Abstract
Contrast enhanced ultrasound (CEUS) has been widely implemented in clinical practice because of the enormous quantity of information it provides, along with its low cost, reproducibility, minimal invasiveness, and safety of the second-generation ultrasound contrast agents. To overcome the limitation of CEUS given by the subjective evaluation of the contrast enhancement behaviour, quantitative analysis of contrast kinetics with generation of time-intensity curves has been introduced in recent years. The quantification of perfusion parameters [named as dynamic-CEUS (D-CEUS)] has several applications in gastrointestinal neoplastic and inflammatory disorders. However, the limited availability of large studies and the heterogeneity of the technologies employed have precluded the standardisation of D-CEUS, which potentially represents a valuable tool for clinical practice in management of gastrointestinal diseases. In this article, we reviewed the evidence exploring the application of D-CEUS in gastrointestinal diseases, with a special focus on liver, pancreas, and inflammatory bowel diseases.
Keywords: Quantitative perfusion analysis, Gastrointestinal diseases, Time-intensity curve, Multiparametric ultrasound
Core Tip: Contrast-enhanced ultrasound (CEUS) has been widely implemented in clinical practice in recent years. Despite its several advantages, the qualitative evaluation of this exam and the lack of objectivity could lead to variability between different operators and ultrasound equipments. Dynamic-CEUS (D-CEUS) with the measurement of perfusion parameters is aimed at overcoming this important limitation. The purpose of this review is to explore the usefulness of D-CEUS in gastroenterological diseases.
INTRODUCTION
Contrast enhanced ultrasound (CEUS) has been widely implemented in clinical practice as a result of the enormous quantity of information it provides, along with its low cost, reproducibility, minimal invasiveness, and safety of the second-generation ultrasound contrast agents (UCAs)[1-4]. Despite its numerous advantages, one of the most significant limitations of CEUS is the subjective evaluation of contrast enhancement related behaviour of the explored tissues[5]. In recent years, dynamic-CEUS (D-CEUS) has been explored to overcome this limitation.
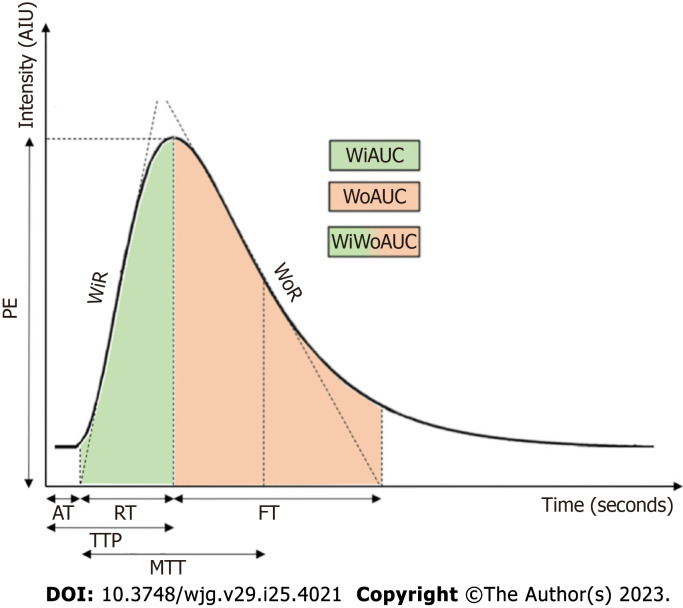
D-CEUS represents the quantitative analysis of UCA-kinetics in a specific region of interest (ROI)[6]. This technique allows two types of analysis in the examined tissue: Disruption-replenishment analysis and wash-in/wash out analysis[7]. The first analysis consists in the evaluation of microbubbles replacement after destroying them with high mechanical index. Requiring the continuous intravenous infusion over five to twenty minutes of UCA, the disruption-replenishment analysis are infrequently used due of their complex methodology[8]. Consequently, the second form of analysis is more frequently employed in clinical practice. It consists of measuring the average intensity of a ROI following a bolus injection of UCA and generating a time-intensity curve (TIC). Hence, multiple parameters are derived from the TIC to quantitatively characterize the different stages of the wash-in and wash-out phases. The fundamental parameters derived from TIC are summarized in Table 1[9,10] and a schematic representation of TIC is shown in Figure 1. Generally, these parameters are obtained from different softwares and might consequently have varying nomenclature but can be divided into two categories: Amplitude parameters and time parameters. These criteria reflect various vascularization features: Amplitude parameters are mainly related to blood volume in the ROI, while blood flow is mostly correlated with time parameters[11]. Tracking microbubbles circulation provides the spatial representation of blood flow patterns and the derivation of parametric values of tissue perfusion since microbubbles strictly remain within the vasculature compartment[12].
Table 1.
Dynamic contrast-enhanced ultrasound parameters in time-intensity curve
|
Abbreviation
|
Parameter
|
Definition
|
Unit
|
| AT | Arrival time | Time from administration of UCA to the beginning of the curve | s |
| AUC or WiWoAUC | Area under the curve or wash-in and wash-out area under the curve | Total area under the curve | AIU |
| FT | Fall time | Time from PE to point where tangent of descending curve across x-axis | s |
| IMAX or MI | Maximum intensity | Maximum intensity of the curve | AIU |
| MTT | Mean transit time | Mean time taken by contrast to pass through the ROI | s |
| PE | Peak enhancement | Maximum intensity of the curve | AIU |
| PI | Peak intensity | Maximum intensity of the curve | AIU |
| Pw | Slope coefficient of wash-in | Coefficient of the enhancement wash-in slope | AIU × s |
| RT | Rise time | Time from PE to point where tangent of ascending curve across x-axis | s |
| TPI or TTP or TP | Time to peak | Time from the beginning of the curve to peak | s |
| WiAUC | Wash-in area under the curve | AUC from the beginning of the curve to PE | AIU × s |
| WoAUC | Wash-out area under the curve | AUC from the PE to the end of the curve | AIU × s |
| WiR | Wash-in rate | Tangent at the ascending part of the curve | AIU × s |
| WoR | Wash-out rate | Tangent at the descending part of the curve | AIU × s |
AIU: Absolute intensity unit; ROI: Region of interest; UCA: Ultrasound contrast agent.
Figure 1.
Time-intensity curve and derived parameters. AIU: Absolute intensity unit; AT: Arrival time; FT: Fall time; MTT: Mean transit time; PE: Peak enhancement; s: Second; TTP: Time to peak; WiAUC: Wash-in area under the curve; WiR: Wash-in rate; WiWoAUC: Wash-in and wash-out area under the curve; WoAUC: Wash-out area under the curve; WoR: Wash-out rate; RT: Rise time.
Examining the pros and cons, D-CEUS is a widely accessible, radiation-free, non-nephrotoxic and cost-effective technique that allows objective enhancement quantification, image comparison, real-time evaluation of the microcirculation perfusion by a strictly intravascular blood pool agent. This is crucial after the introduction of updated response evaluation criteria in solid tumor (RECIST) criteria based on tumour perfusion as D-CEUS potentially enables the monitoring of changes in vascularization even shortly after tumor treatment[13,14]. According to current European Federation for Ultrasound in Medicine and Biology recommendations, D-CEUS is useful for quantifying tumor enhancement objectively, to characterize focal lesions and evaluate the therapeutic response[7]. In contrast, D-CEUS should ideally be uniform regardless of the ultrasound equipment, data collecting, and analysis software, as different approaches and technical issues may influence the results' validity. Lastly, the technical limitations of the method must be addressed, particularly in the abdomen, where intestinal, respiratory, and probe motion artefacts could make this exam challenging, as well as the patient's ability to accomplish the instructions based on his mental and physical state[15].
The first D-CEUS examination was performed on oncological renal illness more than two decades ago[16]. Since then, this technique has spread to several medical specialties, especially in the gastroenterological setting and not only for oncological diseases. In this review we summarize the evidence exploring the application of D-CEUS in gastrointestinal diseases.
LIVER DISEASES
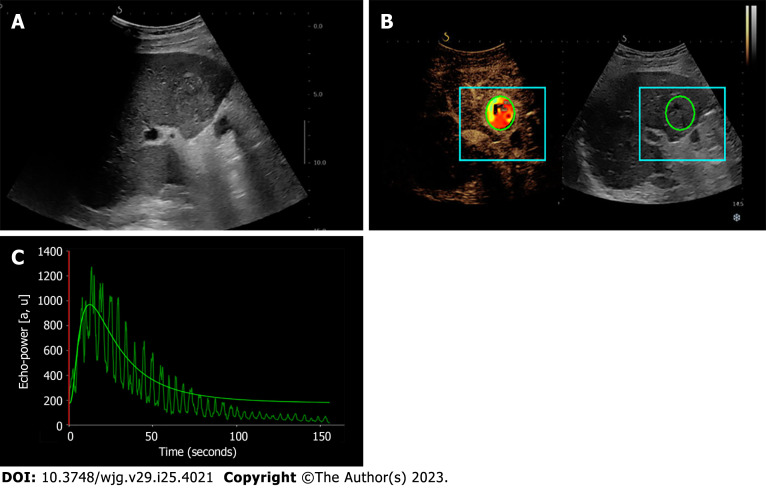
In liver diseases, D-CEUS has been explored primarily for its usefulness in characterizing focal liver lesions (FLLs). Several applications of D-CEUS in predicting biological behaviour, differential diagnosis, and prognosis have been explored. These studies are summarized in Table 2, and Figure 2 illustrates the use of D-CEUS to characterize liver lesions.
Table 2.
Dynamic contrast-enhanced ultrasound and liver diseases
|
Ref.
|
Study design/number of patients
|
Object of D-CEUS
|
Population/groups
|
Machine/UCA/software
|
Significant results (P < 0.05)
|
| Wildner et al[31], 2022 | Prospective/17 | Melanoma liver metastasis | Patients with melanoma liver metastasis treated with sorafenib/Responders’ vs non-responders | Sequoia 512/SonoVue/Qontrast | Increase of MTT and TTP is associated with response to treatment and prognosis |
| Gu et al[30], 2022 | Retrospective/97 | HCC | Patients with HCC underwent thermal ablation | Acuson Sequoia, Phillip EpiQ7/SonoVue/VueBox | WiAUC, WoAUC, and WiWoAUC ratios between HCC and surrounding parenchyma before ablation were predictors of survival |
| Huang et al[18], 2022 | Prospective/120 | HCC | Patients with HCC underwent biopsy/Low-Ki-67 vs high-Ki-67 | Logiq E9/Sonazoid/NovoUltrasound Kit | PE difference between HCC and distal liver parenchyma was different in the Kupffer phase |
| Li et al[22], 2022 | Retrospective/31 | HCC | Patients with HCC underwent surgery/MVI-positive vs MVI-negative | Phillip EpiQ7/Sonazoid/built-in auto contrast software | None of the D-CEUS parameters was related to MVI |
| Zocco et al[28], 2013 | Prospective/46 | Liver parenchyma | Cirrhotic patient underwent HVPG/clinically significant portal hypertension vs severe portal hypertension | iU22/SonoVue/QLAB | Negative correlation between PI, Pw and HVPG. Positive correlation with MTT. AUROC of 1.00 for PI < 23.3 AIU to predict clinically significant portal hypertension |
| Dong et al[21], 2021 | Retrospective/16 | HCC | Patients with HCC underwent surgery/MVI-positive vs MVI-negative | Acuson Oxana, Logiq E9, Siemens Acuson Sequoia/SonoVue, Lumason/VueBox | WiAUC and WoAUC were higher in MVI positive group |
| Schwarz et al[24], 2021 | Retrospective/139 | Focal liver lesion | Patients with diagnosed focal liver lesion/malignant versus benign | Acuson Sequoia, S2000 or S3000 and Phillip EpiQ7/SonoVue/VueBox | RT and late phase ratio were different between malignant and benign liver lesion |
| Xuan et al[19], 2021 | Retrospective/128 | HCC | Patients with HCC underwent biopsy or surgery/highly-differentiated vs moderate-differentiated vs poorly-differentiated | -/SonoVue/- | RT and MTT increased from poorly- to moderate- to highly-differentiated. Enhancement rates decreased from poorly- to moderate- to highly-differentiated |
| Amadori et al[32], 2018 | Prospective/37 | CRC Liver metastasis | Patients underwent chemotherapy/chemotherapy vs chemotherapy plus bevacizumab | iU22 vision 2008/SonoVue/QLAB | Reduction of PI and AUC and increase of TPI correlated with higher PFS in chemotherapy plus bevacizumab group |
| Wildner et al[25], 2019 | Prospective/148 | Focal liver lesion | Patients with focal liver lesion and subsequent final diagnosis/HCC, CCC, PCA, CRC, BC, MM, FNH | Sequoia 512/SonoVue/VueBox | Higher PE and WiWoAUC in HCC than CRC. Lower Relative intensity signal for PCA and CRC compared to HCC at 30 and 120 s after PE |
| Mogensen et al[33], 2017 | Prospective/12 | CRC liver metastasis | Patients underwent chemotherapy/chemotherapy vs chemotherapy plus bevacizumab | Logiq E9/SonoVue/VueBox | Early changes of PE correlate with tumor shrinkage at CT scan |
| Zocco et al[28], 2013 | Prospective/28 | HCC | Patients treated with sorafenib/Responders’ vs non-responders | iU22/SonoVue/QLAB | PI, Pw and AUC 10% decrease correlate with response to therapy. AUC 10% decrease and increased/unchanged TPI and MTT are associated with longer survival. Decrease of Pw is associated with PFS |
| Zhan et al[20], 2019 | Prospective/35 | HCC | Patients with HCC underwent microwaves ablation | Acuson Sequia/Sonovue/SonoLiver | Positive correlation between MVD, VEGF and IMAX; negative correlation between MVD and TTP. TTP was an independent predictor of OS |
| Wildner et al[26], 2014 | Prospective/43 | HCC, ICC | Patient with proven HCC and ICC | Acuson Sequoia 512/SonoVue/VueBox | FT and MTT were lower in ICC than HCC. Relative signal intensity was lower in ICC than HCC in all time point after PE |
| Frampas et al[69], 2013 | Prospective/19 | HCC | Patients with HCC treated with sorafenib or sunitinib/RECIST progressor vs non-progressor | Aplio XV/SonoVue/Vascular Recognition Imaging” mode | AUC decrease ≥ 40% correlated with RECIST non-progression |
| Lassau et al[29], 2011 | Prospective/42 | HCC | Patients with HCC treated with Bevacizumab | Aplio scanner/SonoVue/Contrast Harmonic Imaging-Quantification software | AUC, WiAUC, WoAUC and TPI decrease correlate with tumor response. TPI decrese correlate with PFS. AUC and WoAC decrease correlate with OS |
AIU: Arbitrary intensity unit; AUC: Area under the curve; AUROC: Area under the receiver operating characteristic; BC: Breast cancer; CCC: Cholangiocarcinoma; CRC: Colorectal cancer; CT: Computed tomography; D-CEUS: Dynamic contrast-enhanced ultrasound; FNH: Focal nodular hyperplasia; HCC: Hepatocellular carcinoma; HVPG: Hepatic vein portal pressure; MM: Malignant melanoma; MTT: Mean transit time; MVD: Microvascular density; MVI: Microvascular invasion; OS: Overall survival; PCA: Pancreatic adenocarcinoma; PE: Peak enhancement; PFS: Progression free survival; PI: Peak intensity; Pw: Slope coefficient of wash-in; RECIST: Response evaluation criteria in solid tumors; RT: Rise time; TPI: Time to peak intensity; TTP: Time to peak; UCA: Ultrasound contrast agent; WiAUC: Wash-in area under the curve; WoAUC: Wash-out area under the curve; WiWoAUC: Wash-in and wash-out area under the curve.
Figure 2.
Dynamic contrast-enhanced ultrasound and time-intensity curves of the liver. A: Hypoechoic mass (hepatocellular carcinoma) of IV liver segment visualized in B-mode ultrasound; B and C: Contrast-enhanced ultrasound with corresponding time-intensity curve of the liver mass.
Biological behaviour
The vascular structure of a tumour lesion is closely associated with microscopic features such as the degree of differentiation, the proliferation index and growth rate, the presence of necrosis, the angiogenesis and the vascular invasion[17]. Therefore, the analysis of vascularization parameters could be different according to each of the aforementioned characteristics.
Huang et al[18] investigated the correlation between perfusion parameters and Ki-67 in hepatocellular carcinoma (HCC) patients. Prospective analysis of one hundred twenty patients showed that the peak enhancement (PE) difference between HCC and distal liver parenchyma in the Kupffer phase was significantly higher in low Ki-67 (< 10%) group compared to high Ki-67 (≥ 10%) group, probably due to lower concentration of Kupffer cells in poorly differentiated neoplasms[18]. Supporting this, differences in D-CEUS parameters were found among HCC differentiation classes. Specifically, rise time (RT) and time to peak (TTP) demonstrated a significant positive correlation with differentiation degree, whereas enhancement rate was significantly higher in lesions with less differentiation[19].
Regarding the pure vascular features of tumors, Zhan et al[20] demonstrated that histological determined microvessel density and vascular endothelial grow factor stain positively correlated with maximum intensity (MI), while microvessel density negative correlated with TTP[20]. This suggested that D-CEUS parameters could provide a reliable characterization of the microvascular scaffold of lesions. In another study Dong et al[21] perform a retrospective analysis of D-CEUS characteristics of HCC to investigate the capability to predict microvascular invasion in a cohort of 16 patients who underwent subsequent surgery. They found that wash-in area under the curve (WiAUC) and wash-out area under the curve (WoAUC) were significantly higher in microvascular invasion positive group (P < 0.05), especially when the ROI was positioned in the marginal area of the lesion. This phenomenon is likely to be attributed to the formation of arteriovenous fistulas during vascular invasion, which leads to an increase in blood flow[21]. In contrast, using different UCA, Li et al[22] found no correlation between quantitative parameters and microvascular invasion in thirty-one resected HCCs[22]. One of the most significant differences between second-generation UCAs is their resistance to US wave pressure, which could explain this apparent contradiction in results[23].
This evidence suggests that D-CEUS may serve as a biomarker of the biological behaviour and microscopic characteristics of HCC, detecting the abnormal vascularization characteristics that developed as the disease progressed.
Differential diagnosis
Quantitative analysis of perfusion parameters demonstrated a promising potential to distinguish between benign and malignant FLLs. In a retrospective study including one hundred and thirty-nine FLLs of which forty-four benign and ninety-five malignant, benign lesions showed a significantly higher late-phase ratio (ratio between signal intensities of lesion and surrounding tissue in late phase, LPR) compared to malignant counterpart, showing an area under the curve (AUC) of 0.9, with maximal sensitivity (100%) but low specificity (56.8%). Interestingly, the difference in LPR remains significant also comparing hypoechoic haemangiomas to malignant lesions, suggesting the ability to distinguish a real wash-out from other phenomena. Although RT demonstrated a lower AUC (0.58) for distinguishing benign from malignant lesions, it demonstrated outstanding accuracy (AUC: 0.91) when applied to the distinction between haemangioma and malignancy. Furthermore, considering only benign lesions, haemangioma and adenoma displayed longer mean RT values than other benign lesions[24]. This suggests that quantitative analysis could increase the diagnostic accuracy between benign liver lesions and in challenging situations, such as benign lesions with moderate hyperenhancement (e.g., thrombosed haemangioma) or modest hypoenhancement in late phase (e.g., certain subtypes of hepatic adenoma).
D-CEUS might be helpful to differentiate hypervascular tumours like HCC from other malignant liver lesions that are predominantly necrotic and hypovascular. Wildner et al[25], analysing D-CEUS parameters in HCC and different secondary liver lesions showed that PE normalized for parenchyma signal and wash-in-wash-out area under the curve (WiWoAUC) were significantly higher in HCC compared to colorectal cancer (CRC) metastasis and relative signal intensity at 30 and 120 s after PE was significantly lower for pancreatic adenocarcinoma and CRC liver metastasis compared to HCC[25]. These results clearly suit to the hypervascular nature of HCC in contrast to the more necrotic and weakly centrally vascularized secondary liver lesions of other primitive cancers. While arterial phase parameters are significantly different between HCC and CRC metastatic liver masses, their applicability to differentiate HCC from other primary intrahepatic malignancies is unfitted. Previously, the same authors had investigated the differences between HCC and intrahepatic cholangiocellular carcinoma (ICC) showing no significant differences in arterial phase parameters while ICC group showed lower values of mean transit time (MTT) and fall time in portal and venous phase. Furthermore, relative signal intensity was significantly lower in ICC compared to HCC in all time points after PE at 40 s, 80 s, 100 s and 120 s[26]. Actually, the main difference between HCC and ICC at CEUS is the early and marked wash-out in portal phase for ICC[4]. Objective quantification of the wash-out phenomenon using D-CEUS could improve the diagnostic accuracy of differentiating lesions with similar portal and late phase hypoenhancement.
Prognosis prediction
One of the most promising applications of D-CEUS is the assessment of liver tumour response to treatment, particularly in HCC where chemotherapy regimens are mostly based on vascular-targeting agents[27]. To this purpose, Zocco et al[28] investigated the role of D-CEUS to early detect vascular changes in HCC patients treated with sorafenib and to predict response to therapy and prognosis. The results showed that a decrease in AUC, peak intensity (PI), and slope of wash-in (Pw) between T0 (baseline) and T1 (after fifteen days of therapy) was significantly associated with response to therapy assessed with RECIST criteria after two months of treatment. Furthermore, 10% decrease in AUC was significantly associated with longer survival as increased/unchanged of time to PI (Tp) and MTT, while a Pw reduction was significantly associated with progression-free survival (PFS)[28]. Similar results were obtained by Lassau et al[29] considering patients with advanced HCC treated with bevacizumab. D-CEUS was performed before treatment and at days 3, 7, 14, and 60 after treatment; and every 2 mo thereafter. Interestingly, the results showed that very early changes in D-CEUS characteristics correlated with response to therapy and prognosis. Particularly, the decreases in AUC, AUC during wash in, AUC during washout, and time to PI (TPI) at day 3 were significantly associated with RECIST response at 2 mo. Furthermore, PI, AUC and AUC during washout changes at day 3 were correlated with PFS and overall survival (OS)[29]. These results suggest that effects of antiangiogenetic treatments can be early assessed quantifying the perfusion parameters and could allow for a tempestive intervention when relative prognosis is unfavourable.
D-CEUS could also have a role in predicting the prognosis of HCC patients who received loco-regional treatments. In HCC patients undergoing microwave ablation, TTP evaluated before the procedure was confirmed as an independent predictor of OS[20]. Similarly, the WiAUC, the WoAUC and the WiWoAUC ratio between HCC lesion and surrounding liver parenchyma evaluated before thermal ablation were significantly associated with survival[30]. As a consequence, quantitative perfusion evaluation might provide additional information useful to plan treatment procedures.
Considering non-HCC malignant liver lesions, different studies evaluated D-CEUS modifications to predict early response to therapies. In patients with liver metastasis of melanoma treated with sorafenib, TTP and MTT increased significantly in responders group at 15 and 56 d assessment[31]. Furthermore, CRC-metastatic patients treated with chemotherapy plus bevacizumab showed changes in derived PI, TPI and AUC at day 15 that were significantly correlated with PFS, however these modifications were not related with tumor response or survival[32]. In contrast, Mogensen et al[33] observed a significant association between PE early variation and computed tomography (CT) dimensional tumour decrease in patients treated with chemotherapy plus bevacizumab[33]. Variability in these results could be explained by limitations of 2-dimensional imaging techniques used in all the discussed studies. In the future, 3-dimensional D-CEUS might provide a more accurate evaluation of entire tumor features[34].
Non-oncological hepatic application of D-CEUS
Despite most studies primarily focused on neoplastic liver disease, the application of quantitative analysis of perfusion parameters has also been explored in chronic liver disease. The TICs of the liver parenchyma can provide information’s of intrahepatic blood flow and, indirectly, of portal vein pressure, either that could be altered by liver fibrosis.
In the past, different studies evaluated transit time between vessels to estimate the intrahepatic blood flow and to assess liver fibrosis stage. It was showed that hepatic vein transit time decreased as severity of histologically proved chronic hepatopathy increased, thus allowing diagnosis of severe fibrosis with an accuracy of 79%[35,36]. In addition, hepatic vein arrival time and intrahepatic transit time were correlated with hepatic venous pressure gradient (HVPG), with an area under the receiver operating characteristic (AUROC) of 0.97 for HVPG > 10 mmHg and 0.94 for HVPG > 12 mmHg, respectively[37,38].
Recently, perfusion parameters analysis of liver parenchyma showed a decrease of amplitude-parameters and an increase of time-dependent parameters according to grade of portal pressure assessed with HVPG. Interestingly, PI resulted significantly negative correlated with portal hypertension and showed high accuracy (100% for both specificity and sensitivity) to predict clinical significant portal hypertension using a cut-off of 23.3 dB in patients with liver cirrhosis[39].
PANCREATIC DISEASES
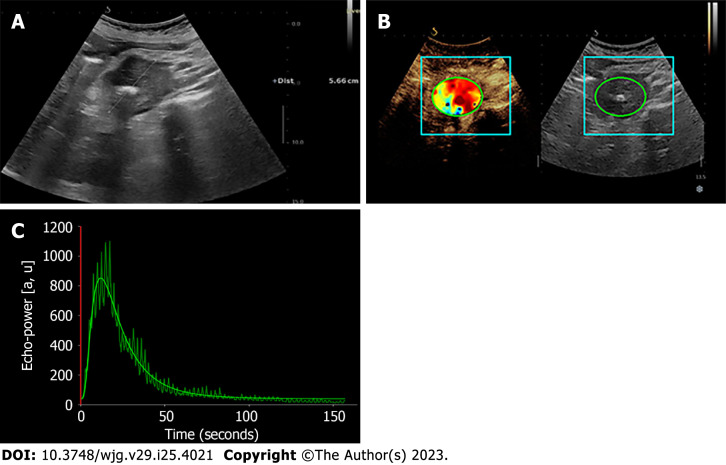
Existing evidence about the usefulness of D-CEUS for pancreatic diseases is very limited and is resumed in Table 3. Characterization and differential diagnosis of benign and malignant pancreatic lesions are the focus of the available research. Figure 3 depicts an example of D-CEUS in pancreatic disease.
Table 3.
Dynamic contrast-enhanced ultrasound and pancreatic diseases
|
Ref.
|
Study design/number of patients
|
Object of D-CEUS
|
Population/groups
|
Machine/UCA/Software
|
Significant results (P < 0.05)
|
| Yang et al[47], 2023 | Retrospective/42 | pNET | Patients with histopathologically proved pNET/G1, G2, G3, pNEC | Acuson Sequoia, Acuson Oxana2/SonoVue/VueBox | rPE, rMTT and rAUC were higher in pNETs G1/G2 than G3/pNECs |
| Zhang et al[45], 2020 | Prospective/11 | LAPC | Patient with LAPC underwent chemoradiotherapy | Acuson Oxana2/SonoVue/SonoLiver | MI decreased after chemoradiotherapy |
| Vitali et al[43], 2015 | Prospective/20 | PC, focal AIP | Patients with diagnosis of AIP vs histologically proved PC | Acuson Sequoia 512, S200/SonoVue/VueBox | The difference in PE (dPE) between lesion and surrounding parenchyma in AIP was lower compared to dPE in PC |
| D’Onofrio et al[40], 2014 | Prospective/10 | Suspected PDAC | Patients with suspected and then histologically proved PDAC | Acuson S2000/SonoVue/VueBox | PE and ascending curve values were different between lesion and adjacent parenchyma |
| Kersting et al[42], 2009 | Prospective/60 | Undefined pancreatic lesion | Patients with undefined pancreatic lesion underwent biopsy/PDAC vs CP | Sonoline Elegra/SonoVue/Axius ACQ | TTP and AT were longer in PDAC compared to focal masses in CP |
AIP: Autoimmune pancreatitis; AT: Arrival time; CP: Chronic pancreatitis; D-CEUS: Dynamic contrast-enhanced ultrasound; LAPC: Local advanced pancreatic cancer; MI: Maximum intensity; PC: Pancreatic cancer; PDAC: Pancreatic ductal adenocarcinoma; PE: Peak enhancement; pNET: Pancreatic neuroendocrine tumor; pNEC: Pancreatic neuroendocrine carcinoma; rAUC: Relative area under the curve; rMTT: Relative mean transit time; rPE: Relative peak enhancement; TTP: Time to peak; UCA: Ultrasound contrast agent.
Figure 3.
Dynamic contrast-enhanced ultrasound and time-intensity curves of the pancreas. A: Hypoechoic mass (adenocarcinoma) of pancreatic head in B-mode ultrasound; B and C: Contrast-enhanced ultrasound with corresponding time-intensity curve of the pancreatic lesion.
One of the first studies regarding the perfusion analysis of pancreatic cancer was conducted by D’Onofrio et al[40]. Prospectively, ten patients with suspected pancreatic ductal adenocarcinoma (PDAC) (as confirmed by histology) underwent CEUS with subsequent quantitative perfusion analysis. The results showed a significant difference in PE and ascending curve between PDAC and normal pancreatic parenchyma, providing an objective quantification of enhancement for the assessment of pancreatic lesion[40].
Chronic pancreatitis (CP), localized autoimmune pancreatitis (AIP), and paraduodenal pancreatitis can present CT and magnetic resonance imaging abnormalities identical to PDAC and, vice versa, potentially resectable malignant lesions can be misdiagnosed due to their similarities with benign masses[41]. In such instances, D-CEUS may reveal the pathophysiological differences between highly vascularized inflammatory lesions and essentially necrotic malignant masses, enabling a correct differentiation between benign lesions and cancer. To compare CP to PDAC, Kersting et al[42] performed D-CEUS in sixty undetermined pancreatic lesions that were histologically characterized as PDAC (fourty-five) or inflammatory lesion in CP (fifteen). The grouped analysis of TICs showed that TTP and arrival time were significantly prolonged in PDAC compared to CP. On the contrary, no differences were detected in MI and AUC between the two pathological conditions[42]. Regarding AIP, D-CEUS with quantitative analysis has the potential to make pre-operative differential diagnosis between focal-type AIP and PDAC non-invasively. Vitali et al[43] compared D-CEUS parameters of three patients with focal AIP with seventeen PDAC patients. Specifically, the difference between PE of PDAC and circumjacent normal parenchyma was significantly lower as compared to AIP. Significant was also the difference between wash in index perfusion (WiPI = WiAUC/RT) of PC and AIP[43]. In another study, TICs of AIP lesions showed delayed and higher enhancement compared to PDAC. Among all CEUS perfusion parameters, ratio of PE, WiAUC, wash-in rate (WiR), WiPI, WoAUC, WiWoAUC, and wash-out rate (WoR) between pancreatic lesion and surrounding normal pancreatic tissue were significantly higher in AIP lesions than PDAC lesions[44]. In cases of diffuse AIP, quantitative perfusion analysis is not suggested since there is no healthy parenchyma for comparison, which is necessary to enhance the comparability of the results regardless of exam- or patient-related factors[42].
Similarly to perfusion analysis for malignant liver lesions, D-CEUS could be considered to evaluate the response to therapies in PDAC. Recently, Zhang et al[45] investigated the role of D-CEUS to monitor the response to chemoradiotherapy in eleven patients with local advanced pancreatic cancer. They performed D-CEUS at baseline and after four weeks of therapy and analyzed the variation of TICs and related parameters. The rising and falling slope rates of TICs diminished after four weeks, and the percentage of MI decreased significantly compared to the surrounding normal parenchyma[45]. Since MI is related to tumour microvascular density, its reduction is coupled with a decrease in lesion blood perfusion, and the quantification of this consequence might reflect the objective efficacy of chemotherapy.
Lastly, D-CEUS could provide information in other types of pancreatic tumors. It has been proven that pancreatic neuroendocrine tumors (pNETs) with different histopathological grades have differences in tumor microvascular perfusion[46], therefore Yang et al[47] analyzed the correlation between perfusion analysis and histopathological grades of pNETs. In forty-two patients, the TICs shape of grade 1 (G1)/grade (G2) lesions were significantly different compared to TICs of grade 3 (G3)/pancreatic neuroendocrine carcinomas (pNECs). Significant differences were revealed at relative RT, relative MTT and relative AUC which were higher in G1/G2 than G3/pNECs. ROC analysis showed that relative AUC had the higher accuracy to distinguish the two groups[47]. The D-CEUS analysis and quantitative parameters have the potential value to non-invasively predict the biological behaviour of pNETs.
INFLAMMATORY BOWEL DISEASES
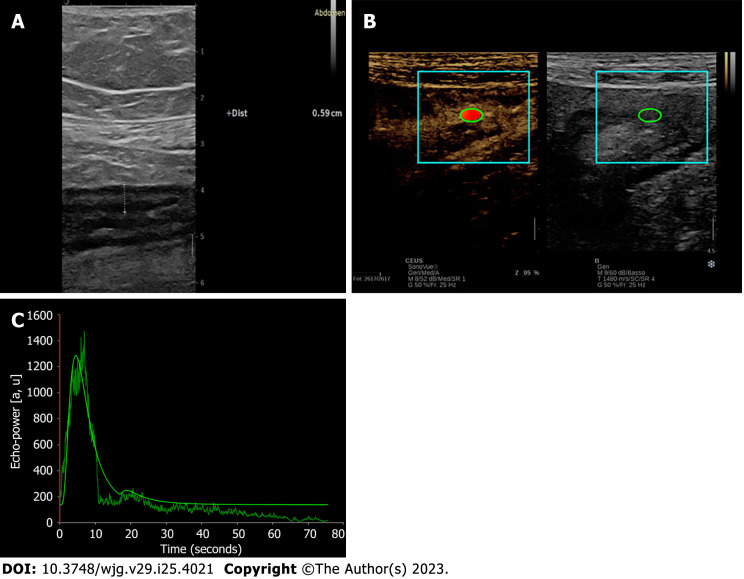
Table 4 summarizes the evidence regarding the role of D-CEUS in inflammatory bowel diseases, and Figure 4 depicts a TIC derived from intestinal wall examination. These studies are mainly focused on Crohn’s disease (CD).
Table 4.
Dynamic contrast-enhanced ultrasound and inflammatory bowel diseases
|
Ref.
|
Study design/number of patients
|
Object of D-CEUS
|
Population/groups
|
Machine/UCA/Software
|
Significant results (P < 0.05)
|
| Laterza et al[60], 2021 | Prospective/44 | CD | Patients with CD treated with anti-TNFα/responders vs non-responders | iU22/SonoVue/QLAB | Correlation between decrease in PI, AUC, Pw, and MTT and response to therapy |
| Goertz et al[57], 2018 | Prospective/18 | CD and UC | Patients with CD or UC treated with vedolizumab/responders vs non-responders | Acuson S2000/SonoVue/VueBox | WiR was lower in responders’ group after 14 wk |
| Wilkens et al[56], 2018 | Retrospective/104 | CD | Patients with severe CD underwent CEUS/normal vs atypical intensity decline on CEUS | iU22/Definity/QLAB | AUC, wash-out time, and intensity at 60s and 120s were higher in atypical decline group and this correlated with bad outcomes |
| Quaia et al[53], 2017 | Prospective/65 | CD | Patients with CD with terminal ileal loop stricture histologically characterized/inflammatory vs fibrostenotic disease | iU22/SonoVue/VueBox | PE, WiR, WiPI, AUC, WiAUC and WoAUC were higher in inflammatory group compared to fibrostenotic gruoup. TTP was not different between the two groups |
| Medellin-Kowalewski et al[55], 2016 | Retrospective/127 | CD | Patients with CD underwent US and CEUS | iU22/Definity/QLAB | PE correlate with wall thickness |
| Quaia et al[61], 2016 | Prospective/50 | CD | Patient with CD underwent medical treatment/responders vs non-responders | iU22/SonoVue/VueBox | Changes in PE, WiR, WoR, WiPI, AUC, WiAUC, and WoAUC from baseline to six weeks after therapy differed between responders and non-responses |
| Socaciu et al[58], 2015 | Prospective/38 | CD and UC | Patients with CD or CU candidate for medical treatment | Logiq 7/SonoVue/SonoLiver | Logarithm of AUC correlated with endoscopic improvement in both diseases |
| Saevik et al[59], 2014 | Prospective/14 | CD | Patients with CD started medical treatment/remission vs treatment failure | Logiq E9/SonoVue/VueBox | PE, WiR, WoR, and WiAUC were different between two groups at 1 mo of treatment |
| Romanini et al[50], 2014 | Prospective/33 | CD | Patients with CD undergoing colonoscopy and biopsy | Sequoia 512/SonoVue/Qontrast | Correlation between high vascular density and Peak% and regional blood flow |
| Ripollés et al[51], 2013 | Prospective/25 | CD | Patients with CD undergoing elective bowel resection/inflammatory vs fibrostenotic disease | Aplio 80/SonoVue/Software in Aplio 80 system | The percentage of increase in contrast enhancement of the bowel wall in inflammatory lesions was greater than fibrotic lesions |
| Nylund et al[52], 2013 | Prospective/33 | CD | Patient with CD underwent surgery or medical treatment/inflammatory vs fibrostenotic disease | Logiq E9/SonoVue/Custom software | Blood flow and blood volume were higher in the medical group compared to surgery group |
| Girlich et al[54], 2012 | Prospective/11 | UC | Patients with UC undergoing endoscopy | Logiq E9/SonoVue/Qontrast | Negative correlation between TTP/Peak% and histopathological score |
| Girlich et al[49], 2011 | Prospective/20 | CD | Patients with planned bowel surgery due to CD | Logiq 9/SonoVue/Qontrast | Negative correlation between TTP and histopathological score. Positive correlation with single items of the score |
| Girlich et al[48], 2009 | Prospective/20 | CD | Patients with active CD vs healthy volunteers | Logiq 9/SonoVue/Qontrast | Higher PE and regional blood volume and shorter TTP in CD |
AUC: Area under the curve; CD: Crohn’s disease; CEUS: Contrast-enhanced ultrasound; D-CEUS: Dynamic contrast-enhanced ultrasound; MTT: Mean transit time; PE: Peak enhancement; PI: Peak intensity; Pw: Slope coefficient of wash-in; TNF-α: Tumor necrosis factor-α; TTP: Time to peak; UC: Ulcerative colitis; UCA: Ultrasound contrast agent; US: Ultrasound; WiAUC: Wash-in area under the curve; WiPi: Wash-in ratio index; WiR: Wash-in rate; WoR: Wash-out rate.
Figure 4.
Dynamic contrast-enhanced ultrasound and time-intensity curves of the bowel wall. A: B-mode ultrasound features of ileal bowel wall thickening in Crohn’s disease; B and C: Contrast-enhanced ultrasound with corresponding time-intensity curve of the bowel wall thickening.
D-CEUS and inflammatory/fibrotic disease
TICs represent the perfusion of a tissue, and we would expect a difference in perfusion parameters not only when comparing fibrosis to inflammation, but also when considering various degrees of inflammation.
Girlich et al[48] described for the first time the difference in perfusion parameters between CD and healthy gut. As expected, the differences were substantially with significantly higher PE and regional blood volume, and longer TTP of thickness bowel wall of CD patients[48]. In another study, the same authors investigated the correlation between perfusion parameters and histopathological characteristics of the gut wall in surgically treated CD patients, confirming that TTP was negatively correlated with the histological inflammatory score[49]. Similarly, a higher PE, regional blood flow and regional blood volume, and shorter TTP were significantly correlated with high vascular density defined as the presence of more than two hundred sixty five vessels per field on histological examination[50]. On the contrary, Ripollés et al[51] showed a non-significant association of TTP with inflammatory or fibrosis histological score, however they used different histological scores and had longer time between CEUS and surgery (34.5 ± 17.3 vs 4.7 ± 4.7 d)[51].
To assess the differences between fibrotic or inflammatory CD, Nylund et al[52] compared sixteen patients with fibrotic disease undergoing surgery to seventeen patients with medically treated active inflammatory disease. In inflammatory disease, the blood volume and blood flow were significantly higher compared to fibrotic disease, while MTT was not significantly different between the two groups. Interestingly, blood volume/bowel wall thickness ratio showed a high accuracy to predict surgery[52]. Similarly, Quaia et al[53] found a significant difference in blood volume related parameters (PE, AUC, WiAUC, WoAUC, WiR and, WiPI) between fibrotic strictures and inflammatory strictures among patients with CD, with the latter having higher values. However, TTP showed no significant differences between the two groups[53]. These findings imply that D-CEUS can detect the microvascular differences between fibrotic and inflammatory tissue and allows non-invasive differentiation of CD phenotypes.
Regarding ulcerative colitis (UC), only one prospective study investigated the relationship between perfusion parameters and histopathological findings. In this study, TTP/Peak(%) showed a strong negative correlation with histopathological inflammatory activity score[54].
D-CEUS and clinical outcomes
Given the negative correlation with inflammation, Peak% > 40.5% and TTP < 35 seconds had a high positive predictive value (94%) and a high negative predictive value (92.3%) for active disease, respectively[50].
It has also been shown that the evaluation of PE and AUC is a useful tool to assessing the severity of CD when the ultrasound global assessment and colorDoppler imaging criteria are indeterminate. Particularly, PE > 23 dB showed a sensitivity and specificity of 90% and 89.5%, respectively, to distinguish moderate from severe disease[55]. Using this cut-off to identify patients with severe disease, Wilkens et al[56] investigated the clinical outcomes in twenty patients with severe disease in whom they observed an atypical, prolonged intestinal washout due to the stuck bubble phenomenon (AUC > 20000 AIU) compared to patient with severe disease and AUC < 20000 AIU (control group). They found a significative higher rate of surgery and a trend toward more combination therapies in study group. Interestingly, they ascribed the stuck bubbles phenomena to the attachment of microbubbles to active leukocytes on the endothelium; if confirmed, this might be utilized as a tool for targeted therapy[56].
Assessment of perfusion parameters is important for identifying disease status, as well as monitoring and predicting the efficacy of therapies. Indeed, D-CEUS measurements changed significantly between clinical- and endoscopic-assessed responders and non-responders with CD and UC following treatments[57,58]. In a prospective study, Saevik et al[59] included fourteen patients with acute CD who started treatment with steroids or tumor necrosis factor-α (TNF-α) inhibitors. CEUS was performed before starting therapy and at one, three and twelve months. At one month, the differences between patients who achieved clinical remission and those who had treatment failure during the follow-up period were significant. Particularly, PE, WiAUC, WiR and WoR was significantly lower in effective treatment group[59]. The reduction in perfusion parameters could be related to a decrease in inflammation and, thus, a treatment response. Recently, it was demonstrated that the reduction in PI, AUC, Pw, and MTT was higher in patients responding to anti-TNF-α therapy after two weeks than in patients who relapsed within six months of treatment initiation, who displayed not only a lower early reduction in perfusion parameters but also an increase in PI after twelve weeks[60]. Changes in quantitative perfusion analysis parameters between baseline and six weeks after therapy initiation distinguished responders from non-responders defined by clinical and endoscopic evaluation at twelve weeks[61]. This highlights the potential for D-CEUS to detect therapy-induced modifications in the pathologic bowel wall and support clinicians in disease management.
OTHER GASTROINTESTINAL DISEASES
The investigation of perfusion parameters with D-CEUS could be an informative tool in the diagnosis and prognosis of other gastrointestinal diseases, such as gastric cancer[62]. In a prospective study including forty-three patients with advanced gastric cancer, Joo et al[63] showed a good feasibility of CEUS (88.4%) and a significant difference in PI and AUC according to differentiation status of the tumor. Localization in the upper stomach and an ulcerated phenotype were the limiting variables for D-CEUS feasibility[63]. Regarding the CRC, the difference in AUC was significantly related with tumor necrosis and T stage[64], suggesting a possible role of D-CEUS in predicting tumor microenvironment characteristics and behavior. Furthermore, dynamic contrast enhanced endoscopic ultrasound (D-CE-EUS) showed a significant correlation between RT and vessel density in patients with left side colonic tumors[65], indicating that perfusion analysis might be useful to predict outcomes of antiangiogenic treatment as Lassau et al[66] has showed in a multicentric study including over one thousand D-CEUS evaluations in more than five hundred patients with solid tumor treated with antiangiogenic therapy[66]. In particular, D-CEUS blood volume related parameters showed significant early changes in gastrointestinal stromal tumours treated with masitinib, predicting positron emission tomography-CT outcome[67].
Concerning non-neoplastic bowel diseases, different studies described the CEUS aspects of various inflammatory bowel diseases, including abscesses, acute appendicitis, diverticulitis, vascular bowel disease, such as intestinal ischemia, and graft vs host disease[68]. To the best of our knowledge, no specific study employing D-CEUS in the aforementioned conditions has been published yet; however, it would be desirable to investigate the potential usefulness of quantitative perfusion analysis in predicting pathological features and prognosis also in this field.
CONCLUSION
The quantification of perfusion parameters in CEUS has several applications in gastrointestinal neoplastic and inflammatory disorders. Everything that is visible with ultrasound can be measured, and this has allowed D-CEUS to be employed within the pancreas and digestive system in addition to the liver evaluation. The objective assessment of tissue perfusion is crucial for the evaluation of all disorders in which the vascular component plays a key pathophysiological role, such as malignant tumours and inflammatory bowel disease. However, the limited availability of large studies and the heterogeneity of the technologies employed have precluded the standardization of this approach which potentially represents a valuable tool for clinical practice in management of gastrointestinal diseases.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 26, 2023
First decision: May 13, 2023
Article in press: June 5, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Lu Q, China S-Editor: Fan JR L-Editor: A P-Editor: Xu ZH
Contributor Information
Mattia Paratore, Medicina Interna e Gastroenterologia, CEMAD Digestive Disease Center, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy.
Matteo Garcovich, Medicina Interna e Gastroenterologia, CEMAD Digestive Disease Center, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy. matteogarcovich@yahoo.it.
Maria Elena Ainora, Medicina Interna e Gastroenterologia, CEMAD Digestive Disease Center, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy.
Laura Riccardi, Medicina Interna e Gastroenterologia, CEMAD Digestive Disease Center, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy.
Antonio Gasbarrini, Medicina Interna e Gastroenterologia, CEMAD Digestive Disease Center, Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy.
Maria Assunta Zocco, Medicina Interna e Gastroenterologia, CEMAD Digestive Disease Center, Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy.
References
- 1.Braden B, Ignee A, Hocke M, Palmer RM, Dietrich C. Diagnostic value and clinical utility of contrast enhanced ultrasound in intestinal diseases. Dig Liver Dis. 2010;42:667–674. doi: 10.1016/j.dld.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2015;34:3–18. doi: 10.14366/usg.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D'Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version) Ultraschall Med. 2018;39:e2–e44. doi: 10.1055/a-0586-1107. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D'Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579–2604. doi: 10.1016/j.ultrasmedbio.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Chiorean L, Tana C, Braden B, Caraiani C, Sparchez Z, Cui XW, Baum U, Dietrich CF. Advantages and Limitations of Focal Liver Lesion Assessment with Ultrasound Contrast Agents: Comments on the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines. Med Princ Pract. 2016;25:399–407. doi: 10.1159/000447670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fröhlich E, Muller R, Cui XW, Schreiber-Dietrich D, Dietrich CF. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med. 2015;34:179–196. doi: 10.7863/ultra.34.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344–351. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 8.Arditi M, Frinking PJ, Zhou X, Rognin NG. A new formalism for the quantification of tissue perfusion by the destruction-replenishment method in contrast ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53:1118–1129. doi: 10.1109/tuffc.2006.1642510. [DOI] [PubMed] [Google Scholar]
- 9.Leen E, Averkiou M, Arditi M, Burns P, Bokor D, Gauthier T, Kono Y, Lucidarme O. Dynamic contrast enhanced ultrasound assessment of the vascular effects of novel therapeutics in early stage trials. Eur Radiol. 2012;22:1442–1450. doi: 10.1007/s00330-011-2373-2. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich CF, Dong Y, Froehlich E, Hocke M. Dynamic contrast-enhanced endoscopic ultrasound: A quantification method. Endosc Ultrasound. 2017;6:12–20. doi: 10.4103/2303-9027.193595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5:53–58. doi: 10.1007/s11523-010-0136-7. [DOI] [PubMed] [Google Scholar]
- 12.Saini R, Hoyt K. Recent developments in dynamic contrast-enhanced ultrasound imaging of tumor angiogenesis. Imaging Med. 2014;6:41–52. doi: 10.2217/iim.13.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson JM, Williams R, Tremblay-Darveau C, Sheeran PS, Milot L, Bjarnason GA, Burns PN. Dynamic contrast enhanced ultrasound for therapy monitoring. Eur J Radiol. 2015;84:1650–1657. doi: 10.1016/j.ejrad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Schäfer S, Nylund K, Sævik F, Engjom T, Mézl M, Jiřík R, Dimcevski G, Gilja OH, Tönnies K. Semi-automatic motion compensation of contrast-enhanced ultrasound images from abdominal organs for perfusion analysis. Comput Biol Med. 2015;63:229–237. doi: 10.1016/j.compbiomed.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Lassau N, Couanet D, Angevin E, Mesrati F, Leborgne S, Garofano A, Leboulaire C, Dupouy N, Laplanche A. Phase II trial of thalidomide in renal-cell carcinoma. Ann Oncol. 2002;13:1029–1035. doi: 10.1093/annonc/mdf213. [DOI] [PubMed] [Google Scholar]
- 17.Santhakumar C, Gane EJ, Liu K, McCaughan GW. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol Int. 2020;14:947–957. doi: 10.1007/s12072-020-10104-3. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Zhou P, Li S, Li K. Prediction of the Ki-67 marker index in hepatocellular carcinoma based on Dynamic Contrast-Enhanced Ultrasonography with Sonazoid. Insights Imaging. 2022;13:199. doi: 10.1186/s13244-022-01320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xuan Z, Wu N, Li C, Liu Y. Application of contrast-enhanced ultrasound in the pathological grading and prognosis prediction of hepatocellular carcinoma. Transl Cancer Res. 2021;10:4106–4115. doi: 10.21037/tcr-21-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan Y, Zhou F, Yu X, Luo F, Liu F, Liang P, Cheng Z, Han Z, Yu J. Quantitative dynamic contrast-enhanced ultrasound may help predict the outcome of hepatocellular carcinoma after microwave ablation. Int J Hyperthermia. 2019;35:105–111. doi: 10.1080/02656736.2018.1483533. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Qiu Y, Yang D, Yu L, Zuo D, Zhang Q, Tian X, Wang WP, Jung EM. Potential application of dynamic contrast enhanced ultrasound in predicting microvascular invasion of hepatocellular carcinoma. Clin Hemorheol Microcirc. 2021;77:461–469. doi: 10.3233/CH-201085. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Han X, Li L, Su C, Sun J, Zhan C, Feng D, Cheng W. Dynamic Contrast-Enhanced Ultrasonography with Sonazoid for Diagnosis of Microvascular Invasion in Hepatocellular Carcinoma. Ultrasound Med Biol. 2022;48:575–581. doi: 10.1016/j.ultrasmedbio.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Barr RG, Huang P, Luo Y, Xie X, Zheng R, Yan K, Jing X, Xu H, Fei X, Lee JM. Contrast-enhanced ultrasound imaging of the liver: a review of the clinical evidence for SonoVue and Sonazoid. Abdom Radiol (NY) 2020;45:3779–3788. doi: 10.1007/s00261-020-02573-9. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz S, Clevert DA, Ingrisch M, Geyer T, Schwarze V, Rübenthaler J, Armbruster M. Quantitative Analysis of the Time-Intensity Curve of Contrast-Enhanced Ultrasound of the Liver: Differentiation of Benign and Malignant Liver Lesions. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11071244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wildner D, Schellhaas B, Strack D, Goertz RS, Pfeifer L, Fiessler C, Neurath MF, Strobel D. Differentiation of malignant liver tumors by software-based perfusion quantification with dynamic contrast-enhanced ultrasound (DCEUS) Clin Hemorheol Microcirc. 2019;71:39–51. doi: 10.3233/CH-180378. [DOI] [PubMed] [Google Scholar]
- 26.Wildner D, Pfeifer L, Goertz RS, Bernatik T, Sturm J, Neurath MF, Strobel D. Dynamic contrast-enhanced ultrasound (DCE-US) for the characterization of hepatocellular carcinoma and cholangiocellular carcinoma. Ultraschall Med. 2014;35:522–527. doi: 10.1055/s-0034-1385170. [DOI] [PubMed] [Google Scholar]
- 27.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Zocco MA, Garcovich M, Lupascu A, Di Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME, Ponziani F, Caracciolo G, Rapaccini GL, Landolfi R, Siciliano M, Pompili M, Gasbarrini A. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014–1021. doi: 10.1016/j.jhep.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Lassau N, Koscielny S, Chami L, Chebil M, Benatsou B, Roche A, Ducreux M, Malka D, Boige V. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification--preliminary results. Radiology. 2011;258:291–300. doi: 10.1148/radiol.10091870. [DOI] [PubMed] [Google Scholar]
- 30.Gu DY, Zhang Y, Hu JX, Qin HY, Lu X, He GB, Shang L. The value of contrast-enhanced ultrasound quantitative parameters in the prognosis prediction of hepatocellular carcinoma after thermal ablation: a retrospective cohort study. J Gastrointest Oncol. 2022;13:2522–2531. doi: 10.21037/jgo-22-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildner D, Heinzerling L, Scheulen ME, Kaempgen E, Schuler G, Strobel D, Janka R, Neurath MF, Sturm J, Knieling F. Assessment of sorafenib induced changes in tumor perfusion of uveal melanoma metastases with dynamic contrast-enhanced ultrasound (DCE-US) J Cancer Res Clin Oncol. 2022;148:955–965. doi: 10.1007/s00432-021-03666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amadori M, Barone D, Scarpi E, Oboldi D, Amadori E, Bandi G, Rossi A, Ferroni F, Ragazzini A, Casadei Gardini A, Frassineti GL, Gavelli G, Passardi A. Dynamic contrast-enhanced ultrasonography (D-CEUS) for the early prediction of bevacizumab efficacy in patients with metastatic colorectal cancer. Eur Radiol. 2018;28:2969–2978. doi: 10.1007/s00330-017-5254-5. [DOI] [PubMed] [Google Scholar]
- 33.Mogensen MB, Hansen ML, Henriksen BM, Axelsen T, Vainer B, Osterlind K, Nielsen MB. Dynamic Contrast-Enhanced Ultrasound of Colorectal Liver Metastases as an Imaging Modality for Early Response Prediction to Chemotherapy. Diagnostics (Basel) 2017;7 doi: 10.3390/diagnostics7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Kaffas A, Sigrist RMS, Fisher G, Bachawal S, Liau J, Wang H, Karanany A, Durot I, Rosenberg J, Hristov D, Willmann JK. Quantitative Three-Dimensional Dynamic Contrast-Enhanced Ultrasound Imaging: First-In-Human Pilot Study in Patients with Liver Metastases. Theranostics. 2017;7:3745–3758. doi: 10.7150/thno.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim AK, Patel N, Eckersley RJ, Goldin RD, Thomas HC, Cosgrove DO, Taylor-Robinson SD, Blomley MJ. Hepatic vein transit time of SonoVue: a comparative study with Levovist. Radiology. 2006;240:130–135. doi: 10.1148/radiol.2401041517. [DOI] [PubMed] [Google Scholar]
- 36.Staub F, Tournoux-Facon C, Roumy J, Chaigneau C, Morichaut-Beauchant M, Levillain P, Prevost C, Aubé C, Lebigot J, Oberti F, Galtier JB, Laumonier H, Trillaud H, Bernard PH, Blanc JF, Sironneau S, Machet F, Drouillard J, de Ledinghen V, Couzigou P, Foucher P, Castéra L, Tranquard F, Bacq Y, d'Altéroche L, Ingrand P, Tasu JP. Liver fibrosis staging with contrast-enhanced ultrasonography: prospective multicenter study compared with METAVIR scoring. Eur Radiol. 2009;19:1991–1997. doi: 10.1007/s00330-009-1313-x. [DOI] [PubMed] [Google Scholar]
- 37.Kim MY, Suk KT, Baik SK, Kim HA, Kim YJ, Cha SH, Kwak HR, Cho MY, Park HJ, Jeon HK, Park SY, Kim BR, Hong JH, Jo KW, Kim JW, Kim HS, Kwon SO, Chang SJ, Baik GH, Kim DJ. Hepatic vein arrival time as assessed by contrast-enhanced ultrasonography is useful for the assessment of portal hypertension in compensated cirrhosis. Hepatology. 2012;56:1053–1062. doi: 10.1002/hep.25752. [DOI] [PubMed] [Google Scholar]
- 38.Jeong WK, Kim TY, Sohn JH, Kim Y, Kim J. Severe portal hypertension in cirrhosis: evaluation of perfusion parameters with contrast-enhanced ultrasonography. PLoS One. 2015;10:e0121601. doi: 10.1371/journal.pone.0121601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon R. Complications After Pancreaticoduodenectomy. Surg Clin North Am. 2021;101:865–874. doi: 10.1016/j.suc.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 40.D'Onofrio M, Canestrini S, Crosara S, De Robertis R, Pozzi Mucelli R. Contrast enhanced ultrasound with quantitative perfusion analysis for objective characterization of pancreatic ductal adenocarcinoma: A feasibility study. World J Radiol. 2014;6:31–35. doi: 10.4329/wjr.v6.i3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. 2020;20:52. doi: 10.1186/s40644-020-00324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kersting S, Konopke R, Kersting F, Volk A, Distler M, Bergert H, Saeger HD, Grützmann R, Bunk A. Quantitative perfusion analysis of transabdominal contrast-enhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology. 2009;137:1903–1911. doi: 10.1053/j.gastro.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 43.Vitali F, Pfeifer L, Janson C, Goertz RS, Neurath MF, Strobel D, Wildner D. Quantitative perfusion analysis in pancreatic contrast enhanced ultrasound (DCE-US): a promising tool for the differentiation between autoimmune pancreatitis and pancreatic cancer. Z Gastroenterol. 2015;53:1175–1181. doi: 10.1055/s-0041-103847. [DOI] [PubMed] [Google Scholar]
- 44.Qiu YJ, Zhao GC, Shi SN, Zuo D, Zhang Q, Dong Y, Lou WH, Wang WP. Application of dynamic contrast enhanced ultrasound in distinguishing focal-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Clin Hemorheol Microcirc. 2022;81:149–161. doi: 10.3233/CH-221390. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Wu L, Yang D, Qiu Y, Yu L, Dong Y, Wang WP. Clinical application of dynamic contrast enhanced ultrasound in monitoring the treatment response of chemoradiotherapy of pancreatic ductal adenocarcinoma. Clin Hemorheol Microcirc. 2020;75:325–334. doi: 10.3233/CH-190786. [DOI] [PubMed] [Google Scholar]
- 46.Khanna L, Prasad SR, Sunnapwar A, Kondapaneni S, Dasyam A, Tammisetti VS, Salman U, Nazarullah A, Katabathina VS. Pancreatic Neuroendocrine Neoplasms: 2020 Update on Pathologic and Imaging Findings and Classification. Radiographics. 2020;40:1240–1262. doi: 10.1148/rg.2020200025. [DOI] [PubMed] [Google Scholar]
- 47.Yang DH, Cheng J, Tian XF, Zhang Q, Yu LY, Qiu YJ, Lu XY, Lou WH, Dong Y, Wang WP. Prediction of Pathological Grades of Pancreatic Neuroendocrine Tumors Based on Dynamic Contrast-Enhanced Ultrasound Quantitative Analysis. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girlich C, Jung EM, Iesalnieks I, Schreyer AG, Zorger N, Strauch U, Schacherer D. Quantitative assessment of bowel wall vascularisation in Crohn's disease with contrast-enhanced ultrasound and perfusion analysis. Clin Hemorheol Microcirc. 2009;43:141–148. doi: 10.3233/CH-2009-1228. [DOI] [PubMed] [Google Scholar]
- 49.Girlich C, Jung EM, Huber E, Ott C, Iesalnieks I, Schreyer A, Schacherer D. Comparison between preoperative quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound and operative macroscopic findings and results of histopathological scoring in Crohn's disease. Ultraschall Med. 2011;32:154–159. doi: 10.1055/s-0029-1245398. [DOI] [PubMed] [Google Scholar]
- 50.Romanini L, Passamonti M, Navarria M, Lanzarotto F, Villanacci V, Grazioli L, Calliada F, Maroldi R. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur J Radiol. 2014;83:1317–1323. doi: 10.1016/j.ejrad.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn's disease: a comparison with surgical histopathology analysis. J Crohns Colitis. 2013;7:120–128. doi: 10.1016/j.crohns.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Nylund K, Jirik R, Mezl M, Leh S, Hausken T, Pfeffer F, Ødegaard S, Taxt T, Gilja OH. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn's disease. Ultrasound Med Biol. 2013;39:1197–1206. doi: 10.1016/j.ultrasmedbio.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Quaia E, Gennari AG, van Beek EJR. Differentiation of Inflammatory from Fibrotic Ileal Strictures among Patients with Crohn's Disease through Analysis of Time-Intensity Curves Obtained after Microbubble Contrast Agent Injection. Ultrasound Med Biol. 2017;43:1171–1178. doi: 10.1016/j.ultrasmedbio.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Girlich C, Schacherer D, Jung EM, Klebl F, Huber E. Comparison between quantitative assessment of bowel wall vascularization by contrast-enhanced ultrasound and results of histopathological scoring in ulcerative colitis. Int J Colorectal Dis. 2012;27:193–198. doi: 10.1007/s00384-011-1300-y. [DOI] [PubMed] [Google Scholar]
- 55.Medellin-Kowalewski A, Wilkens R, Wilson A, Ruan J, Wilson SR. Quantitative Contrast-Enhanced Ultrasound Parameters in Crohn Disease: Their Role in Disease Activity Determination With Ultrasound. AJR Am J Roentgenol. 2016;206:64–73. doi: 10.2214/AJR.15.14506. [DOI] [PubMed] [Google Scholar]
- 56.Wilkens R, Wilson A, Burns PN, Ghosh S, Wilson SR. Persistent Enhancement on Contrast-Enhanced Ultrasound Studies of Severe Crohn's Disease: Stuck Bubbles? Ultrasound Med Biol. 2018;44:2189–2198. doi: 10.1016/j.ultrasmedbio.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Goertz RS, Klett D, Wildner D, Atreya R, Neurath MF, Strobel D. Quantitative contrast-enhanced ultrasound for monitoring vedolizumab therapy in inflammatory bowel disease patients: a pilot study. Acta Radiol. 2018;59:1149–1156. doi: 10.1177/0284185117752032. [DOI] [PubMed] [Google Scholar]
- 58.Socaciu M, Ciobanu L, Diaconu B, Hagiu C, Seicean A, Badea R. Non-Invasive Assessment of Inflammation and Treatment Response in Patients with Crohn's Disease and Ulcerative Colitis using Contrast-Enhanced Ultrasonography Quantification. J Gastrointestin Liver Dis. 2015;24:457–465. doi: 10.15403/jgld.2014.1121.244.chr. [DOI] [PubMed] [Google Scholar]
- 59.Saevik F, Nylund K, Hausken T, Ødegaard S, Gilja OH. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:2029–2037. doi: 10.1097/MIB.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laterza L, Ainora ME, Garcovich M, Galasso L, Poscia A, Di Stasio E, Lupascu A, Riccardi L, Scaldaferri F, Armuzzi A, Rapaccini GL, Gasbarrini A, Pompili M, Zocco MA. Bowel contrast-enhanced ultrasound perfusion imaging in the evaluation of Crohn's disease patients undergoing anti-TNFα therapy. Dig Liver Dis. 2021;53:729–737. doi: 10.1016/j.dld.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Quaia E, Sozzi M, Angileri R, Gennari AG, Cova MA. Time-Intensity Curves Obtained after Microbubble Injection Can Be Used to Differentiate Responders from Nonresponders among Patients with Clinically Active Crohn Disease after 6 Weeks of Pharmacologic Treatment. Radiology. 2016;281:606–616. doi: 10.1148/radiol.2016152461. [DOI] [PubMed] [Google Scholar]
- 62.Li T, Lu M, Song J, Wu P, Cheng X, Zhang Z. Improvement to ultrasonographical differential diagnosis of gastric lesions: The value of contrast enhanced sonography with gastric distention. PLoS One. 2017;12:e0182332. doi: 10.1371/journal.pone.0182332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joo I, Kim SH, Lee DH, Han JK. Dynamic Contrast-Enhanced Ultrasound of Gastric Cancer: Correlation with Perfusion CT and Histopathology. Korean J Radiol. 2019;20:781–790. doi: 10.3348/kjr.2018.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhuang H, Yang ZG, Wang ZQ, Wang XD, Chen HJ, Zhang YC, Luo Y. Features of time-intensity curve parameters of colorectal adenocarcinomas evaluated by double-contrast enhanced ultrasonography: initial observation. Eur J Radiol. 2012;81:677–682. doi: 10.1016/j.ejrad.2011.01.075. [DOI] [PubMed] [Google Scholar]
- 65.Malmstrøm ML, Săftoiu A, Riis LB, Hassan H, Klausen TW, Rahbek MS, Gögenur I, Vilmann P. Dynamic contrast-enhanced EUS for quantification of tumor perfusion in colonic cancer: a prospective cohort study. Gastrointest Endosc. 2018;87:1530–1538. doi: 10.1016/j.gie.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, Taieb S, Aziza R, Sarran A, Labbe-Devilliers C, Gallix B, Lucidarme O, Ptak Y, Rocher L, Caquot LM, Chagnon S, Marion D, Luciani A, Feutray S, Uzan-Augui J, Coiffier B, Benastou B, Koscielny S. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49:794–800. doi: 10.1097/RLI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lassau N, Chami L, Koscielny S, Chebil M, Massard C, Benatsou B, Bidault S, Cioffi A, Blay JY, Le Cesne A. Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinib. Invest New Drugs. 2012;30:765–771. doi: 10.1007/s10637-010-9592-2. [DOI] [PubMed] [Google Scholar]
- 68.Medellin A, Merrill C, Wilson SR. Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom Radiol (NY) 2018;43:918–933. doi: 10.1007/s00261-017-1399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frampas E, Lassau N, Zappa M, Vullierme MP, Koscielny S, Vilgrain V. Advanced Hepatocellular Carcinoma: early evaluation of response to targeted therapy and prognostic value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary results. Eur J Radiol. 2013;82:e205–e211. doi: 10.1016/j.ejrad.2012.12.004. [DOI] [PubMed] [Google Scholar]