Abstract
The World Health Organization stated that 1.6 million deaths worldwide were caused by contact with chemicals and toxins in 2019. In the same year, the Centers for Disease Control and Prevention stated that natural toxins caused 3960 deaths. Myrtus communis, also known as common Myrtle, is a flowering plant native to the Mediterranean region. Myrtle has been traditionally used to treat diarrhea, inflammation, bleeding, headache, pulmonary and skin diseases. This review was performed to assess Myrtle's protective and therapeutic efficacy against various chemical, natural, and radiational noxious. Multiple databases such as PubMed, Web of Sciences, and Scopus were investigated without publication time limitation. Recent studies have demonstrated its potential as a protective agent against both natural and chemical toxins. One of Myrtle's most significant protective properties is its high antioxidant content. Studies have shown that the antioxidant properties of Myrtle can protect against harmful substances such as heavy metals, pesticides, and other environmental toxins. Additionally, Myrtle has anti‐inflammatory properties that can help reduce the damage caused by long‐term exposure to toxins. The anti‐inflammatory and antimicrobial properties of Myrtle have also proven effective in alleviating gastrointestinal conditions such as gastric ulcers.
Keywords: anti‐inflammatory, antioxidant, Myrtle, Myrtus communis, toxin
1. INTRODUCTION
Myrtaceae is a family of woody flowering plants with approximately 5500 species divided into 144 genera and 17 tribes. In this family, the tribe Myrteae includes half of the family's biodiversity, with 51 genera and approximately 2500 species found primarily in the Neotropics. Myrtus is the only genus found in Europe, North Africa, Asia, and especially in the Mediterranean region of southern Europe as far west as Macaronesia (Madeira and the Azores), the Saharan mountains, and as far east as western Asia (Hosseini et al., 2023; Migliore et al., 2012; Thornhill et al., 2015; Vasconcelos et al., 2017; Yahyazadeh et al., 2021).
One of the most mentioned types of Myrtus in traditional books is Myrtus communis, also referred to as “Murt” or “Murd” (Mahboubi, 2017). The Mediterranean shrub M. communis, also known as common Myrtle, is native to the Mediterranean region. The plant is 2.4–3 m tall, with branches forming a close full head that is densely covered in leaves (Sumbul et al., 2011). Fruits are small and dark, with small green leaves (Asif et al., 2011). The evergreen leaves range in length from 2 to 5 cm. It has a bitter taste owing to its astringent properties (Alipour et al., 2014; Gortzi et al., 2008; Özkan & Güray, 2009). Flowers are star‐shaped, white or pinkish in color, and extremely fragrant (Charles, 2012). There are several seeds in the round blue‐black berry fruit. Insects pollinate the flowers, and birds that eat the berries spread the seeds (Figure 1) (Satyavati et al., 1987).
FIGURE 1.

Myrtus communis (This photo was taken by Jonathan Billinger, near to Huntley, Gloucestershire, England, licensed for reuse under Creative Commons License; Attribution‐ShareAlike 4.0 International [CC BY‐SA 4.0]).
It also has other active constituents like polyphenols, anthocyanins, and vitamins, which are shown in Figure 2 (Çakmak et al., 2021; Correddu et al., 2019; Hacıseferoğulları et al., 2012; Hennia et al., 2018; Messaoud & Boussaid, 2011; Ruffoni et al., 2009; Tuberoso et al., 2006). Because of its medicinal and aromatic effects, it is used for the treatment of patients in traditional medicine. Its medicinal uses are because of essential oils and compounds found in its leaves and fruit (Usai et al., 2018). The essential oil is extracted by hydro‐distillation from leaves and employed in the flavor and perfume industries. Myrtle berries and leaves are mostly used in the production of sweet liquor with digestive characteristics (Nuvoli & Spanu, 1996). Many studies have confirmed its antioxidant, antibacterial, and antifungal properties (Chalchat et al., 2010; Özcan & Akgül, 1995; Özcan & Boyraz, 2000; Özcan et al., 2015; Saǧdıç & Özcan, 2003). Myrtle essential oil has antimicrobial, insecticidal, antioxidant, and hepatoprotective properties (Hennia et al., 2019).
FIGURE 2.
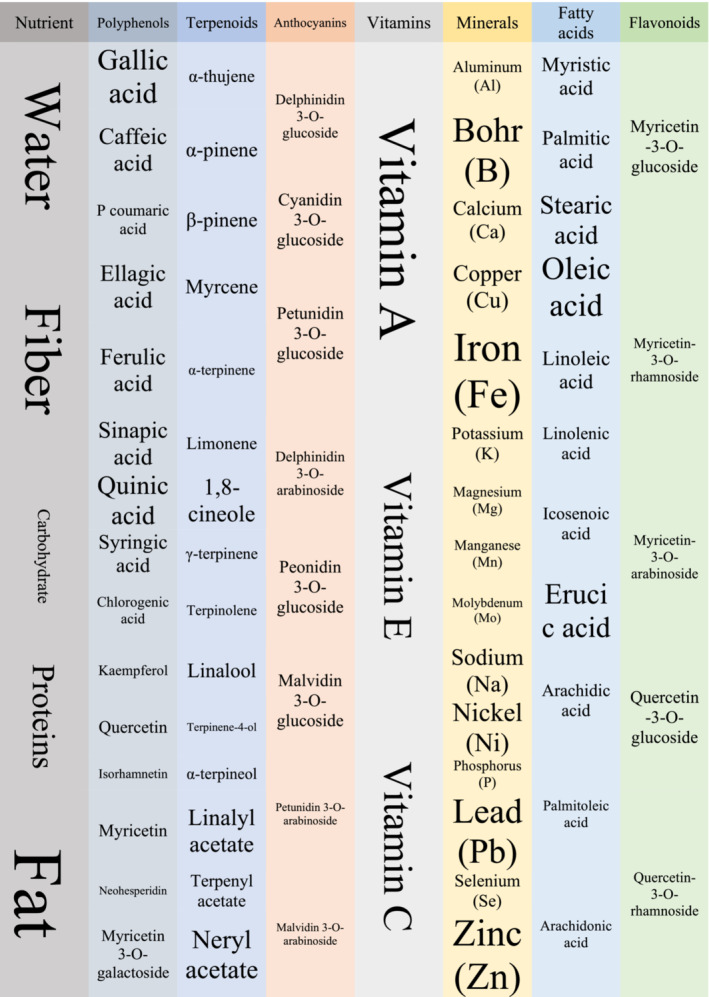
Active constituents of Myrtus communis.
In traditional healers, Myrtle has been used to treat diarrhea, peptic ulcers, hemorrhoids, inflammation, uterine bleeding, headache, palpitation, leukorrhea, urethritis, epistaxis, conjunctivitis, excessive perspiration, pulmonary and skin diseases (Alipour et al., 2014; Mobli et al., 2015). In addition, because of their astringent, tonic, and antiseptic properties, Myrtle leaves have been used to treat wounds and digestive and urinary system disorders (Alipour et al., 2014; Sisay & Gashaw, 2017). Although berries decoctions were used for bathing babies with inflamed skin and leaves and berries decoctions in painful washing, the majority of the berries are utilized to manufacture the distinctive Myrtle liqueur derived by the hydroalcoholic extraction of the berries (Alipour et al., 2014; Fadda et al., 2017; Montoro et al., 2006).
We live in a world where our health is influenced by several elements, including climate, food, and toxins, via the multiple routes of administration. Toxic agents can have a variety of negative health impacts based on their physical, chemical, or biological characteristics, resulting in organ dysfunction. The seriousness of the harm is determined by exposure doses and durations, as well as individual factors such as age, diet, illness, pregnancy, and other relevant factors (Yahyazadeh et al., 2021). Toxins are biomolecules produced primarily for defensive purposes by bacteria, fungi, insects, plants, and vertebrate and invertebrate animals. These molecules cause harm to other organisms through inhalation, injection, ingestion, or absorption (Dorner & Rummel, 2015). The consequences of these toxins are frequently irreversible, resulting in permanent health harm. Toxins significantly impact health, food, and security (Janik et al., 2019).
In 2019, the World Health Organization (WHO) reported that 1.6 million deaths worldwide were caused by exposure to chemicals and toxins. In the same year, the Centers for Disease Control and Prevention reported that 3960 deaths were caused by natural toxins (Yahyazadeh et al., 2021).
This review will explain the treatment of different diseases, which are induced by various toxins, with Myrtle.
2. METHOD
A comprehensive search has been performed from Scopus, ScienceDirect, Web of Sciences, and PubMed databases without date limitation from inception to the first of April 2023. In this review article, all in vitro and in vivo studies were considered. In addition, the following medical keywords were investigated alone or in combinations: “embryotoxicity, genotoxicity, hematological toxicity, hepatotoxicity, ototoxicity, pulmonary toxicity, radiotoxicity, retinotoxicity, or skin phototoxicity” “toxic, toxicity, nephrotoxic, radiation, cardiotoxic, hepatotoxic, mycotoxins, pesticides, or cardiotoxicity” “neurotoxic, fumonisins, natural toxins, chemical toxicity, cardiotoxins, neurotoxins, nephrotoxins, ochratoxin, aflatoxins” “venoms, bacterial toxins, lipopolysaccharides (LPS), chemical agents induced toxicity, metals, cadmium, titanium dioxide, lead, or 6‐hydroxydopamine” “1‐methyl‐4‐phenylpyridinium (MPP), amyloid‐beta, dieldrin, chlorpyrifos, gentamicin, carbon tetrachloride, thioacetamide, diethyl nitrosamine, or azathioprine” “cisplatin, naphthalene, carbon tetrachloride, cardiotoxic agent, isoproterenol, ethanol, hydrogen peroxide, penicillic acid, or ergosterol” “radiation‐induced toxicity, ultraviolet, heavy metals, chromium, mercury, aluminum, doxorubicin, bleomycin, or diclofenac sodium” “thiourea, carbendazim, dextran sulfate sodium, carbon tetrachloride, phorbol, strychnine, 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MTPT), streptozotocin (STZ)” “acetaminophen, hepatotoxins, or liver” with “M. communis.”
3. FINDINGS AND DISCUSSION
We have provided explanations about various chemical and natural toxins and the protective effect of Myrtle against them. The promising results of Myrtle have been reported in different in vitro and in vivo studies (Tables 1 and 2).
TABLE 1.
In vivo protective effects of Myrtus communis against toxins and noxious agents.
| Toxins/noxious | Models | Constituents | Results | References |
|---|---|---|---|---|
| Aflatoxin B1 | Chicken | Myrtle essential oil (500 mg/kg) for 35 days | ↓AST | (Saei et al., 2013) |
| ↓ALP | ||||
| ↓ALT | ||||
| Alcohol | Rats | Myrtle aqueous extract (105 and 175 mg/kg) and methanolic extract (93 and 154 mg/kg) | ↓Gastric ulcer index | (Sumbul et al., 2010) |
| ↓Gastric juice volume | ||||
| ↑Gastric ph | ||||
| Aluminum chloride and D‐galactose | Rats | Myrtle extract (100–200 mg/kg) for 90 days | ↓Aβ | (Yalman et al., 2022) |
| ↓8‐OHdG | ||||
| ↓Acetylcholinesterase activity | ||||
| ↑Neprilysin | ||||
| ↑SOD | ||||
| Arsenic | Rats | Myrtle leaf extract (3 mg/mL) | ↑P53 gene expression | (Naji et al., 2018) |
| ↑PGE level | ||||
| Bleomycin | Rats | Myrtle leaf extract (50 mg/kg) for 14 days | ↓Lipid peroxidation | (Samareh Fekri et al., 2018) |
| Carbon tetrachloride | Rats | Essential oil of Myrtle (250 mL/kg) for 14 days | ↑LDH | (Ben Hsouna et al., 2019) |
| ↓TG | ||||
| ↑HDL‐Ch | ||||
| ↓T‐Ch | ||||
| ↑LDL‐Ch | ||||
| ↑Atherogenic index | ||||
| ↓TBARS | ||||
| ↓PCO | ||||
| ↑SOD | ||||
| ↑CAT | ||||
| ↑GPx | ||||
| Carrageenan | Mice | Myrtucommulone (0.5, 1.5, and 4.5 mg/kg) for 4 h | ↓ICAM‐1 | (Rossi et al., 2009) |
| ↓P‐selectin | ||||
| ↓TNF‐α | ||||
| ↓IL‐1β | ||||
| ↓Nitrotyrosine | ||||
| ↓PAR | ||||
| ↓MPO activity | ||||
| Castor oil | Rats | Myrtle berry seeds extract (25, 50, and 100 mg/kg) | ↓MDA | (Jabri, Rtibi, et al., 2016) |
| ↑GSH | ||||
| ↑SOD | ||||
| ↑CAT | ||||
| ↑GPx | ||||
| ↓Wet fecal weight | ||||
| ↓Wet fecal no. | ||||
| Cerulein | Rats | Myrtle leaf extract (100 mg/kg) for 14 days | ↓Serum lipase level | (Ozbeyli et al., 2020) |
| ↓Amylase level | ||||
| ↓MDA | ||||
| ↑GSH | ||||
| ↓MPO | ||||
| ↓Pancreatic ROS level | ||||
| ↓IL‐1β | ||||
| ↓IL‐6 | ||||
| ↑IL‐10 | ||||
| ↓Pancreatic edema index | ||||
| Croton oil | Rats | Myrtle essential oil (1 and 2 mL/kg) for 10 days | ↓MPO activity | (Maxia et al., 2011) |
| ↓TNF‐α | ||||
| ↓IL‐6 | ||||
| Cypermethrin | Rats | Myrtle leaf extract (1 mL [50 g/L]) for 30 days | ↓Blood glucose | (Berroukche et al., 2017) |
| ↓GPT | ||||
| ↑Glutamic oxaloacetic | ||||
| ↑Transaminase alkaline phosphatase | ||||
| Double ligatures with suture silk | Rats | Myrtle leaf extract (50 mg/kg) for 28 days | ↓Plasma total bilirubin | (Sen et al., 2016) |
| ↓Direct bilirubin | ||||
| ↓Alanine aminotransferase | ||||
| ↓Aspartate aminotransferase | ||||
| ↓Tumor necrosis factor α | ||||
| ↓Interleukin‐1β | ||||
| Ethanol | Rats | Myrtle berries seed extract (25, 50, and 100 mg/kg) for 2 months | ↑Hb | (Jabri et al., 2018) |
| ↑Ht | ||||
| ↓MCV | ||||
| ↑MCHC | ||||
| ↑Plt | ||||
| ↓WBC | ||||
| ↓MDA | ||||
| ↑SOD | ||||
| ↑GPx | ||||
| ↑CAT | ||||
| ↑GSH | ||||
| ↑SH‐groups | ||||
| ↓TNF‐α | ||||
| ↓IL‐6 | ||||
| ↓IL‐8 | ||||
| ↓IL‐1β | ||||
| Ethanol/HCl | Rats | Myrtle essential oil (250, 500, and 1000 mg/kg) for 21 days | ↓UI | (Mansour et al., 2022) |
| ↓UP | ||||
| ↑GV | ||||
| ↑Gastric pH | ||||
| ↓NO production | ||||
| ↓MDA | ||||
| ↑SOD activity | ||||
| ↑CAT | ||||
| ↑GPx | ||||
| Extremely low frequency magnetic fields (ELFMF) | Rats | Myrtle extract (injected 0.5 mg/kg) | ↑FRAP values | (Seif et al., 2019) |
| ↓Plasma POC | ||||
| ↓MetHb | ||||
| ↓Hemichrome | ||||
| Goldblatt's 2K1C | Rats | Myrtle extract (100 mg/kg) for 9 weeks | ↓Serum osteopontin levels | (Cevikelli‐Yakut et al., 2020) |
| ↑IL‐10 | ||||
| ↓Hippocampal MMP‐13 | ||||
| ↓CD36 expression | ||||
| ↓Neprilysin levels | ||||
| ↓AChE activity | ||||
| Hot water | Rats | Myrtle ethanol extract (100 mg/kg) twice a day for 48 h | ↓MDA | (Ozcan et al., 2020) |
| ↑GSH | ||||
| ↑CAT | ||||
| ↑GST | ||||
| ↑SOD | ||||
| Hot water | Rats | Myrtle leaves extract (100 mg/kg) for 2 days | ↑GSH | (Ozcan et al., 2019) |
| ↓MDA | ||||
| ↑SOD | ||||
| ↑CAT | ||||
| ↑Skin NO levels | ||||
| ↑TF activity | ||||
| Hydatid cysts protoscoleces | Mice | Essential oil of Myrtle (0.05, 0.1, 0.2, and 0.4 mg/kg) for 14 days | ↑Mortality of protoscoleces | (Mahmoudvand et al., 2016) |
| Iron | Mice | Myrtle leaf extract (50 and 100 mg/kg) for 4 weeks | ↓Total serum iron | (Eslami et al., 2018) |
| ↓Serum Fe3+ | ||||
| ↓Serum AST | ||||
| ↓Serum ALT | ||||
| ↓Serum ALP | ||||
| Liver ischemia–reperfusion | Rats | Myrtle extract | ↓AST | (Salouage et al., 2010) |
| ↓ALT | ||||
| ↑MEGX | ||||
| Monosodium glutamate and acrylamide | Rats | Myrtle leaf extract (300 mg/kg) for 6 weeks | ↑Bcl‐2 | (Hassan et al., 2020) |
| ↓Apoptosis | ||||
| ↓PD‐1 | ||||
| Paracetamol | Rats | Aqueous extract of Myrtle leaves (200 and 400 mg/kg) for 10 days | ↓SGPT | (Rupesh et al., 2011) |
| ↓SGOT | ||||
| ↑CAT | ||||
| ↓ALP | ||||
| ↑SOD | ||||
| ↓LPO | ||||
| ↓Total bilirubin | ||||
| Silver nanoparticles | Mice | Hydrolyzable tannin fraction of Myrtus communis (50, 100, and 200 mg/kg) for 90 days | ↓Serum AST | (Tavakoli et al., 2020) |
| ↓Serum ALT | ||||
| ↓Serum ALP | ||||
| ↑White blood cells | ||||
| ↑Red blood cells | ||||
| ↑Lymphocytes | ||||
| ↑Hb | ||||
| Streptozotocin | Rats | Myrtle leaf extract (100 mg/kg) for 4 weeks | ↓Blood glucose levels | (Kadıoğlu Yaman et al., 2020) |
| ↓Ache activities | ||||
| ↑Hippocampal CHAT activity | ||||
| ↑Neprilysin levels | ||||
| ↑α7‐nAChR | ||||
| ↑PSA‐NCAM | ||||
| ↑BDNF expressions | ||||
| Streptozotocin | Rats | Ethanol extract of Myrtle (0.25, 0.5, and 1 g/kg) for 14 days | ↓ALT | (Aggul et al., 2022) |
| ↓AST | ||||
| ↓MDA | ||||
| ↓Blood glucose levels | ||||
| ↑GSH levels | ||||
| ↑SOD activities | ||||
| Streptozotocin | Rats | Myrtle fruit hydro‐alcoholic extract | ↓Serum glucose | (Tas et al., 2018) |
| ↓Serum lipid | ||||
| ↓MDA | ||||
| ↑Insulin | ||||
| ↑Paraoxonase arylesterase | ||||
| ↑SOD | ||||
| ↑Whole blood GSH‐Px | ||||
| Streptozotocin | Rats | Myrtle extract | ↓5‐LOX and 15‐LOX | (El‐Bana et al., 2017) |
| ↓Lipoxin A4 | ||||
| ↓TNF‐α | ||||
| ↑Insulin | ||||
| Surgery | Rats | Myrtle berry seeds extract (25, 50, and 100 mg/kg) | ↑Gastric juice pH | (Jabri, Tounsi, et al., 2016) |
| ↓MDA | ||||
| ↑GSH | ||||
| ↑SOD | ||||
| ↑CAT | ||||
| ↑GPx | ||||
| ↓Hydrogen peroxide | ||||
| ↓Free iron | ||||
| ↓Calcium | ||||
| Surgery | Rats | Myrtle leaves essential oil (50 mg/kg) for 7 days | ↑CAT | (Jabri, Hajaji, et al., 2016) |
| ↑SOD | ||||
| ↑GPx | ||||
| ↓Lipid peroxidation | ||||
| Toxoplasma gondii | Mice | Essential oil of Myrtle (100, 200, and 300 mg/kg/day) for 3 weeks | ↓Tissue cyst | (Shaapan et al., 2021) |
| ↓Diameter of tissue cyst | ||||
| ↑IFN‐γ | ||||
| ↑IL‐12 |
TABLE 2.
In vitro protective effects of Myrtus communis against toxins and noxious agents.
| Toxins | Models | Constituents | Results | References |
|---|---|---|---|---|
| Hydrogen peroxide | K562 cell line | Leaf extract (0.4 mg/mL) for 2 h | ↑MDA production | (Ines et al., 2012) |
| ↑CAT | ||||
| ↓GPx1 | ||||
| ↑GSS | ||||
| ↑HMOX2 | ||||
| ↑TXN | ||||
| ↑TXNRD1 | ||||
| ↑AOE372 | ||||
| ↑XRCC1 | ||||
| ↑LIG4 | ||||
| ↑POLD2 | ||||
| ↑XRCC5 | ||||
| ↑GADD45A | ||||
| ↑RPA3 | ||||
| ↑XPA | ||||
| ↑hMSH2 | ||||
| ↑RPA2 | ||||
| ↑TDG | ||||
| ↑PCNA | ||||
| ↑DDIT3 | ||||
| ↑ERCCI | ||||
| HUVEC cell line | Myrtle extract | ↑HIF_1α expression | (Raeiszadeh et al., 2018) | |
| ↑VEGF | ||||
| ↓COX‐2 | ||||
| ↓iNOS | ||||
| K562 cell line | Two compound of Myrtle extract | ↓Lipid peroxidation | (Hayder et al., 2008) | |
| ↓MDA | ||||
| ↑AOE372 expression | ||||
| ↑TXN expression | ||||
| ↓GPX1 expression | ||||
| ↓SEPW1 expression | ||||
| ↑XPC expression | ||||
| ↑LIG4 expression | ||||
| ↑RPA3 expression | ||||
| ↑PCNA expression | ||||
| ↑DDIT3 expression | ||||
| ↓POLD1 expression | ||||
| ↓XRCC expression | ||||
| ↓MPG expression | ||||
| ↓PARP expression | ||||
| ↓SHC1 expression | ||||
| IL‐1β | BEAS‐2B | Myrtle essential oil | ↓IL‐6 | (Gülbol Duran & Terzi, 2021) |
| ↓IL‐10 | ||||
| ↓NFkB | ||||
| LPS | Macrophages | Myrtle essential oil | ↓NO production | (Bouzabata et al., 2015) |
| KM48 and KM149 cell line | Myrtle essential oil | ↓AHL | (Myszka et al., 2020) | |
| ↓PQS | ||||
| ↓mRNA level of the algU gene | ||||
| ↓Exopolysaccharide synthesis | ||||
| ↑Expression of the mucA gene | ||||
| Hydatid cyst protoscolices | Myrtle extract (5, 50, and 100 mg/mL) for 4 h | ↑Activity of caspase 3, 8, and 9 | (Shahnazi et al., 2017) | |
| Human macrophages | Myrtle leaf extract | ↓Superoxide production | (Soomro et al., 2019) | |
| ↓NO production | ||||
| ↓Hydrogen peroxide production | ||||
| ↓NFκB phsphrolation | ||||
| A549 cell line | Myrtle Essential oil (from 31.25 to 200 μg/mL) for 24 h | ↑Caspase 3 gene expression | (Bilgic & Duran, 2020) | |
| ↑Caspase 9 gene expression | ||||
| ↑P21 gene expression |
3.1. Aflatoxin
Aflatoxin, a very poisonous mycotoxin, can withstand freezing and cooking even in extreme temperatures. A variety of aflatoxins are produced by the fungi Aspergillus flavus, Aspergillus nomiu, and Aspergillus parasiticus, which grow in a wide range of temperatures (from 12 to 42°C) (Hosseini et al., 2023; O'Riordan & Wilkinson, 2008; Yahyazadeh et al., 2021). However, the ideal growing temperature range is between 20 and 30°C (Murphy et al., 2006). The fact that aflatoxin can be produced during preharvest and postharvest storage is one of the main problems with the substance. The primary aflatoxin species include types B1, B2, G1, and G2, with B1 being the most dangerous (Ghali et al., 2009). Aflatoxin contamination in humans occurs through the consumption of foods such as eggs, wheat, maize, milk, and dairy products (Mohammadi, 2011). In both acute and chronic situations, it produces nephrotoxicity, hepatotoxicity, immunotoxicity, carcinogenicity, and mutagenicity (Abdel‐Hamid & Firgany, 2015; Barton et al., 2001; Benigni & Bossa, 2011; Meissonnier et al., 2008). In order to produce the trans‐8,9‐dihydro‐8‐(2,6‐diamino‐4‐oxo‐3,4 dihydropyrimid‐5‐ylformamido)‐9‐hydroxy aflatoxin B1 adduct, exo‐8,9‐epoxide, a DNA alkylating agent, is attached to the N7 of guanine (Li, Brown, et al., 2015). This is what gives aflatoxin its carcinogenic properties. Moreover, human cytochrome P450‐3A4.62 partially metabolizes aflatoxin (Bren et al., 2007). Thus, according to the WHO, diseases caused by aflatoxin must be prevented or treated in order to lessen their burden and side effects (Ochieng et al., 2013).
According to the results of their experiment on chicken, Saei et al. concluded that the use of Myrtle essential oil as a treatment reduces the harmful effects of aflatoxin on various aspects, including serum glucose, creatinine (CREA), cholesterol (CHOL), alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) concentration, feed intake, and body weight gain. Serum AST, ALT, and ALP are highly sensitive markers utilized for diagnosing hepatic damage due to their release into the circulation after cellular damage as cytoplasmic enzymes. A decrease in growth rate is the most significant effect of aflatoxin on poultry. The protective properties of this compound are especially evident in growth performance. These findings suggest that the essential oil of Myrtle may be utilized in chickens to prevent the consequences of aflatoxins in contaminated feed, providing a foundation for further exploration of the relationship between Myrtle essential oil and protection against aflatoxin toxicity. This could potentially improve the health, safety, and quality of poultry products (Saei et al., 2013).
3.2. Aluminum
Aluminum is a harmful heavy metal that affects the skeletal, hematopoietic, and respiratory systems (Nayak, 2002). Adenyl cyclase, alkaline phosphatase, and acetylcholinesterase are all inhibited by this metal (Niedworok & Fijałkowski, 2004). As a reactive oxygen species (ROS) producer, aluminum also causes DNA alkylation (El‐Demerdash, 2004).
In a study conducted by Yalman et al., the protective effects of Myrtle against the toxicity caused by aluminum chloride and D‐galactose, which caused Alzheimer's induction in rats, were evaluated. The treatment with Myrtle was found to lower the levels of amyloid beta (Aβ) and 8‐hydroxy‐2‐deoxyguanosine (8‐OHdG), while increasing the levels of neprilysin and superoxide dismutase (SOD). The authors proposed that the protective and therapeutic effects of Myrtle may stem from its phenolic compounds. These results indicate that Myrtle can positively impact cognitive and neuronal functions through its ability to prevent the breakdown of acetylcholine and protect against oxidative stress (Yalman et al., 2022).
3.3. Arsenic
Arsenic is widely spread in farmland soil and groundwater, and arsenic concentrations in soil and river water reach the WHO standard limit of 10 μg/L in many areas worldwide (Min et al., 2021; Missimer et al., 2018). Many studies in recent decades have demonstrated arsenic‐induced immunotoxicity, nephrotoxicity, reproductive toxicity, and other negative effects in animals (Hall, 2002; Shao et al., 2018). Over the last few years, evidence has accumulated that excessive arsenic intake can cause a variety of toxic effects, including liver injury, abnormal metabolism, and cardiovascular disease toxicity (Chen et al., 2019; Zhong et al., 2020). Because of the high affinity of the liver, the detoxification organ, for heavy metals, the liver is the main toxicity target of arsenic (Liu et al., 2020).
Naji et al. study the protective effect of Myrtle leaves on decreasing the toxicity of arsenic trichloride in the rat. Myrtle contains abundant quantities of linoleic acid, octane 3,5‐dimethyl, oleic acid, and other compounds. These substances function to combat the harmful effects of toxicants by activating distinct cellular pathways, such as programmed cell death. This results in safeguarding cells from the toxicological properties of heavy metals. Arsenic trichloride increases the PGE2 levels and protein activity of the p53 gene, reducing gene expression. The utilization of Myrtle, either on its own or combined with arsenic trichloride, can enhance the levels of PGE and improve the condition (Naji et al., 2018).
3.4. Bleomycin
Chemotherapeutic medications are used to treat neoplastic disorders such as testicular carcinoma, ovarian cancer, and malignant pleural effusions. Bleomycin (BLM), a chemotherapeutic drug, is a member of the glycopeptide group derived from the bacteria Streptomyces verticillus (Claussen & Long, 1999). This medication is hazardous to healthy organs such as the lungs (Umezawa et al., 1966). BLM therapy has also been related to the dissection of the double strand of DNA in the presence of iron and oxygen via the generation of reactive nitrogen species and ROS (Della Latta et al., 2015).
In an in vivo study, the Myrtle methanolic extract effects on BLM‐induced pulmonary fibrosis in rats were investigated. After treatment with Myrtle leaf extract for 14 days at the dose of 50 mg/kg, lipid peroxidation (LPO) was reduced in BLM‐induced pulmonary fibrosis. The results showed the anti‐inflammatory effects of Myrtle methanolic extract against lung fibrosis. The extract from Myrtle could remarkably inhibit inflammation and fibrosis of the lung parenchyma through preventive and therapeutic methods. This positive effect can be attributed to tissue inflammation reduction and oxidative stress inhibition (Samareh Fekri et al., 2018).
3.5. Carbon tetrachloride
Several investigations have found that carbon tetrachloride, a common laboratory reagent and industrial use, causes liver damage (Li, Chen, et al., 2015; Weber et al., 2003). Carbon tetrachloride has been shown to alter lipid profiles, stress oxidative markers, total protein, high‐density lipoprotein (HDL), liver enzymes, inflammatory markers, hepatocytes, and fiber segmentation in the liver (Cogliati et al., 2011; Ebeid et al., 2015). Chronic carbon tetrachloride exposure also causes genotoxicity and DNA fragmentation in rats (Alkreathy et al., 2014).
The use of Myrtle essential oil prior to carbon tetrachloride treatment was able to effectively prevent the rise in hepatic markers and lipid levels in rats. Additionally, this particular substance helped improve biochemical and histological factors compared to the group treated solely with carbon tetrachloride. These findings suggest that Myrtle may contain beneficial compounds capable of combating the harmful effects of carbon tetrachloride intoxication and potentially serving as an effective preventative measure against liver damage. The Myrtle essential oil's contents not only safeguard the plasma membrane's integrity but also enhance the liver's regenerative and reparative ability. These findings imply that the Myrtle essential oil compound has hepatoprotective effects against oxidative stress caused by carbon tetrachloride in rats. This is substantiated by the reduction of thiobarbituric acid reactive substances levels in the liver tissue and liver marker enzymes in the serum (Hsouna et al., 2019).
3.6. Carrageenan
Carrageenan‐induced paw edema is a common method for screening anti‐inflammatory activity (Winter et al., 1962). It is a sensitive method for nonsteroidal anti‐inflammatory drugs (NSAIDs) and has long been used to study new NSAIDs (Willoughby & DiRosa, 1972). In addition, carrageenan‐induced inflammation is useful in detecting oral anti‐inflammatory agents (Vinegar et al., 1969). Developing edema induced by carrageenan injection causes an acute and local inflammatory response. In the early phase (0–1 h), histamine, serotonin, and bradykinin are the first mediators elaborated, and prostaglandins and various cytokines such as interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), interleukin‐10 (IL‐10), and tumor necrosis factor α (TNF‐α) are implicated in the second phase (Crunkhorn & Meacock, 1971).
In a study conducted by Rossi et al. using this model, they found that myrtucommulone has high anti‐inflammatory properties (Maxia et al., 2011). Myrtucommulone is a nonprenylated acyl phloroglucinol contained in Myrtle leaves (Rosa et al., 2003). In their study, this compound was able to reduce the level of inflammatory mediators such as TNF‐α and IL‐1β.
3.7. Cerulein
The most well‐studied and commonly used experimental model for acute edematous pancreatitis is cerulein‐induced pancreatitis. Supramaximal pancreatic stimulation with cerulein, cholecystokinin (CCK) analog, causes intra‐acinar activation of trypsinogen in rats (Hofbauer et al., 1998). This decapeptide induces smooth muscles and increases digestive secretions, leading to notable parenchymal fibrosis and pancreatitis criteria (Kim, 2008; Wu et al., 2021).
Ozbeyli et al. studied the effects of Myrtle on acute pancreatitis (AP). Rats were divided into four groups (each one had eight rats): saline‐pretreated control, Myrtle‐pretreated control, saline‐pretreated AP, and Myrtle‐pretreated AP group. Myrtle and saline were administered orally daily (100 mg/kg). After 2 weeks, in cerulein‐induced AP rats, inflammatory factors and cellular and oxidative damage had increased. On the other hand, the administration of Myrtle caused anti‐inflammatory and antioxidant effects, such as a reduction in MDA levels and MPO activity and an elevation in GSH levels. Also, pancreatic ROS release was decreased by Myrtle treatment. The results indicate helpful treatment to protect the pancreas (Ozbeyli et al., 2020).
3.8. Cypermethrin
Pesticides, such as insecticides, can create severe harm to organisms even at low concentrations, and long‐term exposure can result in genetic disorders and physiological abnormalities that shorten lifespan (Bernardes et al., 2015; Wojciechowska et al., 2016; Zacharia, 2011). Cypermethrin is a widely used insecticide around the world. Cypermethrin is a broad‐spectrum chemical pyrethroid group insecticide used in agriculture for pest control and crop loss prevention (Seven et al., 2022). Its widespread use has the potential to have a variety of toxic properties on nontarget organisms (Grewal et al., 2010). Cypermethrin is a highly poisonous chemical that can be inhaled, ingested, and absorbed through the skin. Skin irritation, numbness and tingling, itching and burning sensations in the eyes, loss of bladder control, seizures, and eventually death can result from exposure (Sangha et al., 2011; Sharma et al., 2018).
The results of a study by Berroukche et al. showed that the use of the treatment with Myrtle leaves decoction (50 g/L) partially improved the condition of animals. In addition, it reduced the harmful effects of the free radicals produced by cypermethrin toxicity. Furthermore, the application of Myrtle caused a reduction in GPT activity and an elevation in ALP activity (Berroukche et al., 2017).
3.9. Ethanol
It is proven that ethanol has a toxic effect on the gastrointestinal system (GIS). It can cause irritation and inflammation in GIS. In addition, chronic consumption of ethanol can cause more serious damage to the GIS, including ulcers, bleeding, and even cancer (Bode & Bode, 1997).
Sumbul et al. studied the anti‐ulcer effect of Myrtle extract on alcohol‐induced, pyloric ligation‐induced, and indomethacin‐induced gastric ulceration in rats. Treatment with Myrtle extract significantly reduced ulcer index and gastric juice volume. Also, it showed an increase in the pH of gastric juice and mucus secretion as compared to the toxic control. The findings indicate that Myrtle can be a potential substance for the treatment of gastric ulcers (Sumbul et al., 2010).
Mansour et al. studied the gastroprotective effect of microencapsulated Myrtle essential oil (MMEO) against acute gastric lesions. Rats were randomly divided into six groups. To induce acute gastric lesions, rats were treated with acidified ethanol solution. They found no mortality in rats after 3 weeks of MMEO administration. In addition, MMEO treatment reduced inflammation and submucosal edema. Treatment with MMEO raised pH levels and volume of gastric juice of stomach and reduced gastric lesions (Mansour et al., 2022).
3.10. Extremely low‐frequency magnetic fields (ELFMF)
The International Agency for Research on Cancer has classified ELFMF as a cancer‐causing agent in biological systems. There is evidence that alterations in energy levels can impact the generation and recombination of free radicals (Güler et al., 2012; Timmel et al., 1998).
Seif et al. investigated the protective properties of Myrtle extract against the oxidative effects of ELFMF in adult male rats. The extract improved the erythrocytes' capacity and plasma capacity to handle oxidative circumstances when subjected to ELFMF (Seif et al., 2019).
3.11. Paracetamol
Paracetamol, also known as acetaminophen, is a commonly used antipyretic with a history of liver toxicity when blood levels rise above therapeutic levels (Watkins et al., 2006). Toxicity from paracetamol has recently become more difficult to determine due to the increased use of combination medications containing paracetamol, such as over‐the‐counter cold medicine or prescription pain relievers (Dougherty & Klein‐Schwartz, 2012; Graudins, 2014; Kirschner et al., 2016). Paracetamol was discovered to cause liver damage in the original studies of the mechanisms of toxicity by being converted by hepatic cytochrome P450 enzymes to a minor but toxic intermediate metabolite (Jollow et al., 1973; Mitchell, Jollow, Potter, Davis, et al., 1973; Mitchell, Jollow, Potter, Gillette, et al., 1973; Potter et al., 1973).
A study by Pasumarthi Phaneendra et al. investigated the hepatoprotective effects of aqueous extract of Myrtle leaves (200 and 400 mg/kg). The most accurate indicators of liver injury are serum SGPT, SGOT, ALP, and bilirubin. An increase in the activity of these markers in this investigation demonstrated that paracetamol‐induced hepatocellular injury had occurred.
The formulation's efficacy in restoring the damaged liver's normal functional status was demonstrated by a reduction in increased plasma activity of these enzymes that paracetamol‐treated rats developed. The outcomes imply that the extract has a substantial hepatoprotective effect as it prevented paracetamol‐induced lipid peroxidation when administered. The extraction also significantly increased the amounts of antioxidant enzymes and decreased the oxidative damage caused by paracetamol, demonstrating its antioxidant potential. This investigation unequivocally showed that the formulation had strong hepatoprotective effects in preventing paracetamol‐induced liver damage in rats. These effects may be attributable to the formulation's antioxidative and free radical‐scavenging characteristics Rupesh et al. (Rupesh et al., 2011).
3.12. Silver nanoparticles
The application of silver nanoparticles in biomedicine is on the rise; however, their potential toxicity has not received adequate attention. The liver acts as an effector against inflammation brought on by silver nanoparticles by imposing changes on the level of functional liver enzymes (AST, ALP, and ALT), which also stimulates anomalies in liver function. To investigate the sub‐chronic toxicity of silver nanoparticles in mice and allay this worry, researchers modified silver nanoparticles with a hydrolyzable tannin fraction from Myrtle. The findings showed that treatment with MC‐AgNPs reduced liver dysfunction in mice (ALT/AST/ALP values), particularly ALP, and the AST enzyme levels were comparable to those in the control group. The study concluded that AgNPs could reduce changes in AST, ALT, and ALP, which may be due to the tannins present in the Myrtle extract as beneficial phytochemical components (Tavakoli et al., 2020).
3.13. Streptozotocin
Streptozotocin (STZ) was discovered in a strain of the gram‐positive bacterium Streptomyces achromogenes found in soil (Singaram et al., 1979). This medicine is a glucosamine–nitrosourea substance that is especially toxic to the insulin‐producing beta‐cells of the pancreas in mammals; It is used to treat certain cancers of the islets of Langerhans (Rakhshandeh et al., 2022). In medical research, STZ is used to create a diabetes model (Isaev et al., 2018; Szkudelski, 2001). STZ is an alkylating chemical that is widely used to induce hyperglycemia in experimental animals due to its cytotoxicity effect on pancreatic beta cells via DNA damage induction and its stimulation of the inflammatory response (Alkhedaide, 2019).
In a study, Tas et al. investigated the potential health benefits of Myrtle in both normal and diabetic rats. The study found that a hydroalcoholic extract of the fruit caused a significant reduction in blood glucose levels and elevated serum insulin levels in diabetic rats. These may arise because M. communis fruit contains biologically active phytochemicals, including flavonoids and other phenolic compounds. This may work by increasing insulin production (enhancing pancreatic function), decreasing insulin resistance (speeding up glucose uptake and metabolism), or decreasing intestinal glucose absorption. These effects were not observed in normal rats. The study suggests that Myrtle may have potential therapeutic applications for diabetes and related conditions (Tas et al., 2018). In another in‐vivo study, the Myrtle effect on ovariectomized diabetes rats was investigated. All rats were divided into five groups. STZ was administered 1 week after ovariectomy to induce diabetes. Administration of Myrtle extract decreased blood glucose levels. It also had positive effects on the cognitive function of rats. These findings suggest that Myrtle could be a treatment for cognitive function pathologies (Kadıoğlu Yaman et al., 2020).
In a 2021 study, the effects of ethanol extract of Myrtle for oxidative stress in STZ‐induced diabetes rats were investigated. Rats were orally administered 3 different doses of ethanol extract of Myrtle berries (0.25, 0.5, and 1 g/kg) for 2 weeks. At the end of the experiment, ALT, AST, MDA, and blood glucose levels of the rats significantly decreased while significant increases in GSH levels and SOD activities were observed (Aggul et al., 2022).
Khodaie and his colleagues investigated the wound healing effect of aqueous extract of Myrtle fruit in diabetes. In this study, 60 rats were divided into six groups. Diabetes was induced in them using streptozotocin. Seven days after disease induction, wounds were created on the dorsolateral surface of rats. Rats were treated with aqueous Myrtle extract and gel containing it for 21 days. Myrtle extract increased the speed of wound healing in diabetic rats. It was also found in histological tests that the plant extract decreases tissue's inflammatory cells and increases collagen and angiogenesis. The results show that the aqueous extract of Myrtle fruit improves the speed of wound healing in diabetes (Khodaie et al., 2021).
3.14. Toxoplasma gondii
Toxoplasmosis is one of the most widespread zoonotic parasitic diseases caused by the intracellular parasite T. gondii (Mose et al., 2020). Considering the clinical appearance of toxoplasmosis, the disease does not cause any definite signs in healthy people, whereas a severe and even lethal one can be observed in immunocompromised individuals (Elfadaly et al., 2012). In an in vivo study, 48 male mice were divided into control (C) and experimental (Ex) groups. Each group is subdivided into three subgroups. Except for C1, all mice in all groups were infected with T. gondii after 3 weeks of treatment. Oral administration of Myrtle essential oil showed a reduction in the number and diameter of tissue cysts.
Furthermore, a study assessed the mRNA levels of various innate immunity mediators, including IFN‐γ and IL‐12, by quantitative real‐time PCR as the immune system, particularly cellular immunity, is one of the most crucial mechanisms for controlling toxoplasmosis. The findings showed that although IFN‐γ and IL‐12 mRNA levels were enhanced in all experimental groups of mice, a substantial rise was observed in mice treated with MCEO (M. communis essential oil) at the doses of Ex2 and Ex3 of MCEO when compared to control groups. According to the research, the decrease in parasite load in the infected mice treated with MCEO, which led to the control of T. gondii infection, may be related to the improved immune function of the tested mice, particularly their innate immune system (Shaapan et al., 2021).
The main pharmacological effects of Myrtle are illustrated in Figure 3.
FIGURE 3.
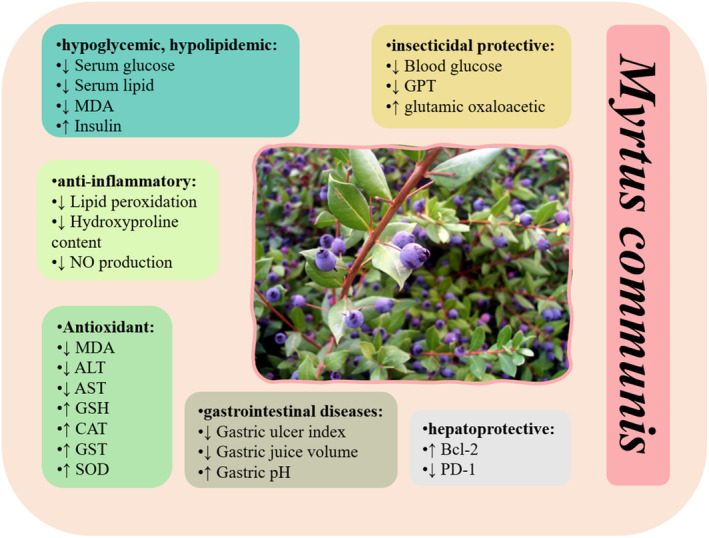
Pharmacological effects of Myrtus communis.
4. CONCLUSION
M. communis, also known as Myrtle, is a medicinal plant that has been used for centuries for its therapeutic properties. Recent studies have demonstrated its potential as a protective agent against both natural and chemical toxins. In addition, the plant contains several bioactive compounds, such as flavonoids, tannins, and essential oils, which provide a basis for its many health‐promoting effects.
One of Myrtle's most significant protective properties is its high antioxidant content. Studies have shown that the antioxidant properties of Myrtle can protect against harmful substances such as heavy metals, pesticides, and other environmental toxins. Additionally, Myrtle has anti‐inflammatory properties that can help reduce the damage caused by long‐term exposure to toxins. Chemical toxins can cause inflammation, which can lead to organ damage, and this is where the anti‐inflammatory properties of Myrtle can help alleviate the resulting damage. The anti‐inflammatory and antimicrobial properties of Myrtle have also proven effective in alleviating gastrointestinal conditions such as gastric ulcers.
Another significant benefit of the Myrtle plant is its hepatoprotective properties. The liver is a vital organ in the body responsible for filtering toxins and keeping the body healthy. Studies have shown that consuming Myrtle can protect the liver against chemical toxins which can help it function more efficiently and prevent damage. Finally, the multipurpose nature of Myrtle means it can be used to treat a wide range of health conditions caused by exposure to toxins. The plant has been traditionally used to treat several conditions, including respiratory and digestive illnesses, infections, and wounds. In addition, recent studies show that Myrtle may also have a preventive effect against cancer, thanks to its ability to inhibit the growth of tumor cells. In conclusion, Myrtle is a promising natural remedy for treating and preventing various toxicological disorders. However, more research is needed to unlock its full potential and develop targeted therapies.
AUTHOR CONTRIBUTIONS
Mohammad Mahdi Dabaghi, Mohammad Saleh Fadaei, Hesan Soleimani Roudi, Vafa Baradaran Rahimi, and Vahid Reza Askari wrote the first draft of the manuscript. All authors contributed to writing the project and read and approved the final manuscript submission. This study has been done by the authors mentioned in this article, and the authors will bear all responsibilities related to the contents of this article.
FUNDING INFORMATION
No funding information provided.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This is a review article. Ethical approval is not required for the study.
ACKNOWLEDGMENTS
The authors are indebted to the reviewers for their valuable comments which helped improve this article's quality.
Dabbaghi, M. M. , Fadaei, M. S. , Soleimani Roudi, H. , Baradaran Rahimi, V. , & Askari, V. R. (2023). A review of the biological effects of Myrtus communis . Physiological Reports, 11, e15770. 10.14814/phy2.15770
Mohammad Mahdi Dabaghi, Mohammad Saleh Fadaei, and Hesan Soleimani Roudi share co‐first authorships.
DATA AVAILABILITY STATEMENT
No data were used to support this study.
REFERENCES
- Abdel‐Hamid, A. A. , & Firgany, A. E.‐D. L. (2015). Vitamin E supplementation ameliorates aflatoxin B1‐induced nephrotoxicity in rats. Acta Histochemica, 117(8), 767–779. [DOI] [PubMed] [Google Scholar]
- Aggul, A. G. , Demir, G. M. , & Gulaboglu, M. (2022). Ethanol extract of Myrtle (Myrtus communis L.) berries as a remedy for streptozotocin‐induced oxidative stress in rats. Applied Biochemistry and Biotechnology, 194(4), 1645–1658. 10.1007/s12010-021-03753-z [DOI] [PubMed] [Google Scholar]
- Alipour, G. , Dashti, S. , & Hosseinzadeh, H. (2014). Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytotherapy Research, 28(8), 1125–1136. [DOI] [PubMed] [Google Scholar]
- Alkhedaide, A. Q. (2019). Anti‐inflammatory effect of Juniperus procera extract in rats exposed to streptozotocin toxicity. Anti‐Inflammatory & Anti‐Allergy Agents in Medicinal Chemistry, 18(1), 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkreathy, H. M. , Khan, R. A. , Khan, M. R. , & Sahreen, S. (2014). CCl4 induced genotoxicity and DNA oxidative damages in rats: Hepatoprotective effect of Sonchus arvensis . BMC Complementary and Alternative Medicine, 14, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif, H. , Akram, M. , Uddin, S. , Hasan, Z. U. , Sami, A. , Iqbal, A. , Tauseef, U. , & Bari, A. (2011). Myrtus communis Linn. (pharmacological activity). Journal of Medicinal Plants Research, 5(26), 6257–6259. [Google Scholar]
- Barton, C. C. , Barton, E. X. , Ganey, P. E. , Kunkel, S. L. , & Roth, R. A. (2001). Bacterial lipopolysaccharide enhances aflatoxin B1 hepatotoxicity in rats by a mechanism that depends on tumor necrosis factor α. Hepatology, 33(1), 66–73. [DOI] [PubMed] [Google Scholar]
- Ben Hsouna, A. , Dhibi, S. , Dhifi, W. , Mnif, W. , Ben Nasr, H. , & Hfaiedh, N. (2019). Chemical composition and hepatoprotective effect of essential oil from Myrtus communis L. flowers against CCL(4)‐induced acute hepatotoxicity in rats. RSC Advances, 9(7), 3777–3787. 10.1039/c8ra08204a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni, R. , & Bossa, C. (2011). Mechanisms of chemical carcinogenicity and mutagenicity: A review with implications for predictive toxicology. Chemical Reviews, 111(4), 2507–2536. [DOI] [PubMed] [Google Scholar]
- Bernardes, M. F. F. , Pazin, M. , Pereira, L. C. , & Dorta, D. J. (2015). Impact of pesticides on environmental and human health. In Ana Cristina A., & Gustavo S. (Eds.), Toxicology studies (pp. 195–233). IntechOpen. 10.5772/59710 [DOI] [Google Scholar]
- Berroukche, A. , Terras, M. , Labani, A. , & Felali, A. (2017). Effects of Myrtus communis leaves decoction on biochemical and hematological disorders induced by cypermethrin chronic toxicity in rats. Journal of Intercultural Ethnopharmacology, 6, 385–390. 10.5455/jice.20171028110108 [DOI] [Google Scholar]
- Bilgic, N. , & Duran, G. G. (2020). Chemical composition of Myrtus communis L. and Proapoptotic effects on the A549 cell line. Journal of Essential Oil‐Bearing Plants., 23, 1283–1295. 10.1080/0972060X.2020.1866681 [DOI] [Google Scholar]
- Bode, C. , & Bode, J. C. (1997). Alcohol's role in gastrointestinal tract disorders. Alcohol Health and Research World, 21(1), 76–83. [PMC free article] [PubMed] [Google Scholar]
- Bouzabata, A. , Cabral, C. , Gonçalves, M. J. , Cruz, M. T. , Bighelli, A. , Cavaleiro, C. , Casanova, J. , Tomi, F. , & Salgueiro, L. (2015). Myrtus communis L. as source of a bioactive and safe essential oil. Food and Chemical Toxicology, 75, 166–172. 10.1016/j.fct.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Bren, U. , Guengerich, F. P. , & Mavri, J. (2007). Guanine alkylation by the potent carcinogen aflatoxin B1: Quantum chemical calculations. Chemical Research in Toxicology, 20(8), 1134–1140. [DOI] [PubMed] [Google Scholar]
- Çakmak, M. , Bakar, B. , Özer, D. , Geckil, H. , Karatas, F. , & Saydam, S. (2021). Investigation of some biochemical parameters of wild and cultured Myrtus communis L. fruits subjected to different conservation methods. Journal of Food Measurement and Characterization, 15, 983–993. [Google Scholar]
- Cevikelli‐Yakut, Z. A. , Ertas, B. , Sen, A. , Koyuncuoglu, T. , Yegen, B. C. , & Sener, G. (2020). Myrtus communis improves cognitive impairment in renovascular hypertensive rats. Journal of Physiology and Pharmacology, 71(5), 665–677. 10.26402/jpp.2020.5.07 [DOI] [PubMed] [Google Scholar]
- Chalchat, J.‐C. , Figueredo, G. , Özcan, M. M. , & Ünver, A. (2010). Effect of hydrodistilation and microwave distillation extraction methods on chemical compositions of essential oil of pickling herb and myrtle plants. South‐Western Journal of Horticulture, Biology and Environment, 1(2), 133–141. [Google Scholar]
- Charles, D. J. (2012). Antioxidant properties of spices, herbs and other sources. Springer Science & Business Media. [Google Scholar]
- Chen, R. , Wang, Q. , Zhao, L. , Yang, S. , Li, Z. , Feng, Y. , Chen, J. , Ong, C. N. , & Zhang, H. (2019). Lomatogonium rotatum for treatment of acute liver injury in mice: A metabolomics study. Metabolites, 9(10), 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen, C. A. , & Long, E. C. (1999). Nucleic acid recognition by metal complexes of bleomycin. Chemical Reviews, 99(9), 2797–2816. [DOI] [PubMed] [Google Scholar]
- Cogliati, B. , Da Silva, T. C. , Aloia, T. P. A. , Chaible, L. M. , Real‐Lima, M. A. , Sanches, D. S. , Matsuzaki, P. , Hernandez‐Blazquez, F. J. , & Dagli, M. L. Z. (2011). Morphological and molecular pathology of CCL4‐induced hepatic fibrosis in connexin43‐deficient mice. Microscopy Research and Technique, 74(5), 421–429. [DOI] [PubMed] [Google Scholar]
- Correddu, F. , Maldini, M. , Addis, R. , Petretto, G. L. , Palomba, M. , Battacone, G. , Pulina, G. , Nudda, A. , & Pintore, G. (2019). Myrtus communis liquor byproduct as a source of bioactive compounds. Food, 8(7), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunkhorn, P. , & Meacock, S. C. (1971). Mediators of the inflammation induced in the rat paw by carrageenin. British Journal of Pharmacology, 42(3), 392–402. 10.1111/j.1476-5381.1971.tb07124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Latta, V. , Cecchettini, A. , Del Ry, S. , & Morales, M. (2015). Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacological Research, 97, 122–130. [DOI] [PubMed] [Google Scholar]
- Dorner, B. G. , & Rummel, A. (2015). Preface biological toxins—Ancient molecules posing a current threat (Vol. 7, pp. 5320–5321). MDPI. [Google Scholar]
- Dougherty, P. P. , & Klein‐Schwartz, W. (2012). Unexpected late rise in plasma acetaminophen concentrations with change in risk stratification in acute acetaminophen overdoses. The Journal of Emergency Medicine, 43(1), 58–63. [DOI] [PubMed] [Google Scholar]
- Ebeid, H. , Gibriel, A. A. , Al‐Sayed, H. , Elbehairy, S. , & Motawe, E. (2015). Hepatoprotective and antioxidant effects of wheat, carrot, and mango as nutraceutical agents against CCl4‐induced hepatocellular toxicity. Journal of the American College of Nutrition, 34(3), 228–231. [DOI] [PubMed] [Google Scholar]
- El‐Bana, M. A. , Medhat, D. , Ashour, M. N. , Diab, Y. , & Hussein, J. (2017). Myrtus communis extract attenuates atherosclerosis in streptozotocin—Induced diabetic rats. Bioscience Research, 14(2), 257–264. https://www.scopus.com/inward/record.uri?eid=2‐s2.0‐85031100084&partnerID=40&md5=5fb3cc1daa367e15f54b7ee837300f07 [Google Scholar]
- El‐Demerdash, F. M. (2004). Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. Journal of Trace Elements in Medicine and Biology, 18(1), 113–121. [DOI] [PubMed] [Google Scholar]
- Elfadaly, H. A. , Hassanain, M. A. , Shaapan, R. M. , Barakat, A. M. , & Toaleb, N. I. (2012). Serological and hormonal assays of murine materno‐fetal Toxoplasma gondii infection with emphasis on virulent strains. World Journal of Medical Sciences, 7(4), 248–254. [Google Scholar]
- Eslami, S. , Ebrahimzadeh, M. A. , & Biparva, P. (2018). Green synthesis of safe zero valent iron nanoparticles by Myrtus communis leaf extract as an effective agent for reducing excessive iron in iron‐overloaded mice, a thalassemia model. RSC Advances, 8(46), 26144–26155. 10.1039/c8ra04451a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda, A. , Palma, A. , D'Aquino, S. , & Mulas, M. (2017). Effects of myrtle (Myrtus communis L.) fruit cold storage under modified atmosphere on liqueur quality. Journal of Food Processing and Preservation, 41(1), e12776. [Google Scholar]
- Ghali, R. , Belouaer, I. , Hdiri, S. , Ghorbel, H. , Maaroufi, K. , & Hedilli, A. (2009). Simultaneous HPLC determination of aflatoxins B1, B2, G1 and G2 in Tunisian sorghum and pistachios. Journal of Food Composition and Analysis, 22(7–8), 751–755. [Google Scholar]
- Gortzi, O. , Lalas, S. , Chinou, I. , & Tsaknis, J. (2008). Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. European Food Research and Technology, 226, 583–590. [Google Scholar]
- Graudins, A. (2014). Overdose with modified‐release paracetamol (Panadol Osteo®) presenting to a metropolitan emergency medicine network: A case series. Emergency Medicine Australasia, 26(4), 398–402. [DOI] [PubMed] [Google Scholar]
- Grewal, K. , Sandhu, G. , Kaur, R. , Brar, R. , & Sandhu, H. (2010). Toxic impacts of cypermethrin on behavior and histology of certain tissues of albino rats. Toxicology International, 17(2), 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülbol Duran, G. , & Terzi, M. Y. (2021). Investigation of anti‐inflammatory effects of Myrtus communis L. essential oil on IL‐1β induced human bronchial epithelial cell line: Experimental study. Journal of Medical Sciences, 41, 470–477. 10.5336/medsci.2021-81607 [DOI] [Google Scholar]
- Güler, G. , Tomruk, A. , Ozgur, E. , Sahin, D. , Sepici, A. , Altan, N. , & Seyhan, N. (2012). The effect of radiofrequency radiation on DNA and lipid damage in female and male infant rabbits. International Journal of Radiation Biology, 88(4), 367–373. [DOI] [PubMed] [Google Scholar]
- Hacıseferoğulları, H. , Özcan, M. M. , Arslan, D. , & Ünver, A. (2012). Biochemical compositional and technological characterizations of black and white myrtle (Myrtus communis L.) fruits. Journal of Food Science and Technology, 49, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A. H. (2002). Chronic arsenic poisoning. Toxicology Letters, 128(1–3), 69–72. [DOI] [PubMed] [Google Scholar]
- Hassan, H. A. , El‐Kholy, W. M. , El‐Sawi, M. R. F. , Galal, N. A. , & Ramadan, M. F. (2020). Myrtle (Myrtus communis) leaf extract suppresses hepatotoxicity induced by monosodium glutamate and acrylamide through obstructing apoptosis, DNA fragmentation, and cell cycle arrest. Environmental Science and Pollution Research International, 27(18), 23188–23198. 10.1007/s11356-020-08780-7 [DOI] [PubMed] [Google Scholar]
- Hayder, N. , Bouhlel, I. , Skandrani, I. , Kadri, M. , Steiman, R. , Guiraud, P. , Mariotte, A. M. , Ghedira, K. , Dijoux‐Franca, M. G. , & Chekir‐Ghedira, L. (2008). In vitro antioxidant and antigenotoxic potentials of myricetin‐3‐o‐galactoside and myricetin‐3‐o‐rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicology in Vitro, 22, 567–581. 10.1016/j.tiv.2007.11.015 [DOI] [PubMed] [Google Scholar]
- Hennia, A. , Miguel, M. G. , & Nemmiche, S. (2018). Antioxidant activity of Myrtus communis L. and Myrtus nivellei batt. & Trab. Extracts: A brief review. Medicines (Basel), 5(3), 89. 10.3390/medicines5030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennia, A. , Nemmiche, S. , Dandlen, S. , & Miguel, M. G. (2019). Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. Journal of Essential Oil Research, 31(6), 487–545. [Google Scholar]
- Hofbauer, B. , Saluja, A. , Lerch, M. , Bhagat, L. , Bhatia, M. , Lee, H. , Frossard, J. , Adler, G. , & Steer, M. (1998). Intra‐acinar cell activation of trypsinogen during caerulein‐induced pancreatitis in rats. The American Journal of Physiology, 275(2), G352–G362. [DOI] [PubMed] [Google Scholar]
- Hosseini, A. , Alipour, A. , Baradaran Rahimi, V. , & Askari, V. R. (2023). A comprehensive and mechanistic review on protective effects of kaempferol against natural and chemical toxins: Role of NF‐kappaB inhibition and Nrf2 activation. BioFactors, 49(2), 322–350. 10.1002/biof.1923 [DOI] [PubMed] [Google Scholar]
- Hsouna, A. B. , Dhibi, S. , Dhifi, W. , Mnif, W. , & Hfaiedh, N. (2019). Chemical composition and hepatoprotective effect of essential oil from Myrtus communis L. flowers against CCL4‐induced acute hepatotoxicity in rats. RSC Advances, 9(7), 3777–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ines, S. , Ines, B. , Wissem, B. , Mohamed, B. S. , Nawel, H. , Dijoux‐Franca, M. G. , Kamel, G. , & Leïla, C. G. (2012). In vitro antioxidant and antigenotoxic potentials of 3,5‐O‐di‐galloylquinic acid extracted from Myrtus communis leaves and modulation of cell gene expression by H2O2 . Journal of Applied Toxicology, 32(5), 333–341. 10.1002/jat.1655 [DOI] [PubMed] [Google Scholar]
- Isaev, N. K. , Genrikhs, E. E. , Voronkov, D. N. , Kapkaeva, M. R. , & Stelmashook, E. V. (2018). Streptozotocin toxicity in vitro depends on maturity of neurons. Toxicology and Applied Pharmacology, 348, 99–104. [DOI] [PubMed] [Google Scholar]
- Jabri, M. A. , Hajaji, S. , Marzouki, L. , El‐Benna, J. , Sakly, M. , & Sebai, H. (2016). Human neutrophils ROS inhibition and protective effects of Myrtus communis leaves essential oils against intestinal ischemia/reperfusion injury. RSC Advances, 6, 16645–16655. 10.1039/c5ra26085j [DOI] [Google Scholar]
- Jabri, M. A. , Marzouki, L. , & Sebai, H. (2018). Myrtle berries seeds aqueous extract abrogates chronic alcohol consumption‐induced erythrocytes osmotic stability disturbance, haematological and biochemical toxicity. Lipids in Health and Disease, 17(1), 94. 10.1186/s12944-018-0746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabri, M. A. , Rtibi, K. , Ben‐Said, A. , Aouadhi, C. , Hosni, K. , Sakly, M. , & Sebai, H. (2016). Antidiarrhoeal, antimicrobial and antioxidant effects of myrtle berries (Myrtus communis L.) seeds extract. The Journal of Pharmacy and Pharmacology, 68(2), 264–274. 10.1111/jphp.12505 [DOI] [PubMed] [Google Scholar]
- Jabri, M. A. , Tounsi, H. , Rtibi, K. , Marzouki, L. , Sakly, M. , & Sebai, H. (2016). Ameliorative and antioxidant effects of myrtle berry seed (Myrtus communis) extract during reflux‐induced esophagitis in rats. Pharmaceutical Biology, 54(9), 1575–1585. 10.3109/13880209.2015.1107748 [DOI] [PubMed] [Google Scholar]
- Janik, E. , Ceremuga, M. , Saluk‐Bijak, J. , & Bijak, M. (2019). Biological toxins as the potential tools for bioterrorism. International Journal of Molecular Sciences, 20(5), 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow, D. , Mitchell, J. , Potter, W. , Davis, D. , Gillette, J. , & Brodie, B. (1973). Acetaminophen‐induced hepatic necrosis. II. Role of covalent binding in vivo. Journal of Pharmacology and Experimental Therapeutics, 187(1), 195–202. [PubMed] [Google Scholar]
- Kadıoğlu Yaman, B. , Çevik, Ö. , Yalman, K. , Ertaş, B. , Şen, A. , & Şener, G. (2020). Myrtus communis subsp. communis improved cognitive functions in ovariectomized diabetic rats. Gene, 744, 144616. 10.1016/j.gene.2020.144616 [DOI] [PubMed] [Google Scholar]
- Khodaie, S. A. , Emadi, F. , Naseri, M. , Kamalinejad, M. , Riahi, S. M. , Alijaniha, F. , & Roghani, M. (2021). The effect of Myrtus communis aqueous extract‐containing gel on wound healing in streptozotocin‐induced diabetic rats. Current Drug Discovery Technologies, 18(4), 542–547. 10.2174/1570163817666200712163956 [DOI] [PubMed] [Google Scholar]
- Kim, H. (2008). Cerulein pancreatitis: Oxidative stress, inflammation, and apoptosis. Gut and Liver, 2(2), 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner, R. I. , Rozier, C. M. , Smith, L. M. , & Jacobitz, K. L. (2016). Nomogram line crossing after acetaminophen combination product overdose. Clinical Toxicology, 54(1), 40–46. [DOI] [PubMed] [Google Scholar]
- Li, L. , Brown, K. L. , Ma, R. , & Stone, M. P. (2015). DNA sequence modulates geometrical isomerism of the trans‐8, 9‐dihydro‐8‐(2, 6‐diamino‐4‐oxo‐3, 4‐dihydropyrimid‐5‐yl‐formamido)‐9‐hydroxy aflatoxin B1 adduct. Chemical Research in Toxicology, 28(2), 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Chen, Y. , Ye, W. , Tao, X. , Zhu, J. , Wu, S. , & Lou, L. (2015). Blockade of CCN4 attenuates CCl4‐induced liver fibrosis. Archives of Medical Science, 11, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zhao, H. , Wang, Y. , Guo, M. , Mu, M. , & Xing, M. (2020). Arsenic (III) and/or copper (II) induces oxidative stress in chicken brain and subsequent effects on mitochondrial homeostasis and autophagy. Journal of Inorganic Biochemistry, 211, 111201. [DOI] [PubMed] [Google Scholar]
- Mahboubi, M. (2017). Effectiveness of Myrtus communis in the treatment of hemorrhoids. Journal of Integrative Medicine, 15(5), 351–358. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand, H. , Fallahi, S. , Mahmoudvand, H. , Shakibaie, M. , Harandi, M. F. , & Dezaki, E. S. (2016). Efficacy of Myrtus communis L. to inactivate the hydatid cyst Protoscoleces. Journal of Investigative Surgery, 29(3), 137–143. 10.3109/08941939.2015.1088601 [DOI] [PubMed] [Google Scholar]
- Mansour, R. B. , Beji, R. S. , Wasli, H. , Zekri, S. , Ksouri, R. , Megdiche‐Ksouri, W. , & Cardoso, S. M. (2022). Gastroprotective effect of microencapsulated Myrtus communis essential oil against ethanol/HCl‐induced acute gastric lesions. Molecules, 27(5), 1566. 10.3390/molecules27051566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxia, A. , Frau, M. A. , Falconieri, D. , Karchuli, M. S. , & Kasture, S. (2011). Essential oil of Myrtus communis inhibits inflammation in rats by reducing serum IL‐6 and TNF‐alpha. Natural Product Communications, 6(10), 1545–1548. [PubMed] [Google Scholar]
- Meissonnier, G. M. , Pinton, P. , Laffitte, J. , Cossalter, A.‐M. , Gong, Y. Y. , Wild, C. P. , Bertin, G. , Galtier, P. , & Oswald, I. P. (2008). Immunotoxicity of aflatoxin B1: Impairment of the cell‐mediated response to vaccine antigen and modulation of cytokine expression. Toxicology and Applied Pharmacology, 231(2), 142–149. [DOI] [PubMed] [Google Scholar]
- Messaoud, C. , & Boussaid, M. (2011). Myrtus communis berry color morphs: A comparative analysis of essential oils, fatty acids, phenolic compounds, and antioxidant activities. Chemistry & Biodiversity, 8(2), 300–310. [DOI] [PubMed] [Google Scholar]
- Migliore, J. , Baumel, A. , Juin, M. , & Médail, F. (2012). From Mediterranean shores to central Saharan mountains: Key phylogeographical insights from the genus Myrtus. Journal of Biogeography, 39(5), 942–956. [Google Scholar]
- Min, D. , Wu, J. , Cheng, L. , Liu, D.‐F. , Lau, T.‐C. , & Yu, H.‐Q. (2021). Dependence of arsenic resistance and reduction capacity of Aeromonas hydrophila on carbon substrate. Journal of Hazardous Materials, 403, 123611. [DOI] [PubMed] [Google Scholar]
- Missimer, T. M. , Teaf, C. M. , Beeson, W. T. , Maliva, R. G. , Woolschlager, J. , & Covert, D. J. (2018). Natural background and anthropogenic arsenic enrichment in Florida soils, surface water, and groundwater: A review with a discussion on public health risk. International Journal of Environmental Research and Public Health, 15(10), 2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, J. , Jollow, D. , Potter, W. , Davis, D. , Gillette, J. , & Brodie, B. (1973). Acetaminophen‐induced hepatic necrosis. I. Role of drug metabolism. Journal of Pharmacology and Experimental Therapeutics, 187(1), 185–194. [PubMed] [Google Scholar]
- Mitchell, J. , Jollow, D. , Potter, W. , Gillette, J. , & Brodie, B. (1973). Acetaminophen‐induced hepatic necrosis. IV. Protective role of glutathione. Journal of Pharmacology and Experimental Therapeutics, 187(1), 211–217. [PubMed] [Google Scholar]
- Mobli, M. , Qaraaty, M. , Amin, G. , Haririan, I. , Hajimahmoodi, M. , & Rahimi, R. (2015). Scientific evaluation of medicinal plants used for the treatment of abnormal uterine bleeding by Avicenna. Archives of Gynecology and Obstetrics, 292, 21–35. [DOI] [PubMed] [Google Scholar]
- Mohammadi, H. (2011). A review of aflatoxin M1, milk, and milk products. In Guevara‐González R. G. (Ed.), Aflatoxins‐biochemistry and molecular biology (pp. 397–414). InTech. [Google Scholar]
- Montoro, P. , Tuberoso, C. I. , Piacente, S. , Perrone, A. , De Feo, V. , Cabras, P. , & Pizza, C. (2006). Stability and antioxidant activity of polyphenols in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur. Journal of Pharmaceutical and Biomedical Analysis, 41(5), 1614–1619. [DOI] [PubMed] [Google Scholar]
- Mose, J. M. , Kagira, J. M. , Kamau, D. M. , Maina, N. W. , Ngotho, M. , & Karanja, S. M. (2020). A review on the present advances on studies of toxoplasmosis in Eastern Africa. BioMed Research International, 2020, 7135268. 10.1155/2020/7135268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, P. A. , Hendrich, S. , Landgren, C. , & Bryant, C. M. (2006). Food mycotoxins: An update. Journal of Food Science, 71(5), R51–R65. [Google Scholar]
- Myszka, K. , Sobieszczańska, N. , Olejnik, A. , Majcher, M. , Szwengiel, A. , Wolko, Ł. , & Juzwa, W. (2020). Studies on the anti‐proliferative and anti‐quorum sensing potentials of Myrtus communis L. essential oil for the improved microbial stability of salmon‐based products. LWT, 127, 109380. 10.1016/j.lwt.2020.109380 [DOI] [Google Scholar]
- Naji, H. A. , Rhiyf, A. G. , & Al‐Zebeeby, A. (2018). Protective features of Myrtus communis leaves against the genotoxic effects of arsenic in wistar rats. Journal of Pharmaceutical Sciences and Research, 10(11), 2921–2923. [Google Scholar]
- Nayak, P. (2002). Aluminum: Impacts and disease. Environmental Research, 89(2), 101–115. [DOI] [PubMed] [Google Scholar]
- Niedworok, J. , & Fijałkowski, P. (2004). Effect of long‐term aluminium chloride intoxication on selected biochemical parameters and oxidative—Antioxidative balance in experimental animals. Polish Journal of Environmental Studies, 13(1), 41–43. [Google Scholar]
- Nuvoli, F. , & Spanu, D. (1996). Analisi e prospettive economiche dell'utilizzazionze industriale del mirto. Rivista Italiana EPPOS, 12, 231–236. [Google Scholar]
- Ochieng, P. J. , Okun, D. , Runo, S. , Njagi, N. , & Murage, J. (2013). Public health strategies for preventing aflatoxin exposure. British Journal of Cancer, 45(1), 1661–8556. [Google Scholar]
- O'Riordan, M. J. , & Wilkinson, M. G. (2008). A survey of the incidence and level of aflatoxin contamination in a range of imported spice preparations on the Irish retail market. Food Chemistry, 107(4), 1429–1435. [Google Scholar]
- Ozbeyli, D. , Sen, A. , Cilingir Kaya, O. T. , Ertas, B. , Aydemir, S. , Ozkan, N. , Yuksel, M. , & Sener, G. (2020). Myrtus communis leaf extract protects against cerulein‐induced acute pancreatitis in rats. Journal of Food Biochemistry, 44(2), e13130. 10.1111/jfbc.13130 [DOI] [PubMed] [Google Scholar]
- Özcan, M. , & Akgül, A. (1995). Antioxidant activity of extracts and essential oils from Turkish spices on sunflower oil. Acta Alimentaria (Budapest), 24(1), 81–90. [Google Scholar]
- Özcan, M. , & Boyraz, N. (2000). Antifungal properties of some herb decoctions. European Food Research and Technology, 212, 86–88. [Google Scholar]
- Özcan, M. , Uyar, B. , & Ünver, A. (2015). Antibacterial effect of myrtle (Myrtus communis L.) leaves extract on microorganisms. Archiv für Lebensmittelhygiene, 66(1), 18–21. [Google Scholar]
- Ozcan, O. , Ipekci, H. , Alev, B. , Ustundag, U. V. , Ak, E. , Sen, A. , Alturfan, E. E. , Sener, G. , Yarat, A. , Cetinel, S. , & Akbay, T. T. (2019). Protective effect of Myrtle (Myrtus communis) on burn induced skin injury. Burns: Journal of the International Society for Burn Injuries, 45(8), 1856–1863. 10.1016/j.burns.2019.07.015 [DOI] [PubMed] [Google Scholar]
- Ozcan, O. , Ipekci, H. , Alev, B. , Ustundag, U. V. , Sen, A. , Emekli‐Alturfan, E. , Sener, G. , Yarat, A. , & Tunali‐Akbay, T. (2020). The effect of Myrtus communis L. ethanol extract on the small intestine and lungs in experimental thermal burn injury. Journal of Thermal Biology, 93, 102685. 10.1016/j.jtherbio.2020.102685 [DOI] [PubMed] [Google Scholar]
- Özkan, A. M. G. , & Güray, Ç. G. (2009). A mediterranean: Myrtus communis L.(myrtle). In Morel J. P. & Mercuri A. M. (Eds.), Plants and culture: Seeds of the cultural heritage of Europe (pp. 159–168). Edipuglia. [Google Scholar]
- Potter, W. , Davis, D. , Mitchell, J. , Jollow, D. , Gillette, J. , & Brodie, B. (1973). Acetaminophen‐induced hepatic necrosis. III. Cytochrome P‐450‐mediated covalent binding in vitro. Journal of Pharmacology and Experimental Therapeutics, 187(1), 203–210. [PubMed] [Google Scholar]
- Raeiszadeh, M. , Esmaeili‐Tarzi, M. , Bahrampour‐Juybari, K. , Nematollahi‐mahani, S. N. , Pardakhty, A. , Nematollahi, M. H. , Mehrabani, M. , & Mehrabani, M. (2018). Evaluation the effect of Myrtus communis L. extract on several underlying mechanisms involved in wound healing: An in vitro study. South African Journal of Botany, 118, 144–150. doi: 10.1016/j.sajb.2018.07.006 [DOI] [Google Scholar]
- Rakhshandeh, H. , Rajabi Khasevan, H. , Saviano, A. , Mahdinezhad, M. R. , Baradaran Rahimi, V. , Ehtiati, S. , Etemad, L. , Ebrahimzadeh‐Bideskan, A. , Maione, F. , & Askari, V. R. (2022). Protective effect of Portulaca oleracea on streptozotocin‐induced type I diabetes‐associated reproductive system dysfunction and inflammation. Molecules, 27(18), 6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, A. , Deiana, M. , Casu, V. , Corona, G. , Appendino, G. , Bianchi, F. , Ballero, M. , & Dessì, M. A. (2003). Antioxidant activity of oligomeric acylphloroglucinols from Myrtus communis L. Free Radical Research, 37(9), 1013–1019. 10.1080/10715760310001595739 [DOI] [PubMed] [Google Scholar]
- Rossi, A. , Di Paola, R. , Mazzon, E. , Genovese, T. , Caminiti, R. , Bramanti, P. , Pergola, C. , Koeberle, A. , Werz, O. , Sautebin, L. , & Cuzzocrea, S. (2009). Myrtucommulone from Myrtus communis exhibits potent anti‐inflammatory effectiveness in vivo. The Journal of Pharmacology and Experimental Therapeutics, 329(1), 76–86. 10.1124/jpet.108.143214 [DOI] [PubMed] [Google Scholar]
- Ruffoni, B. , Mascarello, C. , & Savona, M. (2009). In vitro propagation of ornamental Myrtus (Myrtus communis). In Jain S., & Ochatt S. (Eds.), Protocols for in vitro propagation of ornamental plants (pp. 257–269). Springer. [DOI] [PubMed] [Google Scholar]
- Rupesh, K. M. , Phaneendra, P. , Bodhanapu, S. , Rahiman, F. O. M. , Mohamed, N. K. , & Tamizmani, T. (2011). Antioxidant and hepatoprotective activity of the aqueous extract of Myrtus communis (myrtle) Linn. Leaves. Pharmacology Online, 1, 1083–1090. https://www.scopus.com/inward/record.uri?eid=2‐s2.0‐79957491848&partnerID=40&md5=de97b2c1e87f280043b7ef825cff8754 [Google Scholar]
- Saei, M. M. , Sadeghi, A. A. , & Ahmadvand, H. (2013). The effect of Myrtus communis oil extract on growth performance, serum biochemistry and humoral immune responses in broiler chicks fed diet containing aflatoxin B1. Archives Animal Breeding, 56(1), 842–850. [Google Scholar]
- Saǧdıç, O. , & Özcan, M. (2003). Antibacterial activity of Turkish spice hydrosols. Food Control, 14(3), 141–143. [Google Scholar]
- Salouage, I. , Klouz, A. , Ferchichi, H. , Charfi, R. , Ouanes, L. , Boussaid, M. , & Lakhal, M. (2010). Effect of Myrtus communis L. on an experimental model of a rat liver ischemia‐reperfusion. Acta Horticulturae, 853, 379–382. [Google Scholar]
- Samareh Fekri, M. , Mandegary, A. , Sharififar, F. , Poursalehi, H. R. , Nematollahi, M. H. , Izadi, A. , Mehdipour, M. , Asadi, A. , & Samareh Fekri, M. (2018). Protective effect of standardized extract of Myrtus communis L. (myrtle) on experimentally bleomycin‐induced pulmonary fibrosis: Biochemical and histopathological study. Drug and Chemical Toxicology, 41(4), 408–414. 10.1080/01480545.2018.1459670 [DOI] [PubMed] [Google Scholar]
- Sangha, G. , Kaur, K. , Khera, K. , & Singh, B. (2011). Toxicological effects of cypermethrin on female albino rats. Toxicology International, 18(1), 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyavati, G. , Raina, M. , & Sharma, M. (1987). Medicinal plants of India (Vol. 2). Indian Council of Medical Research. [Google Scholar]
- Seif, F. , Reza Bayatiani, M. , Ansarihadipour, H. , Habibi, G. , & Sadelaji, S. (2019). Protective properties of Myrtus communis extract against oxidative effects of extremely low‐frequency magnetic fields on rat plasma and hemoglobin. International Journal of Radiation Biology, 95(2), 215–224. [DOI] [PubMed] [Google Scholar]
- Sen, A. , Ozkan, S. , Recebova, K. , Cevik, O. , Ercan, F. , Kervancıoglu Demirci, E. , Bitis, L. , & Sener, G. (2016). Effects of Myrtus communis extract treatment in bile duct ligated rats. Journal of Surgical Research, 205(2), 359–367. 10.1016/j.jss.2016.06.094 [DOI] [PubMed] [Google Scholar]
- Seven, B. , Yalçin, E. , & Acar, A. (2022). Investigation of cypermethrin toxicity in Swiss albino mice with physiological, genetic and biochemical approaches. Scientific Reports, 12(1), 11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaapan, R. M. , Al‐Abodi, H. R. , Alanazi, A. D. , Abdel‐Shafy, S. , Rashidipour, M. , Shater, A. F. , & Mahmoudvand, H. (2021). Myrtus communis essential oil; anti‐parasitic effects and induction of the innate immune system in mice with Toxoplasma gondii infection. Molecules, 26(4), 819. 10.3390/molecules26040819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnazi, M. , Azadmehr, A. , Jondabeh, M. D. , Hajiaghaee, R. , Norian, R. , Aghaei, H. , Saraei, M. , & Alipour, M. (2017). Evaluating the effect of Myrtus communis on programmed cell death in hydatid cyst protoscolices. Asian Pacific Journal of Tropical Medicine, 10(11), 1072–1076. 10.1016/j.apjtm.2017.10.010 [DOI] [PubMed] [Google Scholar]
- Shao, Y. , Zhao, H. , Wang, Y. , Liu, J. , Li, J. , Luo, L. , & Xing, M. (2018). The apoptosis in arsenic‐induced oxidative stress is associated with autophagy in the testis tissues of chicken. Poultry Science, 97(9), 3248–3257. [DOI] [PubMed] [Google Scholar]
- Sharma, A. , Yadav, B. , Rohatgi, S. , & Yadav, B. (2018). Cypermethrin toxicity: A review. Journal of Forensic Sciences & Criminal Investigation, 9, 555767. [Google Scholar]
- Singaram, S. , Lawrence, R. S. , & Hornemann, U. (1979). Studies on the biosynthesis of the antibiotic streptozotocin (streptozocin) by Streptomyces achromogenes var. streptozoticus feeding experiments with carbon‐14 and tritium labelled precursors. The Journal of Antibiotics, 32(4), 379–385. [DOI] [PubMed] [Google Scholar]
- Sisay, M. , & Gashaw, T. (2017). Ethnobotanical, ethnopharmacological, and phytochemical studies of Myrtus communis Linn: A popular herb in Unani system of medicine. Journal of Evidence‐Based Complementary and Alternative Medicine, 22(4), 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro, S. , Mesaik, M. A. , Shaheen, F. , Khan, N. , Halim, S. A. , Ul‐Haq, Z. , Ali Siddiqui, R. , & Choudhary, M. I. (2019). Inhibitory effects of myrtucommuacetalone 1 (MCA‐1) from Myrtus communis on inflammatory response in mouse macrophages. Molecules, 25(1), 13. 10.3390/molecules25010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbul, S. , Ahmad, M. A. , Asif, M. , & Akhtar, M. (2011). Myrtus communis Linn.—A review. Indian Journal of Natural Products and Resources, 2(4), 395–402. [Google Scholar]
- Sumbul, S. , Ahmad, M. A. , Asif, M. , Saud, I. , & Akhtar, M. (2010). Evaluation of Myrtus communis Linn. Berries (common myrtle) in experimental ulcer models in rats. Human & Experimental Toxicology, 29(11), 935–944. 10.1177/0960327110364154 [DOI] [PubMed] [Google Scholar]
- Szkudelski, T. (2001). The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research, 50(6), 537–546. [PubMed] [Google Scholar]
- Tas, S. , Tas, B. , Bassalat, N. , & Jaradat, N. (2018). In‐vivo, hypoglycemic, hypolipidemic and oxidative stress inhibitory activities of Myrtus communis L. fruits hydroalcoholic extract in normoglycemic and streptozotocin‐induced diabetic rats. Biomedical Research, 29(13), 2727–2734. [Google Scholar]
- Tavakoli, S. , Ebrahimzadeh, M. A. , Habibi, E. , Biparva, P. , Mohammadi, H. , Zahedi Mazandarani, A. , Vafaeinejad, S. , Ziar, A. , & Eslami, S. (2020). Sub‐chronic intraperitonealy toxicity assessments of modified silver nanoparticles capped coated Myrtus communis‐derived the hydrolyzable tannins in a mice model. Nanomedicine Research Journal, 5(3), 288–297. [Google Scholar]
- Thornhill, A. H. , Ho, S. Y. , Külheim, C. , & Crisp, M. D. (2015). Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Molecular Phylogenetics and Evolution, 93, 29–43. [DOI] [PubMed] [Google Scholar]
- Timmel, C. R. , Till, U. , Brocklehurst, B. , Mclauchlan, K. A. , & Hore, P. J. (1998). Effects of weak magnetic fields on free radical recombination reactions. Molecular Physics, 95(1), 71–89. [Google Scholar]
- Tuberoso, C. I. , Barra, A. , Angioni, A. , Sarritzu, E. , & Pirisi, F. M. (2006). Chemical composition of volatiles in Sardinian myrtle (Myrtus communis L.) alcoholic extracts and essential oils. Journal of Agricultural and Food Chemistry, 54(4), 1420–1426. 10.1021/jf052425g [DOI] [PubMed] [Google Scholar]
- Umezawa, H. , Suhara, Y. , Takita, T. , & Maeda, K. (1966). Purification of bleomycins. The Journal of Antibiotics, 19(5), 210–215. [PubMed] [Google Scholar]
- Usai, M. , Marchetti, M. , Culeddu, N. , & Mulas, M. (2018). Chemical composition of myrtle (Myrtus communis L.) berries essential oils as observed in a collection of genotypes. Molecules, 23(10), 2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos, T. N. , Proença, C. E. , Ahmad, B. , Aguilar, D. S. , Aguilar, R. , Amorim, B. S. , Campbell, K. , Costa, I. R. , De‐Carvalho, P. S. , & Faria, J. E. (2017). Myrteae phylogeny, calibration, biogeography and diversification patterns: Increased understanding in the most species rich tribe of Myrtaceae. Molecular Phylogenetics and Evolution, 109, 113–137. [DOI] [PubMed] [Google Scholar]
- Vinegar, R. , Schreiber, W. , & Hugo, R. (1969). Biphasic development of carrageenin edema in rats. The Journal of Pharmacology and Experimental Therapeutics, 166(1), 96–103. [PubMed] [Google Scholar]
- Watkins, P. B. , Kaplowitz, N. , Slattery, J. T. , Colonese, C. R. , Colucci, S. V. , Stewart, P. W. , & Harris, S. C. (2006). Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: A randomized controlled trial. JAMA, 296(1), 87–93. [DOI] [PubMed] [Google Scholar]
- Weber, L. W. , Boll, M. , & Stampfl, A. (2003). Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Critical Reviews in Toxicology, 33(2), 105–136. [DOI] [PubMed] [Google Scholar]
- Willoughby, D. A. , & DiRosa, M. (1972). Studies on the mode of action of non‐steroid anti‐inflammatory drugs. Annals of the Rheumatic Diseases, 31(6), 540. 10.1136/ard.31.6.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, C. A. , Risley, E. A. , & Nuss, G. W. (1962). Carrageenin‐induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proceedings of the Society for Experimental Biology and Medicine, 111, 544–547. 10.3181/00379727-111-27849 [DOI] [PubMed] [Google Scholar]
- Wojciechowska, M. , Stepnowski, P. , & Gołębiowski, M. (2016). The use of insecticides to control insect pests. Invertebrate Survival Journal, 13(1), 210–220. [Google Scholar]
- Wu, C. , Li, M. , & Chen, W. (2021). Characteristics of gut microbiota in cerulein‐induced chronic pancreatitis. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 14, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyazadeh, R. , Baradaran Rahimi, V. , Yahyazadeh, A. , Mohajeri, S. A. , & Askari, V. R. (2021). Promising effects of gingerol against toxins: A review article. BioFactors, 47(6), 885–913. [DOI] [PubMed] [Google Scholar]
- Yalman, K. , Şen, A. , Çevik, Ö. , Kadioğlu‐Yaman, B. , Ertaş, B. , Yildiz, S. , & Şener, G. (2022). Investigation of the protective and therapeutic efficacy of Myrtus communis extract in aluminum chloride and D‐galactose‐induced Alzheimer's disease in rats. Journal of Research in Pharmacy, 26(5), 1363–1374. [Google Scholar]
- Zacharia, J. I. (2011). Physical and chemical properties of pesticides. In Stoytcheva M. (Ed.), Pesticides in the modern world‐trends in pesticides analysis (pp. 1–20). Intech Open. [Google Scholar]
- Zhong, G. , Wan, F. , Yan, H. , Ning, Z. , Wang, C. , Li, Y. , Pan, J. , Tang, Z. , Yang, Z. , & Huang, R. (2020). Methionine sulfoxide reductases are related to arsenic trioxide‐induced oxidative stress in mouse liver. Biological Trace Element Research, 195, 535–543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


