Abstract
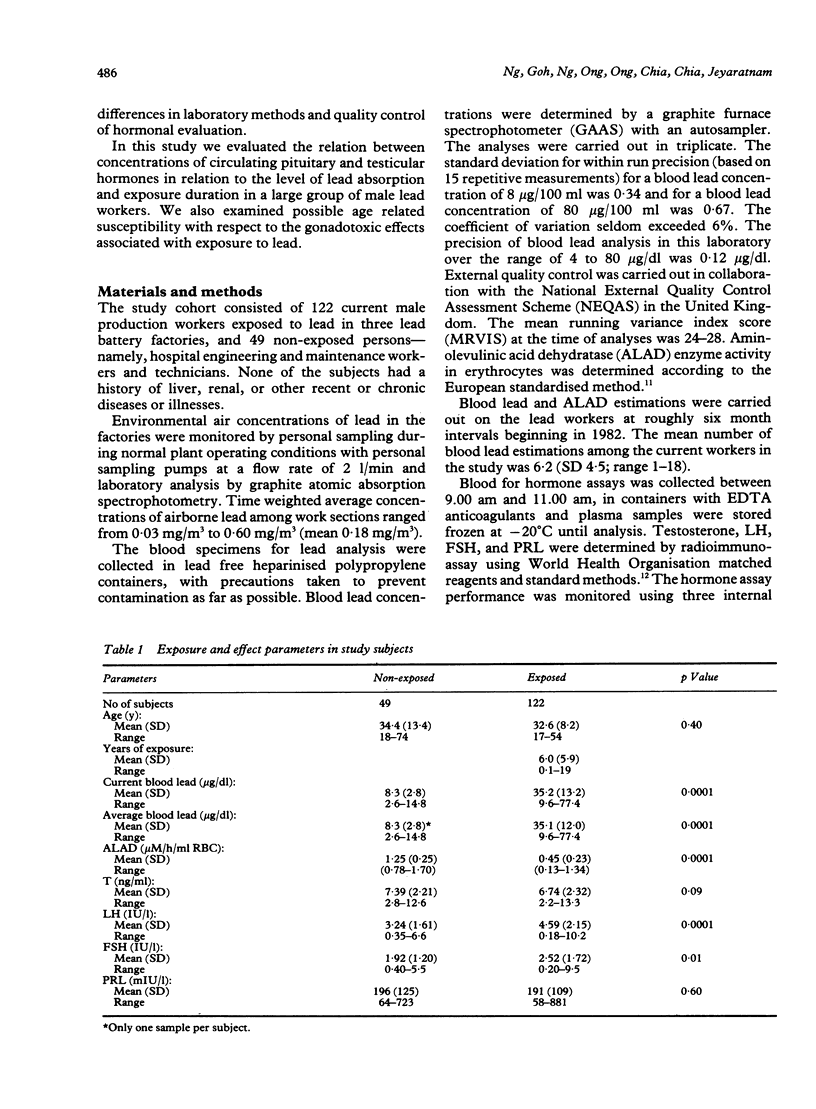
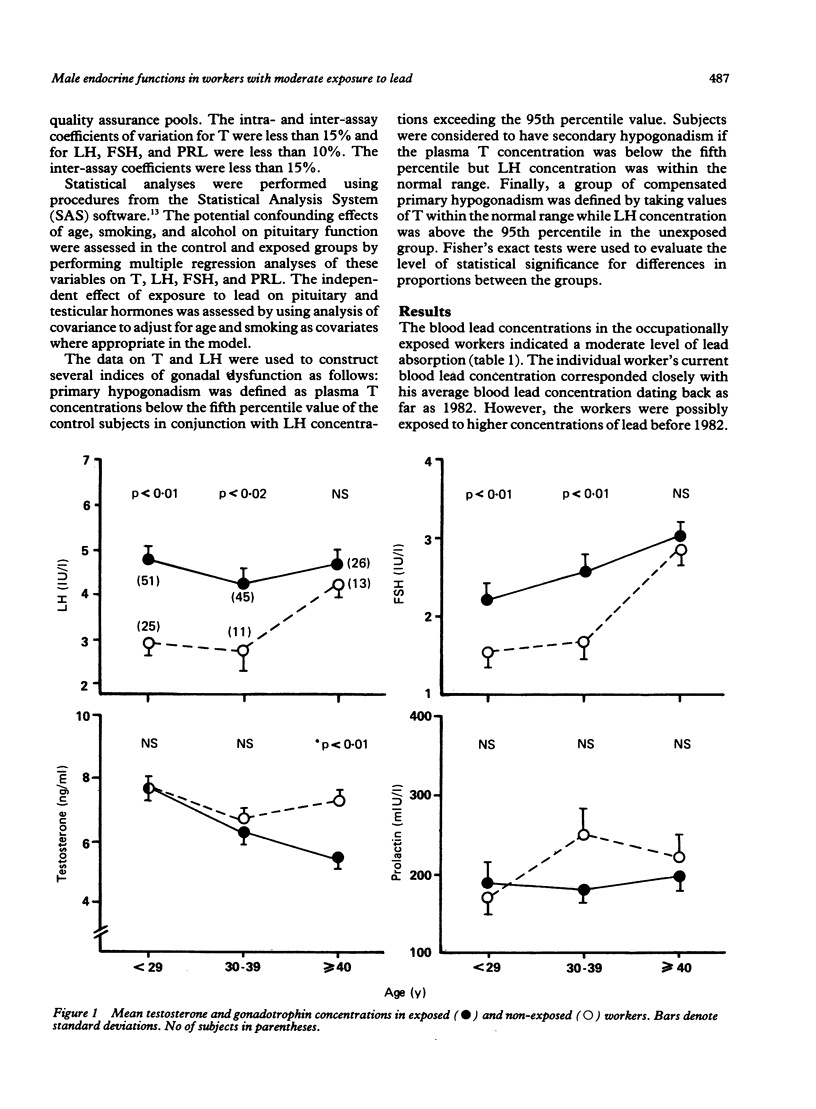
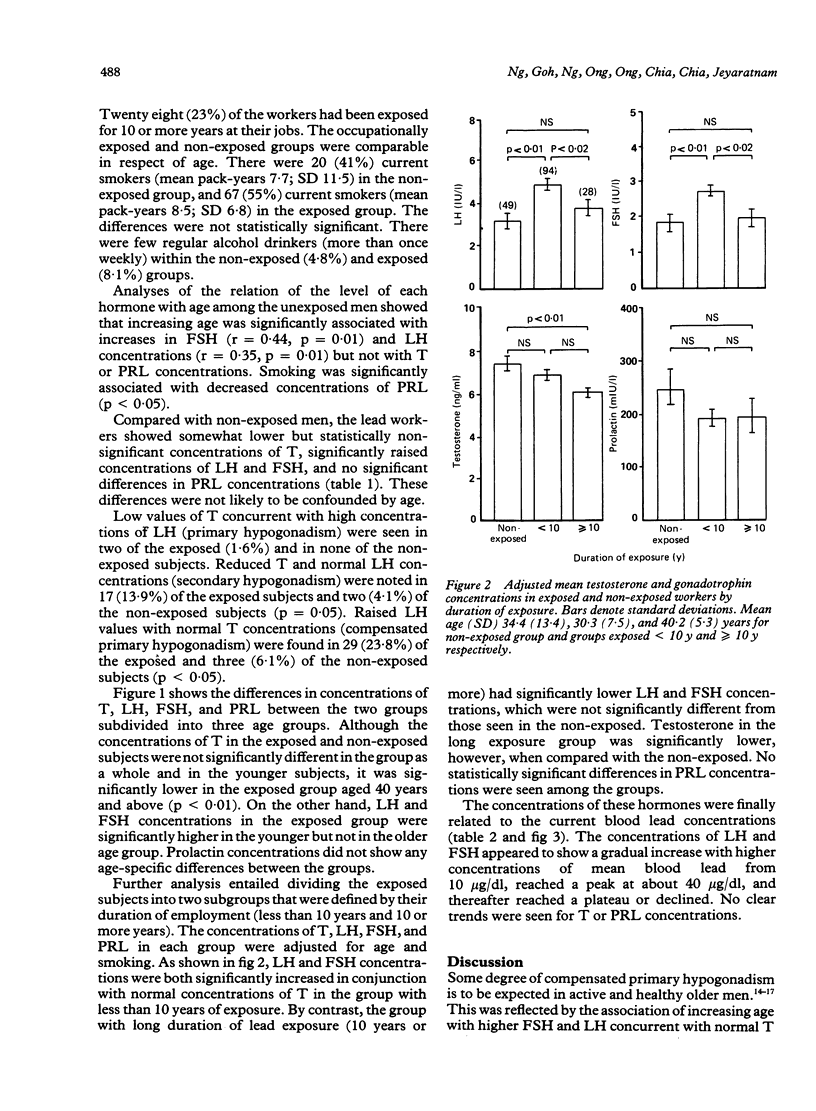
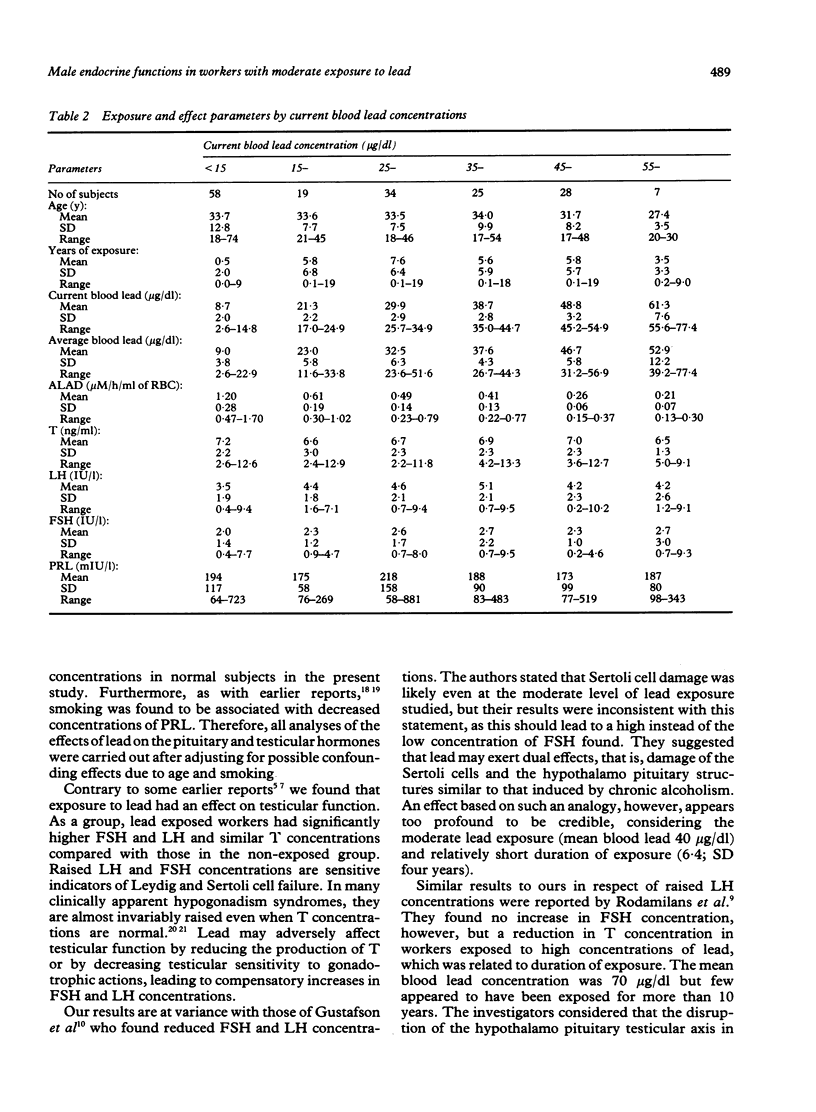
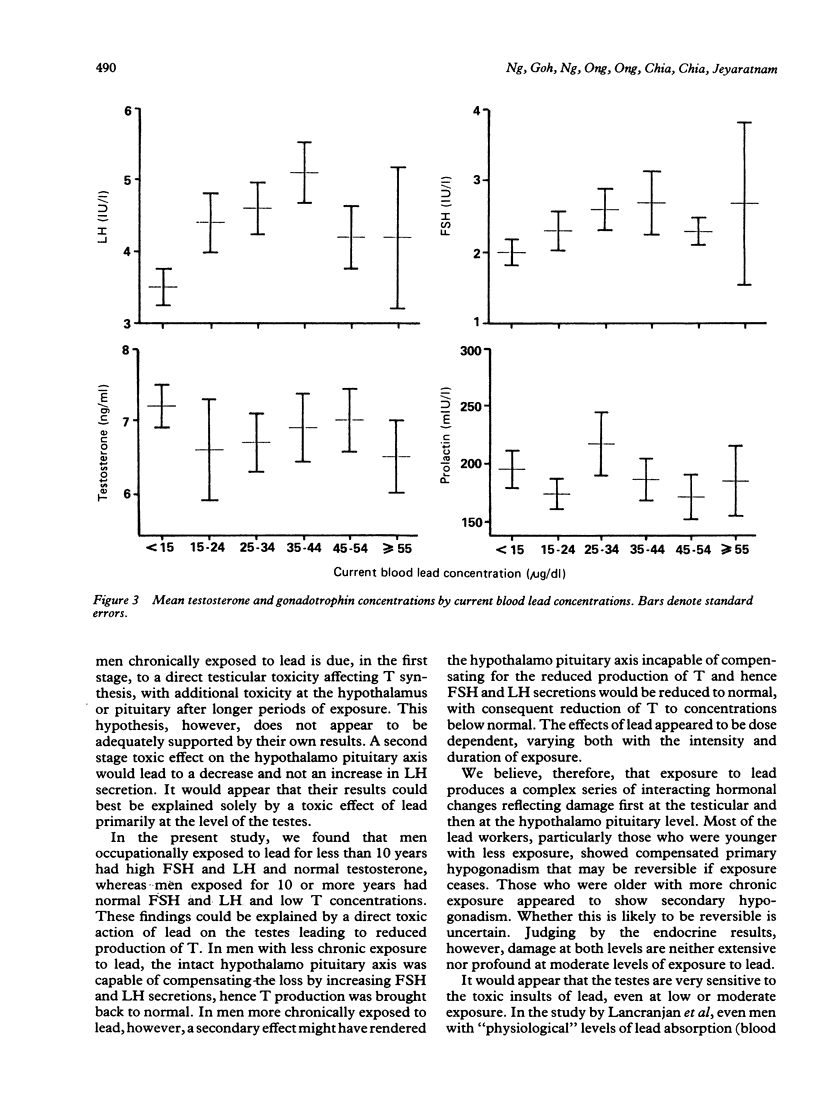
Evidence for the effect of occupational exposure to lead on the male endocrine system is conflicting. This study evaluated the primary (testicular) and secondary (hypothalamo pituitary testicular) effects of exposure to lead in 122 current lead workers and 49 non-exposed workers. The mean current blood lead concentration was 35.2 (range 9.6-77.4) micrograms/dl in the exposed workers, and 8.3 (range 2.6-14.8) micrograms/dl in the non-exposed workers. Concentrations of plasma luteinising hormone (LH) and follicle stimulating hormone (FSH) were both significantly higher in the exposed workers, but testosterone (T) was not significantly different between the two groups. In older exposed workers, however (greater than or equal to 40 years), plasma T concentrations were significantly lower, but LH and FSH concentrations were not significantly different. Compared with non-exposed workers, those exposed for less than 10 years had significantly raised LH and FSH and normal T concentrations whereas those exposed for 10 or more years had significantly lower T, and normal LH and FSH concentrations. The concentrations of LH and FSH showed a moderate increase in relation to blood lead concentrations in the range of 10 micrograms/dl to 40 micrograms/dl and thereafter reached a plateau or declined. No apparent trend for plasma T concentrations occurred. No significant difference in prolactin (PRL) concentration was noted. It is concluded that moderate exposure to lead was associated in dose related fashion with small but measurable changes in male endocrine functions that reflected both primary and secondary effects of lead on the testes and the hypothalamo pituitary testicular axis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assennato G., Paci C., Baser M. E., Molinini R., Candela R. G., Altamura B. M., Giorgino R. Sperm count suppression without endocrine dysfunction in lead-exposed men. Arch Environ Health. 1986 Nov-Dec;41(6):387–390. doi: 10.1080/00039896.1986.9935784. [DOI] [PubMed] [Google Scholar]
- Berlin A., Schaller K. H. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem. 1974 Aug;12(8):389–390. [PubMed] [Google Scholar]
- Blake C. A., Sawyer C. H. Nicotine blocks the suckling-induced rise in circulating prolactin in lactating rats. Science. 1972 Aug 18;177(4049):619–621. doi: 10.1126/science.177.4049.619. [DOI] [PubMed] [Google Scholar]
- Cullen M. R., Kayne R. D., Robins J. M. Endocrine and reproductive dysfunction in men associated with occupational inorganic lead intoxication. Arch Environ Health. 1984 Nov-Dec;39(6):431–440. doi: 10.1080/00039896.1984.10545877. [DOI] [PubMed] [Google Scholar]
- Deslypere J. P., Vermeulen A. Leydig cell function in normal men: effect of age, life-style, residence, diet, and activity. J Clin Endocrinol Metab. 1984 Nov;59(5):955–962. doi: 10.1210/jcem-59-5-955. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Andersson K., Eneroth P., Härfstrand A., Agnati L. F. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroendocrinology. 1989;14(1-2):19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Gustafson A., Hedner P., Schütz A., Skerfving S. Occupational lead exposure and pituitary function. Int Arch Occup Environ Health. 1989;61(4):277–281. doi: 10.1007/BF00381426. [DOI] [PubMed] [Google Scholar]
- Harman S. M., Tsitouras P. D., Costa P. T., Blackman M. R. Reproductive hormones in aging men. II. Basal pituitary gonadotropins and gonadotropin responses to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1982 Mar;54(3):547–551. doi: 10.1210/jcem-54-3-547. [DOI] [PubMed] [Google Scholar]
- Lancranjan I., Popescu H. I., GAvănescu O., Klepsch I., Serbănescu M. Reproductive ability of workmen occupationally exposed to lead. Arch Environ Health. 1975 Aug;30(8):396–401. doi: 10.1080/00039896.1975.10666733. [DOI] [PubMed] [Google Scholar]
- Neaves W. B., Johnson L., Porter J. C., Parker C. R., Jr, Petty C. S. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984 Oct;59(4):756–763. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- Rodamilans M., Osaba M. J., To-Figueras J., Rivera Fillat F., Marques J. M., Pérez P., Corbella J. Lead toxicity on endocrine testicular function in an occupationally exposed population. Hum Toxicol. 1988 Mar;7(2):125–128. doi: 10.1177/096032718800700203. [DOI] [PubMed] [Google Scholar]
- Sparrow D., Bosse R., Rowe J. W. The influence of age, alcohol consumption, and body build on gonadal function in men. J Clin Endocrinol Metab. 1980 Sep;51(3):508–512. doi: 10.1210/jcem-51-3-508. [DOI] [PubMed] [Google Scholar]
- Steinberger E. Current status of studies concerned with evaluation of toxic effects of chemicals on the testes. Environ Health Perspect. 1981 Apr;38:29–33. doi: 10.1289/ehp.813829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Brogan W. C., 3rd Some actions of lead on the sperm and on the male reproductive system. Am J Ind Med. 1983;4(1-2):127–134. [PubMed] [Google Scholar]


