Abstract
Thioredoxin, a redox active protein, has been previously demonstrated to be essential for growth of the anoxygenic photosynthetic bacterium Rhodobacter sphaeroides. In the present study, the involvement of thioredoxin in the formation of the photosynthetic apparatus of R. sphaeroides WS8 was investigated by construction and analysis of a mutant strain disrupted for the chromosomal trxA copy and carrying a plasmid-borne copy of trxA under the control of the hybrid ptrc promoter inducible by IPTG (isopropyl-β-d-thiogalactopyranoside). This strain was viable in the absence of IPTG but was affected in pigmentation. When shifted from high to low oxygen tension conditions, the trxA mutant showed a reduced bacteriochlorophyll content in comparison to that of the wild type. Although thioredoxin is able to regulate aminolevulinic acid (ALA) synthase (the first enzyme of the tetrapyrrole biosynthetic pathway) activity by a dithiol-disulfide exchange, our mutant strain exhibited a level of ALA synthase activity identical to that of the wild type, suggesting that thioredoxin is involved in other steps to regulate the synthesis of the photosynthetic apparatus. Accordingly, we showed that the trxA mutation affects the oxygen-regulated expression of the puf operon encoding the pigment-binding proteins of the light-harvesting and reaction center complexes. Upon transition from aerobic to semiaerobic growth conditions, the maximal puf mRNA level was found to be 40 to 50% lower in the mutant strain than in the wild type. The stability of the puf transcripts was identical in both strains grown under low oxygen tension, indicating that the role of thioredoxin in regulating puf expression occurs at the transcriptional level.
In the facultative photosynthetic bacteria of the genus Rhodobacter, composition and assembly of the photosynthetic apparatus are tightly regulated by light intensity and oxygen tension (41). When the oxygen tension is reduced below a threshold level, the bacteria start to synthesize the pigments and pigment-binding proteins which together form the photosynthetic spectral complexes, localized to the invaginations of the inner cytoplasmic membrane. The last decade has seen important progress in understanding the regulatory pathways that coordinate photosynthesis gene expression by the identification of a growing number of regulatory factors. Several circuits are involved in the oxygen-regulated expression of genes encoding photopigment-biosynthetic enzymes (bch and crt genes) and apoproteins for light-harvesting and reaction center complexes (puc, puf, and puh operons) (for a review, see reference 5). One regulatory pathway involves the transcription factor CrtJ in Rhodobacter capsulatus (30), also called PpsR in Rhodobacter sphaeroides (17), which is responsible for aerobic repression of bch, crt, and puc gene expression. CrtJ has been demonstrated to bind to a conserved palindrome sequence with a higher affinity under oxidizing conditions (31). Photosynthesis gene expression is also controlled by an anaerobic activation circuit. Particularly, the signal transduction system composed of a sensor kinase, RegB, and a response regulator, RegA, in R. capsulatus (35) as well as its counterpart PrrB-PrrA in R. sphaeroides (15, 16) is involved in high-level expression of the puf, puh, and puc operons in response to a reduction in oxygen tension. In addition, activation of photopigment and puc expression requires the trans-acting factor encoded by the appA gene (18), and the mgpS locus is implicated in transcriptional activation of puc and puf operons (33). The anaerobic regulator FnrL is also required for anoxygenic photosynthetic growth of R. sphaeroides and acts at the upstream FNR consensus sequence to mediate oxygen control of the puc operon expression (40).
regB mutants exhibit a residual level of transcription regulation in response to changes in oxygen tension (5), suggesting the existence of an additional redox sensor(s) in the complex molecular mechanisms governing the oxygen-regulated formation of the photosynthetic apparatus.
Thioredoxin, a small heat-stable protein, has a remarkable protein disulfide oxidoreductase activity and ubiquitous distribution (20), suggesting that it plays a crucial role in cellular functions. Particularly, as a redox active protein, thioredoxin is a good candidate to participate in redox signalling pathways involved in photosynthesis regulation in the purple sulfur bacteria.
In plants, the ferredoxin-thioredoxin system (including chloroplastic thioredoxins f and m) is responsible for light-mediated enzyme regulation in oxygenic photosynthesis by a selective thiol redox control (8, 9, 12). Thioredoxin has also been proposed to be involved in redox regulation of the translational activator modulating the synthesis of photosystem II reaction center D1 protein in Chlamydomonas reinhartii (13).
In the facultative phototrophic bacterium R. sphaeroides Y, the thioredoxin system (containing thioredoxin associated with NADPH-thioredoxin reductase) was originally characterized by its ability to regulate δ-aminolevulinic acid (ALA) synthase activity in vitro by a dithiol-disulfide exchange (11). Given that ALA synthase is the first enzyme of the bacteriochlorophyll biosynthetic pathway, this function suggested the involvement of thioredoxin in the oxygen regulation of bacteriochlorophyll synthesis at a posttranslational level by a thiol redox control.
Although dispensable in Escherichia coli, thioredoxin is essential for photosynthetic growth of the obligate phototrophic cyanobacterium Anacystis nidulans (24), as well as for growth of the facultative heterotrophic cyanobacterium Synechocystis sp. strain PCC 6803 (25) and for R. sphaeroides growth by aerobic and anaerobic respiration (29). The essential function of R. sphaeroides thioredoxin involves its oxidoreductase activity. In addition, in contrast to E. coli thioredoxin, thioredoxin purified from R. sphaeroides was demonstrated to have a glutathione-disulfide oxidoreductase, suggesting its ability to act in GSH-dependent processes and reflecting the multiple functions it can serve in vivo (29).
In the present study, we have investigated the putative role of thioredoxin in regulation of photosynthesis metabolism by constructing an R. sphaeroides strain disrupted for the chromosomal trxA copy and containing a plasmid-borne copy of trxA under the control of the hybrid promoter ptrc inducible by IPTG (isopropyl-β-d-thiogalactopyranoside).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. R. sphaeroides strains were grown at 32°C in a malate minimal salt medium (14), supplemented with appropriate antibiotics when necessary. Growth under low oxygen tension (1 to 2% oxygen) was performed by incubating 40 ml of culture in 50-ml flasks under gentle agitation. For aerobic cultivation (20% oxygen), 100-ml cultures were vigorously shaken in 500-ml baffled flasks.
TABLE 1.
Bacteria strains and plasmids
| Organism and strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli | ||
| HB101 | supE44 hsdS20 (rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 6 |
| SM10 | recA thi thr leu; chromosomal RP4-2 (Tc::Mu) | 37 |
| R. sphaeroides | ||
| WS8 | Wild type | 38 |
| WRKTX1 | WS8(pRKSMTX1); see Fig. 1 | This study |
| TK1 | WS8 trxA::Kmr(pRKSMTX1); see Fig. 1 | This study |
| HemAT1 | 2.4.1 derivative; hemA::Ω Kmr and hemT::Ω Smr Spr | 26 |
| Plasmids | ||
| pBlueScript | pUC19, f1 ori, T7 and T3 promoters | 36 |
| pSUP203 | pBR325 derivative, Mob+ Apr Cmr Tcr | 37 |
| pUC4KSAC | Apr, source of the Kmr cassette | 4 |
| pUTC51 | 1.9-kb PstI fragment containing trxA gene under the control of ptrc promoter and lacIq cloned into pKK233-2 | 3 |
| pHP45Ω | Source of the Ω Smr Spr cassette | 32 |
| pRK415 | Mob+ TcrlacZα IncP | 21 |
| pRKSM | 1.95-kb SmaI Ω Smr/Spr cassette cloned into pRK415 | This study |
| pRKSMTX1 | 1.9-kb PstI trxA fragment of pUTC51 cloned into pRKSM; see Fig. 1 | This study |
| pWTX1 | 800-bp HindIII-EcoRI trxA fragment with its own promoter cloned into pRK415 | This study |
| pRKECTX | 1.87-kb PstI-HindIII fragment containing E. coli trxA gene under the control of ptrc promoter and lacIq cloned into pRK415 | This study |
| pUTC80 | pSUP203::3.4-kb EcoRI trxAΩKmr fragment; see Fig. 1 | 29 |
| pBSPUF1 | 349-bp pufBA PCR fragment cloned into pBlueScript | This study |
E. coli strains were grown aerobically at 37°C in Luria-Bertani (LB) medium (34). Antibiotics were added to growth media at the following concentrations: ampicillin, 200 μg/ml for growth of E. coli; tetracycline, 10 μg/ml for E. coli and 2 μg/ml for R. sphaeroides; kanamycin, 100 μg/ml for E. coli and 20 μg/ml for R. sphaeroides; and streptomycin, 25 μg/ml for R. sphaeroides.
Conjugation techniques.
Plasmid DNA was mobilized into R. sphaeroides strains by diparental conjugation with SM10 as the E. coli donor (37) and as described previously (29).
Nucleic acid manipulations.
Plasmids and DNA fragments were isolated, treated with modifying enzymes, and electrophoretically analyzed by standard techniques (34). pBPUF1 (Table 1) was constructed by ligating the phosphorylated and blunt-ended PCR-amplified pufBA 349-bp fragment into the dephosphorylated EcoRV-digested pBlueScript vector (Stratagene). PCR amplification of the pufBA 349-bp fragment was carried out with primers 5′PUFB (5′-GGAGGATAGCATGGCTGATAA-3′) and 3′PUFA (5′-TTACTCGGCGACGGCGACGC-3′) in accordance with the R. sphaeroides puf DNA sequence previously determined (22) and with genomic DNA as a template. Amplification was achieved by denaturing at 96°C for 1 min, primer annealing at 49°C for 2 min, and primer extension at 72°C for 1 min, repeated for 30 cycles, by using the Vent Polymerase (Biolabs). Total RNA was isolated from R. sphaeroides by the method of Nieuwlandt et al. (27) with the following modification: an additional step of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol) purification was included before ethanol precipitation. Electrophoresis, transfer of RNA to a nylon membrane (Biodyne B; Pall), 32P labelling of DNA fragments, Northern blot hybridization, and quantification of RNA bands have been described elsewhere (28).
Bacteriochlorophyll, protein, and enzymatic analyses.
To determine the bacteriochlorophyll content, the cells were sedimented and resuspended in acetone-methanol (7:2, vol/vol). After a 5-min spin in a microcentrifuge, the absorbance of the supernatant at 770 nm was determined.
For ALA synthase assays, crude cell extracts were prepared from semiaerobically grown log-phase cells. Cultures were harvested by centrifugation at 12,000 × g for 10 min. The cell pellets were suspended in 2 ml of inner cytoplasmic membrane buffer (0.1 M NaH2PO4, 0.01 M EDTA [pH 7.6]). After sonication with a Bandelin SONOPULS GM 70 at a 50% duty cycle for 5 min, crude cell extracts were obtained by centrifugation at 12,000 × g for 15 min to remove cell debris. ALA synthase activity was measured essentially by the method of Burnham (10) with the following modifications: succinyl-coenzyme A (Sigma) was added to a final concentration of 0.2 mM rather than being generated in the reaction mixture, and a 15-min reaction time was used for the formation of ALA.
Measurement of the reduced form of thioredoxin was performed by analyzing the reduction of DTNB [5,5′-dithiobis(2-nitrobenzoic acid)] (39) in the presence of crude cell extracts at 23°C. The assay mixtures contained 100 mM Tris-HCl (pH 8.0), 2 mM EDTA, 0.1 mg of bovine serum albumin per ml, and 0.5 mM DTNB in a final volume of 500 μl. The reaction was initiated by the addition of 10 or 20 μl of crude cell extracts, and the reduction of DTNB was monitored at 412 nm [ɛ412(DTNB) = 13,600 M−1 cm−1] on a Perkin-Elmer spectrophotometer. The activity of Trx(SH)2 was expressed as A412 units: 1 U corresponds to an increase in A412 of 1.00 min−1.
For immunological detection of thioredoxin, total proteins from crude cell extracts were electrophoresed in a sodium dodecyl sulfate–14% polyacrylamide gel electrophoresis gel and transferred to an Immobilon-P membrane (Millipore). Western blotting with anti-E. coli thioredoxin antibodies (IMCO Corporation Ltd. AB) was performed by using the Western Exposure Chemiluminescent Detection system (Clontech).
The protein concentration was determined by the method of Bradford (7) with bovine serum albumin as a standard.
RESULTS
Construction and phenotypic properties of R. sphaeroides WS8 trxA::Km(pRKSTX1).
Given that thioredoxin is required for an essential metabolic function in R. sphaeroides growth (29), and to further investigate its involvement in photosynthesis regulation, we have constructed an R. sphaeroides strain disrupted for the chromosomal trxA copy and harboring an additional copy of trxA expressed in trans from an inducible promoter, as illustrated in Fig. 1.
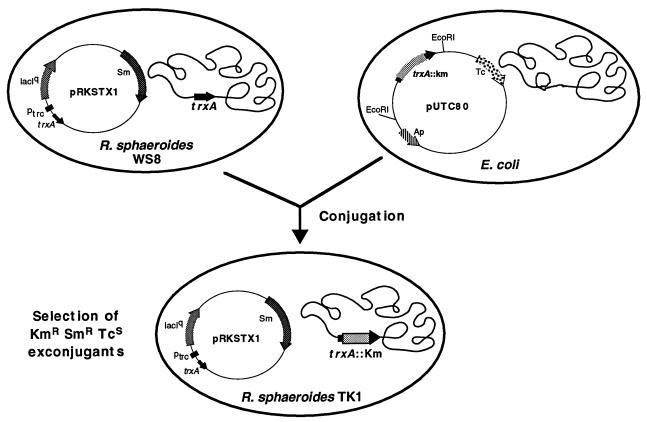
FIG. 1.
Strategy for mutagenesis of trxA. Plasmid pUTC80, which carries a trxA copy inactivated by insertion of the Kmr cassette (Table 1), was transferred by conjugation to R. sphaeroides WS8 harboring pRKSTX1 (Table 1) in which the trxA coding region is under the control of the ptrc promoter inducible by IPTG. The exconjugants were selected on minimal malate medium supplemented with kanamycin and streptomycin.
First, the SmaI fragment of the Ω-Sm/Sp cassette was cloned into the unique EcoRV site of pRK415 (21), resulting in the pRKSM plasmid. Then, a 1.9-kb PstI fragment isolated from pUTC51 (Table 1) and containing the trxA coding region placed under the control of the hybrid ptrc promoter (2), itself under the control of the lacIq repressor and therefore inducible by IPTG, was ligated to pRKSM previously linearized by PstI. The resulting plasmid, named pRKSTX1 (Fig. 1), was transferred to the R. sphaeroides WS8 wild-type strain by diparental conjugation as described in Materials and Methods. The WS8(pRKSTX1) strain was then subjected to site-specific mutagenesis by transferring plasmid pUTC80 (Table 1), a derivative of the suicide vector pSUP203 (37) containing a 2.1-kb trxA region inactivated by the Kmr cassette, from pUC4KSAC (4) and selecting for exconjugants that have integrated the Kmr into the chromosome by homologous recombination. In the pUTC80 construct, the Kmr cassette was flanked by 1,500 bp upstream and 600 bp downstream of the trxA DNA region. The Kmr Smr exconjugants were then screened for a double-crossover event by selection of Tcs clones, which were subsequently analyzed in Southern hybridization experiments (data not shown) with either the trxA gene or the Kmr cassette as a probe. Among the three clones (2% of the Kmr Smr exconjugants) which showed a hybridization signal corresponding to exchange of the trxA chromosomal copy with the pUTC80 copy inactivated by the insertion of the Kmr cartridge, one was named TK1 and further analyzed.
Although our previous studies suggested that trxA is transcribed into a monocistronic transcript (28), we also sequenced about 400 nt downstream of the trxA gene and performed computer analysis for codon usage and GC content. Our analysis suggests that expression of no other translated reading frame within 400 nt downstream of trxA is affected by the insertion of the Kmr cassette within trxA. Computer analysis predicted the presence of a translated open reading frame which is oriented in the opposite direction to that of trxA. This open reading frame showed no significant similarity to those encoding known proteins (data not shown).
TK1 was viable in the absence of the inducer IPTG and exhibited a growth rate under low oxygen tension identical to that of the wild-type parental strain, indicating that the basal level of trxA gene expression in trans under repression conditions was sufficient for the essential function of R. sphaeroides thioredoxin. However, TK1 appeared to be less pigmented than the wild-type parental strain when cultivated under low oxygen tension conditions and without IPTG addition to the culture medium and showed higher doubling times than the wild-type strain under high oxygen tension (150 ± 5 min [mean ± standard deviation] for TK1; 120 ± 5 min for WS8) and during photosynthetic growth (250 ± 10 min for TK1; 190 ± 10 min for WS8). Spectral analysis of crude extracts of this strain was performed. Light harvesting I-specific (870 nm) and light harvesting II-specific (800 to 850 nm) absorbances of this strain were slightly lower than those of the wild type (data not shown). In addition, attempts to replace the R. sphaeroides plasmid-borne trxA copy by its E. coli counterpart by transferring plasmid pRKECTX (Table 1) into strain TK1 failed, indicating that E. coli thioredoxin cannot replace R. sphaeroides thioredoxin in its fundamental function.
Analysis of the thioredoxin expression level by immunochemical detection and of the redox status of thioredoxin in mutant strain TK1.
To ascertain that phenotypic properties of strain TK1 are due to the altered level of trxA gene expression compared to that in the wild-type parental strain, WS8, the thioredoxin content in R. sphaeroides cells grown either aerobically or under low oxygen tension was analyzed by Western immunoblotting (Fig. 2).
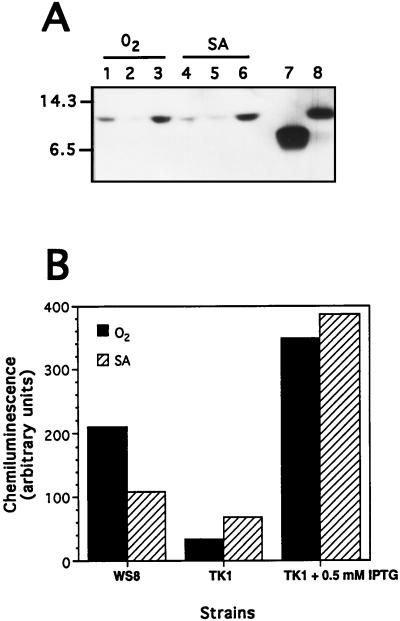
FIG. 2.
(A) Immunochemical detection of thioredoxin from cell extracts of the wild-type WS8 strain (lanes 1 and 4) and the thioredoxin mutant TK1 cultivated without (lanes 2 and 5) or with (lanes 3 and 6) 0.5 mM IPTG. Strains were grown until the end of the exponential phase (A680 = 0.9 to 1) under either high oxygen tension (O2) or low oxygen tension (SA). Cell extracts (50 μg of total proteins) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting with anti-E. coli Trx-specific antibodies and chemiluminescent detection. Lanes 7 and 8 were loaded with 50 ng of E. coli and 1 μg of purified R. sphaeroides Trx, respectively. (B) Quantification of chemiluminescence of Trx bands by densitometric scanning of resultant X-ray films.
For aerobic growth conditions, the intensity of the thioredoxin band corresponding to cell extracts prepared from strain TK1 subjected to IPTG induction (Fig. 2A, lane 3; Fig. 2B) was 150 to 160% of that observed for the wild-type strain (Fig. 2A, lane 1; Fig. 2B). The cell extracts obtained from strain TK1 cultivated in the absence of IPTG exhibit only about 20% of the wild-type level of thioredoxin (Fig. 2A, lane 2; Fig. 2B).
Under semiaerobic growth conditions, the mutant strain had 50 to 60% (Fig. 2A, lane 5; Fig. 2B) of the wild-type level of thioredoxin (Fig. 2A, lane 4; Fig. 2B). When IPTG was added to the culture medium for growth of strain TK1, the thioredoxin level (Fig. 2A, lane 6; Fig. 2B) was about 360% of that observed for the wild type.
These results demonstrate that strain TK1 exhibits a low level of thioredoxin expression due to the repression of its plasmid-borne trxA copy by the lacIq product on the ptrc promoter. Induction by IPTG during cultivation of strain TK1 leads to about the same level of thioredoxin under both low and high oxygen tension conditions, while in wild-type strain WS8, the thioredoxin level is about two times higher under high oxygen tension than under low oxygen tension conditions, in accordance with previous mRNA analyses (28).
To further define the redox status of thioredoxin in mutant strain TK1, we measured the content of the reduced form of thioredoxin by analyzing the capability of crude cell extracts to reduce DTNB as described in Materials and Methods. As shown in Table 2, crude extracts prepared from semiaerobically grown cells of the mutant strain TK1 cultivated under repression conditions exhibit a DTNB-reducing activity about 2.3-fold lower than that observed for wild-type strain WS8. This result suggests that the content of Trx(SH)2 in the mutant strain is about two times lower than that in the wild-type strain, which is in accordance with the relative level of thioredoxin in these two strains, as mentioned above. Crude extracts prepared from TK1 grown in the presence of IPTG show a 1.7-fold lower DTNB-reducing activity than that of the wild-type strain (Table 2), suggesting that although IPTG led to an important increase in the level of thioredoxin as mentioned before, it did not allow an increase to the same extent in the level of reduced thioredoxin.
TABLE 2.
Analysis of DTNB reduction activity
| Straina | Protein concnb (mg/ml) | DTNB reduction activityc (U/mg of protein) |
|---|---|---|
| WS8 | 3 | 2.9 |
| TK1−d | 3.45 | 1.25 |
| TK1+d | 3.7 | 1.7 |
Strains were grown under low oxygen tension (1 to 2% O2) as described in Materials and Methods.
Protein concentration was determined as described by Bradford (7).
DTNB reduction activity was measured in cell extracts as described in Materials and Methods. One unit of activity equals an increase in A412 of 1.00 min−1 at 23°C.
Strain TK1 was cultivated either in the absence (−) or presence (+) of 0.5 mM IPTG.
Effects of altered level of thioredoxin on bacteriochlorophyll content.
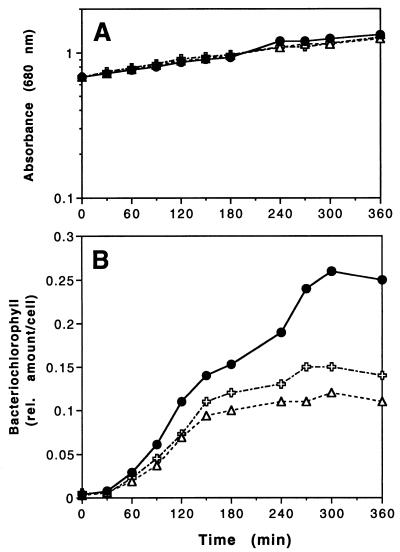
To further characterize the phenotypic properties of strain TK1, we analyzed its bacteriochlorophyll content after shifting the cells from growth under high oxygen tension to growth under low oxygen tension, with and without the addition of IPTG. This strain showed some increase in bacteriochlorophyll content, but the maximal level obtained 5 h after the oxygen shift was only 42% of that observed for the corresponding wild-type strain, WS8 (Fig. 3B), while the growth rate was identical for both strains (Fig. 3A). The addition of 0.5 mM IPTG to growth medium for cultivation of strain TK1 led to a higher bacteriochlorophyll content, but the maximal level observed corresponds to 58% of the wild-type bacteriochlorophyll level, indicating that induction of the plasmid-borne trxA copy by IPTG only partially restored the wild-type bacteriochlorophyll level.
FIG. 3.
Bacterial density (A) and bacteriochlorophyll content (B) from cultures of wild-type strain WS8 (•) and mutant TK1 without (▵) and with ( ) 0.5 mM IPTG after reduction of oxygen tension at time 0. The relative amount of bacteriochlorophyll per cell was calculated as described in Materials and Methods. The experiment was repeated several times with very similar results in regard to relative bacteriochlorophyll levels. The results for one representative measurement are shown.
These results suggest that the altered level of thioredoxin expression in strain TK1 affects the process of photosynthetic apparatus formation in R. sphaeroides, either on the level of bacteriochlorophyll biosynthesis pathway or on the level of formation (or regulation of synthesis) of structural proteins of spectral complexes.
Strain TK1 exhibits a wild-type ALA synthase activity.
ALA synthase catalyzes the formation of 5-aminolevulinic acid, the first step in bacteriochlorophyll biosynthesis in Rhodobacter. Given that R. sphaeroides thioredoxin was previously demonstrated to activate in vitro ALA synthase by a dithiol-disulfide exchange (11), the reduced bacteriochlorophyll level observed in strain TK1 may be due to an alteration in ALA synthase activity.
We therefore analyzed the bacteriochlorophyll content of strain TK1 supplemented with 0.1 mM ALA after shifting the cells from high oxygen tension conditions to semiaerobic growth conditions. We observed that cells from strain TK1 exhibit the same pattern of bacteriochlorophyll incorporation independently of the presence or absence of ALA in the growth medium (data not shown). Therefore, we conclude that ALA cannot restore the wild-type bacteriochlorophyll level in strain TK1.
On the other hand, ALA synthase activity was measured as described in Materials and Methods in crude extracts of semiaerobically grown cells. We observed that strain TK1 presented the same level of ALA synthase activity as the wild-type strain did (Table 3), while the negative control, strain HemAT1 (disrupted for both the hemA and hemT genes, which encode ALA synthase isozymes) (26) had a negligible ALA synthase activity (Table 3). These results together suggest that the low level of thioredoxin expression in our mutant strain has no effect on ALA synthase activity and that the reduced bacteriochlorophyll level in strain TK1 may reflect the involvement of thioredoxin in other levels of photosynthesis regulation.
TABLE 3.
Analysis of ALA synthase activity
| Straina | Protein concnb (mg/ml) | ALA synthase activityc (U/mg of protein) |
|---|---|---|
| WS8 | 3.28 | 50 |
| TK1 | 2.86 | 52 |
| HemAT1d | 3.07 | 1.5 |
Strains were grown under low oxygen tension (1 to 2% O2) as described in Materials and Methods.
Protein concentration was determined as described by Bradford (7).
ALA synthase activity was measured in cell extracts as described in Materials and Methods. One unit of activity equals 1 μmol of ALA h−1 at 37°C.
See Table 1 for genotypic properties.
Effect of oxygen on the expression of the puf operon in mutant strain TK1.
The pigment-binding proteins of R. sphaeroides and R. capsulatus are encoded by the polycistronic puf and puc operons whose expression is highly regulated by oxygen tension (for a review, see reference 41). Particularly, the levels of puc and puf mRNA are regulated by transcription activation when the oxygen tension is reduced and there is an increase in stability of some mRNA segments at low oxygen tension (for a review, see reference 23).
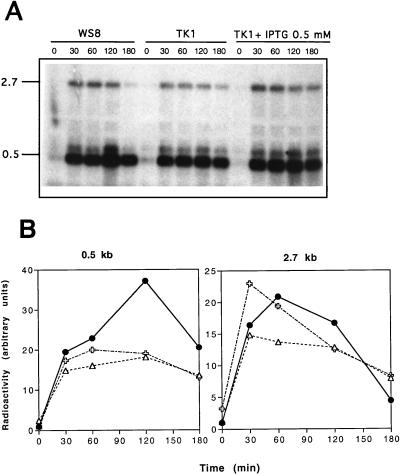
To investigate the effect of altered expression of thioredoxin on oxygen-regulated synthesis of pigment-binding proteins, we analyzed the expression level of the puf operon in the wild type, WS8, and in strain TK1 by Northern blot hybridization after a shift from high to low oxygen tension conditions. A 349-bp HindIII-PstI fragment of plasmid pBPUF1 (Table 1) was used as probe to detect the 2.7-kb pufBALMX mRNA and the more stable 0.5-kb pufBA mRNA segment. While the level of the 2.7-kb pufBALMX mRNA increased about 20-fold 60 min after the decrease in oxygen tension in cultures of the wild-type strain, WS8, it increased only by about 10- to 13-fold at the same time in cultures of the mutant strain, TK1 (Fig. 4). Similarly, the level of the 0.5-kb pufBA mRNA, which increased 35- to 42-fold 120 min after the oxygen tension was lowered in strain WS8, showed only a 6- to 8-fold increase in strain TK1 at the same time. The maximal levels of the 2.7-kb pufBALMX mRNA and of the 0.5-kb pufBA mRNA reached only about 50 to 60% of the maximal level observed for strain WS8. The addition of IPTG to cultures of strain TK1 led to maximal increases of about eight- and threefold for the 2.7-kb pufBALMX and the 0.5-kb pufBA mRNA levels, respectively (Fig. 4). In addition, the 0.5-kb pufBA mRNA level at time 0 was two times higher in mutant strain TK1 than in wild-type strain WS8, suggesting that the trxA mutant fails to totally repress the expression of the puf operon under high oxygen tension conditions. To ascertain that the differences observed in the induction level of puf mRNAs species are specific and not due to variations in the amount of total RNA of the samples, we systematically stripped the Northern blots and reprobed with a DNA probe specific to 16S rRNA. No significant change of the 16S rRNA level was observed (data not shown), indicating that differences in the puf mRNA transcript levels between wild-type strain WS8 and mutant strain TK1 are not due to differences in amount of total RNA.
FIG. 4.
(A) Northern hybridization analysis of the puf transcripts in R. sphaeroides cells shifted from high to low oxygen tension conditions. Total RNA (10 μg) was electrophoresed, blotted, and hybridized to a 349-bp HindIII-PstI pufBA fragment isolated from pBPUF1 (Table 1). Molecular size markers in kilobases are indicated on the left. The numbers above each lane indicate the time (minutes) after the shift. (B) Quantification of radioactivity of puf mRNA bands with a laser densitometer (PhosphorImager; Molecular Dynamics). Symbols: •, strain WS8; ▵ and , strain TK1 grown without and with 0.5 mM IPTG, respectively. Panel B shows the results of quantification of the Northern blot shown in panel A. Very similar results were obtained when the experiment was repeated, and the average values for the experiments are given in the text.
To know whether the trxA mutation in strain TK1 affects puf operon expression either at the transcriptional or RNA stability level, we determined the puf mRNA half-lives from R. sphaeroides cells cultivated under low oxygen tension conditions. We have observed similar puf mRNA half-lives of about 10 and 30 min for the 2.7- and 0.5-kb puf mRNA species, respectively, for both strains (data not shown), suggesting that involvement of thioredoxin in puf operon expression occurs at the transcriptional level.
DISCUSSION
In facultative photosynthetic microorganisms such as R. sphaeroides, the regulation of photosynthetic apparatus formation involves a signal transduction pathway of a redox cascade in response to changes in oxygen tension and/or redox potential (1). Although involvement of a redox signal in oxygen-dependent control of photosynthesis gene expression is clearly established by numerous data, the precise molecular reactions by which these redox signals operate (particularly, the way and hierarchy of interactions mediated by regulatory factors) are not yet clear.
Thioredoxin is capable of regulating protein activity by a thiol redox control and therefore is a good candidate to participate in such redox signalling pathways occurring in photosynthesis processes.
Since the thioredoxin-negative mutant is lethal in R. sphaeroides (29), we have constructed strain TK1 with a disruption for the chromosomal trxA copy and harboring a plasmid-borne trxA gene inducible by IPTG (Fig. 1) and used it to study the role of thioredoxin in photosynthetic complex formation. Examination of the phenotype of strain TK1 revealed that a reduced level of thioredoxin expression results in reduced bacteriochlorophyll incorporation after the oxygen tension was decreased (Fig. 3). Given that mutant strain TK1 exhibited a growth rate identical to that of the wild type, this result indicates that wild-type expression of thioredoxin is specifically required for synthesis and assembly of a functional photosynthetic apparatus. Induction by IPTG of the plasmid-borne trxA copy from strain TK1, which led to a threefold increase in the level of thioredoxin protein compared to that in the wild-type strain (Fig. 2), could restore the wild-type phenotype only partially. The fact that the level of the reduced form of thioredoxin in strain TK1 subjected to IPTG induction was still lower than that of the wild type suggests that not only the absolute level of thioredoxin but also its redox state in the cell plays a critical role in bacteriochlorophyll incorporation. Since we did not coexpress the gene for thioredoxin reductase together with the trxA gene, it is not surprising that the level of reduced thioredoxin is not increased to the same extent as the level of total thioredoxin after IPTG addition. However, one would expect to reach similar levels of reduced thioredoxin after the addition of IPTG to cultures of strain TK1 as in wild-type strain WS8. It is conceivable that the thioredoxin redox system is well balanced in wild-type cells and that unbalanced expression of its components is responsible for the low levels of reduced thioredoxin that were observed in strain TK1 after IPTG addition. Further investigations of the regulated expression of the thioredoxin redox system components are required to test this hypothesis.
Although thioredoxin has been previously demonstrated to activate in vitro ALA synthase activity (11), the first enzyme of the bacteriochlorophyll biosynthetic pathway, mutant strain TK1 exhibits ALA synthase activity identical to that of the wild type, indicating that the altered level of trxA expression in this strain is sufficient for full ALA synthase activity but affects other pathways in the regulatory network governing photosynthesis gene expression. Accordingly, we demonstrate that strain TK1 fails to maximally induce expression of the puf operon (encoding the pigment-binding proteins of the light harvesting I and reaction center complexes and PufQ and PufX) in response to a reduction in oxygen tension. In addition, the trxA mutant also shows a decreased level of puf operon repression under high oxygen tension conditions as deduced from its higher level of 0.5-kb puf mRNA compared to that of the wild type. Induction of thioredoxin expression in strain TK1 by IPTG increased the puf operon activation after reduction of oxygen tension but still could only partially restore the wild-type phenotype.
These results together suggest that thioredoxin, as a transducer of redox potential generated by the decrease in oxygen tension, may participate in the redox cascade that coordinates photosynthesis gene expression. On the basis of its catalytic properties, thioredoxin can be expected to be redox intermediate which more probably could regulate protein function (by a thiol redox control) rather than directly acting on gene expression. Particularly, thioredoxin may act by posttranslational thiol redox control of the biological activity of a transcriptional factor(s) involved in puf operon expression in response to changes in oxygen tension. A good candidate for thioredoxin targets in this regulation mechanism is the AppA factor. AppA is a trans-acting factor involved in activation of photopigments and pigment-binding protein expression and is characterized by an unusual cysteine cluster at the carboxy terminus (18). Thus, one or more of these cysteines are potential regulatory residues that could be targeted by thioredoxin. Indeed, if activation of AppA needs a reduced form (reduction of the cysteine cluster), thioredoxin is a good candidate to reduce the disulfide bonds formed between the SH groups of the cysteine cluster. In other respects, thioredoxin may also modulate the redox-sensing capabilities of the transcription factor CrtJ, which is responsible for aerobic repression of several photosynthesis genes (30) and whose binding to the bchC promoter region is redox sensitive (31). A recent study of its homolog in R. sphaeroides, PpsR, suggests that AppA may be involved in controlling its redox-sensitive repressor activity, either by direct interaction of AppA with PpsR or through interactions with other mediators (19). Thioredoxin is a good candidate for such redox mediators.
In addition, the different phenotypic properties observed between the wild-type strain and strain TK1 subjected to trxA gene induction by IPTG suggest that not only the thioredoxin level but also the redox status of thioredoxin in the cells plays a crucial role in the control of R. sphaeroides photosynthetic apparatus formation.
While our results clearly demonstrate that the wild-type level of thioredoxin expression is required for maximal puf operon expression, the precise mechanisms of action of thioredoxin and the identity of the targets of thioredoxin remain to be resolved.
ACKNOWLEDGMENTS
This work was supported by Deutsche Forschungsgemeinschaft (DFG KL563/7-1) and Fonds der Chemischen Industrie.
REFERENCES
- 1.Allen J F, Alexciev K, Håkansson G. Regulation by redox signalling. Curr Biol. 1995;5:869–872. doi: 10.1016/s0960-9822(95)00176-x. [DOI] [PubMed] [Google Scholar]
- 2.Amann E, Brosius J, Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983;25:167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- 3.Assemat K, Alzari P M, Clément-Métral J D. Conservative substitutions in the hydrophobic core of Rhodobacter sphaeroidesthioredoxin produce distinct functional effects. Prot Sci. 1995;4:2510–2516. doi: 10.1002/pro.5560041207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci USA. 1985;82:4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer C E, Bird T H. Regulatory circuits controlling photosynthesis gene expression. Cell. 1996;85:5–8. doi: 10.1016/s0092-8674(00)81074-0. [DOI] [PubMed] [Google Scholar]
- 6.Bolivar F, Backman K. Plasmids of Escherichia colias cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan B B. Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol. 1980;31:341–374. [Google Scholar]
- 9.Buchanan B B. Regulation of CO2assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Arch Biochem Biophys. 1991;288:1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- 10.Burnham B F. δ-Aminolevulinic acid synthase. Methods Enzymol. 1970;17:195–204. [Google Scholar]
- 11.Clément-Métral J D. Activation of ALA synthetase by reduced thioredoxin in Rhodopseudomonas Y. FEBS Lett. 1979;101:116–120. doi: 10.1016/0014-5793(79)81307-1. [DOI] [PubMed] [Google Scholar]
- 12.Cséke C, Buchanan B B. Regulation of the formation and utilization of photosynthate in leaves. Biochim Biophys Acta. 1986;853:43–63. [Google Scholar]
- 13.Danon A, Mayfield P. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- 14.Drews G. Mikrobiologisches Praktikum. Berlin, Germany: Springer-Verlag; 1983. [Google Scholar]
- 15.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eraso J M, Kaplan S. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J Bacteriol. 1995;177:2695–2706. doi: 10.1128/jb.177.10.2695-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomelsky M, Kaplan S. Genetic evidence that PpsR from Rhodobacter sphaeroides 2.4.1 functions as a repressor of puc and bchFexpression. J Bacteriol. 1995;177:1634–1637. doi: 10.1128/jb.177.6.1634-1637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomelsky M, Kaplan S. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides2.4.1. J Bacteriol. 1995;177:4609–4618. doi: 10.1128/jb.177.16.4609-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomelsky M, Kaplan S. Molecular genetic analysis suggesting interactions between AppA and PpsR in regulation of photosynthesis gene expression in Rhodobacter sphaeroides2.4.1. J Bacteriol. 1997;179:128–134. doi: 10.1128/jb.179.1.128-134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 21.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 22.Kiley P J, Donohue T J, Havelka W A, Kaplan S. DNA sequence and in vitro expression of the B875 light-harvesting polypeptides of Rhodobacter sphaeroides. J Bacteriol. 1987;169:742–750. doi: 10.1128/jb.169.2.742-750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klug G. Regulation of expression of photosynthesis genes in anoxygenic photosynthetic bacteria. Arch Microbiol. 1993;159:397–404. doi: 10.1007/BF00288584. [DOI] [PubMed] [Google Scholar]
- 24.Muller G D, Buchanan B B. Thioredoxin is essential for photosynthetic growth. The thioredoxin m gene of Anacystis nidulans. J Biol Chem. 1989;264:4008–4014. [PubMed] [Google Scholar]
- 25.Navarro F, Florencio F J. The cyanobacterial thioredoxin gene is required for both photoautotrophic and heterotrophic growth. Plant Physiol. 1996;111:1067–1075. doi: 10.1104/pp.111.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidle E L, Kaplan S. 5-Aminolevulinic acid availability and control of spectral complex formation in HemA and HemT mutants of Rhodobacter sphaeroides. J Bacteriol. 1993;175:2304–2313. doi: 10.1128/jb.175.8.2304-2313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieuwlandt D T, Palmer J R, Armbruster D T, Kuo Y-P, Oda W, Daniels C J. A rapid procedure for the isolation of RNA from Haloferax volcanii. In: DasSarma S, Fleischmann E M, editors. Archaea: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 161–162. [Google Scholar]
- 28.Pasternak C, Assemat K, Breton A M, Clément-Métral J D, Klug G. Expression of the thioredoxin gene (trxA) in Rhodobacter sphaeroidesY is regulated by oxygen. Mol Gen Genet. 1996;250:189–196. doi: 10.1007/BF02174178. [DOI] [PubMed] [Google Scholar]
- 29.Pasternak C, Assemat K, Clément-Métral J D, Klug G. Thioredoxin is essential for Rhodobacter sphaeroidesgrowth by aerobic and anaerobic respiration. Microbiology. 1997;143:83–91. doi: 10.1099/00221287-143-1-83. [DOI] [PubMed] [Google Scholar]
- 30.Ponnampalam S N, Buggy J J, Bauer C E. Characterization of an aerobic repressor that coordinately regulates bacteriochlorophyll, carotenoid, and light harvesting-II expression in Rhodobacter capsulatus. J Bacteriol. 1995;177:2990–2997. doi: 10.1128/jb.177.11.2990-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponnampalam S N, Bauer C E. DNA binding characteristics of CrtJ. A redox-responding repressor of bacteriochlorophyll, carotenoid, and light harvesting-II gene expression in Rhodobacter capsulatus. J Biol Chem. 1997;272:18391–18396. doi: 10.1074/jbc.272.29.18391. [DOI] [PubMed] [Google Scholar]
- 32.Prentki P, Krisch H. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 33.Sabaty M, Kaplan S. mgpS, a complex regulatory locus involved in the transcriptional control of the puc and puf operons in Rhodobacter sphaeroides2.4.1. J Bacteriol. 1996;178:35–45. doi: 10.1128/jb.178.1.35-45.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sganga M W, Bauer C E. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell. 1992;68:945–954. doi: 10.1016/0092-8674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- 36.Short J M, Fernandez J M, Sorge J A, Huse W D. λ ZAP: a bacteriophage λ expression vector with in vivoexcision properties. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivogenetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 38.Sistrom W R. Transfer of chromosomal genes mediated by plasmid R68.45 in Rhodopseudomonas sphaeroides. J Bacteriol. 1977;131:526–532. doi: 10.1128/jb.131.2.526-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slaby I, Holmgren A. Structure and enzymatic functions of thioredoxin refolded by complementation of two tryptic peptide fragments. Biochemistry. 1979;18:5584–5591. doi: 10.1021/bi00592a010. [DOI] [PubMed] [Google Scholar]
- 40.Zeilstra-Ryalls J H, Kaplan S. Role of the fnrL gene in photosystem gene expression and photosynthetic growth of Rhodobacter sphaeroides2.4.1. J Bacteriol. 1998;180:1496–1503. doi: 10.1128/jb.180.6.1496-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeilstra-Ryalls J, Gomelsky M, Eraso J M, Yeliseev A, O’Gara J, Kaplan S. Control of photosystem formation in Rhodobacter sphaeroides. J Bacteriol. 1998;180:2801–2809. doi: 10.1128/jb.180.11.2801-2809.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]