Abstract
Gastric cancer with Virchow’s lymph node metastasis (LNM) is not indicated for initial curative surgery. Although there have been some case reports of curative resections after pre-operative treatment, including immune checkpoint inhibitors (ICIs), there is no consensus regarding the optimal timing of surgery. We describe a rare case of initially unresectable gastric cancer treated preoperatively with nivolumab combined chemotherapy, which achieved a pathologically complete response. An 82-year-old man was referred for gastric cancer treatment. Contrast-enhanced computed tomography revealed stomach wall thickening and swollen left supraclavicular LN. This gastric cancer was assessed as unresectable due to the presence of Virchow’s LNM; therefore, chemotherapy and ICI using S-1 plus oxaliplatin plus nivolumab were administered. After three courses of treatment, the primary tumor and Virchow’s LN showed a marked reduction in size. The patient underwent Virchow’s LNM resection as a preliminary step to determine indications for curative surgery. A pathological examination revealed no viable cancer cells were found inside the resected LN. The patient underwent distal gastrectomy. Pathological examination revealed complete degeneration of the primary tumor and regional LN without residual carcinoma. The patient did not receive adjuvant chemotherapy and survived with no evidence of recurrence for one year after the initial treatment.
Keywords: gastric cancer, Virchow’s lymph metastasis, complete response, pre-operative treatment, nivolumab
Plain Language Summary
We describe a rare case of initially unresectable gastric cancer treated preoperatively with nivolumab combined chemotherapy, which achieved a pathologically complete response. An 82-year-old man was referred for gastric cancer treatment. Contrast-enhanced computed tomography revealed stomach wall thickening and swollen left supraclavicular LN. This gastric cancer was assessed as unresectable due to the presence of Virchow’s LNM; therefore, chemotherapy and ICI using S-1 plus oxaliplatin plus nivolumab were administered. After three courses of treatment, the primary tumor and Virchow’s LN showed a marked reduction in size. The patient underwent Virchow’s LNM resection as a preliminary step to determine indications for curative surgery. A pathological examination revealed no viable cancer cells were found inside the resected LN. The patient underwent distal gastrectomy. Pathological examination revealed complete degeneration of the primary tumor and regional LN without residual carcinoma. The patient did not receive adjuvant chemotherapy and survived with no evidence of recurrence for one year after the initial treatment.
Introduction
Gastric cancer was the fifth most common malignancy and the second leading cause of cancer-related deaths worldwide in 2018.1 In particular, there is no indication for initial surgical resection for stage IV gastric cancer, and the prognosis of these patients remains poor, with their median survival time reported to be 8.8–14.1 months.2,3 Recently, the efficacy in prolonging the prognosis for unresectable gastric cancer patients by immune checkpoint inhibitors (ICIs) have been demonstrated.4–6 Although there have been some case reports of conversion surgery after pre-operative treatment, including ICIs, for stage IV gastric cancer,7–9 there is no consensus regarding the optimal timing of curative gastrectomy. In this case, the indication for conversion surgery in a patient with stage IV gastric cancer was determined based on prior pathological diagnosis of unresectable factors. Moreover, the present study presents a rare case of initially unresectable gastric cancer with Virchow’s lymph node metastasis (LNM) treated preoperatively with nivolumab combined chemotherapy, which achieved a pathologically complete response.
Case Report
An 82-year-old man was referred for the treatment of gastric cancer. The patient had abdominal pain, nausea, and vomiting for several weeks. He had no medical or family history of gastric cancer. Physical examination revealed mild anemia in the palpebral conjunctiva. The serum levels of carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA19-9), and Krebs von den lungen-6 (KL-6) were within the normal limits, but the serum levels of hemoglobin and albumin declined to 10.1 g/dL and 3.2 g/dL, respectively. The Helicobacter pylori antibody test result was positive in the blood.
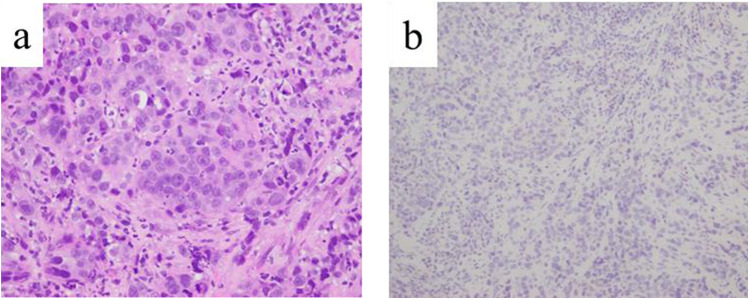
Esophagogastroduodenoscopy (EGD) revealed an irregular ulcerative tumor in the middle to lower region of the stomach, and the pyloric ring was constricted such that the EGD could barely pass through it (Figure 1). Endoscopically, the lesion was diagnosed as a type 3 advanced gastric cancer. Pathological examination of the endoscopic biopsy specimen from this region indicated poorly differentiated adenocarcinoma, and immunostaining for human epidermal growth factor type 2 (HER2) was negative (Figure 2a and b). Microsatellite instability (MSI) test results were MSI-stable In addition, the result of the combined positive score (CPS), which indicates the expression of the programmed cell death-ligand 1, was more than 5%.
Figure 1.
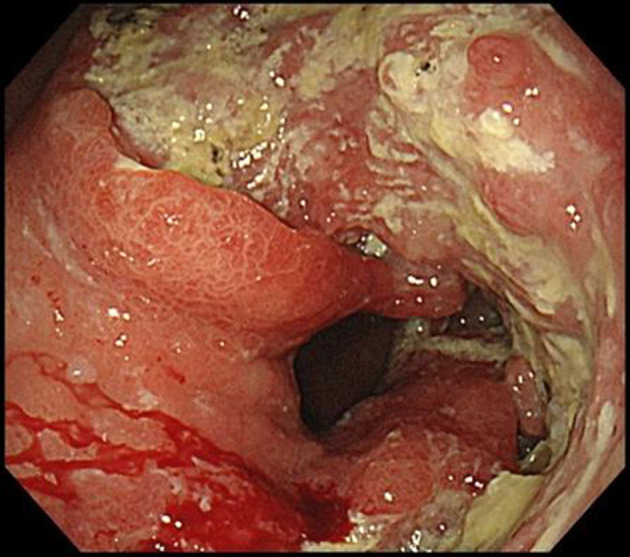
Endoscopic finding before treatment. On esophagogastroduodenoscopy before treatment, an irregular ulcerative tumor in the middle to lower stomach and the pyloric ring was constricted so that the scope could barely pass through it.
Figure 2.
Histopathological findings of biopsied primary gastric cancer. (a) On hematoxylin and eosin staining, alveolar and solid proliferation of tumor cells with hyperchromatic and enlarged nuclei with occasional glandular lumina were detected. (b) On immunostaining, the tumor cells were negative for HER2.
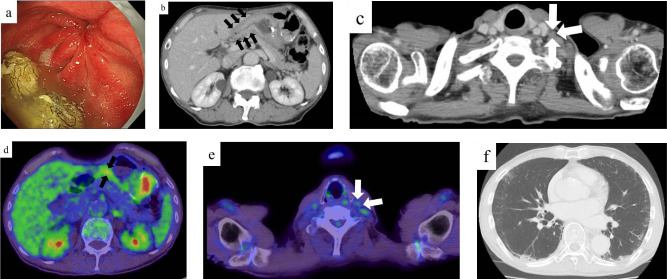
Contrast-enhanced computed tomography (CT) revealed thickening of the wall of the middle to lower stomach and a swollen left supraclavicular LN (Figure 3a and b). However, no distant metastasis to any other organs, including the liver, lung, peritoneal dissemination, or paraaortic LN, was observed. The patient did not undergo PET-CT at this time.
Figure 3.
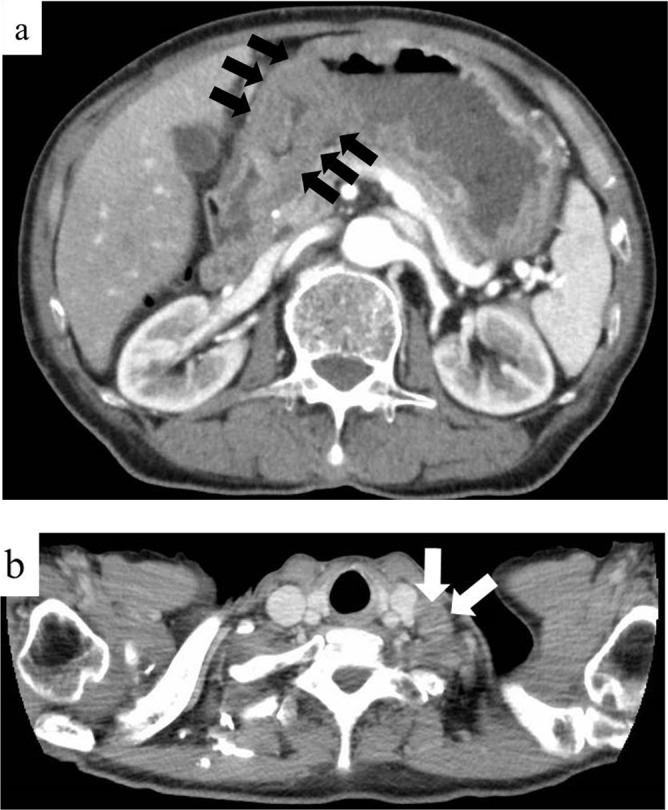
Radiological findings before treatment. (a and b) On contrast-enhanced computed tomography before treatment, a thickening of the wall of the middle to lower stomach (black arrows) and a swollen left supraclavicular lymph node (white arrows) were detected.
Initially, gastric cancer was classified as unresectable because of Virchow’s LNM, and a laparoscopic gastrojejunostomy with partial gastric partitioning was performed to improve the gastric obstruction. Subsequently, the patient received chemotherapy combined with ICI treatment using S-1 plus oxaliplatin (SOX) plus nivolumab fifteen days after gastrojejunostomy with partial gastric partitioning. Anthropometric measurements were as follows: height: 177.5 cm, weight: 63.6 kg, and body surface area: 1.79 m². The patient was administered S-1 (120 mg/body/day) orally on days 1–14, a 0.5-h intravenous infusion of nivolumab at a dose of 360 mg/body, and then a 2-h intravenous infusion of oxaliplatin at a dose of 130 mg/m2, at 3-week intervals. After the first course, the patient experienced an adverse event of fatigue (grade 2), so the doses of S-1 and oxaliplatin were reduced from the next course. S-1 (100 mg/body/day) was administered orally on days 1–14, a 0.5-h intravenous infusion of nivolumab at a dose of 360 mg/body, followed by a 2-h intravenous infusion of oxaliplatin at a dose of 100 mg/m2. The patient did not experience any grade 2 or higher adverse event during or after the second course of treatment.
After three courses of treatment, EGD revealed that the primary gastric cancer had shrunk, and no mucosal irregularities were observed (Figure 4a). Pathological examination of the endoscopic biopsy specimen revealed regenerative atypia without malignant features. Contrast-enhanced CT detected decreased gastric wall thickening and reduced size of the swollen Virchow’s LN (Figure 4b and c). PET-CT showed that mild accumulation was still present in the stomach (SUVmax 3.33); however, there was no abnormal accumulation in Virchow’s LN (Figure 4d and e). As an adverse effect, chest CT revealed grade 1 interstitial pneumonia (Figure 4f), and the serum level of KL-6 increased to 1162 U/mL, but no clinical respiratory symptoms were present.
Figure 4.
Endoscopic and radiological findings after pre-operative treatment. (a) On esophagogastroduodenoscopy after 3 courses of pre-operative treatment, an irregular ulcerative tumor had shrunk, mucosal irregularities had disappeared, and scarring was observed. (b and c) On contrast-enhanced computed tomography after 3 courses of pre-operative treatment, a thickening of the gastric wall (black arrows) and the size of swollen left supraclavicular lymph node (white arrows) were decreased. (d and e) On PET-CT after 3 courses of pre-operative treatment, a mild accumulation was still present in the stomach (SUVmax 3.33: black arrows) and there was no abnormal accumulation in a left supraclavicular lymph node (white arrows). (f) On plain chest computed tomography after 3 courses of pre-operative treatment, a mild ground glass opacity was detected.
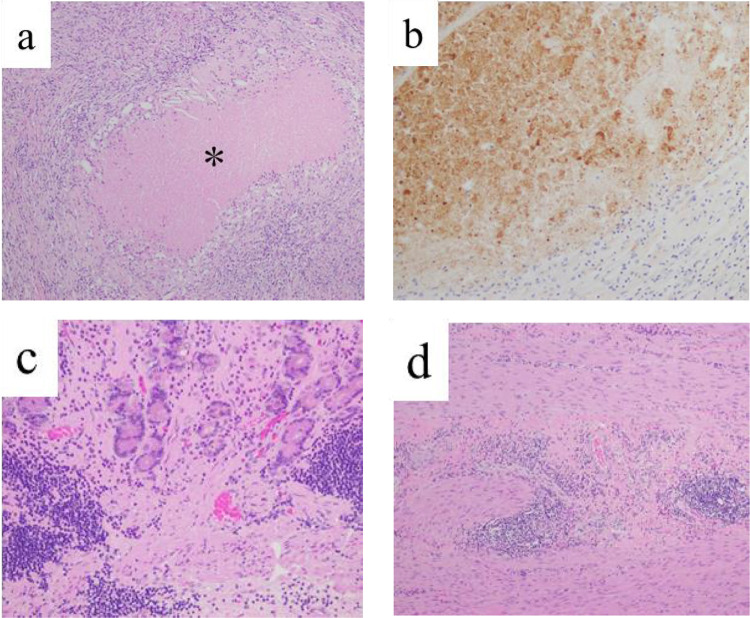
At this point, we determined that continued SOX plus nivolumab therapy was at risk for exacerbation of interstitial pneumonia. First, the patient underwent Virchow’s LN resection as a preliminary step to determine indications for curative resection. Thus, if Virchow’s LN is cancer-negative, it will be determined that the unresectable factor is controlled, and curative conversion surgery will be performed, but if not, drug therapy will be continued. Histopathological examination revealed that the insides of the resected Virchow’s LNs were entirely necrotic, and histiocytes were conspicuous around them. Furthermore, these necrotic parts were positive for AE1/AE3 staining and were thought to be previously present with metastasis from epithelial malignancy but are now thought to be only ghostly cancerous cells. No viable cancer cells were found inside the resected LN (Figure 5a and b).
Figure 5.
Histopathological findings of Virchow’s lymph nodes and primary gastric cancer by the resected specimen. (a) On hematoxylin and eosin staining of the resected Virchow’s lymph nodes, there is coagulation necrosis (*) surrounded with histiocyte. There are no viable cancer cells. (b) On immunohistochemical staining for cytokeratin (clone AE1/AE3) of the resected Virchow’s lymph nodes, the necrotic regions were stained, indicate of necrotic change of cancer cells. (c, d) On hematoxylin and eosin staining of the resected stomach, mucosa (c) and muscularis propria (d) failed to show any viable cancer cell.
Therefore, in the second phase, the patient underwent robot-assisted distal gastrectomy with D2 regional LN dissection because on preoperative PET-CT, a mild accumulation was still present in the stomach (SUVmax 3.33). Histopathological examination revealed complete degeneration of the primary tumor and regional LN without any residual carcinoma (Figure 5c and d), and the histological response of the tumor was categorized as grade 3.
The patient had no post-operative complications, survived without a recurrence for one year after the initial treatment, and did not receive adjuvant chemotherapy. Therefore, a treatment strategy that evaluates unresectable factors pathologically in advance to determine the indications for curative conversion surgery may be useful for initially unresectable gastric cancer after pre-operative treatment.
Discussion
Gastric cancer is the fifth most common malignant neoplasm and the second leading cause of cancer-related deaths worldwide.1 In particular, the incidence and mortality rate of gastric cancer are highest in Eastern Asia. In Japan, gastric cancer is still the third most common malignant cancer and the third leading cause of cancer-related deaths.10 Although surgical resection is usually the most suitable treatment for resectable gastric cancer, there is no indication for gastrectomy in patients with distant metastases, including liver, lung, peritoneal dissemination, and distant LN. Therefore, chemotherapy is the standard treatment for stage IV gastric cancer. Patients with stage IV gastric cancer still have a poor prognosis, with a five-year overall survival rate of 8.8 to 14.9%.2,11 These survival rates were still unsatisfactory. A left supraclavicular LN was first described by Rudolf Ludwig Karl Virchow as a frequent metastasis from gastric cancer.12 Subsequently, a left supraclavicular LN is called Virchow’s LN, and metastasis to this site is considered the final stage of lymphatic progression of cancer, with a very poor prognosis.
The prognostic significance of non-curative surgery for reducing the tumor burden of stage IV gastric cancer has been denied in previous clinical trials.13 However, with the progression of anticancer drug therapy, some case reports state that curative surgery for stage IV gastric cancer becomes possible after pre-operative treatment.7–9,14–16 An international multicenter retrospective study verified the significance of conversion surgery and found that the median survival time of all resected patients who received pre-operative treatment for stage IV gastric cancer was 36.7 months. Those of R0, R1, and R2 resection were 56.6, 25.8, and 21.7 months, respectively.17 In addition, the incidence of Clavien–Dindo grade 3 or higher post-operative complication and mortality rates in conversion surgery patients were 10.3 and 0.2%, respectively.17 These results were relatively favorable and have been considered a treatment option for stage IV gastric cancer when R0 resection is possible after pre-operative treatment.18
However, there is still no clear consensus on conversion surgery for stage IV gastric cancer, and the optimal criteria and duration of pre-operative treatment for which conversion surgery should be performed remain unclear. Moreover, it has not yet been concluded at this time whether curative resection or continued chemotherapy for stage IV gastric cancer after pre-operative treatment is superior, and comparative clinical trials (The RENAISSANCE trial) are being conducted,19 the results of which should be watched closely.
In recent years, a small number of metastases in a small number of metastatic organs is called oligo metastasis. This case is thought to be an oligo metastasis. In gastric cancer, the efficacy of radical resection after preoperative treatment has been suggested for a small number of localized aortic lymph node metastases.20 In addition, the significance of surgical resection has been suggested for single liver metastasis.21,22 Although there are no comprehensive reports on Virchow’s LNM, considering the same as aortic lymph node metastasis, radical resection after preoperative treatment as in this case may lead to improved prognosis.
In patients with gastric obstruction, there are some options for improving gastric obstruction: gastrojejunostomy, stents, naso-enteric nutrition tubes, and gastrostomy. Gastrojejunostomy is considered to be preferable to stenting for gastric obstruction if the patient has a certain amount of survival time and is able to tolerate the surgery, and the site of gastric cancer is amenable to anastomosis.23,24 In addition, previous literatures reported that up to 50% of patients who underwent gastrojejunostomy developed delayed gastric emptying postoperatively and also showed that the addition of gastric partitioning improved postoperative gastric emptying.25–27 Therefore, we think that gastrojejunostomy with partial or complete gastric partitioning is currently the first choice for patients with gastric obstruction. In addition, decompression via a nasogastric tube or gastrostomy should be considered for patients with a fairly limited prognosis.18
In recent years, ICIs have been shown to prolong the prognosis of various unresectable malignant tumors.28–30 Concerning gastric cancer, it was reported that nivolumab, which is a fully human IgG4 monoclonal antibody targeting programmed cell death 1, improved the prognosis.4–6 First, nivolumab monotherapy as third-line therapy improved overall survival time compared to placebo treatment in patients with previously treated unresectable gastric cancer.4 Subsequently, nivolumab with chemotherapy as the first-line therapy offered a prognostic benefit compared with chemotherapy alone.5,6 Therefore, the combination of nivolumab plus chemotherapy is currently recommended as a first-line treatment for HER2-negative unresectable gastric cancer with a CPS of more than 5%.31 Some case reports of conversion surgery use these agents.7–9 However, the prognostic effect of pre-operative treatment using chemotherapy plus ICI for stage IV gastric cancer is still unclear, as with chemotherapy alone.
Since surgical resection is a local treatment, we believe that distant unresectable factors need to be controlled in advance when performing conversion surgery. This is because patients with active distant metastases are not candidates for local treatment and require systemic therapy. In this case, imaging studies revealed that Virchow’s LN decreased in size, and no abnormal accumulation was detected at the same site. Therefore, confirmation that Virchow’s LNM was pathologically cancer-negative by pre-operative treatment was performed prior to curative gastrectomy. This was because we think that if a pathological complete response is obtained in the largest metastatic lesion, there is a high possibility that all other microscopic metastases that may be present have been controlled and disappeared; therefore, primary resection will lead to complete curative treatment. However, we believe that this is a necessary but insufficient condition for conversion surgery. This factor strictly describes only the disappearance of aggregated cancer and does not indicate the absence of microscopic cancer cells in the veins or lymphatics. It was thought that if the serum levels of CEA and CA19-9, which were reported to be associated with prognosis in gastric cancer,32,33 were elevated at the time of pre-treatment, it would be one of the judgment indicators. However, these tumor markers also do not sensitively reflect the existence of a microscopic disease. Recently, it was reported that the detection of minimum residual disease using blood-derived circulating tumor DNA (ctDNA) effectively predicted the prognosis of gastric cancer.21,22,34,35 Moreover, for colorectal cancer, a prospective study using ctDNA to predict recurrence and indications for adjuvant therapy in curative resected colorectal cancer is being conducted.36 However, at present, there is no insurance approval for using ctDNA as a predictive marker for gastric cancer patients in routine clinical practice in Japan. It may be a future task to examine the usefulness of such technology and to establish factors that predict the appropriate timing to shift from systemic therapy to local treatment. However, at this time, a multistep process that evaluates unresectable distant factors and determines curative resection is considered desirable in patients with stage IV gastric cancer. In previous reports, conversion surgery was performed based on the improvement in distant metastases on imaging studies.7–9,14–16
Curative gastrectomy and adjuvant chemotherapy prolong the prognosis of patients with resected stage II and III gastric cancer compared to curative surgery alone37–40 and have become standard treatments. In addition, in stage IV gastric cancer patients, adjuvant chemotherapy is weakly recommended in the Japanese gastric cancer treatment guidelines.18 However, there is no clear evidence regarding the indications for adjuvant therapy in patients with stage IV gastric cancer who eventually achieve pathological complete remission after chemotherapy and ICI. However, as adjuvant chemotherapy has certain adverse effects,37–40 it is necessary to assess the benefits and harms of adjuvant treatment. In this case, a good prognosis was obtained without adjuvant therapy; however, the indication of adjuvant therapy for such cases may be an issue for future consideration.
In conclusion, the present study reports a rare case of initially unresectable gastric cancer with Virchow’s LNM showing a pathologically complete response to pre-operative chemotherapy combined with ICI. A treatment strategy that evaluates distant metastasis, aiding in the decision on whether to perform curative conversion surgery, may be effective for unresectable gastric cancer.
Abbreviations
CEA, Carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CT, computed tomography; EGD, Esophagogastroduodenoscopy; ICI, immune checkpoint inhibitor; LNM, lymph node metastasis; PET-CT, positron emission tomography-CT.
Ethics Statement
Written informed consent was obtained from the patient for the publication of this report and accompanying images. Institutional ethics committee approval was not required to publish this manuscript. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author Contributions
Wataru Izumo contributed to conception and design. Wataru Izumo, Kei Hosoda, Hidekazu Kuramochi, Go Nakajima, Shinsuke Maeda, Shunichi Ito, Yoji Nagashima and Michio Itabashi contributed to the development of methodology and data acquisition. Wataru Izumo, Kei Hosoda, Yoji Nagashima, and Michio Itabashi contributed to the analysis and interpretation of data, writing, review, and revision of the manuscript. All authors have read and approved the final manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Wataru Izumo, Kei Hosoda, Hidekazu Kuramochi, Go Nakajima, Shinsuke Maeda, Shunichi Ito, Yoji Nagashima, and Michio Itabashi declare no conflicts of interest.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. [DOI] [PubMed] [Google Scholar]
- 2.Kim SG, Seo HS, Lee HH, et al. Comparison of the Differences in Survival Rates Between the 7th and 8th Editions of the AJCC TNM Staging System for Gastric Adenocarcinoma: a Single-Institution Study of 5507 Patients in Korea. J Gastric Canc. 2017;17:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148. [DOI] [PubMed] [Google Scholar]
- 4.Boku N, Ryu MH, Kato K, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2022;23:234–247. [DOI] [PubMed] [Google Scholar]
- 5.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto R, Arigami T, Matsushita D, et al. Conversion surgery for stage IV gastric cancer with a complete pathological response to nivolumab: a case report. World J Surg Oncol. 2020;18:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumamoto T, Tomita T, Hojo Y, et al. Pathological Complete Response and Successful Conversion Surgery After Nivolumab Therapy for Stage IV Oesophagogastric Junction Cancer. Vivo. 2021;35:2247–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai P, Rao X, Zhang X, et al. Complete Remission of a Patient With Metastatic Gastric Cancer Treated With Nivolumab Combined With Chemotherapy After Palliative Surgery. Front Immunol. 2022;13:908558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vital Statistics Japan (Ministry of Health, Labour and Welfare). Center for Cancer Control and Information Services, National Cancer Center, Japan Web site. Available from: https://ganjoho.jp/reg_stat/statistics/dl/index.html. Accessed July 11, 2023.
- 11.Nashimoto A, Akazawa K, Isobe Y, et al. Gastric Cancer Treated in 2002 in Japan: 2009 Annual Report of the JGCA Nationwide Registry. Gastric Canc. 2013;16:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virchow RLK. Zur diagnose der krebse im unterleibe. Med Reform. 1848;45:248. [Google Scholar]
- 13.Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Am J Surg. 2008;196:100–113. [DOI] [PubMed] [Google Scholar]
- 14.Izumo W, Furukawa K, Katsuragawa H, et al. A case of advanced gastric cancer with tumor embolus in the portal vein and liver metastasis responding to S-1 plus cisplatin chemotherapy. Nihon Shokakibyo Gakkai Zasshi. 2014;111:2131–2139. [PubMed] [Google Scholar]
- 15.Terashima T, Yamashita T, Takabatake H, et al. Successful second conversion surgery after trastuzumab deruxtecan for recurrent HER2-positive gastric cancer. Clin J Gastroenterol. 2023. doi: 10.1007/s12328-023-01764-3 [DOI] [PubMed] [Google Scholar]
- 16.Iwazawa T, Kinuta M, Yano H, et al. An oral anticancer drug, TS-1, enabled a patient with advanced gastric cancer with Virchow’s metastasis to receive curative resection. Gastric Cancer. 2002;5:96–101. [DOI] [PubMed] [Google Scholar]
- 17.Holen KD, Klimstra DS, Hummer A, et al. International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann Gastroenterol Surg. 2021;6:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021. Gastric Cancer. Kanehara Publishing Co, Ltd; 2021. [Google Scholar]
- 19.Al-Batran SE, Goetze TO, Mueller DW, et al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction - a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer. 2017;17:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–660. [DOI] [PubMed] [Google Scholar]
- 21.Marker SR, Mikhail S, Malietzis G, et al. Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: systematic Review and Pooled Analysis. Ann Surg. 2016;263:1092–1101. [DOI] [PubMed] [Google Scholar]
- 22.Kodera Y, Fujitani K, Fukushima N, et al. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. 2014;17:206–212. [DOI] [PubMed] [Google Scholar]
- 23.Bian SB, Shen WS, Xi HQ, et al. Palliative Therapy for Gastric Outlet Obstruction Caused by Unresectable Gastric Cancer: a Meta-analysis Comparison of Gastrojejunostomy with Endoscopic Stenting. Chin Med J. 2016;129:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed O, Lee JH, Thompson CC, et al. AGA Clinical Practice Update on the Optimal Management of the Malignant Alimentary Tract Obstruction: expert Review. Clin Gastroenterol Hepatol. 2021;19:1780–1788. [DOI] [PubMed] [Google Scholar]
- 25.Oida T, Mimatsu K, Kawasaki A, et al. Modified Devine exclusion with vertical stomach reconstruction for gastric outlet obstruction: a novel technique. J Gastrointest Surg. 2009;13:1226–1232. [DOI] [PubMed] [Google Scholar]
- 26.Ramos MFKP, Barchi LC, de Oliveira RJ, et al. Gastric partitioning for the treatment of malignant gastric outlet obstruction. World J Gastrointest Oncol. 2019;11:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorusso D, Giliberti A, Bianco M, et al. Stomach-partitioning gastrojejunostomy is better than conventional gastrojejunostomy in palliative care of gastric outlet obstruction for gastric or pancreatic cancer: a meta-analysis. J Gastrointest Oncol. 2019;10:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J. 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Japanese Gastric Cancer Association. Comment of Japanese gastric cancer treatment guidelines committee. Available fron: chromeextension://efaidnbmnnnibpcajpcglclefindmkaj/http://www.jgca.jp/pdf/news202112_1.pdf. Accessed July 11, 2023.
- 32.Song YX, Huang XZ, Gao P, et al. Clinicopathologic and prognostic value of serum carbohydrate antigen 19-9 in gastric cancer: a meta-analysis. Dis Markers. 2015;2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng K, Yang L, Hu B, et al. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal A, van Grieken NCT, Palsgrove DN, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun. 2020. doi: 10.1038/s41467-020-14310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slagter AE, Vollebergh MA, Caspers IA, et al. Prognostic value of tumor markers and ctDNA in patients with resectable gastric cancer receiving perioperative treatment: results from the CRITICS trial. Gastric Cancer. 2022;25:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi H, Nakamura Y, Kotani D, et al. CIRCULATE-Japan: circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 2021;112:2915–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. [DOI] [PubMed] [Google Scholar]
- 38.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida K, Kodera Y, Kochi M, et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol. 2019;37:1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SH, Lim DH, Sohn TS, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial. Ann Oncol. 2021;32:368–374. [DOI] [PubMed] [Google Scholar]