Abstract
Over the past two decades, the escalating prescription of opioid medications for pain management has culminated in a widespread opioid epidemic, significantly impacting public health, social dynamics, and economic stability. The urgent need for improved treatments for opioid addiction necessitates a deeper understanding of its biological underpinnings, with genetic variations playing a crucial role in individual susceptibility to opioid use disorder (OUD) and influencing clinical practices.
In this study, we leverage the genetic diversity of four rat strains (ACI/N, BN/NHsd, WKY/N, and F344/N) to examine the contribution of genetic factors to oxycodone metabolism and addiction-like behaviors. We used the extended access to intravenous oxycodone self-administration procedure (12 h/day, 0.15 mg/kg/injection) to comprehensively characterize oxycodone-related behaviors and pharmacokinetics. We measured escalation of oxycodone self-administration, motivation for drug consumption, tolerance to the analgesic effects of oxycodone, withdrawal-induced hyperalgesia, and oxycodone-induced respiratory depression. Additionally, we examined oxycodone-seeking behavior after four weeks of withdrawal by reintroducing the animals to environmental and cue stimuli previously associated with oxycodone self-administration.
The findings revealed notable strain differences in several behavioral measures, including oxycodone metabolism. Intriguingly, BN/NHsd and WKY/N strains exhibited similar drug intake and escalation patterns but displayed significant disparities in oxycodone and oxymorphone metabolism. Minimal sex differences were observed within strains, primarily relating to oxycodone metabolism.
In conclusion, this study identifies strain differences in the behavioral responses and pharmacokinetics associated with oxycodone self-administration in rats, providing a robust foundation for identifying genetic and molecular variants associated with various facets of the opioid addiction process.
Keywords: opioid use disorder, oxycodone, rat, self-administration, sex, strain
1. Introduction
Over the past few decades, the prescription of opioid medications, such as oxycodone (e.g., Oxycontin®, Roxycodone®, Oxectar®), has led to a significant increase in misuse, contributing to a national epidemic with devastating consequences on public health, social, and economic welfare. It is estimated that approximately 10-15% of individuals prescribed opioid painkillers go on to misuse them, resulting in over 2 million Americans annually being diagnosed with a prescription opioid use disorder (SAMHSA, 2022). While opioid medications are effective in treating acute pain and providing relief for some chronic pain patients, they pose a challenge for healthcare providers due to the risk of addiction and other side effects, such as respiratory depression (Moore et al., 2013). A better understanding of the risk factors, including genetic factors, contributing to the increased likelihood of developing a prescription opioid use disorder is crucial.
Opioid use disorder (OUD) is a heterogeneous disorder that varies in presentation and only develops in a subset of individuals using opioids. Animal models with multiple behavioral endpoints can help model various aspects related to OUD in humans (Adinoff et al., 1990; Briand et al., 2008; George et al., 2008; George et al., 2012; Vendruscolo et al., 2012). Our research group has recently begun characterizing individual differences in addiction-like behaviors related to oxycodone and other drugs of abuse in N/NIH Heterogeneous stock (HS) rats (Carrette et al., 2021; Kallupi et al., 2020; Sedighim et al., 2021), an outbred strain created by intercrossing eight inbred rat strains for over 75 generations. The high recombinant nature of HS rats allows for the fine-mapping of genetic loci to precise intervals, significantly reducing the number of potential causative genes within each region (Rat Genome et al., 2013; Valdar et al., 2006). This genetic diversity makes HS rats an ideal resource for studies analogous to human GWASs. Our previous research has demonstrated high individual and cellular variability in response to opioids in the HS rat population (Carrette et al., 2021; Kallupi et al., 2020), with animals classified as high or low addicted based on an Addiction Index computed by merging several behavioral endpoints (escalation of intake, motivation, tolerance, hyperalgesia).
Opioid self-administration and the psychoactive effects of opioids in rodents, similar to OUD in humans, have a heritable component. Inbred strain studies have identified strain differences in various aspects of response to opioids, including baseline heroin self-administration, (Garcia-Lecumberri et al., 2011; Martin et al., 1999; Sanchez-Cardoso et al., 2009), escalation of heroin self-administration (Picetti et al., 2012), preferred opioid dosage (Picetti et al., 2012), acute reactivity to opioid withdrawal (Cobuzzi and Riley, 2011; Kest et al., 2009), antinociceptive effect of opioids (Sudakov et al., 1996), opioid-induced conditioned place preference (Guitart et al., 1992), brain levels of proenkephalin (Martin et al., 1999), and μ opioid receptor regulation (Petruzzi et al., 1997). Furthermore, the founder strains used for HS rats exhibit significant strain differences in the anesthetic and analgesic effects of prescription opioids (Avsaroglu et al., 2007).
In this study, we conducted a comprehensive characterization of addiction-like behaviors in the four available inbred rat strains used to generate the HS rat population (ACI/N, BN/NHsd, F344/N, WKY/N) with the goal to evaluate differences related to the pharmacokinetics and/or metabolism of oxycodone and various oxycodone-related behaviors to better understand genetic differences associated with vulnerability to developing an OUD. Rats self-administered oxycodone for three weeks under extended access conditions, allowing us to measure escalation of drug intake and motivation to obtain the drug using fixed and progressive ratio schedules of reinforcement. We assessed hyperalgesia during acute withdrawal using a mechanical paw withdrawal task and tolerance to the analgesic effects of oxycodone using a tail immersion test. Opioid-induced respiratory depression was evaluated at the beginning and end of the behavioral paradigm using a pulse oximeter. Lastly, we measured oxycodone seeking during protracted withdrawal (4 weeks) by re-exposing the animals to the same environment and cues previously associated with oxycodone self-administration.
2. Materials and Methods
A schematic of the experimental design is represented in Figure 1.
Figure 1.
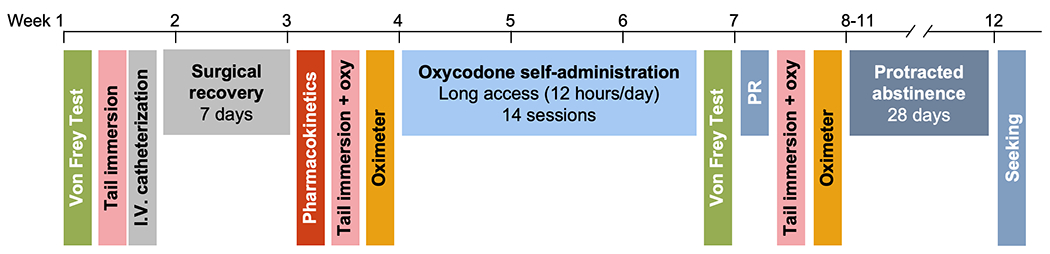
Experimental timeline showing when procedures occurred throughout the course of the experiment.
2.1. Subjects
F344/N (n=12 males, n=10 females), BN/NHsd (n= 8 males, n=10 females), and ACI/N rats (n=8 males, n=9 females) were obtained from Hybrid Rat Diversity Panel at Medical College of Wisconsin (Milwaukee, WI, USA) and WKY/N rats (n= 11 males, n=8 females) were obtained from Charles River. Experiments began when rats were 10-14 weeks old and weighed 140-220g (females) or 250-360g (males). Rats had access to water and standard laboratory chow (PJ Noyes Company, Lancaster, NH, USA) ad libitum in their home cage and in the self-administration chambers. Rats were housed in a temperature- (20-22°C) and humidity-controlled (45-55%) environment on a 12h/12h light cycle, with lights on at 4 pm. All the procedures were performed in accordance with the ARRIVE guidelines (Kilkenny et al., 2010), adhered National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
2.2. Drugs
Oxycodone (Spectrum, New Brunswick, NJ) was dissolved in 0.9% sterile sodium chloride. During self-administration, 0.15 mg/kg was delivered intravenously (i.v.) in 0.1 ml volume per each infusion. For tail immersion, 0.45 mg/kg was delivered i.v. at a volume of 0.3 ml (equivalent of 3 infusions in the self-administration experiment). For oximeter studies, 1 mg/kg was administered i.v. at a volume of 0.1 ml/kg. The doses were selected based on our previous studies (Carrette et al., 2021; Kallupi et al., 2020; Kimbrough et al., 2020).
2.3. Behavioral procedures
2.3.1. Intravenous catheterization
Rats were anesthetized with vaporized isoflurane (2-5%) and intravenous catheters were aseptically inserted into the right jugular vein and the guide cannula was implanted in the mid-scapular region using procedures previously described (de Guglielmo et al., 2017a; de Guglielmo et al., 2017b). Catheters were made of Micro-Renathane tubing (14 cm, 0.023-inch inner diameter, 0.037-inch outer diameter; Braintree Scientific, Braintree, MA, USA) attached to guide cannula (Plastics One, Roanoke, VA, USA) that had been bent at a 90-degree angle. They were then embedded in dental acrylic and anchored with mesh (1 mm thick, 2 cm diameter). Antibiotic (330 mg/kg cefazolin, i.m.) and analgesic (2.5 mg/kg flunixin meglumine, s.c.) were administered immediately following surgery, and analgesic was provided daily for 5 days. Rats were allowed seven days for recovery prior to any self-administration. They were monitored and flushed daily with 0.2 mL of heparinized saline (10 U/ml of heparin sodium; American Pharmaceutical Partners, Schaumberg, IL, USA) in 0.9% bacteriostatic sodium chloride (Hospira, Lake Forest, IL, USA) that contained 262 mg/ml of cefazolin. Catheter patency was evaluated prior to the final self-administration session using methohexital (5 mg/kg), and rats that failed the patency test were excluded from the study.
2.3.2. Operant self-administration
Self-administration was performed in standard operant chambers (Med Associates, St. Albans, VT, USA) that were enclosed in lit, sound-attenuating, ventilated environmental cubicles. Each chamber was equipped with two retractable levers and a cue light above each lever. Drug solutions were delivered through catheter tubing line, which was connected to a swivel on a counterbalance arm, and a syringe pump. Chow and a block of wood (3x3x3cm) were placed in each chamber daily. The experimental sessions commenced with the insertion of both levers into the chamber, accompanied by an intermittent tone (7 kHz, 70 dB) that persisted throughout the session. Responses on the right (active) lever were reinforced on a fixed ratio (FR) 1 schedule, with completion of the FR resulting in the delivery of 0.1 ml of drug solution over a 6 s period and the initiation of a 20 s timeout, signaled by the illumination of the cue light above the lever. Responses on the left (inactive) lever and on the right lever during the timeout were recorded but had no scheduled consequences. Rats underwent 14 long access sessions (12 hours each, five days per week) under the FR1 schedule of reinforcement. The first four sessions were considered acquisition, while the remaining 10 sessions were designated for escalation. Following the 14 FR1 sessions, rats participated in a single progressive ratio (PR) test to measure the motivation of the different strains to earn on infusion of oxycodone. The PR response requirement increased as follows: 1, 1, 2, 2, 3, 3, 4, 4, 5, 5, 6, 6, 7, 7, 8, 8, 9, 9, 10, 10, 11, 12, 13, 14, 15, 16, 17, etc (Kallupi et al., 2020; Schlosburg et al., 2013; Vendruscolo et al., 2012). The same cues (intermittent tone and cue light during the timeout) were present during the PR session, and the session ended after the 60-minute limited hold or 12-hour maximum session duration elapsed, whichever came first.
2.3.3. Oxycodone Seeking Test
After four weeks of withdrawal, during which the rats were left undisturbed in their home cages, they were reintroduced to the operant chamber under the same stimulus conditions (intermittent tone and cue light during the timeout) (Figure 1). However, no drug was delivered contingent upon lever pressing. Drug-paired stimuli presentation occurred under a fixed ratio (FR) 1 schedule with a 20-second timeout on the active lever. Responses on the inactive lever were recorded but had no scheduled consequences.
2.3.4. Mechanical Nociceptive Von-Frey Test
Paw withdrawal in response to a dynamic plantar aesthesiometer (Ugo Basile, Varese, Italy) was tested for mechanical sensitivity a previously reported (Kallupi et al., 2020). The force required and amount of time to paw retraction was recorded for three measures for each hind paw. Measurements were obtained at baseline (before self-administration began), and during acute withdrawal (10-12h after an oxycodone session) (Figure 1). Data are expressed as force (g) for baseline and as percent change of force (g) from each individual baseline.
2.3.5. Tail immersion Test
Time to withdraw tail from a warm water bath was measured at three timepoints: baseline (no oxycodone), before self-administration (with oxycodone) and 2-5 days after the final self-administration session (with oxycodone (Figure 1)). These timepoints were selected to obtain a measure a pain sensitivity, sensitivity to oxycodone, and tolerance to oxycodone, respectively. As previously described (de Guglielmo et al., 2014), rats were restrained in a soft tissue pocket and the distal half of the tail was dipped into a 52°C water bath. The latency to withdrawal the tail from the water bath was measured, and a 10 s cut-off time was implemented to avoid tissue damage. On sessions where oxycodone was administered, 0.45 mg/kg was administered i.v. 15-min before tail immersion test.
2.3.6. Pulse Oximeter
MouseOx Plus (Starr Life Sciences, Oakmont, PA) was used to measure oxygen saturation before and after i.v. administration of oxycodone at baseline and after self-administration (Figure 1). Rats’ necks were shaved before the MouseOx Plus cuff was placed around the neck. Rats were placed into a clean cage and baseline measures of oxygen saturation were taken for 5 min. Then 1 mg/kg oxycodone was administered i.v. and data were recorded for 10 min.
2.4. Pharmacokinetic studies
2.4.1. Collection
The oxycodone pharmacokinetic studies were performed after the implantation of the intravenous catheters (see Figure 1). Baseline blood was collected from the tail prior to i.v. administration of 1 mg/kg oxycodone. Blood was collected after puncturing the tail with a 20-gauge needle into EDTA tubes 5, 15, 30, and 120 minutes after administration. Whole blood (0.1-0.2 ml/timepoint) was spun at 3000G for 10 min to collect plasma, which was frozen at −80 degrees until sample preparation.
2.4.2. Samples preparation
Five μL of internal standard solution at 100 ppb was added to 50 μL of plasma collected from the tail vein, and samples were vortex-mixed for 30 s. 100 μL of ACN were then added and the samples were kept at 4°C for 10 minutes before centrifugation for 8 min at 8000rpm at 4°C. The supernatant was collected and subsequently dried down using the CentriVap. Samples were then reconstituted with 100 μL of water:MeOH (90:10 v/v) and vortexed for 10 s. The samples were then cleaned up using μSPE Oasis HLB Prime μElution plate (Waters Corp, Milford, MA, USA). The activation of the μSPE was carried out with 200 μL of MeOH, conditioning was performed by flushing 200 μL of water three times. 100 μL of samples were then loaded on the SPE μElution plate. Washing and elution were achieved using 200 μL of water, and 100 μL of MeOH respectively. The eluate was collected and dried down through the CentriVap, samples were then reconstituted with 50 μL of water:MeOH (90:10 v/v) and vortexed for 10 s, sonicated in a bath for 5min and then transferred in a vial and placed into the autosampler for the LC-MS/MS analyses.
2.4.3. LC-MS/MS method
The selected compounds were analyzed by a 1200 LC system from Agilent (Agilent Technologies, Santa Clara, CA, USA) equipped with autosampler, vacuum degasser, and column oven. The chromatographic separation was carried out using a Poroshell EC 120 C18 column (2.1x100mm) packed with 2.7 μm particles obtained from Agilent. The mobile phases were as follows: water with 0.1% of Formic acid (phase A) and methanol (phase B). A linear gradient was applied, consisting of an initial isocratic step for 0.5 min at 10% of phase B, then was increased from 10 to 50% in 3 min, at 5min was the mobile phase B was increased up to 95% and hold for 1.5min, the system was then equilibrated for 3 min at the initial conditions. The flow rate of the mobile phase was set at 300 μL/min; the entire flow reached the ion source. The LC column was hold at 40°C. Identification and quantification of the analytes were carried using a triple quadrupole mass spectrometer 6460A QQQ from Agilent (Agilent Technologies, Santa Clara, CA, USA) equipped with a Agilent Jet Stream high-sensitivity ion source operating in positive ionization (PI) for all analytes with the following parameters: gas Temperature 325°C, gas flow 9 L/min, nebulizer pressure 30psi, Sheat gas Temperature 350 °C, Sheat gas flow 11 L/min, capillary voltage 3750 V, Nozzle Voltage 500V. A dynamic multiple reaction monitoring 9dMRM) method was used to observe all the transition of interest. In Supplemental Table 1 precursor ion mass, product ion mass, retention times (RT), fragmentor voltages, collision cell energies (CE), and cell accelerator (CA) voltages are reported for each transition.
2.5. Statistical Analysis
Pharmacokinetic data were analyzed using a two-factor, repeated measures (time) ANOVA, with Tukey’s post hoc analysis, when appropriate. Cmax and AUC comparisons were analyzed using a one-way ANOVA with Tukey’s post-hoc analysis. Other pharmacokinetic measures (C0, Ke, t1/2) were obtained by fitting data (excluding the 0- and 5-minute time periods) to a non-linear function, specifically the one-phase decay function. Self-administration sessions and Z score escalation were analyzed using a two-factor, repeated measures (sessions) ANOVA with Tukey’s post-hoc analysis when appropriate. Mean number of reinforcers for the last three sessions, total reinforcers during acquisition, average z score, progressive ratio number of reinforcers, and mean number of reinstatement responses were analyzed using a one-factor ANOVA with Tukey’s multiple comparisons test, when appropriate. One sample t and Wilcoxon tests were used to determine whether there was a decrease in paw withdrawal latency in the von Frey test during withdrawal, compared to baseline measures. A one-way ANOVA with Tukey’s multiple comparisons was used to compare the effects between strains for the percent of baseline paw withdrawal threshold and the tail withdrawal latency without oxycodone. A two-way repeated measures ANOVA was used to analyze the tail immersion data with oxycodone and oxygen saturation data with Bonferroni post-hoc analysis. Sex differences were analyzed within strain. FR1 self-administration and oxygen saturation data were analyzed with two-factor, repeated measures (session) ANOVA with Bonferroni’s multiple comparisons, when appropriate. The Cmax, mean number of reinforcers, progressive ratio, reinstatement, and von Frey data were analyzed with unpaired t-tests. All data were analyzed using GraphPad Prism 9.
3. Results
3.1. Pharmacokinetics
Plasma levels of oxycodone and three of its metabolites, oxymorphone, noroxycodone, and 6β-oxycodol were measured 5, 15, 30, and 120 minutes after intravenous administration of 1 mg/kg of oxycodone (Figure 2). For oxycodone plasma levels, there was a significant main effect of time F(4,88) =134.8; p<0.0001, strain F(3,88)=3.014; p=0.03 and time x strain interaction F(12,352)=2.363; p=0.006 (Fig 2A). When examining the peak oxycodone levels (Cmax), there was a significant effect of strain (F(3,84)=3.705; p=0.015). Tukey’s post-hoc analysis demonstrated that WKY/N rats had significantly greater oxycodone Cmax compared to BN/NHsd (p=0.014, Fig. 2B). There were significant main effects of time (F(4,84)=47.91; p<0.0001), strain (F(3,84)=3.851; p=0.0124), and an interaction time x strain (F(12,336)=2.167; p=0.013) for oxymorphone levels (Fig 2C). When looking at the oxymorphone Cmax, there was a significant effect of strain (F(3,84=3.46; p=0.020). Tukey’s post-hoc analysis demonstrated that BN/NHsd rats had significantly greater peak plasma levels of oxymorphone compared to F344/N (p=0.017, Fig. 2D). No differences in terms of total plasma levels or Cmax of noroxycodone (F(12,336)=1.631; p=0.081, and F(3,84)=1.13; p=0.34, respectively) (Fig. 2E, F) were detected. For 6β-oxycodol, there were main effects of time (F(4,60)=54.56; p<0.0001), strain (F(3,60)=3.273; p=0.027), and an interaction time x strain (F(12,240)=2.53; p=0.037). The Tukey’s post-hoc analysis demonstrated some strain differences in 6β-oxycodol levels, particularly at the 30 min timepoint (ACI/N vs WKY/N, p = 0.04) and at the 120 min timepoint (F344/N vs BN/NHsd, p = 0.039, Fig. 2E). However, no differences in the Cmax of 6β-oxycodol were detected (F(3,60)=2.32; p=0.084) (Fig. 2F). There was no significant difference in extrapolated concentration at timepoint 0 (C0), area under the curve (AUC), elimination constant (Ke), and half-life (t1/2) between the four strains (Table 1). However, there were significant trends for C0 (F(3,70)=2.40; p=0.075) and AUC (F(3,70)=2.73; p=0.051).
Figure 2.
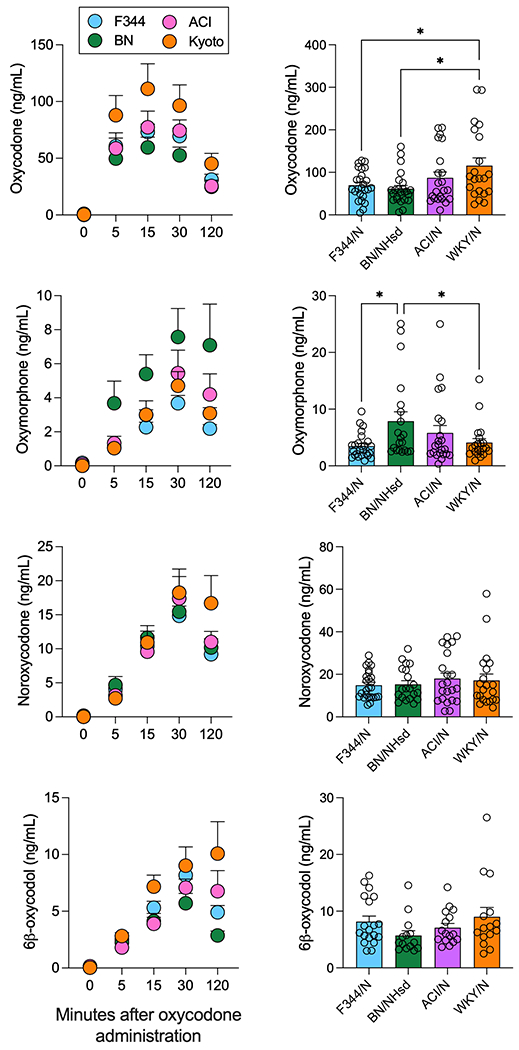
Pharmacokinetics of oxycodone and three of its major metabolites. Plasma levels (expressed in ng/ml) of oxycodone (panels A, B), oxymorphone (panels C, D), noroxycodone (panels E, F), 6β-oxycodol (panels G, H), shown as a time course after 1 mg/kg intravenous oxycodone administration (panels A, C, E, G) or as peak concentration (Cmax) (panels B, D, F, H). Data for the four strains, F344/N (blue), BN/NHsd (green), ACI/N (pink), and WKY/N (orange), are shown as mean ± S.E.M. * indicate post-hoc tests of p < 0.05
Table 1.
Oxycodone pharmacokinetics analysis. The extrapolated concentration at timepoint 0 (C0), area under the curve (AUC), elimination constant (Ke), and half-life (t1/2) are shown for each of the four strains. Data are presented as mean ± S.E.M.
| F344/N | BN/NHsd | ACI | WKY/N | |
|---|---|---|---|---|
| C0 (ng/mL) | 87.2 ± 5.6 | 72.8 ± 10.5 | 97.8 ± 16.7 | 130 ± 25.3 |
| AUC | 6433 ± 636 | 4094 ± 714 | 6462 ± 915.2 | 9149 ± 1695 |
| Ke | 0.0092 ± 0.0010 | 0.0127 ± 0.0014 | 0.0113 ± 0.0011 | 0.0097 ± 0.0012 |
| t1/2 (min) | 83.8 ± 9.3 | 62.1 ± 11.8 | 74.7 ± 10.0 | 66.1 ± 7.0 |
3.2. Self-administration, motivation, and seeking
When analyzing all 14 days of self-administration, there were significant main effects of session (F(13, 72) = 83.16; p<0.0001), strain (F(3, 72) = 4.121; p=0.007) and session x strain interaction (F(39, 936) = 1.432; p=0.043) (Fig 3A). Notably, the Tukey’s post-hoc analysis revealed strain differences in the initial days of self-administration. In addition, a significant effect was observed when analyzing the mean number of reinforcers for the last three FR1 sessions (F(3, 72) = 3.229; p=0.024); however, the post-hoc analysis did not detect strain differences in the number of infusions received (Fig 3B), with only a trend toward significance between F344/N and WKY/N strains (p=0.056). Responding on the inactive lever was low in nearly all rats throughout the study and there was no significant interaction between strain and session day (F(39, 835) = 0.8907; p=0.663, Supplemental Figure 1A). We then conducted an in-depth analysis of the cumulative numbers of rewards obtained during the final self-administration session, focusing on the number of reinforcers earned during each 10-minute bin within the 12-hour session. Our findings reveal that all the rats maintained consistent patterns of intake throughout the entire session, signifying high rates of intake across all strains (Fig 3C).
Figure 3.
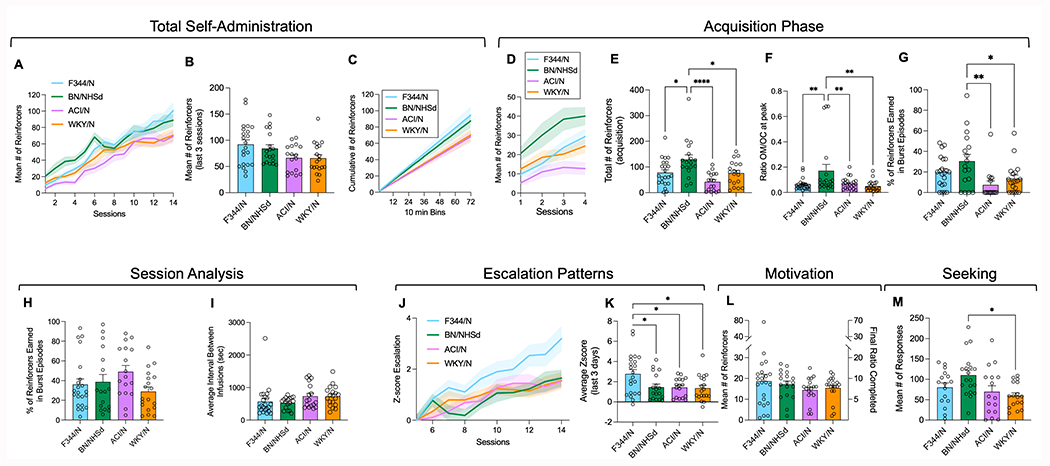
Self-administration of oxycodone. Number of 0.15 mg/kg/injection oxycodone reinforcers earned by F344/N (blue), BN/NHsd (green), ACI/N (pink), and WKY/N (orange) rats across all 14 sessions (panel A), the mean of the last three sessions (panel B) and the cumulative number of reinforcers in the last session (panel C). Number of reinforcers earned across the four acquisition sessions (by session) (panel D), and the total number of reinforcers earned during acquisition (panel E). Ratio of Cmax of oxycodone to Cmax oxymorphone (panel F) and percentage of reinforcers earned during burst episodes in session 4 of the acquisition phase (panel G). Percentage of reinforcers earned during burst episodes (panel H) and average interval between rewards in the last session (panel I). Escalation Z score, beginning with session 5, for each strain of rats (panel J) as well as the average escalation Z score for the last three sessions (panel K). Number of reinforcers earned, and the corresponding final ratio completed during the progressive ratio session, as a measure of motivation (panel L). Number of responses made during the reinstatement test after 4 weeks of protracted withdrawal as a measure of seeking behavior (panel M). Data are shows as mean ± S.E.M. and include individual subjects for the bar graphs. Post hoc analyses indicated significant differences between strains: * p < 0.05; ** p < 0.01; **** p < 0.001
We employed a two-way ANOVA to examine the cumulative number of rewards, which uncovered a significant time x strain interaction (F(213, 4757) = 2.252; p<0.0001). Despite this interaction, the subsequent Tukey’s post-hoc analysis did not reveal any significant strain differences at any of the bins analyzed (Fig 3C).
When focusing on the acquisition phase by examining the first four days of oxycodone self-administration, main effects of session (F(3, 72) = 26.86; p<0.0001) and strain (F(3, 72) = 7.586; p=0.0002) were detected, without a session x strain interaction (Figure 3D). BN/NHsd rats exhibited significantly greater oxycodone intake compared to other strains during this phase F(3, 72) = 7.586; p=0.0002 (Figure 3E, p=0.02 vs F344/N, p<0.0001 vs ACI/N, and p=0.02 vs WKY/N after Tukey’s post hoc). These rats also demonstrated a higher oxymorphone/oxycodone ratio at peak during the pharmacokinetic study (F(3, 72) = 5.108; p=0.002) followed by Tukey’s post hoc (Figure 3F, p<0.01 vs F344/N, ACI/N and WKY/N).
To further explore the relationship between metabolism and self-administration patterns, we examined the rewards obtained during burst episodes, defined as a train of drug infusions that continues as long as there is less than 90 seconds between infusions. The one-way ANOVA demonstrated a significant difference between strains at the end of the acquisition phase (F(3, 72) = 4.663; p=0.005). The subsequent Tukey’s post hoc analysis demonstrated that BN/NHsd rats earned significantly more rewards during burst episodes compared to ACI/N and WKY/N rats (Figure 3G).
Interestingly, no differences were observed at the end of the self-administration phase in terms of the percentage of rewards obtained during burst episodes (F(3, 72) = 1.811; p=0. 59, Fig. 3H), or the average interval between infusions (F(3, 72) = 1.589; p=0. 19, Fig. 3I). These findings suggest that after 14 extended access sessions, the rate of oxycodone intake within a session remains consistent across all strains, regardless of any metabolism differences.
An escalation score was computed by analyzing post-acquisition data of oxycodone self-administration (sessions 5-14, Figures 3J, K). For the escalation score we normalized the number of reinforcers earned during each session to the number of reinforcers earned in session 5 using a Z-score (), where χ is the raw value (number reinforcers earned given session), μ is the mean of reinforcers for each strain during session 5, and σ is the standard deviation for each strain during session 5 (Figure 3J). Analysis of the daily escalation scores showed significant main effects of session (F(3, 72) = 26.96; p<0.0001) and strain (F(3, 72) = 3.611; p=0.0172) as well as session x strain interaction (F(27, 648) = 1.496; p=0.05) (Figure 3F). F344/N rats showed a significantly higher daily escalation score than other strains from session 8 onwards (p <0.05 vs other strains). This finding was further supported by analysis of the total escalation score showing significantly higher values for the F344/N rats versus the other strains (F(3, 72) = 4.398; p=0.0083; p<0.05 vs the other strain after Tukey’s post hoc test, Figure 3K).
In the progressive ratio test, no differences in reinforcers earned between strains were observed (F(3, 72) = 0.7219; p=0.5421, Figure 3L). Inactive lever responding was low and did not differ by strain (F(3, 73) = 2.335; p=0.081) (Supplemental Figure 1B). However, during the seeking test, strain differences in drug-seeking behavior emerged after re-exposure to oxycodone-associated cues F(3, 64) = 3.914; p=0.0125), with BN/NHsd rats responding more than WKY/N (p=0.0107 after Tukey’s post hoc analysis, Figure 3M). Inactive lever responding was relatively low, although there was a main effect of strain (F(3, 60) = 3.260; p=0.028) and Tukey’s post-hoc analysis revealed BN/NHsd rats made more responses than ACI/N rats (p=0.036) (Supplemental Figure 1C).
3.3. Paw withdrawal thresholds and hyperalgesia
Using the von Frey test, we observed significant differences between strains in terms of paw withdrawal threshold at baseline and during acute withdrawal from oxycodone self-administration (Fig 4A). There were significant main effects of strain (F(3, 72) = 11.21; p<0.0001) and time (F(1, 72) = 171.9; p<0.0001), without an interaction between strain and time (F(3, 72) = 1.602; p=0.19).
Figure 4.
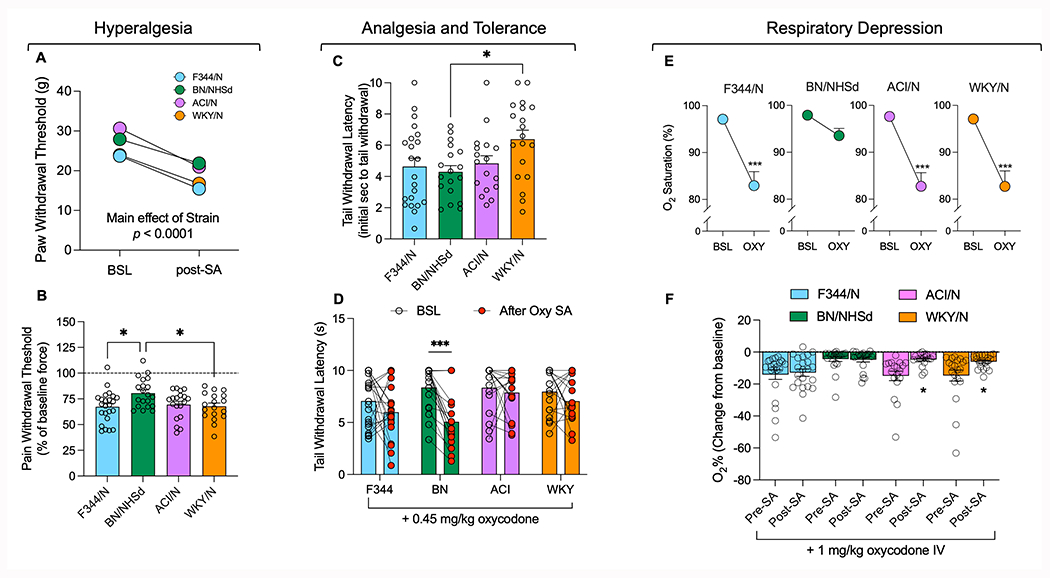
Hyperalgesia, analgesia, tolerance, and respiratory depression. Paw withdrawal threshold in the four strains of rats at baseline (prior to self-administration) and during acute withdrawal (after 14 self-administration sessions), expressed as force (panel A). Paw withdrawal force during acute withdrawal after self-administration is expressed as a function of the baseline paw withdrawal force, as a measure of hyperalgesia (panel B). To measure sensitivity to a noxious stimulus, tail withdrawal latency during the warm water tail immersion test before self-administration and without oxycodone administration is shown in panel C. The tail withdrawal latency after 0.45 mg/kg oxycodone administration both at baseline (before self-administration) and after 14 sessions of oxycodone self-administration are shown as measures of sensitivity to and tolerance to oxycodone, respectively (panel D). Oxygen saturation levels before and after administration of 1 mg/kg oxycodone, taken before self-administration began, as proxies of respiratory depression (panel E). The percent decrease in oxygen saturation after oxycodone administration compared to baseline immediately prior is shown for the oximeter studies conducted before and after 14 self-administration sessions (panel F). Statistical significance between strains is represented as * for p < 0.05 or *** for p < 0.001 in panels B and C. Statistical significance between tests conducted before and after oxycodone self-administration is represented as * for p < 0.05 or *** for p<0.001in panels D and F. Statistically significant differences between oxygen saturation levels before and after acute oxycodone administration is presented as *** for p < 0.001 in panel E.
When data were analyzed as a percentage of reduction versus baseline force (Figure 4B), all rat strains exhibited significant hyperalgesia, with paw withdrawal threshold decreasing during acute withdrawal from oxycodone self-administration compared to baseline levels (F344/N: t(23)=10.55, p=<0.0001; BN/NHsd: t(19)=6.187, p<0.0001; ACI/N: t(18)=9.736, p<0.0001; WKY/N: t(17)=10.36, p<0.0001, Figure 4B). The extent of hyperalgesia varied by strain F(3, 72) = 3.964; p=0.0110, with BN/NHsd rats displaying less withdrawal-induced hyperalgesia (as evidenced by a higher paw withdrawal threshold, Fig 4B) than F344/N (p=0.0141) and WKY/N rats (p=0.0345).
3.4. Analgesia and Tolerance
In the tail immersion test, we measured the latency time of tail withdrawal from a water bath at three timepoints: baseline, after an i.v. injection of 0.45 mg/kg of oxycodone (the equivalent of three reinforcers during self-administration), and at the end of the self-administration phase following an i.v. injection of 0.45 mg/kg of oxycodone (Figure 4C, D). At baseline, we detected strain differences in response to the tail immersion test F(3,72)=3.238; p=0.0271). Tukey’s post hoc test revealed that WKY/N rats had increased tail withdrawal latency compared to BN/NHsd rats (p < 0.05, Figure 4C). We then evaluated the development of tolerance to the analgesic effects of oxycodone in the four strains by repeating the tail immersion test following an injection of 0.45 mg/kg of oxycodone before and after the self-administration phase. A two-way ANOVA showed significant main effects of strain F(3, 72) = 3.094; p=0.032, time F(1, 72) = 15.47; p=0.0002), and strain x time interaction F(3, 72) = 2.941; p=0.039. Tukey’s post hoc test demonstrated that only BN/NHsd rats developed tolerance to the analgesic effects of oxycodone over the course of the self-administration phase (p<0.001 vs pre-SA, Fig 4D).
3.5. Oxycodone-induced respiratory depression
Oxygen saturation was measured before and after a 1 mg/kg oxycodone infusion at two timepoints: before (Figure 4E) and after (Figure 4F) self-administration began. Main effects of time F(1, 72) = 71.19; p<0.0001 and strain F(3, 72) = 4.205; p=0.0083), as well as a time x strain interaction F(3, 72) = 3.196; p=0.0818), were observed before self-administration. Tukey’s post hoc analysis revealed that a 1 mg/kg i.v. oxycodone infusion decreased oxygen saturation in all strains (p<0.001, Fig 4E) except for BN/NHsd.
We then analyzed the results of the same experiment performed after the self-administration phase and compared the data with the baseline responses to oxycodone. A two-way ANOVA showed main effects of time F(1, 72) = 9.57; p=0.0022 and strain F(1, 72) = 5.31; p=0.0028) and a time x strain interaction F(3, 72) = 3.196; p=0.049. Post hoc analysis revealed that only the ACI/N and the WKY/N developed tolerance to the respiratory depression effects of oxycodone (p<0.05 vs Pre-SA, Fig 4F), while the F344/N did not. Finally, the BN/NHsd remained insensitive to the respiratory depression effects of oxycodone.
3.6. Principal component analysis
A Principal Component Analysis (PCA) was conducted to explore the underlying structure of the dataset and identify key factors driving strain and sex differences in oxycodone addiction-like behavior and metabolism in rats. The analysis yielded eigenvalues greater than 1 for the first five principal components, with the first two principal components (PC1 and PC2) accounting for 40.3% of the total variance (Figure 5A). PC1 had an eigenvalue of 2.772 and explained 25.20% of the variance, while PC2 had an eigenvalue of 1.661 and explained 15.10% of the variance (Figure 5A). The PCA results revealed that PC1 was mainly associated with oxycodone intake, escalation, acquisition, progressive ratio, and maximum oxycodone concentration, while PC2 was primarily related to oxycodone metabolism, seeking, tolerance, hyperalgesia, and baseline O2 levels (Figure 5B). The visualization of the scores for each rat on PC1 and PC2 demonstrated some separation between the strains, indicating that the specific combinations of oxycodone-related behaviors and metabolism differed among the strains (Figure 5C).
Figure 5.
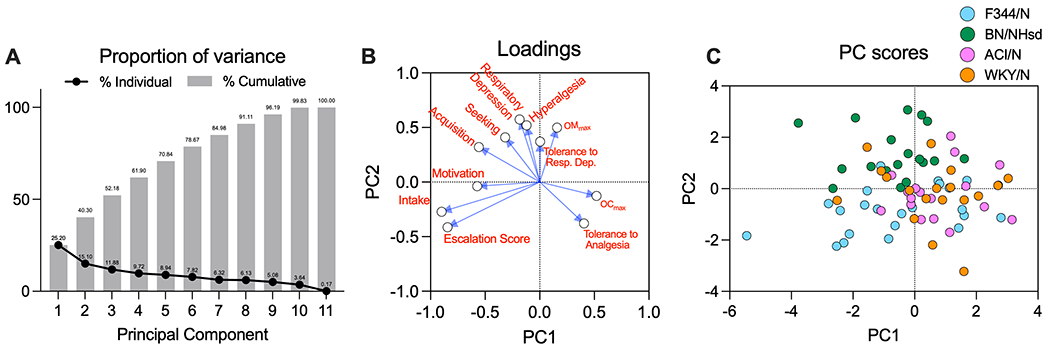
Principal Component Analysis (PCA) of the behavioral and metabolism data. The cumulative proportion of variance is represented as a bar chart. Proportion of variance explained by each principal component (PC) indicated by the line plot (panel A). Loadings plot showing the contribution of each variable to PC1 and PC2. Each variable is represented by a point and labeled accordingly. The closer a variable’s point is to the axes, the stronger its association with the corresponding PC (panel B). Scatter plot of PC scores for individual rats, with each point representing a rat and colored by strain (panel C). The x-axis corresponds to PC1 scores, and the y-axis corresponds to PC2 scores. The plot illustrates the separation between strains in the two-dimensional space defined by PC1 and PC2.
3.7. Sex differences
Sex comparisons were conducted for primary data across various strains, including oxycodone pharmacokinetics, reinforcers earned during fixed ratio 1 (FR1) self-administration sessions, reinforcers earned during progressive ratio tests, responses during reinstatement tests, oxygen saturation in respiratory depression experiments, paw withdrawal threshold as a measure of hyperalgesia, and tail withdrawal latency in tail immersion tests (Figure 6). Sex differences were observed in the pharmacokinetics of oxycodone across all strains (Figures 6A, B, C, D). Significant time x sex interactions for oxycodone plasma levels were found in F344/N F(4, 84) = 2.647; p=0.0389), ACI/N F(4, 80) = 3.526; p=0.010), and WKY/N rats F(4, 76) = 3.329; p=0.0144).
Figure 6.
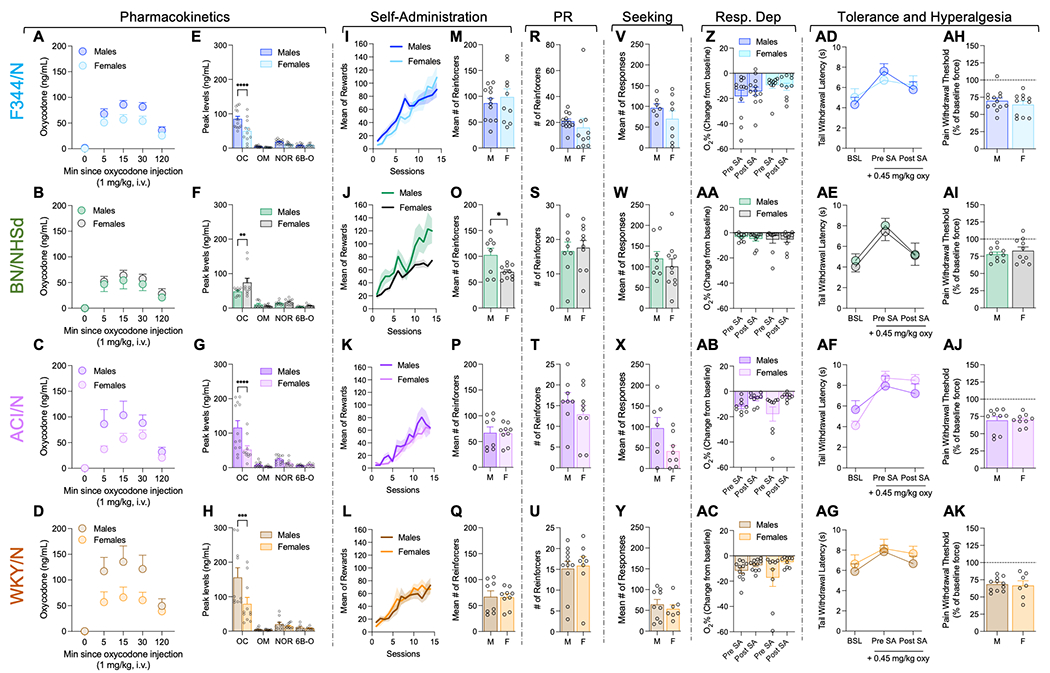
Sex differences in self-administration and other oxycodone-related behaviors, separated by each strain. The pharmacokinetics data show the time course for oxycodone (panels A-D) and Cmax for oxycodone (OC), oxymorphone (OM), noroxycodone (NOR), and 6β-oxycodol (6β-O) (panels E-H) for each strain, separated by sex. The mean number of reinforcers earned across the 14 self-administration sessions (panels I-L) and the mean number of reinforcers earned over the last 3 sessions (panels M-Q). Number of reinforcers earned during the progressive ratio schedule of reinforcement is shown in panels R-U. The number of drug-seeking responses made during the reinstatement tests is shown in panels V-Y Change in oxycodone-induced decrease in oxygen saturation between oximeter tests conducted before and after self-administration are shown in panels Z-AC. The trail withdrawal latency in the warm water tail immersion test is shown for the three tests- one baseline (without oxycodone) and two tests with oxycodone, conducted before self-administration and after the 14 self-administration sessions (panels AD-AG). Finally, the paw withdrawal threshold data from the von Frey test are shown as a percent of baseline force, as a measure of withdrawal-induced hyperalgesia (panels AH-AK). All data are shown as mean ± S.E.M. Statistical significance between males and females is represented as * for p < 0.05, *** for p < 0.001, or **** for p < 0.0001.
Upon examining the peak plasma levels of oxycodone and its metabolites, significant sex differences were detected in all strains for the maximum concentration (Cmax) of oxycodone (Figures 6E, F, G, H). Specifically, male rats exhibited higher peak concentrations of oxycodone in F344/N (significant sex x metabolite interaction F(3,86) = 5.886; p=0.001 followed by post hoc comparison, p<0.001 vs females, Fig 6E), ACI/N (significant sex x metabolite interaction F(3,74) = 5.073; p=0.003 followed by post hoc comparison, p<0.001 vs females, Fig 5G), and WKY/N (significant sex x metabolite interaction F(3,70) = 4.258; p=0.008 followed by post hoc comparison, p<0.001 vs females, Fig 6H). In contrast, female rats displayed higher peak concentrations of oxycodone in BN/NHsd rats (significant interaction sex x metabolite F(3,66) = 2.943; p=0.039 followed by post hoc comparison, p<0.01 vs females, Fig 6F). Further analysis of the pharmacokinetic data found main effects of sex for C0 (F(1,66) = 7.85; p=0.007) and AUC (F(1,66) = 5.34; p=0.025) (Supplemental Table 2). Additionally, there was a significant sex x strain interaction for Ke (F(3,63) = 3.63; p=0.018), and post-hoc analyses revealed WKY/N rats had a slower elimination rate than BN/NHsd rats (p=0.014) (Supplemental Table 1).
Despite the observed sex differences in oxycodone pharmacokinetics, a sex difference in oxycodone intake was only noted in the BN/NHsd strain (Fig 6J, O), without sex-related differences in oxycodone self-administration detected in the other strains (Fig. 6I, K, L, M, P, Q). Analysis of self-administration data in the BN/NHsd strain revealed a significant session x strain interaction F(13,208)=2.514; p = 0.003 (Fig 6J). A t-test of the average oxycodone infusions during the last three days demonstrated that male BN/NHsd rats self-administered significantly more oxycodone compared to females t(16) = 2.613; p < 0.05 (Fig 6O).
No sex differences were detected in other behavioral measures tested, including motivation (progressive ratio, Figures 6R, S, T, U), oxycodone-seeking (Figures 6V, W, X, Y), oxycodone-induced respiratory depression (oxygen saturation, Figures 6Z, AA, AB, AC), tolerance (tail immersion test, Figures 6AD, AE, AF, AG), and withdrawal-induced hyperalgesia (mechanical sensitivity in von Frey test, Figures 6AH, AI, AJ, AK).
4. Discussion
In our recent work with outbred animals, we have shown that HS rats exhibit substantial individual differences in key addiction-related behavioral measures, including escalation of intake, progressive ratio responding (motivation), development of tolerance to the analgesic effects of opioids, and development of hyperalgesia during withdrawal (Carrette et al., 2021). These differences correlate with variations at the cellular (Kallupi et al., 2020), molecular (Carrette et al., 2022), and behavioral levels (Sedighim et al., 2021). The primary focus of the present study was to identify the inbred strains that contribute to some of the behavioral phenotypes observed in HS rats by performing a comprehensive analysis of oxycodone metabolism and oxycodone addiction-like behaviors in four of the founder strains of the HS population (F344/N, BN/NHsd, ACI/N, and WKY/N). We evaluated strain and sex differences in the pharmacokinetics of oxycodone, oxycodone self-administration and escalation, motivation to obtain oxycodone, oxycodone seeking, withdrawal-induced hyperalgesia, sensitivity or tolerance to oxycodone, and oxycodone-induced respiratory depression.
The pharmacokinetic results highlighted significant strain differences in the maximum plasma oxycodone concentration, with WKY/N rats attaining notably higher levels compared to other strains (Fig 2A, B). In contrast, BN/NHsd rats exhibited a lower concentration of plasma oxycodone, but higher concentrations of plasma oxymorphone following the injection of 1 mg/kg of oxycodone i.v. (Fig 2C, D). A recent study (Babalonis et al., 2021) reported that oxymorphone is 12.5-14 times more potent than oxycodone in behavioral measures associated with drug liking, which may account for the strain differences observed during the acquisition phase of our self-administration study. Specifically, BN/NHsd rats demonstrated a faster acquisition rate in comparison to other strains (Fig 3D, E), which could be linked to their higher oxymorphone/oxycodone plasma level ratio relative to the other strains (Fig 3F). This finding implies that the concentration of oxymorphone, metabolically derived from oxycodone, may play a role in the initial rewarding effects of i.v. oxycodone. Furthermore, BN/NHsd rats earned significantly more rewards during burst episodes compared to ACI and WKY rats during the acquisition phase (Fig 3G). This disparity in burst episode rewards could be ascribed to the unique pharmacokinetics observed in BN/NHsd rats. Two possible explanations for this phenomenon include: 1) the lower levels of oxycodone in BN/NHsd rats’ blood may result in a diminished sensation of the drug’s effects, thereby encouraging more burst episodes; or 2) the elevated levels of oxymorphone in their blood may render oxycodone more rewarding for BN/NHsd rats, leading to an increase in burst episodes.
It is worth noting that the curves obtained in our experiments display an absorption period, which is uncommon for i.v. injections. This deviation from the classical first-order kinetic decrease in plasma concentrations warrants further consideration of certain factors that might have contributed to the observed absorption period in our study. One potential factor is the difference in administration and collection sites, with i.v. administration through the jugular vein and blood collection from the tail. This discrepancy might have resulted in a brief delay in the appearance of oxycodone in the tail blood samples due to the time required for the drug to distribute throughout the systemic circulation. Additionally, the puncture method used for blood collection might have caused localized vasoconstriction or altered blood flow, potentially affecting the drug’s distribution to the tail and leading to the observed absorption period. Another possible factor is the presence of residual oxycodone in the catheter tube after administration, causing a slower diffusion of the drug into the bloodstream. This residual oxycodone might have contributed to the observed absorption period in our study. In future studies, we will consider implementing additional controls to minimize the residual volume in the catheter and ensure complete delivery of the drug, such as flushing the catheter with saline following drug administration.
Interestingly, the differences in oxycodone metabolism did not impact the total amount of oxycodone consumed over the self-administration phase, as indicated by the absence of strain differences in oxycodone intake during the last three days of the protocol (Fig 3A, B, C). Moreover, these differences did not affect the motivation to obtain oxycodone (Fig. 3L). However, we detected strain differences in the escalation score, with F344/N rats showing a steeper slope in their escalation curve compared to other strains (Fig. 3J, K). This increased vulnerability to escalation could be related to previous data demonstrating prominent opioid-induced activation of CDK5, a regulator of striatal dopaminergic signaling involved in the behavioral plasticity underlying addiction to various abused drugs (Goulding et al., 2019; Nestler et al., 2001), in F344/N rats compared to other strains (Salas et al., 2013).
We also detected strain differences when the rats were re-exposed to the cues previously associated with oxycodone after 4 weeks of withdrawal. In this oxycodone seeking test, the BN/NHsd rats showed higher oxycodone seeking behavior than the other three strains, as demonstrated by the significantly higher number of responses on the oxycodone-associated lever (Fig 3M).
At the end of the extended access of oxycodone self-administration phase all four strains tested developed opioid withdrawal-induced hyperalgesia, as demonstrated by the lower paw withdrawal threshold compared to their baseline pre-self-administration (Fig 4B). The severity of hyperalgesia was lower for the BN/NHsd. This is in contrast with the results of the tolerance study with the tail immersion test (Fig 4D), which demonstrated that the BN/NHsd was the only strain that developed tolerance to the analgesic effects of oxycodone.
Lastly, we performed a longitudinal assessment of the respiratory depression effects of oxycodone in the four strains by injecting a bolus dose of oxycodone i.v. before and after the self-administration phase and recorded the changes in oxygen saturation. At baseline, all the strains except for BN/NHsd showed respiratory depression after the oxycodone injection (Figure 4E). After a history of extended access of oxycodone self-administration, ACI/N and WKY/N developed tolerance to the oxycodone-induced reduction of oxygen saturation while the F344/N did not and the BN/NHsd remained insensitive to it (Fig 4F).
We also conducted a Principal Component Analysis (PCA) to explore the underlying structure of the dataset and identify key factors driving strain and sex differences in oxycodone addiction-like behavior and metabolism in rats. Our findings revealed complex relationships between various aspects of opioid response, including metabolism, intake, hyperalgesia, respiratory depression, and tolerance. The relationship between the concentrations of oxycodone and its metabolite was complex, with positive and inverse associations observed across the principal components. The positive correlation between peak oxycodone (OCmax) and oxymorphone (OMmax) levels along PC1 is consistent with the expected relationship between a drug and its metabolite, as higher concentrations of oxycodone would typically result in higher levels of its metabolite, oxymorphone. However, the inverse relationship along PC2 highlights the potential influence of other factors on this association that will require further investigation. Our analysis also revealed similarities in the loadings of oxycodone-induced respiratory depression and oxycodone-induced hyperalgesia, suggesting that these variables share a similar pattern and tend to vary together across the rats in the dataset. This might indicate that these two variables share common underlying biological mechanisms or pathways that influence their relationship with the other variables in the dataset. Furthermore, the PCA results showed that tolerance to analgesic effects and tolerance to respiratory depression have loadings in opposite directions on the principal components, suggesting that these two aspects of opioid tolerance may not exhibit the same pattern of variation across the rats in the dataset, thus might be influenced by different factors or mechanisms. It is essential to recognize that tolerance to various effects of opioids may not develop uniformly, and the relationship between different aspects of tolerance can be complex (Altarifi and Negus, 2015; Cecchi et al., 2008; Grecksch et al., 2006; Solomon et al., 1987). Future research should continue to explore the factors contributing to the development of tolerance to different opioid effects, as well as potential therapeutic strategies that could help mitigate the risks associated with opioid use while preserving their analgesic benefits.
While our study has highlighted the pharmacokinetic differences between strains and their influence on addiction-like behaviors, it is also essential to consider the potential role of neural circuit mechanisms in contributing to the observed behavioral phenomena. The mesolimbic dopamine system, which includes dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), is known to play a crucial role in mediating the rewarding effects of drugs of abuse, including opioids (Di Chiara and Imperato, 1988; Koob and Volkow, 2010). Strain differences in this system have been identified, such as variations in amphetamine-stimulated locomotor activity, dopamine synthesis, and dopamine release in the nucleus accumbens (Bowling et al., 1993), as well as differences in the density of dopamine receptors and transporters in the NAc and VTA (Kosten and Ambrosio, 2002), and could potentially influence the rewarding effects of oxycodone and the propensity to develop addiction-like behaviors. Additionally, differences in the responsiveness of dopaminergic neurons to opioids have been observed (Cadoni and Di Chiara, 2000).
Other neural circuits and neurotransmitter systems may also contribute to the strain differences observed in our study. The endogenous opioid system, which includes mu, delta, and kappa opioid receptors and their endogenous ligands, has been implicated in mediating various aspects of opioid addiction, including tolerance, dependence, and withdrawal (Kieffer and Evans, 2009). Strain differences in the expression and function of these opioid receptors could potentially influence the behavioral response to oxycodone (Belknap et al., 1993). Moreover, the glutamatergic and GABAergic systems within the corticostriatal circuitry have also been implicated in the development and expression of opioid addiction-like behaviors (Kalivas and Volkow, 2005). Strain differences in the expression and function of glutamate and GABA receptors, as well as their interactions with the dopaminergic and opioid systems, could contribute to the observed variations in oxycodone addiction-like behaviors (Gass et al., 2009).
The serotonergic system has also been implicated in the modulation of opioid addiction-like behaviors. Serotonin (5-HT) can influence the rewarding effects of opioids, as well as modulate opioid-induced tolerance, dependence, and withdrawal (Berrocoso and Mico, 2009). Strain differences in the expression and function of 5-HT receptors and transporters in brain regions involved in opioid addiction could contribute to the observed variations in oxycodone addiction-like behaviors (Kosten et al., 2007).
Another important factor to consider is the role of stress and the hypothalamic-pituitary-adrenal (HPA) axis in opioid addiction. Strain differences in HPA axis function and stress responsiveness have been reported (Kabbaj et al., 2000), and these differences could potentially influence vulnerability to opioid addiction. For example, alterations in corticotropin-releasing factor (CRF) signaling within the extended amygdala have been implicated in the development of opioid dependence and withdrawal (Koob, 2008). Strain differences in CRF receptor expression and function could contribute to the observed variations in oxycodone addiction-like behaviors (Stevenson et al., 2009).
In summary, a comprehensive understanding of the factors driving strain differences in oxycodone addiction-like behaviors requires considering the complex interplay between pharmacokinetic factors and various neural circuit mechanisms. Future studies should aim to elucidate the interactions between these factors and identify potential therapeutic targets for the treatment of opioid use disorders.
We then assessed sex differences in all the measures tested within each strain of rats. Major sex differences were found in the pharmacokinetics of oxycodone with females showing higher peaks of oxycodone plasma levels than males in the F344/N, ACI/N and WKY/N strains. Interestingly, the opposite was observed in the BN/NHsd strain, with males showing higher peak concentrations of oxycodone plasma levels than females, consistent with previous data in Sprague Dawley rats (Chan et al., 2008). No sex differences were observed in the peak concentrations of the other metabolites. Higher plasma levels of oxycodone in females did not result in higher self-administration rates. However, in the BN/NHsd strain, the males that had higher plasma levels of oxycodone showed significantly higher self-administration rates compared to females.
The literature is inconsistent with regard to sex differences in oxycodone self-administration. Some studies suggest male and female Sprague Dawley rats self-administered similar amounts under extended access procedures (Guha et al., 2022), although some show female Sprague Dawley or Wistar rats self-administer more oxycodone (Kimbrough et al., 2020; Mavrikaki et al., 2017), and we have reported male heterogeneous stock rats self-administering more than females (de Guglielmo et al., 2019). Together, these results and ours suggest there may be interactions between pharmacokinetics, sex, and strain that play a role in the rates of oxycodone self-administration. No other significant sex differences were observed in the rest of the behavioral measures tested, except for a non-significant trend (p=0.06) of higher oxycodone seeking in male ACI/N rats compared to females, which is in contrast with previous literature in Sprague Dawley rats that showed higher relapse-related effects in females (Guha et al., 2022).
Overall, these findings offer a foundation for the identification of genetic and molecular variants that underlie different aspects of the opioid addiction process, highlight the complexity of the relationships between various aspects of opioid response and underscore the importance of studying multiple behavioral endpoints in response to drug administration, as sensitivity in one assay may not transfer to other assays. A better understanding of the factors driving strain and sex differences in oxycodone addiction-like behavior and metabolism can help inform the development of more targeted and effective interventions for opioid use disorders.
Supplementary Material
5. Funding and Disclosure:
This work was supported by the National Institute on Drug Abuse [DA051972 and DA056602 to FT, CB and GdG, DA044451 to OG and DA053443 to RM-F], by the National Institute on Alcohol Abuse and Alcoholism [T32 AA007456 to MRD, AA026999, AA028549 and AA006420 to RM-F] by the Brain & Behavior Research Foundation [2020 NARSAD Young Investigator Award to GdG] and from the Preclinical Addiction Research Consortium (PARC) at the University of California San Diego. The authors declare no competing interests.
7. References
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW, 1990. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry 47, 325–330. [DOI] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS, 2015. Differential tolerance to morphine antinociception in assays of pain-stimulated vs. pain-depressed behavior in rats. Eur J Pharmacol 748, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avsaroglu H, van der Sar AS, van Lith HA, van Zutphen LF, Hellebrekers LJ, 2007. Differences in response to anaesthetics and analgesics between inbred rat strains. Lab Anim 41, 337–344. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Comer SD, Jones JD, Nuzzo P, Lofwall MR, Manubay J, Hatton KW, Whittington RA, Walsh SL, 2021. Relative potency of intravenous oxymorphone compared to other micro opioid agonists in humans - pilot study outcomes. Psychopharmacology (Berl) 238, 2503–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Riggan J, O’Toole LA, 1993. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 112, 352–358. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA, 2009. Cooperative opioid and serotonergic mechanisms generate superior antidepressant-like effects in a mice model of depression. Int J Neuropsychopharmacol 12, 1033–1044. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT, 1993. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32, 885–893. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Seeman P, Robinson TE, 2008. Cocaine self-administration produces a persistent increase in dopamine D2 High receptors. Eur Neuropsychopharmacol 18, 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G, 2000. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur J Pharmacol 387, R23–25. [DOI] [PubMed] [Google Scholar]
- Carrette LL, de Guglielmo G, Kallupi M, Maturin L, Brennan M, Boomhower B, Conlisk D, Sedighim S, Tieu L, Fannon MJ, Velarde N, Kononoff J, Kimbrough A, Simpson S, Smith LC, Shankar K, Ramirez F Jr., Chitre AS, Lin B, Polesskaya O, Solberg Woods LC, Palmer AA, George O, 2021. The cocaine and oxycodone biobanks, two repositories from genetically diverse and behaviorally characterized rats for the study of addiction. eNeuro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrette LLG, Corral C, Boomhower B, Brennan M, Crook C, Ortez C, Shankar K, Simpson S, Maturin L, Solberg Woods LC, Palmer AA, de Guglielmo G, George O, 2022. Leptin Protects Against the Development and Expression of Cocaine Addiction-Like Behavior in Heterogeneous Stock Rats. Front Behav Neurosci 16, 832899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H, 2008. Differential responses to morphine-induced analgesia in the tail-flick test. Behav Brain Res 194, 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD, Smith MT, 2008. Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol 35, 295–302. [DOI] [PubMed] [Google Scholar]
- Cobuzzi JL, Riley AL, 2011. Spontaneous withdrawal in opiate-dependent Fischer 344, Lewis and Sprague-Dawley rats. Pharmacol Biochem Behav 98, 28–34. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G, Ciccocioppo R, 2017a. Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology (Berl) 234, 223–234. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Stopponi S, Demopulos G, Gaitanaris G, Ciccocioppo R, 2014. Analgesic tolerance to morphine is regulated by PPARgamma. Br J Pharmacol 171, 5407–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O, 2019. Dopamine D(3) Receptor Antagonism Reverses the Escalation of Oxycodone Self-administration and Decreases Withdrawal-Induced Hyperalgesia and Irritability-Like Behavior in Oxycodone-Dependent Heterogeneous Stock Rats. Front Behav Neurosci 13, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Matzeu A, Kononoff J, Mattioni J, Martin-Fardon R, George O, 2017b. Cebranopadol Blocks the Escalation of Cocaine Intake and Conditioned Reinstatement of Cocaine Seeking in Rats. J Pharmacol Exp Ther 362, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A, 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Torres I, Martin S, Crespo JA, Miguens M, Nicanor C, Higuera-Matas A, Ambrosio E, 2011. Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol 25, 783–791. [DOI] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF, 2009. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 34, 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF, 2008. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33, 2474–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF, 2012. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A 109, 18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, de Guglielmo G, Carrette LLG, George O, Contet C, 2019. Systemic Administration of the Cyclin-Dependent Kinase Inhibitor (S)-CR8 Selectively Reduces Escalated Ethanol Intake in Dependent Rats. Alcohol Clin Exp Res 43, 2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecksch G, Bartzsch K, Widera A, Becker A, Hollt V, Koch T, 2006. Development of tolerance and sensitization to different opioid agonists in rats. Psychopharmacology (Berl) 186, 177–184. [DOI] [PubMed] [Google Scholar]
- Guha SK, Alonso-Caraballo Y, Driscoll GS, Babb JA, Neal M, Constantino NJ, Lintz T, Kinard E, Chartoff EH, 2022. Ranking the contribution of behavioral measures comprising oxycodone self-administration to reinstatement of drug-seeking in male and female rats. Front Behav Neurosci 16, 1035350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ, 1992. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse 12, 242–253. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H, 2000. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci 20, 6983–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND, 2005. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Carrette LLG, Kononoff J, Solberg Woods LC, Palmer AA, Schweitzer P, George O, de Guglielmo G, 2020. Nociceptin attenuates the escalation of oxycodone self-administration by normalizing CeA-GABA transmission in highly addicted rats. Proc Natl Acad Sci U S A 117, 2140–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Smith SB, Schorscher-Petcu A, Austin JS, Ritchie J, Klein G, Rossi GC, Fortin A, Mogil JS, 2009. Gnao1 (G alphaO protein) is a likely genetic contributor to variation in physical dependence on opioids in mice. Neuroscience 162, 1255–1264. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Evans CJ, 2009. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology 56 Suppl 1, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8, e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kononoff J, Simpson S, Kallupi M, Sedighim S, Palomino K, Conlisk D, Momper JD, de Guglielmo G, George O, 2020. Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacology (Berl) 237, 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2008. A role for brain stress systems in addiction. Neuron 59, 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E, 2002. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology 27, 35–69. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Haile CN, 2007. Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behav Neurosci 121, 380–388. [DOI] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E, 1999. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res 821, 350–355. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E, 2017. Oxycodone self-administration in male and female rats. Psychopharmacology (Berl) 234, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A, Derry S, Eccleston C, Kalso E, 2013. Expect analgesic failure; pursue analgesic success. Bmj 346, f2690. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW, 2001. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A 98, 11042–11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzi R, Ferraro TN, Kurschner VC, Golden GT, Berrettini WH, 1997. The effects of repeated morphine exposure on mu opioid receptor number and affinity in C57BL/6J and DBA/2J mice. Life Sci 61, 2057–2064. [DOI] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ, 2012. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology (Berl) 220, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rat Genome S, Mapping C, Baud A, Hermsen R, Guryev V, Stridh P, Graham D, McBride MW, Foroud T, Calderari S, Diez M, Ockinger J, Beyeen AD, Gillett A, Abdelmagid N, Guerreiro-Cacais AO, Jagodic M, Tuncel J, Norin U, Beattie E, Huynh N, Miller WH, Koller DL, Alam I, Falak S, Osborne-Pellegrin M, Martinez-Membrives E, Canete T, Blazquez G, Vicens-Costa E, Mont-Cardona C, Diaz-Moran S, Tobena A, Hummel O, Zelenika D, Saar K, Patone G, Bauerfeind A, Bihoreau MT, Heinig M, Lee YA, Rintisch C, Schulz H, Wheeler DA, Worley KC, Muzny DM, Gibbs RA, Lathrop M, Lansu N, Toonen P, Ruzius FP, de Bruijn E, Hauser H, Adams DJ, Keane T, Atanur SS, Aitman TJ, Flicek P, Malinauskas T, Jones EY, Ekman D, Lopez-Aumatell R, Dominiczak AF, Johannesson M, Holmdahl R, Olsson T, Gauguier D, Hubner N, Fernandez-Teruel A, Cuppen E, Mott R, Flint J, 2013. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat Genet 45, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas E, Alonso E, Polanco MJ, Cano MV, Ruiz-Gayo M, Alguacil LF, 2013. Differential regulation of CDK5 and c-Fos expression by morphine in the brain of Lewis and Fischer 344 rat strains. Neuroscience 230, 151–156. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, Miguens M, Del Olmo N, Garcia-Lecumberri C, Ambrosio E, 2009. Strain differences between Lewis and Fischer 344 rats in the modulation of dopaminergic receptors after morphine self-administration and during extinction. Neuropharmacology 57, 8–17. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Whitfield TW Jr., Park PE, Crawford EF, George O, Vendruscolo LF, Koob GF, 2013. Long-term antagonism of kappa opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci 33, 19384–19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedighim S, Carrette LLG, Venniro M, Shaham Y, de Guglielmo G, George O, 2021. Individual differences in addiction-like behaviors and choice between cocaine versus food in Heterogeneous Stock rats. Psychopharmacology 238, 3423–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RE, Wasserman EA, Gebhart GF, 1987. Tolerance to antinociceptive effects of morphine without tolerance to its effects on schedule-controlled behavior. Psychopharmacology (Berl) 92, 327–333. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW, 2009. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology 34, 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakov SK, Borisova EV, Lyupina YV, 1996. Influence of inheritance and fostering on sensitivity to effects of morphine on nociception and locomotor activity in two inbred rat strains. Neuropharmacology 35, 1131–1134. [DOI] [PubMed] [Google Scholar]
- Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, Taylor MS, Rawlins JN, Mott R, Flint J, 2006. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet 38, 879–887. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32, 7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


