Abstract
OBJECTIVE
Meningioma is the most common primary intracranial neoplasm. Only 1%–3% of meningiomas are malignant according to the 2016 WHO criteria (WHO grade III). High-grade meningiomas present specific gene expression signatures indicating aggressive growth or recurrence. However, changes in gene expression and in neuroinflammatory gene expression signatures in WHO grade III meningiomas and during progression from WHO grade I or II to grade III are unknown.
METHODS
The authors used a NanoString targeted gene expression panel with focus on 787 genes relevant in meningioma pathology and neuroinflammatory pathways to investigate patients with grade III meningiomas treated at Rigshospitalet from 2000 to 2020 (n = 51). A temporal dimension was added to the investigation by including samples from patients’ earlier grade I and II meningiomas and grade III recurrences (n = 139 meningiomas). The authors investigated changes in neuroinflammatory gene expression signatures in 1) grade I meningiomas that later transformed into grade III meningiomas, and 2) grade III meningiomas compared with nonrecurrent grade I meningiomas.
RESULTS
The authors’ data indicate that FOXM1, TOP2A, BIRC5, and MYBL2 were enriched and the HOTAIR regulatory pathway was enriched in grade III meningiomas compared with nonrecurrent grade I meningiomas. They discovered a separation of malignant and benign meningiomas based only on genes involved in microglia regulation with enrichment of P2RY12 in grade I compared with grade III meningiomas. Interestingly, FOXM1 was upregulated in premalignant grade I meningioma years before the grade III transformation.
CONCLUSIONS
The authors found gene expression changes in low-grade meningiomas that predated histological transformation to grade III meningiomas. Neuroinflammation genes distinguished grade III from grade I meningiomas.
Keywords: malignant meningioma, gene expression, neuroinflammation, oncology
MENINGIOMAS are the most common primary intracranial neoplasms, accounting for 37% of all primary intracranial neoplasms.1 Classification is based on histopathological characteristics according to the 2016 WHO Classification of Tumors of the Central Nervous System.2 The recently published 2021 WHO classification3 introduces the TERT promoter mutation4,5 and CDKN2A/B homozygous deletion6 as additional, independent criteria for a grade 3 diagnosis. The majority of meningiomas (approximately 80%) are slow-growing, indolent grade I meningiomas, but a subset of patients (1%–3%) either present with de novo grade III meningioma (patients with a primary malignant meningioma) or develop a grade III meningioma after a previous grade I or II meningioma (patients with a secondary malignant meningioma).7 Grade I meningiomas may thereby be completely benign and remain benign during follow-up (herein defined as benign grade I) or recur and undergo transformation to grade II and then grade III meningioma or directly to grade III (herein defined as premalignant grade I). Despite the high morbidity and mortality following a grade III meningioma diagnosis, attempts to develop targeted, personalized adjuvant treatment options are hindered because of limited knowledge of the phenotypes, underlying molecular mechanisms, and the rarity of this disease.
Gene expression profiling has defined molecular subgroups. The most aggressive subtype was characterized by loss of the DREAM complex, a master regulator of the cell cycle.8 Surprisingly, driver mutations or alterations did not differentiate subgroups of anaplastic meningiomas, while differential gene expression did.9 Targeted gene expression analyses have suggested a 36-gene signature that is associated with risk of recurrence among all meningiomas.10 However, several key comparisons within grade III meningiomas remain to be explored with novel therapeutic development in sight.
There is limited knowledge in transcriptional changes across multiple recurrences, particularly in premalignant grade I or II meningiomas. Moreover, the neuroinflammatory profile in meningiomas and tumor-associated macrophages (TAMs) has shown relevance only recently.11,12 TAM infiltration is decreased in aggressive meningiomas characterized as molecular subgroups with specific features.13 Moreover, inhibition of immunosuppressive myeloid cells attenuates meningioma growth14 and aggressive meningiomas have upregulated anti-inflammatory TAMs.12,15 In this context, investigation of neuroinflammation in contrasting meningioma phenotypes is warranted. We analyzed the gene expression profile in meningioma tissue from 51 patients with grade III meningiomas treated at Rigshospitalet between 2000 and 2020, including previous benign meningiomas and later grade III recurrences. The patients were matched to 51 patients with benign grade I meningiomas known to have no recurrences despite long-term follow-up (Fig. 1).
FIG. 1.
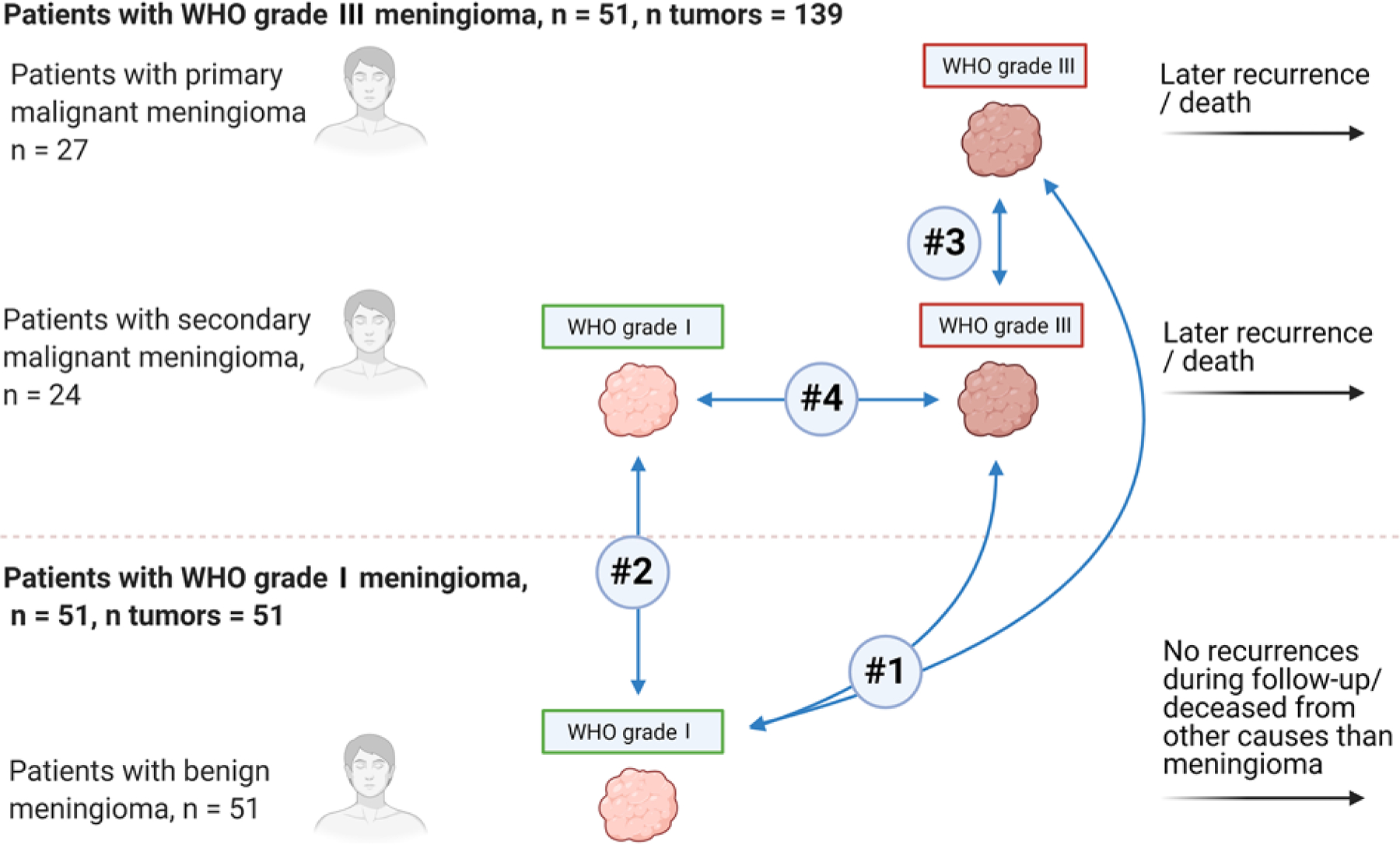
Overview of patients and meningiomas and the four pre hoc planned comparisons. Analysis 1 compares the first (earliest presenting) grade III meningiomas (n = 51) with benign grade I meningiomas from controls (n = 51). Analysis 2 compares the first premalignant grade I meningioma (n = 9) with benign grade I meningioma from controls (n = 51). Analysis 3 comprises the first grade III meningioma from patients with primary grade III meningioma (n = 27) compared with the first grade III meningiomas from patients with secondary malignant meningiomas (n = 24). Analysis 4 is within patients with secondary grade III meningioma: the last premalignant tumor (grade I or II) compared with the first grade III meningioma within individual patients.
We present a targeted gene expression analysis focusing on key neuroinflammatory pathways and genes previously suggested to be involved in meningioma biology to study 1) if premalignant grade I meningiomas differ from benign grade I meningiomas that do not grade-transform or recur, 2) if neuroinflammation profiles are different between grade I and III tumors, and 3) if previously suggested molecular features of aggressive growth and recurrence can be validated.
Methods
Patient Population and Sample Selection
The background population included all patients treated surgically for meningioma at the Copenhagen University Hospital between March 2000 and December 2020. Fifty-one patients with grade III meningiomas were identified in the pathology database by the SNOMED code M95303. Because of recurrences, patients underwent 1–10 surgeries, and all surgical samples were identified using the Danish social security number system (n = 151). We previously published an in-depth, granular clinical analysis of the cohort.16 We retrieved paraffin-embedded samples from 139 (92%) of the 151 surgeries. Patients with secondary grade III meningiomas had undergone previous surgery for grade I and/or grade II meningiomas that later recurred as grade III. We defined grade I and II meningiomas that later progressed to grade III as “premalignant meningiomas.”
All meningiomas were reevaluated by a senior neuropathologist according to the 2016 WHO criteria. Patients with grade III meningiomas were matched on sex, location (skull base or non–skull base), age at diagnosis (± 5 years), and tumor sample age (± 5 years) with patients who had a grade I meningioma without recurrence (benign grade I meningiomas, n = 51). Recurrence status was checked in benign controls and defined as no growth on control MRI scans for a minimum of 5 years and no new neurological symptoms during the entire follow-up time available (a minimum 5 years). In 1 case, matching on sex was not possible. For all patients, age, sex, meningioma sample age, WHO grade, number of surgeries, anatomical localization, and histological subtype were noted. Ethics approval was given by the Regional Research Ethics Committees, Capital Region of Denmark, and exemption from patient consent was granted.
RNA Extraction and Quality
RNA was extracted from formalin-fixed paraffin-embedded tumor blocks using the RNeasy mini kit (Qiagen). All blocks had > 70% tumor tissue present evaluated on H&E-stained slides and were generally high in tumor cell content. A Bioanalyzer 2100 with the 6000 RNA Nano assay (Agilent Technologies) was used to evaluate the concentration, quality (the percentage of RNA fragments > 300 nucleotides [DV300]), and RNA integrity number (RIN) of extracted RNA. A Nanodrop spectrophotometer (Thermo Fisher Scientific) was used to validate the concentration and purity of extracted RNA (wavelength absorbance ratio A260/280). All samples had an absorbance ratio of approximately 2.0, the median RIN was 2.3 (range 1.4–5.3), and 44% of samples had a DV300 > 50%. RNA input was scaled based on the DV300 value [100 ng × (100/DV300)] making the input range from 200 to 350 ng in total (NanoString Inc., MAN-10050-03).
NanoString nCounter Analyses and Normalization
The nCounter neuroinflammation panel from NanoString covering 770 genes, including 13 housekeeping genes, was used to generate RNA expression data. We customized the panel by adding 30 targets known in previous literature to be involved in meningioma pathophysiology9,17 (Supplementary Material S1). RNA was hybridized with biotin-labeled capture probes for 18–19 hours at 65°C using the nCounter XT CodeSet gene expression panel assay (NanoString Inc., MAN-10056-03). Samples were loaded to the nCounter preparation station. Excess capture probes and reporter probes were removed, and the hybridized RNA was loaded onto a cartridge and immobilized for imaging on the nCounter Digital Analyzer. Using nSolver 4.0 (NanoString Inc.), the technical success of the experiment was assessed by hybridization effectiveness and an overall quality control check. Binding density flags were present in 8 samples; these were rerun successfully at a lower input.
Run-to-run variability was normalized with 6 positive and 8 negative RNA transcripts included in the CodeSet. Sample-to-sample variability was normalized using counts from the 13 included housekeeping genes with the GeNorm Algorithm and the NanoStringNorm package in R.18 RNA from one sample was run in 8 different batches to evaluate a possible batch effect.
Statistical Analyses
Pre hoc, four key comparisons of the targeted gene expression profile were planned (Fig. 1).
In analysis 1, grade I meningiomas from benign controls were compared with meningiomas from patients with grade III meningiomas. In patients with subsequent grade III recurrences, the first grade III meningioma was used.
In analysis 2, grade I meningiomas from benign controls were compared with premalignant grade I meningiomas. Nine patients presented with a premalignant grade I meningioma and were included in the analysis. In patients with more than one premalignant grade I meningioma, the first was used.
Analysis 3 was within patients with grade III meningioma. We compared grade III meningiomas from patients with primary malignant meningioma with grade III meningiomas in patients with secondary malignant meningioma. In patients with multiple grade III recurrences, the first was used.
Analysis 4 was within patients with secondary grade III meningiomas. We compared the last premalignant meningioma (if multiple grade I or II recurrences were available) with the first grade III meningioma to investigate changes in gene expression on either side of the “grade switch.”
To investigate differences of gene expression we used a probabilistic index (PBI) regression model. In analyses 1, 2, and 3, the PBI is the probability that the gene expression in the tumor of a patient in one group is higher than the gene expression in the tumor of a patient in the other group. The regression analyses were adjusted for age at date of tumor biopsy and sex. Hence, the PBI regression model is a generalization of the Mann-Whitney-Wilcoxon rank test, which allowed us to control for patient demographics. In analysis 4 (Fig. 1), we accounted for the fact that two measurements are coming from the same individual, and we employed the Wilcoxon signed-rank test (formulas available in Supplementary Material S2); p values were adjusted using the Benjamini-Hochberg correction method. Differentially expressed genes (DEGs) based on the PBI had an adjusted p value < 0.05.
Heatmaps and Pathway Analyses
Qlucore Omics Explorer version 3.7 (Qlucore AB) was used to generate heatmaps and 2-way unsupervised hierarchical clustering (UHCL) based on DEG identified from t-tests in analyses 1, 2, and 3. For all analyses, the following filters were applied: variance > 0.1, p < 0.05, and > 2-fold change in gene expression, except analysis 1, in which the variance filter was set to > 0.2. Analysis 1 was extended by subgrouping genes according to biofunctions relevant in neuroinflammation (e.g., microglia and cytokines) as annotated by NanoString, and by assessing heatmaps based on the genes in the respective neuroinflammatory pathway.
DEGs from t-tests in analysis 1 were loaded into Ingenuity Pathway Analysis (Qiagen),19 and data were assessed in the Ingenuity Knowledge Base. For canonical pathways, a −log(p value) > 3 and z-scores > 2 or < −2 were set as thresholds for significant activation or inhibition, respectively.
Results
DEGs in WHO Grade III Meningiomas and Grade I Meningiomas
An overview of patients with grade III meningiomas and benign controls can be seen in Table 1. A comparison of the 51 first grade III meningiomas and the 51 benign grade I meningiomas from controls yielded 429 DEGs based on the PBI (Fig. 2A). The top 5 upregulated genes with the highest PBI in grade III cases were TOP2A, MELK, RAD51, BIRC5, and PTTG1. Among benign grade I meningiomas from benign controls, the top 5 upregulated genes based on PBI were SLC44A1, ENPP6, PINK1, AGO4, and TNFRSF25. A heatmap and UHCL based on 78 (36 up and 42 down) DEGs, all included in the 429 DEGs identified in the PBI model (Figs. 3 and 4), showed almost complete separation of grade I and III meningiomas. We expanded the analysis and constructed heatmaps of the genes involved in the respective pathways as annotated by NanoString (Table 2). Genes involved in microglia regulation clearly separated benign grade I and grade III meningiomas (Supplementary Material S3).
TABLE 1.
Clinical characteristics of patients with WHO grade III meningioma and benign controls
| Patients w/ Grade III Meningioma (n = 51)* | Benign Controls (n = 51) | |
|---|---|---|
| Surgeries, n | 151 | 51 |
| Age at op, median (range) | 61 (10–87) | 60 (29–85) |
| Male sex, n (%) | 25 (49.0) | 24 (47.1) |
| Skull base location, n (%) | 9 (17.6) | 8 (15.7) |
| Age of sample in yrs, median (range)† | 8.2 (0.4–24.6) | 10.8 (6.3–21.2) |
| Histology, n (%) | ||
| Anaplastic | 42 (82.4) | 0 (0) |
| Papillary features | 4 (7.8) | 0 (0) |
| Rhabdoid features | 5 (9.8) | 0 (0) |
| Meningothelial, fibrous, transitional | 0 (0) | 50 (98.0) |
| Angiomatous | 0 (0) | 1 (2.0) |
Data are based on the first (earliest presenting) WHO grade III meningioma.
Sample age was calculated from last follow-up date.
FIG. 2.
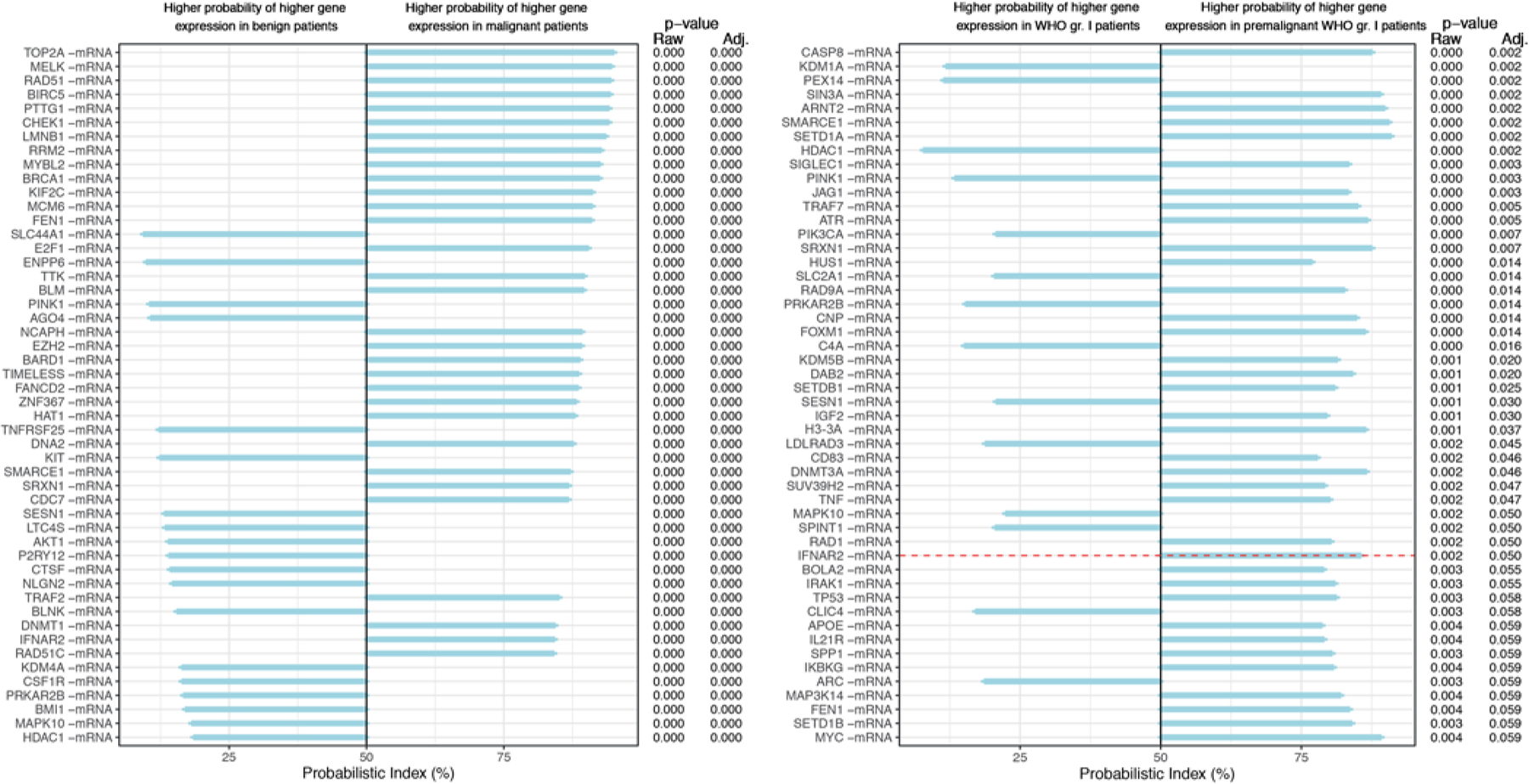
PBIs, p values, and adjusted p values for analyses 1 (left) and 2 (right). Left: In analysis 1 the first grade III meningiomas (n = 51) were compared with benign grade I meningiomas from controls (n = 51). The genes are ranked by magnitude of PBI, and the top 50 of the 429 genes (adjusted p < 0.05) are shown. Right: In analysis 2, the first premalignant grade I meningiomas (n = 9) were compared to benign grade I meningiomas (n = 51). Genes are ranked according to adjusted p value, and 37 genes have an adjusted p < 0.05 (border marked with a dotted red horizontal line). Adj. = adjusted; gr. = grade.
FIG. 3.
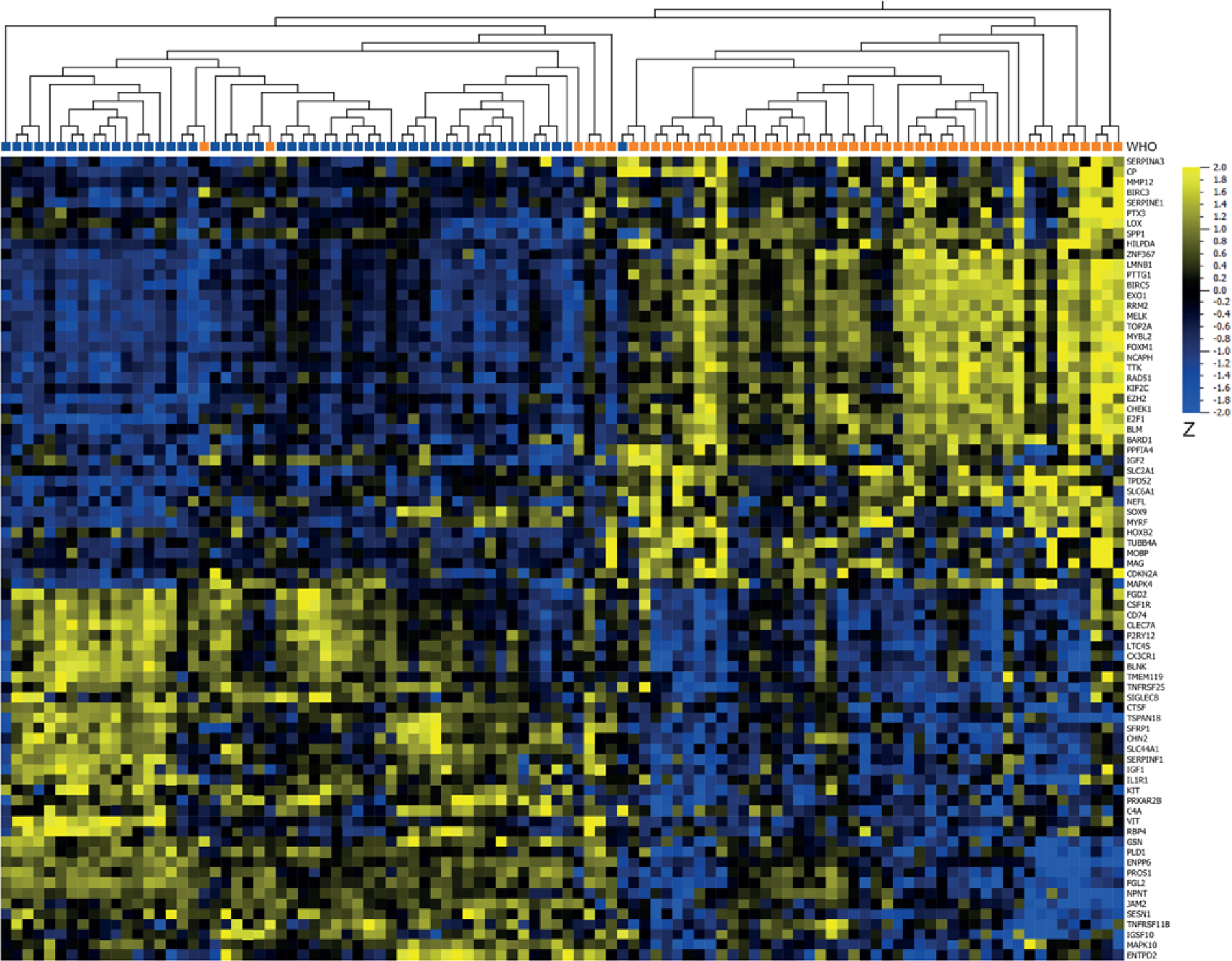
Heatmap and UHCL of DEGs from t-tests comparing mRNA expression between 51 WHO grade III meningiomas (orange) and 51 WHO grade I meningiomas from controls (blue). The analysis yielded 78 DEGs out of 787 investigated genes with a > 2-fold change in gene expression, p < 0.05, and q = 0.08 (36 upregulated in grade III [yellow] and 42 downregulated in grade III [blue]).
FIG. 4.
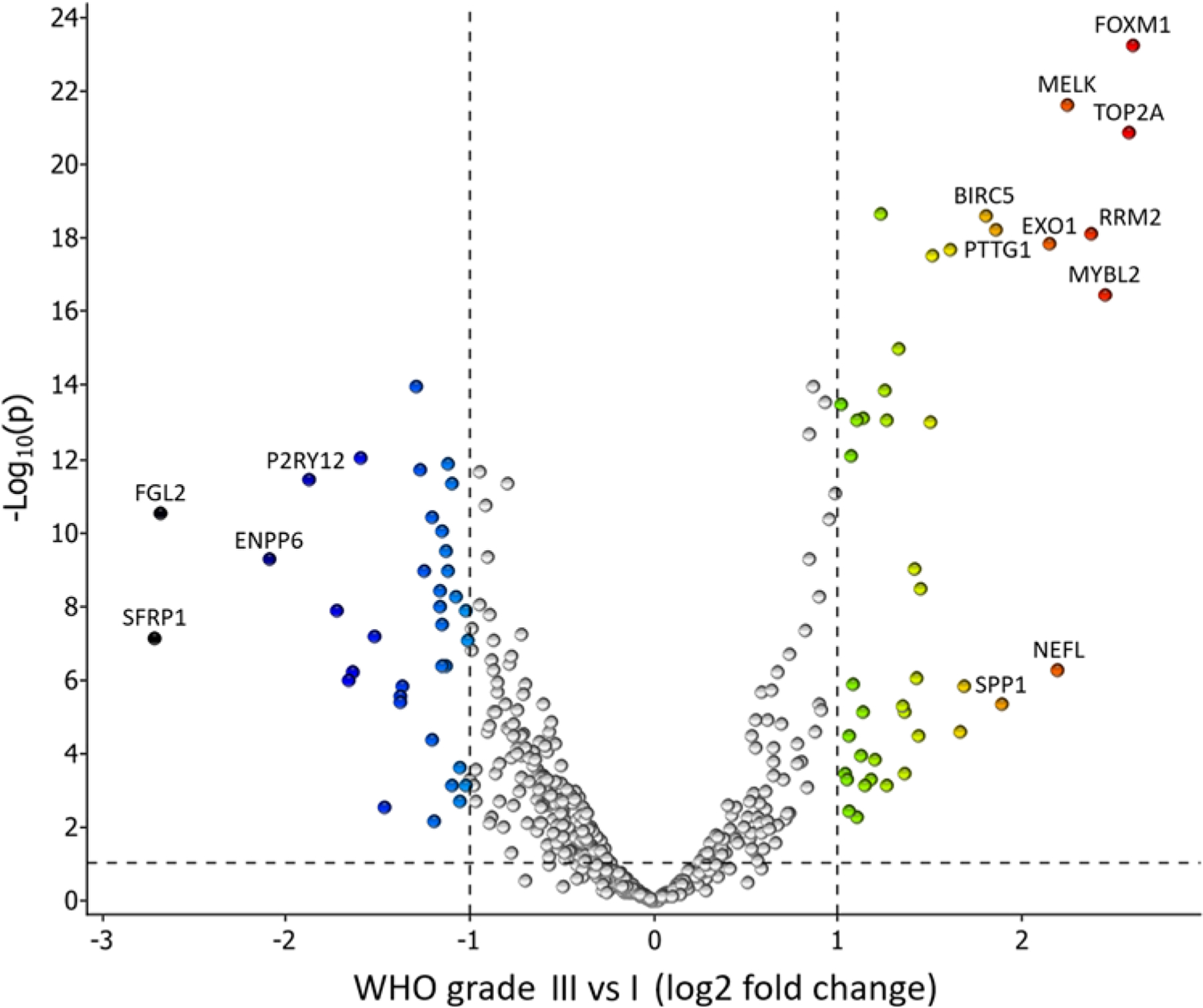
Volcano plot of DEGs from t-tests comparing mRNA expression between 51 WHO grade III meningiomas and 51 WHO grade I meningiomas from controls. Dashed lines indicate cutoff values for differential expression. Annotated genes have a log2 fold change > 1.8.
TABLE 2.
Genes sorted by neuroinflammatory pathways in analysis 1 comparing WHO grade III meningiomas with WHO grade I meningiomas from benign controls
| Pathway (annotated by NanoString) | Genes Annotated to Pathway (n) | DEG w/in Pathway* | Genes† |
|---|---|---|---|
| Microglia‡ | 187 | 28 | Top 5 downregulated: P2RY12, NPNT, CX3CR1, JAM2, IGF1 Top 5 upregulated: RRM2, SPP1, LMNB1, TPD52, SLC2A1 |
| Cytokine‡ | 117 | 8 | Downregulated: CSFR1, TNFRSF25, CX3CR1, KIT, IL1R1 Upregulated: BIRC3, NEFL |
| Inflammatory signaling | 102 | 3 | Downregulated: CD74, FGL2 Upregulated: BIRC3 |
| Innate immune system | 144 | 2 | Downregulated: MAPK10 Upregulated: BIRC3 |
| Oligodendrocyte | 27 | 5 | Downregulated: ENPP6, GSN Upregulated: MAG, MOBP, MYRF |
| Adaptive immune response | 130 | 9 | Downregulated: KIT, BLNK, CD74, MAPK10, CTSF, SIGLEC8 Upregulated: NEFL, KIF2C, TUBB4A |
| Astrocyte | 55 | 7 | Downregulated: C4A, ENTPD2 Upregulated: SLC6A1, SERPIN3A, CP, SOX9, PTX3 |
| Neuron | 81 | 5 | Downregulated: None Upregulated: TUBB4A, PPFIA4, SLC6A1, TPD52, NEFL |
For all analyses, q < 0.09 and > 2-fold change in gene expression.
Up-/downregulation in grade III meningiomas compared with grade I meningiomas from benign controls.
Heatmaps of the microglia- and cytokine-associated DEGs in analysis 1 can be seen in Supplementary Material S3.
Among canonical pathways (Supplementary Material S4), the biological pathway most significantly enriched (p value and z-score combined) by genes in the data set was the HOTAIR Regulatory Pathway [−log(p value) = 3.68, z-score = 2.24] with EZH2, FOXM1, JAM2, MMP12, and SPP1 involved.
Premalignant WHO Grade I Tumors Show an Aggressive Gene Expression Profile
The gene expression signature from 9 premalignant grade I meningiomas was compared with meningiomas from benign controls. A total of 37 DEGs were identified based on the PBI. The top 5 genes with the highest PBI (sorted by adjusted p value) in premalignant grade I meningiomas compared with the 51 benign grade I meningiomas were CASP8, SIN3A, ARNT2, SMARCE1, and SETD1A. The list also included FOXM1 (adjusted p value = 0.014), H3-3A (adjusted p = 0.037), and TRAF7 (adjusted p = 0.005) (Fig. 2B). The top 5 genes with the lowest PBI (sorted by adjusted p value) included KDM1A, PEX14, HDAC1, PINK1, and PIK3CA. UHCL and the heatmap showed 21 DEGs (Fig. 5). Consensus between the PBI model (nonparametric, generalization of Mann-Whitney-Wilcoxon rank test) and quantification in the Qlucore software (parametric, t-test) was found in the following 7 genes: IGF2, FOXM1, ARNT2, TNF, and SIGLEC1 (upregulated) and PRKAR2B and C4A (downregulated).
FIG. 5.
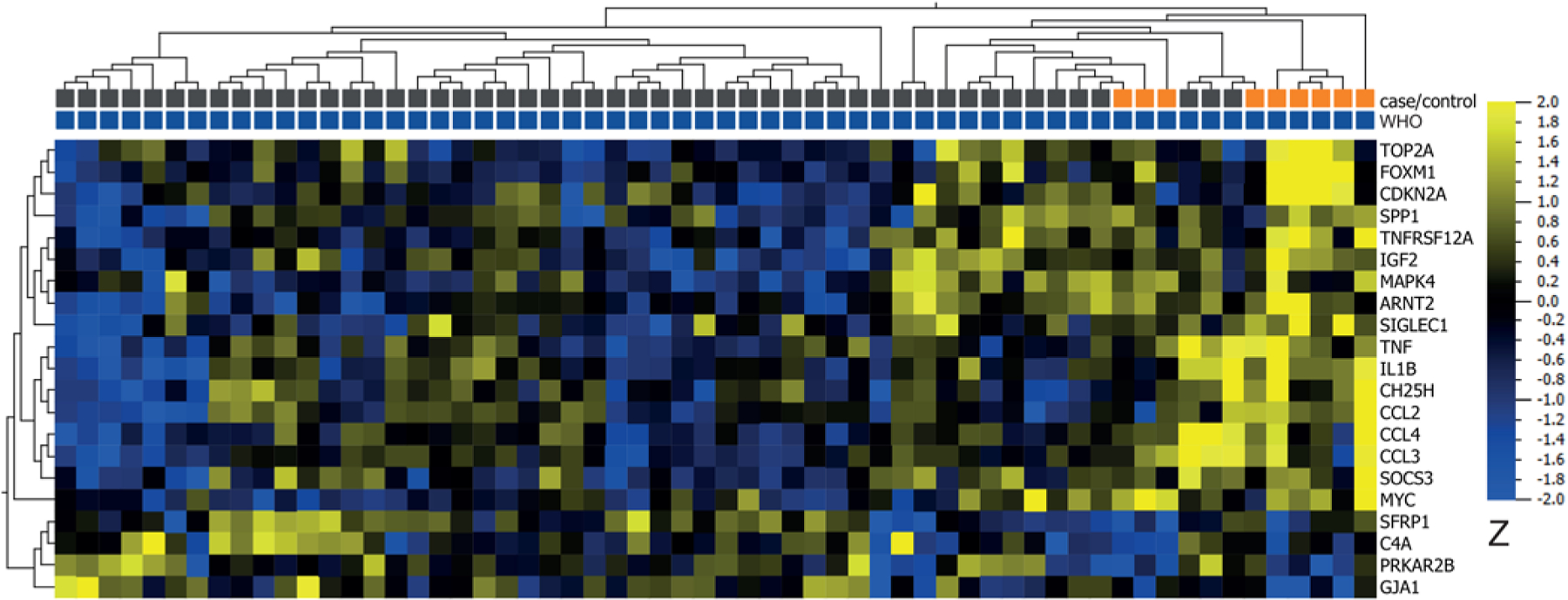
Heatmap and UHCL of DEGs from t-tests comparing mRNA expression between 9 premalignant grade I meningiomas (orange) and 51 benign grade I meningiomas (gray) (analysis 2). The analysis yielded 21 DEGs out of 787 investigated genes with a > 2-fold change in gene expression, p value < 0.05, and q = 0.20 (17 upregulated in premalignant grade I [yellow] and 4 downregulated in premalignant grade I [blue]).
No DEGs Identified in Secondary and Primary WHO Grade III Meningioma
A comparison of grade III meningiomas from patients with secondary (n = 24) and primary (n = 27) malignant meningioma showed no DEGs based on the PBI (Supplementary Material S5). T-tests yielded 5 DEGs, among these MYBL2. However, the q value was 0.51 (Supplementary Material S6). Comparison of rhabdoid/papillary and anaplastic histology (heatmap and UHCL) is shown in Supplementary Material S7.
DEGs at Grade Switching
A comparison at grade switch within patients with secondary malignant meningioma yielded 119 DEGs. The top 5 genes (sorted by p value) with a positive median of the difference between the first grade III tumor and the last premalignant tumor, and thus upregulated, were MYBL2, TOPBP1, PTTG1, RRM2, and NCAPH. The top 5 genes with a negative median of the difference were ENPP6, P2RY12, FGL2, CSF1R, and BIK (Supplementary Material S5).
Gene Expression Trajectories Across Recurrences
The gene expression trajectories of FOXM1, TOP2A, and P2RY12 across recurrences among patients with malignant meningiomas are shown in Supplementary Material S8. We observed large intra- and interpatient variability across the trajectories, but a trend toward a rise in FOXM1 and TOP2A expression was seen while P2RY12 expression diminished.
Discussion
We detected upregulation of transcripts defining a malignant phenotype already in premalignant, histological grade I meningiomas. Further analyses showed differences of neuroinflammatory gene expression profiles between grade I and III meningiomas. Our findings also corroborated previous reports that gene expression signatures in meningiomas with aggressive phenotypes are enriched for genes involved in cell-cycle regulation and mitosis.10
Clinical Implications and Targets for Precision Medicine
Personalized follow-up and adjuvant treatment to minimize morbidity and optimize healthcare utilization could avoid unnecessary treatment and imaging. In this context, 1%–3% of all meningiomas that are histologically diagnosed as grade I meningiomas according to the WHO 2016 classification are known to undergo malignant transformation and finally recur as secondary malignant meningiomas, which carry dismal prognoses.7 Early identification of this subgroup would provide a treatment window to optimize outcomes via additional surgery, closer follow-up, or adjuvant therapies. Molecular markers that define aggressive clinical courses comprise patients with loss of 1p,20 TERT promoter mutations,4,5 intermediate or malignant methylation classes,21 CDNK2A homozygous deletion,6 or high Ki-67 labeling index.22,23 Only 5.4%–15% of meningiomas harbor the TERT promoter mutation,4,5 and < 5% harbor CDKN2A/B alterations; thus, molecular markers for improved prognostic precision are needed in the remaining cases. Our data revealed a distinctive signature of grade III meningiomas already in premalignant grade I meningiomas. Benign grade I meningiomas that remained benign could thus be differentiated from premalignant grade I meningiomas that progressed to grade III. Early identification of the latter would allow tailored adjuvant treatment and follow-up. Our findings are in line with targeted gene expression data,10 and here it is indicated that changes in RNA expression can happen years before phenotypic transformation. Premalignant grade I tumors had upregulation of FOXM1 years before malignant transformation (Figs. 2B and 5). Previously, Viaene et al. identified differences in whole-transcriptome analyses in grade I meningiomas that later progressed to grade II (n = 3 paired cases) compared with grade I meningiomas without grade progression and additionally evaluated differences in gene expression among all meningioma grades.24 In comparison with our findings, they reported separate clustering of grade I progressing and grade I nonprogressing meningiomas. This supports our proof-of-concept finding that detectable changes in gene expression precede a phenotypical transformation to grade III. We did not find any direct matches between the 7 genes expressed differentially between grade I meningiomas that progressed to grade III (analysis 2) and those that did not, and those reported by Viaene et al. in their comparison of grade I progressing versus grade I nonprogressing meningiomas. In their analyses, TNF-related genes, IGF1, and SIGLE-C11 were upregulated in progressing grade I meningiomas. Moreover, they compared nonpaired grade I and III meningiomas and found, as we did, upregulation of FOXM1, EZH2, CHEK1, BIRC5, TOP2A, and MYBL2 in grade III meningiomas. They reported differential expression of GREM2, SNORA46, and SNORA48 among all meningioma grades. These genes were not included in our study, which compromises comparability of these genes. Discrepancies may reflect different methodologies (full transcriptome vs targeted gene expression) and, importantly, different biological hypotheses. Viaene et al. studied progression to grade II, while we analyzed grade III meningiomas. Different transcripts may be implicated in transformation to grade II compared with grade III.
The central role of FOXM1 in aggressive meningioma biology is not a new finding.10,17,25 Our data set corroborates its high expression in a large cohort of grade III meningiomas and unprecedentedly describes its upregulation in premalignant grade I meningiomas in a unique series of meningiomas that progressed from grade I to grade III. The 2.6-fold difference of FOXM1 expression between grade III and I meningiomas in our data might indicate FOXM1 as a pharmacological target in humans. The FOXM1 inhibitor Siomycin-A has been shown to diminish meningioma volume and growth in mouse models,25 although inhibition is complicated by posttranslational modifications, kinetics, and clinical factors.26,27 FOXM1 inhibitors are therefore not yet available for human use, despite our data and previous data. Moreover, high expression of CHEK1 (encoding Checkpoint kinase 1, Chk1) in grade III versus grade I meningiomas was evident in our material. Chk1 inhibitors are used either as monotherapy or in combination with genotoxic therapies in clinical and preclinical studies.28,29 So far, no clinical trials have explored their use in aggressive meningioma. The potential of targeted therapy based on upregulated transcripts in benign meningioma to prevent aggressive transformation is not known, although our findings and other recent data suggest investigating transcripts such as EZH2, CHEK1, and FOXM1 for preclinical testing.
The gene expression of FOXM1 and TOP2A varied extensively across recurrences but appeared to be higher in malignant than benign tumors (Supplementary Material S8). Our study has shown that NanoString technology is suitable for gene expression studies in archived formalin-fixed paraffin-embedded meningioma tissue, as degraded RNA can be compensated through increasing input. The gene expression profiles could support screening profiles to quickly identify meningiomas at high risk of malignant transformation and allow potentially curative measures before transformation. Following prospective validation and protein analyses, upregulated transcripts could be included in a prognostic, targeted gene expression panel, and inclusion of neuroinflammatory markers could be predictive for assessing outcome of future treatment targeting protumorigenic macrophages.
Neuroinflammatory Signatures and Two Contrasting Meningioma Phenotypes
We found differences in genes involved in microglia regulation between grade I and III meningiomas (Table 2 and Supplementary Material S3), which were in concordance with novel findings of an immunogenic meningioma subtype.13 P2RY1230,31 had a high PBI of expression in grade I tumors from benign controls. Furthermore, P2RY12 and CX3CR132 had a > 1.5-fold lower expression in WHO grade III compared with benign grade I meningiomas. P2RY12 is considered a microglia-specific marker even under pathological conditions33 and is associated with the M1 proinflammatory subtype.34 CX3CR1 is a chemokine receptor for fractalkine, which is, among other features, secreted by damaged neurons to recruit microglia.31 Our results suggest that migrating microglia may have a role in proimmunogenic meningiomas, although the postulated microglial specificity of P2RY12 must be further established.
FGL2 and SFRP1 had a > 2-fold change in the benign grade 1 meningiomas compared with the grade 3 meningiomas. The precise antineoplastic functions of most studied genes are not known, while FGL235 and SFRP1 have specified antineoplastic or proneoplastic functions. The SFRP family modulates Wnt-signaling and epigenetic silencing of SFRP leads to Wnt-pathway activation;36 our results agree with earlier findings of high SFRP1 expression in tumors with low FOXM1 expression in meningiomas.17 FGL2 is a membrane-bound or secreted protein expressed by macrophages, T cells, and tumor cells.35 In glioma, it confers poor prognosis,37 and FGL2 may promote tumor progression by expanding tumor-supportive M2-macrophages.37,38 Its possible role in meningioma is, however, far from established, and FGL2 can instead recruit inflammatory cells in other malignancies.35 We did not quantify different cells, and higher FGL2 expression could reflect large numbers of macrophages in benign meningiomas.11 Moreover, the premalignant grade I meningiomas showed upregulation of SIGLEC1. Recently, an autoregulatory loop between cancer cells and TAMs, involving TNF-alpha and SIGLEC1, generated protumoral TAMs and an immunosuppressive environment in breast cancer.39 Our data imply the same functional relation in meningiomas, although causality must be addressed in functional studies. Protumorigenic polarization of macrophages seems to be an early event in malignant transformation. Importantly, inhibition of the CSF1-CSF1R axis, an important regulator of macrophage phenotype, could diminish meningioma growth in a murine model.14 Thus, the characterization and manipulation of protumorigenic TAMs in meningioma is pertinent for future treatment. In contrast, anti-inflammatory treatment may have negative effects since aggressive meningioma phenotypes appear to be associated with attenuated inflammatory markers here and in previous literature.12,15
The HOTAIR regulatory pathway was significantly affected by DEGs in the Ingenuity Pathway Analysis (Supplementary Material S4). We investigated DEGs downstream in the regulatory pathway, not HOTAIR expression. HOTAIR is a long noncoding RNA that modulates gene expression by interaction with chromatin remodeling complexes and specifically the PRC2 complex.40 The PRC2 complex balances proliferation versus differentiation.41 Atypical meningiomas exhibit upregulation of the catalytic subunit EZH2 of the PRC2 complex,42 a finding reproduced in grade III meningiomas in our data. Deregulation in either direction can have an oncogenic effect and a possible effect on the threshold of transcriptional activation of genes controlling proliferation such as CDKN2A, a known biomarker in meningioma.6,43 E2F is a part of the repressive DREAM complex regulating cell cycle,44 and loss of the repressive function characterizes aggressive meningiomas with aggressive phenotypes.8
Patients with secondary malignant meningioma had higher expression (Supplementary Material S3) of MYBL2,10 among others, in the first sample from a grade III meningioma compared with the last premalignant grade I or II meningioma sample, while P2RY1234 and FGL237 were downregulated, possibly enabling or marking the malignant switch.
Within the grade III meningiomas, we found no difference in gene expression between the first grade III meningiomas from patients with primary (n = 27) and secondary (n = 24) malignant meningiomas. Data on whether overall survival differs between primary and secondary grade III tumors are contradictory,16,45 and molecular or phenotypic differences remain unknown. Anaplastic versus rhabdoid/papillary seemed to cluster; however, the q value was 0.31 (Supplementary Material S7). The gene expression profile did not differentiate tumors with rhabdoid/papillary features with and without concomitant anaplasia or high mitotic index, although the material was small.
Limitations and Strengths
Our study was limited by the number of malignant meningiomas and the retrospective design. Pathological diagnoses were validated to agree with 2016 WHO grading, but we could not completely exclude previous misclassification with omission of eligible cases. Our benign controls had a 5-year follow-up; the criteria strongly suggested a benign phenotype but did not completely rule out later recurrence. We did not have data on precise steroid dosages before surgery, which could have influenced the neuroinflammation signature. We had no reason, however, to suspect a large dosage discrepancy between the two groups. Clinical data were used for characterization of the contrasting phenotypes in the cohort (Table 1), but in-depth clinical data from the patients with grade III meningiomas were not compared with gene expression for statistical reasons with our study design. The risk of false-positive findings would be unacceptably high, with additional clinical outcomes and more granular phenotypes. We preselected the four most important analyses according to hypotheses and thus did not perform an exploratory investigation that would have required a validation cohort. The retrospective nature of data also limited causal interpretations of gene expression changes or patterns in association with previous radiotherapy, outcome, or survival. A targeted gene expression panel with a neuroinflammatory focus was chosen as 1) a predefined set of genes and hypotheses minimized the statistical risk of false-positive findings, 2) the sample quality was not expected to support a full transcriptome analysis with a median RIN of 2.3, and 3) we defined a need to increase knowledge of neuroinflammatory genes in contrasting phenotypes of meningioma. We did not analyze protein synthesis that would have resulted from upregulated mRNA transcripts. Immunohistochemical analysis is warranted to localize gene products and quantitative analyses such as Western blot for quantification; moreover, proteomics can depict relevant protein expression. Logical next steps are targeted analysis of immunohistochemical expression and quantification of, for example, FOXM1 in meningioma and more exploratory studies of proteins with proteomics methodologies.
Despite its limitations, the study had unusual strengths. The study included samples from several stages of disease from an unselected population of meningioma patients treated in Eastern Denmark, and the sample size was comparatively large for malignant meningiomas. It was still possible to obtain tissues from repeated surgeries during malignant transformation, and we had detailed clinical information from malignant meningiomas and benign controls. The cohort was thereby uniquely suitable to study gene expression signatures of malignant phenotypes and malignant transformation over time in a hypothesis-driven manner within the preplanned analyses 1–4. Because of this study design, we refrained from dividing patients into discovery and validation cohorts and employed all data for testing the primary statistical hypotheses. Prospective validation of premalignant and malignant gene signatures is needed, preferably across multiple institutions, and the causal relation between upregulated transcripts and malignant transformation can be analyzed in experimental settings such as murine knockout models.
Conclusions
Our results from an unselected population of 51 patients with WHO grade III meningiomas showed that premalignant WHO grade I meningiomas had a gene expression signature different from that of benign WHO grade I meningiomas and similar to WHO grade III meningiomas years before histopathological transformation to a malignant phenotype. Moreover, we found different neuroinflammatory profiles in benign and malignant meningiomas, particularly among RNA transcripts involved in microglia regulation. Taken together, results from our targeted gene expression analyses show novel features that can allow early detection of malignant phenotypes.
Supplementary Material
Acknowledgments
A.D.M. and T.M. received a grant from The Danish Cancer Society (R278-A16459). A.D.M. received a grant from The Research Fund of Rigshospitalet, Copenhagen University Hospital. T.M. is funded by The Lundbeck Foundation (R266-2017-4029). The Bio- and GenomeBank Denmark is acknowledged for biological material and for data regarding handling and storage. We thank the laboratory scientists in the Department of Pathology, Rigshospitalet, Copenhagen, for their technical expertise and help.
ABBREVIATIONS
- DEG
differentially expressed gene
- DV300
percentage of RNA fragments > 300 nucleotides
- PBI
probabilistic index
- RIN
RNA integrity number
- TAM
tumor-associated macrophage
- UHCL
unsupervised hierarchical clustering
Footnotes
Disclosures
Dr. Heegaard: consultant for Sanofi, Leo Pharma, Santen, and Thea. Dr. Litman: funded by LEO Pharma.
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Supplementary Material. https://thejns.org/doi/suppl/10.3171/2022.7.JNS22585.
References
- 1.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis D, Ohgaki H, Wiestler O, Cavenee W. WHO Classification of Tumours of the Central Nervous System 4th ed. International Agency for Research on Cancer, World Health Organization; 2016. [Google Scholar]
- 3.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier AD, Stenman A, Svahn F, et al. TERT promoter mutations in primary and secondary WHO grade III meningioma. Brain Pathol. 2021;31(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirian C, Duun-Henriksen AK, Juratli T, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91(4):378–387. [DOI] [PubMed] [Google Scholar]
- 6.Sievers P, Hielscher T, Schrimpf D, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fountain DM, Young AMH, Santarius T. Malignant meningiomas. Handb Clin Neurol 2020;170:245–250. [DOI] [PubMed] [Google Scholar]
- 8.Patel AJ, Wan YW, Al-Ouran R, et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci U S A. 2019;116(43):21715–21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collord G, Tarpey P, Kurbatova N, et al. An integrated genomic analysis of anaplastic meningioma identifies prognostic molecular signatures. Sci Rep. 2018;8(1):13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WC, Vasudevan HN, Choudhury A, et al. A prognostic gene-expression signature and risk score for meningioma recurrence after resection. Neurosurgery 2020;88(1):202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proctor DT, Huang J, Lama S, Albakr A, Van Marle G, Sutherland GR. Tumor-associated macrophage infiltration in meningioma. Neurooncol Adv 2019;1(1):vdz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borch JDS, Haslund-Vinding J, Vilhardt F, Maier AD, Mathiesen T. Meningioma—brain crosstalk: a scoping review. Cancers (Basel) 2021;13(17):4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassiri F, Liu J, Patil V, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597(7874):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeung J, Yaghoobi V, Miyagishima D, et al. Targeting the CSF1/CSF1R axis is a potential treatment strategy for malignant meningiomas. Neuro Oncol. 2021;23(11):1922–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haslund-Vinding J, Møller JR, Ziebell M, Vilhardt F, Mathiesen T. The role of systemic inflammatory cells in meningiomas. Neurosurg Rev 2022;45(2):1205–1215. [DOI] [PubMed] [Google Scholar]
- 16.Maier AD, Mirian C, Haslund-Vinding J, et al. Granular clinical history and outcome in 51 patients with primary and secondary malignant meningioma. J Neurosurg. Published online March 11, 2022. doi: 10.3171/2022.1.JNS212723 [DOI] [PubMed] [Google Scholar]
- 17.Vasudevan HN, Braunstein SE, Phillips JJ, et al. Comprehensive molecular profiling identifies FOXM1 as a key transcription factor for meningioma proliferation. Cell Rep. 2018;22(13):3672–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: a language and environment for statistical computing. R Foundation for Statical Computing Accessed August 3, 2022. https://www.r-project.org/
- 19.Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansson CM, Buckley PG, Grigelioniene G, et al. Comprehensive genetic and epigenetic analysis of sporadic meningioma for macro-mutations on 22q and micro-mutations within the NF2 locus. BMC Genomics. 2007;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 2017;18(5): 682–694. [DOI] [PubMed] [Google Scholar]
- 22.Mirian C, Skyrman S, Bartek J Jr, et al. The Ki-67 Proliferation index as a marker of time to recurrence in intracranial meningioma. Neurosurgery 2020;87(6):1289–1298. [DOI] [PubMed] [Google Scholar]
- 23.Daniela Maier A, Brøchner CB, Bartek J Jr, et al. Mitotic and proliferative indices in WHO Grade III meningioma. Cancers (Basel) 2020;12(11):3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viaene AN, Zhang B, Martinez-Lage M, et al. Transcriptome signatures associated with meningioma progression. Acta Neuropathol Commun. 2019;7(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Park KJ, Ryu BK, et al. Forkhead box M1 (FOXM1) transcription factor is a key oncogenic driver of aggressive human meningioma progression. Neuropathol Appl Neurobiol 2020;46(2):125–141. [DOI] [PubMed] [Google Scholar]
- 26.Liao GB, Li XZ, Zeng S, et al. Regulation of the master regulator FOXM1 in cancer. Cell Commun Signal. 2018;16(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesnokov MS, Halasi M, Borhani S, et al. Novel FOXM1 inhibitor identified via gene network analysis induces autophagic FOXM1 degradation to overcome chemoresistance of human cancer cells. Cell Death Dis 2021;12(7):704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu Z, Oleinick NL, Zhang J. ATR/CHK1 inhibitors and cancer therapy. Radiother Oncol. 2018;126(3):450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JM, Nair J, Zimmer A, et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. Lancet Oncol 2018;19(2): 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes SE, Hollopeter G, Yang G, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. [DOI] [PubMed] [Google Scholar]
- 31.Zujovic V, Benavides J, Vigé X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29(4):305–315. [PubMed] [Google Scholar]
- 32.Li G, Hattermann K, Mentlein R, Mehdorn HM, Held-Feindt J. The transmembrane chemokines CXCL16 and CX3CL1 and their receptors are expressed in human meningiomas. Oncol Rep. 2013;29(2):563–570. [DOI] [PubMed] [Google Scholar]
- 33.Keane L, Cheray M, Blomgren K, Joseph B. Multifaceted microglia—key players in primary brain tumour heterogeneity. Nat Rev Neurol. 2021;17(4):243–259. [DOI] [PubMed] [Google Scholar]
- 34.Zhu C, Kros JM, van der Weiden M, Zheng P, Cheng C, Mustafa DA. Expression site of P2RY12 in residential microglial cells in astrocytomas correlates with M1 and M2 marker expression and tumor grade. Acta Neuropathol Commun. 2017;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J, Yan J, Rao G, et al. The duality of Fgl2-secreted immune checkpoint regulator versus membrane-associated procoagulant: therapeutic potential and implications. Int Rev Immunol. 2016;35(4):325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukui T, Kondo M, Ito G, et al. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24(41):6323–6327. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, Kong LY, Hu J, et al. FGL2 as a multimodality regulator of tumor-mediated immune suppression and therapeutic target in gliomas. J Natl Cancer Inst. 2015;107(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan J, Zhao Q, Gabrusiewicz K, et al. FGL2 promotes tumor progression in the CNS by suppressing CD103+ dendritic cell differentiation. Nat Commun. 2019;10(1):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassetta L, Fragkogianni S, Sims AH, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588–602.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laugesen A, Højfeldt JW, Helin K. Role of the polycomb repressive complex 2 (PRC2) in transcriptional regulation and cancer. Cold Spring Harb Perspect Med. 2016;6(9):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harmancı AS, Youngblood MW, Clark VE, et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2017;8:14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams EA, Santagata S, Wakimoto H, et al. Distinct genomic subclasses of high-grade/progressive meningiomas: NF2-associated, NF2-exclusive, and NF2-agnostic. Acta Neuropathol Commun. 2020;8(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadasivam S, DeCaprio JA. The DREAM complex: master coordinator of cell cycle-dependent gene expression. Nat Rev Cancer. 2013;13(8):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peyre M, Gauchotte G, Giry M, et al. De novo and secondary anaplastic meningiomas: a study of clinical and histomolecular prognostic factors. Neuro Oncol. 2018;20(8):1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


