Abstract
Background:
The resolution of inflammation is an active process mediated by specialized lipid mediators called lipoxins and resolvins. Periodontal ligament fibroblasts (PDLFs) play a significant role in periodontal regeneration. The purpose of the current study was to determine the impact of resolvin D1 (RvD1) on human PDLF cell wound healing and proliferation, receptor expression (G-protein-coupled receptor 32 [GPR32] and formyl peptide receptor 2 [ALX/FPR2]), and cytokine expression and release.
Methods:
PDLFs were stimulated with interleukin-1β (IL-1β) (500 pg/ml) with and without RvD1 (100 nM). RvD1 receptor expression was determined by quantitative real-time polymerase chain reaction (qPCR), immunofluorescence microscopy, and fluorescence-activated cell sorting. Wound closure was measured by a scratch assay, and proliferation was determined by bromodeoxyuridine incorporation. Interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemoattractant protein-1, cyclooxygenase-2, matrix metalloproteinases-1, −2, and −3 (MMP-1, −2, and −3), tissue inhibitors of metalloproteinases-1 and −2 (TIMP-1 and −2), prostaglandin E2, and osteoprotegerin (OPG) gene expression and production were measured using qPCR and Western blotting, multiplex immunoassay, and enzyme-linked immunosorbent assay.
Results:
PDLF expressed GPR32 and ALX/FPR2. RvD1 reversed IL-1β-induced inhibition of wound healing and proliferation of PDLF. IL-1β also induced the production of proinflammatory cytokines and MMPs. This effect was reversed by RvD1. RvD1 reversed IL-1β–induced inhibition of TIMP-1, TIMP-2, and OPG.
Conclusion:
The data suggested that RvD1 has a pro-wound healing, proliferative, and anti-inflammatory impact on the PDLF that favors periodontal regeneration.
Keywords: aspirin-triggered resolvin D1, cytokine, fibroblast, inflammation, periodontal disease, regeneration
1 |. INTRODUCTION
Periodontal disease is an infectious-inflammatory pathology where the host response to commensal organisms is responsible for the destruction of tooth-supporting tissues, including periodontal ligament and bone.1,2 Periodontal regeneration is a complete restoration of the function and morphology of the lost attachment between tissues.3 Clinically, periodontal regeneration is often challenging; uncontrolled inflammation is considered important in the failure to regenerate lost tissues.4 Wound healing and regeneration of the periodontal tissues are regulated by the primary cells of the periodontal ligament, the periodontal ligament fibroblasts (PDLFs). In addition to their function as key structural cells producing extracellular matrix and regulating tissue turnover, PDLF can produce and release cytokines and proinflammatory mediators such as interleukin-6 (IL-6), interleukin-8 (IL-8), prostaglandin E2 (PGE2), and matrix metalloproteinases (MMPs),5–15 which participate in the local inflammatory response triggered by bacteria.7 Thus, modulation of PDLF inflammatory phenotype is an important goal in predictable periodontal regeneration.
The outcomes of acute inflammation vary from complete resolution to the progression into chronic inflammation, scarring, and fibrosis.16 Resolution of inflammation is an active process that is mediated by local lipid mediators (lipoxins and resolvins) synthesized during the resolution phase of acute inflammation.17 Topically applied resolvins promoted periodontal regeneration in animal models with a pleiotropic impact on cells of the periodontium, especially fibroblasts and osteoblasts.18 Antifibrotic actions on murine fibroblasts have also been reported.19
D series resolvins (RvD1-RvD4) are derived from docosahexaenoic acid (DHA). When an inflammatory event is initiated by infection or physical injury, RvDs, similar to the other pro-resolution lipid mediators, help the tissue return to homeostasis.20 RvD1 has been recently shown to restore regulatory T cells/T helper 17 cells (Treg/Th17) imbalance in systemic lupus erythmatosus.21 RvD1 actions are receptor-mediated through binding to (GPR32) and formyl peptide receptor 2 (ALX/FPR2). GPR32 is an orphan G-protein-coupled receptor (GPCR) with seven transmembrane domains expressed by phagocytic leukocytes, mainly macrophages/monocytes, and polymorphonuclear leukocytes (PMNs). GPR32 binding to RvD1 or lipoxin A4 (LXA4) induces β-arrestin interaction with the receptor.22,23 ALX/FPR2 is a chemotactic GPCR27–29 that is also abundant on the surface of phagocytic leukocytes.24 ALX/FPR2 is a dual-action receptor whose downstream response is dependent on the type of its ligand. Upon activation with N-formyl-methionine-leucyl-phenylalanine (fMLP), ALX/FPR2 induces migration and calcium mobilization in human monocytes and neutrophils and is involved in inflammatory and host defense responses.25–27 However, when the same receptor is activated by ligands such as annexin 1, LXA4, or RvD1,itexhibitsanti-inflammatoryactionssuchasinhibiting leukocyte migration and promoting the resolution of inflammation.28–30
RvD1 was shown to counteract the toxic actions of Porphyromonas gingivalis on human gingival fibroblasts31 and inhibit the production of PGE2 induced by IL-1β and tumor necrosis factor-α (TNF-α) while increasing fibroblast growth factor release by PDLF.32 However, the mechanism of this activity in the context of periodontal inflammation and regeneration is poorly understood. Therefore, we hypothesized that RvD1 will enhance PDLF function during periodontal inflammation and promote a phenotypic shift in the PDLF gene and protein expression and function that favors clinically observed periodontal regeneration induced by these molecules. Therefore, the purpose of the current study was to determine the impact of RvD1 on human PDLF cell scratch closure and proliferation, RvD1 receptor expression (GPR32 and ALX/FPR2), and PDLF cytokine expression and release.
2 |. MATERIALS AND METHODS
2.1 |. Primary human periodontal ligament fibroblast cell culture
Primary human PDLF were purchased and cultured according to the manufacturer’s protocol.* The cells arrived from the manufacturer in vials containing approximately 6.75×105 cells pervial, with about 97% viability, 38% seeding efficiency, and 19-h doubling time. The cells were in passage three and confirmed by the manufacturer to be sterile, mycoplasma-free, and HIV, hepatitis B virus, and hepatitis C virus negative. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 0.1 mM non-essential amino acids, 100 IU/ml penicillin and 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B at 37°C and 5% CO2 in 100% humidity until they achieved 80% confluence. Cells were brought to quiescence 1 day before experiments by reducing FBS concentration to 0.1%. The medium was changed every 2 days, and cells were used between passages 5 and 8. In all experiments, PDLF were treated with vehicle control (0.04% ethanol), 17(S)-RvD1† (100 nM), human recombinant IL-1β‡ (500 pg/ml), or a combination of IL-1β and RvD1.
2.2 |. Messenger RNA extraction, reverse transcription, and quantitative real-time polymerase chain reaction (qPCR)
Primary human PDLF were plated at an initial density of 1.6 × 105 cells/well in 6-well plates and allowed to grow to 80% confluence, starved overnight, and treated with the experimental conditions for 4h. RNA was extracted using a ribonucleic acid (RNA) isolation reagent protocol§; a total of 2.5 μg of messenger RNA (mRNA) of each sample were reverse-transcribed complementary deoxyribonucleic acid (cDNA) and amplified using High Capacity cDNA Reverse Transcription Kit.** Thermal cycler conditions were 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min, and the cycle ended by bringing the temperature down to 4°C. The cDNA was stored at −20°C when not in use for real-time qPCR. One microliter of cDNA of each sample was added to 19 μl of qPCR reaction mix in a 96-well qPCR plate. qPCR probes†† were used for the following targets: IL-6 (Hs00985639_m1), IL-8 (Hs00174103_m1), monocyte chemoattractant protein-1 (MCP-1) (Hs00234140_m1), cyclooxygenase-2 (COX-2) (Hs00153133_m1), MMP-1 (Hs00899658_m1), MMP-2 (Hs01548727_m1), MMP-3 (Hs00968305_m1), GPR32 (Hs01102536_s1), ALX/FPR2 (Hs02759175_s1), tissue inhibitor of metalloproteinase-1 (TIMP-1) (Hs00171558_m1), TIMP-2 (Hs00234278_m1), and osteoprotegerin (OPG) (Hs00900358_m1). Glyceraldehyde-3-phosphate dehydrogenase, GAPDH (Hs99999905_m1), was used as an endogenous control housekeeping gene. The qPCR reactions were run in a Real-Time PCR System.‡‡ All samples were run in technical duplicates and biological triplicates. Data were analyzed using the ΔΔCt method. The mRNA levels of all targets were normalized to the mRNA levels of GAPDH and the control group.
2.3 |. Immunofluorescence and confocal microscopy
Periodontal ligament fibroblasts cells were plated at an initial density of 4 × 104 cells/well in a 24-well plate, grown to 80% confluence, starved overnight, and treated with the experimental conditions for 24 h. Cells were fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature and blocked with 5% normal goat serum in phosphate-buffered saline (PBS) for 1 h. Cells were incubated overnight with rabbit primary antibodies against GPR32,§§ ALX/FPR2,*** or isotype in 5% goat serum in PBS. All wells were incubated with Alexa Fluor® (568)-conjugated goat anti-rabbit IgG secondary antibody††† in PBS for 2 h. Images were acquired using a fluorescent microscope equipped with a camera.‡‡‡
2.4 |. Fluorescence-activated cell sorting (FACS)
PDLF cells were seeded in 75-cm2 culture flasks (Corning; Tewksbury, MA, USA) at an initial density of 106 cells/flask and grown to 80% confluence, starved overnight, and treated with experimental conditions for 24 h. Cells were trypsinized and fixed with 4% PFA on ice for 30 min. About 4 × 106 cells were used for each experimental condition. Cells from each experimental condition were equally divided into four microcentrifuge tubes; two tubes from each condition were blocked for 1 h with antibody buffer (5% normal goat serum in PBS containing 0.03% sodium azide), stained with rabbit polyclonal anti-human GPR32 primary antibody or isotype in antibody buffer.§§§ Fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody was used.**** The other two tubes were incubated with phycoerythrin (PE)-conjugated mouse anti-human ALX/FPR2 monoclonal antibody or isotype†††† in FACS buffer (0.5% bovine serum albumin and 0.03% sodium ozonide (NaO3) in PBS). The fractions of cells expressing GPR32 and ALX/FPR2 proteins were assessed by measuring fluorescence on a cell sorter‡‡‡‡ using a computer software.§§§§
2.5 |. Scratch assay
Primary human PDLF cells were seeded in the different experimental conditions at 8 × 104 cells/well in 12-well plates and were allowed to grow to 80% confluence before they were starved overnight. A 2- to 3-mm–wide scratch was created by dragging a rubber policeman across a PDLF monolayer in a 12-well plate.17 Cells were then treated according to their assigned experimental condition for 9 days with media changes every 3 days. Two differential interference contrast (DIC) images for each well were acquired using an inverted phase microscope equipped with a camera***** under 2× objective (a total magnification of 20×) at baseline, 3 days, 6 days, and 9 days. An image analysis software††††† was used to calculate the scratch area covered by new migrating PDLF cells. To assess cell viability at all time points, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was used in the pilot phase of these experiments. Cell viability was at least 95% in all experimental conditions.
2.6 |. 5-Bromo-2′-deoxyuridine DNA synthesis analysis
To assess cell proliferation, a 5-Bromo-2′-deoxyuridine (BrdU) DNA synthesis assay‡‡‡‡‡ was used according to the manufacturer’s instructions. The optical density change in wells was determined at a wavelength of 450 nm in a spectrophotometer. The magnitude of the proliferative activity of PDLF cells in each well is directly proportional to the amount of color change in each well. Due to the limited number of cells and the limited amount of growth medium in each well rendered, image acquisition and quantification were not performed to avoid the potential risk of interrupting the growth of the PDLF cells and compromising the integrity of the assay results.
2.7 |. Western blot analyses
The intracellular protein levels of IL-6, IL-8, MCP-1, COX-2, and OPG were assessed by Western blot analyses. Cells were plated at an initial density of 1.6 × 105 cells/well in 6-well plates. When the cells became approximately 80% confluent, they were starved overnight and treated with the experimental conditions described above for 6 h. Samples containing 60 μg of protein were subjected to Sodium dodecyl-sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% milk Tris-buffered saline with 0.1% Tween 20 detergent (milk–TBST) for 1 h at room temperature, incubated with primary mouse anti-human IL-6, IL-8, and MCP-1 monoclonal antibodies§§§§§, and goat anti-human COX-2 polyclonal antibody,****** and mouse anti-human β-actin monoclonal antibody†††††† in 5% milk–TBST overnight at 4°C, washed with TBST, incubated with secondary horseradish peroxidase-conjugated rabbit anti-mouse‡‡‡‡‡‡ or rabbit anti-goat§§§§§§ antibodies, washed, and visualized with enhanced luminol-based chemiluminescent western blotting detection reagents.******* Stripping of membranes for 20 min at room temperature was performed with a stripping buffer††††††† before reincubating with various primary antibodies.
2.8 |. Multiplex immunoassay
PDLF cells were plated at an initial density of 4 × 104 cells/well in a 24-well plate, grown to 80% confluence, starved overnight, and treated with experimental conditions for 24 h. Supernatants were then collected and stored at −80°C until later use. The levels of cytokines and chemokines (IL-6, IL-8, and MCP-1), MMP-1, MMP2, MMP-3, TIMP-1, and TIMP-2 in the supernatants were determined using commercially available multiplex assay kits‡‡‡‡‡‡‡ according to the manufacturer’s instructions. The plates were read using a suspension array system§§§§§§§.
In separate experiments, PDLF cells were plated at an initial density of 1.6 × 105 cells/well in 6-well plates and starved overnight when approximately 80% confluent. Then, the cells were treated with the experimental conditions for 6 h. Total cell protein was then harvested using a commercial cell lysis buffer optimized for multiplex immunoassay kits with added protease inhibitor (1:100)******** and stored at −80°C until later use. Using commercially available kits, these lysates were used to measure intracellular protein concentrations of MMP-1, MMP-2, MMP-3, TIMP-1, and TIMP-2.
2.9 |. Enzyme-linked immunosorbent assay
Conditioned medium was collected as described above. The levels of PGE2†††††††† and OPG‡‡‡‡‡‡‡‡ in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA) using commercial kits per manufacturers’ instructions.
2.10 |. Statistical analysis
A two-tailed, unpaired Student t-test or analysis of variance with post hoc correction for multiple analyses (Bonferroni adjustment) was used for data analysis. The α-level was set at 0.05 for statistical significance. All values are expressed as mean ± standard error of the mean (SEM) (n ≥ 3) from three independent experiments.
3 |. RESULTS
3.1 |. PDLF express RvD1 receptors
To determine whether the impact of RvD1 on regeneration occurred through direct actions on PDLF, the expression of receptors for RvD1 was assessed. PDLF expressed the RvD1 receptors GPR32 and ALX/FPR2. The expression of the GPR32 (1.3- to 1.5-fold) and ALX (2- to 3-fold) was significantly upregulated with RvD1 treatment, IL-1β treatment, or both (p < 0.05) (Figure 1A,B). Immunofluorescence further confirmed that PDLF expressed GPR32 and ALX/FPR2 receptors (Figure 1C). FACS analysis was used to determine the expression and the fraction of PDLF expressing RvD1 surface receptor proteins. In the control group, 54.6 ± 1.1% of PDLF expressed ALX/FPR2 protein. The fraction of cells expressing the receptor was increased from 21.4% to 66.3% ± 1.2% in RvD1-treated cells, from 18.3% to 64.6% ± 1.1% in IL-1β–treated cells, and from 36.6% to 74.6% ± 1.3% when treated with IL-1β and RvD1 (Figure 1D). PDLF cells expressed GPR32 protein at lower levels. When compared with resting PDLF [Mean Fluorescence Intensity (MFI) = 371.3 ± 7.5], GPR32 expression by PDLF was upregulated when cells were treated with RvD1 (MFI = 499.7 ± 12.1), IL-1β (MFI = 510.8 ± 9.6), or both (MFI = 704.2 ± 23.8; Figure 1E).
FIGURE 1.
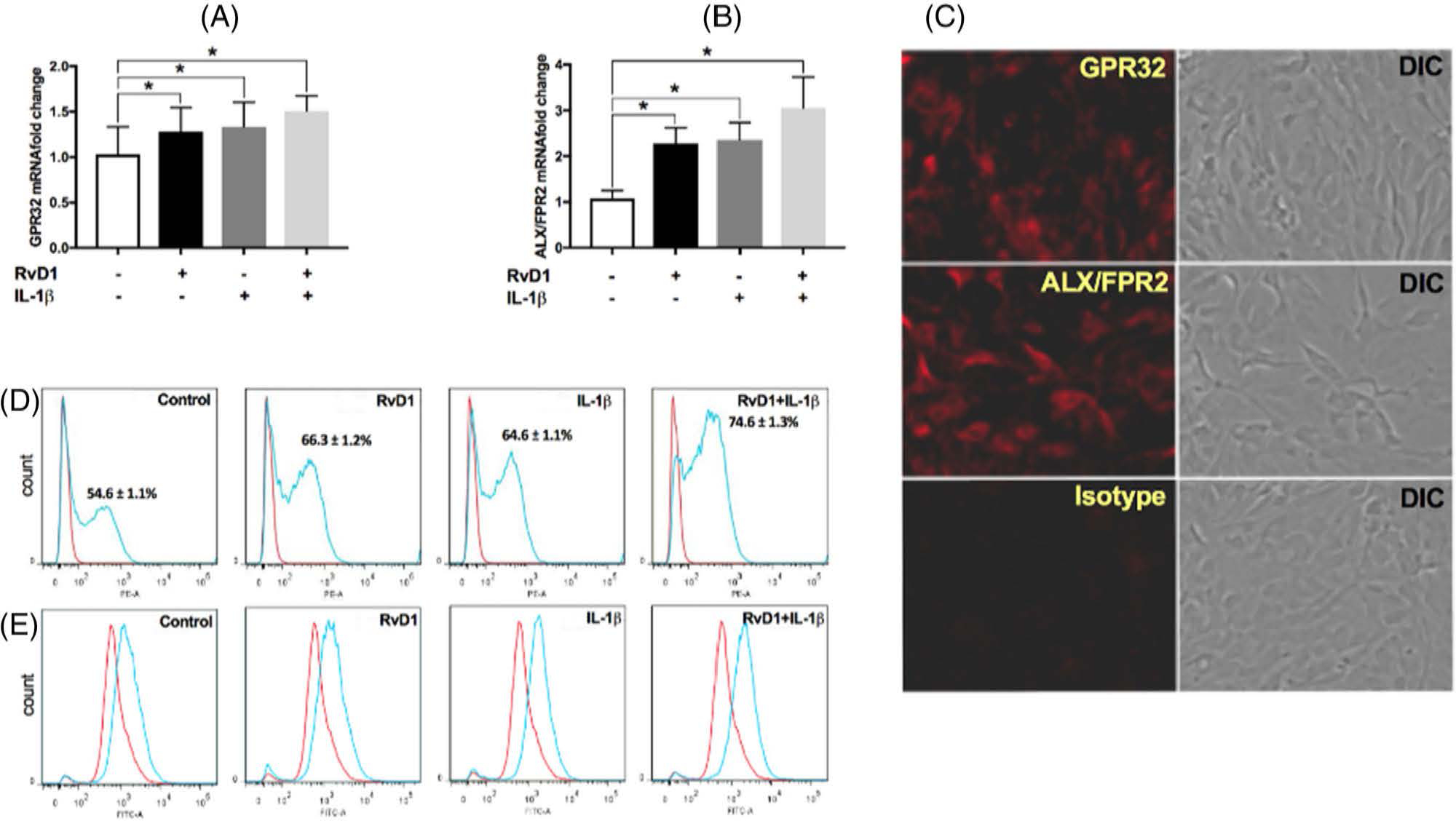
Resolvin D1 (RvD1) receptor expression. (A and B) Expression of RvD1 receptor genes (G-protein-coupled receptor 32 [GPR32] and formyl peptide receptor 2 [ALX/FPR2]) was assessed under the same assigned experimental conditions using real-time quantitative polymerase chain reaction. (C) Periodontal ligament fibroblasts (PDLFs) were fixed and stained with anti-human GPR32 (1:200) and anti-human ALX/FPR2 (1:200) antibodies followed by fluorochrome-conjugated secondary antibodies (1:250) and then assessed by immunofluorescence. (D) Receptor protein expression on the cell surface was assessed by staining with a PE-conjugated anti-human ALX/FPR2 (1:200) or isotype control (1:200, red line) using fluorescence-activated cell sorting (FACS) analysis. (E) PDLFs stained with an anti-human GPR32 primary antibody (1:200) or isotype control (1:200, red line) followed by a fluorescein isothiocyanate-conjugated secondary antibody (1:200) was assessed by FACS. Data are presented as mean ± SEM of three (A and C–G) or five (B) independent experiments. ALX/FPR2, formyl peptide receptor 2; DIC, differential interference contrast; GPR32, G-protein-coupled receptor 32; IL-1β, interleukin-1β; RvD1, resolving D1. *p < 0.05.
3.2 |. RvD1 promotes PDLF scratch closure and proliferation
To assess the actions of RvD1 on wound healing and proliferation, PDLF were treated with IL-1β to simulate inflammatory conditions and with 100 nM RvD1 to determine the impact on cell function. Differential interference contrast images of scratches were analyzed over time. RvD1 alone did not affect the scratch closure of unstimulated PDLF. IL-1β significantly inhibited scratch closure. The addition of RvD1 to IL-1β–treated PDLF resulted in the rescue of the PDLF scratch closure rate and scratch closure (p < 0.05) (Figure 2A,B). To investigate the impact of RvD1 on PDLF proliferation under the same conditions, BrdU incorporation was measured at 24 h. RvD1 alone did not change the proliferative activity of unstimulated PDLF, but IL-1β significantly inhibited PDLF proliferation that was rescued by RvD1 (p < 0.05) (Figure 2C).
FIGURE 2.
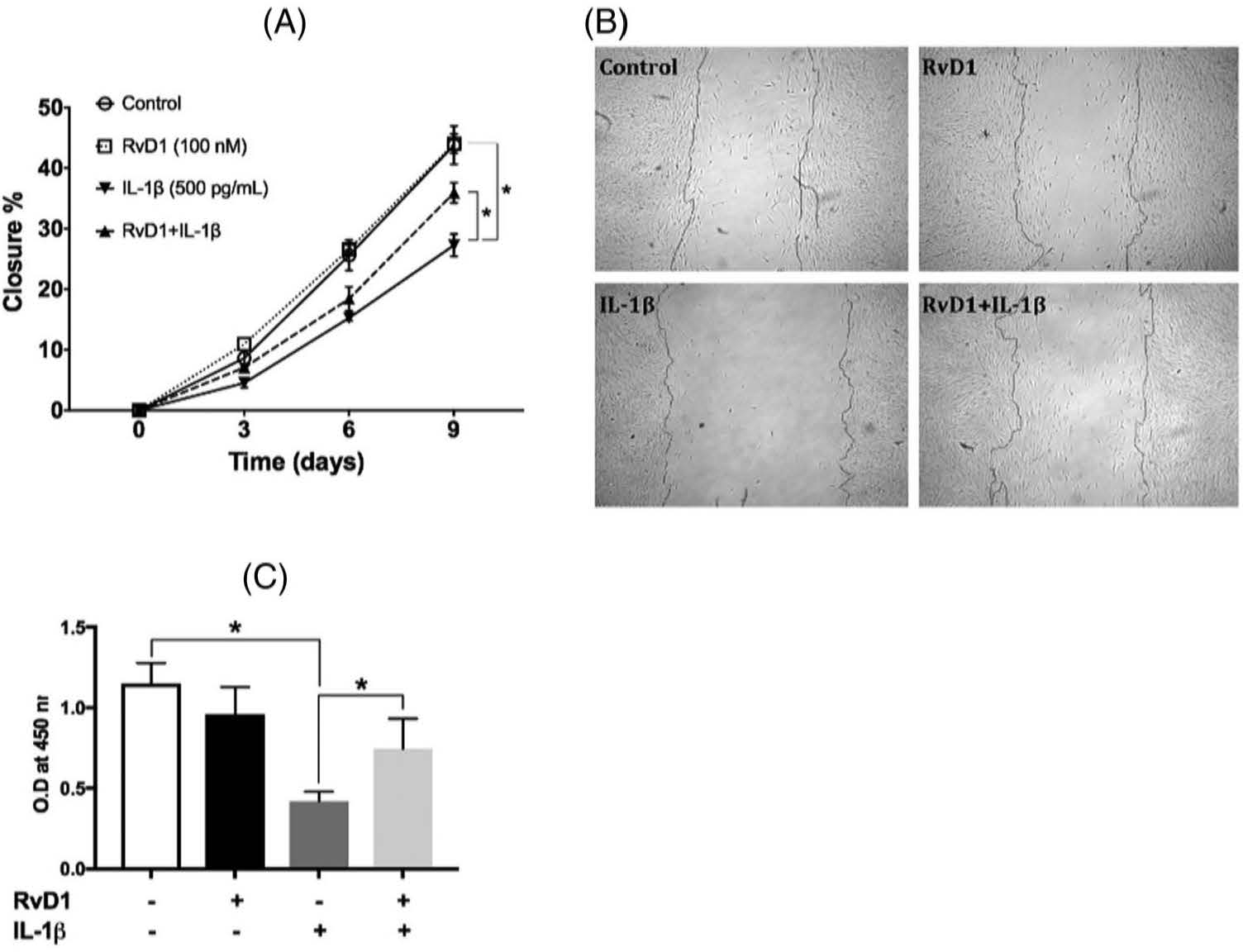
Scratch closure and proliferation of the periodontal ligament fibroblasts (PDLFs) in response to resolvin D1 (RvD1). (A) A monolayer of PDLFs scratched with a rubber policeman was treated with vehicle, RvD1 (100 nM), or interleukin-1β (500 pg/ml) or with RvD1 followed by interleukin-1β. Scratch closure was measured at days 0 (baseline), 3, 6, and 9. (B) Representative scratch images on day 9. (C) PDLFs were treated with the same conditions, and the proliferative response was measured using the BrdU assay at 24 h. IL-1β, interleukin-1β; O.D, optical density; RvD1, resolving D1. *difference is statistically significant, p < 0.05
3.3 |. RvD1 did not alter IL-1β–induced upregulation in the gene expression of inflammatory mediators and MMP
To investigate the impact of RvD1 on IL-6, IL-8, MCP-1, COX-2, and MMP-1, −2, and −3 gene expression, mRNA levels were assessed using real-time qPCR (Figure 3). COX-2 was studied to determine the intracellular downstream mechanisms through which PGE2 production might become affected. IL-1β induced a robust increase in the mRNA levels of IL-6 (216 ± 40-fold), IL-8 (236 ± 39-fold), MCP-1 (19 ± 1.3-fold), and COX-2 (42 ± 12-fold). IL-1β also induced a considerable upregulation in the expression of MMP-1 (7.0 ± 1.6-fold) and MMP-3 (27.0 ± 4.2-fold). The addition of RvD1 did not significantly change the IL-1β–induced increase in the expression of these genes (Figure 3A,E,G). The MMP-2 gene expression levels remained unchanged in all experimental conditions (Figure 3F). Gene expression levels of TIMP-1, TIMP-2, and OPG also did not significantly change (Figures 3H,J).
FIGURE 3.
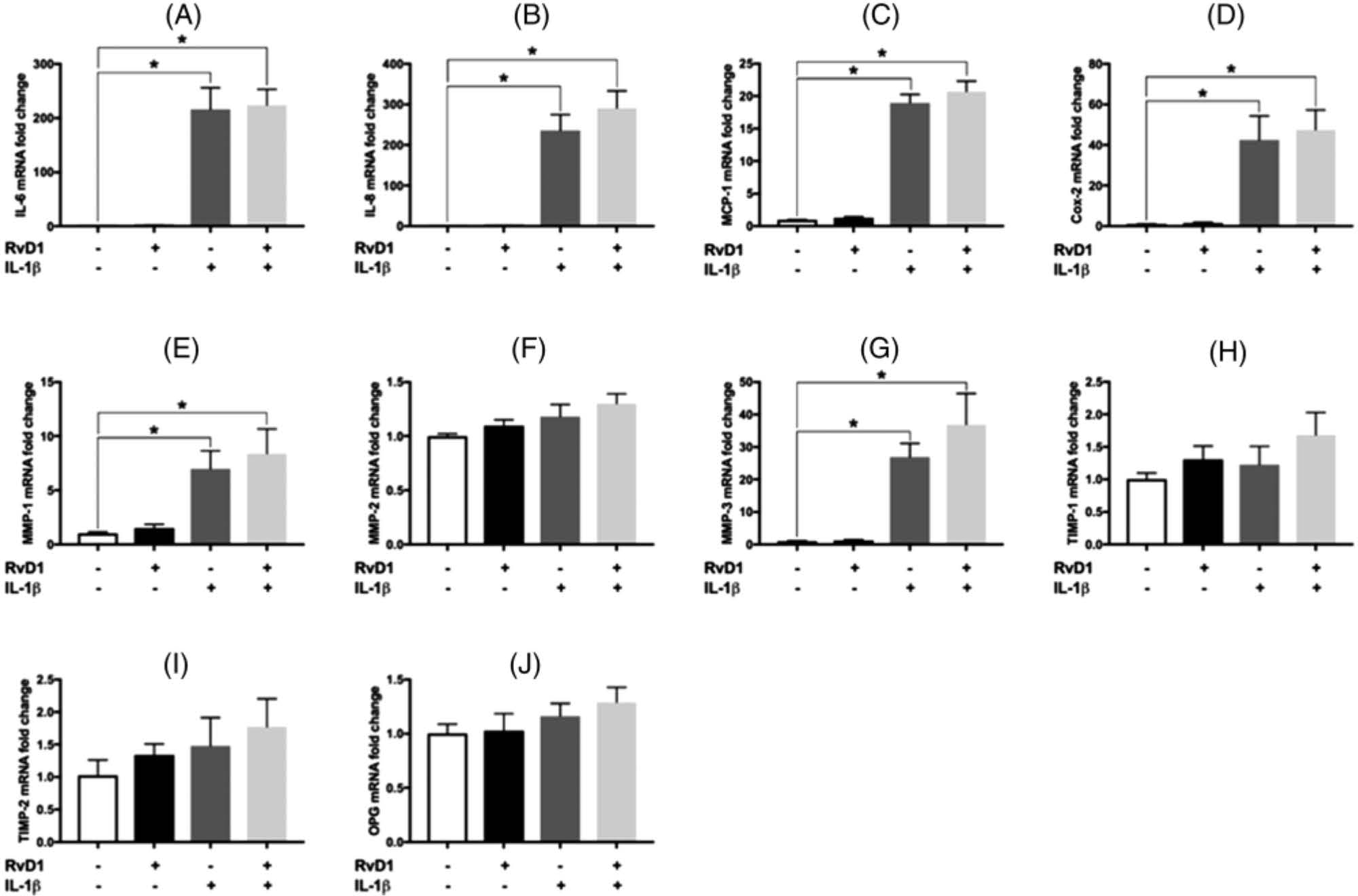
Gene expression levels of interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), cyclooxygenase-2 (COX-2), matrix metalloproteinase (MMP)-1, MMP-2, MMP-3, tissue inhibitors of metalloproteinase (TIMP)-1, TIMP-2, and osteoprotegerin (OPG) by periodontal ligament fibroblasts. Under the same assigned experimental conditions as in Figures 1 and 2, mRNA was harvested, and the expression levels of (A) IL-6, (B) IL-8, (C) MCP-1, (D) COX-2, (E) MMP-1, (F) MMP-2, (G) MMP-3, (H) TIMP-1, (I) TIMP-2, and (J) OPG genes were assessed using qPCR. Data are presented as mean ± SEM of three independent experiments. IL, interleukin; MCP, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; OPG, osteoprotegerin; RvD1, resolving D1; TIMP, tissue inhibitors of metalloproteinases. *p < 0.05.
3.4 |. RvD1 reduces IL-1β–induced increases in intracellular protein levels of IL-6, IL-8, and MCP-1 but not COX-2
The intracellular protein concentrations of IL-6, IL-8, MCP-1, and COX-2 produced by PDLF were investigated by Western blot analyses; intracellular protein concentrations of MMP-1, −2, and −3 were determined using multiplex immunoassay. IL-1β upregulated the intracellular protein levels of IL-6, IL-8, MCP-1, COX-2, MMP-1, and MMP-3 (Figure 4). RvD1 significantly reduced the IL-1β–induced increase in the protein levels of IL-6, IL-8, MCP-1, MMP-1, and MMP-3 (all p < 0.05) (Figure 4C,E,G,K). However, the IL-1β stimulated an increase in the intracellular protein levels of COX-2 and was not affected by RvD1 (Figure 4D,K). The intracellular protein levels of MMP-2 did not change in any experimental condition (Figure 4F). The intracellular levels of the TIMP-1 protein did not change(Figure4H);RvD1reversedtheIL-1β–induced inhibition of intracellular protein levels of TIMP-2 and OPG (Figure 4I,J,L).
FIGURE 4.
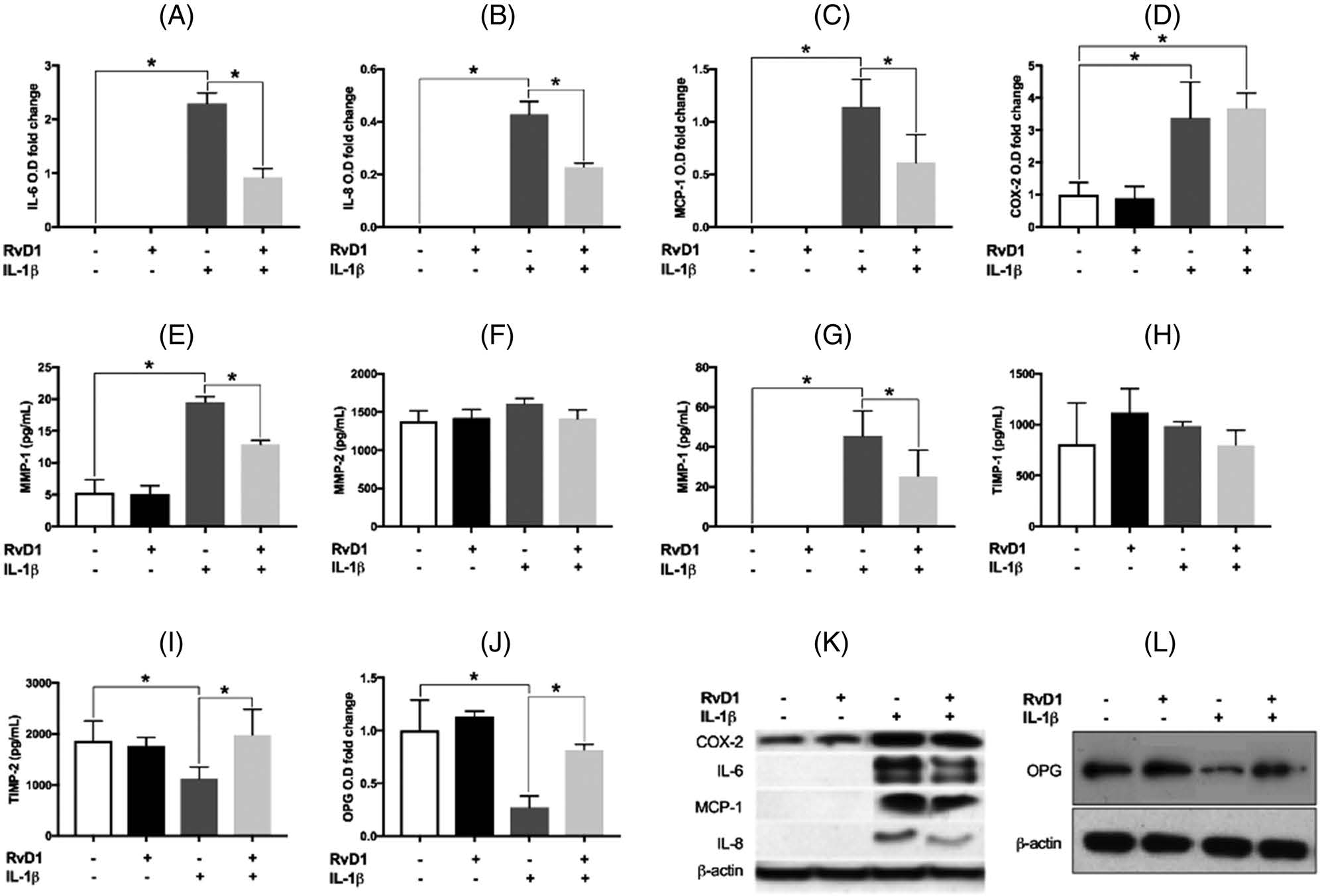
Intracellular protein levels of interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), cyclooxygenase-2 (COX-2), matrix metalloproteinase (MMP)-1, MMP-2, MMP-3, tissue inhibitors of metalloproteinase (TIMP)-1, TIMP-2, and osteoprotegerin (OPG) are produced by periodontal ligament fibroblasts. Under the same treatment conditions, cell lysates were harvested, and the intracellular protein levels of (A) IL-6, (B) IL-8, (C) MCP-1, (D) COX-2, and (J) OPG were assessed by Western blot. The intracellular protein levels of (E) MMP-1, (F) MMP-2, (G) MMP-3, (H) TIMP-1, and (I) TIMP-2 were determined using multiplex immunoassay. Representative images of the Western blot analyses of (K) IL-6, IL-8, MCP-1, and COX-2, and of (L) OPG. Data are presented as mean ± SEM of three independent experiments. COX-2, cyclooxygenase-2; IL, interleukin; MCP, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; O.D, optical density; OPG, osteoprotegerin; RvD1, resolving D1; TIMP, tissue inhibitors of metalloproteinases. *p < 0.05.
3.5 |. RvD1 reverses IL-1β–induced production of inflammatory mediators and MMP
To investigate the actions of RvD1 on the IL-1β–induced production of IL-6, IL-8, MCP-1, PGE2, and MMP-1, −2, and −3, PDLFs were treated as above for 24 h; and IL-6, IL-8, MCP-1, and MMP-1, −2, and −3 were measured in the supernatants using multiplex immunoassay (Figure 5). PGE2 production was measured using ELISA. Stimulation with IL-1β markedly increased the production of IL-6, IL-8, MCP-1, PGE2, MMP-1, and MMP-3. RvD1 significantly reduced the IL-1β–induced increase in the production of cytokines and MMPs (all p < 0.05) (Figures 5A–E,G). RvD1 reduced MMP-2 levels only with IL-1β, but the production of MMP-2 by PDLF was not affected by IL-1β or RvD1 alone (Figure 5F).
FIGURE 5.
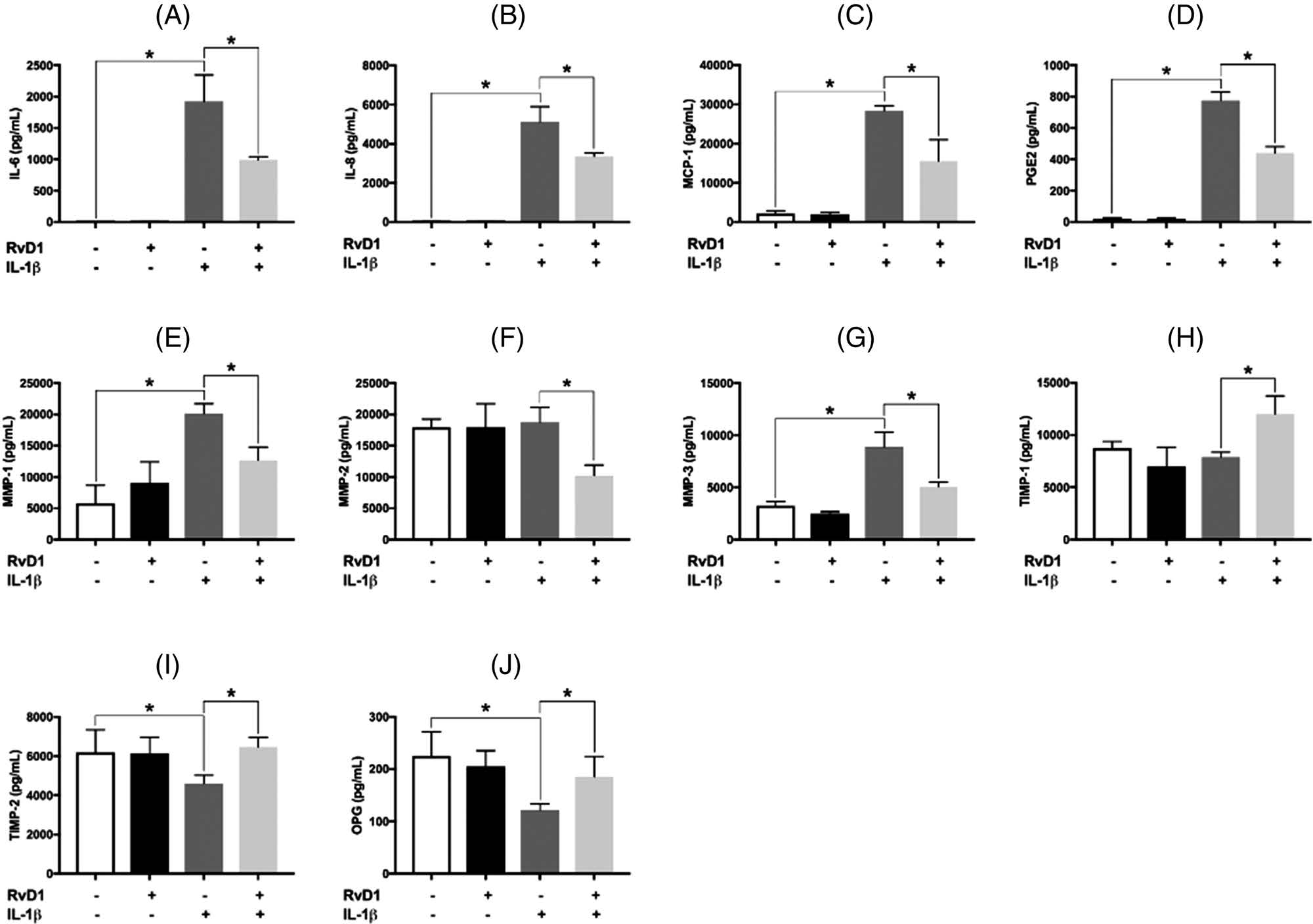
Production of interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), prostaglandin E2 (PGE2), matrix metalloproteinase (MMP)-1, MMP-2, MMP-3, tissue inhibitors of metalloproteinase (TIMP)-1, TIMP-2, and osteoprotegerin (OPG) by periodontal ligament fibroblasts. Under the same assigned experimental conditions as in Figure 1, the levels of (A) IL-6, (B) IL-8, (C) MCP-1, (E) MMP-1, (F) MMP-2, (G) MMP-3, (H) TIMP-1, and (I) TIMP-2 in supernatants were then determined using multiplex immunoassay. The levels of (D) PGE2 and (J) OPG were measured using ELISA. Data are presented as mean ± SEM of three independent experiments. IL, interleukin; MCP, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; O.D, optical density; OPG, osteoprotegerin; PGE2, prostaglandin E2; RvD1, resolving D1; TIMP, tissue inhibitors of metalloproteinases. *p < 0.05.
The levels of TIMP-1, TIMP-2, and OPG in the supernatants were determined by a multiplex immunoassay. As with MMP-2, IL-1β + RvD1 increased the levels of TIMP-1; IL-1β or RvD1 alone did not affect the levels of TIMP-1 (Figure 5H). TIMP-2 and OPG production decreased (about 20% and 50%, respectively) when PDLF was stimulated with IL-1β. RvD1 restored TIMP-2 and OPG production to levels similar to unstimulated controls (Figure 5I,J).
4 |. DISCUSSION
Tissue morphology and function can only be restored after injury when acute inflammation is followed by active resolution of inflammation.33,34 In addition to being the main source of the extracellular matrix of the periodontal ligament, PDLFs are active players in response to inflammation and immune regulation, producing significant levels of cytokines. Thus, in addition to inflammatory cells, stromal cells intensify the inflammatory response to infectious agents.6,35–37 PDLF are also key for periodontal regeneration. Clinical and laboratory studies have shown that inflammation interferes with wound healing and tissue regeneration.16,38,39 Importantly, in an inflammatory milieu, PDLF may contribute to the overall amplification and chronicity of inflammation. In this study, RvD1 restored wound healing and proliferation properties of PDLF while resolving the inflammatory phenotype of these cells. This regulation of the PDLF phenotype suggested that RvD1 may have a key role in wound healing and tissue regeneration and its known impact on inflammatory cells (neutrophils and macrophages). Thus, modulation of the PDLF phenotype from one associated with inflammation amplification to regeneration presents a potential target for the promotion of periodontal tissue regeneration.
RvD1 is known to bind to two receptors (GPR32 and ALX/FPR2).22 In previous work by our group, we reported the expression of these RvD1 receptors by murine fibroblasts.19 In the current study, we showed for the first time, to our knowledge, that human PDLF expressed GPR32 and ALX/FPR2. The expression of these receptors was upregulated in the presence of RvD1 and IL-1β, and the result of treatment with a combination of both was additive. The current study also suggested that the amount of IL-1β–induced proinflammatory mediators and MMP-1 and MMP-3 significantly decreased when cells were treated with RvD1. This finding agreed with previous work that showed RvD1 reversed IL-1β–induced and TNF-α–induced increases in the production of PGE2 by PDLF32 and with work on other fibroblasts. RvD1 inhibited the proinflammatory cytokine production by primary human lung fibroblasts stimulated with IL-1β and cigarette smoke extract.14 A recent study on the actions of resolvin E1 (RvE1) and RvD1 on rat cardiac fibroblasts stimulated with E. coli lipopolysaccharide (LPS) demonstrated that RvD1 (100 nM) prevented the LPS-induced increase in proinflammatory mediators such as IL-6, TNF-α, MCP-1, IL-10, and pro-IL-1β.15 These findings suggest a consistent fibroblast response of various phenotypes to inflammation and a consistent effect of RvD1 in modulating those responses.
N-formyl-methionine-leucyl-phenylalanine is known to have proinflammatory chemotactic effects on monocytes and neutrophils through ALX/FPR2 receptor binding.25–27 To our knowledge, no research has yet been conducted to study the potential actions of fMLP on PDLFs. Since the current study shows that PDLF expresses ALX/FPR2 receptor, it may be plausible to hypothesize that fMLP stimulates PDLF function through ALX/FPR2 receptor binding. However, more research will be needed to explore such a possibility.
Alveolar bone resorption associated with periodontal disease results from elevated RANKL/OPG ratios40 and is PGE2-dependent.16 In the current study, the observed inhibition of excessive IL-1β–induced production of PGE2 and MMPs and the production of TIMP-1, TIMP-2, and OPG suggested that RvD1 may have a protective role in the prevention of tissue destruction and bone loss associated with periodontal disease. These data may explain our previous in vivo findings,18,41 where resolvins prevented ligature-induced periodontal ligament destruction in rabbits and induced complete regeneration of soft and hard tissues lost to periodontitis.18 Our current data also suggested that RvD1 directly impacted structural periodontal cells, such as PDLF. This finding was supported by another study,42 which demonstrated direct proresolution actions of resolvins on osteoblasts. The findings from the current study suggest that PDLF osteogenicity may be enhanced by RvD1. However, further studies are needed to test this hypothesis.
In the current study, we also investigated the mechanisms underlying RvD1 actions. Although gene expression levels of IL-6, IL-8, MCP-1, COX-2, MMP-1, and MMP-3 were robustly upregulated by stimulation with IL-1β, treatment of PDLF with RvD1 did not significantly impact the upregulation of IL-1β–stimulated gene expression. On the other hand, RvD1 significantly reduced the IL-1β–induced increases in the intracellular protein levels of IL-6, IL-8, MCP-1, and MMP-1 and −3, suggesting a post-transcriptional targeting of RvD1 actions. The protein levels of COX-2 were not affected by RvD1; however, RvD1 may have reversed the enzymatic activity of COX-2 rather than altering its protein concentration. Further research is needed to investigate the potential effects of RvD1 on COX-2 enzymatic activity. The observed actions of RvD1 on the extracellular levels of MMP-2 may be due to an RvD1-stimulated increase in the degradation rates of this enzyme. Inhibition of TIMP-2 intracellular protein concentrations by IL-1β was reversed by RvD1, suggesting that RvD1 actions on TIMP-2 production occur at the post-transcriptional level. Finally, our data showed that RvD1 abrogated IL-1β–induced inhibition of OPG intracellular protein levels, suggesting that RvD1 actions on OPG production also occur at the post-transcriptional level. While the current study elucidated various mechanisms involved, future studies are needed to fully understand the mechanisms involved in RvD1 actions on PDLF cells.
5 |. CONCLUSIONS
Our data suggested that RvD1 has key anti-inflammatory and pro-regeneration roles regulating PDLF actions, thus promoting wound healing and regeneration of periodontal ligament tissues. Unraveling the pleiotropic actions of resolvins in the regulation of inflammation and downstream functions in wound healing and tissue regeneration will contribute to the development of novel treatment modalities for periodontal disease.
ACKNOWLEDGMENTS
The current study was supported by the National Institute of Dental and Craniofacial Research grants (grant nos.DE025020 and DE028353). The authors declare that they have no conflicts of interest to report.
Footnotes
Lonza, Bend, OR, catalog no. CC-7049.
Cayman Chemical, Ann Arbor, MI.
R&D Systems; Minneapolis, MN.
TRIzol, Ambion RNA, Grand Island, NY.
Thermofisher Scientific, Waltham, MA.
TaqMan; Thermofisher Scientific, Waltham, MA.
StepOne Plus; Thermofisher Scientific, Waltham, MA.
Sigma Aldrich Corp, St. Louis, MO, catalog no. SAB4501270.
Abcam, Cambridge, MA, catalog no. ab203129.
Invitrogen, Grand Island, NY, Catalog no. A-11011.
Zeiss Axio; Zeiss, Thornwood, NY
Sigma Aldrich Corp, St. Louis, MO, catalog no. SAB4501270.
Abcam, Cambridge, MA, catalog no. ab6717.
Santa Cruz Biotechnology Inc, Dallas, TX, catalog no. sc-57141.
BD FACSAria™ II; BD Biosciences, San Jose, CA.
FlowJo 7.6.4; Tree Star, Inc., Ashland, OR.
Olympus CK40; PixeLINK, Ottawa, ON, Canada.
ImageJ; NIH, Bethesda, MD
EMD Millipore Corporation, Billerica, MA.
Santa Cruz Biotechnology, Inc, Dallas, TX, catalog nos. sc-130326, sc-8427, sc-32771, respectively.
Abcam, Cambridge, MA, catalog no. ab23672.
Cell Signaling Technology Inc, Danvers, MA, catalog no. 3700.
Cell Signaling Technology Inc, Danvers, MA, catalog no. 7606.
Santa Cruz Biotechnology, Inc, Dallas, TX, catalog nos. sc-2354.
Amersham Biosciences, Amersham, UK.
Thermofisher Scientific, Waltham, MA.
EMD Millipore Corporation, Billerica, MA.
Bio-Plex 200; Bio-Rad Laboratories, Inc., Hercules, CA.
EMD Millipore Corporation, Billerica, MA.
Neogen, Lansing, MI.
eBioscience, San Diego, CA.
REFERENCES
- 1.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000;64:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwell H Position paper: guidelines for periodontal therapy. Committee Res Sci. 2001;72:1624–1628. [DOI] [PubMed] [Google Scholar]
- 3.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontol. 2000;51:208–219. [DOI] [PubMed] [Google Scholar]
- 4.Van Dyke TE, Hasturk H, Kantarci A, et al. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. 2015;94:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards D, Rutherford RB. The effects of interleukin 1 on collagenolytic activity and prostaglandin-E secretion by human periodontal-ligament and gingival fibroblast. Arch Oral Biol. 1988;33:237–243. [DOI] [PubMed] [Google Scholar]
- 6.Saito S, Ngan P, Saito M, Lanese R, Shanfeld J, Davidovitch Z. Interactive effects between cytokines on PGE production by human periodontal ligament fibroblasts in vitro. J Dent Res. 1990;69:1456–1462. [DOI] [PubMed] [Google Scholar]
- 7.Fracon RN, Teofilo JM, Satin RB, Lamano T. Prostaglandins and bone: potential risks and benefits related to the use of nonsteroidal anti-inflammatory drugs in clinical dentistry. J Oral Sci. 2008;50:247–252. [DOI] [PubMed] [Google Scholar]
- 8.Hou LT, Liu CM, Liu BY, Lin SJ, Liao CS, Rossomando EF. Interleukin-1beta, clinical parameters and matched cellular-histopathologic changes of biopsied gingival tissue from periodontitis patients. J Periodontal Res. 2003;38:247–254. [DOI] [PubMed] [Google Scholar]
- 9.Salvi GE, Brown CE, Fujihashi K, et al. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J Periodontal Res. 1998;33:212–225. [DOI] [PubMed] [Google Scholar]
- 10.Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86:306–319. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal R, Ghobrial IM, Roodman GD. Chemokines in multiple myeloma. Exp Hematol. 2006;34:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161. [DOI] [PubMed] [Google Scholar]
- 13.Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30:23–33. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao HM, Sapinoro RE, Thatcher TH, et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS ONE. 2013;8:e58258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salas-Hernandez A, Espinoza-Perez C, Vivar R, et al. ResolvinD1 and E1 promote resolution of inflammation in rat cardiac fibroblast in vitro. Mol Biol Rep. 2021;48:57–66. [DOI] [PubMed] [Google Scholar]
- 16.Kumar V, Abbas AK, Aster JC. Robbins and Cotran pathologic basis of disease. xvi. [Google Scholar]
- 17.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasturk H, Kantarci A, Goguet-Surmenian E, et al. ResolvinE1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. [DOI] [PubMed] [Google Scholar]
- 19.Herrera BS, Kantarci A, Zarrough A, Hasturk H, Leung KP, VanDyke TE. LXA4 actions direct fibroblast function and wound closure. Biochem Biophys Res Commun. 2015;464:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng T, Ding S, Liu S, Li X, Tang X, Sun L. Resolvin D1 improves the Treg/Th17 imbalance in systemic lupus erythematosus through miR-30e-5p. Front Immunol. 2021;12:668760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnamoorthy S, Recchiuti A, Chiang N, et al. ResolvinD1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnardottir H, Thul S, Pawelzik SC, et al. The resolvin D1 receptor GPR32 transduces inflammation resolution and atheroprotection. J Clin Invest. 2021:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belisle B, Abo A. N-Formyl peptide receptor ligation induces rac-dependent actin reorganization through Gbeta gamma subunits and class Ia phosphoinositide 3-kinases. J Biol Chem. 2000;275:26225–26232. [DOI] [PubMed] [Google Scholar]
- 25.Gao JL, Murphy PM. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem. 1993;268:25395–25401. [PubMed] [Google Scholar]
- 26.Mills JS, Miettinen HM, Cummings D, Jesaitis AJ. Characterization of the binding site on the formyl peptide receptor using three receptor mutants and analogs of Met-Leu-Phe and Met-Met-Trp-Leu-Leu. J Biol Chem. 2000;275:39012–39017. [DOI] [PubMed] [Google Scholar]
- 27.Shen W, Li B, Wetzel MA, et al. Down-regulation of the chemokine receptor CCR5 by activation of chemotactic formyl peptide receptor in human monocytes. Blood. 2000;96:2887–2894. [PubMed] [Google Scholar]
- 28.Perretti M, Getting SJ, Solito E, Murphy PM, Gao JL. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am J Pathol. 2001;158:1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maderna P, Godson C. Taking insult from injury: lipoxins and lipoxin receptor agonists and phagocytosis of apoptotic cells. Prostaglandins Leukot Essent Fatty Acids. 2005;73:179–187. [DOI] [PubMed] [Google Scholar]
- 30.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaled M, Shibani NA, Labban N, et al. Effects of resolvinD1 on cell survival and cytokine expression of human gingival fibroblasts. J Periodontol. 2013;84:1838–1846. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa M, Zarrough A, Bolstad AI, et al. Resolvin D1 protects periodontal ligament. Am J Physiol Cell Physiol. 2013;305:C673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. [DOI] [PubMed] [Google Scholar]
- 34.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. [DOI] [PubMed] [Google Scholar]
- 35.Fukushima H, Jimi E, Okamoto F, Motokawa W, Okabe K. IL-1-induced receptor activator of NF-kappa B ligand in human periodontal ligament cells involves ERK-dependent PGE2 production. Bone. 2005;36:267–275. [DOI] [PubMed] [Google Scholar]
- 36.Nokhbehsaim M, Deschner B, Winter J, et al. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1beta in vitro. Clin Oral Investig. 2012;16:275–283. [DOI] [PubMed] [Google Scholar]
- 37.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 38.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCulloch CA. Basic considerations in periodontal wound healing to achieve regeneration. Periodontol 2000. 1993;1:16–25. [PubMed] [Google Scholar]
- 40.Garlet GP, Cardoso CR, Silva TA, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21:12–20. [DOI] [PubMed] [Google Scholar]
- 41.Hasturk H, Kantarci A, Ohira T, et al. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. [DOI] [PubMed] [Google Scholar]
- 42.Gao L, Faibish D, Fredman G, et al. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol. 2013;190:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]


