Abstract
In the present research, the structural, electronic and optical properties of transition metal dichalcogenide-doped transition metal oxides MoS2-doped-V2O5 with various doping concentrations (x = 1–3%) of MoS2 atoms are studied by using first principles calculation. The generalized gradient approximation Perdew–Burke–Ernzerhof simulation approach is used to investigate the energy bandgap (Eg) of orthorhombic structures. We examined the energy bandgap (Eg) decrement from 2.76 to 1.30 eV with various doping (x = 1–3%) of molybdenum disulfide (MoS2) atoms. The bandgap nature shows that the material is a well-known direct bandgap semiconductor. MoS2 doping (x = 1–3%) atoms in pentoxide (V2O5) creates the extra gamma active states which contribute to the formation of conduction and valance bands. MoS2-doped-V2O5 composite is a proficient photocatalyst, has a large surface area for absorption of light, decreases the electron-hole pairs recombination rate and increases the charge transport. A comprehensive study of optical conductivity reveals that strong peaks of MoS2-doped-V2O5 increase in ultraviolet spectrum region with small shifts at larger energy bands through increment doping x = 1–3% atoms of MoS2. A significant decrement was found in the reflectivity due to the decrement in the bandgap with doping. The optical properties significantly increased by the decrement of bandgap (Eg). Two-dimensional MoS2-doped-V2O5 composite has high energy absorption, optical conductivity and refractive index, and is an appropriate material for photocatalytic applications.
Keywords: two-dimensional MoS2-doped-V2O5 composite, MoS2 doping, optical conductivity, photocatalytic application
1. Introduction
Two-dimensional transition metal oxides (TMOs) and transition metal dichalcogenide (TMDs) composites are advanced and novel nanocomposites due to their unique optical, physical and chemical properties, that is dependent on the size as smaller nanoparticles are exhibiting quantum nature [1,2]. The excessive extinction coefficient, tune capacity, shape and dimension make them fairly traumatic in many fields, e.g. digital devices, nano-medicine and photocatalytic applications [3]. The chemical composition of MoS2, the valance shell of ‘S’ with −2 which is comfortable for oxidation, and C-S bonding atoms increase the stability of nanocomposite [4]. To boost the photocatalytic application of semiconductor photocatalysts, there are three foremost parameters that should be followed. Firstly, the increases of excitation wavelength, secondly decreases of electron-hole pair recombination, and lastly third very major point the increment of active sites around the surface edges for absorbance of the photon of light. The surface area for absorbance of light can be increased by following the strategies: firstly by inclusion with cations or anions, secondly by doping with metals element and at third factor by improving the structure nature of semiconductor photocatalysts to increase their surface area or porosity to enhance the photocatalytic application [5]. The MoS2 and V2O5 composite enrich the family with a large surface area with increased active sites around the surface edges for absorbance of the photon of light and decrease the recombination rate of electron-hole pair. MoS2-doped-V2O5 (V/MOS) composite is not only used as a two-dimensional photocatalyst, but also in sensors [6], electrochemistry [7], as a photo-anode in the sensitized solar cell [8], batteries [9] and photocatalytic application [10].
Vanadium is a rare earth element and it has a diversity of valance states. Among the family of oxides, V2O5 (vanadium pentoxide) has a proficient stable oxide structure and is mostly used in electrochemical instruments [11], thermoelectric devices [12], gas sensors [13], two-dimensional circuit board [14], oxidization accelerators and catalysis applications [15]. V2O5 is a prominent TMO belonging to group 5, d-block with unique properties such as 20 K boiling points and 936 K melting points. In the proficient advanced oxidation photocatalyst progression, the first step is electron-hole pair (ehv) creation during separation. These produced electron-hole pairs move to the V/MOS semiconductor surface and increase the oxidation and reduction of absorbed photons separately [16]. The lifetime of electrons and hole generation influence redox reaction [17]. The hole (hv) comprises sufficient to oxidize various organic pollutants, furthermore, hydroxyl group in the water system. The hole can oxidize through various organic pollutants by creating in water; moreover, the electrons in the conduction band interrelate with the oxygen of water and create superoxide radicals that further interact with OH− ion to form radical. This is a strong oxidizing candidate to the pollutant through oxidation of the organic molecule [18].
Two-dimensional V/MOS architectures are also greatly attractive in the field of energy harvesting and the environment. Photocatalysis is a proficient method for photo-anode, dye degradation, wastewater purification and water splitting [19]. Two-dimensional TMDs-doped-TMOs play a momentous role in the degradation of pharmaceuticals in industrial wastewater due to their low energy bandgap, less toxicity, best dispersibility etc. Nowadays, the discarding of pharmaceutical devastate has become a important concern worldwide. Separating various medications from wastewater effluents is often unsuccessful with convectional wastewater and biological treatments. However, pharmaceutical substances such as antibiotics, hormones, steroids and other drugs can neither be separated from effluent nor be destroyed by biological treatment [20]. As a consequence, heterogeneous photocatalysis has boosted as a promising technique for reducing the adverse effects of industrial consumption. Sunlight-active materials used as catalysts for destroying such pollutants are a realistic choice [21–23].
MoS2 is loaded on V2O5, whether the utmost valance state of V+5 and easily oxidized S2− can hasten to electron during the reaction. There is no theoretical and experimental research on the structural electronic and optical behaviour of two-dimensional MoS2-doped-V2O5 with doping x = 1–3% atoms of MoS2 in the available literature. As a result, the primary aim of the present work is to provide some significant authentic information to the presented data on electronic, structural and optical properties of MoS2-doped-V2O5 using density functional theory through Cambridge Serial Total Energy Package (CASTEP) software simulation. MoS2-doped-V2O5 have high optical conductivity and are more consistent and appropriate surface area materials for photocatalytic applications. Two-dimensional MoS2-doped-V2O5 is used to determine appropriate and efficient photocatalytic applications. The results indicate that there is a decrement trend in the energy bandgap from 2.77 to 0.0 eV with doping x = 1–3% atoms of MoS2. MoS2-doped-V2O5 has high optical conductivity, is more consistent and has an appropriate surface area; it enhances photocatalytic activity. MoS2-doped-V2O5 has high optical conductivity as well as absorbance, is more consistent and is appropriate material for photocatalytic applications.
2. Computational methodology
The crystal structure of TMO (V2O5) pentoxide material with space group Fdd2 (no. 43) was used in the CASTEP program; moreover, before the analysis of the characteristics the geometry was optimized. CASTEP software, based on density functional theory (DFT), was executed on the accomplishment of our first-principles calculations. The bandgap and electronic exchange correlation have been deliberated by using generalized gradient approximation (GGA) and Perdew–Burke–Ernzerhof (PBE) parametrization method. The ultra-soft pseudo-potential was used to investigate the electrostatic interaction between ionic core and valance electrons. The atomic electronic configuration of vanadium (V2) and oxygen (O5) are and , respectively. The novel ionic states creation is the result of nuclei interaction with the internal core and outer side electron. The outermost electrons in the MoS2-doped-V2O5 composite interact with the core electron, then the ions-electron potential converges. Brillouin zone integration is simulated through customized K-points; furthermore, these K-points meshes build by 1 × 1 × 1 on the Monkhorst pack grid for optimization of the crystal structure. During the complete optimization, the total energy keeps constant at 1 × 10−5 eV. Moreover, the convergence assessment of applied forces to the MoS2 atoms is set aside at −2.283 eV/Å. The geometry is optimized through the CASTEP algorithm with doping x = 1–3% atoms of MoS2 in V2O5.
3. Results and discussion
3.1. Structural analysis
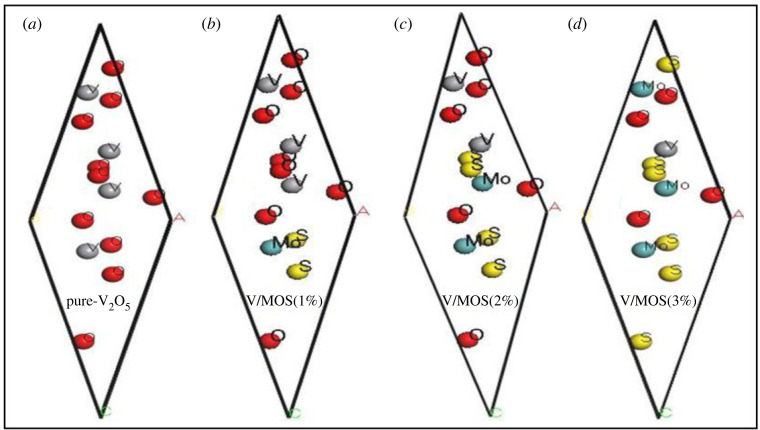
The supercell 1 × 1 × 1 of MoS2-doped-V2O5 is shown in figure 1. The orthorhombic structure of MoS2-doped-V2O5 with space group Fdd2 (no. 43) [24–28]. The crystal lattice parameters of two-dimensional MoS2-doped-V2O5 is oriented through a = 11.044, b = 10.315, c = 5.700 and angles α = 81.723°, β = 67.562°, γ = 30.715° are shown in table 1. The overall energy of MoS2-doped-V2O5 crystal is maintained low level by estimating equilibrium lattice parameters with the help of the Monkhorst pack grid, and results are obtained throughout a wide range of lattice parameter values. The decrement in lattice parameters values with various doping (x = 1–3%) atoms of molybdenum disulfide (MoS2) as a result there is presented a strong attraction between MoS2 and V2O5 atoms. Furthermore, doping with (x = 1–3%) MoS2 atoms, the decrement in supercell volume varies from 282.25 to 123.71 Å as shown in table 2. This indicates super-cells of MoS2-doped-V2O5 are remarkably shrunk as a result of decrement in the energy bandgap (Eg).
Figure 1.
Supercell cell structure of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
Table 1.
The lattice constants/angles of the supercell of MoS2-doped-V2O5.
| material | lattice constant Å/ angles |
binding energy (eV/atom) | ||
|---|---|---|---|---|
| pure V2O5 | a = 11.044 | b = 10.315 | c = 5.700 | −2.283 eV |
| angles | α = 81.723° | β = 67.562° | γ = 30.715° | |
| space group | Fdd2 (no. 43) | |||
Table 2.
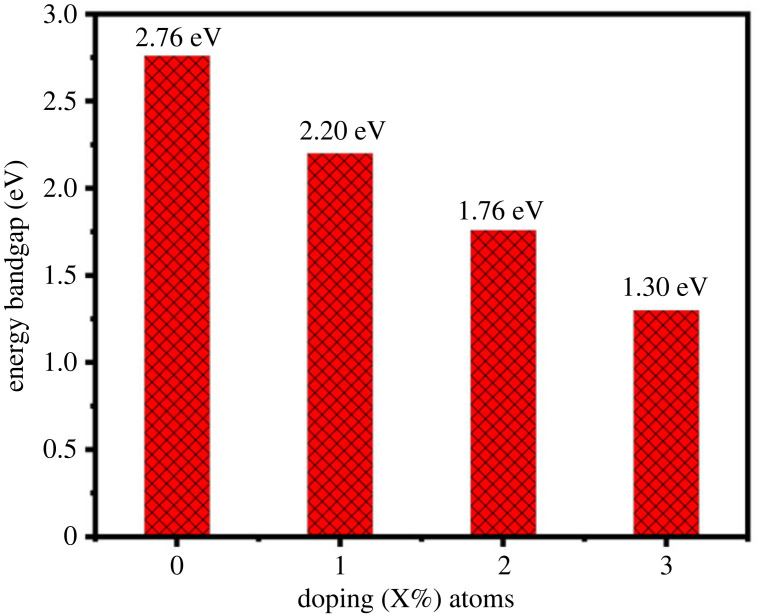
The energy bandgap (Eg) and volume of super cells of two-dimensional MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
| compound | volume (A)3 | bandgap (eV) |
|---|---|---|
| pure V2O5 | 282.25 | 2.76 |
| MoS2-doped-V2O5 composite (x = 1%) | 201.71 | 2.20 |
| MoS2-doped-V2O5 composite (x = 2%) | 140.12 | 1.76 |
| MoS2-doped-V2O5 composite (x = 3%) | 123.71 | 1.30 |
3.2. Electronic band structures and density of states
The band structure of a crystal is relating to energy eigenvalues and plays a vital role to understand energy Fermi (EF) levels which determine whether the composite is conductor, semi-conductor or a insulator. There are two types of bands first one is conduction band (CB) which is located at the accurately above the energy fermi level (EF) as well as valance band (VB) which is located below the EF.
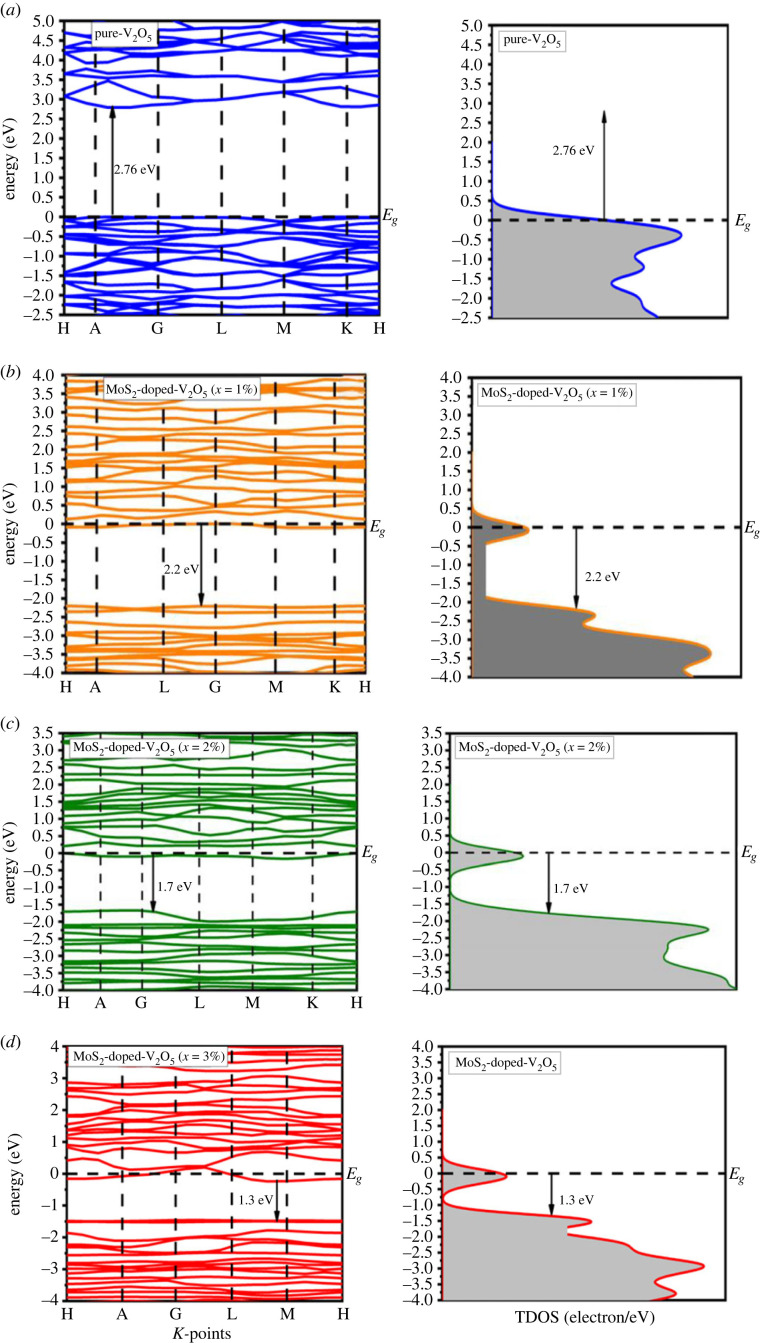
Figure 2 shows that the upper position of valance band maxima (VBM) and lower position of conduction band minima (CBM) are accurately positioned at the same point ‘G’ represents that MoS2-doped-V2O5 eminent direct semiconductor. In the current study, the direct bandgap is reported with various doping (x = 1–3%) atoms of MoS2. The band structures of two-dimensional MoS2-doped-V2O5 composite are shown in figure 2a–d; these are obtained by doping (x = 1–3%) atoms of MoS2. The minimum bandgap of 1.3 eV is obtained at 3% MoS2 atoms. A decrement trend in the MoS2-doped-V2O5 band structures can be examined through increased doping x = 1–3% atoms as shown in figure 3. The bandgap values and volume of super cells are decreasing as shown in table 2. Due to a decrease in the volume of super-cells with increased doping atoms (x = 1–3%) of MoS2 in V2O5, the CB moves toward the Fermi level (EF) along the G symmetry, which is the main reason for the reduction of the energy bandgap (Eg) of the MoS2-doped-V2O5 materials. The bandgap structures revealed that two-dimensional material appears in semiconductors within direct bandgap nature. These findings show that two-dimensional MoS2-doped-V2O5 is a suitable material for photocatalytic applications.
Figure 2.
(a–d) Band structures of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
Figure 3.
Bandgap doping concentration (X%) atoms of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite.
The electronic bandgap represented per unit energy is measured through total density of states although ions' contribution of various band structures is analysed by partial density of states. The total density of states (TDOS) and partial density of states (PDOS) are used to explicate the decrement in the bandgap. The Fermi level , which is indicated by a dotted line, is set at the peak of the valence band. Figure 4a–d shows with increased doping (x = 1–3% atoms), its value tends to decrease from 20 to the minimum value of 16 at 3%. This characteristic peak shifts towards the right through the doping concentration of 3%. Moreover, in p states, the number of gamma states increases as the doping atoms increase. As a consequence, p states represent more major contributions than s and d states, as shown in figure 4a–d. By increasing doping (x = 1–3%) atoms, the hybridization of p-d states increases as a result of decrement in an energy bandgap (Eg). These results indicate that the MoS2-doped-V2O5 composite is a suitable material for photocatalytic applications.
Figure 4.
(a–d) PDOS and TDOS of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
When through increased x = 1–3% atoms the VB moves near a lower energy level, although the CB shifts towards the lower energy, which is the basic motive for the decrement of the bandgap. With the help of all observations, we can conclude that the PDOS shifts towards minimum energies as a consequence of doping x = 1–3% atoms, leading to a decrement in the bandgap, which also implies the optical properties described below.
3.3. Optical properties
The optical properties of (two-dimensional) TMOs and TMDs composites are fascinating and have noteworthy photocatalytic applications. The complex dielectric parameters explain the response of two-dimensional MoS2-doped-V2O5 composite (x = 1%, 2% and 3%) to an electric field. It is divided into two components, real dielectric function (RDF) and imaginary dielectric function (IDF), and is dependent on the crystal's optical band structure.
The optical properties of two-dimensional MoS2-doped-V2O5 composite may be described with the help of electronic structure moreover relative permittivity, refractive index, coefficient of absorption, reflectivity as well as energy loss function. These properties are very helpful in signifying the viability and suitability of two-dimensional structured MoS2-doped-V2O5 material in photocatalytic applications. All of the optical conductivity properties are the result of the interaction of valance electrons among core inner electrons of MoS2-doped-V2O5 composite and waves (electromagnetic waves). All these properties are connected, and the complex dielectric function can be used which is given in [25,29,30].
| 3.1 |
To study the optical response to the effect of doping (x = 1–3%) atoms of MoS2 on RDF and IDF of the dielectric function can be measured through the following equations (3.2) and (3.3) [31,32]:
| 3.2 |
| 3.3 |
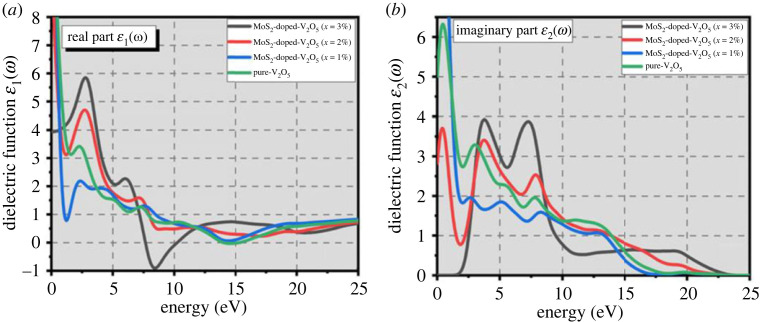
The dielectric constants RDF and IDF present the relative permittivity of the two-dimensional MoS2-doped-V2O5 composite. If we take the term dielectric literally, it shows how much an electric field is permitted to go through MoS2-doped-V2O5 composite at different doping x = 1–3% atoms. This mostly exhibits how much polarization MoS2-doped-V2O5 composite can be tolerated at doping atoms. As a result, an ideal electrical conductor should have zero value because no field can be there within conductor boundaries. The RDF gives information regarding the polarization intensity of MoS2-doped-V2O5 at different percentages of doping atoms. The main peak of dielectric function at 1% atom at 3.2 eV and at 3% it reaches 5.8 eV as shown in figure 5a,b. It seems to decrease towards a high value of 7.5 eV when with various doping (x = 1–3% atoms) of MoS2, respectively. The optical characteristics are interlinked to energy dissipation and are affected by IDF function. The IDF is shown in figure 5b. As doping (x = 1–3%) atoms of MoS2 increased, IDF peaks shift slightly to the lower value, and energy also shifted towards higher values. All peaks slightly move toward higher energy when doping increases. These dielectric results show that two-dimensional MoS2-doped-V2O5 is a suitable material for photocatalytic applications.
Figure 5.
Dielectric functions (a) RDF and (b) IDF of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
3.4. Refractive index and extinction
By vigilant investigation of the extinction coefficient k(w) and refractive index n(w) of two-dimensional MoS2-doped-V2O5 transition material the absorbance of electromagnetic (EM) radiation and the calculation of optical transparency is shown in figure 6a,b. The behaviour of refractive index n(w) and extinction coefficient k(w) in the range of 0–50 eV with doping (x = 1–3% atoms) of MoS2 of two-dimensional MoS2-doped-V2O5 composite.
Figure 6.
(a) Refractive index (n) and (b) extinction coefficient of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
At 0 eV two-dimensional MoS2-doped-V2O5 has a refractive index (n) of 1.5, 2, 2.3 and 2.5 with pure and doping (x = 1–3% atoms) of MoS2, respectively. The refractive move toward decreasing radically with energy eV up to 5 eV. The refractive index (n) fluctuates from 0 to 5 in the range of energy 0 to 50 eV outstanding to the diverse frequencies of the inner-transition band. The lower refractive index shows smaller polarization in the eminent energy range. The following equations are used to calculate the additional optical factors such as reflectivity, absorption, refractive index and energy loss [15,33–36]:
| 3.4 |
| 3.5 |
| 3.6 |
| 3.7 |
| 3.8 |
| 3.9 |
| 3.10 |
| 3.11 |
| 3.12 |
| 3.13 |
3.5. Absorbance and energy loss
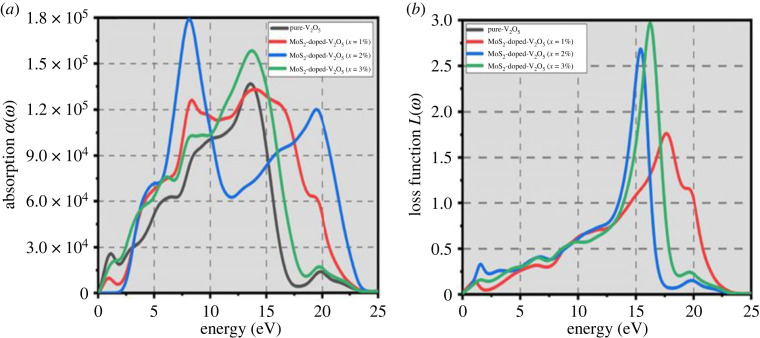
The absorption of any material is capably connected to the capability of absorbance luminescent EM radiation in disparity to photons with appropriate energy , furthermore, the energy loss function describes the energy dissipation when the material comes into range with incident photons as shown in figure 7b. The absorbance of two-dimensional MoS2-doped-V2O5 is increasing by doping (x = 1%, 2% and 3%) atoms of MoS2 as shown in figure 7a. The photon energy increase through increasing doping (x = 1%, 2% and 3%) atoms of MoS2, and the absorption coefficient of MoS2-doped-V2O5 rises, achieving 1.8 × 105 cm−1 at 5 eV with 3%. The results indicate that two-dimensional MoS2-doped-V2O5 has high optical conductivity as well as absorbance and is a more consistent and appropriate material for photocatalytic applications.
Figure 7.
(a) Absorption and (b) energy loss function of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
3.6. Optical conductivity and reflectivity
Optical conductivity describes the conductance of photogenerated electrons by the photoelectric phenomenon. Electromagnetic radiation EM is used to break the bonding of particles. Figure 8a–d shows the optical conductance of MoS2-doped-V2O5 composite in the range of 0 to 60 eV. The real peaks of optical conductance initiate from the origin point and touch its maximum conductivity values of 1.7, 2, 2.5 and 3.5 cm−1 at the energy range 0 to 20 eV with pure V2O5 and increasing doping (x = 1%, 2% and 3%) atoms of MoS2. The real optical conductivity of two-dimensional MoS2-doped-V2O5 composite initially increases from the starting zero point and at 5 eV and suddenly decreases till 30 eV.
Figure 8.
(a–d) Optical conductivity of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
On the other hand, imaginary optical conductivity shows the largest value at 5 eV: 1.7, 1.6, 1.4 and 1.6 cm−1 for pure V2O5 and doping (x = 1%, 2% and 3%) atoms of MoS2. The optical conductivity is increasing with the increased doping concentration of MoS2. These results of optical conductivity indicate that two-dimensional MoS2-doped-V2O5 is appropriate material for photocatalytic applications.
The reflectivity of any material can be used to examine its surface behaviour. The reflectivity surface behaviour of two-dimensional MoS2-doped-V2O5 composite at different doping (x = 1%, 2% and 3%) atoms of MoS2 is shown in figure 9. At 0 eV, the value of reflectivity for MoS2-doped-V2O5 material is 0 for pure V2O5, 0.32 at 1% atom, 0.15 at 2% atom and 0.7 at 3% atom, respectively. These values show that the reflectivity peak slightly shifts towards the higher values of frequency as doping concentration increases. These results of reflectivity indicate that two-dimensional MoS2-doped-V2O5 composite is a suitable material for photocatalytic applications.
Figure 9.
Reflectivity of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%).
3.7. Photocatalytic mechanism
Figure 10 schematic diagram shows that the sunlight conciliates photo-degradation application of two-dimensional MoS2-doped-V2O5 composite. Two-dimensional MoS2-doped-V2O5 composite with a large surface area absorbs the waste material more proficiently through active surface sites as equation (3.14). Under the sunlight, revelation two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 electrons and holes in the CB and VB correspondingly as equation (3.15). Organic particle becomes excited in the exposure of sunlight, and it moves from HOMO toward LUMO of pollutant level. Furthermore, electrons are moved from the VB to the CB of two-dimensional MoS2-doped-V2O5 and increase electron density with increased doping (x = 1%, 2% and 3% atoms). The photo-captured electrons are positioned in the conduction level of MoS2-doped-V2O5 renovating the oxygen molecule into super-oxide radical as equation (3.16). At a similar moment, hydroxyl radical produced the holes in the valance level through water splitting molecules as equations (3.17) and (3.18). Both superoxide and hydroxyl (unsaturated form) changed the pollutant into CO2 and H2O as equation (3.19). Underexposure of sunlight photocatalytic activity of organic waste pollutants by MoS2-doped-V2O5 is given below [37–39].
| 3.14 |
| 3.15 |
| 3.16 |
| 3.17 |
| 3.18 |
| 3.19 |
Figure 10.
Schematic diagram of the photocatalytic mechanism of two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite (x = 1%, 2% and 3%) under sunlight.
The computed results show that under visible light, active sites of the MoS2-doped-V2O5 composite may be proficient to make excellent photocatalytic activity. Two-dimensional (TMDs-doped-TMOs) MoS2-doped-V2O5 composite semi-conductor is appropriate material for photocatalytic applications.
4. Conclusion
In the present research, the structural, electronic and optical properties of TMDs-doped-TMOs MoS2-doped-V2O5 with various doping (x = 1–3% atoms) of MoS2 is studied by using first principles calculation. The GGA-PBE simulation approach is used to investigate orthorhombic structures. It is examined that the energy bandgap (Eg) decrement from 2.76 to 1.3 eV with direct semiconductor nature through various doping (x = 1–3% atoms) of MoS2. MoS2 doping (x = 1–3% atoms) in pentoxide (V2O5) creates the extra gamma active states which contribute to the formation of conduction and valance bands. MoS2-doped-V2O5 is a proficient composite for photocatalysis, has a large surface area for light absorption, decreases the electron-hole pairs recombination rate and increases the charge transport. A comprehensive study of optical conductivity reveals that strong peaks of MoS2-doped-V2O5 increase in ultraviolet spectrum region with small shifts at larger energy bands through increment doping x = 1–3% atoms of MoS2. A significant decrement was found in the reflectivity due to the decrement in the bandgap with doping x = 1–3% atoms of MoS2. The optical properties significantly increased by the decrement of Eg. Two-dimensional MoS2-doped-V2O5 composite has high energy absorption, optical conductivity, and refractive index and is an appropriate material for photocatalytic applications.
Acknowledgement
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The data are provided in the electronic supplementary material [40].
Authors' contributions
M.H.J.: conceptualization, formal analysis, investigation, methodology, software, writing—original draft, writing—review and editing; M.S.b.R.: writing—review and editing; M.Z.H.B.M.: writing—review and editing; M.A.B.A.: supervision, writing—review and editing; Z.I.Z.: writing—review and editing; A.M.F.: writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work. This research is supported by Universiti Tun Hussein Onn Malaysia through grant Tier-1 (Q524). This work was funded by the Fundamental Research Grant Scheme awarded by the Ministry of Higher Education Malaysia (FRGS/1/2019/STG07/UTHM/02/5 (FRGS K171)).
References
- 1.Kumar U, Mishra KA, Kushwaha AK, Cho SB. 2022. Bandgap analysis of transition-metal dichalcogenide and oxide via machine learning approach. J. Phys. Chem. Solids 171, 110973. ( 10.1016/j.jpcs.2022.110973) [DOI] [Google Scholar]
- 2.Choudhary N, et al. 2018. Two-dimensional transition metal dichalcogenide hybrid materials for energy applications. Nano Today 19, 16-40. ( 10.1016/j.nantod.2018.02.007) [DOI] [Google Scholar]
- 3.Chang L, Sun Z, Hu YH. 2021. 1T phase transition metal dichalcogenides for hydrogen evolution reaction. Electrochem. Energy Rev. 4, 194-218. ( 10.1007/s41918-020-00087-y) [DOI] [Google Scholar]
- 4.Rasmussen FA, Thygesen KS. 2015. Computational 2D materials database: electronic structure of transition-metal dichalcogenides and oxides. J. Phys. Chem. C 119, 13 169-13 183. ( 10.1021/acs.jpcc.5b02950) [DOI] [Google Scholar]
- 5.Sahoo R, Singh M, Rao TN. 2021. Cover feature: a review on the current progress and challenges of 2D layered transition metal dichalcogenides as Li/Na-ion battery anodes (ChemElectroChem 13/2021). ChemElectroChem 8, 2355-2355. ( 10.1002/celc.202100692) [DOI] [Google Scholar]
- 6.Ren R. 2018. Synthesis and characterization of transition metal oxide and dichalcogenide nanomaterials for energy and environmental applications. PhD dissertation, The University of Wisconsin-Milwaukee. [Google Scholar]
- 7.Jyothi MS, Nayak V, Reddy KR, Naveen S, Raghu AV. 2019. Nanophotocatalysis and environmental applications. In Materials and technology, p. 83. New York, NY: Springer. [Google Scholar]
- 8.Jinendra U, Bilehal D, Nagabhushana BM, Reddy KR, Reddy CV, Raghu AV. 2019. Template-free hydrothermal synthesis of hexa ferrite nanoparticles and its adsorption capability for different organic dyes: comparative adsorption studies, isotherms and kinetic studies. Mater. Sci. Energy Technol. 2, 657-666. ( 10.1016/j.mset.2019.08.005) [DOI] [Google Scholar]
- 9.Jinendra U, Kumar J, Nagabhushana BM, Raghu AV, Bilehal D. 2019. Facile synthesis of CoFe2O4 nanoparticles and application in removal of malachite green dye. Green Mater. 7, 137-142. ( 10.1680/jgrma.18.00054) [DOI] [Google Scholar]
- 10.Kannan K, Radhika D, Reddy KR, Raghu AV. 2021. Gd3 + and Y3 + co-doped mixed metal oxide nanohybrids for photocatalytic and antibacterial applications. Nano Express 2, 010014. [Google Scholar]
- 11.Bonaccorso F, et al. 2015. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 347, 6217. ( 10.1126/science.1246501) [DOI] [PubMed] [Google Scholar]
- 12.Roslan MS, Haider Z, Chaudhary KT. 2020. Characterization of the impact of nanoparticles on micro strain in 2D graphene synthesized by arc discharge plasma. Mater. Today Commun. 25, 101285. ( 10.1016/j.mtcomm.2020.101285) [DOI] [Google Scholar]
- 13.Wang D, Dang X, Tan B, Zhang Q, Zhao H. 2022. 3D V2O5-MoS2/rGO nanocomposites with enhanced peroxidase mimicking activity for sensitive colorimetric determination of H2O2 and glucose. Spectrochim. Acta A Mol. Biomol. Spectrosc. 269, 120750. ( 10.1016/j.saa.2021.120750) [DOI] [PubMed] [Google Scholar]
- 14.Sajid MM, et al. 2020. Preparation and characterization of vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Surfaces Interfaces 19, 100502. ( 10.1016/j.surfin.2020.100502) [DOI] [Google Scholar]
- 15.Chandrappa GT, Steunou N, Cassaignon S, Bauvais C, Livage J. 2003. Hydrothermal synthesis of vanadium oxide nanotubes from V2O5 gels. Catal. Today 78, 85-89. ( 10.1016/S0920-5861(02)00298-5) [DOI] [Google Scholar]
- 16.Jameel MH, et al. 2023. Effect of external static pressure on structural, electronic, and optical properties of 2-D hetero-junction-MoS2 for a photocatalytic applications: a DFT study. Opt. Quantum Electron. 2, 1-14. ( 10.1007/s11082-023-04853-2) [DOI] [Google Scholar]
- 17.Zhang X, Teng SY, Loy ACM, How BS, Leong WD, Tao X. 2020. Transition metal dichalcogenides for the application of pollution reduction: a review. Nanomaterials 10, 6. ( 10.3390/nano10061012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Bremner SP, Ho-baillie A. 2018. Assessment of 2D materials and transition metal oxides as carrier selective contacts for silicon solar cells.
- 19.Raveesha R, et al. 2022. Synthesis and characterization of novel thiazole derivatives as potential anticancer agents: molecular docking and DFT studies. Comput. Toxicol. 21, 100202. ( 10.1016/j.comtox.2021.100202) [DOI] [Google Scholar]
- 20.Pramodh B, et al. 2021. Synthesis, spectral characterization, crystal structure and theoretical investigation of (E)-3-(4-bromothiophen-2-yl)-1-(5-bromothiophen-2-yl)prop-2-en-1-one. Chem. Data Collect. 31, 100587. ( 10.1016/j.cdc.2020.100587) [DOI] [Google Scholar]
- 21.Fakharuddin A, et al. 2021. Robust inorganic hole transport materials for organic and perovskite solar cells: insights into materials electronic properties and device performance. Sol. RRL 5, 1-44. ( 10.1002/solr.202000555) [DOI] [Google Scholar]
- 22.Cui Q, et al. 2016. Ultrathin and atomically flat transition-metal oxide: promising building blocks for metal-insulator electronics. ACS Appl. Mater. Interfaces 8, 34 552-34 558. ( 10.1021/acsami.6b11302) [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y, et al. 2021. Recent advances and perspectives of MoS2-based materials for photocatalytic dyes degradation: a review. Colloids Surfaces A Physicochem. Eng. Asp. 611, 125836. ( 10.1016/j.colsurfa.2020.125836) [DOI] [Google Scholar]
- 24.Huang S, Chen C, Tsai H, Shaya J, Lu C. 2018. Photocatalytic degradation of thiobencarb by a visible light-driven MoS2 photocatalyst. Sep. Purif. Technol. 197, 147-155. ( 10.1016/j.seppur.2018.01.009) [DOI] [Google Scholar]
- 25.Wu M-h, Li L, Liu N, jin Wang D, cheng Xue Y, Tang L. 2018. Molybdenum disulfide (MoS2) as a co-catalyst for photocatalytic degradation of organic contaminants: a review. Process Saf. Environ. Prot. 118, 40-58. ( 10.1016/j.psep.2018.06.025) [DOI] [Google Scholar]
- 26.Ahamad T, Naushad M, Al-Saeedi SI, Almotairi S, Alshehri SM. 2020. Fabrication of MoS2/ZnS embedded in N/S doped carbon for the photocatalytic degradation of pesticide. Mater. Lett. 263, 127271. ( 10.1016/j.matlet.2019.127271) [DOI] [Google Scholar]
- 27.Ramsha K, Adeel R, Sofia J, Rahim J, Muhammad AA, Mohammad M. 2018. Synthesis and characterization of MoS2/TiO2 nanocomposites for enhanced photocatalytic degradation of methylene blue under sunlight irradiation. Key Eng. Mater. 778, 137-143. ( 10.4028/www.scientific.net/KEM.778.137) [DOI] [Google Scholar]
- 28.Mustafa MU, Agam MA, Md Juremi NR, Mohamad F, Wibawa J, Ali AH. 2011. Physical and chemical changes of polystyrene nanospheres irradiated with laser. AIP Conf. Proc. 1347, 67-71. ( 10.1063/1.3586956) [DOI] [Google Scholar]
- 29.Jameel MH, et al. 2023. A comparative DFT study of electronic and optical properties of Pb/Cd-doped LaVO4 and Pb/Cd-LuVO4 for electronic device applications. Comput. Condens. Matter 34, e00773. ( 10.1016/j.cocom.2022.e00773) [DOI] [Google Scholar]
- 30.Jameel MH, et al. 2022. First principal calculations to investigate structural, electronic, optical, and magnetic properties of Fe3O4 and Cd-doped Fe2O4. Comput. Condens. Matter 30, e00629. ( 10.1016/j.cocom.2021.e00629) [DOI] [Google Scholar]
- 31.Abbas N, et al. 2023. A comparative study of structural, vibrational mode, optical and electrical properties of pure nickel selenide (NiSe) and Ce-doped NiSe nanoparticles for electronic device applications. Phys. B Condens. Matter 649, 414471. ( 10.1016/j.physb.2022.414471) [DOI] [Google Scholar]
- 32.Jameel MH, et al. 2021. First principal calculations of electronic, optical and magnetic properties of cubic K1-xYxNbO3(Y=Fe, Ni). Phys. Scr. 96, 125839. ( 10.1088/1402-4896/ac198d) [DOI] [Google Scholar]
- 33.Beaula Ruby Kamalam M, et al. 2021. Direct sunlight-driven enhanced photocatalytic performance of V2O5 nanorods/ graphene oxide nanocomposites for the degradation of Victoria blue dye. Environ. Res. 199, 111369. ( 10.1016/j.envres.2021.111369) [DOI] [PubMed] [Google Scholar]
- 34.Saleem S, et al. 2022. Modification in structural, optical, morphological, and electrical properties of zinc oxide (ZnO) nanoparticles (NPs) by metal (Ni, Co) dopants for electronic device applications. Arab. J. Chem. 15, 103518. ( 10.1016/j.arabjc.2021.103518) [DOI] [Google Scholar]
- 35.Dadigala R, Bandi R, Gangapuram BR, Dasari A, Belay HH, Guttena V. 2019. Fabrication of novel 1D/2D V2O5/g-C3N4 composites as Z-scheme photocatalysts for CR degradation and Cr (VI) reduction under sunlight irradiation. J. Environ. Chem. Eng. 7, 102822. ( 10.1016/j.jece.2018.102822) [DOI] [Google Scholar]
- 36.Povar I, Zinicovscaia I, Spinu O, Pintilie B. 2019. Thermodynamic stability areas of polyvanadates of alkaline earth metals. J. Chem. 2019, 7091781. ( 10.1155/2019/7091781) [DOI] [Google Scholar]
- 37.Mishra A, et al. 2020. Rapid photodegradation of methylene blue dye by rGO- V2O5 nano composite. J. Alloys Compd. 842, 155746. ( 10.1016/j.jallcom.2020.155746) [DOI] [Google Scholar]
- 38.Gao XT, et al. 2018. V2O5 nanoparticles confined in three−dimensionally organized, porous nitrogen−doped graphene frameworks: flexible and free−standing cathodes for high performance lithium storage. Carbon N. Y. 140, 218-226. ( 10.1016/j.carbon.2018.08.060) [DOI] [Google Scholar]
- 39.Pooseekheaw P, Thongpan W, Panthawan A, Kantarak E, Sroila W, Singjai P. 2020. Porous V2O5/TiO2 nanoheterostructure films with enhanced visible-light photocatalytic performance prepared by the sparking method. Molecules 25, 1-11. ( 10.3390/molecules25153327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jameel MH, Sufi bin Roslan M, Bin Mayzan MZH, Agam MAB, Zaki ZI, Fallatah AM. 2023. Investigation of structural, electronic and optical properties of two-dimensional MoS2-doped-V2O5 composites for photocatalytic application: a density functional theory study. Figshare. ( 10.6084/m9.figshare.c.6747560) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are provided in the electronic supplementary material [40].