Abstract
In the present study, the effect of coffee leaf extract (CLE) on in vitro enzyme inhibition was studied. Furthermore, its impact on the high-fat diet (HFD)-induced obese mice (C57BL/6) at the levels of 100 and 200 mg/kg body weight along with positive control (orlistat) and the normal group maintained with starch-fed diet (SFD) was observed. CLE had significant α amylase and lipase enzyme inhibitory properties. In HFD-induced obese mice, treatment with CLE significantly reduced the body weight gain. The investigation demonstrated that CLE administration lowered blood glucose, total cholesterol, total triglycerides and LDL levels while increasing the HDL levels. It reduced the development of fatty liver by reducing hepatic fat accumulation and decreased the fat cell size in the adipose tissue. Further, CLE significantly increased the liver antioxidant enzyme activities and lowered the levels of hepatotoxicity markers in the serum when compared to the HFD-fed mice. The treatment also downregulated the mRNA expression of lipogenic transcription factors (SREBP-1c, CEBP-α) and enzymes (ACC, FAS) than HFD. Overall, the results indicate that coffee leaves have anti-obesity potential and can be used as functional ingredients in the development of innovative products for managing lifestyle disorders such as obesity.
Keywords: Coffee leaves, Histopathology, Lipogenic genes, Obesity, Serum lipid profile
Introduction
The hallmarks of metabolic syndrome, a group of metabolic disorders, are hyperglycemia, hypertension, hypertriglyceridemia, etc. Obesity is one of the important chronic metabolic disorders increasing at an alarming rate worldwide due to diet and lifestyle changes. It is observed to increase the incidence of type 2 diabetes, develops insulin resistance, hypertension and cardiovascular diseases (Geetha et al. 2022). Increased levels of serum total cholesterol, low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG), combined with a decrease in high-density lipoprotein cholesterol (HDL-C), are the major characteristics of obesity-associated hyperlipidemia. Diet plays a significant role in the development of obesity. Human obesity has been linked to dietary fat intake, and experimental studies have also suggested that a high-fat diet majorly contributes to increased oxidative stress (Ibrahim et al. 1997).
One of the key factors contributing to the development of obesity is oxidative stress which occurs due to an imbalance in the antioxidants and reactive oxygen species (ROS) in the body. ROS have a high chemical reactivity and can cause substantial oxidative damage to cells when present in concentrations above a threshold level of the normal functioning of cells (Chan et al. 2006; Pang et al. 2007). The human body has developed a highly complicated antioxidant defense system to protect cells and organs from ROS. It comprises multiple endogenous antioxidant enzymes such as glutathione peroxidase, superoxide dismutase and catalase. Besides that diets rich in antioxidants are the exogenous components functioning synergistically and interactively to neutralize free radicals (Block 1991; Wang and Luo 2007). Long-term consumption of foods with a high energy density promotes an aberrant lipid metabolism with higher levels of TC and TG according to epidemiological and experimental investigations (Prentice and Jebb 2003, Yang et al. 2019). Therefore, modifying the regulatory network of transcription factors related to lipid metabolism could be a useful strategy to reduce lipid build-up and consequent obesity-related conditions.
Locals in the countries where coffee plants are traditionally cultivated have been drinking tea-like beverages made from coffee leaves for more than 200 years (Patay et al. 2016). According to recent studies, coffee leaves are known to have anti-hypertensive and anti-inflammatory properties and they are shown to increase insulin levels and decrease insulin resistance in metabolic syndrome rats (Chen et al. 2019; Widyastuti et al. 2020; Ji et al. 2021). The abundance of bioactive components in coffee leaves has recently drawn attention to their health advantages for humans. The phytochemicals found in coffee leaves include alkaloids, flavonoids, phenolics, xanthonoid, phytosterols, amino acids, tannins, anthocyanins and terpenes (Campa et al. 2012; Chen et al. 2018). Chlorogenic acids (CGAs), sinapic acids, caffeic acids and p-coumaric acids are the main phenolic acids found in coffee leaves along with rutin, quercetin and its glycosides, while caffeine, trigonelline, theobromine and theophylline are the chief alkaloids from coffee leaves. These compounds in coffee leaves have already been known for antioxidant, anti-inflammatory, anti-diabetic, anti-hypertensive effects, etc. (Martins et al. 2014; Patil et al. 2022b). As a result, coffee leaves have the potential to be a unique therapeutic beverage and act as an alternative to traditional tea consumption and herbal teas to control lifestyle problems like obesity.
Therefore, in the current study, we have assessed the effect of coffee leaf extract on in vitro enzyme inhibition and in vivo serum lipid profile, blood glucose levels, antioxidant enzyme activities, serum hepatotoxicity markers and gene expression of key lipogenic enzymes in high-fat diet-induced C57BL/6 obese mice.
Materials and methods
Chemicals
Orlistat was obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The raw materials required for the preparation of the animal diet were acquired from HI-MEDIA (Mumbai, India) and casein from Nimish Corporation, Mumbai. Kits for plasma biochemical analysis were obtained from Agappe Diagnostics Ltd. (Kerala state, India). cDNA synthesis kit was obtained from Takara Bio Inc. (Shiga, Japan #RR037A).
Processing of coffee leaves and preparation of coffee leaf extracts (CLE)
The intact coffee leaves were sorted from the infected/damaged leaves and washed with clean water. After that, the leaves were blanched for 30 s followed by soaking them in cold water for 30 min. The leaves were then dried in a cross-flow drier (45 ± 2 °C) for 5–6 h, powdered and stored in airtight containers. For the preparation of coffee leaf extracts, 10 g dried leaf powder was brewed with 100 ml water (80–85 °C) for 30 min, filtered and allowed to freeze dry. The obtained freeze-dried powder was carefully collected and stored in an amber-colored air-tight container at − 20 °C until further use. The freeze-dried powder was freshly weighed and dissolved in water every time for preparation of the required dosage during animal experimentation.
α-Amylase and lipase inhibitory activity
With a few minor modifications, α-amylase inhibitory activity was carried out as described by Kwon et al. (2006). 100 µl of varying concentrations of CLE and 400 µl of 0.1 M phosphate buffer saline (pH 6.9) were added to the test tubes, followed by 500 µl of 1% starch solution (substrate) and 500 µl of α-amylase enzyme solution (2 mg/ml). After incubation for 10 min in the dark (27 °C ± 1), 1 ml of glucose reagent (M/s Agappe Diagnostics Ltd, India) was added and the tubes were further incubated for 30 min. Absorbance was then measured at 505 nm using a spectrophotometer.
The estimation of lipase activity was done by the method described by Weibel et al. (1987) with certain modifications. Firstly, to prepare the substrate emulsion, lecithin (1 mM), cholesterol (0.1 mM), bovine serum albumin (15 mg/ml), NaCl (100 mM), CaCl2 (1 mM) and 2 mM Tris–HCl (pH 8) were mixed with olive oil and ultra-sonicated. The mentioned composition of the emulsion was chosen to closely resemble the in vivo conditions. Orlistat a known lipase inhibitor was used as a positive control and was prepared in dimethyl (DMSO) at a concentration of 2 mg/ml. Briefly, to 1.5 ml of substrate solution varying concentrations of coffee leaf extracts were added. Subsequently, porcine pancreatic lipase (EC# 3.1.1.3) at a concentration of 1 mg/ml was added to initiate the reaction. The reaction mixtures were then incubated for 15 min at 37 °C followed by the addition of 1.5 ml ethanol to terminate the reaction. The amount of fatty acid liberated was assessed by titrating against 0.02 N NaOH.
Animal experiment
A male C57BL/6 mouse strain was used for the present study. It is a widely used model for studying diet-induced obesity as it closely parallels the progression of obesity and its related complications in humans (Kleinert et al. 2018). The experimental procedures and procurement of male mice (C57BL/6, 20–22 g, 6–8 weeks old) conformed to clearance from the institutional animal ethical committee (IAEC/216/2021). Sincere attempts were taken to reduce the suffering, and the numbers of animals were maintained to get the statistical data comparisons. Polycarbonate cages were used to house the mice and upheld at 12 h light/dark cycle with ad libitum water and food.
-
Animal grouping
After acclimatization for 1 week, the animals were divided into five groups (n = 6): (1) starch-fed diet (SFD), (2) high-fat diet (HFD), (3) HFD with positive control (PC) orlistat (10 mg/kg body weight), (4) HFD with CLE1 (100 mg/kg body weight) and (5) HFD with CLE2 (200 mg/kg body weight). The dosage of the coffee leaf extract (CLE) was based on the amount of total polyphenols (TP) present in them as coffee leaves are a rich source of polyphenols, especially chlorogenic acid (CGA). The TP in the freeze-dried powder of the CLE was determined, and the dosage was selected based on the previous literature (Singh et al. 2020). A daily dose (200 μl) of CLE and orlistat was given to the animals through oral gavaging. The animals were fed with AIN-76 diet (American Institute of Nutrition). The detailed diet composition is given in Table 1.
-
Evaluation
The body weight of the animals was recorded weekly throughout the experiment. Fecal matter was collected for fat estimation during the experiment. Mice were killed after 90 days of treatment. They were euthanized by carbon dioxide inhalation, and the blood was drawn by cardiac puncture. The collected blood was then subjected to serum separation. The liver was harvested, washed, weighed and stored at − 80 °C until further analysis. Samples of liver and adipose tissues for histological studies were fixed in 10% formalin.
Table 1.
AIN-76 diet composition
| Ingredients (g/kg) | SFD (g) | 30% HFD (g) |
|---|---|---|
| Starch | 653 | 453 |
| Casein | 200 | 200 |
| Vitamin mix | 10 | 10 |
| Mineral mix | 35 | 35 |
| Choline chloride | 2 | 2 |
| DL Methionine | 0.5 | 0.5 |
| Groundnut oil | 100 | 100 |
| Lard | – | 200 |
SFD starch-fed diet, HFD high-fat diet
Fecal fat content
Fat content from the fecal matter collected every week was estimated according to the methods of Geetha et al. (2022) from 1 g of lyophilized fecal matter and expressed as g/100 g.
Blood glucose and serum lipid profile
The blood glucose levels and lipid profile (TG, cholesterol, LDL-C and HDL-C) were analyzed in the overnight fasted and killed animals. The animals underwent overnight fasting, next day blood was drawn from the tail vein, under anesthesia before the killing, and the blood glucose was estimated using a glucometer (M/s ARKRAY Healthcare Pvt. Ltd). The total triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LD-C) were analyzed in the serum collected, as per the instructions provided in the respective kits (M/s Agappe Diagnostics Ltd., India).
Intraperitoneal glucose tolerance test (IPGTT) and area under curve (AUC)
IPGTT was performed 1 week before killing the animals. After overnight fasting, d-glucose (2 g/kg body weight) was administered intraperitoneally in sterile saline. Blood was drawn from the tail vein to determine the blood glucose levels at 0 min (before administration of glucose) and 30, 60 and 120 min (after administration of glucose) using a glucometer (M/s ARKRAY Healthcare Pvt. Ltd.). The values are presented in the form of AUC (Andrikopoulos et al. 2008).
Histological analysis of liver and adipose tissues
The liver and adipose tissue samples were fixed in paraffin and sectioned (4–10 µm) after being preserved in 10% formalin. Further, the sections were deparaffinized with 100% xylenol, hydrated with ethanol at progressively lower concentrations and stained with H&E (Talawar et al. 2020). After that, the stained sections were examined at 10 × magnification and digital images were taken under a light microscope.
Serum hepatotoxicity markers
Hepatotoxicity markers such as serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase enzymes (ALP) and creatinine in the serum were estimated using the commercially available kits from M/s Agappe Diagnostics Ltd., India.
Antioxidant enzyme activities
Catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) antioxidant enzyme activities were examined in the protein extract from approximately 100 mg of liver tissues, and activities were represented in terms of units/mg of protein (Aebi 1984; Flohé and Günzler 1984; Flohi and Tting 1984).
RNA extraction, cDNA synthesis and gene expression analysis
Following the procedure outlined by Billing et al. (2017), total RNA (100 mg, RNA later processed) was extracted from animal liver tissues. Nano-drop was used to quantify the RNA, and according to the instructions indicated in the cDNA synthesis kit (Takara Bio Inc., Shiga, Japan #RR037A), 1 μg of RNA was used for synthesizing cDNA.
Following the instructions given by the manufacturer (Takara Bio Inc., Shiga, Japan), gene expression of CEBP-α (CCAT-enhancer-binding protein-alpha), SREBP-1c (sterol regulatory element-binding protein-1c), ACC (Acetyl Coenzyme A Carboxylase) and FAS (fatty acid synthase) was performed using CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). In brief, the reaction mixture contained cDNA, forward primer, reverse primer, SYBR 2X buffer and double-distilled water to a total volume of 20 μl. The qPCR was performed for 40 cycles, using the following conditions for each cycle: 95 °C for 5 min, 95 °C for 10 s, 60 °C for 20 s and 72 °C for 30 s. After the normalization with β-actin, the gene expression was assessed as per the method. The acquired normalized values were shown as mean from triplicate samples of three independent runs.
Procurement of the primer sequences for the genes was done from Bioserve Biotechnologies Pvt. Ltd., India. Primer sequences used for PCR analysis were as follows: SREBP-1c, TGACCCGGCTATTCCGTGA (forward) and CTGGGCTGAG CAATACAGTTC (reverse); CEBP-α, TCACTTGCAGTTCCAGATCG (forward) and TTGACCAAGGAGCTCTCAGG (reverse); ACC, ATGGGCGGAATGGTCTCTTTC (forward) and TGGGGACCTTGTCTTCATCAT (reverse); and FAS, GGAGGTGGTGATAGCCGGTAT (forward) and TGGGTAATCCATAGAGCCCAG (reverse).
Statistical analysis
From at least three independent experiments, all the data were expressed as means with standard deviations (± SD) with p < 0.05 using Microsoft Excel software 2013 (Microsoft Corporation, Redmond, Washington, USA) and Graph Pad Prism software version 7.0 (Graph Pad Prism Inc., San Diego, California). One-way analysis of variance (ANOVA) was used to analyze the experimental data, and Duncan’s multiple range test (DMRT) was used to evaluate sample differences.
Results and discussion
In vitro anti-obesity potential of CLE
Firstly, to investigate the anti-obesity potential of CLE in vitro enzyme inhibitory experiments were carried out which include the α-amylase and lipase inhibitory assay. We have assessed the α-amylase and lipase-inhibiting properties of CLE in comparison with acarbose and orlistat as the positive control, known drugs for the respective enzyme inhibition. The amount of extract needed to inhibit 50% of the enzyme activity is known as the IC50 value. The results revealed that the CLE could inhibit α-amylase and showed an IC50 value of 120.86 ± 2.56 µg in comparison with its standard acarbose having an IC50 value of 40.9 ± 0.4 µg. Lipase inhibition assay displayed an IC50 value of 211.89 ± 1.96 µg when compared to the standard drug orlistat which had an IC50 value of 158.37 ± 1.2 µg demonstrating that CLE in its native form can lower fat absorption by suppressing lipase activity.
Coffee leaves are known to have antioxidant as well as anti-hyperglycemic properties (Nurmasari et al. 2020). Hyperglycemia is one of the key factors involved in the development of obesity; therefore, suppressing hyperglycemia can help in preventing the onset of obesity and its related complications. There are numerous approaches to control hyperglycemia one of which comprises the inhibition of hydrolyzing enzymes such as α-amylase. It is an important enzyme of the digestive system that hydrolyzes polysaccharides into smaller oligosaccharides and therefore inhibition of this enzyme will cause a delay in the breakdown of polysaccharides from food and its subsequent absorption in the body. Lipase helps in the absorption of fat by acting on the triglycerides and breaking them down into monoglycerides and fatty acids. These free fatty acids act as a substrate for the synthesis of glucose which further increases the blood glucose levels. Our studies show that coffee leaf extract could significantly inhibit the activities of α-amylase as well as lipase. However, further in vivo evidence is required to elucidate the mode of action and prove its anti-obesity potential.
Effect of CLE on body weight and fecal fat in high-fat diet-induced obese mice
C57BL6 mice are one of the ideal models to study obesity. In the present study, obesity is induced by the supplementation of a high-fat diet to C57BL6 mice which is characterized by an increase in body weight. Figure 1a illustrates the images of mice from five different experimental groups where the change in body weight is visibly clear, while Fig. 1b represents changes in the weight of the mice during an experimental period of 90 days. The body weight of the animals in the HFD group was increased from 21.68 to 37.4 g, while for the SFD group, it was increased from 20.4 to 33.4 g. A continuous increase in body weight was observed in the group fed with HFD compared to the control group (SFD). PC, CLE1 and CLE2 had final body weights of 32.7 g, 33.03 g and 31.4 g, respectively, which is similar to SFD.
Fig. 1.

Effect of coffee leaf extract administration on a body weight, b body weight during the experimental duration, c body weight gain of high-fat-fed C57BL6 mice. *Data values are means ± SD (n = 6). SFD starch-fed diet, HFD high-fat diet, PC positive control (orlistat; 10 mg/kg body weight), CLE1 coffee leaf extract (100 mg/kg body weight), CLE2 coffee leaf extract (200 mg/kg body weight)
All the groups exhibited gain in body weight during the experimental period (Fig. 1c). The HFD group showed the highest body weight gain compared to the other groups, whereas treatment with PC and CLE robustly lowered the body weight gain when compared to the HFD. It can also be observed that the experimental groups had a dose-dependent effect on the body weights. The results indicate that CLE had a stronger impact on body weight reduction which could be as a result of reduced absorption of fat from the diet due to the presence of bioactives with lipase inhibitory properties. It might also result from preventing fat buildup in the adipose tissue, and therefore, further exploration of the mode of action is necessary. Similarly, excretion of fecal fat was observed to be more in the PC (10.52 ± 1.44%) and experimental groups (CLE1: 8.86 ± 1.05% and CLE2: 9.2 ± 1.11%) as compared to the HFD group (7.51 ± 0.56%) as the reduction in absorption of fat from the diet leads to bulkier fecal matter.
Effect of CLE on blood glucose and serum lipid profile
As shown in Fig. 2, blood glucose levels in the HFD group were higher than those in the SFD group, While CLE significantly lowered the glucose levels more than HFD. Hyperglycemia can generate free radicals increasing oxidative stress by glycation of proteins and auto-oxidation and potentially weaken the antioxidative machinery by glycating the enzymes that neutralize free radicals (Ceriello 2000; Jakus 2000). Our studies demonstrate that CLE controlled the blood glucose levels and reduced hyperglycemia in high-fat-induced obese mice.
Fig. 2.

Influence of coffee leaf extract administration on blood glucose levels of high-fat-fed obese mice. *Data values are means ± SD (n = 6). Columns not sharing common alphabets differ significantly at p < 0.05. SFD starch-fed diet, HFD high-fat diet, PC positive control (orlistat; 10 mg/kg body weight), CLE1 coffee leaf extract (100 mg/kg body weight), CLE2 coffee leaf extract (200 mg/kg body weight)
Changes in serum lipid profile are represented in Fig. 3. Consumption of dietary fat is seen to alter the serum lipid levels as mice in the HFD group displayed a significant increase in the levels of serum TC, TG and LDL in comparison with the SFD group, whereas the serum HDL level of HFD group was lower than SFD. CLE administration showed a significant decrease in the serum TC, TG and LDL levels and an increase in the serum HDL levels in a dose-dependent manner. One of the important causes of metabolic syndrome is excessive fat/high-calorie diet consumption resulting in increased cholesterol and triglyceride levels in the body (Golan et al. 1998). Hypertriglyceridemia is known to cause other complications such as diabetes mellitus, hypertension and cardiovascular diseases (Grundy and Denke 1990). Dyslipidemia with high TG, LDL, TG/HDL-C ratio, TC/HDL-C ratio is seen in people with metabolic syndrome (Castellani 2004).
Fig. 3.
Changes in serum lipid profile of high-fat-fed obese mice on coffee leaf extract administration. *Data values are means ± SD (n = 6). Columns not sharing common alphabets differ significantly at p < 0.05. SFD starch-fed diet, HFD high-fat diet, PC positive control (orlistat; 10 mg/kg body weight), CLE1 coffee leaf extract (100 mg/kg body weight), CLE2 coffee leaf extract (200 mg/kg body weight)
In our study, administration of CLE restored the serum lipid profile to normal. This might be because of the synergistic effects of phenolics and alkaloids found in coffee leaves. Chlorogenic acid a major phenolic found in coffee leaves is known to play a significant role in maintaining the body’s lipid and glucose metabolism (Pimpley et al. 2020). Similarly, other bioactives from coffee leaves may also contribute to this effect (Patil et al. 2022b). Thus, consumption of coffee leaf extracts or products developed from it would lower the risk of metabolic syndrome by increasing the HDL-C and reducing the TG and TC concentrations.
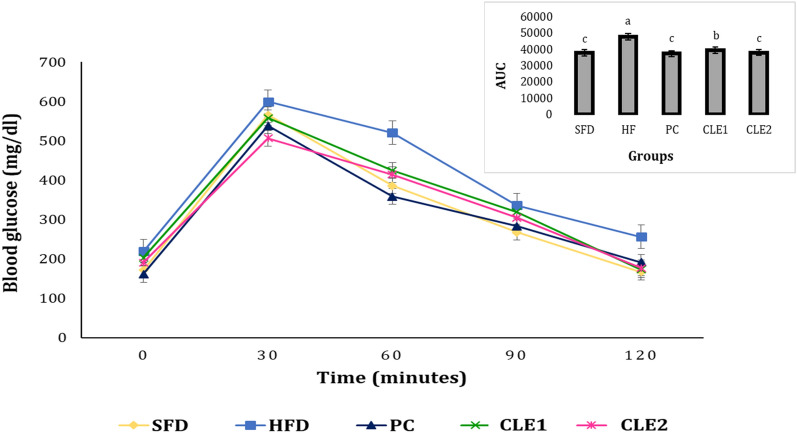
IPGTT and AUC
IPGTT measures the clearance of an intraperitoneally injected glucose load from the body and detects disturbances in glucose metabolism. It is very important to study glucose tolerance in obese conditions as being overweight can cause hyperglycemia. Figure 4 shows blood glucose levels at various time intervals after administration of D-glucose (2 g/kg body weight) up to 120 min, and AUC is also used to present the values. The blood glucose levels were highest at 30 min after administration of intraperitoneal glucose and reduced in a time-dependent manner. It was found that CLE-treated groups were able to bring their blood sugar levels to normal and reach baseline early when compared to the HFD group. The change in the AUC of glucose levels also indicates the same where the AUC of HFD is higher than the SFD and experimental groups. Thus, glucose intolerance was observed more in the HFD group and treatment with CLE decreased intolerance and improved glucose sensitivity. Regulation of fasting and postprandial hyperglycemia is crucial for keeping blood sugar levels under control because hyperglycemia causes insulin resistance and beta cell damage, which is known as glucotoxicity (Luquet et al. 2005). The current findings suggest that CLE may provide a suitable therapeutic approach to managing metabolic syndrome as it has a protective effect against the onset of obesity-related high blood glucose and insulin resistance.
Fig. 4.
Effect of coffee leaf extract administration on intraperitoneal glucose tolerance test (IPGTT) with area under curve (AUC). *Data values are means ± SD (n = 6). Columns/means in the line graph not sharing common alphabets differ significantly (p < 0.05). SFD starch-fed diet, HFD high-fat diet, PC positive control (orlistat; 10 mg/kg body weight), CLE1 coffee leaf extract (100 mg/kg bod weight), CLE2 coffee leaf extract (200 mg/kg body weight)
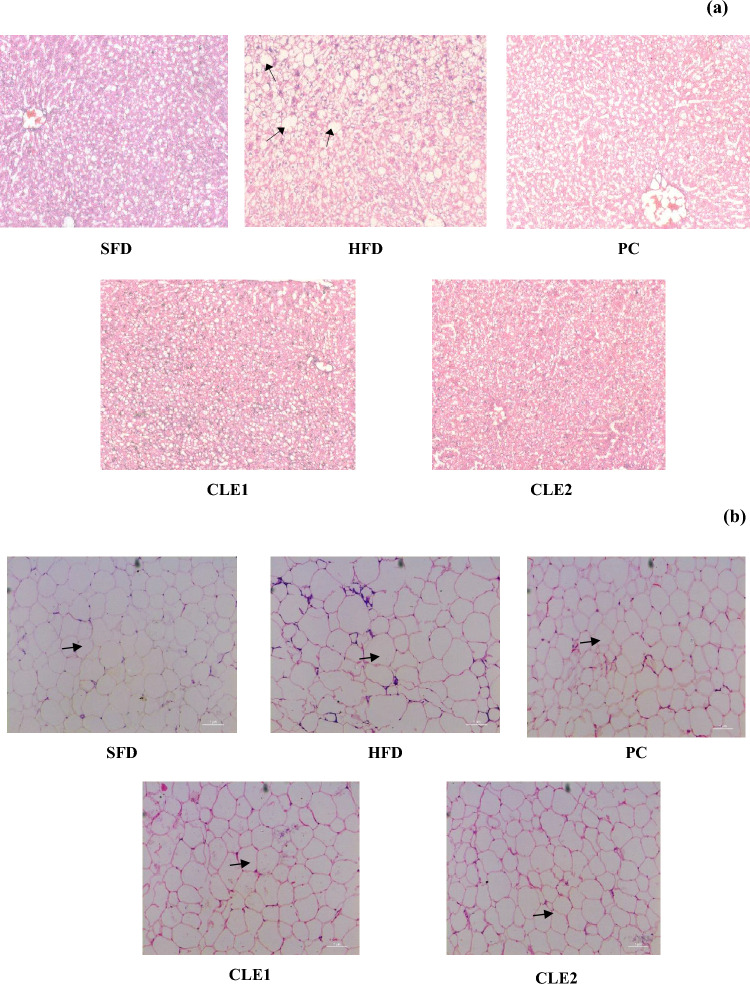
Histological changes in liver and adipose tissues
Figure 5a and b displays histological analysis of the liver and adipose tissues, respectively. The liver sections from the SFD group displayed normal histology, whereas lipid droplet (vacuole-like structure) accumulation is seen in the HFD group as indicated by the black arrows. The PC and experimental groups displayed a reduced number of lipid droplets as compared to the HFD group indicating that CLE reduces the accumulation of fat in liver cells. Inflammation brought on by hepatic fat accumulation leads to an imbalance in lipid metabolism and a fatty liver, which are linked to metabolic syndrome, insulin resistance and obesity (Rohla and Weiss 2014).
Fig. 5.
Influence of coffee leaf extract treatment on histology of a liver and b adipose tissues. *SFD starch-fed diet, HFD high-fat diet, PC positive control (orlistat; 10 mg/kg body weight), CLE1 coffee leaf extract (100 mg/kg body weight), CLE2 coffee leaf extract (200 mg/kg body weight)
The H&E staining of the adipose tissue shows that the HFD group contained fat cells that were noticeably larger than those of the SFD group as denoted by the black arrows. In the PC and experimental group, the adipose tissue histology was similar to the control group as they had smaller fat cells than those of the HFD group. Excessive adipose tissue is linked to increased fat storage and adipose cell hypertrophy, which raises glucose tolerance, fasting glucose levels, and lipid profiles, and also activates several inflammatory markers (Bansal et al. 2012). These findings imply that CLE inhibits HFD-induced hyper-adiposity, which reduces insulin resistance and a persistent low-grade inflammatory state.
Effect of CLE on serum levels of hepatotoxicity markers and liver antioxidant enzyme activities
Serum hepatotoxicity markers The SGPT, SGOT, ALP and creatinine levels are represented in Table 2. The concentration of these metabolites was significantly higher in the HFD group than in the SFD group, while PC and CLE-treated groups showed reduced SGOT, SGPT, ALP and creatinine levels up to the values of the SFD group. This indicates that there is no damage to the vital organs. Hussain et al. (2021) revealed that a polyphenol-enriched complex plant extract reduced serum levels of SGOT and SGPT in high-fat diet-induced obese mice. Similarly, in a study investigating the effects of Xylopia parviflora seed and Aframomum citratum fruit extract on diet-induced obesity, the levels of plasma SGOT, SGPT, ALP and creatinine indicated no liver and kidney damage (Nwakiban et al. 2022).
Table 2.
Effect of CLE administration on hepatotoxicity markers in the serum and antioxidant enzyme activities in the liver
| SFD | HFD | PC | CLE1 | CLE2 | |
|---|---|---|---|---|---|
| Serum hepatotoxicity markers | |||||
| SGPT (U/L) | 8.615 ± 0.77c | 24.391 ± 1.14a | 13.698 ± 1.09b | 11.146 ± 1.00bc | 9.89 ± 0.80c |
| SGOT (U/L) | 12.056 ± 1.60d | 21.176 ± 1.68a | 17.921 ± 0.66b | 15.685 ± 0.79c | 14.387 ± 1.38c |
| ALP (U/L) | 15.925 ± 3.69e | 50.856 ± 3.01a | 25.077 ± 2.88d | 35.913 ± 2.12b | 30.125 ± 2.56c |
| Creatinine (mg/dL) | 0.721 ± 0.10c | 1.14 ± 0.15a | 0.818 ± 0.11c | 0.913 ± 0.12b | 0.659 ± 0.10d |
| Liver antioxidant enzyme activities | |||||
| GPx (unit activity/min/mg protein) | 71.721 ± 9.97c | 41.011 ± 5.87d | 80.013 ± 4.67a | 70.179 ± 6.76c | 74.376 ± 8.03b |
| SOD (U/mg of protein) | 0.594 ± 0.08c | 0.412 ± 0.06d | 0.721 ± 0.05ab | 0.704 ± 0.02b | 0.739 ± 0.07a |
| Catalase (U/mg of protein) | 2.927 ± 0.33b | 0.913 ± 0.11c | 5.233 ± 0.27a | 2.707 ± 0.30b | 2.922 ± 0.22b |
SFD starch-fed diet, HFD high-fat diet, PC positive control (orlistat; 10 mg/kg body weight), CLE1 coffee leaf extract (100 mg/kg body weight), CLE2 coffee leaf extract (200 mg/kg body weight)
*Data values are means ± SD (n = 6). Rows not sharing common alphabets differ significantly at p < 0.05
Serum glutamic pyruvic transaminase (SGPT) and serum glutamic-oxaloacetic transaminase (SGOT) are primarily found in the liver. These enzymes catalyze the transfer of amino groups between amino acids and α-keto acids. Alkaline phosphatase enzyme (ALP) is yet another key liver enzyme that acts as a catalyst in important processes for the normal functioning of the body. Thus, elevated levels of these enzymes in the serum indicate damage to the liver tissue. Creatinine is a waste product of creatinine (supplying energy to muscles) which is removed from the body by the kidneys. Hence, elevated levels of creatinine in the serum indicate damage to the kidney.
During obesity condition, there is an increase in hepatic fat accumulation, which results in elevated levels of these biomarkers, which in turn causes the development of fatty liver disease and hepatic inflammation (Rohla and Weiss 2014). Lipid accumulation in the liver often leads to the development of non-alcoholic fatty liver disease (NAFLD) which is strongly associated with metabolic syndrome and obesity condition. The results from the present investigation indicated the elevation of ALP, SGPT, SGOT and creatinine biomarkers among the HFD group as a consequence of liver and kidney damage condition, whereas treatment with CLE ameliorated the progression of liver and kidney damage by lowering the mentioned hepatotoxicity markers and also improved glucose homeostasis, inflammation and hepatic lipid metabolism in comparison with HFD-induced obese mice.
Liver antioxidant enzyme activities The activities of antioxidant enzymes like SOD, CAT and GPx are depicted in Table 2. A significant decrease in the activities of SOD, CAT and GPx was observed in the liver of the HFD group in comparison with those of the SFD group. It appears that the administration of CLE causes a significant increase in the activities of antioxidant enzymes when compared to the HFD group. Moreover, the antioxidant activity increased in a dose-dependent manner in the treated groups. In a parallel study conducted by De Oliveira et al. (2015), it was observed that a polyphenol-rich Acai seed extract restored the activities of SOD, CAT and GPx in high-fat diet-induced C57BL6 mice. Kim et al. (2013) reported a significant increase in the activities of SOD and GPx following treatment with Allium sativum L. stem extract.
High dietary fat consumption has been linked to several diseases, including diabetes, cardiovascular disease and cerebrovascular disease. A common denominator among them is oxidative stress which occurs when excessive reactive oxygen species (ROS) are formed leading to cell damage. In normal conditions, the production of ROS is balanced with antioxidants in the body and if there is an imbalance between them, oxidative stress will occur. Antioxidant enzymes like SOD, CAT and GPx are the body’s endogenous defense mechanisms preventing cell damage against free radicals and providing a repair mechanism for oxidized membrane components (Yadav et al. 2016; Mirończuk-Chodakowska et al. 2018).
In our study, high-fat diet caused a marked decrease in the SOD, CAT and GPx activities suggesting the depletion of the antioxidant system and thus might enhance the oxidative stress markers. Decreased activity of these enzymes in obesity conditions can lead to the development of various obesity-associated metabolic complications. Administration of CLE could effectively boost the activity of the antioxidant enzymes compared to HFD-fed group. Improved antioxidant defense systems (SOD, CAT and GPx) can help in scavenging free radicals generated during hyperlipidemia and obese conditions (Alshammari et al. 2017). Consumption of antioxidant-rich food helps in improved function of the antioxidant defense system. Therefore, besides the endogenous antioxidant defense system, consuming antioxidant-rich food is also recommended (Lobo et al. 2010). In our previous studies, we have reported coffee leaves as an abundant source of bioactives with significant antioxidant activity (Patil et al. 2022a; Patil and Murthy 2022). The abundance of antioxidant components in CLE might be responsible for inducing the strong antioxidant defense among CFE groups in comparison with the HFD group.
Effect of CLE on hepatic gene expression
We examined the impact of lipid metabolism-related enzymes in the liver on gene expression to determine the mechanism behind the modifications in the serum and hepatic biochemical profile with CLE ingestion (Fig. 6). When compared to the SFD group, the expression of the lipogenic transcription factors CCAT-enhancer-binding protein-alpha (CEBP-α) and sterol regulatory element-binding protein-1c (SREBP-1c) was considerably upregulated in the HFD group. The ACC (Acetyl Coenzyme A Carboxylase) and FAS (fatty acid synthase) mRNA expression was lower in the CLE-treated groups compared to the HFD group.
Fig. 6.
Impact of coffee leaf extract on hepatic gene expression. *Data values are means ± SD (n = 6). Columns not sharing common alphabets differ significantly at p < 0.05. SFD starch-fed diet, HFD high-fat diet, PC positive control (orlistat; 10 mg/kg body weight), CLE1 coffee leaf extract (100 mg/kg body weight), CLE2 coffee leaf extract (200 mg/kg body weight)
Several mechanisms, including hepatic lipid production, breakdown and oxidation, control the changes in the liver and the lipid contents. The expression of genes involved in lipid synthesis is regulated by transcription factors known as sterol regulatory element-binding proteins (SREBPs). SREBP-1c is a critical regulator of hepatic fatty acid production and is primarily expressed in the liver. ACC and FAS are two lipogenic enzymes whose expression is favorably associated with SREBP-1c (Kohjima et al. 2008). Overexpression of ACC and FAS in the liver is strongly linked with hepatic steatosis. Acetyl CoA, a necessary substrate for the production of fatty acids, is converted into malonyl CoA by ACC in the process of fatty acid synthesis. Further, the enzyme FAS uses both acetyl CoA and malonyl CoA in the biosynthesis of fatty acids (Dorn et al. 2010; Das et al. 2022). CLE treatment displayed reduced expression of SREBP-1c, CEBP-α, ACC and FAS compared to the HFD, thus improving the lipid metabolism by suppressing lipogenesis in the liver.
CGA is the major phenolic compound found in coffee leaves along with others such as quercetin, p-coumaric acid and rutin. The anti-obesity effects of CLE can be attributed to these phenolics. Chlorogenic acid has been shown to suppress weight gain from high-fat-diet-related increase in body weight, visceral fat and hepatic-free fatty acids (Santana-Gálvez et al. 2017). A study by Huang et al. (2015) suggests that 5-CQA (the most widely studied CGA) may improve lipid metabolism by modifying the mRNA expression of PPAR-α (peroxisome proliferator-activated receptor α), LXR-α (liver X receptor α) and target genes involved in cholesterol synthesis, fatty acid synthesis, etc. In another study carried out by Cho et al. (2010), CGA improves lipid metabolism properties and exhibits anti-obesity by increasing the fatty acid β-oxidation activity and PPAR-α expression in the liver as well as inhibiting fatty acid synthase (FAS). Quercetin is yet another major phenolic compound found in coffee leaves which is also known to have anti-obesity effects. Quercetin was shown to induce activation of AMPK α and β1 (adenosine monophosphate-activated protein kinase) which increases the inactive ACC by phosphorylating it and thus in turn inhibits adipogenesis (Ahn et al. 2008).
However, CLE being a crude extract the anti-obesity effects of coffee leaves are not only because of the phenolics but can also be attributed to the alkaloids present in them. One such important alkaloid is caffeine. It is known to exhibit its anti-obesity effects by preventing oxidative stress by inhibiting the free radical formation and directly inhibiting lipid peroxide formation (Tellone et al. 2015). Trigonelline is the second-most prevalent alkaloid in coffee leaves. The neuroprotective and hypoglycemic properties of trigonelline are its most well-known effects. Trigonelline has been demonstrated to gradually lower blood glucose levels in streptozotocin and HFD-induced type 2 diabetes mice (Zhou et al. 2013). Since coffee leaves are a consortium of bioactive molecules, the anti-obesity effect of CLE can be due to the synergistic effect of these compounds.
Conclusion
The in vitro enzyme inhibitory assays indicate that coffee leaf extract has considerable α-amylase and lipase, inhibiting properties. The coffee leaf extract seems to play an important role in maintaining the body weight, lipid profile and blood glucose levels in high-fat diet-induced obese mice. It was also observed to increase the antioxidant enzyme levels and decrease the hepatotoxicity markers in vivo, which reduces the chance of obesity caused by high-fat diet and the complications that come along with it. Additionally, it lowered lipogenesis in the liver by reducing the expression of SREBP-1c, CEBP-α, ACC and FAS in the treated groups. Therefore, coffee leaf extract or products developed from it are anticipated to be potential anti-obese ingredients in the functional food industry along with providing sustainability to the coffee industry.
Acknowledgements
The CSIR-CFTRI, Mysore, India, is gratefully acknowledged by the authors for providing essential lab facilities. Ms. Siddhi Patil thanks DBT, New Delhi, for the Research Fellowship award. The Central Instrumentation Facility and pilot plant services of CFTRI are acknowledged by the authors for their assistance.
Author contributions
PSM contributed to conception, execution and writing up of the manuscript. GSK guided animal experiment study as well as data evaluations and suggestions. SP was involved in execution of research work plan, analysis, and data interpretation. MD contributed to analysis and data interpretation.
Funding
The project was supported by Grant no. Q-11/15/2019-R&D, Ministry of Food Processing, Government of India. The part of work was also financially funded by DBT Ministry of Science and Technology, New Delhi, Government of India (GAP-462).
Data availability
All the data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
The manuscript involves animal studies for which the ethics approval has been given below:
Animal ethics approval
Male C57BL/6 mice were used in the experiment (20–22 g). The Institutional Animal Ethics Committee (IAEC/216/2021) of CSIR-CFTRI, Mysore, India, granted clearance for the animal experiments. Sincere attempts were taken to reduce the suffering, and the numbers of animals were maintained to get the statistical data comparisons.
Informed consent
Not applicable (This study does not involve human participants).
Contributor Information
Siddhi Patil, Email: siddhi.patil919@gmail.com, https://www.researchgate.net/scientific-contributions/Siddhi-Patil-2199892422.
Moumita Das, Email: mous.zone12@gmail.com, https://www.researchgate.net/profile/Moumita-Das-23.
G. Suresh Kumar, Email: sureshg@cftri.res.in.
Pushpa S. Murthy, Email: pushpa@cftri.res.in
References
- Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373(4):545–549. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- Alshammari GM, Balakrishnan A, Al-Khalifa A. Antioxidant effect of Alrabian Coffee (Coffea arabica L.) blended with cloves or cardamom in high-fat diet-fed C57BL/6J mice. Trop J Pharm Res. 2017;16(7):1545–1552. doi: 10.4314/tjpr.v16i7.12. [DOI] [Google Scholar]
- Andrikopoulos S, Amy RB, Nadia D, Barbara CF, Joseph P. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Bansal P, Paul P, Mudgal J, Nayak PG, Pannakal ST, Priyadarsini KI, Unnikrishnan MK. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol. 2012;64(6):651–658. doi: 10.1016/j.etp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Billing AM, Hamidane HB, Bhagwat AM, Cotton RJ, Dib SS, Kumar P, Hayat S, et al. Complementarity of SOMAscan to LC-MS/MS and RNA-seq for quantitative profiling of human embryonic and mesenchymal stem cells. J Proteom. 2017;150:86–97. doi: 10.1016/j.jprot.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991 doi: 10.1093/ajcn/53.1.270S. [DOI] [PubMed] [Google Scholar]
- Campa C, Mondolot L, Rakotondravao A, Bidel LPR, Gargadennec A, Couturon E, La Fisca P, Rakotomalala JJ, Jay-Allemand C, Davis AP. A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: biological implications and uses. Ann Bot. 2012;110(3):595–613. doi: 10.1093/aob/mcs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani WJ. Metabolic and nutritional aspects of the atherogenic atypical lipoproteins: lipoprotein(a), remnant lipoproteins, and oxidized low-density lipoprotein. Nutr Res. 2004;24(9):681–693. doi: 10.1016/j.nutres.2004.05.004. [DOI] [Google Scholar]
- Ceriello A. Oxidative stress and glycemic regulation. Metab Clin Exp. 2000;49(2 Suppl. 1):27–29. doi: 10.1016/S0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- Chan SHH, Tai MH, Li CY, Chan JYH. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radical Biol Med. 2006;40(11):2028–2039. doi: 10.1016/j.freeradbiomed.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Chen XM, Ma Z, Kitts DD. Effects of processing method and age of leaves on phytochemical profiles and bioactivity of coffee leaves. Food Chem. 2018;249(September 2017):143–153. doi: 10.1016/j.foodchem.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Chen X, Kaiwen M, David DK. Characterization of phytochemical mixtures with inflammatory modulation potential from coffee leaves processed by green and black tea processing methods. Food Chem. 2019;271(July 2018):248–258. doi: 10.1016/j.foodchem.2018.07.097. [DOI] [PubMed] [Google Scholar]
- Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48(3):937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Das M, Geetha V, Zarei M, Harohally NV, Suresh KG. Modulation of obesity associated metabolic dysfunction by novel lipophilic fraction obtained from Agaricus bisporus. Life Sci. 2022;305(July):120779. doi: 10.1016/j.lfs.2022.120779. [DOI] [PubMed] [Google Scholar]
- Dorn C, Riener MO, Kirovski G, Saugspier M, Steib K, Weiss TS, Gäbele E, Kristiansen G, Hartmann A, Hellerbrand C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int J Clin Exp Pathol. 2010;3(5):505–514. [PMC free article] [PubMed] [Google Scholar]
- Flohé L, Günzler WA. Assays of gluthathione peroxidase. Methods Enzymol. 1984;105:114–120. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Flohi BL, Tting F. [10] Assays. Methods. 1984;105(1975):93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Geetha V, Moumita D, Mehrdad Z, Mayookha VP, Nanishankar VH, Kumar GS. Studies on the partial characterization of extracted glycosaminoglycans from fish waste and its potentiality in modulating obesity through in-vitro and in-vivo. Glycoconj J. 2022;39(4):525–542. doi: 10.1007/s10719-022-10077-5. [DOI] [PubMed] [Google Scholar]
- Golan M, Fainaru M, Weizman A. Role of behaviour modification in the treatment of childhood obesity with the parents as the exclusive agents of change. Int J Obes. 1998;22(12):1217–1224. doi: 10.1038/sj.ijo.0800749. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Denke MA. Dietary influences on serum lipids and lipoproteins. J Lipid Res. 1990;31(7):1149–1172. doi: 10.1016/s0022-2275(20)42625-2. [DOI] [PubMed] [Google Scholar]
- Huang K, Liang X, Zhong Y, He W, Wang Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J Sci Food Agric. 2015;95(9):1903–1910. doi: 10.1002/jsfa.6896. [DOI] [PubMed] [Google Scholar]
- Hussain A, Cho JS, Kim JS, Lee YI. Protective effects of polyphenol enriched complex plants extract on metabolic dysfunctions associated with obesity and related nonalcoholic fatty liver diseases in high fat diet-induced C57bl/6 mice. Molecules. 2021;26(2):1–14. doi: 10.3390/molecules26020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim W, Lee US, Yeh CC, Szabo J, Bruckner G, Chow CK. Oxidative Stress and antioxidant status in mouse liver: effects of dietary lipid, vitamin E and iron. J Nutr. 1997;127(7):1401–1406. doi: 10.1093/jn/127.7.1401. [DOI] [PubMed] [Google Scholar]
- Jakus V. The role of free radicals, oxidative stress and antioxidant systems in diabetic vascular disease Úloha vo Ných Radikálov, Oxidačného Stresu a Antioxidačných Systémov Pri Diabetickej Vaskulárnej Chorobe. Bratisl Lek Listy. 2000;101(10):541–551. [PubMed] [Google Scholar]
- Ji D, Ma H, Chen X. Ultrasonication increases γ-aminobutyric acid accumulation in coffee leaves and affects total phenolic content and angiotensin-converting enzyme inhibitory activity. J Food Process Preserv. 2021;45(10):1–11. doi: 10.1111/jfpp.15777. [DOI] [Google Scholar]
- Kim I, Kim HR, Kim JH, Om AS. Beneficial effects of Allium sativum L. stem extract on lipid metabolism and antioxidant status in obese mice fed a high-fat diet. J Sci Food Agric. 2013;93(11):2749–2757. doi: 10.1002/jsfa.6094. [DOI] [PubMed] [Google Scholar]
- Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14(3):140–162. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21(4):507–511. doi: 10.3892/ijmm.21.4.507. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Vattem DA, Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nut. 2006;15(1):107–118. [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Gaudel C, Holst D, Lopez-Soriano J, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Roles of PPAR delta in lipid absorption and metabolism: a new target for the treatment of type 2 diabetes. Biochim Biophys Acta Mol Basis Dis. 2005;1740(2):313–317. doi: 10.1016/j.bbadis.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Martins SCV, Araújo WL, Tohge T, Fernie AR, DaMatta FM. In high-light-acclimated coffee plants the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield. PLoS One. 2014;9(4):1–11. doi: 10.1371/journal.pone.0094862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63(1):68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Nurmasari W, Anjani G, Almira VG, Putri SE, Pratiw AR. Effects of the administration of brewed robusta coffee leaves on total antioxidant status in rats with high-fat, high- fructose diet-induced metabolic syndrome. Potravinarstvo Slovak J Food Sci. 2020;14(1):258–263. [Google Scholar]
- Nwakiban A, Parfait A, Shivashankara ST, Piazza S, Tchamgoue AD, Beretta G, Dell’agli M, Magni P, Agbor GA, Kuiaté JR, Manjappara UV, Polyphenol-rich extracts of xylopia and aframomum species show metabolic benefits by lowering hepatic lipid accumulation in diet-induced obese mice. ACS Omega. 2022;7(14):11914–11928. doi: 10.1021/acsomega.2c00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira PR, De B, Da Costa CA, De Bem GF, Cordeiro VSC, Santos IB, De Lenize CRM, Carvalho EP, Da Conceição S, et al. Euterpe oleracea Mart.-derived polyphenols protect mice from diet-induced obesity and fatty liver by regulating hepatic lipogenesis and cholesterol excretion. PLoS One. 2015;10(12):1–16. doi: 10.1371/journal.pone.0143721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Yao W, Yang X, Xie C, Liu D, Zhang J, Gao X. Purification, characterization and biological activity on hepatocytes of a polysaccharide from Flammulina velutipes mycelium. Carbohyd Polym. 2007;70(3):291–297. doi: 10.1016/j.carbpol.2007.04.010. [DOI] [Google Scholar]
- Patay ÉB, Bencsik T, Papp N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac J Trop Med. 2016;9(12):1127–1135. doi: 10.1016/j.apjtm.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Patil S, Murthy PS. Drying kinetics, phytochemical profile and antioxidant potentials of Coffea robusta leaves and its valorization as a functional beverage. Waste Biomass Valorization. 2022 doi: 10.1007/s12649-022-01771-4. [DOI] [Google Scholar]
- Patil S, Vedashree M, Murthy PS. Phytochemical profile and antioxidant potential of coffee leaves influenced by green extraction techniques and in vitro bio-accessibility of its functional compounds. J Meas Charact. 2022;16(3):2335–2346. doi: 10.1007/s11694-022-01345-x. [DOI] [Google Scholar]
- Patil S, Vedashree M, Murthy PS. Valorization of coffee leaves as a potential agri-food resource: bio-active compounds, applications and future prospective. Planta. 2022;255(3):1–17. doi: 10.1007/s00425-022-03846-x. [DOI] [PubMed] [Google Scholar]
- Pimpley V, Patil S, Srinivasan K, Desai N, Murthy PS. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep Biochem Biotechnol. 2020;50(10):969–978. doi: 10.1080/10826068.2020.1786699. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Jebb SA. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev. 2003;4(4):187–194. doi: 10.1046/j.1467-789X.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- Rohla M, Weiss TW. Adipose tissue, inflammation and atherosclerosis. Clin Lipidol. 2014;9(1):71–81. doi: 10.2217/clp.13.80. [DOI] [Google Scholar]
- Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22(3):7–9. doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Thrimawithana T, Shukla R, Adhikari B. Managing obesity through natural polyphenols: a review. Future Foods. 2020 doi: 10.1016/j.fufo.2020.100002. [DOI] [Google Scholar]
- Talawar ST, Mohan Kumar AS, Bhaskaragoud G, Mohan Kumar BV, Suresh Kumar G. Wheat bran oil concentrate induced AMPK activation, insulin and lipid homeostasis alleviates adipokines and cytokine in high fat fed C57BL6 mice. J Agric Food Res. 2020;2(July):100080. doi: 10.1016/j.jafr.2020.100080. [DOI] [Google Scholar]
- Tellone E, Galtieri A, Giardina B, Russo A, Bellocco E, Barreca D, Ficarra S. Antioxidant activity of caffeine: a focus on human red blood cells and correlations with several neurodegenerative disorders. Coffee in health and disease prevention. New York: Elsevier Inc; 2015. [Google Scholar]
- Wang Z, Luo D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum makino. Carbohyd Polym. 2007;68(1):54–58. doi: 10.1016/j.carbpol.2006.07.022. [DOI] [Google Scholar]
- Weibel EK, Hadvary P, Hochuli E, Kupfer E, Lengsfeld H. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini I. Producing organism, fermentation, isolation and biological activity. J Antibiot. 1987;40(8):1081–1085. doi: 10.7164/antibiotics.40.1081. [DOI] [PubMed] [Google Scholar]
- Widyastuti N, Anjani G, Almira VG, Putri SE, Pratiwi AR, Prawira-Atmaja MI. The effect of brewed robusta coffee leaves on insulin levels and HOMA-IR index in metabolic syndrome rats. Rom J Diabetes Nutr Metab Dis. 2020;27(1):16–24. doi: 10.46389/rjd-2020-1004. [DOI] [Google Scholar]
- Yadav A, Kumari R, Ashwani Yadav JP, Mishra SS, Prabha S. Antioxidants and its functions in human body—a review. Res Environ Life Sci. 2016;11(November):1328–1331. [Google Scholar]
- Yang D, Hu C, Deng X, Bai Y, Cao H, Guo J, Su Z. Therapeutic effect of chitooligosaccharide tablets on lipids in high-fat diets induced hyperlipidemic rats. Molecules. 2019 doi: 10.3390/molecules24030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhou S, Zeng S. Experimental diabetes treated with trigonelline: effect on β cell and pancreatic oxidative parameters. Fundam Clin Pharmacol. 2013;27(3):279–287. doi: 10.1111/j.1472-8206.2011.01022.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.