In this paper, we introduce an iatrogenesis intervention framework guided by medical innovation, which encompasses the objectives, influences of medical innovation on iatrogenesis, factors affecting various types of iatrogenesis, and strategies for controlling iatrogenesis. Advancements in medical innovation across various domains can bridge the patient safety implementation gap by uncovering, characterizing, refining, accelerating, scaling, extending, and mobilizing the external and internal conditions and factors that influence the control and elimination of iatrogenesis.
Iatrogenesis, or the adverse effects and risks associated with medical interventions, poses a significant challenge in global public health as it ranks as the fifth leading cause of death worldwide and a leading cause of death in numerous countries (1-2). Prior to the coronavirus disease 2019 (COVID-19) pandemic, 2.6 million deaths occurred annually due to safety lapses in hospitals in low-income countries, while nearly 15% of hospital expenditure and activity in developed nations were attributed to addressing treatment safety failures (3).
Iatrogenesis Definition, Current Situation, and International Attention to this Issue
According to the World Health Organization (WHO), “iatrogenesis, often called adverse drug reactions (ADRs), is any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy” (4). Based on this definition, approximately 5%−8% of global deaths are attributed to ADRs, accounting for half of all preventable harm in medical care and yielding an estimated annual cost of 42 billion United States dollars (USD) worldwide (5-6). However, this definition and associated data may underestimate or overlook the impacts of other factors, such as diagnostic procedures, nosocomial infections, and hospital management. Therefore, we propose that iatrogenesis should be more comprehensively defined as any injury or illness resulting from medical care or caused by medical intervention, encompassing diagnostic procedures, treatment methods, healthcare practices, and other relevant factors.
Numerous studies have been conducted to understand the extent, impact, and improvement strategies of iatrogenic harm or iatrogenesis (1,7-8). Innovative technologies are being developed to reduce iatrogenic harm, while also introducing new varieties of iatrogenic errors and events (9-10). Risk assessment tools for iatrogenesis management are evolving, potentially enabling health professionals to better identify patients at higher risk of iatrogenic harm and allowing healthcare providers to implement preventive measures (11-12). Various stakeholders have developed guidelines and frameworks to address iatrogenesis management (6,13-14). Innovations in patient care models may help mitigate iatrogenic risks and consequences (15-16).
Given the significant impact of iatrogenesis on population health, the 72nd World Health Assembly, convened in Switzerland in May 2019, adopted a resolution designating September 17th as World Patient Safety Day. This initiative aims to disseminate the concept of patient safety and foster global collaboration to enhance patient safety.
In light of the COVID-19 pandemic, the first theme of World Patient Safety Day highlighted the safety of health workers who protect us all while facing a 5 to 10 times higher risk of contracting COVID-19 compared to the general population (17).
Unfortunately, vulnerable populations are most susceptible to iatrogenesis — especially those with multiple conditions and medication requirements. These disparities exacerbate global health inequalities and hinder the attainment of the Sustainable Development Goals (SDGs).
The Underlying Factors Contributing to the Issues and the Obstacles Encountered During their Resolution Process
As global demand for health care continues to rise due to factors such as aging populations, climate change, and emerging infectious diseases, promoting safe medical practices has become an urgent and challenging task. Patient safety within the healthcare system is a complex, multi-disciplinary, and multi-level dynamic issue. Externally, it involves macro-level elements such as policy systems (particularly health insurance policies and health laws and regulations), economic development, technological progress, cultural beliefs, social environments, and natural ecosystems. At the meso level, it encompasses family, organizational, and social support, while at the micro level, it relates to individual knowledge, beliefs, and behavior patterns.
Internally, patient safety is the cumulative and comprehensive outcome of unsafe medical practices on patients and even on healthy populations, such as adverse vaccine reactions. Iatrogenesis spans from outside the healthcare institutions to within them. The probability, extent, and types of iatrogenesis are connected to the preventable harms at each stage or process of health management and medical practices accompanying various health statuses, including disease, disability, and death. On one hand, iatrogenic risks stem from the medical intervention itself, such as ADRs or complications from surgical procedures (1,3,6). On the other hand, iatrogenesis can result from errors or mistakes made by healthcare personnel during medical activities, such as negligence, nosocomial infections due to improper protection, and drug safety issues arising from irrational drug use (1,3,6).
Internal and external factors interact to form a complex network of causes and mechanisms affecting avoidable harm, causing iatrogenesis to manifest in diverse ways among patients and healthcare workers. For instance, WHO estimates that 50% of prescribed and marketed medications are unsuitable, and 50% of patients administer these medications improperly, emphasizing the need to address medication iatrogenesis risk (18). Contributing factors may include inaccurate or delayed diagnoses, carelessness by patients and healthcare workers, lack of health awareness, insufficient high-quality medicines, shortcomings in the drug regulatory system, and inappropriate incentives and constraints within health insurance and health services. These issues may exist or coexist across primary care, hospital, and rehabilitation systems, interacting with various stakeholders, information systems, manufacturers, and management platforms.
Addressing iatrogenesis is not solely a matter of disease management, but rather involves tackling the underlying avoidable health disparities and medical costs. This is particularly important as the world faces challenges such as limited health resources, funding for pandemic response, global aging, and climate change. The challenge of iatrogenesis, which is more complex than other diseases, necessitates a comprehensive framework to guide all aspects of the work.
The Complexity of the Innovation
According to the third edition of the Oslo Manual, “Innovation is the implementation of a new or significantly improved product (good or service), process, marketing method, or organizational method in business practices, workplace organization, or external relations” (19). In the field of medicine, innovation is specifically defined as “a new type of diagnostic, therapeutic, or screening health technology that is in its first stage of commercialization, marketing, or promotion and has been validated for its effectiveness and safety in clinical research” (20). Considering the relationship between the nature of innovation and the scope of change is relevant since the definition of innovation includes notions of novelty and change (21).
Generally, medical innovation can be described as any new tests, devices, drugs, vaccines, procedures, plans, management measures, or systems developed for disease prevention, diagnosis, or treatment, with the primary goal of enhancing health levels and health system efficiency. In order to optimize the utilization efficiency of health resources, identifying disruptive innovations from incremental innovations is crucial (21). In the healthcare field, disruptive innovations have the greatest potential to significantly impact expenditures, particularly through changes in medical strategies, care pathways, or even health pathways that encompass care, prevention, social support, and medical-welfare accompaniment, as well as home return or care assistance (21).
The Impact of Medical Innovation on Iatrogenic Outcomes
Medical innovation is inextricably linked to iatrogenesis, and the definition and scope of iatrogenesis continue to evolve due to advancements in the medical field. On one hand, medical innovation has decreased healthcare costs related to chronic lifelong diseases and transformed conditions such as breast cancer, HIV/AIDS, heart disease, and lung cancer from being synonymous with a death sentence (22). Research involving 30 developing and high-income countries revealed that from 1960 to 1997, new therapies contributed to a 45% increase in life expectancy; this contribution rose to 73% between 2000 and 2009 (23).
On the other hand, the integration of technologies such as the Internet, the Internet of Things, big data, and artificial intelligence into medical and healthcare services and management has given rise to new forms of iatrogenesis. These emerging forms are evident in remote diagnosis and treatment, internet-based self-diagnosis and treatment models, variable online medical resources, and commercial information.
In our opinion, medical errors can be effectively reduced, and patients’ health can be maximally restored through medical innovation at all levels. Hence, we propose an iatrogenesis intervention framework from the standpoint of medical innovation (Figure 1).
Figure 1.
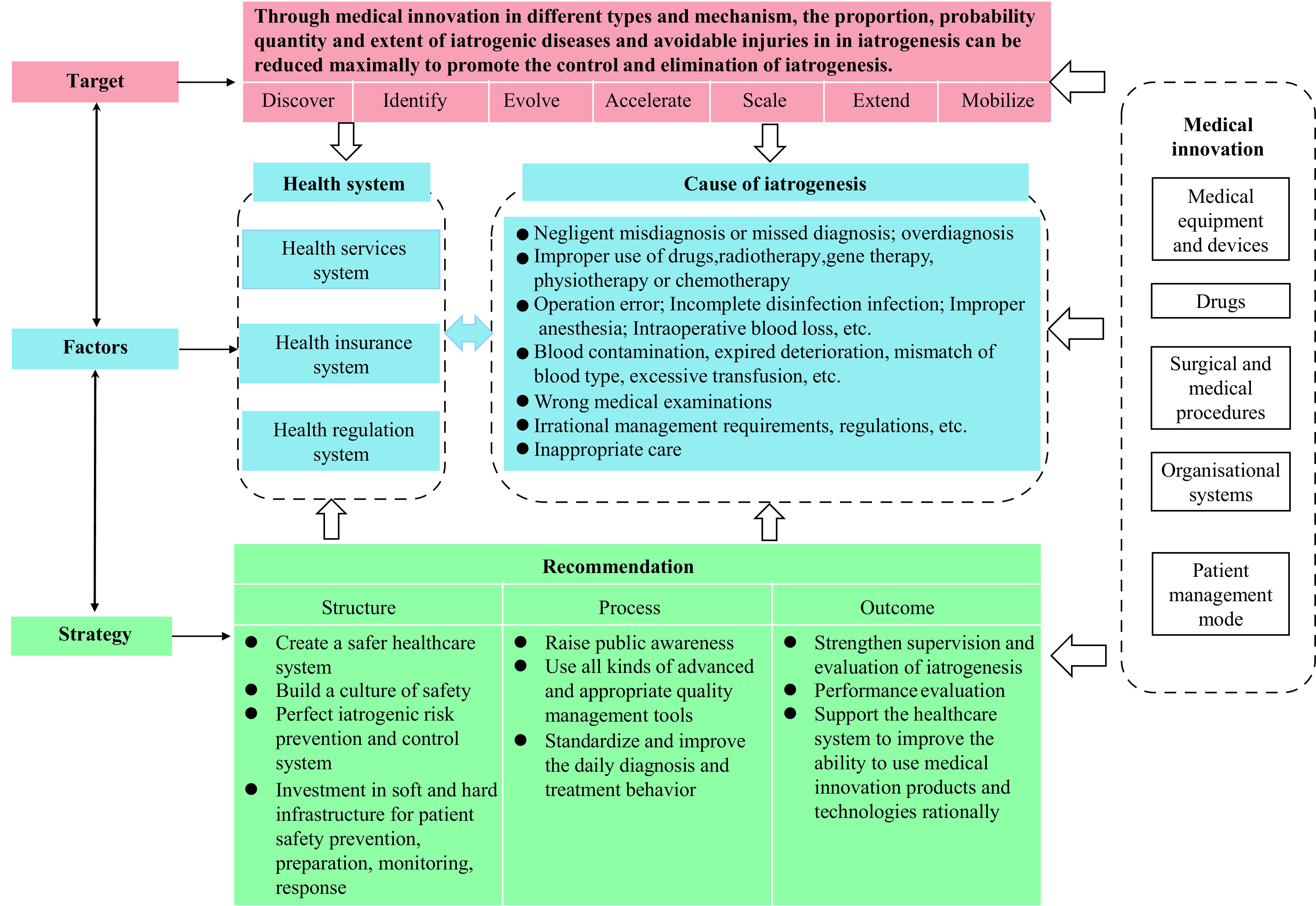
Framework for iatrogenesis intervention from the perspective of medical innovation.
Currently, there is a lack of consensus regarding the categorization of medical innovations, which are often classified into various types, including medical equipment and devices, pharmaceuticals, surgical procedures, organizational systems, and patient management strategies (21).
Innovations in medical equipment and devices can enhance detection, improvement, evolution, acceleration, scalability, precision, medical operation efficiency, and patient safety monitoring. Additionally, innovations in pharmaceuticals, surgical procedures, and medical practices can help evolve, accelerate, scale, and extend the prevention and elimination of iatrogenic injuries caused by factors beyond the control of medical professionals at a given time.
Furthermore, innovations in organizational systems and patient management, such as strengthening business guidance and industry supervision, enhancing medication safety education for patients and their families, can help identify, classify, and mitigate various types of iatrogenesis. These innovations can improve the control and eradication of iatrogenesis and act simultaneously on iatrogenic risk prevention and control at different stages, maximizing the positive impact of narrowing implementation gaps in patient safety.
The management of iatrogenesis can be significantly enhanced through medical innovation. By focusing on patient safety, usability, and the integration of technology into healthcare procedures, medical innovations can reduce the likelihood of unforeseen negative outcomes. Developments in medical imaging, machine learning, and artificial intelligence can increase diagnostic accuracy and minimize diagnostic errors. Innovative monitoring tools, wearable sensors, and remote monitoring technologies enable continuous tracking of health indicators and other measures.
Evaluating and Implementing Medical Innovations in Iatrogenesis Management
Medical innovation, while offering potential benefits, may also introduce new risks. As such, it is crucial to establish a risk-benefit evaluation mechanism for medical innovation products, technologies, or systems. This would enable the identification and adoption of suitable innovations for controlling, mitigating, and eliminating medical risks.
In an effort to manage risks associated with medical innovation and expand its benefits to a greater number of individuals, we propose a structure-process-outcome framework for integration in iatrogenic management. In the structure dimension, increasing government investment in both software and hardware for patient safety prevention, preparation, monitoring, and response is recommended. This involves fostering collaboration among various stakeholders. Additionally, the implementation of relevant risk control measures through continuous medical innovations is necessary.
In the process dimension, stakeholders should focus on raising public awareness, employing appropriate management tools, standardizing and enhancing daily diagnostic and treatment procedures, utilizing benefit-risk assessment methods to guide clinical practices, and maximizing the appropriate usage of risk assessment tools to minimize medical risks while increasing the degree, speed, and scope of benefits to the public. In the outcome dimension, policymakers should work towards strengthening iatrogenic disease and injury reporting and learning systems, establishing a “quality first” performance management mechanism, and improving quality control and operational mechanisms. Supporting and investing in the healthcare system to enhance the ability to utilize innovative products and technologies, and involving patients and their families in data reporting and service evaluations, are also crucial steps in achieving these goals. This will ultimately lead to the establishment of a closed-loop system for innovative products and technologies, improving healthcare outcomes and the effectiveness and efficiency of service delivery.
In conclusion, the development and appropriate application of medical innovation can contribute to the prevention and control of iatrogenesis in a more efficient, effective, and affordable manner.
References
- 1.Peer R, Shabir N Iatrogenesis: a review on nature, extent, and distribution of healthcare hazards. J Fam Med Primary Care. 2018;7(2):309–14. doi: 10.4103/jfmpc.jfmpc_329_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamna M, Dilip C, Ajmal M, Linu Mohan P, Shinu C, Jafer CP, et al A prospective study on adverse drug reactions of antibiotics in a tertiary care hospital. Saudi Pharm J. 2014;22(4):303–8. doi: 10.1016/j.jsps.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. The Montreux Charter on Patient Safety galvanizes action to address avoidable harm in health care. 2023. https://www.who.int/news/item/28-02-2023-the-montreux-charter-on-patient-safety-galvanizes-action-to-address-avoidable-harm-in-health-care. [2023-4-20].
- 4.WHO Meeting on International Drug Monitoring: the Role of National Centres (1971: Geneva, Switzerland), World Health Organization. International drug monitoring: the role of national centres, report of a WHO meeting [held in Geneva from 20 to 25 September 1971]. World Health Organization. 1972. https://apps.who.int/iris/handle/10665/40968. [2023-4-20].
- 5.Verma R, Vasudevan B, Pragasam V Severe cutaneous adverse drug reactions. Med J Armed Forces India. 2013;69(4):375–83. doi: 10.1016/j.mjafi.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. WHO calls for urgent action by countries for achieving medication without harm. 2022. https://www.who.int/news/item/16-09-2022-who-calls-for-urgent-action-by-countries-for-achieving-medication-without-harm. [2023-4-20].
- 7.Onder G, Van Der Cammen T J M, Petrovic M, Somers A, Rajkumar C Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing. 2013;42(3):284–91. doi: 10.1093/ageing/aft038. [DOI] [PubMed] [Google Scholar]
- 8.Ligi I, Arnaud F, Jouve E, Tardieu S, Sambuc R, Simeoni U Iatrogenic events in admitted neonates: a prospective cohort study. Lancet. 2008;371(9610):404–10. doi: 10.1016/S0140-6736(08)60204-4. [DOI] [PubMed] [Google Scholar]
- 9.Palmieri PA, Peterson LT, Ford EW Technological iatrogenesis: new risks force heightened management awareness. J Healthcare Risk Manage. 2007;27(4):19–24. doi: 10.1002/jhrm.5600270405. [DOI] [PubMed] [Google Scholar]
- 10.Mathioudakis NN, Abusamaan MS, Shakarchi AF, Sokolinsky S, Fayzullin S, McGready J, et al Development and validation of a machine learning model to predict near-term risk of iatrogenic hypoglycemia in hospitalized patients. JAMA Network Open. 2021;4(1):e2030913. doi: 10.1001/jamanetworkopen.2020.30913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanfilippo S, Michaud V, Wei JQ, Bikmetov R, Turgeon J, Brunetti L Classification and assessment of medication risk in the elderly (Care): use of a medication risk score to inform patients’ readmission likelihood after hospital discharge. J Clin Med. 2021;10(17):3947. doi: 10.3390/jcm10173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franck LS, Scoppettuolo LA, Wypij D, Curley MAQ Validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain. 2012;153(1):142–8. doi: 10.1016/j.pain.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdouni R, Reyburn-Orne T, Youssef TH, Haddad IY, Gerkin RD Impact of a standardized treatment guideline for pediatric iatrogenic opioid dependence: a quality improvement initiative. J Pediatr Pharmacol Ther. 2016;21(1):54–65. doi: 10.5863/1551-6776-21.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott IA, Gray LC, Martin JH, Mitchell CA Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125(6):529–37.e4. doi: 10.1016/j.amjmed.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Schwappach DLB, Wernli M Medication errors in chemotherapy: incidence, types and involvement of patients in prevention. A review of the literature. Eur J Cancer Care. 2010;19(3):285–92. doi: 10.1111/j.1365-2354.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez SE, Conrad DA, Marcus-Smith MS, Reed P, Watts C Patient-centered innovation in health care organizations. A conceptual framework and case study application. Health Care Manage Rev. 2013;38(2):166–75. doi: 10.1097/HMR.0b013e31825e718a. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Protecting the health workers who protect us all. 2020. https://www.who.int/news-room/feature-stories/detail/protecting-the-health-workers-who-protect-us-all. [2023-4-20].
- 18.World Health Organization. The safety of medicines in public health programmes: pharmacovigilance, an essential tool. World Health Organization. 2006. https://apps.who.int/iris/handle/10665/43384.[2023-4-20].
- 19.OECD. OECD Oslo manual 3rd edition, 2005. [2023-4-20].
- 20.Instruction n°DGOS/PF4/2014/33 du 28 janvier 2014 relative au programme de recherche translationnelle, au programme hospitalier de recherche clinique, au programme de recherche médico-économique, au programme de recherche sur la performance du système de soins, au programme de recherche infirmière et paramédicale. http://circulaire.legifrance.gouv.fr/index.php?action=afficherCirculaire&hit=1&r=37900. [2023-4-20].
- 21.Dubromel A, Geffroy L, Aulagner G, Dussart C Assessment and diffusion of medical innovations in France: an overview. J Mark Access Health Policy. 2018;6(1):1458575. doi: 10.1080/20016689.2018.1458575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood JC. The value of saving lives: policies for patient access to life-saving therapies must keep pace with biomedical innovation. Pharm Technol 2016;40(5):12. https://www.biopharminternational.com/view/image-analysis-algorithm-for-therapeutic-mab-aggregate-analysis.
- 23.Lichtenberg FR. Pharmaceutical innovation and longevity growth in 30 developing and high-income countries, 2000-2009. 2012. https://www.nber.org/papers/w18235. [2023-4-20].


