Abstract
The peptidoglycan cortex of endospores of Bacillus species is required for maintenance of spore dehydration and dormancy, and the structure of the cortex may also allow it to function in attainment of spore core dehydration. A significant difference between spore and growing cell peptidoglycan structure is the low degree of peptide cross-linking in cortical peptidoglycan; regulation of the degree of this cross-linking is exerted by d,d-carboxypeptidases. We report here the construction of mutant B. subtilis strains lacking all combinations of two and three of the four apparent d,d-carboxypeptidases encoded within the genome and the analysis of spore phenotypic properties and peptidoglycan structure for these strains. The data indicate that while the dacA and dacC products have no significant role in spore peptidoglycan formation, the dacB and dacF products both function in regulating the degree of cross-linking of spore peptidoglycan. The spore peptidoglycan of a dacB dacF double mutant was very highly cross-linked, and this structural modification resulted in a failure to achieve normal spore core dehydration and a decrease in spore heat resistance. A model for the specific roles of DacB and DacF in spore peptidoglycan synthesis is proposed.
Peptidoglycan (PG) is the structural element of the bacterial cell wall which determines cell shape and which resists the turgor pressure within the cell. The bacterial endospores produced by species of Bacillus, Clostridium, and several other bacterial genera are modified cells that are able to survive long periods and extreme conditions in a dormant, relatively dehydrated state. The PG wall within the endospore is required for maintenance of the dehydrated state (10, 11), which is the major determinant of spore heat resistance (2, 17, 22). Spore PG appears to be comprised of two distinct though contiguous layers. The thin inner layer, the germ cell wall, appears to have a structure similar to that of the vegetative wall and serves as the initial cell wall of the germinated spore (1, 20, 21, 31). The thicker outer layer, the spore cortex, has a modified structure which may determine its ability to carry out roles specific to the spore, and is rapidly degraded during spore germination (1, 20, 35, 37). The most dramatic of the cortex structural modifications results in partial cleavage or complete removal of ∼75% of the peptide side chains from the glycan strands. Loss of these peptides limits the cross-linking potential of the PG and results in the formation of only one peptide cross-link per 35 disaccharide units in the spore PG, compared to one peptide cross-link per 2.3 to 2.9 disaccharide units in the vegetative PG (1, 20, 36). This low degree of cross-linking has been predicted to give spore PG a flexibility that allows it to have a role in attainment of spore core dehydration (14, 34) in addition to its clear role in maintenance of dehydration. We are studying the structure and mechanism of synthesis of spore PG in an attempt to discern the roles of this structure and its individual components in determining spore properties.
A family of proteins called the penicillin-binding proteins (PBPs) polymerizes PG on the external surface of the cell membrane (reviewed in reference 7). The high-molecular-weight (high-MW) members of this family (generally ≥60 kDa) carry the transglycosylase and transpeptidase activities involved in polymerization and cross-linking of the glycan strands. The low-MW PBPs have commonly been found to possess d,d-carboxypeptidase activity. This activity can remove the terminal d-alanine of the peptide side chains and thereby prevent the side chain from serving as a donor in the formation of a peptide cross-link. Analysis of the B. subtilis genome reveals six low-MW PBP-encoding genes: dacA (33), dacB (4), dacC (19), dacF (38), pbpE (23), and pbpX (accession no. Z99112). The four dac gene products exhibit very high sequence similarity to proven d,d-carboxypeptidases, and this activity has been demonstrated in vitro for the dacA and dacB products, PBP5 (12) and PBP5* (32), respectively. The sequences of the pbpE and pbpX products are more distantly related, and no activity has yet been established or ruled out for them.
PBP5 is the major penicillin-binding and d,d-carboxypeptidase activity found in vegetative cells (12). Although dacA expression declines significantly during sporulation, a significant amount of PBP5 remains during the time of spore PG synthesis (29). A dacA-null mutation results in no obvious effects on vegetative growth, sporulation, spore characteristics, or spore germination (3, 33). However, loss of PBP5 does result in a reduction of cleavage of peptide side chains from the tetrapeptide to the tripeptide form in the spore PG (20). PBP5* is expressed only during sporulation and only in the mother cell compartment of the sporangium, under the control of the RNA polymerase ςE subunit (4, 5, 28, 29). A dacB-null mutation leading to loss of this d,d-carboxypeptidase results in a fourfold increase in the effective cross-linking of the spore PG (1, 20, 22). This structural change is accompanied by only slight decreases in spore core dehydration and heat resistance (3, 22). The suspected d,d-carboxypeptidase activities of the products of the dacC and dacF genes have not been demonstrated. The latter two genes are expressed only during the postexponential growth phase: dacC is expressed during early stationary phase under the control of ςH (19) and dacF is expressed only within the forespore under the control of ςF (27, 38). Null mutations effecting either gene result in no obvious phenotype and no change in spore PG structure (19, 38).
The multiplicity of these proteins in sporulating cells and the lack of effect of loss of some of them suggested redundancy of function among these proteins, a situation observed previously with PBPs of a high-MW class (25, 30, 39). In order to examine this possibility we have constructed mutants lacking multiple low-MW PBPs and have examined their sporulation efficiency, spore PG structure, spore heat resistance and wet density, and spore germination and outgrowth. The present study demonstrates a role for the dacF gene product in synthesis of spore PG, and we also present a model for the roles of the dacB and dacF gene products in spore PG formation.
MATERIALS AND METHODS
Bacterial strains, growth, sporulation, spore germination, and spore properties.
All B. subtilis strains described in Table 1 are derivatives of strain 168. Growth rates were determined and sporulation was carried out in 2×SG medium containing no antibiotics (13) at 37°C. Spores were purified by washing with water as previously described (18) and were stored in H2O at 4°C. Microscopic examination indicated that all spore preparations were >98% free of vegetative cells, sporulating cells, or germinated spores. Dipicolinic acid (DPA) was measured as described previously (18). Spore viability was assayed by plating dilutions of untreated spores on Luria broth plates (16). Spore chloroform resistance was assayed by vortex mixing of spores in a 10% (vol/vol) suspension of chloroform for 1 min prior to dilution and plating. For determination of spore heat resistance, identical samples of purified spores were heated in H2O at 85°C for various times, and survivors on Luria broth plates were enumerated (16). For determination of germination rates, spores were heat activated in H2O at 70°C for 30 min prior to germination in 2× YT medium (16 g of tryptone, 10 g of yeast extract, and 5 g of NaCl per liter) containing 4 mM l-alanine at 37°C. Spore protoplast wet density was determined by using metrizoic acid or Nycodenz (Sigma) gradients as previously described (15, 22). Dormant spores were permeabilized (incubation in 50 mM Tris HCl [pH 8.0], 8 M urea, 1% sodium dodecyl sulfate, 50 mM dithiothreitol for 60 min at 37°C) and washed five times in H2O prior to density determinations such that the gradient material could permeate the spore coats and cortex (15, 22).
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotypea | Constructionb | Reference or source |
|---|---|---|---|
| PS832 | Wild type | Lab stock | |
| PS1900 | dacA::Cm | 20, 33 | |
| PS1901 | dacF::Cm | 20, 38 | |
| PS2066 | ΔdacB | 22 | |
| PS2324 | dacC::Sp | 19 | |
| PS2419 | dacA::Cm dacC::Sp | PS2324→PS1900 | This study |
| PS2420 | dacA::Cm ΔdacB | PS1900→PS2066 | This study |
| PS2421 | ΔdacB dacF::Cm | PS1901→PS2066 | This study |
| PS2423 | ΔdacB dacC::Sp | PS2324→PS2066 | This study |
| DPVB1 | dacC::Sp dacF::Cm | PS1901→PS2324 | This study |
| DPVB6 | dacA::Sp | pDPV54→PS832 | This study |
| DPVB7 | dacA::Sp dacF::Cm | pDPV54→PS1901 | This study |
| DPVB9 | ΔdacA::Cm | pDPV55→PS832 | This study |
| DPVB10 | ΔdacA | Exigrant of DPVB9 | This study |
| DPVB14 | dacA::Cm ΔdacB dacC::Sp | PS2324→PS2420 | This study |
| DPVB15 | dacA::Sp ΔdacB dacF::Cm | DPVB6→PS2421 | This study |
| DPVB16 | ΔdacA dacF::Cm | PS1901→DPVB10 | This study |
| DPVB17 | ΔdacA dacC::Sp dacF::Cm | PS2324→DPVB16 | This study |
| DPVB18 | ΔdacB dacC::Sp dacF::Cm | PS2324→PS2421 | This study |
Cm, chloramphenicol resistance; Sp, spectinomycin resistance.
An arrow indicates the transformation of the donor plasmid or chromosomal DNA into the recipient strain.
Strain construction.
For construction of new dacA mutations, primers complementary to positions 80806 to 80829 and 82494 to 82516 in the dacA sequence (accession no. D26185) were used to PCR amplify the gene. The 1,710-bp PCR product was cut with StuI and SacI at sites which occur upstream and downstream of the gene, respectively, and a 1,654-bp fragment was inserted into HincII-SacI-digested pUC19 to produce pDPV51. A Spr cassette, the 1,148-bp PstI fragment of pDG1726 (9), was inserted into a unique NsiI site at codon 31 of dacA in pDPV51 to produce pDPV54. B. subtilis PS832 was transformed with ScaI-linearized pDPV54 with selection for Spr. Recombination of the Spr cassette into the genomic copy of dacA by a double-crossover event to produce strain DPVB6 was verified by Southern blotting. A deletion removing codons 7 to 159 of dacA (resulting mutant termed ΔdacA) was constructed by digesting pDPV51 with HincII and ligating to produce pDPV53. A 1,200-bp EcoRI-SphI fragment from pDPV53 containing ΔdacA was inserted into EcoRI-SphI-digested pJH101 (6) to produce pDPV55. This plasmid was transformed into PS832, and selection for Cmr gave transformants in which the plasmid had inserted into the dacA locus by a single crossover event. These Cmr transformants were screened by Southern blotting to identify one, DPVB9, in which a gene conversion event resulted in both copies of dacA having the internal deletion. DPVB9 was grown nonselectively through ∼40 generations and then plated nonselectively for single colonies. The resulting colonies were screened for chloramphenicol sensitivity. One chloramphenicol-sensitive isolate, DPVB10, was demonstrated by Southern blotting to have lost the inserted plasmid and to have a single copy of the dacA with the internal deletion in its chromosome.
Spore PG structure determination.
Spore PG was extracted and digested with a muramidase (Mutanolysin; Sigma) and muropeptides were analyzed by reversed-phase high-performance liquid chromatography (HPLC) as described previously (1, 20). For some samples the cross-linking of Mutanolysin-solubilized muropeptides was determined by amino acid analysis as described previously (24). The amount of diaminopimelic acid (Dpm) detected prior to and following 1-fluoro-2,4-dinitrobenzene (FDNB) treatment was normalized to the amount of glutamic acid detected in each sample. Amino acid analysis was carried out as previously described (8). The HPLC system used for PG structure and amino acid analyses consisted of a Waters 600E controller and pump, a Waters 486 UV detector, and a Powerchrom hardware and software system (ADInstruments Inc.) on an Apple PowerMacintosh 5400 computer, used for signal integration.
RESULTS
Growth and sporulation of dac mutant strains.
The growth rates and sporulation efficiencies of each of the single dac mutant strains have been previously reported (19, 22, 33, 38). In all cases the vegetative growth rates and the sporulation efficiencies are indistinguishable from those of the wild-type strain. Since dacA is the only one of the four genes expressed during vegetative growth, it was expected that in no case would loss of additional dac gene products have an effect on the growth rate, and this was indeed the case for all the strains analyzed (data not shown). When cultures were assayed for the production of chloroform-resistant spores 24 h after the initiation of sporulation, it was found that all mutant strains, except those lacking both dacB and dacF, sporulated as efficiently as the wild type, producing approximately 109 spores/ml (data not shown). Double and triple mutants lacking both dacB and dacF appeared to produce as many spores as the wild type within the first 12 h of sporulation (>90% of cells produced spores based upon microscopic observation). However, these spores lost viability during further incubation, and only 10 to 35% of the spores of these mutants were able to produce colonies after 24 h at 37°C. During the time that these spores were purified by repeated washing in H2O at 4°C they retained this level of viability; a suspension of dacB dacF spores at a particular optical density produced only 10% as many colonies as a similar suspension of wild-type spores.
Heat resistance and wet density of dac mutant spores.
Purified spores produced by the wild type and each of the mutant strains were also tested for their heat resistance (Table 2). As observed previously, the only single dac mutation that resulted in an alteration in spore heat resistance was in dacB (3, 19, 22, 38). No combination of dac mutations that did not include dacB, even the dacA dacC dacF triple mutant, had any effect on spore heat resistance. Among dacB mutants, the additional loss of dacA, dacC, or both produced no change in spore heat resistance. However, disruption of dacF in a dacB mutant did result in a more dramatic loss of heat resistance. Once again, the further loss of dacA or dacC produced no additional change in spore heat resistance.
TABLE 2.
Properties of dac mutant sporesa
| Strain(s) | Genotype | Heat resistance (D85 [min])b | Protoplast wet density (difference from wild type [g/ml])c |
|---|---|---|---|
| PS832 | Wild type | 34 | |
| PS1900, DPVB6, DPVB10d | dacA | 29 | 0 |
| PS2066 | dacB | 9 | −0.004 |
| PS2324 | dacC | 27 | 0 |
| PS1901 | dacF | 27 | −0.002 |
| PS2420 | dacA dacB | 8 | −0.002 |
| PS2419 | dacA dacC | 27 | −0.002 |
| DPVB7 | dacA dacF | 28 | 0.002 |
| PS2423 | dacB dacC | 9 | −0.004 |
| PS2421 | dacB dacF | 5 | −0.017 |
| DPVB1 | dacC dacF | 27 | 0 |
| DPVB14 | dacA dacB dacC | 11 | 0 |
| DPVB15 | dacA dacB dacF | 4 | −0.024 |
| DPVB17 | dacA dacC dacF | 27 | 0 |
| DPVB18 | dacB dacC dacF | 5 | −0.012 |
Values are averages of determinations for three or more independent spore preparations.
Time at 85°C when a 10-fold drop in spore viability occurred. Variation in these values among experiments was 10 to 40% for each strain. However, within each experiment the relative degrees of heat resistance among the strains were the same.
The average protoplast wet density for the wild type was 1.369 g/ml. Due to the difficulty in determining absolute densities across a gradient, the variation in measured values among experiments was 0.7 to 2% for each strain. This range is equivalent to the difference seen between the wild type and the most severely affected strain. However, between experiments the differences in densities between the wild type and any particular strain were relatively constant. It is these differences which are reported.
Each of these three dacA strains was analyzed at least two times, and no differences were observed between the three strains. The effects of the different dacA mutations in multiple mutants are assumed to be equivalent.
The relative dehydration of the spore core has previously been shown to be directly related to spore heat resistance (2, 17, 22). Spore core dehydration was measured as spore wet density by equilibrium density gradient centrifugation. Prior to density determination the spores were chemically permeabilized to allow penetration of the gradient matrix through the spore coats and peptidoglycan layers. This allows specific assay of the density of the spore core and has no effect on the heat resistance of the spores (15, 22). In no case did loss of dacA, dacC, or both have any significant effect on spore core dehydration (Table 2, changes of ≤0.004 g/ml). The dacF mutation also had no large effect on spore core dehydration, even in a dacA dacC dacF triple mutant, until it was combined with a dacB mutation (Table 2). As we found previously (22), the reduced heat resistance of dacB spores is accompanied by little or no change in spore core dehydration (Table 2). However, the reduced heat resistance of these spores is explained by the fact that they begin to take up water during heating, with an accompanying loss of heat resistance (22). The dacB dacF double and triple mutant spores did have a significantly reduced spore core wet density (Table 2) (changes of >0.01 g/ml), even in the absence of heating.
PG structure in dac mutant spores.
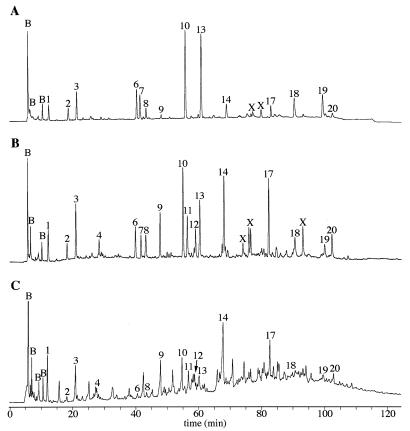
Previous work has shown that changes in spore heat resistance are often accompanied by changes in spore PG structure (1, 20). Consequently, PG was extracted from purified spores of the wild-type strain and the various dac mutants and was digested with a muramidase, and the resulting muropeptides were analyzed by reversed-phase HPLC (Fig. 1; Table 3). As observed previously, single mutations in dacC and dacF produced no significant alteration in the observed muropeptide profiles, while a mutation in dacA resulted in a twofold decrease in the number of tripeptide side chains (1, 19, 20) (Table 3). A dacB mutation produces the largest effects, a nearly twofold increase in the number of tetrapeptide side chains with a corresponding decrease in l-Ala side chains and a two- to threefold increase in the percentage of side chains that are cross-linked (1, 20) (Fig. 1B; Table 3). Strains containing combinations of these mutations in most cases produced muropeptide profiles resembling a combination of those produced by the parent strains. Strains with combinations of dacA, dacC, and dacF mutations showed only the reduction of tripeptide side chains characteristic of the dacA mutation, and dacA dacB double mutants had an exact combination of the muropeptide alterations produced by the two single mutations (Table 3).
FIG. 1.
HPLC separation of spore PG muropeptides. Muropeptides produced by muramidase digestion of purified PG from spores of PS832 (wild-type) (A), PS2066 (ΔdacB) (B), and PS2421 (ΔdacB dacF::Cm) (C) were separated by using a methanol gradient system (20). Peaks labeled B are buffer components seen in control samples. Peaks are numbered as previously described (20). Peaks 1, 2, and 3 are disaccharides with tripeptide (DS-TriP), alanine, and tetrapeptide (DS-TP) side chains, respectively. Peaks 10 and 13 are tetrasaccharides with tetrapeptide (TS-TP) and alanine side chains, respectively. Peaks 18 and 19 are hexasaccharides with tetrapeptide (HS-TP) and alanine side chains, respectively. Peaks 8, 9, 14, 17, and 20 are cross-linked DS-TriP–DS-TP, DS-TP–DS-TP, DS-TP–TS-TP, TS-TP–TS-TP, and TS-TP–HS-TP, respectively. Peaks 6, 7, 11, and 12 are reduction products of peaks 13, 10, 14, and 17, respectively. Peaks labeled X are also reduction products of muramic-δ-lactam-containing muropeptides; corrections for this reduction were performed in calculation of PG structural parameters (20).
TABLE 3.
Structural parameters of PG from dac mutant sporesa
| Genotype | % of muramic acid with side chain ofb:
|
% of Dpm in cross-links analyzed by:
|
||||
|---|---|---|---|---|---|---|
| Lactam | l-Ala | TP | TriP | HPLC | FDNB | |
| Wild type | 50c | 26 ± 1 | 23 ± 2 | 1.5 ± 0.1 | 13 ± 1 | 31 (1) |
| dacA | 49 ± 1 | 25 ± 4 | 25 ± 3 | 0.8 ± 0.2 | 12 ± 2 | 34 (1) |
| dacB | 47 ± 1 | 12 ± 1 | 43 ± 1 | 2.2 ± 0.1 | 36 ± 2 (30)d | 57 (1) |
| dacC | 51c | 26 ± 2 | 23 ± 3 | 1.4 ± 0.1 | 13 ± 3 | 31 (1) |
| dacF | 51 ± 1 | 28 ± 2 | 20 ± 2 | 1.4 ± 0.2 | 13 ± 1 | 30 (2) |
| dacA dacB | 46 ± 1 | 11 ± 2 | 47 ± 2 | 1.0 ± 0.1 | 37 ± 3 (28)d | 59 (2) |
| dacA dacC | 50c | 26 ± 3 | 24 ± 3 | 0.7 ± 0.1 | 14 ± 2 | 32 (2) |
| dacA dacF | 50 ± 1 | 26 ± 1 | 23 ± 2 | 0.9 ± 0.1 | 13 ± 1 | 31 (1) |
| dacB dacC | 46 ± 1 | 11 ± 1 | 45 ± 2 | 2.1 ± 0.2 | 36 ± 2 (28)d | 59 (2) |
| dacB dacFe | 40 ± 1 | 6 ± 4 | 57 ± 8 | 4.8 ± 0.4 | 33 ± 2 (23)d | 72 (4) |
| dacC dacF | 51c | 30 ± 1 | 18 ± 1 | 1.3c | 12 ± 1 | 32 (1) |
| dacA dacB dacC | 44 ± 2 | 12 ± 2 | 54 ± 4 | 1.8 ± 0.9 | 38 ± 3 (21)d | 60 (2) |
| dacA dacB dacFe | 42 ± 2 | 9 ± 3 | 54 ± 3 | 2.5 ± 0.3 | 26 ± 5 (15)d | 60 (2) |
| dacA dacC dacF | 51c | 29 ± 2 | 20 ± 2 | 0.7 ± 0.1 | 13 ± 3 | 29 (2) |
| dacB dacC dacFe | 39 ± 2 | 9 ± 4 | 52 ± 6 | 5.4 ± 0.6 | 34 ± 2 (27)d | 76 (2) |
Data are averages of analyses of 3 to 10 independent spore preparations (strain numbers as indicated in Table 2) with errors of 1 standard deviation except for cross-linking determinations using FDNB, for which the number of independent determinations is given in parentheses.
Abbreviations: Lactam, muramic-δ-lactam; TP, tetrapeptide; TriP, tripeptide.
These values showed no significant deviation among the samples assayed.
The values in parentheses are for effective cross-links; the remaining cross-links involve a peptide that has been cleaved from the muramic acid by a muramic acid-l-alanine amidase (1, 20, 21).
Values for all dacB dacF strains determined by HPLC analyses were calculated by using only identified peaks and ignoring the elevated baseline. Since the latter area undoubtedly includes a large amount of cross-linked muropeptides, the cross-linking values determined by HPLC are certainly underestimates.
The exceptions to this trend were dacB dacF strains (Fig. 1C; Table 3), as loss of dacF produced a dramatic change in the spore PG structure above that produced by the dacB mutation. Most notable was the appearance of a large number of novel muropeptide peaks eluting in the later part of the gradient (Fig. 1C [60 to 110 min]), in the region where high-mass muropeptides elute (note also the elevated baseline in Fig. 1C compared to those in Fig. 1A and B). These novel peaks and the elevated baseline were observed in several independent muropeptide preparations from dacB dacF spores as well as from spores of the triple mutants lacking these two genes. We calculated some spore PG structural parameters for these strains by using data for only the identifiable peaks (Table 3). However, it is important that these calculations do not take into account a large percentage of the muropeptides present in unidentified peaks late in the gradient. In general the peaks eluting in the latter half of the gradient include muropeptides containing multiple muramic-δ-lactam residues, hexasaccharides, and cross-linked compounds (1, 20). Reduction of a muropeptide containing two muramic-δ-lactam residues can give rise to eight different products (there are two possible reduction products for each lactam residue) and possibly eight different peaks (1, 20, 37). Our interpretation of the dacB dacF double-mutant muropeptide chromatogram is that there was a very high level of peptide cross-linking, giving rise to trimer and possibly tetramer muropeptides. The very large number of reduction products for such compounds results in a large number of small peaks eluting late in the HPLC gradient and thus the elevated baseline seen in Fig. 1C. To verify this postulated high level of cross-linking we used FDNB modification of Dpm residues in the peptide side chains, followed by amino acid analysis to estimate cross-linking (24). This method is not as accurate as the HPLC muropeptide analysis (1, 20, 24), but it does provide values useful for comparison of the relative cross-linking between samples. The cross-linking values obtained for dacB dacF double- and triple-mutant spore PG were in most cases significantly higher than those produced by the dacB single-mutant spore PG (Table 3). We attribute the slightly lower cross-linking value for the dacA dacB dacF triple mutant (Table 3) to the instability of these spores and potential degradation of some of the spore PG.
Germination and outgrowth of dac mutant spores.
Modification of cortical PG structure can result in alteration of not only spore heat resistance but also spore germination properties (1, 20, 24). Consequently, we tested each of the purified spore preparations for their germination and outgrowth (data not shown). All of the spore preparations were able to initiate spore germination, as evidenced by a decrease in optical density of spore suspensions, with kinetics similar to those of wild-type spores. Spores of strains with combinations of mutations in dacA, dacC, and dacF also commenced outgrowth with kinetics indistinguishable from those of the wild type, while dacB spores were delayed in outgrowth, as observed previously (22). All the double and triple dac mutant spores that contained a dacB mutation exhibited a similar delay in outgrowth. For mutants lacking both dacB and dacF the delay in spore outgrowth was in some cases even greater; this is likely due to the low viability of these spores.
DISCUSSION
Analysis of the phenotype and PG structure of spores produced by strains containing multiple dac mutations has allowed us to determine the roles and potential redundancies of the multiple carboxypeptidases present during B. subtilis sporulation. As observed previously, a dacA mutation had only a very slight effect on spore PG structure but no effect on spore phenotype (3, 20, 33). This change in PG structure was also seen in spores of multiple dac mutants lacking dacA but was never correlated with any obvious phenotype. A mutation in dacC also had no effect on spore phenotype and no effect on spore PG structure (19). Even in combination with mutations in dacF or dacB, no change in spore PG structure or phenotype due to a dacC mutation could be observed.
The effects of a dacB mutation include a large increase in PG cross-linking (1, 20, 22) which is associated with an inability to maintain spore core dehydration upon heating and therefore a slight loss in spore heat resistance (22). No significant effect of a dacF mutation was previously found on spore PG structure (1, 20, 22), dehydration (22), or heat resistance (22, 38). Apparently, DacF is at least partially redundant with DacB, such that it is only in the absence of DacB that a function for DacF can be observed. Spores of dacB dacF mutant strains exhibited a variety of phenotypes different from those of dacB spores, including lower viability, higher PG cross-linking, higher core water content, and decreased heat resistance. It is likely that all of these differences are the result of the loss of DacF carboxypeptidase activity, which would lead to an increase in the number of cortical PG side chains available for cross-link formation above that in the dacB strain. If the increased cross-linking in dacB dacF spores results in the inability to achieve or maintain normal spore core dehydration, reduced spore heat resistance would be a direct result. Loss of viability, even at a low temperature, may also be a result of increased spore core water content, as increased hydration of spore core macromolecules may result in a decrease in their stability and/or an increased rate of spontaneous damage.
Interestingly, spm mutant spores have a similar defect in spore core dehydration, yet they do not lose viability as fast or to as great a degree as the dacB dacF double-mutant spores (22). This may be due to the type of defect that results in the increased spore water content. The spm mutant spores have a relatively normal spore PG structure (22), while the altered structure of the dacB dacF spore PG may render the spore more susceptible to loss of some additional protective factor. This additional factor was not DPA, as the double-mutant spores retained wild-type levels of DPA throughout their purification (data not shown). The spore germination machinery must be relatively insensitive to the dacB dacF mutant spores’ loss of viability and altered PG structure, as they retained the ability to initiate germination and apparently to degrade cortex PG. However, the delay in outgrowth of dacB and dacB dacF mutant spores could be related to a slowed degradation of the highly cross-linked spore PG (22).
The significant sequence similarity and apparent partial functional redundancy of the dacB and dacF products suggests that they both have d,d-carboxypeptidase activities that are involved in regulating spore PG cross-linking. Why is there no obvious effect of the dacF mutation alone and what might be the purpose of these redundant activities? The lack of phenotypic change associated with a dacF mutation could be partially due to a significant difference in the abundance of the DacB and DacF proteins. While studies directly comparing the expression of these two dac genes have not been carried out, two lines of evidence suggest that dacB is more strongly expressed than dacF. Data on the expression of transcriptional and translational fusions of the two genes to lacZ indicate that dacB-lacZ fusions are expressed at a level 10- to 50-fold higher than the level of endogenous B. subtilis β-galactosidase activity, while dacF-lacZ expression is only 2- to 5-fold higher than the endogenous activity (22, 38). However, interpretation of these results is complicated by differences in growth media and strain backgrounds. The second line of evidence suggesting greater expression of dacB than dacF involves detection of the proteins with labeled β-lactams. DacB is easily detected by standard procedures using radio- or fluorescence-labeled β-lactams (3, 26, 29), while multiple attempts to clearly identify DacF by these procedures have failed (26). However, the inability to visualize DacF by these methods could be due to poor binding of the β-lactams by DacF, rapid decay of the complex, or poor recovery of the inner forespore membrane.
It is interesting that dacB is expressed only in the mother cell compartment of the developing sporangium (4, 28) while dacF is expressed only in the forespore compartment (27). Most of the spore PG is believed to be synthesized from the mother cell side of the developing spore wall (31). If the dacB and dacF products are each transported across the membrane into the intermembrane space in which the spore PG is synthesized, then they may have greatly different opportunities for acting upon the nascent spore PG. DacF may only have access to the limited amount of PG synthesized from the forespore side. This portion of the spore PG is generally thought to represent the germ cell wall PG (31). In contrast, the dacB product, PBP5*, may have access to the much greater amount of PG synthesized from the mother cell side, generally thought to represent the spore cortex. This model would explain the large effect on spore PG structure produced by a dacB mutation and the insignificant effect of a dacF mutation. However, the structure of the PG of dacB dacF spores suggests that this model is overly simplistic. If DacF acted only on the germ cell wall then it should affect only muropeptides which do not contain muramic-δ-lactam, as this modification appears to be confined to the cortical PG (1, 20, 21). However, in the dacB dacF mutant spores there is clearly an increase in cross-linking of muramic-δ-lactam-containing muropeptides over that seen in dacB spores, indicating that DacF can indeed participate in cortical PG synthesis.
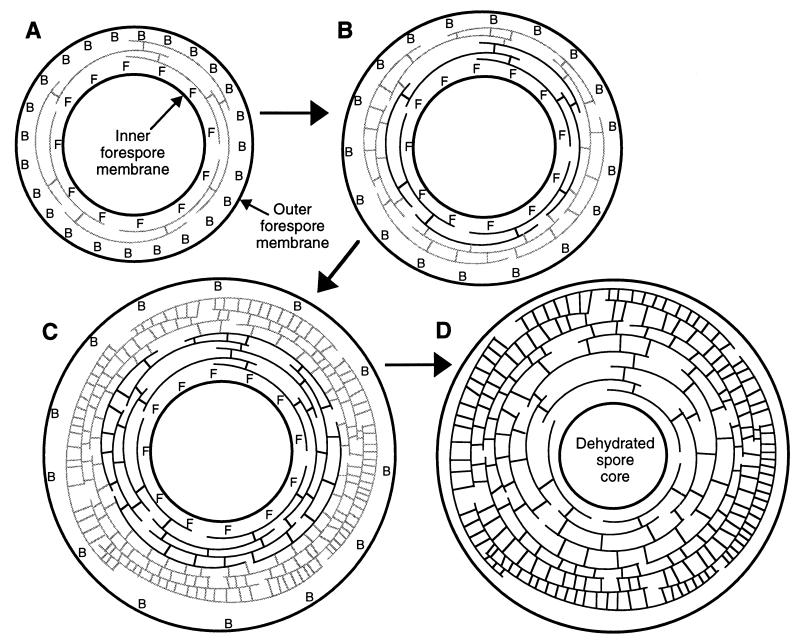
We propose another model for the effects of d,d-carboxypeptidases on spore PG structure, in which a gradient of peptide cross-linking is produced during cortex synthesis (Fig. 2). A cortex in which the innermost layers are loosely cross-linked and the outer layers are much more highly cross-linked might have the mechanical properties suggested by the anisotropically expansive cortex model of spore core dehydration proposed by Warth (34). The presence of both PBP5* and DacF at the initiation of spore PG synthesis would result in low cross-linking in the innermost layers of the spore cortex, closest to the inner forespore membrane. As cortex synthesis progresses outward, the effect of DacF diminishes due to its association with the inner forespore membrane or a decrease in its enzymatic activity through protein instability and/or decreased expression. At a certain point only PBP5* would be determining the degree of PG cross-linking. If the gradient of PG cross-linking were to continue after this point, then the relative effect of PBP5* would have to decrease over time. This may be a function of reduced expression of dacB during later sporulation, as transcription of dacB begins and peaks early during sporulation and decreases dramatically around the time of initiation of cortex synthesis (28). The activity of PBP5* may therefore decrease relative to the rate of cortex PG synthesis, through protein instability or through expansion of the surface area over which cortex synthesis is taking place, resulting in a gradient of cross-linking independent of DacF.
FIG. 2.
Schematic representation of a model for spore PG synthesis and spore core dehydration. Only the developing forespore is shown; the forespore is contained within the mother cell cytoplasm during sporulation. (A) DacB is expressed in the mother cell and appears on the outer forespore membrane while DacF is expressed in the forespore and appears on the inner forespore membrane; DacB is expressed at a level higher than is DacF. Both of these d,d-carboxypeptidases act on the first layers of spore PG synthesized, resulting in a very low level of peptide cross-linking. (B and C) Additional layers of PG are synthesized from the mother cell side (adjacent to the outer forespore membrane). DacB specific activity progressively decreases due to protein degradation and/or expansion of the surface area for PG synthesis. DacF is too distant to affect the cross-linking of these layers. DacF specific activity may also decrease, but this is not a necessary feature of the model. PG cross-linking increases as d,d-carboxypeptidase activity decreases, resulting in a gradient of cross-linking across the span of the spore cortex PG. (D) The cortex PG swells due to its overall low level of cross-linking. The swelling is predominantly inward due to the gradient of cross-linking, resulting in a decrease in the volume and water content of the spore core. Shaded arcs and short lines connecting arcs represent newly synthesized PG strands and peptide cross-links, respectively. Solid arcs and connecting lines represent PG and cross-links produced during the earlier stages of synthesis. Abbreviations: B, DacB molecule; F, DacF molecule.
This model can explain the lack of effect of a dacF mutation alone on spore PG structure. In a dacF mutant the innermost layers of the cortex might be slightly more cross-linked (such a slight increase was observed previously [20]), but PBP5* by itself may be able to produce a gradient of cross-linking which is sufficient to allow normal spore formation. In a dacB mutant the activity of DacF could produce a gradient of cross-linking within the innermost layers of the cortex, but the remainder of the cortex would have the high cross-linking observed (1, 20, 22) (Table 3). This slight cross-linking gradient may allow the attainment of normal spore core dehydration but might be insufficient to allow maintenance of core dehydration upon heating (22). The potential difference in the expression levels for dacB and dacF has no effect on this model, since the important factors for generation of a gradient of cross-linking are the spatial separation of the two gene products and some decay of PBP5* activity over time. The dacB dacF double mutant’s spores would have a high degree of cross-linking throughout the cortex, essentially nullifying the cortex’s ability to participate in spore core dehydration. The remaining degree of core dehydration observed in the double mutant spores may be attributable to another spore dehydration mechanism(s). In order to obtain definitive evidence for this model of cortex PG synthesis, a gradient of PG cross-linking within the spore cortex PG needs to be demonstrated directly. This will require analysis of spore PG structure throughout its synthesis, and these analyses are in progress.
ACKNOWLEDGMENTS
This work was supported by grants GM19698 (to P.S.) and GM56695 (to D.L.P) from the National Institutes of Health and by funds from Virginia Tech.
We acknowledge the technical assistance of Laura Koller in the completion of this work. We thank R. Schuch, P. J. Piggot, T. Murray, and L. B. Pedersen for supplying strains.
REFERENCES
- 1.Atrih A, Zöllner P, Allmaier G, Foster S J. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaman T C, Gerhardt P. Heat resistance of bacterial spores correlated with protoplast dehydration, mineralization, and thermal adaptation. Appl Environ Microbiol. 1986;52:1242–1246. doi: 10.1128/aem.52.6.1242-1246.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan C E, Gustafson A. Mutagenesis and mapping of the gene for a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992;174:5430–5435. doi: 10.1128/jb.174.16.5430-5435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan C E, Ling M-L. Isolation and sequence analysis of dacB, which encodes a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992;174:1717–1725. doi: 10.1128/jb.174.6.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan C E, Strominger J L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci USA. 1976;73:1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari F A, Nguyen A, Lang D, Hoch J A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983;154:1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghuysen J-M. Serine β-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 8.González-Castro M J, López-Hernández J, Simal-Lozano J, Oruña-Concha M J. Determination of amino acids in green beans by derivitization with phenylisothiocyanate and high-performance liquid chromatography with ultraviolet detection. J Chromatogr Sci. 1997;35:181–185. [Google Scholar]
- 9.Guérout-Fleury A-M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–337. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 10.Imae Y, Strominger J L. Relationship between cortex content and properties of Bacillus sphaericus spores. J Bacteriol. 1976;126:907–913. doi: 10.1128/jb.126.2.907-913.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshikawa T, Beaman T C, Pankratz H S, Nakashio S, Corner T R, Gerhardt P. Resistance, germination, and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984;159:624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence P J, Strominger J L. Biosynthesis of the peptidoglycan of bacterial cell walls. The reversible fixation of radioactive penicillin G to the d-alanine carboxypeptidase of Bacillus subtilis. J Biol Chem. 1970;245:3660–3666. [PubMed] [Google Scholar]
- 13.Leighton T J, Doi R H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971;254:3189–3195. [PubMed] [Google Scholar]
- 14.Lewis J C, Snell N S, Burr H K. Water permeability of bacterial spores and the concept of a contractile cortex. Science. 1960;132:544–545. doi: 10.1126/science.132.3426.544. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay J A, Beaman T C, Gerhardt P. Protoplast water content of bacterial spores determined by bouyant density sedimentation. J Bacteriol. 1985;163:735–737. doi: 10.1128/jb.163.2.735-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 17.Nakashio S, Gerhardt P. Protoplast dehydration correlated with heat resistance of bacterial spores. J Bacteriol. 1985;162:571–578. doi: 10.1128/jb.162.2.571-578.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson W L, Setlow P. Sporulation, germination, and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons Ltd.; 1990. pp. 391–450. [Google Scholar]
- 19.Pedersen L B, Murray T, Popham D L, Setlow P. Characterization of dacC, which encodes a new low-molecular-weight penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1998;180:4967–4973. doi: 10.1128/jb.180.18.4967-4973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popham D L, Helin J, Costello C E, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popham D L, Helin J, Costello C E, Setlow P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popham D L, Illades-Aguiar B, Setlow P. The Bacillus subtilis dacB gene, encoding penicillin-binding protein 5*, is part of a three-gene operon required for proper spore cortex synthesis and spore core dehydration. J Bacteriol. 1995;177:4721–4729. doi: 10.1128/jb.177.16.4721-4729.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popham D L, Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis pbpE operon, which codes for penicillin-binding protein 4* and an apparent amino acid racemase. J Bacteriol. 1993;175:2917–2925. doi: 10.1128/jb.175.10.2917-2925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popham D L, Setlow P. The cortical peptidoglycan from spores of Bacillus megaterium and Bacillus subtilis is not highly cross-linked. J Bacteriol. 1993;175:2767–2769. doi: 10.1128/jb.175.9.2767-2769.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popham D L, Setlow P. Phenotypes of Bacillus subtilis mutants lacking multiple class A high-molecular-weight penicillin-binding proteins. J Bacteriol. 1996;178:2079–2085. doi: 10.1128/jb.178.7.2079-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popham, D. L., and P. Setlow. Unpublished data.
- 27.Schuch R, Piggot P J. The dacF-spoIIA operon of Bacillus subtilis, encoding ςF, is autoregulated. J Bacteriol. 1994;176:4104–4110. doi: 10.1128/jb.176.13.4104-4110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson E B, Hancock T W, Buchanan C E. Transcriptional control of dacB, which encodes a major sporulation-specific penicillin-binding protein. J Bacteriol. 1994;176:7767–7769. doi: 10.1128/jb.176.24.7767-7769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sowell M O, Buchanan C E. Changes in penicillin-binding proteins during sporulation of Bacillus subtilis. J Bacteriol. 1983;153:1331–1337. doi: 10.1128/jb.153.3.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki H, Nishimura Y, Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci USA. 1978;75:664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tipper D J, Linnet P E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976;126:213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd J A, Bone E J, Ellar D J. The sporulation-specific penicillin-binding protein 5a from Bacillus subtilis is a dd-carboxypeptidase in vitro. Biochem J. 1985;230:825–828. doi: 10.1042/bj2300825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todd J A, Roberts A N, Johnstone K, Piggot P J, Winter G, Ellar D J. Reduced heat resistance of mutant spores after cloning and mutagenesis of the Bacillus subtilis gene encoding penicillin-binding protein 5. J Bacteriol. 1986;167:257–264. doi: 10.1128/jb.167.1.257-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warth A D. Mechanisms of heat resistance. In: Dring G J, Ellar D J, Gould G W, editors. Fundamental and applied aspects of bacterial spores. London, England: Academic Press, Inc.; 1985. pp. 209–225. [Google Scholar]
- 35.Warth A D, Strominger J L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972;11:1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- 36.Warth A D, Strominger J L. Structure of the peptidoglycan from vegetative cell walls of Bacillus subtilis. Biochemistry. 1971;10:4349–4358. doi: 10.1021/bi00800a001. [DOI] [PubMed] [Google Scholar]
- 37.Warth A D, Strominger J L. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci USA. 1969;64:528–535. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J-J, Schuch R, Piggot P J. Characterization of a Bacillus subtilis operon that includes genes for an RNA polymerase ς factor and for a putative dd-carboxypeptidase. J Bacteriol. 1992;174:4885–4892. doi: 10.1128/jb.174.15.4885-4892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yousif S Y, Broome-Smith J K, Spratt B G. Lysis of Escherichia coli by β-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985;131:2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]