered statistically significant (P value less than 0.05, assuming that the OVF follows a chi-squared distribution with one degree of freedom). Continuous covariates were evaluated on cefepime clearance by the power relationship as shown in equation 1, while categorical covariates were evaluated by the linear equation shown in equation 2.
| (1) |
| (2) |
where L is liter, h is hour, and CLTV is typical value of clearance, θ is theta.
Monte Carlo simulations.
Monte Carlo simulations for patients not on CVVHD were performed using 10,000 virtual subjects using the mrgsovle package, version 1.0.6, in R (46). Cefepime doses were simulated up to 6,000 mg with dosing intervals of q8h, q12h, and q24h and CI for 7 days. Plasma protein binding of 20% was assumed. Creatinine clearance was simulated ranging from 0 to 150 mL/min. Pharmacodynamics targets of 65% T>MIC and 100% T>2×MIC with a PTA of 90% described in the steady state were selected. To assess the risk for neurotoxicity, a logistic regression model developed by Boschung-Pasquier et al. (equation 3) was used, where cefepime was administered three times a day (2 g every 8 h), with dosing adjusted for patients with an eGFR of 50 mL/min/1.73 m2 (22).
| (3) |
In this study, the risk of neurotoxicity was evaluated based on the trough and average concentrations. Neurotoxicity of <10% was considered low (optimal), a value between 10 and 20% was considered medium (the maximum acceptable range), and beyond 20% was deemed a high risk of neurotoxicity.
Data availability.
The data that support the findings of this study are available from the corresponding author upon request.
ACKNOWLEDGMENT
The Higher Education Commission of Pakistan provided financial support in the form of a Ph.D. scholarship for Muhammad Bilal through the German Academic Exchange Service (DAAD). The study's design, data collection and analysis, and the choice to submit the work for publication were all independent of the sponsor. We acknowledge support for the article processing charge from the DFG (German Research Foundation, 491454339).
REFERENCES
- 1.Cordero J, Lopez J, Lora J, Martinez J, Mercado L, Vega R, Oliva M, Leudo D. 2022. Development of neurotoxicity syndrome associated with the use of cefepime. Health Sci J 16:921. [Google Scholar]
- 2.Pais GM, Chang J, Barreto EF, Stitt G, Downes KJ, Mohammad AH, Lesnicki E, Panchal V, Bruzzone M, Argyle Bumanglag V, Burke SN, Marc Scheetz H, Scheetz MH. 2022. Clinical pharmacokinetics and pharmacodynamics of cefepime. Clin Pharmacokinet 61:929–953. doi: 10.1007/s40262-022-01137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim PW, Wu Y, Cooper C, Rochester G, Valappil T, Wang Y, Kornegay C, Nambiar S. 2010. Meta-analysis of a possible signal of increased mortality associated with cefepime use. Clin Infect Dis 51:381–389. doi: 10.1086/655131. [DOI] [PubMed] [Google Scholar]
- 4.Cunha BA, Gill MV. 1995. Cefepime. Med Clin North Am 79:721–732. doi: 10.1016/s0025-7125(16)30035-9. [DOI] [PubMed] [Google Scholar]
- 5.Donà V, Scheidegger M, Pires J, Furrer H, Atkinson A, Flury BB. 2019. Gradual in vitro evolution of cefepime resistance in an ST131 Escherichia coli strain expressing a plasmid-encoded CMY-2 β-lactamase. Front Microbiol 10:1–8. doi: 10.3389/fmicb.2019.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaucaire G. 1999. Clinical activity of cefepime in severe infections. Clin Microbiol Infect 5:S6–S14. doi: 10.1111/j.1469-0691.1999.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 7.European Committee on Antimicrobial Susceptibility Testing. 2023. Breakpoint tables for interpretation of MICs and zone diameters, version 13.0.
- 8.Bristol-Myers Squibb Company. 2003. Product information: Maxipime®, cefepime hydrochloride for injection. Bristol-Myers Squibb Company, Princeton, NJ. [Google Scholar]
- 9.Roos JF, Bulitta J, Lipman J, Kirkpatrick CMJ. 2006. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J Antimicrob Chemother 58:987–993. doi: 10.1093/jac/dkl349. [DOI] [PubMed] [Google Scholar]
- 10.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. 2010. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother 54:1111–1116. doi: 10.1128/AAC.01183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamoto MP, Nakahiro RK, Chin A, Bedikian A. 1993. Cefepime clinical pharmacokinetics. Clin Pharmacokinet 25:88–102. doi: 10.2165/00003088-199325020-00002. [DOI] [PubMed] [Google Scholar]
- 12.Turnidge JD. 1998. The pharmacodynamics of beta-lactams. Clin Infect Dis 27:10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 13.Nicasio AM, Ariano RE, Zelenitsky SA, Kim A, Crandon JL, Kuti JL, Nicolau DP. 2009. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 53:1476–1481. doi: 10.1128/AAC.01141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 15.Aitken SL, Altshuler J, Guervil DJ, Hirsch EB, Ostrosky-Zeichner LL, Ericsson CD, Tam VH. 2015. Cefepime free minimum concentration to minimum inhibitory concentration (fCmin/MIC) ratio predicts clinical failure in patients with Gram-negative bacterial pneumonia. Int J Antimicrob Agents 45:541–544. doi: 10.1016/j.ijantimicag.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shaer MH, Neely MN, Liu J, Cherabuddi K, Venugopalan V, Rhodes NJ, Klinker K, Scheetz MH, Peloquin CA. 2020. Population pharmacokinetics and target attainment of cefepime in critically ill patients and guidance for initial dosing. Antimicrob Agents Chemother 64:e00745-20. doi: 10.1128/AAC.00745-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul M, Yahav D, Fraser A, Leibovici L. 2006. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 57:176–189. doi: 10.1093/jac/dki448. [DOI] [PubMed] [Google Scholar]
- 18.Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. 2007. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 7:338–348. doi: 10.1016/S1473-3099(07)70109-3. [DOI] [PubMed] [Google Scholar]
- 19.Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EFM, Rabinstein AA. 2013. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care 17:R264. doi: 10.1186/cc13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alpay H, Altun Ö, Biyikli K. 2004. Cefepime-induced non-convulsive status epilepticus in a peritoneal dialysis patient. Pediatr Nephrol 19:445–447. doi: 10.1007/s00467-003-1333-8. [DOI] [PubMed] [Google Scholar]
- 21.Durand-Maugard C, Lemaire-Hurtel A-S, Gras-Champel V, Hary L, Maizel J, Prud'homme-Bernardy A, Andréjak C, Andréjak M. 2012. Blood and CSF monitoring of cefepime-induced neurotoxicity: nine case reports. J Antimicrob Chemother 67:1297–1299. doi: 10.1093/jac/dks012. [DOI] [PubMed] [Google Scholar]
- 22.Boschung-Pasquier L, Atkinson A, Kastner LK, Banholzer S, Haschke M, Buetti N, Furrer DI, Hauser C, Jent P, Que YA, Furrer H, Babouee Flury B. 2020. Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect 26:333–339. doi: 10.1016/j.cmi.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Lamoth F, Buclin T, Pascual A, Vora S, Bolay S, Decosterd LA, Calandra T, Marchetti O. 2010. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob Agents Chemother 54:4360–4367. doi: 10.1128/AAC.01595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huwyler T, Lenggenhager L, Abbas M, Ing Lorenzini K, Hughes S, Huttner B, Karmime A, Uçkay I, von Dach E, Lescuyer P, Harbarth S, Huttner A. 2017. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect 23:454–459. doi: 10.1016/j.cmi.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Dellinger RP, Levy M, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Aitken L, Al Rahma H, Bernard GR, Biban P, Bion JF, Calandra T, Carcillo JA, Clemmer TP, Divatia JV, Du B, Fujishima S, Gando S, Bruch CG, Guyatt G, Hazelzet JA, Hirasawa H, Hollenberg SM, Jacobi J, Jenkins I, Jimenez E, Jones AE, Kacmarek RM, Kern W, Koh SO, Kotani J, Machado F, Marini J, The Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup , et al. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kois AK, Gluck JA, Nicolau DP, Kuti JL. 2022. Pharmacokinetics and time above the MIC exposure of cefepime in critically ill patients receiving extracorporeal membrane oxygenation (ECMO). Int J Antimicrob Agents 60:106603. doi: 10.1016/j.ijantimicag.2022.106603. [DOI] [PubMed] [Google Scholar]
- 27.Cheng V, Abdul-Aziz MH, Burrows F, Buscher H, Corley A, Diehl A, Jakob SM, Levkovich BJ, Pellegrino V, Que YA, Reynolds C, Rudham S, Wallis SC, Welch SA, Zacharias D, Roberts JA, Shekar K, Fraser JF. 2021. Population pharmacokinetics of cefepime in critically ill patients receiving extracorporeal membrane oxygenation (an ASAP ECMO study). Int J Antimicrob Agents 58:106466. doi: 10.1016/j.ijantimicag.2021.106466. [DOI] [PubMed] [Google Scholar]
- 28.Delattre IK, Musuamba FT, Jacqmin P, Taccone FS, Laterre PF, Verbeeck RK, Jacobs F, Wallemacq P. 2012. Population pharmacokinetics of four β-lactams in critically ill septic patients comedicated with amikacin. Clin Biochem 45:780–786. doi: 10.1016/j.clinbiochem.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes NJ, Grove ME, Kiel PJ, O'Donnell JN, Whited LK, Rose DT, Jones DR, Scheetz MH. 2017. Population pharmacokinetics of cefepime in febrile neutropenia: implications for dose-dependent susceptibility and contemporary dosing regimens. Int J Antimicrob Agents 50:482–486. doi: 10.1016/j.ijantimicag.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, Fraser GL. 2017. Cefepime-induced neurotoxicity: a systematic review. Crit Care 21:276. doi: 10.1186/s13054-017-1856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyner JL. 2012. Assessment and diagnosis of renal dysfunction in the ICU. Chest 141:1584–1594. doi: 10.1378/chest.11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonckheere S, De Neve N, De Beenhouwer H, Berth M, Vermeulen A, Van Bocxlaer J, Colin P. 2016. A model-based analysis of the predictive performance of different renal function markers for cefepime clearance in the ICU. J Antimicrob Chemother 71:2538–2546. doi: 10.1093/jac/dkw171. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz S, Minville V, Asehnoune K, Virtos M, Georges B, Fourcade O, Conil J-M. 2015. Screening of patients with augmented renal clearance in ICU: taking into account the CKD-EPI equation, the age, and the cause of admission. Ann Intensive Care 5:49. doi: 10.1186/s13613-015-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehmann L, Zoller M, Minichmayr IK, Scharf C, Huisinga W, Zander J, Kloft C. 2019. Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents 54:309–317. doi: 10.1016/j.ijantimicag.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 36.Verhave JC, Fesler P, Ribstein J, Du Cailar G, Mimran A. 2005. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis 46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Matthews I, Kirkpatrick C, Holford N. 2004. Quantitative justification for target concentration intervention – parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol 58:8–19. doi: 10.1111/j.1365-2125.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo H, Kim YK, Park S, Kim H, Lee D-H. 2023. Population pharmacokinetics and Monte Carlo simulation of cefepime in critically ill patients with hospital-acquired/ventilator-associated pneumonia. Infect Chemother 55:29–41. doi: 10.3947/ic.2022.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tessier PR, Nicolau DP, Onyeji CO, Nightingale CH. 1999. Pharmacodynamics of intermittent- and continuous-infusion cefepime alone and in combination with once-daily tobramycin against Pseudomonas aeruginosa in an in vitro infection model. Chemotherapy 45:284–295. doi: 10.1159/000007198. [DOI] [PubMed] [Google Scholar]
- 40.Vondracek TG. 1995. Beta-lactam antibiotics: is continuous infusion the preferred method of administration? Ann Pharmacother 3:1. [DOI] [PubMed] [Google Scholar]
- 41.Grant EM, Kuti JL, Nicolau DP, Nightingale C, Quintiliani R. 2002. Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin-tazobactam program in a large community teaching hospital. Pharmacotherapy 22:471–483. doi: 10.1592/phco.22.7.471.33665. [DOI] [PubMed] [Google Scholar]
- 42.Suttels V, Andre P, Thoma Y, Veuve F, Decosterd L, Guery B, Buclin T. 2022. Therapeutic drug monitoring of cefepime in a non-critically ill population: retrospective assessment and potential role for model-based dosing. JAC Antimicrob Resist 4:dlac043. doi: 10.1093/jacamr/dlac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurkacz M, Dobrek L, Wiela-Hojeńska A. 2021. Antibiotics and the nervous system—which face of antibiotic therapy is real, Dr. Jekyll (neurotoxicity) or Mr. Hyde (neuroprotection)? Molecules 26:7456. doi: 10.3390/molecules26247456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zander J, Maier B, Suhr A, Zoller M, Frey L, Teupser D, Vogeser M. 2015. Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation. Clin Chem Lab Med 53:781–791. [DOI] [PubMed] [Google Scholar]
- 45.Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ (ed). 2018. N7 users guides https://nonmem.iconplc.com/nonmem743/guides.
- 46.Baron KT. 2022. Models, mrgsolve: simulate from ODE-based. R version 4.2.1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.