Abstract
Aims
Wolff–Parkinson–White (WPW) syndrome is a conduction disorder characterized by an accessory electrical pathway between the atria and ventricles, which may predispose to supraventricular tachycardia (SVT) and sudden cardiac death. It can be seen as an isolated finding or associated with structural heart disease. Our aims were to determine the prevalence of a WPW pattern in a large and unselected cohort of neonates and to describe the electro- and echocardiographic characteristics as well as the natural history during early childhood.
Methods and results
Electrocardiograms and echocardiograms of neonates (aged 0–30 days) from a large, prospective, population-based cohort study were included. Neonates with a WPW pattern were identified and matched 1:4 to controls. Localization of the accessory pathway was assessed by different algorithms. Among 17 489 neonates, we identified 17 (76% boys) with a WPW pattern consistent with a prevalence of 0.1%. One neonate had moderate mitral regurgitation while other echocardiographic parameters were similar between cases and controls (all P > 0.05). The accessory pathways were primarily predicted to be left-sided. At follow-up (available in 14/17 children; mean age 3.2 years) the pre-excitation pattern persisted in only four of the children and none of the children had experienced any episodes of SVT.
Conclusion
The prevalence of a WPW pattern in our cohort of unselected neonates was 0.1%. The WPW pattern was more frequent in boys and generally not associated with structural heart disease, and the accessory pathways were primarily left-sided. At follow-up, the WPW pattern had disappeared in most of the children suggesting either an intermittent nature or that normalization occurs.
Clinical Trial Registration
Copenhagen Baby Heart, NCT02753348.
Keywords: Wolff–Parkinson–White, Electrocardiography, Echocardiography, Newborns, Supraventricular tachycardia
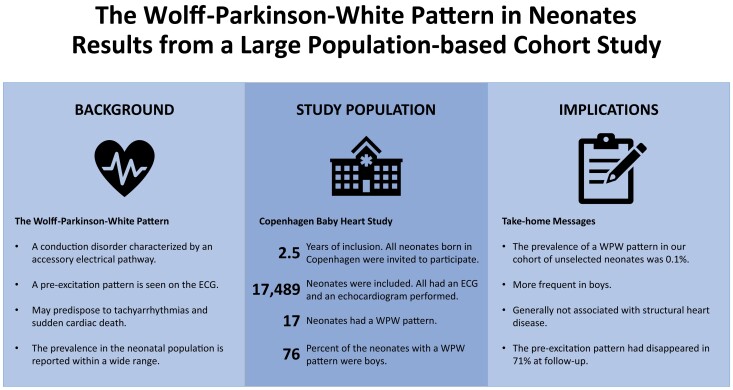
Graphical Abstract
Graphical Abstract.
What’s new?
The prevalence of a WPW pattern was 0.1% in a large and unselected cohort of neonates.
The WPW pattern was more frequent in boys and generally not associated with structural heart disease.
At follow-up investigations, the WPW pattern had disappeared in most of the children, suggesting either an intermittent nature of the ventricular pre-excitation or that normalization occurs.
Introduction
Wolff–Parkinson–White (WPW) syndrome is a cardiac conduction system disorder characterized by an accessory electrical pathway between the atria and ventricles. The condition was first reported in 1915, followed by a publication by Wolff et al. in 1930.1,2 Electrical conduction through the accessory pathway causes early ventricular activation and results in typical electrocardiographic (ECG) features such as a short PR interval, initial slurring of the QRS complex, known as a delta-wave, and QRS prolongation. The clinical implications range from no cardiac symptoms to recurrent paroxysmal supraventricular tachycardia (SVT), syncope, and sudden cardiac death.3–6 The ECG pattern of ventricular pre-excitation in the absence of any clinical cardiovascular symptoms is referred to as a WPW pattern.7,8 In previous studies involving both children and adults, the prevalence of a WPW pattern has been reported within a wide range from 0.03% to 0.5%.3,5,7,9–18 Estimating the prevalence of a WPW pattern in the general population is difficult, highly dependent on the applied methodology, and challenged by the fact that individuals with a WPW pattern often are asymptomatic. Furthermore, the pre-excitation pattern may be less pronounced, intermittent, or concealed.7 In particular, in the youngest age group, the diagnosis is often made as an incidental finding, or only in neonates with severe symptoms.3 For most patients with a WPW pattern, the accessory pathway exists as an isolated electrophysiological abnormality, but has in 20–37% of neonatal cohorts been associated with congenital cardiac defects, most frequently Ebstein’s anomaly, but also congenitally corrected transposition of the great arteries, ventricular septal defects, and hypertrophic cardiomyopathy.3,4,9,18–20 However, most of the studies investigating the prevalence of a WPW pattern, or the association with structural heart diseases, were based on cohorts of symptomatic and/or hospitalized individuals potentially introducing a degree of referral bias, with the possibility of an underestimation of the prevalence of a WPW pattern and an overestimation of the associated risk of structural heart disease.
The aims of this study were to determine the prevalence of a WPW pattern in a large and unselected cohort of neonates, to describe electrocardiographic and echocardiographic characteristics in these neonates as well as findings at follow-up in early childhood.
Methods
The Copenhagen Baby Heart Study
The Copenhagen Baby Heart Study is a multicentre, prospective, population-based cohort study. All neonates from April 2016 to October 2018 born in the three largest maternity wards in the Copenhagen area—Rigshospitalet, Herlev Hospital, and Hvidovre Hospital—were offered inclusion in the study.21,22 The three hospitals are public, provide healthcare free of charge, and serve a diverse population with mixed socioeconomic background. As part of the study, electrocardiography, echocardiography, post-ductal pulse oximetry, and blood sampling were performed during the first month of life. Weight, height, and gestational age were registered, and the body size area was calculated according to the formula by Haycock et al.23
The study followed the Helsinki declaration and was approved by the Regional Ethics Committee (H-16001518) and the Danish Data Protection Agency (I-suite 04546, HGH-2016-53). Written consent was obtained from parents.
Electrocardiograms
Electrocardiograms were recorded with a MAC 5500 HD system (GE ECG System, Milwaukee, USA) with paper speed of 25 mm/sec, sensitivity at 10 mm/mV, sample rate of 500 Hz, and bandwidth filter of 0.16–150 Hz. We recorded lead I, II, III, aVR, aVL, aVF, V1, and in most cases V6. The ECGs were recorded while the neonate was calm or sleeping. All tracings were acquired digitally, and ECG intervals, amplitudes, axes, etc. were automatically analysed using GE’s Marquette 12SL ECG Analysis Program and stored in a widely available ECG management system (MUSE, Version 8, GE Healthcare, Milwaukee, USA).
To detect the neonates with a WPW pattern, we manually reviewed all the ECGs with any one of the following ECG characteristics: PR interval <85 ms (or undetermined PR interval), QRS duration >62 ms, or an QRS axis between 0 and -90°. Using this approach, the 2000 ECGs with the lowest PR interval and the longest QRS duration, respectively, and 5166 ECGs in total were identified and manually reviewed. All the ECGs suspected of having a WPW pattern were reviewed by three medical doctors experienced in paediatric ECGs (MMP, AP, and AHC) to confirm or reject the presence of a WPW pattern. Neonates with a WPW pattern were systematically offered repeated cardiac evaluation, including ECG and echocardiography. Identified cases were matched 1:4 with controls based on sex, age, gestational age, and weight at examination. Controls had normal ECGs and echocardiograms. Medical records of all the neonates with a neonatal WPW pattern were reviewed, and during the follow-up examination, the parents were asked whether their child had experienced any cardiac symptoms. No neonates were excluded from the study due to either arrhythmias or the presence of structural heart disease.
To evaluate the location of the accessory pathway, we applied nine different published algorithms—devised from both paediatric and adult cohorts—on our cohort of neonates with a WPW pattern.24–32 The algorithms are based on 12-lead ECGs and use QRS polarity, polarity of the delta-wave, or R/S amplitude ratios to characterize the location of the accessory pathway and to distinguish between four and nine different anatomical regions. A predicted location was defined as left-sided if all, or the majority of the used algorithms, had predicted the accessory pathway as left-sided. The localization algorithms were re-applied on the follow-up ECGs in cases with a persisting pre-excitation pattern.
Echocardiograms
Transthoracic echocardiograms (TTE) were obtained with Vivid E9 ultrasound equipment (GE Healthcare, Horten, Norway) and performed by physicians or sonographers trained in paediatric echocardiography. Standard subxiphoid, apical, left parasternal, and suprasternal views were acquired with 12 and 6 MHz cardiac sector transducers. Measurements were obtained in accordance with the American Society of Echocardiography’s guidelines for paediatric echocardiography.33 All raw data (cine loops and measurements) were acquired using EchoPac software (GE Healthcare, Horten, Norway). Congenital heart disease was defined as any TTE-detected structural and/or functional abnormality requiring clinical follow-up, that is, interatrial communications, persistent ductus arteriosus, and trivial valve regurgitation were not considered abnormal. Echocardiographic measurements investigated in the present study were left ventricular internal diameters in end-systole and end-diastole [left ventricular internal diameter end-systole (LVIDs) and left ventricular internal diameter end-diastole (LVIDd)] and interventricular septum and posterior wall thicknesses in end-diastole [interventricular septal diameter (IVSD) and left ventricular posterior wall diameter end-diastole (LVPWd)], all measured in the 2D parasternal long axis (PLAX) view. Left atrial (LA) diameter was measured in PLAX by M-mode across the aortic root in end-systole. Fractional shortening (FS) was determined as (LVIDd−LVIDs)/LVIDd and left ventricular ejection fraction (LVEF) as [end-diastolic volume (EDV)−end-systolic volume]/EDV, all based on Teichholz et al.’s formulae.34 Peak flow velocities during early diastole (MV E velocity) and atrial contraction (MV A velocity) as well as main pulmonary artery (MPA) flow velocity were measured according to guidelines.35
Statistical analysis
Baseline characteristics and ECG parameters for the neonates are presented as median values and inter-quartile ranges (IQR), or absolute numbers with percentages. Comparisons between the neonates with a WPW pattern and the matched controls were performed with the Wilcoxon rank sum test for continuous variables and χ2 or Fisher’s exact tests for binary/categorical variables. Box plots were used to graphically depict the electrocardiographic parameters for the neonates with a WPW pattern vs. the matched controls. R statistical software v. 4.1.0 (Boston, MA, USA) was used for statistical analysis. A P-value <0.05 was considered significant.
Results
Study population
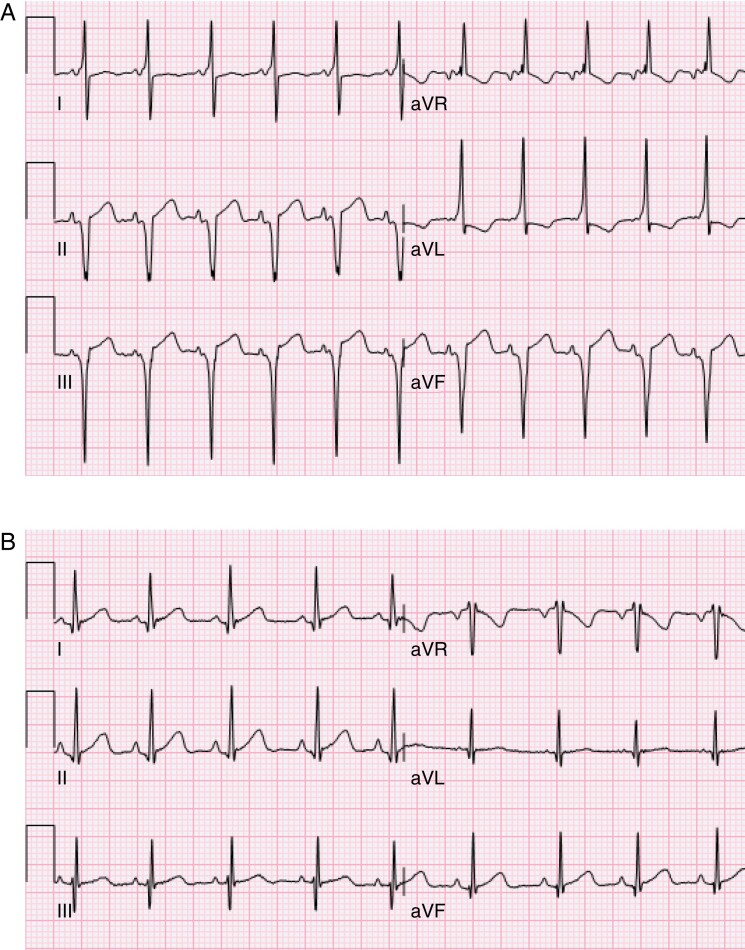
The overall study population consisted of 17 489 neonates (52% boys). Of these, 5166 ECGs were manually assessed, and there was consensus of a WPW pattern in 17 of the neonates (ECG example shown in Figure 1). The prevalence of a WPW pattern in our cohort of unselected neonates was therefore 0.1% with a predominance of boys (n = 13, 76%). None of the children had experienced any episodes of SVT during the follow-up period (median age 3.2 years at follow-up). Baseline characteristics for the study population were divided into three groups: the overall study population, neonates with a WPW pattern, and matched controls (Table 1). Median values for age, gestational age, weight, height, and body surface area were similar in all three groups (all P > 0.05). The mean gestational age for the neonates with a WPW pattern was 279 days (range 252–295 days), with one neonate being born pre-term at a gestational age of 36 + 0 weeks. The maternal ethnic composition for the cohort was 92.3% Caucasian, 3.4% Asian, 1.5% Middle Eastern, 1% Black, and 1.8% other/mixed (data available in 15 713 mothers).
Figure 1.
(A) Electrocardiogram from a 10-day-old boy showing a short PR interval, distinct delta-waves, QRS prolongation, and a QRS axis of −82°. (B) Electrocardiogram from the same boy at follow-up investigation at the age of 2 years without a pre-excitation pattern.
Table 1.
Baseline characteristics
| All (n = 17 489) | WPW (n = 17) | Matched controls (n = 68) | P-valuea | P-valueb | |
|---|---|---|---|---|---|
| Sex, male | 9034 (52%) | 13 (76%) | 52 (76%) | NS | 0.05 |
| Age, days | 11 (7–14) | 10 (7–15) | 11 (7–14) | NS | NS |
| Gestational age, days | 281 (274–287) | 281 (270–288) | 278 (271–284) | NS | NS |
| Weight, kg | 3.6 (3.3–4.0) | 3.5 (3.4–3.9) | 3.7 (3.2–4.0) | NS | NS |
| Height, cm | 52 (51–54) | 53 (52–54) | 52 (51–54) | NS | NS |
| Body surface area, cm2 | 2332 (2191–2482) | 2297 (2242–2434) | 2348 (2172–2504) | NS | NS |
Categorical variables are displayed as absolute numbers (percentages) and continuous variables as medians (inter-quartile ranges). Significant values are marked with bold.
NS, not significant; WPW, Wolff–Parkinson–White.
Comparison between the neonates with a WPW pattern and the matched controls.
Comparison between the neonates with a WPW pattern and the overall study population.
Electrocardiographic findings
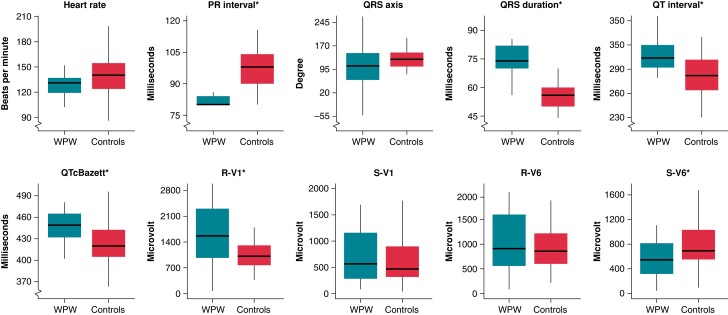
Comparing neonates with a WPW pattern with matched controls (Figure 2 and Table 2), we found a significant shorter PR interval (80 vs. 98 ms), longer QRS duration (74 vs. 56 ms), QT interval (304 vs. 282 ms), and QTcBazett interval (449 vs. 420 ms), likely reflecting the delta-wave duration, as well as a higher maximum amplitude in R-V1 (R-V1; 1562 vs. 1010 µV), and a lower maximum amplitude in S-V6 (S-V6; 546 vs. 693 µV) in neonates with a WPW pattern (all P < 0.05). There were no significant differences in heart rate, QRS axis, maximum amplitude in S-V1 (S-V1), and maximum amplitude in R-V6 (R-V6) between neonates with a WPW pattern and matched controls (all P > 0.05). If defining the normal QRS axis as within the interval 77–204°, we found that six (38%) of the neonates with a WPW pattern and none (0%) of the matched controls had an abnormal QRS axis (P < 0.00001).36 Based on the QRS axis, we found that the distribution was 56% vs. 90% for right axis deviation (+91 to +180°, P < 0.01), 13% vs. 4% for extreme axis deviation (+181 to +270°, P > 0.05), 25% vs. 0% for left axis deviation (0 to −90°, P < 0.01), and 6% vs. 6% for ‘normal axis’ (+1 to +90°, P > 0.05) for neonates with a WPW pattern and matched controls, respectively.
Figure 2.
Box plots of ECG parameters of neonates with a WPW pattern and matched controls. Electrocardiographic parameters with significant differences between the groups are marked with *. The horizontal line in the middle is the median value, the box depicts the inter-quartile range (IQR), and the whiskers 1.5 times the IQR. WPW, Wolff–Parkinson–White.
Table 2.
Electrocardiographic and echocardiographic characteristics of neonates with a WPW pattern and matched controls
| WPW (n = 17) | Matched controls (n = 68) | P-value | |
|---|---|---|---|
| Heart rate, bpm | 131 (119–137) | 140 (124–154) | NS |
| PR interval, ms | 80 (80–84) | 98 (90–104) | <0.001 |
| QRS axis, degrees | 106 (61–147) | 127 (104–148) | NS |
| QRS duration, ms | 74 (70–82) | 56 (50–60) | <0.00001 |
| QT interval, ms | 304 (292–320) | 282 (264–302) | <0.00001 |
| QTcBazett, ms | 449 (432–465) | 420 (405–442) | <0.00001 |
| Max amplitude R-V1, µV | 1562 (971–2304) | 1010 (765–1307) | <0.05 |
| Max amplitude S-V1, µV | 566 (280–1159) | 468 (314–898) | NS |
| Max amplitude R-V6, µV | 932 (571–1640) | 878 (615–1251) | NS |
| Max amplitude S-V6, µV | 546 (317–815) | 693 (551–1032) | <0.05 |
| Fractional shortening, % | 29.2 (27.9–32.4) | 31.1 (29.1–34.0) | NS |
| Ejection fraction, % | 59.0 (57.0–63.8) | 61.8 (58.9–65.6) | NS |
| IVSD, mm | 2.5 (2.3–2.7) | 2.3 (2.1–2.7) | NS |
| LVPWd, mm | 2.0 (1.9–2.3) | 2.0 (1.8–2.4) | NS |
| LVIDd, mm | 20.5 (19.1–20.9) | 20.1 (18.7–21.5) | NS |
| LVIDs, mm | 14.0 (13.3–14.8) | 13.8 (12.9–14.7) | NS |
| LA diameter, cm | 11.3 (10.0–12.2) | 12.1 (10.9–13.3) | NS |
| MV A velocity, m/s | 0.50 (0.47–0.59) | 0.55 (0.48–0.64) | NS |
| MV E velocity, m/s | 0.56 (0.50–0.62) | 0.60 (0.51–0.69) | NS |
| MPA flow velocity, m/s | 0.81 (0.75–0.89) | 0.89 (0.79–0.96) | NS |
Data displayed as medians (inter-quartile ranges). Significant values are marked with bold.
bpm, beats per minute; IVSD, interventricular septal diameter; LA, left atrium; LVIDd, left ventricular internal diameter end-diastole; LVIDs, left ventricular internal diameter end-systole; LVPWd, left ventricular posterior wall diameter end-diastole; MPA, main pulmonary artery; MV A, trans-mitral atrial peak inflow velocity; MV E, trans-mitral early peak inflow velocity; NS, not significant; WPW, Wolff–Parkinson–White.
We found a predominance of accessory pathways with a predicted location in the left side of the heart (n = 10–17; 59–100%, depending on the algorithm applied; Table 3). All the 13 boys (100%) and three of the four girls (75%) had a predicted left-sided accessory pathway. The algorithms devised on paediatric cohorts predicted that 14–17 of the neonates (82–100%) had a left-sided accessory pathway, whereas the adult algorithms predicted that 10–17 (59–100%) of the neonates had a left-sided accessory pathway.
Table 3.
Comparison of different algorithms to predict the location of the accessory pathways in our cohort of neonates with a WPW pattern
| Characteristics of the algorithms | Results when applied on our cohort | ||||
|---|---|---|---|---|---|
| Mean age | Number of locations | Left-sided | Right-sided | Postero-/mid-septal | |
| Taguchi et al.24 | 48 ± 18 years (unavailable) | 5 | 17 (100%) | 0 (0%) | 0 (0%) |
| LA/LL: 11 | |||||
| LPL/LP: 6 | |||||
| Arruda et al.25 | 32 ± 15 years (9–78 years) | 9 | 15 (88%) | 2 (12%) | 0 (0%) |
| LL/LAL: 13 | RA/RAL: 1 | ||||
| LP/LPL: 2 | RL: 1 | ||||
| Boersma et al.26 | 13 ± 4 years (0–18 years) | 6 | 16 (94%) | 1 (6%) | 0 (0%) |
| LL: 11 | RPS: 1 | ||||
| LPS: 5 | |||||
| Li et al.27 | 13.6 ± 3.4 years (3–18 years) | 4 | 14 (82%) | 3 (18%) | 0 (0%) |
| LFW: 14 | RF: 2 | ||||
| RS: 1 | |||||
| Iturralde et al.28 | 31.6 ± 15.5 years (4 months−70 years) | 5 | 15 (88%) | 2 (12%) | 0 (0%) |
| LPL/LAS: 9 | RASP: 1 | ||||
| LIP/LI: 6 | RIP/RI: 1 | ||||
| Baek et al.29 | 11.7 ± 3.9 years (3.8–25 years) | 6 | 17 (100%) | 0 (0%) | 0 (0%) |
| LFW: 10 | |||||
| LPS: 7 | |||||
| Xie et al.30 | 36 ± 16 years (9–71 years) | 9 | 10 (59%) | 6 (35%) | 1 (6%) |
| LPL: 6 | RP: 6 | MS: 1 | |||
| LAL: 4 | |||||
| d’Avila et al.32 | Unavailable | 8 | 16 (94%) | 0 (0%) | 1 (6%) |
| LL: 10 | PS: 1 | ||||
| LPS: 6 | |||||
| Milstein et al.31 | 34 ± 21 years (unavailable) | 4 | 12 (71%) | 0 (0%) | 5 (29%) |
| LL: 12 | PS: 5 | ||||
The age specifies the mean age (range) of the study population the algorithm was devised on.
LA, left anterior; LAL, left anterolateral; LAS, left anterosuperior; LFW, left free wall; LI, left inferior; LIP, left inferior paraseptal; LL, left lateral; LP, left posterior; LPL, left posterolateral; LPS, left posteroseptal; MS, mid-septal; PS, posteroseptal; RA, right anterior; RAL, right anterolateral; RASP, right anterosuperior paraseptal; RF, right free wall; RI, right inferior; RIP, right inferior paraseptal; RL, right free wall; RP, right posterior; RPS, right posterioseptal; RS, right septum.
Among the 17 neonates with a WPW pattern, a follow-up ECG was available in 14 [82% boys, median age 3.2 (2.8–3.6 IQR) years at follow-up]. Of these, a persistent pre-excitation pattern was found in four (29%, 75% boys) of the children. Applying the localization algorithms on these follow-up ECGs showed consistent predictions in three (left-sided in two; postero-/mid-septal in one) but altered prediction in one (initially predicted as left-sided but right-sided at follow-up).
Echocardiographic findings
One of the neonates with a WPW pattern had moderate mitral regurgitation without Barlow’s disease, prolapse, or parachute mitral valve; the left ventricle was mildly dilated with a FS of 26%; there was no identified congenital heart disease. All the other neonates with a WPW pattern had structurally and functionally normal hearts; in specific, there were no cases of Ebstein’s anomaly. Echocardiographic measurements of the left ventricle (IVSD, LVPWd, LVIDd, LVIDs, LVEF, and FS), LA diameter, trans-mitral flow velocities (MV E and A), and MPA flow velocity did not differ significantly between the neonates with a WPW pattern and the matched controls (all P > 0.05; Table 2).
Discussion
In the present large, population-based cohort study with ECGs from 17 489 neonates, we found a prevalence of a WPW pattern of 0.1% with a male predominance of 76%. One neonate with a WPW pattern had moderate mitral regurgitation while all the other neonates with a WPW pattern had structurally normal hearts without significant differences in the echocardiographic measurements compared with controls. Most of the accessory pathways were predicted as left-sided, and at follow-up, the pre-excitation pattern only persisted in four (29%) of the children. None of the children had experienced any episodes of SVT during follow-up.
Estimating the ‘true’ prevalence of a WPW pattern is challenging, and previous studies from Japan11 (n = 137), Belgium12 (n = unavailable), Canada15 (n = 33), and the United States5,13,14,16–18 (n = 48–8733) have reported prevalences in a range of 0.03–0.5%. This wide range may be explained by multiple factors, in particular, differences in study design: inclusion of symptomatic vs. asymptomatic cases, hospitalized vs. non-hospitalized individuals, different age groups studied, geographical and ethnic dissimilarities, and cohorts of men-only in some studies. Additionally, the phenomenon of intermittent or concealed pre-excitation further adds to the difficulties in reliably estimating the prevalence particularly in the youngest population due to their inability to communicate symptoms. Our finding of a prevalence of a WPW pattern of 0.1% in an unselected and asymptomatic cohort of neonates, however, is consistent with most frequently reported prevalences. Nevertheless, a potential underestimation of the prevalence in our cohort cannot be completely ruled out as neonates often have a high heart rate, where the pre-excitation pattern may be more subtle and thus difficult to visually verify or completely concealed due to heart rate–dependent conduction through the accessory pathway.7 Furthermore, the pre-excitation pattern in neonates with a left-lateral pathway may also be very subtle or concealed as the anterograde route for conduction, i.e. the time required for the electrical impulse to travel from the sinus node to the atrial insertion of the accessory pathway, is longer than the time required to reach the atrioventricular (AV) node.37 We also observed an over-representation of males, which is a common observation4–6,12,13,18–20,38 with the majority of studies reporting upward of 61–66% of patients being males;4,19,38–40 we are only aware of one study from Japan (n = 137) that reported a higher prevalence of a WPW pattern among females (58%).11
One feature of the WPW pattern is its often intermittent nature, which may include an overt pre-excitation pattern with beat-to-beat normalization, or a phenotype that may be concealed for long time periods, despite a firmly established diagnosis based on an initial standard 12-lead ECG.5 Previous studies with cohorts of paediatric and adult WPW patients, using either repeated ECG recordings and/or Holter monitoring, found intermittent pre-excitation in up to 67% of the patients.10,20,40,41 Reasons for this intermittent or absolute loss of manifest ventricular pre-excitation are poorly understood. Accessory pathways are thought to be a congenital condition with a greater prevalence in neonates.7,9 Several studies from the United States4,19,39 investigating patients diagnosed with WPW syndrome in childhood (n = 90–446, aged 0–20 years), reported spontaneous loss of ventricular pre-excitation in up to 36% of the study populations, suggesting loss of anterograde conduction. An Italian study42 found that SVT became non-inducible in a similar percentage of WPW patients (n = 212, aged 7–63 years), suggesting loss of retrograde conduction as well.7 The patients who lost the pre-excitation pattern had a longer anterograde effective refractory period and a slower conduction over the accessory pathway than patients with a consistent pre-excitation pattern.42,43 Furthermore, considering that both the AV node and the accessory pathway are known to be dynamic structures that undergo maturational changes, it is likely that the changes in the preferred route for the electrical impulses are related to alterations in the conduction time and the refractoriness of these cardiac structures.44,45 An important note is, however, that intermittent or concealed ventricular pre-excitation does not exclude patients from developing SVT, and cardiac arrest can be the first clinical manifestation of a WPW pattern.7,9
The optimal management of children with WPW syndrome is still controversial, with some physicians aiming at a conservative approach while others advocate for early ablation, however, as the ablation techniques have evolved radiofrequency catheter ablation is increasingly being performed in children.46 Accurate prediction of the location of accessory pathways based on ECG characteristics is an important first step before the radiofrequency catheter ablation and facilitates optimal planning of mapping and ablation strategies. Also, patient counselling on expected procedural outcome, including risk of pacemaker, can often be estimated based on the location of the accessory pathway. Several algorithms with reported high sensitivity and specificity have been developed for the adult patient population, but these algorithms appear to be less applicable in children, particularly for right-sided accessory pathways.27 Therefore, algorithms specifically for children and youths have more recently been devised.26,27,29 Applying published algorithms24,25,28,30–32 on our neonatal cohort, we found a predominance of left-sided accessory pathways consistent with findings in most previous studies.6,26,38,41 However, none of the algorithms were specifically developed for neonates, and in most of the algorithms, the initial step is based on the amplitudes of the R- and S-waves in lead V1; neonates typically have tall R-waves in V1 likely explaining the high number of predicted left-sided pathways in our cohort. Furthermore, we did not have any invasive electrophysiological data on the neonates to confirm the localizations, or to test for multiple accessory pathways.
In the present study, most of the neonates with a WPW pattern had structurally normal hearts. However, previous studies from Italy38 (n = 184, aged 8–12 years), Denmark6 (n = 362, aged 0–80 years), and the United States4 (n = 446, aged 0–20 years) found associated heart diseases in 1.6, 3.6, and 9.0%, respectively. Some studies even reported associated heart diseases in up to 37% of patients (n = 27–140, aged 0–82 years).3,18–20,39 Likely explanations for the variable prevalences of associated heart disease between different studies include differences in study design, inclusion of asymptomatic vs. symptomatic patients, variable definitions of structural heart disease, etc. In addition, we cannot rule out that the low rate of associated heart disease in our cohort may to some extent be caused by selection bias towards healthier neonates. In developed countries, severe structural cardiac abnormalities are often identified at the foetal ultrasound, resulting in either termination of the pregnancy or hospitalization shortly after birth, making participation in our study less likely or not feasible.
There are several other limitations to the current study. We only had one ECG tracing per child, as well as only one follow-up ECG. The neonatal ECGs were recorded with eight leads instead of 12, and no Holter monitoring or exercise stress test was performed. Furthermore, we cannot completely rule out that some neonates with very subtle ECG findings or an intermittent WPW pattern have not been detected. Lastly, the results may not be generalizable to populations with different ethnic distributions.
Conclusion
The prevalence of a WPW pattern in our cohort of unselected neonates from the general population was 0.1%. The WPW pattern was more frequent in boys and generally not associated with structural heart disease, and the accessory pathways were mostly left-sided. A striking observation was that the WPW pattern in most of the children could not be reproduced on follow-up ECGs at a mean age of 3 years, suggesting either that the ventricular pre-excitation pattern has an intermittent nature or that normalization had occurred.
Acknowledgements
We sincerely thank all the participants of the Copenhagen Baby Heart Study for their contribution.
Contributor Information
Maria Munk Pærregaard, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark.
Joachim Hartmann, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark.
Anne-Sophie Sillesen, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark.
Christian Pihl, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark.
Sofie Dannesbo, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark; Department of Cardiology, The Capital Regions Unit for Inherited Cardiac Diseases, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Inge Lehmanns Vej 7, DK-2100 Copenhagen, Denmark.
Thilde Olivia Kock, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark.
Adrian Pietersen, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark.
Anna Axelsson Raja, Department of Cardiology, The Capital Regions Unit for Inherited Cardiac Diseases, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Inge Lehmanns Vej 7, DK-2100 Copenhagen, Denmark.
Kasper Karmark Iversen, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark.
Henning Bundgaard, Department of Cardiology, The Capital Regions Unit for Inherited Cardiac Diseases, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Inge Lehmanns Vej 7, DK-2100 Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark.
Alex Hørby Christensen, Department of Cardiology, Herlev–Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Copenhagen, Denmark; Department of Cardiology, The Capital Regions Unit for Inherited Cardiac Diseases, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Inge Lehmanns Vej 7, DK-2100 Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark.
Funding
This work was supported by the Danish Heart Foundation (Grant 20-R140-A9569-22168, MMP), the Danish Children’s Heart Foundation (Grant 19-R112-A5248-26048, MMP), Snedkermester Sophus Jacobsen and wife Astrid Jacobsens foundation, Murermester Lauritz Peter Christensen and wife Kirsten Sigrid Christensens foundation, The Research Council at Herlev–Gentofte Hospital, The Health Foundation Denmark, The Independent Research Fund Denmark (Grant 0134-00363B, AHC), and Toyota–Fonden, Denmark.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
- 1. Wilson FN. A case in which the vagus influenced form of the ventricular complex of the electrocardiogram. Ann Noninvasive Electrocardiol 2002;7:154–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolff L, Parkinson J, White PD. Bundle-branch block with short P-R interval in healthy young people prone to paroxysmal tachycardia. Am Heart J 1930;5:685–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chambers S, Jnah A, Newberry D. The pathophysiology, diagnosis, and management of Wolff–Parkinson–White syndrome in the neonate. Adv Neonatal Care 2021;21:178–88. [DOI] [PubMed] [Google Scholar]
- 4. Cain N, Irving C, Webber S, Beerman L, Arora G. Natural history of Wolff-Parkinson-White syndrome diagnosed in childhood. Am J Cardiol 2013;112:961–5. [DOI] [PubMed] [Google Scholar]
- 5. Averill KH, Fosmoe RJ, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects: IV. Wolff-Parkinson-White syndrome. Am J Cardiol Elsevier1960;6:108–29. [DOI] [PubMed] [Google Scholar]
- 6. Borregaard R, Lukac P, Gerdes C, Moller D, Mortensen PT, Pedersen Let al. Radiofrequency ablation of accessory pathways in patients with the Wolff-Parkinson-White syndrome: the long-term mortality and risk of atrial fibrillation. Europace 2015;17:117–22. [DOI] [PubMed] [Google Scholar]
- 7. Cohen MI, Triedman JK, Cannon BC, Davis AM, Drago F, Janousek Jet al. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm Elsevier2012;9:1006–24. [DOI] [PubMed] [Google Scholar]
- 8. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist Cet al. 2019 ESC guidelines for the management of patients with supraventricular tachycardiaThe task force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC): developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 9. Triedman JK. Management of asymptomatic Wolff–Parkinson–White syndrome. Heart BMJ Publishing Group Ltd2009;95:1628–34. [DOI] [PubMed] [Google Scholar]
- 10. Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J 2001;142:530–6. [DOI] [PubMed] [Google Scholar]
- 11. Inoue K, Igarashi H, Fukushige J, Ohno T, Joh K, Hara T. Long-term prospective study on the natural history of Wolff-Parkinson-White syndrome detected during a heart screening program at school. Acta Paediatr Oslo Nor 2000; 89:542–5. [DOI] [PubMed] [Google Scholar]
- 12. De Bacquer D. Prevalences of ECG findings in large population based samples of men and women. Heart 2000;84:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janson CM, Millenson ME, Okunowo O, Dai D, Christmyer Z, Tan RBet al. Incidence of life-threatening events in children with Wolff-Parkinson-White syndrome: analysis of a large claims database. Heart Rhythm 2022;19:642–7. [DOI] [PubMed] [Google Scholar]
- 14. Hiss RG, Lamb LE. Electrocardiographic findings in 122,043 individuals. Circulation 1962;25:947–61. [DOI] [PubMed] [Google Scholar]
- 15. Manning GW. An electrocardiographic study of 17,000 fit, young Royal Canadian Air Force aircrew applicants. Am J Cardiol 1960;6:70–5. [DOI] [PubMed] [Google Scholar]
- 16. Swiderski J, Lees MH, Nadas AS. The Wolff-Parkinson-White syndrome in infancy and childhood. Heart 1962;24:561–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hejtmancik MR, Herrmann GR. The electrocardiographic syndrome of short P-R interval and broad QRS complexes: a clinical study of 80 cases. Am Heart J 1957;54:708–21. [DOI] [PubMed] [Google Scholar]
- 18. Tsao S, Deal BJ. Management of symptomatic Wolff–Parkinson–White syndrome in childhood. Prog Pediatr Cardiol 2013;35:7–15. [Google Scholar]
- 19. Deal BJ, Keane JF, Gillette PC, Garson A. Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: management and follow-up. J Am Coll Cardiol 1985;5:130–5. [DOI] [PubMed] [Google Scholar]
- 20. Hindman MC, Last JH, Rosen KM. Wolff-Parkinson-White syndrome observed by portable monitoring. Ann Intern Med 1973;79:654–63. [DOI] [PubMed] [Google Scholar]
- 21. Sillesen A-S, Raja AA, Pihl C, Vøgg ROB, Hedegaard M, Emmersen Pet al. Copenhagen Baby Heart Study: a population study of newborns with prenatal inclusion. Eur J Epidemiol 2019;34:79–90. [DOI] [PubMed] [Google Scholar]
- 22. Pærregaard MM, Hvidemose SO, Pihl C, Sillesen A-S, Parvin SB, Pietersen Aet al. Defining the normal QT interval in newborns: the natural history and reference values for the first 4 weeks of life. Europace 2021;23:278–86. [DOI] [PubMed] [Google Scholar]
- 23. Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 1978;93:62–6. [DOI] [PubMed] [Google Scholar]
- 24. Taguchi N, Yoshida N, Inden Y, Yamamoto T, Miyata S, Fujita Met al. A simple algorithm for localizing accessory pathways in patients with Wolff-Parkinson-White syndrome using only the R/S ratio. J Arrhythmia 2014;30:439–43. [Google Scholar]
- 25. Arruda MS, McClelland JH, Wang X, Beckman KJ, Widman LE, Gonzalez MDet al. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol 1998;9:2–12. [DOI] [PubMed] [Google Scholar]
- 26. Boersma L, García-Moran E, Mont L, Brugada J. Accessory pathway localization by QRS polarity in children with Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol 2002;13:1222–6. [DOI] [PubMed] [Google Scholar]
- 27. Li H-Y, Chang S-L, Chuang C-H, Lin M-C, Lin Y-J, Lo L-Wet al. A novel and simple algorithm using surface electrocardiogram that localizes accessory conduction pathway in Wolff-Parkinson-White syndrome in pediatric patients. Acta Cardiol Sin 2019;35:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iturralde P, Araya-Gomez V, Colin L, Kershenovich S, de Micheli A, Gonzalez-Hermosillo JA. A new ECG algorithm for the localization of accessory pathways using only the polarity of the QRS complex. J Electrocardiol 1996;29:289–99. [DOI] [PubMed] [Google Scholar]
- 29. Baek SM, Song MK, Uhm J-S, Kim GB, Bae EJ. New algorithm for accessory pathway localization focused on screening septal pathways in pediatric patients with Wolff-Parkinson-White syndrome. Heart Rhythm 2020;17:2172–9. [DOI] [PubMed] [Google Scholar]
- 30. Xie B, Heald SC, Bashir Y, Katritsis D, Murgatroyd FD, Camm AJet al. Localization of accessory pathways from the 12-lead electrocardiogram using a new algorithm. Am J Cardiol 1994;74:161–5. [DOI] [PubMed] [Google Scholar]
- 31. Milstein S, Sharma AD, Guiraudon GM, Klein GJ. An algorithm for the electrocardiographic localization of accessory pathways in the Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol PACE1987;10:555–63. [DOI] [PubMed] [Google Scholar]
- 32. d'Avila A, Brugada J, Skeberis V, Andries E, Sosa E, Brugada P. A fast and reliable algorithm to localize accessory pathways based on the polarity of the QRS complex on the surface ECG during sinus rhythm. Pacing Clin Electrophysiol PACE1995;18:1615–27. [DOI] [PubMed] [Google Scholar]
- 33. Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AKet al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465–95. [DOI] [PubMed] [Google Scholar]
- 34. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976;37:7–11. [DOI] [PubMed] [Google Scholar]
- 35. Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2006;19:1413–30. [DOI] [PubMed] [Google Scholar]
- 36. Pærregaard MM, Kock J, Pihl C, Pietersen A, Iversen KK, Bundgaard Het al. The evolution of the neonatal QRS axis during the first four weeks of life. Neonatology 2021;118:155–62. [DOI] [PubMed] [Google Scholar]
- 37. Teo WS, Klein GJ, Yee R, Leitch JW, Murdock CJ. Significance of minimal preexcitation in Wolff-Parkinson-White syndrome. Am J Cardiol 1991;67:205–7. [DOI] [PubMed] [Google Scholar]
- 38. Santinelli V, Radinovic A, Manguso F, Vicedomini G, Gulletta S, Paglino Get al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol 2009;53:275–80. [DOI] [PubMed] [Google Scholar]
- 39. Perry JC, Garson A. Supraventricular tachycardia due to Wolff-Parkinson-White syndrome in children: early disappearance and late recurrence. J Am Coll Cardiol 1990;16:1215–20. [DOI] [PubMed] [Google Scholar]
- 40. Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJet al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993;87:866–73. [DOI] [PubMed] [Google Scholar]
- 41. Kiger ME, Mccanta AC, Tong S, Schaffer M, Runciman M, Collins KK. Intermittent versus persistent Wolff-Parkinson-White syndrome in children: electrophysiologic properties and clinical outcomes. Pacing Clin Electrophysiol 2016;39:14–20. [DOI] [PubMed] [Google Scholar]
- 42. Pappone C, Santinelli V, Rosanio S, Vicedomini G, Nardi S, Pappone Aet al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol 2003;41:239–44. [DOI] [PubMed] [Google Scholar]
- 43. Klein GJ, Gulamhusein SS. Intermittent preexcitation in the Wolff-Parkinson-White syndrome. Am J Cardiol Elsevier 1983;52:292–6. [DOI] [PubMed] [Google Scholar]
- 44. Cohen MI, Wieand TS, Rhodes LA, Vetter VL. Electrophysiologic properties of the atrioventricular node in pediatric patients. J Am Coll Cardiol 1997;29:403–7. [DOI] [PubMed] [Google Scholar]
- 45. Chang R-KR, Wetzel GT, Shannon KM, Stevenson WG, Klitzner TS. Age- and anesthesia-related changes in accessory pathway conduction in children with Wolff-Parkinson-White syndrome. Am J Cardiol 1995;76:1074–6. [DOI] [PubMed] [Google Scholar]
- 46. Hernández-Madrid A, Hocini M, Chen J, Potpara T, Pison L, Blomström-Lundqvist Cet al. How are arrhythmias managed in the paediatric population in Europe? Results of the European Heart Rhythm survey. Europace 2014;16:1852–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.