Abstract
The purpose of this study is to compare the levels of maternal serum pregnancy–associated plasma protein-A at the first trimester in pregnancies complicated by impaired placental diseases, such as preeclampsia (PE), intrauterine fetal growth restriction (IUGR), and gestational hypertension (GH), with those in pregnancies without the development of any of these outcomes to expand the knowledge of how this protein behaves in the different impaired placental diseases. This current work is an observational study based on a prospective cohort. Pregnancy-associated plasma protein-A was measured in 422 patients who had completed maternal-perinatal outcomes. Comparisons of pregnancy characteristics and the biomarker between outcome groups (PE, IUGR, gestational hypertension, and not impaired placental outcomes) were analyzed. PAPP-A MoM in the IUGR (0.8 IQR: 0.6–0.9) and GH groups (0.5 IQR: 0.3–1.4) compared to the PE group (1.06 IQR: 0.66–1.52) was significantly lower (p < 0.005). Pregnant women who developed early-onset PE (1.11 IQR 1.08–1.18) presented significant differences with the IUGR group (0.83 IQR: 0.59–0.98; p = 0.002) and those who developed preterm-PE (1.19 IQR: 0.66–1.58; p = 0.045). The results demonstrate that the levels of PAPP-A at first trimester in the sample of women who developed PE, and specially term-PE, were higher than those in women who developed GH or IUGR. The GH group had the lowest PAPP-A values in this sample of pregnant women. Research in a population with a high prevalence of preeclampsia is still lacking and deserves more extended studies to define if these patients could have different rates of PAPP-A.
Keywords: Pregnancy-associated Plasma Protein-A (PAPP-a), Preeclampsia, IUGR, Gestational Hypertension, Hypertensive Disorders of Pregnancy
Introduction
Health in obstetric involves two patients: mother and fetus. For decades, the World Health Organization (WHO) has been working to reduce morbidity and mortality in obstetrics, improving quality prenatal care and introducing strategies to decrease the severity of obstetric diseases. One of the most dangerous illnesses during pregnancy is PE. PE is an obstetric pathology that is classified in the group of illnesses that have an inappropriate process of placentation. Consequently, these women suffer from different diseases related to vasoconstriction and hypertensive disorders and from gestational hypertension to PE, and it can also affect the fetus, which receives fewer amounts of nutrients and oxygen during pregnancy, reducing its potential growth [1–5].
Hypertensive disorders of pregnancy affect 5–8% of pregnant women worldwide each year and are among the obstetric diseases with the worst gestational outcomes, with high maternal-perinatal morbidity and mortality [6–10], secondary to maternal and fetal complications such as IUGR (42). The prevalence of PE is around 2–4% worldwide [4, 11]; however, Colombia has a prevalence of PE of 6–8%, and it was the first or second cause of maternal mortality in the last years [5, 12, 13]. The prevalence of gestational hypertension (GH), also among hypertensive disorders of pregnancy, is around 2–4%, and its relationship with biomarkers has been less studied [14, 15].
The similar physiopathology of these diseases is the cause of which prediction models for PE, GH, and IUGR involve the same biomarker at first trimester of pregnancy, such as the maternal serum pregnancy-associated plasma protein-A (PAPP-A). This biomarker is produced by the placental tissue, and the improper trophoblastic invasion in pregnant women, affected by impaired placental diseases, generates a diminution of its levels, due to the decrease in placental function [14–19]. PAPP-A began to be used in biochemical screening for trisomies 21, 18, and 13. Because of its relation to placental function, it became a target of study about screening for PE and IUGR. These trials could determine that low-serum levels of PAPP-A were associated with a higher incidence of PE and IUGR. Since this discovery, PAPP-A biomarker is part of the model of early screening for PE and IUGR at first trimester of pregnancy that is used nowadays [7–9, 18, 20–23].
The precise early counselling, applying prediction models, could decrease maternal and fetal morbidity and mortality through pharmacological prevention by a low dose of acetylsalicylic acid (ASA) beginning before fourteen gestational weeks [24]. These models that combined maternal demographic characteristics and biophysical and biochemical markers of impaired placentation can identify a high proportion of pregnancies at risk of PE and/or IUGR [25–30]. However, one of the limitations of these studies, which were used to obtain the screening combined test, have been made in populations with lower incidence of PE than in other countries, such as Colombia [4].
This study aims to compare the levels of PAPP-A at first trimester in pregnancies complicated with PE, IUGR, and GH and of those without the development of any impaired disease.
Materials and Methods
Study Population
This is an observational study based on a prospective cohort of an ongoing research study about diagnostic validation of hypertensive disorders’ biomarkers in Colombian pregnant women. A total of 566 patients were included between November 2013 and August 2017, and three main institutions took part in this multicenter study, Ecodiagnóstico El Bosque Diagnostic Unit Centre, El Bosque Clinic, and South Kennedy Hospital (including West Health Services Unit) at Bogotá (Colombia). The population of interest was single pregnancies who had completed maternal-perinatal outcomes, and the exclusion criteria were threatened abortion, major fetal anomalies, fetal chromosomopathies, and maternal age below 14 years old.
Maternal medical history was taken at the inclusion time with a special request of different hypertension risk factors, and, for physical examination, maternal blood pressure and weight were measured. In addition, pregnancy-associated plasma protein-A (PAPP-A) was taken at the same time of the first-trimester scan for chromosomal abnormalities.
Maternal and fetal outcomes were collected by clinical records to group patients according to the presented impaired placenta illness at the delivery.
Maternal History
Searched maternal history included maternal age, racial origin (Caucasian, Afro-Caribbean, Asian, and mixed), economic level, smoking habit, method of conception, personal chronic pathologies (hypertension, systematics lupus erythematosus, renal pathology, antiphospholipid syndrome, and diabetes mellitus), and also personal and family history of PE and IUGR. Also, parity was asked, and, if it was not the first pregnancy, the new paternity was investigated and registered. Regarding the early preventive measures such as ASA intake, this is a blind study without any type of medical intervention by the research, so this was not considered.
Weight, height, and mean arterial pressure (MAP) were measured by calibrated equipment. Blood pressure was taken by automated devices (Microlife, BP A100 Plus, Taipéi, Taiwán) twice in both arms to calculate MAP.
Maternal serum biomarkers were measured using the DELFIA XPRESS (PerkinElmer, Inc.). To standardize according to maternal and pregnancy characteristics, every biomarker’s value was converted to a multiple of the expected normal median (MoM) specific to a pregnancy of the same gestational age, maternal weight, racial origin, smoking habit, method of conception, and parity as it’s described in the previous scientific literature [17, 19, 26].
Outcome Measures
Obstetric records collected by clinical history were examined to establish four groups according to impaired placentation disease (PE, IUGR, and GH) and patients without the development of these outcomes. Posteriorly, in order to know if there would be different results if PE disease appears before or after 34 weeks and before or after 37 weeks, a new analysis was done with five groups. Firstly, patients were grouped in gestations without the presence of impaired placentation disease or normal, PE after 37 weeks (term PE), PE before 37 weeks (preterm PE), IUGR, and GH. Lastly, patients were grouped in normal gestations, PE after 34 weeks (late-onset PE), PE before 34 weeks (early-onset PE), IUGR, and GH [15].
The definitions of PE and gestational hypertension were taken from the last Task Force on Hypertension in Pregnancy [31, 32]: the systolic blood pressure should be 140 mm Hg or more, and/or the diastolic blood pressure should be 90 mm Hg or more, on at least two occasions 4 h apart, after 20 weeks of gestation in previously normotensive women. In the case of PE, pregnant women should have proteinuria of 300 mg or more in 24 h or present any criteria of severe PE in addition.
The criteria to establish IUGR diagnosis was a fetal or neonatal weight below the third percentile for gestational age or below the 10th percentile and alteration of fetoplacental circulation [33, 34].
Women who had a diagnosis of PE and IUGR were included in the group of PE because in both cases, the cause of finalization of pregnancy was PE.
Ethical Considerations
El Bosque University Ethical Committee agreed with this study, so the written informed consent was signed by every pregnant woman who participated in the study. In addition, the ethical principles for human research from the Helsinki Declaration and the Colombian Resolution 8430 of 1993 were considered in this study [35, 36]. The privacy of each patient was respected throughout the study.
Statistical Analysis
Comparisons of pregnancy characteristics and the biomarker between outcome groups were analyzed, conducted both in mean and median, by the Student’s t test for variables with Gaussian distribution, Mann–Whitney U test for variables without Gaussian distribution, and square Ji Pearson’s test for categorical variables (adjusted significance level p < 0.05 and p < 0.01). Shapiro–Wilk test (p > 0.05, normality) was used to determine Gaussian distribution.
The statistical software package SPSS 24.0 (IBM SPSS Statistics for Windows, Version 24.0; IBM Corp., Armonk, NY, USA) was used for all data analyses.
Results
Pregnancy-associated plasma protein-A was analyzed in 422 single pregnancies. Of these, 360 patients had a normal pregnancy (without development of impaired placentation disease), 32 (7.6%, CI95% 5.3–10.41) developed PE (PE), 14 (3.3% CI95% 1.6–5.0) developed IUGR, and 16 (3.8% CI95% 2.0–5.6) developed GH.
The mean age was 27.4 ± 6.5 years old, and most of the patients were of mixed race (96.4%); only 7 pregnant women (1.7%) were Afro-American, and 8 (1.9%) were Caucasian. Most of the pregnant women had a low-middle socioeconomic level: 46.9% of patients had a low level and 48.3% middle.
A total of 156 (37%) were nulliparous, and 266 (63%) were multiparous; from this group of multiparous women, 37.5% (n = 100) had a new couple (new paternity). A proportion of 7.1% of women smoked previously or during pregnancy. At first trimester, the mean body mass index (BMI) was 24.6 ± 4 kg/m2, and only in 7 pregnant women (1.6%), the BMI was more than 35 kg/m2. The mean MAP at first trimester was within normal limits in every patient (80 ± 7 mmHg).
The percentage of pregnant women who had history of any chronic disease was 3.3%: 8 (1.9%) had chronic hypertension, 3 (0.7%) were diabetic, 1 (0.2%) had systematic erythematous lupus, 1 (0.2%) had antiphospholipid antibody syndrome, and 2 (0.5%) had chronic nephropathy. Regarding previous history of PE and IUGR, of these 266 multiparous, 31 (11.7%) had PE in previous pregnancies, and 15 (5.6%) had had a baby with IUGR previously. A total of 77 women (18.2%) had family history of PE (mother and/or sisters), and 29 (6.2%) had family history of IUGR.
The mean gestational age and the mean neonatal birth weight were 38 weeks plus 3 days ± 1 week plus 5 days and 3012.3 ± 464.9 g, respectively.
Table 1 shows the comparison of sociodemographic and clinical data between groups according to outcomes. No statistical differences were found (p > 0.05).
Table 1.
Demographic characteristics of outcome groups
| Characteristics | Statistics | No impaired placentation disease | Preeclampsia | Intrauterine growth restriction | Gestational hypertension |
|---|---|---|---|---|---|
| (n = 360) | (n = 32) | (n = 16) | (n = 14) | ||
| Maternal age (years) | Mean (SD) | 27.34 (6.5) | 28.13 (6.96) | 26.69 (6.36) | 27.64 (7.55) |
| Median (IQR) | 27 (23–32) | 29 (21–34) | 26 (22–32) | 27 (22–34) | |
| Racial origin | Mixed, n (%) | 345 (95.8) | 32 (100) | 16 (100) | 14 (100) |
| Socioeconomic level | High, n (%) | 18 (5) | 2 (6.3) | 0 (0) | 0 (0) |
| Low, n (%) | 170 (47.2) | 12 (37.5) | 8 (50) | 8 (57.1) | |
| Middle, n (%) | 172 (47.8) | 18 (56.3) | 8 (50) | 6 (42.9) | |
| Smoking habit | Yes, n (%) | 26 (7.2) | 2 (6.3) | 2 (12.5) | 0 (0) |
| Chronic diseases | Yes, n (%) | 9 (2.5) | 4 (12.5) | 1 (6.3) | 0 (0) |
| Chronic Hypertension | Yes, n (%) | 6 (1.6) | 2 (6.2) | 2 (12.5) | - |
| Familiar history of PE | Yes, n (%) | 62 (17.2) | 11 (34.4) | 1(6.3) | 3 (21.4) |
| Personal history of PE | Yes, n (%) | 22 (6.1) | 5 (15.6) | 1 (6.3) | 3 (21.4) |
| Familiar history IUGR | Yes, n (%) | 22 (6.1) | 6 (18.8) | 1 (6.3) | 0 (0) |
| Personal history of IUGR | Yes, n (%) | 10 (2.8) | 1 (3.1) | 2 (12.5) | 2 (14.3) |
| Parity | First pregnancy, n (%) | 133 (36.9) | 12 (37.5) | 8 (50) | 3 (21.4) |
| Multiparous, new paternity, n (%) | 84 (23.3) | 9 (28.1) | 4 (25) | 3 (21.4) | |
| Multiparous, no new paternity, n (%) | 143 (39.7) | 11 (34.4) | 4 (25) | 8 (57.1) | |
| Body mass index (BMI) | Mean (SD) | 24.59 (3.93) | 24.95 (4.96) | 23.02 (2,75) | 27.56 (5.16) |
| Median (IQR) | 24.1 (21.8–26.6) | 23.69 (21.7–28.0) | 22.9 (21–24) | 26.9 (23.3–31.7) | |
| Gestational age (weeks) at the moment of inclusion | Mean (SD) | 12.75 (0.69 | 12.67 (0.67) | 12.74 (0.77) | 13.03 (0.48) |
| Median (IQR) | 12.6 (12.2–13.3) | 12.6 (12.4–13.2) | 12.9 (12.2–13.3) | 13.1 (12.6–13.4) | |
| Fetal CRL (mm) at the moment of inclusion | Mean (SD) | 66.23 (9.46) | 65.02 (8.46) | 65.49 (9.60) | 69.99 (7.86) |
| Median (IQR) | 65.6 (59–73.6) | 66 (60.3–71.4) | 66.1 (58.2–70.5) | 69.9 (65–78) | |
| Gestational age (weeks) at delivery | Mean (SD) | 38.70 (1.54) | 36.79 (2.45) | 36.56 (2.49) | 38.36 (1.10) |
| Median (IQR) | 39.00 (38.00–39.06) | 37.00(36.0–39.0) | 37.35 (36.42–37.57) | 38.45 (37.5–39.02) | |
| Mean neonatal birth weight (g) | Mean (SD) | 3031.83 (423.98) | 2619.97(689.47) | 2073.76 (407.87) | 3024.83 (366.71) |
| Median (IQR) | 3070.00 (2830–3320) | 2860.0 (2395–3187.5) | 2242.5 (2056.25–2391.25) | 3085.0 (2730–3251.25) |
PE, preeclampsia; IUGR, intrauterine growth restriction; CRL, crown rump length
The distribution of levels of PAPP-A at first trimester in the studied patients are described in Fig. 1 and Table 2. Comparisons between groups were done. Firstly, (a) no impaired placentation outcome in contrast with those with a pathological outcome such as PE, IUGR, and pregnant women with GH. A second analysis (b) compared the pregnant women whose babies were diagnosed with IUGR with patients who developed PE or GH; and finally, a third analysis (c) was done to compare patients with GH with those pregnant women who developed PE. The results of the IUGR and GH groups (0.83 IQR: 0.59–0.98; 0.51 IQR: 0.34–1.45, respectively) were significantly lower compared to the PE group (1.06 IQR: 0.66–1.52); p = 0.041; p = 0.042, respectively. No other significant differences were found.
Fig. 1.
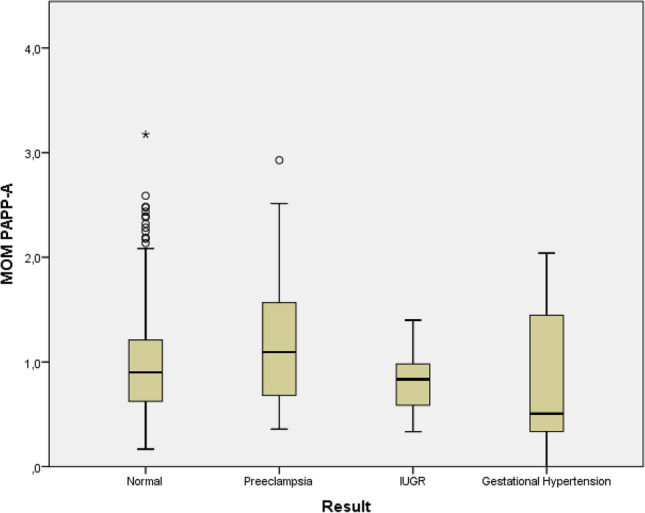
Box-and-whisker plots of multiple of median values (MOM) of PAPP-A. In the normal (no impared placentation disease), preeclampsia, IUGR (intrauterine growth restriction) and gestational hypertension groups
Table 2.
PAPP-A of outcome groups
| Characteristics | Statistics | No impaired placentation disease | Preeclampsia | Intrauterine growth restriction | Gestational hypertension |
|---|---|---|---|---|---|
| (n = 360) | (n = 32) | (n = 16) | (n = 14) | ||
| MoM PAPP-A | Mean (SD) | 1.00 (0.50) | 1.15 (0.63) | 0.79 (0.29) | 0.79 (0.65) |
| Median (IQR) | 0.90 (0.63–1.21) | 1.06 (0.66–1.52) (b*)(c*) | 0.83 (0.59–0.98) | 0.51 (0.34–1.45) |
(a) Comparison with patients without impaired placentation disease. (b) Comparison with patients with development of intrauterine growth restriction. (c) Comparison with patients with gestational hypertension. There was no statistical significance with (a). *Significant at p < 0.05. **Significant at p < 0.01. SD, standard deviation; IQR, interquartile range
Different results were found when the PE group was divided into two groups, depending on presentation of the disease after o before the 34th week. Only the patients of the subgroup of pregnant women who developed early-onset PE (1.11 IQR 1.08–1.18) presented significant differences from the IUGR group (0.83 IQR: 0.59–0.98); p = 0.002 (Table 3).
Table 3.
PAPP-A of outcome groups insolated PE into early-onset PE and late-onset PE ≥ 34w
| Characteristics | Statistics | No impaired placentation disease | Early-onset PE, < 34w | Late-onset PE, ≥ 34w | Intrauterine growth restriction | Gestational hypertension |
|---|---|---|---|---|---|---|
| (n = 360) | (n = 5) | (n = 27) | (n = 16) | (n = 14) | ||
| MoM PAPP-A | Mean (SD) | 1.00 (0.50) | 1.14 (0.11) | 1.15 (0.68) | 0.79 (0.29) | 0.79 (0.65) |
| Median (IQR) | 0.90 (0.63–1.21) | 1.11 (1.08–1.18) (b**) | 0.92 (0.60–1.58) | 0.83 (0.59–0.98) | 0.51 (0.34–1.45) |
(a) Comparison with normal, (b) comparison with intrauterine growth restriction, (c) comparison with gestational hypertension, (d) comparison with PE≥34w. There was no statistical significance with (a), (c), and (d). *Significant at p < 0.05. **Significant at p < 0.01. PE, preeclampsia; SD, standard deviation; IQR, interquartile range
Dividing the PE group into two subgroups depending on the development of the disease before or after 37th week, statistical significance was found comparing pregnant women who developed term PE (1.19 IQR: 0.66–1.58) with the IUGR group; p = 0.045 (Table 4). Two patients in the group of PE developed also IUGR. The value of PAPP-A in the group of PE plus IUGR was very low compared with other groups (mean 0.6 (SD 0.6); median 0.6 (IQR 0.5–0.6)).
Table 4.
PAPP-A of outcome groups, insolated into preterm PE and late PE
| Characteristics | Statistics | No impaired placentation disease | Preterm preeclampsia, < 37w | Late preeclampsia, ≥ 37w | Intrauterine growth restriction | Gestational hypertension |
|---|---|---|---|---|---|---|
| (n = 360) | (n = 19) | (n = 13) | (n = 16) | (n = 14) | ||
| MoM PAPP-A | Mean (SD) | 1.00 (0.50) | 1.07 (0.60) | 1.27 (0.68) | 0.79 (0.29) | 0.79 (0.65) |
| Median (IQR) | 0.90 (0.63–1.21) | 1.04 (0.65–1.26) | 1.19 (0.66–1.58) (b*) | 0.83 (0.59–0.98) | 0.51 (0.34–1.45) |
(a) Comparison with normal, (b) comparison with intrauterine growth restriction, (c) comparison with gestational hypertension, (d) comparison with PE ≥ 37w. There was no statistical significance with (a), (c), and (d). *Significant at p < 0.05. **Significant at p < 0.01. SD, standard deviation; IQR, interquartile range
Discussion
Impaired placental disease is the new name that is currently used to call those pregnancy illnesses which have inappropriate placentation. For decades ago, clinical trials have been able to confirm that preeclampsia (PE), especially early-onset PE, and intrauterine growth restriction (IUGR) have a similar physiopathologic origin: the impaired placentation [1–3].
Trials about this group of obstetric diseases have focused on physiopathology, and there is enough evidence that one of the most important biomarkers for the prediction of PE and IUGR is the PAPP-A [37]; moreover, the screening combined test from the Fetal Medicine Foundation (FMF) group, which uses this gestational protein, is, nowadays, the most accurate methods of prognostication of the pregnancy at first trimester [2, 22, 24, 26, 27, 30, 38–40]. This model is also used to predict other hypertensive disorders of pregnancy, such as GH [14]. The predictive ability of this protein is also evaluated at the third trimester, but its efficiency decreases out of the first trimester of pregnancy [41].
Nearly one quarter of maternal deaths in Latin America are associated with hypertensive disorders during pregnancy, and Colombia is one of the countries with the highest incidence of PE [13]. This is the most important reason for researching this topic in pregnant Colombian women and investigating which would be the most accurate screening test to counsel preventive treatment in those women who need it [4, 5, 7, 12].
The results of this study, done in a high prevalence of preeclampsia population, also show the low level of PAPP-A in the groups which developed GH or IUGR compared to the group of patients without any impaired placentation outcomes. However, it does not occur when the PE group is analyzed. These results of pregnant Colombian women, curiously, demonstrate that the level of PAPP-A at first trimester in the sample of women who developed PE was higher than that when they only developed GH or IUGR. Most of the trials have focused on the prediction of PE and current models establish the use of PAPP-A to screen PE, finding lower levels of PAPP-A in patients who develop PE or IUGR [17, 18, 20, 22, 42]. However, some studies in other populations report similar results to this work. Saruhan et al. investigated if PAPP-A levels at first trimester were associated with adverse pregnancy outcomes, concluding that PAPP-A was not useful to prognosticate adverse outcomes [42]. Also, Ragnhild et al. reported higher PAPP-A values in the group of patients who had severe PE than those in the group of patients who only developed GH [43]. This is similar to the result of this study when the PE group is separated into patient with developing of PE before or after 34 weeks of pregnancy. Nevertheless, it is not the same when the subgroups of PE are separated using the limit of 37 weeks of pregnancy. It seems that the results of our study could be consistent with the work of Ragnhild et al.
In contrast, the patients who developed GH presented very low levels of PAPP-A. Most of the previous research had not found results like that [19, 21, 44, 45] and reported that PAPP-A is lowest in patients that developed early-onset PE and lower than that in patients who only had gestational hypertension. However, some studies found lower values of PAPP-A in patients with GH than those in the group of patients with PE like this work in Colombian pregnant women [38].
Levels of PAPP-A in this study are only similar to that in other international works about this biomarker in the outcome group of IUGR [23, 28, 37]. Surprisingly, the PAPP-A value in the group of patients who did not develop any impaired placentation disease was lower than in other studies [19, 21, 28, 43–46].
This work has used an unselected population with a high proportion of comorbidities (3.3%) and a high incidence of hypertensive disorders of pregnancy (10.9%), mainly PE (7.6%). Some ethnic origins are more prone to suffering hypertensive disorders during pregnancy, but because of that, this study employed data of MoMs to compare outcome groups, in order to standardize the population’s characteristics. Therefore, the results of PAPP-A in the PE group are unexpected, although other research groups have reported results that concluded that PAPP-A losses efficacy when it is used in populations with high comorbidity [43].
The most remarkable result of our work is the data of PAPP-A in Colombian women who developed PE, not only because it seems that there is no statistical significance compared to women who did not develop any impaired placentation disease but because the mean value of PAPP-A in these patients are upper than the mean value of patients without adverse outcomes. This could not be sustained by any of the theories about how the impair trophoblast invasion process is related to a low serum PAPP-A as in other works, where lower levels than the fifth percentile are reported to increase the risk of developing impaired placentation disease: intrauterine growth restriction (adjusted odds ratio, 2.9; 95% confidence interval [CI], 2.0–4.1) and preeclampsia (adjusted odds ratio, 2.3; 95% CI, 1.6–3.3) [17]. The findings of many studies have confirmed that the serum concentrations of PAPP-A are decreased at 11 to 14 weeks of gestation in women who develop hypertensive disorders during pregnancy, and it is more evident in those pregnant women who have early-onset PE. This study also separated women with early-onset PE and late-onset PE to review the data of the PE group, and this subanalysis could confirm that the group of early-onset PE presents the highest data of PAPP-A on this classification, although the group that presented the greatest level of PAPP-A was the term PE group. In addition, few previous papers describe the levels of PAPP-A higher than 1 MoMs, as this work reported [43]. These findings create a great doubt about the normal ranges of this biomarker in populations where PE has a higher incidence. Moreover, it raises the question if it is correct to use the normal limit of the range of PAPP-A basis on studies in European population. Further publications of the completed research will be written to be able to define why serum PAPP-A is so high in the PE group of Colombian pregnant women.
One of the most important hypotheses about the reason of this finding is related to treatment with ASA since first trimester of pregnancy. The complications of the hypertensive disorders of pregnancy can be reduced with an adequate intake of ASA in the high-risk population [24, 47]. This ongoing research study is a double-blind, and each patient was treated by their obstetrician without any commentary or recommendation before the screening realized at first trimester of pregnancy by the investigator staff. Currently, most of the patients who have high risk of PE uses ASA during pregnancy, and it is not ethical to avoid this intake. Because of that, there is the hypothesis that women who have high risk of PE perhaps did not develop it because they took ASA during pregnancy. This treatment could have changed the outcome findings, so the conclusions must be very careful when outcomes are measured; perhaps final outcomes had been more serious if this prophylactic treatment would not have ordered at the beginning of the pregnancy [48].
The main limitations of this study were that we incurred a random error because of the small sample size. Further, a selection bias was observed, given that the patients included in this study corresponded to a specific population of Bogotá. However, this work presents first findings of an ambitious research in a population where chronic illnesses related to hypertension diseases during pregnancy have a high prevalence. To study the behavior of these diseases in this type of population has huge importance because the improvement of screening and prevention strategies could decrease maternal and perinatal morbidity and mortality worldwide.
In conclusion, the results of this study demonstrate that the levels of PAPP-A at first trimester in the sample of women who developed GH and IUGR were low. Remarkably, the GH group had the lowest PAPP-A values in this sample of pregnant women. PAPP-A levels in the patients who developed PE were remarkably higher than those in most of works reported before, and this could have clinical implications that need clarification to improve the screening of hypertensive diseases during pregnancy.
Acknowledgements
We thank the Universidad El Bosque, Ecodiagnóstico El Bosque SAS, Fundación Salud Bosque—Clínica El Bosque and Subred Sur Occidente ESE USS Kennedy for their participation in the study.
Author Contribution
All authors contributed to the study’s conception and design. Obtaining financing was achieved by Ximena Carolina Romero. Data acquisition was in charge of Ximena Carolina Romero Sara Rincón and Montserrat Uriel. Material preparation and statistical analysis were performed by Edgar Antonio Ibáñez and Nydia Alexandra Rojas. The first draft of the manuscript was written by Montserrat Uriel, and all authors commented on the previous versions of the manuscript. Sara Rincón checked the English translation. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Colombia Consortium This work was supported by the Universidad El Bosque (grant number: PCI-2013–472).
Data availability
All data is safeguarded by the El Bosque Research Group of Maternal Fetal Medicine and Gynecology, Universidad El Bosque, Bogotá, Colombia.
Code Availability (Software Application or Custom Code)
Not applicable.
Declarations
Ethical Approval
All procedures performed in studies involving human participants were under the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Universidad El Bosque (No PCI-2013–472).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All individual participants included in the study signed informed consent regarding publishing their data.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Montserrat Uriel, Email: urielmonserrat@gmail.com, Email: muriel@unbosque.edu.co.
Ximena Carolina Romero Infante, Email: romeroximena@unbosque.edu.co.
References
- 1.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 2.Panaitescu AM, Akolekar R, Kametas N, Syngelaki A, Nicolaides KH. Impaired placentation in women with chronic hypertension who develop pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50(4):496–500. doi: 10.1002/uog.17517. [DOI] [PubMed] [Google Scholar]
- 3.Figueras F, Gratacos E, Rial M, Gull I, Krofta L, Lubusky M, et al. Revealed versus concealed criteria for placental insufficiency in an unselected obstetric population in late pregnancy (RATIO37): randomised controlled trial study protocol. BMJ Open. 2017;7(6):e014835. doi: 10.1136/bmjopen-2016-014835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(Suppl 1):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 5.Ray JG, Wanigaratne S, Park AL, Bartsch E, Dzakpasu S, Urquia ML. Preterm preeclampsia in relation to country of birth. J Perinatol. 2016;36(9):718–722. doi: 10.1038/jp.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40(3):213–220. doi: 10.1038/hr.2016.126. [DOI] [PubMed] [Google Scholar]
- 7.Romero Infante XC, Uriel M, Porras Ramírez A, Rincón FS. Comparison of preeclampsia and fetal growth restriction screenings at first trimester in a high-risk population. J Obstet Gynaecol Res. 2021;47(2):765–773. doi: 10.1111/jog.14605. [DOI] [PubMed] [Google Scholar]
- 8.Romero Infante XC, Uriel M, Rincón Franco S, Ibáñez Pinilla EA, Rojas NA. First trimester placental growth factor in maternal blood and placenta related disorders. J Matern Fetal Neonatal Med. 2021;1:1–8. doi: 10.1080/14767058.2021.1960966. [DOI] [PubMed] [Google Scholar]
- 9.Guzmán YN, Uriel M, Ramírez AP, Romero XC. Uterine artery pulsatility index as a pre-eclampsia predictor in the 3 trimesters in women with singleton pregnancies. Rev Bras Ginecol Obstet. 2021;43(12):904–910 . doi: 10.1055/s-0041-1740273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez Ardila A, Uriel M, Rico Mendoza FA, Romero Infante XC. Prevalencia de morbilidad materna extrema en unidad de cuidados intensivos de una institución prestadora de servicios de salud de tercer nivel en Bogotá D.C. Acta Colombiana de Cuidado Intensivo. 2022;22(2):81–87. 10.1016/j.acci.2021.04.003.
- 11.Magee LA, Nicolaides KH, von Dadelszen P. Preeclampsia. N Engl J Med. 2022;386(19):1817–1832. doi: 10.1056/NEJMra2109523. [DOI] [PubMed] [Google Scholar]
- 12.Herrera JA, Herrera-Medina R, Herrera-Escobar JP, Nieto-Díaz A. Reduction of maternal mortality due to preeclampsia in Colombia–an interrupted time-series analysis. Colomb Med (Cali) 2014;45(1):25–31 . doi: 10.25100/cm.v45i1.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero XC, Gutiérrez AM, Rojas NA, Ramírez A, Aldana J, Eslava M, Pérez BD, Forero CA, Uriel M, Camacho Rodríguez B. Incidence of hypertensive disorders in pregnancy and clinical demographic characteristics in pregnant women in three institutions in Bogotá, D. C., Colombia. Investig Segur Soc Salud. 2018;20(2):21–30.
- 14.Antwi E, Amoakoh-Coleman M, Vieira DL, Madhavaram S, Koram KA, Grobbee DE, Agyepong IA, Klipstein-Grobusch K. Systematic review of prediction models for gestational hypertension and preeclampsia. PLoS One. 2020;15(4):e0230955. doi: 10.1371/journal.pone.0230955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, Kihara AB, Di Renzo GC, Romero R, D'Alton M, Berghella V, Nicolaides KH, Hod M. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(Suppl 1):1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akolekar R, Minekawa R, Veduta A, Romero XC, Nicolaides KH. Maternal plasma inhibin A at 11–13 weeks of gestation in hypertensive disorders of pregnancy. Prenat Diagn. 2009;29(8):753–760. doi: 10.1002/pd.2279. [DOI] [PubMed] [Google Scholar]
- 17.Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein-A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87(4):1762–1767. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]
- 18.Staboulidou I, Galindo A, Maiz N, Karagiannis G, Nicolaides KH. First-trimester uterine artery Doppler and serum pregnancy-associated plasma protein-a in preeclampsia and chromosomal defects. Fetal Diagn Ther. 2009;25(3):336–339. doi: 10.1159/000235880. [DOI] [PubMed] [Google Scholar]
- 19.Poon LC, Akolekar R, Lachmann R, Beta J, Nicolaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010;35(6):662–670. doi: 10.1002/uog.7628. [DOI] [PubMed] [Google Scholar]
- 20.Poon LC, Maiz N, Valencia C, Plasencia W, Nicolaides KH. First-trimester maternal serum pregnancy-associated plasma protein-A and pre-eclampsia. Ultrasound Obstet Gynecol. 2009;33(1):23–33. doi: 10.1002/uog.6280. [DOI] [PubMed] [Google Scholar]
- 21.Poon LC, Stratieva V, Piras S, Piri S, Nicolaides KH. Hypertensive disorders in pregnancy: combined screening by uterine artery Doppler, blood pressure and serum PAPP-A at 11–13 weeks. Prenat Diagn. 2010;30(3):216–223. doi: 10.1002/pd.2440. [DOI] [PubMed] [Google Scholar]
- 22.Poon LC, Syngelaki A, Akolekar R, Lai J, Nicolaides KH. Combined screening for preeclampsia and small for gestational age at 11–13 weeks. Fetal Diagn Ther. 2013;33(1):16–27. doi: 10.1159/000341712. [DOI] [PubMed] [Google Scholar]
- 23.Spencer K, Cowans NJ, Avgidou K, Molina F, Nicolaides KH. First-trimester biochemical markers of aneuploidy and the prediction of small-for-gestational age fetuses. Ultrasound Obstet Gynecol. 2008;31(1):15–19. doi: 10.1002/uog.5165. [DOI] [PubMed] [Google Scholar]
- 24.O'Gorman N, Wright D, Rolnik DL, Nicolaides KH, Poon LC. Study protocol for the randomised controlled trial: combined multimarker screening and randomised patient treatment with ASpirin for evidence-based PREeclampsia prevention (ASPRE) BMJ Open. 2016;6(6):e011801. doi: 10.1136/bmjopen-2016-011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon LC, Karagiannis G, Leal A, Romero XC, Nicolaides KH. Hypertensive disorders in pregnancy: screening by uterine artery Doppler imaging and blood pressure at 11–13 weeks. Ultrasound Obstet Gynecol. 2009;34(5):497–502. doi: 10.1002/uog.7439. [DOI] [PubMed] [Google Scholar]
- 26.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33(1):8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 27.Tan MY, Koutoulas L, Wright D, Nicolaides KH, Poon LCY. Protocol for the prospective validation study: 'Screening programme for pre-eclampsia' (SPREE) Ultrasound Obstet Gynecol. 2017;50(2):175–179. doi: 10.1002/uog.17467. [DOI] [PubMed] [Google Scholar]
- 28.Spencer K, Yu CK, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;25(10):949–953. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 29.Wright D, Gallo DM, Gil Pugliese S, Casanova C, Nicolaides KH. Contingent screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2016;47(5):554–559. doi: 10.1002/uog.15807. [DOI] [PubMed] [Google Scholar]
- 30.O'Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, Wright A, et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks' gestation. Ultrasound Obstet Gynecol. 2017;49(6):751–755. doi: 10.1002/uog.17399. [DOI] [PubMed] [Google Scholar]
- 31.Hypertension in pregnancy Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 32.Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol. 2011;204(4):288–300. doi: 10.1016/j.ajog.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 34.Martins JG, Biggio JR, Abuhamad A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012) Am J Obstet Gynecol. 2020;223(4):B2–B17. doi: 10.1016/j.ajog.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 35.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 36.Resolución 8430 de 1993. Bogotá D.C. (Colombia) República de Colombia Ministerio de Salud. 1993. https://minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/RESOLUCION-8430-DE-1993.pdf.
- 37.Hoseini MS, Sheibani S, Sheikhvatan M. The evaluating of pregnancy-associated plasma protein-A with the likelihood of small for gestational age. Obstet Gynecol Sci. 2020;63(3):225–230. doi: 10.5468/ogs.2020.63.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Audibert F, Boucoiran I, An N, Aleksandrov N, Delvin E, Bujold E, Rey E. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am J Obstet Gynecol. 2010;203(4):383.e1–8. doi: 10.1016/j.ajog.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51(6):743–750. doi: 10.1002/uog.19039. [DOI] [PubMed] [Google Scholar]
- 40.O'Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M, et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks' gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49(6):756–760. doi: 10.1002/uog.17455. [DOI] [PubMed] [Google Scholar]
- 41.Birdir C, Droste L, Fox L, Frank M, Fryze J, Enekwe A, et al. Predictive value of sFlt-1, PlGF, sFlt-1/PlGF ratio and PAPP-A for late-onset preeclampsia and IUGR between 32 and 37 weeks of pregnancy. Pregnancy Hypertens. 2018;12:124–128. doi: 10.1016/j.preghy.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Saruhan Z, Ozekinci M, Simsek M, Mendilcioglu I. Association of first trimester low PAPP-A levels with adverse pregnancy outcomes. Clin Exp Obstet Gynecol. 2012;39(2):225–228. [PubMed] [Google Scholar]
- 43.Skråstad RB, Hov GG, Blaas HG, Romundstad PR, Salvesen KÅ. A prospective study of screening for hypertensive disorders of pregnancy at 11–13 weeks in a Scandinavian population. Acta Obstet Gynecol Scand. 2014;93(12):1238–1247. doi: 10.1111/aogs.12479. [DOI] [PubMed] [Google Scholar]
- 44.Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53(5):812–818. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 45.Park FJ, Leung CH, Poon LC, Williams PF, Rothwell SJ, Hyett JA. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. Aust N Z J Obstet Gynaecol. 2013;53(6):532–539. doi: 10.1111/ajo.12126. [DOI] [PubMed] [Google Scholar]
- 46.Sonek J, Krantz D, Carmichael J, Downing C, Jessup K, Haidar Z, et al. First-trimester screening for early and late preeclampsia using maternal characteristics, biomarkers, and estimated placental volume. Am J Obstet Gynecol. 2018;218(1):126.e1–126.e13. doi: 10.1016/j.ajog.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Rolnik DL, Wright D, Poon LCY, Syngelaki A, O'Gorman N, de Paco MC, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50(4):492–495. doi: 10.1002/uog.18816. [DOI] [PubMed] [Google Scholar]
- 48.Vallejo GM, Uriel M, Porras-Ramírez A, Romero XC. Could aspirin treatment modify the assessment of the uterine arteries? Rev Bras Ginecol Obstet. 2022;44(3):231–237. doi: 10.1055/s-0042-1742411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is safeguarded by the El Bosque Research Group of Maternal Fetal Medicine and Gynecology, Universidad El Bosque, Bogotá, Colombia.
Not applicable.


