Highlights
-
•
Successful outbreak response requires timely supplemental immunization activities.
-
•
There are numerous sources of delayed response beyond the vaccine approval process.
-
•
Several countries met multiple target indicators for successful outbreak response.
Keywords: Polio eradication, Oral polio vaccine, Circulating vaccine-derived poliovirus, Polio outbreak response
Abstract
Background
Trivalent oral poliovirus vaccine (tOPV) was globally replaced with bivalent oral poliovirus vaccine (bOPV) in April 2016 (“the switch”). Many outbreaks of paralytic poliomyelitis associated with type 2 circulating vaccine-derived poliovirus (cVDPV2) have been reported since this time. The Global Polio Eradication Initiative (GPEI) developed standard operating procedures (SOPs) to guide countries experiencing cVDPV2 outbreaks to implement timely and effective outbreak response (OBR). To assess the possible role of compliance with SOPs in successfully stopping cVDPV2 outbreaks, we analyzed data on critical timelines in the OBR process.
Methods
Data were collected on all cVDPV2 outbreaks detected for the period April 1, 2016 and December 31, 2020 and all outbreak responses to those outbreaks between April 1, 2016 and December 31, 2021. We conducted secondary data analysis using the GPEI Polio Information System database, records from the U.S. Centers for Disease Control and Prevention Polio Laboratory, and meeting minutes of the monovalent OPV2 (mOPV2) Advisory Group. Date of notification of circulating virus was defined as Day 0 for this analysis. Extracted process variables were compared with indicators in the GPEI SOP version 3.1.
Results
One hundred and eleven cVDPV2 outbreaks resulting from 67 distinct cVDPV2 emergences were reported during April 1, 2016-December 31, 2020, affecting 34 countries across four World Health Organization Regions. Out of 65 OBRs with the first large-scale campaign (R1) conducted after Day 0, only 12 (18.5%) R1s were conducted by the target of 28 days after Day 0. Of the 89 OBRs with the second large-scale campaign (R2) conducted after Day 0, 30 (33.7%) R2s were conducted by the target of 56 days after Day 0. Twenty-three (31.9%) of the 72 outbreaks with isolates dated after Day 0 were stopped within the 120-day target.
Conclusion
Since “the switch”, delays in OBR implementation were evident in many countries, which may be related to the persistence of cVDPV2 outbreaks >120 days. To achieve timely and effective response, countries should follow GPEI OBR guidelines.
1. Introduction
With the last detected case of type 2 indigenous wild poliovirus (WPV2) in 1999, the Global Certification Commission certified WPV2 eradication in 2015. Still, at the time of certification, cases of type 2 paralytic poliomyelitis continued due to use of the type 2 oral poliovirus vaccine (OPV). To reduce the risk of outbreaks from circulating vaccine-derived poliovirus type 2 (cVDPV2) in communities with low vaccination coverage, synchronized global replacement of trivalent OPV (tOPV, containing all three serotypes of live Sabin stains) with bivalent OPV (bOPV, containing Sabin types 1 and 3) was implemented in April 2016 (“the switch”) [1]. Following the switch, outbreaks of cVDPV2 associated with use of tOPV prior to the switch were detected; this transmission was expected to be stopped with two targeted vaccination campaigns using monovalent Sabin OPV type 2 (mOPV2). Over the years since the tOPV-bOPV switch, newly seeded outbreaks following mOPV2 campaigns have emerged in expanding geographies [2].
Outbreaks caused by cVDPVs are characterized by evidence of person-to-person transmission in the community, sometimes with prolonged undetected circulation and trans-boundary international spread, and represent a major threat to polio eradication [3], [4], [5], [6], [7]. The increasing number of cVDPV2 outbreaks has been ascribed to the reduction in population intestinal immunity to poliovirus type 2 among children born after the switch [7] and deficiencies in the timeliness, scope, and quality of outbreak response. The Global Polio Eradication Initiative (GPEI) and governments of affected countries are responsible for stopping these cVDPV2 outbreaks. To offer guidance to these countries, GPEI developed standard operating procedures (SOPs) for timely and effective outbreak response (OBR), including supplemental immunization activities (SIAs) [5].
The OBR process commences with the detection and sequence analysis of cVDPV2 by laboratories of the Global Polio Laboratory Network and subsequent notification of the health authorities of the affected country and World Health Organization (WHO) offices at country, regional, and headquarter levels. The International Health Regulations (IHR) (2005) stipulate that detections must be immediately reported by national authorities to the IHR focal point at the respective WHO regional office [8]. Within 72 h of confirmation of an outbreak, the country should declare the outbreak a national public health emergency (PHE), complete a risk assessment (RA), and develop a response plan for surveillance, vaccination through SIAs, and social mobilization [4].
The development of an RA and response plan is done in coordination with the Outbreak Preparedness and Response Team (OPRT; succeeded in 2022 by the Outbreak Response and Preparedness Group [ORPG]) of GPEI and WHO regional offices. The purpose of the RA is to review virologic and epidemiologic characteristics of the newly detected virus; assess the immunization and surveillance performance in the affected geography; determine the level of risk for further national or international spread; assess country capacity to implement the OBR; and provide a vaccine request for response. The RA and vaccine requests are reviewed by the mOPV2 Advisory Group (AG; succeeded by the OPV2 Advisory Group) to advise the WHO Director-General (DG) on vaccine release. The AG further advises the country on clarifying the RA, especially concerning appropriateness of the OBR with regard to geographical scope, timing, and vaccine needs. With the 2019 restart of tOPV production for OBR in countries with co-circulation of cVDPV2 and wild poliovirus type 1 (WPV1) or cVDPV types 1 or 3, the mOPV2 AG has also advised on release of tOPV since 2019.
The AG then responds to the final RA and OPV2 request with approval or deferral. If the AG approves the vaccine request, it recommends to the WHO DG to approve the release of mOPV2/tOPV from global stockpiles. When the DG approves the request, the UNICEF Supply Division facilitates the release and prompt shipment of OBR vaccine to the affected country. Since there is no limit to the number of outbreaks (defined as unique genetic emergence groups) that can occur in each country, a given response may address one or more outbreaks. As outlined in the SOPs, OBR SIAs should include a rapid response vaccination (Round 0 [R0]) followed by two larger-scale high-quality SIAs (Rounds 1 and 2 [R1 and R2]) and mop-up vaccination as needed within specified timelines. The purpose of initiating timely SIAs of sufficient scope and quality is to stop polio outbreaks by Day 120 after notification of cVDPV2 detection.
Rapid OBR SIAs are critical in controlling cVPDV2 outbreaks by promptly raising type 2 population immunity. Any break in the chain of OBR steps and timelines following notification of virus detection could jeopardize the success of the response, leading to ongoing transmission and further spread of the virus. Failure to comply with SOPs could occur by delays in notification of test results, approval and shipping of vaccines, or the quality of SIA implementation and post-campaign activities. To assess the possible role of lack of compliance with the SOPs in the persistence of cVDPV2 outbreaks globally, we analyzed data on critical steps and timelines in the OBR process, starting from notification of detection of cVDPV2 to the post-response SIA period, and summarized the success of each OBR in stopping transmission by the 120-day target date.
2. Methods
2.1. Data collection
2.1.1. Data sources and definitions
The GPEI Polio Information System (POLIS) is a standardized global data depository of all AFP cases and poliovirus detections, key surveillance indicators, and OBR activities that is available to all GPEI partners and modeling collaborators. POLIS served as the principal source of data used to assess compliance of cVDPV2 outbreak response with outbreak response SOPs. Data were collected covering the period following the global switch, April 1, 2016-December 31, 2020. Data collection for the outbreak response period was extended by one year (April 1, 2016-December 31, 2021) to capture comprehensive response measures and outcomes for outbreaks confirmed in late 2020.
Data from POLIS were extracted into an Excel spreadsheet for analysis. Variables extracted included country of cVDPV2 detection; genetic emergence group; date of onset or collection of first/index poliovirus isolate; date of onset or collection of most recent poliovirus isolate; sample source for identification of the first cVDPV2 detection (an AFP case, environmental surveillance (ES) sample or community/contact isolate); and number and sample source of cVDPV2 viruses detected for each emergence group, outbreak, and country.
Other variables extracted included the number of nucleotide substitutions in the region coding for the surface VP1 protein relative to the Sabin parent strain for the index and subsequent viruses in an emergence, date of notification of detection of first emergent virus to WHO Headquarters, in-country or international spread of the emergent virus, date of declaration of PHE, number and dates (beginning and end) of OBR mOPV2/tOPV SIA rounds, preparation of type 2 vaccine(s) used during each SIA, and detection of cVDPV2 isolates 14 days or more following the end of R2 (defined as breakthrough infections). All outbreaks were defined on a country basis.
Additional sources of data included records from the U.S. Centers for Disease Control and Prevention Polio Laboratory (Atlanta, GA, USA), for date of notification of circulating virus; mOPV2 AG meeting minutes, for date of country submission of risk assessments and date of AG approval/meetings following RA submissions; and the WHO mOPV2 AG Secretariat tracker, for date of mOPV2 approval by the WHO DG.
Genetic emergence groups were defined as cVDPV strains that emerged from OPV use and have no relationship to any previously identified VDPVs globally on the basis of virus sequence data. The emergence groups were named based on country and first administrative level (province, state) of first detection and numbered sequentially as necessary.
An outbreak was characterized as detection of cVDPV2 with community level transmission as demonstrated by detection in more than one human or two separate detections from the environment more than 60 days apart, as indicated in the OBR guidelines [5]. A virus or viruses identified in a new country but genetically linked to a particular cVDPV2 emergence group first identified in another country were classified as a distinct outbreak from the same emergence group detected in the original country. The date of first detection of the outbreak was determined by either the date of onset of paralysis for the index AFP case, or by the date of sample collection for positive ES samples, targeted healthy children, or AFP case-contacts. The date of notification of circulating virus was regarded as Day 0 of the outbreak (for purposes of OBR) and defined as evidence of person-to-person transmission following identification of one or more additional isolates (from AFP cases or ES samples) associated with the same VDPV2 emergence or re-classifications of prior ambiguous or pending viruses.
The end of an outbreak was considered as 1) the last date of collection of positive ES sample or a community member or AFP-contact sample or 2) the date of onset of latest AFP case, with at least six months of adequate surveillance indicators. In countries with multiple outbreaks, certain response activities were conducted for an existing outbreak before new outbreaks were detected in the same geography and generated negative values for the time intervals; those negative values were excluded from calculations. All definitions are summarized in Table 1.
Table 1.
Description of the WHO standard operating procedure (SOP) indicators, timelines, and outbreak measures for response to VDPV events and outbreaks — worldwide, 2016–2020.
| Term | Description1 | Recommended Time from Day 0 per SOPs |
|---|---|---|
| SOP Indicator | ||
| Notification to WHO headquarters (HQ) of first virus detection | Date of notification to WHO HQ of first virus detected, either from an AFP case, a positive environmental sample, or a positive contact of AFP case. | Not included in SOPs but measured in this study. |
| Day 0: Notification of circulating virus | Date of receipt of Polio Laboratory Network sequencing report by WHO HQ. Classification of circulating virus may follow detection of two or more related viruses in-country. | |
| Previously isolated VDPVs, classified as ambiguous or pending classification, may be reclassified as circulating (i.e., an outbreak) if another related poliovirus is detected indicating evidence of transmission. | ||
| Public Health Emergency (PHE) declaration2 | Date national government declares PHE in a non-endemic country. If outbreaks in-country remain open, additional PHE declarations are not needed for subsequent cVDPV2 outbreaks. |
3 days |
| Round 0 (R0) Start | Beginning of initial response of rapid, focused, and small-scale round; maximize quality in high-risk areas near detection. Conduct rapid (<14 days), focused response of 100,000–400,000 children. | 14 days |
| Round 1 (R1) Start | Beginning of increased response size (1–4 million children) in order to improve chance of rapidly stopping transmission. | 28 days |
| Round 2 (R2) End | Completion of increased response size (1–4 million children) in order to improve chance of rapidly stopping transmission. | 56 days |
| Time to stop an outbreak | Number of days to stop an outbreak per SOPs, i.e., date of last collection of a positive environmental sample or virus isolation or onset of paralysis from Day 0. | 120 days |
| Outcome measure | ||
| Breakthrough infection | Detection of AFP case, environmental isolate, or evidence of ongoing transmission 14 days or more after the completion of the second round of SIAs. | |
| Number of mOPV2 response rounds | Number of SIA rounds with mOPV2 use by December 31, 2021. Count includes rounds utilizing mOPV2 alone, mOPV2 in conjunction with IPV, or tOPV in Afghanistan and Pakistan. Excludes mop-ups. | |
| International spread | Emergence exported to other countries. | |
| Most recent isolation in country | Date of most recent cVDPV2 detection (by December 31, 2020). | |
| Outbreak closure | Date of outbreak closure announced by WHO Regional Office based on Outbreak Response Assessment (OBRA) recommendation. Requires quality surveillance and no detections of the poliovirus of the outbreak serotype for at least six months. |
Abbreviations: PHE, Public Health Emergency.
1According to Standard Operating Procedures for Responding to a Poliovirus Event or Outbreak (2020).
2PHE is declared for the first cVDPV2 outbreak in country.
2.1.2. Source of analysis variables
Variables extracted were compared with SOP indicators. During 2015–2020, four versions of the SOPs were released by GPEI; version 1 was released in 2015 and version 3.1 in 2020. There were no major changes in the indicators in updates to version 1; indicators and timelines used here were from version 3.1 relevant to the entire reporting period (Table 1).
2.1.3. Definitions of response timelines
The SOPs direct that within three days of notification of circulating virus (Day 0), a country should declare a cVDPV2 outbreak as a national PHE [5]. However, if WPV1 is endemic in a country, as in Afghanistan and Pakistan, a country does not need to declare a separate PHE for the cVDPV2 outbreak. If a country is not endemic for WPV1, but cVDPV2 outbreaks are already occurring within its borders, and a new emergence or importation occurs, the country can opt to apply a previous PHE declaration to the new outbreak.
Before or following a declaration of a PHE, an affected country should draft an RA and a vaccine request to respond to the outbreak. If an RA lacks certain key elements, it is considered an initial RA and returned to the relevant country team with feedback, and the final RA is expected to be resubmitted to the AG within the next 24 h. The AG is to approve or defer the final RA and mOPV2 request within 48 h of submission. If the AG approves the vaccine request and advises the DG to approve and release mOPV2 or tOPV from global stockpiles, the WHO DG is to decide on approval within 24 h. The SOPs further direct that R0 should begin by Day 14, R1 should start by Day 28, and R2 should end by Day 56, all as counted from the Day 0 notification date.
The operational definition of breakthrough infection has changed over time and been inconsistently applied in deliberations. Certain countries regard cVDPV2 isolates detected on or after 14 days following the end of R2 as breakthrough cases, while other countries consider isolates detected on or after 21 or 28 days following the end of R2 as breakthrough cases. For this study, breakthrough infection was defined as the detection of a cVDPV2 isolate 14 or more days following the end of R2, as designated in GPEI SOP version 3.1, either from AFP cases or from ES samples.
2.1.4. Inclusion and exclusion criteria of SIA response rounds
This study evaluated OBR SIAs conducted during April 1, 2016-December 31, 2021, in response to cVDPV2 outbreaks occurring during April 1, 2016-December 31, 2020, by date of AFP case onset or specimen collection. Response rounds utilizing mOPV2 alone, mOPV2 in conjunction with inactivated poliovirus vaccine (IPV), or tOPV in response to concomitant WPV1 and cVDPV2 circulation in Afghanistan and Pakistan, were included in the analysis. All mOPV2/tOPV SIAs conducted as national immunization days (covering an entire country), sub-national immunization days (covering a portion of a country), and case responses (campaigns in response to a positive isolate) were included; mOPV2/tOPV mop-ups (small scale immunization activities in areas of poor coverage) were excluded from consideration [9]. OBRs to cVDPV1 and cVDPV3 outbreaks are coordinated directly by the GPEI ORPG and were not included.
Following the development of the novel type 2 OPV (nOPV2) as a more genetically stable alternative to Sabin OPV2, WHO provided Emergency Use Listing for nOPV2 in November 2020 [10], [11]. While countries have subsequently increased the use of nOPV2 to respond to cVDPV2 outbreaks since March 2021, the approval and release process for nOPV2 use did not follow a similar process. Therefore, the OBR timelines for nOPV2 SIAs are beyond the scope of this study.
2.2. Data analysis
Annual incidence and prevalence of cVDPV2 emergences, number of outbreaks by country, and the number of viruses detected by sample source were analyzed using Excel. Time intervals for each SOP indicator were calculated between Day 0 and milestones specified in the SOPs, including dates of OBR rounds (R0, R1, and R2). Descriptive statistics of time intervals were calculated, including medians, minimums, maximums, and interquartile ranges (IQRs). A complete epidemiologic description of the outbreaks is found in a separate publication [7].
3. Results
3.1. Post-switch cVDPV2 emergences, outbreaks, and virus detections
3.1.1. Emergences, outbreaks, and virus detections by year, worldwide
One hundred and eleven cVDPV2 outbreaks, resulting from 67 cVDPV2 emergences, were reported from 34 countries, with AFP case onset or samples collected during April 1, 2016-December 31, 2020 (Table 2). Among the 3168 confirmed cVDPV2 viruses that were detected during this period, 1614 (51%) were isolated from specimens from AFP cases, 953 (30%) from ES samples, and 601 (19%) from specimens from healthy community members or AFP case-contacts. The largest annual increase in new emergences occurred during 2019 with 40 incident emergences in 14 incident countries and affecting five additional countries. While only 14 new emergences were detected the following year, 2020 saw the largest number of reported isolations (1977), with 1079 (55%) from confirmed AFP case samples, 612 (31%) from ES samples, and 286 (14%) from specimens taken from healthy community members or AFP case-contacts.
Table 2.
cVDPV2 emergences and viruses identified after the switch from trivalent OPV to bivalent OPV, by time-period — worldwide, April 2016 – December 2020.
| Period |
No. cVDPV2 Emergences |
No. Countries Affected |
No. Confirmed AFP Cases | No. Confirmed ES Isolates | No. Confirmed Healthy or Community Contacts | No. Total Viruses | ||
|---|---|---|---|---|---|---|---|---|
| Prevalent | Incident | Prevalent | Incident1 | |||||
| Apr 2016 – Dec 2016 | 3 | 3 | 2 | 2 | 2 | 5 | 2 | 9 |
| Jan 2017 – Dec 2017 | 5 | 4 | 4 | 3 | 96 | 3 | 62 | 161 |
| Jan 2018 – Dec 2018 | 8 | 6 | 7 | 5 | 71 | 81 | 75 | 227 |
| Jan 2019 – Dec 2019 | 44 | 40 | 19 | 14 | 366 | 252 | 176 | 794 |
| Jan 2020 – Dec 2020 | 35 | 14 | 30 | 13 | 1079 | 612 | 286 | 1977 |
| Apr 2016 – Dec 2020 | 672 | 67 | 342 | 342 | 1614 | 953 | 601 | 3168 |
Abbreviations: AFP, Acute Flaccid Paralysis; ES, Environmental Surveillance samples.
Includes countries with new emergences, new outbreaks, and new infections by spread from other countries.
Sum of first five rows is not equivalent to the last row of the column.
3.1.2. Emergences, outbreaks, and virus detections by WHO region
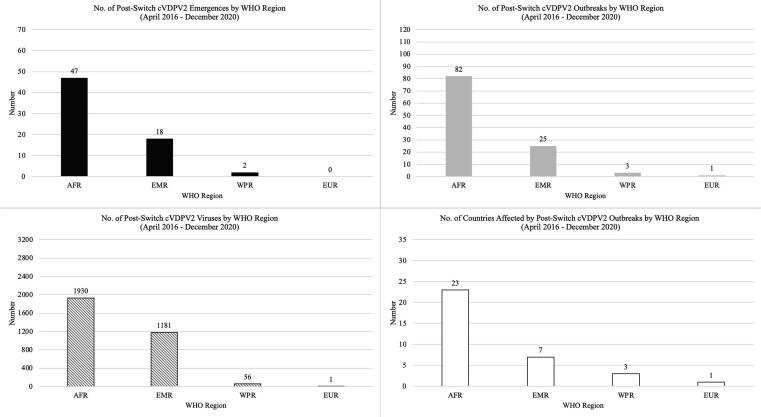
The 47 emergences, 82 outbreaks, and 1930 viruses detected in 23 countries in the African Region accounted for 70.1%, 73.9%, 60.9% and 67.6% of the global post-switch cVDPV2 emergences, outbreaks, virus detections, and countries affected, respectively (Fig. 1). With multiple outbreaks in the Democratic Republic of the Congo (15) and the Central African Republic (8), the Central Africa sub-regional block had the largest number of emergences (n = 25) and outbreaks (n = 38) within the African Region (Table 3, Fig. 2). Due to the large international spread and prolonged transmission of the Nigeria-Jigawa-1 emergence (NIE-JIS-1), West Africa accounted for the largest number of viruses (n = 952, 49.3%) and countries affected (n = 12) in the African Region.
Fig. 1.
Number of cVDPV2 emergences, outbreaks, viruses detected, and countries affected after the switch from trivalent OPV to bivalent OPV, by WHO region, 2016–2020.
Table 3.
cVDPV2 outbreaks and viruses detected after the switch from trivalent OPV to bivalent OPV, by WHO region and country — worldwide, 2016–2020.
| WHO Region & Subregion | Country | No. Outbreaks | No. Confirmed AFP Cases | No. Confirmed ES Isolates | No. Confirmed Healthy or Community Contacts | No. Total Viruses Detected |
|---|---|---|---|---|---|---|
| AFR | 82 | 977 | 483 | 470 | 1930 | |
| Central | 38 | 498 | 67 | 253 | 818 | |
| Angola | 5 | 141 | 21 | 22 | 184 | |
| Cameroon | 4 | 7 | 13 | 4 | 24 | |
| CAR | 8 | 25 | 17 | 44 | 86 | |
| Chad | 3 | 112 | 14 | 23 | 149 | |
| Congo (Rep.) | 3 | 2 | 1 | 2 | 5 | |
| DRC | 15 | 211 | 1 | 158 | 370 | |
| Eastern & Southern | 16 | 103 | 17 | 40 | 160 | |
| Ethiopia | 10 | 50 | 7 | 16 | 73 | |
| Kenya1 | 2 | 0 | 2 | 1 | 3 | |
| Mozambique | 1 | 1 | 0 | 2 | 3 | |
| South Sudan | 1 | 50 | 8 | 19 | 77 | |
| Zambia | 2 | 2 | 0 | 2 | 4 | |
| West | 28 | 376 | 399 | 177 | 952 | |
| Benin | 1 | 11 | 7 | 0 | 18 | |
| Burkina Faso | 2 | 66 | 0 | 12 | 78 | |
| Côte d'Ivoire | 2 | 64 | 158 | 24 | 246 | |
| Ghana | 1 | 30 | 55 | 26 | 111 | |
| Guinea | 1 | 44 | 1 | 1 | 46 | |
| Liberia | 1 | 0 | 7 | 2 | 9 | |
| Mali | 2 | 52 | 4 | 3 | 59 | |
| Niger2 | 2 | 21 | 9 | 13 | 43 | |
| Nigeria | 12 | 61 | 157 | 80 | 298 | |
| Senegal | 1 | 0 | 1 | 0 | 1 | |
| Sierra Leone | 1 | 10 | 0 | 6 | 16 | |
| Togo | 2 | 17 | 0 | 10 | 27 | |
| EMR | 25 | 622 | 437 | 122 | 1181 | |
| Afghanistan | 3 | 308 | 182 | 37 | 527 | |
| Egypt | 1 | 0 | 1 | 0 | 1 | |
| Iran (Islamic Republic of) | 1 | 0 | 3 | 0 | 3 | |
| Pakistan | 15 | 158 | 179 | 16 | 353 | |
| Somalia | 3 | 23 | 58 | 15 | 96 | |
| Sudan | 1 | 59 | 14 | 11 | 84 | |
| Syrian Arab Republic | 1 | 74 | 0 | 43 | 117 | |
| EUR | 1 | 1 | 0 | 0 | 1 | |
| Tajikistan | 1 | 1 | 0 | 0 | 1 | |
| WPR | 3 | 14 | 33 | 9 | 56 | |
| China | 1 | 1 | 1 | 3 | 5 | |
| Malaysia | 1 | 0 | 8 | 0 | 8 | |
| Philippines | 1 | 13 | 24 | 6 | 43 | |
| All Regions | 111 | 1614 | 953 | 601 | 3168 |
Abbreviations: AFP, Acute Flaccid Paralysis; AFR, African Region; CAR, Central African Republic; DRC, Democratic Republic of the Congo; EMR, Eastern Mediterranean Region; ES, Environmental Sample; EUR, European Region; WPR, Western Pacific Region.
Two outbreaks of SOM-BAN-1 in Kenya: 1st outbreak: 2018; 2nd outbreak: 2020–2021.
Two outbreaks of NIE-JIS-1 in Niger: 1st outbreak: 2018–2019; 2nd outbreak: 2020.
Fig. 2.
Number of cVDPV2 emergences, outbreaks, viruses detected, and countries affected after the switch from trivalent OPV to bivalent OPV, by subregional block — WHO African Region, 2016–2020.
The Eastern Mediterranean Region experienced the second largest number of cVDPV2 emergences (n = 18), outbreaks (n = 25), viruses detected (n = 1181), and countries affected (n = 7), primarily stemming from the spread of the Pakistan-Gilgit Baltistan-1 emergence (PAK-GB-1) across Pakistan and Afghanistan and exportation to the Islamic Republic of Iran (positive ES samples, without AFP cases) (Table 3, Fig. 1, Supplementary Table 1). The spread of PAK-GB-1 from Afghanistan to Tajikistan resulted in one cVDPV2 outbreak in the European Region in 2020. The Western Pacific Region experienced two emergences resulting in three outbreaks affecting three countries, with 56 viruses detected.
3.1.3. Outbreaks, cases, and virus detections by country
Post-switch cVDPV2 outbreaks affected 34 countries across the African, Eastern Mediterranean, European, and Western Pacific Regions (Table 3). Among the cVDPV2 outbreak-affected countries, seven countries experienced more than three outbreaks during April 2016-December 2020: Cameroon (n = 4), Angola (5), Central African Republic (8), Ethiopia (10), Nigeria (12), the Democratic Republic of the Congo (15), and Pakistan (15). The remaining 27 countries experienced one to three outbreaks during the same period: 16 countries had a single outbreak, seven countries had two outbreaks, and four countries had three outbreaks. While Afghanistan confronted only three cVDPV2 outbreaks, the country experienced the largest number of confirmed AFP cases during the observation period with 308 cases. Other countries with more than 100 confirmed AFP cases during this period include Chad (n = 112), Angola (141), Pakistan (158), and the Democratic Republic of the Congo (211).
3.1.4. Spread and duration of cVDPV2 emergences
Of the 67 unique cVDPV2 genetic emergences that were detected during April 2016-December 2020, 18 (26.9%) spread beyond the country of emergence during this period (Supplementary Table 1). NIE-JIS-1 spread to 13 countries outside Nigeria; the Chad-N’Djamena-1 emergence (CHA-NDJ-1) spread to six other countries; and the PAK-GB-1 emergence spread to three other countries. The Angola-Luanda-1 emergence (ANG-LUA-1), the Central African Republic-Bangui-1 (CAF-BNG-1) emergence, the Somalia-Banadir-1 emergence (SOM-BAN-1), and the Togo-Savanes-1 emergence (TOG-SAV-1) each spread to two other countries. Eleven emergences spread to only one country outside the respective country of origin.
NIE-JIS-1 and SOM-BAN-1 exhibited the longest duration of circulation with over 1000 days between the first and latest isolates by December 31, 2020; both continued to circulate in 2021 and 2022. In addition, NIE-JIS-1, with 892 virus isolates, was the largest emergence by virus count, given extensive ES in Nigeria. Other emergences with over 100 viruses detected by December 31, 2020, included SOM-BAN-1 (101); Angola-Huila-1 (ANG-HUI-1, 107); Syria-Deir Al-Zour-1 (SYR-DEI-1, 117); Democratic Republic of the Congo-Kasai-3 (DRC-KAS-3, 186); Afghanistan-Nangarhar-1 (AFG-NGR-1, 210); CHA-NDJ-1 (294); and PAK-GB-1 (616). Outbreaks in countries with ES demonstrated proportionally larger virus isolate counts compared with countries with no or limited ES (only AFP surveillance detections).
3.2. Outbreak response
3.2.1. Initial responses: timeliness and SOP compliance
Among the 34 countries that were affected by cVDPV2 outbreaks during 2016–2020, two (Afghanistan and Pakistan) were endemic for WPV transmission. Among the 32 affected countries that were not WPV-endemic, 27 (84.4%) declared a PHE (Egypt, the Islamic Republic of Iran, Republic of the Congo, Sierra Leone, and Tajikistan did not declare cVDPV2 outbreak PHEs). While six countries declared PHEs before Day 0 based on preliminary laboratory reports, 21 of the 27 countries declared PHEs following Day 0 (notification of circulating virus). Of these, a PHE was declared at a median of 16 days after Day 0 (interquartile range [IQR] 4 to 63 days), and only five (23.8%) met the target date of 3 days (Table 4, Table 5).
Table 4.
Range, median, and interquartile range of duration of initial response among post-switch cVDPV2 outbreaks — worldwide, 2016– 2021.
| Indicator | Target (Days) | N1 | Minimum (Days) | Q1 (Days) | Median (Days) | Q3 (Days) | Maximum (Days) |
|---|---|---|---|---|---|---|---|
| Day 0 to declaration of PHE | 3a | 212 | 0 | 4 | 16 | 63 | 263 |
| Day 0 to initial RA submission to AG | N/A | 54 | 0 | 6 | 16 | 41 | 231 |
| Initial RA submission to AG to final RA submission to AG | 1b | 91 | 0 | 0 | 0 | 2 | 119 |
| Final RA submission to AG approval | 2b | 91 | 0 | 0 | 0 | 2 | 10 |
| AG approval to DG approval | 1b | 68 | 0 | 1 | 2 | 4 | 39 |
| First DG approval to start of first SIA | 42 | 2 | 12 | 30 | 77 | 227 | |
| R0 start | 10b | 19 | 2 | 11 | 12 | 20 | 146 |
| For outbreak responses without R0: R1 start | 24b | 23 | 7 | 44 | 62 | 94 | 227 |
| SIAs | 101 | 9 | 29 | 58 | 98 | 459 | |
| R0 start to R2 end | 42a | 53 | 24 | 60 | 87 | 117 | 459 |
| For outbreak responses without R0: R1 start to R2 end | 28a | 483 | 9 | 17 | 29 | 51 | 108 |
Abbreviations: AG, Advisory Group; DG, Director-General of WHO; PHE, Public Health Emergency; RA, Risk Assessment; SIA, Supplementary Immunization Activity.
Negative values stemming from activities conducted prior to a current outbreak were excluded from calculations. These values often originated in countries with multiple outbreaks.
Declaration of PHE for first outbreak in country. Among the 32 non-endemic countries (excludes Afghanistan and Pakistan), five countries did not declare PHEs for any cVDPV2 outbreaks in their respective jurisdictions, and 6 declared a PHE before Day 0 (official notification of a cVDPV) based on preliminary laboratory reports.
Includes tOPV administered in response to WPV1 and cVDPV2 outbreaks in Afghanistan and Pakistan.
SOP target.
Target not in SOPs but expected operationally.
Table 5.
Compliance with cVDPV2 outbreak response SOP indicators after the switch from trivalent OPV to bivalent OPV, by WHO region —worldwide, 2016–2020.
| Indicator | Target (Days) |
Regional Compliance12 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
AFR |
EMR | EUR3 | WPR | All Regions | |||||
| Central | Eastern & Southern | West | Total | ||||||
| Day 0 to declaration of PHE4 | 3a | 50% (2/4) | 50% (2/4) | 0% (0/9) | 24% (4/17) | 0% (0/2) | N/A | 50% (1/2) | 24% (5/21) |
| Initial RA submission to AG to final RA submission to AG |
1b | 77% (24/31) | 69% (9/13) | 67% (16/24) | 72% (49/68) | 76% (16/21) | N/A | 100% (2/2) | 74% (67/91) |
| Final RA submission to AG approval |
2b | 94% (29/31) | 54% (7/13) | 83% (20/24) | 82% (56/68) | 62% (13/21) | N/A | 100% (2/2) | 78% (71/91) |
| AG approval to DG approval | 1b | 30% (7/23) | 50% (5/10) | 63% (10/16) | 45% (22/49) | 53% (9/17) | N/A | 50% (1/2) | 47% (32/68) |
| First DG approval to start of first SIA (Outbreak responses with R0) |
10b | 10% (1/10) | 33% (1/3) | 60% (3/5) | 28% (5/18) | N/A | N/A | 0% (0/1) | 26% (5/19) |
| First DG approval to start of first SIA (Outbreak responses without R0) |
24b | 0% (0/3) | 0% (0/3) | 29% (2/7) | 15% (2/13) | 22% (2/9) | N/A | 0% (0/1) | 17% (4/23) |
| Day 0 to R0 start | 14a | 27% (3/11) | 0% (0/6) | 0% (0/7) | 13% (3/24) | 0% (0/1) | N/A | 0% (0/1) | 12% (3/26) |
| Day 0 to R1 start5 (R1 + R2 only outbreak responses) |
28a | 0% (0/5) | 0% (0/3) | 20% (2/10) | 11% (2/18) | 36% (4/11) | N/A | 0% (0/1) | 20% (6/30) |
| Day 0 to R1 start5 (All outbreak responses) |
28a | 22% (5/23) | 0% (0/9) | 11% (2/18) | 14% (7/50) | 39% (5/13) | N/A | 0% (0/2) | 19% (12/65) |
| Day 0 to R2 end5 | 56a | 31% (9/29) | 17% (2/12) | 32% (7/22) | 29% (18/63) | 50% (12/24) | N/A | 0% (0/2) | 34% (30/89) |
| R0 start to R2 end5 | 42a | 18% (4/22) | 0% (0/12) | 9% (1/11) | 11% (5/45) | 0% (0/7) | N/A | 0% (0/1) | 9% (5/53) |
| R1 start to R2 end5 (Outbreak responses without R0) |
28a | 62% (8/13) | 0% (0/3) | 46% (6/13) | 48% (14/29) | 50% (9/18) | N/A | 0% (0/1) | 48% (23/48) |
| Outbreak length (Day 0 to most recent isolate/onset) |
120a | 39% (9/23) | 25% (2/8) | 14% (3/21) | 27% (14/52) | 44% (7/16) | 0% (0/1) | 67% (2/3) | 32% (23/72) |
Abbreviations AFR, African Region; AG, Advisory Group; DG, Director-General of WHO; EMR, Eastern Mediterranean Region; EUR, European Region; PHE, Public Health Emergency; RA, Risk Assessment; SIA, Supplementary Immunization Activity; WPR, Western Pacific Region.
Percentage of compliant outbreak responses in region = number of outbreak responses in region compliant with target indicator/number of all outbreak responses in region applicable to indicator. Rounded to nearest integer.
Negative values stemming from activities conducted prior to a current outbreak were excluded from calculations. These values often originated in countries with multiple outbreaks.
The sole country in the European Region to face a cVDPV2 outbreak between April 2016 and December 2020 – Tajikistan – did not declare a public health emergency nor organize any mOPV2 response rounds. However, Tajikistan conducted nOPV2 SIAs in May, June, and August 2021 in response to PAK-GB-1.
Declaration of PHE for first outbreak in country. Among the 32 non-endemic countries (excludes Afghanistan and Pakistan), five countries did not declare PHEs for any cVDPV2 outbreaks in their respective jurisdictions.
Includes tOPV administered in response to WPV1 and cVDPV2 outbreaks in Afghanistan and Pakistan.
SOP target.
Target not in SOPs but expected operationally.
Following submission of the final risk assessment (n = 91) supporting mOPV2/tOPV use, the AG generally approved requests the same day (IQR 0–2 days) (Table 4); 71 (78.0%) of the requests were approved within the 2-day target (Table 5). Approval from the WHO DG for release (n = 68) typically followed AG approval by a median of two days (IQR:1–4) (Table 4); 32 (47.1%) were approved by the 1-day target (Table 5).
3.2.2. OBR SIAs: timeliness and SOP compliance
Countries subsequently conducted their first SIA at a median of 29.5 days following DG approval (n = 42), with the swiftest response two days and the slowest response 227 days following DG approval (Table 4). The median times from DG approval to the start of R0 and R1 SIAs were 12 and 62 days, respectively (Table 4); only 26% and 17% of R0 and R1 SIAs, respectively, were started within their respective target dates of 10 and 24 days (Table 5). Various SIAs, such as the initial R0 campaigns for Ethiopia-Oromia-3 (ETH-ORO-3) in Ethiopia and NIE-JIS-1 in Côte d’Ivoire, were delayed for months following their respective DG approvals in the first quarter of 2020 due to disruptions in implementation caused by the COVID-19 pandemic [12].
Overall, countries responded to outbreaks with a median of four mOPV2/tOPV SIAs (data not shown). The number of OBR SIAs ranged from 0 to 23 (IQR:3–6) and included rounds from previous outbreaks covering the same geographical scope, mOPV2 activities with concurrent administration of IPV, and tOPV SIAs for outbreaks in Afghanistan and Pakistan.
Of the 111 outbreaks, 10 (9.0%) were not responded to with mOPV2 nor tOPV, but rather IPV or nOPV2 and not covered by the AG process. Therefore, these outbreaks are not included in some of the analyses. The SIA response to China-Sichuan-1 (CHN-SIC-1) in China (index virus sample collection dated April 18, 2018) included only IPV. Responses to nine outbreaks, with dates of first detection during July–December 2020 (index case onset or sample collection indicated in parentheses) included only nOPV2 rounds and were delayed until March 2021 or later: [ANG-HUI-1 (September 8, 2020), ANG-LUA-1 (October 12, 2020), and CAF-BNG-1 (December 16, 2020) in Republic of the Congo; CHA-NDJ-1 in Ethiopia (December 28, 2020); NIE-JIS-1 in Liberia (October 5, 2020); NIE-JIS-1 in Senegal (December 24, 2020); NIE-JIS-1 in Sierra Leone (October 27, 2020); PAK-GB-1 in Tajikistan (November 22, 2020); and Nigeria-Zamfara State-1 (NIE-ZAS-1) in Nigeria (July 26, 2020)]. Although the DG had approved the release of mOPV2 for Liberia NIE-JIS-1 and Republic of the Congo ANG-HUI-1 in August 2020 and November 2020, respectively, these mOPV2 responses were ultimately canceled and amended to nOPV2 responses for March 2021 (Liberia) and May 2021 (Republic of the Congo).
For 101 (91%) of the 111 outbreaks, mOPV2 or tOPV was used as the OBR vaccine. Most of these responses (n = 53, of 101) included all three SOP-suggested initial rounds: R0, R1, and R2. The median initial response time for these OBRs, from the start of R0 to the end of R2, was 87 days (IQR:60–117) (Table 4). Conversely, 48 outbreaks included only R1 and R2 mOPV2 rounds for initial OBR, with a median initial response time, from the start of R1 to the end of R2, of 29 days (IQR:17–51) (Table 4). Among the 53 OBRs with rapid initial rounds, 26 R0s were conducted after the notification of circulating virus (Day 0) with a median of 21.5 days from Day 0 to the start of R0 (IQR:16–32). Only three (11.5%) of these 26 responses were conducted within the targeted 14 days of Day 0 (Table 5). Similarly, among the 48 OBRs without R0, 30 responses commenced with R1 following Day 0 with a median of 90 days from Day 0 to the start of R1 (IQR:29–156); only six (20%) of these OBRs complied with the targeted 28-day maximum interval between Day 0 and start of R1. Thus, among the 56 OBRs (26 R0 and 30 R1, above) to conduct mOPV2 response rounds following Day 0 notification, the median response time from Day 0 to first response was 32 days (IQR:20–99; Table 4).
In addition to the 56 outbreak responses that included first rounds after Day 0, nine outbreak responses included effective R0s preceding Day 0 (due to previous outbreaks in-country) and R1s shortly after Day 0. Among these 65 OBRs with R1 conducted after Day 0 (56 responses after Day 0 and nine with R0 preceding Day 0 but R1 after Day 0), R1 began a median of 51 days from Day 0 (IQ:30–95). Among these 65 responses, only 12 (18.5%) of R1s were conducted within 28 days from Day 0 (Table 5). The time interval between Day 0 and the end of the second large-scale campaign (R2) was a median of 79 days (IQR:38–154) (data not shown). Overall, only 30 (33.7%) of the 89 OBRs with R2 following Day 0 were completed by Day 56 (Table 5). Half (8 of 16) of the delays surrounding initial rounds conducted more than 100 days after Day 0 stemmed from campaign suspensions in the first half of 2020 attributable to the COVID-19 pandemic.
Regarding termination of circulation, the median number of days from Day 0 to last virus isolation (as of December 2021) among the 72 outbreaks with first isolates dated after Day 0 was 166.5 (IQR: 106–307 days; range: 11–1248 days); only 23 (31.9%) met the 120-day GPEI timeline (Table 5). Seven (43.8%) of the 16 applicable OBRs in the Eastern Mediterranean Region ceased virus circulation by Day 120, while only 14 (26.9%) of the 52 applicable outbreak responses in the African Region achieved the same target. Furthermore, only 7 (9.7%) of these 72 outbreaks were controlled within 30 days after the 120-day timeline for stopping the outbreak, and 18 (25%) of them continued for six months or more beyond the target, i.e., past Day 300. Outbreaks with all viruses isolated prior to Day 0 (n = 38) (negative values) and with indeterminant dates of Day 0 (n = 1) were excluded from these calculations.
Fifty-six (55.4%) of the 101 cVDPV2 outbreaks with an mOPV2 response that began on or before December 31, 2020, experienced a breakthrough case 14 days or more following the end of R2 requiring additional SIA activities.
4. Discussion
This analysis focused on the timeliness of different steps taken in response to cVDPV2 outbreaks compared to recommended timelines in the SOPs. The considerable variability among and within countries, many with COVID-19-related interruptions, makes it very difficult to generalize. Regardless, it is notable that only 34% (30/89) of outbreaks in this period achieved the response targets within 56 days, and only 32% (23/72) were able to stop outbreaks within the 120-day target (Table 5). Of 30 outbreaks in which response targets were met within 56 days, 77% (23/30) were stopped within 120 days. Conversely, when response targets were not met within 56 days, 49% (29/59) were stopped within 120 days (data not shown).
Five of the listed events (cVDPV-A and NIE-SOS-2 in Nigeria, RDC-HLO-1 in Democratic Republic of the Congo, SYR-DEI-1 in Syria, SOM-BAN-1 in Somalia) were outbreaks where transmission clearly or possibly began prior to the switch and were only recognized after the switch. This lag was expected and was part of the rationale for the creation of the mOPV2 stockpile and cVDPV2 OBR SOPs before the switch [13]. Three of five pre-switch emergence cVDPV2 outbreaks and two new peri-switch emergences detected during 2016–2017 were stopped by the end of 2017. This trend began to change in 2018 with the first detection of emergences that clearly originated from mOPV2 use. Even with this changing epidemiology, newly emerged and detected outbreaks in 2018 were stopped within a year with a few notable exceptions. This course changed dramatically in 2019 as characterized by many emergences in a small number of countries without a clear link to the use of mOPV2 in the affected areas, making 2019 the peak year for incident emergences. The reasons for this increase in 2019 remain unclear.
The large number of outbreaks over the period of study may be the result of the known risks associated with delays in implementing SIAs in affected areas and the spread of virus to new areas during those delays. In addition, some outbreaks continued in specific areas despite OBR activities, clearly indicating the deficiencies in vaccinating all children in those instances. These outbreaks occurred during a period when the increasing time since the switch led to an accumulating number of children who had never encountered type 2 OPV that increased the likelihood of more rapid spread of cVDPV2.
Management of the OBR process needed to adjust for several factors that were extremely similar to OBR activities prior to the switch but address a few novel issues that were the direct consequence of the switch. Since Sabin mOPV2 was the only tool initially available to respond to a cVDPV2 outbreak and the entire premise of the switch was to remove OPV2 from use, access was tightly controlled; only the WHO DG could release mOPV2/tOPV2 on the advice of the AG. Thus, country response from detection of the first virus to the preparation of a risk assessment needed to proceed rapidly to conduct initial SIAs and stop the outbreak as quickly as possible. Accordingly, when we examined this process, the initial RA was submitted a median of 16 days after Day 0 and requested vaccine was released by the DG a median of 2 days after that. The DG released vaccine within 8 days of initial RA submission for 75% of the outbreaks reported during April 1, 2016-December 31, 2020.
The key elements of an appropriate OBR SIA should involve a timely process, an appropriately designed scope based on a risk assessment, and effective implementation to achieve high coverage. There were some key areas where delays were evident. The first area was the time from detection of the first virus of the outbreak to notification of circulating virus (Supplemental Table 1). Normally, this process was initiated by the detection of a newly designated cVDPV2 and notification to the national and WHO authorities. The challenge is that this identification timeline is affected by gaps in surveillance, as evidenced by the large genetic divergence of an index virus from the parental Sabin type 2 vaccine strain for certain emergences (Supplemental Table 1). With an approximate 10 nucleotide substitutions per year, this divergence indicates that several emergences went undetected for much more than a year from their originating OPV2 dose. A second way that notification delay occurred was in the establishment of evidence of local circulation with viruses detected through ES. In the early post-switch period, most VDPV2 viruses detected through ES sampling never resulted in an outbreak notification (circulation defined in the SOPs as two positive samples from the same site ≥60 days apart) due to successive detections at shorter intervals. In the few cases where an outbreak was so detected, a 60-day delay resulted. As population immunity decreased with time, this definition was relaxed in newly affected geographies and outbreak responses were considered more rapidly in those instances, although the SOPs were not formally revised.
The recognition of an outbreak triggers immediate planning for a response. Hence, a second category of delays concerns the time from OBR planning until the SIAs commence. These delays could be due to multiple factors including preparation of an adequate RA, delays in vaccine requests, limitations on global vaccine stockpile supplies, delays in implementation during part of 2020 due to COVID-19, and the deliberate outbreak response delays while waiting for availability of nOPV2. Delays would accumulate in some specific OBRs where initial SIAs failed to stop transmission.
As can be seen from our results, median times in general are close to the established guidelines. However, as is evident from the overall and interquartile ranges for some of these steps, notable outliers often occurred. There are also some prominent differences between regions. It has not been possible to identify any consistent or general reason for many of these delays, but it does denote that some outbreaks that continued and spread were not responded to in a timely manner, yet other responses with delays were promptly stopped. The inherent risks of transmission are different between and within countries and may also explain some of these discordant results.
Various additional factors affected timeliness of outbreak responses. For example, the initial outbreak response to NIE-JIS-1 in Côte d’Ivoire (index case onset of paralysis dated September 24, 2019) was delayed by 120 days between the initial risk assessment submission and AG approval. This delay was first due to multiple independent importations from Ghana that provided no evidence of local circulation based on the absence of genetic relatedness of the ES samples. Weighing the risks of introducing mOPV2 into the OPV2 naïve population and the limited global mOPV2 stockpile at the time, the AG did not approve the early RA pending further developments in virus detection in Côte d’Ivoire. Once evidence of local circulation was established, an updated RA submission with target population miscalculations and an exhausted global mOPV2 supply further delayed release until replenished.
Among several limitations of our study, we did not attempt to examine the specific details of each OBR and the means by which those aspects affect success in stopping outbreaks. Other than timeliness, multiple other critical factors could determine the effectiveness of any specific response. The effectiveness of OBR SIAs, as indicated by coverage, can be highly variable and difficult to assess; we did not assess any indicators of OBR SIA quality. Robust field and laboratory surveillance with minimal delays is critical to estimate the likely location of virus transmission at the time of a response. The more uncertainty there is about the geography of transmission, the larger the response should be. However, the larger the response, the more likely mOPV2 is to create a new cVDPV2 emergence (a longer perimeter of the OBR zone). These risks can be mitigated by high coverage during OBR SIAs to markedly reduce the number of susceptible children remaining and limit circulation of Sabin-related virus. Conversely, failure to achieve high coverage can lead to new emergence outbreaks in the OBR zone and contribute to clustering of outbreaks in specific countries.
Key limitations of this study were in the completeness and quality of the data available. These limitations are readily attributable to the challenges of linking epidemiologic, decision management, laboratory, and immunization data recorded at different times in different databases, often with discrepancies or missing variables. The authors declare that there may be remaining errors that could not be resolved. Missing data for some key performance measures imply that some specific elements cannot be weighted in interpretation. Because of these limitations, it was not possible nor prudent to address causality related to outcome. With limited ability to compare populations within and between countries, it is not readily possible to compare factors affecting the high variability between genetic emergences and determine reasons for persistence, spread, or swift termination of certain emergences and outbreaks compared to others. Even within populations, why some countries have variable results between outbreaks is not readily addressable. In addition, it was not possible to address the effects of the COVID-19 pandemic on the outbreak response process after OBR SIAs resumed due to insufficient information on program and staffing disruptions by country.
This study has attempted to address the decision-making process of polio SIA responses to cVDPV2 outbreaks and the timeliness of the initial immunization response during the first four plus years post-switch. This study has included both the extent and heterogeneity of cVDPV2 outbreak spread and duration, as well as program performance indicators. There is little indication of systemic OBR flaws, but the variability in meeting SOP targets is an indication of the opportunities for more consistent and improved performance of future OBR management.
5. Funding.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
6. Data Availability Statement.
Data that support the findings of this study are available from the corresponding author upon reasonable request.
7. Disclaimer
The views expressed are solely those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
CRediT authorship contribution statement
Roopa Darwar: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Oladayo Biya: Methodology, Investigation, Writing – original draft, Project administration. Sharon A. Greene: Conceptualization, Methodology, Investigation, Writing – review & editing. Jaume Jorba: Methodology, Investigation, Writing – review & editing. Mohammad Al Safadi: Investigation, Writing – review & editing. Richard Franka: Writing – review & editing. Eric Wiesen: Writing – review & editing. Elias Durry: Writing – review & editing. Mark A. Pallansch: Conceptualization, Methodology, Investigation, Writing – original draft, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge Mary Alleman, Wasan Al-Tamimi, Elizabeth Davlantes, Djebo Gourmanon, Steve Mwangi, Dhoud Samba, Kourouma Sory, and Margaret Werner for providing dates of various SIAs and Adam Kraus and Neha Sikka for developing the main data abstraction framework.
Footnotes
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization or UNICEF or Bill and Melinda Gates Foundation. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.02.060.
Contributor Information
Roopa Darwar, Email: rdarwar@cdc.gov.
Oladayo Biya, Email: ybn2@cdc.gov.
Sharon A. Greene, Email: SGreene@cdc.gov.
Jaume Jorba, Email: jfj6@cdc.gov.
Mohammad Al Safadi, Email: alsafadim@who.int.
Richard Franka, Email: rpf5@cdc.gov.
Eric Wiesen, Email: ejw2@cdc.gov.
Elias Durry, Email: exd4@cdc.gov.
Mark A. Pallansch, Email: map1@cdc.gov.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, Rubin J, Garon J, Harris J, Hyde T, Wassilak S, Patel M, Nandy R, Chang-Blanc D, Immunization Systems Management Group of the Global Polio Eradication Initiative. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine — Worldwide, 2016. Morbidity and Mortality Weekly Report, 2016; 65(35): 934–938. https://www.jstor.org/stable/24858956. [DOI] [PubMed]
- 2.Kalkowska D.A., Pallansch M.A., Cochi S.L., Kovacs S.D., Wassilak S.G., Thompson K.M. Updated characterization of post-OPV cessation risks: Lessons from 2019 serotype 2 outbreaks and implications for the probability of OPV restart. Risk Anal. 2021;41(2):320–328. doi: 10.1111/risa.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns C.C., Diop O.M., Sutter R.W., Kew O.M. Vaccine-derived polioviruses. J Infect Dis. 2014;210(suppl_1):S283–S293. doi: 10.1093/infdis/jiu295. [DOI] [PubMed] [Google Scholar]
- 4.Jorba J., Diop O.M., Iber J., Henderson E., Zhao K., Quddus A., et al. Update on vaccine-derived poliovirus outbreaks—worldwide, January 2018–June 2019. Morb Mortal Wkly Rep. 2019;68(45):1024. doi: 10.15585/mmwr.mm6845a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Standard operating procedures: responding to a poliovirus event or outbreak – version 4; 2020. p. 25. Retrieved from https://apps.who.int/iris/handle/10665/363627.
- 6.Lopalco P.L. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol Infect. 2017;145(3):413–419. doi: 10.1017/S0950268816002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macklin G.R., O’Reilly K.M., Grassly N.C., Edmunds W.J., Mach O., Krishnan R.S.G., et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science. 2020;368(6489):401–405. doi: 10.1126/science.aba1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. International Health Regulations (2005), 2nd ed.; 2008. Retrieved from http://www.who.int/ihr/publications/9789241596664/en.
- 9.Global Polio Eradication Initiative Polio Information System. SIA data dictionary; 2020. Retrieved from https://extranet.who.int/polis/DocumentsComponent/GetDocument?fileId=593.
- 10.Global Polio Eradication Initiative. nOPV2: clinical development summary; 2020. Retrieved from https://polioeradication.org/wp-content/uploads/2021/02/nOPV2-Clinical-Development-Summary-1.29-EN.pdf.
- 11.World Health Organization. First ever vaccine listed under WHO emergency use; 2020. Retrieved from https://www.who.int/news/item/13-11-2020-first-ever-vaccine-listed-under-who-emergency-use.
- 12.Global Polio Eradication Initiative. Call to action to support COVID-19 response; 2020. Retrieved from https://polioeradication.org/wp-content/uploads/2020/04/POB-COVID-19-Statement-20200402.pdf.
- 13.Thompson K.M., Pallansch M.A., Duintjer Tebbens R.J., Wassilak S.G., Kim J.H., Cochi S.L. Preeradication vaccine policy options for poliovirus infection and disease control. Risk Anal. 2013;33(4):516–543. doi: 10.1111/risa.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Data will be made available on request.