ABSTRACT
Nanoparticle-based antibiotic delivery systems are essential in combating antibiotic-resistant bacterial infections arising from acquired resistance and/or biofilm formation. Here, we report that the ceftazidime-decorated gold nanoparticles (CAZ_Au NPs) can effectively kill clinical ceftazidime-avibactam-resistant Enterobacteriaceae with various resistance mechanisms. Further study of underlying antibacterial mechanisms suggests that CAZ_Au NPs can damage the bacterial cell membrane and increase the level of intracellular reactive oxygen species. Moreover, CAZ_Au NPs show great potential in inhibiting biofilm formation and eradicating mature biofilms via crystal violet and scanning electron microscope assays. In addition, CAZ_Au NPs demonstrate excellent performance in improving the survival rate in the mouse model of abdominal infection. In addition, CAZ_Au NPs show no significant toxicity at bactericidal concentrations in the cell viability assay. Thus, this strategy provides a simple way to drastically improve the potency of ceftazidime as an antibiotic and its use in further biomedical applications.
KEYWORDS: Enterobacteriaceae, ceftazidime-avibactam, antimicrobial resistance, gold nanoparticles
INTRODUCTION
The evolution of bacterial resistance to antibiotics leads humanity to a situation reminiscent of the “preantibiotic era” (1). Antimicrobial resistance poses a pressing global public health crisis, resulting in the deaths of at least 1.27 million people worldwide and being associated with nearly 5 million fatalities in 2019, according to a report released in the Lancet (2). Carbapenem-resistant Enterobacteriaceae (CRE) are the principal pathogens among antimicrobial-resistant bacteria (3). CRE infections are difficult to treat. Babiker et al. collected clinical data related to CRE infections at a local hospital from 2000 to 2017, and their findings showed that the 1-year mortality rate for CRE infections, including Escherichia coli, Enterobacter spp., and Klebsiella pneumoniae, was as high as 49.2% (566 of 1,319) (4). Most antibiotics are ineffective against CRE, with the exception of tigecycline, colistin, eravacycline, meropenem-vaborbactam, imipenem-relebactam, ceftazidime-avibactam (CZA) (5), and so on.
CZA is a combination of the third-generation cephalosporin ceftazidime (CAZ) and a novel non-β-lactam β-lactamase inhibitor avibactam (AVI) available since 2015 (6). CAZ inhibits cell wall synthesis of Gram-negative bacteria by binding to the penicillin-binding proteins, thus showing broad-spectrum antibacterial activity. The antibacterial activity of CAZ can be expanded by AVI, particularly against Ambler classes A (e.g., extended-spectrum β-lactamases [ESBLs], K. pneumoniae carbapenemases [KPCs]), C (e.g., AmpC β-lactamases), and some class D (e.g., oxacillinase [OXA]-48) enzyme-producing strains (7). Although CZA has excellent in vitro activity against CRE, there has been a gradual increase in studies reporting CZA resistance among CRE worldwide (8–12). Bacteria may develop resistance to CZA via three primary mechanisms: (i) enzymatic resistance causing antibiotic inactivation; (ii) chemical modification of the antibiotic target or the expression of an alternative target; and (iii) alterations in cell permeability or expression of efflux pumps. Among these, mechanism A is the most common cause of CZA resistance (7). CZA does not exhibit bactericidal activity against CRE that produce Ambler class B enzymes (e.g., new delhi metallo-β-lactamase [NDM], verona integron-encoded metallo-β-lactamase [VIM], imipenemase [IMP]) and some class D enzymes (e.g., OXA-23) (13). Furthermore, CZA resistance may depend on the mutations at different β-lactamase sites (e.g., KPC-33 [variant of β-lactamase KPC-2, D179Y] [14], KPC-71 [variant of β-lactamase KPC-2, Ins182-183 S] [15], KPC-88 [variant of β-lactamase KPC-2, D176Y] [16], CMY-42 [CMY-2 variant AmpC β-lactamase, V231S] [17]). In addition, an overproduction of efflux pumps (16), the modification and low expression of outer membrane proteins (e.g., OmpK37 mutation [18], low expression of OmpC and OmpF [19]) can also result in CZA resistance.
Considering the increase in CZA resistance and its multiple underlying mechanisms, it is urgently required to effectively treat CZA-resistant Enterobacteriaceae infection. The process of developing new medications is characterized by high costs, protracted development times, and low success rates (20). However, compared to this, reengineering antibiotics is a simpler task and has a broad range of applications, especially in combating antimicrobial resistance (21). With the rapid development of nanotechnology, nanomaterials have been comprehensively applied in modern society (22). In the field of nanomedicine, metal nanoparticles such as Ag, Au, and Zn have shown impressive properties such as low in vivo toxicity and bactericidal activity (23–25). Metal nanoparticles have provided possible solutions to bacterial resistance with the help of mechanisms such as the formation of irreversible pores, interference with the efflux pump, disruption of cell membranes, and direct interaction with bacterial macromolecules (26–28). Among various metal nanoparticles, gold nanoparticles (Au NPs) are distinctive because of their small size, uncomplicated surface chemistry, and availability of surface modification (29). Several studies have suggested the use of Au NPs to deliver antibiotics such as penicillin (30), kanamycin (31), imipenem (32), meropenem (32), cefoxitin (33), and colistin (34). However, these studies continue to present four main problems: (i) antibiotic-resistant bacteria that correspond to the delivered antibiotic are not used; (ii) the antibiotic resistance mechanism is not clear or comprehensive; (iii) in vivo experiments have not been performed; and (iv) the selection of controls often lacks Au NPs without any decoration.
Thus, this study aims to examine the efficacy of ceftazidime-decorated gold nanoparticles (CAZ_Au NPs) in killing CZA-resistant Enterobacteriaceae with different resistance mechanisms. CAZ_Au NPs were prepared, characterized, and examined for their antibacterial activities against various CZA-resistant Enterobacteriaceae.
RESULTS AND DISCUSSION
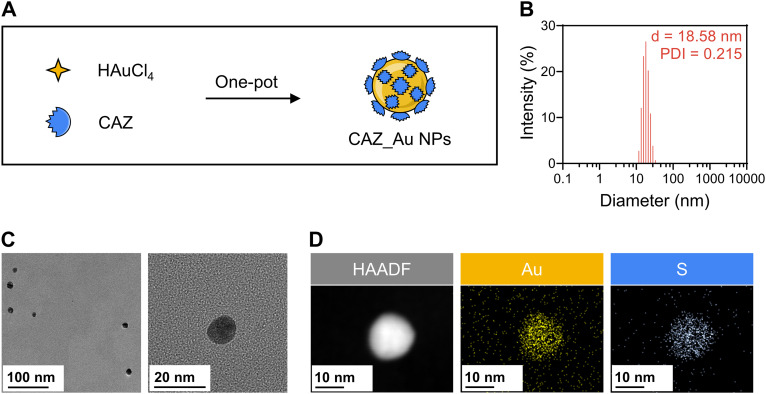
Synthesis and characterization of CAZ_Au NPs.
Tetrachloroauric acid (HAuCl4) is most commonly used to prepare Au NPs. This process, which employs a direct one-step reaction, requires a reductive environment that is created by reducing agents such as citric acid or NaBH4 (35). This synthesis method, called the one-pot method, is a simple, fast, and low-cost approach for the construction of organic or inorganic functional nanoparticles (36). One-pot synthesis via antibiotics reduces the likelihood of any unwanted antibacterial outcomes (33). Studies also report that the amine group in β-lactam antibiotics takes part in the reduction (37), which provides the theoretical basis for our study.
In our study, CAZ_Au NPs were successfully synthesized by reducing HAuCl4 with excessive CAZ (Fig. 1A). Because of the specific reducibility of CAZ, the Au3+ in HAuCl4 could be reduced to Au0 to form CAZ_Au NPs via the Au−N bond. The residual CAZ was removed via dialysis. Dynamic light scattering (DLS) showed that the average size was 18.58 nm and the polydispersity index (PDI) was 0.215 (Fig. 1B), which indicated a small size and homogeneous dispersion, respectively. Transmission electron microscopy (TEM) showed that the nanoparticles were round with uniform sizes of approximately 11 nm (Fig. 1C). The DLS size was larger than the TEM size. This could be because TEM determined the dry particle size, whereas DLS reflected the hydrodynamic size (38). It was noticeable that nanoparticles of smaller sizes exhibited improved antimicrobial activity (39). Thus, the antimicrobial activity of CAZ_Au NPs was well anticipated. Furthermore, energy dispersive spectroscopy (EDS) confirmed that CAZ_Au NPs contained gold and sulfur, which were homogeneously distributed over the nanoparticles (Fig. 1D). Sulfur existed only among the raw materials in ceftazidime, further indicating the successful synthesis of CAZ_Au NPs. Additionally, NaBH4 was chosen to reduce Au3+ to Au0, forming Au NPs that were used in further studies as the control.
FIG 1.
Synthesis and characterizations of ceftazidime-decorated gold nanoparticles (CAZ_Au NPs). (A) Scheme for the synthesis of CAZ_Au NPs. (B) Size distribution intensity plot generated by dynamic light scattering (DLS) for CAZ_Au NPs. (C) Representative transmission electron microscopy (TEM) images of CAZ_Au NPs. (D) TEM mapping of CAZ_Au NPs. PDI, polydispersity index; d, diameter. HAADF, high-angle annular dark-field.
In further studies evaluating the antibacterial activity, antibiofilm activity, and biosafety, due to the difficulty in measuring the concentration of CAZ in the CAZ_Au NPs after dialysis, the concentration of CAZ_Au NPs was determined based on the concentration of gold. The methodology used by Li et al. was referenced (40), in which the same component was fixed at the same concentration in various formulations through dilution for subsequent studies. Similarly, the consistent concentration of Au in both CAZ_Au NPs and Au NPs was ensured through dilution. Additionally, the potential concentration of CAZ in CAZ_Au NPs before dialysis was calculated based on the dilution ratio, allowing for the establishment of the CAZ group accordingly. It should be noted that dialysis removed the free CAZ, and thus, the concentration of CAZ in the CAZ group represented the maximum possible concentration of CAZ in CAZ_Au NPs. For example, when the concentration of CAZ_Au NPs was 1 μg/mL, it could contain up to 2.8 μg/mL of CAZ, and when the concentration of CAZ_Au NPs was 4 μg/mL, it could contain up to 11.1 μg/mL of CAZ. Furthermore, because CAZ was usually combined with AVI for clinical use (41), the CZA group was also set up. The CZA group constituted the addition of 4 μg/mL (in vitro) (42) or 8 mg/kg (in vivo) (43) of AVI based on the CAZ group.
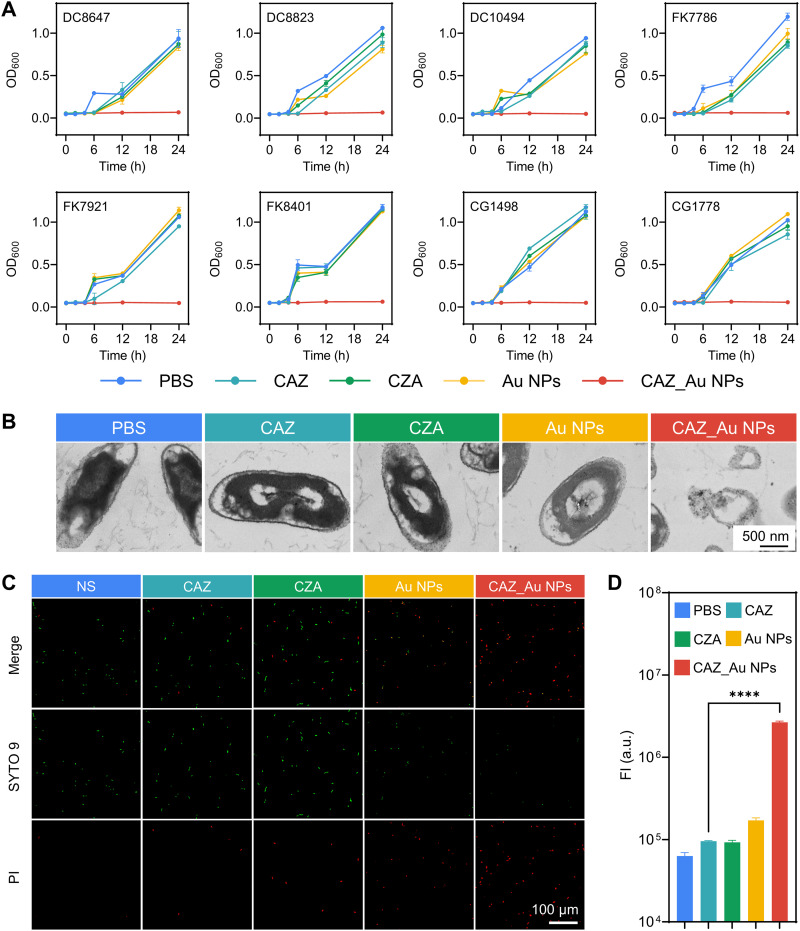
Study of in vitro antibacterial properties and mechanisms.
As shown in Table 1, 34 nonduplicate CZA-resistant Enterobacteriaceae strains were collected, including 9 E. coli, 16 K. pneumoniae, and 9 Enterobacter cloacae strains. The mechanisms of resistance to CZA were verified using PCR and sequencing. Partial data were confirmed from our previous study (11, 12, 44). The main resistance mechanism was the production of Ambler class B β-lactamases (NDM-1, NDM-5, IMP). Moreover, other resistance mechanisms such as OXA-23, KPC-33, KPC-71, KPC-88, CMY-42, and OmpK37 mutation and low expression of OmpC and OmpF were included. CAZ_Au NPs demonstrated excellent antibacterial activity against all strains with the MIC ranging from 1 to 4 μg/mL (containing up to 2.8 to 11.1 μg/mL CAZ) with the exception of KPC-71 and KPC-88 strains, whose MIC was 8 μg/mL (containing up to 22.2 μg/mL CAZ). The MIC values of Au NPs, CAZ, and CZA were ≥256, 512, and 512/4 μg/mL, respectively (except for KPC mutant strains), indicating that these agents did not have significant standalone antibacterial activity. The KPC mutant strains showed MIC values ≥256 μg/mL for Au NPs, MIC values ranging from 32 μg/mL to 256 μg/mL for CAZ, and MIC values ranging from 32/4 to 256/4 μg/mL for CZA. This indicated that CAZ_Au NPs had better bactericidal effects against these KPC mutant strains than CZA. To conduct further in vitro investigation of antibacterial properties and mechanisms, the concentration of CAZ_Au NPs was determined using the MIC values. The concentrations of CAZ, CZA, and Au NPs were previously noted. Some randomly selected strains were subjected to a growth curve assay to examine the effect of CAZ_Au NPs on the growth kinetics of CZA-resistant strains. As shown in Fig. 2A, bacteria exposed to CAZ, CZA, and Au NPs displayed a killing curve comparable to phosphate-buffered saline (PBS), which all showed significant growth. While CAZ_Au NPs effectively inhibited bacterial growth. Meanwhile, to observe the change in bacterial structures after various treatments, the TEM assay was performed, and DC10494 was randomly selected as the experimental strain. The TEM images showed that CAZ_Au NPs induced bacterial lysis, whereas the bacterial structures in other groups were intact (Fig. 2B). The change in cell membrane permeability and reactive oxygen species (ROS) might be the mechanism underlying the antibacterial properties of CAZ_Au NPs (28); thus, we performed experiments to corroborate this notion. DC10494 was randomly selected as the experimental strain. The changes in cell membrane permeability were further investigated via live/dead staining assays and visualized using confocal laser scanning microscopy (CLSM). In this experiment, the bacteria exhibiting cell membrane damage were marked with propidium iodide (PI) that emits red fluorescence, whereas those with intact cell membranes were stained using the green fluorescent probe SYTO 9. As shown in Fig. 2C, the red fluorescence was weak in the normal saline (NS) group, indicating that most bacteria had intact cell membranes after 4 h of incubation at 37°C without any drug treatment. The bacterial suspension treated with CAZ and CZA showed a slightly higher intensity of red fluorescence, indicating that CAZ and CZA could increase the cell membrane permeability of some bacteria. In contrast, bacteria treated with CAZ_Au NPs showed highly intense red fluorescence and a slight green fluorescence, suggesting that most of the bacterial cell membranes were damaged. We also found that treatment with Au NPs could increase the cell membrane permeability of most bacteria in NS, which agreed with the above-mentioned hypothesis. Therefore, the ability of CAZ_Au NPs to alter the permeability of the cell membrane is likely to be conferred by Au NPs. With regard to ROS detection, we found that after exposure for 2 h at 37°C, Au NPs and CAZ_Au NPs could significantly increase the ROS level compared to the PBS group (Fig. 2D), which also corresponds to the above-mentioned hypothesis. Between Au NPs and CAZ_Au NPs, the effect of CAZ_Au NPs was more prominent, which explains its excellent antibacterial properties. In addition, to explore whether CAZ and Au NPs exhibited a synergistic antibacterial effect that resulted in excellent antibacterial properties of CAZ_Au NPs, a checkerboard assay was conducted. As shown in Table S1, over a wide concentration range, a combination of CAZ and Au NPs did not enhance antibacterial effects, suggesting that antibacterial property of CAZ_Au NPs was attributed to “decoration” rather than “synergy”.
TABLE 1.
CZA resistance and antimicrobial susceptibility of the CAZ, CZA, Au NPs, and CAZ_Au NPsa
| Species | Strains | Antimicrobial resistance mechanism | MIC (μg/mL) |
|||
|---|---|---|---|---|---|---|
| CAZ | CZA | Au NPs | CAZ_Au NPs | |||
| E. coli | DC8466 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 1 |
| DC8647 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 2 | |
| DC8823 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 1 | |
| DC8896 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 2 | |
| DC9016 | NDM-5 | ≥512 | ≥512/4 | ≥256 | 4 | |
| DC10494 | NDM-5 | ≥512 | ≥512/4 | ≥256 | 4 | |
| DC10957 | NDM-5 | ≥512 | ≥512/4 | ≥256 | 4 | |
| DC11712 | CMY-42 | ≥512 | ≥512/4 | ≥256 | 2 | |
| DC11722 | CMY-42 | ≥512 | ≥512/4 | ≥256 | 2 | |
| K. pneumoniae | FK6709 | OXA-23 | ≥512 | ≥512/4 | ≥256 | 1 |
| FK7079 | IMP | ≥512 | ≥512/4 | ≥256 | 2 | |
| FK7112 | OmpK37 mutation | ≥512 | ≥512/4 | ≥256 | 1 | |
| FK7513 | NDM-5 | ≥512 | ≥512/4 | ≥256 | 1 | |
| FK7710 | NDM-5 | ≥512 | ≥512/4 | ≥256 | 1 | |
| FK7786 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 2 | |
| FK7921 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 1 | |
| FK7978 | NDM-5 | ≥512 | ≥512/4 | ≥256 | 1 | |
| FK8401 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 1 | |
| FK8696 | KPC-33 | 256 | 256/4 | ≥256 | 1 | |
| FK3746-HR | KPC-71 | 128 | 128/4 | ≥256 | 8 | |
| FK3831-HR | KPC-88 | 32 | 32/4 | ≥256 | 8 | |
| FK6299-HR | KPC-71 | 128 | 128/4 | ≥256 | 8 | |
| FK6304-HR | KPC-88 | 64 | 64/4 | ≥256 | 8 | |
| FK6506-HR | KPC-71 | 128 | 128/4 | ≥256 | 8 | |
| FK6625-HR | KPC-88 | 64 | 64/4 | ≥256 | 8 | |
| E. cloacae | CG1381 | OXA-23 | ≥512 | ≥512/4 | ≥256 | 1 |
| CG1400 | Low expression of OmpC and OmpF | ≥512 | ≥512/4 | ≥256 | 4 | |
| CG1498 | IMP | ≥512 | ≥512/4 | ≥256 | 4 | |
| CG1574 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 2 | |
| CG1591 | IMP | ≥512 | ≥512/4 | ≥256 | 2 | |
| CG1593 | IMP | ≥512 | ≥512/4 | ≥256 | 2 | |
| CG1608 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 2 | |
| CG1737 | NDM-5 | ≥512 | ≥512/4 | ≥256 | 4 | |
| CG1778 | NDM-1 | ≥512 | ≥512/4 | ≥256 | 2 | |
The table indicates the mechanisms of CZA resistance and antimicrobial susceptibility of the CAZ, CZA, Au NPs, and CAZ_Au NPs against the 34 clinical isolates used in this study. Au NP, gold nanoparticle; CAZ, ceftazidime; CAZ_Au NP, ceftazidime-decorated gold nanoparticle; CZA, ceftazidime-avibactam; HR, heteroresistance; OXA, oxacillinase; NDM, new delhi metallo-β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; IMP, imipenemase.
FIG 2.
CAZ_Au NPs induce bacterial growth inhibition and lysis via the elevation of cell membrane permeability and reactive oxygen species (ROS). (A) Growth curve of ceftazidime-avibactam (CZA)-resistant Enterobacteriaceae after various treatments. (B) Representative TEM images indicating the morphologies of CZA-resistant Enterobacteriaceae after various treatments. (C) Representative CLSM images showing the cell membrane permeability of CZA-resistant Enterobacteriaceae after various treatments. (D) Fluorescence intensity analysis of intracellular ROS in CZA-resistant Enterobacteriaceae after various treatments by using a ROS assay kit. NS, normal saline; OD600, optical density at 600 nm; PBS, phosphate-buffered saline; PI, propidium iodide.
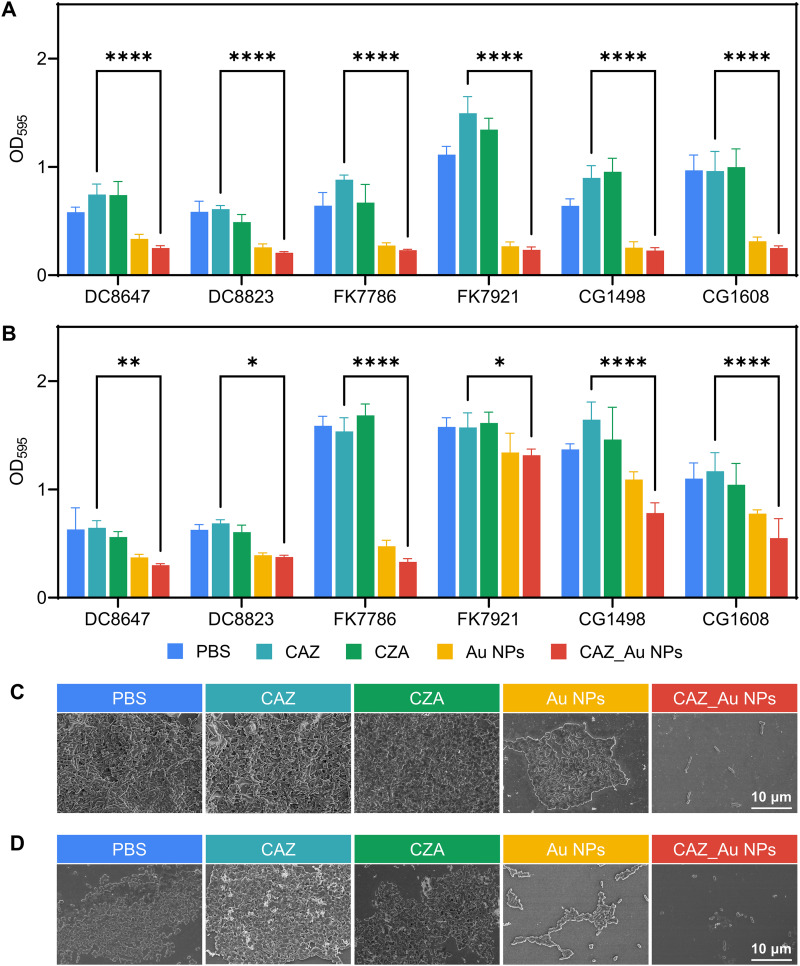
Study of in vitro antibiofilm properties.
Biofilms are a collection of bacteria and their extracellular polymeric products. Bacteria living in biofilms are 10 to 1,000 times more resistant to standard antibiotic treatments than those living in planktonic environments (45). This indicates the importance of antibiofilm treatments, and novel antibiofilm agents are urgently required. Many nanoparticles have been reported for their antibiofilm properties (46–48).
Here, the antibiofilm properties of CAZ_Au NPs were investigated via the crystal violet and scanning electron microscope (SEM) assays. The concentration of CAZ_Au NPs was determined based on the 1/2 MIC values. The concentrations of CAZ, CZA, and Au NPs have been previously noted. Fig. 3 (A and B) shows the results of the crystal violet assay for biofilm-inhibition and -eradication effects. These figures indicated that biofilms treated with Au NPs and CAZ_Au NPs were significantly inhibited and eradicated. An SEM assay was performed to further characterize these biofilm-inhibition and -eradication effects. FK7079 was randomly selected as the experimental strain. The SEM assay showed results that were similar to the crystal violet assay, revealing that bacterial groups treated with PBS, CAZ, and CZA exhibited a thick biofilm, whereas those treated with Au NPs showed significant eradication of the biofilm. Furthermore, only dispersed individual bacteria were observed in the CAZ_Au NPs treatment group. Overall, the results of this study indicated that Au NPs and CAZ_Au NPs could inhibit biofilm formation and eradicate mature biofilms.
FIG 3.
CAZ_Au NPs inhibit the form of biofilm and eradicate mature biofilm ex vivo. (A) The biofilm-inhibitory effects of various treatments by measuring absorbance at 595 nm of crystal violet-stained CZA-resistant Enterobacteriaceae biofilms. (B) Biofilm-eradication effects of the various treatments by measuring absorbance at 595 nm (OD595) of crystal violet-stained CZA-resistant Enterobacteriaceae biofilms. (C) Representative scanning electron microscope (SEM) images indicating the biofilm-inhibitory effects of CZA-resistant Enterobacteriaceae after various treatments. (D) Representative SEM images demonstrating the biofilm-eradication effects of CZA-resistant Enterobacteriaceae after various treatments.
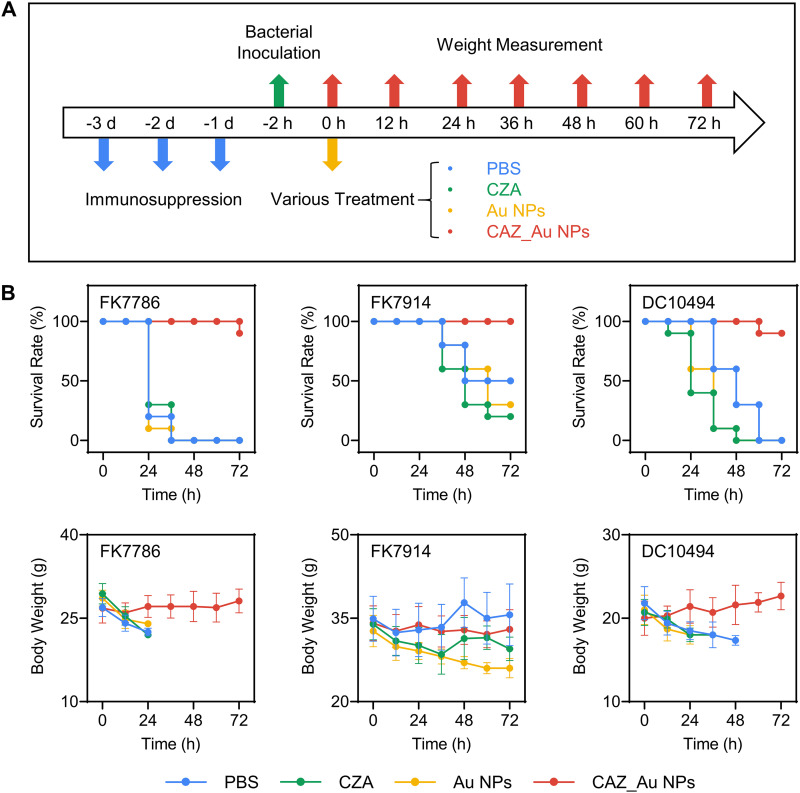
Study of in vivo antibacterial properties.
CZA-resistant Enterobacteriaceae strains were not found to be lethal in healthy mice, which might be attributed to their low virulence (49, 50). Thus, an immune deficiency mouse model was established to simulate the infection of these bacteria in clinical immunocompromised patients (51–53). FK7786, FK7914, and DC10494 were randomly selected as experimental strains. The schematic of the in vivo experiment is shown in Fig. 4A. Briefly, cyclophosphamide (200 μL, 150 mg/kg) was injected into the mouse abdominal cavity for 3 days to establish an immune deficiency model. Subsequently, a bacterial suspension (200 μL, 1 × 105 CFU) was injected into the abdominal cavities of the mice. Considering that CAZ has been often used in combination with AVI for the treatment of clinical infections (41), the mice were randomly divided into four groups of 10, including the PBS group, CZA group, Au NP group, and CAZ_Au NP group. After 2 h, the corresponding agents were injected into the abdominal cavities, and the mice were weighed every 12 h. The concentration of CAZ_Au NPs was 10 mg/kg, and the concentration of CZA and Au NPs had been noted before. The volume injected per mouse was 200 μL. As shown in Fig. 4B, mice treated with CAZ_Au NPs had a survival rate of 100 or 90% after 72 h. On the contrary, mice infected with FK7786 and DC10494 and treated with PBS, CZA, and Au NPs died after 72 h. Mice infected with FK7914 showed a survival rate of only ≤50% when treated with PBS, CZA, and Au NPs for 72 h. These results demonstrated the excellent effects of CAZ_Au NPs in enhancing the survival rate in the mouse model of abdominal infection. As for body weights, it was observed that the differences in the weights of mice in the same group were unavoidable, and mice with low body weights were more likely to die. Therefore, it made more sense to analyze changes in body weight before the mice died. The body weights of infected mice treated with PBS, CZA, and Au NPs decreased significantly. The group treated with CAZ_Au NPs showed low to no reduction in body weights, demonstrating their strong therapeutic potential.
FIG 4.
CAZ_Au NPs improve the survival for mice with intraperitoneal infection caused by CZA-resistant Enterobacteriaceae. (A) Workflow of experimental processing. (B) Survival rate and body weight of CZA-resistant Enterobacteriaceae-infected mice after various treatments at different time points.
In vitro biocompatibility.
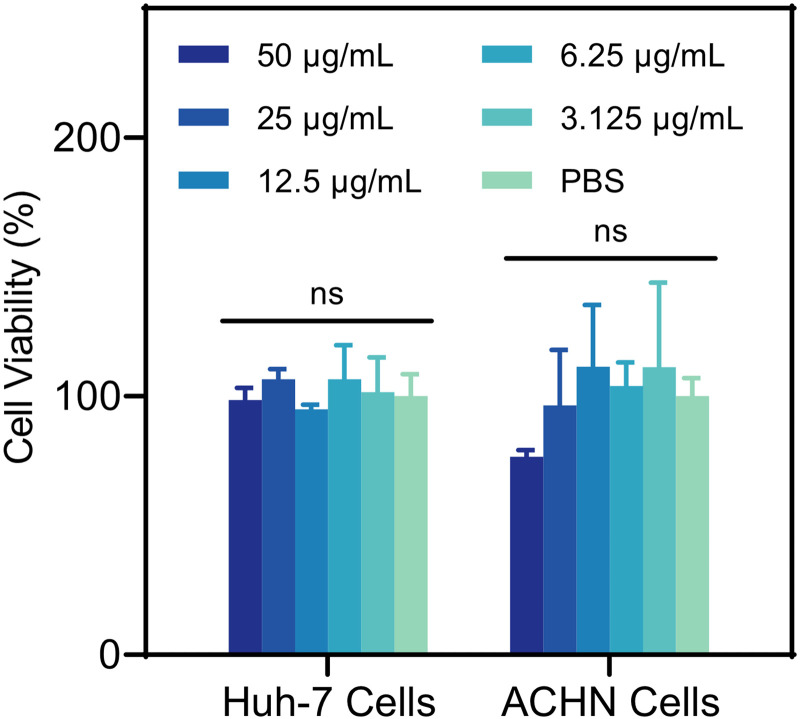
To initially evaluate the safety of CAZ_Au NPs in vivo, an in vitro cell viability experiment was conducted. Considering that drug metabolism is primarily related to the liver and kidney (54, 55), the Huh-7 cell line (origin: human liver) and the ACHN cell line (origin: human kidney) were selected. As seen in Fig. 5, there was no significant variation in cell viability between CAZ_Au NPs of different concentrations and PBS after treatment of 24 h, which meant biosafety at bactericidal concentrations.
FIG 5.
Cell viability of the Huh-7 and ACHN cells treated with different concentrations of CAZ_Au NPs tested by using the CCK-8 kit for 24 h. ns, not significant.
CONCLUSION
Using nanoparticles to deliver antibiotics to combat antimicrobial resistance is an excellent strategy in today’s context. In this study, CAZ_Au NPs were successfully synthesized and characterized, which showed small size and homogeneity. We found that CAZ_Au NPs could efficiently kill CZA-resistant Enterobacteriaceae with various resistance mechanisms by damaging the bacterial membrane and inducing intracellular ROS generation. Furthermore, CAZ_Au NPs could inhibit biofilm formation and eradicate mature biofilms. Moreover, CAZ_Au NPs showed excellent performance in improving the survival rate in the mouse model of abdominal infection. Importantly, CAZ_Au NPs showed no significant toxicity to liver and kidney cells at bactericidal concentrations. Owing to the impressive antibacterial, antibiofilm, and biosafety properties, CAZ_Au NPs showed a very appealing application prospect. The use of novel nanotechnology enhances the potency of CAZ and extends its applicability. The clinical application of CAZ_Au NPs in preclinical studies and clinical trials should be explored in the future. Lastly, we believe that Au NPs can serve as a universal platform to deliver other drugs to mitigate antibiotic resistance.
MATERIALS AND METHODS
Materials.
The main materials used in this study and their corresponding manufacturers are listed in Table S2.
Bacterial isolates.
A total of 34 nonduplicate clinical CZA-resistant Enterobacteriaceae strains were isolated, including 28 strains obtained from the First Affiliated Hospital of Wenzhou Medical University (Zhejiang, China). Additionally, six strains (KPC-71 and KPC-88) were previously selected by our team as CZA-heteroresistant strains according to the population analysis profile methodology published by Band et al. (56). All isolates were identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS; bioMérieux, Lyon, France) according to the manufacturer’s protocols and demonstrated stable resistance even after continuous passaging. The National Center for Clinical Laboratory provided the quality control strain E. coli ATCC 25922.
Preparation of CAZ_Au NPs and Au NPs.
CAZ_Au NPs and Au NPs were prepared as previously described, with minor changes (57). Briefly, CAZ (0.05 mmoL), Tween 80 (30 mg), and triethylamine (50 μL) were dissolved in 10 mL ice-cold water for 20 min by ultrasonication to obtain a mixture. Then, HAuCl4 (0.05 mmol, 200 μL) was added to the mixture dropwise under vigorous stirring (1,000 rpm) in an ice-cold water bath. The mixture was continuously stirred for the next 2 h. The solution turned brown, and CAZ_Au NPs were formed. Furthermore, the NaBH4 reduction method was used to prepare the Au NPs without any decoration. HAuCl4 (0.05 mmol) and Tween 80 (30 mg) were mixed in 10 mL of ice-cold water for 10 min in an ice-water bath. NaBH4 (6 mg, dissolved in 2 mL ice-cold water) was added into the mixture dropwise under vigorous stirring (1,000 rpm). The color of the solution changed immediately to brown, and the solution was stirred for another 2 h. To remove any unreacted compounds, CAZ_Au NPs and Au NPs underwent dialysis in double-distilled water for 1 day and were sterilized by filtering through a 0.22-μm filter.
Characterization of CAZ_Au NPs.
The size and dispersibility of the nanoparticle were assessed using a DLS instrument (Malvern Zetasizer Nano ZS90, England). The morphologies and elements were characterized by TEM/EDS (JEOL JEMF200, Japan).
Antimicrobial susceptibility testing.
The MIC was determined by the broth microdilution method. The MIC values of CAZ and CZA were measured according to the latest Performance Standards for Antimicrobial Susceptibility Testing published by the America Clinical and Laboratory Standards Institute. The MIC values of Au NPs and CAZ_Au NPs were determined as previously described with some modifications (57). Briefly, a bacterial density of 1 × 106 CFU/mL with Au NPs or CAZ_Au NPs of different concentrations was prepared in 100 μL of Luria-Bertani (LB) medium. The growth of bacteria was determined by visual inspection after incubating for 16 to 20 h at 37°C.
Growth curve.
The growth curve experiment was performed similarly to antimicrobial susceptibility testing. First, 200 μL of LB medium with a bacterial density of 1 × 106 CFU/mL was inoculated with CAZ, CZA, Au NPs, or CAZ_Au NPs of corresponding concentrations. The absorbance at 600 nm was measured at 0, 2, 4, 6, 12, and 24 h using a microplate reader.
Bacterial morphology observation by TEM.
The bacteria treated with PBS, CAZ, CZA, Au NPs, and CAZ_Au NPs were fixed by 2.5% glutaraldehyde and 0.1% osmic acid and dehydrated with increasing concentrations of ethanol (30, 50, 70, 80, 90, 95, and 100%). The samples were then cut into slices and stained with 0.2% lead citrate and 2% uranyl acetate.
SYTO 9/PI staining assay.
Bacteria were treated with NS, CAZ, CZA, Au NPs, and CAZ_Au NPs at 37°C for 4 h and stained with SYTO 9 (0.5 μM) and PI (3.0 μM) according to the manufacturer’s protocol. The samples were observed under a confocal microscope (Nikon A1R-SIM-STORM, Japan) at excitation wavelengths of 488 and 561 nm and emission wavelengths of 530 (green) and 617 (red) nm.
ROS detection.
As per the manufacturer’s protocols, 500 μL of bacterial suspensions (with 106 CFU/mL) were incubated with 10 μM dichloro-dihydro-fluorescein diacetate (DCFH-DA) probes at 37°C for 45 min in the dark to load cells with probes. It was then washed three times with 500 μL PBS to remove the free probes. After that, the DCFH-DA-loaded bacteria were treated with 500 μL PBS, CAZ, CZA, Au NPs, or CAZ_Au NPs at 37°C for 2 h, respectively. Bacteria treated with PBS were used as the negative control. The bacterial suspension after drug treatment was divided into black 96-well opaque plates with 150 μL/well. The fluorescence intensity was detected using a microplate reader at the excitation and emission wavelengths of 488 and 535 nm, respectively.
Checkerboard assay.
The checkerboard assay was performed as previously described with some modifications (58). Briefly, a bacterial density of 1 × 106 CFU/mL with Au NPs or CAZ of different concentrations single or in combination was prepared. The growth of bacteria was determined by visual inspection after incubating for 16 to 20 h at 37°C.
Crystal violet assay.
To determine the inhibition of biofilm formation, a bacterial density of 1 × 106 CFU/mL with CAZ, CZA, Au NPs, or CAZ_Au NPs of corresponding concentrations was added to 200 μL of LB medium. After incubating at 37°C for 24 h, the biofilms were gently washed and dried, and 200 μL of 1.0% crystal violet was added. After staining for 15 min, the biofilms were washed and dried. To dissolve crystal violet, 200 μL of 95% ethanol and 5% acetic acid were added. A clean 96-well plate was used to transfer the dissolved crystal violet. The absorbance at 600 nm was measured by a microplate reader. To determine the eradication of mature biofilms, a bacterial density of 1 × 106 CFU/mL was added to 200 μL of LB medium and incubated at 37°C for 24 h. The biofilms were then gently washed. After that, 200 μL of PBS containing CAZ, CZA, Au NPs, or CAZ_Au NPs of corresponding concentrations was added. After incubating for 24 h, a crystal violet assay was performed similarly to the inhibition of the biofilm formation experiment.
SEM assay.
Silicon wafers (3 × 3 mm) were put into 24-well plates to provide a surface for biofilm formation. To determine the inhibition of biofilm formation, 1 mL of LB medium with a bacterial density of 1 × 106 CFU/mL and CAZ, CZA, Au NPs, or CAZ_Au NPs of corresponding concentrations was incubated at 37°C for 24 h. Then, the silicon wafers were rinsed thrice with PBS, fixed with ice-cold 2.5% glutaraldehyde for 15 min, and dehydrated for 10 min in increasing concentrations of ethanol (30, 50, 70, 80, 90, and 100%). The samples were air-dried, gold-sprayed, and then observed by SEM (Hitachi SU8010, Japan). To determine the eradication of mature biofilms, a bacterial density of 1 × 106 CFU/mL was added to 1 mL of LB medium and incubated at 37°C for 24 h. Then, the silicon wafers were gently washed. After that, 1 mL of PBS containing CAZ, CZA, Au NPs, or CAZ_Au NPs of corresponding concentrations was added. After incubating for 24 h, a SEM assay was performed similarly to the inhibition of the biofilm formation experiment.
In vivo antibacterial activity.
Male ICR mice (6 to 8 weeks old, specific pathogen-free [SPF]) were obtained from Vital River (Zhejiang, China). The animal study was reviewed and approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (approval SYXK 2021-0017). All animal research was performed in compliance with Wenzhou Laboratory Animal Welfare and Ethics standards. The workflow is mentioned in the Results and Discussion section. At the end of the experiment, the surviving mice were euthanized.
Cell viability assay.
Huh-7 cells and ACHN cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum and incubated at 37°C in a 5% CO2 incubator. Then, 10 μL of CAZ_Au NPs of different concentrations were added into 100 μL cell suspension (2,000 cells). After incubating for 24 h, 10 μL of CCK-8 reagent was added to each well, and the plate was incubated at 37°C in the dark for 1 h. The absorbance at 450 nm was measured using a microplate reader. The percentage of cell viability was calculated as follows: cell viability (%) = (absorbance of sample − absorbance of medium)/(absorbance of negative control − absorbance of medium) × 100.
Statistical analysis.
The data are expressed as means ± standard deviation from at least three independent experiments. Statistical analysis was performed by one-way analysis of variance (ANOVA), and P values <0.05 were considered statistically significant (indicated with *), 0.01 (indicated with **), 0.001 (indicated with ***), and 0.0001 (indicated with ****). GraphPad Prism 9.4 software was used to perform the statistical analysis.
ACKNOWLEDGMENT
We declare no conflict of interest.
This work was funded by grant 2022E10022 from the Key Laboratory of Clinical Laboratory Diagnosis and Translational Research of Zhejiang Province.
Footnotes
Supplemental material is available online only.
Contributor Information
Tieli Zhou, Email: wyztli@163.com.
Lijiang Chen, Email: 29340442@qq.com.
REFERENCES
- 1.Mathé-Hubert H, Amia R, Martin M, Gaffé J, Schneider D. 2022. Evolution of bacterial persistence to antibiotics during a 50,000-generation experiment in an antibiotic-free environment. Antibiotics 11:451. doi: 10.3390/antibiotics11040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antimicrobial Resistance Collaborators. 2022. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Noriega E, Garza-González E, Bocanegra-Ibarias P, Paz-Velarde BA, Esparza-Ahumada S, González-Díaz E, Pérez-Gómez HR, Escobedo-Sánchez R, León-Garnica G, Morfín-Otero R. 2022. A case-control study of infections caused by Klebsiella pneumoniae producing New Delhi metallo-β-lactamase-1: predictors and outcomes. Front Cell Infect Microbiol 12:867347. doi: 10.3389/fcimb.2022.867347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babiker A, Clarke LG, Saul M, Gealey JA, Clancy CJ, Nguyen MH, Shields RK. 2021. Changing epidemiology and decreased mortality associated with carbapenem-resistant Gram-negative bacteria, 2000–2017. Clin Infect Dis 73:e4521–e4530. doi: 10.1093/cid/ciaa1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doi Y. 2019. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis 69:S565–S575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiore M, Alfieri A, Di Franco S, Pace MC, Simeon V, Ingoglia G, Cortegiani A. 2020. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant pathogens: a systematic review and network meta-analysis. Antibiotics 9:388. doi: 10.3390/antibiotics9070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Wang J, Wang R, Cai Y. 2020. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist 22:18–27. doi: 10.1016/j.jgar.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Li K, Dong H, Ren D, Gong D, Jiang F, Shi C, Li J, Zhang Q, Yan W, Li Y. 2021. Ceftazidime-avibactam resistance in Klebsiella pneumoniae sequence type 11 due to a mutation in plasmid-borne blakpc-2 to blakpc-33, in Henan, China. Infect Drug Resist 14:1725–1731. doi: 10.2147/IDR.S306095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carattoli A, Arcari G, Bibbolino G, Sacco F, Tomolillo D, Di Lella FM, Trancassini M, Faino L, Venditti M, Antonelli G, Raponi G. 2021. Evolutionary trajectories toward ceftazidime-avibactam resistance in Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother 65:e0057421. doi: 10.1128/AAC.00574-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Xiao L, Hong D, Zhao Y, Hu X, Shi S, Chen F. 2022. Epidemiology of resistance of carbapenemase-producing Klebsiella pneumoniae to ceftazidime-avibactam in a Chinese hospital. J Appl Microbiol 132:237–243. doi: 10.1111/jam.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L, Zhao Y, Yao Z, Zhang X, Zheng S, Chen L, Zhang Y, Wang L, Zhou T, Cao J. 2022. Rapid Resa ceftazidime-avibactam Enterobacterales NP test: rapid detection of ceftazidime-avibactam susceptibility in Enterobacterales. J Clin Microbiol 60:e0000422. doi: 10.1128/jcm.00004-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng W, Liao W, Zhao Y, Wang L, Shu H, Jia H, Chen T, Zhang Y, Zhou T, Wu Q. 2022. A selective medium for screening ceftazidime/avibactam resistance in carbapenem-resistant Enterobacterales. Front Microbiol 13:956044. doi: 10.3389/fmicb.2022.956044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu T, Guo Y, Ji Y, Wang B, Zhou K. 2022. Epidemiology and mechanisms of ceftazidime–avibactam resistance in Gram-negative bacteria. Engineering 11:138–145. doi: 10.1016/j.eng.2020.11.004. [DOI] [Google Scholar]
- 14.Wang C, Zhao J, Liu Z, Sun A, Sun L, Li B, Lu B, Liu Y, Cao B. 2021. In vivo selection of imipenem resistance among ceftazidime-avibactam-resistant, imipenem-susceptible Klebsiella pneumoniae isolate with KPC-33 carbapenemase. Front Microbiol 12:727946. doi: 10.3389/fmicb.2021.727946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Ke H, Wu W, Tu Y, Zhou H, Yu Y. 2021. Molecular mechanisms driving the in vivo development of KPC-71-mediated resistance to ceftazidime-avibactam during treatment of carbapenem-resistant Klebsiella pneumoniae infections. mSphere 6:e0085921. doi: 10.1128/mSphere.00859-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang M, Sun B, Huang Y, Liu C, Wang Y, Ren Y, Zhang Y, Wang Y, Mu D. 2022. Diversity of ceftazidime-avibactam resistance mechanism in KPC2-producing Klebsiella pneumoniae under antibiotic selection pressure. Infect Drug Resist 15:4627–4636. doi: 10.2147/IDR.S371285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hentschke M, Kotsakis SD, Wolters M, Heisig P, Miriagou V, Aepfelbacher M. 2011. CMY-42, a novel plasmid-mediated CMY-2 variant AmpC β-lactamase. Microb Drug Resist 17:165–169. doi: 10.1089/mdr.2010.0137. [DOI] [PubMed] [Google Scholar]
- 18.Gaibani P, Re MC, Campoli C, Viale PL, Ambretti S. 2020. Bloodstream infection caused by KPC-producing Klebsiella pneumoniae resistant to ceftazidime/avibactam: epidemiology and genomic characterization. Clin Microbiol Infect 26:516.e1–516.e4. doi: 10.1016/j.cmi.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Masi M, Vergalli J, Ghai I, Barba-Bon A, Schembri T, Nau WM, Lafitte D, Winterhalter M, Pagès JM. 2022. Cephalosporin translocation across enterobacterial OmpF and OmpC channels, a filter across the outer membrane. Commun Biol 5:1059. doi: 10.1038/s42003-022-04035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N, Chaput L, Villoutreix BO. 2021. Virtual screening web servers: designing chemical probes and drug candidates in the cyberspace. Brief Bioinform 22:1790–1818. doi: 10.1093/bib/bbaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverman SM, Moses JE, Sharpless KB. 2017. Reengineering antibiotics to combat bacterial resistance: click chemistry [1,2,3]-triazole vancomycin dimers with potent activity against MRSA and VRE. Chemistry 23:79–83. doi: 10.1002/chem.201604765. [DOI] [PubMed] [Google Scholar]
- 22.Guo L, He N, Zhao Y, Liu T, Deng Y. 2020. Autophagy modulated by inorganic nanomaterials. Theranostics 10:3206–3222. doi: 10.7150/thno.40414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negut I, Grumezescu V, Grumezescu AM. 2018. Treatment strategies for infected wounds. Molecules 23:2392. doi: 10.3390/molecules23092392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández Martínez SP, Rivera González TI, Franco Molina MA, Bollain Y Goytia JJ, Martínez Sanmiguel JJ, Zárate Triviño DG, Rodríguez Padilla C. 2019. A novel gold calreticulin nanocomposite based on chitosan for wound healing in a diabetic mice model. Nanomaterials 9:75. doi: 10.3390/nano9010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mane PC, Chaudhari RD, Shinde MD, Kadam DD, Song CK, Amalnerkar DP, Lee H. 2017. Designing ecofriendly bionanocomposite assembly with improved antimicrobial and potent on-site Zika virus vector larvicidal activities with its mode of action. Sci Rep 7:15531. doi: 10.1038/s41598-017-15537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okkeh M, Bloise N, Restivo E, De Vita L, Pallavicini P, Visai L. 2021. Gold nanoparticles: can they be the next magic bullet for multidrug-resistant bacteria? Nanomaterials 11:312. doi: 10.3390/nano11020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, Choi I. 2019. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci 20:2468. doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makabenta JMV, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. 2021. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol 19:23–36. doi: 10.1038/s41579-020-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YC, Yang YC, Yang KC, Shieh HR, Wang TY, Hwu Y, Chen YJ. 2014. Pegylated gold nanoparticles induce apoptosis in human chronic myeloid leukemia cells. Biomed Res Int 2014:182353. doi: 10.1155/2014/182353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang P, Pageni P, Rahman MA, Bam M, Zhu T, Chen YP, Nagarkatti M, Decho AW, Tang C. 2019. Gold nanoparticles with antibiotic-metallopolymers toward broad-spectrum antibacterial effects. Adv Healthc Mater 8:e1800854. doi: 10.1002/adhm.201800854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patil T, Khot V, Pandey-Tiwari A. 2022. Single-step antibiotic-mediated synthesis of kanamycin-conjugated gold nanoparticles for broad-spectrum antibacterial applications. Lett Appl Microbiol 75:913–923. doi: 10.1111/lam.13764. [DOI] [PubMed] [Google Scholar]
- 32.Shaker MA, Shaaban MI. 2017. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: in vitro antibacterial study. Int J Pharm 525:71–84. doi: 10.1016/j.ijpharm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Alafnan A, Rizvi SMD, Alshammari AS, Faiyaz SSM, Lila ASA, Katamesh AA, Khafagy ES, Alotaibi HF, Ahmed ABF. 2022. Gold nanoparticle-based resuscitation of cefoxitin against clinical pathogens: a nano-antibiotic strategy to overcome resistance. Nanomaterials 12:3643. doi: 10.3390/nano12203643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller SE, Bell CS, Mejias R, McClain MS, Cover TL, Giorgio TD. 2016. Colistin-functionalized nanoparticles for the rapid capture of Acinetobacter baumannii. J Biomed Nanotechnol 12:1806–1819. doi: 10.1166/jbn.2016.2273. [DOI] [PubMed] [Google Scholar]
- 35.Lázár I, Szabó HJ. 2018. Prevention of the aggregation of nanoparticles during the synthesis of nanogold-containing silica aerogels. Gels 4:55. doi: 10.3390/gels4020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi Y. 2016. Pot economy and one-pot synthesis. Chem Sci 7:866–880. doi: 10.1039/c5sc02913a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boomi P, Ganesan R, Prabu Poorani G, Jegatheeswaran S, Balakumar C, Gurumallesh Prabu H, Anand K, Marimuthu Prabhu N, Jeyakanthan J, Saravanan M. 2020. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int J Nanomed 15:7553–7568. doi: 10.2147/IJN.S257499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moradian C, Rahbarizadeh F. 2021. PE38-based gene therapy of HER2-positive breast cancer stem cells via VHH-redirected polyamidoamine dendrimers. Sci Rep 11:15517. doi: 10.1038/s41598-021-93972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turakhia B, Chikkala S, Shah S. 2019. Novelty of bioengineered iron nanoparticles in nanocoated surgical cotton: a green chemistry. Adv Pharmacol Sci 2019:9825969. doi: 10.1155/2019/9825969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Piao YZ, Chen H, Shi K, Dai J, Wang S, Zhou T, Le AT, Wang Y, Wu F, Ma R, Shi L, Liu Y. 2023. Dynamic covalent nano-networks comprising antibiotics and polyphenols orchestrate bacterial drug resistance reversal and inflammation alleviation. Bioact Mater 27:288–302. doi: 10.1016/j.bioactmat.2023.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkin SD, Abid S, Foster M, Bose M, Keller A, Hollaway R, Sader HS, Greenberg DE, Finklea JD, Castanheira M, Jain R. 2018. Multidrug-resistant Pseudomonas aeruginosa from sputum of patients with cystic fibrosis demonstrates a high rate of susceptibility to ceftazidime-avibactam. Infect Drug Resist 11:1499–1510. doi: 10.2147/IDR.S173804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu W, Xiong L, Luo Q, Chen Y, Ji J, Ying C, Liu Z, Xiao Y. 2021. In vitro activity comparison of ceftazidime-avibactam and aztreonam-avibactam against bloodstream infections with carbapenem-resistant organisms in China. Front Cell Infect Microbiol 11:780365. doi: 10.3389/fcimb.2021.780365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Medicines Agency. 2020. European public assessment report (EPAR) for Zavicefta: summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf. Accessed 23 November 2020.
- 44.Liu S, Huang N, Zhou C, Lin Y, Zhang Y, Wang L, Zheng X, Zhou T, Wang Z. 2021. Molecular mechanisms and epidemiology of carbapenem-resistant Enterobacter cloacae complex isolated from Chinese patients during 2004–2018. Infect Drug Resist 14:3647–3658. doi: 10.2147/IDR.S327595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciofu O, Moser C, Jensen PØ, Høiby N. 2022. Tolerance and resistance of microbial biofilms. Nat Rev Microbiol 20:621–635. doi: 10.1038/s41579-022-00682-4. [DOI] [PubMed] [Google Scholar]
- 46.Oves M, Rauf MA, Hussain A, Qari HA, Khan AAP, Muhammad P, Rehman MT, Alajmi MF, Ismail IIM. 2019. Antibacterial silver nanomaterial synthesis from Mesoflavibacter zeaxanthinifaciens and targeting biofilm formation. Front Pharmacol 10:801. doi: 10.3389/fphar.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Zhou J, Yuan L, Wu F, Xie L, Yan X, Li H, Li Y, Shi L, Hu R, Liu Y. 2023. Neighboring carboxylic acid boosts peroxidase-like property of metal-phenolic nano-networks in eradicating Streptococcus mutans biofilms. Small 19:e2206657. doi: 10.1002/smll.202206657. [DOI] [PubMed] [Google Scholar]
- 48.Benoit DSW, Sims KR, Jr, Fraser D. 2019. Nanoparticles for oral biofilm treatments. ACS Nano 13:4869–4875. doi: 10.1021/acsnano.9b02816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennequin C, Robin F. 2016. Correlation between antimicrobial resistance and virulence in Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis 35:333–341. doi: 10.1007/s10096-015-2559-7. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Zhao G, Chao X, Xie L, Wang H. 2020. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. IJERPH 17:6278. doi: 10.3390/ijerph17176278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang YL, Yang JJ, Ni W. 2022. Immunomodulatory effects of sinensetin on macrophage and cyclophosphamide-induced immunosuppression in mice. Pharmazie 77:147–151. [DOI] [PubMed] [Google Scholar]
- 52.Ahlmann M, Hempel G. 2016. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol 78:661–671. doi: 10.1007/s00280-016-3152-1. [DOI] [PubMed] [Google Scholar]
- 53.Song X, Liu L, Peng S, Liu T, Chen Y, Jia R, Zou Y, Li L, Zhao X, Liang X, Tang H, Yin Z. 2022. Resveratrol regulates intestinal barrier function in cyclophosphamide-induced immunosuppressed mice. J Sci Food Agric 102:1205–1215. doi: 10.1002/jsfa.11458. [DOI] [PubMed] [Google Scholar]
- 54.Almazroo OA, Miah MK, Venkataramanan R. 2017. Drug metabolism in the liver. Clin Liver Dis 21:1–20. doi: 10.1016/j.cld.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Shen JX, Youhanna S, Zandi Shafagh R, Kele J, Lauschke VM. 2020. Organotypic and microphysiological models of liver, gut, and kidney for studies of drug metabolism, pharmacokinetics, and toxicity. Chem Res Toxicol 33:38–60. doi: 10.1021/acs.chemrestox.9b00245. [DOI] [PubMed] [Google Scholar]
- 56.Band VI, Hufnagel DA, Jaggavarapu S, Sherman EX, Wozniak JE, Satola SW, Farley MM, Jacob JT, Burd EM, Weiss DS. 2019. Antibiotic combinations that exploit heteroresistance to multiple drugs effectively control infection. Nat Microbiol 4:1627–1635. doi: 10.1038/s41564-019-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Zheng W, Li S, Zhong L, Jiang X. 2022. Aminophenol-decorated gold nanoparticles for curing bacterial infections. Nano Lett 22:3576–3582. doi: 10.1021/acs.nanolett.1c04968. [DOI] [PubMed] [Google Scholar]
- 58.Lin Y, Zhang Y, Liu S, Ye D, Chen L, Huang N, Zeng W, Liao W, Zhan Y, Zhou T, Cao J. 2021. Quercetin rejuvenates sensitization of colistin-resistant Escherichia coli and Klebsiella pneumoniae clinical isolates to colistin. Front Chem 9:795150. doi: 10.3389/fchem.2021.795150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00262-23-s0001.docx, DOCX file, 0.02 MB (18.4KB, docx)
Supplemental material. Download aac.00262-23-s0002.docx, DOCX file, 0.02 MB (18KB, docx)