Abstract
Combined left ventricular assist device (LVAD) support and pharmacological management of the failing heart can induce reversal of maladaptive cardiac remodelling leading to normalisation of cardiac structure and recovery of cardiac function. The purpose of this study was to compare the gene expression profiles of recovered and non-recovered LVAD patients in order to identify mechanisms underlying the recovery process and differences which may determine outcome. Myocardial expression of 54 genes chosen for their potential role in heart failure and tissue repair was measured using quantitative PCR at the time of LVAD implantation and again at explantation (recovery, n=13) or transplantation (non-recovery, n=5). Patients who went on to recover had higher levels of Giα2, EPAC2 and lower levels of IGF2 at the time of LVAD implant compared to patients who failed to recover. During recovery, expression of BNP, IL-1β, VWF and SFRP1 was decreased whilst RGS4 increased. Expression of IGF1 and pro-fibrotic genes was coordinated during recovery. Correlation analysis identified a novel co-regulation of SFRP1 and βMHC in myocardium. In summary, the gene expression profile underlying recovery is complex and comprises both regression and exacerbation of elements of the pathological gene program. Modulation of Giα2, EPAC2, RGS4 and SFRP1 indicates that inhibition of cAMP signalling may potentiate recovery prior to treatment whilst enhanced cAMP and Wnt signalling may underlie recovery during LVAD support.
Keywords: Quantitative PCR, Reverse remodelling, LVAD, cAMP signalling, Wnt signalling, IGF1
Introduction
Heart failure is a complex disorder characterized by abnormalities in cardiac function, structure, biochemistry and gene expression. The maladaptive rearrangements in cardiac structure are collectively termed ‘cardiac remodelling’ and culminate in left ventricular dilatation and eventual organ failure [1]. Until recently, it was widely believed that cardiac remodelling associated with heart failure was irreversible; however, studies of left ventricular assist device (LVAD) supported patients have shown this is not necessarily the case [2–4]. LVAD support has become linked with a phenomenon called ‘reverse remodelling’ where many of the pathological events associated with heart failure, such as cellular hypertrophy, regress towards normal parameters [5, 6]. On occasion, these changes may be accompanied by improvements in cardiac function sufficient to wean the patients off LVAD support without the need for transplantation [2–4, 7–9]. A combined approach of mechanical unloading and pharmacological management has increased the frequency of sustained recovery following LVAD support to greater than 70% [10].
Attempts to understand the mechanisms underlying the recovery process have been hampered by the paucity of tissue available for research, generally a 1–2 mm3 biopsy taken at the time of LVAD explantation following recovery. In particular, methods of global gene expression profiling, such as microarray analysis [11] and more recently high throughput sequencing techniques, are more difficult to apply in this context. As an alternative approach, we have adopted the use of quantitative polymerase chain reactions (qPCR) analysis to deliver precise measurements of a selection of key genes chosen for their potential relevance in reverse remodelling and myocardial repair. Making use of methods for maximising yield [12], we present here the analysis of a wide spectrum of genes analysed in the specific context of myocardial recovery. Genes chosen for analysis include those associated with growth and repair mechanisms, inflammation, extracellular matrix (ECM) remodelling and cell signalling. New data is compared to pre-existing data from the same patient group in order to derive a global overview of changes in gene expression. The data reveal novel insight into underlying mechanisms and highlight differences at outset which affect outcome.
Materials and Methods
Study Population
The study was approved by the Royal Brompton and Harefield ethical review committee and informed consent was obtained from patients. The investigation conforms to the principles outlined in the Declaration of Helsinki. The study population comprised 18 patients with medically refractory dilated cardiomyopathy (DCM) who required LVAD support due to deteriorating clinical status with evidence of secondary organ dysfunction in the context of low cardiac output despite maximal medical treatment. Patients with evidence of ischaemic cardiomyopathy or myocarditis were specifically excluded. All patients were on inotrope support at the time of implantation and five required an intra-aortic balloon pump (IABP). During the period of LVAD support, patients received a two-stage drug regime designed to promote recovery by maximising reverse remodelling in the first instance (β1/β2-blocker (carvedilol), angiotensin converting enzyme (ACE) inhibitor (lisinopril), angiotensin receptor I antagonist (losartan) and aldosterone antagonist (spironolactone)). Patients were subsequently switched to a selective β1 blocker (bisoprolol) and given the β2-agonist, clenbuterol, with a view to stimulate hypertrophy and improve cardiac function [10]. Echocardiography and exercise testing were performed regularly during treatment to monitor the recovery process. In cases where set of defined morphological and functional criteria were met [10], the patient was considered to have recovered, and the LVAD was explanted. Patients who did not meet the criteria for recovery underwent cardiac transplantation. The patient data and outcomes are summarised in Fig. 1. In 13 cases, sufficient cardiac function was recovered to allow LVAD explantation without the need for transplantation (Fig. 1a). Recovery was sustained in these patients as shown by ejection fractions (EF) at 1, 2 and 5 years post-explantation of 65±9%, 67±6% and 63± 11%, respectively. In five cases, cardiac function was not recovered, and the patients were transplanted (Fig. 1b). Left ventricular samples were taken from patients at the time of LVAD implantation and explantation and snap-frozen in liquid nitrogen and stored at −80°C. In addition to the implant and explant samples from recovered and non-recovered patients, a further 35 ventricular samples were available for mRNA quantification. These included samples taken from donor organs used for transplantation (n=8), donor organs unsuitable for transplantation (n=9), patients with stable heart failure at the time of transplantation (n=10) and patients with rapidly deteriorating heart failure at the time of LVAD implant or explant (n=8). Data collected from these samples was used in correlation analyses.
Fig. 1.
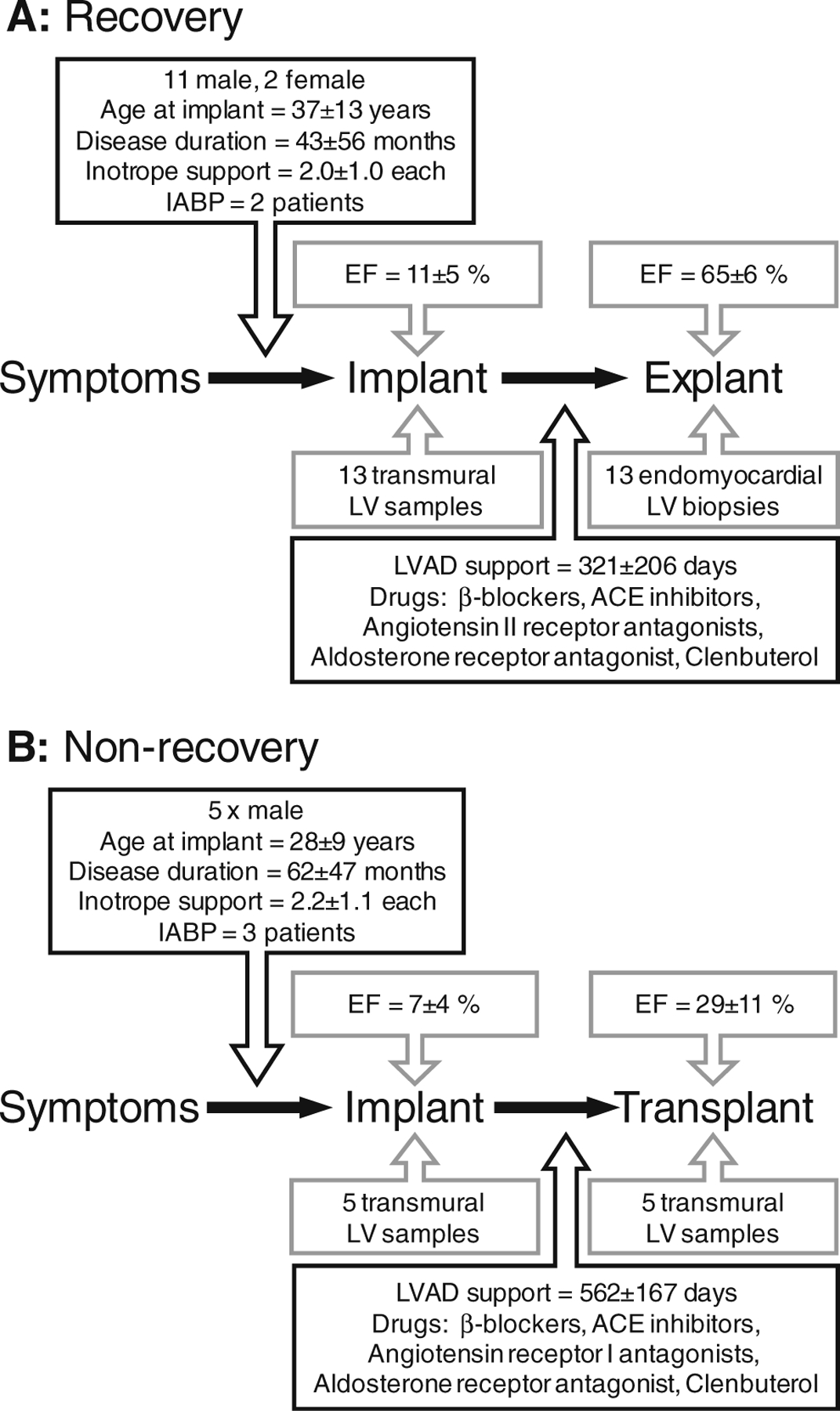
Summary of key data for (a) recovery patients (n=13) and (b) non-recovery patients (n=5). LVAD left ventricular assist device, LV left ventricle, EF ejection fraction, ACE angiotensin converting enzyme
qPCR Analysis
For qPCR analysis, total RNA was extracted, DNasetreated and quantified using a protocol optimised for maximal recovery from myocardial biopsies [12]. cDNA was prepared using a maximum of 50 ng of total RNA per 10 ul reaction volume with random hexamers and diluted to the RNA equivalent of 2 ng/μl with filter-sterilised water. qPCR was performed on the ABI Prism 7,700 using Taqman chemistry and 3 μl of diluted cDNA. Target gene expression was normalised to 18S ribosomal RNA (rRNA) levels and analysed using the comparative Ct method. No significant difference in 18S rRNA levels was observed between groups. Assay details are given in Online resource 1.
Statistical Analysis
All statistical analyses were performed using Graphpad Prism 4 software. Data are given as mean±standard error of the mean except where stated. Non-parametric statistical tests were used throughout, and p≤0.05 was taken as significant except where stated.
Results
In order to explore the molecular mechanisms that characterize reverse cardiac remodelling, we measured the mRNA levels of 54 genes involved in different key biological processes at the time of LVAD implant and compared them to levels at LVAD explant in each patient. Of all the genes measured, mRNA levels of only ten altered significantly with recovery (Table 1). We previously reported decreased expression of genes associated with ECM remodelling (tissue inhibitor of matrix metalloproteinase 4 (TIMP4)), creatine synthesis (glycine amidinotransferase (GATM)), cell signalling (follistatin-like 3 (FSTL3) and exchange factor directly activated by cAMP 2 (EPAC2)) and cardiac morphogenesis (heart and neural crest derivatives expressed transcript 2 (HAND2)) at explant in patients who recovered following treatment [11, 13–16]. The data presented here identifies further alterations in markers of cAMP signalling (regulator of G-protein signalling-4 (RGS4)) as well as markers of disease severity (brain natriuretic peptide (BNP) and interleukin-1β (IL-1β)), endothelium (von Willebrand factor (VWF)) and a Wnt signalling antagonist (secreted frizzled-related protein-1 (SFRP1)). Specifically, mRNA levels of BNP, IL-1β, VWF and SFRP1 were found to be decreased at explant in patients who recovered whilst levels of RGS4 were increased (median fold changes, BNP=0.19-fold, IL-1β=0.28-fold, VWF=0.66-fold, SFRP1=0.36-fold and RGS4=2.10-fold at explant in recovery; p≤0.03; Fig. 2a–e). These changes appear to be specific to recovery, since no significant variation was detected for these genes in patients who did not recover following treatment (Fig. 2a–e). We used a correlation analysis to test if individual changes in any of these genes were related to the duration of LVAD support or clenbuterol administration and found no significant associations (data not shown). To identify associations between gene expression and cardiac function, we directly compared mRNA abundance of BNP, IL-1β, VWF, SFRP1 and RGS4 against the available clinical data for all patients (EF, pulmonary capillary wedge pressure (PCWP), cardiac index (CI), left ventricular end-systolic diameter, left ventricular end-diastolic diameter, age at implantation and time from first symptom to implantation). We found no association between gene expression and clinical parameters measured at implant. However, at explant, SFRP1 mRNA abundance correlated negatively with CI (Fig. 2f), implying that better cardiac performance is associated with lower myocardial expression of this gene.
Table 1.
Summary of analyses in recovered (R) and non-recovered (NR) patients
| Gene | R Exp/Imp | NR Exp/Imp | Implant R/NR | Gene | R Exp/Imp | NR Exp/Imp | Implant R/NR |
|---|---|---|---|---|---|---|---|
| Cardiac hypertrophy and failure markers | ITGAX | – | – | – | |||
| BNP | ↓ | – | – | CD68 | – | – | – |
| ANP | – | – | – | RANTES | – | – | – |
| αMHC | – | – | – | Growth factors | |||
| βMHC | – | – | – | SDF1 | – | – | – |
| ACTA1 | – | – | – | IGF1a | –b | – | – |
| ACTA2 | – | – | – | IGF2 | – | – | ↓ |
| ECM remodelling markers | Endothelial related markers | ||||||
| MMP1a | – | – | – | PECAM1 | – | – | – |
| MMP2a | – | – | – | VWF | ↓ | – | – |
| MMP8a | – | – | – | nNOS | – | – | – |
| MMP9a | – | – | – | Array hitsc | |||
| MMP11a | – | – | – | GATMa | ↓ | – | – |
| MMP14a | – | – | – | SFRP1 | ↓ | – | – |
| TIMP1a | – | – | – | TBX2 | – | – | – |
| TIMP2a | – | – | – | EPAC2a | ↓ | – | ↑ |
| TIMP3a | – | – | – | Transcription factors | |||
| TIMP4a | ↓ | – | – | GATA4 | – | – | – |
| Pro-fibrotic markers | HAND1 | – | – | – | |||
| COL1A1a | – | – | ↓ | HAND2a | ↓ | – | – |
| COL3A1a | – | – | – | KLF15 | – | – | – |
| FNa | – | – | – | Follistatins | |||
| TGFβ1a | – | – | ↓ | FSTa | – | – | – |
| CTGF | – | – | – | FSTL1a | – | – | |
| THY1a | – | – | ↓ | FSTL3a | ↓ | – | – |
| Inflammation markers | β-adrenergic receptors | ||||||
| IL-1β | ↓ | – | – | ADRB1 | – | – | – |
| IL-6 | – | – | – | ADRB2 | – | – | – |
| TNFα | – | – | – | G-protein related | |||
| IL-10 | – | – | – | Giα2 | – | – | ↑ |
| IL-18 | – | – | – | RGS3 | – | – | – |
| TLR4 | – | – | – | RGS4 | ↑ | – | – |
| MCP1 | – | – | – | ||||
Fig. 2.
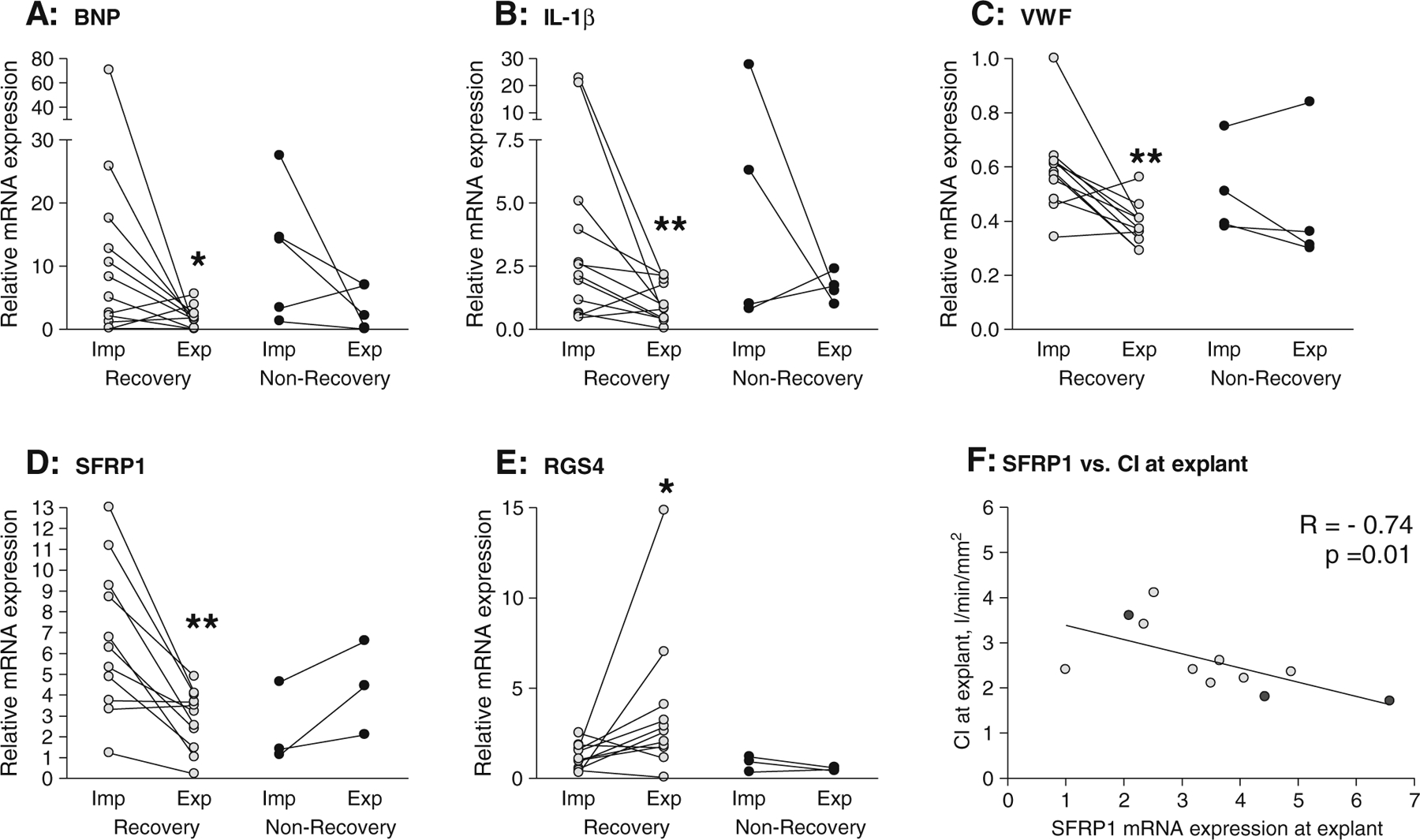
Individual changes in mRNA expression of a BNP, b IL-1β, c VWF, d SFRP1, and e RGS4 in recovery and non-recovery and f correlation between gene expression and cardiac index (CI) at explant. qPCR was used to quantify mRNA levels in left ventricular samples taken at the time of LVAD implant (Imp) and explant (Exp) in recovered and non-recovered patients. Differences between implant and explant were tested using non-parametric paired t tests; *p<0.05 and **p <0.01. Correlations were assessed using Spearman’s rank correlation test
The second aim of this study was to identify myocardial markers which may predict whether a patient will recover or not. Of the 54 genes examined, only six were differentially expressed at the time of LVAD implant in recovered and non-recovered patients (Table 1). These included the pro-fibrotic markers collagen IαI (COL1A1), thymus cell antigen 1 (THY1; a cardiac fibroblast marker [17]) and transforming growth factor β1 (TGFβ1), whose myocardial expression at implant is low in patients who do recover [13]. In this analysis, we further show that at the time of LVAD implant patients who subsequently recover have lower levels of insulin-like growth factor-2 (IGF2) compared to non-recovery patients (0.49 ±0.05-fold, p=0.01; Fig. 3a) and higher levels of EPAC2 and the inhibitory G-protein Giα2 (2.50 ±0.33-fold and 2.14±0.20-fold respectively, p<0.05; Fig. 3b–c). The elevated expression of Giα2 may be of particular significance as within the recovery patients there was a clear correlation between implant expression levels and subsequent cardiac function as determined by lower PCWP measured at explant (Fig. 3d) and better EF measured at 1, 3 and 5 years post-explant (Fig. 3e).
Fig. 3.
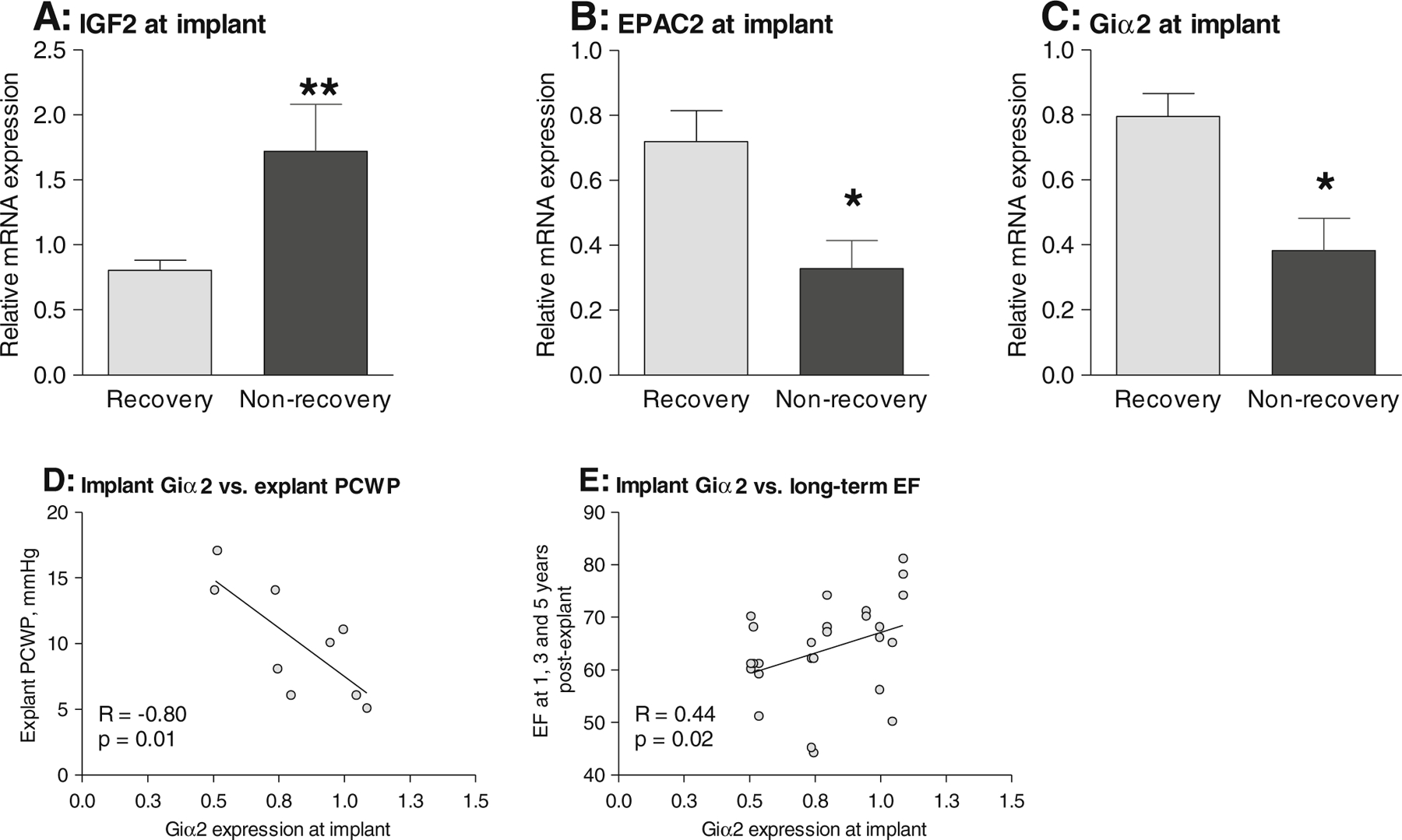
Comparison of implant mRNA expression of a IGF2, b EPAC2, c Giα2 in recovery and non-recovery. d Correlation between implant Giα2 expression and pulmonary capillary wedge pressure (PCWP) at explant in recovery patients. e Correlation between implant Giα2 expression and ejection fraction (EF) measured at 1, 3 and 5 years post-explant in recovery patients. qPCR was used to quantify mRNA levels in left ventricular samples taken at the time of LVAD implant in recovered and non-recovered patients. Differences between recovered and non-recovered patients were tested using non-parametric unpaired t tests; *p<0.05 and **p<0.01. Correlations were assessed using Spearman’s rank correlation test
One advantage of quantifying a large number of genes in the same samples is the opportunity to examine the mRNA profiles en masse in order to identify networks of coordinated gene expression. To do this, we correlated expression of all 54 genes against each other and further increased the power of the analysis by including expression data measured in an additional 35 ventricular samples taken from failing and non-failing hearts. We applied a Bonferroni correction to reduce the number of false positives and only correlations with p≤0.001 were considered significant. To visualise the results, data were colour-coded according to statistical significance and direction/type of correlation as shown in Fig. 4 with genes identified as altered during recovery analysed in more detail in Fig. 5. The data reveal several points of interest. First, of the genes which were altered during recovery, there was little similarity between the correlation patterns, suggesting that the molecular processes they participate in are probably independently regulated during recovery (Fig. 5a). Within these patterns, SFRP1 expression correlated with HAND2 (R=0.45, p=0.0001, n=68) and GATM (R=0.43, p=0.0003, n=68). BNP expression correlated with FSTL3 (R=0.67, p < 0.0001, n=6) and with markers of heart failure and fibrosis. IL-1β expression was associated with other inflammatory genes and with ECM modulators, but not with ECM components or markers of heart failure, suggesting that inflammation and cardiac remodelling in these patients may not be co-regulated events. Detailed comparison of genes which distinguish recovery patients from non-recovery patients at the time of LVAD implantation revealed the expected tight associations among COL1A1, TGFβ1 and THY1 (Fig. 5b). Although IGF2 expression correlated with THY1, there was little or no overlap of the correlation patterns for IGF2, EPAC2 and Giα2 with the fibrotic genes, suggesting that they may represent independent processes associated with recovery.
Fig. 4.
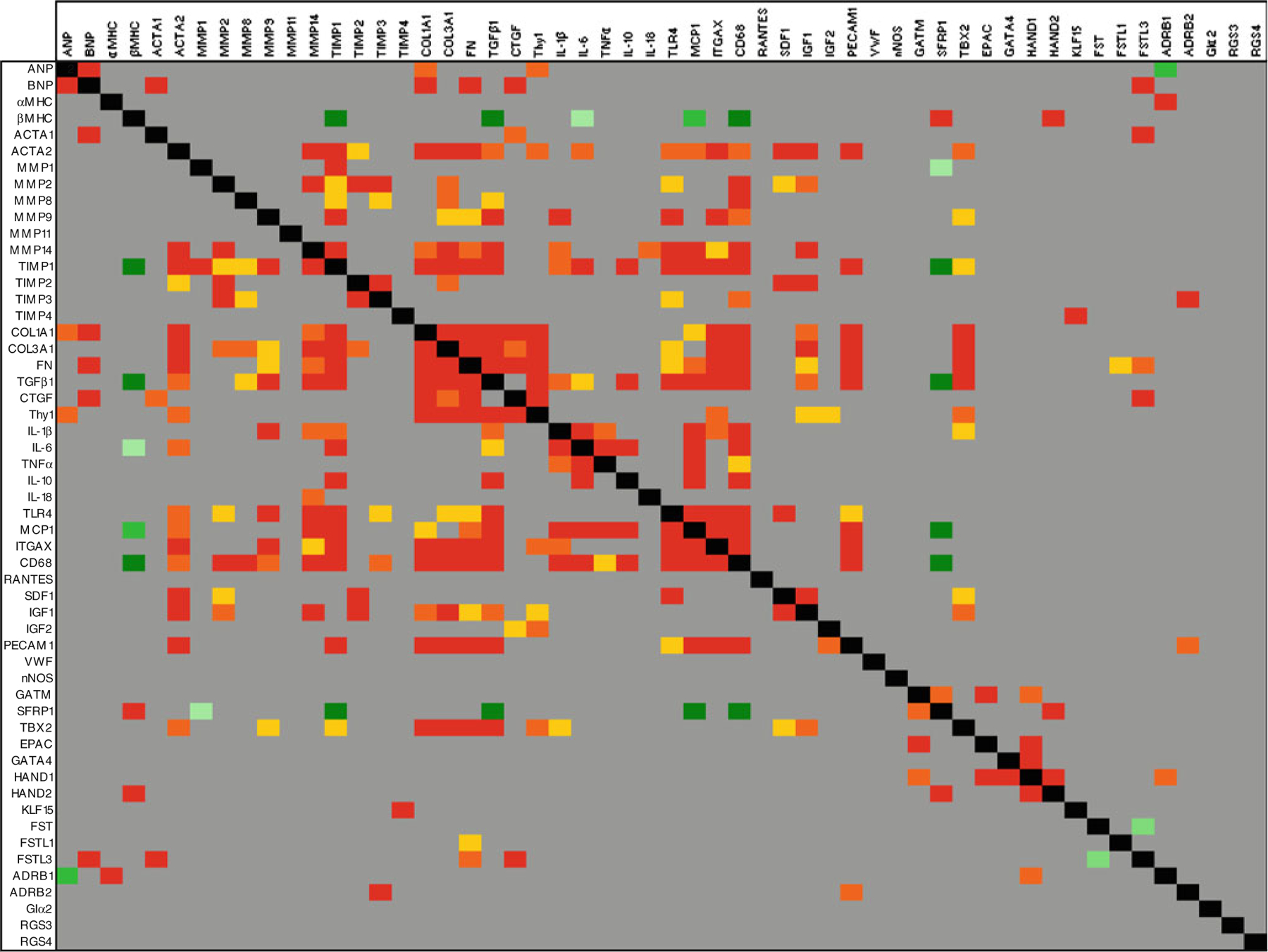
A meta-analysis of mRNA expression in human myocardium. qPCR was used to measure mRNA from 54 genes in 71 cDNA samples prepared using ventricular tissue taken from donor organs used for transplantation (n=8), donor organs unsuitable for transplantation (n=9), patients with stable heart failure at the time of transplantation (n=10), patients with rapidly deteriorating heart failure at the time of LVAD implant (n=26) and explant (n=18). Correlation was assessed using Spearman’s rank correlation test and the normalised expression levels, where dCt = target gene Ct—18S Ct. Red, orange and yellow blocks represent positive correlations where p≤0.001, p≤0.0005 and p≤0.0001, respectively. Dark green, mid-green and light green blocks represent negative correlations where p≤0.001, p≤0.0005 and p≤0.0001, respectively. Grey blocks indicate gene pairs which do not correlate significantly, and black blocks indicate the perfect correlation of a gene against itself
Fig. 5.
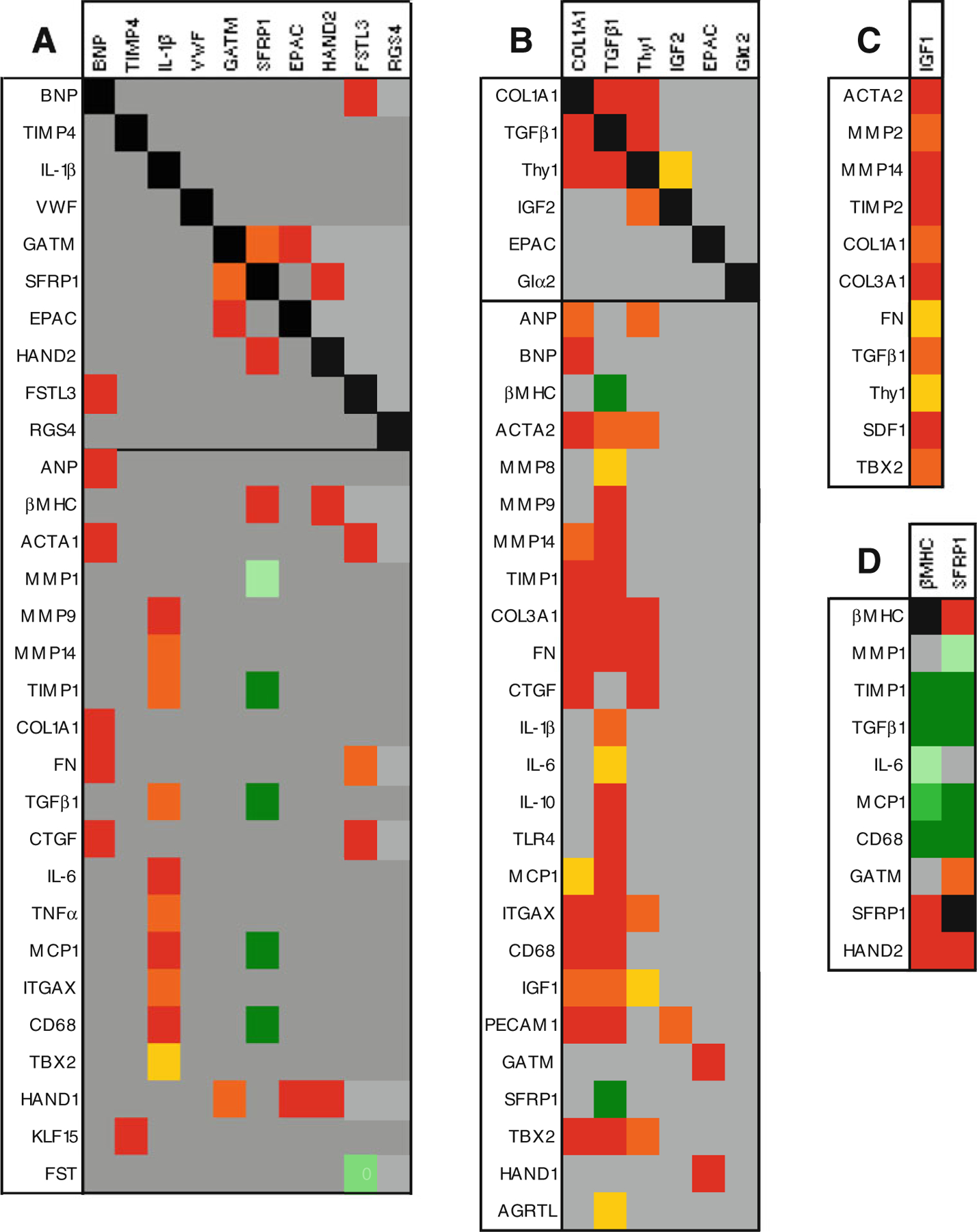
Correlation fingerprints of a genes altered in recovery, b genes which differentiate recovered patients from non-recovered at LVAD implant, c IGF1 and d SFRP1 and βMHC. For ease of comprehension and to condense the dataset, only genes which correlated with the genes examined in a, b c, or d are shown
We previously reported complex regulation of IGF1 during recovery whereby patients with high IGF1 levels at implant show decreased expression during LVAD support and patients with low IGF1 levels at implant show increased expression (Online resource 2a) and noted a potential association with fibrotic markers [13, 18]. Here, we further explored the correlation profile of IGF1 during recovery and found that genes which correlate significantly with IGF1 are predominantly associated with fibrosis, namely matrix metalloproteinase 2 (MMP2), MMP14, TIMP2, COL1A1, collagen IIIαI (COL3A1), fibronectin (FN), TGFβ1, THY1 and smooth muscle actin α2 (ACTA2). Other genes with significant IGF1 correlations included stromal cell-derived factor 1 (SDF1) and T-box 2 (TBX2; Fig. 5c). To determine whether expression of these genes was influenced by IGF1, we segregated the recovery patients according to IGF1 expression at implant and re-analysed the data (Fig. 6; Online resource 2). This comparison revealed that recovery patients with low levels of IGF1 at implant also have significantly lower levels of MMP14, COL1A1, COL3A1, TGFβ1 and THY1 at implant than patients with high IGF1 (Fig. 6a; Online resource 2). Moreover, the increase in IGF1 expression during LVAD support seen in recovery patients with low IGF1 levels at implant is accompanied by significant increases in MMP2, TIMP2, COL1A1, COL3A1, FN, TGFβ1 and THY1 (Fig. 6b; Online resource 2). Similarly, the general trend in recovery patients who decrease IGF1 expression during LVAD support is for expression of these genes to also decrease. The change in expression of these genes during LVAD support is such that recovery patients with low IGF1 at implant have greater mRNA levels of MMP2, TIMP2, COL1A1, COL3A1, TGFβ1 and THY1 at explant (Fig. 6c; Online resource 2). There was no significant effect of segregating ACTA2, SDF1 and TBX2 according to IGF1 expression at implant (Online resource 2j, k, l).
Fig. 6.
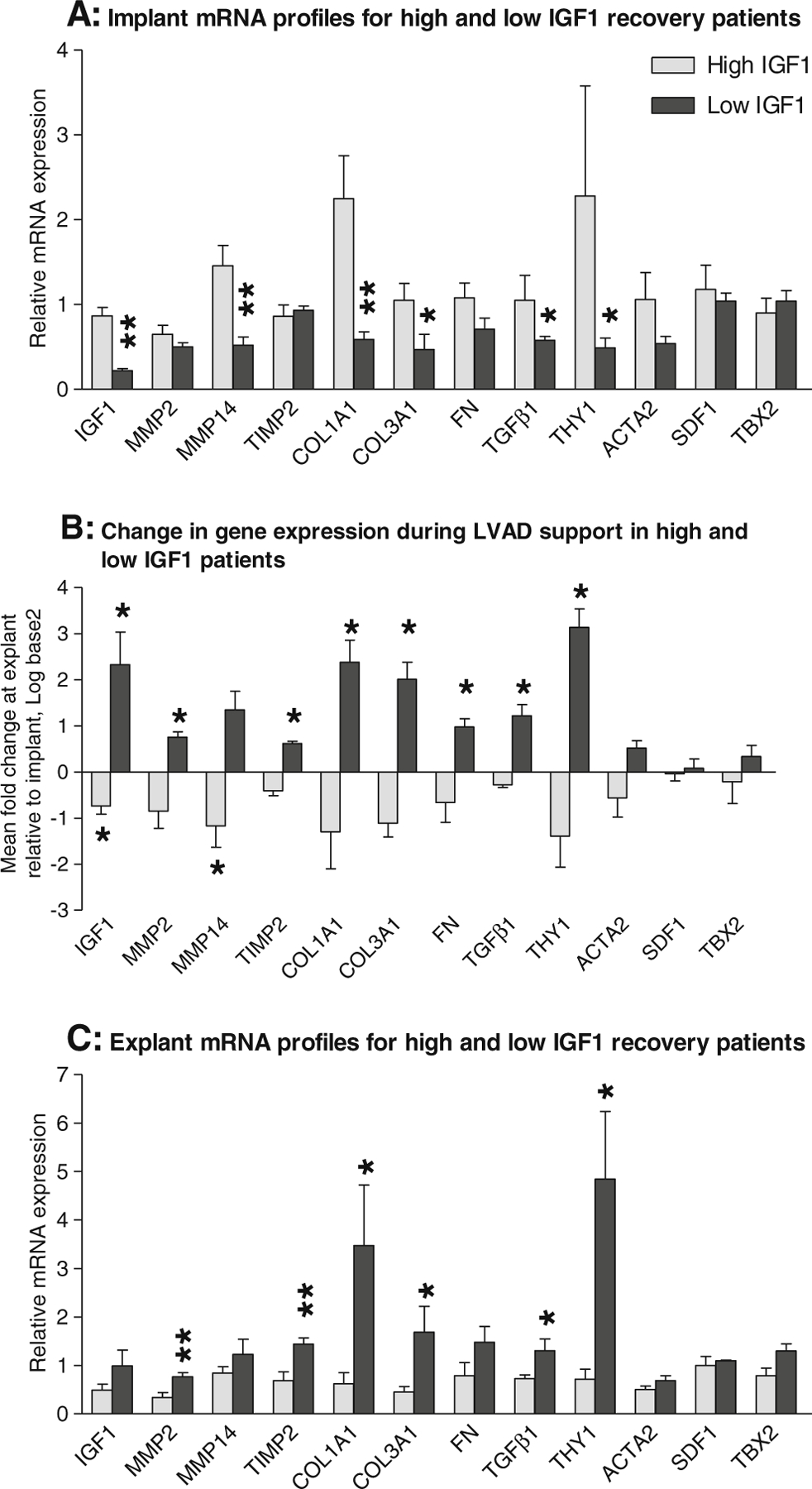
Gene expression profiles in high and low IGF1 recovery patients. a Comparison of the gene expression profiles at implant. b Comparison of the changes in gene expression during LVAD support. c Comparison of the gene expression profiles at explant. qPCR was used to measure mRNA levels in left ventricular myocardium collected at the time of LVAD implant and explant in up to 13 recovery patients. The data were sub-grouped according to the patient’s IGF1 status. In (b), data are presented as log base 2 of the mean fold change in expression at explant compared to implant±SEM and tested using a non-parametric paired t test. In (a) and (c), data are presented as the mean relative expression levels±SEM and tested using a non-parametric unpaired t test
Finally, we used the correlation analysis to identify previously unknown networks of coordinated gene expression in myocardium and noted that SFRP1 and β myosin heavy chain (βMHC) have conspicuously similar correlation profiles (Fig. 5d). Both SFRP1 and βMHC correlate tightly with each other (R=0.50, p<0.0001, n=67) and with HAND2 (R=0.45 and R=0.51 respectively, p≤0.0001, n≥65). Moreover, both genes correlate negatively with fibrosis and inflammation markers, including TIMP1 (R=−0.52 and R=−0.53 respectively, p≤0.0001, n≥64), TGFβ1 (R=−0.52 and R=−0.49 respectively, p≤0.0001, n ≥68), monocyte chemotactic protein 1 (MCP1; R=−0.49 and R=−0.43 respectively, p≤0.0002, n≥68) and CD68 (R=−0.53 and R=−0.45 respectively, p≤0.0001, n≥68).
Discussion
The process of reverse remodelling induced by LVAD support in end-stage heart failure has been widely studied since the phenomenon was first described [5, 6]. Many of the characteristic features of the failing heart are shown to normalise following mechanical unloading, and yet despite this, the majority of LVAD patients do not recover cardiac function and require transplantation. Our studies of LVAD recipients are unique in that they specifically examine patients who show sustained clinical recovery following LVAD support combined with pharmacological management and stimulation of the β2-adrenergic receptor pathway as opposed to those undergoing conventional LVAD support as a bridge-to-transplantation. Here, we report that repression of BNP, IL-1β, VWF, SFRP1 and induction of RGS4 comprise a specific transcriptional profile underlying reverse remodelling leading to recovery. The induction of both BNP and IL-1β is an established event in the pathogenesis of heart failure [1, 19], and down-regulation of these genes during recovery represents a normalisation of the pathological expression profile. The reduction in myocardial BNP expression is mirrored by a reduction in serum BNP levels previously reported in the same patients [10]. However, myocardial levels of BNP and IL-1β were also generally lower at explant in non-recovered patients, and down-regulation of BNP is a typical feature of mechanical unloading which is regularly observed in conventional LVAD patients [20, 21]. Thus, while the repression of BNP and IL-1β in myocardium may be a necessary for recovery, it is not sufficient.
A novel finding of this study is the repression of SFRP1 during recovery. SFRP1 is a secreted protein able to directly bind Wnt protein, thereby antagonising both canonical and non-canonical Wnt signalling pathways [22]. Here, SFRP1 is significantly increased in LVAD recipients compared to normal donor controls (data not shown) and down-regulation of SFRP1 during LVAD support indicates that Wnt signalling may be suppressed in failure and activated during the process of recovery. The significance of this observation is reflected in the literature where Wnt signalling has been identified as one of the cardiac development pathways reactivated in heart failure and assigned a pivotal role in cardiac remodelling with potential for therapeutic intervention [23]. Specifically, cardioprotective effects have been established for canonical Wnt signalling whereby inactivation of GSKβ, a downstream target of Wnt signalling, leads to cytosolic and nuclear accumulation of β-catenin and reduced scar following myocardial infarction (MI) [24]. Similarly, injection of constitutively active β-catenin into the border zone post-MI led to reduced scar [25]. During canonical Wnt signalling, GSKβ is inactivated leading to cytoplasmic accumulation of β-catenin and its subsequent nuclear translocation where it interacts with Tcf/Lef transcription factors and initiates a transcription program which includes cell survival and proliferation. MCP-1 is a direct transcriptional target of β-catenin and Tcf [26] and the inverse correlation between SFRP1 and MCP1 expression we observe here is consistent with SFRP1 modulating the cardioprotective canonical Wnt pathway during recovery.
For SFRP1, the correlation pattern reveals positive associations with βMHC, GATM and HAND2 and negative associations with MMP1, TIMP1, TGFβ1, MCP1 and CD68, indicating that these genes may be Wnt responsive. Within this list, the putative targets of repression by Wnt signalling are involved in signalling and transcription, whereas the putative targets of induction are broadly pro-fibrotic and markers of macrophages. The positive correlation between SFRP1 and βMHC expression was unexpected. In human heart failure, ventricular levels of βMHC mRNA increase moderately [27], but we found no correlation between βMHC and expression of other heart failure markers suggesting independent regulation of these genes. Instead, expression of βMHC and SFRP1 correlated tightly, and both genes had very similar correlation patterns suggesting potential repression of βMHC by Wnt signalling. Interestingly, although βMHC expression is unaltered in the recovery patients, SFRP1 is significantly downregulated, and this is co-incident with significant but complex alterations in mRNA and protein expression of sarcomeric and non-sarcomeric cytoskeletal proteins [28, 29].
Our data show decreased VWF and increased RGS4 expression in the myocardium of LVAD recipients who are successfully explanted following recovery. Expression of both VWF and RGS4 is significantly increased in LVAD recipients compared to normal donor controls (data not shown). VWF is a large circulating glycoprotein with an essential role in haemostasis which is selectively expressed in endothelial cells and megakaryocytes [30]. Circulating levels of VWF have been shown to be of importance in the context of LVAD support [31]. The down-regulation of myocardial VWF seen in our recovery patients most likely represents an inhibition of local VWF available for secretion rather than a decrease reduction in endothelial cell numbers per se as expression of the other endothelial marker measured (platelet/endothelial cell adhesion molecule 1 (PECAM1)) was unchanged. Whilst the downregulation of BNP, IL-1β, SFRP1 and VWF all constitute a reversal of a gene program associated with pathology, the induction of RGS4 may represent the augmentation of a protective response initiated by the failing heart since levels are already high in heart failure [32] and RGS4 signalling can attenuate cardiac hypertrophy [33]. The RGS proteins comprise a family of at least 25 proteins which inactivate the α-subunits of G-proteins thereby terminating G-protein signalling [34]. RGS4 is a negative regulator of G-protein signalling mediated by Gαi/o and Gαq/11. Giα2 is increased in heart failure [32], and we saw no further change during LVAD support of either recovered or non-recovered patients. The induction of RGS4 might therefore be expected to increase intracellular cAMP content. This response may be specific to recovery because we see no change in RGS4 in non-recovered patients and others see no change in conventional LVAD recipients [35]. The induction of RGS4 occurs alongside the repression of EPAC2 [11]. EPACs are PKA-independent downstream effectors of cAMP signalling able to regulate cardiomyocyte contraction and hypertrophy [36]. The complementary regulation of RGS4 and EPAC2 during LVAD support would be expected to favour activation of PKA-dependent cAMP signalling in the recovered patients thereby implicating this pathway as a candidate mechanism in the recovery process.
The potential significance of cAMP signalling as a reparative mechanism in myocardium is further underlined by the observation that expression of EPAC2 and Giα2 is higher at implant in patients who recover compared to those who fail to recover. Giα2 is a negative regulator of cAMP signalling which intervenes upstream of cAMP to inhibit cAMP synthesis leading to decreased cAMP content [37]. It is generally held that decreased cAMP content is an important event in the pathogenesis of DCM; however, it remains to be established whether this constitutes a pathological mechanism or a compensatory adaptation [37]. Our observations that Giα2 is increased in recovery patients before the start of treatment and positively correlates with long-term recovered cardiac function support the idea that decreased cAMP content in heart failure is a compensatory adaptation. Thus, the potential to recover may correspond to decreased intracellular cAMP content at outset. In the event that cAMP signalling is activated, the up-regulation of EPAC2 may favour the PKA-independent branch of the cascade. These observations may be useful clinically since it is possible to modulate cAMP levels and EPAC activity pharmacologically and the rate of successful explanation could be improved by priming patients for recovery using these enhancers.
Studies of IGF1 and ECM remodelling during reverse remodelling seen in conventional LVAD recipients have yielded conflicting results [5, 6]. Here, we observe coordinated expression of IGF1 and pro-fibrotic genes comprising two alternate profiles underlying the recovery process. Specifically, during recovery patients with high mRNA levels of IGF1 and pro-fibrotic markers at implant down-regulate these genes during recovery, whilst patients with low levels of IGF1 and pro-fibrotic markers at implant up-regulate these genes. IGF1 exerts numerous beneficial effects on myocardium and has been implicated as a central regulator of repair and regeneration in both cardiac and skeletal muscle, being able to curb both inflammatory and fibrotic responses and enhance recruitment of local and circulating stem cells [38, 39]. In contrast, fibrosis is an established feature of maladaptive cardiac remodelling thought to preclude recovery during LVAD support [1, 40]. It is surprising therefore that recovery is achievable when patients either have comparatively high levels of pro-fibrotic markers at outset or induce a pro-fibrotic response during unloading. Given that fibrosis is an obstacle to recovery, it would follow that high IGF1/pro-fibrotic patients benefit most from combined LVAD support and pharmacological management, whilst the low IGF1/pro-fibrotic patients are most likely to recover spontaneously. In the latter group, potential stimuli of IGF1 up-regulation include mechanical unloading as seen in the rat model of heterotopic transplantation [41] and clenbuterol induction of IGF1 in cardiac fibroblasts [42]. Further work is required to establish how the observed patterns of pro-fibrotic gene expression relate to histological measurements of fibrosis and to determine whether IGF1 is coordinating or tracking the fibrotic response during myocardial recovery.
The primary limitation of this study is that it exclusively examines the mRNA expression profile. Detailed protein and enzymatic analyses are technically constrained in the context of recovery because the amount of sample taken at the time of successful explantation is insufficient to complete the work. We believe that qPCR and microarray technologies are the most productive techniques to use with such samples [11, 13–15, 18, 43] but recognise that caution should be exercised when interpreting the results of gene expression analyses, not least in the context of fibrosis or cell signalling.
In summary, this study has compared the myocardial mRNA profiles of 54 genes in the context of clinical recovery and non-recovery following LVAD support and found that the expression profile underlying recovery is complex and comprises both regression and exacerbation of elements of the pathological gene program. The expression profile indicates reduced cAMP signalling as a candidate mechanism which potentiates myocardial recovery prior to treatment. Conversely, increased cAMP signalling alongside enhanced Wnt signalling are implicated as potential mechanisms of myocardial recovery during LVAD support.
Supplementary Material
Acknowledgements
This work was supported by grants from Thoratec Corporation, The Royal Brompton and Harefield Charitable Trustees, the Magdi Yacoub Institute, Heart Research UK and by the National Institutes of Health Research Cardiovascular Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College.
Contributor Information
Leanne Elizabeth Felkin, Heart Science Centre, National Heart and Lung Institute, Imperial College London, Hill End Road, Harefield, Middlesex UB9 6JH, UK.
Enrique A. Lara-Pezzi, Heart Science Centre, National Heart and Lung Institute, Imperial College London, Hill End Road, Harefield, Middlesex UB9 6JH, UK, Cardiovascular Development Biology Department, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Melchor Fernández Almagro 3, 28029 Madrid, Spain
Jennifer L. Hall, Lillehei Heart Institute, University of Minnesota, Minneapolis, MN 55455, USA
Emma J. Birks, Heart Science Centre, National Heart and Lung Institute, Imperial College London, Hill End Road, Harefield, Middlesex UB9 6JH, UK, Department of Heart Failure, Transplantation and Mechanical Support, Jewish Hospital, Louisville, KY 40202, USA
Paul J. R. Barton, Heart Science Centre, National Heart and Lung Institute, Imperial College London, Hill End Road, Harefield, Middlesex UB9 6JH, UK, NIHR Cardiovascular Biomedical Research Unit, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London SW3 6NP, UK
References
- 1.Mann DL, & Bristow MR (2005). Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation, 111, 2837–2849. [DOI] [PubMed] [Google Scholar]
- 2.Frazier OH, & Myers TJ (1999). Left ventricular assist system as a bridge to myocardial recovery. The Annals of Thoracic Surgery, 68, 734–741. [DOI] [PubMed] [Google Scholar]
- 3.Muller J, Wallukat G, Weng YG, et al. (1997). Weaning from mechanical cardiac support in patients with idiopathic dilated cardiomyopathy. Circulation, 96, 542–549. [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Beniaminovitz A, Levin H, et al. (1998). Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation, 98, 2383–2389. [DOI] [PubMed] [Google Scholar]
- 5.Birks E, & George R (2010). Molecular changes occurring during reverse remodelling following left ventricular assist device support. Journal of Cardiovascular Translational Research, 3, 635–642. [DOI] [PubMed] [Google Scholar]
- 6.Razeghi P, Myers TJ, Frazier OH, & Taegtmeyer H (2002). Reverse remodeling of the failing human heart with mechanical unloading. Emerging concepts and unanswered questions. Cardiology, 98, 167–174. [DOI] [PubMed] [Google Scholar]
- 7.Farrar DJ, Holman WR, McBride LR, et al. (2002). Long-term follow-up of thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. The Journal of Heart and Lung Transplantation, 21, 516–521. [DOI] [PubMed] [Google Scholar]
- 8.Simon MA, Kormos RL, Murali S, et al. (2005). Myocardial recovery using ventricular assist devices: prevalence, clinical characteristics, and outcomes. Circulation, 112, I–32. [DOI] [PubMed] [Google Scholar]
- 9.Dandel M, Weng Y, Siniawski H, et al. (2005). Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation, 112, I37–I45. [DOI] [PubMed] [Google Scholar]
- 10.Birks EJ, Tansley PD, Hardy J, et al. (2006). Left ventricular assist device and drug therapy for the reversal of heart failure. The New England Journal of Medicine, 355, 1873–1884. [DOI] [PubMed] [Google Scholar]
- 11.Hall JL, Birks EJ, Grindle S, et al. (2007). Molecular signature of recovery following combination left ventricular assist device (LVAD) support and pharmacologic therapy. European Heart Journal, 28, 613–627. [DOI] [PubMed] [Google Scholar]
- 12.Felkin LE, Taegtmeyer AB, & Barton PJ (2006). Real-time quantitative polymerase chain reaction in cardiac transplant research. Methods in Molecular Biology, 333, 305–330. [DOI] [PubMed] [Google Scholar]
- 13.Felkin LE, Lara-Pezzi E, George R, et al. (2009). Expression of extracellular matrix genes during myocardial recovery from heart failure after left ventricular assist device support. The Journal of Heart and Lung Transplantation, 28, 117–122. [DOI] [PubMed] [Google Scholar]
- 14.Cullen ME, Yuen AH, Felkin LE, et al. (2006). Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: potential implications for local creatine synthesis. Circulation, 114, I16–I20. [DOI] [PubMed] [Google Scholar]
- 15.Breckenridge RA, Zuberi Z, Gomes J, et al. (2009). Overexpression of the transcription factor Hand1 causes predisposition towards arrhythmia in mice. Journal of Molecular and Cellular Cardiology, 47, 133–141. [DOI] [PubMed] [Google Scholar]
- 16.Lara-Pezzi E, Terracciano CM, Soppa GK, et al. (2009). A gene expression profile of the myocardial response to clenbuterol. Journal of Cardiovascular Translational Research, 2, 191–197. [DOI] [PubMed] [Google Scholar]
- 17.Hudon-David F, Bouzeghrane F, Couture P, & Thibault G (2007). Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. Journal of Molecular and Cellular Cardiology, 42, 991–1000. [DOI] [PubMed] [Google Scholar]
- 18.Barton PJR, Felkin LE, Birks EJ, et al. (2005). Myocardial insulin-like growth factor-I gene expression during recovery from heart failure after combined left ventricular assist device and clenbuterol therapy. Circulation, 112, 46–I52. [DOI] [PubMed] [Google Scholar]
- 19.Birks EJ, Latif N, Owen VJ, et al. (2001). Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation, 104, I233–I240. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn M, Voss M, Mitko D, et al. (2004). Left ventricular assist device support reverses altered cardiac expression and function of natriuretic peptides and receptors in end-stage heart failure. Cardiovascular Research, 64, 308–314. [DOI] [PubMed] [Google Scholar]
- 21.Bruggink AH, de Jonge N, van Oosterhout MFM, et al. (2006). Brain natriuretic peptide is produced both by cardiomyocytes and cells infiltrating the heart in patients with severe heart failure supported by a left ventricular assist device. The Journal of Heart and Lung Transplantation, 25, 174–180. [DOI] [PubMed] [Google Scholar]
- 22.Kawano Y, & Kypta R (2003). Secreted antagonists of the Wnt signalling pathway. Journal of Cell Science, 116, 2627–2634. [DOI] [PubMed] [Google Scholar]
- 23.Bergmann MW (2010). Wnt signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circulation Research, 107, 1198–1208. [DOI] [PubMed] [Google Scholar]
- 24.Kaga S, Zhan L, Altaf E, & Maulik N (2006). Glycogen synthase kinase-3β/β-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. Journal of Molecular and Cellular Cardiology, 40, 138–147. [DOI] [PubMed] [Google Scholar]
- 25.Hahn JY, Cho HJ, Bae JW, et al. (2006). β-Catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. The Journal of Biological Chemistry, 281, 30979–30989. [DOI] [PubMed] [Google Scholar]
- 26.Mestdagt M (2006). Transactivation of MCP-1/CCL2 by β-catenin/TCF-4 in human breast cancer cells. International Journal of Cancer, 118, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowes BD, Minobe W, Abraham WT, et al. (1997). Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. Journal of Clinical Investigation, 100, 2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birks EJ, Hall JL, Barton PJR, et al. (2005). Gene profiling changes in cytoskeletal proteins during clinical recovery following left ventricular assist device (LVAD) support. Circulation, 112, I57–I64. [DOI] [PubMed] [Google Scholar]
- 29.Latif N, Yacoub MH, George R, et al. (2007). Changes in sarcomeric and non-sarcomeric cytoskeletal proteins and focal adhesion molecules during clinical myocardial recovery after left ventricular assist device support. The Journal of Heart and Lung Transplantation, 26, 230–235. [DOI] [PubMed] [Google Scholar]
- 30.Vischer UM (2006). von Willebrand factor, endothelial dysfunction, and cardiovascular disease. Journal of Thrombosis and Haemostasis, 4, 1186–1193. [DOI] [PubMed] [Google Scholar]
- 31.Miller LW (2010). The development of the von Willebrand syndrome with the use of continuous flow left ventricular assist devices: a cause-and-effect relationship. Journal of the American College of Cardiology, 56, 1214–1215. [DOI] [PubMed] [Google Scholar]
- 32.Owen VJ, Burton PBJ, Mullen AJ, et al. (2001). Expression of RGS3, RGS4 and Gi alpha 2 in acutely failing donor hearts and end-stage heart failure. European Heart Journal, 22, 1015–1020. [DOI] [PubMed] [Google Scholar]
- 33.Tokudome T, Kishimoto I, Horio T, et al. (2008). Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation, 117, 2329–2339. [DOI] [PubMed] [Google Scholar]
- 34.Wieland T, & Mittmann C (2003). Regulators of G-protein signalling: multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacology & Therapeutics, 97, 95–115. [DOI] [PubMed] [Google Scholar]
- 35.Takeishi Y, Jalili T, Hoit BD, et al. (2000). Alterations in Ca2+ cycling proteins and G α q signaling after left ventricular assist device support in failing human hearts. Cardiovascular Research, 45, 883–888. [DOI] [PubMed] [Google Scholar]
- 36.Gloerich M, & Bos JL (2010). Epac: defining a new mechanism for cAMP action. Annual Review of Pharmacology and Toxicology, 50, 355–375. [DOI] [PubMed] [Google Scholar]
- 37.Movsesian MA (2004). Altered cAMP-mediated signalling and its role in the pathogenesis of dilated cardiomyopathy. Cardiovascular Research, 62, 450–459. [DOI] [PubMed] [Google Scholar]
- 38.Musaro A, Giacinti C, Borsellino G, et al. (2004). Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proceedings of the National Academy of Sciences of the United States of America, 101, 1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santini MP, Tsao L, Monassier L, et al. (2007). Enhancing repair of the mammalian heart. Circulation Research, 100, 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruckner BA, Razeghi P, Stetson S, et al. (2004). Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. The Journal of Heart and Lung Transplantation, 23, 36–42. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Ying J, Razeghi P, et al. (2006). Atrophic remodeling of the transplanted rat heart. Cardiology, 105, 128–136. [DOI] [PubMed] [Google Scholar]
- 42.Bhavsar PK, Brand NJ, Felkin LE, et al. (2010). Clenbuterol induces cardiac myocyte hypertrophy via paracrine signalling and fibroblast-derived IGF-1. Journal of Cardiovascular Translational Research, 3, 688–695. [DOI] [PubMed] [Google Scholar]
- 43.Lara-Pezzi E, Felkin LE, Birks EJ, et al. (2008). Expression of follistatin-related genes is altered in heart failure. Endocrine, 149, 5822–5827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


