Abstract
Using odors to find food and mates is one of the most ancient and highly conserved behaviors. Arthropods from flies to moths to crabs use broadly similar strategies to navigate toward odor sources—such as integrating flow information with odor information, comparing odor concentration across sensors, and integrating odor information over time. Because arthropods share many homologous brain structures—antennal lobes for processing olfactory information, mechanosensors for processing flow, mushroom bodies (or hemi-ellipsoid bodies) for associative learning, and central complexes for navigation, it is likely that these closely related behaviors are mediated by conserved neural circuits. However, differences in the types of odors they seek, the physics of odor dispersal, and the physics of locomotion in water, air, and on substrates mean that these circuits must have adapted to generate a wide diversity of odor-seeking behaviors. In this review, we discuss common strategies and specializations observed in olfactory navigation behavior across arthropods, and review our current knowledge about the neural circuits subserving this behavior. We propose that a comparative study of arthropod nervous systems may provide insight into how a set of basic circuit structures has diversified to generate behavior adapted to different environments.
Keywords: Olfaction, Navigation, Arthropod, Insect, Neural circuits
Introduction
Odor is a fundamental signal used to locate resources across the animal kingdom. Food sources, be they flowers, fruits, decaying corpses, or live humans, emit chemical signatures that can be transported long distances on wind or water currents and tracked back to their sources to find food. Like food, hospitable sites for egg laying also release chemical cues useful for localization. Finally, many animals release or deposit specific chemicals—pheromones—to enable conspecifics to find important locations in the environment. These include both volatile pheromones released to attract mates, and chemical trails that can be followed by conspecifics to a previously located food source.
Arthropods—whether they’re ants at a picnic or mosquitoes on a summer evening—are famously good at tracking odors. Arthropoda is an enormous and varied phylum, with members found across even the most inhospitable environments. Behavioral studies across arthropod species have highlighted both common and divergent strategies in odor-seeking behavior. The arthropod brain has a highly conserved structure, with dedicated regions for processing odor and flow stimuli, learning temporal associations between scents and food rewards, and representing navigational variables to guide locomotion. However, the nature of the odors that arthropod species encounter and seek, the structure of the odor signals they follow, and the physical constraints of moving are highly variable across ecosystems, driving adaptive changes in sensation and locomotion. In this review, we highlight common features of olfactory navigation behavior that have been observed across arthropod species, and review current knowledge about the neural circuit structures that likely support these behaviors. We further describe key differences in behavior across species and speculate on possible changes in neural circuit structure and function that might underlie them. We argue that a comparative study of olfactory navigation behavior and circuitry across arthropods is likely to yield important insight into how nervous systems have diversified to adapt to different environments, and describe tools and resources that will be required to pursue this research program.
The complexity of natural odor signals
Odor signals are highly dependent on their environments and differ in both their chemistry and physical structure. One of the largest differences occurs between odor signals in air versus water: volatility determines if a molecule will become gaseous and be dispersed in air, while solubility determines if a molecule may be dispersed in water. Terrestrial plant odors, which are appetitive for many insects, are often molecules such as monoterpenes, aliphatic acids, aromatic alcohols and esters, all of which are relatively insoluble (Raguso 2008; Shields and Hildebrand 2001). Similarly, moth pheromones are generally insoluble fatty alcohols and polyunsaturated hydrocarbons (Zhang and Löfstedt 2015). Finally, CO2, a common insect cue released by prey, fermentation, and vegetation, is a volatile gas. In contrast, appetitive odorants for crustaceans are generally molecules like dissolved free amino acids, amines, nucleotides, and peptides, which are readily soluble in water, but not volatile (Derby and Thiel 2014).
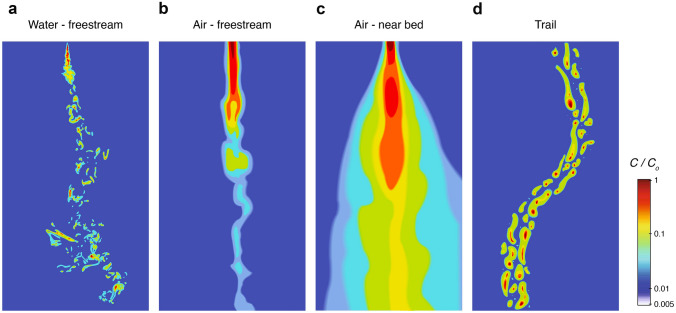
The physical structure of odor signals also differs between air and water (Fig. 1). Odors that disperse into the environment form structured plumes, which are shaped by both advective transport (i.e., odor motion via flow) and diffusive transport (i.e., odor motion via molecular diffusion, Crimaldi and Koseff 2001; Crimaldi et al. 2002; Connor et al. 2018; Celani et al. 2014). This creates structural differences between plumes in water and air. In water, advection dominates, resulting in filamentous plumes with fairly high odorant concentrations within each filament, even distant from the source (Fig. 1a). By contrast, plumes in air are subject to more diffusive transport, resulting in odor plumes with a more graded structure (Fig. 1b). In both cases, plume structure is shaped by turbulence, which varies with distance from a substrate, or “boundary layer” (Crimaldi et al. 2002). In the layer closest to the surface, flow is slow and odors form gradients that vary minimally over time (Fig. 1c). In contrast, in free stream conditions, odor forms temporally intermittent plumes with more filamentous structure. In a viscous substrate, odor does not form a plume but rather a smoother gradient (Louis et al. 2008). This generates clear differences in the sensory landscapes experienced by organisms moving through fluids (i.e., swimming or flying) compared to those moving on a substrate or within a medium such as larvae.
Fig. 1.
Odor dispersal is driven by features of the environment. a Odors in water form filamentous plumes with packets of high odor concentration found downstream of the plume. b In air, increased diffusion generates odor plumes with a graded structure. c Plumes along substrates, close to a boundary layer, form orderly gradients. d Odor trails are deposited on a substrate and can be broken up by weathering. All images depict instantaneous odor structure. Colors represent normalized concentration as a fraction of source concentration.
(Adapted from Weissburg 2000, Webster and Weissburg 2009, Connor et al. 2018, Draft et al. 2018)
Features of the landscape can also shape the spatial and temporal structure of odor plumes. As fluid moves over obstructions, it sheds eddies, which can dramatically impact odor dispersal—for example, gaps between odor filament encounters are much longer in forests compared to fields, allowing plumes to meander over greater distances in fields (Murlis et al. 2000). Eddy formation is also a function of the speed of the fluid, and as wind speeds and turbulence vary with time of day and position within a forest canopy, odor plumes become wider-ranging depending on time of day and canopy location (Thistle et al. 2004; DePasquale et al. 2022). The size of eddies is also driven by the viscosity of the fluid, meaning that eddies in air are much larger than those in water (0.1–1 mm in aquatic habitats, 1–10 mm in air).
In contrast to air or water-borne plumes, chemical trails are deposited directly on a substrate (Draft et al. 2018; Jinn et al. 2020) and typically do not fluctuate due to turbulence (Fig. 1d). Nevertheless, such trails can be disrupted by wind or water currents, by odorant evaporation, or by the movement of other animals (Jinn et al. 2020). Trails are often narrow, meaning the animal will experience large odor concentration changes as it moves and searches along the trail. Thus, a need to process dynamically changing odor stimuli is likely common across olfactory environments, although the precise dynamics, and their relationship to animal movement, will be specific to each type of “odor landscape” (Crimaldi et al. 2022).
Diverse strategies for determining odor source direction and location
A fundamental challenge in olfactory navigation is that the direction of an odor source is often not clear from instantaneous measurements of odor concentration. As described above, odor signals tend to be highly dynamic and variable in both space and time, meaning that navigators often must integrate instantaneous measurements of odor, or rely on non-olfactory cues, to make reasonable inferences about odor source location. Several strategies such as flow taxis, bilateral sensory comparisons, and integration of measurements over time, appear across many species to facilitate such inference (Fig. 2). Even in environments where odors form orderly gradients, navigators such as larval Drosophila are known to employ temporal integration (Gershow et al. 2012; Schulze et al. 2015; Gomez-Marin et al. 2011; Gepner et al. 2015) as well as spatial active search strategies such as head-casting (Gomez-Marin et al 2011) to compensate for shallow or noisy signals (Louis et al. 2008; Gomez-Marin et al 2011).
Fig. 2.
Animals use diverse strategies for odor source localization. a Flow taxis is a common strategy for navigating turbulent plumes, as flow direction represents a more reliable signal of source direction than local concentration. b Spatial comparisons across antennae facilitate the detection of odor gradients and odor motion based on concentration and timing differences. c Integration of plume dynamics over time can be used to infer odor source location based on reliable plume statistics. d Many of these strategies imply the use of spatial memory to integrate sensory observations with movement of the antennae or body. For example, an ant using measurements over successive antennal sweeps to follow an odor trail must integrate local measurements of odor with knowledge of antennal motion to identify trail direction. Thus, a variety of cues, requiring different levels of computation, play a role in localizing an odor source
A widespread strategy for finding the source direction in more turbulent environments is to measure the flow direction (Fig. 2a). As odors are typically transported by wind or water currents, average flow direction represents a more reliable signal of odor source location than instantaneous concentration. Flow taxis has been observed in moths (Kennedy and Marsh 1974), mosquitos (Dekker and Carde 2011), flying and walking Drosophila (Budick and Dickinson 2006; Alvarez-Salvado et al. 2018; van Breugel and Dickinson 2014), cockroaches (Willis and Avondet 2005), tsetse flies (Gibson et al 1991), blue crabs (Zimmer-Faust et al. 1995; Page et al. 2011a, 2011b), crayfish (Kozlowski et al. 2003), and lobsters (Moore et al. 1991), among others. Animals standing on a substrate can estimate the flow direction from mechanosensory deflections of their antennae (Alvarez-Salvado et al. 2018; Suver et al. 2019), while free-flying or swimming animals must combine optic flow with mechanosensory information to estimate the direction of ambient wind or water-flow (Kennedy 1940; Rutkowski et al. 2011; van Breugel and Dickinson 2014; van Breugel et al. 2022). This is because in flight and swimming, only changes in flow can be detected through mechanosensation; steady-state flow instead transports the animal itself, resulting in an optic flow signal displaced from the animal’s intended direction of movement (Kennedy 1940; Rutkowski et al. 2011; van Breugel et al. 2022). Flow direction can be measured instantaneously, or can be integrated over time and stored to obtain a more reliable estimate of a noisy variable (Willis and Avondet 2005; Grunbaum and Willis 2015).
Comparisons across sensors are another common strategy for determining odor source direction (Fig. 2b). Walking Bombyx mori moths use antennal comparison of odor timing and intensity to set heading (Takasaki et al. 2012), as do crayfish (Kraus-Epley and Moore 2002) and cockroaches (Bell and Tobin 1981, 1982). Blue crabs have been shown to use comparisons across both leg and antennal chemosensors to navigate (Keller et al. 2003; Page et al. 2011a, b). Although fruit flies have very closely spaced antennae, recent work has shown that flies can use cross-antennal comparisons to locate a male producing the pheromone cVA when within 5 mm of the source (Taisz et al. 2022), and that bilateral comparisons contribute to food odor-localization in flies, though they are not required (Duistermars et al. 2009; Wasserman et al. 2012; Louis et al. 2008). A recent study has suggested that odor motion may also be extracted through bilateral sensor comparison, and serve as a cue for predicting the centerline of a plume (Kadakia et al. 2022). Cross-sensor comparisons may also occur along the length of an antenna, such as in the American cockroach (Periplaneta Americana). In this species, central neurons exhibit spatial tuning for locations along the antenna (Nishino et al. 2018; Paoli et al. 2020), and the total length of antennae is a greater determinant of successful odor source tracking than the presence of bilateral sensors (Lockey and Willis 2015). Finally, active movements of the antennae or legs can provide additional directional or spatial information, but require the animal to keep track of its movements to interpret the resulting odor signals. For example, ants tracking a chemical trail will sweep their antennae across the trail to determine which direction to go (Draft et al. 2018), and larvae will sweep their heads from side to side, ultimately selecting the direction with the largest concentration gradient (Gomez-Marin et al. 2011). Together these studies point to a range of different computations involving comparisons across sensors on different parts of the body, as well as computations that require integration and storage of sensor position information with odor information.
Although the precise dynamics of odor plumes are chaotic and therefore unpredictable, the statistical properties of odor plumes are reproducible and contain information that animals could use to navigate (Boie et al. 2018; Victor et al. 2019; Crimaldi et al. 2022). Odor plumes in air decrease in concentration with longitudinal distance from the plume source and become more sparse with lateral distance (Crimaldi et al. 2002; Connor et al. 2018). Odor motion preferentially occurs laterally from the plume center (Fig. 2c, Kadakia et al. 2022). Moreover, sources that are separated from one another create plumes that are decorrelated in time, allowing for separation of sources at a distance (Ackels et al. 2021). Evidence from several species suggests that rapid plume fluctuations—which are characteristic of the plume center—can promote more effective upwind navigation (Demir et al. 2020; Baker et al. 1985, 1990). Overall, the insect olfactory system appears to be adapted for high temporal resolution (Szyszka et al. 2014), facilitating the measurement of high odor encounter frequencies. Thus, temporal cues in the odor plume as well as motion signals may help navigating animals locate the plume center.
Many of the strategies described above either require or are aided by different forms of memory. For example, measuring the statistics of odor encounters requires temporal integration (Fig. 2c). Active strategies for measuring concentration gradients, such as head casting and antennal sweeping, require an animal to integrate information about the position of its body or sensors with the timing of odor encounters to extract direction information (Fig. 2d). Additionally, animals may integrate odor encounters with body motion information as they move through the world. Supporting this idea, ants can use learned olfactory cues to walk at a direction offset from the wind direction toward their nest (Steck et al. 2010), and models that incorporate spatial memory have proven effective in allowing artificial agents to navigate towards odor sources (Grunbaum and Willis 2015). Some insects such as bees form explicit spatial memories of where food sources were encountered and can navigate between them using novel routes, suggesting a form of vector memory (Le Moël et al. 2019). Together these observations point to a role for spatial memory processes in olfactory navigation, as well as the integration of sensory information with self-motion information to generate representations of the olfactory environment. Thus, different forms of memory, ranging from simple integration over time, to sensory-motor integration, to vector memory may play a role in olfactory navigation in diverse species.
Although classical work on olfactory navigation sought to isolate single cues or algorithms used to find an odor source, more recent work has emphasized the combination of multiple cues to more robustly navigate in complex environments (Alvarez Salvado et al. 2018; Jayaram et al. 2022). Experimental approaches for measuring the integration of various cues, and computational models for how these cues are integrated are likely to prove fruitful in understanding how arthropods are able to find food and mates reliably despite the complexity and ambiguity of natural odor signals. A deeper understanding of the neural circuit structures underlying navigation, as discussed below, will allow us to understand how diverse direction cues are effectively integrated for robust navigation.
Common features of odor-seeking behavior and species-specific variations
Although arthropods use a wide array of locomotor strategies, from peristaltic waves in larvae (Clark et al. 2018), to walking in adult insects (Büschges et al. 2008), to flight (Sane 2003) and swimming (Zhang et al. 2014), classic behavioral work in moths, fruit flies, mosquitoes, and crabs has identified core olfactory navigation behaviors found across many arthropods. These include ‘surging,’ in which an organism responds to an appetitive odor by navigating in a defined direction; ‘searching’, or casting behavior driven by the loss of odor; and ‘stopping’, or pausing to obtain more information about the odor source direction (Fig. 3). These high-level behavioral components are observed in many arthropods despite differences in the mode of locomotion. However, details of how these components are utilized vary across organisms.
Fig. 3.
High-level components of olfactory navigation are conserved across species. a Arthropods use a combination of surging, searching, and stopping to navigate to an odor source. b Surging consists of relatively straight, directional movements, that generally persist while contacts with odor are frequent. In different species, surging may be driven by different sensory signals (e.g., pulsed or constant plumes), and occur with different speeds due to locomotor differences. c Searching, or “casting”, follows loss of the plume and consists of increases in turning and path curvature. As with surging, the dynamics of searching vary between species—flying Manduca (left) perform the highly stereotyped casts typical of pheromone tracking in moths, while walking Drosophila (right) perform irregular searching on loss of odor. d Stopping may occur when odor contacts are infrequent, and can allow for evidence accumulation via active search (Drosophila larval head casting, left) or through observations over time (Callinectes adult, right)
One of the most common behavioral strategies arthropods employ is surging. Surging consists of movement that is typically straighter and faster than baseline locomotion, in a defined direction (Fig. 3b). In many cases, the direction of the surge is defined by flow, as discussed above (David et al. 1983; van Breugel et al. 2014; Alvarez-Salvado et al. 2018; Demir et al. 2020). In other cases, the surge direction is determined from cross-antennal comparisons (Takasaki et al. 2012; Draft et al. 2018). Additionally, while surging behaviors in most arthropods are informed by flow sensing, in larval Drosophila, who occupy a very low-flow environment, surging behavior is also seen in response to concentration gradients (Gershow et al. 2012). Surging is nearly always evoked by the presence of attractive odor, but the duration of surges and the stimulus dynamics required to evoke surging differ across species. In moths, surges are transient and pulsed pheromone is required to maintain upwind progress (Baker et al. 1985, 1990). In contrast, in both flying and walking Drosophila, sustained upwind progress occurs as long as an attractive odor (apple cider vinegar or ethanol) is present. The dynamics required to elicit upwind progress can depend on odor as well as species. In flying A. aegypti mosquitoes, CO2 will only evoke strong upwind flight when presented as a filamentous plume, while skin odors can evoke the same behavior when presented as a broad, homogenous plume (Dekker and Cardé 2011; Dekker et al. 2005). A similar difference has recently been observed in Drosophila, where vinegar produces upwind walking when presented continuously, while CO2 must be pulsed to evoke upwind movement (Zocchi et al. 2022). Thus, the same behavior may be driven by different odor dynamics across species and even odors.
Upon losing contact with odor, many arthropods exhibit some form of searching to facilitate finding the lost plume. A common feature of these behaviors is a change in the statistics of turning relative to both baseline locomotion and the straighter surges; although, as with surging, the details of these turning behaviors differ across species and locomotor modes (Fig. 3c). For example, many insects perform ‘casts’, crosswind motions with or without upwind displacement (Kennedy et al. 1974; van Bruegel et al. 2014). In flying moths, casts consist of extremely regular side to side motions in the plane parallel to the ground (Kuenen and Carde 1994; David et al. 1983). In some moth species, casting occurs even in homogenous clouds of odor (Kennedy et al. 1981), while in others, casting occurs in response to loss of the plume (Vickers and Baker 1994; Kennedy 1983; Takasaki et al. 2012). Like moths, casts in flying mosquitoes occur at stereotyped angles and velocity relative to the wind, and are initiated by loss of contact with the odor plume (Dekker and Carde 2011). Flying Drosophila exhibit crosswind casts of variable speed and duration which are stronger at higher odorant concentrations and weaken over time with successive odor encounters (Van Bruegel and Dickinson 2014; Pang et al. 2018). However, walking flies instead show a non-directional local search upon odor loss (Alvarez-Salvado et al. 2018). Walking cockroaches (P.americana) and silk moths (B.mori) also exhibit cast-like turning upon loss of odor, but these casts are much more variable in their timing and structure than those of moths (Willis and Avondet 2005; Takasaki et al. 2012). Casting behavior is also observed during trail following upon loss of the odor trail in both ants (Draft et al. 2018) and copepods (Weissburg et al. 1998) but is notably absent from the olfactory behavior of many walking crustaceans such as lobsters (Moore et al 1991), blue crabs (Page et al. 2011a, b), and crayfish (Kozlowski et al. 2003).
Stopping or pausing is an often-overlooked component of navigation. Stopping, which consists of the cessation of forward motion, has generally been considered an endpoint of navigation (i.e., abandonment of the plume on loss of odor), rather than a bona fide navigation strategy. However, evidence from walking arthropods—insect and crustacean—suggests stopping plays an integral role in evidence accumulation (Fig. 3d). Crawling Drosophila use stops to perform head casts, which actively sample the environment to correct course (Gomez-Marin et al. 2011). Ants also pause to perform additional antennal sweeps of a trail (Draft et al. 2018), suggesting that pausing is a common strategy that allows animals to obtain additional information about the likely direction of the odor source. Observations on pausing during plume tracking support this hypothesis. Blue crabs exhibit increased stop frequency as the turbulence of an odor plume increases (Weissburg and Zimmer-Faust 1994); similarly, crayfish stop with increasing frequency as odor pulse rate decreases (Kozlowski et al. 2003). Recent work in walking Drosophila has shown that stop frequency evolves dynamically over time—odor filament encounters produce a transient decrease in the rate of stopping, which eventually decays back to baseline (Demir et al. 2020). These findings suggest that across species, the statistics of stopping are driven by an organism’s ability to integrate information about its environment (Rigolli et al. 2022).
Ecological mechanisms underlying variations in olfactory navigation behavior
This survey of olfactory navigation behaviors suggests that a set of common high-level behaviors—surging, casting, and pausing—are evoked by different stimuli and stimulus dynamics across species. How might we understand these behavioral motifs as a product of the olfactory environments encountered by an animal? Weissburg (2000) proposed a unifying framework to describe how the reliability of olfactory signals in different environments might shape navigation strategies. This model proposes two factors impacting navigation strategies—a temporal integration factor, describing an animal’s capacity to compute time-averaged plume statistics, and a spatial integration factor characterizing the ability to instantaneously measure the statistics of a plume across space. These factors are influenced both by the dynamics of the environment (the width of an odor plume and the size of the smallest eddies in the plume, which are both related to Reynolds number) and by an organism’s own anatomy and behavior (e.g., span of antennae and speed of locomotion). For example, flying moths with high movement speeds and narrowly spaced antennae would have low spatial and temporal integration capacities, suggesting that wind direction may be a more salient signal of odor source than any features of the plume itself. On the other hand, walking lobsters have a low sampling rate and therefore less effective temporal integration, but much larger capacity for spatial integration due to the length of their antennae, making strategies dependent on bilateral sensing or other comparisons across space more salient.
New tools for detailed, high throughput quantification of olfactory behaviors may allow scientists to test and expand on this intriguing hypothesis. In particular, the literature reviewed above suggests that animals adopt active strategies to increase their capacity to collect and integrate information about a plume. Modeling approaches suggest that insect-like searching behaviors arise as an optimal solution to navigating a turbulent plume through a Bayesian inference process (Rigolli et al. 2022). Capitalizing on technological innovations to capture the fine details of navigation in arthropods adapted to a range of environments may reveal guiding principles for how these high-level behavioral strategies have evolved to match the statistics of odor plumes across different environments.
Neural circuits for olfactory navigation
Why are common behavioral motifs observed across organisms with highly divergent body plans, and how do species-specific variations in olfactory navigation behavior arise? Efforts to address this question will require a detailed understanding of the neural circuitry that gives rise to olfactory navigation behavior. While decades of work have investigated olfactory and navigational circuits across a range of insects (Heinze and Homberg 2008; Namiki and Kanzaki 2014; Martin et al. 2015) and crustaceans (Ache and Derby 1985), major breakthroughs have occurred in recent years due to the power of Drosophila as a model for genetic dissection of neural circuits (Guo et al. 2019). In the following sections, we discuss what is currently known about neural circuits for detecting and processing odor and flow information, synthesizing these information streams to generate navigational commands, and translating these commands into locomotor behavior (Fig. 4). Our discussion will focus on recent work using Drosophila, but include important contributions from other arthropod models. Finally, we will consider what is known about the evolution and development of these circuit structures, and describe new tools and technologies that will enable comparisons of circuit structure and function across species. We hypothesize that such a comparative approach will help reveal which features of circuit organization are essential and which vary across species.
Fig. 4.
Odor and wind processing pathways in Drosophila. a Glomeruli in the antennal lobe integrate signals from antennal olfactory sensory neurons, and pass this information to two associative centers, the mushroom body and the lateral horn, which compute learned and innate valence, respectively. A subset of mushroom body and lateral horn outputs are combined with wind information in the fan-shaped body of the central complex to generate navigational signals. These signals are passed to the premotor lateral accessory lobe and ultimately drive behavior through motor neurons in the ventral nerve cord. Other mushroom body and lateral horn outputs bypass the central complex to drive behavior through direct pathways to the LAL or other descending inputs to the ventral nerve cord. b Stretch receptive Johnston’s organ neurons (JONs) that project to the antennal mechanosensory and motor center (AMMC) respond to displacement of the antennae due to wind. This information is passed to the wedge, where inputs from the two antennae are integrated to generate a representation of wind direction. Other WED neuron carry displacement information to the LAL and then to central complex. In the fan-shaped body of the central complex, wind direction and odor value information is integrated. This information is thought to descend via the LAL to drive activity in the VNC. c Anatomically distinct circuits for odor (pink) and wind (blue) sensing converge in navigation centers (purple, detailed in Fig. 5) to drive movement toward an odor source
Olfactory circuits
Arthropod olfaction is initiated by a large set of olfactory receptor neurons (ORNs) in the antennae (Fig. 4a). ORNs express olfactory receptors that allow them to transduce odors into electrical signals (Vosshall et al. 1999, 2000; De Bruyne et al. 1999, 2001; Hallem et al. 2004; Hallem and Carlson 2006; Silbering et al. 2011). Arthropods use many receptor families as olfactory receptors, most notably the olfactory receptor (ORs) family, which are found only in insects (Vosshall et al. 2000; Hallem and Carlson 2006), and the ionotropic receptors (IRs), which are found more broadly in all protostomes, including arthropods (Croset et al. 2010; Rytz et al. 2013; Corey et al. 2013; Kozma et al. 2020a,b). Both of these receptors function as ion channels (Sato et al. 2008; Wicher et al. 2008; Benton et al. 2009), and require co-expression of a pore-forming co-receptor (orco for ORs, and IR8a or IR25a for IRs), and a more variable “tuning” receptor that confers odorant-specificity (Larsson et al. 2004; Benton et al. 2006; Abuin et al. 2011; Del Marmol and Ruta 2021). As in many sensory systems, ORNs show strong selectivity for specific odors at low concentrations, and become less selective at higher concentrations (de Bruyne et al. 1999, 2001; Hallem et al. 2006). Canonical accounts found that each olfactory receptor neuron expressed a single tuning OR, accounting for the differences in odor tuning across ORN types (Vosshall et al. 2000; Couto et al. 2005; Hallem et al. 2004). However, recent studies in mosquitoes (Herre et al. 2022) as well as flies (Task et al. 2022) have called this model into question, suggesting much greater overlap of receptor expression in individual ORNs. Additional receptor families, such as ppk channels, TRP channels, and gustatory receptors (GRs) have also been shown to act as chemoreceptors in subsets of arthropod neurons (Joseph and Carlson 2015). Activation of ORNs has been shown to produce navigational phenotypes in both larval and adult flies (Schulze et al. 2015; Gepner et al. 2015; Matheson et al. 2022), with different patterns of ORN activation preferentially driving different patterns of locomotor output (Jung et al. 2015; Matheson et al. 2022).
In most arthropod olfactory systems, axons of ORNs that express the same receptor type converge on a smaller number of projection neurons (PNs) in structures known as glomeruli (Couto et al. 2005; Loesel et al. 2013). Some olfactory specialists, such as moths, exhibit “macroglomeruli” for pheromone odors that may play a role in boosting signal strength for these ethologically meaningful odors, as well as in detecting specific pheromone blends (Rospars and Hildebrand 2000; Belmabrouk et al. 2011). Olfactory coding is further refined by local circuitry—mostly composed of inhibitory interneurons—that performs functions such as gain control (Root et al. 2008; Olsen et al. 2008) and decorrelation of olfactory receptor input (Olsen et al. 2010). Local circuitry can also contribute to spatial comparisons. Although fly ORNs project to both hemispheres, these synapses have asymmetric weights, resulting in encoding of cross-antenna intensity differences at the PN level (Gaudry et al. 2013). A recent study identified a single inhibitory interneuron that amplifies cross-antennal differences to produce a spatial code for the direction of the pheromone cVA at short distances (Taisz et al. 2022). Antennal lobe interneurons are highly variable in their morphology even across individuals in one species (Chou et al. 2010) and express a wide variety of peptides that can modulate olfactory processing, for example in response to hunger state (Ignell et al. 2009; Root et al. 2011; Ko et al. 2015; Lizbinski et al. 2018); variations in inhibitory connectivity are one site where differences in the spatial or temporal selectivity of navigation behavior across species might arise.
From the primary olfactory lobe (the antennal lobe in insects), odor information is carried to higher order olfactory areas by a variety of projection neurons (PNs). Uniglomerular PNs carry information from a single glomerulus—reflecting mostly input from one receptor type—while multi-glomerular PNs carry information from groups of glomeruli (Berck et al. 2016; Liang et al. 2013; Vogt et al. 2021). While projection neurons target a number of regions (such as the posterior lateral protocerebrum and anterior ventro-lateral protocerebrum) in different species, (Tanaka et al. 2012; Loesel et al. 2013), the primary targets of olfactory information in insects are the mushroom body and the lateral horn (Bates et al. 2020; Schlegel et al. 2021) (Fig. 4a).
The mushroom body (MB) is an associative structure implicated in olfactory learning. Many years of experiments support this role, starting from the observation that chemical ablation of the mushroom body abolishes learned odor aversion, but not innate aversion (de Belle and Heisenberg 1994). Olfactory projection neurons synapse on to a subset of the input neurons of the mushroom body, which are known as Kenyon Cells (KCs). KCs are the most numerous cell type in the insect brain (2000–50,000 per brain in flies, Caron et al. 2013, 180,000 per brain in bees, Sachse and Gallizia 2006), and are thought to decorrelate and sparsify the representation of odors to facilitate odor discrimination and identification (Turner et al. 2008; Honegger et al. 2011). Sparsification is achieved in part through a large inhibitory neuron, known as APL, that innervates most of the mushroom body (Papadopoulou et al. 2011). KCs make synaptic connections onto a much smaller set of mushroom body output neurons (MBONs) in a highly structured series of compartments within the mushroom body (Aso et al. 2014a, b; Owald et al. 2015). Each compartment is innervated by its own dopaminergic neuron (DANs), which controls plasticity of the KC— > MBON synapse (Liu et al. 2012; Hige et al. 2015; Cohn et al. 2015; Aso et al. 2016). DANs encode a variety of innate valence cues such as sweet taste, sugar input, shock, and odors, as well as features of locomotion (Cohn et al. 2015; May et al. 2020; Siju et al. 2020; Vrontou et al. 2021; Zolin et al. 2021; Kato et al. 2022). They are therefore though to function as the unconditioned stimulus in classical associative plasticity.
MBONs themselves are thought to link the valence of an odor to an appropriate motor output (Strube-Bloss et al. 2011; Owald et al. 2015). Activation of one set of MBONs has been shown to promote attraction, while another set promotes aversion (Aso et al. 2014b). Several MBONs have been implicated in odor-guided food search on different timescales (Tsao et al. 2018; Sayin et al. 2019). One MBON, labeled by MB077B, shows activity that correlates with upwind running (Handler et al. 2019). More recently, different MBONs were shown to promote upwind/downwind movement or path straightening (Matheson et al. 2022), which might suggest an involvement in olfactory or visual navigation, respectively. Intriguingly, activation of certain MBON groups—such as the alpha lobe cluster MB052B—can produce multiple components of olfactory navigation behavior, including upwind running during stimulation, and search behavior at stimulus offset, suggesting that the MB output encodes high-level behavioral “suites” that promote attraction or aversion. At a minimum, this architecture enables arthropods to avoid or approach odors based on previous experience of rewards and punishments. However, recurrent connections between mushroom body neurons may enable more complicated forms of learning, such as reward prediction-based learning, extinction, second-order conditioning, and context-dependent conditioning (Eschbach et al. 2020). KC— > MBON synaptic plasticity also depends critically on the timing of KC activity relative to DAN activity (Gerber et al. 2014; Handler et al. 2019; Devineni et al. 2021), and different MBON compartments appear to encode memories on different timescales (Hige et al. 2015; Aso and Rubin 2016). Thus, the mushroom body has been proposed to act as an “incentive circuit” that integrates internal state information and odors over multiple timescales to promote goal-directed behavior (Gkanias et al. 2022). Variations in MBON connectivity, neuromodulation, or plasticity rules are another likely site where species-specific differences in the timing and duration of olfactory behaviors might arise.
Olfactory projection neurons also target the lateral horn (LH). Thought to perform innate olfactory processing, the lateral horn is a much more complex structure than the mushroom body, with 496 morphologically identified cell types in Drosophila, and complex forms of connectivity (Schlegel et al. 2021). Broadly, recordings from lateral horn neurons support the idea that it clusters odors according to valence (Strutz et al. 2014; Das Chakraborty et al. 2022). Lateral horn odor responses are thought to be more stereotyped than those of mushroom body output neurons (Fisek and Wilson 2014; Jeanne et al. 2018; Frechter et al. 2019; Kohl et al. 2013). In contrast to the mushroom body, only a small number of lateral horn outputs produce identifiable behavioral outputs (Dolan et al. 2019). In particular, one cluster of lateral horn outputs, called LHAD1b2, has been shown to produce approach behavior (Dolan et al. 2019) as well as upwind navigation (Matheson et al. 2022), and is required for some forms of odor attraction (Boehm et al. 2022), while a second cluster called aSP-g controls female receptivity during courtship (Taisz et al. 2022). The AD1b2 cluster of LH output neurons also receives axo-axonal input from mushroom body output neurons (Dolan et al. 2019; Schlegel et al. 2021), suggesting that these two pathways interact to shape behavior. Like the alpha lobe MBON cluster, activation of AD1b2 neurons produces an entire “suite” of behaviors related to attraction, including upwind navigation during stimulation, and search behavior triggered by the loss of stimulation (Matheson et al. 2022). In both the lateral horn and the mushroom body, neurons that produce navigational phenotypes do not show responses dependent on wind direction (Matheson et al. 2022), suggesting that the outputs of these regions represents an odor value signal that is translated into navigational and locomotor commands by downstream circuitry.
Flow-direction circuits
As described above, a key component of olfactory navigation in many species is the use of air or water-flow cues to provide a directional cue for navigation. In many terrestrial arthropods, such as flies (Álvarez-Salvado et al. 2018; Suver et al. 2019), ants (Wolf and Wehner 2000), and cockroaches (Bell and Kramer 1979), wind direction is sensed by the antennae. Wind from different directions differentially displaces the two antennae, generating a code for wind direction that can be read out by central neurons (Yorozu et al. 2009; Suver et al. 2019; Okubo et al. 2020). These displacements are detected by a chordotonal organ known as Johnson’s organ, which is composed of an array of stretch receptors arranged in bowl-like shape around the joint to detect displacement (Gopfert and Robert 2002; Kamikouchi et al. 2006). Primary stretch receptor neurons, known as Johnson’s organ neurons (JONs), are tuned for both direction and dynamics, and provide wind direction information to a region known as the antennal mechanosensory and motor center (AMMC, Kamikouchi et al. 2009; Matsuo et al. 2014). Stabilizing the antennae abolishes orientation to wind in these species, demonstrating that these mechanosensory signals are the only source of wind direction information for wind-guided navigation (Bell and Kramer 1979; Wolf and Wehner 2000; Alvarez-Salvado et al. 2018).
In flies, several components of the central circuitry that processes wind direction information has recently been elucidated (Fig. 4b). From the AMMC, a set of projection neurons carry information to the nearby wedge (WED, Kamikouchi et al. 2009; Matsuo et al. 2014). Recorded AMMC projection neurons respond primarily to deflections of the ipsilateral antenna. In contrast, the WED contains neurons that encode the difference between antennal deflections (Patella and Wilson 2018; Suver et al. 2019). From the WED, wind direction information is sent both to the antler (ANT, Suver et al. 2019) and to the lateral accessory lobe (LAL, Okubo et al. 2020). Two paths from the LAL carry wind direction information to the fan-shaped body, a part of the central complex (described in detail below). One path carries wind information to the ellipsoid body compass (Okubo et al. 2020), while a parallel path carries wind information to columnar neurons of the fan-shaped body (Currier et al. 2020). These two wind inputs to the central complex appear to have different formats. In the ellipsoid body, neurons represent wind direction as a “compass” with different neurons responding to different directions of wind (Okubo et al. 2020). In contrast, fan-shaped body inputs encode wind direction as the activity of a set of orthogonal “basis vectors,” located at ± 45° to the fly’s midline (Currier et al. 2020). How these two representations function together in wind-guided navigation is not yet clear.
A second group of terrestrial arthropods, including crickets, locusts, cockroaches, and mantis (Jacobs et al. 2008; Boyan and Ball 1990), detect wind using a large collection of hair follicles on two structures on the abdomen known as cerci. Each cercus contains between 750 and 2000 mechanosensory hairs (Jacobs et al. 2008; Boublil et al. 2021), each tuned to specific wind directions and frequencies. Sensory neurons innervating these hairs project into the terminal abdominal ganglion to generate a spatial map of horizontal air direction. Aquatic arthropods, such as crayfish (Tautz et al. 1981; Masters et al. 1982) and lobster (Vedel and Clarac 1976; Laverack 1964), detect water currents using two types of hairs distributed along the antennae. The first type of hair responds to motion in the surrounding water (e.g., by a predator), while the second responds to bending of the antennae due to water flow (Tautz et al. 1981; Masters et al. 1982). The brain circuits that process flow signals from these mechanoreceptors are currently unknown. In the future, it will be interesting to investigate whether the various structures that detect flow information in different arthropods send convergent information to highly conserved structures such as the central complex.
Navigation circuits
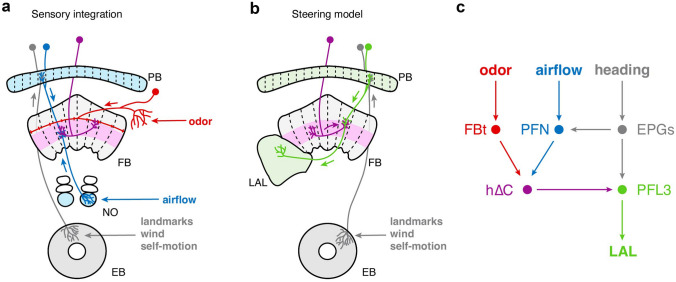
The central complex is an ancient structure thought to form the navigation center of the arthropod brain, and has recently been implicated in olfactory navigation (Matheson et al. 2022, Fig. 5). Early studies of mutant flies with disrupted central complex morphology suggest a role for this structure in visual navigation and control of locomotion (Strauss and Heisenberg 1993). The discovery of a map of sky polarization within the central complex of locusts solidified its role in visually guided navigation (Heinze and Homberg 2007). In recent years, functional studies from genetically identified neurons in Drosophila, and connectomic reconstruction of this structure in both flies (Hulse et al. 2021) and bees (Sayre et al. 2021) have provided new insights into its organization and function. A key feature of the central complex is a global heading signal caried by the compass neurons of the ellipsoid body (EB) (Seelig and Jayaraman 2015). This signal can be influenced by visual cues (Omoto et al. 2017; Sun et al. 2017), and mechano-sensory cues about wind direction (Okubo et al. 2020), both of which are carried by different types of ring neurons (Hulse et al. 2021), as well as by self-motion cues carried by neurons known as PENs (Green and Maimon 2017; Turner-Evans et al. 2017). This heading signal is thought to be maintained by recurrent connections within the ellipsoid body that form a ring attractor (Kim et al. 2017), and is propagated to the downstream fan-shaped body through ∆7 neurons of the protocerebral bridge (PB, Lyu et al. 2022; Hulse et al. 2021).
Fig. 5.
Olfactory navigation circuits within the central complex. The fan-shaped body has been proposed to integrate odor and wind direction signals to generate a goal-direction signal for navigation. a Compass neurons (EPGs, gray) carry a multimodal representation of heading and provide input to the fan-shaped body (FB) through the protocerebral bridge (PB). Columnar inputs to the fan-shaped body (PFNs, blue) receive heading input from the PB and airflow information from the noduli (NO). Tangential inputs to the fan-shaped body (red) carry non-directional odor information. Local neurons (h∆C, purple) receive input from both columnar neurons and tangential neurons and show odor-gated wind-direction tuned signals. b PFL3 neurons (green) receive phase-shifted heading information from the PB and indirect input from local neurons. They are proposed to control steering through their outputs to the LAL. c FB circuits integrate odor, airflow, and heading representation to direct navigation.
(Adapted from Hulse et al. 2021; Lyu et al. 2022; Lu et al. 2022; Currier et al. 2020; Matheson et al. 2022.)
The fan-shaped body (FB) is a highly conserved region of the central complex and may play a role in setting navigational goals. The fan-shaped body receives two main types of inputs: columnar neurons (known as PFNs) and tangential neurons (FBt, Fig. 5a). Columnar neurons have been shown to encode spatial direction cues such as wind direction (Currier et al. 2020), optic flow (Stone et al. 2017; Lyu et al. 2022), and self-motion (Lu et al. 2022). Current data suggest that columnar neurons receive sensory inputs from their projections in the noduli (NO), and a heading input through the protocerebral bridge (Lyu et al. 2022; Lu et al. 2022; Currier et al. 2020). These two inputs allow columnar neurons to encode information as vectors, where the angle is given by the bump inherited from the compass, and the magnitude is given by the sensory input (Lyu et al. 2022; Lu et al. 2022; Hulse et al. 2021). In contrast, tangential neurons appear to mostly encode non-spatial “context” cues such as odor (Matheson et al. 2022), tastants (Sareen et al. 2021), and sleep state information (Donlea et al. 2014). Thus, an appealing model is that the architecture of the fan-shaped body allows for non-spatial context cues (encoded by tangential neurons) to select spatial and navigational trajectories encoded by columnar inputs (Honkanen et al. 2019; Hulse et al. 2021; Matheson et al 2022).
Columnar and tangential neurons are connected by a set of local or pontine neurons. One of these groups, called hΔC, was recently shown to encode an odor-gated wind direction signal (Matheson et al. 2022). Silencing of these neurons decreased the persistence of navigation behavior, while sparse activation of these neurons could produce goal-directed running in a reproducible but arbitrary direction. These data suggest that this fan-shaped body representation of odor may be involved in setting a persistent goal direction during olfactory navigation, which would be required for surging behavior. However, other circuits are likely involved in the initial orientation toward odor, as well as in offset-evoked search behavior. While the function of most fan-shaped body local neurons and tangential neurons has not yet been determined, the connectivity patterns present in fan-shaped body local neurons suggest that they may be used in general for performing vector computations on the inputs encoded by columnar neurons (Hulse et al. 2021). A second group of local neurons, h∆B, were recently shown to compute an allocentric heading-direction signal (Lyu et al. 2022; Lu et al. 2022), suggesting that one function of fan-shaped body local neurons may be to perform computations in allocentric space. Finally, another group of local/output neurons called FC1 were recently shown to encode a goal direction in the context of orientation relative to a visual target (menotaxis, Pires et al. 2022). Thus, a common function of local neurons may be to encode diverse goals in allocentric space.
Like the mushroom body, the fan-shaped body appears to have a much smaller number of outputs than inputs. A notable output tract from the fan-shaped body to the LAL is formed by the PFL3 neurons (Fig. 5b; Stone et al. 2017; Hulse et al. 2021; Rayshubskiy et al. 2020). Intriguingly, PFL3 neurons receive phase-shifted information from the compass system in the ellipsoid body, producing two representations of heading that are each shifted by ~ 90° in the two hemispheres of the brain. Numerous modeling studies of the FB have hypothesized that this connectivity pattern uniquely positions PFL3 neurons to steer insects toward goal directions (Stone et al. 2017; LeMoel et al. 2019; Hulse et al. 2021; Goulard et al. 2021; Sun et al. 2021; Matheson et al. 2022). In these models, PFL3 neurons compare a goal direction, represented as a localized bump of activity across the FB, with the current heading of the fly, represented in the compass. The two shifted representations of heading in PFL3 neurons allow them to compute whether a left or right turn would bring the fly in line with its goal heading. PFL3 neurons provide input to LAL descending neurons that have been shown to drive turning (see below). Thus, PFL3 neurons have been proposed to integrate goal direction information and drive turning to maintain a stable goal-directed heading (Fig. 5c; Matheson et al. 2022; Stone et al. 2017; Sun et al. 2021; Goulard et al. 2021; Le Moël et al. 2019). Recent experimental findings support this model, and provide additional detail on how representations in central complex are “read-out” to produce goal-directed steering (Pires et al. 2022; Westeinde et al. 2022). Given emerging evidence that the fan-shaped body play a role in setting navigational goals, this area may be a possible neural substrate of the various “surge” behaviors observed in arthropod taxa. The diverse sensory inputs to the fan-shaped body may allow for goal directions to be computed based on flow, comparisons between sensors, or spatial memory. Investigating how diverse goal directions are computed in the fan-shaped body will be a fruitful area for future research.
Pre-motor and motor circuits
Directed locomotion for navigation requires descending input from the brain to the ventral nerve cord (Fig. 4c). In the fly, there are around 350 descending neurons (DNs) per hemisphere, about 100 of which are now genetically accessible (Namiki et al 2018). A major question in descending motor control is whether motor actions are evoked by a small number of “command-like neurons”, or whether they are governed by population activity. Current data provide evidence for both models. Some behaviors, such as backwards walking, can be evoked by a single descending neuron, called “moonwalker” that is both necessary and sufficient for this behavior (Bidaye et al. 2014). In contrast, control of walking appears to be distributed across a population of DNs. At least three DNs in flies have been directly implicated in control of walking and turning: DNa01, DNa02, and DNp09 (Chen et al. 2018; Rayshubskiy et al. 2020; Bidaye et al. 2020). However, a recent imaging study of all DNs outside the SEZ found that ~ 60% participated in walking (Aymanns et al. 2022), consistent with a population code for control of walking and turning. Flight maneuvers also appear to be encoded at a population level. A population of 30 DNs were recently described that modulate wing beat amplitude and frequency to cause turns or straight trajectories of various speeds (Namiki et al. 2022).
In addition to controlling forward velocity and turning, the LAL may also play a role in generating more complex patterns of locomotor behavior. In moths, both descending and contralateral projection neurons, have been shown to respond to pheromone (Kanzaki et al. 1991; Namiki et al. 2014). Several of these neurons exhibit striking “flip-flop” activity, in which odor causes the neurons to switch their activity from high-firing rate to low or vice-versa (Olberg 1983; Kanzaki et al. 1994; Kanzaki and Mishima 1996; Namiki et al. 2014). Flip-flop activity in some descending neurons is correlated with zig-zagging head-movements (Kanzaki and Mishimi 1996) which are also observed during odor-offset-evoked casting behavior (Kanzaki et al. 1992). The LAL receives direct olfactory input from the mushroom body (Rayshubskiy et al. 2020; Li et al 2020; Scaplen et al. 2021) and from the superior protocerebrum (Namiki et al 2014). Thus, one hypothesis is that the casting or search component of olfactory navigation may be generated at the level of the LAL based on direct input from the mushroom body/lateral horn, rather than in the central complex. Consistent with this idea, studies in flies observed offset search when stimulating olfactory neurons in the mushroom body and lateral horn, but not when stimulating at the level of the fan-shaped body (Matheson et al. 2022).
Descending neurons can also control stopping—a behavior associated with gathering additional information during olfactory search (Demir et al. 2020). In flies, a group of neurons known as Pair 1 evoke stopping (Lee et al. 2021), although it is not known whether these neurons receive olfactory input. Another DN in larvae, known as PDM-DN, has been shown to evoke stop-turns and is required for proper olfactory navigation (Tastekin et al. 2018). Thus, DNs may be one locus where pausing behavior is initiated to obtain additional information about the odor environment. However, more experimental work will be required to understand how these DNs fit into complete sensory-motor circuits.
Evolution and development of neural circuits across arthropods
Despite over 600 million years of evolution, the basic structures of the arthropod brain appear to be highly conserved (Loesel et al. 2013). Olfactory lobes that receive input from the olfactory receptor neurons in the first antenna are a common feature of arthropod brains (Schmidt and Mellon 2010; Sombke et al. 2011), despite immense variations in the size and morphology of the antennae themselves (Elgar et al. 2018; Derby 2021). These are generally divided into glomeruli and innervated by both local interneurons and projection neurons (Loesel et al. 2013). Mushroom bodies are found across insects (Strausfeld et al. 2009), although the inputs to this structure can differ across species. The mushroom body receives primarily olfactory input in some species (Li et al. 2020), and primarily visual input in others (Lin and Strausfeld 2012) and can persist in the absence of olfactory input (Strausfeld et al. 2009). In crustaceans, a structure known as the hemi-ellipsoid body receives input from the olfactory lobes (Wolff et al. 2017), and has recently been suggested to be homologous to the mushroom body, as they share many structural and molecular features (Wolff et al. 2015, 2017; Strausfeld et al. 2020).
The central complex (or “central body”) has been observed in diverse arthropod lineages, including insects, crustaceans, chelicerates (spiders, scorpions, horseshoe crabs), onychophorans (velvet worms), and chilopods (centipedes) (Homberg 2008; Loesel et al. 2013; Thoen et al. 2017; Chou et al. 2022). While the gross morphology of this structure differs across clades, many of its basic cell types and connectivity motifs appear to be well-conserved at least between flies and bees (Hulse et al. 2021; Sayre et al. 2021). Functional components of the central complex are structurally conserved across arthropods, although with morphological differences. The ellipsoid body forms a separate donut-shaped structure in flies, while in other insects, and crustaceans, it forms the lower division of the fan-shaped central body (Thoen et al. 2017; Sayre et al. 2021; Chou et al. 2022). The ellipsoid body and the lower division of the central body share strong GABA immunoreactivity (Homberg et al. 1999), likely arising from the ring neurons that carry sensory input to this structure (Heinze and Homberg 2008; el Jundi et al. 2014; Omoto et al. 2017; Fisher et al. 2019; Kim et al. 2019).
A lateral accessory lobe or lateral complex is also found in many insect species (Namiki and Kanzaki 2016). Descending neurons originating in the LAL have been shown to influence steering in both moths (Kanzaki and Mishima 1996) and flies (Chen et al. 2018; Rayshubskiiy et al. 2020). The LAL receives ascending input from the ventral nerve cord in both locusts (Homberg 1994) and crickets (Zorovic and Hedwig 2011). LAL-like structures have been observed in several crustaceans species, including stomatopods, crayfish, water fleas, and isopods (Thoen et al. 2017; Utting et al. 2000; Kress et al. 2016; Kenning and Harzsch 2013).
Both the conservation and the evolutionary variation observed across arthropod brain structures must ultimately arise from developmental processes. Significant changes in central brain structures occur between the larval and adult forms of Drosophila. For example, in larvae, the antennal lobe has a similar structure but many fewer glomeruli and receptors (Berck et al. 2016; Bates et al. 2020), the mushroom body is simplified (Truman et al. 2022), and the central complex is severely reduced or absent (Reibli et al. 2013; Andrade et al. 2019), though a LAL remains (Cardona et al. al 2009). Despite significant remodeling of the nervous system during metamorphosis (Truman et al. 2022), intriguing correspondences between larval and adult neurons have been observed. For example, the same neurons (moonwalker/mooncrawler) produce backward walking in adults (Bidaye et al. 2014), and backward crawling in larvae (Carreira-Rosario et al. 2018), despite striking differences in the physics and control of locomotion. Likewise, Pair 1 neurons produce stopping in both adults and larvae (Lee et al. 2021). These studies suggest that central control of high-level behaviors may be preserved even when the details of locomotion and body plan are completely different.
Conserved central brain structures, such as the mushroom body and central complex, arise from conserved stem cells, called neuroblasts, that proliferate to generate specific conserved cell types (Urbach and Technau 2004; Kunz et al. 2012; Yang et al. 2013; Yu et al. 2013; Walsh and Doe 2017). For example, different Kenyon cell types are generated sequentially from mushroom body neuroblasts (Lee and Luo 1999), and the columns of the central complex arise from four medial neuroblasts, called DM1-4, which decussate to form the characteristic scaffold of the fan-shaped body (Andrade et al. 2019; Sullivan et al. 2019). The generation of different cell types is controlled by the expression of a series of temporally restricted transcription factors and RNA binding proteins (Ren et al. 2017; Sullivan et al. 2019). Changes to the timing of these developmental programs results in the formation of a functional, but incomplete, central complex in the larval flour beetle (Koniszewski et al. 2016; Farnworth et al. 2020). Likewise, differences in the timing of mushroom body development relative to hatching vary across species (Farris and Sinakevitch 2003). Increases in both the number of neural progenitors, and the time over which these progenitors divide, contributes to the formation of some of the largest mushroom bodies among all insects in honeybees (Farris et al. 1999). Thus, changes to genes regulating neuroblast number, the timing of cell proliferation, and the expression of temporal transcription factors that specify cell identity, may drive the diversification of neural circuits to support behavior in diverse environments. Tools based on these genes may allow for experimental access to conserved cell types in diverse organisms (Farnworth et al. 2020), and for manipulation of circuit structures with consequences for behavior.
Toward a comparative approach to olfactory navigation
In this review, we have tried to summarize our current state of knowledge about the diversity of olfactory navigation behaviors in arthropods and the potential circuit mechanisms underlying them. Several themes emerge from this review. First, despite differences in physiology across species and plume dynamics across environments, many arthropods rely on shared sensory strategies (flow detection, spatial comparison, short-term memory) to determine which way to go when searching for an odor source, and move with a conserved set of high-level behavioral features (surging, searching, pausing). Second, arthropods rarely rely on a single sensory cue or strategy to find an odor source, but rather appear to be able to integrate many sources of information coherently. Finally, the arthropod brain is built on a scaffold of conserved structures (antennal lobes, mushroom bodies, central complex), many of which have highly structured and conserved connectivity. How can the same brain scaffold be adapted to enable animals to effectively navigate toward odor goals in diverse environments, using diverse modes of locomotion? Given the ubiquity of olfactory navigation behavior in arthropods, its crucial role in animal survival and reproduction, and the conservation of underlying brain structures described above, we propose that addressing this question through a comparative study of the neural basis of olfactory navigation is likely to provide great insight into the organization and “functional logic” of neural circuit assembly.
How do species-specific differences in olfactory navigation arise? The most notable differences between species include which odors are innately attractive or aversive, what temporal patterns of odor fluctuation are required to drive movement upwind or upstream, and the characteristic frequencies and timescales of behavioral features such as surging, searching, and stopping. Thus far, the most progress has been made in understanding cross-species differences in odor preferences, which are mostly driven by changes in the repertoire of olfactory receptors and the structure of peripheral olfactory circuits. For example, specialization by Drosophilia sechellia on the acidic noni fruit has been linked to increased sensitivity to noni odor in a specific ORN, OR22a, an increased number of OR22a neurons, and enlargement of the corresponding glomerulus (Auer et al. 2020). More generally, the OR olfactory receptor family, which are unique to insects, have been shown to broadly respond to esters and alcohols (including hydrophobic or amphipathic esters and alcohols), while the IR family of olfactory receptors, which are expressed across arthropods, are selective for water-soluble acids and amines (Silbering et al. 2011). Differences in the selectivity of behavior for specific temporal patterns of odor stimulation have been linked to differences in the representation of odors, either with transient or persistent responses, at the level of the antennal lobe (Zocchi et al. 2022). However, whether central circuits for olfactory navigation differ across species, and how these circuit changes might be related to differences in behavior is not known. In Drosophila species, evolution of odor preference during courtship behavior has been linked to changes in central, rather than peripheral, odor selectivity (Seeholzer et al. 2018). Many central circuit modifications could underlie differences in the odor selectivity or dynamics of search behavior across organisms, including changes in neuron number, neuronal connectivity, synapse dynamics, and peptide and peptide receptor expression. A deeper understanding of the role of central neural circuits in olfactory navigation will be critical to make sense of how species-specific variations have emerged.
What tools and resources will be needed to more fully understand how the arthropod brain generates olfactory navigation behavior? A critical first step will be quantitative comparisons of search and navigation behavior across species in similar behavioral paradigms with well-controlled sensory stimuli. The explosion of tools for high throughput tracking and quantification of behavior using methods based on deep learning makes these previously challenging approaches newly possible (Mathis et al. 2018; Pereira et al. 2019). Complete connectomes for the fly brain and ventral nerve cord are in progress and will likely be made public soon (Dorkenwald et al. 2022; Phelps et al. 2021). Complementary work on the connectomes of other arthropods will allow us to understand which pathways are conserved and which vary across evolution. However, functional studies that ask how representations of odor, flow, locomotion, and environment change across each of the regions described here will also be required to understand how the arthropod brain effectively integrates different types of information to produce coherent goal-directed behavior. New tools for fast imaging across brain areas (Aimon et al. 2019), and for sparse imaging within developmentally defined populations (Isaacman-Beck et al. 2020), will be particularly useful in this regard. In addition, tools for labeling and manipulating conserved cell types across species will be needed. A promising avenue here are the newly developing tools to access cells based on developmental lineage and the expression of conserved transcription factors (Farnworth et al. 2020). Such tools would be more likely to generalize across species than current genetic drivers which are based on the expression of regulatory regions upstream of brain-expressed genes (Pfeiffer et al. 2008; Tirian and Dickson 2017). Moreover, they might allow for the manipulation of functionally related cohorts of neurons, and ultimately an understanding of how neural circuits evolve through changes in developmental pathways.
Conclusions
Olfactory navigation is an ancient behavior that is critical for the survival and reproduction of most arthropod species. While the odors that drive attraction, the physical structure of odor plumes and trails, and the modes of locomotion differ greatly across arthropods, many of the core computations and behavioral motifs involved in getting to an odor source appear remarkably conserved. New tools for comparing the behavior, cell types, and organization of neural circuits across species have renewed interest in comparative approaches to understanding neural function. We propose that such comparative study of olfactory navigation in arthropods is likely to elucidate fundamental principles of neural circuit development, organization, and evolution.
Acknowledgements
This work was supported by NSF 2014217 (neuronex: Odor2Action), Brain Initiative RF1 NS127129, and NIDCD DC017979 to K.I.N.
Author contributions
TJS, AJL, and KIN wrote the manuscript. AJL made the figures with input from TJS and KIN.
Data availability
No new data were generated during this study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Theresa J. Steele and Aaron J. Lanz contributed equally to this work.
References
- Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69(1):44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ache BW, Derby CD. Functional organization of olfaction in crustaceans. Trends Neurosci. 1985;8:356–360. doi: 10.1016/0166-2236(85)90122-5. [DOI] [Google Scholar]
- Ackels T, Erskine A, Dasgupta D, Marin AC, Warner T, Tootoonian S, Fukunaga I, Harris JJ, Schaefer AT. Fast odour dynamics are encoded in the olfactory system and guide behaviour. Nature. 2021;593(7860):558–563. doi: 10.1038/s41586-021-03514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimon S, Katsuki T, Jia T, Grosenick L, Broxton M, Deisseroth K, Sejnowski TJ, Greenspan RJ. Fast near-whole–brain imaging in adult Drosophila during responses to stimuli and behavior. Plos Biol. 2019;7(2):e2006732. doi: 10.1371/journal.pbio.2006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Salvado E, Licata AM, Connor EG, McHugh MK, King BM, Stavropoulos N, Victor JD, Crimaldi JP, Nagel KI. Elementary sensory-motor transformations underlying olfactory navigation in walking fruit-flies. Elife. 2018;7:e37815. doi: 10.7554/eLife.37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade IV, Riebli N, Nguyen BC, Omoto JJ, Cardona A, Hartenstein V. Developmentally arrested precursors of pontine neurons establish an embryonic blueprint of the Drosophila central complex. Curr Biol. 2019;29(3):412–425. doi: 10.1016/j.cub.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Rubin GM. Dopaminergic neurons write and update memories with cell-type-specific rules. Elife. 2016;5:e16135. doi: 10.7554/eLife.16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo TT, Dionne H, Abbott LF, Axel R, Tanimoto H, Rubin GM. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guérin G, Plaçais PY, Robie AA, Yamagata N, Schnaitmann C, Rowell WJ, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer TO, Khallaf MA, Silbering AF, Zappia G, Ellis K, Álvarez-Ocaña R, Arguello JR, Hansson BS, Jefferis GS, Caron SJ, Knaden M. Olfactory receptor and circuit evolution promote host specialization. Nature. 2020;579(7799):402–408. doi: 10.1038/s41586-020-2073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymanns F, Chen CL, Ramdya P. Descending neuron population dynamics during odor-evoked and spontaneous limb-dependent behaviors. Elife. 2022;11:e81527. doi: 10.7554/eLife.81527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TC, Willis MA, Haynes KF, Phelan PL. A pulsed cloud of sex pheromone elicits upwind flight in male moths. Physiol Ent. 1985;10(3):257–265. doi: 10.1111/j.1365-3032.1985.tb00045.x. [DOI] [Google Scholar]
- Baker TC (1990) Upwind flight and casting flight: complementary phasic and tonic systems used for location of sex pheromone sources by male moth. In: Proc 10th Int Symp Olf Taste, Oslo, pp 18–25
- Bates AS, Schlegel P, Roberts RJ, Drummond N, Tamimi IF, Turnbull R, Zhao X, Marin EC, Popovici PD, Dhawan S, Jamasb A. Complete connectomic reconstruction of olfactory projection neurons in the fly brain. Curr Biol. 2020;30(16):3183–3199. doi: 10.1016/j.cub.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell WJ, Kramer E. Search and anemotactic orientation of cockroaches. J Insect Physiol. 1979;25(8):631–640. doi: 10.1016/0022-1910(79)90112-4. [DOI] [Google Scholar]
- Bell WJ, Tobin TR. Orientation to sex pheromone in the American cockroach: analysis of chemo-orientation mechanisms. J Insect Physiol. 1981;27(8):501–508. doi: 10.1016/0022-1910(81)90036-6. [DOI] [Google Scholar]
- Bell WJ, Tobin TR. Chemo-orientation. Biol Rev. 1982;57(2):219–260. doi: 10.1111/j.1469-185X.1982.tb00369.x. [DOI] [Google Scholar]
- Belmabrouk H, Nowotny T, Rospars JP, Martinez D. Interaction of cellular and network mechanisms for efficient pheromone coding in moths. PNAS. 2011;108(49):19790–19795. doi: 10.1073/pnas.1112367108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4(2):e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berck ME, Khandelwal A, Claus L, Hernandez-Nunez L, Si G, Tabone CJ, Li F, Truman JW, Fetter RD, Louis M, Samuel AD. The wiring diagram of a glomerular olfactory system. Elife. 2016 doi: 10.7554/eLife.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaye SS, Machacek C, Wu Y, Dickson BJ (2014) Neuronal control of Drosophila walking direction. Science 344(6179):97–101. 10.1126/science.1249964 [DOI] [PubMed]
- Salil S, Meghan B, Laturney AK, Yuejiang C, Till L, Ansgar B, Kristin B, Scott Two brain pathways initiate distinct forward walking programs in Drosophila. Neuron. 2020;108(3):469–485. doi: 10.1016/j.neuron.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm AC, Friedrich AB, Hunt S, Bandow P, Siju KP, De Backer JF, Claussen J, Link MH, Hofmann TF, Dawid C, Kadow IC. A dopamine-gated learning circuit underpins reproductive state-dependent odor preference in Drosophila females. Elife. 2022;11:e77643. doi: 10.7554/eLife.77643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boie SD, Connor EG, McHugh M, Nagel KI, Ermentrout GB, Crimaldi JP, Victor JD. Information-theoretic analysis of realistic odor plumes: What cues are useful for determining location? PLoS Comp Biol. 2018;14(7):e1006275. doi: 10.7554/eLife.77643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boublil BL, Diebold CA, Moss CF. Mechanosensory hairs and hair-like structures in the animal kingdom: specializations and shared functions serve to inspire technology applications. Sensors. 2021;21(19):6375. doi: 10.3390/s21196375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyan GS. Ball EE (1990) Neuronal organization and information processing in the wind-sensitive cercal receptor/giant interneurone system of the locust and other orthopteroid insects. Prog Beurobiol. 1990;35(3):217–243. doi: 10.1016/0301-0082(90)90028-F. [DOI] [PubMed] [Google Scholar]
- Budick SA, Dickinson MH. Free-flight responses of Drosophila melanogaster to attractive odors. J Exp Biol. 2006;209(15):3001–3017. doi: 10.1242/jeb.02305. [DOI] [PubMed] [Google Scholar]
- Büschges A, Akay T, Gabriel JP, Schmidt J. Organizing network action for locomotion: insights from studying insect walking. Brain Res Rev. 2008;57(1):162–171. doi: 10.1016/j.brainresrev.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Cardona A, Larsen C, Hartenstein V. Neuronal fiber tracts connecting the brain and ventral nerve cord of the early Drosophila larva. J Comp Neurol. 2009;515(4):427–440. doi: 10.1002/cne.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron SJ, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497(7447):113–117. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Rosario A, Zarin AA, Clark MQ, Manning L, Fetter RD, Cardona A, Doe CQ. MDN brain descending neurons coordinately activate backward and inhibit forward locomotion. Elife. 2018;7:e38554. doi: 10.7554/eLife.38554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celani A, Villermaux E, Vergassola M. Odor landscapes in turbulent environments. Phys Rev X. 2014;4(4):041015. doi: 10.1103/PhysRevX.4.041015. [DOI] [Google Scholar]
- Chakraborty SD, Chang H, Hansson BS, Sachse S. Higher-order olfactory neurons in the lateral horn support odor valence and odor identity coding in Drosophila. Elife. 2022;11:e74637. doi: 10.7554/eLife.74637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Spletter ML, Yaksi E, Leong J, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neuro. 2010;13(4):439–449. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou A, Sayre ME, Lin C, Cronin TW. Neuroanatomy of stomatopod central complexes offers putative neural substrate for oriented behaviors in crustaceans. bioRxiv. 2022 doi: 10.1101/2022.06.10.495695. [DOI] [Google Scholar]
- Clark MQ, Zarin AA, Carreira-Rosario A, Doe CQ. Neural circuits driving larval locomotion in Drosophila. Neural Dev. 2018;13(1):1. doi: 10.1186/s13064-018-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Lin CL, Hermans MC, Denis V, Florian F, Michael A, Anthony U, Cammarato MH, Pavan D, Ramdya Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nat Commun. 2018;9(1):4390. doi: 10.1038/s41467-018-06857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R, Morantte I, Ruta V. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell. 2015;163(7):1742–1755. doi: 10.1016/j.cell.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor EG, McHugh MK, Crimaldi JP. Quantification of airborne odor plumes using planar laser-induced fluorescence. Exp Fluids. 2018;59(9):1–1. doi: 10.1007/s00348-018-2591-3. [DOI] [Google Scholar]
- Corey EA, Bobkov Y, Ukhanov K, Ache BW. Ionotropic crustacean olfactory receptors. PLoS ONE. 2013;8(4):e60551. doi: 10.1371/journal.pone.0060551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15(17):1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Crimaldi JP, Koseff JR. High-resolution measurements of the spatial and temporal scalar structure of a turbulent plume. Exp Fluids. 2001;31(1):90–102. doi: 10.1007/s003480000263. [DOI] [Google Scholar]
- Crimaldi JP, Wiley MB, Koseff JR. The relationship between mean and instantaneous structure in turbulent passive scalar plumes. J Turbul. 2002;3(1):014. doi: 10.1088/1468-5248/3/1/014. [DOI] [Google Scholar]
- Crimaldi J, Lei H, Schaefer A, Schmuker M, Smith BH, True AC, Verhagen JV, Victor JD. Active sensing in a dynamic olfactory world. J Comp Neurol. 2022;50(1):1–6. doi: 10.1007/s10827-021-00798-1. [DOI] [PubMed] [Google Scholar]
- Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, Gibson TJ, Benton R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6(8):e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier TA, Matheson AM, Nagel KI. Encoding and control of orientation to airflow by a set of Drosophila fan-shaped body neurons. Elife. 2020;9:e61510. doi: 10.7554/eLife.61510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CT, Kennedy JS, Ludlow AR. Finding of a sex pheromone source by gypsy moths released in the field. Nature. 1983;303(5920):804–806. doi: 10.1038/303804a0. [DOI] [Google Scholar]
- De Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263(5147):692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- De Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J Neurosci. 1999;19(11):4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30(2):537–552. doi: 10.1016/S0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Dekker T, Cardé RT. Moment-to-moment flight manoeuvres of the female yellow fever mosquito (Aedes aegypti L.) in response to plumes of carbon dioxide and human skin odour. J Exp Biol. 2011;214(20):3480–3494. doi: 10.1242/jeb.055186. [DOI] [PubMed] [Google Scholar]
- Dekker T, Geier M, Cardé RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol. 2005;208(15):2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- Del Mármol J, Yedlin MA, Ruta V. The structural basis of odorant recognition in insect olfactory receptors. Nature. 2021;597(7874):126–131. doi: 10.1038/s41586-021-03794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir M, Kadakia N, Anderson HD, Clark DA, Emonet T. Walking Drosophila navigate complex plumes using stochastic decisions biased by the timing of odor encounters. Elife. 2020;9:e57524. doi: 10.7554/eLife.57524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale A, Hogan JD, Guadamuz Araya C, Dominy NJ, Melin AD. Aeroscapes and the sensory ecology of olfaction in a tropical dry forest. Front Ecol Evol. 2022;10:347. doi: 10.3389/fevo.2022.849281. [DOI] [Google Scholar]
- Derby CD. The crustacean antennule: a complex organ adapted for lifelong function in diverse environments and lifestyles. Biol Bull. 2021;240(2):67–81. doi: 10.1086/713537. [DOI] [PubMed] [Google Scholar]
- Derby C, Thiel M. Nervous systems and control of behavior. Oxford: Oxford University Press; 2014. [Google Scholar]
- Devineni AV, Deere JU, Sun B, Axel R. Individual bitter-sensing neurons in Drosophila exhibit both ON and OFF responses that influence synaptic plasticity. Curr Biol. 2021;31(24):5533–5546. doi: 10.1016/j.cub.2021.10.020. [DOI] [PubMed] [Google Scholar]
- Dolan MJ, Frechter S, Bates AS, Dan C, Huoviala P, Roberts RJ, Schlegel P, Dhawan S, Tabano R, Dionne H, Christoforou C, et al. Neurogenetic dissection of the Drosophila lateral horn reveals major outputs, diverse behavioural functions, and interactions with the mushroom body. Elife. 2019;8:e43079. doi: 10.7554/eLife.43079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Pimentel D, Miesenböck G. Neuronal machinery of sleep homeostasis in Drosophila. Neuron. 2014;81(4):860–872. doi: 10.1016/j.neuron.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorkenwald S, McKellar CE, Macrina T, Kemnitz N, Lee K, Lu R, Wu J, Popovych S, Mitchell E, Nehoran B, Jia Z. FlyWire: online community for whole-brain connectomics. Nat Methods. 2022;19(1):119–128. doi: 10.1038/s41592-021-01330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draft RW, McGill MR, Kapoor V, Murthy VN. Carpenter ants use diverse antennae sampling strategies to track odor trails. J Exp Biol. 2018;221(22):jeb185124. doi: 10.1242/jeb.185124. [DOI] [PubMed] [Google Scholar]
- Duistermars BJ, Chow DM, Frye MA. Flies require bilateral sensory input to track odor gradients in flight. Curr Biol. 2009;19(15):1301–1307. doi: 10.1016/j.cub.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claire EA, Michael F, Winding CM, Mei SM, Rebecca S, Katharina A, Javier E, Tomoko VA, Ohyama AS, Bertram T, Gerber RD, Fetter JW, Ashok T, Albert LK, Marta C, Zlatic Recurrent architecture for adaptive regulation of learning in the insect brain. Nature Neuroscience. 2020;23(4):544–555. doi: 10.1038/s41593-020-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Jundi B, Pfeiffer K, Heinze S, Homberg U. Integration of polarization and chromatic cues in the insect sky compass. J Comp Physiol A. 2014;200(6):575–589. doi: 10.1007/s00359-014-0890-6. [DOI] [PubMed] [Google Scholar]
- Elgar MA, Zhang D, Wang Q, Wittwer B, Pham HT, Johnson TL, Freelance CB, Coquilleau M. Focus: ecology and evolution: insect antennal morphology: the evolution of diverse solutions to odorant perception. Yale J Biol Med. 2018;91(4):457. [PMC free article] [PubMed] [Google Scholar]
- Farnworth MS, Eckermann KN, Bucher G. Sequence heterochrony led to a gain of functionality in an immature stage of the central complex: a fly–beetle insight. PLoS Biol. 2020;18(10):e3000881. doi: 10.1371/journal.pbio.3000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SM, Sinakevitch I. Development and evolution of the insect mushroom bodies: towards the understanding of conserved developmental mechanisms in a higher brain center. Arthropod Struct Dev. 2003;32(1):79–101. doi: 10.1016/S1467-8039(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Farris SM, Robinson GE, Davis RL, Fahrbach SE. Larval and pupal development of the mushroom bodies in the honey bee, Apis mellifera. J Comp Neurol. 1999;414(1):97–113. doi: 10.1002/(SICI)1096-9861(19991108)414:1<97::AID-CNE8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Fişek M, Wilson RI. Stereotyped connectivity and computations in higher-order olfactory neurons. Nat Neurosci. 2014;17(2):280–288. doi: 10.1038/nn.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvette E, Fisher Jenny Lu, Isabel DR, Wilson I. Sensorimotor experience remaps visual input to a heading-direction network. Nature. 2019;576(7785):121–125. doi: 10.1038/s41586-019-1772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechter S, Bates AS, Tootoonian S, Dolan MJ, Manton J, Jamasb AR, Kohl J, Bock D, Jefferis G. Functional and anatomical specificity in a higher olfactory centre. Elife. 2019;8:e44590. doi: 10.7554/eLife.44590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry Q, Hong EJ, Kain J, de Bivort BL, Wilson RI. Asymmetric neurotransmitter release enables rapid odour lateralization in Drosophila. Nature. 2013;493(7432):424–428. doi: 10.1038/nature11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner R, Skanata MM, Bernat NM, Kaplow M, Gershow M. Computations underlying Drosophila photo-taxis, odor-taxis, and multi-sensory integration. Elife. 2015;4:e06229. doi: 10.7554/eLife.06229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B, Yarali A, Diegelmann S, Wotjak CT, Pauli P, Fendt M. Pain-relief learning in flies, rats, and man: basic research and applied perspectives. Learn Mem. 2014;21(4):232–252. doi: 10.1101/lm.032995.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershow M, Berck M, Mathew D, Luo L, Kane EA, Carlson JR, Samuel AD. Controlling airborne cues to study small animal navigation. Nat Methods. 2012;9(3):290–296. doi: 10.1038/nmeth.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, Packer MJ, Steullet P, Brady J. Orientation of tsetse flies to wind, within and outside host odour plumes in the field. Physiol Entomol. 1991;16(1):47–56. doi: 10.1111/j.1365-3032.1991.tb00542.x. [DOI] [Google Scholar]
- Evripidis Gkanias Li, Yan McCurdy Michael, Nitabach N, Barbara W. An incentive circuit for memory dynamics in the mushroom body of Drosophila melanogaster. eLife. 2022;11:e75611. doi: 10.7554/eLife.75611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marin A, Stephens GJ, Louis M. Active sampling and decision making in Drosophila chemotaxis. Nat Commun. 2011;2(1):1. doi: 10.1038/ncomms1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. The mechanical basis of Drosophila audition. J Exp Biol. 2002;205(9):1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- Goulard R, Buehlmann C, Niven JE, Graham P, Webb B. A unified mechanism for innate and learned visual landmark guidance in the insect central complex. PLoS Comp Biol. 2021;17(9):e1009383. doi: 10.1371/journal.pcbi.1009383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Adachi A, Shah KK, Hirokawa JD, Magani PS, Maimon G. A neural circuit architecture for angular integration in Drosophila. Nature. 2017;546(7656):101–106. doi: 10.1038/nature22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünbaum D, Willis MA. Spatial memory-based behaviors for locating sources of odor plumes. Movement Ecol. 2015;3(1):1–21. doi: 10.1186/s40462-015-0037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Pan Y, Gong Z. Recent advances in the genetic dissection of neural circuits in Drosophila. Neurosci Bull. 2019;35(6):1058–1072. doi: 10.1007/s12264-019-00390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125(1):143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117(7):965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Handler A, Graham TG, Cohn R, Morantte I, Siliciano AF, Zeng J, Li Y, Ruta V. Distinct dopamine receptor pathways underlie the temporal sensitivity of associative learning. Cell. 2019;178(1):60–75. doi: 10.1016/j.cell.2019.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315(5814):995–997. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Neuroarchitecture of the central complex of the desert locust: intrinsic and columnar neurons. J Comp Neurol. 2008;511(4):454–478. doi: 10.1002/cne.21842. [DOI] [PubMed] [Google Scholar]
- Herre M, Goldman OV, Lu TC, Caballero-Vidal G, Qi Y, Gilbert ZN, Gong Z, Morita T, Rahiel S, Ghaninia M, Ignell R. Non-canonical odor coding in the mosquito. Cell. 2022;185(17):3104–3123. doi: 10.1016/j.cell.2022.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hige T, Aso Y, Modi MN, Rubin GM, Turner GC. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron. 2015;88(5):985–998. doi: 10.1016/j.neuron.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U. Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J Comp Physiol A. 1994;175(5):597–610. doi: 10.1007/BF00199481. [DOI] [Google Scholar]
- Homberg U. Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Struct Dev. 2008;37(5):347–362. doi: 10.1016/j.asd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Homberg U, Vitzthum H, Müller M, Binkle U. Immunocytochemistry of GABA in the central complex of the locust Schistocerca gregaria: identification of immunoreactive neurons and colocalization with neuropeptides. J Comp Neurol. 1999;409(3):495–507. doi: 10.1002/(SICI)1096-9861(19990705)409:3<495::AID-CNE12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Honegger KS, Campbell RA, Turner GC. Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J Neurosci. 2011;31(33):11772–11785. doi: 10.1523/JNEUROSCI.1099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen A, Adden A, da FreitasSilva J, Heinze S. The insect central complex and the neural basis of navigational strategies. J Exp Biol. 2019;222(Suppl_1):jeb188854. doi: 10.1242/jeb.188854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse BK, Haberkern H, Franconville R, Turner-Evans DB, Takemura S, Wolff T, Noorman M, Dreher M, Dan C, Parekh R, Hermundstad AM, et al. A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and context-dependent action selection. Elife. 2021;10:e66039. doi: 10.7554/eLife.66039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignell R, Root CM, Birse RT, Wang JW, Nässel DR, Winther ÅM. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. PNAS. 2009;106(31):13070–13075. doi: 10.1073/pnas.0813004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacman-Beck J, Paik KC, Wienecke CF, Yang HH, Fisher YE, Wang IE, Ishida IG, Maimon G, Wilson RI, Clandinin TR. SPARC enables genetic manipulation of precise proportions of cells. Nat Neurosci. 2020;23(9):1168–1175. doi: 10.1038/s41593-020-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GA, Miller JP, Aldworth Z. Computational mechanisms of mechanosensory processing in the cricket. J Exp Biol. 2008;211(11):1819–1828. doi: 10.1242/jeb.016402. [DOI] [PubMed] [Google Scholar]
- Jayaram V, Kadakia N, Emonet T. Sensing complementary temporal features of odor signals enhances navigation of diverse turbulent plumes. Elife. 2022;11:e72415. doi: 10.7554/eLife.72415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne JM, Fişek M, Wilson RI. The organization of projections from olfactory glomeruli onto higher-order neurons. Neuron. 2018;98(6):1198–1213. doi: 10.1016/j.neuron.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn J, Connor EG, Jacobs LF. How ambient environment influences olfactory orientation in search and rescue dogs. Chem Senses. 2020;45(8):625–634. doi: 10.1093/chemse/bjaa060. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Carlson JR. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet. 2015;31(12):683–695. doi: 10.1016/j.tig.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Hueston C, Bhandawat V. Odor-identity dependent motor programs underlie behavioral responses to odors. Elife. 2015;4:e11092. doi: 10.7554/eLife.11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadakia N, Demir M, Michaelis BT, DeAngelis BD, Reidenbach MA, Clark DA, Emonet T. Odour motion sensing enhances navigation of complex plumes. Nature. 2022;611(7937):754–761. doi: 10.1038/s41586-022-05423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikouchi A, Shimada T, Ito KE. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol. 2006;499(3):317–356. doi: 10.1002/cne.21075. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458(7235):165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- Kanzaki R, Ikeda A. Morphology and physiology of pheromone-triggered flip-flopping descending interneurons of the male silkworm moth, Bombyx mori. In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and taste XI. Tokyo: Springer; 1994. pp. 851–851. [Google Scholar]
- Kanzaki R, Mishima T. Pheromone-triggered ‘Flipflopping’neural signals correlate with activities of neck motor neurons of a male moth, Bombyx mori. Zool Sci. 1996;13(1):79–87. doi: 10.2108/zsj.13.79. [DOI] [Google Scholar]
- Kanzaki R, Arbas EA, Hildebrand JG. Physiology and morphology of protocerebral olfactory neurons in the male moth Manduca sexta. J Comp Physiol A. 1991;168(3):281–298. doi: 10.1007/BF00198348. [DOI] [PubMed] [Google Scholar]
- Kanzaki R, Sugi N, Shibuya T. Self-generated zigzag turning of Bombyx mori males during pheromone-mediated upwind walking. Zool Sci. 1992;9(3):515–527. doi: 10.34425/zs000983. [DOI] [Google Scholar]
- Kato A, Ohta K, Okanoya K, Kazama H. Dopaminergic neurons dynamically update sensory values during navigation. bioRxiv. 2022 doi: 10.1101/2022.08.17.504092. [DOI] [PubMed] [Google Scholar]
- Keller TA, Powell I, Weissburg MJ. Role of olfactory appendages in chemically mediated orientation of blue crabs. Mar Ecol Prog Ser. 2003;261:217–231. doi: 10.3354/meps261217. [DOI] [Google Scholar]
- Kennedy JS. The visual responses of flying mosquitoes. Proc Zool Soc Lond. 1940 doi: 10.1111/j.1096-3642.1940.tb00831.x. [DOI] [Google Scholar]
- Kennedy JS. Zigzagging and casting as a programmed response to wind-borne odour: a review. Physiol Entomol. 1983;8(2):109–120. doi: 10.1111/j.1365-3032.1983.tb00340.x. [DOI] [Google Scholar]
- Kennedy JS, Marsh D. Pheromone-regulated anemotaxis in flying moths. Science. 1974;184(4140):999–1001. doi: 10.1126/science.184.4140.999. [DOI] [PubMed] [Google Scholar]
- Kennedy JS, Ludlow AR, Sanders CJ. Guidance of flying male moths by wind-borne sex pheromone. Physiol Entomol. 1981;6(4):395–412. doi: 10.1111/j.1365-3032.1981.tb00655.x. [DOI] [Google Scholar]
- Kenning M, Harzsch S. Brain anatomy of the marine isopod Saduria entomon Linnaeus, 1758 (Valvifera, Isopoda) with special emphasis on the olfactory pathway. Front Neuroanat. 2013;7:32. doi: 10.3389/fnana.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Rouault H, Druckmann S, Jayaraman V (2017) Ring attractor dynamics in the Drosophila central brain. Science 356(6340):849–853. 10.1126/science.aal4835 [DOI] [PubMed]
- Soo S, Kim AM, Sandro H, Romani LF, Vivek A, Jayaraman Generation of stable heading representations in diverse visual scenes. Nature. 2019;576(7785):126–131. doi: 10.1038/s41586-019-1767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko KI, Root CM, Lindsay SA, Zaninovich OA, Shepherd AK, Wasserman SA, Kim SM, Wang JW. Starvation promotes concerted modulation of appetitive olfactory behavior via parallel neuromodulatory circuits. Elife. 2015;4:e08298. doi: 10.7554/eLife.08298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155(7):1610–1623. doi: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koniszewski ND, Kollmann M, Bigham M, Farnworth M, He B, Büscher M, Hütteroth W, Binzer M, Schachtner J, Bucher G. The insect central complex as model for heterochronic brain development—background, concepts, and tools. Dev Genes Evol. 2016;226(3):209–219. doi: 10.1007/s00427-016-0542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski C, Voigt R, Moore PA. Changes in odour intermittency influence the success and search behaviour during orientation in the crayfish (Orconectes rusticus) Mar Freshw Behav Physiol. 2003;36(2):97–110. doi: 10.1080/1023624031000136279. [DOI] [Google Scholar]
- Kozma MT, Ngo-Vu H, Rump MT, Bobkov YV, Ache BW, Derby CD. Single cell transcriptomes reveal expression patterns of chemoreceptor genes in olfactory sensory neurons of the Caribbean spiny lobster, Panulirus argus. BMC Genomics. 2020;21(1):1–9. doi: 10.1186/s12864-020-07034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma MT, Ngo-Vu H, Wong YY, Shukla NS, Pawar SD, Senatore A, Schmidt M, Derby CD. Comparison of transcriptomes from two chemosensory organs in four decapod crustaceans reveals hundreds of candidate chemoreceptor proteins. PLoS ONE. 2020;15(3):e0230266. doi: 10.1371/journal.pone.0230266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus-Epley KE, Moore PA. Bilateral and unilateral antennal lesions alter orientation abilities of the crayfish, Orconectes rusticus. Chem Senses. 2002;27(1):49–55. doi: 10.1093/chemse/27.1.49. [DOI] [PubMed] [Google Scholar]
- Kress T, Harzsch S, Dircksen H. Neuroanatomy of the optic ganglia and central brain of the water flea Daphnia magna (Crustacea, Cladocera) Cell Tiss Res. 2016;363(3):649–677. doi: 10.1007/s00441-015-2279-4. [DOI] [PubMed] [Google Scholar]
- Kuenen LP, Carde RT. Strategies for recontacting a lost pheromone plume: casting and upwind flight in the male gypsy moth. Physiol Entomol. 1994;19(1):15–29. doi: 10.1111/j.1365-3032.1994.tb01069.x. [DOI] [Google Scholar]
- Kunz T, Kraft KF, Technau GM, Urbach R. Origin of Drosophila mushroom body neuroblasts and generation of divergent embryonic lineages. Development. 2012;139(14):2510–2522. doi: 10.1242/dev.077883. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Laverack MS. The antennular sense organs of Panulirus argus. Comp Biochem Physiol. 1964;13(4):301–321. doi: 10.1016/0010-406X(64)90026-X. [DOI] [PubMed] [Google Scholar]
- Le Moël F, Stone T, Lihoreau M, Wystrach A, Webb B. The central complex as a potential substrate for vector based navigation. Front Psychol. 2019;10:690. doi: 10.3389/fpsyg.2019.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Doe CQ. A locomotor neural circuit persists and functions similarly in larvae and adult Drosophila. Elife. 2021;10:e69767. doi: 10.7554/eLife.69767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126(18):4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Li F, Lindsey JW, Marin EC, Otto N, Dreher M, Dempsey G, Stark I, Bates AS, Pleijzier MW, Schlegel P, Nern A. The connectome of the adult Drosophila mushroom body provides insights into function. Elife. 2020;9:e62576. doi: 10.7554/eLife.62576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Li Y, Potter CJ, Yizhar O, Deisseroth K, Tsien RW, Luo L. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79(5):917–931. doi: 10.1016/j.neuron.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Strausfeld NJ. Visual inputs to the mushroom body calyces of the whirligig beetle Dineutus sublineatus: modality switching in an insect. J Comp Neurol. 2012;520(12):2562–2574. doi: 10.1002/cne.23158. [DOI] [PubMed] [Google Scholar]
- Liu C, Plaçais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488(7412):512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- Lizbinski KM, Marsat G, Dacks AM. Systematic analysis of transmitter coexpression reveals organizing principles of local interneuron heterogeneity. Eneuro. 2018 doi: 10.1523/ENEURO.0212-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockey JK, Willis MA. One antenna, two antennae, big antennae, small: total antennae length, not bilateral symmetry, predicts odor-tracking performance in the American cockroach Periplaneta americana. J Exp Biol. 2015;218(14):2156–2165. doi: 10.1242/jeb.117721. [DOI] [PubMed] [Google Scholar]
- Loesel R, Wolf H, Kenning M, Harzsch S, Sombke A. Architectural principles and evolution of the arthropod central nervous system. In: Minelli A, editor. Arthropod biology and evolution. Berlin, Heidelberg: Springer; 2013. pp. 299–342. [Google Scholar]
- Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB. Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci. 2008;11(2):187–199. doi: 10.1038/nn2031. [DOI] [PubMed] [Google Scholar]
- Lu J, Behbahani AH, Hamburg L, Westeinde EA, Dawson PM, Lyu C, Maimon G, Dickinson MH, Druckmann S, Wilson RI. Transforming representations of movement from body-to world-centric space. Nature. 2022;601(7891):98–104. doi: 10.1038/s41586-021-04191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu C, Abbott LF, Maimon G. Building an allocentric travelling direction signal via vector computation. Nature. 2022;601(7891):92–97. doi: 10.1038/s41586-021-04067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JP, Guo P, Mu L, Harley CM, Ritzmann RE. Central-complex control of movement in the freely walking cockroach. Curr Biol. 2015;25(21):2795–2803. doi: 10.1016/j.cub.2015.09.044. [DOI] [PubMed] [Google Scholar]
- Masters WM, Aicher B, Tautz J, Markl H. A new type of water vibration receptor on the crayfish antenna. J Comp Physiol. 1982;149(3):409–422. doi: 10.1007/BF00619156. [DOI] [Google Scholar]
- Matheson AM, Lanz AJ, Licata AM, Currier TA, Syed MH, Nagel KI. A neural circuit for wind-guided olfactory navigation. Nat Commun. 2022;13(1):1–21. doi: 10.1038/s41467-022-32247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci. 2018;21(9):1281–1289. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- Matsuo E, Yamada D, Ishikawa Y, Asai T, Ishimoto H, Kamikouchi A. Identification of novel vibration-and deflection-sensitive neuronal subgroups in Johnston’s organ of the fruit fly. Frontiers Physiol. 2014;5:179. doi: 10.3389/fphys.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CE, Rosander J, Gottfried J, Dennis E, Dus M. Dietary sugar inhibits satiation by decreasing the central processing of sweet taste. Elife. 2020;9:e54530. doi: 10.7554/eLife.54530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PA, Atema J, Gerhardt GA. Fluid dynamics and microscale chemical movement in the chemosensory appendages of the lobster, Homarus americanus. Chem Senses. 1991;16(6):663–674. doi: 10.1093/chemse/16.6.663. [DOI] [Google Scholar]
- Murlis J, Willis MA, Cardé RT. Spatial and temporal structures of pheromone plumes in fields and forests. Physiol Entomol. 2000;25(3):211–222. doi: 10.1046/j.1365-3032.2000.00176.x. [DOI] [Google Scholar]
- Shigehiro NR, Kanzaki Comparative neuroanatomy of the lateral accessory lobe in the insect brain. Front Physiol. 2016;7:244. doi: 10.3389/fphys.2016.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki S, Iwabuchi S, Pansopha Kono P, Kanzaki R (2014) Information flow through neural circuits for pheromone orientation. Nat Commun 5:5919. 10.1038/ncomms6919 [DOI] [PubMed]
- Namiki S, Dickinson MH, Wong AM, Korff W, Card GM. The functional organization of descending sensory-motor pathways in Drosophila. Elife. 2018 doi: 10.7554/eLife.34272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki S, Ros IG, Morrow C, Rowell WJ, Card GM, Korff W, Dickinson MH. A population of descending neurons that regulates the flight motor of Drosophila. Curr Biol. 2022;32(5):1189–1196. doi: 10.1016/j.cub.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino H, Iwasaki M, Paoli M, Kamimura I, Yoritsune A, Mizunami M. Spatial receptive fields for odor localization. Curr Biol. 2018;28(4):600–608. doi: 10.1016/j.cub.2017.12.055. [DOI] [PubMed] [Google Scholar]
- Okubo TS, Patella P, D’Alessandro I, Wilson RI. A neural network for wind-guided compass navigation. Neuron. 2020;107(5):924–940. doi: 10.1016/j.neuron.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olberg RM. Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth. Bombyx Mori J Comp Physiol. 1983;152(3):297–307. doi: 10.1007/BF00606236. [DOI] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452(7190):956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66(2):287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto JJ, Keleş MF, Nguyen BC, Bolanos C, Lovick JK, Frye MA, Hartenstein V. Visual input to the Drosophila central complex by developmentally and functionally distinct neuronal populations. Curr Biol. 2017;27(8):1098–1110. doi: 10.1016/j.cub.2017.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, Waddell S. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 2015;86(2):417–427. doi: 10.1016/j.neuron.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page JL, Dickman BD, Webster DR, Weissburg MJ. Getting ahead: context-dependent responses to odorant filaments drive along-stream progress during odor tracking in blue crabs. J Exp Biol. 2011;214(9):1498–1512. doi: 10.1242/jeb.049312. [DOI] [PubMed] [Google Scholar]
- Page JL, Dickman BD, Webster DR, Weissburg MJ. Staying the course: chemical signal spatial properties and concentration mediate cross-stream motion in turbulent plumes. J Exp Biol. 2011;214(9):1513–1522. doi: 10.1242/jeb.049304. [DOI] [PubMed] [Google Scholar]
- Pang R, van Breugel F, Dickinson M, Riffell JA, Fairhall A. History dependence in insect flight decisions during odor tracking. PLoS Comp Biol. 2018;14(2):e1005969. doi: 10.1371/journal.pcbi.1005969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli M, Nishino H, Couzin-Fuchs E, Galizia CG. Coding of odour and space in the hemimetabolous insect Periplaneta americana. J Exp Biol. 2020;223(3):jeb218032. doi: 10.1242/jeb.218032. [DOI] [PubMed] [Google Scholar]
- Papadopoulou M, Cassenaer S, Nowotny T, Laurent G. Normalization for sparse encoding of odors by a wide-field interneuron. Science. 2011;332(6030):721–725. doi: 10.1126/science.1201835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patella P, Wilson RI. Functional maps of mechanosensory features in the Drosophila brain. Curr Biol. 2018;28(8):1189–1203. doi: 10.1016/j.cub.2018.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira TD, Aldarondo DE, Willmore L, Kislin M, Wang SS, Murthy M, Shaevitz JW. Fast animal pose estimation using deep neural networks. Nat Methods. 2019;16(1):117–125. doi: 10.1038/s41592-018-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, Mungall C. Tools for neuroanatomy and neurogenetics in Drosophila. PNAS. 2008;105(28):9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps JS, Hildebrand DG, Graham BJ, Kuan AT, Thomas LA, Nguyen TM, Buhmann J, Azevedo AW, Sustar A, Agrawal S, Liu M. Reconstruction of motor control circuits in adult Drosophila using automated transmission electron microscopy. Cell. 2021;184(3):759–774. doi: 10.1016/j.cell.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires PM, Abbott LF, Maimon G. Converting an allocentric goal into an egocentric steering signal. bioRxiv. 2022 doi: 10.1101/2022.11.10.516026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso RA. Wake up and smell the roses: the ecology and evolution of floral scent. Ann Rev Ecol Evol Syst. 2008 doi: 10.1146/annurev.ecolsys.38.091206.095601. [DOI] [Google Scholar]
- Rayshubskiy A, Holtz SL, D’Alessandro I, Li AA, Vanderbeck QX, Haber IS, Gibb PW, Wilson RI. Neural circuit mechanisms for steering control in walking Drosophila. bioRxiv. 2020 doi: 10.1101/2020.04.04.024703. [DOI] [Google Scholar]
- Ren Q, Yang CP, Liu Z, Sugino K, Mok K, He Y, Ito M, Nern A, Otsuna H, Lee T. Stem cell-intrinsic, seven-up-triggered temporal factor gradients diversify intermediate neural progenitors. Curr Biol. 2017;27(9):1303–1313. doi: 10.1016/j.cub.2017.03.047. [DOI] [PubMed] [Google Scholar]
- Riebli N, Viktorin G, Reichert H. Early-born neurons in type II neuroblast lineages establish a larval primordium and integrate into adult circuitry during central complex development in Drosophila. Neural Dev. 2013;8(1):1–7. doi: 10.1186/1749-8104-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigolli N, Reddy G, Seminara A, Vergassola M. Alternation emerges as a multi-modal strategy for turbulent odor navigation. Elife. 2022;11:e76989. doi: 10.7554/eLife.72196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nässel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59(2):311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145(1):133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospars JP, Hildebrand JG. Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem Senses. 2000;25(2):119–129. doi: 10.1093/chemse/25.2.119. [DOI] [PubMed] [Google Scholar]
- Rutkowski AJ, Miller MM, Quinn RD, Willis MA. Egomotion estimation with optic flow and air velocity sensors. Biol Cyber. 2011;104(6):351–367. doi: 10.1007/s00422-011-0440-z. [DOI] [PubMed] [Google Scholar]
- Rytz R, Croset V, Benton R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Bio. 2013;43(9):888–897. doi: 10.1016/j.ibmb.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Sachse S, Galizia CG. Topography and dynamics of the olfactory system. Cambridge: MIT Press; 2006. pp. 251–273. [Google Scholar]
- Sane SP. The aerodynamics of insect flight. J Exp Biol. 2003;206(23):4191–4208. doi: 10.1242/jeb.00663. [DOI] [PubMed] [Google Scholar]
- Sareen P, McCurdy LY, Nitabach MN. A neuronal ensemble encoding adaptive choice during sensory conflict. Nat Commun. 2021;12(1):1–13. doi: 10.1038/s41467-021-24423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452(7190):1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Sayin S, De Backer JF, Siju KP, Wosniack ME, Lewis LP, Frisch LM, Gansen B, Schlegel P, Edmondson-Stait A, Sharifi N, Fisher CB, et al. A neural circuit arbitrates between persistence and withdrawal in hungry Drosophila. Neuron. 2019;104(3):544–558. doi: 10.1016/j.neuron.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre ME, Templin R, Chavez J, Kempenaers J, Heinze S. A projectome of the bumblebee central complex. Elife. 2021;10:e68911. doi: 10.7554/eLife.68911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaplen KM, Talay M, Fisher JD, Cohn R, Sorkaç A, Aso Y, Barnea G, Kaun KR. Transsynaptic mapping of Drosophila mushroom body output neurons. Elife. 2021;10:e63379. doi: 10.7554/eLife.63379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel P, Bates AS, Stürner T, Jagannathan SR, Drummond N, Hsu J, Capdevila LS, Javier A, Marin EC, Barth-Maron A, Tamimi IF, et al. Information flow, cell types and stereotypy in a full olfactory connectome. Elife. 2021;10:e66018. doi: 10.7554/eLife.66018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Mellon D. Neuronal processing of chemical information in crustaceans. In: Breithaupt T, Thiel M, editors. Chemical communication in crustaceans. New York: Springer; 2010. pp. 123–147. [Google Scholar]
- Schulze A, Gomez-Marin A, Rajendran VG, Lott G, Musy M, Ahammad P, Deogade A, Sharpe J, Riedl J, Jarriault D, Trautman ET. Dynamical feature extraction at the sensory periphery guides chemotaxis. Elife. 2015 doi: 10.7554/eLife.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeholzer LF, Seppo M, Stern DL, Ruta V. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature. 2018;559(7715):564–569. doi: 10.1038/s41586-018-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig JD, Jayaraman V. Neural dynamics for landmark orientation and angular path integration. Nature. 2015;521(7551):186–191. doi: 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields VD, Hildebrand JG. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J Comp Physiol A. 2001;186(12):1135–1151. doi: 10.1007/s003590000165. [DOI] [PubMed] [Google Scholar]
- Siju KP, Štih V, Aimon S, Gjorgjieva J, Portugues R, Kadow IC. Valence and state-dependent population coding in dopaminergic neurons in the fly mushroom body. Curr Biol. 2020;30(11):2104–2115. doi: 10.1016/j.cub.2020.04.037. [DOI] [PubMed] [Google Scholar]
- Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, Benton R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31(38):13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombke A, Harzsch S, Hansson BS. Organization of deutocerebral neuropils and olfactory behavior in the centipede Scutigera coleoptrata (Linnaeus, 1758) (Myriapoda: Chilopoda) Chem Senses. 2011;36(1):43–61. doi: 10.1093/chemse/bjq096. [DOI] [PubMed] [Google Scholar]
- Steck K, Knaden M, Hansson BS. Do desert ants smell the scenery in stereo? Anim Behav. 2010;79(4):939–945. doi: 10.1016/j.anbehav.2010.01.011. [DOI] [Google Scholar]
- Stone T, Webb B, Adden A, Weddig NB, Honkanen A, Templin R, Wcislo W, Scimeca L, Warrant E, Heinze S. An anatomically constrained model for path integration in the bee brain. Curr Biol. 2017;27(20):3069–3085. doi: 10.1016/j.cub.2017.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ, Sinakevitch I, Brown SM, Farris SM. Ground plan of the insect mushroom body: functional and evolutionary implications. J Comp Neurol. 2009;513(3):265–291. doi: 10.1002/cne.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ, Wolff GH, Sayre ME. Mushroom body evolution demonstrates homology and divergence across Pancrustacea. Elife. 2020 doi: 10.7554/eLife.52411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13(5):1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube-Bloss MF, Nawrot MP, Menzel R. Mushroom body output neurons encode odor–reward associations. J Neurosci. 2011;31(8):3129–3140. doi: 10.1523/JNEUROSCI.2583-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz A, Soelter J, Baschwitz A, Farhan A, Grabe V, Rybak J, Knaden M, Schmuker M, Hansson BS, Sachse S. Decoding odor quality and intensity in the Drosophila brain. Elife. 2014;3:e04147. doi: 10.7554/eLife.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis F, Sullivan Timothy L, Warren Chris Q. Temporal identity establishes columnar neuron morphology connectivity and function in a Drosophila navigation circuit. eLife. 2019;8:e43482. doi: 10.7554/eLife.43482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SA, Romain N, Hod F, Dana Eric R, Schreiter Loren L, Karel L, Svoboda Douglas S, Kim Ann M, Vivek H, Jayaraman V. Neural signatures of dynamic stimulus selection in Drosophila. Nat Neurosci. 2017;20(8):1104–1113. doi: 10.1038/nn.4581. [DOI] [PubMed] [Google Scholar]
- Sun X, Yue S, Mangan M. How the insect central complex could coordinate multimodal navigation. Elife. 2021;10:e73077. doi: 10.7554/eLife.73077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suver MP, Matheson AM, Sarkar S, Damiata M, Schoppik D, Nagel KI. Encoding of wind direction by central neurons in Drosophila. Neuron. 2019;102(4):828–842. doi: 10.1016/j.neuron.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka P, Gerkin RC, Galizia CG, Smith BH. High-speed odor transduction and pulse tracking by insect olfactory receptor neurons. PNAS. 2014;111(47):16925–16930. doi: 10.1073/pnas.1412051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taisz I, Donà E, Münch D, Bailey SN, Morris WJ, Meechan KI, Stevens KM, Varela I, Gkantia M, Schlegel P, Ribeiro C, et al. Generating parallel representations of position and identity in the olfactory system. bioRxiv. 2022 doi: 10.1101/2022.05.13.491877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki T, Namiki S, Kanzaki R. Use of bilateral information to determine the walking direction during orientation to a pheromone source in the silkmoth Bombyx mori. J Comp Physiol A. 2012;198(4):295–307. doi: 10.1007/s00359-011-0708-8. [DOI] [PubMed] [Google Scholar]
- Task D, Lin CC, Vulpe A, Afify A, Ballou S, Brbic M, Schlegel P, Raji J, Jefferis G, Li H, Menuz K, et al. Chemoreceptor co-expression in Drosophila melanogaster olfactory neurons. Elife. 2022;11:e72599. doi: 10.7554/eLife.72599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastekin I, Khandelwal A, Tadres D, Fessner ND, Truman JW, Zlatic M, Cardona A, Louis M. Sensorimotor pathway controlling stopping behavior during chemotaxis in the Drosophila melanogaster larva. Elife. 2018;7:e38740. doi: 10.7554/eLife.38740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz J, Masters WM, Aicher B, Markl H. A new type of water vibration receptor on the crayfish antenna. J Comp Physiol. 1981;144(4):533–541. doi: 10.1007/BF01326838. [DOI] [Google Scholar]
- Thistle HW, Peterson H, Allwine G, Lamb B, Strand T, Holsten EH, Shea PJ. Surrogate pheromone plumes in three forest trunk spaces: composite statistics and case studies. Forest Sci. 2004;50(5):610–625. doi: 10.1093/forestscience/50.5.610. [DOI] [Google Scholar]
- Thoen HH, Marshall J, Wolff GH, Strausfeld NJ. Insect-like organization of the stomatopod central complex: functional and phylogenetic implications. Front Behav Neurosci. 2017;11:12. doi: 10.3389/fnbeh.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirian L, Dickson BJ. The VT GAL4, LexA, and split-GAL4 driver line collections for targeted expression in the Drosophila nervous system. bioRxiv. 2017 doi: 10.1101/198648. [DOI] [Google Scholar]
- Truman JW, Price J, Miyares RL, Lee T. Metamorphosis of memory circuits in Drosophila reveal a strategy for evolving a larval brain. bioRxiv. 2022 doi: 10.1101/2022.06.09.495452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CH, Chen CC, Lin CH, Yang HY, Lin S. Drosophila mushroom bodies integrate hunger and satiety signals to control innate food-seeking behavior. Elife. 2018;7:e35264. doi: 10.7554/eLife.35264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2008;99(2):734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- Turner-Evans D, Wegener S, Rouault H, Franconville R, Wolff T, Seelig JD, Druckmann S, Jayaraman V. Angular velocity integration in a fly heading circuit. Elife. 2017;6:e23496. doi: 10.7554/eLife.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach R, Technau GM. Neuroblast formation and patterning during early brain development in Drosophila. BioEssays. 2004;26(7):739–751. doi: 10.1002/bies.20062. [DOI] [PubMed] [Google Scholar]
- Utting M, Agricola HJ, Sandeman R, Sandeman D. Central complex in the brain of crayfish and its possible homology with that of insects. J Comp Neurol. 2000;416(2):245–261. doi: 10.1002/(SICI)1096-9861(20000110)416:2<245::AID-CNE9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- van Breugel F, Dickinson MH. Plume-tracking behavior of flying Drosophila emerges from a set of distinct sensory-motor reflexes. Curr Biol. 2014;24(3):274–286. doi: 10.1016/j.cub.2013.12.023. [DOI] [PubMed] [Google Scholar]
- van Breugel F, Jewell R, Houle J. Active anemosensing hypothesis: how flying insects could estimate ambient wind direction through sensory integration & active movement. J R Soc Interface. 2022;19(193):20220258. doi: 10.1098/rsif.2022.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel JP, Clarac F. Hydrodynamic sensitivity by cuticular organs in the rock lobster Palinurus vulgar is. morphological and physiological aspects. Mar Freshw Behav Physiol. 1976;3(4):235–251. doi: 10.1080/10236247609378514. [DOI] [Google Scholar]
- Vickers NJ, Baker TC. Reiterative responses to single strands of odor promote sustained upwind flight and odor source location by moths. PNAS. 1994;91(13):5756–5760. doi: 10.1073/pnas.91.13.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Boie SD, Connor EG, Crimaldi JP, Ermentrout GB, Nagel KI. Olfactory navigation and the receptor nonlinearity. J Neurosci. 2019;39(19):3713–3727. doi: 10.1523/JNEUROSCI.2512-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Zimmerman DM, Schlichting M, Hernandez-Nunez L, Qin S, Malacon K, Rosbash M, Pehlevan C, Cardona A, Samuel AD. Internal state configures olfactory behavior and early sensory processing in Drosophila larvae. Sci Adv. 2021;7(1):eabd6900. doi: 10.1126/sciadv.abd6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96(5):725–736. doi: 10.1016/S0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102(2):147–159. doi: 10.1016/S0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Vrontou E, Groschner LN, Szydlowski S, Brain R, Krebbers A, Miesenböck G. Response competition between neurons and antineurons in the mushroom body. Curr Biol. 2021;31(22):4911–4922. doi: 10.1016/j.cub.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KT, Doe CQ. Drosophila embryonic type II neuroblasts: origin, temporal patterning, and contribution to the adult central complex. Development. 2017;144(24):4552–4562. doi: 10.1242/dev.157826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S, Lu P, Aptekar JW, Frye MA. Flies dynamically anti-track, rather than ballistically escape, aversive odor during flight. J Exp Biol. 2012;215(16):2833–2840. doi: 10.1242/jeb.072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Weissburg MJ. The hydrodynamics of chemical cues among aquatic organisms. Annu Rev Fluid Mech. 2009;41(1):73–90. doi: 10.1146/annurev.fluid.010908.165240. [DOI] [Google Scholar]
- Weissburg MJ. The fluid dynamical context of chemosensory behavior. Biol Bull. 2000;198(2):188–202. doi: 10.2307/1542523. [DOI] [PubMed] [Google Scholar]
- Weissburg MJ, Zimmer-Faust RK. Odor plumes and how blue crabs use them in finding prey. J Exp Biol. 1994;197(1):349–375. doi: 10.1242/jeb.197.1.349. [DOI] [PubMed] [Google Scholar]
- Weissburg MJ, Doall MH, Yen J. Following the invisible trail: kinematic analysis of mate-tracking in the copepod Temora longicornis. Phil Trans R Soc Lond Ser B: Biol Sci. 1998;353(1369):701–712. doi: 10.1098/rstb.1998.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westeinde EA, Kellogg E, Dawson PM, Lu J, Hamburg L, Midler B, Druckmann S, Wilson RI. Transforming a head direction signal into a goal-oriented steering command. bioRxiv. 2022 doi: 10.1101/2022.11.10.516039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D, Schäfer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452(7190):1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Willis MA, Avondet JL. Odor-modulated orientation in walking male cockroaches Periplaneta americana, and the effects of odor plumes of different structure. J Exp Biol. 2005;208(4):721–735. doi: 10.1242/jeb.01418. [DOI] [PubMed] [Google Scholar]
- Wolf H, Wehner R. Pinpointing food sources: olfactory and anemotactic orientation in desert ants, Cataglyphis fortis. J Exp Biol. 2000;203(5):857–868. doi: 10.1242/jeb.203.5.857. [DOI] [PubMed] [Google Scholar]
- Wolff GH, Strausfeld NJ. Genealogical correspondence of mushroom bodies across invertebrate phyla. Curr Biol. 2015;25(1):38–44. doi: 10.1016/j.cub.2014.10.049. [DOI] [PubMed] [Google Scholar]
- Wolff GH, Thoen HH, Marshall J, Sayre ME, Strausfeld NJ. An insect-like mushroom body in a crustacean brain. Elife. 2017;6:e29889. doi: 10.7554/eLife.29889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Awasaki T, Yu HH, He Y, Ding P, Kao JC, Lee T. Diverse neuronal lineages make stereotyped contributions to the Drosophila locomotor control center, the central complex. J Comp Neurol. 2013;12:2645–2662. doi: 10.1002/cne.23339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, Anderson DJ. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458(7235):201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Awasaki T, Schroeder MD, Long F, Yang JS, He Y, Ding P, Kao JC, Wu GY, Peng H, Myers G. Clonal development and organization of the adult Drosophila central brain. Curr Biol. 2013;23(8):633–643. doi: 10.1016/j.cub.2013.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Löfstedt C. Moth pheromone receptors: gene sequences, function, and evolution. Frontiers Ecol Evol. 2015;3:105. doi: 10.3389/fevo.2015.00105. [DOI] [Google Scholar]
- Zhang C, Guy RD, Mulloney B, Zhang Q, Lewis TJ. Neural mechanism of optimal limb coordination in crustacean swimming. PNAS. 2014;111(38):13840–13845. doi: 10.1073/pnas.1323208111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer-Faust RK, Finelli CM, Pentcheff ND, Wethey DS. Odor plumes and animal navigation in turbulent water flow: a field study. Biol Bull. 1995;188(2):111–116. doi: 10.2307/1542075. [DOI] [PubMed] [Google Scholar]
- Zocchi D, Emily SY, Hauser V, O’Connell TF, Hong EJ. Parallel encoding of CO2 in attractive and aversive glomeruli by selective lateral signaling between olfactory afferents. Curr Biol. 2022;32(19):4225–4239. doi: 10.1016/j.cub.2022.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolin A, Cohn R, Pang R, Siliciano AF, Fairhall AL, Ruta V. Context-dependent representations of movement in Drosophila dopaminergic reinforcement pathways. Nat Neurosci. 2021;24(11):1555–1566. doi: 10.1038/s41593-021-00929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorović M, Hedwig B. Processing of species-specific auditory patterns in the cricket brain by ascending, local, and descending neurons during standing and walking. J Neurophysiol. 2011;105(5):2181–2194. doi: 10.1152/jn.00416.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated during this study.