Abstract
Reconstruction of posterior lamellar eyelids remains challenging due to their delicate structure, highly specialized function, and cosmetic concerns. Current clinically available techniques for posterior lamellar reconstruction mainly focus on reconstructing the contour of the eyelids. However, the posterior lamella not only provides structural support for the eyelid but also offers a smooth mucosal surface to facilitate globe movement and secrete lipids to maintain ocular surface homeostasis. Bioengineered posterior lamellar substitutes developed via acellular or cellular approaches have shown promise as alternatives to current therapies and encouraging outcomes in animal studies and clinical conditions. Here, we provide a brief reference on the current application of autografts, biomaterials, and tissue‐engineered substitutes for posterior lamellar eyelid reconstruction. We also shed light on future challenges and directions for eyelid regeneration strategies and offer perspectives on transitioning replacement strategies to regeneration strategies for eyelid reconstruction in the future.
Keywords: conjunctiva, eyelid reconstruction, posterior lamella, regenerative medicine, tarsal plate, tissue engineering
1. INTRODUCTION
The eyelids play critical roles in protecting the globe, maintaining ocular surface hydration, and expressing emotions. Anatomically, the eyelids contain two layers, namely, the anterior lamella and the posterior lamella 1 (Figure 1a). The eyelids not only physically provide protective coverage for the cornea and the globe but also serve important roles in maintaining ocular health and preserving visual integrity. Esthetically, the frame of the eye is distinctively defined by the position and shape of the eyelids. Thus, eyelid reconstruction must reconstruct both structure and function of the eyelids and restore an esthetically acceptable appearance with minimal surgical morbidities. To achieve these goals, we previously proposed that eyelid defects should be reconstructed following the “like for like” reconstructive principle and the surgeon should take into consideration the characteristics of the defects, including the thickness, size, and location. 2 By following these principles, numerous well‐developed surgical techniques, including free skin grafts and tissue flaps, have been applied to repair skin or myocutaneous defects in anterior lamellar reconstruction, with satisfying functional and cosmetic outcomes in clinical practice. 3 , 4 , 5 However, due to the unique structural and functional properties of the eyelid, as well as the lack of histologically ideal donor tissues, current clinically available surgical techniques have shown limited clinical success in posterior lamellar reconstruction 6 , 7 , 8 (Figure 2).
FIGURE 1.

Illustration of the anatomical structure of the eyelid. (a) Eyelid. (b) Conjuctiva. (c) Meibomian gland. (d) Tear flim.
FIGURE 2.

Illustration of the surgical strategies of eyelid reconstruction. FTSG, full thickness skin graft.
The current strategies for posterior lamellar reconstruction mainly depend on the defect size. In general, small defects involving less than 25% of the eyelid width are preferably repaired by direct closure. Moderate defects involving 25%–50% of the eyelid width can also be reconstructed by primary closure in combination with cantholysis or local tarsoconjunctival flap transfer. For the defects involving at least 50% of the eyelid width, free composite autografts, Hughes tarsoconjunctival flaps, Tenzel semicircular flaps, tarsal transposition flaps, or combinational techniques are usually required to provide primary coverage and additional lubrication for the globe. 1 , 2 , 9 However, these reconstructive techniques are aimed at replacing the defective tissue with similar autologous tissue to restore the contour of the eyelid. And there is no treatment option capable of restoring the natural function of the eyelid. The development of restorative, instead of palliative, management strategies for eyelid defects in terms of both function and structure requires extensive surgical knowledge and versatility; thus, there is interest in tissue engineering‐based therapies.
Bioengineering represents the future of reconstructive surgery and is emerging as a promising alternative for eyelid reconstruction (Figure 3). Ideally, bioengineered posterior lamellar substitutes should restore key structures and functions of the eyelid without additional donor‐site morbidities or postoperative complications. To date, many innovative native tissue grafts, 10 , 11 biomaterials, 12 , 13 and bioengineered tissues 14 , 15 have been proposed as posterior lamellar substitutes, some of which have been clinically used for eyelid reconstruction and have yielded promising preliminary results. 11 , 14 , 15 The bioengineering of posterior lamellar substitutes can be categorized into two distinct approaches: (1) acellular approaches, in which natural or synthetic acellular biomaterials are transplanted into defect areas to act as bioscaffolds for guiding tissue regeneration in vivo by leveraging endogenous cells and microenvironments (Figure 4a); and (2) cellular approaches, in which bioscaffolds are precellularized in vitro to mimic specific functions of the target tissue and then transplanted to promote tissue regeneration (Figure 4b). It appears that the presence of functional cells within the bioscaffold before implantation is particularly important for successful eyelid reconstruction, and strategies based on cellular approaches have indeed achieved promising results in preclinical studies. 16 , 17 , 18 However, these laboratory technologies are still in the progress of translation from bench to bedside, which will face many challenges. In this review, the eyelid anatomy will be briefly presented first to provide context for the requirements of ideal posterior lamellar substitutes, followed by an overview of current biomaterials and tissue engineering strategies for posterior lamellar eyelid reconstruction. Finally, the ongoing challenges and future directions of eyelid regeneration strategies will be discussed, and some perspectives will be offered on transitioning replacement strategies to regeneration strategies for eyelid reconstruction in the future.
FIGURE 3.

The three basic elements in eyelid tissue engineering.
FIGURE 4.

Schematic diagram of acellular and cellular approaches for posterior lamellar reconstruction. (a) Acellular approach. (b) Cellular approach. ADMs, acellular dermal matrixes; DAM, decellularized amniotic membrane; PDC, porcine decellularized conjunctiva; PE, porous polyethylene; PLGA, poly(lactic‐co‐glycolic) acid; PPF, poly(propylene fumarate).
2. EYELID ANATOMY: FOCUSING ON THE POSTERIOR LAMELLAR
The eyelid is essentially a bilamellar structure that composed of the anterior and posterior lamella (Figure 1a). The former consists of the skin and the orbicularis oculi muscle, providing blood supply to the lamellar structures. The eyelid skin is thin and lacks subcutaneous fat. It is highly elastic and able to facilitate eyelid movement. The orbicularis oculi muscle, consisting of the preseptal, orbital, and pretarsal subunits, is responsible for eyelid closure. By using the laxity of adjacent tissues, local skin or myocutaneous flaps are used to reconstruct skin and muscle defects of the anterior lamella. 2
The posterior lamellar comprises the palpebral conjunctiva and the tarsal plate, which form histological foundations of the two primary functions of the posterior lamellar, that is, structural support and corneal protection via a mucosal surface. The tarsal plate is a semilunar‐shaped structure approximately 25 mm in length and 1 mm in thickness, while the height of the tarsal plate ranges from 8 to 12 mm in the upper eyelid and 4 to 5 mm in the lower eyelid. 19 , 20 The tarsal plate is a distinct transitional connective tissue which possesses the characteristics of both cartilage and dense fibrous connective tissue. 21 , 22 Histologically, the tarsal plate is not a truly fibrocartilaginous structure 23 , 24 but consists mainly of abundant meibomian glands secreting meibum and extracellular matrix (ECM) surrounding the fibroblasts. The ECM of the tarsal plate mainly contains collagen (Col) I, Col III, Col IV, aggrecan, and tenascin, along with various glycosaminoglycans. The interactions between collagen and other ECM components contribute to the rigidity and mechanical strength of the eyelid. 24 , 25 , 26 The meibomian glands are individual branched alveolar sebaceous glands consisting of duct systems and secretory acini. The ducts extend to the entire length of the tarsal plate and open at the free margin of the eyelid. Histologically, a meibomian gland consists of stratified squamous keratinized ductal epithelial cells and meibocytes arranged as functional secretory units called acini 27 (Figure 1c). The meibum, an essential composition of the tear film, is spread out when meibomian gland ducts are compressed by the transverse muscle fibers during blinking. Meibomian gland dysfunctions can result in ocular and eyelid discomfort, ocular surface diseases such as evaporative dry eye. 28
The conjunctiva, which arises from the corneoscleral limbus and overlies the inner surface of the eyelid, comprises a nonkeratinized stratified columnar and stratified squamous epithelium interspersed with goblet cells and a vascularized basement membrane composed of laminin and Col IV. 29 The epithelium is composed of stratified squamous nongoblet cells (90%–95%), goblet cells (5%–10%), and occasional lymphocytes and melanocytes 30 (Figure 1b). Soluble mucins can be secreted by the goblet cells, which serve as a crucial component of the tear film to bathe the ocular surface (Figure 1d). Conjunctival loss or scarring that can result from conjunctival damage or ocular surface disease may lead to eyelid misalignment, limited eye movement, diplopia, and dry eye symptoms. 31 In addition, it should be noted that the conjunctiva can spontaneously re‐epithelialize upon injury, as both stratified squamous nongoblet and goblet cells can be regenerated continuously by conjunctival stem cells, 32 inspiring tissue engineering strategies using conjunctival stem cells to repair conjunctival defects.
Collectively, the posterior lamella is a delicate bilamellar structure that serves two primary functions: mechanical support for the eyelid and sufficient lubrication of the cornea. Specifically, the tarsal plate provides mechanical support, maintains the appearance of the eyelid, and prevents abnormalities such as corneal exposure and eyelid retraction, making it an essential part of physical appearance and eyelid functions. The conjunctiva provides a mucosal surface that allows smooth globe movement; additionally, the goblet cells within the conjunctiva and the meibomian glands within the tarsal plate secrete mucins and lipids, respectively, which stabilize the tear film to maintain ocular surface homeostasis. To achieve functional eyelid reconstruction, an ideal posterior lamellar substitute should have the following characteristics: (1) mechanical strength: the posterior lamellar substitute should be a thin, elastic and stable construct that possesses considerable mechanical strength to maintain a semilunar shape, match the contour of the globe, and stabilize eyelid movement; (2) a smooth epithelialized inner surface: the construct should support efficient epithelial repopulation and stratification to mimic the conjunctiva; and (3) secretory functions: the substitute should produce sebaceous and/or mucin‐like substances to stabilize the tear film and prevent dry eye.
3. AUTOLOGOUS TISSUE GRAFTS
3.1. Tarsoconjunctival, tarsal, and tarsomarginal grafts
Autografts that could provide physical support and/or a mucosal layer have been applied in clinical practice for posterior lamella reconstruction (Figure 5). According to the three main features of the posterior lamella, the tarsoconjunctival graft is the golden standard for posterior lamella reconstruction and can be used to reconstruct the defects involving less than 75% of the whole eyelid width. 33 , 34 , 35 Compared with tarsoconjunctival flaps, such as the Hughes flap, 36 , 37 Cutler‐Beard flap, 38 , 39 and Mustarde lower lid switch flap, 40 , 41 free tarsoconjunctival grafts can reconstruct the defect eyelid in a single‐stage surgery, which is particularly suitable for elderly patients after malignant eyelid tumor resection. Tarsal grafts can provide posterior lamellar replacement without the conjunctival lining. When used for repairing full‐thickness defects, free tarsoconjunctival or tarsal grafts are often used in combination with other vascularized flaps. 42 , 43 As the free tarsal graft lacks conjunctival lining, it is usually left alone to allow gradual re‐epithelialization through the migration of the marginal conjunctiva.
FIGURE 5.
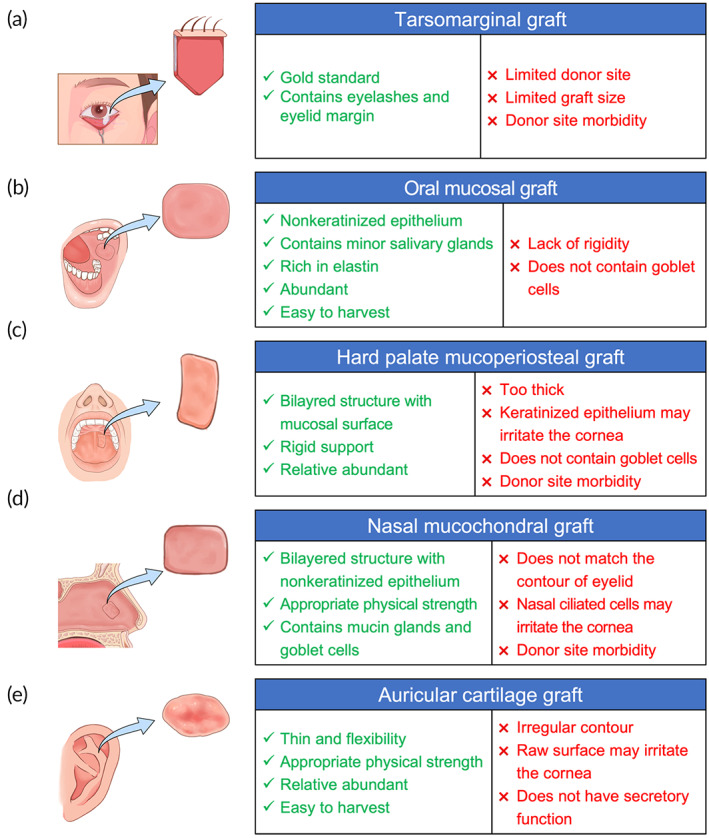
Summary of different autografts for posterior lamella eyelidreconstruction. (a) Tarsomarginal graft. (b) Oral mucosal graft. (c) Hard palate mucoperiosteal graft. (d) Nasal mucochondral graft. (e) Auricular cartilage graft.
Tarsomarginal graft is a composite graft, which is composed of the tarsal plate, conjunctiva, lid margin, and eyelashes (Figure 5a). Currently, tarsomarginal grafts of One‐fourth and even one‐third of the whole eyelid width can be obtained by primary closure. 44 Tarsomarginal grafts are suitable for eyelid defect reconstruction in various areas, including medial, central, or lateral defects in both lower and upper eyelids, particularly, the defects involving the eyelid margin, as these grafts preserve the native structures of the lid margin. As a free graft, the tarsomarginal graft should be covered with a local myocutaneous flap or a distal axial flap to ensure sufficient blood supply, which allows simultaneous transplantation of not only one but also two even three tarsomarginal grafts harvested from different donor eyelids. The main limitations of these autologous grafts include small graft size, eyelid retraction, scar formation, and donor eyelid morbidity, 45 which hinder their clinical application. 44 , 45 The postoperative complication rate for free tarsoconjunctival graft implantation has been reported to be as high as 43% in the East Asian population 35 and 84% in the Caucasian population. 46 Ectropion and entropion are the most common complications in the lower and upper eyelids, respectively.
3.2. Nontarsoconjunctival grafts
To overcome the constraints of posterior lamella‐derived autografts, mucous membrane autografts harvested from other areas, including the lip, 47 , 48 buccal mucosa, 49 , 50 gingival alveolar mucosa, 51 hard palate, 52 , 53 and nasal septum, 54 , 55 have been developed as replacements.
3.2.1. Oral mucosa grafts
The oral mucosa consists of vascular connective tissue named the lamina propria and nonkeratinized stratified squamous avascular epithelium 56 (Figure 5b). Oral mucosal grafts obtained from different parts of the oral cavity have variable features. Oral mucosal grafts from the lip and bucca are rich in elastin, which confers resistance to shearing and compression, and are highly vascularized, which facilitates integration of the graft once transplanted. Thus, buccal and labial mucosal grafts are typically used to replace the conjunctiva or as a lining for other tarsal substitutes, 57 such as auricular cartilage grafts 49 , 58 and amniotic membrane grafts. 59 , 60 Oral mucosal grafts can be harvested in forms of oral mucosal membrane grafts and minor salivary gland grafts, the latter one has been used to treat severe dry eye disease. The mucosal membrane can serve as a bioscaffold to support epithelial cells proliferation and growth. In addition, as shown by in vitro studies, epithelial stem/progenitor cells that exist in oral mucosal grafts can re‐epithelialize the tarsal plate and stabilize the ocular surfaces. 61 , 62 However, the mucous membranes do not contain goblet cells, which may lead to corneal irritation and dry eye symptoms. 63 Hence, simultaneous minor salivary gland grafting may be warranted to solve this problem. 64
3.2.2. Hard palate mucoperiosteal grafts
The hard palate mucosa that composed of a mucosal surface and a dense fibrous connective tissue layer has a histological structure similar to that of the posterior lamella (Figure 5c). Hard palate mucoperiosteal grafts, taken from the center of the hard palate, can provide both rigid structural support and a moist mucosal surface for the eyelid, making them an optimal substitute for the reconstruction of the posterior lamella. 53 , 65 , 66 However, the hard palate mucosa is a firm tissue featuring keratinized stratified squamous epithelium. Furthermore, long‐term observation revealed that the persistence of orthokeratosis and/or parakeratosis may last for years following transplantation, which may result in irritation of the cornea and cause pain. 67 Additionally, the donor area of hard palate mucoperiosteal grafts is usually left alone for secondary healing, which may cause possible hemorrhage, donor‐site discomfort, and difficulties in eating. The gingival mucosa is a firm, thick, and keratinized mucosa that extends to the nonkeratinized alveolar mucosa, which is relatively loose and mobile. Grafts harvested from the gingival alveolar region consist of a segment of gingival mucosa 2 mm in height, with the remainder comprising alveolar mucosa. This transitional structure allows gingival alveolar mucosa grafts to be suitable for repairing posterior lamellar defects that involve the eyelid margin. The marginal palpebral area can be replaced by the tight gingival pyart of the graft to achieve a rigid and stable structure, and the nonkeratinized alveolar part can ensure a nonirritating posterior lamellar surface. 51
3.2.3. Nasal mucosal grafts
Nasal mucosal grafts harvested from the nasal septum, 68 , 69 turbinate, 67 or ala 55 bear a close histological resemblance to the tarsoconjunctiva. Furthermore, the nasal mucosa contains not only a large number of goblet cells but also subepithelial mucin glands; therefore, it is able to provide substitute mucin. 67 Through a tight connection to the eyelid, septal mucochondral grafts can provide firmness as a tarsal substitute and a stable eyelid margin (Figure 5d). After thinning of the cartilage, grafts will curl up toward the mucosa side, matching the contour of the tarsal plate and achieving satisfying eyelid support and cosmetic outcomes. 70 Nasal mucosal grafts harvested from the inner part of the nasal ala can avoid the risk of septal perforation and hemorrhage. 55 In addition, composite mucosal grafts should be harvested from beyond the nasal hair area because conjunctival or corneal irritation may be caused by any remaining nasal hair. 71
3.2.4. Auricular cartilage grafts
Auricular cartilage grafts are thin elastic cartilage with suitable flexibility and appropriate physical strength (Figure 5e). Due to their lower metabolic rate and fewer vascular requirements, auricular cartilage grafts remain viable for many years after implantation. Thus, reconstruction of the posterior lamella with auricular cartilage grafts leads to a cosmetic contour and adequate support without significant graft absorption or shrinkage. 72 , 73 When auricular cartilage grafts are used for tarsoconjunctiva replacement, the conjunctival surface is missing and usually left for reepithelization, but the rough surface of the grafts may result in corneal irritation. 74 Adding an oral mucosal graft for coverage could help solve this problem. 49 , 58
Although various autografts have been introduced for clinical posterior lamellar reconstruction, the lack of donor area, risk of disease exacerbation, donor‐site morbidity, and incomplete functional matching have hindered the clinical application of autologous tissues and have stimulated the exploration of appropriate acellular or cellular bioengineered substitutes for posterior lamellar reconstruction.
4. ACELLULAR APPROACHES
Acellular approaches aim to repair defective eyelid tissue and restore its key functions with natural or synthetic biomaterials. The ideal biomaterial for posterior lamellar repair should be a thin and stable matrix with high biocompatibility and have the ability to be integrated into the peripheral tarsus without provoking inflammatory responses and, importantly, should mimic the biological functions and physical structures of the natural ECM to support cell survival, proliferation, and growth. Currently, three major types of biomaterials are used for posterior lamellar reconstruction: decellularized ECM, natural polymers, and synthetic polymers.
4.1. Decellularized matrixes
Studies have demonstrated that the amniotic membrane can support epithelialization by offering a basement membrane and an avascular stromal matrix similar to the native conjunctiva. Furthermore, amniotic membrane possesses anti‐angiogenic, anti‐inflammatory, and anti‐scarring properties, making it the most widespread substitute for conjunctival replacement. 75 , 76 Clinically, decellularized amniotic membrane grafts are placed directly over the bare tarsus up to the eyelid margin 77 , 78 or applied in combination with other techniques for posterior lamellar reconstruction. 79 , 80 As a scaffold, the implanted decellularized amniotic membrane can support the growth of surrounding conjunctiva‐derived cells, including conjunctival nongoblet epithelial cells and goblet cells, 78 , 81 and is then absorbed within 6 months without scarring. 82 Derived materials, such as freeze‐dried amniotic membrane, 75 air‐dried dehydrated amniotic membrane, 83 and urea‐de‐epithelialized amniotic membrane, 84 have been developed to optimize the processes of amniotic membrane sterilization and storage and to better preserve the basement membrane structures, growth factor contents, and stroma matrix components. Although good functional and esthetic outcomes have been reported, the limited availability, variable standards for preparation, inconsistent tissue properties, and risk of infectious disease transmission continue to make the use of decellularized amniotic grafts problematic. 84
In addition to decellularized amniotic membranes, decellularized porcine and human conjunctival grafts have also been tested for conjunctival defect reconstruction in animal experiments 85 , 86 and clinical studies. 85 In clinical cases, transplanted decellularized porcine conjunctival grafts were integrated with autologous conjunctiva without graft disintegration or fibroplasia and were completely epithelialized at 2 weeks. 85 In addition, other decellularized biomembranes, such as decellularized bovine or porcine pericardium 10 , 87 and decellularized adipose‐derived mesenchymal stromal cell matrix, 88 have also shown promising results in facilitating the adhesion of goblet cells and conjunctival epithelial cells in conjunctival substitute engineering.
Acellular dermal matrixes originated from human (AlloDerm®, BellaDerm®), 89 , 90 , 91 porcine (Endurage®), 11 , 92 , 93 , 94 and bovine (Surgimen®) 94 , 95 dermis provide off‐the‐shelf materials to replace the tarsal plate because of relatively low immunogenicity and good histocompatibility. 96 Structurally, the ADM is a sturdy but flexible flat sheet comprising cross‐linked collagen matrix with a dermal surface and a basement membrane surface. The crosslinking process makes the graft resistant to absorption and decomposition, while its flexibility facilitates it to conform to ocular surface contours, and the collagen matrix provides a natural surface for epithelial migration and the infiltration of fibroblasts. 97 , 98 The efficiency and safety profiles of human, porcine, or bovine acellular dermal matrixes applied as lower eyelid spacer grafts have been well demonstrated in a series of level II and III clinical studies. 14 , 89 , 90 , 94 These acellular dermal matrix grafts can be inserted below the tarsal plate as a spacer to increase eyelid height and provide structural support after releasing the lower eyelid retractors 7 and have shown outcomes in augmenting the height of retracted lower eyelids comparable to those of autologous auricular cartilage grafts and hard palate grafts. 94 In clinical scenarios, tarsal plate defects are often accompanied by conjunctival deficiency, and reconstruction of tarsal plate defects with acellular dermal matrix grafts often requires a local conjunctival flap or other autografts for reconstruction of the inner layer lining. 11 , 99 , 100 For patients with insufficient conjunctival tissue, an acellular dermal matrix graft can be transplanted to the defective posterior lamella with the dermal surface oriented toward the wound bed and the basement membrane facing the ocular surface, which allows full conjunctival epithelialization over the graft surface within 3–6 weeks postoperatively. 101 However, the main drawback limiting the widespread use of ADM grafts is graft contraction and resorption, especially for grafts originating from humans. 102 The mean graft contraction rate has been reported to be 57% for acellular dermal matrix grafts versus 16% for hard palate mucosal grafts. 103
Another clinically available acellular membrane for tarsal plate reconstruction is TarSys®, which is decellularized porcine small intestinal submucosa containing Col I, Col III, Col IV, and associated glycosaminoglycans. 104 , 105 However, as TarSys® is a xenogeneic product, concerns due to the risk of infectious disease transmission and reported immunogenic inflammatory‐related complications remain. 106 , 107 , 108
4.2. Natural polymers
Natural polymer scaffolds sourced from ECM components have been widely used for conjunctival defect repair due to their high degree of biocompatibility; such scaffolds include Col I, 109 chitosan, 110 and keratin. 111 Among them, Col I, the most abundant protein in the conjunctival matrix, is the most commonly used. It has been demonstrated to successfully support not only conjunctival epithelial cell proliferation but also differentiation in vitro. 112 In vivo, collagen‐based substitutes have been shown to promote conjunctival regeneration with complete epithelization, minor fibrosis, and minimal wound contracture. 109 , 113 However, untreated collagen hydrogels are opaque and exhibit poor mechanical stability because they are made of loosely packed collagen fibers and thus differ from the native conjunctival stroma, which limits their surgical applicability. Therefore, plastic compressed collagen gels 109 , 112 and vitrified collagen membranes 113 have been developed to simulate collagen fibril arrangement in normal conjunctiva and provide enhanced mechanical strength. In vitro studies have demonstrated that plastic compressed collagen gels and vitrified collagen membranes support conjunctival epithelial cell proliferation and phenotype development. 112 , 113 Once implanted into the conjunctival defect area in rabbit models, these conjunctival equivalents engineered from both collagen‐based materials promoted conjunctival regeneration by supporting rapid re‐epithelization as well as sufficient goblet cell repopulation while minimizing wound contracture and fibrosis. 109 , 113 Other natural ECM proteins, such as gelatin, 114 , 115 elastin, 116 and their combinations, 81 have also been tested in the context of conjunctival equivalent engineering. Despite their high biocompatibility, natural polymers are still unable to completely mimic the components of the conjunctival ECM and have unpredictable degradation rates in vivo, which leads to greater uncertainty in their reconstructive effects. 117
4.3. Synthetic polymers
Synthetic polymeric materials have certain physical geometries, chemical compositions, and biological properties; they are produced following standardized manufacturing processes and do not require decellularization procedures. Synthetic polymeric materials for conjunctival reconstruction should have the following properties: (1) processability into thin films with a controlled thickness; (2) biocompatibility to support cell adhesion, proliferation, and differentiation; and (3) biodegradability after implantation. A variety of synthetic polymers, including poly(acrylic acid) (PAA), 116 poly(ε‐caprolactone) (PCL), 116 , 118 , 119 poly(lactic acid) (PLA), 12 poly(l‐lactic acid‐co‐ε‐caprolactone) (PLCL), 120 and poly(vinyl alcohol) (PVA), 116 have been tested in conjunctival repair studies. Among them, scaffolds engineered from PCL, 121 PLA, 12 and their derivatives 122 have been used in conjunctival repair and have been shown to support goblet cell growth in animal models. The United States Food and Drug Administration has already approved the clinical application of PCL. Therefore, various modified PCL scaffolds have attracted intensive attention in conjunctival equivalent engineering due to their great potential for clinical translation. 116
Additionally, synthetic polymers, such as porous polyethylene (PE), 13 , 104 , 123 poly(3‐hydroxybutyrate‐co‐3‐hydroxyhexanoate) (PHBHHx), 124 poly(propylene fumarate) (PPF), 125 , 126 poly(lactic‐co‐glycolic) acid (PLGA), 18 and PCL, 16 have also been used in tarsal plate reconstruction in recent studies. Among them, only porous high‐density PE (Medpor®) has been used as an eyelid spacer for treating lower eyelid retraction in clinical practice. 123 However, this material should be used sparingly in eyelid surgery, due to high postoperative complication rates, such as poor stability, implant exposure, abnormal skin contours, and unexplained pain. 127 Other synthetic scaffolds are available and have been investigated for tarsal reconstruction in animal models. However, poor tissue tolerance impedes their clinical translation, and inflammatory tissue responses have been observed around the implants. 7
The common advantages of these polymers are good biodegradability, rigidity, and moldability. However, compared with scaffolds derived from natural tissues, synthetic polymers have relatively low biocompatibility and usually have hydrophobic features because of the lack of natural cell recognition sites, 7 , 128 which limits their clinical translation. These limitations have prompted attempts to modify their biological characteristics by combining several differential biomaterials to make scaffolds with properties more closely resembling those of the natural tarsal plate. For example, with its excellent mechanical integrity and cytocompatibility, the biodegradable biopolymer PLA is widely used in the biomedical field. However, this material is hydrophobic, which is unfavorable for cell adhesion and growth. 128 It has been considered a novel direction of tissue engineering to blend PLA with other biomaterials with better wettability, which could result in better tissue recovery. Yan et al. 12 developed a PLA electrospun nanofibrous membrane scaffold for conjunctival repair, which was coated with cellulose nanofibrils to improve hydrophilicity, coated with silk peptide to promote conjunctival epithelial cell proliferation, and loaded with levofloxacin to prevent postoperative infection. By promoting the restoration of conjunctival structure and function, this scaffold yielded favorable therapeutic effects after transplantation in a rabbit conjunctival defect model. 12 In addition, to improve the hydrophobicity and modulate the biomechanical property of pure PPF polymer, a degradable and mechanically adjustable scaffold was synthesized by copolymerizing PPF with 2‐hydroxyethyl methacrylate (HEMA), a widely used hydrophilic monomer. The PPF‐HEMA scaffold showed no cytotoxicity in vitro, elicited less inflammatory response and showed better tissue infiltration than the acellular dermal matrix control in tarsal plate reconstruction in rabbits. 125 This copolymerized scaffold was then further modified by depositing a soft collagen/chitosan layer onto the PPF‐HEMA layer to fabricate a biomimetic biphasic scaffold. In a rabbit transconjunctival defect model, this biphasic scaffold allowed tissue to grow inward and maintained the contour of the eyelid, while the collagen/chitosan phase was able to support re‐epithelialization with functional palpebral conjunctiva. 126
5. CELLULAR APPROACHES
Although numerous “smart” biomimetic biomaterials with the potential to guide tissue regeneration have been developed for posterior lamellar reconstruction, 12 , 126 biomaterial‐guided tissue regeneration extensively relies on the surrounding tissue microenvironment, which may not be suitable in some clinical scenarios, especially in cases of local inflammation, systemic autoimmune diseases, poor vascular beds, or massive or even complete loss of posterior lamellar tissue. Thus, acellular approaches are usually accompanied by fibrotic tissue formation, which can restore the mechanical support function of the eyelid but does not restore the secretory function and barely induces reepithelialization. Recently, progress has been made in the engineering of a cellular graft that can restore both the structural and functional properties of the posterior lamellar, especially lipid secretion and the smooth mucosal surface.
5.1. Tissue‐engineered tarsal plate equivalents
In 2019, Dai et al. 18 engineered a composite construct consisting of PLGA sponge, bone marrow‐derived mesenchymal stem cells (BMSCs), plasmid DNA which encodes transforming growth factor‐β1, and fibrin gel. When the construct was grafted into the tarsal plate defects in a rabbit model, the defects showed both structural and functional reconstruction 8 weeks postimplantation, as demonstrated by sufficient ECM deposition and meibomian gland acinar unit formation within the implants. 18 Although the origin of the meibomian glands in this study remains controversial, restoration of the lipid secretion function of the tarsus opened a new avenue in posterior lamellar tissue engineering. With the same aim of engineering a tarsal graft with lipid secretion function, Chen et al. 16 developed biomimetic tarsal plate substitutes by coating a 3D‐bioprinted PCL scaffold with decellularized matrixes of adipose derived mesenchymal stem cells to improve hydrophilicity and cell adhesion properties of PCL‐based materials. In vitro studies revealed that this biomimetic scaffold had excellent cytocompatibility and supported SZ95 sebocyte proliferation. More importantly, 1 month after subcutaneous dorsal implantation into nude mice, the seeded SZ95 sebocytes showed good proliferation with structures similar to the meibomian gland acinar and an intact epithelial layer. Meanwhile, the seeded SZ95 sebocytes secreted abundant neutral lipids that were widely distributed within the scaffold. This study suggests that the lipid secretory function of tarsal plate substitutes could be restored by seeding lipid‐secreting cells onto bioscaffolds. However, orthotopic tarsal defect reconstruction studies are required to further test the secretory function of the regenerated meibomian glands.
5.2. Tissue‐engineered conjunctival equivalents
As far as we know, no study has investigated cellular approaches to repair palpebral conjunctival defects so far. Notably, numerous studies on bulbar conjunctival tissue engineering have demonstrated encouraging results in the promotion of reepithelialization and reduction of scarring in both animal models 17 , 32 , 120 and clinical trials 59 , 129 (Table 1). Considering that the palpebral conjunctiva has structures and functions similar to those of the bulbar conjunctiva, 30 strategies for engineering bulbar conjunctival equivalents are translatable for engineering palpebral conjunctiva equivalents.
TABLE 1.
Cellular approaches for conjunctival reconstruction.
| Study | Seed cells | Scaffold | Highlight in vitro results | Highlight in vivo results |
|---|---|---|---|---|
| Conjunctival source | ||||
| Wu et al. (2020) 32 | Human and rabbit total, p75−, p75+, and p75++ CjECs | AM |
‐Human p75++ CjECs showed the strongest proliferation potential. ‐Human p75++ CjECs formed the thickest conjunctival epithelium with 8–10 cell layers and the highest density of MUC5AC+ cells. |
‐Large conjunctiva defect model in rabbits. ‐Conjunctiva epithelium engineered from rabbit p75++ CjECs achieved more complete reepithelization and more goblet cells than that engineered from control cells. |
| Hong et al. (2018) 133 | Human CjECs | PLGA membrane, PET membrane |
‐Human CjECs formed epithelium with two to three cell layers in both groups. ‐PET group showed higher MUC5AC expression, while PLGA group showed higher CK19 and Ki67 expression. |
‐Corneal defect model in rabbits. ‐PLGA‐based epithelial sheets achieved a faster and more complete reepithelization but without goblet cells compared to the PET scaffolds. |
| Witt et al. (2020) 17 | Human CEx, CjECs | PDC |
‐CEx cultured on PDC formed a thicker epithelium with MUC5AC+ and PAS+ cells. ‐No goblet cells were observed in the cultured CjECs. |
‐Conjunctival defect in rabbits. ‐PDC + CEx constructs showed preserved progenitor cell properties and higher goblet cell numbers than control groups. |
| Bandeira et al. (2019) 84 | Human CEx | uHAM | ‐CEx cultured on uHAM formed multilayered stratified epithelium with MUC5AC+ cells after 3–4 weeks of expansion. |
‐Clinical study on ocular surface reconstruction. ‐uHAM + CEx constructs repaired large epithelial defects without lesion recurrence in two patients throughout the follow‐up. |
| Nonconjunctival mucosal source | ||||
| Komai et al. (2021) 15 | Human oral mucosal explant | AM | / |
‐Retrospective cohort study in fornix reconstruction involved 16 eyes of 15 patients. ‐Mean follow‐up was 102.1 ± 46 months. −5‐year overall fornix‐reconstruction success probability was 79.6% ± 10.7%, and success probability for thermal/chemical injury and OCP was 100% and 53.3% ± 24.8%, respectively. |
| Wang et al. (2019) 129 | Human limbal stem cells | AM | / |
‐Retrospective case series in conjunctival sac reconstruction involved 36 eyes of 36 patients. ‐Mean follow‐up was 17.4 ± 3.9 months. ‐Symblepharon was completely relieved in 30 eyes 3 months after surgery. ‐No symblepharon recurrence during the 3‐month follow‐up period. |
| Kobayashi et al. (2015) 130 | Human nasal, oral, corneal, conjunctival mucosal cells | AM |
‐All cell types formed epithelium with four to five layers of CK3+ and CK13+ cells. ‐MUC5AC+ cells were only detected in cultured nasal mucosal cells. |
‐Conjunctival defect model in rabbits. ‐Transplanted CEMNS were well adopted to conjunctival defects and contained goblet cells at Day 14. |
| Other epithelial source | ||||
| Yang et al. (2015) 141 | Human amniotic epithelial cells | Decellularized AM |
‐Amniotic epithelial cells that was induced with decellularized conjunctival matrix for 5 days expressed CK4, CK13, and MUC5AC. ‐Differentiated amniotic epithelial cells cultured on decellularized AM for 14 days formed four to five cell layers. |
‐Conjunctival defect model in rabbits. ‐Engineered conjunctiva was covered with two to five layers of cells and contained MUC5AC+ cells, while fresh AM only had a single layer of epithelia. |
| Lu et al. (2011) 164 | Monkey epidermal progenitor cells | Denuded AM | ‐Epidermal progenitor cells cultured on AM formed a multilayered epithelium without MUC5AC+ cells. |
‐Conjunctival defect model in in rhesus monkey. ‐Autologous cultivated epidermal progenitor cells formed several layers of epithelial cells with similar appearance to those of normal conjunctival epithelium. ‐No goblet cells were detected in the sheets. |
Abbreviation: AM, amniotic membrane; CEMNS, cultured epithelial mucosal nasal sheet; CEx, conjunctival explant; CjECs, conjunctival epithelial cells; OCP, ocular cicatricial pemphigoid; PAS, periodic acid Schiff; PDC, porcine decellularized conjunctiva; PET, polyester; PLGA, poly(lactic‐co‐glycolic) acid; uHAM, urea‐de‐epithelialized human amniotic membrane.
To meet the needs of conjunctival repair, a tissue‐engineered conjunctival epithelial substitute should have good surgical manipulability, excellent biocompatibility, and low inflammatory properties. Histologically, it needs to be a stratified epithelium composed of squamous epithelial cells, goblet cells, and stem/progenitor cells. By following the principles of tissue engineering, a conjunctival equivalent with a multilayered stratified epithelium and individual interspersed goblet cells can be developed in vitro by cultivating conjunctival explants on a matrix that has supportive niche features to facilitate adhesion, proliferation, as well as differentiation of epithelial and goblet cells under defined conditions. Various biomaterials for direct conjunctival replacement, as mentioned above, ranging from decellularized matrixes to artificial synthetic polymers, could possibly be adapted for conjunctival equivalent engineering, such as decellularized amniotic membranes, 32 , 130 decellularized porcine conjunctiva, 17 decellularized porcine pericardium, 10 fibrin, 131 , 132 collagen, 112 , 113 PLGA, 133 PCL, 118 , 134 polyester, 133 and 3D‐printed membranes based on a blend of previously described biomaterials. 81 These biomaterials act as carrier scaffolds of epithelial cells during in vitro culture. However, as mentioned earlier, the main problems with current synthetic scaffolds include difficult surgical manageability, poor biocompatibility, and inadequate biomechanical strength in vivo.
A wide range of cell types has been used for developing bioengineered conjunctival equivalents. Conjunctival epithelial cells are the most frequently used cells for conjunctival equivalent engineering. Conjunctival epithelial cells cultured on plastic compressed collagen gels, 112 and amniotic membranes 84 retained their characteristic cuboidal shape with mucin expression, indicating that these substrata may influence the differentiation of goblet cells. Multilayered conjunctival epithelial cell organization was achieved by culturing human conjunctival epithelial cells on electrospun bioscaffolds consisting of PCL and decellularized tissue matrix. 134 Furthermore, goblet cell repopulation, a hallmark of conjunctival epithelium, was observed in bioengineered conjunctival equivalents developed from conjunctival epithelial cells 120 , 133 , 135 and conjunctival stem cells. 136 However, the use of conjunctival epithelial cells and conjunctival stem cells requires expansion in vitro, which in turn requires a stem cell niche‐like matrix or feeder cell support before cultivation on a presumptive substrate. 137 , 138 Furthermore, the isolation, expansion, and preservation of conjunctival epithelial cells as well as conjunctival stem cells in vitro require xenobiotic components that limit their clinical application. Thus, engineering stable, stratified, and goblet cell‐rich constructs can be achieved by explant culture of small conjunctival samples on decellularized conjunctiva 17 , 135 or amniotic membrane. 139 , 140 Such conjunctival explants can form a thicker epithelium with more MUC5AC‐positive goblet cells on decellularized porcine conjunctiva than conjunctival epithelial cells. 17 A possible interpretation of the superiority of conjunctival explants over conjunctival epithelial cells is that the conjunctival stem cell populations within the conjunctival explants could maintain their undifferentiated state longer as they are located closer to their niche environment.
Conjunctival epithelial equivalents containing goblet cells have been engineered from nonconjunctival cell sources, such as nasal mucosal epithelial cells, 130 which harbor goblet cells, and amniotic epithelial cells, which are able to differentiate into conjunctival epithelial cells and goblet cells under specific conditions. 141 For patients with a large conjunctival deficiency or scarring, oral mucosal epithelial cells are considered as an alternative source of seed cells for conjunctival epithelial equivalent engineering strategies and have been applied in clinical practice in the form of a cultivated oral mucosal epithelium transplantation technique for ocular surface reconstruction 59 , 61 and forniceal reconstruction. 15 Although promising results have been achieved in terms of high rates of successful forniceal reconstruction, the engineered oral epithelium lacks goblet cells, which limits its usefulness for conjunctival reconstruction.
6. DISCUSSION AND FUTURE PERSPECTIVES
6.1. Future avenues: From reparative to regenerative surgery
Although the injured conjunctiva is able to reepithelialize spontaneously, the accompanying fibrosis can lead to forniceal shortening and even ankyloblepharon, followed by corneal diseases and/or dry eye diseases, which may lead to ultimate vision loss.
Numerous techniques have been developed for posterior lamellar reconstruction over the past decades; however, the eyelid reconstruction techniques currently available in clinical practice are essentially replacement strategies. When this type of strategy is implemented, autografts or cell‐free biomaterials are implanted into the defect area to provide mechanical support. These biomaterials are easily harvested and relatively inexpensive, but high rates of complications have also been reported, such as graft contraction and shrinkage, 102 graft exposure, 98 and cyst formation. 14 Most importantly, due to the lack of posterior lamellar cellular components, these grafts barely provide a smooth epithelialized surface and restore secretory function. Although some studies have reported that implanted decellularized matrixes could support cell growth both in vitro 84 and in vivo, 85 concomitant fibrosis rather than complete reepithelization was observed on the surface of the biomaterials upon implantation in animal models. 10 , 109 Cellular approaches aim to restore the full function of the posterior lamella by implanting a tissue‐engineered tarsal or conjunctival equivalent into the defect area, which can be referred to as “regenerative surgery” and represents a future direction in this field. For example, by seeding immortalized human SZ95 sebocytes onto a scaffold, a tarsal plate substitute capable of secreting neutral lipids both in vitro and in vivo was successfully engineered. 16 Moreover, by culturing conjunctival epithelial cells on vitrified collagen membrane, conjunctival equivalents that could greatly promote goblet cell repopulation as well as minimize fibrosis and wound contracture was successfully developed. 113 These results, at least partially, reproduced the secretory function of the human eyelid. However, the field remains in its infancy, and extensive investigation is required in the future.
6.2. Future challenges to functional eyelid reconstruction
6.2.1. Bridging the gap with 3D bioprinting technology
The human tarsal plate has three key characteristics: a semilunar shape, a thickness of 1 mm, and a certain degree of mechanical strength. Thus, tarsal plate substitutes should possess similar features to fit clinical conditions. However, neither native nor bioengineered tarsal plate substitutes that are currently available fully demonstrate these characteristics. For example, auricular cartilage grafts are thin, relatively abundant, and mechanically similar to the native tarsus and thus have been widely used in clinical conditions and have provided satisfactory esthetics and appropriate support for the eyelid without apparent shrinkage or absorption in several studies. 2 , 8 However, the contour of native auricular cartilage does not fit the contour of the globe, resulting in corneal irritation. 74 Porous Medpor® is a biocompatible and rigid material that can be easily molded into an ultrathin and curved eyelid spacer for the treatment of eyelid retraction. 123 , 142 However, porous Medpor® eyelid spacers show low tissue tolerance, with an implant exposure rate as high as 76.5%, 143 which significantly hinders their widespread use.
The development of 3D bioprinting technology has the potential to bridge this gap. This technology involves depositing cell‐loaded biomaterials layer by layer in predetermined architecture to produce artificial multicellular tissues and/or organs with biomimetic microstructures. 144 , 145 Recently, bioprinted tracheal, 146 , 147 human nasal cartilage, 148 , 149 and long bone 150 , 151 , 152 constructs have been successfully engineered. These bioprinted constructs exhibit considerable mechanical strength for maintaining specific geometric shapes or bearing loads, contain tissue‐specific cells for generating functional tissues in vivo, and even have well‐designed microstructures to serve special functions, such as promoting angiogenesis 153 , 154 and guiding tissue regeneration. 155 , 156 , 157 3D cell bioprinting technology allows personalized designs, precise preparation procedures, and on‐demand creation within a short time frame, 144 , 158 making the fabrication of shape‐customized and mechanically strengthened tarsal substitutes possible. In addition, 3D bioprinting technology has been used in conjunctival equivalent engineering. For example, 3D‐bioprinted gelatin‐based membranes have been reported to offer necessary mechanical and physical properties required for effective conjunctival defect reconstruction. 81 Compared to the amniotic membrane, the 3D‐printed membrane had a higher density of goblet cells on the regenerated epithelium and had a more predictable degradation pattern, less scar formation, and minor inflammatory responses in vivo. 81 Another study demonstrated the successful application of bioprinted hydrogel microconstructs loaded with rabbit conjunctival stem cells for conjunctival regeneration. 159 The bioprinted microconstructs favorably mimicked the biochemical and biomechanical properties of the native stem cell niche matrix of the conjunctiva. Therefore, they were capable of maintaining their stem cell phenotype and effectively supporting conjunctival stem cell growth and differentiation into squamous epithelial cells as well as goblet cells. With the use of 3D bioprinting, posterior lamellar substitutes could be successfully fabricated with the required mechanical properties and composite structures, and various types of seed cells can be fully expected in the future.
6.2.2. Focusing on secretory function
The restoration of secretory function remains another great challenge in eyelid reconstruction. Goblet cells within the conjunctiva and meibomian glands within the tarsus are responsible for secreting lipids and mucins, respectively, which participate in tear film formation and maintain ocular surface homeostasis. 160 , 161 Engineering tarsal plates and conjunctival equivalents able to secrete lipids and/or mucins presents a potential strategy to solve this problem. Stem cells involved in the development of meibomian glands have not been well demonstrated thus far, posing an obstacle to meibomian gland regeneration. Recently, a stem/progenitor cell population expressing Krox20, a zinc finger transcription factor, was identified as a crucial driver of the development and homeostasis of the meibomian gland, 162 shedding light on the identification of meibomian gland stem cells and the subsequent construction of meibomian gland substitutes. In addition to regenerating the meibomian glands, engineering tarsal equivalents by directly seeding lipid‐secreting sebocytes onto scaffolds presents another feasible approach. 16 However, sebocytes are terminally differentiated cells with limited self‐renewal capability, and the amounts and types of secreted lipids and the durability of the lipid secretion function still require further in‐depth investigation. Although goblet cells account for only 5%–10% of cells of the conjunctival epithelium, they play an irreplaceable role in lubrication, immunomodulation, and ocular surface homeostasis. 31 Conjunctival stem cells are able to differentiate into goblet cells and conjunctival epithelial cells under physiological conditions, but this process often fails to reappear in tissue‐engineered conjunctival equivalents. 30 , 163 For example, Witt et al. preexpanded human conjunctival epithelial cells on porcine decellularized conjunctiva for 14 days in vitro, and a stratified epithelium with an average thickness of 15.15 μm was formed, but no goblet cells were found in the epithelium. 17 Methods to increase goblet cell numbers have mainly focused on directing resident stem cells toward goblet cell differentiation. 139
6.2.3. Bioengineering a bilayered construct for transconjunctival reconstruction
Anatomically, the native posterior lamella of the eyelid is a bilayered structure, with each layer exhibiting unique structures and functions. In clinical conditions, posterior lamellar defects are often accompanied by conjunctival insufficiency. Thus, the ideal bioengineered posterior lamellar substitute should also be a bilayered hybrid, with one lamella providing mechanical strength and the other providing a smooth epithelial surface. However, most recent advances in posterior lamellar reconstruction meet one of the three requirements by using a single tarsal or conjunctival substitute, 6 , 11 , 66 undoubtably achieving inferior reconstructive outcomes. Recently, a biphasic scaffold composed of a hard PPF–HEMA layer and a soft collagen/chitosan layer was developed to mimic the bilayered structure of the native posterior lamella. 126 Two weeks after implantation into tarsoconjunctival defects in a rabbit model, the histological findings demonstrated that the hard, porous PPF–HEMA layer supported the shape of the eyelid and allowed the ingrowth of surrounding tissue, while the soft collagen/chitosan sponge layer promoted cell migration and conjunctival regeneration, 126 suggesting the potential of this construct for tarsoconjunctival repair. However, similar to other acellular approaches, concerns regarding the efficiency of restoring secretory function and a smooth epithelial lining due to the lack of cellular components remain with this approach. Unfortunately, to our knowledge, no bilayered cellular substitutes for posterior lamellar reconstruction have been reported in the literature. By using integrated techniques, such as 3D bioprinting and advanced biomaterials, engineering a bilayered cellular substitute for posterior lamellar reconstruction is feasible and may solve the remaining problems.
7. CONCLUSION
In conclusion, posterior lamellar reconstruction remains a great challenge because of the delicate bilayer structure and complicated functions of the eyelid. An ideal posterior lamellar substitute should exhibit three features: sufficient mechanical strength, a smooth epithelial surface, and lipid secretion function. However, currently available clinical autografts and techniques for posterior lamellar reconstruction do not fully meet these requirements, which has stimulated the exploration of acellular and cellular bioengineered substitutes for posterior lamellar reconstruction. Acellular approaches via replacing defective posterior lamellar tissue with biomimetic biomaterials have demonstrated promising outcomes in both experimental studies and clinical conditions. Cellular approaches aim to promote posterior lamellar tissue regeneration by implanting living tissue‐engineered grafts, but these methods are still in their infancy. Future studies on biphasic scaffold design and the extensive use of 3D printing technology will facilitate the design of successful tissue‐engineered bilayer cellular substitutes with secretory functions for eyelid reconstruction.
AUTHOR CONTRIBUTIONS
Yuxin Yan: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); resources (equal); writing – original draft (equal). Qiumei Ji: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); visualization (equal); writing – review and editing (equal). Rao Fu: Data curation (equal); formal analysis (equal); investigation (equal). Chuanqi Liu: Data curation (equal); investigation (equal); methodology (equal). Jing Yang: Data curation (equal); investigation (equal); methodology (equal). Xiya Yin: Data curation (equal); investigation (equal); methodology (equal). Qingfeng Li: Conceptualization (equal); data curation (equal); project administration (equal); resources (equal); writing – review and editing (equal). Ru‐Lin Huang: Conceptualization (equal); data curation (equal); project administration (equal); resources (equal); funding acquisition (equal); writing‐ review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10497.
ACKNOWLEDGMENTS
This research was supported by grants from the National Natural Science Foundation of China (81871571 to Ru‐Lin Huang), the Shanghai Pujiang Program (2019PJD023 to Ru‐Lin Huang), and the Shanghai Municipal Key Clinical Specialty (shslczdzk00901 to Ru‐Lin Huang).
Yan Y, Ji Q, Fu R, et al. Biomaterials and tissue engineering strategies for posterior lamellar eyelid reconstruction: Replacement or regeneration? Bioeng Transl Med. 2023;8(4):e10497. doi: 10.1002/btm2.10497
Yuxin Yan and Qiumei Ji contributed equally to this work.
Contributor Information
Qingfeng Li, Email: dr.liqingfeng@shsmu.edu.cn.
Ru‐Lin Huang, Email: huangrulin@sjtu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chang EI, Esmaeli B, Butler CE. Eyelid reconstruction. Plast Reconstr Surg. 2017;140(5):724e‐735e. [DOI] [PubMed] [Google Scholar]
- 2. Yan Y, Fu R, Ji Q, et al. Surgical strategies for eyelid defect reconstruction: a review on principles and techniques. Ophthalmol Ther. 2022;11(4):1383‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaca EE, Surek C, Klosowiak J, Dumanian GA, Alghoul MS. Neurotized free platysma flap for functional eyelid reconstruction: a cadaveric study of anatomical feasibility. Plast Reconstr Surg. 2020;145(4):1049‐1057. [DOI] [PubMed] [Google Scholar]
- 4. Malik MM, Vahdani K. Lower eyelid reconstruction: a new classification incorporating the vertical dimension. Plast Reconstr Surg. 2020;145(4):877e‐878e. [DOI] [PubMed] [Google Scholar]
- 5. Segal KL, Nelson CC. Periocular Reconstruction. Facial Plast Surg Clin North Am. 2019;27(1):105‐118. [DOI] [PubMed] [Google Scholar]
- 6. Fin A, De Biasio F, Lanzetta P, Mura S, Tarantini A, Parodi PC. Posterior lamellar reconstruction: a comprehensive review of the literature. Orbit. 2019;38(1):51‐66. [DOI] [PubMed] [Google Scholar]
- 7. Park E, Lewis K, Alghoul MS. Comparison of efficacy and complications among various spacer grafts in the treatment of lower eyelid retraction: a systematic review. Aesthet Surg J. 2017;37(7):743‐754. [DOI] [PubMed] [Google Scholar]
- 8. Jennings E, Krakauer M, Nunery WR, Aakalu VK. Advancements in the repair of large upper eyelid defects: a 10‐year review. Orbit. 2021;40(6):470‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alghoul MS, Bricker JT, Vaca EE, Purnell CA. Lower eyelid reconstruction: a new classification incorporating the vertical dimension. Plast Reconstr Surg. 2019;144(2):443‐455. [DOI] [PubMed] [Google Scholar]
- 10. Chen F, Deng J, Luo L, et al. Crosslinked decellularized porcine pericardium as a substrate for conjunctival reconstruction. Stem Cells Int. 2022;2022:7571146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Custer PL, Maamari RN. Porcine dermal matrix sandwich graft for lower eyelid reconstruction. Orbit. 2021;40(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 12. Yan D, Zhang S, Yu F, et al. Insight into levofloxacin loaded biocompatible electrospun scaffolds for their potential as conjunctival substitutes. Carbohydr Polym. 2021;269:118341. [DOI] [PubMed] [Google Scholar]
- 13. Xu P, Feng X, Zheng H, et al. A tarsus construct of a novel branched polyethylene with good elasticity for eyelid reconstruction in vivo. Regen Biomater. 2020;7(3):259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tao JP, Aakalu VK, Wladis EJ, et al. Bioengineered acellular dermal matrix spacer grafts for lower eyelid retraction repair: a report by the American Academy of ophthalmology. Ophthalmology. 2020;127(5):689‐695. [DOI] [PubMed] [Google Scholar]
- 15. Komai S, Inatomi T, Nakamura T, et al. Long‐term outcome of cultivated oral mucosal epithelial transplantation for fornix reconstruction in chronic cicatrising diseases. Br J Ophthalmol. 2021;106(10):1355‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen L, Yan D, Wu N, et al. 3D‐printed poly‐caprolactone scaffolds modified with biomimetic extracellular matrices for tarsal plate tissue engineering. Front Bioeng Biotechnol. 2020;8:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Witt J, Dietrich J, Mertsch S, Schrader S, Spaniol K, Geerling G. Decellularized porcine conjunctiva as an alternative substrate for tissue‐engineered epithelialized conjunctiva. Ocul Surf. 2020;18(4):901‐911. [DOI] [PubMed] [Google Scholar]
- 18. Dai Y, Jin K, Feng X, Ye J, Gao C. Regeneration of different types of tissues depends on the interplay of stem cells‐laden constructs and microenvironments in vivo. Mater Sci Eng C Mater Biol Appl. 2019;94:938‐948. [DOI] [PubMed] [Google Scholar]
- 19. Coban I, Sirinturk S, Unat F, Pinar Y, Govsa F. Anatomical description of the upper tarsal plate for reconstruction. Surg Radiol Anat. 2018;40(10):1105‐1110. [DOI] [PubMed] [Google Scholar]
- 20. Kim YS, Hwang K. Shape and height of tarsal plates. J Craniofac Surg. 2016;27(2):496‐497. [DOI] [PubMed] [Google Scholar]
- 21. DeParis SW, Zhu AY, Majumdar S, et al. Effects of collagen crosslinking on porcine and human tarsal plate. BMC Ophthalmol. 2019;19(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun MT, O'Connor AJ, Wood J, Casson R, Selva D. Tissue engineering in ophthalmology: implications for eyelid reconstruction. Ophthalmic Plast Reconstr Surg. 2017;33(3):157‐162. [DOI] [PubMed] [Google Scholar]
- 23. Gao Q, Xu P, Hu S, Ye J. The micro‐structure and biomechanics of eyelid tarsus. J Biomech. 2022;133:110911. [DOI] [PubMed] [Google Scholar]
- 24. Sun MT, Pham DT, O'Connor AJ, et al. The biomechanics of eyelid tarsus tissue. J Biomech. 2015;48(12):3455‐3459. [DOI] [PubMed] [Google Scholar]
- 25. Ugradar S, Le A, Lesgart M, Goldberg RA, Rootman D, Demer JL. Biomechanical and morphologic effects of collagen cross‐linking in human Tarsus. Transl Vis Sci Technol. 2019;8(6):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith TM, Suzuki S, Sabat N, Rayner CL, Harkin DG, Chirila TV. Further investigations on the crosslinking of tarsal collagen as a treatment for eyelid laxity: optimizing the procedure in animal tissue. Ophthalmic Plast Reconstr Surg. 2019;35(6):600‐603. [DOI] [PubMed] [Google Scholar]
- 27. Dietrich J, Garreis F, Paulsen F. Pathophysiology of Meibomian glands ‐ an overview. Ocul Immunol Inflamm. 2021;29(4):803‐810. [DOI] [PubMed] [Google Scholar]
- 28. Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11 S):S20‐S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Makuloluwa AK, Hamill KJ, Rauz S, et al. The conjunctival extracellular matrix, related disorders and development of substrates for conjunctival restoration. Ocul Surf. 2021;5:S1542‐0124(21)00050‐1. [DOI] [PubMed] [Google Scholar]
- 30. Eidet JR, Dartt DA, Utheim TP. Concise review: comparison of culture membranes used for tissue engineered conjunctival epithelial equivalents. J Funct Biomater. 2015;6(4):1064‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swamynathan SK, Wells A. Conjunctival goblet cells: ocular surface functions, disorders that affect them, and the potential for their regeneration. Ocul Surf. 2020;18(1):19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu N, Yan C, Chen J, et al. Conjunctival reconstruction via enrichment of human conjunctival epithelial stem cells by p75 through the NGF‐p75‐SALL2 signaling axis. Stem Cells Transl Med. 2020;9(11):1448‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tenland K, Berggren J, Engelsberg K, et al. Successful free Bilamellar eyelid grafts for the repair of upper and lower eyelid defects in patients and laser speckle contrast imaging of revascularization. Ophthalmic Plast Reconstr Surg. 2021;37(2):168‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pham CM, Heinze KD, Mendes‐Rufino‐Uehara M, Setabutr P. Single‐stage repair of large full thickness lower eyelid defects using free tarsoconjunctival graft and transposition flap: experience and outcomes. Orbit. 2022;41(2):178‐183. [DOI] [PubMed] [Google Scholar]
- 35. Ominato J, Oyama T, Cho H, Shiozaki N, Eguchi K, Fukuchi T. Evaluation of the postoperative course of east Asian eyelid reconstruction with free tarsoconjunctival graft transplantation: a Japanese single‐centre retrospective study. JPRAS Open. 2022;33:6‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hishmi AM, Koch KR, Matthaei M, Bolke E, Cursiefen C, Heindl LM. Modified Hughes procedure for reconstruction of large full‐thickness lower eyelid defects following tumor resection. Eur J Med Res. 2016;21(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McKelvie J, Ferguson R, Ng SGJ. Eyelid reconstruction using the “Hughes” tarsoconjunctival advancement flap: long‐term outcomes in 122 consecutive cases over a 13‐year period. Orbit. 2017;36(4):228‐233. [DOI] [PubMed] [Google Scholar]
- 38. Rahmi D, Mehmet B, Ceyda B, Sibel O. Management of the large upper eyelid defects with cutler‐beard flap. J Ophthalmol. 2014;2014:424567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bengoa‐Gonzalez A, Laslau BM, Martin‐Clavijo A, Mencia‐Gutierrez E, Lago‐Llinas MD. Reconstruction of upper eyelid defects secondary to malignant tumors with a newly modified cutler‐beard technique with tarsoconjunctival graft. J Ophthalmol. 2019;2019:6838415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang YC, Dai HY, Xing X, Lv C, Zhu J, Xue CY. Pedicled lower lid‐sharing flap for full‐thickness reconstruction of the upper eyelid. Eye (Lond). 2014;28(11):1292‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamashita K, Yotsuyanagi T, Sugai A, et al. Full‐thickness total upper eyelid reconstruction with a lid switch flap and a reverse superficial temporal artery flap. J Plast Reconstr Aesthet Surg. 2020;73(7):1312‐1317. [DOI] [PubMed] [Google Scholar]
- 42. Yazici B, Ozturker C, Cetin EA. Reconstruction of large upper eyelid defects with bilobed flap and tarsoconjunctival graft. Ophthalmic Plast Reconstr Surg. 2020;36(4):372‐374. [DOI] [PubMed] [Google Scholar]
- 43. Rajak SN, Malhotra R, Selva D. The ‘over‐the‐top’ modified cutler‐beard procedure for complete upper eyelid defect reconstruction. Orbit. 2019;38(2):133‐136. [DOI] [PubMed] [Google Scholar]
- 44. Vimont T, Arnaud D, Rouffet A, Giot JP, Florczak AS, Rousseau P. Hubner's tarsomarginal grafts in eyelid reconstruction: 94 cases. J Stomatol Oral Maxillofac Surg. 2018;119(4):268‐273. [DOI] [PubMed] [Google Scholar]
- 45. Toft PB. Reconstruction of large upper eyelid defects with a free tarsal plate graft and a myocutaneous pedicle flap plus a free skin graft. Orbit. 2016;35(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 46. Hawes MJ, Jamell GA. Complications of tarsoconjunctival grafts. Ophthalmic Plast Reconstr Surg. 1996;12(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 47. Yoshitatsu S, Shiraishi M. A modified method for upper eyelid reconstruction with innervated orbicularis oculi myocutaneous flaps and lower lip mucosal grafts. JPRAS Open. 2021;28:131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh S, Narang P, Mittal V. Labial mucosa grafting for lid margin, anterior lamellar, and posterior lamellar correction in recurrent cicatricial entropion. Orbit. 2021;40(4):301‐305. [DOI] [PubMed] [Google Scholar]
- 49. Jin MJ, Gao Y. Using buccal mucosa and auricular cartilage with a local flap for full‐thickness defect of lower eyelid. J Craniofac Surg. 2021;32(7):e660‐e661. [DOI] [PubMed] [Google Scholar]
- 50. Talwar B, Khader M, Monis PL, Aftab A. Surgical treatment of recurrent lower eyelid sebaceous gland carcinoma and reconstruction with Pedicled nasolabial and non‐vascularised buccal mucosal flaps. J Maxillofac Oral Surg. 2019;18(4):536‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baltu Y. Posterior lamellar reconstruction of the lower eyelid with a Gingivoalveolar mucosal graft. J Craniofac Surg. 2018;29(4):1017‐1019. [DOI] [PubMed] [Google Scholar]
- 52. Wang W, Meng H, Yu S, Liu T, Shao Y. Reconstruction of giant full‐thickness lower eyelid defects using a combination of palmaris longus tendon with superiorly based nasolabial skin flap and palatal mucosal graft. J Plast Surg Hand Surg. 2021;55(3):147‐152. [DOI] [PubMed] [Google Scholar]
- 53. Yue H, Tian L, Bi Y, Qian J. Hard palate Mucoperiosteal transplantation for defects of the upper eyelid: a pilot study and evaluation. Ophthalmic Plast Reconstr Surg. 2020;36(5):469‐474. [DOI] [PubMed] [Google Scholar]
- 54. Lee JH, Woo SS, Shin SH, et al. Upper eyelid reconstruction using a combination of a nasal septal chondromucosal graft and a Fricke flap: a case report. Arch Craniofac Surg. 2021;22(4):204‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lemaitre S, Levy‐Gabriel C, Desjardins L, et al. Outcomes after surgical resection of lower eyelid tumors and reconstruction using a nasal chondromucosal graft and an upper eyelid myocutaneous flap. J Fr Ophtalmol. 2018;41(5):412‐420. [DOI] [PubMed] [Google Scholar]
- 56. Saluja G, Patel BC, Gupta P. Mucous membrane graft. StatPearls. 2022. [PubMed] [Google Scholar]
- 57. Grixti A, Malhotra R. Oral mucosa grafting in periorbital reconstruction. Orbit. 2018;37(6):411‐428. [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto N, Ogi H, Yanagibayashi S, et al. Eyelid reconstruction using Oral mucosa and ear cartilage strips as Sandwich grafting. Plast Reconstr Surg Glob Open. 2017;5(4):e1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gopakumar V, Agarwal S, Srinivasan B, Krishnakumar S, Krishnan UM, Iyer G. Clinical outcome of autologous cultivated oral mucosal epithelial transplantation in ocular surface reconstruction. Cornea. 2019;38(10):1273‐1279. [DOI] [PubMed] [Google Scholar]
- 60. Ngowyutagon P, Prabhasawat P, Chirapapaisan C, et al. Successful ocular surface reconstruction in complete ankyloblepharon with the simple oral mucosal epithelial transplantation technique: a case report. Cornea. 2021;40(11):1482‐1486. [DOI] [PubMed] [Google Scholar]
- 61. Sotozono C, Inatomi T, Nakamura T, et al. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 2014;92(6):e447‐e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rootman DB, Kim MJ, Aldave AJ, Douglas R, Hwang C, Goldberg R. Ocular surface, fornix, and eyelid rehabilitation in Boston type I keratoprosthesis patients with mucous membrane disease. Ophthalmic Plast Reconstr Surg. 2015;31(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 63. Mai C, Bertelmann E. Oral mucosal grafts: old technique in new light. Ophthalmic Res. 2013;50(2):91‐98. [DOI] [PubMed] [Google Scholar]
- 64. Wakamatsu TH, Sant'Anna A, Cristovam PC, Alves VAF, Wakamatsu A, Gomes JAP. Minor salivary gland transplantation for severe dry eyes. Cornea. 2017;36(Suppl 1):S26‐S33. [DOI] [PubMed] [Google Scholar]
- 65. Ding J, Ma X, Xin Y, Li D. Correction of lower eyelid retraction with hard palate graft in the anophthalmic socket. Can J Ophthalmol. 2018;53(5):458‐461. [DOI] [PubMed] [Google Scholar]
- 66. Hendriks S, Bruant‐Rodier C, Lupon E, Zink S, Bodin F, Dissaux C. The palatal mucosal graft: the adequate posterior lamellar reconstruction in extensive full‐thickness eyelid reconstruction. Ann Chir Plast Esthet. 2020;65(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 67. Weinberg DA, Tham V, Hardin N, et al. Eyelid mucous membrane grafts: a histologic study of hard palate, nasal turbinate, and buccal mucosal grafts. Ophthalmic Plast Reconstr Surg. 2007;23(3):211‐216. [DOI] [PubMed] [Google Scholar]
- 68. Kececi Y, Bali ZU, Ahmedov A, Yoleri L. Angular artery Island flap for eyelid defect reconstruction. J Plast Surg Hand Surg. 2020;54(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 69. Eser C, Kesiktas E, Gencel E, Tabakan I, Yavuz M. Total or near‐total lower eyelid defect reconstruction using malar myocutaneous bridge and nasojugal flaps and septal chondromucosal graft. Ophthalmic Plast Reconstr Surg. 2016;32(3):225‐229. [DOI] [PubMed] [Google Scholar]
- 70. Santos G, Goulao J. One‐stage reconstruction of full‐thickness lower eyelid using a Tripier flap lining by a septal mucochondral graft. J Dermatolog Treat. 2014;25(5):446‐447. [DOI] [PubMed] [Google Scholar]
- 71. Cristofari S, Rem K, Revol M, Atlan M, Stivala A. Reconstruction of full‐thickness lower lid defects using Texier's procedure: retrospective assessment of the indications. J Oral Maxillofac Surg. 2019;77(2):433‐439. [DOI] [PubMed] [Google Scholar]
- 72. Pushker N, Modaboyina S, Meel R, Agrawal S. Auricular skin‐cartilage sandwich graft technique for full‐thickness eyelid reconstruction. Indian J Ophthalmol. 2022;70(4):1404‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barrancos C, Garcia‐Cruz I, Ventas‐Ayala B, Sales‐Sanz M. The addition of a conjunctival flap to a posterior lamella auricular cartilage graft: a technique to avoid corneal complications. Eur J Ophthalmol. 2021;31(4):2165‐2170. [DOI] [PubMed] [Google Scholar]
- 74. Suga H, Ozaki M, Narita K, et al. Comparison of nasal septum and ear cartilage as a graft for lower eyelid reconstruction. J Craniofac Surg. 2016;27(2):305‐307. [DOI] [PubMed] [Google Scholar]
- 75. Walkden A. Amniotic membrane transplantation in ophthalmology: an updated perspective. Clin Ophthalmol. 2020;14:2057‐2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sabater‐Cruz N, Figueras‐Roca M, Gonzalez Ventosa A, Padro‐Pitarch L, Tort J, Casaroli‐Marano RP. Current clinical application of sclera and amniotic membrane for ocular tissue bio‐replacement. Cell Tissue Bank. 2020;21(4):597‐603. [DOI] [PubMed] [Google Scholar]
- 77. Naxer S, Horn M, Schittkowski M. Processed amniotic membrane for conjunctival reconstruction in complex strabismus surgery. Strabismus. 2018;21:1‐7. [DOI] [PubMed] [Google Scholar]
- 78. Agraval U, Rundle P, Rennie IG, Salvi S. Fresh frozen amniotic membrane for conjunctival reconstruction after excision of neoplastic and presumed neoplastic conjunctival lesions. Eye (Lond). 2017;31(6):884‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ding J, Hou Z, Li Y, Lu N, Li D. Eyelid and fornix reconstruction in abortive cryptophthalmos: a single‐center experience over 12 years. Eye (Lond). 2017;31(11):1576‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reed DS, Giles GB, Johnson A, et al. Acute reconstruction of periorbital trauma resulting in eyelid anterior lamella loss with simultaneous full‐thickness skin grafting and amniotic membrane grafting: a case report. Mil Med. 2022;187(1–2):e246‐e249. [DOI] [PubMed] [Google Scholar]
- 81. Dehghani S, Rasoulianboroujeni M, Ghasemi H, et al. 3D‐printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: an in vitro & in vivo study. Biomaterials. 2018;174:95‐112. [DOI] [PubMed] [Google Scholar]
- 82. Miyakoshi A, Nishida Y, Tanaka A, Hayashi A. Histological equivalence of a hyper‐dry amniotic membrane and the Ambio2TM after implantation in the rabbit conjunctiva. Ophthalmic Res. 2020;63(4):423‐426. [DOI] [PubMed] [Google Scholar]
- 83. Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting‐a review. Cell Tissue Bank. 2017;18(2):193‐204. [DOI] [PubMed] [Google Scholar]
- 84. Bandeira F, Yam GH, Fuest M, et al. Urea‐de‐epithelialized human amniotic membrane for ocular surface reconstruction. Stem Cells Transl Med. 2019;8(7):620‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhao L, Jia Y, Zhao C, et al. Ocular surface repair using decellularized porcine conjunctiva. Acta Biomater. 2020;101:344‐356. [DOI] [PubMed] [Google Scholar]
- 86. Witt J, Mertsch S, Borrelli M, et al. Decellularised conjunctiva for ocular surface reconstruction. Acta Biomater. 2018;67:259‐269. [DOI] [PubMed] [Google Scholar]
- 87. Huang D, Xu B, Yang X, Xu B, Zhao J. Conjunctival structural and functional reconstruction using acellular bovine pericardium graft (Normal GEN(R)) in rabbits. Graefes Arch Clin Exp Ophthalmol. 2016;254(4):773‐783. [DOI] [PubMed] [Google Scholar]
- 88. Yan D, Yan C, Yu F, et al. Exploitation of human mesenchymal stromal cell derived matrix towards the structural and functional restoration of the ocular surface. Biomater Sci. 2020;8(17):4712‐4727. [DOI] [PubMed] [Google Scholar]
- 89. Kim KY, Woo YJ, Jang SY, Lee EJ, Yoon JS. Correction of lower eyelid retraction using acellular human dermis during orbital decompression. Ophthalmic Plast Reconstr Surg. 2017;33(3):168‐172. [DOI] [PubMed] [Google Scholar]
- 90. Bee YS, Alonzo B, Ng JD. Review of AlloDerm acellular human dermis regenerative tissue matrix in multiple types of oculofacial plastic and reconstructive surgery. Ophthalmic Plast Reconstr Surg. 2015;31(5):348‐351. [DOI] [PubMed] [Google Scholar]
- 91. Scruggs JT, McGwin G Jr, Morgenstern KE. Use of noncadaveric human acellular dermal tissue (BellaDerm) in lower eyelid retraction repair. Ophthalmic Plast Reconstr Surg. 2015;31(5):379‐384. [DOI] [PubMed] [Google Scholar]
- 92. McGrath LA, Hardy TG, McNab AA. Efficacy of porcine acellular dermal matrix in the management of lower eyelid retraction: case series and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2020;258(9):1999‐2006. [DOI] [PubMed] [Google Scholar]
- 93. Grumbine FL, Idowu O, Kersten RC, Deparis SW, Vagefi MR. Correction of lower eyelid retraction with en glove placement of porcine dermal collagen matrix implant. Plast Reconstr Surg. 2019;143(3):743‐746. [DOI] [PubMed] [Google Scholar]
- 94. Barmettler A, Heo M. A prospective, randomized comparison of lower eyelid retraction repair with autologous auricular cartilage, bovine acellular dermal matrix (Surgimend), and porcine acellular dermal matrix (Enduragen) spacer grafts. Ophthalmic Plast Reconstr Surg. 2018;34(3):266‐273. [DOI] [PubMed] [Google Scholar]
- 95. Sun J, Liu X, Zhang Y, et al. Bovine acellular dermal matrix for levator lengthening in thyroid‐related upper‐eyelid retraction. Med Sci Monit. 2018;24:2728‐2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Symbas J, McCord C, Nahai F. Acellular dermal matrix in eyelid surgery. Aesthet Surg J. 2011;31(7 Suppl):101 S‐7 S. [DOI] [PubMed] [Google Scholar]
- 97. Chermnykh E, Kalabusheva E, Vorotelyak E. Extracellular matrix as a regulator of epidermal stem cell fate. Int J Mol Sci. 2018;19(4):1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dailey RA, Marx DP, Ahn ES. Porcine dermal collagen in lower eyelid retraction repair. Ophthalmic Plast Reconstr Surg. 2015;31(3):233‐241. [DOI] [PubMed] [Google Scholar]
- 99. Eah KS, Sa HS. Reconstruction of large upper eyelid defects using the reverse Hughes flap combined with a sandwich graft of an acellular dermal matrix. Ophthalmic Plast Reconstr Surg. 2021;37(3 S):S27‐S30. [DOI] [PubMed] [Google Scholar]
- 100. Vahdani K, Siapno DL, Lee JH, Woo KI, Kim YD. Long‐term outcomes of acellular dermal allograft as a tarsal substitute in the reconstruction of extensive eyelid defects. J Craniofac Surg. 2018;29(5):1327‐1331. [DOI] [PubMed] [Google Scholar]
- 101. Park SJ, Kim Y, Jang SY. The application of an acellular dermal allograft (AlloDerm) for patients with insufficient conjunctiva during evisceration and implantation surgery. Eye (Lond). 2018;32(1):136‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Teo L, Woo YJ, Kim DK, Kim CY, Yoon JS. Surgical outcomes of porcine acellular dermis graft in anophthalmic socket: comparison with oral mucosa graft. Korean J Ophthalmol. 2017;31(1):9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sullivan SA, Dailey RA. Graft contraction: a comparison of acellular dermis versus hard palate mucosa in lower eyelid surgery. Ophthalmic Plast Reconstr Surg. 2003;19(1):14‐24. [DOI] [PubMed] [Google Scholar]
- 104. Liao SL, Wei YH. Correction of lower lid retraction using tarSys bioengineered grafts for graves ophthalmopathy. Am J Ophthalmol. 2013;156(2):387‐392 e1. [DOI] [PubMed] [Google Scholar]
- 105. Borrelli M, Unterlauft J, Kleinsasser N, Geerling G. Decellularized porcine derived membrane (Tarsys(R)) for correction of lower eyelid retraction. Orbit. 2012;31(3):187‐189. [DOI] [PubMed] [Google Scholar]
- 106. Mancera N, Schneider A, Margo CE, Bajric J. Inflammatory reaction to decellularized porcine‐derived xenograft for lower eyelid retraction. Ophthalmic Plast Reconstr Surg. 2019;35(4):e95‐e97. [DOI] [PubMed] [Google Scholar]
- 107. Kim HJ, Grossniklaus HE, Wojno TH. A cyst‐like foreign body reaction to porcine decellularized membrane (TarSys). Ophthalmic Plast Reconstr Surg. 2014;30(4):e100‐e102. [DOI] [PubMed] [Google Scholar]
- 108. Munday WR, Klett Z, McNiff JM, Ko C. Foreign body giant cell reaction to tarSys xenograft. J Cutan Pathol. 2014;41(10):771‐774. [DOI] [PubMed] [Google Scholar]
- 109. Witt J, Borrelli M, Mertsch S, Geerling G, Spaniol K, Schrader S. Evaluation of plastic‐compressed collagen for conjunctival repair in a rabbit model. Tissue Eng Part A. 2019;25(15–16):1084‐1095. [DOI] [PubMed] [Google Scholar]
- 110. Sun MT, O'Connor AJ, Milne I, et al. Development of macroporous chitosan scaffolds for eyelid Tarsus tissue engineering. Tissue Eng Regen Med. 2019;16(6):595‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Borrelli M, Joepen N, Reichl S, et al. Keratin films for ocular surface reconstruction: evaluation of biocompatibility in an in‐vivo model. Biomaterials. 2015;42:112‐120. [DOI] [PubMed] [Google Scholar]
- 112. Drechsler CC, Kunze A, Kureshi A, et al. Development of a conjunctival tissue substitute on the basis of plastic compressed collagen. J Tissue Eng Regen Med. 2017;11(3):896‐904. [DOI] [PubMed] [Google Scholar]
- 113. Zhou H, Lu Q, Guo Q, et al. Vitrified collagen‐based conjunctival equivalent for ocular surface reconstruction. Biomaterials. 2014;35(26):7398‐7406. [DOI] [PubMed] [Google Scholar]
- 114. Sharifi S, Islam MM, Sharifi H, et al. Tuning gelatin‐based hydrogel towards bioadhesive ocular tissue engineering applications. Bioact Mater. 2021;6(11):3947‐3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rose JB, Pacelli S, Haj AJE, et al. Gelatin‐based materials in ocular tissue engineering. Materials (Basel). 2014;7(4):3106‐3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. He M, Storr‐Paulsen T, Wang AL, et al. Artificial polymeric scaffolds as extracellular matrix substitutes for autologous conjunctival goblet cell expansion. Invest Ophthalmol Vis Sci. 2016;57(14):6134‐6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stoppel WL, Ghezzi CE, McNamara SL, Black LD 3rd, Kaplan DL. Clinical applications of naturally derived biopolymer‐based scaffolds for regenerative medicine. Ann Biomed Eng. 2015;43(3):657‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Baradaran‐Rafii A, Biazar E, Heidari‐keshel S. Cellular response of limbal stem cells on polycaprolactonen scaffolds for ocular epithelial regeneration. Curr Eye Res. 2016;41(3):326‐333. [DOI] [PubMed] [Google Scholar]
- 119. Sharma S, Gupta D, Mohanty S, Jassal M, Agrawal AK, Tandon R. Surface‐modified electrospun poly(epsilon‐caprolactone) scaffold with improved optical transparency and bioactivity for damaged ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2014;55(2):899‐907. [DOI] [PubMed] [Google Scholar]
- 120. Yao Q, Zhang W, Hu Y, et al. Electrospun collagen/poly(L‐lactic acid‐co‐epsilon‐caprolactone) scaffolds for conjunctival tissue engineering. Exp Ther Med. 2017;14(5):4141‐4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shin YJ, Lee HI, Kim MK, et al. Biocompatibility of nanocomposites used for artificial conjunctiva: in vivo experiments. Curr Eye Res. 2007;32(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 122. Yao Q, Hu Y, Yu F, Zhang W, Fu Y. A novel application of electrospun silk fibroin/poly(l‐lactic acid‐co‐epsilon‐caprolactone) scaffolds for conjunctiva reconstruction. RSC Adv. 2018;8(33):18372‐18380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Garibaldi DC, Robinson MR, Lee SS, et al. New corticosteroid‐eluting porous polyethylene implant for the management of lower eyelid retraction: a pilot study. Ophthalmic Plast Reconstr Surg. 2006;22(6):424‐429. [DOI] [PubMed] [Google Scholar]
- 124. Zhou J, Peng SW, Wang YY, Zheng SB, Wang Y, Chen GQ. The use of poly(3‐hydroxybutyrate‐co‐3‐hydroxyhexanoate) scaffolds for tarsal repair in eyelid reconstruction in the rat. Biomaterials. 2010;31(29):7512‐7518. [DOI] [PubMed] [Google Scholar]
- 125. Gao Q, Hu B, Ning Q, et al. A primary study of poly(propylene fumarate)‐2‐hydroxyethyl methacrylate copolymer scaffolds for tarsal plate repair and reconstruction in rabbit eyelids. J Mater Chem B. 2015;3(19):4052‐4062. [DOI] [PubMed] [Google Scholar]
- 126. Xu P, Gao Q, Feng X, et al. A biomimetic Tarso‐conjunctival biphasic scaffold for eyelid reconstruction in vivo. Biomater Sci. 2019;7(8):3373‐3385. [DOI] [PubMed] [Google Scholar]
- 127. Mavrikakis I, Francis N, Poitelea C, Parkin B, Brittain P, Olver J. Medpor lower eyelid spacer: does it biointegrate? Orbit. 2009;28(1):58‐62. [DOI] [PubMed] [Google Scholar]
- 128. Li G, Zhao M, Xu F, et al. Synthesis and biological application of polylactic acid. Molecules. 2020;25(21):5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang T, Shan F, Zhou Q, Shi W, Xie L. Allogeneic cultivated limbal epithelial sheet transplantation in reconstruction of conjunctival sac after chemical and thermal burns. Front Med (Lausanne). 2021;8:697264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kobayashi M, Nakamura T, Yasuda M, et al. Ocular surface reconstruction with a tissue‐engineered nasal mucosal epithelial cell sheet for the treatment of severe ocular surface diseases. Stem Cells Transl Med. 2015;4(1):99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Garcia‐Posadas L, Soriano‐Romani L, Lopez‐Garcia A, Diebold Y. An engineered human conjunctival‐like tissue to study ocular surface inflammatory diseases. PLoS One. 2017;12(3):e0171099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Safinaz MK, Norzana AG, Hairul Nizam MH, et al. The use of autologous fibrin as a scaffold for cultivating autologous conjunctiva in the treatment of conjunctival defect. Cell Tissue Bank. 2014;15(4):619‐626. [DOI] [PubMed] [Google Scholar]
- 133. Hong S, Yun JH, Kim ES, Kim JS, Tchah H, Hwang C. Human conjunctival epithelial sheets grown on poly(lactic‐co‐glycolic) acid membranes and cocultured with human Tenon's fibroblasts for corneal repair. Invest Ophthalmol Vis Sci. 2018;59(3):1475‐1485. [DOI] [PubMed] [Google Scholar]
- 134. Bosworth LA, Doherty KG, Hsuan JD, et al. Material characterisation and stratification of conjunctival epithelial cells on electrospun poly(epsilon‐caprolactone) fibres loaded with decellularised tissue matrices. Pharmaceutics. 2021;13(3):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kasbekar S, Kaye SB, Williams RL, Stewart RMK, Leow‐Dyke S, Rooney P. Development of decellularized conjunctiva as a substrate for the ex vivo expansion of conjunctival epithelium. J Tissue Eng Regen Med. 2018;12(2):e973‐e982. [DOI] [PubMed] [Google Scholar]
- 136. Bertolin M, Breda C, Ferrari S, et al. Optimized protocol for regeneration of the conjunctival epithelium using the cell suspension technique. Cornea. 2019;38(4):469‐479. [DOI] [PubMed] [Google Scholar]
- 137. Toth E, Beyer D, Zsebik B, Vereb G, Takacs L. Limbal and conjunctival epithelial cell cultivation on contact lenses‐different affixing techniques and the effect of feeder cells. Eye Contact Lens. 2017;43(3):162‐167. [DOI] [PubMed] [Google Scholar]
- 138. Schrader S, Tuft SJ, Beaconsfield M, Borrelli M, Geerling G, Daniels JT. Evaluation of human MRC‐5 cells as a feeder layer in a xenobiotic‐free culture system for conjunctival epithelial progenitor cells. Curr Eye Res. 2012;37(12):1067‐1074. [DOI] [PubMed] [Google Scholar]
- 139. Tian L, Qu M, Wang Y, et al. Inductive differentiation of conjunctival goblet cells by gamma‐secretase inhibitor and construction of recombinant conjunctival epithelium. Exp Eye Res. 2014;123:37‐42. [DOI] [PubMed] [Google Scholar]
- 140. Eidet JR, Utheim OA, Raeder S, et al. Effects of serum‐free storage on morphology, phenotype, and viability of ex vivo cultured human conjunctival epithelium. Exp Eye Res. 2012;94(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 141. Yang SP, Yang XZ, Cao GP. Conjunctiva reconstruction by induced differentiation of human amniotic epithelial cells. Genet Mol Res. 2015;14(4):13823‐13834. [DOI] [PubMed] [Google Scholar]
- 142. Khalil AA, Yassine S, Torbey JS, Mehanna CJ, Alameddine RM. Intraocular migration of porous polyethylene malar implant used for Treacher Collins eyelid reconstruction. Ophthalmic Plast Reconstr Surg. 2021;37(2):e73‐e75. [DOI] [PubMed] [Google Scholar]
- 143. Lin CW, Liao SL. Long‐term complications of different porous orbital implants: a 21‐year review. Br J Ophthalmol. 2017;101(5):681‐685. [DOI] [PubMed] [Google Scholar]
- 144. Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. [DOI] [PubMed] [Google Scholar]
- 145. Luo Y, Lin X, Huang P. 3D bioprinting of artificial tissues: construction of biomimetic microstructures. Macromol Biosci. 2018;18(6):e1800034. [DOI] [PubMed] [Google Scholar]
- 146. Park JH, Ahn M, Park SH, et al. 3D bioprinting of a trachea‐mimetic cellular construct of a clinically relevant size. Biomaterials. 2021;279:121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Park JH, Yoon JK, Lee JB, et al. Experimental tracheal replacement using 3‐dimensional bioprinted artificial trachea with autologous epithelial cells and chondrocytes. Sci Rep. 2019;9(1):2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lan X, Liang Y, Vyhlidal M, et al. In vitro maturation and in vivo stability of bioprinted human nasal cartilage. J Tissue Eng. 2022;13:20417314221086368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lan X, Liang Y, Erkut EJN, et al. Bioprinting of human nasoseptal chondrocytes‐laden collagen hydrogel for cartilage tissue engineering. FASEB J. 2021;35(3):e21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fitzpatrick V, Martin‐Moldes Z, Deck A, et al. Functionalized 3D‐printed silk‐hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials. 2021;276:120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Camarero‐Espinosa S, Moroni L. Janus 3D printed dynamic scaffolds for nanovibration‐driven bone regeneration. Nat Commun. 2021;12(1):1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hann SY, Cui H, Esworthy T, et al. Dual 3D printing for vascularized bone tissue regeneration. Acta Biomater. 2021;123:263‐274. [DOI] [PubMed] [Google Scholar]
- 153. Yan Y, Chen H, Zhang H, et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials. 2019;190‐191:97‐110. [DOI] [PubMed] [Google Scholar]
- 154. Daly AC, Pitacco P, Nulty J, Cunniffe GM, Kelly DJ. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials. 2018;162:34‐46. [DOI] [PubMed] [Google Scholar]
- 155. Daghrery A, Ferreira JA, Xu J, et al. Tissue‐specific melt electrowritten polymeric scaffolds for coordinated regeneration of soft and hard periodontal tissues. Bioact Mater. 2023;19:268‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Li C, Kuss M, Kong Y, et al. 3D printed hydrogels with aligned microchannels to guide neural stem cell migration. ACS Biomater Sci Eng. 2021;7(2):690‐700. [DOI] [PubMed] [Google Scholar]
- 157. Tayebi L, Rasoulianboroujeni M, Moharamzadeh K, Almela TKD, Cui Z, Ye H. 3D‐printed membrane for guided tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2018;84:148‐158. [DOI] [PubMed] [Google Scholar]
- 158. Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2020;4(4):370‐380. [DOI] [PubMed] [Google Scholar]
- 159. Zhong Z, Deng X, Wang P, et al. Rapid bioprinting of conjunctival stem cell micro‐constructs for subconjunctival ocular injection. Biomaterials. 2021;267:120462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197:108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Periman LM, Perez VL, Saban DR, Lin MC, Neri P. The immunological basis of dry eye disease and current topical treatment options. J Ocul Pharmacol Ther. 2020;36(3):137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tchegnon E, Liao CP, Ghotbi E, et al. Epithelial stem cell homeostasis in Meibomian gland development, dysfunction, and dry eye disease. JCI Insight. 2021;6(20):e151078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Makuloluwa AK, Hamill KJ, Rauz S, et al. Biological tissues and components, and synthetic substrates for conjunctival cell transplantation. Ocul Surf. 2021;22:15‐26. [DOI] [PubMed] [Google Scholar]
- 164. Lu R, Zhang X, Huang D, et al. Conjunctival reconstruction with progenitor cell‐derived autologous epidermal sheets in rhesus monkey. PLoS One. 2011;6(11):e25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


