How plant roots cope with the soil complexity and integrate heterogeneous conditions into development, defence, and metabolism remains unclear. Structured microfluidic devices now enable controlled generation of complex microenvironments for microscopy-based root studies.
Keywords: Biosensors, complex conditions, 3D printing, lab-on-a-chip, live imaging, microfluidics, root–microbe interactions, synthetic environments
Abstract
When interacting with the environment, plant roots integrate sensory information over space and time in order to respond appropriately under non-uniform conditions. The complexity and dynamic properties of soil across spatial and temporal scales pose a significant technical challenge for research into the mechanisms that drive metabolism, growth, and development in roots, as well as on inter-organismal networks in the rhizosphere. Synthetic environments, combining microscopic access and manipulation capabilities with soil-like heterogeneity, are needed to elucidate the intriguing antagonism that characterizes subsurface ecosystems. Microdevices have provided opportunities for innovative approaches to observe, analyse, and manipulate plant roots and advanced our understanding of their development, physiology, and interactions with the environment. Initially conceived as perfusion platforms for root cultivation under hydroponic conditions, microdevice design has, in recent years, increasingly shifted to better reflect the complex growth conditions in soil. Heterogeneous micro-environments have been created through co-cultivation with microbes, laminar flow-based local stimulation, and physical obstacles and constraints. As such, structured microdevices provide an experimental entry point into the complex network behaviour of soil communities.
Introduction
The first point of contact of plants with their soil environment is their root system, and as such root function has critical implications for the whole organism, decisive for nutrient uptake, carbon sequestration, and interaction with microorganisms (Fita et al., 2015; Barkaoui et al., 2016; Fry et al., 2018; Robinson et al., 2018; Ober et al., 2021), while also rapidly responding and plastically acclimating their architecture to various stress factors (Gruber et al., 2013; Gilroy et al., 2014; Keinath et al., 2015; Stanley et al., 2018; Correa et al., 2019). Roots shape the soil environment through the establishment of the rhizosphere, by increasing the bioavailability of mineral nutrients, influencing soil structure, and providing a specific carbon-rich niche for adapted microbial communities (Guyonnet et al., 2018; Canarini et al., 2019; Correa et al., 2019; Lucas et al., 2019).
Root–environment interactions are characterized by spatial and temporal heterogeneity stemming from the level of a single soil particle up to the whole root system, with fluctuations from seconds to days (Aleklett et al., 2018). The biological processes involved act on drastically different spatial scales—from subcellular protein function to cellular processes such as the perception of environmental stimuli, second messenger signalling to organ development, and restructuring of the root system architecture (Fig. 1) (Kazan, 2013; Fichman and Mittler, 2020; Karlova et al., 2021). Elucidating root traits, and how roots integrate sensory information leading to a strategic growth pattern, requires a fundamental understanding of the mechanisms underlying the integration of the complex soil environment into plant physiology and development from the micro- to macroscale and from seconds to days.
Fig. 1.
Relative scale of microfluidics applications, targets, and readouts. Microfluidic devices can be designed to address questions at multiple scales, from subcellular (~µm) to the rhizosphere (~cm), targeting a variety of processes, from protein localization to root system development. Illustration images, from left to right: Sakai et al., 2019; Finkbeiner et al., 2022; Yanagisawa et al., 2017; Stanley et al., 2018, the Open Access licence covering this article does not apply to this image; Aufrecht et al., 2022. A detailed list of devices, including time scales and length scales, appears in Table 1. Images are reproduced with permission of their respective copyright holders.
The challenge of root analysis across spatial and temporal scales has been met with the development of sophisticated root phenotyping systems, allowing the coupling of genotypic profiles to root phenotypes (Clark et al., 2020). These approaches aim to overcome the major limitation of accessing the ‘hidden half’ of plants (Atkinson et al., 2019), which is the visual inaccessibility of the soil matrix, that traditionally requires extracting the root system. As such, approaches such as the ‘GloRoot’, bioluminescence-based in situ tracking of root development in thin-soil sheets, such as rhizotrons (Rellán-Álvarez et al., 2015), or the utilization of microcomputed tomography (µ-CT) (Atkinson et al., 2019; Teramoto et al., 2020), have enabled imaging root systems in their native environment. While these systems allow minimally invasive access to root system architecture and high-throughput phenotyping, the spatial and temporal resolution is limited, and cannot tap into microscale or rapid processes, critical to fully grasp the complexity of the rhizosphere. Multifaceted approaches that include high-resolution imaging techniques (Clark et al., 2020), genetic tools such as genetically encoded biosensors (recently reviewed in Sadoine et al., 2021), and manipulation of environmental conditions, remain incompatible with a native soil environment. This is due to the fact that traditional microscopic techniques cannot be used and, furthermore, environmental conditions cannot be precisely controlled. Commonly, microscopic studies of root systems have therefore turned to accessible but artificially homogeneous agar-based or hydroponic growth of plants (Gregory et al., 2009; Asao, 2012). While allowing high-resolution imaging, agar-based setups typically lack the structural, chemical, and biotic diversity posed by a native soil environment. Therefore, studies of root systems compromise between having a controlled system, imaging capabilities, or the ability to mimic soil-like conditions.
In recent years, there has been a transfer of microfluidic technology (Box 1) from the fields of chemical analysis, medical diagnostics, and mammalian cell biology into plant science (Yanagisawa et al., 2021). The development of new structured microdevices has met the challenge of stimulating and imaging roots at high spatiotemporal resolution. Microfluidics harnesses the laminar flow-dominated fluid dynamics (Box 2) of minuscule volumes, and possibilities of rapid design and prototyping to generate defined controlled environments. Since the 2010s with the development of the first Arabidopsis thaliana-adapted approaches (Meier et al., 2010; Grossmann et al., 2011; Parashar and Pandey, 2011; Busch et al., 2012) micro- to milli-fluidic devices have advanced in the range of accessible species, precise environmental control, possibilities of co-cultivation with microbial communities, and available scaffolds (Fig. 1). Novel technologies such as 3D printing now move the boundaries of accessibility and turn an originally exotic technique into a potential backbone for an advanced ‘soil-on-a-chip’ toolkit for root–environment studies, a concept proposed by Stanley et al. (2016), that not only improves existing capabilities but also enables experimental designs which were not feasible until now. An overview of root-adapted microfluidic devices, including details about the relevant time scales, length scales, and number of samples, is shown in Table 1.
Box 1. Fabrication and production of microdevices.
Microfluidic devices are typically fabricated by casting PDMS [poly(dimethylsiloxane)] into a mould carrying the specific design features, such as microchannels or microchambers, needed for the experiment, a process called soft lithography (Xia and Whitesides, 1998). The mould is most commonly manufactured through photolithography techniques, which provide extremely high feature resolution at a sub-micrometre scale. This technique, however, has the disadvantage of being costly and requiring specific expertise.
Currently, soft lithography (called ‘soft’ because it uses elastomeric materials, such as PDMS) is the most common solution for the fabrication of microfluidic devices, even though it has been pointed out that PDMS has its own drawbacks—its processing requires relatively long times, it is difficult to seal, and it swells when exposed to many organic solvents. The identification of both materials and procedures that might lead to affordable, upscalable production of microfluidic devices, though, is still an open challenge (Battat et al., 2022).
Photolithography can be replaced by 3D printing for the fabrication of the mould—3D printers are not only more affordable and accessible (lowering the setup cost from US$80 000 to US$1000–20 000) but can also provide a fairly high resolution, ranging from tens to hundreds of micrometres. 3D printing also offers an advantage in terms of turnaround time, which can be <2 h, compared with ~24 h for soft lithography (Bhattacharjee et al., 2016).
Furthermore, microfluidic devices have been fabricated by cutting features from a silicon sheet and enclosing it between two coverslips (Bertrand-Rakusová et al., 2020); in this case, the spatial resolution of the features is not comparable with moulded PDMS, but still satisfactory for certain applications. Alternatively, commercial pre-designed microfluidic chambers can be bought for reasonable prices, but have the drawback of not being customizable in the design.
Box 2. Microfluidic flow dynamics/parameters.
The term microfluidics indicates both the science that studies the behaviour of fluids in micro-sized channels and the technological fabrication of micro-devices, embedding chambers, and channels (typical dimensions on the order of tens to hundreds of micrometres) for the confinement or flow of fluids at the microscale (typical volumes on the order of 10–3–10–6 μm–3). The use of fluids in such conditions offers a number of advantages, for example the ability to use small, controlled amounts of reagents or samples, high resolution and sensitivity in both the measurements and the manipulation of the samples, low costs, and short times for analysis, allowing high throughput. Furthermore, fluids at such scales show a behaviour very different from at the macro-scale, thanks to the typically small Reynolds numbers involved: the Reynolds number is a dimensionless quantity, which predicts flow patterns, related to the density and viscosity of the fluid and to the typical velocity and characteristic dimension of the flow. Systems with a high Reynolds number, typically macroscopic, exhibit turbulent flows, with particles moving irregularly. Systems with a low Reynolds number, typically microscopic, exhibit laminar flow, with particles moving in parallel in smooth straight trajectories. In this latter case, different fluids stay perfectly separated as mixing does not occur, thus allowing control of the location and movement of reagents, suspensions, or cells in the channels (see Figure for an explanatory sketch).
Turbulent versus laminar flows: in the left panel, two different fluids entering a middle channel from two sides give rise to a turbulent flow (high Reynold’s number), with the red and blue fluids mixing together. The same geometry in a microsized channel (right panel) would lead to a laminar flow with the two fluid components remaining perfectly separated (no mixing).
Table 1.
Overview of root-adapted microfluidic devices displaying plants per device, spatial resolution, and reported time span of the specimen on the device
| Device | Reference | Organism | Plants per device | Resolution | Time span |
|---|---|---|---|---|---|
| Multi-laminar flow device | Meier et al. (2010) | A. thaliana | 1 | µm | 1 d |
| Plant-in chip | Parashar and Pandey (2011) | A. thaliana | 8 | μm | 4 d |
| RootChip | Grossmann et al. (2011) | A. thaliana | 8 | μm | 3 d |
| RootArray | Busch et al. (2012) | A. thaliana | 64 | μm | 3 d |
| RootChip16 | Jones et al. (2014) | A. thaliana | 16 | μm | 5 d |
| dfRootChip | Stanley et al. (2018) | A. thaliana | 5 | μm | 3 d |
| Plant Array Chip | Park et al. (2017) | A. thaliana | 400 | mm | 10 d |
| TRIS Device | Massalha et al. (2017) | A. thaliana | 9 | μm | 5 d |
| EcoFab | Sasse et al. (2018) | B. dystachion | 1 | μm | 21 d |
| vRootChip | Fendrych et al. (2018) | A. thaliana | 4 | μm | 3 d |
| Porous network device | Aufrecht et al. (2017) | A. thaliana | 1 | μm | 4 d |
| Foldable Chip Array | Song et al. (2019 | N. tabacum | 4 | μm | 10 d |
| Petaloid Root-growth chip | Chai et al. (2019) | O. sativa | 1 | mm | 11 d |
| RootChip-8S | Denninger et al. (2019) | A. thaliana | 8 | μm | 5 d |
| RMI-chip | Noirot-Gros et al. (2020) | P. tremuloides | 6 | μm | 10 d |
| Coverslip-based microfluidic device | Singh et al. (2021) | A. thaliana | 3 | μm | 5 d |
| Rhizosphere-on-a-chip | Aufrecht et al. (2022) | B. dystachion | 1 | 12 d | |
| RootTrapr | Suwanchaikasem et al. (2022) | C. sativa | 1 | mm | 14 d |
| Microfluidic device | Dai et al. (2022) | O. sativa | 1 | μm |
Reflecting soil-like heterogeneity in structured microdevices
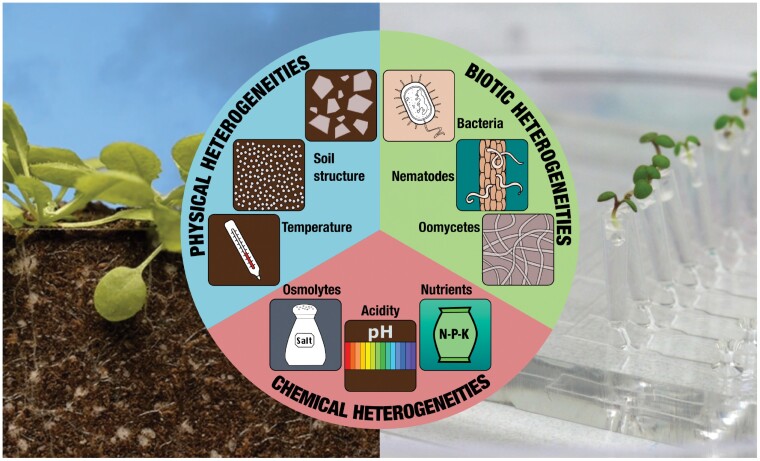
Soil, including the rhizosphere, represents the complex interplay of the lithosphere, hydrosphere, atmosphere, and biosphere (Totsche et al., 2010). This results in an environment that involves dynamic and spatially heterogeneous physical, chemical, and biological factors (Fig. 2). Soil-mimicking microdevices therefore need to account for the spatiotemporal heterogeneity of various stimuli of the soil environment (Fig. 3).
Fig. 2.
Schematic representation of environmental heterogeneities in the root–soil interplay—namely physical (soil structure and temperature), chemical (osmolytes, nutrients, acidity), and biotic (bacteria, nematodes, filamentous eukaryotes such as oomycetes). While these factors are entangled and not easily separable in real soil, microfluidics and soil-mimicking microdevices allow the control of each one of them and investigate the response of the root system with a precision ranging from the micro- to macro-scale and on time scales ranging from seconds to days. (Left image © Wim van Egmond, still from a time-lapse made in collaboration with Gerlinde De Deyn, Wageningen University and Research 2020. This image is reproduced with permission and is not covered by an Open Access licence).
Fig. 3.
Range of chemical, physical, and biotic parameters of root–soil interactions addressed in structured microdevices. (A) Generation of asymmetric environmental conditions through bilaminar flow in the dual-flow RootChip (Stanley et al., 2018), with permission; the Open Access licence covering this article does not apply to this image. (B) Integration of a pillar-based matrix into a EcoFab to simulate the particulate soil matrix (Jabusch et al., 2021). (C) Assessing multi-organismic interaction between fungi and bacteria on a heterogenous soil-like chip (Mafla-Endara et al., 2021). (D) In situ imaging of ROS production of mature rice roots. Reprinted with permission from Dai et al. (2022). Diel fluctuation of extracellular reactive oxygen species production in the rhizosphere of rice. Environmental Science & Technology 56, 9075–9082. Copyright 2022 American Chemical Society, the Open Access licence covering this article does not apply to this image. (E) Root growth in a soil-like matrix (Aufrecht et al., 2022), and root colonization of Populus tremoloides by Pseudomonas fluorescens on the RMI chip (Noirot-Gros et al., 2020).
Physical heterogeneity
Soil is a matrix of particulate matter including inorganic and organic compounds associated as suspended particles, colloids, and rock, which again define channels, pores, and aggregates of scales between micrometres and millimetres (Totsche et al., 2010). This matrix is filled with gaseous, liquid, and solid phases, and therefore presents a spatially heterogeneous environment with diverse mechanical properties, and associated fluid dynamics (Aleklett et al., 2018). Roots are responsive to mechanical stimuli associated with the soil structure by thigmotropic object avoidance and changes in branching patterns (Dunbabin et al., 2013; Kolb et al., 2017; Ponce et al., 2017; Correa et al., 2019).
‘Transparent soil’ technology has been developed in order to reconstitute soil structure in visually accessible experimental setups. This refers to optically water-like artificial particles utilized to form a soil-like particulate matrix. As such, the polymer Nafion, a synthetic ionomer, has been proven to be particularly useful due to its refractive index (RI) of n=1.228–1.310 (Bayani Ahangar et al., 2019), which closely matches the RI of water [and most experimentally used plant media; n(water)=1.333] and thus renders the material virtually invisible in liquid-filled cuvettes or chambers. Hence, Nafion particles can emulate soil particles, while minimizing shading or optical distortions during the microscopy of fluorescent biological specimens (Downie et al., 2012). Nafion matrices have been used not only to image root system development, but also to perform high-resolution confocal imaging of root–microbe interactions between A. thaliana and the enterobacterium Escherichia coli, allowing the capture of in situ microcolony formation (Downie et al., 2012). In the field of soil microbiology, Nafion particles have also been introduced into microfluidic setups to track the influence of fluctuating environmental conditions on bacterial communities through stable isotope labelling (Sharma et al., 2020). In a similar fashion, biopolymer-based hydrogels such as calcium/magnesium alginate have been used to track the root development of Glycine max and associated pH changes in the surrounding substrate (Ma et al., 2019). In comparison, hydrogel-based beads are more cost-efficient and customizable regarding their composition, while Nafion particles represent a reusable matrix of impenetrable particles of great shape diversity.
Root-adapted microfluidic devices did not initially hold particulate matrices of any kind, but rather utilized linear channels for guiding root growth, or utilized unstructured chambers (Meier et al., 2010; Grossmann et al., 2011; Parashar and Pandey, 2011; Gao et al., 2018; Massalha et al., 2019). A network of channels featuring a variety of different structural elements was first employed for the investigation of the tip growth of Camellia pollen tubes, allowing the measurement of growth responses upon the encounter of obstacles, bends, or constrictions (Agudelo et al., 2012). The dual-flow RootChip was the first device for primary roots of Arabidopsis seedlings that demonstrated the possibility to use micropillars as structural guides for root growth direction in a bi-laminar flow (Stanley et al., 2018) (Fig. 3A). Another device adapted for taproots, a microfluidic environment for root colonization studies, displayed not only the use of pillars as a simulation of a soil matrix but also the possibility for sub-compartmentalization along the microfluidic device (Aufrecht et al., 2018). Similarly, the introduction of pillars was seen in the Ecosystem Fabrication (EcoFab) concepts, which originally introduced sand or small-sized soil particles (Gao et al., 2018; Jabusch et al., 2021) (Fig. 3B). The employment of granular media algorithms trained on native sandy soils was then utilized first in the field of soil microecology to design soil matrix-like masks for poly(dimethylsiloxane) (PDMS) devices, that were initially used to track biofilm development influenced by pore hydrodynamics (Aufrecht et al., 2019). Algorithm-generated soil-like matrices could thus show the role of rheotaxis, current-oriented motility to the present fluid flow, which determined the initial adherence of bacterial cells by influencing their translocation. In a rather reductionist approach, different types of geometric structures present in soil were integrated on-chip to track hyphal growth, particle transportation, and foraging behaviour of fungi, bacteria, and protists (Mafla-Endara et al., 2021) (Fig. 3C). The transfer of algorithm-based designs of soil-mimicking PDMS matrices was lastly seen with the development of rhizosphere-on-a-chip, which utilized a similar approach to that of Aufrecht et al. (2018) for a liquid-filled PDMS matrix for the growth of Brachypodium distachyon (Aufrecht et al., 2022) (Fig. 3E). In the rhizosphere-on-a-chip, Aufrecht et al. could display that a granular environment leads to the constitution of specific chemical niches in surrounding substrates, in this case through the enrichment of amino acids by exudation. Asymmetric concentrations of carbon compounds could further be driving factors of the spatial distribution of interacting bacteria and chemical profiles of the surrounding soil. The rhizosphere-on-a-chip device therefore highlights the potential of soil-mimicking devices to disentangle the interaction between heterogeneous granular matrices and the root’s activity, while retaining a controlled experimental platform and visual access.
Mechanical stimuli are not the only physical factors shaping the interaction in the rhizosphere. Similarly, soil displays dynamic temperature gradients, with the upper soil layers following variations in solar radiation and deeper layers exhibiting a more constant temperature (Singh and Sharma, 2017). Temperature gradients have already been achieved in microfluidic devices adapted for chemical research utilizing asymmetric hot and cold fluid streams (Mao et al., 2002). To study the cold-mediated inhibition of Arabidopsis seed germination, a PDMS-based phenotyping system was developed by pairing an inclined PDMS block with a thermo-electric cooling plate, generating a temperature gradient in the growth channels engraved into the PDMS block (Wang et al., 2017).
The representation of soil structures on chips and in microdevices has advanced not only through the development of transparent soil but also through the algorithm-assisted design of PDMS masks to reflect granulated patterns of soil. In this way, spatially heterogeneous environments can be created while preserving microscopic access.
Chemical heterogeneity
The chemical heterogeneity of soil originates from the mineral and organic composition of the soil matrix, as well as from the influence of the organisms present and the plant root itself, releasing bioactive compounds and organic matter. The chemical profile of the surrounding soil matrix includes compounds acting as nutrients, stress elicitors, and signal molecules including phytohormones and volatiles modulating cellular functions. These conditions display spatiotemporal variation and challenge the root system with locally asymmetric conditions, which raises the question of how local stimuli and temporal dynamics affect the overall acclimation of root systems.
The development of the RootChip, a fully integrated system with on-chip cultivation of multiple seedlings and micromechanical valves for perfusion control, was aimed at the temporal dynamics of growth conditions. Rapid exchange of the root microenvironment was achieved by applying pulsed treatments to A. thaliana roots in a linear channel with a root-circumferential laminar flow (Grossmann et al., 2011). This system was used for live-cell imaging under pulsed treatment with glucose and galactose, for which transport kinetics could be determined using genetically encoded biosensors. Pulsed treatments with short delay times are a particular strength of microfluidic perfusion systems due to the small dead volumes. The rapid switching of solutions was critical in a biosensor-based quantitative assessment of intracellular Zn2+ concentration in Arabidopsis vacuoles (Lanquar et al., 2014), the characterization of the biosensors ABACUS for abscisic acid (ABA) (Jones et al., 2014), and CerTN-L15 for calcium in planta (Denninger et al., 2014), or the discovery of rapid, non-genomic growth inhibition upon auxin application (Fendrych et al., 2018). The simplified version RootChip-8S, replacing the on-chip valve system with external flow control (Denninger et al., 2019; Guichard et al., 2020), has contributed to a wider dissemination of the technique and enabled, among others, the testing of computational models of mechanisms underlying gibberellin gradients in growing roots (Rizza et al., 2021). Recently, pulsed perfusion with concentrated osmolytes enabled the quantitative description of cell wall elasticity in the root elongation zone and helped discover a cytokinin-dependent mechanism that modulates cell wall mechanics and thus limits cell expansion and promotes cell differentiation (Liu et al., 2022).
Besides temporal control over the chemical conditions, spatial heterogeneity or even localized treatments are an important, yet more challenging aspect of approaching soil-emulating growth conditions. Laminar flow, the predominant flow regime in microfluidic environments, can help to create gradients or asymmetric conditions with sharp boundaries (Stanley et al., 2018; Convery and Gadegaard, 2019) (Fig. 3A).
Therefore, the first functionality integrated into a root-adapted microfluidic device was the localized treatment of roots. The device design by Meier and colleagues used three parallel laminar streams to allow a local application of a liquid treatment to an A. thaliana root segment of 10–800 µm (Meier et al., 2010). A central liquid stream carrying an auxin derivative was focused perpendicular to the root by two lateral streams that carried an auxin transport inhibitor, triggering localized emergence of root hairs at the treated site. While truly pioneering plant microfluidics, the downsides of this early device included the potentially invasive manual mounting of a single root within the treatment chamber and the limited range within which the focused stream could target the root.
The dual-flow RootChip aimed at spatial asymmetry by generating a bi-laminar flow laterally of the centrally confined root via two separate inlets (Stanley et al., 2018). This setup enabled the study of asymmetric acclimation of root hair growth to locally different concentrations of phosphate. The experiments demonstrated that root hairs are able to rapidly respond to local nutrient conditions with a non-genomic, cell-autonomous regulation of growth rate and duration.
Previously mentioned approaches focused on the taproot system of A. thaliana, but proved incompatible with the fibrous root systems of monocots. An approach to adapt such microdevices to monocots can be seen in the petaloid chamber root growth chip for the cultivation of the fibrous root system of Oryza sativa, with five separate chambers connected to a central column holding the seedling (Chai et al., 2019). Each chamber could be separately filled with either a solid or liquid medium, allowing the generation of independent conditions for each root. This setup was utilized to assess the root system architecture if challenged with differing degrees of water availability by polyethylene glycol (PEG) 6000 treatment, in which the roots primarily elongated in chambers of higher water availability.
The RootTrapr, designed based on the Ecosystem Fabrication setup (Gao et al., 2018), could assess the influence of the defence-stimulating elicitor chitosan on Cannabis sativa and allowed the display of an ABA-related reduction in lateral root formation, as well as a multi-omics analysis of the plant’s response (Suwanchaikasem et al., 2022).
Microfluidic chips have not only enabled the assessment of the effects of chemical stimulants and asymmetric chemical conditions but can also be utilized to sample and assess chemical changes originating from the plant’s activity. In general, microfluidic platforms can be used as an alternative cultivation platform for the ex situ analysis of metabolites and exudates. For example, the EcoFab was used to grow B. distachyon in synthetic media and sterile soil extract, during which root phenotypes were captured throughout the growth period. This enabled a metabolic analysis of phosphate content and exudate profile (Sasse et al., 2019), revealing distinct metabolic responses to the complex soil extract and increased root hair length. In situ analysis on-chip has also been achieved: a microfluidic cultivation platform for O. sativa was fitted with an integrated nutrient sensor, which allowed the non-invasive tracking of nitrate and phosphate uptake from the medium for up to 15 d (Jiang et al., 2017). Further, a microfluidic imaging chamber confining a mature root of O. sativa based on the readout of a redox-sensitive dye allowed the display of a day and night cycle-dependent production of reactive oxygen species (ROS) (Dai et al., 2022) (Fig. 3D). Lastly, these diurnal patterns of ROS production were linked to an interplay in the rhizosphere between bacterial respiration and oxygen release by the root system.
While these systems were confined to specific nutrients and chemical compounds, a further advancement was the direct coupling of LC/MS to microfluidic habitats for root growth. This was achieved by integrating nanoporous polyester track-etched (PETE) membranes at specific positions into a microfluidic growth system for a wheat seedling’s root, which allowed metabolic exchange with an integrated microfluidic sampling system (Patabadige et al., 2019). The setup enabled dynamic tracking of root exudation at two spatially separated positions, and the detection of saccharides and amino acids characteristic of root exudates. This approach was further advanced by coupling a soil-like PDMS matrix on-chip for growing B. distachyon and a PETE membrane to liquid micro-junction surface sampling probe mass spectrometry (LMJ-SSP-MS), which allowed the capture of the spatial distribution of amino acid exudation at a high resolution (Cahill et al., 2020; Aufrecht et al., 2022) (Fig. 3E). LMJ-SSP-MS itself is a microfluidic technique, that was designed to allow chemical sampling on a running microfluidic biology-on-a-chip device; in this way a liquid junction generated between the sampling device and the porous membrane is utilized to extract nanolitre to microlitre volumes out of the biology-on-a chip device (Cahill et al., 2020). As such, microfluidic technology cannot only aid the simulation of soil-like conditions but also expand the available sampling and analytical toolset.
Biotic heterogeneity
Soil is a biodiverse and densely populated ecosystem, enabled by the variety of available chemical substrates and structures. A single gram of soil contains on average 1010 bacterial cells, 102–104 cells of eukaryotic protists, 10–20 m of fungal hyphae, and ~10 nematodes (Roesch et al., 2007; Geisen et al., 2018; Das and Dash, 2019; Lehmann et al., 2020; van den Hoogen et al., 2020). The various species can act as pathogens, parasites, commensals, and mutualists, while also providing essential ecosystem services by driving nutrient cycling and mineralization, and influencing soil structure (Forster, 1990; Adl and Gupta, 2006; Figueiredo et al., 2016; Seppey et al., 2017). The plant’s innate immune system, cell wall composition, and deposition of organic matter further filter the soil biota which are present into a specific rhizosphere community, that characterizes the root-influenced soil (Durán et al., 2018; Canarini et al., 2019; Knights et al., 2021).
On the smallest scale, and most abundant, are bacterial species (Osiro et al., 2012). Bacterial association in the rhizosphere can range from structured communities filtered into the rhizosphere to biofilm coverage of the root’s surface, and endophytes invading root tissues (Bai et al., 2015; Deng et al., 2019; Knights et al., 2021) and also being widespread causes of plant disease (Huang et al., 2020). Microfluidic bioreactor designs have been deployed in bacterial research since the early 2000s, independently of plant systems (Heo et al., 2003; Balagaddé et al., 2005; Grünberger et al., 2012; Burmeister et al., 2019). Using the above-described dual-flow-RootChip, the micropillars guiding the root proved suitable for trapping green fluorescent protein (GFP)-expressing Pseudomonas fluorescens along the Arabidopsis root (Stanley et al., 2018). A device specifically targeted at root–microbe interaction is the Tracking Root Interaction System (TRIS), which was used to perform live-cell imaging of the first 6 h of colonization by Bacillus subtilis NCIB 3610 (Massalha et al., 2017). This setup enabled access to spatio-temporal patterns of colonization and could identify the elongation zone as an area of initial association with the root. In addition, a basic consortium of E. coli and B. subtilis was tested and could display displacement of E. coli by B. subtilis from the root’s surface. Similarly, a microfluidic device was employed to track colonization by P. fluorescencs SWB25 on Populus tremoloides roots and B. subtilis NCIB 3610 on O. sativa roots in a setup termed an RMI (root–microbe interactions) chip (Noirot-Gros et al., 2020) (Fig. 3F). While species of the B. subtilis species complex and pseudomonads are regularly isolated from the rhizosphere and include agronomically relevant species (Fan et al., 2017), these are only one of many taxa represented in the rhizosphere. The next step in reflecting structured communities of the rhizosphere is the transfer of native rhizosphere strains. As such, endophytic and rhizosphere-associated strains isolated from the P. tremoloides microbiome were assessed for their spatiotemporal dynamics of root colonization on A. thaliana in a microfluidic habitat, which over the course of a 4 d co-cultivation period allowed the correlation of inoculum density with the formation and size of endophytic microcolonies (Aufrecht et al., 2018). With comprehensive collections of rhizosphere microbiota strains of A. thaliana and further species such as Lotus japonicus, the next frontier is the integration of simplified but taxonomically representative synthetic communities into root-adapted microfluidics (Bai et al., 2015; Wippel et al., 2021); this is currently preceded by multi-species experiments performed in microfluidic setups outside of plant research (Alnahhas et al., 2019).
Eukaryotic species including oomycetes, fungi, nematodes, and various taxa of protists are another major constituent of the soil biota and source of plant-protective and pathogenic species (Richards et al., 2006; Giraldo and Valent, 2013). Zoospores of the oomycete Phytophthora sojae and sugar beet nematode were the first pathogenic species combined with A. thaliana in an early microfluidic setup, the plant-in-chip device (Parashar and Pandey, 2011). The plant-in-chip targeted the establishment of pathogen–root interaction and could thus display the 1–2 h period in which nematodes were attracted to the root and then enabled the invasion of root tissue 2–3 d post-inoculation to be followed. Zoospores were found to initially accumulate close to the root tip around 2 hours post-inoculation (hpi), forming a cluster, from which hyphae grew out and penetrated the plant’s tissue from 6 hpi onward (Parashar and Pandey, 2011). These experiments displayed the potential of microfluidic habitats to target, in particular, early stages of association with eukaryotic plant pathogens. For fungal species, a variety of microfluidic devices have been published (Richter et al., 2022), while for protozoan species the field of microfluidic soil-like approaches is just emerging (Gaines et al., 2019). A multi-species integration including protists, fungi, and nematodes in a soil-like structured environment assessed the dynamics of soil–structure–organism interaction (Mafla-Endara et al., 2021) (Fig. 3F). The establishment of such multi-species or multi-kingdom setups in soil-like microdevices with plant roots has yet to be shown.
Conclusion and outlook
Root–soil interactions result from multi-scale processes, with length scales ranging from micrometres to centimetres, and time scales ranging from milliseconds to days. Processes on larger length and time scales are experimentally accessible with in situ phenotyping setups such as rhizotrons, the GloRoot platform, or µ-CT (Rellán-Álvarez et al., 2015; Atkinson et al., 2019). However, these systems are less suitable to address the micrometre-scaled, rapid dynamics that are a major constituting factor of root–soil interactions. Due to their versatility, microfluidic devices facilitate the simulation of specific environmental conditions, provide access to rapid micrometre-scaled processes, and allow, dependent on the device and species, high-resolution imaging from days to weeks (Stanley et al., 2018; Sasse et al., 2019). Consequently, microfluidics can contribute to a broad range of research areas from prokaryotic interactors to root system architecture (Table 1).
Root microdevices have greatly advanced since the beginning of the 2010s in reconstituting soil-like conditions. Structural scaffolds have moved from linear unstructured chambers and channels to PDMS matrices based on the particulate structure of native soils (Grossmann et al., 2011; Gao et al., 2018; Stanley et al., 2018; Jabusch et al., 2021; Mafla-Endara et al., 2021; Aufrecht et al., 2022). Furthermore, the range of species has greatly expanded from the dicot A. thaliana to a variety of monocots including O. sativa, B. distachyon, wheat, and non-model species such as C. sativa (Chai et al., 2019; Sasse et al., 2019; Aufrecht et al., 2022; Suwanchaikasem et al., 2022).
Progress in accessing chemical interactions with the rhizosphere has primarily been made by including advanced microfluidic chemical analysis tools and sensors into microfluidic cultivation chambers, enabling in situ analysis at high spatio-temporal resolution (Patabadige et al., 2019; Cahill et al., 2020; Aufrecht et al., 2022), thus offering novel possibilities to the study of exudation and signalling. As such, root-adapted microdevices paired with advances in visualization of morphological changes of tissues, namely morphodynamics, could further underline the integration of environmental conditions into the reshaping of root architecture and the transition between local and systemic responses based on tracking of signalling dynamics. The capabilities of in situ analysis of the plant’s status and also environmental properties could be further supported by the integration of bioelectronics into PDMS-based microdevices including nutrient sensors, spectrometric readouts, and measurements of electric field changes, but also the capabilities of localized stimulation (Dufil et al., 2022). The coupling of bioelectronics and microfluidics in fully integrated, miniaturized arrays of lab-on-a-chip technology will probably reduce the need for external analysis hardware.
Microbial species define the rhizosphere and contribute to plant performance and resilience. Microbial interactors, mostly bacterial species, have therefore also been included in root-adapted microfluidics (Massalha et al., 2019; Noirot-Gros et al., 2020; Jabusch et al., 2021), and have also expanded from model species such as E. coli and B. subtilis to native rhizosphere strains (Aufrecht et al., 2018). Whether a full reflection of taxonomically diverse rhizosphere communities, as seen in the usage of synthetic communities in the study of rhizosphere microbiology (Bai et al., 2015; Thiergart et al., 2020; Wippel et al., 2021), can be established in microdevices, is yet to be shown. Despite the representation of bacterial species, filamentous eukaryotes containing major plant pathogens and symbionts have not seen a similar representation in microdevices, even in early tests with oomycetes (Parashar and Pandey, 2011). Fungal pathogens and symbionts in particular would be prime targets for the future direction of soil-like microdevices similar to the root–bacteria interaction assessing temporal dynamics, potential influences of environmental stress, and utilizing advances in highly resolved chemical analysis to track nutrient trading during symbiosis.
Despite a wide range of potential applications, the utilization of microfluidics in plant research is still limited as only a few designs are used repeatedly, notably the EcoFab and RootChip devices (Denninger et al., 2014, 2019; Jones et al., 2014; Lanquar et al., 2014; Fendrych et al., 2018; Stanley et al., 2018; Rizza et al., 2019; Sasse et al., 2019; Hani et al., 2021; Kuang et al., 2022; Liu et al., 2022; Suwanchaikasem et al., 2022). Conventional methods of producing microfluidics, namely soft lithography, are a limiting factor by being costly and slow, and therefore not allowing rapid prototyping and in-house manufacturing. The integration of 3D printing into microfluidic manufacturing since the 2010s is moving this barrier (Ho et al., 2015; Waheed et al., 2016). The EcoFab concept builds on 3D-printed casting moulds for PDMS, as also the multi-petaloid chamber, and even fully 3D-printed microdevices have been tested in association with plants (Gao et al., 2018; Chai et al., 2019; Guichard et al., 2021, Preprint; Moussus and Meier, 2021). While, 3D-printing techniques provide access to rapid prototyping and enable full in-house production capabilities for many research labs, resolution is still a restriction for a full replacement of soft lithography. However, through the use of high-resolution production techniques such as two-photon or triplet–triplet annihilation polymerization, submicrometre resolutions could become achievable and affordable in the near future (Limberg et al., 2022).
In addition to the complexity of production, the usability of microdevices can also depend on active liquid handling capabilities. Consequently, the complexity of devices has reduced through their design cycle. For example, the RootChip device transitioned from an on-chip liquid handling to a linear channel design with external liquid handling (Grossmann et al., 2011; Stanley et al., 2018; Guichard et al., 2020). Similarly, the EcoFab device and its associated community have focused on a modular design and components easily adapted to different experimental questions (Gao et al., 2018). The complexity of the readouts leads to a further drawback, by restricting the throughput of microfluidic devices. Most devices display sample numbers in the range of 5–20, with only few exceptions exceeding that (RootArray, PlantArrayChip) (Table 1). Studies on root–environment interactions using microfluidics face the challenge of achieving a modular reconstruction of soil complexity towards an idealistic soil-on-a-chip device while ensuring sufficient throughput, accessibility, and ease of use.
Although microfluidic devices have helped improve controllability compared with traditional environments such as agar plates or hydroponics, they still suffer from common issues. One example is the illumination of the root system during growth, for which a range of side effects have been reported including changes in nutrient uptake, biotic interactions, root exudation, and root development (van Gelderen et al., 2018; Cabrera et al., 2022). To avoid inherent biases for the study of roots under light exposure, several studies have included light shielding of agar plates (Xu et al., 2013; Silva-Navas et al., 2015). Such concepts have also been developed for microfluidic devices, such as a shielding insert for the RootTrapr device (Suwanchaikasem et al., 2022). Similarly, for certain stresses such as drought, only proxies can be utilized in a liquid-based system, for example the application of osmolytes such as PEG, mannitol, sorbitol, or salt. Such proxies lower the water activity but also differ in their specific effect and do not constitute the full complexity of drought stress under field conditions (Dubois and Inzé, 2020). Consequently, standardizing best practices between traditional cultivation systems and microfluidic devices will be a necessary step for the field of root–environment studies.
Finally, beyond their application for root–environment studies, the versatility of structured microdevices has the potential to help uncover mechanisms underlying the integration of sensory information over space and time in root growth under non-uniform conditions (Meroz, 2021).
Acknowledgements
We apologize to all colleagues whose work could not be included in this review due to space limitations. We would like to thank Juliette Roué for helpful discussions.
Contributor Information
Christian-Frederic Kaiser, Institute of Cell and Interaction Biology, Heinrich-Heine-University Düsseldorf, Universitätsstrasse 1, D-40225 Düsseldorf, Germany; CEPLAS - Cluster of Excellence on Plant Sciences, Heinrich-Heine-University Düsseldorf, Universitätsstrasse 1, D-40225 Düsseldorf, Germany.
Alessia Perilli, School of Plant Sciences and Food Security, Tel Aviv University, Tel Aviv, Israel.
Guido Grossmann, Institute of Cell and Interaction Biology, Heinrich-Heine-University Düsseldorf, Universitätsstrasse 1, D-40225 Düsseldorf, Germany; CEPLAS - Cluster of Excellence on Plant Sciences, Heinrich-Heine-University Düsseldorf, Universitätsstrasse 1, D-40225 Düsseldorf, Germany.
Yasmine Meroz, School of Plant Sciences and Food Security, Tel Aviv University, Tel Aviv, Israel.
Ivan Reyna-Llorens, Centre for Research in Agricultural Genomics (CRAG), Spain.
Conflict of interest
The authors have no conflicts to declare.
Funding
Research in the Grossmann lab is supported by the German Research Foundation (DFG Heisenberg Professorship; grant nos GR4559/4-1, GR4559/5-1, and CRC1208 project A14) and Germany’s Excellence Strategy (CEPLAS—EXC-2048/1—project ID 390686111).
Research in the Meroz lab is supported by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 824074 (GrowBot), the Israel Science Foundation Grant (1981/ 14), the Human Frontier Science Program, reference no. RGY0078/2019, and Tel Aviv University Breaktrough Innovative Research Grant.
References
- Adl MS, GuptaVS.. 2006. Protists in soil ecology and forest nutrient cycling. Canadian Journal of Forest Research 36, 1805–1817. [Google Scholar]
- Agudelo CG, Sanati A, Ghanbari M, Packirisamy M, GeitmannA.. 2012. A microfluidic platform for the investigation of elongation growth in pollen tubes. Journal of Micromechanics and Microengineering 22, 115009. [Google Scholar]
- Aleklett K, Kiers ET, Ohlsson P, Shimizu TS, Caldas VE, HammerEC.. 2018. Build your own soil: exploring microfluidics to create microbial habitat structures. The ISME Journal 12, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnahhas RN, Winkle JJ, Hirning AJ, Karamched B, Ott W, Josić K, BennettMR.. 2019. Spatiotemporal dynamics of synthetic microbial consortia in microfluidic devices. ACS Synthetic Biology 8, 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao T, ed. 2012. Hydroponics: a standard methodology for plant biological researches. Rijeka, Croatia: InTech. [Google Scholar]
- Atkinson JA, Pound MP, Bennett MJ, WellsDM.. 2019. Uncovering the hidden half of plants using new advances in root phenotyping. Current Opinion in Biotechnology 55, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufrecht JA, Fowlkes JD, Bible AN, Morrell-Falvey J, Doktycz MJ, RettererST.. 2019. Pore-scale hydrodynamics influence the spatial evolution of bacterial biofilms in a microfluidic porous network. PLoS One 14, e0218316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufrecht J, Khalid M, Walton CL, Tate K, Cahill JF, RettererST.. 2022. Hotspots of root-exuded amino acids are created within a rhizosphere-on-a-chip. Lab on a Chip 22, 954–963. [DOI] [PubMed] [Google Scholar]
- Aufrecht JA, Ryan JM, Hasim S, Allison DP, Nebenführ A, Doktycz MJ, RettererST.. 2017. Imaging the root hair morphology of Arabidopsis seedlings in a two-layer microfluidic platform. Journal of Visualized Experiments 126, 55971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufrecht JA, Timm CM, Bible A, Morrell-Falvey JL, Pelletier DA, Doktycz MJ, RettererST.. 2018. Quantifying the spatiotemporal dynamics of plant root colonization by beneficial bacteria in a microfluidic habitat. Advanced Biosystems 2, 1800048. [Google Scholar]
- Bai Y, Müller DB, Srinivas G, et al. 2015. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528, 364–369. [DOI] [PubMed] [Google Scholar]
- Balagaddé FK, You L, Hansen CL, Arnold FH, QuakeSR.. 2005. Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science 309, 137–140. [DOI] [PubMed] [Google Scholar]
- Barkaoui K, Roumet C, VolaireF.. 2016. Mean root trait more than root trait diversity determines drought resilience in native and cultivated Mediterranean grass mixtures. Agriculture, Ecosystems & Environment 231, 122–132. [Google Scholar]
- Battat S, Weitz DA, WhitesidesGM.. 2022. An outlook on microfluidics: the promise and the challenge. Lab on a Chip 22, 530–536. [DOI] [PubMed] [Google Scholar]
- Bayani Ahangar S, Bellur K, Medici E, Tajiri K, Allen JS, ChoiCK.. 2019. Optical properties and swelling of thin film perfluorinated sulfonic-acid ionomer. ECS Transactions 92, 197–204. [Google Scholar]
- Bertrand-Rakusová H, Chebli Y, GeitmannA.. 2020. Silicone chambers for pollen tube imaging in microstructured in vitro environments. Methods in Molecular Biology 2160, 211–221. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee N, Urrios A, Kang S, FolchA.. 2016. The upcoming 3D-printing revolution in microfluidics. Lab on a Chip 16, 1720–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister A, Hilgers F, Langner A, et al. 2019. A microfluidic co-cultivation platform to investigate microbial interactions at defined microenvironments. Lab on a Chip 19, 98–110. [DOI] [PubMed] [Google Scholar]
- Busch W, Moore BT, Martsberger B, et al. 2012. A microfluidic device and computational platform for high-throughput live imaging of gene expression. Nature Methods 9, 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera J, Conesa CM, PozoJC.. 2022. May the dark be with roots: a perspective on how root illumination may bias in vitro research on plant–environment interactions. New Phytologist 233, 1988–1997. [DOI] [PubMed] [Google Scholar]
- Cahill JF, Khalid M, Retterer ST, Walton CL, KerteszV.. 2020. In situ chemical monitoring and imaging of contents within microfluidic devices having a porous membrane wall using liquid microjunction surface sampling probe mass spectrometry. Journal of the American Society for Mass Spectrometry 31, 832–839. [DOI] [PubMed] [Google Scholar]
- Canarini A, Kaiser C, Merchant A, Richter A, WanekW.. 2019. Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Frontiers in Plant Science 10, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai HH, Chen F, Zhang SJ, Li YD, Lu ZS, Kang YJ, YuL.. 2019. Multi-chamber petaloid root-growth chip for the non-destructive study of the development and physiology of the fibrous root system of Oryza sativa. Lab on a Chip 19, 2383–2393. [DOI] [PubMed] [Google Scholar]
- Clark NM, Van den Broeck L, Guichard M, et al. 2020. Novel imaging modalities shedding light on plant biology: start small and grow big. Annual Review of Plant Biology 71, 789–816. [DOI] [PubMed] [Google Scholar]
- Convery N, GadegaardN.. 2019. 30 years of microfluidics. Micro and Nano Engineering 2, 76–91. [Google Scholar]
- Correa J, Postma JA, Watt M, WojciechowskiT.. 2019. Soil compaction and the architectural plasticity of root systems. Journal of Experimental Botany 70, 6019–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Wu B, Chen B, Ma B, ChuC.. 2022. Diel fluctuation of extracellular reactive oxygen species production in the rhizosphere of rice. Environmental Science & Technology 56, 9075–9082. [DOI] [PubMed] [Google Scholar]
- Das S, Dash HR, eds. 2019. Microbial diversity in the genomic era. London: Elsevier/Academic Press. [Google Scholar]
- Deng Y, Chen H, Li C, et al. 2019. Endophyte Bacillus subtilis evade plant defense by producing lantibiotic subtilomycin to mask self-produced flagellin. Communications Biology 2, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denninger P, Bleckmann A, Lausser A, Vogler F, Ott T, Ehrhardt DW, Frommer WB, Sprunck S, Dresselhaus T, GrossmannG.. 2014. Male–female communication triggers calcium signatures during fertilization in Arabidopsis. Nature Communications 5, 4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denninger P, Reichelt A, Schmidt VAF, Mehlhorn DG, Asseck LY, Stanley CE, Keinath NF, Evers J-F, Grefen C, GrossmannG.. 2019. Distinct RopGEFs successively drive polarization and outgrowth of root hairs. Current Biology 29, 1854–1865. [DOI] [PubMed] [Google Scholar]
- Downie H, Holden N, Otten W, Spiers AJ, Valentine TA, DupuyLX.. 2012. Transparent soil for imaging the rhizosphere. PLoS One 7, e44276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, InzéD.. 2020. Plant growth under suboptimal water conditions: early responses and methods to study them. Journal of Experimental Botany 71, 1706–1722. [DOI] [PubMed] [Google Scholar]
- Dufil G, Bernacka-Wojcik I, Armada-Moreira A, StavrinidouE.. 2022. Plant bioelectronics and biohybrids: the growing contribution of organic electronic and carbon-based materials. Chemical Reviews 122, 4847–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbabin VM, Postma JA, Schnepf A, Pagès L, Javaux M, Wu L, Leitner D, Chen YL, Rengel Z, DiggleAJ.. 2013. Modelling root–soil interactions using three-dimensional models of root growth, architecture and function. Plant and Soil 372, 93–124. [Google Scholar]
- Durán P, Thiergart T, Garrido-Oter R, Agler M, Kemen E, Schulze-Lefert P, HacquardS.. 2018. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 175, 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Blom J, Klenk H-P, BorrissR.. 2017. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an ‘Operational Group B. amyloliquefaciens’ within the B. subtilis species complex. Frontiers in Microbiology 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M, Akhmanova M, Merrin J, Glanc M, Hagihara S, Takahashi K, Uchida N, Torii KU, FrimlJ.. 2018. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nature Plants 4, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman Y, MittlerR.. 2020. Rapid systemic signaling during abiotic and biotic stresses: is the ROS wave master of all trades? The Plant Journal 102, 887–896. [DOI] [PubMed] [Google Scholar]
- Figueiredo M do VB, Bonifacio A, Rodrigues AC, de AraujoFF.. 2016. Plant growth-promoting rhizobacteria: key mechanisms of action. In: Choudhary DK, Varma A, eds. Microbial-mediated induced systemic resistance in plants. Singapore: Springer Singapore, 23–37. [Google Scholar]
- Finkbeiner T, Manz C, Raorane ML, Metzger C, Schmidt-Speicher L, Shen N, Ahrens R, Maisch J, Nick P, GuberAE.. 2022. A modular microfluidic bioreactor to investigate plant cell–cell interactions. Protoplasma 259, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fita A, Rodríguez-Burruezo A, Boscaiu M, Prohens J, VicenteO.. 2015. Breeding and domesticating crops adapted to drought and salinity: a new paradigm for increasing food production. Frontiers in Plant Science 6, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster SM. 1990. The role of microorganisms in aggregate formation and soil stabilization: types of aggregation. Arid Soil Research and Rehabilitation 4, 85–98. [Google Scholar]
- Fry EL, Evans AL, Sturrock CJ, Bullock JM, BardgettRD.. 2018. Root architecture governs plasticity in response to drought. Plant and Soil 433, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines A, Ludovice M, Xu J, Zanghi M, Meinersmann RJ, Berrang M, Daley W, BrittonD.. 2019. The dialogue between protozoa and bacteria in a microfluidic device. PLoS One 14, e0222484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sasse J, Lewald KM, Zhalnina K, Cornmesser LT, Duncombe TA, Yoshikuni Y, Vogel JP, Firestone MK, NorthenTR.. 2018. Ecosystem Fabrication (EcoFAB) protocols for the construction of laboratory ecosystems designed to study plant–microbe interactions. Journal of Visualized Experiments 134, 57170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisen S, Mitchell EAD, Adl S, et al. 2018. Soil protists: a fertile frontier in soil biology research. FEMS Microbiology Reviews 42, 293–323. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi W-G, Toyota M, Devireddy AR, MittlerR.. 2014. A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends in Plant Science 19, 623–630. [DOI] [PubMed] [Google Scholar]
- Giraldo MC, ValentB.. 2013. Filamentous plant pathogen effectors in action. Nature Reviews. Microbiology 11, 800–814. [DOI] [PubMed] [Google Scholar]
- Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, YoungIM.. 2009. Root phenomics of crops: opportunities and challenges. Functional Plant Biology 36, 922. [DOI] [PubMed] [Google Scholar]
- Grossmann G, Guo W-J, Ehrhardt DW, Frommer WB, Sit RV, Quake SR, MeierM.. 2011. The RootChip: an integrated microfluidic chip for plant science. The Plant Cell 23, 4234–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber BD, Giehl RFH, Friedel S, von WirénN.. 2013. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiology 163, 161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünberger A, Paczia N, Probst C, Schendzielorz G, Eggeling L, Noack S, Wiechert W, KohlheyerD.. 2012. A disposable picolitre bioreactor for cultivation and investigation of industrially relevant bacteria on the single cell level. Lab on a Chip 12, 2060–2068. [DOI] [PubMed] [Google Scholar]
- Guichard M, Bertran Garcia de Olalla E, Stanley CE, GrossmannG.. 2020. Microfluidic systems for plant root imaging. Methods in Cell Biology 160, 381–404. [DOI] [PubMed] [Google Scholar]
- Guichard M, Holla S, Wernerová D, Grossmann G, MininaEA.. 2021. RoPod, a customizable toolkit for non-invasive root imaging, reveals cell type-specific dynamics of plant autophagy. bioRxiv 10.1101/2021.12.07.471480 [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonnet JP, Cantarel AAM, Simon L, HaicharFEZ.. 2018. Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecology and Evolution 8, 8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hani S, Cuyas L, David P, et al. 2021. Live single-cell transcriptional dynamics via RNA labelling during the phosphate response in plants. Nature Plants 7, 1050–1064. [DOI] [PubMed] [Google Scholar]
- Heo J, Thomas KJ, Seong GH, CrooksRM.. 2003. A microfluidic bioreactor based on hydrogel-entrapped E. coli: cell viability, lysis, and intracellular enzyme reactions. Analytical Chemistry 75, 22–26. [DOI] [PubMed] [Google Scholar]
- Ho CMB, Ng SH, Li KHH, YoonY-J.. 2015. 3D Printed microfluidics for biological applications. Lab on a Chip 15, 3627–3637. [DOI] [PubMed] [Google Scholar]
- Huang W, Reyes-Caldas P, Mann M, Seifbarghi S, Kahn A, Almeida RPP, Béven L, Heck M, Hogenhout SA, CoakerG.. 2020. Bacterial vector-borne plant diseases: unanswered questions and future directions. Molecular Plant 13, 1379–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabusch LK, Kim PW, Chiniquy D, et al. 2021. Microfabrication of a chamber for high-resolution, in situ imaging of the whole root for plant–microbe interactions. International Journal of Molecular Sciences 22, 7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ali MA, Jiao Y, Yang B, DongL.. 2017. In-situ, real-time monitoring of nutrient uptake on plant chip integrated with nutrient sensor. 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS). Kaohsiung: IEEE, 289–292. [Google Scholar]
- Jones AM, Danielson JA, ManojKumar SN, Lanquar V, Grossmann G, FrommerWB.. 2014. Abscisic acid dynamics in roots detected with genetically encoded FRET sensors. eLife 3, e01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R, Boer D, Hayes S, TesterinkC.. 2021. Root plasticity under abiotic stress. Plant Physiology 187, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K. 2013. Auxin and the integration of environmental signals into plant root development. Annals of Botany 112, 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath NF, Waadt R, Brugman R, Schroeder JI, Grossmann G, Schumacher K, KrebsM.. 2015. Live cell imaging with R-GECO1 sheds light on flg22- and chitin-induced transient [Ca2+]cyt patterns in Arabidopsis. Molecular Plant 8, 1188–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights HE, Jorrin B, Haskett TL, PoolePS.. 2021. Deciphering bacterial mechanisms of root colonization. Environmental Microbiology Reports 13, 428–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb E, Legué V, Bogeat-TriboulotM-B.. 2017. Physical root–soil interactions. Physical Biology 14, 065004. [DOI] [PubMed] [Google Scholar]
- Kuang W, Sanow S, Kelm JM, Müller Linow M, Andeer P, Kohlheyer D, Northen T, Vogel JP, Watt M, ArsovaB.. 2022. N-dependent dynamics of root growth and nitrate and ammonium uptake are altered by the bacterium Herbaspirillum seropedicae in the cereal model Brachypodium distachyon. Journal of Experimental Botany 73, 5306–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar V, Grossmann G, Vinkenborg JL, Merkx M, Thomine S, FrommerWB.. 2014. Dynamic imaging of cytosolic zinc in A rabidopsis roots combining FRET sensors and RootChip technology. New Phytologist 202, 198–208. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Zheng W, Ryo M, Soutschek K, Roy J, Rongstock R, Maaß S, RilligMC.. 2020. Fungal traits important for soil aggregation. Frontiers in Microbiology 10, 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg DK, Kang J-H, HaywardRC.. 2022. Triplet–triplet annihilation photopolymerization for high-resolution 3D printing. Journal of the American Chemical Society 144, 5226–5232. [DOI] [PubMed] [Google Scholar]
- Liu S, Strauss S, Adibi M, et al. 2022. Cytokinin promotes growth cessation in the Arabidopsis root. Current Biology 32, 1974–1985. [DOI] [PubMed] [Google Scholar]
- Lucas M, Schlüter S, Vogel H-J, VetterleinD.. 2019. Roots compact the surrounding soil depending on the structures they encounter. Scientific Reports 9, 16236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Shi Y, Siemianowski O, Yuan B, Egner TK, Mirnezami SV, Lind KR, Ganapathysubramanian B, Venditti V, CademartiriL.. 2019. Hydrogel-based transparent soils for root phenotyping in vivo. Proceedings of the National Academy of Sciences, USA 116, 11063–11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafla-Endara PM, Arellano-Caicedo C, Aleklett K, Pucetaite M, Ohlsson P, HammerEC.. 2021. Microfluidic chips provide visual access to in situ soil ecology. Communications Biology 4, 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Yang T, CremerPS.. 2002. A microfluidic device with a linear temperature gradient for parallel and combinatorial measurements. Journal of the American Chemical Society 124, 4432–4435. [DOI] [PubMed] [Google Scholar]
- Massalha H, Korenblum E, Malitsky S, Shapiro OH, AharoniA.. 2017. Live imaging of root–bacteria interactions in a microfluidics setup. Proceedings of the National Academy of Sciences, USA 114, 4549–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massalha H, Korenblum E, Shapiro O, AhroniA.. 2019. Tracking root interactions system (TRIS) experiment and quality control. Bio-protocol 9, e3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M, Lucchetta EM, IsmagilovRF.. 2010. Chemical stimulation of the Arabidopsis thaliana root using multi-laminar flow on a microfluidic chip. Lab on a Chip 10, 2147–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroz Y. 2021. Plant tropisms as a window on plant computational processes. New Phytologist 229, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Moussus M, MeierM.. 2021. A 3D-printed Arabidopsis thaliana root imaging platform. Lab on a Chip 21, 2557–2564. [DOI] [PubMed] [Google Scholar]
- Noirot-Gros M-F, Shinde SV, Akins C, Johnson JL, Zerbs S, Wilton R, Kemner KM, Noirot P, BabniggG.. 2020. Functional imaging of microbial interactions with tree roots using a microfluidics setup. Frontiers in Plant Science 11, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober ES, Alahmad S, Cockram J, et al. 2021. Wheat root systems as a breeding target for climate resilience. Theoretical and Applied Genetics 134, 1645–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiro D, Filho RB, Assis OBG, Jorge LA, ColnagoLA.. 2012. Measuring bacterial cells size with AFM. Brazilian Journal of Microbiology 43, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar A, PandeyS.. 2011. Plant-in-chip: microfluidic system for studying root growth and pathogenic interactions in Arabidopsis. Applied Physics Letters 98, 263703. [Google Scholar]
- Park Y-H, Lee N, Choi G, ParkJ-K.. 2017. Plant array chip for the germination and growth screening of Arabidopsis thaliana. Lab on a Chip 17, 3071–3077. [DOI] [PubMed] [Google Scholar]
- Patabadige DEW, Millet LJ, Aufrecht JA, Shankles PG, Standaert RF, Retterer ST, DoktyczMJ.. 2019. Label-free time- and space-resolved exometabolite sampling of growing plant roots through nanoporous interfaces. Scientific Reports 9, 10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce G, Corkidi G, Eapen D, Lledías F, Cárdenas L, CassabG.. 2017. Root hydrotropism and thigmotropism in Arabidopsis thaliana are differentially controlled by redox status. Plant Signaling & Behavior 12, e1305536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellán-Álvarez R, Lobet G, Lindner H, et al. 2015. GLO-Roots: an imaging platform enabling multidimensional characterization of soil-grown root systems. eLife 4, e07597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TA, Dacks JB, Jenkinson JM, Thornton CR, TalbotNJ.. 2006. Evolution of filamentous plant pathogens: gene exchange across eukaryotic kingdoms. Current Biology 16, 1857–1864. [DOI] [PubMed] [Google Scholar]
- Richter F, Bindschedler S, Calonne-Salmon M, Declerck S, Junier P, StanleyCE.. 2022. Fungi-on-a-Chip: microfluidic platforms for single-cell studies on fungi. FEMS Microbiology Reviews 46, fuac039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza A, Tang B, Stanley CE, Grossmann G, Owen MR, Band LR, JonesAM.. 2021. Differential biosynthesis and cellular permeability explain longitudinal gibberellin gradients in growing roots. Proceedings of the National Academy of Sciences, USA 118, e1921960118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza A, Walia A, Tang B, JonesAM.. 2019. Visualizing cellular gibberellin levels using the nlsGPS1 Förster resonance energy transfer (FRET) biosensor. Journal of Visualized Experiments 143, 58739. [DOI] [PubMed] [Google Scholar]
- Robinson H, Kelly A, Fox G, Franckowiak J, Borrell A, HickeyL.. 2018. Root architectural traits and yield: exploring the relationship in barley breeding trials. Euphytica 214, 151. [Google Scholar]
- Roesch LFW, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD, Daroub SH, Camargo FAO, Farmerie WG, TriplettEW.. 2007. Pyrosequencing enumerates and contrasts soil microbial diversity. The ISME Journal 1, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoine M, Ishikawa Y, Kleist TJ, Wudick MM, Nakamura M, Grossmann G, Frommer WB, HoC-H.. 2021. Designs, applications, and limitations of genetically encoded fluorescent sensors to explore plant biology. Plant Physiology 187, 485–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Charlot F, Le Saux T, Bonhomme S, Nogué F, Palauqui J-C, FattaccioliJ.. 2019. Design of a comprehensive microfluidic and microscopic toolbox for the ultra-wide spatio-temporal study of plant protoplasts development and physiology. Plant Methods 15, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse J, Martinoia E, NorthenT.. 2018. Feed your friends: do plant exudates shape the root microbiome? Trends in Plant Science 23, 25–41. [DOI] [PubMed] [Google Scholar]
- Sasse J, Kant J, Cole BJ, et al. 2019. Multilab EcoFAB study shows highly reproducible physiology and depletion of soil metabolites by a model grass. New Phytologist 222, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppey CVW, Singer D, Dumack K, Fournier B, Belbahri L, Mitchell EAD, LaraE.. 2017. Distribution patterns of soil microbial eukaryotes suggests widespread algivory by phagotrophic protists as an alternative pathway for nutrient cycling. Soil Biology and Biochemistry 112, 68–76. [Google Scholar]
- Sharma K, Palatinszky M, Nikolov G, Berry D, ShankEA.. 2020. Transparent soil microcosms for live-cell imaging and non-destructive stable isotope probing of soil microorganisms. eLife 9, e56275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Navas J, Moreno-Risueno MA, Manzano C, Pallero-Baena M, Navarro-Neila S, Téllez-Robledo B, Garcia-Mina JM, Baigorri R, Gallego FJ, del PozoJC.. 2015. D-Root: a system for cultivating plants with the roots in darkness or under different light conditions. The Plant Journal 84, 244–255. [DOI] [PubMed] [Google Scholar]
- Singh RK, SharmaRV.. 2017. Numerical analysis for ground temperature variation. Geothermal Energy 5, 22. [Google Scholar]
- Singh G, Pereira D, Baudrey S, Hoffmann E, Ryckelynck M, Asnacios A, ChaboutéM.. 2021. Real-time tracking of root hair nucleus morphodynamics using a microfluidic approach. The Plant Journal 108, 303–313. [DOI] [PubMed] [Google Scholar]
- Song ZX, Chai HH, Chen F, Yu L, FangC.. 2019. A foldable chip array for the continuous investigation of seed germination and the subsequent root development of seedlings. Micromachines 10, 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley CE, Grossmann G, Casadevall i Solvas X, deMelloAJ.. 2016. Soil-on-a-Chip: microfluidic platforms for environmental organismal studies. Lab on a Chip 16, 228–241. [DOI] [PubMed] [Google Scholar]
- Stanley CE, Shrivastava J, Brugman R, Heinzelmann E, van Swaay D, GrossmannG.. 2018. Dual-flow-RootChip reveals local adaptations of roots towards environmental asymmetry at the physiological and genetic levels. New Phytologist 217, 1357–1369. [DOI] [PubMed] [Google Scholar]
- Suwanchaikasem P, Idnurm A, Selby-Pham J, Walker R, BoughtonBA.. 2022. Root-TRAPR: a modular plant growth device to visualize root development and monitor growth parameters, as applied to an elicitor response of Cannabis sativa. Plant Methods 18, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto S, Takayasu S, Kitomi Y, Arai-Sanoh Y, Tanabata T, UgaY.. 2020. High-throughput three-dimensional visualization of root system architecture of rice using X-ray computed tomography. Plant Methods 16, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiergart T, Durán P, Ellis T, et al. 2020. Root microbiota assembly and adaptive differentiation among European Arabidopsis populations. Nature Ecology & Evolution 4, 122–131. [DOI] [PubMed] [Google Scholar]
- Totsche KU, Rennert T, Gerzabek MH, Kögel-Knabner I, Smalla K, Spiteller M, VogelH.. 2010. Biogeochemical interfaces in soil: the interdisciplinary challenge for soil science. Journal of Plant Nutrition and Soil Science 173, 88–99. [Google Scholar]
- van den Hoogen J, Geisen S, Wall DH, et al. 2020. A global database of soil nematode abundance and functional group composition. Scientific Data 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen K, Kang C, PierikR.. 2018. Light signaling, root development, and plasticity. Plant Physiology 176, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed S, Cabot JM, Macdonald NP, Lewis T, Guijt RM, Paull B, BreadmoreMC.. 2016. 3D printed microfluidic devices: enablers and barriers. Lab on a Chip 16, 1993–2013. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang H, Wang Y, DongL.. 2017. Generation of temperature gradient on microfluidic plant chip for high-throughput plant phenotyping. 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS). Los Angeles, CA, USA: IEEE, 398–401. [Google Scholar]
- Wippel K, Tao K, Niu Y, et al. 2021. Host preference and invasiveness of commensal bacteria in the Lotus and Arabidopsis root microbiota. Nature Microbiology 6, 1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, WhitesidesGM.. 1998. Soft lithography. Angewandte Chemie International Edition 37: 550–575. [DOI] [PubMed] [Google Scholar]
- Xu W, Ding G, Yokawa K, Baluška F, Li Q-F, Liu Y, Shi W, Liang J, ZhangJ.. 2013. An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. Scientific Reports 3, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N, Kozgunova E, Grossmann G, Geitmann A, HigashiyamaT.. 2021. Microfluidics-based bioassays and imaging of plant cells. Plant and Cell Physiology 62, 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N, Sugimoto N, Arata H, Higashiyama T, SatoY.. 2017. Capability of tip-growing plant cells to penetrate into extremely narrow gaps. Scientific Reports 7, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]