Abstract
Background:
Serological evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been reported in white-tailed deer (WTD) in the United States and Canada. Even though WTD are susceptible to SARS-CoV-2 infection, there is no evidence of infection by this virus in other mammalian species that might interact with WTD in nature. Similar to WTD, feral swine are widely distributed and generally occupy the same range as WTD in Texas. The objective of this study was to determine the prevalence of SARS-CoV-2 neutralizing antibody in WTD during 2020 and 2021 and determine the prevalence of SARS-CoV-2 neutralizing antibody in feral swine during 2018 (prepandemic period) and from March 2020 to February 2021 (pandemic period) in Travis County, Texas.
Materials and Methods:
Sera samples were collected from hunter-killed WTD and feral swine during the prepandemic and pandemic period and tested for SARS-CoV-2 antibody by a plaque reduction neutralization assay in Vero cells.
Results:
SARS-CoV-2 antibody was not detected in any of the 166 feral swine sera samples, including 24 samples collected during the prepandemic and 142 samples collected during the pandemic period. Furthermore, SARS-CoV-2 antibody was not detected in the 115 WTD samples collected during late 2020, but antibody was detected in WTD in early 2021.
Conclusions:
The results indicated that SARS-CoV-2 infection of WTD occurred during early 2021 in Travis County, Texas, but serological evidence of SARS-CoV-2 infection was not detected in the feral swine samples collected from the same locality and during the same time period of the collection of WTD samples.
Keywords: neutralizing antibody, SARS-CoV-2, feral swine, white-tailed deer, Texas
Introduction
White-tailed deer (WTD; Odocoileus virginianus) is one of the most abundant and widely distributed large ruminant mammalian species in North America, including Texas (Gray, 2013). Furthermore, Texas hosts the highest population of feral swine (Sus scrofa), which is one of the most destructive invasive vertebrate species and causes millions of dollars losses in agriculture every year (Lewis et al., 2019).
Serological evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in WTD was first reported during early 2021 in the mid-west and Texas (Chandler et al., 2021; Palermo et al., 2022). Subsequent studies indicated that SARS-CoV-2 infection was common among WTD throughout much of the United States and in selected areas in Canada (Hale et al., 2022; Pickering et al., 2022). Serosurveys for evidence of SARS-CoV-2 infection among feral swine have not been reported even though they occupy the same range as WTD in Texas (Mapston, 2012). As a follow-up to the initial observations that WTD were being infected with SARS-CoV-2 in Texas (Palermo et al., 2022), this study was conducted to further determine the temporal pattern of infection in WTD during 2020 and 2021 and to determine if feral swine were being infected with SARS-CoV-2 during 2018 (prepandemic period) and from March 2020 to February 2021 (pandemic period) in Travis County, Texas.
Materials and Methods
Sample collection
A total of 257 blood samples were collected from hunter-harvested WTD (n = 115) and feral swine (n = 142) from October to December 2020 and March 2020 to February 2021 at four sites (A–D) located within Travis County Texas, respectively (Palermo et al., 2020). The present study was conducted in accordance with Scientific Permit SPR-0801-168 issued by the Texas Parks and Wildlife Department, Austin, Texas. Also, a subset of 24 blood samples (17 females and 7 males) that were collected previously in the same areas from adult feral swine in 2018 was used as prepandemic control samples. Date and collection location, age, and sex were recorded in each animal (WTD or feral swine). All the blood samples were obtained by postmortem cardiac puncture at a central collecting station and transported to the laboratory for processing. Samples were centrifuged at 1200 g at 4°C for 10 to 15 min and then the sera were stored in aliquots of 1–2 mL at −20°C until tested for SARS-CoV-2 neutralizing antibody at different times.
SARS-CoV-2 neutralization test
Sera samples obtained from WTD and feral swine were tested for neutralizing antibody to SARS-CoV-2 by a plaque reduction neutralization assay in Vero cells (Palermo et al., 2022). In brief, the serum samples were heat treated at 56°C for 30 min and then diluted 1:5 in EMEM (Eagle's minimum essential medium). Each diluted sample was mixed with 40–50 plaque-forming units (PFUs) of SARS-CoV-2 (USA-WA1/2020 strain) and incubated at 37°C for 1 h. SARS-CoV-2 antibody positive and negative sera were included in the neutralization assays as controls.
Virus and serum mixtures were inoculated onto confluent monolayers of Vero E6 cells propagated in 12-well plates and then a 0.8% agarose was added onto the cell monolayer and the cells were incubated for 2 days at 37°C and then stained with a 0.03% neutral red solution to visualize the dead foci of cells as plaques. The virus dose was determined as the mean number of PFU recorded on 12-well cells infected with 40–50 PFU based on the testing of the viral dose dilution and negative control. The PFUs were counted and if the sera dilution (1:10) reduced ≥80% of the virus dose, the sample was considered positive for antibody.
Results
All sera samples collected from feral swine, including 24 samples in 2018, and 142 samples collected from March 2020 to February 2021 during the pandemic period were negative for SARS-CoV-2 neutralizing antibody (Table 1). Furthermore, SARS-CoV-2 antibody was not detected in 115 WTD serum samples collected during late 2020, including 40 samples collected in October, 45 in November, and 30 in December for a total of 115 antibody-negative samples. However, as previously reported (Palermo et al., 2022), SARS-CoV-2 antibody was detected in 37% (20/54) of the WTD sampled during January–February 2021 (Table 2).
Table 1.
Description of the White-Tailed Deer and Feral Swine Samples Collected in Travis County, Texas from March 2020 to February 2021
| N (%) | |
|---|---|
| Feral swine | |
| Age | |
| Juvenile | 92 (64.8) |
| Adult | 50 (35.2) |
| Sex | |
| Male | 67 (47.2) |
| Female | 75 (52.8) |
| WTD | |
| Age (year) | |
| 0.5 | 15 (13) |
| 1.5 | 38 (33) |
| 2.5 | 14 (12.2) |
| 3.5 | 21 (18.3) |
| ≥4.5 | 27 (23.5) |
| Sex | |
| Male | 67 (58.3) |
| Female | 48 (41.7) |
WTD, white-tailed deer.
Table 2.
SARS-CoV-2 Neutralization Antibody Prevalence Rate in White-Tailed Deer and Feral Swine Serum Samples Collected in Travis County, Texas from March 2020 to February 2021
| |
WTD |
Feral swine |
|---|---|---|
| % (No/total samples) | % (No/total samples) | |
| March–September 2020 | NA | 0 (0/71) |
| October–December 2020 | 0 (0/115) | 0 (0/58) |
| January–February 2021 | 37 (20/54)a | 0 (0/13) |
Prevalence rate determined previously (Palermo et al., 2022).
NA, no samples available; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion and Conclusions
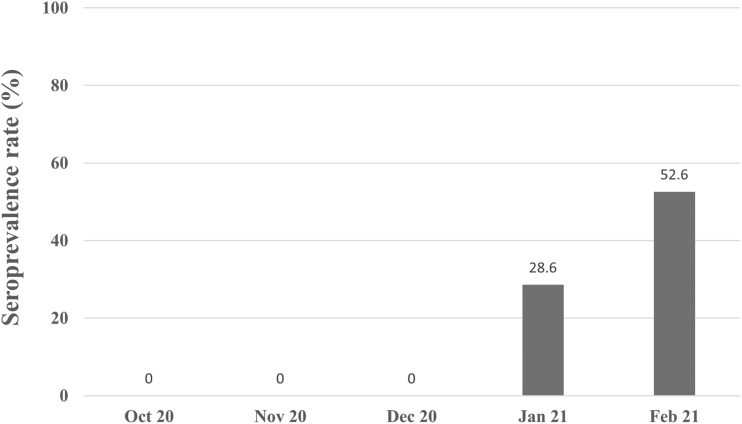
Our study represented the first serological surveillance for SARS-CoV-2 in WTD and feral swine in Texas during the first year of the coronavirus disease 2019 (COVID-19) pandemic. The lack of SARS-CoV-2 antibody in the 115 WTD samples collected in late 2020 (October to December 2020) suggested that SARS-CoV-2 infection did not occur among WTD and provided insight regarding the pattern of SARS-CoV-2 infection during the COVID-19 pandemic. The data complement a previous study where SARS-CoV-2 antibody in WTD was detected during January 2021 (28.6%, 10/35) and increased significantly (p < 0.05) through February 2021 (52.6%, 10/19) (Fig. 1) (Palermo et al., 2022). Thus, suggesting that seroconversion to SARS-CoV-2 infection among WTD from Travis County occurred in early 2021.
FIG. 1.
SARS-CoV-2 neutralizing antibodies trend in white-tailed deer from October 2020 to February 2021 in Travis County, Texas.
Seroconversions coincided with the peak number of COVID-19 human cases in Travis County in January, 2021 (Austin Public Health, 2021) suggesting that the source of infection among WTD were infected humans. Interestingly, the B.1.1.7 (Alpha) variant represented 6% of the SARS-CoV-2 variants among humans by January 2021 and increased to 23% by the end of February 2021 in Texas (Hodcroft, 2021). Similarly, another study reported a high rate of SARS-CoV-2 antibody among captive WTD in Texas during January 2021 (Hamer et al., 2022). Also, studies in Iowa indicated that the SARS-CoV-2 antibody rates in WTD increased from 5.2% in October 2020 to 89% in December 2020, and suggested that humans were also the source of SARS-CoV-2 infection (Kuchipudi et al., 2022).
Coronaviruses (CoV) have been reported in domestic and wild ruminants, including WTD (Tsunemitsu et al., 1995) and are antigenically and genetically similar to Bovine CoV and Human CoV OC43 (Alekseev et al., 2008; Vijgen et al., 2005). Interestingly, in a serosurvey for CoV antibody in WTD from Ohio (Tsunemitsu et al., 1995), 6.6% (2/30) of the WTD serum samples cross-reacted to Bovine CoV strains by indirect immunofluorescence assays but failed to neutralize those Bovine CoV strains, possibly due to the Bovine-like CoV isolated from WTD (WTD CoV) had substitutions in the region-binding domain of the spike protein that could have limited the host receptor affinity (Alekseev et al., 2008). However, as there is currently limited research in WTD CoV, it cannot be ruled out that unknown CoV might be circulating in WTD and cross-react with SARS-CoV-2, and it warrants further research.
Serological evidence of SARS-CoV-2 infection was not detected in any of the 166 feral swine sera samples collected during the pre- and COVID-19 pandemic period from the same area where samples were collected from WTD in Travis County, Texas. However, the lack of SARS-CoV-2 antibody among feral swine could have been associated with the limited number of samples (n = 13) collected and tested during early 2021. A similar lack of serological evidence to SARS-CoV-2 infection was reported in farmed pigs from the Netherlands (Sikkema et al., 2022).
These results are supported by previous experimental studies that showed pigs were not susceptible to SARS-CoV-2 infection and was thought to be due to an early apoptosis mechanism, detected in porcine epithelial cells, which prevented SARS-CoV-2 replication (Nelli et al., 2021; Vergara-Alert et al., 2021). Further SARS-CoV-2 serosurveys among WTD and feral swine should be conducted due to the possibility of the emergence of SARS-CoV-2 variants that could affect the health of humans, domestic animals, and wildlife.
Acknowledgments
The authors thank John D. Cornelius, Jim Mobley, Kevin Cagle, Philip Cagle, Beau Bush, Susan Bush, Bryan Rugh, and James Ashley for collecting data and specimens for this study.
Authors' Contributions
P.M.P. was the leader who designed the study and performed the laboratory testing of the animals' samples and prepared the article; J.O. assisted with the laboratory testing of the samples and data analysis; D.M.W. assisted with the design, preparation of the article, and provided funding for the study; and J.C.M. collected and processed the samples from the animals and assisted with the design of the study and preparation of the article. All authors approved the final version of the article.
Author Disclosure Statement
No conflicting financial interests exist.
Funding Information
This research was funded by the Office Research and Sponsored Projects and by a Grant 2U54MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH).
References
- Alekseev KP, Vlasova AN, Jung K, et al. Bovine-like coronaviruses isolated from four species of captive wild ruminants are homologous to bovine coronaviruses, based on complete genomic sequences. J Virol 2008;82(24):12422–12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin Public Health 2021: Available from: https://austin.maps.arcgis.com/apps/dashboards/f8b42573e2df477b87be64bb69574b8e [Last accessed: December 20, 2022].
- Chandler JC, Bevins SN, Ellis JW, et al. SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proc Natl Acad Sci U S A 2021;118(47):e2114828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SS. 2013. Performance Report Federal Aid Project No. W-127-r-20. Big Game Research and Surveys. Available from: https://tpwd.texas.gov/huntwild/wild/research/highlights/taxa/publications/Gray_2013_PronghornDiseases.pdf (pp. 1–26) [Last accessed: December 20, 2022].
- Hale VL, Dennis PM, McBride DS, et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature 2022;602(7897):481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Nunez C, Roundy CM, et al. Persistence of SARS-CoV-2 neutralizing antibodies longer than 13 months in naturally infected, captive white-tailed deer (Odocoileus virginianus), Texas. Emerg Microbes Infect 2022;11(1):2112–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft E. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. 2021. Available from: https://covariants.org [Last accessed: February 24, 2023].
- Kuchipudi SV, Surendran-Nair M, Ruden RM, et al. Multiple spillovers and onward transmission of SARS-Cov-2 in free-living and captive White-tailed deer (Odocoileus virginianus). Proc Natl Acad Sci U S A 2022;119(6):e2121644119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS, Corn JL, Mayer JJ, et al. Historical, current, and potential population size estimates of invasive wild pigs (Sus scrofa) in the United States. Biol Invasions 2019;21(7):2373–2384. [Google Scholar]
- Mapston ME. Feral hogs in Texas; 2012. Available from: https://tfsweb.tamu.edu/uploadedFiles/TFSMain/Manage_Forest_and_Land/Wildlife_Management/Non-Game/Feral_hogs_TCE.pdf [Last accessed: December 20, 2022].
- Nelli RK, Phadke KS, Castillo G, et al. Enhanced apoptosis as a possible mechanism to self-limit SARS-CoV-2 replication in porcine primary respiratory epithelial cells in contrast to human cells. Cell Death Discov 2021;7(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo PM, Orbegozo J, Morrill JC, et al. Serological evidence of West Nile virus infection in white-tailed deer in Central Texas. Vector Borne Zoonotic Dis 2020;20(11):850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo PM, Orbegozo J, Watts DM, et al. SARS-CoV-2 neutralizing antibodies in White-Tailed deer from Texas. Vector Borne Zoonotic Dis 2022;22(1):62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MV, Martins M, Falkenberg S, et al. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol 2021;95(11):e00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering B, Lung O, Maguire F, et al. Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission. Nat Microbiol 2022;7(12):2011–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema RS, Tobias T, Oreshkova N, et al. Experimental and field investigations of exposure, replication and transmission of SARS-CoV-2 in pigs in the Netherlands. Emerg Microbes Infect 2022;11(1):91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H, el-Kanawati ZR, Smith DR, et al. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J Clin Microbiol 1995;33(12):3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara-Alert J, Rodon J, Carrillo J, et al. Pigs are not susceptible to SARS-CoV-2 infection but are a model for viral immunogenicity studies. Transbound Emerg Dis 2021;68(4):1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L, Keyaerts E, Moës E, et al. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol 2005;79(3):1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]