Abstract
Background
the aim of this study was to retrospectively operationalise the World Guidelines for Falls Prevention and Management (WGFPM) falls risk stratification algorithm using data from The Irish Longitudinal Study on Ageing (TILDA). We described how easy the algorithm was to operationalise in TILDA and determined its utility in predicting falls in this population.
Methods
participants aged ≥50 years were stratified as ‘low risk’, ‘intermediate’ or ‘high risk’ as per WGFPM stratification based on their Wave 1 TILDA assessments. Groups were compared for number of falls, number of people who experienced one or more falls and number of people who experienced an injury when falling between Wave 1 and Wave 2 (approximately 2 years).
Results
5,882 participants were included in the study; 4,521, 42 and 1,309 were classified as low, intermediate and high risk, respectively, and 10 participants could not be categorised due to missing data. At Wave 2, 17.4%, 43.8% and 40.5% of low-, intermediate- and high-risk groups reported having fallen, and 7.1%, 18.8% and 18.7%, respectively, reported having sustained an injury from falling.
Conclusion
the implementation of the WGFPM risk assessment algorithm was feasible in TILDA and successfully differentiated those at greater risk of falling. The high number of participants classified in the low-risk group and lack of differences between the intermediate and high-risk groups may be related to the non-clinical nature of the TILDA sample, and further study in other samples is warranted.
Keywords: risk, falls, stratification, longitudinal, prediction, older people
Key Points
We applied the World Guidelines for Falls’ risk stratification algorithm to data of the Irish Longitudinal Study on Ageing.
The prevalence of reported falls at follow-up was significantly higher in those at ‘high risk’ than those at ‘low risk’.
There may not be a clinically useful differentiation between those categorised at ‘intermediate risk’ and those at ‘high risk’.
The algorithm produced a sensitivity score of 39.5% and specificity of 82.0% for predicting one or more falls at follow-up.
Background
The second largest cause of unintentional injury-related deaths worldwide is due to falls [1], and annually in Ireland, more than 7,000 people aged 65 years and over require hospitalisation due to a fall [2]. In England, fragility fractures alone are estimated to cost health and social care services £1.1 billion annually [3]. Several interventions exist that can reduce the risk of falling, most notably comprehensive geriatric assessment [4] and exercise interventions [5]. However, as with all healthcare, there are limited resources, and one challenge is making sure that people who would benefit from these types of interventions, receive them.
Multiple guidelines aiming to reduce falls and improve post-fall management have been published over the last decade. Many have suggested methods or measures to enhance identification of those at high risk of a first fall or recurrent falls. A raft of algorithms or assessment tools exist, with the aim to help busy clinicians rapidly assess and interpret risk in order to inform the acute and long-term management of individuals at risk of falling. However, due to the complex multifactorial nature of falls, there is little consensus on the best approach [6].
The 2022 World Guidelines for Falls Prevention and Management (WGFPM) [7] include a falls risk stratification algorithm. The algorithm stratifies community-dwelling older adults into ‘low risk’, ‘intermediate risk’ and ‘high risk’ of falling. The tool is designed to be used either during opportunistic case finding such as annual health visits or when individuals present to healthcare professionals with a fall or related injury. Having robust tools at hand for all health and social care professionals will ensure they are empowered to proactively and effectively prevent and manage falls to reduce likelihood of occurrence and premature morbidity and mortality, and reduce health and social care costs.
The aim of this study was to retrospectively operationalise the WGFPM falls risk stratification algorithm using data from The Irish Longitudinal Study on Ageing (TILDA). In this population, we described how easy the algorithm was to operationalise, and determined its utility in predicting falls.
Methods
Setting
We analysed data from TILDA, a population-based longitudinal study that collects information on the health, economic and social circumstances of community-dwelling adults aged 50 years or over in Ireland. Wave 1 of the study (baseline) took place between October 2009 and July 2011, and subsequent data were collected approximately 2 yearly (Wave 2: February 2012 to March 2013). At each wave, participants completed a computer-assisted personal interview conducted by trained social interviewers in the participants’ own home, and a self-completion questionnaire, which they completed in their own time. Waves 1 and 3 also included a detailed health assessment conducted by trained research nurses at a dedicated health centre or in the participants’ own home. Individuals with cognitive impairment or dementia who could not provide written informed consent to participate in the study were excluded [8]. The full cohort profile has been described elsewhere [9].
Participants
For the main analysis, we included TILDA Wave 1 participants aged 50 years or over, who completed the health assessment (at home or at a health assessment centre) and who had self-reported falls information at TILDA Wave 1 (2010).
Procedure
The WGFPM falls risk stratification algorithm has two points of entry: opportunistic case finding and adults presenting to healthcare with a fall or related injury. For the purpose of this study, the TILDA Wave 1 health assessment was treated as opportunistic case finding, and the entry point of ‘adults presenting to healthcare with a fall or related injury’ was not used. Table 1 shows the variables in the risk stratification algorithm and the variables that these were matched to from the TILDA Wave 1 health assessment.
Table 1.
Application of the WGFPM risk stratification algorithm to the TILDA dataset
| WGFPM risk stratification algorithm | Variable used for operationalisation with the TILDA data set | |
|---|---|---|
| Assess falls in past 12 months (fall in last 12 months or positive answer to 3KQ) |
|
Participants were asked the following:
|
|
Participants were asked the following:
|
|
|
Participants were asked the following:
|
|
| Assess fall severity (answering ‘yes’ to one criteria is sufficient to satisfy severity criteria) |
|
If participants reported having fallen, they were asked the following:
|
|
If participants reported having fallen, they were asked the following:
|
|
|
Clinical Frailty Scale (CFS) scored using the CFS Decision Tree [10], and operationalised by TILDA [11]. A score of ≥5 considered ‘frail’ | |
|
No comparative TILDA variable | |
|
If participants reported having fallen, they were asked the following:
|
|
| Assess gait & balance | Gait speed ≤0.8 m/s or Timed Up and Go (TUG) > 15 seconds | TUG >15 seconds |
aIf answered ‘slightly unsteady’ or ‘very unsteady’ to either of the two questions, the criteria were considered satisfied.
Analysis
Data were analysed with R software [12]. Descriptive statistics were presented as mean with standard deviation (± SD), median with interquartile range (IQR) or count with percentage (%).
All participants were stratified as low, intermediate or high risk according to the WGFPM risk stratification algorithm at Wave 1. Using data from Wave 2 (approximately 2 years after Wave 1), the groups were compared for number of falls between Wave 1 and Wave 2, number of people who experienced one or more falls between Wave 1 and Wave 2, and number of people who experienced an injury when falling between Wave 1 and Wave 2. To test for differences between groups, the Kruskal–Wallis test was used for the number of falls between Wave 1 and Wave 2. The Chi-squared test or Fisher’s exact test (if ≤10 participants in a group) were used for the categorical outcomes. All post hoc tests used Bonferroni adjustment for multiple comparisons.
The sensitivity and specificity of the WGFPM falls risk stratification algorithm in predicting people with one or more falls between Wave 1 and Wave 2 were calculated using the following definitions:
– True positives: participants categorised as ‘high risk’ who reported having fallen between Wave 1 and Wave 2;
– True negatives: participants categorised as ‘low risk’ who reported no falls between Wave 1 and Wave 2;
– False positives: participants categorised as ‘high risk’ who reported no falls between Wave 1 and Wave 2;
– False negatives: participants categorised as ‘low risk’ who reported having fallen between Wave 1 and Wave 2.
Sensitivity and specificity were calculated either by ignoring the ‘intermediate risk’ group, or by combining the intermediate- and high-risk groups.
In addition, sensitivity analysis was conducted by limiting the analysis to those who were 65 years or older at their Wave 1 assessment.
Ethics
Ethical approval for each wave was obtained from the Faculty of Health Sciences Research Ethics Committee at Trinity College Dublin, Ireland (Wave 1, reference: ‘The Irish Longitudinal Study on Ageing’, date of approval: May 2008; Wave 2, reference: ‘The Irish Longitudinal Study on Ageing’, date of approval: October 2011). All participants provided written informed consent.
Results
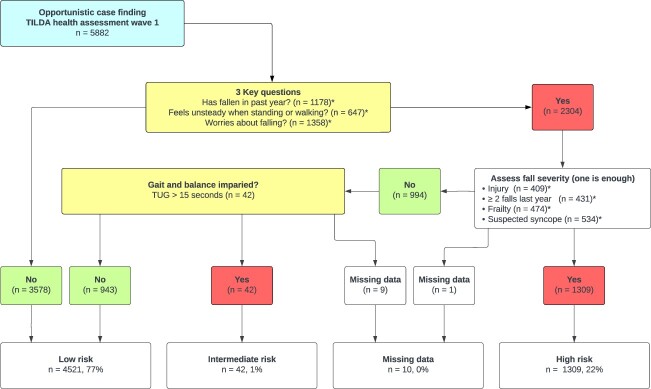
TILDA Wave 1 recruited a total of 8,173 participants aged 50 years or older, of whom 5,891 completed a health assessment (5,031 in a dedicated health centre, 860 in their own home). Of these, 5,882 had self-reported falls information. Figure 1 shows the process of categorising participants as ‘low’ (n = 4,521), ‘intermediate’ (n = 42) and ‘high’ (n = 1,309) risk of falls. Due to missing data, we were unable to categorise 10 participants. A description of the participants in each category is presented in Table 2.
Figure 1.
Operationalisation of the World Guidelines for Falls Prevention and Management risk stratification algorithm in Wave 1 of The Irish Longitudinal Study on Ageing. * number indicates a positive response/criterion, participants can have multiple positive responses/criteria.
Table 2.
Sample characteristics by risk category at Wave 1
| Variable | All participants | Low risk | Intermediate risk | High risk | Missing | P-value for difference between low-, intermediate- and high-risk groups |
P-value for difference between groups: L = Low I = Intermediate H = High |
|---|---|---|---|---|---|---|---|
| Number of participants | 5,882 | 4,521 (76.9%) | 42 (0.7%) | 1,309 (22.3%) | 10 (0.2%) | ||
| Mean age | 63.2 (±9.3) | 62.1 (±8.7) | 77.3 (±8.0) | 66.2 (±10.3) | 65.6 (±10.4) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P < 0.001 |
| Female | 3,182 (54.1%) | 2,360 (52.2%) | 23 (54.8%) | 797 (60.9%) | 2 (20.0%) | P < 0.001 | L vs. I: P = 1 L vs. H: P < 0.001 I vs. H: P = 1 |
| Median number of falls in past year | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.0 (0.0–2.0) | 0.0 (0.0–0.75) | P < 0.001 | L vs. I: P = 0.035 L vs. H: p < 0.001 I vs. H: p < 0.001 |
| Mean BMI | 28.7 (±5.1) | 28.5 (±4.9) | 30.6 (±5.7) | 29.2 (±5.6) | 29.1 (±6.5) | P < 0.001 | L vs. I: P = 0.039 L vs. H: P = 0.002 I vs. H: P = 0.308 |
| Education up to primary school level | 1,540 (26.2%) | 1,074 (23.8%) | 23 (54.8%) | 437 (33.4%) | 6 (60.0%) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 0.005 |
| Education to secondary school level | 2,413 (41.0%) | 1,902 (42.1%) | 14 (33.3%) | 493 (37.7%) | 4 (40.0%) | ||
| Education to tertiary/higher level | 1,927 (32.8%) | 1,543 (34.1%) | 5 (11.9%) | 379 (29.0%) | 0 (0.0%) | ||
| Median number of chronic conditions | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 2.0 (1.0–3.8) | 2.0 (1.0–3.0) | 2.0 (0.2–3.0) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 1 |
| Median Clinical Frailty Scale Score | 2.0 (2.0–4.0) | 2.0 (2.0–3.0) | 3.5 (3.0–4.0) | 4.0 (2.0–6.0) | 4.0 (2.0–4.0) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 0.68 |
| MI | 271 (4.6%) | 189 (4.2%) | 4 (9.5%) | 77 (5.9%) | 1 (10.0%) | P = 0.009 | L vs. I: P = 0.299 L vs. H: P = 0.039 I vs. H: P = 0.938 |
| HF | 58 (1.0%) | 31 (0.7%) | 2 (4.8%) | 25 (1.9%) | 0 (0.0%) | P < 0.001 | L vs. I: P = 0.109 L vs. H: P < 0.001 I vs. H: P = 0.611 |
| Stroke or TIA | 207 (3.5%) | 102 (2.3%) | 7 (16.7%) | 97 (7.4%) | 1 (10.0%) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 0.112 |
| Cancer | 365 (6.2%) | 273 (6.0%) | 3 (7.1%) | 88 (6.7%) | 1 (10.0%) | P = 0.577 | |
| Median number of medications | 2.0 (0.0–4.0) | 2.0 (0.0–3.0) | 4.0 (3.0–7.2) | 3.0 (1.0–6.0) | 0.5 (0.0–3.8) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 0.085 |
| Anti-depressants | 389 (6.6%) | 216 (4.8%) | 5 (11.9%) | 167 (12.8%) | 1 (10.0%) | P < 0.001 | L vs. I: P = 0.151 L vs. H: P < 0.001 I vs. H: P = 1 |
| Anti-cholinergics | 245 (4.2%) | 135 (3.0%) | 3 (7.1%) | 107 (8.2%) | 0 (0.0%) | P < 0.001 | L vs. I: P = 0.361 L vs. H: P < 0.001 I vs. H: P = 1 |
| Anti-hypertensives | 2,163 (36.8%) | 1,542 (34.1%) | 27 (64.3%) | 590 (45.1%) | 4 (40.0%) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 0.042 |
| Fear of falling—not afraid | 4,521 (76.9%) | 3,897 (86.2%) | 11 (26.2%) | 608 (46.4%) | 5 (50.0%) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 0.160 |
| Fear of falling—somewhat afraid | 1,040 (17.7%) | 522 (11.5%) | 25 (59.5%) | 489 (37.4%) | 4 (40.0%) | ||
| Fear of falling—very afraid | 318 (5.4%) | 100 (2.2%) | 6 (14.3%) | 211 (16.1%) | 1 (10.0%) | ||
| Mean grip strength (kg) | 27.1 (±9.8) | 28.0 (±9.8) | 20.0 (±6.3) | 24.0 (±9.5) | 28.2 (±8.9) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P = 0.032 |
| Median IADL difficulties | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | P < 0.001 | L vs. I: P = 1 L vs. H: P < 0.001 I vs. H: P = 0.002 |
| Mean TUG time (seconds) | 9.2 (±3.8) | 8.6 (±1.9) | 19.3 (±7.6) | 11.0 (±6.5) | 8.2 (n = 1) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P < 0.001 |
| Median MOCA score | 25.0 (23.0–27.0) | 26.0 (23.0–28.0) | 20.0 (17.0–25.0) | 25.0 (22.0–27.0) | 22.0 (18.0–23.0) | P < 0.001 | L vs. I: P < 0.001 L vs. H: P < 0.001 I vs. H: P < 0.001 |
Data presented as mean and standard deviation (± SD), median and interquartile range (IQR), or count and percentage (%). BMI = body mass index; MI = myocardial infarction; HF = heart failure; TIA = transient ischaemic attack; IADL = instrumental activities of daily living; TUG = Timed Up and Go; MOCA = Montreal Cognitive Assessment. P-values were calculated using Kruskal–Wallis test for continuous outcomes and Chi-squared test or Fisher exact test (if ≤10 participants in a group) for categorical outcomes. All post hoc tests with Bonferroni adjustment.
Table 3 presents the differences between participants in the different falls risk categories at Wave 2. All groups had attrition in participants, though this was greatest in the ‘intermediate risk’ group, in part explained by deaths. There were no differences between the intermediate- and high-risk groups in terms of number of falls reported, number of participants who had fallen or number of participants who had sustained an injury from falling. Ignoring the intermediate-risk group, the WGFPM algorithm as applied to TILDA produced a sensitivity score of 39.5% and specificity of 82.0% for predicting one or more falls between Wave 1 and Wave 2 in this cohort. Alternatively, combining participants in the intermediate- and high-risk groups the WGFPM algorithm produced a sensitivity score of 40.2% and specificity of 81.5%. Results for the sensitivity analysis restricting age to ≥65 years at wave 1 are presented in Table 4.
Table 3.
Differences between risk categories at Wave 2
| Variable | Low risk | Intermediate risk | High risk | P-value (between groups) | P-value (between specific risk groups) | |||
|---|---|---|---|---|---|---|---|---|
| Low vs. intermediate | Low vs. high | Intermediate vs. high | ||||||
| Number of participants at Wave 1 | 4,521 | 42 | 1,309 | – | – | – | – | |
| Missing at Wave 2 | 342 (7.6%) | 10 (23.8%) | 131 (10.0%) | P < 0.001 | P = 0.003 | P = 0.017 | P = 0.026 | |
| Died before Wave 2 | 55 (1.2%) | 5 (11.9%) | 50 (3.8%) | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.075 | |
| Missing for unknown reason | 287 (6.3%) | 5 (11.9%) | 81 (6.2%) | P = 0.316 | – | – | – | |
| Number of participants at Wave 2 follow-up | No age restriction | 4,179 | 32 | 1,178 | – | – | – | – |
| ≥65 years at Wave 1 | 1,506 | 29 | 599 | – | – | – | – | |
| Median number of falls between Wave 1 and Wave 2 | No age restriction | 0.0 (0.0–0.0) | 0.0 (0.0–1.2) | 0.0 (0.0–1.0) | P < 0.001 | P < 0.001 | P < 0.001 | P = 1 |
| ≥65 years at Wave 1 | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | P < 0.001 | P = 0.001 | P < 0.001 | P = 1 | |
| Participants reporting one or more falls between Wave 1 and Wave 2 | No age restriction | 729 (17.4%) | 14 (43.8%) | 477 (40.5%) | P < 0.001 | P < 0.001 | P < 0.001 | P = 1 |
| ≥65 years at Wave 1 | 302 (20.1%) | 13 (44.8%) | 272 (45.4%) | P < 0.001 | P < 0.001 | P = 0.003 | P = 1 | |
| Participants reporting an injury sustained from a fall between Wave 1 and Wave 2 | No age restriction | 295 (7.1%) | 6 (18.8%) | 220 (18.7%) | P < 0.001 | P = 0.071 | P < 0.001 | P = 1 |
| ≥65 years at Wave 1 | 132 (8.8%) | 5 (17.2%) | 134 (22.4%) | P < 0.001 | P = 0.52 | P < 0.001 | P = 1 | |
Data presented as count with percentage (%) or median and interquartile range (IQR). P-values were calculated using Kruskal–Wallis test for continuous outcomes and Chi-squared test or Fisher exact test (if ≤10 participants in a group) for categorical outcomes. All post hoc tests with Bonferroni adjustment.
Table 4.
Sensitivity and specificity analyses, ignoring or combining the intermediate-risk group
| Age | True positives | True negatives | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|
| Intermediate-risk group removed from analysis | No age restriction | 477 | 3,792 | 39.5% | 82.0% | 73.2% |
| ≥65 years at Wave 1 | 272 | 1,204 | 47.4% | 78.6% | 70.1% | |
| Combined intermediate- and high-risk groups | No age restriction | 491 | 3,792 | 40.2% | 81.5% | 72.9% |
| ≥65 years at Wave 1 | 285 | 1,204 | 48.5% | 77.8% | 69.8% |
Discussion
The aim of this study was to retrospectively operationalise the WGFPM falls risk stratification algorithm using data from TILDA. An increase in incidence of falls and injuries resulting from falls was associated with WGFPM-derived intermediate- and high-risk groups, compared to low risk. The latter implies that the WGFPM risk stratification algorithm successfully identified those at greater risk of falling when using the opportunistic case finding method. However, in this analysis from TILDA, the utility of the intermediate-risk group stratification was less certain, given the small numbers the algorithm identified and that this group did not appear to be significantly different in terms of falls risk from the high-risk group. Of particular note at Wave 2 was the relatively high number of people in the low-risk group who reported having fallen since Wave 1, and a smaller but significant number of participants reported having sustained injuries falling.
The WGFPM recommendation for the ‘intermediate risk’ group is for tailored exercises, education on falls risk prevention and reassessment in 1 year’s time. This is contrasted by recommendations of multifactorial risk assessment, individualised tailored interventions and reassessment in 30 to 90 days’ time for those in the high-risk group [7]. In our TILDA-based study, the ‘intermediate risk’ group was very small and not significantly different to the high-risk group in terms of the number of falls or injurious falls reported at Wave 2. From inspection of Table 2, on average the ‘intermediate risk’ participants appeared to be older, have spent less time in formal education, and were slower, weaker and had lower cognitive scores than the high-risk group, which may justify amalgamation with the high-risk group in terms of potential benefit from interventions.
Given the retrospective nature of the data available, we can only make limited inferences as to why opportunistic case finding identified such a small proportion of the TILDA cohort as at intermediate risk. We postulate this may be the result of the cut-off point in the Timed Up and Go (TUG) (>15 seconds). Normative TUG data for older adults have been reported as 8.1 seconds (95%: 7.1–9.0) for those aged 60–69 years, 9.2 seconds (95% CI: 8.2–10.2) for 70–79 years and 11.3 seconds (95% CI: 10.0–12.7) for 80–99 years [13]. In the previous TILDA study by Savva et al. [14], the median TUG time for the 7.7% of Wave 1 participants who were aged ≥65 years and had physical frailty was still <15 seconds. A TUG time of more than 15 seconds in the absence of frailty, suspected syncope, or 2 or more falls, or an injury resulting from a fall in the previous year, may be expected to represent a small sample of the population.
Despite its suggested benign label, the ‘low risk’ group in TILDA still had a relatively high percentage of participants reporting falls and injuries from falls at Wave 2. Indeed, approximately 60% of people reporting injuries from falls at Wave 2 came from the low-risk group. Conversely, only 40% of the high-risk group went on to have a further fall at Wave 2, which implies that the algorithm resulted in a relatively large proportion of false positives. Ignoring the ‘intermediate risk’ group (no age restriction), the WGFPM algorithm produced a sensitivity score of 39.5% and specificity of 82.0% in this TILDA cohort (assuming ‘high risk’ is interpreted as expected to fall and ‘low risk’ as not expected to fall). These figures are comparable to the sensitivity and specificity reported by Burns et al. [15], 45.3% and 83.4%, respectively, based on asking if participants had fallen in the past year (an affirmative response interpreted as being expected to fall). In our described TILDA cohort, this same ‘fallen in the past year’ question had a sensitivity of 36.7% and specificity of 85.1%, suggesting that the more extensive WGFPM algorithm did not add much accuracy internally.
The ability to accurately predict risk of subsequent falls may be affected by the dichotomisation of continuous variables in the WGFPM algorithm (e.g. number of falls, frailty scales, TUG), which is likely to reduce the power of the prediction [16], although there may be benefits to simplifying the risk assessment tool to maximise ease of use. Other risk assessment tools that rely on more advanced computation such as the FRAT-UP [17] or the prediction model by Dormosh et al. [18] provide individualised predicted probabilities or risk of falls on a continuous scale, both of which have been externally validated and show reasonable discriminative power [19, 20]. Dormosh et al. [18] reported sensitivity of 62% and specificity of 70% in their model; however, the feasibility of these methods compared to the approach used by the WGFPM algorithm may be significantly lower where resources are limited.
The impact of screening for falls risk is not fully understood and screening should not be necessarily assumed as ‘doing no harm’ [21, 22]. Whether being classified as high risk may cause a fear of falling, a known risk factor of falling, is not clear. Being classified as high risk may increase anxiety and has been shown to be stigmatising, as it may threaten an individual’s identity and autonomy [23–25]. However, it may also lead to lifestyle changes such as avoidance of activities perceived as high risk [26]. Similarly, for some individuals wrongly classified as low risk, this may exacerbate denial of falls risk, which is a known barrier to participation in falls prevention interventions [27]. In other fields, like cancer, where risk stratification to inform eligibility for an intervention is more widely embedded, it would seem that risk stratification prior to being offered an intervention is generally acceptable if people feel it will benefit those who need it most, improve efficiency/resource allocation and reduce false positives [28].
The present study has several limitations. The implementation of the WGFPM risk assessment algorithm was feasible in TILDA, albeit not fully (e.g. the lying on the floor/unable to get up question was not available; gait speed was not available in the full cohort). It should be noted that WGFPM falls risk stratification algorithm also recommended the alternative use of a gait speed cut-off of <0.8 m/s, which we did not assess. Being at high risk of falls and falling are not analogous, and describing people in the high-risk group who did not fall as ‘false positives’ may be disingenuous. Individuals at high risk of falling may have mitigated for their falls risk through lifestyle restrictions and behavioural modifications and may still be considered at high risk of falling. Similarly, ‘false positives’ may be explained by individuals having participated in falls prevention interventions and by Wave 2 no longer being classified as ‘high risk’. While details of the latter are not available, TILDA has observed that falling status is dynamic over time [29]. We did consider various outcomes as a measure of ‘falls risk’; however, we wished to focus on the clinical interpretation of the tool. We felt that any cut-off is relatively arbitrary, and the clinical relevance of distinguishing between different cut-offs (e.g. ‘1 or more falls’, ‘2 or more falls’ or ‘3 or more falls’) was unclear.
TILDA Wave 1 household response rate was 62% [30], and as in other longitudinal studies, healthy persons may be over-represented. Our TILDA sample only included community-dwelling individuals, and although at Wave 1 participants living with dementia were excluded, recall bias is possible. The retrospective self-reported nature of falls is liable to recollection and social desirability bias. Previous research has shown that falls tend to be under-reported when relying on verbal prompts and memory, and under-reporting has been attributed to problems recalling falls, stigma attached to falling and differences in how falls are defined and perceived (e.g. what constitutes ‘a fall’) [31, 32]. However, we do not have reasons to believe that this potential bias affected participants classified as low or high risk differently. The WGFPM algorithm is likely targeted at a higher average age than that of our main analysis, which focused on participants aged 50 years or over, and causes of falls may change with age. Yet, the accuracy of the algorithm did not improve when age of participants at Wave 1 was restricted to 65 years or older (Table 4).
The follow-up time of approximately 2 years differed to the recommendations of the WGFPM [7] of reassessment within a year for all participants. It is likely that this increased follow-up period exacerbated inaccuracies in recollections of falls. Finally, there were significantly more participants missing at Wave 2 from the high- and intermediate-risk groups than the low-risk group. It is possible that these may represent frailer individuals whose absence may have led to a reduced estimation of the incidence of falls between Wave 1 and Wave 2.
Conclusion
In TILDA, the WGFPM risk assessment algorithm successfully differentiated those at greater risk of falling when using the opportunistic case finding method. However, the utility of the intermediate-risk group was unclear and may not provide a clinically useful differentiation with the high-risk group. The high number of participants classified in the low-risk group and lack of differences between the intermediate- and high-risk groups may be related to the non-clinical nature of the TILDA sample. Further study in other samples is warranted, including in relation to the WGFPM-recommended actions and interventions.
Contributor Information
Peter Hartley, Discipline of Medical Gerontology, School of Medicine, Trinity College Dublin, Dublin, Ireland; Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK; Department of Physiotherapy, Cambridge University Hospital NHS Foundation Trust, Cambridge, UK.
Faye Forsyth, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK.
Scott Rowbotham, Department of Physiotherapy, The Queen Elizabeth Hospital King’s Lynn NHS Foundation Trust, King’s Lynn, UK.
Robert Briggs, Discipline of Medical Gerontology, School of Medicine, Trinity College Dublin, Dublin, Ireland; Mercer’s Institute for Successful Ageing, St James’s Hospital, Dublin, Ireland.
Rose Anne Kenny, Discipline of Medical Gerontology, School of Medicine, Trinity College Dublin, Dublin, Ireland; Mercer’s Institute for Successful Ageing, St James’s Hospital, Dublin, Ireland.
Roman Romero-Ortuno, Discipline of Medical Gerontology, School of Medicine, Trinity College Dublin, Dublin, Ireland; Mercer’s Institute for Successful Ageing, St James’s Hospital, Dublin, Ireland; Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland.
Declaration of Conflicts of Interest
R.A.K. was a co-author of the 2022 publication of the World Falls Guidelines (https://doi.org/10.1093/ageing/afac205).
Declaration of Sources of Funding
TILDA is funded by Atlantic Philanthropies, Irish Life and the Irish Department of Health. P.H. is supported by Homerton College and the Health Foundation’s grant to the University of Cambridge for The Healthcare Improvement Studies Institute (THIS Institute). THIS Institute is supported by the Health Foundation, an independent charity committed to bringing about better health and healthcare for people in the UK. F.F. is funded by the Evelyn Trust. R.R.-O. is funded by a grant from Science Foundation Ireland under grant number 18/FRL/6188. The funders had no role in the conduct of the research or preparation of the article, study design, the collection, analysis and interpretation of data, writing of the report or the decision to submit the paper for publication.
Data Availability Statement
TILDA provides access to the datasets for research use through anonymised publicly accessible dataset files, and through an on-site Hot Desk Facility. The publicly accessible dataset files are hosted by the Irish Social Science Data Archive based in University College Dublin, and the Interuniversity Consortium for Political and Social Research (ICPSR) based in the University of Michigan. Researchers wishing to access the data must complete a request form, available on either the ISSDA or ICPSR website.
References
- 1. Step Safely: Strategies for Preventing and Managing Falls across the Life-Course. Geneva: World Health Organization, 2021. [Google Scholar]
- 2. HSE, NCAOP, DOHC . Strategy to Prevent Falls and Fractures in Ireland’s Ageing Population: Report of the National Steering Group on the Prevention of Falls in Older People and the Prevention and Management of Osteoporosis throughout Life. Dublin, Ireland: Health Service Executive, 2008. Available online: https://www.hse.ie/eng/services/publications/olderpeople/strategy-to-prevent-falls-and-fractures-in-irelands-ageing-population---full-report.pdf (accessed 23 October 2022). [Google Scholar]
- 3. Great Britain . Office for Health Improvement and Disparities. Falls: Applying all our Health. London: GOV.UK, 2022. [Google Scholar]
- 4. Veronese N, Custodero C, Demurtas J et al. Comprehensive geriatric assessment in older people: an umbrella review of health outcomes. Age Ageing 2022; 51: afac104. 10.1093/ageing/afac104. [DOI] [PubMed] [Google Scholar]
- 5. Sherrington C, Fairhall NJ, Wallbank GK et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev 2019; 2019: CD012424. 10.1002/14651858.CD012424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meekes WM, Korevaar JC, Leemrijse CJ, van de Goor IA. Practical and validated tool to assess falls risk in the primary care setting: a systematic review. BMJ Open 2021; 11: e045431. 10.1136/bmjopen-2020-045431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montero-Odasso M, van der Velde N, Martin FC et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing 2022; 51: afac205. 10.1093/ageing/afac205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kearney PM, Cronin H, O'Regan C et al. Cohort Profile: The Irish Longitudinal Study on Ageing. Int J Epidemiol 2011; 40: 877–84. [DOI] [PubMed] [Google Scholar]
- 9. Donoghue OA, McGarrigle CA, Foley M, Fagan A, Meaney J, Kenny RA. Cohort Profile Update: The Irish Longitudinal Study on Ageing (TILDA). Int J Epidemiol 2018; 47: 1398l. 10.1093/ije/dyy163. [DOI] [PubMed] [Google Scholar]
- 10. Theou O, Perez-Zepeda MU, van der Valk AM, Searle SD, Howlett SE, Rockwood K. A classification tree to assist with routine scoring of the clinical frailty scale. Age Ageing 2021; 50: 1406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Halloran AM, Hartley P, Moloney D, McGarrigle C, Kenny RA, Romero-Ortuno R. Informing patterns of health and social care utilisation in Irish older people according to the clinical frailty scale. HRB Open Res 2021; 4: 54. 10.12688/hrbopenres.13301.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 13. Bohannon R. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther 2006; 29: 64–8. [DOI] [PubMed] [Google Scholar]
- 14. Savva GM, Donoghue OA, Horgan F, O'Regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci 2013; 68: 441–6. [DOI] [PubMed] [Google Scholar]
- 15. Burns ER, Lee R, Hodge SE, Pineau VJ, Welch B, Zhu M. Validation and comparison of fall screening tools for predicting future falls among older adults. Arch Gerontol Geriatr 2022; 101: 104713. 10.1016/j.archger.2022.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006; 332: 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cattelani L, Palumbo P, Palmerini L et al. FRAT-up, a web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res 2015; 17: e41. 10.2196/jmir.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dormosh N, Schut MC, Heymans MW, van der Velde N, Abu-Hanna A. Development and internal validation of a risk prediction model for falls among older people using primary care electronic health records. J Gerontol A Biol Sci Med Sci 2022; 77: 1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palumbo P, Klenk J, Cattelani L et al. Predictive performance of a fall risk assessment tool for community-dwelling older people (FRAT-up) in 4 European cohorts. J Am Med Dir Assoc 2016; 17: 1106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dormosh N, Heymans MW, van der Velde N et al. External validation of a prediction model for falls in older people based on electronic health records in primary care. J Am Med Dir Assoc 2022; 23: 1691–1697.e3. [DOI] [PubMed] [Google Scholar]
- 21. Gray JA, Patnick J, Blanks RG. Maximising benefit and minimising harm of screening. BMJ 2008; 336: 480–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organisation . WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organisation, 2020. [Google Scholar]
- 23. Anderson H, Stocker R, Russell S et al. Identity construction in the very old: a qualitative narrative study. PloS One 2022; 17: e0279098. 10.1371/journal.pone.0279098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gardiner S, Glogowska M, Stoddart C, Pendlebury S, Lasserson D, Jackson D. Older people’s experiences of falling and perceived risk of falls in the community: a narrative synthesis of qualitative research. Int J Older People Nurs 2017; 12: e12151. 10.1111/opn.12151. [DOI] [PubMed] [Google Scholar]
- 25. Hanson HM, Salmoni AW, Doyle PC. Broadening our understanding: approaching falls as a stigmatizing topic for older adults. Disabil Health J 2009; 2: 36–44. [DOI] [PubMed] [Google Scholar]
- 26. Ellmers TJ, Wilson MR, Norris M, Young WR. Protective or harmful? A qualitative exploration of older people’s perceptions of worries about falling. Age Ageing 2022; 51: afac067. 10.1093/ageing/afac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yardley L, Bishop FL, Beyer N et al. Older people’s views of falls-prevention interventions in six European countries. Gerontologist 2006; 46: 650–60. [DOI] [PubMed] [Google Scholar]
- 28. Dennison RA, Boscott RA, Thomas R et al. A community jury study exploring the public acceptability of using risk stratification to determine eligibility for cancer screening. Health Expect 2022; 25: 1789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartley P, Forsyth F, O'Halloran A, Kenny RA, Romero-Ortuno R. Eight-year longitudinal falls trajectories and associations with modifiable risk factors: evidence from the Irish Longitudinal Study on Ageing (TILDA). Age Ageing 2023; 52. 10.1093/ageing/afad037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donoghue O, Foley M, Kenny RA. Cohort Maintenance Strategies Used by the Irish Longitudinal Study on Ageing (TILDA). Dublin: The Irish Longitudinal Study on Ageing, 2017. Available from: https://tilda.tcd.ie/publications/reports/pdf/Report_CohortMaintenance.pdf. [Google Scholar]
- 31. Hannan MT, Gagnon MM, Aneja J et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston study. Am J Epidemiol 2010; 171: 1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freiberger E, de Vreede P. Falls recall—limitations of the most used inclusion criteria. Eur Rev Aging Phys Act 2011; 8: 105–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
TILDA provides access to the datasets for research use through anonymised publicly accessible dataset files, and through an on-site Hot Desk Facility. The publicly accessible dataset files are hosted by the Irish Social Science Data Archive based in University College Dublin, and the Interuniversity Consortium for Political and Social Research (ICPSR) based in the University of Michigan. Researchers wishing to access the data must complete a request form, available on either the ISSDA or ICPSR website.