Abstract
Purpose
Recombinant erythropoietin (EPO) administered for traumatic brain injury (TBI) may increase short-term survival, but the long-term effect is unknown.
Methods
We conducted a pre-planned long-term follow-up of patients in the multicentre erythropoietin in TBI trial (2010–2015). We invited survivors to follow-up and evaluated survival and functional outcome with the Glasgow Outcome Scale-Extended (GOSE) (categories 5–8 = good outcome), and secondly, with good outcome determined relative to baseline function (sliding scale). We used survival analysis to assess time to death and absolute risk differences (ARD) to assess favorable outcomes. We categorized TBI severity with the International Mission for Prognosis and Analysis of Clinical Trials in TBI model. Heterogeneity of treatment effects were assessed with interaction p-values based on the following a priori defined subgroups, the severity of TBI, and the presence of an intracranial mass lesion and multi-trauma in addition to TBI.
Results
Of 603 patients in the original trial, 487 patients had survival data; 356 were included in the follow-up at a median of 6 years from injury. There was no difference between treatment groups for patient survival [EPO vs placebo hazard ratio (HR) (95% confidence interval (CI) 0.73 (0.47–1.14) p = 0.17]. Good outcome rates were 110/175 (63%) in the EPO group vs 100/181 (55%) in the placebo group (ARD 8%, 95% CI 3 to 18%, p = 0.14). When good outcome was determined relative to baseline risk, the EPO groups had better GOSE (sliding scale ARD 12%, 95% CI 2–22%, p = 0.02). When considering long-term patient survival, there was no evidence for heterogeneity of treatment effect (HTE) according to severity of TBI (p = 0.85), presence of an intracranial mass lesion (p = 0.48), or whether the patient had multi-trauma in addition to TBI (p = 0.08). Similarly, no evidence of treatment heterogeneity was seen for the effect of EPO on functional outcome.
Conclusion
EPO neither decreased overall long-term mortality nor improved functional outcome in moderate or severe TBI patients treated in the intensive care unit (ICU). The limited sample size makes it difficult to make final conclusions about the use of EPO in TBI.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07141-5.
Keywords: Erythropoietin, Neurological outcome, Traumatic brain injury
Take-home message
| The use of erythropoietin in patients treated in the intensive-care unit after severe or moderate traumatic brain injury does not improve very long-term outcome, even though uncertainty remains, and further studies are needed. |
Introduction
Traumatic brain injury (TBI) is a significant medical problem that mainly affects a younger population with high mortality, morbidity and treatment costs [1, 2]. Limited medical treatment options exist beyond supportive intensive care aimed at decreasing secondary brain injury [3]. The secondary injury process includes cerebral edema, resulting in decreased cerebral blood flow and ischemia [3]. Erythropoietin (EPO) has been shown in experimental studies to have many protective effects in this setting, and systematic reviews show possible signals of lower mortality in patients treated with EPO [4]. Potential mechanisms of EPO in cerebral injury models include reduction of apoptosis, inhibition of inflammation, and restoration of cerebral blood flow [5, 6].
Between 2010 and 2015, the Erythropoietin in Traumatic Brain Injury (EPO-TBI) trial randomized patients with moderate-to-severe TBI to receive up to three doses of recombinant erythropoietin 1 week apart during care in the intensive care-unit (ICU) [7, 8]. No difference was found in the primary outcome of functional outcome at 6 months measured with the Glasgow Outcome Scale-Extended (GOSE), but a statistically significant difference in survival (secondary outcome) was found. However, the follow-up time was 6 months, and the optimal follow-up time in TBI patients remains unknown. In the current study, we performed an extended follow-up of patients included in the EPO-TBI trial, hypothesizing that EPO might improve long-term outcome after TBI.
Methods
This study was a long-term follow-up of patients included in the EPO-TBI study, which was conducted between 2010 and 2015 and published in 2015. It was a randomized-controlled trial performed at hospitals in Australia, New Zealand, Saudi Arabia, France, Finland, Ireland and Germany [7]. Patients receiving treatment in an ICU with moderate or severe TBI based on the Glasgow Coma Scale (GCS) score were screened and included if all inclusion and no exclusion criteria were met [8]. Randomization was stratified by site. These patients received either weekly doses of 40,000 IU of subcutaneous epoetin alfa (Eprex Janssen-Cilag Pty Ltd, Titusville, NJ, USA) or a placebo (0.9% sodium chloride). Deep venous thrombosis was screened for with twice-weekly ultrasound of the legs. Data for calculating the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT-TBI) risk for poor six-month outcome, the Injury Severity Score (ISS), the Abbreviated Injury Scale (AIS) and the Acute Physiology and Chronic Health Evaluation (APACHE) score were obtained prospectively in all included patients. Six ISS body regions were assessed: head or neck, face, thorax, abdominal or pelvic contents, the extremities, or pelvic girdle and external. The patients were followed up until 6 months after the event with determination of functional outcome with the GOSE, survival and quality of life (QoL). QoL was evaluated with the visual analog scale (VAS) on the EuroQol Group 5-Dimension Self-Report Questionnaire (EuroQol 5D-5L), ranging from 0 to 100, with higher scores indicating a better health status [9].
Outcomes
For this study, we measured survival of all the patients and invited the survivors to take part in a long-term follow-up study including an assessment of functional outcome and QoL. Given the prolonged study duration, the follow-up timepoint varied from three to eight years. The survivors were contacted and invited to take part in the study by phone or letter as appropriate, including a reminder. An outcome assessor blinded to the treatment interviewed the patients to determine the GOSE and QoL. The secondary neurological outcomes were assessed with the GOSE at the longest follow-up and QoL was assessed among the survivors [10]. We analyzed neurological outcome dichotomized into a good (GOSE 5–8) or a poor outcome (GOSE 1–4). In addition, we analyzed the effect of the intervention on the sliding dichotomy of the GOSE scale [11]. First reported by Murray et al. in 2005, the sliding dichotomy scale is designed to ensure that the definition of “good” outcome is tailored to each individual patient’s baseline prognosis on entry into the trial. This was achieved by dividing patient baseline IMPACT score into tertiles (WORST, MEDIUM, GOOD). To achieve a good outcome on the sliding scale, patients from the WORST tertile needed to achieve a GOSE at follow-up > 2, and patients in the MEDIUM tertile of baseline risk need to achieve a GOSE > 4, whereas patients in the GOOD tertile needed to achieve a GOSE > 6. The study protocol underwent ethical review at all participating sites [details provided in the electronic supplemental material (ESM)].
Data collection
The original study used a web-based case record form that included detailed data on the patient characteristics, the injury mechanism, pre-hospital care, and the immediate hospital management [8]. Admission computed tomography (CT) scans were reviewed and the IMPACT-TBI risk for poor 6-month outcome was calculated [12]. The ISS scores were recorded by trained assessors blinded to patient treatment and outcome. Long-term neurological outcome data and QoL were recorded on outcome assessment forms similar to those used in the original study.
Statistical analysis
The statistical analysis plan for this post hoc study was embedded in the original published study plan [13] with sample size predetermined by the parent trial. The primary outcome (patient survival) was analyzed using Cox proportional hazards regression fitting a shared frailty model with individual sites as a clustered variable. Proportional hazards assumptions were confirmed using loglog survival plots and by fitting time-dependent interactions between treatment and log(time). Multivariable sensitivity analyses were performed using covariate adjustment for region, TBI severity, and baseline risk (extended IMPACT-TBI) [13, 14]. Binomial secondary outcomes were analyzed using generalized linear mixed-effects models with a binomial distribution and a log link to facilitate relative risks (95% confidence interval [CI]) and an identify link to facilitate risk differences (95% CI). Quality-of-life measures were analyzed using linear mixed-effects models and presented as mean difference (95% CI). All secondary analyses used robust errors to account for clustering by site.
Subgroup analyses were performed considering the following groups of interest:
Severe (GCS 3–8) or moderate TBI (GCS 9–12)
Intracranial mass lesion (Marshall CT scan classification V or VI) or no intracranial mass lesion (Marshall CT scan classification I, II, III, or IV)
Patients with and without multi-trauma defined as the presence of an AIS of 3 (serious) or higher in two or more ISS body regions or an extracranial ISS score (ISS excluding the head and neck AIS) of higher than six. This approach is consistent with international reviews and we used this same approach in our previously published subgroup analysis [15–17].
Subgroup analyses were reported using forest plots, with heterogeneity between subgroups determined by fitting an interaction term between the treatment and the subgroup. To determine the representativeness of our sample, comparisons with original trial participants not included in the analyses were performed using Chi-square tests for equal proportions, analysis of variance for normally distributed data and Kruskal–Wallis tests otherwise, with results reported as frequencies (%), means (standard deviation), or medians (interquartile range), respectively. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and a two-sided p-value of 0.05 was used to indicate statistical significance.
Ethical assessment, consent, and trial registration
Ethical approval was obtained at all the EPO-TBI study sites. For the follow-up, the patient’s next of kin or legal representative gave informed consent for participation according to local requirements. The original EPO-TBI trial was registered at ClinicalTrials.gov (NCT00987454), the Australian and New Zealand Clinical Trials Registry (ACTRN12609000827235), and European Drug Regulatory Authorities Clinical Trials (011-005235-22). This follow-up study was registered at ClinicalTrials.gov in 2017 (NCT03061565).
Results
Included patients
The original study included 603 patients, of whom 116 were treated in countries that were unable to take part in the follow-up, mainly due to the coronavirus disease 2019 (COVID-19) pandemic (France, Ireland and Germany). Of the 487 eligible patients, 131 were lost to follow-up, leaving 356 patients in the study (Fig. 1). Data on QoL were collected from 259 patients. The baseline injury characteristics in the patients with data on long-term functional outcome, survival, and those lost to follow-up are shown in Supplemental Table 1. There were some differences in the injury characteristics of patients included and not included in the analysis. Data on long-term survival were available in 487 patients and data on functional outcome in 356 patients. Of the 356 included patients, 175 received EPO and 181 received the placebo. The groups were well balanced with regards to baseline characteristics (Table 1).
Fig. 1.
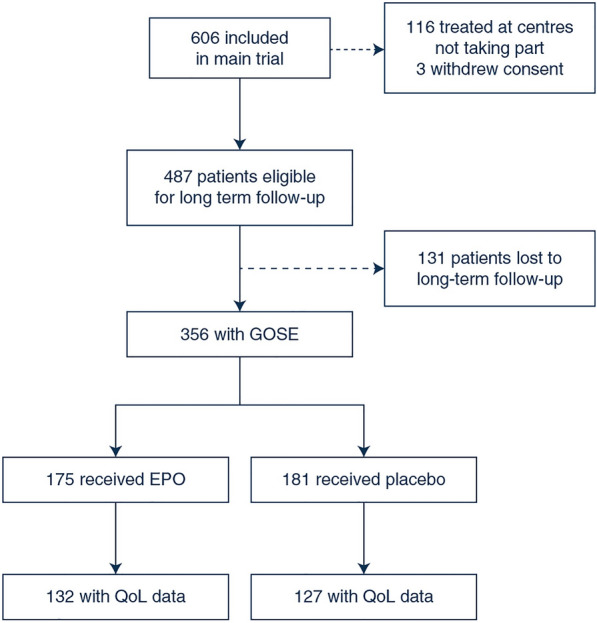
Flow chart of included patients. GOS Glasgow Outcome Scale-Extended, QOL quality of life
Table 1.
Baseline characteristics of patients included in the long-term follow-up
| All patients (n = 356) |
Erythropoietin (n = 175) |
Placebo (n = 181) |
|
|---|---|---|---|
| Age (years)—Median [IQR] | 27.7 [21.4–47.4] | 28.2 [20.7–46.8] | 27.5 [21.5–47.7] |
| Female sex—n (%) | 66 (18) | 30 (17) | 36 (20) |
| Cause of injury—n (%) | |||
| Motor vehicle accident | 158 (44) | 73 (42) | 85 (47) |
| Motorcycle | 41 (12) | 23 (13) | 18 (10) |
| Bicycle | 9 (2) | 4 (2) | 5 (3) |
| Pedestrian | 38 (11) | 19 (11) | 19 (11) |
| Fall/jump | 75 (21) | 42 (24) | 33 (18) |
| Hit by object | 25 (7) | 11 (6) | 14 (8) |
| Other | 10 (3) | 3 (2) | 7 (4) |
| Severe Traumatic Brain Injury—n (%) | 267 (75) | 131 (75) | 136 (75) |
| Moderate Traumatic Brain Injury—n (%) | 89 (25) | 44 (25) | 45 (25) |
| Pupillary response—n (%) | |||
| Both reacting | 293 (82) | 142 (81) | 151 (83) |
| Dilated and both non-reactive | 1 (0.3) | 0 (0) | 1 (1) |
| Both non-reactive | 27 (8) | 11 (6) | 16 (9) |
| One non-reactive | 31 (9) | 20 (11) | 11 (6) |
| Unknown | 4 (1) | 2 (1) | 2 (1) |
| Hypotension—n (%) | 124 (35) | 65 (37) | 59 (33) |
| Hypoxia—n (%) | 77 (22) | 40 (23) | 37 (20) |
| Haemoglobin at baseline (g/L)—Mean (STD) | 118 (21.1) | 119 (20.4) | 117 (21.7) |
| APACHE II Score—Mean (STD) | 20.6 (7.1) | 21 (7.4) | 20.1 (6.7) |
| Injury Severity Score—Mean (STD) | 27 (10) | 27.3 (9.3) | 26.7 (10.6) |
| IMPACT-TBI probability of poor outcome—Mean (STD) | 0.46 (0.23) | 0.47 (0.24) | 0.46 (0.23) |
| Abbreviated Injury Head Score—Median [IQR] | 4 [3–5] | 4 [3–5] | 4 [3–5] |
| Marshall CT classification—n (%) | |||
| I | 13 (4) | 8 (5) | 5 (3) |
| II | 216 (61) | 108 (62) | 108 (60) |
| III | 47 (13) | 21 (12) | 26 (14) |
| IV | 12 (3) | 6 (3) | 6 (3) |
| V | 8 (3) | 3 (2) | 5 (3) |
| VI | 60 (17) | 29 (17) | 31 (17) |
| Time from injury to randomization, hr—Mean (STD) | 17.9 (5.6) | 18 (5.34) | 17.8 (5.85) |
| Time from injury to first dose, hr—Mean (STD) | 19.2 (5.3) | 19.2 (5.1) | 19.1 (5.6) |
APACHE acute physiology and chronic health evaluation, CT computed tomography, IMPACT-TBI International Mission for Prognosis and Analysis of Clinical Trials in traumatic brain injury
Long-term mortality
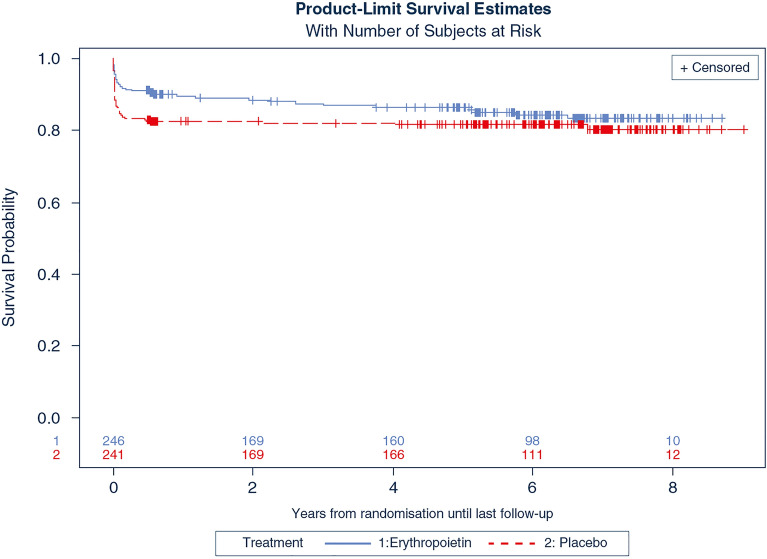
The median time from randomisation to death or last known time alive was 5.7 years for EPO (interquartile range (IQR) 0.6–6.9 years) and 5.3 years (IQR 0.6–6.7 years) for placebo (p = 0.71). Of the 487 patients in our cohort, 63 patients had died by day 180 [EPO 22/246 (9%) vs placebo 41/241 (17%) p = 0.008]. After 2 years, an additional 17 patients had died with mortality status unknown for a further 74 patients. [EPO 35/203 (17.2%) vs 45/210 (21.4%) p = 0.28]. The Kaplan–Meier survival plot is shown in Fig. 2. The unadjusted HR for mortality for EPO compared to placebo was 0.73 (95% CI 0.47–1.14, p = 0.17). The adjusted HR for EPO was 0.68 (95% CI 0.43–1.06, p = 0.08). The univariate HRs of all included subgroups are shown in Fig. 3 and the adjusted HRs in Supplemental Fig. 1 in the ESM. The Kaplan–Meier survival plots of the subgroups are shown in the ESM (Supplemental Figs. 2–9). There was no significant difference in the effect of EPO compared to placebo based on TBI severity and the presence of an intracranial mass lesion (Fig. 2 and Supplemental Fig. 1). The effect of EPO compared to the placebo on long-term mortality in the patients with and without multi-trauma are shown in Fig. 3 and Supplemental Fig. 1. While the risk of death was significantly lower in EPO patients with multi-trauma compared to placebo (p < 0.05), there was no significant evidence of heterogeneity of treatment effect due to any of the two definitions of multi-trauma (Interaction p values 0.13 and 0.08) (Fig. 3).
Fig. 2.
Long-term survival in patients with moderate or severe traumatic brain injury and treated with erythropoietin (EPO) or placebo [hazard ratio EPO vs placebo 0.73, 95% confidence interval 0.47–1.14)
Fig. 3.
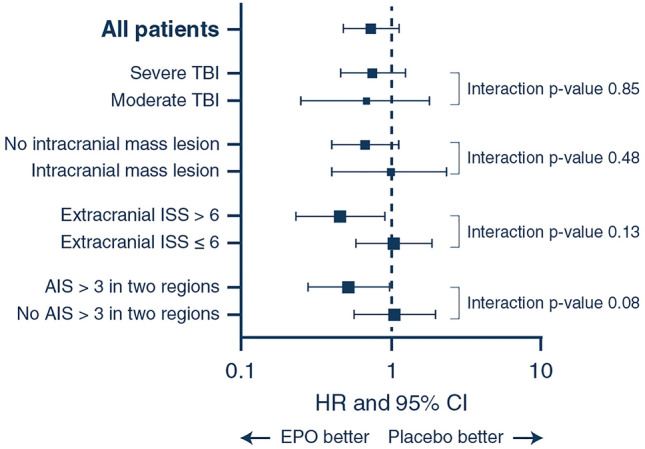
Survival analysis shown as hazard ratios and 95% confidence intervals in all patients included in the EPO-TBI trial. AIS abbreviated injury scale, CI confidence interval, HR hazard ratio, ISS injury severity score, TBI traumatic brain injury
Long-term neurological outcome
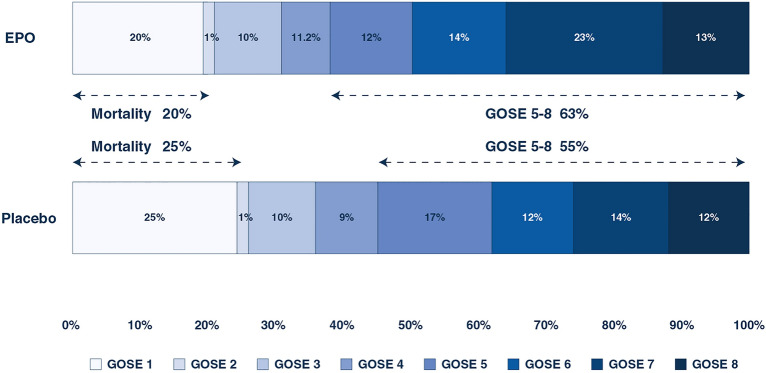
The median time to GOSE assessment was 6.4 years (IQR 5.6–7.1) in the EPO group and 6.2 (IQR 5.3–7.2) a non-significant difference (p = 0.31). The long-term neurological outcomes in the patients treated with EPO and the placebo are shown in Fig. 4. The proportion of patients with a good neurological outcome at follow-up was 110/175 (63%) in the EPO-treated patients and 100/181 (55%) in the placebo-treated patients (Table 2), a non-significant difference (p = 0.14). In the patients with severe TBI, the good outcome rate was 57% in the EPO-treated patients and 50% in the placebo-treated patients (Table 2) (p = 0.27). In the patients with moderate TBI, the good outcome rates were similar. The interaction p-values for heterogeneity of treatment (THE) effect due to TBI severity or presence of a mass lesion was not significant (Table 2). The adjusted analysis showed no difference in outcome between EPO and the placebo for the proportion of good outcomes. When analyzing GOSE as a sliding scale, there was a significantly better outcome with the use of EPO compared to the placebo (Table 2). The analysis adjusting for TBI severity with the IMPACT model showed no difference in the proportion of patients with a good outcome (odds ratio (OR) 1.6, 95% CI 0.97–2.6, p = 0.06) with the use of EPO compared to the placebo. EPO appeared to be associated with a trend for better neurological outcome compared to the placebo in the patients with multi-trauma based on an AIS score of higher than three in two regions compared to those with isolated TBI, as shown in Table 2, but the p values for heterogeneity were not significant.
Fig. 4.
Distribution of neurological long-term outcome by the Glasgow Outcome Scale-Extended in those 356 patients treated with erythropoietin or placebo with complete data on functional outcome. EPO erythropoietin, GOSE Glasgow Outcome Scale-Extended. The median time to GOSE assessment was 6.4 years (IQR 5.6–7.1) in the EPO group and 6.2 (IQR 5.3–7.2) a non-significant difference (p = 0.31)
Table 2.
Long-term Glasgow Outcome Scale, Extended and quality-of-life data in 356 traumatic brain injury patients including subgroups treated with erythropoietin or placebo
| Erythropoietin | Placebo | Relative risk (95% CI) |
Risk difference % (95% CI) |
p value | Interaction p-value |
|
|---|---|---|---|---|---|---|
| Secondary outcomes | ||||||
| Good outcome (GOSE 5–8) | 110/175 (63%) | 100/181 (55%) | 1.14 (0.96–1.35) | 7.6% ( 2.6% to 17.8%) | 0.14 | |
| Good outcome sliding scale | 112/175 (64%) | 94/181 (52%) | 1.23 (1.03–1.47) | 12.1% (1.9% to 22.2%) | 0.02 | |
| Good outcome in subgroups | ||||||
| Severe TBI | 71/124 (57%) | 68/135 (50%) | 1.14 (0.91—1.43) | 6.9% (− 5.2% to 19%) | 0.27 | 0.89 |
| Moderate TBI | 39/51 (76%) | 32/46 (70%) | 1.10 (0.86—1.40) | 6.9% (− 10.8% to 24.6%) | 0.44 | |
| Intracranial mass lesion | 16/32 (50%) | 19/36 (53%) | 0.95 (0.6–1.51) | 2.8% ( 26.6% to 21%) | 0.82 | 0.33 |
| No intracranial mass lesion | 94/143 (66%) | 81/145 (56%) | 1.18 (0.98 1.42) | 9.9% ( 1.3% to 21.1%) | 0.09 | |
| Extracranial ISS > 6 | 64/97 (66%) | 56/100 (56%) | 1.18 (0.94–1.48) | 10% ( 3.6% to 23.5%) | 0.15 | 0.13 |
| Extracranial ISS ≤ 6 | 46/78 (59%) | 44/81 (54%) | 1.09 (0.83–1.43) | 4.7% ( 10.7% to 20%) | 0.55 | |
| AIS > 3 in two regions | 51/76 (67%) | 39/80 (49%) | 1.38 (1.05–1.81) | 18.4% (3.1% to 33.6%) | 0.02 | 0.08 |
| No AIS > 3 in two regions | 59/99 (60%) | 61/101 (60%) | 0.99 (0.79–1.24) | 0.8% ( 14.4% to 12.8%) | 0.91 | |
| Quality of life | ||||||
| EQ 5D-5L utility score, Mean (STD) | 0.776 (0.297) | 0.713 (0.352) | Adjusted mean difference 0.07 (95% CI 0.0–0.15) | 0.12 | ||
| EQ Visual Analog Scale, Mean (STD) | 77.2 (19.8) | 74.6 (22.1) | Adjusted mean difference 3.03 (95% CI 1.94 to 8.00) | 0.32 | ||
AIS abbreviated injury scale, EQ 5D-5L EuroQol Group 5-Dimension Self-Report Questionnaire, CI confidence interval, GOSE Glasgow Outcome Scale-Extended, ISS injury severity score, TBI traumatic brain injury
Quality of life
The EQ-5D utility scores were higher in the EPO-treated groups than in the placebo-treated groups, but the difference was not significant (Table 2). Similar findings were also seen for the EQ VAS scores between the groups. The QOL results are shown in Table 2 and the Supplemental Figs. 10 and 11.
Discussion
In this long-term follow-up of patients with moderate or severe TBI treated with either EPO or a placebo, we found no significant difference in long-term neurological outcome or survival.
We found no evidence of heterogeneity for the treatment effect of EPO in the a priori defined subgroups the severity of TBI, presence of an intracranial mass lesion or whether the patient had multi-trauma in addition to TBI. The point estimated appeared to favor EPO compared to placebo in patients with multi-trauma, but this should be interpreted with caution since these were not pre-defined subgroups of the original trial. The limited sample size suggests a further need to study the use of EPO in patients with TBI and multi-trauma.
Our findings are in line with systematic reviews suggesting beneficial effects and lack of harm from the use of EPO in patients with TBI and major trauma [4, 18]. However, the studies conducted thus far may have had small sample sizes. A new study to confirm or refute the 8% difference in good outcome rates between EPO and a placebo would need to include 1600 patients, while a study that would confirm or refute the 5% difference in mortality would require 2300 patients. We are currently conducting a trial titled EPO Trauma that aims to enrol 2500 patients and provide more robust answers about the effect of EPO on both functional outcome and mortality (NCT04588311).
Interestingly, EPO appeared slightly more effective in the patients with multi-trauma in addition to TBI and those without mass lesions. However, there were limited numbers of patients in these subgroups and these findings may have been due to chance. In addition, the multi-trauma subgroups were defined after the EPO-TBI study was completed and these analysis should be seen as exploratory [7, 17]. Nonetheless, it is possible that the disease mechanism differs slightly in patients with TBI with and without major trauma to other organs. Experimental effects of EPO have included increases in brain tissue oxygen and restoration of cerebral blood flow with correction of tissue ischemia [5]. It may be that occult ischemia is a more important injury mechanism in patients with more severe diffuse injury than in those with evacuated mass lesions. Another possible mechanism could be attenuation of the blood–brain-barrier disruption, which is a known mechanism of ischemic brain injury resulting in edema. It is also possible that systemic inflammation, a contributor to multi-organ failure after multi-trauma is somehow blunted by EPO [19].
We note some changes in patient outcomes over time after TBI. Mortality increased, but on the other hand, the proportion of patients with a good neurological outcome slightly improved. Nonetheless, the long-term mortality rate of 16% in our study is lower than that found in an observational study from the US [20]. Indeed, in the multi-center Transforming Research and Clinical Knowledge (TRACK) in TBI study in the United States of America, 12-month mortality was 31% in the severe TBI patients and 13% in the moderate TBI patients.
In the current study, around 20% of the patients had a poor neurological outcome—defined as a GOSE of 2–4 at long-term follow-up, which highlights the longstanding effects of TBI. This is in line with a French study including a follow-up spanning 8 years, where a poor outcome based on the GOSE was seen in 20% of the patients [21]. This also aligns with data from the TRACK-TBI study, in which poor functional outcome was seen in around 20% of the patients at 12 months in both the moderate and severe TBI groups.
Few studies have assessed long-term QoL after TBI. In the current study, long-term QoL appeared comparable to that found in a recent Dutch study on long-term QoL after injury [22, 23]. This study including almost 5000 patients with various types of trauma requiring hospital care, the mean EQ-5D utility score at two years was 0.79. Factors associated with poor QoL included poor pre-injury QoL, frailty, and female sex. The presence of TBI was associated with cognitive problems. Overall, the scores seen in this study seem comparable to other types of brain injury such as out-hospital cardiac arrest based on patients included in the Targeted Temperature management trial published in 2021, where the EQ-5D based on the VAS was around 0.75. There was a non-significant difference in EQ-5D utility scores between the EPO- and placebo-treated patients. At the 6-months follow-up, the difference in QoL appeared to be slightly higher [24]. Whether this difference is due to chance remains to be determined in larger studies. However, EQ-5D has been shown to be able to detect differences in health status in certain neurological conditions, such as stroke [25]. Finally, the EQ-5D scores seen in the long term were similar to those obtained at 6 months.
Strengths and limitations
This study’s strengths include its multi-center setting, randomized design, and the fact that all the analysis was conducted according to a pre-planned analysis plan in the same way as in the original study. All the outcome assessors were blinded to the treatment intervention. In addition, this was the longest follow-up included in any randomized interventional trial focusing on TBI.
Nonetheless, we recognize certain limitations. Due to the COVID-19 pandemic and local organizational problems, we were unable to include all the countries that took part in the original EPO-TBI trial. In addition, due to differences the workload for obtaining site specific research approvals and research contracts, there was also a considerable variability in the timing of the follow-up. In addition, despite considerable efforts, many patients were lost to followup. This decreased the sample size and may have influenced the results. We note some differences in the clinical characteristics of those included and not included in this follow-up. However, we did not find any difference in the baseline characteristics of the EPO- and placebo-treated patients in both the included and excluded cohorts. Finally, the use of EPO may be associated with side effects. Even though our primary study did not show any difference in major complications, such as thrombotic events, we cannot comment on whether these occurred at a later stage, since these were not followed up [26].
Conclusion
The use of EPO compared to a placebo did not improve overall long-term outcomes or QoL in patients with moderate or severe TBI and in any of the a priori defined subgroups. The limited sample size makes it difficult to make the final conclusions about the use of EPO in TBI and further studies are required.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The EPO-TBI Investigators, the ANZICS Clinical Trials Group: Colin McArthur Lynette Newby: Auckland City Hospital, New Zealand; Frank Van Haren, Shakira Spiller, Mary Nourse, Josie Russell Brown: Canberra Hospital, Australia; Seton Henderson, Jan Mehrtens: Christchurch Hospital, New Zealand; David Silverman, Robyn Hutchinson: Dunedin Hospital, New Zealand; Brent Richards, Mandy Tallott, Jonathan Field: Gold Coast University Hospital, Australia; Markus Skrifvars, Heikki Vartiala, Marianne Eliasson, Mika Koivikko: Helsinki University Central Hospital, Finland; Peter Harrigan, Miranda Hardie, Adam Tolfree: John Hunter Hospital, Australia; Yaseen Arabi, Samir Haddad, Marwan Al Kishi, Ahmad Deeb, Shmeylan Al Harbi, Lolowa Al-Swaidan, Turki Al Moammar, Juliet Lingling, Shella Caliwag, Hanie Richi, Asma Al Jandan: King Abdulaziz Medical City, Kingdom of Saudi Arabia; Stepani Bendel, Sari Rahikainen: Kuopio University Hospital, Finland; Victor Tam, Jacqui Robinson: Liverpool Hospital, Australia; Louise Cole, Leonie Weisbrodt, Rebecca Gresham, Maria Nikas, Anne Richie: Nepean Hospital, Australia; Richard Strickland, Justine Rivett, Sonya Kloeden, Stephanie O’Connor: Royal Adelaide Hospital, Australia; David Cooper, Richard McAllister: Royal Hobart Hospital, Australia; Deborah Barge, Jeffrey Presneill: Royal Melbourne Hospital, Australia; Simon Finfer, Elizabeth Yarad, Simon Bird, Anne O’Connor, Naomi Hammond, Frances Bass: Royal North Shore Hospital, Australia; Melanie Boardman, Sharon Waterson: Royal Perth Hospital, Australia; David Gattas, Heidi Buhr: Royal Prince Alfred Hospital, Australia; Priya Nair, Claire Reynolds, Robyn Tantau: St Vincent’s Hospital Sydney, Australia; David James Cooper, Jasmin Board, Shirley Vallance, Phoebe McCracken, Meredith Young: The Alfred, Australia; Geoffrey Gordon, Stephen Reeves, Sonja Brennan: The Townsville Hospital, Australia; Paul Young, Anna Hunt, Nina Beehre, Hannah Smellie: Wellington Regional Hospital, New Zealand; Vineet Nayyar, Christina Whitehead, Jing Kong, George Bonovas: Christina Whitehead, Jing Kong, George Bonovas, Westmead Hospital, Australia.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. EPO-TBI was supported by grants from the National Health and Medical Research Council of Australia (Grant No. 545902) and the Transport Accident Commission of Victoria (Grant No. D162). MBS received personal research funding from Medicinska Understodsforeningen Liv och Halsa, Finska Lakaresallskapet and Svenska Kulturfonden. AN received support from the Health Research Board of Ireland.
Data availability
The study data will be made available on reasonable request.
Declarations
Conflicts of interest
MBS reports speaker fees from BARD Medical (Ireland). All other authors report that they have no relevant conflicts of interest.
Footnotes
The members of the “EPO-TBI Investigators and the ANZICS Clinical Trials Group” was processed under acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Markus B. Skrifvars, Email: markus.skrifvars@hus.fi
the EPO-TBI Investigators, the ANZICS Clinical Trials Group:
Colin McArthur Lynette Newby, Frank Van Haren, Shakira Spiller, Mary Nourse, Josie Russell Brown, Seton Henderson, Jan Mehrtens, David Silverman, Robyn Hutchinson, Brent Richards, Mandy Tallott, Jonathan Field, Markus Skrifvars, Heikki Vartiala, Marianne Eliasson, Mika Koivikko, Peter Harrigan, Miranda Hardie, Adam Tolfree, Yaseen Arabi, Samir Haddad, Marwan Al Kishi, Ahmad Deeb, Shmeylan Al Harbi, Lolowa Al-Swaidan, Turki Al Moammar, Juliet Lingling, Shella Caliwag, Hanie Richi, Asma Al Jandan, Stepani Bendel, Sari Rahikainen, Victor Tam, Jacqui Robinson, Victor Tam, Sharon Micallef, Louise Cole, Leonie Weisbrodt, Rebecca Gresham, Maria Nikas, Anne Richie, Richard Strickland, Justine Rivett, Sonya Kloeden, Stephanie O’Connor, David Cooper, Richard McAllister, Deborah Barge, Jeffrey Presneill, Simon Finfer, Elizabeth Yarad, Simon Bird, Anne O’Connor, Naomi Hammond, Frances Bass, Melanie Boardman, Sharon Waterson, David Gattas, Heidi Buhr, Priya Nair, Claire Reynolds, Robyn Tantau, David James Cooper, Jasmin Board, Shirley Vallance, Phoebe McCracken, Meredith Young, Geoffrey Gordon, Stephen Reeves, Sonja Brennan, Paul Young, Anna Hunt, Nina Beehre, Hannah Smellie, Vineet Nayyar, Christina Whitehead, Jing Kong, George Bonovas, Christina Whitehead, Jing Kong, and George Bonovas
References
- 1.Raj R, Bendel S, Reinikainen M, Hoppu S, Laitio R, Ala-Kokko T, Curtze S, Skrifvars MB. Costs, outcome and cost-effectiveness of neurocritical care: a multi-center observational study. Crit Care. 2018;22:225. doi: 10.1186/s13054-018-2151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raj R, Bendel S, Reinikainen M, Hoppu S, Luoto T, Ala-Kokko T, Tetri S, Laitio R, Koivisto T, Rinne J, Kivisaari R, Siironen J, Higgins A, Skrifvars MB. Temporal trends in healthcare costs and outcome following ICU admission after traumatic brain injury. Crit Care Med. 2018;46:e302–e309. doi: 10.1097/CCM.0000000000002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocchetti N, Carbonara M, Citerio G, Ercole A, Skrifvars MB, Smielewski P, Zoerle T, Menon DK. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16:452–464. doi: 10.1016/S1474-4422(17)30118-7. [DOI] [PubMed] [Google Scholar]
- 4.French CJ, Glassford NJ, Gantner D, Higgins AM, Cooper DJ, Nichol A, Skrifvars MB, Imberger G, Presneill J, Bailey M, Bellomo R. Erythropoiesis-stimulating agents in critically Ill trauma patients: a systematic review and meta-analysis. Ann Surg. 2017;265:54–62. doi: 10.1097/SLA.0000000000001746. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, Lu M, Pool C, Heavner G, Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, Bailey M, Cooper DJ, Duranteau J, Huet O, Mak A, McArthur C, Pettila V, Skrifvars M, Vallance S, Varma D, Wills J, Bellomo R, Investigators E-T, Group ACT Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet. 2015;386:2499–2506. doi: 10.1016/S0140-6736(15)00386-4. [DOI] [PubMed] [Google Scholar]
- 8.Nichol A, French C, Little L, Presneill J, Cooper DJ, Haddad S, Duranteau J, Huet O, Skrifvars M, Arabi Y, Bellomo R, Investigators E-T, the A, New Zealand Intensive Care Society Clinical Trials G Erythropoietin in traumatic brain injury: study protocol for a randomised controlled trial. Trials. 2015;16:39. doi: 10.1186/s13063-014-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 10.Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma. 1998;15:587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- 11.Murray GD, Barer D, Choi S, Fernandes H, Gregson B, Lees KR, Maas AI, Marmarou A, Mendelow AD, Steyerberg EW, Taylor GS, Teasdale GM, Weir CJ. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J Neurotrauma. 2005;22:511–517. doi: 10.1089/neu.2005.22.511. [DOI] [PubMed] [Google Scholar]
- 12.Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, Mushkudiani NA, Choi S, Maas AI. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J Neurotrauma. 2007;24:270–280. doi: 10.1089/neu.2006.0029. [DOI] [PubMed] [Google Scholar]
- 13.Presneill J, Little L, Nichol A, French C, Cooper DJ, Haddad S, Duranteau J, Huet O, Skrifvars M, Arabi Y, Bellomo R, Investigators E-T, Group ACT Statistical analysis plan for the Erythropoietin in Traumatic Brain Injury trial: a randomised controlled trial of erythropoietin versus placebo in moderate and severe traumatic brain injury. Trials. 2014;15:501. doi: 10.1186/1745-6215-15-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JD, Maas AI. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher N, Balogh ZJ. The definition of polytrauma: the need for international consensus. Injury. 2009;40(Suppl 4):S12–22. doi: 10.1016/j.injury.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Pape HC, Lefering R, Butcher N, Peitzman A, Leenen L, Marzi I, Lichte P, Josten C, Bouillon B, Schmucker U, Stahel P, Giannoudis P, Balogh Z. The definition of polytrauma revisited: an international consensus process and proposal of the new 'Berlin definition'. J Trauma Acute Care Surg. 2014;77:780–786. doi: 10.1097/TA.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 17.Skrifvars MB, Bailey M, French C, Presneill J, Nichol A, Little L, Duranteau J, Huet O, Haddad S, Arabi Y, McArthur C, Cooper DJ, Bellomo R, investigators E-T, the ACTG Erythropoietin in patients with traumatic brain injury and extracranial injury-A post hoc analysis of the erythropoietin traumatic brain injury trial. J Trauma Acute Care Surg. 2017;83:449–456. doi: 10.1097/TA.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Wang AJ, Chen Y, Zhao G, Jiang Z, Wang X, Shi D, Zhang T, Sun B, He H, Williams Z, Hu K. Efficacy and safety of erythropoietin for traumatic brain injury. BMC Neurol. 2020;20:399. doi: 10.1186/s12883-020-01958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkose S, Ozgurer A, Bulut M, Koksal O, Ozdemir F, Ozguc H. Relationships between markers of inflammation, severity of injury, and clinical outcomes in hemorrhagic shock. Adv Ther. 2007;24:955–962. doi: 10.1007/BF02877699. [DOI] [PubMed] [Google Scholar]
- 20.McCrea MA, Giacino JT, Barber J, Temkin NR, Nelson LD, Levin HS, Dikmen S, Stein M, Bodien YG, Boase K, Taylor SR, Vassar M, Mukherjee P, Robertson C, Diaz-Arrastia R, Okonkwo DO, Markowitz AJ, Manley GT, Investigators T-T. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol. 2021;78:982–992. doi: 10.1001/jamaneurol.2021.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruet A, Bayen E, Jourdan C, Ghout I, Meaude L, Lalanne A, Pradat-Diehl P, Nelson G, Charanton J, Aegerter P, Vallat-Azouvi C, Azouvi P. A detailed overview of long-term outcomes in severe traumatic brain injury eight years post-injury. Front Neurol. 2019;10:120. doi: 10.3389/fneur.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Munter L, Geraerds A, de Jongh MAC, van der Vlegel M, Steyerberg EW, Haagsma JA, Polinder S. Prognostic factors for medical and productivity costs, and return to work after trauma. PLoS One. 2020;15:e0230641. doi: 10.1371/journal.pone.0230641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Munter L, Polinder S, Havermans RJM, Steyerberg EW, de Jongh MAC. Prognostic factors for recovery of health status after injury: a prospective multicentre cohort study. BMJ Open. 2021;11:e038707. doi: 10.1136/bmjopen-2020-038707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knott RJ, Harris A, Higgins A, Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, Bailey M, Cooper DJ, Duranteau J, Huet O, Mak A, McArthur C, Pettila V, Skrifvars MB, Vallance S, Varma D, Wills J, Bellomo R. Cost-effectiveness of erythropoietin in traumatic brain injury: a multinational trial-based economic analysis. J Neurotrauma. 2019;36:2541–2548. doi: 10.1089/neu.2018.6229. [DOI] [PubMed] [Google Scholar]
- 25.Payakachat N, Ali MM, Tilford JM. Can the EQ-5D detect meaningful change? A systematic review. Pharmacoeconomics. 2015;33:1137–1154. doi: 10.1007/s40273-015-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skrifvars MB, Bailey M, Presneill J, French C, Nichol A, Little L, Duranteau J, Huet O, Haddad S, Arabi Y, McArthur C, Cooper DJ, Bellomo R, investigators E-T, the ACTG Venous thromboembolic events in critically ill traumatic brain injury patients. Intensive Care Med. 2017;43:419–428. doi: 10.1007/s00134-016-4655-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data will be made available on reasonable request.