Abstract
Impulsive choice, often characterized by excessive preference for small, short-term rewards over larger, long-term rewards, is a prominent feature of substance use and other neuropsychiatric disorders. The neural mechanisms underlying impulsive choice are not well understood, but growing evidence implicates nucleus accumbens (NAc) dopamine and its actions on dopamine D2 receptors (D2Rs). Because several NAc cell types and afferents express D2Rs, it has been difficult to determine the specific neural mechanisms linking NAc D2Rs to impulsive choice. Of these cell types, cholinergic interneurons (CINs) of the NAc, which express D2Rs, have emerged as key regulators of striatal output and local dopamine release. Despite these relevant functions, whether D2Rs expressed specifically in these neurons contribute to impulsive choice behavior is unknown. Here, we show that D2R upregulation in CINs of the mouse NAc increases impulsive choice as measured in a delay discounting task without affecting reward magnitude sensitivity or interval timing. Conversely, mice lacking D2Rs in CINs showed decreased delay discounting. Furthermore, CIN D2R manipulations did not affect probabilistic discounting, which measures a different form of impulsive choice. Together, these findings suggest that CIN D2Rs regulate impulsive decision-making involving delay costs, providing new insight into the mechanisms by which NAc dopamine influences impulsive behavior.
Subject terms: Motivation, Reward
Introduction
Choosing between different reward options requires consideration of their respective costs and benefits. For instance, increasing delay costs can diminish the subjective value of a reward, leading to a preference for immediate rewards [1]. The degree of “discounting” of future rewards, typically measured in humans and animals using delay discounting tasks [2], varies widely among healthy individuals. However, delay discounting can become maladaptive, leading to an excessive bias towards proximal, often less valuable rewards. Indeed, excessive impulsive choice is strongly implicated in substance use disorders (SUDs), attention-deficit hyperactivity disorder (ADHD), schizophrenia and other neuropsychiatric illnesses, as well as in obesity [3–6]. Excessive delay discounting also correlates with risky sexual behavior and overall lack of health monitoring and poor treatment compliance [3, 7]. It is not surprising that delay discounting has been proposed as a trans-disease and trans-diagnostic process, reflecting its potential as a candidate treatment target [3, 8]. However, the underlying neural substrates and cellular mechanisms remain to be fully understood.
Neuroimaging studies in humans and neuropharmacological and lesion studies in rodents suggest a critical involvement of the nucleus accumbens (NAc) in impulsive choice. Activation of the human ventral striatum, which comprises the NAc, correlates with the subjective value of delayed rewards [9, 10]. Lesions of the NAc core subregion in rats reduce preference for large, delayed rewards without affecting sensitivity to reward magnitude [11, 12], although partial inactivation of the NAc core can decrease delay discounting [13].
The activity of midbrain dopamine neurons has similarly been implicated in delay-based decision-making [14, 15]. Given the dense dopaminergic innervation of the NAc and the high prevalence of excessive choice impulsivity in disorders that feature ventral striatal dysfunction [3, 16], NAc dopamine has been suspected as a key modulator of the region’s role in impulsive choice. While dopamine denervation in the NAc failed to alter delay discounting [17], more recent work has demonstrated that phasic dopamine release in the NAc in vivo encodes delay-related costs and the changing subjective value of rewards [18, 19]. Furthermore, optogenetically-evoked NAc dopamine release specifically alters delay-based, but not magnitude-based choices [19].
Various cross-species studies suggest that dopamine D2 receptors (D2Rs) are critical mediators of dopamine’s actions in these behaviors. Systemic blockade of D2Rs, but not D1 receptors, reduces the value of delayed rewards in rats [20], suggesting a causal link between impulsive choice and D2R function. Because such approaches are likely to engage with D2Rs expressed brain-wide, the specific contribution of D2Rs within the NAc remains unclear. Positron emission tomography (PET) imaging findings indicate that low D2R availability in the NAc core in rats is correlated with increased impulsivity in a delay discounting task [21, 22]. A similar correlation has been reported in ventral striatum of pathological gamblers [16], but whether D2R expression in the NAc leads to impulsive choice involving delayed rewards is unresolved. While neuroimaging and pharmacological studies provide strong support for a role of NAc D2Rs in impulsive choice, they lack the resolution necessary to identify the specific cellular substrates and mechanisms of dopamine-D2R actions. This is especially relevant in the NAc, where D2Rs are widely expressed in spiny projection neurons (SPNs), cholinergic interneurons (CINs), and in presynaptic dopaminergic and glutamatergic axon terminals and can have distinct cellular signaling outcomes [23]. Moreover, given the greater relative abundance of SPNs, observations made with global approaches may obscure important cellular and behavioral contributions of D2Rs expressed in sparser neuronal populations.
Among these, CINs — the main source of acetylcholine in striatum [24, 25] — emerge as an intriguing candidate substrate for impulsive behavior. First, CINs influence striatal output by modulating cortico-striatal plasticity in SPNs [26, 27], which are thought to play key roles in action selection and reward valuation [28, 29]. Second, CINs not only powerfully control local dopamine release [30–32], but their cue-evoked firing activity and acetylcholine release is, in turn, sensitive to dopamine actions on D2Rs [33–35]. Third, recent work involving systemic administration of cholinergic receptor agonists and antagonists has suggested a complex involvement of acetylcholine in delay and probabilistic discounting tasks [36–39]. However, whether D2R function in NAc CINs is critical to impulsive choice has not been investigated.
We recently reported that selective D2R overexpression in NAc CINs impairs learning to suppress responding in a Go/No-Go task when an inhibitory response was required [34]. While this finding is consistent with increased action impulsivity, it is unknown whether NAc CIN D2Rs contribute to impulsive choice behaviors. To this end, we used region- and cell type-selective approaches to alter D2R expression in NAc CINs. We found that higher D2R levels in these neurons increase impulsive choice, but only when it involved temporal, but not probabilistic costs. This effect was not associated with altered sensitivity to reward magnitude or impairments in timing. These findings suggest a novel interaction between NAc dopamine and acetylcholine in mediating delay-based impulsive choice.
Materials and methods
Please see Supplementary Material for additional methods
Mice
Adult male and female hemizygous ChAT-Cre mice (B6.FVB(Cg)-Tg(Chat-cre)GM60Gsat/Mmucd, GENSAT; MMRRC stock no. 030869-UCD) [40], backcrossed > ten generations to C57BL/6 J background, were used in D2R overexpression experiments. For knockout experiments, mice were generated from crosses of hemizygous ChAT-IRES-Cre (ChATtm1(cre)Lowl/MwarJ; JAX stock no. 031661) to Drd2loxP/loxP (Drd2tm1.1Mrub/J, JAX stock no. 020631) [41] mice. The ChAT-IRES-Cre/Drd2loxP progeny were then crossed to Drd2loxP/loxP to generate ChAT-IRES-Cre/Drd2loxP/loxP (CIN-D2KO) and Drd2loxP/loxP(WT controls). Mice averaged approximately 6 months of age and were age-matched across groups. Body weights did not significantly differ between groups. Mice were housed in groups of 3–5 per cage on a 12-h light/dark cycle, and all experiments were conducted during the light cycle. All experimental procedures were performed in accordance with NIH guidelines and were approved by Institutional Animal Care and Use Committees of Fordham University and of the New York State Psychiatric Institute.
Surgical Procedures
Mice (>10 weeks) underwent stereotaxic surgical procedures under ketamine and xylazine-induced anesthesia in which they received Cre-dependent double-inverted open-reading frame (DIO) adeno-associated virus (AAVs) bilaterally into the NAc (400 nL/side). Infusions were done using Bregma-based coordinates: AP, + 1.70 mm; ML, ± 1.20 mm; DV, −4.1 mm (from dura) at a rate of 20 nl/s (20 pulses, 5 min). Viruses used: AAV2/9-EF1a-DIO-D2R(S)-P2A-EGFP [34]; AAV2/5-hSyn-DIO-EGFP (Addgene # 50457-AAV5) or AAV2/9-Syn-DIO-EGFP (Addgene # 100043-AAV9). Assignment of AAV was counterbalanced for sex, age, and home cage origin. Behavior experiments began at least 4 weeks following viral infusions, and mice were food-restricted and maintained at 85–90% of their baseline body weight; water was available ad libitum.
Delay Discounting and Probability Discounting Tasks
After successful completion of the criteria for trough and lever press training (see Supplementary Materials and Methods for more information), mice were trained on a delay discounting procedure adapted for mice [42]. Delay discounting sessions began with 10 forced choice trials. In forced choice trials, one of the lever lights appeared for 5 s before the extension of its associated lever; only one lever was presented in forced trials. In forced “delayed” trials, pressing of the corresponding lever led to the large reward after a delay. In “forced immediate” trials, responding on the alternate lever led to a small reward with no delay. The order of forced trials was alternated. Both levers were rewarded on a fixed ratio (FR-1) schedule and retracted following a press. In the remaining 20 “free choice” trials, both levers were extended following 5-s presentation of both lever lights. Levers remained extended until a press on either lever was made. New trials began following a variable intertrial interval (ITI) (mean = 29 s). For the first 14 daily sessions, the delay to the small and large rewards was set to 0 s to assess preference for reward size. These sessions were followed by sessions in which the delay to the large reward was increased across sessions, while the delay to the small reward remained at 0 s. Time delays (2, 4, 6, 8, and 10 s) to the large reward following a lever press were presented in separate sessions (3 sessions for each delay) in ascending order [42].
Following successful completion of trough and lever press training, separate cohorts were trained on the probabilistic discounting task [42]. The initial training phase for this task was identical to that of the delay discounting task. Here, the probability of receiving the large reward was decreased (80, 60, 50, 40, 33, 20%) across sessions. Each probability was presented for 3 consecutive days.
Timing Tasks
A different cohort of mice underwent training on a temporal discrimination task [43]. For discrimination training, mice learned to press one of the two levers after a 2-s tone (“short”) and the other following an 8-s tone (“long”) to earn a reward. The durations of the short and long tones were increased from 2 to 6 s and from 8 to 24 s and original lever assignments were maintained. The same mice were then trained for the peak interval task. Here, trials began with lever extension. Lever presses were only rewarded if they occurred after a fixed interval following lever extension. Each reinforcement was followed by a variable ITI (mean = 30 s) during which the lever remained retracted. New trials were signaled by lever extension. In peak interval training, a target interval of 24 s was used, as described [44]. In peak trials, the lever was extended for 72–96 s, but lever presses had no consequences. Each session consisted of 36 FI-24 s trials and 24 peak trials.
Data analysis
In discounting tasks, the percent of free choice trials in which the large reward option was chosen was determined for each delay or probability by dividing the number of presses on the large reward lever by the total number of presses. The total number of presses always equaled the total number of trials. Data were presented as the average of the last 2 sessions at each delay or probability. Mice that did not achieve ≥50% large choice after averaging the last two sessions of initial choice training (in the absence of delay or probability costs) were excluded from the analysis. Press latency in each trial was calculated as the period from lever extension to the lever press, and session medians were obtained for each lever and for both levers combined. Data are presented as the mean of the median latency. For temporal discrimination, data was expressed as the percentage of correct responses made on a given lever based on the sample duration presented. Peak interval data used for analysis was averaged across the last 5 sessions. Sample sizes were determined by performing statistical power analyses based on effect sizes observed in preliminary data or on similar work in the literature. Statistical analyses were performed using GraphPad Prism 9 (GraphPad) and MATLAB (MathWorks). Data are expressed as mean ± standard error of the mean (SEM). Unpaired two-tailed Student’s t tests were used to compare two-group data. Analyses of behavior data across time/sessions or following progressive changes in delay or probability were done using two-way repeated measures ANOVA. Temporal discrimination data was analyzed using three-way ANOVA, when appropriate. Tukey multiple comparisons tests were used to compare within-group means. For all analyses, p values < 0.05 were considered statistically significant. Investigators were blinded to the genotype throughout behavioral assays and data analysis.
Results
D2R upregulation in NAc CINs increases delay discounting
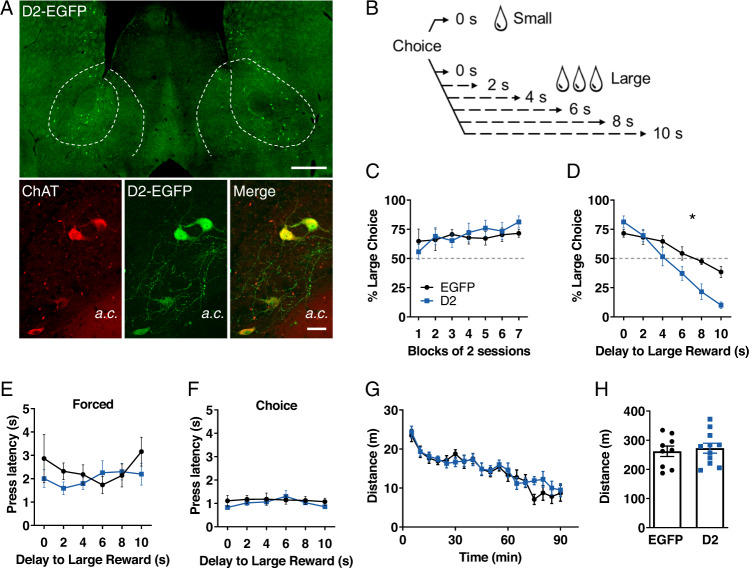
To determine whether increased D2R levels in NAc CINs contribute to impulsive choice, we first delivered Cre-dependent (AAV) expressing D2R-EGFP or EGFP bilaterally into the NAc of ChAT-Cre mice (8 mice/group). This manipulation led to efficient transduction of D2Rs in CINs in the NAc, particularly within the core subregion (Figs. 1A and S1). We have previously demonstrated, using single molecule fluorescent in situ hybridization, that this vector results in 2.4-fold D2R overexpression in NAc CINs [34]. Four weeks after surgery, mice were trained on a delay discounting task adapted to mice [42, 45] that measures the choice between pressing one lever to obtain a small, immediate reward or pressing another lever to obtain a three times larger reward that is presented after increasing delays (Fig. 1B).
Fig. 1. D2R upregulation in NAc CINs increases delay discounting.
A Top, low magnification image of AAV-DIO-D2-EGFP expression in CINs of the NAc (shown within dotted lines; scale = 500 µm). Bottom, higher magnification within the NAc core showing typical CIN morphology and co-localization of AAV-DIO-D2-EGFP and ChAT immunolabeling (a.c., anterior commissure; scale = 30 µm). B Schematic illustration of the delay discounting task. On free choice trials, two lever options are presented, each leading to a small or a large reward. The delay to the large reward is progressively increased across sessions (0–10 s), while the small reward is given with no delay. C In the absence of delays to either reward, EGFP and D2R-overexpressing mice similarly increased preference for the large reward option after 14 training sessions (shown here as blocks of 2 sessions). Delay main effect: F (6, 84) = 2.608, p = 0.0229; virus effect: F (1, 14) = 0.07516, p = 0.7880; virus x delay interaction: F (6, 84) = 1.257, p = 0.2861. D As delays to the large reward were progressively increased, a 2-way RM ANOVA found a significant main effect of delay (F (5, 70) = 43.47, p < 0.0001) as well as a significant virus x delay interaction: F (5, 70) = 6.13, p < 0.0001, * denotes significant interaction. n = 8 mice/group, 5 females, 3 males per group. Data is expressed as the average of the last 2 days at each delay. E, F. D2R upregulation did not alter the median press latency during forced trials (virus effect: F (1, 14) = 0.5845, p = 0.4572; virus x delay interaction: F (5, 70) = 1.131, p = 0.3522) or during free choice trials (virus effect: F (1, 14) = 0.4418, p = 0.5171; virus x delay interaction: F (5, 70) = 0.8204, p = 0.5393). Values expressed as mean of median latencies ± S.E.M. G, H. No significant group changes were observed in either distance traveled in 5-min bins over a 90-min period in an open field using a different cohort of mice (virus effect: F (1, 18) = 0.1850, p = 0.6722; virus x delay interaction: F (17, 306) = 1.087, p = 0.3651), or in mean total distance traveled over 90-min (t (18) = 0.4301, p = 0.6722). EGFP, n = 9 (3 females, 6 males); D2, n = 11 (5 females, 6 males).
We first assessed the percent choices made on the “large” lever in the absence of delays to either lever over the course of two weeks (Fig. 1C). We observed a significant main effect of session block, and Tukey’s multiple comparisons test revealed significant simple main effects within the D2 group (Block 1 vs 5 and 1 vs 7), but not within the EGFP group. However, we found no main effect of virus or a virus x session block interaction, suggesting that D2R upregulation does not alter the sensitivity to the two different reward sizes presented in this task. Following this initial phase, mice experienced increasing delays to the large reward following a lever press, while the small reward continued to be delivered without delay. Delays to the large reward were presented in separate sessions for 3 days each. As seen in Fig. 1D, both groups showed discounting of the large reward as delays increased (decreasing choice of the large reward). This discounting, however, was significantly steeper in D2R-overexpressing mice compared to controls, suggesting that D2R upregulation in NAc CINs increases impulsive choice. These effects did not appear to be sex-specific (Fig. S2A, B). Mice did not show significant alterations in the median latency to press levers in the forced trials at the start of each session or in the free choice trials (Figs. 1E, F and S3). Figure 1G, H show that the same viral manipulation in a different cohort of mice did not alter distance traveled in an open field. These results suggest that D2R upregulation increases intertemporal choice without general alterations in motivation or locomotor activity.
D2R upregulation in NAc CINs does not alter probabilistic discounting
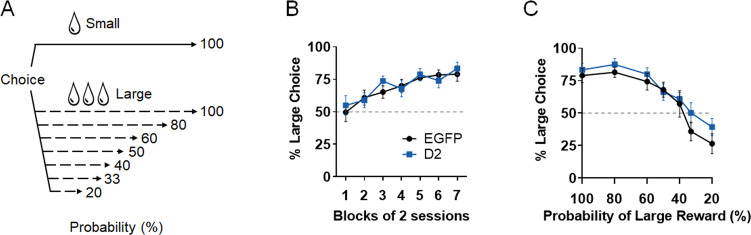
Because rewards obtained after long delays in our delay discounting paradigm could be perceived as being less certain than those obtained after short delays [46, 47], it is possible that the effect of CIN D2R upregulation on delay discounting is largely driven by an enhanced intolerance to reward uncertainty. We addressed this issue using a probabilistic discounting task in which subjects must choose between a small, certain reward or a larger reward delivered with decreasing probability (Fig. 2A). While performance in probability discounting and delay discounting depends on an intact NAc core, these two forms of choice behavior are generally considered dissociable processes [11, 46].
Fig. 2. D2R upregulation in NAc CINs does not alter probabilistic discounting.
A Schematic illustration of probabilistic discounting task. On free choice trials, two lever options are presented, each leading to a small or a large reward. The probability of receiving the large reward is progressively decreased across sessions (100–20%), while the small reward is always given. B With increased training, both EGFP and D2R-overexpressing mice increased preference for the large reward when both options were equally probable to yield reward (100%) probability; session block effect: F (6, 84) = 17.85, p < 0.0001; Tukey’s test showed significant differences between session blocks 1 and 7 for both groups (adjusted p values < 0.0001). No significant main effects of virus (F (1, 14) = 0.08267, p = 0.7779) or virus x session block interactions (F (6, 84) = 0.9243, p = 0.4818) were observed. C. A significant main effect of probability was detected (F(6, 84) = 38.39, p < 0.0001), with both groups showing significant simple main effects within-group (100% vs. 33% or 20%, adjusted p values < 0.0001), but this effect was not significantly different following D2R upregulation (virus: F (1, 14) = 0.8632, p = 0.3686; virus x probability: F (6, 84) = 0.7642, p = 0.6001, n = 8 mice/group). EGFP: 3 males, 5 females; D2: 4 males, 4 females.
We used a variation of the probabilistic discounting paradigm [42] in a different cohort of ChAT-Cre mice overexpressing either D2Rs or EGFP in NAc CINs. Initial training for this task was identical to the delay discounting task, involving two levers that, when pressed, led to a large or a small reward with 100% probability and 0-s delay. Mice from both groups similarly increased their preference for the large reward across these sessions (Fig. 2B). Following this phase, the probability of the small reward remained at 100%, while the large reward progressively decreased across sessions, and % preference for the large, probabilistic reward was determined. Two-way RM ANOVA indicated that while there was a main effect of probability on discounting (decreased large certain reward choices), there was no significant effect of D2R upregulation (Figs. 2C and S2C, D). These results contrast with our delay discounting findings, suggesting that augmented CIN D2R expression preferentially increases impulsive choice behavior involving delayed reinforcement.
D2R upregulation in NAc CINs does not alter timing
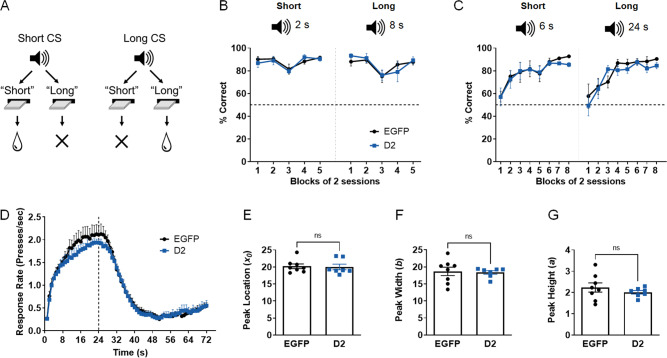
The ability to accurately represent the time it takes to receive a reward following a press is a key behavioral sub-component in delay discounting tasks [48]. Thus, it is conceivable that CIN D2R upregulation results in an overestimation of time intervals, thereby reducing tolerance of delays compared to controls. To test this hypothesis, we first used a temporal discrimination task to determine whether D2R upregulation altered the ability to correctly categorize two auditory tones of different durations as “short” or “long” [43]. A single press on one lever was rewarded following a 2-s (“short”) tone, while pressing the other lever was rewarded following an 8-s (“long”) tone (Fig. 3A, B). The mean percentage of correct responses during “short” or “long” trials across test sessions was not affected by D2R upregulation. To determine whether there might be distortions that are specific to particular time ranges [43], we proportionally increased the tone durations for the previously defined “short” and “long” levers to 6 s and 24 s in the same animals (Fig. 3C). Following the switch to the 6-s (“short”) versus 24-s (“long”) sessions, mice initially exhibited near chance performance in both trial types, likely due to the similarity between the 6-s and the 8-s tone, which was previously mapped to the “long” lever. Discriminative performance improved in both groups of mice with training without an effect of D2R upregulation.
Fig. 3. CIN D2R upregulation in NAc does not alter timing.
A Schematic representation of the temporal discrimination task. In each session, a single response on one of two lever options is rewarded based on the duration of the sample auditory stimulus. Two tone durations are presented in each session. B Mean proportion of correct responses on the corresponding lever in 2-s tone trials (short, left) or 8-s tone trials (long, right) across blocks of 2 sessions was not altered by D2R upregulation. A 3-way ANOVA showed no virus main effect, F (1, 65) = 0.005903, p = 0.9390, and no significant virus x tone duration x session block (F(4, 65) = 0.9819, p = 0.4237) or virus x session block (F (4, 65) = 0.05092, p = 0.9950) or virus x tone duration (F (1, 65) = 0.2230, p = 0.6383) interactions; n = 8 EGFP (7 females, 1 male), 7 D2 (6 females, 1 male). C The duration of tones was proportionally increased to 6 s (short, left) and 24 s (long, right). While there was a significant main effect of session block on discrimination of tone durations (F (7, 104) = 21.52, p < 0.0001), no significant main effect of virus (F (1, 104) = 1.890, p = 0.1721), or virus x tone duration x session block (F (7, 104) = 0.2323, p = 0.9766), virus x session block (F(7, 104) = 0.6384, p = 0.7232), or virus x tone duration (F (1, 104) = 0.07523, p = 0.7844) interactions were found. D Mean lever press rate during peak trials in the final five sessions of the 24-s peak interval task. E–G Mean best-fitting parameters (derived from fitting to Gaussian function) for peak location (timing accuracy), peak width (timing precision), or peak height (peak response rate). No significant differences were observed in any of these parameters using unpaired t tests.
Using the same mice, we then examined whether D2R upregulation impacted the precision and accuracy of timing using a peak interval task. In this procedure, mice initially learned that lever responses are only rewarded if they occur after a fixed interval of 24 s (FI-24). Peak trials, in which the lever is extended but responses are not rewarded, are then introduced randomly with FI trials [44]. Figure 3D shows the response rate during these peak probe trials, averaged over the final 5 sessions, in which control mice showed stable performance in both response rate and timing precision. The response rates and their distribution were similar in both D2R-overexpressing mice and control EGFP mice, with the highest mean response rates near the target of 24 s. For a quantitative analysis of peak trial performance, we fit a Gaussian probability density function to peak trial data from individual mice, as previously done [44, 49].
We found no significant differences in best-fit parameter values for peak location (x0) or peak width (b) suggesting no D2R-mediated alterations in the accuracy and precision in timing 24-s intervals (Fig. 3E, F). Moreover, we did not find alterations in maximal response rate estimates reflected in the peak height parameter (a) (Fig. 3G), suggesting that motivation was not affected, consistent with our previous findings in a progressive ratio task [50]. These results, together with the temporal discrimination data, suggest that timing is not fundamentally altered by increased D2R expression levels in NAc CINs.
Genetic inactivation of CIN D2Rs decreases delay discounting but does not affect probabilistic discounting
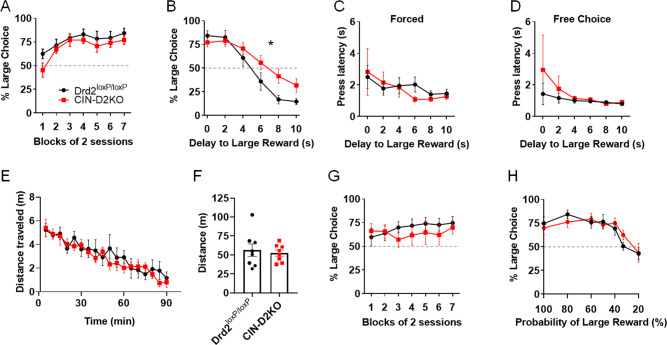
To determine whether D2Rs in CINs are required for impulsive choice, we used ChAT-IRES-Cre x Drd2loxP/loxP (CIN-D2KO) mice, which lack the D2R gene in striatal CINs. This strategy has been routinely used for the study of striatal CIN function and associated behavior [35, 51–53]. While this approach has the potential to impact cells that co-express ChAT and D2R outside the NAc, no studies, to our knowledge, have demonstrated D2R expression in extrastriatal cholinergic neurons. As shown in Fig. 4A, CIN-D2KO did not differ from Drd2loxP/loxP control mice in increasing their preference for the large reward in delay discounting 0 s phase, suggesting that a lack of CIN D2Rs does not alter reward magnitude sensitivity. However, compared to Drd2loxP/loxP mice, CIN-D2KO mice showed decreased delay discounting as evidenced by the greater choice of the large reward option at longer delays (Fig. 4B). This effect did not appear to be sex-specific (Fig. S4). No genotype differences were found in the press latency during forced or free choice trials (Figs. 4C, D and S5). Moreover, no alterations in open field locomotion were observed in a different cohort of mice (Fig. 4E, F).
Fig. 4. Lack of CIN D2Rs decreases delay, but not probabilistic, discounting.
A ChAT-IRES-Cre mice were crossed to Drd2loxP/loxP mice to obtain mice lacking D2Rs in CINs (CIN-D2KO). CIN-D2KO and control Drd2loxP/loxP mice increased preference for the large reward with training in the absence of delays to either reward option (session block effect: F (6, 78) = 9.206, p < 0.0001; Tukey’s test showed significant within session effects in both groups between session blocks 1 and 7 (adjusted p values < 0.001), but no effect of genotype: F (1, 13) = 1.651, p = 0.2213, and no genotype x session block interaction: F (6, 78) = 0.607, p = 0.7237). B Compared to Drd2loxP/loxP mice, CIN-D2KO mice showed greater preference for the large reward at longer delays (decreased delay discounting). Delay effect: F (5, 65) = 72.56, p < 0.0001; genotype x delay: F (5, 84) = 4.756, p < 0.001, * denotes significant interaction. Drd2loxP/loxP, n = 8 (5 females, 3 males); CIN-D2KO, n = 7 (4 females, 3 males). One Drd2loxP/loxP did not reach the criterion of ≥50% large choice on Session Block 7 and was excluded from the analysis. C, D Two-way ANOVA showed a significant main effect of delay on median press latency during forced trials (F (5, 65) = 2.628, p = 0.0317), but no genotype effect: F (1, 13) = 0.05419, p = 0.8195; genotype x delay interaction: F (5, 65) = 0.5731, (p = 0.7203) or during free choice trials (genotype effect: F (1, 13) = 0.5917, p = 0.4555; genotype x delay interaction: F (5, 65) = 0.4477, p = 0.8135). No delay effect was seen during free choice trials (F (5, 65) = 1.349, p = 0.2554). Values expressed as mean of median latencies ± S.E.M. E, F No significant group differences were observed in either distance traveled in 5-min bins over a 90-min period in an open field using a different cohort of mice (genotype effect: F (1, 14) = 0.1786, p = 0.6790; virus x delay interaction: F (17, 238) = 0.7946, p = 0.7946), or in mean total distance traveled over 90-min (t (14) = 0.4226, p = 0.6790). n = 8 mice per group (4 females, 4 males/ group). G Using a third cohort of mice, no significant group differences were found in the preference between reward options during training (when both options were given at 100% probability). Session block effect: F (6, 72) = 0.7816, p = 0.5871; genotype (F (1, 12) = 0.3588, p = 0.5603); genotype x session block interactions (F (6, 72) = 0.9471, p = 0.4672) were observed. H No significant group differences were observed in probabilistic discounting in same cohort as in G (probability effect): F (6, 72) = 14.26, p < 0.0001; genotype x probability: F (6, 72) = 0.9263, p = 0.4814, Drd2loxP/loxP, n = 8 (4 females, 4 males); CIN-D2KO, n = 6 (3 females, 3 males), 1 CIN-D2KO did not reach the criteria of ≥ 50% large choice on Session Block 7 and was excluded from the analysis.
We then tested whether absence of CIN D2Rs would impact probabilistic discounting. Using a new cohort of CIN-D2KO mice, we found no effect of genotype on performance in the initial training, when probability of either choice was 100% (Fig. 4G). Moreover, unlike the effect on delay discounting, CIN-D2KO mice showed no alterations in probabilistic discounting (Figs. 4H and S4). These findings suggest that CIN D2Rs are required for appropriate discounting of delayed rewards, but do not play a role in discounting of probabilistic rewards.
Discussion
Using Cre-mediated recombination with AAV gene transfer, we have found that selectively overexpressing D2Rs in CINs of the adult NAc leads to a significant increase in impulsive choice as measured in a delay discounting task. This effect was not associated with gross alterations in sensitivity to reward magnitude or in the ability to time intervals. In line with these results, CIN-D2KO mice, which lack the Drd2 gene in striatal CINs, showed decreased delay discounting. No alterations in probabilistic discounting, a related form of cost-based decision-making, were observed with either D2R manipulation. Together, these findings indicate that D2R expression levels in CINs powerfully regulate impulsive decision-making involving delay costs.
Brain imaging and autoradiography studies have reported a correlation between lower D2R availability in ventral striatum, especially the NAc core, and higher trait-like motor and choice impulsivity in rats [21, 22]. Whether alterations in NAc D2R levels or function cause impulsive choice behavior, however, has been difficult to demonstrate conclusively. Many of the studies to date have involved acute systemic administration of D2R/D3R pharmacological agents. Some reported increased sensitivity to delay costs with D2R antagonism [20, 54], whereas others showed no effect with either antagonists or agonists [2, 55, 56]. Such discrepancies may relate, to some extent, to the combined impact of these agents on the NAc and relevant extrastriatal regions (e.g., prefrontal cortex, amygdala, and ventral tegmental area or VTA), whose D2R signaling may have varied roles in impulsive decision-making [57–61]. However, the few studies that have performed intra-NAc microinfusions of raclopride, eticlopride or quinpirole have shown no effect of NAc D2Rs in delay discounting [62, 63]. It is conceivable that even within the NAc, concurrent blockade of D2Rs in different cell types may mask their unique contributions. For example, shRNA-mediated knockdown of D2Rs in the VTA increases delay discounting [58]. While VTA dopamine neurons have brain-wide projections, it is plausible that presynaptic D2Rs in dopaminergic afferents to the NAc play a role in limiting impulsive decision-making. In contrast, our findings clearly demonstrate that D2Rs in NAc CINs increase delay discounting. Therefore, it is possible that the global reductions in NAc D2Rs previously observed in higher trait impulsive choice are occurring in SPNs or VTA dopamine axon terminals, which account for most striatal D2Rs. Our data suggest that an unappreciated increase in D2R levels could be simultaneously occurring in CINs and contributing to impulsive phenotypes. Thus, dopamine’s dynamic encoding of reward value in the face of delay costs might entail complex modulation of D2R function across different neuronal populations. We previously showed that increasing D2R levels in NAc CINs significantly increases pauses in CIN firing in response to VTA dopamine terminal stimulation, an effect blocked by a D2R antagonist [34]. If additional CIN D2R receptors promote impulsive choice by perturbing dopamine-to-CIN signaling, then it is conceivable that reducing CIN D2R function pharmacologically, or by other means, could reverse this phenotype.
Growing evidence has also implicated cholinergic neurotransmission in delay discounting. Chronic smokers show greater impulsive choice, suggesting a permissive role for nicotinic acetylcholine receptor (nAChR) function [64, 65]. Likewise, acute nicotine administration in rats leads to enhanced choice impulsivity, an effect that is prevented by nAChR blockade [36, 37]. However, conflicting results have also been described [38, 39]. Because these studies also relied on systemic delivery, the specific brain areas and cell types that are critically involved remain to be defined. The behavioral effects that we observed following selective targeting of NAc CINs clearly identify these neurons as a key node in the neurocircuitry underlying impulsive choice.
In our task design, which has been previously used for mice [42, 45, 66], delays to the large reward were presented across different sessions. In contrast, many studies of delay discounting (primarily in rats) have used within-session shifts in delay, which may require greater moment-to-moment cognitive flexibility compared to across-session designs [2]. Our delay discounting protocol also presents delays in ascending order and without a cue to signal the delay to the large reward. While these are common parameters in rodent delay discounting tasks, the order of delay presentation (ascending vs. descending) or the signaling of delays can considerably influence how acute dopaminergic manipulations impact delay discounting [59, 67–70] (but also see [71]). Whether alterations in CIN D2R expression similarly impact delay discounting in other variations of the task remains to be determined.
Because our task design involves ascending delays, it could be argued that the effects on delay discounting may be, to some extent, due to alterations in learning or adapting to new contingencies. We found no effect of either D2R manipulation on probability discounting, which is measured with the same increasing-cost design as delay discounting, or on learning to categorize interval durations in the timing tasks. Therefore, it is unlikely that general alterations in learning or behavioral flexibility play a major role in the D2R-mediated effects, but changes that are specific to delay discounting cannot be completely ruled out.
Previous work has also shown that individual differences in the degree of delay discounting (e.g., low vs high impulsivity) can influence how brain manipulations, like lesions or pharmacological agents, alter subsequent discounting [59]. Whether the effect of our D2R manipulations depends on individual differences in baseline discounting is unclear given our experimental design but future work could address this question by performing D2R manipulations after mice have been pre-assessed on the delay discounting task.
Performance in both probabilistic discounting and delay discounting tasks depends on an intact NAc core and can be sensitive to D2R blockade or nicotine [11, 20, 39, 72, 73]. However, the manipulations of CIN D2Rs in our study did not affect probabilistic discounting, indicating that these receptors selectively contribute to decision-making involving delay costs. Similar behavioral dissociations have emerged in studies that tested both discounting paradigms [39, 74, 75]. Furthermore, accumulating evidence indicates that certain SUDs are associated with increased delay discounting but not probability discounting [76–78], which has been supported by preclinical literature [79]. Our findings reinforce the notion that distinct cellular mechanisms underlie these different forms of discounting and propose a role for striatal dopamine and acetylcholine interactions in impulsive choice. Although intra-NAc delivery of D2R agonist increases risk-seeking behavior, biasing choice toward larger, probabilistic rewards [80], it is possible that D2R-expressing cell types in the NAc other than CINs play a more prominent role in this behavior. Supporting this hypothesis is the fact that phasic activity in D2R-expressing SPNs is sufficient to decrease risk preference [80]. Whether D2Rs in SPNs mediate probabilistic discounting would need to be directly tested.
Altered sensitivity to reward magnitude can result in impulsive choice if an animal is unable to perceive a large reward as more valuable than a small reward. Neither of our CIN D2Rs manipulations affected discrimination between the large and small reinforcers in sessions involving no delay or probability costs. These findings, which are line with several studies using NAc lesions and systemic D2R or cholinergic receptor drugs [11, 36, 38, 63, 73, 81, 82], suggest that CIN D2Rs are unlikely to modulate delay discounting through alterations in processing the reward magnitude of the choices presented. However, a more subtle effect of CIN D2Rs on reward magnitude sensitivity could be addressed with task variations that systematically adjust reward magnitude [13, 83].
The ability to accurately represent the time interval between a reward-seeking action and reward retrieval is intricately linked to delay intolerance. For example, overestimation of elapsed time could reduce preference for the larger delayed reward [84]. Indeed, individuals deemed impulsive on delay discounting tasks are more prone to timing errors compared to control subjects [85]. Similarly, rats that showed higher timing precision in peak interval and temporal discrimination tasks also show reduced delay-based impulsivity [48, 86]. Multiple studies also support a role of dopamine in timing [87–89]. Transgenic mice that selectively overexpress D2Rs in striatal SPNs since early in development show reduced timing precision in a peak interval task as well as deficits in timing long sample durations in a temporal discrimination paradigm [43, 44]. In contrast, we did not observe an effect of CIN D2R upregulation in either timing task, suggesting that the effect of CIN D2Rs on delay discounting does not involve alterations in the representation of time.
Choices made in delay discounting tasks require a dynamic, subjective assessment of reward value that integrates the magnitude and the changing delay properties of a reward [9]. The NAc appears to be a key site for this integration across species. In humans, neural activity in the ventral striatum during delay discounting is more strongly correlated with subjective value than to objective reward characteristics like magnitude and delay [9]. Furthermore, inactivation of the NAc core in rats decreased discounting only in a task that measured sensitivity to both delay and magnitude but had no effect when these factors were independently adjusted [13]. The cellular mechanisms underlying integration of these reward characteristics in intertemporal choice, however, are not well understood, but they may involve distinct subsets of striatal neurons whose activity is modulated by both reward size and delay [90–92]. Further, cue-evoked activity of a subset of neurons in the dorsal caudate nucleus encodes the temporally discounted value of rewards but not reward magnitude or delay alone [91]. Whether a similar dynamic computation of subjective value occurs in specific subset(s) of NAc neurons during delay discounting tasks remains to be determined, but recent evidence indicates that NAc dopamine may contribute to this process. For instance, cue-evoked dopamine release in NAc not only encodes the relative value of small and large reward options, but also how that value changes with increasing delays [19]. Because cue-evoked dopamine and acetylcholine signals temporally coincide in mouse striatum [34, 35], it is tempting to speculate that D2R-dependent modulation of NAc CINs contributes to integration of reward size and delay information in impulsive decision-making.
Growing evidence suggests that discounting of delayed rewards is a stable, heritable trait that contributes to the etiology and treatment outcomes of various mental health disorders [3, 8, 93]. Despite the important clinical implications, few pharmacological interventions are currently available that are specific to impulsivity and that are based on an understanding of its complex subdomains and underlying neurocircuitry. Our findings identify NAc CIN D2Rs as critical players in the mechanisms of delay-based impulsive choice. This new information refines our current understanding of the contributions of striatal dopamine and acetylcholine to impulsive behavior and raise the possibility that modulation of NAc acetylcholine might hold promise for more targeted treatments for choice impulsivity.
Supplementary information
Acknowledgements
We thank David D’Onofrio, Eric Teboul, Emily Huegler, and Daphne Baker for technical assistance, Katherine Nautiyal for input on behavioral design and analysis, and Christoph Kellendonk and Jonathan Javitch for sharing the D2R viral vector.
Author contributions
JC, JY, RES, JRF and BA and EFG conducted the experiments and data analysis. EFG and JC wrote the manuscript. PDB and BA edited the manuscript. EFG and PDB designed the experiments. EFG supervised the experiments and data analysis.
Funding
This work was supported by K01 MH107648, R01 DA055018 and Fordham University Faculty Research Grants to EFG; JC was supported by a Len Blavatnik STEM Research Fellowship and Fordham Undergraduate Research Grant; PDB and BA were supported by R01 MH068073.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Julianna Cavallaro, Jenna Yeisley.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01608-1.
References
- 1.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 2.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 3.Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134:287–97. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, Hudec KL. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev. 2016;43:162–74. doi: 10.1016/j.cpr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12:213–21. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–69. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Daugherty JR, Brase GL. Taking time to be healthy: Predicting health behaviors with delay discounting and time perspective. Pers Individ Dif. 2010;48:202–07.. [Google Scholar]
- 8.Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB. Can delay discounting deliver on the promise of RDoC? Psychol Med. 2019;49:190–99.. doi: 10.1017/S0033291718001770. [DOI] [PubMed] [Google Scholar]
- 9.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lempert KM, Speer ME, Delgado MR, Phelps EA. Positive autobiographical memory retrieval reduces temporal discounting. Soc Cogn Affect Neurosci. 2017;12:1584–93.. doi: 10.1093/scan/nsx086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 12.da Costa Araújo S, Body S, Hampson CL, Langley RW, Deakin JF, Anderson IM, et al. Effects of lesions of the nucleus accumbens core on inter-temporal choice: further observations with an adjusting-delay procedure. Behav Brain Res. 2009;202:272–7. doi: 10.1016/j.bbr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Moschak TM, Mitchell SH. Partial inactivation of nucleus accumbens core decreases delay discounting in rats without affecting sensitivity to delay or magnitude. Behav Brain Res. 2014;268:159–68. doi: 10.1016/j.bbr.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–24. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28:7837–46. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joutsa J, Voon V, Johansson J, Niemelä S, Bergman J, Kaasinen V. Dopaminergic function and intertemporal choice. Transl Psychiatry. 2015;5:e491–e91. doi: 10.1038/tp.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–82. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- 18.Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic Nucleus Accumbens Dopamine Release Encodes Effort- and Delay-Related Costs. Biol Psychiatry. 2010;68:306–09. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry. 2015;77:903–11.. doi: 10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- 21.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlow RL, Gorges M, Wearn A, Niessen HG, Kassubek J, Dalley JW, et al. Ventral striatal D2/3 receptor availability is associated with impulsive choice behavior as well as limbic corticostriatal connectivity. Int J Neuropsychopharmacol. 2018;21:705–15.. doi: 10.1093/ijnp/pyy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo EF. Disentangling the diverse roles of dopamine D2 receptors in striatal function and behavior. Neurochem Int. 2019;125:35–46. doi: 10.1016/j.neuint.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–8. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- 25.Matamales M, Gotz J, Bertran-Gonzalez J. Quantitative imaging of cholinergic interneurons reveals a distinctive spatial organization and a functional gradient across the mouse striatum. PLoS One. 2016;11:e0157682. doi: 10.1371/journal.pone.0157682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci. 1998;10:3020–3. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–42. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 2020;25:3241–55.. doi: 10.1038/s41380-019-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 31.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell reports. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Cai X, Ritzau-Jost A, Kramer PF, Li Y, Khaliq ZM, et al. An action potential initiation mechanism in distal axons for the control of dopamine release. Science. 2022;375:1378–85.. doi: 10.1126/science.abn0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–5. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- 34.Gallo EF, Greenwald J, Yeisley J, Teboul E, Martyniuk KM, Villarin JM, et al. Dopamine D2 receptors modulate the cholinergic pause and inhibitory learning. Mol Psychiatry. 2022;27:1502–14.. doi: 10.1038/s41380-021-01364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martyniuk KM, Torres-Herraez A, Lowes DC, Rubinstein M, Labouesse MA, Kellendonk C. Dopamine D2Rs coordinate cue-evoked changes in striatal acetylcholine levels. eLife. 2022;11:e76111. doi: 10.7554/eLife.76111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology (Berl) 2011;217:455–73. doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- 37.Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Ozga JE, Anderson KG. Reduction in delay discounting due to nicotine and its attenuation by cholinergic antagonists in Lewis and Fischer 344 rats. Psychopharmacology (Berl) 2018;235:155–68.. doi: 10.1007/s00213-017-4752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez IA, Gilbert RJ, Bizon JL, Setlow B. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology (Berl) 2012;224:489–99. doi: 10.1007/s00213-012-2777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–23. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–8. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nautiyal KM, Wall MM, Wang S, Magalong VM, Ahmari SE, Balsam PD, et al. Genetic and Modeling Approaches Reveal Distinct Components of Impulsive Behavior. Neuropsychopharmacology. 2017;42:1182–91.. doi: 10.1038/npp.2016.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, et al. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav Neurosci. 2009;123:720–30. doi: 10.1037/a0016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–9. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: adolescent-limited and life-persistent patterns of impulsivity. Behav Neurosci. 2011;125:194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClure J, Podos J, Richardson H. Isolating the delay component of impulsive choice in adolescent rats. Front Integr Neurosci. 2014;8:1–9. [DOI] [PMC free article] [PubMed]
- 49.Buhusi CV, Meck WH. Timing for the absence of a stimulus: the gap paradigm reversed. J Exp Psychol Anim Behav Process. 2000;26:305–22. doi: 10.1037//0097-7403.26.3.305. [DOI] [PubMed] [Google Scholar]
- 50.Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS, et al. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat Commun. 2018;9:1086. doi: 10.1038/s41467-018-03272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE, Nelson AB, Ramos M, Del Barrio D, et al. Parkinsonism driven by antipsychotics originates from dopaminergic control of striatal cholinergic interneurons. Neuron. 2016;91:67–78. doi: 10.1016/j.neuron.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Augustin SM, Chancey JH, Lovinger DM. Dual dopaminergic regulation of corticostriatal plasticity by cholinergic interneurons and indirect pathway medium spiny neurons. Cell reports. 2018;24:2883–93.. doi: 10.1016/j.celrep.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bocarsly ME, da Silva e Silva D, Kolb V, Luderman KD, Shashikiran S, Rubinstein M, et al. A mechanism linking two known vulnerability factors for alcohol abuse: heightened alcohol stimulation and low striatal dopamine D2 receptors. Cell reports. 2019;29:1147–63.e5. doi: 10.1016/j.celrep.2019.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology (Berl) 2005;179:587–96. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- 55.Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol. 2011;22:300–11. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernosky-Smith KA, Qiu YY, Feja M, Lee YB, Loughlin B, Li JX, et al. Ventral tegmental area D2 receptor knockdown enhances choice impulsivity in a delay-discounting task in rats. Behav Brain Res. 2018;341:129–34.. doi: 10.1016/j.bbr.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
- 60.Pardey MC, Kumar NN, Goodchild AK, Cornish JL. Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex. J Psychopharmacol. 2013;27:203–12. doi: 10.1177/0269881112465497. [DOI] [PubMed] [Google Scholar]
- 61.Larkin JD, Jenni NL, Floresco SB. Modulation of risk/reward decision making by dopaminergic transmission within the basolateral amygdala. Psychopharmacology (Berl) 2016;233:121–36. doi: 10.1007/s00213-015-4094-8. [DOI] [PubMed] [Google Scholar]
- 62.Yates JR, Bardo MT. Effects of intra-accumbal administration of dopamine and ionotropic glutamate receptor drugs on delay discounting performance in rats. Behav Neurosci. 2017;131:392–405. doi: 10.1037/bne0000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Zuo Y, Yu P, Ping X, Cui C. Role of basolateral amygdala dopamine D2 receptors in impulsive choice in acute cocaine-treated rats. Behav Brain Res. 2015;287:187–95. doi: 10.1016/j.bbr.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 64.Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology (Berl) 2006;186:255–63. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- 65.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 66.Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- 67.Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacology (Berl) 2014;231:85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maguire DR, Henson C, France CP. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology. 2014;87:173–9. doi: 10.1016/j.neuropharm.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- 70.Orsini CA, Mitchell MR, Heshmati SC, Shimp KG, Spurrell MS, Bizon JL, et al. Effects of nucleus accumbens amphetamine administration on performance in a delay discounting task. Behav Brain Res. 2017;321:130–36.. doi: 10.1016/j.bbr.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behav Pharmacol. 2009;20:424–36. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- 72.St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–97. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- 73.Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green L, Myerson J, Ostaszewski P. Amount of reward has opposite effects on the discounting of delayed and probabilistic outcomes. J Exp Psychol Learn Mem Cogn. 1999;25:418–27. doi: 10.1037//0278-7393.25.2.418. [DOI] [PubMed] [Google Scholar]
- 75.Acheson A, Farrar AM, Patak M, Hausknecht KA, Kieres AK, Choi S, et al. Nucleus accumbens lesions decrease sensitivity to rapid changes in the delay to reinforcement. Behav Brain Res. 2006;173:217–28. doi: 10.1016/j.bbr.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 77.Johnson MW, Johnson PS, Herrmann ES, Sweeney MM. Delay and probability discounting of sexual and monetary outcomes in individuals with cocaine use disorders and matched controls. PLoS One. 2015;10:e0128641. doi: 10.1371/journal.pone.0128641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox DJ, Dolan SB, Johnson P, Johnson MW. Delay and probability discounting in cocaine use disorder: Comprehensive examination of money, cocaine, and health outcomes using gains and losses at multiple magnitudes. Exp Clin Psychopharmacol. 2020;28:724–38.. doi: 10.1037/pha0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, et al. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124:470–77. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature. 2016;531:642–6. doi: 10.1038/nature17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steele CC, Peterson JR, Marshall AT, Stuebing SL, Kirkpatrick K. Nucleus accumbens core lesions induce sub-optimal choice and reduce sensitivity to magnitude and delay in impulsive choice tasks. Behav Brain Res. 2018;339:28–38. doi: 10.1016/j.bbr.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balleine B, Killcross S. Effects of ibotenic acid lesions of the Nucleus Accumbens on instrumental action. Behav Brain Res. 1994;65:181–93. doi: 10.1016/0166-4328(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 83.Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci. 2007;27:245–50. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 85.Baumann AA, Odum AL. Impulsivity, risk taking, and timing. Behav Processes. 2012;90:408–14. doi: 10.1016/j.beproc.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marshall AT, Smith AP, Kirkpatrick K. Mechanisms of impulsive choice: I. Individual differences in interval timing and reward processing. J Exp Anal Behav. 2014;102:86–101. doi: 10.1002/jeab.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 88.Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–7. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- 89.Balci F, Ludvig EA, Abner R, Zhuang X, Poon P, Brunner D. Motivational effects on interval timing in dopamine transporter (DAT) knockdown mice. Brain Res. 2010;1325:89–99. doi: 10.1016/j.brainres.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 90.Roesch M, Bryden D. Impact of size and delay on neural activity in the rat limbic corticostriatal system. Front Neurosci. 2011;5:1–13. [DOI] [PMC free article] [PubMed]
- 91.Hori Y, Mimura K, Nagai Y, Fujimoto A, Oyama K, Kikuchi E, et al. Single caudate neurons encode temporally discounted value for formulating motivation for action. eLife. 2021;10:e61248. doi: 10.7554/eLife.61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cai X, Kim S, Lee D. Heterogeneous coding of temporally discounted values in the dorsal and ventral striatum during intertemporal choice. Neuron. 2011;69:170–82. doi: 10.1016/j.neuron.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anokhin AP, Grant JD, Mulligan RC, Heath AC. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol Psychiatry. 2015;77:887–94. doi: 10.1016/j.biopsych.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.