Abstract
Introduction:
We assessed the association between visit-to-visit blood pressure variability (BPV) up to 12 years and subsequent dementia risk, and tested the modifying effect of antihypertensive medications.
Methods:
We studied 2,234 participants from two community-based cohorts of older adults with normal cognition or mild cognitive impairment. Participants were followed through annual assessments for up to 27 years. Visit-to-visit BPV was quantified over 3, 6, 9, and 12 years, respectively.
Results:
Higher systolic BPV (SBPV) during 3, 6, 9, and 12 years was associated with a subsequent increased risk of dementia, with hazard ratios ranging from 1.02 (95% CI: 1.01–1.04) to 1.10 (95% CI: 1.05–1.16). The association between SBPV and dementia risk was stronger among participants not taking calcium channel blockers (p-for interaction<0.05).
Discussion:
Among older adults, long-term exposure to higher visit-to-visit SBPV is associated with increased risk of dementia later in life, and calcium channel blockers may modify this association.
Keywords: blood pressure variability, dementia, antihypertensive medication, calcium channel blockers, older adults
1. Introduction
Elevated blood pressure (BP) in midlife is an established risk factor for late-life dementia1–3. However, the link between late-life BP levels and dementia risk is less clear. Longitudinal studies of late-life BP and dementia risk have often reported null, weak, or non-linear associations1–3. More recent data suggest that higher visit-to-visit BP variability (BPV) is an independent risk factor for stroke4, cardiovascular events5, and cerebral small vessel disease6, beyond mean BP levels. These observations highlight the importance of BPV for brain health and suggest higher BPV as a risk factor for adverse cognitive outcomes. Indeed, longitudinal observational studies have demonstrated an association between higher BPV and increased dementia risk among older adults7–9. However, it remains unclear whether BPV is a risk factor for dementia (e.g. precedes dementia) or merely reflects the prodromal period of dementia affecting autonomic function7, 8. To date, most studies on BPV and dementia have measured BPV during the dementia ascertainment period10–12, raising concerns about reverse causation bias. Furthermore, prior studies have mainly assessed BPV during short periods, while the link between BPV over longer periods and dementia remains unknown.
Despite the ambiguous impact of antihypertensive treatment on dementia risk in older adults1, none of the major randomized clinical trials (RCT) have examined the impact of BPV as a treatment target. Data suggest that while antihypertensive medications decrease BPV in older adults, they are associated with potential drug-class effects. Studies show that calcium channel blockers (CCBs) and non-loop diuretics are most effective in reducing systolic BPV (SBPV), while renin-angiotensin system modulators (RAS) and β-blockers increase SBPV13. Furthermore, CCBs have been shown to reduce the risk of stroke more than expected on the basis of mean BP levels. Such effects of CCBs on stroke risk reduction beyond mean BP levels have been shown to be attributed to the effects of CCBs on reducing BPV4, 14. Whether such effects of antihypertensive treatment are also attributable to the link between BPV and dementia risk is unknown. As such, there remains a knowledge gap on the interaction between antihypertensive treatment and BPV in the context of dementia risk.
Thus, our study examined the association between long-term visit-to-visit BPV for up to 12 years and subsequent dementia risk among community-dwelling older adults in the United States. We further investigated whether antihypertensive treatment and specific drug classes had an impact on the association between BPV and dementia risk.
2. METHODS
2.1. Study Population
The study population consisted of participants from the Rush Memory and Aging Project (MAP) and the Religious Orders Study (ROS), two ongoing community-based cohort studies of aging and cognition in the United States15. An Institutional Review Board of Rush University Medical Center approved both studies. All participants signed an informed consent, an Anatomic Gift Act, and a repository consent that allows their resources to be shared. The two studies had similar study designs, data collection, and recruitment strategies, allowing efficient merging of data into the ROSMAP cohort15. Study designs and recruitment strategies have been described in detail15.
Briefly, the ROS and MAP are prospective cohort studies enrolling older adults (65+ years old) without known dementia that agree to annual clinical evaluation and brain autopsy at deaths. Loss of contact with participants is rare, with an annual follow-up rate of survivors exceeding 90% and 95% for the ROS and MAP cohorts, respectively15, 16. As of 2021, a total of 1,486 participants had enrolled in the ROS and were followed annually for up to 27 years (Figure 1). As of 2021, a total of 2,192 participants had enrolled in the MAP and annually followed for up to 24 years (Figure 1). Across the total ROSMAP population (n=3,678), we excluded participants who were <65 years old at baseline (n=132), had a clinical diagnosis of dementia at one or more of their first three visits (n=416), did not have baseline BP assessment (n = 36), or had less than three years of data on BP (n=663), and those who did not met criteria for inclusion in any of the period-specific analyses (n=197, e.g. no dementia status after visit 2, 5, 8 or 11). Accordingly, 2,234 participants with normal cognition (n=1,778) or mild cognitive impairment (MCI, n=456) were included in our analysis (eFigure 1). Comparison between excluded and included participants at baseline showed that included participants were younger, more frequently White persons, had higher years of education, lower frequency of vascular risk factors and diseases, and lower frequency of MCI (eTable 1).
Figure 1. Schematic diagram of study design.
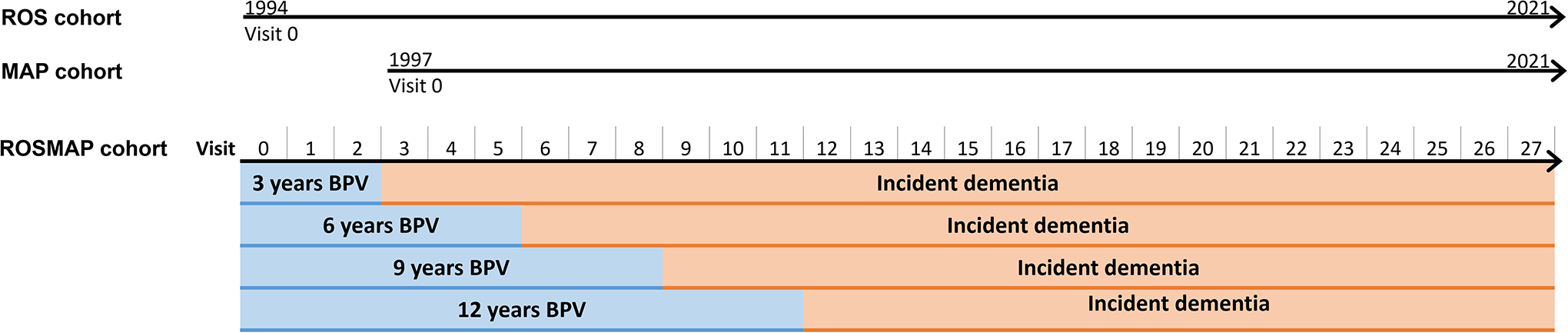
The ROS cohort began recruitment in 1994 and the MAP cohort began recruitment in 1997. In 2021, participants had up to 27 and 21 years of follow-ups, respectively. Given the cohorts had similar study designs, data collection and recruitment strategies, they were merged into the ROSMAP cohort with up to 27 years follow-up. BPV was computed over the first 3, 6, 9 and 12 years. Incident dementia occurred after visit 2, 5, 8 and 11 corresponding to BPV periods, and dementia cases during the first 3, 6, 9 and 12 years were censored in each period-specific analyses, respectively.
2.2. Visit-to-visit blood pressure variability
BP was measured with a sphygmomanometer by a trained research assistant at each annual visit17. Two measurements were taken in the seating position, and after one minute, another measurement was taken in standing position. The mean of these three BP readings were used as the BP value for each visit. The median number of BP assessments during the follow-up period was 9 (interquartile range 6 to 12). Visit-to-visit BPV was primarily defined as the coefficient of variance (CV) in BP (CVBP= inter-individual standard deviation [SD] of BP/mean BP×100). In addition, we assessed visit-to-visit BPV using the SD, and average real variability (ARV), two other commonly used metrics of BPV. All three BPV metrics were highly correlated, with the Pearson correlation coefficients ranging from 0.80 to 0.98 (eTable 2). We primarily focused on visit-to-visit SBPV given its stronger correlation with adverse cardiovascular and brain health outcomes18, 19. We also repeated the main analyses using visit-to-visit diastolic BPV (DBPV) and reported the results in Supplemental Material.
To assess the effect of different BPV duration on dementia risk, we separately calculated BPV during the first 3, 6, 9 and 12 years, respectively (Figure 1). Participants were required to have BP readings available for the baseline visit (visit 0), the last visit (visit 2, 5, 8 or 11), and at least one intermediate visit for each of the periods.
2.3. Clinical diagnosis of dementia
Cognitive status was determined at every annual visit based on a three-stage process including computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and diagnostic classification by a clinician, as detailed previously20. Clinical diagnosis of dementia was based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA)21. In our analysis, the primary outcome of all-cause dementia was defined as persons with probable AD, possible AD or those with other primary cause of dementia with no clinical evidence of AD. The secondary outcomes of pure AD and AD were defined as AD with no other condition contributing to cognitive impairment, and AD with another condition contributing to cognitive impairment, respectively.
2.4. Covariates
Years of education, race, and sex were self-reported. History of smoking, claudication, heart conditions (including heart attack, coronary thrombosis, coronary occlusion and myocardial infarction), hypertension and diabetes mellitus were determined by participants’ self-reports at each visit. History of stroke was based on clinical review of self-reports, neurological exam when available, cognitive testing and interview of participants. Summary scores indicating each participant’s cumulative vascular risk factor burden (a score ranging from 0 to 3 including history of hypertension, diabetes mellitus and smoking) and cumulative vascular disease burden (a score ranging from 0 to 4 including history of stroke, heart conditions and claudication) covering a time frame from baseline to the corresponding cycle were computed as previously described22, 23. Participants supplied all medications prescribed by a doctor, vitamins, supplements, and over-the-counter medicines taken in the 2 weeks prior to the evaluation. Direct visual inspection of all containers of prescription and over-the-counter agents allowed for medication documentation. Medication use was subsequently coded using the Medi-Span Drug Data Base system as previously described24, 25. Antihypertensive medications included angiotensin converting enzyme inhibitors (ACE), β-blockers, CCBs, direct renin inhibitors, diuretics (diuretic alone or in combination with other antihypertensive medication), angiotensin receptor blockers (ARB), alpha blockers (alpha 1 and 2 receptor blockers) and other antihypertensive medications. In our analyses, we grouped ACE and ARB into the RAS modulators group.
2.5. Statistical analysis
The primary analyses focused on the association between SBPV over different durations (3, 6, 9 and 12 years) and risk of dementia using a series of Cox-proportional hazard models. Person-time started from visit 2, 5, 8 or 11 until dementia diagnosis, death, or participant’s last visit, whichever came first. Age of participants at each visit was used as the time scale. Participants with incident dementia during the first three years were not included in the analyses of 3 years BPV, and those with incident dementia during the first 6 years were not included in the analyses of 6 years BPV and dementia risk, and so on. In sensitivity analyses, we employed inverse-probability weights (IPW) to assess the potential for selection bias due to informative censoring26 (eTable 3). Competing risk of death was accounted for by estimating cause-specific hazard ratios (HRs) for death and dementia27. Proportional hazard assumptions were checked graphically, using Schoenfeld residuals and by including an interaction term with time.
We assessed the association between BPV and dementia risk using continuous and categorical values of BPV. The categorical values of BPV were defined according to tertiles of 12 years of BPV to allow for a reasonable number of participants in each of the period-specific analyses. Accordingly, the continuous values of SBPV over each period were divided into three groups of <8%, 8–10% and ≥10%, with the reference group defined as the lowest category.
The Cox-proportional hazard models were first performed in crude unadjusted models (Model 1). In Model 2, multivariable Cox models were performed with adjustments for demographic factors (baseline age, sex, race, and education), cumulative vascular risk factors burden (smoking, hypertension and diabetes mellitus), cumulative vascular diseases burden (stroke, heart disease and claudication), mean BP, and medication use (antihypertensive, cardiac, lipid lowering and mental health medications) from the beginning of the at-risk period (i.e., the end of the corresponding BPV period). There were no missing values in covariates for the main analyses. To account for the potential time-varying effect of medication use during BPV periods, we performed two sensitivity analyses where analyses in Model 2 were repeated after adjustment for 1) medication use at any point during the corresponding BPV periods, and 2) incident medication use during BPV periods by categorizing medication use into 3 groups of absent, incident during the BPV period, and present from the start of the BPV period. To account for missing data in the sensitivity analyses, multiple imputation by chained equations using five imputations was applied.
To assess the effect of antihypertensive medication use, we tested for interaction between period-specific SBPV and any antihypertensive medication use until the corresponding SBPV period. Similarly, to assess the effect of antihypertensive medication classes, we tested for interaction between period-specific SBPV and each antihypertensive medication class until the corresponding SBPV period. Interaction was tested on a multiplicative scale by adding a product term to the Cox-proportional hazard models. For participants who missed data on antihypertensive medication use, analyses were repeated after using the data from the previous visit (<11%) that yielded similar results to the complete case analyses.
In a secondary analysis, we assessed period-specific association between BPV and subtypes of dementia including AD (AD with other contributing factors) and pure AD (AD with no other contributing factor) using Cox proportional hazard models as described above. Similarly, the period-specific analyses were repeated for DBPV, pulse pressure variability (PPV) and other measures of SBPV (SD and ARV). We also assessed the association of individual SBP measures at select visits, and period-specific mean SBP with risk of dementia from fully adjusted models. To assess the effect of number of BP data used in quantification of BPV on the results, we performed a sensitivity analysis by including only participants with maximum number of BP data in each of the period-specific analyses. To account for the slope of SBP, a sensitivity analysis was performed after adjustment for intra-individual slope of SBP (β estimate calculated from linear regression models) during the corresponding BPV periods. To compare long-term versus short-term dementia risk, a sensitivity analyses was performed after excluding dementia cases during the first 5 years following BPV periods.
A p-values <0.05 was considered statistically significant. Statistical analyses were performed in R version 4.1.0 (R Foundation), and SPSS version 28.0 (IBM statistics).
3. RESULTS
3.1. Study population
Baseline characteristics of participants are presented in Table 1. During the median follow-up of 10 years (interquartile range 6–14 years), 668 participants developed dementia, including 641 cases of AD (AD with other contributing factors) and 569 cases of pure AD (AD with no other contributing factor). Mean SBP over the first 3, 6, 9 and 12 years was 134±15 mmHg, 133±13 mmHg, 132±13 mmHg, and 131±12 mmHg, respectively. The mean SBPV over 3, 6, 9 and 12 years was 8.3±5%, 9.2±4%, 10±3%, and 10±3%, respectively. The mean number of SBP readings for the 6-, 9- and 12-years periods was 5.8±0.5 (median 6, IQR=0), 8.5±0.8 (median 9, IQR =1), and 11.1±1.3 (median 12, IQR=1), respectively.
Table 1.
Characteristics of study participants at baseline
| Characteristics | n=2234 |
|---|---|
| Age, years (mean ± SD) | 78 ± 7 |
| Female, n (%) | 1647 (74) |
| Whites, n (%) | 2107 (94) |
| Education, years (mean ± SD) | 16 ± 4 |
| Cognitively normal, n (%) | 1778 (80) |
| Smoking status: | |
| Never, n (%) | 1550 (69) |
| Former, n (%) | 643 (29) |
| Current, n (%) | 41 (2) |
| Hypertension, n (%) | 1098 (49) |
| Diabetes mellitus, n (%) | 247 (11) |
| Claudication, n (%) | 132 (6) |
| Heart diseases, n (%) | 194 (9) |
| Stroke, n (%) | 145 (7) |
| Systolic blood pressure, mmHg (mean ± SD) | 135 ± 18 |
| Diastolic blood pressure, mmHg (mean ± SD) | 75 ± 11 |
| Antihypertensive medications: | 1371 (61) |
| Calcium channel blockers, n (%) | 466 (21) |
| β-blockers, n (%) | 519 (23) |
| Diuretics, n (%) | 692 (31) |
| ACE inhibitors, n (%) | 406 (18) |
| ARBs, n (%) | 224 (10) |
| Cardiac medications*, n (%) | 229 (10) |
| Lipid lowering medication*, n (%) | 722 (32) |
| Mental health medications*, n (%) | 497 (22) |
Lipid lowering medications included statins and lipid lowering nonstatins; cardiac medications included cardiac glycosides, antianginals, and antiarrhythmic medications; and mental health medications included antidepressant, tricyclic antidepressant, insomnia, anti-anxiety, antipsychotic and antimanic medications.
Abbreviations: ACE inhibitors: angiotensin converting enzyme inhibitors, ARBs: angiotensin receptor blockers.
3.2. Risk of all-cause dementia in relation to visit-to-visit BPV, BP and mean BP
In Model 1, participants with SBPV of ≥10% during 3, 6, 9 and 12 years had increased risk of all-cause dementia compared to those with SBPV of <8% (Table 2, Figure 2, p-values <0.05). The magnitude of these associations was the highest for SBPV over 12 years with a HR of 2.26 (95% CI, 1.53–3.33) (Table 2). After full adjustments in Model 2, SBPV ≥10% remained associated with increased risk of dementia, and the highest HRs were for SBPV over 12 years (Table 2, HR [95% CI], 1.75 [1.16–2.66]). In Model 2, each unit increase in SBPV over 3, 6, 9 and 12 years was associated with 1.02-, 1.04-, 1.06- and 1.10-fold increased risk of dementia, respectively (eTable 3, p-values <0.05). The same trend was observed between DBPV over 3, 6 and 9 years and dementia risk, although the results did not reach statistical significance (eTable 4). Finally, higher PPV over 3, 6, 9 and 12 years was associated with increased risk of all-cause dementia, although the HR were smaller and less consistent than those observed for SBPV (eTable 5).
Table 2.
Association of systolic blood pressure variability and risk of all-cause dementia.
| SBPV period | events/at risk | SBPV categories, HR (95% CI) | p for trend | ||
|---|---|---|---|---|---|
| SBPV < 8% |
SBPV 8–10% |
SBPV ≥10% |
|||
| 3 years | 629/2,079 | ||||
| Model 1 | 1 (ref) | 1.23 (0.98, 1.55) | 1.45 (1.21, 1.73) | <0.001 | |
| Model 2 | 1 (ref) | 1.22 (0.97, 1.54) | 1.23 (1.03, 1.47) | 0.018 | |
| 6 years | 435/1432 | ||||
| Model 1 | 1 (ref) | 1.30 (1.01, 1.68) | 1.63 (1.32, 2.02) | <0.001 | |
| Model 2 | 1 (ref) | 1.24 (0.96, 1.60) | 1.37 (1.09, 1.72) | 0.007 | |
| 9 years | 245/878 | ||||
| Model 1 | 1 (ref) | 1.15 (0.83, 1.60) | 1.66 (1.24, 2.23) | <0.001 | |
| Model 2 | 1 (ref) | 1.19 (0.85, 1.66) | 1.42 (1.04, 1.94) | 0.028 | |
| 12 years | 161/539 | ||||
| Model 1 | 1 (ref) | 1.68 (1.11, 2.55) | 2.26 (1.53, 3.33) | <0.001 | |
| Model 2 | 1 (ref) | 1.39 (0.91, 2.14) | 1.75 (1.16, 2.66) | 0.008 | |
Model 1 is unadjusted. Model 2 was adjusted for age at baseline, years of education at baseline, sex, race, and history of vascular risk factors burden (smoking, hypertension and diabetes mellitus), vascular diseases burden (stroke, heart disease and claudication), medication use (antihypertensive, cardiac, lipid lowering, and mental health medications) and mean SBP until the corresponding SBPV period. SBPV was assessed using coefficient of variance. Abbreviations: SBPV: systolic blood pressure variability; HR: hazard ratio; CI: confidence interval.
Figure 2. Kaplan-Meier plots for incident all-cause dementia risk according to blood pressure variability categories.
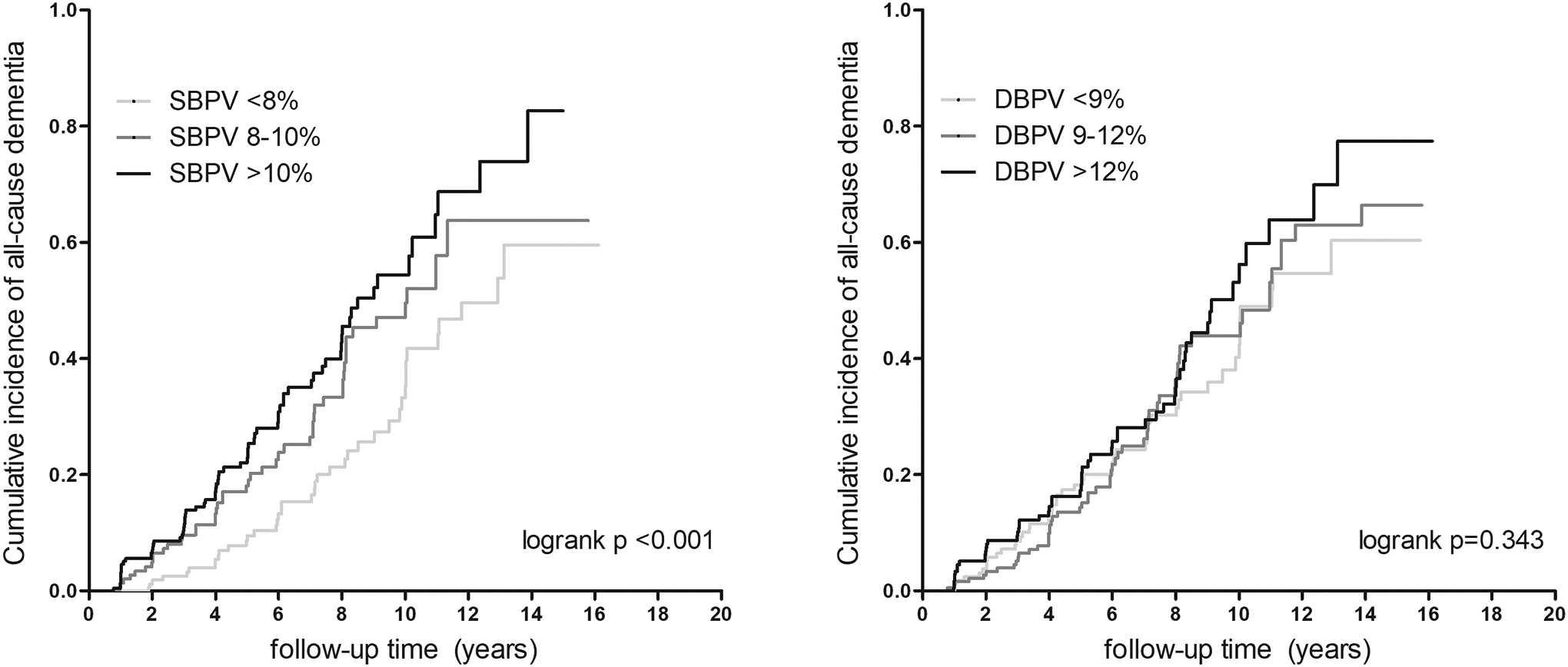
Abbreviations: SBPV: systolic blood pressure variability; DBPV: diastolic blood pressure variability.
We did not find an association between SBP at any of the selected visits, or mean SBP over the first 3, 6, 9 and 12 years and risk of all-cause dementia (Table 3). Categorizing SBP into tertiles did not suggest a non-linear trend between SBP and dementia risk (eTable 6).
Table 3.
Association of SBP and mean SBP with risk of all-cause dementia.
| SBP per 5 mmHg (HR, 95% CI) |
p-value | Mean SBP per 5 mmHg (HR, 95% CI) |
p-value | ||
|---|---|---|---|---|---|
| Visit 0 | 1.02 (0.99,1.05) | 0.056 | |||
| Visit 2 | 1.01 (0.99, 1.03) | 0.495 | 3 years | 1.03 (0.99, 1.06) | 0.053 |
| Visit 5 | 1.01 (0.98, 1.03) | 0.662 | 6 years | 1.04 (0.99, 1.08) | 0.061 |
| Visit 8 | 1.01 (0.97, 1.04) | 0.699 | 9 years | 1.02 (0.96, 1.07) | 0.559 |
| Visit 11 | 1.04 (0.99, 1.09) | 0.055 | 12 years | 1.02 (0.95, 1.10) | 0.555 |
Analyses were adjusted for baseline age, sex, race, education, history of vascular risk factors burden (smoking, hypertension and diabetes mellitus), vascular diseases burden (stroke, heart disease and claudication), and medication use (antihypertensive, cardiac, lipid lowering and mental health medications). Dementia cases were censored before corresponding SBP visits or during mean SBP periods. Abbreviations: SBP: systolic blood pressure; HR: hazard ratios; CI: confidence interval.
3.3. Risk of AD in relation to visit-to-visit BPV
Higher SBPV over all periods was associated with increased risk of AD, with the highest HR for SBPV over 12 years (eTable 7, HR [95% CI], 1.10 [1.04–1.16]). Only higher SBPV over 9 and 12 years was associated with increased risk of pure AD (eTable 7). Similarly, only higher DBPV over 9 and 12 years was associated with increased risk of pure AD (eTable 7).
3.4. Moderating effect of antihypertensive treatment
When assessing the effect of antihypertensive medications, a stronger trend of associations between period-specific SBPV and dementia risk was observed for participants who were not taking antihypertensive medications (eFigure 2). Analyses of antihypertensive medication classes suggested that participants taking CCBs over the first 3 years had lower SBPV compared to those taking other antihypertensive medications (eTable 8). We observed a significant moderating effect of CCBs on the association between SBPV and dementia risk (Figure 3). Specifically, the period-specific association between SBPV and dementia risk was stronger among those who were not taking CCBs during the first three or nine years (Figure 3, p-for interaction <0.05). Adjustment for use of other antihypertensive medications (β-blockers, RAS modulators and diuretics) did not change the results. Also, a similar trend was observed for diuretic and RAS use over longer periods, although it did not reach statistical significance. We did not observe any significant moderating effect for β-blocker use on the period-specific association between SBPV and dementia risk. The number of events and at-risk persons are provided in eTable 9.
Figure 3. Systolic blood pressure variability and dementia risk according to antihypertensive medication classes.
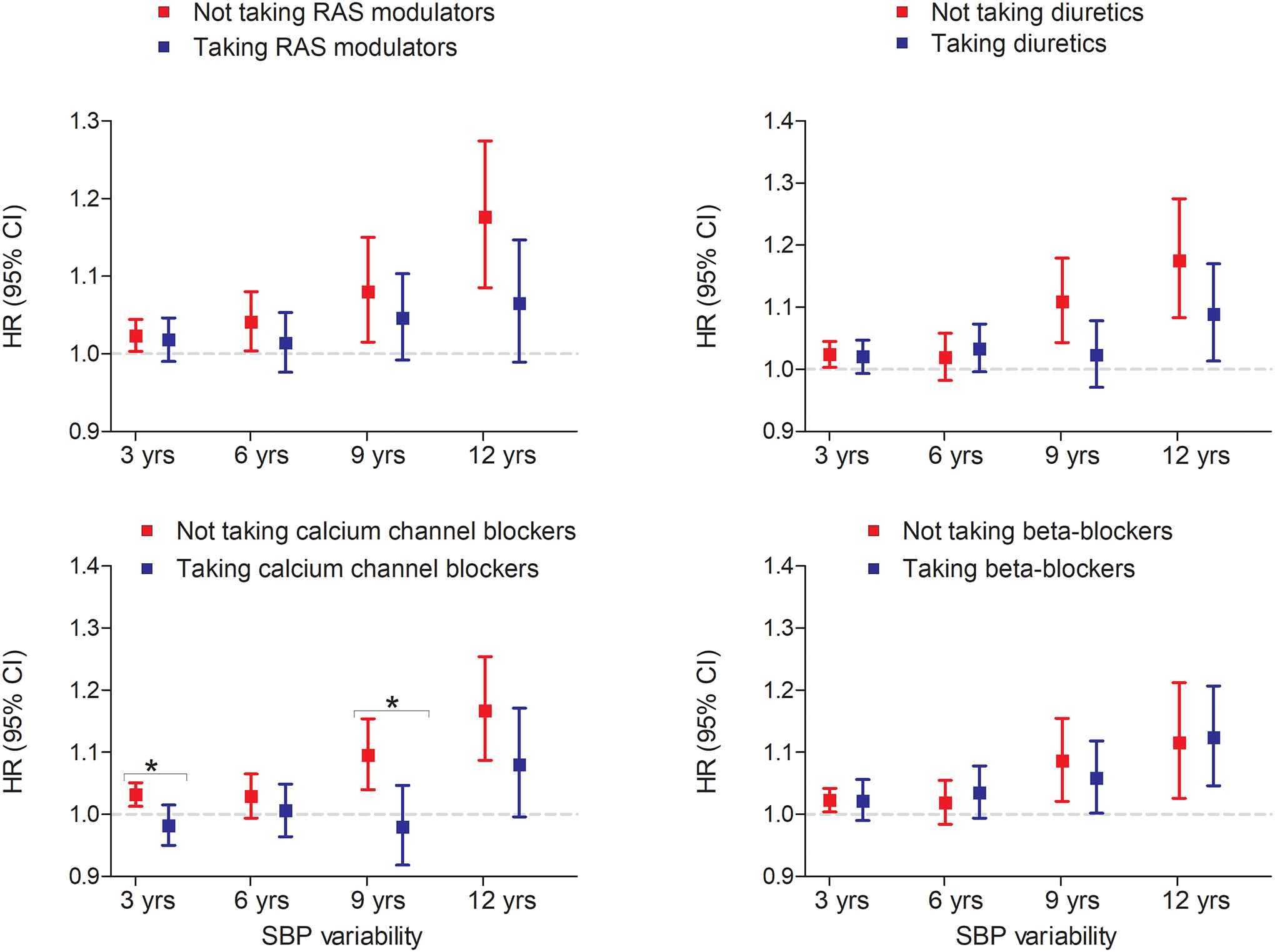
Hazard ratios were calculated using continuous values of systolic blood pressure variability. All analyses were adjusted for age at baseline, years of education at baseline, sex, race, and history of vascular risk factors burden (smoking, hypertension and diabetes mellitus), vascular diseases burden (stroke, heart disease and claudication), medication use (cardiac, lipid lowering, and mental health medications) and mean SBP until the corresponding SBPV period. SBPV was assessed using coefficient of variance. *indicates p for interaction <0.05.
We observed a trend for lower risk of dementia associated with 3 years SBPV among participants who used combination therapy of CCBs and diuretics compared to those who did not take any antihypertensive medication (eFigure 3, p-for interaction>0.05). When using 3 groups of antihypertensive medications (i.e., absent, present during the entire BPV period and occasional use), there was a trend of higher dementia risk among those who used medications occasionally compared to consistent users, especially for β-blockers, although results did not reach statistical significance (eFigure 4).”
3.5. Sensitivity analyses
After application of IPW, the association between SBPV and risk of dementia did not change (eTable 3). Adjustment for medication use at any point during SBPV periods or incident medication use did not essentially change the results (eTable 10 and 11). Cause-specific HRs for all-cause dementia in relation to SBPV were similar to the primary findings, with a similar pattern of associations for all-cause death (eTable 12). Sensitivity analyses after excluding participants with few numbers of BP assessments during follow-up (eTable 13), or adjustment for intra-individual slope of SBP (eTable 14) did not change the results. Other measures of SBPV including SD and ARV yielded similar results to the CV metric (eTable 15). The association between SBPV and longer-term dementia risk was similar to the short-term dementia risk, except for the 12 years SBPV time period where associations were no longer significant after excluding dementia cases in the first 5 years following SBPV (eTable 16).
4. DISCUSSION
Among participants of two prospective community-dwelling cohorts of older adults, we found that higher visit-to-visit SBPV of up to 12 years was associated with increased risk of all-cause dementia and AD later in life, independent of mean SBP and other vascular risk factors. Furthermore, we found that the association between SBPV and dementia risk was stronger among participants not taking CCBs, whereas the association between SBPV and dementia risk was similar across other antihypertensive medication classes.
Our findings align with previous observational cohort studies on the association between visit-to-visit BPV and dementia risk8–11. A recent meta-analysis of 6 longitudinal studies showed that higher visit-to-visit SBPV increases the risk of dementia by 1.11-fold.9 However, the majority of these prior studies did not censor dementia cases that developed during the BPV measurement period, raising concerns about reverse causation bias. Furthermore, these studies assessed BPV during periods spanning up to 6 years, while the effect of more prolonged exposure to BP fluctuations on dementia risk had not been explored. To address these knowledge gaps, we assessed the association between BPV measured up to 12 years and subsequent dementia risk by truncating BP measurements before incident dementia. In addition, we assessed the risk of longer-term dementia risk in relation to BPV by excluding dementia events that occurred 5 years immediately following BPV assessment.
To date, there were only two longitudinal cohort studies that censored dementia cases during the BPV period. The Rotterdam study showed that higher variations in BP were associated with incident dementia, especially when BP variations were measured 15 years preceding incident dementia28. However, in this study BP variations were measured only at 2 sequential visits, while typical BPV measurements utilize a minimum of 3 BP readings. The Korean National Health Insurance System cohort showed that higher visit-to-visit SBPV measured up to 6 years was associated with a subsequent increased risk of all-cause dementia, AD, and vascular dementia29. Our results extend the findings from theses prior studies by showing that among older adults, a prolonged exposure to visit-to-visit SBPV of up to 12 years is associated with short-term and longer-term (i.e., after 5 years) increased dementia risk in later life. Collectively, these results support a temporal link between BPV and dementia risk, suggesting that BPV may be a risk factor for subsequent dementia rather than a manifestation of the clinical course of a prodromal dementia syndrome.
We did not find an association between more conventional measures of BP and incident dementia, including isolated SBP measurements or mean SBP levels, consistent with other studies on BP and dementia risk in older adults1. While prior studies have also suggested reverse or non-linear associations between SBP and dementia risk in older adults1, we did not find such trends between SBP and all-cause dementia. This might be due to the younger age range of our cohort (baseline 65 years old), whereas a reverse link between SBP and dementia risk has often been reported in those 80+ years of age. Therefore, BP levels alone may not be sufficient for accurate dementia risk stratification in older adults, while other measures such as BPV may be more relevant.
Although hypertension is recognized as one of the most important risk factors for dementia, the role of antihypertensive treatment in mitigating dementia risk remains a matter of debate1. To date, most RCTs have not shown significant effects of antihypertensive treatment on incident dementia in older adults1, although in SPRINT MIND there was positive results for MCI and the combined outcome of MCI and probable dementia, respectively1, 30. While post hoc analyses of SPRINT MIND showed higher BPV to be associated with development of dementia despite excellent BP control31, none of the prior RCTs on BP control and cognitive outcomes have considered BPV as a treatment target. Therefore, the role of antihypertensive treatment on BPV and subsequent incident dementia is unclear. Furthermore, data from epidemiological studies on BPV and dementia risk have not focused on utilization of specific antihypertensive medications or drug classes. In this context, our findings suggest that CCBs may modify the relationship between SBPV and dementia risk. More specifically, we showed that SBPV was associated with lower dementia risk among those taking CCBs, an effect that was independent of other antihypertensive medication classes.
In addition, our secondary analyses suggested that the association between SBPV and dementia risk is possibly the lowest among participants on combination therapy of CCBs and diuretics (eFigure 3), although this result needs further validation in larger, well-designed studies. These findings are in line with a previous meta-analysis of RCTs in stroke patients, where it has been shown that CCBs and non-loop diuretics reduce SBPV, whereas β-blockers, ACE, and ARB may increase SBPV13. In the context of dementia, results from the Systolic Hypertension in Europe trial suggested that CCBs were more effective in reducing dementia risk than other antihypertensive medications among older adults32.
Possible mechanisms underlying the link between BPV and dementia are detailed in a previous review by Ma. et al7. Recently, direct involvement of BPV in the neurodegenerative process has been implicated by showing an association between higher SBPV and neurofibrillary tangles and several ischemia-related cerebrovascular lesions18. This is in line with our findings that higher visit-to-visit SBPV is associated with both incident all-cause dementia and AD.
Major strengths of our study include a large sample size, longitudinal design, community-based cohort of older adults, and a long follow-up period of up to 27 years. Furthermore, extensive phenotyping regarding antihypertensive medication enabled us to assess the role of different medication classes. We also investigated the role of various BPV metrics including CV, SD and ARV on dementia risk. Given that there is no gold-standard method to define BPV, CV is a practicable approach for consideration.
We acknowledge limitations of our study. First, the study sample primarily consists of White individuals ≥65 years old, limiting the generalizability of our findings to other race-ethnic and age groups. Second, we measured visit-to-visit BPV using annual measurements while the impact of shorter variations in BPV remains unknown. Third, inclusion of only survivors in the period-specific analyses may have resulted in selection bias. However, results of our IPW analyses do not support this premise. Fourth, this was a clinical-pathologic study comprising of participants with high levels of education, which might have underestimated the true effect estimates. Finally, this was an observational study and we are not able to make any causal inference.
5. CONCLUSION
In summary, the present study shows that long-term exposure to higher visit-to-visit SBPV in older adults is associated with increased risk of dementia in later life. Furthermore, CCBs may moderate the link between SBPV and dementia risk. Collectively, these results emphasize the potential importance of SBPV on brain health among older adults and underscore the need to consider SBPV as a target beyond mean BP levels, for future risk stratification in older adults. In addition, future RCTs of antihypertensive treatment and dementia prevention, in addition to examining BPV as a possible treatment target, should also consider differential drug-class effects in relation to BPV.
Supplementary Material
HIGHLIGHTS.
Among adults aged >65, higher systolic blood pressure variability spanning 3 to 12 years is associated with an increased risk of dementia later in life.
Single blood pressure measurement or mean blood pressure levels does not seem to associate with dementia risk among older adults.
The association between systolic blood pressure variability and dementia risk is stronger among those not taking calcium channel blocker medications.
RESEARCH IN CONTEXT.
Systematic review:
The authors reviewed the literature using traditional (e.g., PubMed) sources. Most prior publications on blood pressure variability (BPV) and dementia risk did not censor dementia cases that developed during the BPV measurement period, or have short durations of follow-up, raising concerns for reverse causation bias. These relevant citations are appropriately cited.
Interpretations:
Our findings show that higher systolic BPV of up to 12 years is associated with an increased risk of all-cause dementia and Alzheimer’s disease later in life, independent of mean BP levels. Furthermore, the association between systolic BPV and dementia risk was stronger among those not taking calcium channel blockers.
Future directions:
Future clinical trials of antihypertensive treatment and dementia prevention should examine the feasibility of BPV measurement as a possible treatment target, while also considering differential drug-class effects. Furthermore, BPV may need to be considered for risk stratification of older adults beyond mean BP levels.
Funding:
ROSMAP is supported by NIA grants (P30AG10161, P30AG72975, R01AG15819, and R01AG17917). ROSMAP resources can be requested at https://www.radc.rush.edu
Conflict of interest:
Dr. Gorelick serves on a data safety and monitoring board for a Novartis funded study of blood pressure lowering therapy and cognition in heart failure.
REFERENCES
- 1.Mahinrad S, Sorond FA, Gorelick PB. Hypertension and cognitive dysfunction: A review of mechanisms, life-course observational studies and clinical trial results. Rev Cardiovasc Med 2021;22(4):1429–1449. [DOI] [PubMed] [Google Scholar]
- 2.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: A review. Curr Hypertens Rep 2017;19(3):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res 2019;124(7):1025–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;375(9718):895–905. [DOI] [PubMed] [Google Scholar]
- 5.Parati G, Lantelme P. Blood pressure variability, target organ damage and cardiovascular events. J Hypertens 2002;20(9):1725–1729. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Song A, Viswanathan A, et al. Blood pressure variability and cerebral small vessel disease: A systematic review and meta-analysis of population-based cohorts. Stroke 2020;51(1):82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Tully PJ, Hofman A, et al. Blood pressure variability and dementia: A state-of-the-art review. Am J Hypertens 2020;33(12):1059–1066. [DOI] [PubMed] [Google Scholar]
- 8.de Heus RAA, Tzourio C, Lee EJL, et al. Association between blood pressure variability with dementia and cognitive impairment: A systematic review and meta-analysis. Hypertension 2021;78(5):1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia P, Lee HWY, Chan JYC, et al. Long-term blood pressure variability increases risks of dementia and cognitive decline: A meta-analysis of longitudinal studies. Hypertension 2021;78(4):996–1004. [DOI] [PubMed] [Google Scholar]
- 10.Alperovitch A, Blachier M, Soumare A, et al. Blood pressure variability and risk of dementia in an elderly cohort, the three-city study. Alzheimers Dement 2014;10(5 Suppl):S330–337. [DOI] [PubMed] [Google Scholar]
- 11.Rouch L, Cestac P, Sallerin B, et al. Visit-to-visit blood pressure variability is associated with cognitive decline and incident dementia: The s.Ages cohort. Hypertension 2020;76(4):1280–1288. [DOI] [PubMed] [Google Scholar]
- 12.van Middelaar T, van Dalen JW, van Gool WA, et al. Visit-to-visit blood pressure variability and the risk of dementia in older people. J Alzheimers Dis 2018;62(2):727–735. [DOI] [PubMed] [Google Scholar]
- 13.Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet 2010;375(9718):906–915. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010;9(5):469–480. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Buchman AS, Boyle PA, et al. Religious orders study and rush memory and aging project. J Alzheimers Dis 2018;64(s1):S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Wilson RS, Arvanitakis Z, et al. Selected findings from the religious orders study and rush memory and aging project. J Alzheimers Dis 2013;33 Suppl 1(0):S397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah RC, Wilson RS, Bienias JL, et al. Relation of blood pressure to risk of incident alzheimer’s disease and change in global cognitive function in older persons. Neuroepidemiology 2006;26(1):30–36. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Blacker D, Viswanathan A, et al. Visit-to-visit blood pressure variability, neuropathology, and cognitive decline. Neurology 2021;96(23):e2812–e2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. Us population data. Arch Intern Med 1993;153(5):598–615. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59(2):198–205. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of alzheimer’s disease: Report of the nincds-adrda work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 22.Boyle PA, Buchman AS, Wilson RS, et al. Association of muscle strength with the risk of alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 2009;66(11):1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal NT, Bienias JL, Bennett DA, et al. The relation of cigarette smoking to incident alzheimer’s disease in a biracial urban community population. Neuroepidemiology 2006;26(3):140–146. [DOI] [PubMed] [Google Scholar]
- 24.Medi-Span PC. Master drug data base documentation manual. Indianapolis, Indiana 1995. [Google Scholar]
- 25.Arvanitakis Z, Schneider JA, Wilson RS, et al. Statins, incident alzheimer disease, change in cognitive function, and neuropathology. Neurology 2008;70(19 Pt 2):1795–1802. [DOI] [PubMed] [Google Scholar]
- 26.van der Wal WM, Geskus RB. Ipw: An r package for inverse probability weighting. Journal of Statistical Software 2011;43:1–23. [Google Scholar]
- 27.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y, Wolters FJ, Chibnik LB, et al. Variation in blood pressure and long-term risk of dementia: A population-based cohort study. PLoS Med 2019;16(11):e1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo JE, Shin DW, Han K, et al. Blood pressure variability and the risk of dementia: A nationwide cohort study. Hypertension 2020;75(4):982–990. [DOI] [PubMed] [Google Scholar]
- 30.Group SMIftSR, Williamson JD, Pajewski NM, et al. Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 2019;321(6):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Havenon A, Anadani M, Prabhakaran S, et al. Increased blood pressure variability and the risk of probable dementia or mild cognitive impairment: A post hoc analysis of the sprint mind trial. J Am Heart Assoc 2021;10(18):e022206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in europe (syst-eur) trial. Lancet 1998;352(9137):1347–1351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


