Abstract
Friedreich's ataxia (FA) is a life-threatening autosomal recessive disorder characterized by neurological and cardiac dysfunction. Arrhythmias and heart failure are the main cause of premature death. From prior studies in murine models of FA, adeno-associated virus encoding the normal human frataxin gene (AAVrh.10hFXN) effectively treated the cardiac manifestations of the disease. However, the therapeutic dose window is limited by high level of human frataxin (hFXN) gene expression associated with toxicity. As a therapeutic goal, since FA heterozygotes have no clinical manifestations of FA, we estimated the level of frataxin (FXN) necessary to convert the heart of a homozygote to that of a heterozygote. In noncardiac cells, FA heterozygotes have 30–80% of normal FXN levels (17.7–47.2 ng/mg, average 32.5 ng/mg) and FA homozygotes 2–30% normal levels (1.2–17.7 ng/mg, average 9.4 ng/mg). Therefore, an AAV vector would need to augment endogenous in an FA homozygote by >8.3 ng/mg. To determine the required dose of AAVrh.10hFXN, we administered 1.8 × 1011, 5.7 × 1011, or 1.8 × 1012 gc/kg of AAVrh.10hFXN intravenously (IV) to muscle creatine kinase (mck)-Cre conditional knockout Fxn mice, a cardiac and skeletal FXN knockout model. The minimally effective dose was 5.7 × 1011 gc/kg, resulting in cardiac hFXN levels of 6.1 ± 4.2 ng/mg and a mild (p < 0.01 compared with phosphate-buffered saline controls) improvement in mortality. A dose of 1.8 × 1012 gc/kg resulted in cardiac hFXN levels of 33.7 ± 6.4 ng/mg, a significant improvement in ejection fraction and fractional shortening (p < 0.05, both comparisons) and a 21.5% improvement in mortality (p < 0.001). To determine if the significantly effective dose of 1.8 × 1012 gc/kg could achieve human FA heterozygote levels in a large animal, this dose was administered IV to nonhuman primates. After 12 weeks, the vector-expressed FXN in the heart was 17.8 ± 4.9 ng/mg, comparable to the target human levels. These data identify both minimally and significantly effective therapeutic doses that are clinically relevant for the treatment of the cardiac manifestations of FA.
Keywords: adeno-associated virus, Friedreich's ataxia, cardiac gene therapy, dose-finding study
INTRODUCTION
Friedreich's ataxia (FA), an autosomal recessive life-threatening disorder for which there is no approved treatment, is the most common inherited ataxia.1 The frataxin (FXN) protein is expressed in the cytoplasm and directed to the mitochondria where it plays a critical role in energy generation.2–7 The majority of FA patients have varying degrees of guanine-adenine-adenine trinucleotide expansion within the first intron of the FXN gene on chromosome 9q214, which causes transcriptional silencing, leading to a reduction in the levels of FXN messenger RNA and protein.8–13 Symptoms of the disorder start at 5–15 years of age.14–17 The neurological manifestations occur first, with ataxia, loss of sensation, and dysarthria. The cardiac disease typically manifests later, at 20–25 years of age.18,19 While progressive neurological disease limits mobility, cardiomyopathy is the cause of death in two thirds of individuals with FA, with heart failure and arrhythmias, the common causes of cardiac-related death.1,18–21
From prior studies in experimental animals, it is known that a cardiotropic adeno-associated virus encoding the normal human frataxin gene (AAVrh.10hFXN) will effectively treat the cardiac manifestations of the disease.22 Perdomini et al.23 showed that intravenous (IV) administration of AAVrh.10hFXN dramatically reversed the cardiac insufficiency phenotype in the muscle creatine kinase (mck)-Cre conditional knockout Fxn mouse model of FA,24 in which the mouse fxn gene is completely deleted from the heart and skeletal muscle. A dose of 5.4 × 1013 gc/kg of AAVrh.10hFXN reversed changes in left ventricular (LV) mass, fractional shortening, survival, and corrected the cardiac biochemical defects.21 The early onset of the cardiac disease was prevented, and heart failure and cardiac remodeling were reversed.
AAVrh.10hFXN-mediated dosing studies have also been carried out in the cardiac-specific partial knockout FXN alpha myosin-heavy chain (αMyhc) mouse model, which expresses less than 50% of the normal murine FXN levels.25 These mice are normal at rest and only have a dysfunctional cardiac phenotype when stressed by the administration of dobutamine or exercise.25 Following IV administration of AAVrh.10hFXN at a dose of 4 × 1012 gc/kg, significant rescue was observed in the cardiac phenotype as indicated by correction of dobutamine stress-induced ejection fraction and fractional shortening and improvement in treadmill performance.25
While AAV-mediated FXN gene therapy is efficacious in these experimental models of cardiac manifestations of FA, the therapeutic window is narrow, with high levels of the human frataxin (hFXN) transgene expression associated with toxicity.26–29 Belbellaa et al.28 demonstrated that IV administration of 2.5 × 1013 gc/kg dose of an AAVrh.10 vector encoding human FXN gene to MCK mice initially showed complete restoration of cardiac function, but the high levels of myocardial hFXN at this dose were ultimately associated with a decline in cardiac function, with cardiomyocyte subcellular disorganization, cell death, and fibrosis. Consistent with these data, Huichalaf et al.29 demonstrated that, in the MCK mouse, doses between 1 × 1013 and 1 × 1014 vg/kg of an AAV9FXN vector with and without codon optimization of the transgene led to FXN-mediated toxicity in the liver and heart.29
Together, these studies lead to the question: What is the minimal effective dose of AAVrh.10hFXN to safely treat the cardiac manifestations of FA? To answer this, we first determined the levels of cardiac FXN necessary to convert a human FA homozygote to FA heterozygote. Then, using this level of cardiac FXN as the minimal therapeutic goal, we assessed two experimental animal models, MCK FXN CRE-conditional knockout mice and normal nonhuman primates (NHPs), to determine the minimum dose of IV administration of AAVrh.10hFXN necessary to convert the cardiac FXN levels of a human FA homozygote to estimated FA heterozygote levels.30
METHODS
Assessment of heart FXN levels was carried out in human heart (n = 5) tissue obtained from autopsies of individuals that died of causes other than FA. AAVrh.10hFXN was administered IV via the tail vein to 7-week-old MCK mice (n = 10/sex/group) at 1.8 × 1011, 5.7 × 1011, and 1.8 × 1012 gc/kg (Supplementary Table S1). The mice underwent weighing and testing of cardiac function in-life and assessment of cardiac hFXN levels following necropsy. AAVrh.10hFXN was administered IV to NHP (n = 4 NHP per dose) at 5.7 × 1011 and 1.8 × 1012 gc/kg, with two NHP receiving phosphate-buffered saline injections (Supplementary Table S2). The NHP underwent assessment of cardiac hFXN levels following necropsy. Detailed methods including statistical analysis are provided in the Supplementary Data.
RESULTS
FXN levels in a non-FA disease heart
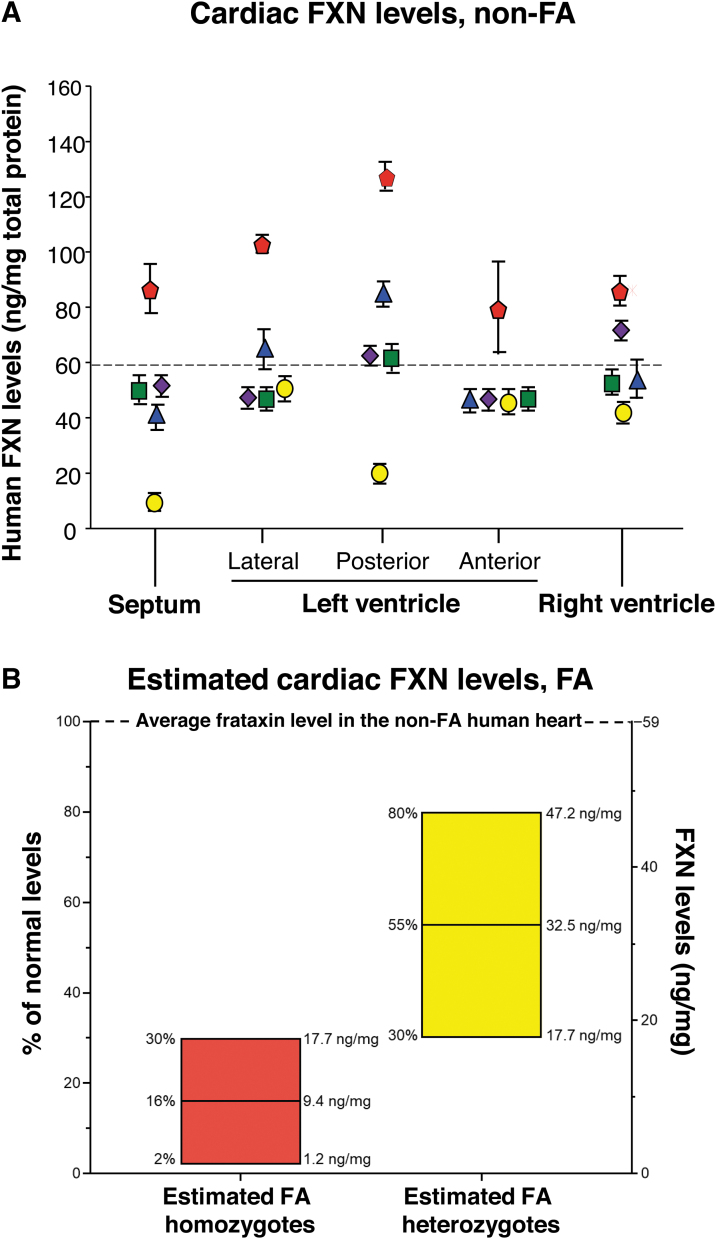
To establish the endogenous FXN levels in the myocardium of non-FA subjects, human cardiac samples were obtained during autopsy from five individuals who did not have FA. The average level of myocardial FXN measured in the heart samples was 59 ± 5 ng/mg protein (Fig. 1A). These data were utilized to estimate the cardiac FXN levels in subjects who are heterozygote and homozygote for FA. Individuals with FA have FXN levels in noncardiac tissues (buccal cells) 2–30% normal (1.2–17.7 ng/mg, mean 16% or 9.4 mg), and heterozygote carriers have FXN protein levels 30–80% normal in noncardiac tissues (17.7–47.2 ng/mg).30 Based on the concept that the percent normal of the homozygote and heterozygote FXN levels in the heart parallel that in noncardiac tissues, using the normal cardiac FXN levels as the guide, we estimated the cardiac FXN levels in FA homozygotes and heterozygotes (Fig. 1B).
Figure 1.
FXN protein levels in the non-FA and FA human heart. (A) Human cardiac FXN levels in individuals who do not have FA. Heart samples from five subjects at autopsy were obtained from the Department of Pathology at Weill Cornell Medicine. For each heart, the septum, left ventricle (including lateral, posterior, and anterior walls), and right ventricle were assessed. Samples were analyzed in triplicate (mean ± SEM). Subject 1 (80-year-old male, White), yellow circle; Subject 2 (75-year-old male, Hispanic), red pentagram; Subject 3 (65-year-old female, African American), blue triangle; Subject 4 (73-year-old female, White), green square; and Subject 5 (72-year-old male, White), purple diamond. Although there was subject to subject variability, on average, FXN levels in the different heart regions were similar (p ≥ 0.06). The mean of all heart samples was 59 ± 5 ng FXN/mg protein (dashed line). (B) FXN levels in human FA homozygote and heterozygote heart estimated using the mean normal levels (A) extrapolated to the heart using the FXN levels in noncardiac tissues in FA homozygous and heterozygotes (see FXN levels in a non-FA disease heart in the Results section and Lazaropoulos et al.30). FA, Friedreich's ataxia; FXN, frataxin; SEM, standard error of the mean.
Efficacy of varying doses of AAVrh.10hFXN in the MCK mouse model of FA cardiomyopathy
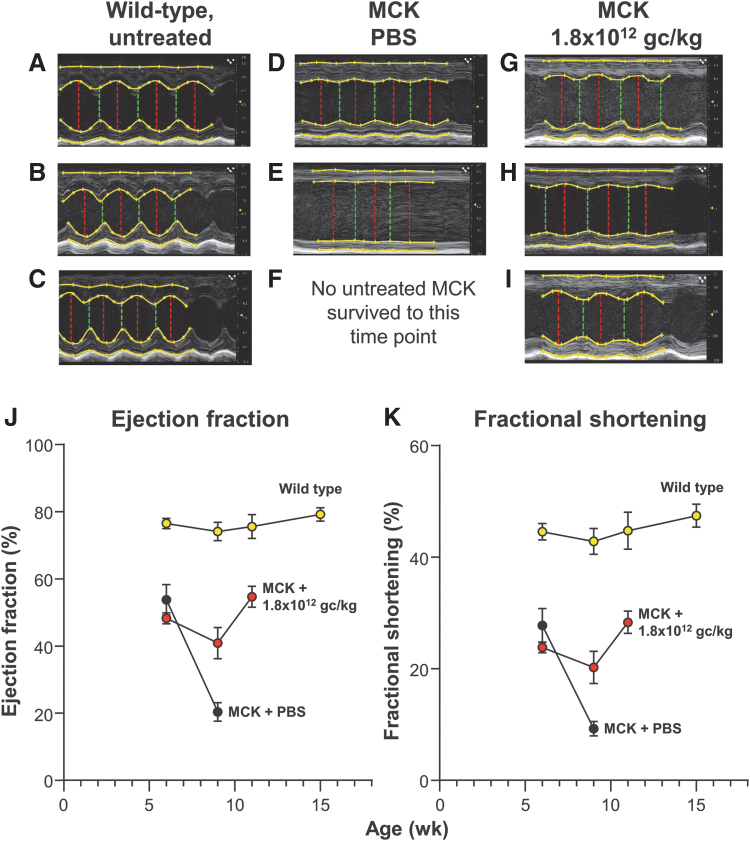
AAVrh.10hFXN was administered IV via the tail vein to 7-week-old MCK mice (n = 10/gender/group) at three dose levels (1.8 × 1011, 5.7 × 1011, and 1.8 × 1012 gc/kg) (Supplementary Table S1). An additional 10/gender MCK and 5/gender wild-type (WT) C57Bl/6J mice were administered vehicle as negative and positive controls, respectively. Administration was performed at 7 weeks of age in MCK mice as this is when the phenotype starts to develop, and there is a measurable difference between these mice and WT mice.24,31 All mice in the study were weighed and monitored regularly, and a subset underwent echocardiographic assessments at scheduled time points. Mice in the study were sacrificed when moribund, and heart samples were collected from half of the animals from each group for measurement of tissue FXN protein levels.
General health
Mice in the study were assessed for respiratory function, alertness, general behavior, reflex responsiveness, and general health. There was a dose response on the general health of the treated mice as evidenced by the age at which the different dose groups required supplemental care. MCK mice that received PBS required supplemental care at age 56 days (7-day post-treatment), 1.8 × 1011 and 5.7 × 1011 gc/kg dose groups at age 66 days (17-day post-treatment), and the 1.8 × 1012 gc/kg dose group at age 76 days (27-day post-treatment).
Body weight
As expected, untreated MCK mice began to lose body weight over time due to rapid disease progression. However, following AAVrh.10hFXN administration, there was a dose-dependent improvement in body weight. Using a repeated-measures analysis of variance over time, there was no improvement in body weight at the low dose of 1.8 × 1011 gc/kg. Within the 5.7 × 1011 gc/kg dose, there was a trend in improvement in body weight compared with controls. For the 1.8 × 1012 gc/kg dose, the body weight increases were significant compared with the PBS-treated controls (p < 0.0001; Fig. 2).
Figure 2.
Body weights of treated MCK mice post-administration. The average life span of untreated MCK mice is 11 weeks.24 As a measure of overall health, MCK mice were weighed at regular intervals after vector or PBS treatment until they became moribund (Supplementary Table S1). The mean weights (g) ± SEM are shown for time of assessment after vector administration, with males (squares) and females (triangles) separately. All mice were treated at 7 weeks of age ±1–2 days. Group 1 mice (1.8 × 1011 gc/kg dose, blue symbols); Group 2 mice (5.7 × 1011 gc/kg dose, green symbols); Group 3 mice (1.8 × 1012 gc/kg, red symbols); Group 4 mice (PBS controls, gray symbols). (A) Males; (B) Females. MCK, muscle creatine kinase (mck)-Cre conditional knockout Fxn mouse model; PBS, phosphate-buffered saline.
Echocardiograms
The low (1.8 × 1011 gc/kg) and medium (5.7 × 1012 gc/kg) doses had no impact on echocardiographic parameters (data not shown). However, by 4-week post-dose, compared with PBS-treated control MCK mice, a single IV administration of 1.8 × 1012 gc/kg AAVrh.10hFXN demonstrated a significant improvement in echocardiographic parameters including ejection fraction and fractional shortening when compared with PBS-treated control MCK mice (p < 0.05, both comparisons; Fig. 3).
Figure 3.
Echocardiographic assessment of cardiac function of FXN-MCK mice and controls following treatment with AAVrh.10hFXN. Mice from each treatment group were monitored over time with echocardiography (see the Methods section). M-mode measurements were recorded for the short axis of the left ventricle at the midventricular level. (A–I) Representative echocardiogram tracings of the left ventricle for the same male mouse at 3 time points (7, 9, and 11 weeks of age, with the 7-week time point just before treatment and the 9- and 11-week assessments post-treatment) from each cohort. Description of the echocardiogram tracings in each panel, top to bottom: Top yellow line in each image traces the epicardial border along the anterior wall of the left ventricle, the second yellow line is a trace of the anterior endocardial wall border from full systole to full diastole. The third yellow line from the top traces the endocardial border of the posterior wall; the bottom yellow line traces the epicardial border of the posterior wall. The top two and bottom two lines together measure the wall thickness of the anterior and posterior wall of the left ventricle, respectively. The vertical red and green lines (left to right in each panel) measure the chamber of the left ventricle at full diastole and full systole, respectively. (A–C) WT untreated mouse control at 41, 64, and 76 days. (D–F) MCK mouse, PBS treated control at 42 and 63 days. (G–I) MCK mouse, 1.8 × 1012 gc/kg dose at 42, 63, and 76 days. No mice in the PBS-treated MCK mouse cohort survived to the 11-week assessment time point. (J, K) Quantitative assessment of ejection fraction and fractional shortening calculated from the M-mode measurements of the short axis. (J) Change in ejection fraction (%) over time of WT C57Bl/6, PBS-treated, and AAVrh.10hFXN (1.8 × 1012 gc/kg)-treated MCK mice. (K) Change in fractional shortening (%) in the same mice. Shown is the mean and standard error of percent change. All measurements were performed at 6 weeks (1 week before treatment), 9, 11, and 15 weeks of age for all surviving mice. Data from males and females were combined for each dosage cohort, shown as mean ± SEM. C57Bl/6 WT mice, yellow circles; MCK mice treated with AAVrh.10hFXN, 1.8 × 1012 gc/kg dose, red circles; and MCK mice with PBS (controls), black circles. AAVrh.10hFXN, adeno-associated virus encoding the normal human frataxin gene; M-mode, motion mode; WT, wild type.
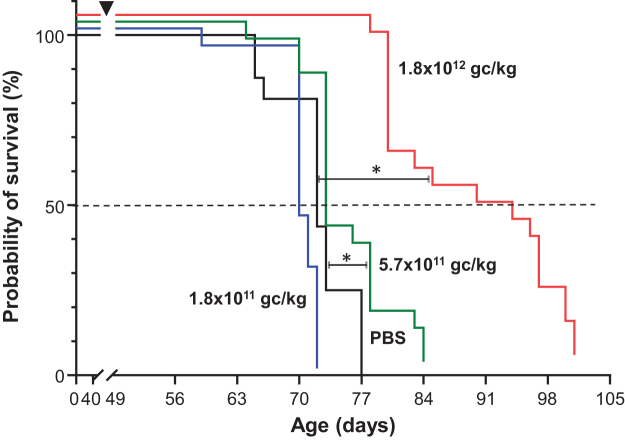
Survival
PBS-treated MCK mice survived an average of 72 days (∼10 weeks), akin to the life span of untreated MCK mice.24 AAVrh.10hFXN administration resulted in a dose-dependent improvement in survival. While there was no improvement in survival with the 1.8 × 1011 gc/kg dose, there was a small but significant improvement in mortality with 5.7 × 1011 gc/kg dose (p < 0.01), and a 21.5% improvement (87.5 vs. 72 days, p < 0.001) compared with untreated controls at the 1.8 × 1012 gc/kg dose (Fig. 4).
Figure 4.
Dose–response survival of MCK mice administered AAVrh.10hFXN. MCK mice were intravenously administered AAVrh.10hFXN at 3 different doses (1.8 × 1011, 5.7 × 1011, and 1.8 × 1012 gc/kg) or PBS at 7 weeks of age (±2 days, at 48–50 days). Mice were euthanized when moribund. Survival plots (Kaplan–Meier) were plotted for each dose cohort, with survival curves slightly offset on y-axis to show all the data. MCK mice with AAVrh.10hFXN, 1.8 × 1011 gc/kg dose, blue line; 5.7 × 1011 gc/kg dose, green line; 1.8 × 1012 gc/kg dose, red line; MCK mice with PBS (controls), black solid line. Black dashed line, 50% survival. Comparison of treated versus PBS survival curves was analyzed with log-rank (Mantel–Cox) test, with log-rank test for trend. *p < 0.01 for 5.7 × 1011 gc/kg dose group and p < 0.0001 for the 1.8 × 1012 gc/kg dose.
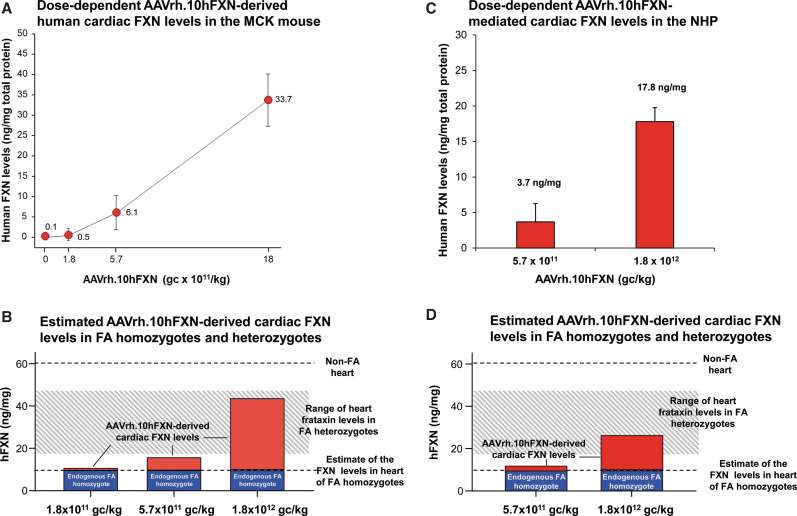
Cardiac FXN expression
A dose-dependent increase in cardiac FXN expression was seen with average increase in FXN levels of 0.5 ± 0.1, 6.1 ± 4.2, and 33.7 ± 6.4 ng/mg, respectively, for 1.8 × 1011, 5.7 × 1011, and 1.8 × 1012 gc/kg of AAVrh.10hFXN administered (Fig. 5A). To establish if the levels observed in MCK mice are in the “therapeutic” range, cardiac FXN levels observed in MCK mice were added to the estimated endogenous FXN levels in FA homozygotes (Fig. 5B; Table 1). When the average FXN level increase at the 5.7 × 1011 and 1.8 × 1012 gc/kg doses was added to the estimated endogenous levels, the estimated increase in cardiac FXN levels would reach 26% and 73% of normal human heart FXN levels, respectively, and would be within the heterozygous range at the 1.8 × 1012 gc/kg dose.
Figure 5.
Dose-dependent cardiac FXN levels following AAVrh.10hFXN treatment of MCK mice (A, B) and NHPs (C, D) and correlation of these to estimated cardiac FXN levels in FA homozygotes and heterozygotes. (A) Dose-dependent human cardiac FXN levels in MCK mice intravenously administered AAVrh.10hFXN at 1.8 × 1011, 5.7 × 1011, and 1.8 × 1012 gc/kg (3 male and 3 female/dose, except the highest dose group 4 male/4 female). PBS-administered mice (3 male/3 female) served as negative control. At sacrifice, hFXN levels in the heart were assessed. The AAV-expressed human FXN levels in the treated mouse hearts (left ventricular wall) were measured in triplicate by ELISA and expressed as ng/mg total protein (mean ± SEM) of male and female combined for each dose group. Human FXN levels are shown versus AAVrh.10hFXN dose (log scale). (B) Estimated FXN cardiac levels in FA homozygotes based on the AAVrh.10FXN dose-dependent cardiac levels achieved in the MCK mice. The human FXN levels obtained in the MCK mouse in (A) were combined with the estimated endogenous human heart FXN levels in FA homozygotes (Fig. 1). The vector-derived hFXN levels observed in MCK mice (red bars) were added to the estimated endogenous cardiac FXN levels of 9.4 ng/mg estimated in FA homozygotes (blue bars), which allows for the estimation of the FXN levels obtained from a given dose of AAVrh.10hFXN. The gray shading represents the FXN level range estimated in FA carriers (heterozygotes). (C) NHP cardiac FXN levels following dose-dependent administration of AAVrh.10hFXN. Shown is the average of FXN protein levels in the heart (5 samples/NHP) assessed by ELISA of n = 4 (2 male, 2 female) NHPs administered 5.7 × 1011 gc/kg and n = 4 (2 male, 2 female) administered 1.8 × 1012 gc/kg. Data shown as mean ± SEM of 5 heart regions combined, for all animals for each dose group, with the mean values above each bar. The human FXN ELISA assay detects endogenous FXN in the NHP heart. The baseline NHP FXN levels were subtracted (using PBS controls levels, mean 45.3 ng/mg obtained from 1 male/1 female). (D) Extrapolation from the NHP data of the dose-dependent levels of FXN that would be achieved in the heart of the average FA patient following administration of AAVrh.10hFXN. The mean human FXN levels quantified in the treated NHPs (C) added to the estimated human FA homozygote data (Fig. 1B) allow for the estimation of the theoretical efficacy of the AAVrh.10hFXN vector dosing. The vector-derived hFXN levels observed in NHP (red bars) were added to the hFXN level baseline of 9.4 ng/mg estimated in FA homozygotes (blue bars). The gray shading represents the FXN level range seen in FA carriers (heterozygotes). NHP, nonhuman primate.
Table 1.
Estimated Total Frataxin Levels Estimated to Be Achieved in the Friedreich's Ataxia Homozygote Heart Following Intravenous Administration of AAVrh.10hFXN
| Experimental model | AAVrh.10hFXN dose (gc/kg) | AAVrh.10hFXN-derived cardiac hFXN levels (ng/mg)a | Estimated average endogenous cardiac FXN level in FA homozygotes (ng/mg)b | Estimated total cardiac FXN levels following AAVrh.10hFXN administration (ng/mg)c | Comparison (ng/mg) to reach the estimated minimal FA heterozygote cardiac level (17.7 ng/mg)d |
|---|---|---|---|---|---|
| MCK mouse | 1.8 × 1011 | 0.5 | 9.4 | 9.9 | −7.8 |
| 5.7 × 1011 | 6.1 | 9.4 | 15.5 | −2.2 | |
| 1.8 × 1012 | 33.7 | 9.4 | 43.1 | +25.4 | |
| NHP | 5.7 × 1011 | 3.7 | 9.4 | 13.1 | −4.6 |
| 1.8 × 1012 | 17.8 | 9.4 | 27.2 | +9.6 |
Using FXN levels in non-FA human cardiac tissue obtained at autopsy, known FXN levels homozygotes and heterozygotes in noncardiac tissues,30 and FXN levels obtained following administration of AAVrh.10hFXN in MCK mice and NHP, this table estimates the total FXN estimated levels associated with each.
Cardiac FXN levels were determined using ELISA from following necropsy in MCK mice or NHPs as described in the Methods section. In NHP, the endogenous NHP FXN levels detected by the hFXN ELISA were subtracted to give levels directly mediated by AAVrh.10hFXN.
The levels of FXN in buccal cells and blood mononuclear cells in FA homozygotes are 2–30% of normal (median 16%, of normal).30 Based on the assumption that the percent normal level in homozygotes is similar in the heart and with the knowledge that normal cardiac tissue has FXN levels of 59 ng/mg, this translates to an estimated cardiac FXN level of 9.4 ng/mg in FA homozygotes.
The addition of the AAVrh.10hFXN-mediated FXN levels with the estimated average endogenous cardiac FXN levels in an FA homozygote results in the estimated total cardiac FXN levels following AAVrh.10hFXN administration.
FA heterozygotes have noncardiac FXN levels 30–80% of normal and are phenotypically normal. Translating this to the normal heart levels of 59 ng/mg leads to an estimation that FA heterozygotes have cardiac FXN levels of 17.7–47.2 ng/mg. Based on these estimates, achieving ≥17.7 ng/mg of normal human myocardial FXN levels should be potentially curative in these patients.
AAVrh.10hFXN, adeno-associated virus encoding the normal human frataxin gene; ELISA, enzym-linked immunosorbent assay; FA, Friedreich's ataxia; hFXN, human frataxin; FXN, frataxin; MCK, muscle creatine kinase (mck)-Cre conditional knockout Fxn mouse model; NHP, nonhuman primate.
Efficacy of varying doses of AAVrh.10hFXN in NHP
AAVrh.10hFXN was administered IV to NHP (n = 4 NHPs, two males/two females per dose) and at two dose levels (5.7 × 1011 and 1.8 × 1012 gc/kg) and two NHPs received PBS injections (one male and one female) (Supplementary Table S2). All NHPs in the study were weighed and monitored regularly, and NHPs were sacrificed at 8-week post-administration, and heart samples were collected from each group for measurement of tissue FXN protein levels. No abnormal findings were recorded during the length of the study. All animals remained healthy and alert until sacrifice.
Cardiac hFXN expression in NHP
The heart samples for NHP administered PBS had endogenous levels of NHP FXN (45.3 ± 2.6 ng/mg) as quantified by the cross-reactivity of the anti-human FXN antibody. After subtracting the endogenous NHP FXN levels, there was an AAVrh.10hFXN-mediated dose-dependent increase in FXN expression with levels of 3.7 and 17.8 ng/mg for the 5.7 × 1011 and 1.8 × 1012 gc/kg doses, respectively (Fig. 5C). To establish if the levels observed in NHP were in the “therapeutic” range, FXN levels observed in NHP were added to the estimated endogenous FXN levels in FA homozygotes and heterozygotes (Table 1; Fig. 5D). At doses of 5.7 × 1011 and 1.8 × 1012 gc/kg, the increase in FXN levels when added to endogenous reached 22% and 46% of normal human heart FXN levels, respectively. The high dose mediated cardiac FXN levels that were estimated to be within the heterozygous range.
DISCUSSION
A well-grounded rationale exists to support the proposed use of gene therapy with AAVrh.10hFXN as a strategy to reverse cardiac manifestation of FA, including: (1) slowly progressive disorder with well-identified target cells (cardiac myocytes); (2) genetic etiology (FXN) and understood pathogenesis; (3) proof-of-concept established in two independent FA cardiac gene knockout models treated with AAVrh.10hFXN.23,25,27 While AAVrh.10hFXN-mediated FXN gene therapy is efficacious in these experimental models, the therapeutic window is narrow, with high levels of the hFXN transgene expression associated with toxicity.28,29 The goal of this study was to establish the cardiac levels of FXN that should yield therapeutic benefit and assess the dose of AAVrh.10hFXN that could achieve those levels, with improvement in cardiac function and overall survival.
To identify the minimal therapeutic dose, we first estimated the target level of FXN in the human heart necessary to effectively treat individuals with FA. Then, using this level of cardiac FXN as the minimal therapeutic goal, we assessed two experimental models, MCK FA conditional knockout mice and WT NHPs, and determined the minimally effective dose of AAVrh.10hFXN necessary to achieve a therapeutic level of normal human FXN in the heart for the treatment of FA.
Proof-of-concept for AAVrh.10hFXN to treat the cardiomyopathy associated with FA
Several studies support the potential of AAVrh.10hFXN to treat the cardiomyopathy associated with FA. The initial efficacy study showed that IV administration of AAVrh.10hFXN dramatically reversed the cardiac phenotype in MCK mice,23 a severe model of FA cardiac disease that completely lacks murine FXN protein in cardiac and skeletal muscle.24,28 This model has progressive disease deterioration with the mice developing LV hypertrophy by 4–5 weeks of age. This is followed by a rapid loss of systolic function starting at 6–7 weeks, leading to a decrease in resting cardiac output. Most MCK mice die by ∼11 weeks of age due to cardiac failure.
The lack of FXN in the heart tissue results in an iron–sulfur (Fe-S) enzyme deficiency that begins before the onset of cardiac dysfunction with subsequent mitochondrial iron accumulation.24 In this study, a dose of 5.4 × 1013 gc/kg of AAVrh.10hFXN reversed changes in LV mass, fractional shortening, survival, and corrected the cardiac biochemical defects.23 Not only was the onset of the disease prevented but also heart failure and cardiac remodeling were reversed. Reversal of the mitochondrial abnormalities and biochemical Fe-S proteins deficits were observed within 1-week post-treatment. The functional cardiac recovery occurred rapidly, with heart function normalizing within 4–5 weeks of treatment.23
A second study was carried out in αMyhc mice.25 These mice use the cardiac-specific, but weak, αMyhc promoter driving CRE-recombinase that acts on CRISPR-added loxP sites to partially delete exon 4 in the FXN gene. The partial FXN deletion creates a mouse with normal cardiac function at rest but manifests a cardiac phenotype with stress (decreased ejection fraction, decreased fractional shortening with dobutamine cardiac stress, decreased ability to exercise) similar to the typical human FA phenotype. In the αMyhc model, IV administration of 4 × 1012 gc/kg of AAVrh.10hFXN was efficacious.25 Although cardiac FXN levels were not measured, the cardiac phenotype was improved as seen by correction of the dobutamine stress-induced ejection fraction and fractional shortening as assessed by echocardiograms, as well as improvement in treadmill performance.
High levels of FXN are associated with toxicity
Subsequent studies carried out in MCK mice focused on determining cardiac FXN levels associated with efficacy and safety.28 IV administration of 2.5 × 1013 gc/kg AAVrh.10hFXN to MCK mice initially showed complete restoration of cardiac function, but the high levels of myocardial hFXN achieved at this dose were ultimately associated with myocardial fibrosis and inflammatory cell infiltration and decline in cardiac function.28 Thus, while increased hFXN protein expression in the myocardium is associated with correction of FA-associated cardiomyopathy in the MCK mouse model,23,27 there is the potential for cardiac toxicity associated with a high level of expression of FXN.28 Consistent with this concept, Huichalaf et al.29 demonstrated that doses between 1 × 1013 and 1 × 1014 vg/kg of an AAV serotype 9 vector expressing hFXN when administered IV in the MCK mouse were associated with toxic overexpression of hFXN in the liver and heart with only a partial and transient correction of the cardiac function.
Establishment of cardiac FXN levels necessary for therapeutic benefit
To estimate the target cardiac FXN levels in FA homozygotes and heterozygotes, we first determined that the average levels of FXN in non-FA heart tissue were 59 ng/mg protein. While there are no published levels of cardiac hFXN in FA homozygotes and heterozygotes, there are measurements of FXN levels in homozygotes and heterozygotes in noncardiac tissues. Lazaropoulos et al.30 demonstrated that the levels of hFXN in buccal cells and blood mononuclear cells in FA homozygotes are 2–30% of normal (median 16%). Based on the assumption that the percent normal levels in FA homozygotes are similar in the heart and with the knowledge that normal cardiac tissue has average levels of 59 ng/mg, this translates to an estimated average cardiac FXN level of 9.4 ng/mg in FA homozygotes.
FA heterozygotes have noncardiac FXN levels 30–80% of normal. Translating this to the normal heart levels of 59 ng/mg leads to an estimation that FA heterozygotes have cardiac FXN levels of 17.7–47.2 ng/mg. Based on these estimates, achieving >17.7 ng/mg of normal human myocardial FXN levels should be potentially therapeutic in FA homozygotes. With the estimate that FA homozygotes have ∼9.4 ng/mg of endogenous cardiac FXN, a dose of AAVrh.10hFXN that provides >8.3 ng/mg of cardiac FXN would yield a minimal therapeutic benefit.
Determination of the minimal AAVrh.10hFXN effective dose in the MCK mouse model of FA
With the establishment of target cardiac hFXN levels likely to yield therapeutic benefit, experiments were carried out in the MCK mouse model testing different doses to assess the FXN levels obtained and their correlation with efficacy parameters. No differences on any efficacy endpoint from controls were observed with the low dose (1.8 × 1011 gc/kg). At the medium dose (5.7 × 1011 gc/kg), there was a marginal improvement in body weight and a small but significant improvement in survival relative to controls. The high dose (1.8 × 1012 gc/kg) was associated with significant improvements in echocardiogram parameters (ejection fraction, fractional shortening), a significant increase in median survival (21.5%), and a significant increase in body weight, at 4-week post-dose.
The 5.7 × 1011 gc/kg dose generated ∼6 ng/mg of cardiac FXN, equivalent to 26% of normal levels when added to the estimated endogenous levels in an FA homozygote heart, resulting in a level close to the estimated lowest level of FA heterozygotes. A dose 1/2 log higher (1.8 × 1012 gc/kg) resulted in ∼34 ng/mg of cardiac FXN, equivalent to 73% of normal levels when added to the estimated endogenous levels in the human FA homozygote heart, resulting in total FXN levels at the higher end of the estimated heterozygote range.
Translation of AAVrh.10hFXN doses from mice to NHPs
In NHP, a dose of 5.7 × 1011 gc/kg produced cardiac FXN levels of ∼4 ng/mg, equivalent to estimated 22% of normal levels when added to the endogenous levels in a homozygote FA heart, resulting in levels close to the lowest heterozygote level. The dose of 1.8 × 1012 gc/kg generated ∼28 ng/mg, equivalent to 46% of normal levels when added to the background levels in a homozygous FA heart, which is well within the heterozygote range.
In summary, using FXN levels from normal human hearts and extrapolating the known FXN levels in noncardiac tissues in FA subjects, we estimated the cardiac FXN levels in the FA homozygote and heterozygote heart. Based on these estimated levels, achieving ≥30% of normal human cardiac FXN levels will result in levels at the low end of the FXN range in FA heterozygotes, levels of cardiac FXN that should provide clinically meaningful benefit to FA patients. Based on data showing improvement in cardiac function in MCK mice and cardiac expression in NHP, we have identified that, when added to endogenous cardiac FXN levels, it is possible to achieve therapeutic cardiac FXN levels with doses of AAVrh.10hFXN in the range of 5.7 × 1011 and 1.8 × 1012 gc/kg, doses 14- to 44-fold lower than the AAVrh.10hFXN dose that Belbellaa et al.28 associated with cardiac toxicity. We estimate that these doses are the minimally effective safe doses to treat the cardiac manifestations of FA.
Supplementary Material
ACKNOWLEDGMENTS
We thank N. Mohamed for editorial support. We also thank Jason Mezey and Mahboubeh Rostami for advice related to statistical analysis, and Nithya Selvan and Anju Nair for thoughtful discussions.
AUTHORs' CONTRIBUTIONS
C.M.-Z.: Study design, data analysis, involved with drafting of the article, carrying out animal study, in-life assessments, necropsies. M.G.: Study design, data analysis, involved with drafting of the article, carrying out in-life assessments especially echocardiograms. A.G.: Carrying out animal study, in-life assessments, necropsies, conducting of study-related assays, data analysis. M.Y.-B.: Carrying out animal study, in-life assessments, necropsies, data analysis. N.G.: Carrying out in-life assessments especially echocardiograms. A.C. and V.K.: Carrying out animal study, in-life assessments, necropsies.
J.B.R.: Study design, carrying out NHP study, data analysis, involved with drafting of the article. B.P.D.: Study design, conducting of study-related assays, data analysis. S.M.K.: Data analysis, involved with drafting of the article. A.B.: Providing human cardiac samples from autopsies and resultant analysis. R.J.R.A. and H.R.M.: Carrying out NHP study, study veterinarian. S.M.: study pathologist; involved with drafting of the article. R.K. and J.A.B.: Study design, involved with drafting of the article. R.G.C.: Study design, study oversight, involved with drafting of the article. D.S.: Study design, study oversight, data analysis, involved with drafting of the article.
AUTHOR DISCLOSURE
D.S., S.M.K., and R.G.C. hold equity in and are consultants to LEXEO. R.K. and J.A.B. are employees of Lexeo Therapeutics.
FUNDING INFORMATION
These studies were supported, in part, by the National Institutes of Health (NIH) R61HL151355 and LEXEO Therapeutics, Inc. Wake Forest Vervet Research Colony is supported, in part, by NIH resource grant P40-OD010965.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Tsou AY, Paulsen EK, Lagedrost SJ, et al. Mortality in Friedreich ataxia. J Neurol Sci 2011;307(1–2):46–49; doi: 10.1016/j.jns.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 2. Koutnikova H, Campuzano V, Foury F, et al. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat Genet 1997;16(4):345–351; doi: 10.1038/ng0897-345 [DOI] [PubMed] [Google Scholar]
- 3. Bencze KZ, Kondapalli KC, Cook JD, et al. The structure and function of frataxin. Crit Rev Biochem Mol Biol 2006;41(5):269–291; doi: 10.1080/10409230600846058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pastore A, Puccio H. Frataxin: A protein in search for a function. J Neurochem 2013;126(Suppl. 1):43–52; doi: 10.1111/jnc.12220 [DOI] [PubMed] [Google Scholar]
- 5. Anzovino A, Lane DJ, Huang ML, et al. Fixing frataxin: ‘Ironing out’ the metabolic defect in Friedreich's ataxia. Br J Pharmacol 2014;171(8):2174–2190; doi: 10.1111/bph.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cook A, Giunti P. Friedreich's ataxia: Clinical features, pathogenesis and management. Br Med Bull 2017;124(1):19–30; doi: 10.1093/bmb/ldx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fox NG, Yu X, Feng X, et al. Structure of the human frataxin-bound iron-sulfur cluster assembly complex provides insight into its activation mechanism. Nat Commun 2019;10(1):2210; doi: 10.1038/s41467-019-09989-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campuzano V, Montermini L, Molto MD, et al. Friedreich's ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996;271(5254):1423–1427. [DOI] [PubMed] [Google Scholar]
- 9. Monros E, Molto MD, Martinez F, et al. Phenotype correlation and intergenerational dynamics of the Friedreich ataxia GAA trinucleotide repeat. Am J Hum Genet 1997;61(1):101–110; doi: 10.1086/513887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pandolfo M. Friedreich ataxia: The clinical picture. J Neurol 2009;256(Suppl. 1):3–8; doi: 10.1007/s00415-009-1002-3 [DOI] [PubMed] [Google Scholar]
- 11. Koeppen AH. Friedreich's ataxia: Pathology, pathogenesis, and molecular genetics. J Neurol Sci 2011;303(1–2):1–12; doi: 10.1016/j.jns.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raman SV, Phatak K, Hoyle JC, et al. Impaired myocardial perfusion reserve and fibrosis in Friedreich ataxia: A mitochondrial cardiomyopathy with metabolic syndrome. Eur Heart J 2011;32(5):561–567; doi: 10.1093/eurheartj/ehq443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blair IA, Farmer J, Hersch S, et al. The current state of biomarker research for Friedreich's ataxia: A report from the 2018 FARA biomarker meeting. Future Sci OA 2019;5(6):Fso398; doi: 10.2144/fsoa-2019-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delatycki MB, Corben LA. Clinical features of Friedreich ataxia. J Child Neurol 2012;27(9):1133–1137; doi: 10.1177/0883073812448230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kearney M, Orrell RW, Fahey M, et al. Pharmacological treatments for Friedreich ataxia. Cochrane Database Syst Rev 2016;2016(8):Cd007791; doi: 10.1002/14651858.CD007791.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lupoli F, Vannocci T, Longo G, et al. The role of oxidative stress in Friedreich's ataxia. FEBS Lett 2018;592(5):718–727; doi: 10.1002/1873-3468.12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delatycki MB, Bidichandani SI. Friedreich ataxia-pathogenesis and implications for therapies. Neurobiol Dis 2019;132:104606; doi: 10.1016/j.nbd.2019.104606 [DOI] [PubMed] [Google Scholar]
- 18. Hanson E, Sheldon M, Pacheco B, et al. Heart disease in Friedreich's ataxia. World J Cardiol 2019;11(1):1–12; doi: 10.4330/wjc.v11.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schadt KA, Friedman LS, Regner SR, et al. Cross-sectional analysis of electrocardiograms in a large heterogeneous cohort of Friedreich ataxia subjects. J Child Neurol 2012;27(9):1187–1192; doi: 10.1177/0883073812448461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Child JS, Perloff JK, Bach PM, et al. Cardiac involvement in Friedreich's ataxia: A clinical study of 75 patients. J Am Coll Cardiol 1986;7(6):1370–1378. [DOI] [PubMed] [Google Scholar]
- 21. Peverill RE, Donelan L, Corben LA, et al. Differences in the determinants of right ventricular and regional left ventricular long-axis dysfunction in Friedreich ataxia. PLoS One 2018;13(12):e0209410; doi: 10.1371/journal.pone.0209410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De BP, Heguy A, Hackett NR, et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther 2006;13(1):67–76; doi: 10.1016/j.ymthe.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 23. Perdomini M, Belbellaa B, Monassier L, et al. Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia. Nat Med 2014;20(5):542–547; doi: 10.1038/nm.3510 [DOI] [PubMed] [Google Scholar]
- 24. Puccio H, Simon D, Cossee M, et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet 2001;27(2):181–186; doi: 10.1038/84818 [DOI] [PubMed] [Google Scholar]
- 25. Salami CO, Jackson K, Jose C, et al. Stress-induced mouse model of the cardiac manifestations of Friedreich's ataxia corrected by AAV-mediated gene therapy. Hum Gene Ther 2020;31(15–16):819–827; doi: 10.1089/hum.2019.363 [DOI] [PubMed] [Google Scholar]
- 26. Vannocci T, Notario Manzano R, Beccalli O, et al. Adding a temporal dimension to the study of Friedreich's ataxia: The effect of frataxin overexpression in a human cell model. Dis Model Mech 2018;11(6):dmm032706; doi: 10.1242/dmm.032706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belbellaa B, Reutenauer L, Monassier L, et al. Correction of half the cardiomyocytes fully rescue Friedreich ataxia mitochondrial cardiomyopathy through cell-autonomous mechanisms. Hum Mol Genet 2019;28(8):1274–1285; doi: 10.1093/hmg/ddy427 [DOI] [PubMed] [Google Scholar]
- 28. Belbellaa B, Reutenauer L, Messaddeq N, et al. High levels of frataxin overexpression lead to mitochondrial and cardiac toxicity in mouse models. Mol Ther Methods Clin Dev 2020;19:120–138; doi: 10.1016/j.omtm.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huichalaf C, Perfitt TL, Kuperman A, et al. In vivo overexpression of frataxin causes toxicity mediated by iron-sulfur cluster deficiency. Mol Ther Methods Clin Dev 2022;24:367–378; doi: 10.1016/j.omtm.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lazaropoulos M, Dong Y, Clark E, et al. Frataxin levels in peripheral tissue in Friedreich ataxia. Ann Clin Transl Neurol 2015;2(8):831–842; doi: 10.1002/acn3.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitnall M, Suryo Rahmanto Y, Sutak R, et al. The MCK mouse heart model of Friedreich's ataxia: Alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc Natl Acad Sci U S A 2008;105(28):9757–9762; doi: 10.1073/pnas.0804261105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.