ABSTRACT
Background:
Smoking is a major predisposing factor for many health problems including cancers, vascular disorders, etc., To quit smoking is the only solution to prevent them. Various medicinal and non-medicinal methods are used worldwide for the same. The present study evaluates the effect of a nicotine free herbal formulation containing ingredients like Mucuna pruriens, Withania somnifera, Bacopa monnieri, etc., for cessation of smoking and its effects on other health parameters related to smoking.
Materials and Methods:
The present study was a placebo controlled, double blind, randomized, and multi-centric clinical study conducted at three clinical sites in India. After ethical approval and informed consent, all participants were given Smotect Tablets or Placebo tablets in a dose of 2 tablets twice daily for 90 days. A total of 103 participants (52 in trial group and 51 in placebo group) completed the study. Evaluation of cessation of smoking was done along with other parameters like measurement of lung capacity, clinical assessment, and laboratory investigations before and after the study.
Results:
A significant reduction in smoking as well as in the alveolar Carbon monoxide (p < 0.05) and Carboxyhemoglobin levels (p < 0.05) were observed with the use of Smotect tablets as compared to placebo over a period of 90 days. Significant improvement was also observed in quality of life, energy and stamina levels, and reduction of stress level. Smotect tablets were found to be safe without causing any adverse effects.
Conclusion:
Smotect Tablets is an effective and safe remedy for cessation of smoking and reducing other effects related to smoking.
KEYWORDS: CO, COHb, de-addiction, Smotect tablets
INTRODUCTION
All over the world, tobacco has been used, in chewable or smoking forms for euphoria, enjoyment, stimulation, or pleasure.[1,2] India is the second largest manufacturer and consumer of tobacco after China.[3] Tobacco smoking is among the major preventable causes of premature deaths worldwide.[4,5] Smoking is known to be injurious to health causing several health hazards including various respiratory tract diseases, cardiovascular diseases, GI tract diseases, liver diseases, neurological diseases, stroke, and cancer.[6-9] Smoking primarily impacts the respiratory tract causing many respiratory tract diseases, including acute and chronic cough, emphysema, COPD, and bronchitis.[8] Nicotine present in smoke causes physical and psychological dependency and is the major factor for tobacco dependence and addiction.[6,7]
As the avoidance of etiological factors is said to be the first line of treatment for any disease, quitting tobacco smoking is the best way to significantly reduce the risk associated with smoking.[7] However, due to the sudden stoppage/quitting smokers can suffer from nicotine withdrawal symptoms like anxiety, irritability, increased eating, dysphoria, hedonic dysregulation, etc.[7] Presently behavioral treatment, Nicotine Replacement Therapy (NRT) and pharmacological treatment are used for smoking cessation.[10,11] Nicotine patches, gums, inhalers, and sprays are some of the options used currently. Some of the quitters also try e-cigarettes that are battery operated devices converting liquid nicotine to vapor. However, NRT has shown to be not a successful option by majority of the smokers. The pharmacological approaches have a beneficial role in alleviating withdrawal symptoms, but they are expensive and possess numerous potential side effects.[12,13] Some quitters also select to go for hypnosis, acupuncture, and counseling.
Considering limitations of the treatment options, there is therefore a need to develop a anti-smoking formulation that is free from the existing drawbacks of the anti-smoking formulations without nicotine. Need is also observed to develop formulation that not only helps in de-addiction of smoking but also treats the ailments and/or side effects caused by smoking.
Smotect Tablets is an herbal formulation comprising of standardized herbal extracts mainly Mucuna pruriens, Withenia somnifera, etc., These ingredients can be supportive in cessation of smoking and reducing other effects related to smoking. Looking at the activities of the ingredients, the present clinical study was planned. The objective of the study was to evaluate the effect of Smotect Tablets, a Nicotine free herbal formulation for cessation of smoking and its effects on other health parameters related to smoking.
MATERIALS AND METHODS
-
Study design, sites –
The present study was a placebo controlled, double blind, randomized, multi-centric clinical study conducted at three clinical sites in India, viz; Ayurved Sanshodhan Vibhag, Ayurved Seva Sangh Hospital, Ganeshwadi, Panchvati, Nashik; KVTR Ayurvedic College, Boradi, Dhule-425 428, and D.Y. Patil School of Ayurveda, Sector 7 Nerul, Navi Mumbai, Maharashtra.
-
Ethical considerations-
Ethical approvals from Institutional ethics committees of all study centers were obtained. The study was registered on Clinical Trials Registry India (CTRI) vide registration number CTRI/2017/06/008790, dated 08/06/2017.
-
Enrolment of participants-
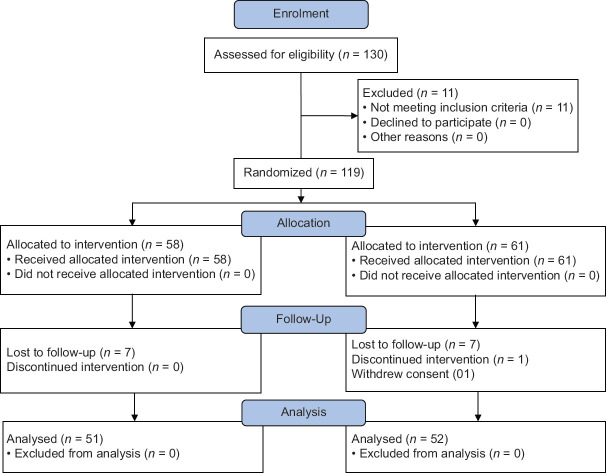
Participants having a history of smoking a minimum of 5 cigarettes daily for at least 3 years and showing a high level of alveolar CO levels attending out-patient department of the study centers were considered for the study. The study was carried out and reported adhering to CONSORT statement [Figure 1].
-
Study duration & Visits:
The total duration of treatment was 3 months (90 days). Patients were asked to visit study site every 30th day for 3 months (90 days).
-
Primary and secondary Outcomes:
The primary outcome of the study was to evaluate the efficacy of Smotect Tablets in smokers by assessing cessation of smoking (reduction or complete giving up). Also, changes in Lung capacity were assessed on spirometer.
The secondary outcomes of study were to evaluate the efficacy of Smotect Tablets in smokers by assessing changes in levels of alveolar CO and COHb, Quality of life (QOL) on WHO-QOL Questionnaire, changes in the Cardiac risk markers (Apolipoprotein A1 and B), serum cortisol level, change in stress level and anxiety on Hamilton Anxiety Rating Scale (HAM-A scale), change in level of energy, stamina, and physical strength on a 7 point scale, global assessment for overall change by the subject and investigator at the end of the study.
Safety and tolerability of study drug were assessed by any occurrence of adverse events (AEs) and adverse drug reactions and change in laboratory parameters such as Liver Function Tests, Renal Function Tests, lipid profile, CBC, ESR, hemoglobin, and urine examinations.
Figure 1.
CONSORT Figure
Selection of participants
Male and female participants between 18 to 70 years of age (both inclusive) having a history of smoking a minimum of 5 cigarettes daily for at least 3 years and showing a high level of alveolar CO levels and who gave written consent and were ready to abide to trial procedures included in the study.
Participants suffering from any major illnesses, uncontrolled hypertension and diabetes, hepatic or renal impairment, central nervous system disorders, and known hypersensitivity to any ingredient of the study drug were excluded from the study. Participants with continuing history of alcohol and/or drug abuse were excluded from the study.
Sample size
Sample size calculation assumed that a sample size of minimum 100 evaluable cases (divided into two groups of minimum 50 participants each in placebo and study drug group) would provide an 80% power to estimate cessation of smoking and change in lung capacity at 5% level of significance at the end of the study.
-
Treatment Groups-
After fulfilling the eligibility criteria participants were randomized to either trial group or placebo as per computer generated randomization list. Subjects were advised to consume given medication in a dose of two Tablets twice daily orally after meals.
-
Study drug
The study was double blinded study and therefore the study product and Placebo Tablets were manufactured and packed in such a way to ensure that the subject and Investigator were blinded from the same. Table 1 provides details of composition of the study product. Unblinding was done at the completion of the study. Placebo Tablets were made using inert materials IP grade—MCC, HPMC, and Talc.
Table 1.
Composition of Smotect Tablet (each film coated tablet contains)
| Ingredient | Scientific Name | Quantity |
|---|---|---|
| Ashvagandha extract | Withania somnifera | 100 mg |
| Amla extract | Emblica officinalis | 50 mg |
| Gokshura extract | Tribulus terrestris | 50 mg |
| Bramhi extract | Bacopa monnieri | 50 mg |
| Yashtimadhu extract | Glycyrhizza glabra | 100 mg |
| Shirisha extract | Albezzia lebbek | 25 mg |
| Shunthi extract | Zingiber officinale | 25 mg |
| Lavang extract | Syzygium aromaticum | 15 mg |
| Kapikacchu extract | Mucuna pruriens | 250 mg |
| Tulsi extract | Ocimum sanctum | 50 mg |
| Haridraextract | Curcuma longa | 50 mg |
Assessment parameters
On baseline visit, participant’s alveolar CO and COHb levels were measured. Participant’s lung capacity was measured using spirometer. After an overnight fasting (10-12 hours), blood samples were collected for laboratory tests viz. CBC, ESR, BSL-Fasting, LFT, RFT, Lipid Profile, Cardiac risk markers (Apo-lipoprotein A1 and B), Total testosterone level and HIV II, urine routine & microscopic & I. Subject’s chest expansion was measured by measuring chest circumference (Axilla and Xiphoid process) and chest diameter (AP and ML).
Participants were asked for their average daily consumption of cigarettes on every 15 days interval. Spirometer test and CO and COHb levels were done to evaluate their lung capacity every month. On monthly basis, subject’s Quality of life on WHO QOL, level of energy, level of stamina, physical strength was evaluated on a 7-point scale. Also, level of stress on VAS and level of anxiety on HAMA scale were evaluated and Subject’s chest expansion was measured.
All the participants were closely monitored for any adverse event, starting with the baseline visit till the end of the study visit.
Plan for statistical analysis
All baseline and demographic data were summarized descriptively. All continuous variables were summarized using mean, standard deviation, and standard error of mean and median. All categorical variables were summarized using frequency and percentages. The primary population for this study was Per-Protocol population. GraphPad InStat Version 3.6 (www.graphpad.com) software was used for statistical analysis of data. Comparison of variables representing categorical data was performed using Chi-square test. All other secondary outcomes were analyzed by applying appropriate statistical methods like paired and unpaired t test, etc., All P values were reported based on two-sided significance test and all the statistical tests were interpreted at least up to 5% level of significance.
RESULTS
Out of 119 recruited participants, 103 participants (52 in trial group and 51 in placebo group) completed the study, while 16 participants dropped out prematurely due to loss to follow-ups [see Figure 1]. All the participants who took even a single dose of study drug were considered for safety evaluation. The average age of participants in trial group was 40.58 ± 14.64 years while the average age of the participants in placebo group was 39.58 ± 13.79 years. There was no significant difference (p > 0.05) in the age between the two groups.
Assessment of primary outcome parameters
-
Number of cigarettes smoked per day by the participants:
At baseline visit, the average numbers of cigarettes smoked per day in trial group were 9.53 ± 6.42 which reduced significantly (p < 0.05) to 6.67 ± 5.54 at the end of 30 days. At the end of 60 days, the same was 5.25 ± 4.10 and further to 4.00 ± 3.43 at the end of 90 days. In the placebo group, the average number of cigarettes smoked per day reduced from 10.04 ± 6.20 to 8.73 ± 5.97, i.e., insignificant reduction (p > 0.05) at the end of 30 days. At the end of 60 days, the reduction was significant and was observed to be 8.00 ± 5.55 and further to 7.09 ± 4.83 at the end of 90 days. On analysis between the groups, the reduction was significantly more (p < 0.05) in trial group as compared to placebo. The details are given in Table 2. At the end of the study, complete cessation of smoking was observed in 12 (23.08%) participants in trial group and 02 (3.92%) participants in placebo group. The details are given in Table 3.
-
Measurement of lung capacity on Spirometry:
Measurement of lung capacity on Spirometry observed that there were no significant changes in FVC, FEV1, and FEV1/FVC in trial group on day 30, 60, and 90 while in the placebo group a significant decrease (p < 0.05) in FVC and FEV1 was observed on day 30, 60, and 90. No significant change was observed on FEV1/FVC value on day 30, 60, and 90 in placebo group. The difference between both the two groups was statistically insignificant (p > 0.05) at all the follow up visits till the end of the study. The details on changes in mean FVC, FEV1, FEV1/FVC ratio are shown in Table 4.
There was a significant increase in FEF50% and FEF25-75% in trial group from baseline to the end the study, i.e., 90 days. However, there was no change on these parameters in placebo group from baseline to the end of the study. The difference between the groups was statistically insignificant (p > 0.05) at the end of the study.
Table 2.
Assessment of Number of Cigarettes Smoked per Day (Cessation of Smoking)
| Groups | Baseline | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|
| TRIAL Group | 9.53±6.42 | 6.67±5.54, P<0.05 | 5.25±4.10, P<0.05 | 4.00±3.43, P<0.05 |
| Placebo Group | 10.04±6.20 | 8.73±5.97, P>0.05 | 8.00±5.55, P<0.05 | 7.09±4.83, P<0.05 |
| Between Group Analysis | P>0.05 | P<0.05 | P<0.05 | P<0.05 |
Table 3.
Distribution of Participants for Cessation of Smoking
| % Range for Smoking Cessation | TRIAL Group | Placebo Group | ||
|---|---|---|---|---|
|
|
|
|||
| Number of Participants (Total 52) | Percentage of Participants | Number of Participants (Total 51) | Percentage of Participants | |
| 100% | 12 | 23.08 | 02 | 03.92 |
| 81-100% | 02 | 03.83 | 00 | 00.00 |
| 61-80% | 18 | 34.61 | 06 | 11.76 |
| 41-60% | 10 | 19.23 | 08 | 15.69 |
| 21-40% | 06 | 11.53 | 06 | 11.76 |
| 0-20% | 00 | 00.00 | 09 | 17.65 |
| Remained Same | 03 | 05.76 | 12 | 23.53 |
| Increased | 01 | 01.92 | 08 | 15.69 |
| Total | 52 | 100% | 51 | 100% |
Table 4.
Assessment of Changes in Spirometer Values (FVC, FEV1, FEV1/FVC ratio)
| Group | Parameter | Baseline | 30 Days | 60 Days | 90 Days |
|---|---|---|---|---|---|
| TRIAL Group | FVC (in L) | 3.40±0.97 | 3.09±1.40 P>0.05 | 3.07±1.40 P>0.05 | 3.15±0.76 P>0.05 |
| FEV1 (in L) | 2.43±0.81 | 2.28±1.19 P>0.05 | 2.34±1.20 P>0.05 | 2.44±0.90 P>0.05 | |
| FEV1/FVC (in %) | 73.86±17.31 | 74.55±34.41 P>0.05 | 77.47±33.92 P>0.05 | 76.13±20.98 P>0.05 | |
| Placebo Group | FVC (in L) | 3.27±0.85 | 3.01±0.86 P<0.05 | 2.95±1.01 P<0.05 | 3.01±0.87 P<0.05 |
| FEV1 (in L) | 2.40±0.81 | 2.19±0.84 P<0.05 | 2.26±0.89 P<0.05 | 2.16±0.91 P<0.05 | |
| FEV1/FVC (in %) | 74.11±16.77 | 73.75±20.07 P>0.05 | 78.51±19.21 P>0.05 | 72.73±21.94 P>0.05 | |
| Between group analysis FVC | P>0.05 | P>0.05 | P>0.05 | P>0.05 | |
| Between group analysis FEV1 | P>0.05 | P>0.05 | P>0.05 | P<0.05 | |
| Between group analysis FEV1/FVC | P>0.05 | P>0.05 | P>0.05 | P>0.05 | |
Assessment of secondary outcome parameters
-
Alveolar CO levels:
A significant reduction in the CO levels was observed in trial group from a baseline of 13.33 ± 7.76 ppm to 12.20 ± 9.88 ppm at the end of 30 days. There was a further reduction (p < 0.05) to 10.76 ± 6.90 ppm at the end of 60 days and 10.63 ± 7.70 ppm at the end of 90 days. There was no significant (p > 0.05) change in the CO levels in placebo group from baseline of 14.00 ± 7.51 ppm to 14.84 ± 8.45 at the end of 30 days. Further on day 60 and day 90 as well the change was found to be non-significant (p > 0.05) as the score was 13.8 ± 7.42 and 13.53 ± 7.50, respectively. On analysis between the groups, it was observed that the reduction in CO levels was significantly higher (p < 0.05) in trial Group as compared to placebo. See Graph 1.
-
Alveolar COHb levels:
The COHb levels in trial group showed a significant reduction from 2.80 ± 1.25% at baseline levels to 2.37 ± 1.27% at the end of 90 days. In the placebo group, the reduction was non-significant from baseline 2.87 ± 1.19 to the end of the study 2.84 ± 1.21. Between groups analysis showed that consumption of trial significantly reduced COHb levels as compared to placebo. See Graph 2.
-
Quality of life (QOL) on WHO QOL Questionnaire:
Assessment of quality of life showed that there was a significant improvement on the physical health score from 19.28 ± 2.44 at baseline visit to 20.49 ± 1.97 on day 90. In placebo group, the mean physical health score showed non-significant change (p > 0.05) from 20.14 ± 2.50 at baseline visit to 20.37 ± 2.29 on day 90. The improvement in trial group was found to be significantly higher as compared to placebo.
Also, in trial group, the mean psychological health domain score improved significantly (p < 0.05) from 19.06 ± 2.65 at baseline visit to 19.86 ± 2.37 on day 90, whereas in placebo group, the mean psychological health domain score showed non-significant change (p > 0.05) from 19.33 ± 2.67 at baseline visit to 19.98 ± 2.40 at day 90. The improvement in trial group was found to be significantly higher as compared to placebo.
-
Changes in the Cardiac risk markers (Apolipoprotein A1 and B):
There was no significant change in the levels of cardiac risk markers from baseline to the end of the study in both the study groups. The levels remained in the normal physiological range at both the visits.
-
Serum cortisol and total testosterone levels:
The mean serum cortisol (μg/dl) and total testosterone (mg/dl) levels were found within normal range in all participants at baseline visit and at the end of the study in both the groups and did not show any significant change from baseline to the end of the study.
-
Change in stress level and anxiety on HAMA scale:
The mean stress score assessed on VAS scale reduced significantly (p < 0.05) from 40.58 ± 17.79 at baseline visit to 29.90 ± 15.12 at the end of 90 days in trial group while the score reduced from 42.16 ± 18.01 to 35.32 ± 17.43 in placebo group which was significant (p < 0.05). Though the reduction in stress levels was better in trial as compared to placebo it was found to be non-significant (p > 0.05). Similarly, HAMA score to assess anxiety showed significant reduction in both the groups after 90 days of treatment. However, there was no significant difference between the two groups. There were no significant changes on various parameters to measure chest circumference in both the groups.
-
Change in level of energy, stamina, and physical strength on a 7-point scale:
The levels of energy, stamina, and physical strength showed a significant improvement (p < 0.05) from baseline to monthly follow-ups in trial group while the change on these parameters was insignificant (p > 0.05) in placebo group. The improvement on energy, stamina, and physical strength was found to be significant in trial group as compared to placebo.
-
Global assessment for overall change by the subject and investigator at the end of the study treatment:
Global assessment for overall change by the physician on CGI-I scale showed that most participants in trial group showed very much to minimal improvement as compared to placebo Also a higher percentage of participants in placebo group either showed no change or worsening of their condition.
Graph 1.
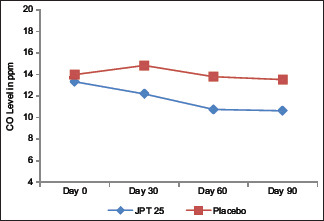
Assessment of Changes in CO levels (in ppm)
Graph 2.
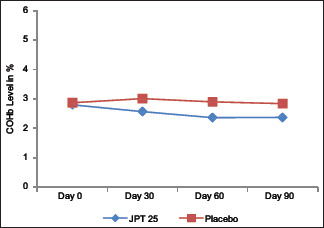
Assessment of Changes in COHb levels (in %)
Assessment of safety parameters
No significant (p > 0.05) changes were observed in laboratory parameters such as CBC, ESR, Hb%, LFTs, RFTs, lipid profile, blood sugar level, and urine examination when compared between baseline visit and day 90 visit in both the study groups. All the laboratory values were within normal range at baseline visit and at the end of the study. No clinically significant change in vitals such as pulse rate, temperature, respiration rate, and blood pressure (systolic and diastolic pressure) was observed from baseline visit to every follow up visits and at the end of the study in both the study groups.
AEs including abdominal discomfort, abdominal pain, fever, cough, backache, and body ache were noted during the trial. In the trial group, 19 participants reported 26 AEs and in placebo group 24 participants reported 31 AEs. None of the AE was found to be related to the study product or procedure. No treatment or procedure or interruption was required in both the study groups to resolve these episodes. Almost all the participants showed excellent to good tolerability to the investigational products.
DISCUSSION
Smoking is considered as the single greatest risk factor that plays role in the incidence of major diseases that cause death due to heart diseases, peripheral vascular diseases, hypertension, lung cancer, diabetes, cancer, etc., There is a wide range of treatment options that have proved effective, including behavioral and pharmacological therapies. The therapies vary widely in their efficacy, their acceptability and it is their cost-effectiveness. Smotect Tablets is an herbal formulation comprising of standardized herbal extracts mainly Mucuna pruriens, Withenia somnifera, etc., which can be supportive in cessation of smoking and reducing other effects related to smoking.
It was observed that ninety days treatment with Smotect tablets significantly reduced the mean number of cigarettes smoked per day as compared to placebo. While 23.08% completely gave up smoking in trial group only 4.0% did so in the placebo group. At the end of the study, only 1.92% of participants in trial group showed increase in smoking frequency compared to 16.00% participants in placebo group. Also, treatment with Smotect tablets showed significant reduction in mean CO level (ppm) and COHb (%) levels. The findings suggest that Smotect tablets may help to reduce dependence on smoking and reduce toxic residues in lungs and restore normal function of respiratory system.
Apart from reporting a significant reduction in craving for cigarette smoking, participants also reported to reduction in stress level (on VAS), anxiety level (as per HAMA scale) and other symptoms such as insomnia, irritability, nervousness, difficulty concentrating and restlessness and improved quality of life and energy, stamina, and physical strength levels compared to placebo. These findings suggest that Smotect tablets were not only helpful to quit smoking but also helped in alleviating the withdrawal symptoms of tobacco smoking.
Smotect tablets is poly herbal combination of 11 standardized herbal extracts, which are helpful to smokers to quit or reduce smoking and to reduce the ill effects and complications of smoking. Mucuna pruriens seed extract contains a high concentration of L-dopa a vital source of dopamine. Neuro-protective action of seeds of Mucuna pruriens has shown to help in restoration of the endogenous monoamine contents including dopamine in the substantia nigra of the brain indicating its dopaminergic action.[14] This dopaminergic action helps to boost energy, elevate mood, and reduce depression. This helps to overcome the urge of smoking over a period and thus helps in cessation of smoking. Withania somnifera (2% withanoloids) is used in patients with nervous exhaustion, insomnia, and debility due to stress.[15] Thus, it helps in withdrawal effects of smoking cessation.
Other ingredients possess anti-anxiety, anti-inflammatory, analgesic, immunomodulator, anti-oxidant, rejuvenator, brain tonic, and stimulant properties. Most of the ingredients are useful in various respiratory diseases due to their bronchodilator, anti-inflammatory, anti-allergic, anti-tussive, and mucolytic actions.[16-25] The synergistic action of these ingredients could have helped in cessation of smoking and to overcome other effects related to smoking.
Most of the AEs reported in both the groups were unrelated to the study drug. The mean values of almost all laboratory parameters were within normal limits at the end of the study. No significant change in any of the vitals parameters was observed during and at the end of the study. Taken together these observations demonstrated that Smotect tablets are safe to use in smokers.
CONCLUSION
Three months of treatment with Smotect tablets helps in cessation of smoking. Smotect tablets helps to improve quality of life of smokers along with improvement in the levels of energy, stamina and physical strength, and reduction in levels of stress. Smotect tablets, a nicotine free herbal composition, can be recommended as a safe and effective remedy for de-addiction of smoking along with improving health-related problems arising due to smoking. Further studies with larger sample size are warranted to establish the efficacy of Smotect tablets especially on various lung functions.
Financial support and sponsorship
The study was sponsored and funded by Project Happiness (Mr. Gurseet Singh).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bruijnzeel AW. Reward processing and smoking. Nicotine Tob Res. 2017;19:661–2. doi: 10.1093/ntr/ntw303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishore K. Monograph of tobacco (Nicotiana tobacum) Indian J Drugs. 2014;2:5–23. [Google Scholar]
- 3.Semwal DK, Mishra SP, Chauhan A, Semwal RB. Adverse health effects of tobacco and role of Ayurveda in their reduction. J Med Sci. 2015;15:139–46. [Google Scholar]
- 4.Mishra S, Joseph RA, Gupta PC, Pezzeck B, Ram F, Sinha DN, et al. Trends in bidi and cigarette smoking in India from 1998 to 2015, by age, gender and education. BMJ Glob Health. 2016;1:e000005. doi: 10.1136/bmjgh-2015-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol Health. 2017;32:1018–36. doi: 10.1080/08870446.2017.1325890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, et al. Tobacco smoking in India: Prevalence, quit-rates and respiratory morbidity. Indian J Chest Dis Allied Sci. 2006;48:37–42. [PubMed] [Google Scholar]
- 7.Benowitz NL. Neurobiology of nicotine addiction: Implications for smoking cessation treatment. Am J Med. 2008;121:S3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Onor IO, Stirling DL, Williams SR, Bediako D, Borghol A, Harris MB, et al. Clinical effects of cigarette smoking: Epidemiologic impact and review of pharmacotherapy options. Int J Environ Res Public Health. 2017;14:1147. doi: 10.3390/ijerph14101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha SP, Bhalla DK, Whayne TF, Jr, Gairola C. Cigarette smoke and adverse health effects: An overview of research trends and future needs. Int J Angiol. 2007;16:77–83. doi: 10.1055/s-0031-1278254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003;18:1053–7. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadgave U, Nagesh L. Nicotine replacement therapy: An overview. Int J Health Sci (Qassim) 2016;10:425–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Buddhadev SG, Buddhadev SS. A review article on phytochemical properties of Tamraparna and its traditional uses. Int J Herbal Med. 2014;2:39–41. [Google Scholar]
- 13.Sood A, Ebbert JO, Prasad K, Croghan IT, Bauer B, Schroeder DR. A randomized clinical trial of St. John's Wort for smoking cessation. J Altern Complement Med. 2010;16:761–7. doi: 10.1089/acm.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rana DG, Galani VJ. Dopamine mediated antidepressant effect of Mucuna pruriens seeds in various experimental models of depression. Ayu. 2014;35:90–7. doi: 10.4103/0974-8520.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma SK, Kumar A. Therapeutic uses of Withania somnifera (Ashwagandha) with a note on withanolides and its pharmacological action. Asian J Pharm Clin Res. 2011;4:1–4. [Google Scholar]
- 16.Uddin Q, Samiulla L, Singh VK, Jamil SS. Phytochemical and pharmacological profile of withania somnifera dunal: A review. J Appl Pharm Sci. 2012;02:170–5. [Google Scholar]
- 17.Dasaroju S, Gottumukkala KM. Current trends in the research of emblica officinalis (Amla): A pharmacological perspective. Int J Pharm Sci Rev Res. 2014;24:150–9. [Google Scholar]
- 18.Prajapati SM, Patel BR. Phyto-pharmacological perspective of Yashtimadhu (Glycyrrhiza Glabra LINN.) – A review. Int J Pharm Biol Arch. 2013;4:833–41. [Google Scholar]
- 19.Verma SC, Vashishth E, Singh R, Kumaril A, Meena AK, Pant P, et al. A review on parts of Albizia lebbeck (L.) Benth. used as ayurvedic drugs. Res J Pharm Tech. 2013;6:1307–13. [Google Scholar]
- 20.Chhatre S, Nesari T, Somani G, Kanchan D, Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev. 2014;8:45–51. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhakat A, Saha S. Review on the traditional and contemporary use of Sunthi (Zingiber officinale Rosc.) and its medical importance in Ayurveda. Int Ayurvedic Med J. 2017;5:3075–81. [Google Scholar]
- 22.Yadav KD, Reddy K. Critical review on pharmacological properties of Brahmi. Int J Ayurvedic Med. 2013;4:92–9. [Google Scholar]
- 23.Pandey G, Madhuri S. Pharmacological activities pharmacological activities of ocimum sanctum (Tulsi): A Review. Int J Pharm Sci Rev Res. 2014;5:61–6. [Google Scholar]
- 24.Krup V, Prakash LH, Harini A. Pharmacological activities of turmeric (Curcuma longa linn): A review. J Homeop Ayurv Med. 2013;2:1167–206. [Google Scholar]
- 25.Kharat RS, Lad MD. Review of pharmacological activities of haridra (Curcuma longa L.) World J Pharma Res. 2016;3:412–23. [Google Scholar]