Abstract
Alterations in glutamatergic and GABAergic function in the medial prefrontal cortex (mPFC) are prevalent in individuals with major depressive disorder, resulting in impaired synaptic plasticity that compromises the integrity of signal transfer to limbic regions. Scopolamine, a non-selective muscarinic receptor antagonist, produces rapid antidepressant-like effects by targeting M1-type acetylcholine receptors (M1R) on somatostatin (SST) interneurons. So far, these effects have been investigated with relatively short-term manipulations, and long-lasting synaptic mechanisms involved in these responses are still unknown. Here, we generated mice with conditional deletion of M1R (M1f/fSstCre+) only in SST interneurons to determine the role of M1R in modulating long-term GABAergic and glutamatergic plasticity in the mPFC that leads to attenuation of stress-relevant behaviors. We have also investigated whether the molecular and antidepressant-like effects of scopolamine could be mimicked or occluded in male M1f/fSstCre+ mice. M1R deletion in SST-expressing neurons occluded the rapid and sustained antidepressant-like effects of scopolamine, as well as scopolamine-induced increases in c-Fos+/CaMKIIα cells and proteins necessary for glutamatergic and GABAergic function in the mPFC. Importantly, M1R SST deletion resulted in resilience to chronic unpredictable stress in behaviors relevant to coping strategies and motivation, and to a lesser extent, in behaviors relevant to avoidance. Finally, M1R SST deletion also prevented stress-induced impairments in the expression of GABAergic and glutamatergic markers in the mPFC. These findings suggest that the antidepressant-like effects of scopolamine result from modulation of excitatory and inhibitory plasticity via M1R blockade in SST interneurons. This mechanism could represent a promising strategy for antidepressant development.
Subject terms: Stress and resilience, Depression
Introduction
Currently available antidepressants have serious limitations for treating major depressive disorder (MDD), including low response rates, a significant number of treatment resistant patients, and a time-lag before there is a therapeutic response. Low doses of scopolamine, a non-selective muscarinic acetylcholine receptor antagonist, can induce rapid and sustained antidepressant effects in patients diagnosed with MDD [1–4].
Behavioral studies of scopolamine action have provided compelling evidence for the recruitment of the cholinergic system in rapid antidepressant responses. Cholinergic neurotransmission controls plasticity both locally and at the circuit-level by coordinating postsynaptic neuronal excitability and firing, as well as presynaptic release of neurotransmitters [5]. Of the five subtypes of metabotropic acetylcholine muscarinic receptors (M1-M5), Gαq-coupled M1R is the most abundant in the prefrontal cortex and is densely expressed in pyramidal neurons [6]. Nevertheless, GABA interneurons also express M1R, including ~60% of somatostatin (SST) cells in the medial prefrontal cortex (mPFC) [7], which exhibit larger depolarizing responses to cholinergic activation than other subtype of interneurons [7–9]. In the neocortex, acetylcholine-induced activation of M1R modulates plasticity at both GABAergic and glutamatergic synapses, thereby regulating activity of excitatory and inhibitory neurons, and influencing network dynamics [5].
Alterations in GABA signaling disrupt network activity, leading to reduced efficiency and precision of glutamatergic excitatory networks, and aberrant information transfer to target regions, resulting in maladaptive states that are thought to contribute to the pathophysiology of MDD [10]. Indeed, both human subjects with MDD and chronically stressed rodents show disruptions in GABA and glutamate function, leading to decreases in mPFC volume, spine density and neuronal atrophy [11–19]. Conversely, rapid-acting antidepressants, including scopolamine, rapidly reverse these functional and structural impairments by promoting synaptogenesis and new spine formation in the mPFC [10, 13, 14, 17–24].
A major question in the field is, what are the initial cellular triggers for rapid antidepressant responses and the long-lasting functional rearrangements that drive sustained effects? Previous studies have shown that fast antidepressant-like effects in rodent models are mediated by an initial decrease in activity of GABA interneurons, notably SST cells, leading to rapid disinhibition of pyramidal neurons and a transient glutamate burst that drives synaptic plasticity. In addition, more recent evidence suggests that adaptive enhancement of GABA signaling may contribute to sustained antidepressant effects by reestablishing optimal excitation and inhibition levels in the mPFC and correct firing patterns in target regions [10, 13, 14, 25–28].
In this study, we generated mice with conditional M1R deletion in SST interneurons (M1f/fSstCre+) as an animal model to study long-term consequences of this manipulation on biochemical markers of GABAergic and glutamatergic synaptic function that lead to attenuation of stress-relevant behavioral outcomes. We investigated whether the antidepressant-like effects of scopolamine can be mimicked (behaviors under baseline or stress conditions) or occluded (scopolamine challenge) by this deficit in M1 function in SST neurons in male M1f/fSstCre+ mice. These studies build on previous experiments showing that an initial recruitment of glutamatergic function is required for the rapid antidepressant-like effects of scopolamine, and extend these studies to investigate adaptive changes in the GABA system that could be involved in attenuation of stress-relevant behaviors.
Materials and methods
Animals
Male transgenic mice and littermates (8–12-week-old) were bred in-house. M1 ‘floxed’ mice were obtained from Dr. Susumu Tonegawa (MIT), and were generated by gene targeting in embryonic stem cells from C57BL6/J mice, as described [29]. Sst-Cre (#013044) mice were obtained from Jackson Laboratories. To generate M1f/fSstCre+ mice (mutant) and M1f/+SstCre- littermates (control), M1 ‘floxed’ mice were crossed with Sst-Cre heterozygous mice to produce M1f/+SstCre- and M1f/fSstCre+ offspring. Then, M1f/+SstCre- and M1f/fSstCre+ mice were bred to generate control and mutant littermate mice used in the experiments. All animals were group-housed with a 12/12 h light-dark cycle and food and water ad libitum. All procedures were conducted in compliance with the National Institute of Health (NIH) guidelines for the care and use of laboratory animals and were approved by the Yale Institutional Animal Care and Use Committee.
Drug administration
Scopolamine (Sigma-Aldrich, 25 µg/kg, i.p.) was dissolved in saline and administered every other day for 3 days, based on previous studies [3, 7, 22, 30], and behavioral testing started 24 h after the last injection. For c-Fos analysis, scopolamine was administered in a single dose (25 µg/kg, i.p.) and animals were perfused 60 min later. Muscarine (Sigma-Aldrich) was dissolved in saline and used for slice electrophysiology at 60 μM [7].
Chronic unpredictable stress
Animals were exposed to a sequence of two random unpredictable stressors per day for 21 days, accordingly to a previously established protocol [14, 22, 31]. Stressors included: wet bedding (overnight), bedding removal (overnight), inverted light-dark cycle, restraint (2 h), water deprivation (overnight), peppermint odor (overnight), cage tilting (overnight), cage shaking (30 min) and isolation (24 h). CUS continued on the days between behavioral tests. To avoid acute effects of stress, animals were not exposed to any stressors on the days of treatments or prior to behavioral tests. Control animals (non-stressed) were handled daily but were not exposed to any stressors.
Behavioral studies
Behavioral studies in mice that did not undergo CUS were conducted using two independent cohorts for separate batteries of tests to avoid the influence of acute stress that could result from multiple testing. Animals were habituated to testing rooms 30 min before each experiment. All behavioral tests were video recorded and conducted between 10 a.m. and 3 p.m. Experiments were scored by an experimenter blind to treatments.
Forced Swim Test (FST)
Mice were placed in a clear cylinder filled with water (25 °C ± 1) and sessions were scored for total immobility time during minutes 2–6, as previously described [19, 22]. For experiments involving scopolamine treatment, mice were exposed to two swim sessions, including a pre-swim session for evaluation of baseline behaviors (before treatment), followed by the FST (after treatment). For experiments involving CUS exposure, mice were only exposed to the FST.
Novelty-suppressed feeding test (NSFT)
Mice were food deprived for 16 h and placed in a dimly lit box (40 × 40 × 25) with a pellet of food in the center; the latency to feed was measured with a time limit of 12 min, as described [22]. Immediately after the test, home cage food intake was measured over a 12 min period as a non-stressed feeding control.
Female urine sniffing test (FUST)
Mice were habituated for 60 min to a sterile cotton-tipped applicator placed in their home cage, as described [22, 32]. Mice were first exposed to a new cotton tip dipped in sterile water and 45 min later mice were exposed to a new cotton tip infused with fresh urine collected from females of the same strain. Urine or water sniffing time was measured over 5 min. Time spent biting the cotton tip was not included in the analysis.
Elevated plus-maze test (EPM)
The EPM was performed in a dimly lit room (60 lux). Animals were placed on the central platform with the head facing one of the enclosed arms of a plus-shaped maze (61 cm arm length, 6.5 cm arm width, 25 cm wall height, 45 cm above the floor). Each animal was allowed to explore the maze for 5 min, as described [33]. Percentage of open arm entries, percentage of time spent in the open arms and the number of enclosed arm entries were analyzed by Anymaze Software (version 4.5, Stoelting, Wood Dale-USA). Animals were only considered to enter an arm when 85% of their body was inside the region.
Sucrose splash test (SUST)
A 10% sucrose solution was squirted onto the dorsal coat of the mouse, as has been described [34]. Grooming time was measured for 5 min.
Locomotor activity (LMA)
LMA was determined using an infrared automated tracking system (Med Associates) during 30 min [22, 31].
Western blotting
Western blotting was conducted as previously described [14, 22, 35], using the synaptosome fraction of mPFC homogenates. Briefly, cells were collected into RIPA lysis buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM NaVO3, 5 mM NaF and 1× protease inhibitor cocktail). For synaptosome preparations, cells were homogenized in a solution containing 0.32 M sucrose, 20 mm Hepes (pH 7.4), 1 mM EDTA, 1× protease inhibitor cocktail, 10 mM NaF and 1 mM NaVO3. Homogenates were then centrifuged at 2800 rpm, supernatants were collected and further centrifuged at 12,000 rpm to obtain a pellet containing crude synaptosomes. Pellets were then resuspended and sonicated in RIPA lysis buffer. Total protein concentrations were measured using a BCA kit (Thermo Scientific, USA). Proteins were detected with primary antibodies for proteins relevant to glutamate (rabbit anti-PSD95 #2507, rabbit anti-GluA1 #13185, rabbit anti-VGLUT1 #12335, 1:1000, Cell Signaling) and GABA signaling (rabbit anti-GAD1 #5305, 1:1000, Cell Signaling; rabbit anti-VGAT #AB5062P, 1:1000, Millipore; rabbit anti-gephyrin #AB5725, 1:500, Millipore), and appropriate secondary antibodies (anti-rabbit, 1:3000 for VGLUT1 and gephyrin, and 1:5000 for all the others, Vector Laboratories), as we have previously validated [14, 22]; labeled bands were visualized with chemiluminescent reagent (ECL, GE Healthcare). Unsaturated and preserved bands were quantified using the ImageJ Software and Image Studio Lite (ver 5.2). GAPDH (#5174 rabbit, 1:5000, Cell Signaling) was used for loading control and normalization.
Immunofluorescence and viral infection efficiency
c-Fos, M1R, SST, CaMKIIα, parvalbumin (PV), VGLUT1 and VGAT immunofluorescence were evaluated using primary antibodies (rabbit anti-c-Fos #ab214672, 1:1000, Abcam; rabbit anti-M1R #RB-Af340, 1:1000, Frontier Institute; mouse anti- CaMKIIα #MA1-048, 1:1000, ThermoFisher; mouse anti-PV #ab277625, 1:1000, Abcam; mouse anti-SST #sc-74556, 1:300, Santa Cruz; guinea-pig anti-VGLUT1 #135304, 1:2000, Synaptic Systems; rabbit anti-VGAT #131002, 1:1000, Synaptic Systems) and appropriate secondary antibodies (AlexaFluor® 488 goat anti-rabbit or anti-mouse, AlexaFluor® 647 goat anti-guinea-pig, anti-mouse or anti-rabbit, 1:1000), as described [14, 22]. For quantification, sections containing the mPFC were analyzed by an experimenter blind to treatments using a confocal microscope to obtain Z-stack image sequences (Leica TSE-SPE). See Supplementary Methods for further details.
Electrophysiological recordings
For whole-cell recordings, coronal slices containing the mPFC were prepared from M1f/fSstCre+ male mice, as described [22, 36]. Pyramidal neurons were visualized using a microscope (40x IR lens) with infrared differential interference contrast (IR/DIC). For visualization of SST cells, SstCre+ (control) and M1f/fSstCre+ (mutant) male mice received infusion of a Cre-recombinase mCherry virus (AAV2-hSyn-DIO-mCherry, #50459, titer ≥ 5 × 1012 vg/ml, Addgene, USA). For this, anesthetized mice (ketamine/xylazine, 100/10 mg/kg) received bilateral infusions of mCherry virus (0.5 µl/side; 0.1 µl/min) into the mPFC (coordinates from bregma: anterior-posterior: +1.9 mm; medial-lateral: ±0.4 mm; dorsal-ventral −2.7 mm) [22]; mice were used after a 2–3 week recovery period. Interneurons expressing mCherry were visualized using the appropriate filter. Inward currents induced by muscarine (60 µM), delivered by y-tube for 15 s, were recorded under voltage-clamp at a holding potential of −70 mV. Postsynaptic currents (PSCs) were measured in continuous single-electrode voltage-clamp mode (3000 Hz low-pass filter) at −65 mV to separate inhibitory (IPSCs) from excitatory postsynaptic currents (EPSCs). Whole-cell recordings were made with an Axoclamp-2B amplifier. See Supplementary Methods for further details.
Statistical analysis
Results were analyzed by Student’s two-tailed t-test and one- or two-way ANOVA, as appropriate, followed by Duncan test, p ≤ 0.05. All distributions were tested for homogeneity of variance using Levene’s test and for normality using the Kolmogorov–Smirnov test. Distributions that were not homogeneous and did not follow a normal distribution were corrected by logarithmic transformation. Distributions that could not be corrected were analyzed by Mann–Whitney or Kruskal–Wallis H test. Sample sizes were chosen based on previous experience with the tests employed and power analyses (Cohen’s d power analysis, > 0.8 effect size) conducted following a pilot study (not shown). For all analyses, we used the SPSS Software (Version 20.0). Each experiment was replicated a minimum of 2 times.
Principal component analysis (PCA) identifies linear combinations of the variables that maximally explain the variance of the data, allowing for a reduction in the dimensionality of the dataset. Here, the behavioral scores from different tests in one of three categories (All Behaviors, Coping/Motivated Behaviors or Avoidance Behaviors) or protein expression levels related to one of two categories (GABA-related or Glutamate-related signaling) were subjected to PCA. The categories were as follows: (1) All Behaviors (FST, SUST, FUST, EPM Poa, EPM Pta and NSFT); (2) Coping/Motivated Behaviors (FST, SUST and FUST), (3) Avoidance Behaviors (NSFT, EPM Poa and EPM Pta), (4) Glutamate Signaling (VGLUT1, PSD95 and GluA1), and (5) GABA Signaling (VGAT, gephyrin and GAD1). Data from each category were subjected to KMO and Bartlett’s test of sphericity to determine appropriateness for PCA. PCA with a varimax rotation was performed on the correlation matrix for each category. The resulting principal components with eigenvalues >1 were selected. In 4 out of 5 categories only one component/factor was identified (for the All Behaviors category two factors were identified, and the one with the maximal eigenvalue was used). For each category analyzed, this resultant principal component/factor score was used to conduct subsequent statistical analysis.
Results
Selective genetic deletion of M1R in SST interneurons
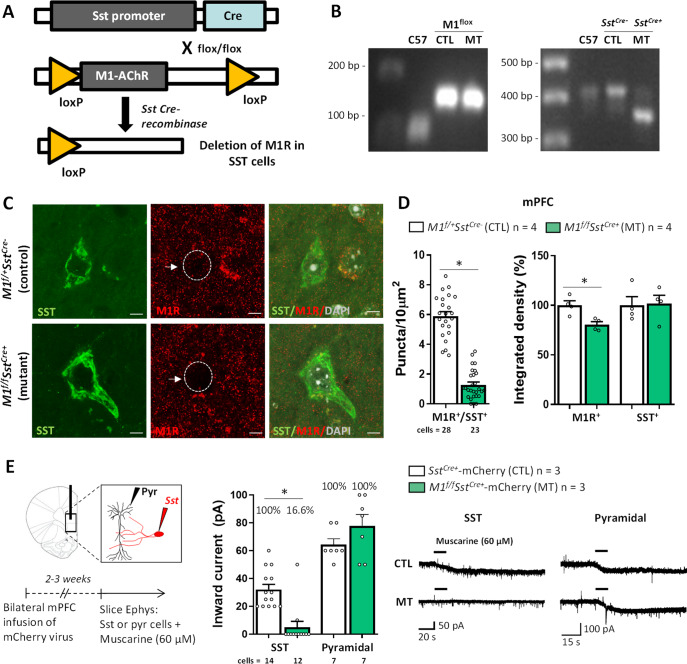
We deleted M1R specifically in SST interneurons using a conditional knockout strategy to investigate the involvement of SST M1R-related mechanisms in stress-relevant behaviors and biochemical markers of GABA and glutamatergic synaptic activity (Fig. 1A, B). In this first set of studies using M1f/fSstCre+ mice we used male mice to validate the model for direct comparison with previous studies investigating the mechanisms of action of scopolamine, which were conducted in male mice [7, 22, 37, 38]. To delete M1R selectively in SST interneurons, we crossed mice expressing Cre-recombinase under control of the SST promoter (SstCre) with mice in which loxP sites were inserted into the M1R gene (floxed M1R mice). To validate the selectivity and efficiency of M1R knockout in SST neurons, we performed double immunohistochemistry for M1R and SST in the mPFC (Fig. 1C, D). The soma of SST cells display punctate M1R staining, as previously reported [7]. The number of M1R puncta that overlap with SST+ soma is significantly reduced in M1f/fSstCre+ mice compared to littermate controls. We also measured overall M1R and SST labeling in the mPFC of M1f/fSstCre+ mice and observed a ~25% reduction in M1R expression with no alteration in SST levels, suggesting that the genetic manipulation effectively reduced M1R expression without altering overall SST labeling. In addition, there was no change in M1R expression in either CaMKIIα or PV cells, indicating that the genetic manipulation was selective to SST interneurons (Supplementary Fig. 1A, B).
Fig. 1. Selective genetic deletion of M1R in somatostatin interneurons.
A Strategy for conditional M1R knockout in somatostatin (SST) interneurons. B Examples of PCR genotyping of M1flox (first gel) and Sst-Cre (second gel) from background (C57BL/6J), control (CTL, M1f/+SstCre-) and mutant (MT, M1f/fSstCre+) strains. C Representative micrographs of M1R expression in the soma of SST+ interneurons in control mice and mice with selective deletion of M1R (M1f/fSstCre+). Scale bar: 5 µm. Magnification: ×100 + ×3 zoom. D M1R puncta (per 10 µm2) co-localized with SST labeling in floxed M1 mice with expression of Cre recombinase in SST interneurons (t49 = 13.43, p < 0.05), and percentage of integrated density of overall M1R (t6 = 3.55, p < 0.05) or SST expression (t6 = 0.14, p > 0.05) in the mPFC of M1f/fSstCre+ and control mice. E Patch-clamp electrophysiology showing muscarine-induced inward currents in SST+ interneurons or pyramidal cells in brain slices from M1f/fSstCre+ and control mice that received bilateral infusion of a Cre-dependent mCherry virus into the mPFC to label SST+ cells (SST: t24 = 4.94, p < 0.05; cells that responded to muscarine, CTL: 100%, MT: 16.66%. Pyramidal: t12 = 1.47, p > 0.05; cells that responded to muscarine, CTL: 100%, MT: 100%). Traces represent inward currents from SST neurons following application of muscarine (60 μM). Each bar represents the mean ± standard error of the mean (S.E.M.). *p ≤ 0.05 compared to the control group, Student’s t-test.
To confirm that functional M1 responses were decreased in SST neurons of SstCre+ and M1f/fSstCre+ mice, muscarine-induced currents were recorded in mPFC slices using whole-cell patch clamp (Fig. 1E). While 100% of SST cells responded to muscarine application in mPFC slices from control animals, only 16.6% of SST cells responded to muscarine in slices from M1f/fSstCre+ mice; in addition, these cells showed a significant reduction in inward currents, suggesting that M1f/fSstCre+ mice lack M1R in SST interneurons as expected. Conversely, 100% of mPFC pyramidal cells responded to muscarine in both M1f/fSstCre+ and control groups (Fig. 1E), and there was no difference in amplitude or frequency of inward currents between groups, indicating that the SST M1R deletion did not affect muscarinic receptor function in pyramidal neurons.
Deletion of M1R in SST interneurons does not change baseline behaviors or synaptic protein levels in the mPFC, but alters PSCs in pyramidal neurons
To determine whether SST M1 knockout altered baseline differences in stress-relevant behaviors, separate cohorts of mice underwent behavioral testing using two independent protocols. In the first protocol, mice were evaluated in forced swim and locomotor activity tests (Supplementary Fig. 2A, B); next, these animals were split into four groups and treated with scopolamine (results described in Fig. 2A). In the second protocol, a separate cohort of animals was tested every other day in the SUST, EPM and NSFT (Supplementary Fig. 2C–E); however, these animals were not used in subsequent experiments with scopolamine treatment since some tests, such as the EPM, can lead to one-trial tolerance. Unstressed M1f/fSstCre+ mice did not display any baseline behavioral change in the LMA, FST, SUST, EPM and NSFT (Supplementary Fig. 2A–E). We also tested the effects of M1 SST deletion on levels of proteins relevant to GABA and glutamate function. Based on previous studies suggesting involvement of mPFC in stress- and scopolamine-induced behavioral responses [22, 37–39], we chose this brain region to characterize synaptic changes. There were no significant changes in the expression of synaptic glutamatergic (VGLUT1, PSD95 and GluA1) or GABAergic (VGAT, gephyrin and GAD1) proteins at baseline (Supplementary Fig. 2F, G).
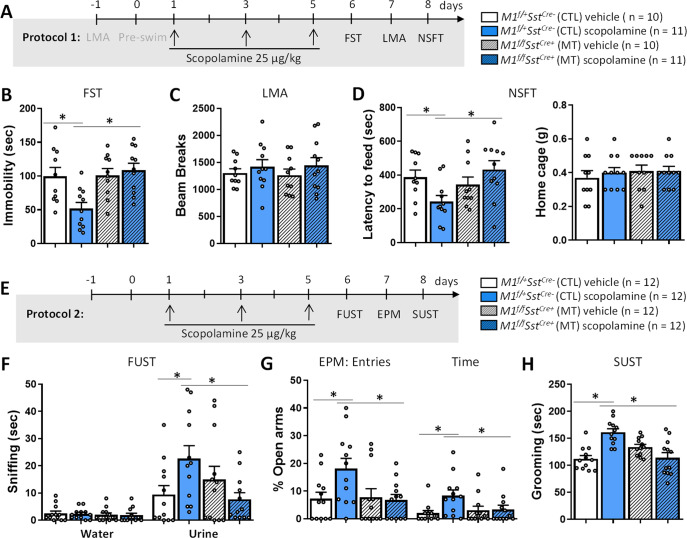
Fig. 2. M1R deletion in SST interneurons blocks the rapid and sustained antidepressant- and anxiolytic-like effects of scopolamine.
A Time course for Protocol 1 and cohorts to test M1f/+SstCre- control (CTL) and M1f/fSstCre+ mutant (MT) mice in tests of stress-responsive behaviors. B Effects of SST M1R deletion on scopolamine-induced behavioral responses in the forced swim test (FST, Fint 1,38 = 6.81, p < 0.05), C locomotor activity (LMA, Fint 1,38 = 0.062, p > 0.05), D novelty-suppressed feeding test (NSFT, Fint 1,38 = 7.08, p < 0.05). E Time course for Protocol 2 and cohorts to test M1f/+SstCre- control (CTL) and M1f/fSstCre+ mutant (MT) mice in tests of stress-responsive behaviors. F female urine sniffing test (FUST, Urine: Fint 1,44 = 6.82, p < 0.05; Water: Fint 1,44 = 0.00, p > 0.05), G elevated plus-maze (EPM, entries: Fint1,44 = 4.26, p < 0.05; time: Fint 1,44 = 3.81, p < 0.05) and H sucrose splash test (SUST, Fint 1,44 = 25.05, p < 0.05). Each bar represents the mean ± standard error of the mean (SEM). *p ≤ 0.05 compared to the control group, treatment*genotype interaction (Fint) evaluated by Two-way ANOVA followed by Duncan test.
We next tested the effects of M1 SST deletion on PSCs recorded from mPFC SST and pyramidal neurons. Whole-cell recordings from SST interneurons revealed no alterations in the frequency or amplitude of EPSCs and IPSCs in mPFC slices from M1f/fSstCre+ mice (Supplementary Fig. 3A–C). There was also no alteration in the EPSC/IPSC (E/I) ratio in these neurons. In contrast, there was a reduction in the frequency of both EPSCs and IPSCs in pyramidal neurons in mPFC slices from M1f/fSstCre+ mice, whereas the E/I frequency ratio remained unaltered (Supplementary Fig. 3D, E). Interestingly, the amplitude of EPSCs was unchanged, whereas there was a decrease in the amplitude of IPSCs, resulting in an overall increase in the E/I amplitude ratio (Supplementary Fig. 3F).
Deletion of M1R in SST interneurons occludes the rapid and sustained antidepressant- and anxiolytic-like effects of scopolamine
We have shown previously that viral-mediated knockdown of M1R in GAD1 or SST interneurons in the mPFC blocks some behavioral effects of scopolamine [7]. We therefore expected that behavioral consequences of scopolamine treatment would be blocked or occluded in male M1f/fSstCre+ mice (Fig. 2). As above, we performed two independent protocols for behavioral testing in separate cohorts of mice (Fig. 2A, E). The first cohort of mice underwent LMA followed by the FST for assessment of baseline behaviors (shown in Supplementary Fig. 2A, B) and were treated with either vehicle or scopolamine (25 μg/kg). We used the same scopolamine regimen (every other day for 3 days) as that used for clinical studies and for our previous preclinical research [3, 7, 30]. Twenty-four hours later, mice were tested in the FST, LMA and NSFT, 24 h apart. In the second protocol (Fig. 2E), a different cohort of mice was treated with either vehicle or scopolamine and tested in the FUST, EPM and SUST, 24 h apart. Scopolamine produced rapid antidepressant- and anxiolytic-like effects in control mice in the FST, NSFT, FUST, EPM and SUST, and these responses were absent in M1f/fSstCre+ mice (Fig. 2B, D, F–H). There was no effect of genotype and/or treatment on locomotor activity, enclosed arm entries in the EPM or home cage feeding in the NSFT (Fig. 2C, D, Supplementary Fig. 4A).
Deletion of M1R in SST interneurons eliminates scopolamine-induced increases in proteins involved in glutamate and GABA function and c-Fos+ cell number in the mPFC
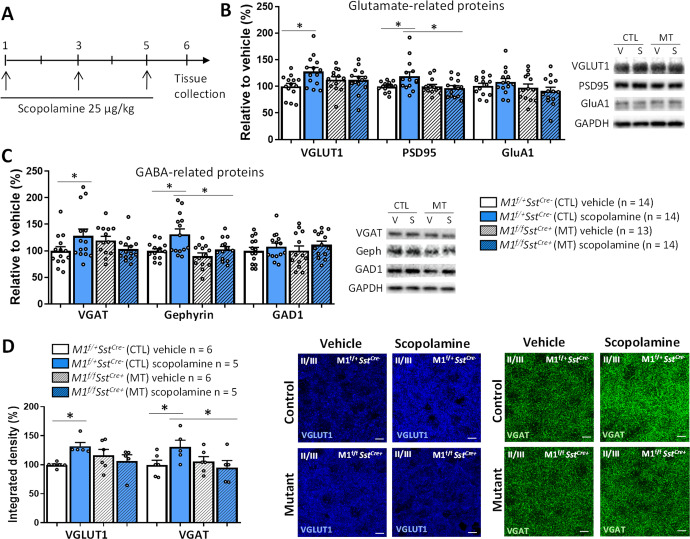
The rapid and sustained antidepressant actions of scopolamine are associated with enhancement of synaptic function via up-regulation of glutamate-related synaptic proteins [19, 22, 38, 40]. However, the effects of scopolamine on the expression of GABAergic markers have not been evaluated. We determined whether scopolamine-induced changes in pre- and postsynaptic molecular markers are observed in M1f/fSstCre+ mice using mPFC synaptoneurosome preparations collected 24 h after scopolamine treatment (25 μg/kg, every other day for 3 days, Fig. 3A). In control mice, scopolamine increased levels of proteins relevant to both glutamate and GABA signaling, such as the presynaptic vesicular transporters VGLUT1 and VGAT, as well as the postsynaptic scaffolding proteins PSD95 and gephyrin, and these effects were not observed in M1f/fSstCre+ mice (Fig. 3B, C).
Fig. 3. M1R deletion in SST interneurons prevents scopolamine-induced increase in GABAergic and glutamatergic protein levels in the mPFC.
A Time course for scopolamine treatment and tissue collection in M1f/+SstCre- control (CTL) and M1f/fSstCre+ mutant (MT) mice. B Effects of SST M1R deletion on scopolamine-induced changes on VGLUT1 (Fint 1,51 = 4.75, p < 0.05), PSD95 (Fint 1,51 = 4.47, p < 0.05) and GluA1 levels (Fint 1,51 = 1.09, p > 0.05), and representative bands of western blot for proteins relevant to glutamate signaling in the mPFC 24 h after treatment. C Effects of SST M1R deletion on scopolamine-induced changes on VGAT (Fint 1,51 = 6.21, p < 0.05), gephyrin (H3 = 8.91, p < 0.05) and GAD1 levels (Fint 1,51 = 0.06, p > 0.05), and representative bands of Western blot for proteins relevant to GABA signaling in the mPFC 24 h after treatment. D Effects of scopolamine on VGLUT1 (H3 = 8.70, p < 0.05) and VGAT levels (Fint 1,18 = 4.44, p < 0.05) in the mPFC of control and M1f/fSstCre+ mice (IL and PL regions, layer II/III), and representative immunofluorescence images (scale bar: 10 µm. Magnification: ×60 + ×2 zoom). Bars represent mean ± standard error of the mean (SEM). *p ≤ 0.05 compared to the control group, treatment*genotype interaction (Fint) evaluated by Two-way ANOVA followed by Duncan test (F value; normal distribution) or Kruskal-Wallis followed by Dunn’s test (H value; non-normal distribution).
Scopolamine-induced increase in VGLUT1 and VGAT levels in control animals and the occlusion of these effects in M1f/fSstCre+ mice was confirmed by immunofluorescence specifically in the PL and IL regions of the mPFC (layer II/III, Fig. 3D). Although the PL and IL send and receive projections to and from different limbic structures, scopolamine has been shown to recruit both PL and IL to exert its behavioral effects [7]. We did not observe differences in the recruitment of these regions in the current study, therefore, results are shown from the two areas combined.
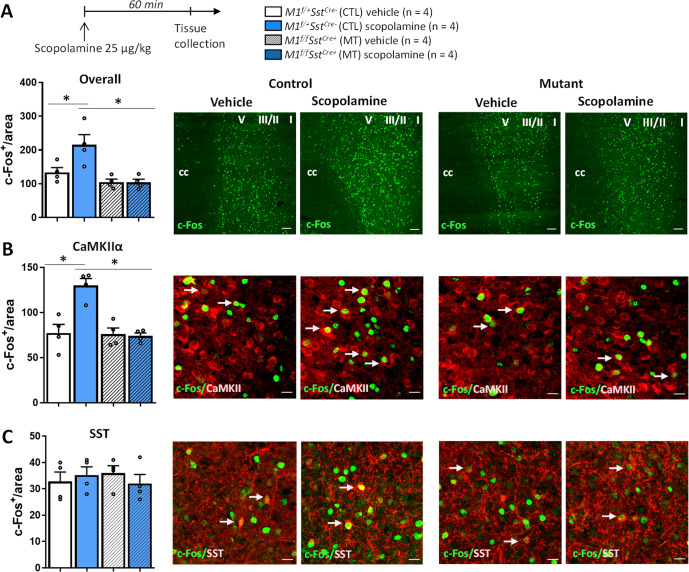
A separate cohort of animals was treated with a single injection of vehicle or scopolamine (25 μg/kg) and animals were perfused 60 min later to evaluate c-Fos expression in the mPFC (PL and IL, Fig. 4A). Scopolamine increased the expression of c-Fos in mPFC and this effect was not observed in M1f/fSstCre+ mice. We next performed additional immunolabeling to investigate whether these alterations occur in glutamatergic (CaMKIIα) or SST cells (Fig. 4B, C). The results indicate that scopolamine increased c-Fos expression specifically in CaMKIIα, but not SST cells, after 1 h of single administration, and this effect was not observed in M1f/fSstCre+ mice.
Fig. 4. M1R deletion in SST interneurons prevents scopolamine-induced increase in c-Fos expression in CaMKIIα (glutamatergic) neurons in the mPFC.
A Time course for scopolamine treatment and perfusion. B Number of c-Fos+ cells in the mPFC of control (CTL) and M1f/fSstCre+ mutant (MT) mice (PL and IL regions; Scale bar: 100 µm. Magnification: ×10) (Fint 1,12 = 5.47, p < 0.05). B Number of c-Fos+ cells that co-localize with CaMKIIα+ neurons in the mPFC of control (CTL) and M1f/fSstCre+ mutant (MT) mice (PL and IL regions; Scale bar: 10 µm. Magnification: ×20, ×2.8 zoom) (Fint 1,12 = 14.42, p < 0.05). C Number of c-Fos+ cells that co-localize with SST+ neurons in the mPFC of control (CTL) and M1f/fSstCre+ mutant (MT) mice (PL and IL regions; Scale bar: 10 µm. Magnification: ×20, ×2.8 zoom) (Fint 1,12 = 0.98, p > 0.05). White arrows indicate examples of co-localization. Bars represent mean ± standard error of the mean (S.E.M.). *p ≤ 0.05 compared to the control group, treatment*genotype interaction (Fint) evaluated by two-way ANOVA followed by Duncan test (F value; normal distribution).
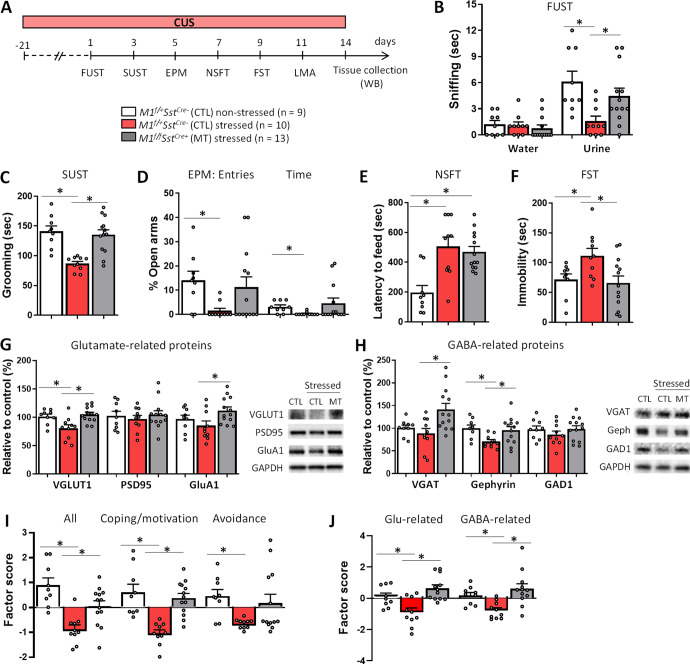
Deletion of M1R in SST interneurons promotes stress resilience and rescues deficits in glutamate and GABA signaling in the mPFC of M1f/fSstCre+ mice
Next, we investigated whether SST M1R deletion could confer resilience to chronic stress using the CUS model [41–43] (Fig. 5A). In control mice, CUS exposure produced depressive- and anxiogenic-like behaviors in the FUST, SUST, EPM, NSFT and FST in comparison to non-stressed mice (Fig. 5B–F). These effects were absent in M1f/fSstCre+ mice, with the exception of the NSFT in which the CUS-induced increase in the latency to feed was maintained (Fig. 5E). No effect was observed on locomotor activity, enclosed arm entries in the EPM or home cage feeding in the NSFT (Supplementary Fig. 4B–D). Following behavioral testing, we collected mPFC punches from these animals to determine whether SST M1R deletion could rescue stress-induced deficits in GABA- and glutamate-related proteins. CUS decreased the expression of proteins relevant to glutamate signaling, such as VGLUT1, and markers of GABA signaling, such as gephyrin (Fig. 5G, H). These stress-induced decreases in glutamate and GABA markers were not observed in mPFC punches from M1f/fSstCre+ mice; in fact, M1f/fSstCre+ mice actually showed increases in GluA1 and VGAT levels in comparison to stressed controls.
Fig. 5. M1R deletion in SST interneurons promotes stress resilience and prevents glutamate- and GABA-related protein deficits in the mPFC of M1f/fSstCre+ mice.
A M1f/+SstCre- control (CTL) and M1f/fSstCre+ mutant (MT) mice were exposed to the chronic unpredictable stress protocol (CUS) and underwent behavioral testing. B Effects of CUS on stress-related behaviors in M1f/fSstCre+ mice, including the female urine sniffing test (FUST, urine: F2,29 = 5.55, p < 0.05; water: F2,29 = 0.37, p > 0.05), C sucrose splash test (SUST, F2,29 = 14.31, p < 0.05), D elevated plus-maze (EPM, entries: H2 = 6.72, p < 0.05; time: H2 = 6.96, p < 0.05), E novelty-suppressed feeding test (NSFT, F2,29 = 10.98, p < 0.05) and F forced swim test (FST, F2,29 = 4.59, p < 0.05). G Western blotting for proteins relevant to glutamatergic signaling, including VGLUT1 (F2,29 = 7.74, p < 0.05), PSD95 (F2,29 = 0.31, p > 0.05) and GluA1 (F2,29 = 3.61, p < 0.05), H or GABAergic signaling, including VGAT (H2 = 7.15, p < 0.05), gephyrin (F2,29 = 4.74, p < 0.05) and GAD1 (F2,29 = 0.85, p > 0.05). I Data from behavioral tests following CUS exposure were grouped into Coping Strategies and Motivated Behaviors (FUST, SUST and FST) or Innate Avoidance (EPM and NSFT), and then transformed into factor scores by principal component analysis (All: F2,29 = 13.70, p < 0.05; Coping: F2,29 = 15.50, p < 0.05; Avoidance: H2 = 5.66, p < 0.05). J Factor analysis of CUS effects on glutamate- (F2,29 = 10.62, p < 0.05) and GABA-related proteins (F2,29 = 6.12, p < 0.05) in M1f/fSstCre+ mice. *p ≤ 0.05 compared to the control group; One-way ANOVA followed by Duncan test (F value, normal distribution) or Kruskal–Wallis followed by Dunn’s test (H value, non-normal distribution).
For better visualization of the results, behavioral tasks were grouped into Coping Strategies and Motivated Behaviors (FUST, SUST and FST), which are sensitive to antidepressant administration, and Innate Avoidance (EPM Poa, EPM Pta and NSFT), sensitive to anxiolytic drugs, and then transformed into factor scores by principal component analysis (Fig. 5I). Following CUS exposure, SST M1R deletion produced more robust effects in Motivation and Coping Strategies than in phenotypes relevant to Innate Avoidance. We also grouped glutamate- or GABA-related proteins into factor scores and observed reversal of CUS-induced impairments in both glutamate and GABA signaling (Fig. 5J).
Discussion
The results shown here support the idea that M1R in SST interneurons is involved in regulating stress-relevant behaviors and synaptic plasticity in response to scopolamine challenge (Supplementary Fig. 5). The current data show that scopolamine acts via SST M1R to produce rapid and sustained antidepressant effects, and to enhance glutamate and GABA signaling in the mPFC. Moreover, selective knockout of M1R in SST interneurons results in plasticity in cortical excitatory and inhibitory synapses that contribute to resilience to chronic stress.
In the mPFC, M1R-mediated cholinergic modulation of both GABAergic and glutamatergic synapses is important for coordination of excitatory and inhibitory activity at the synaptic- and circuit-level, influencing behavioral states [44]. In the current study, SST M1R deletion did not result in baseline alterations in GABAergic and glutamatergic synaptic protein levels in the mPFC, which could explain why these animals did not exhibit baseline behavioral phenotypes. However, a strength of this study is that M1f/fSstCre+ mice displayed resilience to chronic stress and prevented CUS-induced decreases in pre- and postsynaptic markers necessary for glutamate and GABA function in the mPFC of the same animals. These findings suggest that, under stressful conditions, SST M1R deletion renders cortical circuits sensitive to GABA- and glutamate-induced plasticity, which alleviates stress-relevant behaviors. This mechanism is similar to that described for fast antidepressants [10, 13, 14]. Interestingly, behaviors sensitive to antidepressant medications are more sensitive to the SST M1 deletion than behaviors relevant to innate avoidance (notably the NSFT), that are characterized as anxiety-related behaviors. These results suggest that M1R in SST interneurons influence different circuits that are recruited depending on the nature of the task performed.
Imaging and postmortem studies have demonstrated alterations in glutamatergic and GABAergic connectivity in several brain regions in MDD subjects and chronically stressed rodents [10, 23, 45]. It is noteworthy that stress-induced glutamate changes are brain region-specific and have been reported with mixed results. In the PFC and hippocampus of individuals with MDD, a number of studies have shown reductions in glutamate transmission, as well as decreased synaptic markers, dendritic complexity and glutamate receptor subtypes [46–48], while there is evidence of hypertrophy of glutamatergic neurons in the amygdala [49]. On the other hand, more consistent reductions in cortical GABA levels and related proteins have been observed in human subjects with MDD and chronically stressed rodents, notably in levels of SST, GAD1, VGAT and gephyrin, whereas normalization of GABA signaling is associated with remission of depressive symptoms [16–18, 50–53]. In preclinical studies, stress- and depression-related behavioral outcomes produced by GABA deficits have been modeled in transgenic animals. Mice lacking the SST protein (SST-KO) exhibit increased stress-related behaviors, and reduced GAD1 and BDNF gene expression [54]. Interestingly, CUS results in decreased IPSCs in both pyramidal and SST neurons, and this is reversed by rapid antidepressant treatment [14]. The current findings show that SST M1R deletion does not affect PSCs in SST interneurons. However, mPFC pyramidal neurons showed an overall decrease in PSCs frequency and a decrease in IPSCs amplitude, whereas the E/I amplitude was increased. One possible interpretation for these results is that, although overall synaptic inputs onto pyramidal cells are reduced in the mPFC, there is a shift in the E/I amplitude favoring excitation, suggesting that whenever EPSCs occur, there is a higher chance to evoke action potentials due to weakened antagonizing inhibitory inputs. Although this alteration does not influence baseline behaviors significantly, it could select more specific downstream circuits under chronic stress conditions. Since we did not find any alteration in synaptic protein levels at baseline, these results are probably due to rearrangements in presynaptic release probability and postsynaptic strength instead of structural changes (such as spine density). However, because deletion of M1R in all SST neurons throughout development could affect several interconnected networks across the brain leading to complex rearrangements in local and more distant circuits [10, 25], additional physiological cell properties, including evoked PSCs, input resistance and membrane potential in different brain regions may contribute to understanding the stress-relevant phenotypes in these mice.
The antidepressant- and anxiolytic-like effects of scopolamine were evident in control mice but were largely absent in M1f/fSstCre+ mice. These findings are consistent with a previous report that short-term shRNA-mediated knockdown of M1R in SST interneurons, but not principal neurons, in the mPFC prevents the antidepressant-like effects of scopolamine [7]. Likewise, we have previously shown that acute chemogenetic activation of SST interneurons in the mPFC blocks the rapid and sustained antidepressant-like effects of scopolamine, demonstrating that transient inhibition of SST interneurons is an initial cellular trigger for rapid antidepressant effects [22]. However, in contrast to the current study, previous studies involved relatively short-term viral manipulations and did not explore chronic adaptations in synaptic plasticity that are implicated in rapid antidepressant responses and stress resilience.
To date, many studies have focused on enhancement of glutamate-induced plasticity following fast antidepressant responses [22, 23, 32, 55–57]. Although it is thought that scopolamine and ketamine initially inhibit GABA interneurons and disinhibit pyramidal cells in the mPFC, leading to an enhancement of glutamatergic function [22, 23, 56, 58], these drugs also appear to enhance GABA function in cortical areas, which could contribute to sustained antidepressant responses [12, 14, 25, 26, 59]. Interestingly, the rapid antidepressant-like effect of ketamine is accompanied by reversal of deficits in both GABAergic and glutamatergic function in the mPFC of stressed mice. However, the effects of scopolamine on GABA transmission has not been evaluated. Here, we found that scopolamine increased levels of synaptic proteins related to both glutamate- and GABA activity in the mPFC, including VGLUT1 and PSD95, as well as VGAT and gephyrin, respectively, and these effects were blocked in M1f/fSstCre+ mice. Previous studies revealed that VGLUT1 knockdown in the mPFC induces depressive behaviors that are not reversed by scopolamine, indicating that up-regulation of VGLUT1 is required for scopolamine’s effects [40].
In addition, a single dose of scopolamine produces a transient increase in GABA and glutamate cycling in the mPFC of awake rats [24]. It is unclear, however, at what timepoint alterations in the GABA system occur following scopolamine treatment, and whether GABA-mediated plasticity is required for rapid and/or sustained antidepressant responses. One hypothesis is that rapid antidepressant activity requires an initial, time-dependent inhibition of GABA interneurons and a rapid glutamate burst (acute phase), leading to a second, homeostatic network adjustment to re-establish E/I balance in the mPFC via potentiation of GABA function (chronic/sustained phase) [10, 25]. Our results suggest that a single administration of scopolamine increased c-Fos expression specifically in glutamatergic (CaMKIIα) cells 1 h after administration, while no effect was observed in SST interneurons at this timepoint. The lack of c-Fos staining in SST neurons at the 1 h timepoint is consistent with the idea that the initial inhibition of SST interneurons induced by fast-acting antidepressants is rapid and transient [13, 22, 25], whereas homeostatic enhancement of GABA function is delayed (> 1 h) [25].
Taken together, our results suggest that enhancement of both GABA and glutamate plasticity could restore signal integrity in the mPFC, culminating in antidepressant states and stress resilience. Follow-up studies will be needed to map the contribution of other brain regions to these responses and to determine how glutamatergic and GABAergic mechanisms interact to promote rapid and sustained antidepressant effects. In addition, future studies are needed to determine sex-specific effects of SST M1 deletion in female mice. The current results suggest that targeting both excitatory and inhibitory neurotransmitter systems via M1R antagonism in SST interneurons could be an effective approach to treat MDD.
Supplementary information
Acknowledgements
RSD passed away on February 1, 2020. This article is dedicated to RSD in memory of his great mentorship and scientific leadership.
Author contributions
MVF designed the study, performed the experiments, analyzed the data and wrote the manuscript. MW performed the electrophysiological experiments and analyzed the data. X-YL and CL provided technical support and helped to perform the experiments. RSD contributed to experimental design and was involved in data interpretation and analyses. MRP provided scientific input, was involved in data interpretation and analyses, edited and revised the manuscript.
Funding
This study was supported by grant K99MH126098, MH077681, MH105910 and NARSAD Young Investigator Award 2020 (BBRF Foundation, #29063). This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deceased: Ronald S. Duman.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01583-7.
References
- 1.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–9. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–29. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 7.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Investig. 2016;126:2482–94. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence JJ. Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci. 2008;31:317–27. doi: 10.1016/j.tins.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–7. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90. doi: 10.1016/j.neuron.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Port JD, Pazdernik V, Coombes BJ, Vande Voort JL, Frye MA. Racemic ketamine treatment attenuates anterior cingulate cortex GABA deficits among remitters in treatment-resistant depression: a pilot study. Psychiatry Res Neuroimaging. 2022;320:111432. doi: 10.1016/j.pscychresns.2021.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh B, Port JD, Voort JLV, Coombes BJ, Geske JR, Lanza IR, et al. A preliminary study of the association of increased anterior cingulate gamma-aminobutyric acid with remission of depression after ketamine administration. Psychiatry Res. 2021;301:113953. doi: 10.1016/j.psychres.2021.113953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogaca MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13:87. doi: 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosal S, Duman CH, Liu RJ, Wu M, Terwilliger R, Girgenti MJ, et al. Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol Dis. 2019;134:104669. [DOI] [PubMed]
- 15.Godfrey KEM, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. J Psychiatr Res. 2018;105:33–44. doi: 10.1016/j.jpsychires.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–20. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 18.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019;101:774–8. doi: 10.1016/j.neuron.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duman RS. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Res. 2018;7. [DOI] [PMC free article] [PubMed]
- 22.Fogaca MV, Wu M, Li C, Li XY, Picciotto MR, Duman RS. Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol Psychiatry. 2021;26:3277–91.. doi: 10.1038/s41380-020-00916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duman RS, Shinohara R, Fogaca MV, Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry. 2019;24:1816–32. [DOI] [PMC free article] [PubMed]
- 24.Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22:120–6. doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luscher B, Feng M, Jefferson SJ. Antidepressant mechanisms of ketamine: focus on GABAergic inhibition. Adv Pharmacol. 2020;89:43–78. doi: 10.1016/bs.apha.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry. 2016;80:457–68.. doi: 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2017;22:920–30.. doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry. 2017;82:549–59.. doi: 10.1016/j.biopsych.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamsler A, McHugh TJ, Gerber D, Huang SY, Tonegawa S. Presynaptic m1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proc Natl Acad Sci USA. 2010;107:1618–23. doi: 10.1073/pnas.0912540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, et al. Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry. 2018;83:29–37. doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fogaca MV, Fukumoto K, Franklin T, Liu RJ, Duman CH, Vitolo OV, et al. N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019;44:2230–8. doi: 10.1038/s41386-019-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato T, Fogaca MV, Deyama S, Li XY, Fukumoto K, Duman RS. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry. 2018;23:2007–17.. doi: 10.1038/mp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fogaça MV, Campos AC, Coelho LD, Duman RS, Guimaraes FS. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology. 2018;135:22–33. doi: 10.1016/j.neuropharm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Pothula S, Kato T, Liu RJ, Wu M, Gerhard D, Shinohara R, et al. Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol Psychiatry. 2020;26:5097–111. [DOI] [PubMed]
- 35.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–52.. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Investig. 2019;130:1336–49. [DOI] [PMC free article] [PubMed]
- 37.Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE, et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–61.. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–9. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wohleb ES, Gerhard D, Thomas A, Duman RS. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15:11–20. doi: 10.2174/1570159X14666160309114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Li M, Zhou D, Lv D, Liao Q, Lou Z, et al. Vesicular glutamate transporter 1 (VGLUT1)-mediated glutamate release and membrane GluA1 activation is involved in the rapid antidepressant-like effects of scopolamine in mice. Neuropharmacology. 2018;131:209–22.. doi: 10.1016/j.neuropharm.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 41.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 42.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–64. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banasr M, Lepack A, Fee C, Duric V, Maldonado-Aviles J, DiLeone R, et al. Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress. 2017;1:2470547017720459. [DOI] [PMC free article] [PubMed]
- 44.Fernandez de Sevilla D, Nunez A, Buno W. Muscarinic receptors, from synaptic plasticity to its role in network activity. Neuroscience. 2021;456:60–70. doi: 10.1016/j.neuroscience.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Abdallah CG, Jackowski A, Sato JR, Mao X, Kang G, Cheema R, et al. Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol. 2015;25:1082–90. doi: 10.1016/j.euroneuro.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–5. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81:886–97.. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161:368–70. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 51.Goren MZ, Kucukibrahimoglu E, Berkman K, Terzioglu B. Fluoxetine partly exerts its actions through GABA: a neurochemical evidence. Neurochem Res. 2007;32:1559–65. doi: 10.1007/s11064-007-9357-2. [DOI] [PubMed] [Google Scholar]
- 52.Kucukibrahimoglu E, Saygin MZ, Caliskan M, Kaplan OK, Unsal C, Goren MZ. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur J Clin Pharmacol. 2009;65:571–7. doi: 10.1007/s00228-009-0650-7. [DOI] [PubMed] [Google Scholar]
- 53.Dubin MJ, Mao X, Banerjee S, Goodman Z, Lapidus KA, Kang G, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci. 2016;41:E37–45. doi: 10.1503/jpn.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin LC, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015;20:377–87. doi: 10.1038/mp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duman RS, Deyama S, Fogaca MV. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci. 2021;53:126–39.. doi: 10.1111/ejn.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Investig. 2020;130:1336–49.. doi: 10.1172/JCI130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukumoto K, Fogaca MV, Liu RJ, Duman C, Kato T, Li XY, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci USA. 2019;116:297–302. doi: 10.1073/pnas.1814709116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fogaca MV, Fukumoto K, Franklin T, Liu RJ, Duman CH, Vitolo OV, et al. N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019;44:2230–8. [DOI] [PMC free article] [PubMed]
- 59.Perrine SA, Ghoddoussi F, Michaels MS, Sheikh IS, McKelvey G, Galloway MP. Ketamine reverses stress-induced depression-like behavior and increased GABA levels in the anterior cingulate: an 11.7 T 1H-MRS study in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:9–15. doi: 10.1016/j.pnpbp.2013.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.