Abstract
When coresident with the Ti (tumor-inducing) plasmid, the 21-kDa product of the osa gene of the plasmid pSa can suppress crown gall tumorigenesis incited by Agrobacterium tumefaciens. Neither T-DNA processing nor vir (virulence) gene induction is affected by the presence of osa in the bacterium. We used Arabidopsis thaliana root segments and tobacco leaf discs to demonstrate that Osa inhibits A. tumefaciens from transforming these plants to the stable phenotypes of tumorigenesis, kanamycin resistance, and stable β-glucuronidase (GUS) expression. When A. tumefaciens contained osa, the lack of expression of transient GUS activity in infected plant tissues, as well as the lack of systemic viral symptoms following agroinfection of Nicotiana benthamiana by tomato mottle virus, suggested that oncogenic suppression by Osa occurs before T-DNA enters the plant nucleus. The extracellular complementation of an A. tumefaciens virE2 mutant (the T-DNA donor strain) by an A. tumefaciens strain lacking T-DNA but containing a wild-type virE2 gene (the VirE2 donor strain) was blocked when osa was present in the VirE2 donor strain, but not when osa was present in the T-DNA donor strain. These data indicate that osa inhibits VirE2 protein, but not T-DNA export from A. tumefaciens. These data further suggest that VirE2 protein and T-DNA are separately exported from the bacterium. The successful infection of Datura stramonium plants and leaf discs of transgenic tobacco plants expressing VirE2 protein by an A. tumefaciens virE2 mutant carrying osa confirmed that oncogenic suppression by osa does not occur by blocking T-DNA transfer. Overexpression of virB9, virB10, and virB11 in A. tumefaciens did not overcome oncogenic suppression by osa. The finding that the expression of the osa gene by itself, rather than the formation of a conjugal intermediate with pSa, blocks transformation suggests that the mechanism of oncogenic suppression by osa may differ from that of the IncQ plasmid RSF1010.
The Ti plasmid resident in virulent strains of Agrobacterium tumefaciens confers on this soil-borne bacterium the ability to cause crown gall tumors on a wide range of dicotyledonous plants, as well as some monocots and gymnosperms. Generally, when this plasmid coresides with other plasmids, such as RP4 and RK2 and their derivatives, the virulence of A. tumefaciens remains unaffected (10, 20, 28). However, plasmids of the incompatibility groups Q (12) and W (41) severely affect tumorigenicity when coresident with the Ti plasmid in A. tumefaciens. Ward et al. (42) showed that the presence of the IncQ RSF1010 derivative pJW323 inhibited tumor formation on Kalanchoë daigremontiana leaves. This inhibitory effect could be overcome by the coordinate overexpression of the virulence genes virB9, virB10, and virB11. pJW323 inhibited the ability of A. tumefaciens to serve as a proficient VirE2 donor cell in extracellular complementation assays (34), and this inhibition could not be alleviated by overexpressing VirE2 in strains containing pJW323 (2). Although the mechanism of the effect of pJW323 on oncogenic suppression remains unclear, Binns et al. (2) proposed that pJW323 preferentially competes for the putative T-DNA–VirE2 transport apparatus composed of VirB proteins.
Loper and Kado (28) and Farrand et al. (10) showed that the tumorigenicity of A. tumefaciens is suppressed when it harbors the IncW plasmid pSa. This plasmid, originally isolated from Shigella flexneri, is a 38-kbp broad-host-range plasmid that confers resistance to the antibiotics spectinomycin, kanamycin, gentamicin, chloramphenicol, and sulfonamide. The presence of pSa in A. tumefaciens does not cause instability of the Ti plasmid, and oncogenicity is regained when the strain is cured of pSa (10). Moreover, pSa does not prevent conjugative transfer of the Ti plasmid, and when the Ti plasmid is transferred from a pSa-bearing strain to a recipient strain, oncogenicity is conferred upon the recipient (10).
Previous studies localized the oncogenic suppression gene to a 3.1-kbp region of pSa (7). This region contains the osa (oncogenic suppressive activity) gene that is the fourth gene of a four-gene operon. The presence of osa alone in A. tumefaciens is sufficient for suppressing oncogenicity (3, 8).
The amino acid sequence of Osa shares 39% identity and 60% similarity with the FiwA protein (encoded by the fertility inhibition gene, fiwA) of the plasmid RP1 (3). This gene is involved in the inhibition of conjugative transfer of IncW plasmids, such as R388 and pSa (11). The similarity of the amino acid sequences between these two proteins suggested that Osa may inhibit T-DNA transfer from A. tumefaciens to plants, a process that is believed to occur by a conjugative mechanism (22, 25, 37).
In this study, we have investigated the mechanism of oncogenic suppression mediated by osa. Using a number of different assays, we demonstrate that the presence of osa in A. tumefaciens inhibits tumorigenesis not by blocking T-DNA transfer, but rather by inhibiting VirE2 protein export from the bacterium to the plant cell.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The A. tumefaciens strains and plasmids used in this study are listed in Table 1. A. tumefaciens strains were cultured at 30°C in YEP complete or AB minimal medium containing 0.5% sucrose (26) or in medium 523 (23) supplemented with the appropriate antibiotics (rifampin, 10 μg/ml; kanamycin, 25 μg/ml; spectinomycin, 50 μg/ml; tetracycline, 5 μg/ml). Plasmids were introduced into A. tumefaciens by electroporation as described in the Bio-Rad Gene Pulser instruction manual.
TABLE 1.
A. tumefaciens strains and plasmids used in this study
| Strain or plasmida | Relevant characteristics | Antibiotic resistanceb | Source or reference |
|---|---|---|---|
| Strains | |||
| A136 | Nononcogenic strain; lacks pTi | Rifr | 43 |
| A208 | Oncogenic nopaline-type strain; pTiT37 | Rifr | 36 |
| A348 | Oncogenic octopine-type strain; pTiA6 | Rifr | 14 |
| At221 | Tn3-HoHo1 insertion in virE2; A348mx358 | Carr Rifr | 38 |
| At789 | A348(pBISN1) | Kanr Rifr | 31 |
| At793 | A136(pBISN1) | Kanr Rifr | 31 |
| At900 | LBA4404(pSa) | Chlr Genr Kanr Rifr Sper Sulr | This study |
| At901 | LBA4404(pSa::neo) | Chlr Genr Kanr Neor Rifr Sper Sulr | This study |
| At902 | LBA4404(pUCD3960) | Carr Rifr Sper | This study |
| At903 | LBA4404(pUCD5533) | Carr Rifr Sper | This study |
| At904 | At221(pSa) | Carr Chlr Genr Kanr Rifr Sper Sulr | This study |
| At905 | At221(pSa::neo) | Carr Chlr Genr Kanr Neor Rifr Sper Chlr | This study |
| At906 | At221(pUCD3960) | Carr Rifr Sper | This study |
| At907 | At221(pUCD5533) | Carr Rifr Sper | This study |
| At915 | LBA4404(pToMoV-A) | Kanr Rifr | R. Gilbertson |
| At916 | LBA4404(pToMoV-B) | Kanr Rifr | R. Gilbertson |
| At925 | A208(pBISN1, pUCD5542, pSa) | Chlr Genr Kanr Rifr Sper Sulr Tetr | This study |
| At926 | A208(pBISN1, pUCD5542, pUCD3960) | Carr Kanr Rifr Sper Tetr | This study |
| At927 | A208(pBISN1, pUCD5542, pUCD5533) | Carr Kanr Rifr Sper Tetr | This study |
| At928 | A208(pBISN1) | Kanr Rifr | This study |
| At929 | A208(pBISN1, pSa) | Chlr Genr Kanr Rifr Sper Sulr | This study |
| At931 | A208(pBISN1, pUCD3960) | Carr Kanr Rifr Sper | This study |
| At932 | A208(pBISN1, pUCD5533) | Carr Kanr Rifr Sper | This study |
| At935 | A208(pBISN1, pUCD5542) | Kanr Rifr Tetr | This study |
| At985 | At221(pJB31) | Carr Sper Rifr | This study |
| At989 | At221(pBISN1) | Carr Kanr Rifr | This study |
| At990 | At221(pBISN1, pUCD3960) | Carr Kanr Rifr Sper | This study |
| At991 | At221(pBISN1, pUCD5533) | Carr Kanr Rifr Sper | This study |
| At1042 | LBA4404(pToMoV-A, pSa) | Chlr Genr Kanr Rifr Sper Sulr | This study |
| At1043 | LBA4404(pToMoV-B, pSa) | Chlr Genr Kanr Rifr Sper Sulr | This study |
| At1044 | LBA4404(pToMoV-A, pSa::neo) | Chlr Genr Kanr Neor Rifr Sper Sulr | This study |
| At1045 | LBA4404(pToMoV-B, pSa::neo) | Chlr Genr Kanr Neor Rifr Sper Sulr | This study |
| At1046 | LBA4404(pToMoV-A, pUCD3960) | Carr Kanr Rifr Sper | This study |
| At1047 | LBA4404(pToMoV-B, pUCD3960) | Carr Kanr Rifr Sper | This study |
| At1048 | LBA4404(pToMoV-A, pUCD5533) | Carr Kanr Rifr Sper | This study |
| At1049 | LBA4404(pToMoV-B, pUCD5533) | Carr Kanr Rifr Sper | This study |
| At1050 | LBA4301(pToMoV-B, pJK703) | Kanr Neor Rifr | This study |
| At1085 | A208(pJB31, pUCD5542) | Sper Rifr Tetr | This study |
| LBA4301 | rec mutant of A. tumefaciens Ach5; no pTi | Rifr | 24 |
| LBA4404 | Disarmed octopine-type strain; lacks T-DNA | Rifr | 33 |
| Plasmids | |||
| pBISN1 | T-DNA binary vector; nos-nptII and superpromoter-gusA intron gene | Kanr | 31 |
| pJB31 | RSF1010 derivative | Sper | 1 |
| pJK703 | pTiC58 virD::Tn5 | Neor | 35 |
| pSa | Oncogenic suppressive plasmid | Chlr Genr Kanr Sper Sulr | 28 |
| pSa::neo | Neomycin phosphotransferase gene disruption of the osa gene of pSa | Chlr Genr Kanr Neor Sper Sulr | This study |
| pToMoV-A | Dimer of the A component of the ToMoV genome cloned into pCGN1547 | Kanr | R. Gilbertson |
| pToMoV-B | Dimer of the B component of the ToMoV genome cloned into pCGN1547 | Kanr | R. Gilbertson |
| pUCD105 | Stable cloning vector for A. tumefaciens; pTAR ori | Carr Kanr Sper | 41 |
| pUCD2001 | Low-copy cloning vector for A. tumefaciens | Carr Kanr Tetr | 13 |
| pUCD3960 | osa gene under the control of a npt promoter in pUCD105 | Carr Sper | 3 |
| pUCD5533 | Asp718-blunted derivative of pUCD105 | Carr Sper | This study |
| pUCD5542 | virB9, -10, and -11 genes under the control of the virB promoter in a modified pUCD2001 | Tetr | This study |
Laboratory stocks: A, E. W. Nester; At, S. B. Gelvin; LBA, R. A. Schilperoort; UCD, C. I. Kado.
Car, carbenicillin; Chl, chloramphenicol; Gen, gentamicin; Kan, kanamycin; Neo, neomycin; Rif, rifampin; Spe, spectinomycin; Sul, sulfonamide; Tet, tetracycline.
Agroinfection.
A. tumefaciens strains containing the binary vector pCGN1547 with a dimer of the A or the B component of the tomato mottle virus (ToMoV) genome were grown in 523 medium containing the appropriate antibiotics at 30°C overnight. The cells were washed and resuspended in fresh medium to a concentration of 109 cells/ml. Equal volumes of the two strains carrying ToMoV A and B components were mixed, and 40 μl of cells was applied to the cut surface of Nicotiana benthamiana stems. The plants were scored after 2 weeks for the appearance of yellow mottling symptoms on the newly expanding leaves. Squash blot analysis was conducted according to the method of Gilbertson et al. (16, 17). PCR analysis for the detection of viral DNA was conducted according to the method of Gilbertson et al. (18).
Transformation of Arabidopsis roots.
Arabidopsis thaliana ecotype Aa-0 was grown in MS medium as described by Nam et al. (30). Roots of 2-week-old plants were cut into 5-mm segments and placed on MS basal agar medium (per liter: 4.32 g of MS salts, 0.5 g of MES [4-morpholineethanesulfonic acid] [pH 5.7], 0.1 g of myo-inositol, 1 ml of vitamin stock solution [0.5 mg of nicotinic acid per ml, 0.5 mg of pyridoxine per ml, 0.5 mg of thiamine-HCl per ml], 10 g of sucrose, and 0.75% Bactoagar [Difco]). A drop of the appropriate A. tumefaciens strain was applied to the root segments for 10 min, excess bacteria were removed from the plate, and the roots were cocultivated at 25°C for 2 days. For assaying tumor formation, the root segments were rinsed with sterile distilled water containing 100 μg of Timentin (clavulanic acid) per ml, blotted dry on filter paper, and transferred to hormone-free MS basal agar medium containing Timentin (100 μg/ml). The development of tumors on the cut surfaces of the roots was scored after 4 weeks of incubation at 25°C. To assay transformation to kanamycin resistance, the cocultivated root segments were transferred to callus-inducing medium (CIM; containing per liter: 4.3 g of MS minimal salts, 0.5 g of MES [pH 5.7], 1 ml of vitamin stock, 0.1 g of myo-inositol, 20 g of glucose, 0.5 mg of 2,4-dichlorophenoxyacetic acid, 0.3 mg of kinetin, 5 mg of indoleacetic acid, and 0.75% Bactoagar) containing Timentin (100 μg/ml) and kanamycin (150 μg/ml). Nonselected calli were grown on CIM containing Timentin but lacking kanamycin. To assay β-glucuronidase (GUS) activity qualitatively, root segments were collected from the CIM plate after various periods of incubation, blotted onto filter paper, and stained with X-Gluc solution (50 mM NaH2PO4, 10 mM EDTA, 300 mM mannitol, 2 mM X-Gluc [5-bromo-4-chloro-3-indolyl glucuronide] [pH 7.0]) overnight at 37°C. For quantitative GUS assays, the roots were ground in a microcentrifuge tube containing GUS extraction buffer (50 mM Na2HPO4, 5 mM dithiothreitol, 1 mM EDTA, 0.1% Sarkosyl, 0.1% Triton X-100 [pH 7.0]), and the specific GUS activity was determined as described by Jefferson et al. (21).
Tobacco leaf disc infection.
Leaves derived from axenically grown N. tabacum cv. Wisconsin 38 or transgenic tobacco plants constitutively expressing the virE2 gene (kindly provided by Vitaly Citovsky) were used for the source of leaf discs. Overnight liquid cultures of A. tumefaciens were incubated with leaf discs for 10 min. The discs were blotted dry on sterile filter paper and placed on MS basal agar medium for 2 days at 25°C. Depending on the experiment, the leaf discs were transferred to various agar media as follows: (i) on MS basal medium without phytohormones for the generation of tumors; (ii) on CIM containing 100 μg of kanamycin per ml for the selection of kanamycin-resistant calli; and (iii) on CIM lacking kanamycin for growth of nonselected calli. When infected leaf discs were assayed for GUS activity, discs were sampled and stained with X-Gluc beginning 2 days after the start of cocultivation.
Tobacco suspension cell infection.
Nicotiana tabacum BY-2 cells were propagated in Murashige and Skoog medium (Gibco-BRL, Gaithersburg, Md.) containing 3% sucrose, 1 mg of thiamine-HCl per liter, 0.2 mg of 2,4-dichlorophenoxyacetic acid per liter, and 370 mg of KH2PO4 per liter. The suspension cells were incubated at 25°C under continuous light with shaking (140 rpm). A. tumefaciens strains were grown to a density of 2 × 109 cells/ml in AB-glucose medium. Bacterial cells were centrifuged and suspended at a concentration of 109 cells/ml in induction medium (AB salts, 0.5% glucose, 2 mM sodium phosphate, 50 mM MES [pH 5.6], 100 μM acetosyringone), and incubated with gentle shaking for 14 to 18 h at 25°C. The cells were washed and concentrated 50-fold in plant culture medium, and 200 μl of bacterial cells was added to 50 ml of BY-2 cells and cocultivated at 25°C. Two days later, most bacterial cells were washed from the plant cells by repeated centrifugation at 300 rpm (Beckman Sorvall [Newtown, Conn.] GLC-2 clinical centrifuge) for 2 min and resuspension in plant culture medium. The plant cells were finally resuspended in plant culture medium containing Timentin (100 μg/ml) and continuously cultured. Starting from day 2 of cocultivation, plant cells were collected and stained in X-Gluc staining solution overnight at 37°C.
Extracellular complementation of tumorigenesis on Kalanchoë plants.
Leaves of K. daigremontiana plants were washed with sterile water and wounded with a sterile toothpick. Various dilutions of different combinations of A. tumefaciens strains were inoculated into the wound sites. These sites were examined 2 weeks later for tumor formation.
Inoculation of Datura plants.
Stems of young Datura stramonium plants (8 to 10 cm in height) were inoculated by poking the stems with a sterile toothpick harboring a paste of bacterial inoculum. Tumor formation was scored by examination of the inoculated sites 2 to 3 weeks later.
RESULTS
Expression of osa in A. tumefaciens inhibits the stable transformation of plants.
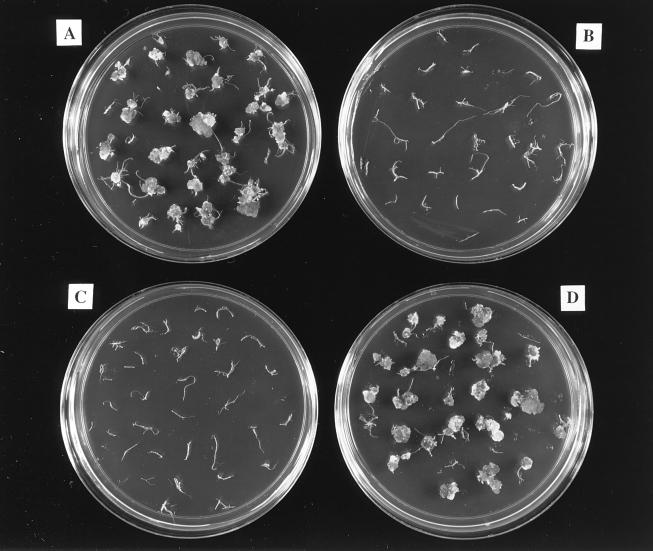
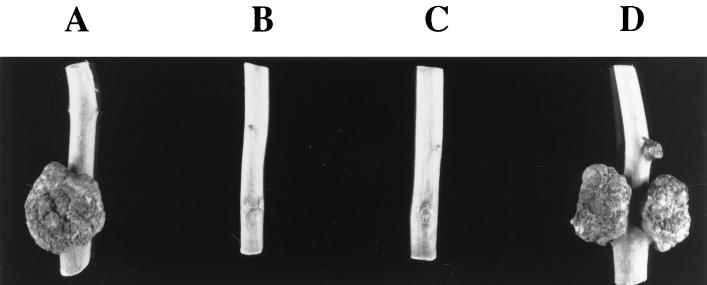
To extend the previous observations that the oncogenic suppressive activity of Osa occurs irrespective of the recipient plant host (10, 28) and to determine whether osa could inhibit the transformation of plants to other stable phenotypes, we infected A. thaliana ecotype Aa-0 root segments and tobacco leaf discs with A. tumefaciens strains containing or lacking pSa or the plasmid pUCD3960 containing just the osa gene under the control of a heterologous kanamycin promoter. In addition, some of these strains contained the plasmid pBISN1, which is a binary vector (31) that contains within the T-DNA a nos-nptII gene and a gusA intron gene under the control of a strong “superpromoter” (32). The presence of pBISN1 in these strains allowed us to monitor transformation to the stable phenotypes of kanamycin resistance and GUS expression, respectively, in addition to the formation of teratomas. We used A. tumefaciens A208 to inoculate sterile Arabidopsis ecotype Aa-0 root segments, because we had previously determined that this combination of bacterial strain and host ecotype resulted in reproducible transformation to strong and predictable stable phenotypes (30). Two days after inoculation, we transferred the root segments to CIM, either with or without kanamycin, or to MS medium lacking hormones. Figure 1 shows that in the absence of the osa gene, A. tumefaciens induced teratomas approximately 1 month after inoculation (Fig. 1A and D). However, A. tumefaciens strains containing the osa gene (either in the plasmid pSa or pUCD3960) failed to induce tumors (Fig. 1B and C). We observed the production of kanamycin-resistant calli only when the inciting A. tumefaciens strain lacked pSa or osa (Fig. 2A and D). We did not obtain kanamycin-resistant calli when the osa gene was resident in the A. tumefaciens strains (Fig. 2B and C). However, on CIM lacking kanamycin, calli developed approximately 1 month after cocultivation with all strains (data not shown). When stained with the chromogenic GUS substrate X-Gluc, only calli derived from root segments infected with A. tumefaciens strains lacking osa showed blue spots (data not shown). These data confirm that the presence of the osa gene in A. tumefaciens not only blocks tumorigenesis, but also blocks the transformation of plant cells to other stable phenotypes, such as kanamycin resistance and stable GUS expression. When we used tobacco leaf discs to conduct similar infections, we reproducibly obtained the same results (data not shown).
FIG. 1.
Teratoma formation on Arabidopsis roots. Sterile Arabidopsis root segments were cocultivated with A. tumefaciens for 2 days and then transferred to MS basal medium lacking phytohormones, but containing antibiotics to prevent the growth of bacterial cells. Teratomas first appeared approximately 2 weeks after infection, and the roots were photographed 1 month after infection. Root segments were infected by A. tumefaciens At928 [A208(pBISN1)] (A), A. tumefaciens At929 [A208(pBISN1, pSa)] (B), A. tumefaciens At931 [A208(pBISN1 plus osa)] (C), or A. tumefaciens At932 [A208(pBISN1 plus vector)].
FIG. 2.
Transformation of Arabidopsis roots to kanamycin resistance. Sterile Arabidopsis root segments were cocultivated with A. tumefaciens for 2 days and then transferred to CIM containing antibiotics to prevent the growth of bacterial cells and to select for transformed plant cells. The roots were photographed 1 month after infection. Root segments were infected by A. tumefaciens At928 [A208(pBISN1)] (A), A. tumefaciens At929 [A208(pBISN1, pSa)] (B), A. tumefaciens At931 [A208(pBISN1 plus osa)] (C), or A. tumefaciens At932 [A208(pBISN1 plus vector)] (D).
osa inhibits the transient transformation of plants by A. tumefaciens.
The data described above indicate that the presence of osa in A. tumefaciens inhibits the stable transformation of plants. It is possible, however, that osa inhibits the integration of T-DNA into the plant genome, but not T-DNA transfer to and transient expression within plant nuclei. We therefore conducted two sets of experiments to test whether osa affects the transient expression of genes introduced into plants by A. tumefaciens.
Agroinfection is inhibited by osa.
The introduction of multimeric forms of both of the components of two-component geminiviruses into plants can result in systemic viral infection (19). When these components are cloned within the T-DNA and introduced into plants by A. tumefaciens, they can be released by homologous recombination. This release presumably requires T-DNA nuclear transport but not T-DNA integration into the plant genome. To determine whether this agroinfection is inhibited by osa, we coinoculated N. benthamiana plants with A. tumefaciens strains containing multimers of the A and B components of ToMoV. The strains additionally contained the osa gene (either on pSa or cloned separately into pUCD3960) or control constructions. Table 2 shows the results of these experiments. Successful agroinfection resulted in the development of yellow mottling symptoms on the upper leaves 14 days after inoculation. We confirmed the systemic spread of the virus by squash blot hybridization and PCR analysis of DNA isolated from the upper leaves, using hybridization probes and primers, respectively, that would detect viral DNA. We never detected viral symptoms in plants inoculated with A. tumefaciens strains containing osa. In addition, we could not detect viral DNA in the upper leaves of these plants. Inoculation of plants with an A. tumefaciens strain with a mutation in virD2 (and therefore unable to transfer T-DNA to plants) similarly did not result in viral symptoms or systemic viral spread. However, A. tumefaciens strains containing a disrupted osa gene (pSa::neo) or a vector lacking osa (pUCD5533) effected agroinfection of these plants. We repeated these experiments three times using five plants of each inoculation group each time. The results of these experiments indicate that (i) osa can completely inhibit agroinfection of N. benthamiana plants by ToMoV, and (ii) osa blocks Agrobacterium-mediated transformation at some step prior to T-DNA integration.
TABLE 2.
Agroinfection of N. benthamiana plants by A. tumefaciens strains containing or lacking osa
| A. tumefaciens strain | Strain description | Determination of viral infection (no. of positive plants/no. inoculated)
|
||
|---|---|---|---|---|
| Systemic symptoms | Squash blot | PCR product | ||
| At915 + At916 | LBA4404(pToMoV-A) + LBA4404(pToMoV-B) | 15/15 | 15/15 | 15/15 |
| At1042 + At1043 | LBA4404(pToMoV-A, pSa) + LBA4404(pToMoV-B, pSa) | 0/15 | 0/15 | 0/15 |
| At1044 + At1045 | LBA4404(pToMoV-A, pSa::neo) + LBA4404(pToMoV-B, pSa::neo) | 15/15 | 15/15 | 15/15 |
| At1046 + At1047 | LBA4404(pToMoV-A, pUCD3960) + LBA4404(pToMoV-B, pUCD3960) | 0/15 | 0/15 | 0/15 |
| At1048 + At1049 | LBA4404(pToMoV-A, pUCD5533) + LBA4404(pToMoV-B, pUCD5533) | 15/15 | 15/15 | 15/15 |
| At915 + At1050 | LBA4404(pToMoV-A) + LBA4301(pToMoV-B, pJK703) | 0/15 | 0/15 | 0/15 |
osa prevents expression of transient GUS activity in infected plant cells.
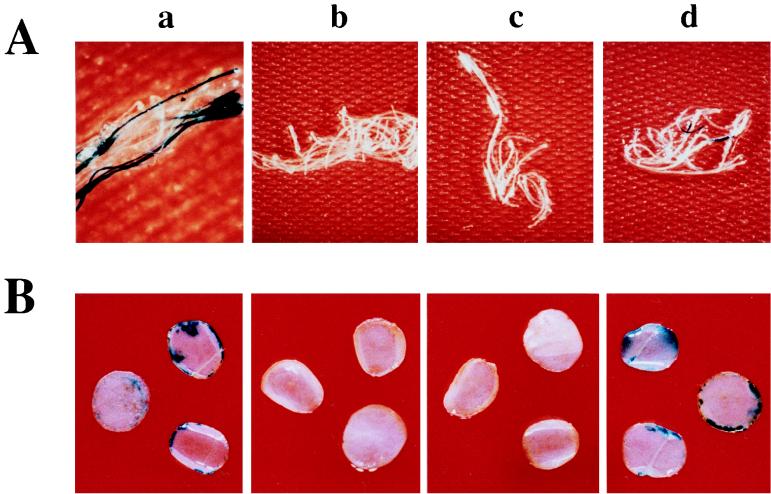
To confirm that osa inhibits the transient expression of T-DNA-encoded genes, we infected Arabidopsis root segments, tobacco leaf discs, and tobacco BY-2 suspension cells with A. tumefaciens A208(pBISN1) either containing or lacking osa. The gusA intron gene in pBISN1 allowed us to assay expression of GUS activity within infected plant cells without expression of GUS activity in the bacteria (27). Figure 3 shows Arabidopsis root segments (Fig. 3A) and tobacco leaf discs (Fig. 3B) stained with X-Gluc 4 days after inoculation with various A. tumefaciens strains. Infection of these plants with strains lacking osa resulted in the production of GUS activity, as indicated by sectors of blue-staining tissue. However, when the A. tumefaciens strains contained either pSa or the osa gene, we never detected GUS activity. We obtained similar results after infecting tobacco BY-2 suspension cells with the A. tumefaciens strains (i.e., when the bacteria lacked osa, 2 to 5% of the tobacco cell clusters stained blue with X-Gluc). Infection of the cells with A. tumefaciens harboring pSa or the osa gene never resulted in detectable GUS activity (data not shown).
FIG. 3.
Transient GUS expression in Arabidopsis roots and tobacco leaf discs. Sterile Arabidopsis root segments (A) or tobacco leaf discs (B) were cocultivated with A. tumefaciens for 2 days and then transferred to medium containing Timentin to prevent the growth of bacterial cells. After 2 additional days, the plant material was washed with 0.9% NaCl and stained with X-Gluc. Photographs were taken with a dissecting microscope. Plant tissue was infected with A. tumefaciens At928 [A208(pBISN1)] (a), A. tumefaciens At929 [A208(pBISN1, pSa)] (b), A. tumefaciens At931 [A208(pBISN1 plus osa)] (c), or A. tumefaciens At932 [A208(pBISN1 plus vector)] (d).
Taken together, the agroinfection and GUS expression data indicate that osa blocks Agrobacterium-mediated transformation at some step prior to nuclear entry of the T-DNA.
osa blocks VirE2 protein but not T-DNA export from A. tumefaciens.
To determine whether osa blocks T-DNA transfer from A. tumefaciens to plant cells, we initially performed extracellular complementation assays. Otten et al. (34) had previously shown that when two avirulent A. tumefaciens strains, one lacking T-DNA (the VirE2 donor) and the other with a mutation in virE2 (the T-DNA donor) are coinoculated into plant wounds, tumors can develop. Control experiments had shown that this complementation is most likely extracellular (i.e., not within A. tumefaciens), because it could occur in the absence of Ti plasmid conjugation between the mutant strains, and that it required bacterial binding to plant cells. These data have been interpreted to indicate the independent transfer of VirE2 protein and the T-DNA–VirD2 complex from A. tumefaciens (2, 40).
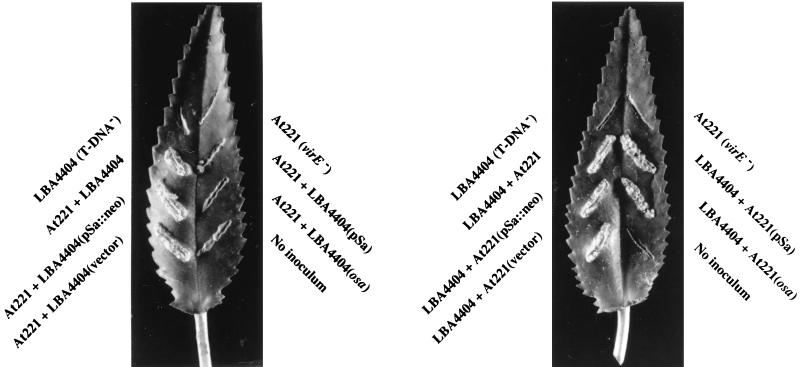
Figure 4 confirms that when coinoculated into Kalanchoë leaves, A. tumefaciens LBA4404 (the VirE2 donor) and At221 (the T-DNA donor) can incite crown gall tumors. Neither strain alone was tumorigenic. When A. tumefaciens At221 containing either pSa or the osa gene was used as the T-DNA donor strain, tumors still resulted. Thus, osa does not block T-DNA transport from the bacterium. When A. tumefaciens LBA4404 containing pSa or the osa gene was used as the VirE2 donor, however, no tumors resulted from coinoculation with A. tumefaciens At221. Oncogenic suppression depended upon the presence of an active osa gene within the bacterium. Disruption of the osa gene within pSa by a neomycin phosphotransferase gene (pSa::neo) permitted A. tumefaciens LBA4404 to serve as a VirE2 donor.
FIG. 4.
Extracellular complementation of A. tumefaciens strains harboring pSa or osa. A. tumefaciens At221 (virE2 mutant, a T-DNA donor) and LBA4404 (a VirE2 donor lacking T-DNA) containing various plasmids were coinoculated onto Kalanchoë leaves. The leaves were photographed after 1 month. The A. tumefaciens strains are LBA4404, T-DNA− VirE2 donor; At221, virE2 mutant T-DNA donor; At900, LBA4404(pSa); At901, LBA4404(pSa::neo); At902, LBA4404 (osa); At903, LBA4404 (vector); At904, At221(pSa); At905, At221(pSa::neo); At906, At221 (osa); and At907, At221 (vector).
These data indicate that osa blocks the transfer of VirE2 protein, but not the T-DNA, from A. tumefaciens to plant cells.
osa does not inhibit tumorigenesis on Datura by an A. tumefaciens virE2 mutant strain.
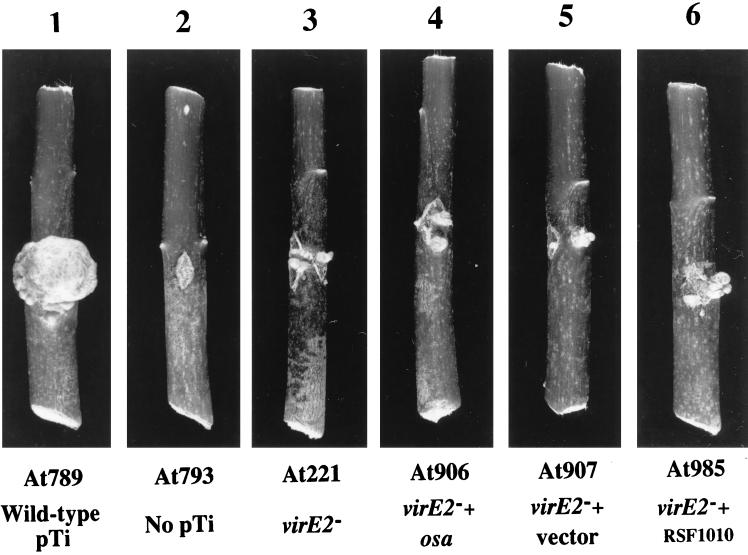
Although virE2 mutant A. tumefaciens strains are avirulent on many host plants (38), they may incite small tumors on highly sensitive hosts such as D. stramonium. When inoculated onto D. stramonium stems, the virE2 mutant A. tumefaciens At221 incited small tumors (approximately 2 to 3 mm in diameter) compared to the large tumors (100 to 150 mm in diameter) incited by wild-type A. tumefaciens At789 (Fig. 5). A. tumefaciens virE2 mutant strains containing the osa gene (At906) or vector sequences alone (At907) both retained the ability to incite small tumors on D. stramonium stems (Fig. 5). These data support the hypothesis that osa does not prevent T-DNA export from A. tumefaciens. Binns et al. (2) previously showed that the oncogenic suppressive IncQ plasmid pJW323 inhibits VirE2 protein export from A. tumefaciens, but inhibits T-DNA export only to a limited extent. Figure 5 confirms one of these observations. A. tumefaciens At221 containing another RSF1010 derivative, pJB31 (At985), could still transfer T-strands and incite small tumors on D. stramonium stems.
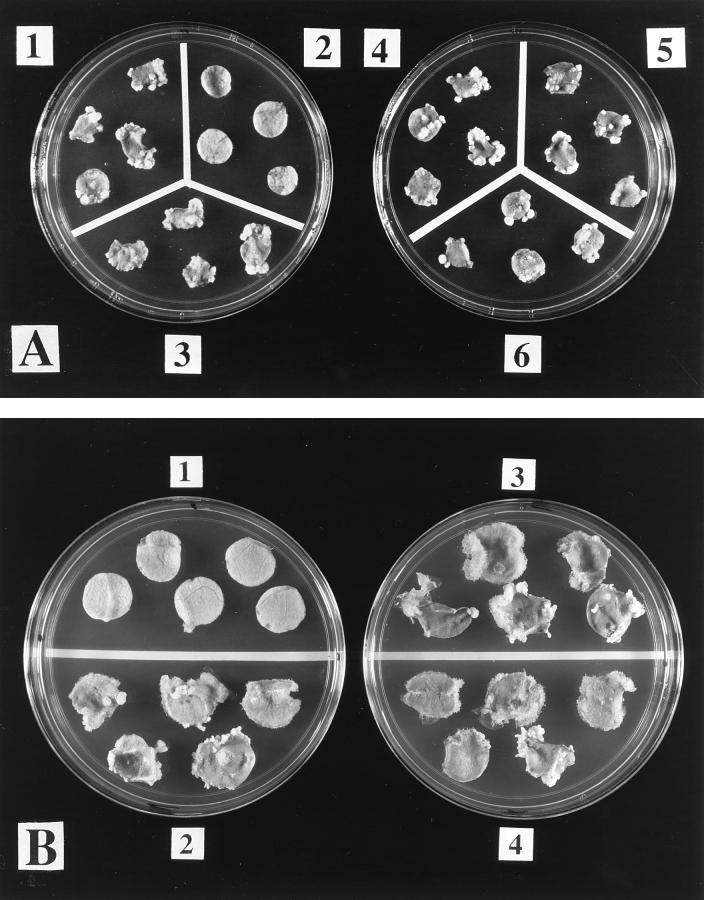
FIG. 5.
Effect of osa on tumorigenesis by the virE2 mutant A. tumefaciens At221. Datura stems were inoculated with various A. tumefaciens strains, and the infected stem sections were photographed 30 days later. Panels: 1, A. tumefaciens At789 (wild-type pTi); 2, A. tumefaciens At793 (no pTi); 3, A. tumefaciens At221 (virE2 mutant); 4, A. tumefaciens At906 (At221 plus osa); 5, A. tumefaciens At907 (At221 plus vector); 6, A. tumefaciens At985 (At221 plus pJB31).
osa does not inhibit tumorigenesis of a virE2 mutant A. tumefaciens strain when inoculated into VirE2-producing transgenic tobacco plants.
The virE2 mutant strain A. tumefaciens At221 cannot incite tumors on wild-type tobacco. Citovsky et al. (5) demonstrated that the same A. tumefaciens strain could induce tumors on transgenic tobacco that produced VirE2 protein. We reasoned that if osa blocks VirE2 protein but not T-DNA transport from A. tumefaciens, a virE2 mutant strain containing osa should retain virulence on VirE2-producing tobacco plants. Figure 6A4 shows that as described by Citovsky et al. (5), A. tumefaciens At221 could induce tumors on VirE2-producing transgenic tobacco leaf discs. A. tumefaciens At221 could not induce tumors on wild-type tobacco leaf discs (data not shown). The presence of osa (Fig. 6A5) or vector sequences (Fig. 6A6) in At221 did not inhibit tumorigenesis by the strain on VirE2-producing tobacco leaf discs. In addition, the RSF1010 derivative pJB31 did not inhibit tumorigenesis by A. tumefaciens At221 on VirE2-producing tobacco plants (Fig. 6A3). Finally, as shown in Fig. 6B2 to B4, neither the presence of the osa gene nor vector sequences could inhibit tumorigenesis by the wild-type strain A. tumefaciens A208 when inoculated onto VirE2-producing transgenic tobacco leaf discs. These data confirm that osa does not inhibit T-strand export from A. tumefaciens and further demonstrate that the IncQ plasmid pJB31 also does not inhibit T-DNA export from the bacterium.
Overexpression of the proteins VirB9, -10, and -11 cannot overcome oncogenic suppression by osa.
Binns et al. (2) previously demonstrated that the oncogenic suppression effect of RSF1010 derivatives could be overcome by overexpression of VirB9, -10, and -11 proteins in A. tumefaciens. These authors hypothesized, therefore, that IncQ plasmids effect oncogenic suppression by competing with VirE2 protein for the virB-encoded protein export apparatus. To determine whether osa likewise competes with VirE2 for the VirB protein export channel, we cloned virB9, -10, and -11 under the control of the virB promoter to create pUCD5542. pUCD5542 could complement a polar virB10 mutant A. tumefaciens strain and restore virulence (data not shown). We introduced pUCD5542 into A. tumefaciens A208(pBISN1) (At935) and A208(pBISN1) containing either pSa (At925), the osa gene (At926), or vector sequences alone (At927) and inoculated these strains onto wounded D. stramonium stems. Figure 7 shows that A. tumefaciens A208(pBISN1) containing pUCD5542 was highly tumorigenic on D. stramonium. However, the presence of additional copies of virB9, virB10, and virB11 in A. tumefaciens A208(pBISN1) could not overcome oncogenic suppression by pSa or by the osa gene. In addition, pUCD5542 could neither overcome oncogenic suppression by pSa or the osa gene on tobacco leaf discs or Kalanchoë leaves, nor could it permit GUS expression (directed by pBISN1) in inoculated tobacco leaf discs (data not shown). However, A. tumefaciens At1085, containing pUCD5542 and pJB31 in the oncogenic strain A208, was also not tumorigenic on Kalanchoë leaves (data not shown). Thus, it is likely that the expression of virB9, virB10, and virB11 with our construction was not strong enough to overcome oncogenic suppression.
FIG. 7.
Infection of D. stramonium stems with A. tumefaciens strains containing multiple copies of virB9, -10, and -11. D. stramonium seedlings were wounded and inoculated with A. tumefaciens A208 containing pBISN1, pUCD5542, and no additional plasmid (At935) (A); pSa (At925) (B); osa (At926) (C); or vector (At927) (D). The stem sections were photographed 1 month after inoculation.
DISCUSSION
In this study, we have investigated the mechanism of oncogenic suppression by the osa gene of the IncW plasmid pSa. Previous studies had shown that when coresident with the Ti plasmid, pSa inhibits tumor formation by A. tumefaciens (28). Oncogenic suppression did not result from a permanent alteration of the host A. tumefaciens strain. When cured of pSa, the bacteria regained their tumorigenic potential (10). Genetic dissection of pSa revealed that a 3.1-kbp region of the plasmid was responsible for oncogenic suppression (7). Further analysis indicated that a single gene from this region, termed osa, was sufficient to block tumorigenesis by A. tumefaciens (3).
Previous studies had indicated that the oncogenic suppression effect of pSa upon A. tumefaciens did not result from a lack of vir gene induction or T-DNA processing (6). In addition, the presence of pSa did not affect the synthesis or membrane localization of VirB2, VirB3, VirB4, VirB9, and VirD4 proteins in the bacterium (3). These results suggested that pSa exerted its oncogenic suppression effect at some point at or after T-DNA transfer. We therefore examined the effect of pSa on later steps of the T-DNA transformation process.
We initially confirmed that pSa or a plasmid containing just the osa gene inhibited tumorigenesis by A. tumefaciens A208 on Arabidopsis root segments. According to these assays, oncogenic suppression was complete, because we never saw the development of tumors on infected Arabidopsis roots. Similar results were reported by Farrand et al. (10). In that study, A. tumefaciens C58 (a nopaline-type strain similar to A. tumefaciens A208) harboring pSa failed to induce tumors on carrot discs or Kalanchoë leaves. However, Farrand et al. reported that A. tumefaciens 1D1 (an octopine-type strain) containing pSa was able to incite, at low efficiency, small and slowly developing tumors on carrot discs, Kalanchoë, sunflower, tomato, and marigold. We also have seen the development of small tumors on D. stramonium infected with A. tumefaciens A277 (an agropine-type supervirulent strain) harboring pSa (24a). This lack of complete oncogenic suppression suggests that pSa reduces tumorigenicity by competing with some aspect of the normal T-DNA transfer process.
Chernin et al. (4) previously reported that the IncW plasmids pSa and R388 suppressed the oncogenicity of A. tumefaciens 1D1 by inhibiting auxin production by the bacterium. Application of the auxin indoleacetic acid to the plants a few days before inoculation restored oncogenicity to A. tumefaciens 1D1(pSa). Because of this report, and because of the involvement of phytohormones in the stable transformation of plant cells to crown gall tumors (15), we investigated the effects of pSa or the osa gene upon the transformation of plant cells to other stable phenotypes. We determined that osa inhibits transformation of plant tissues to kanamycin resistance and the ability to express GUS activity stably. We therefore conclude that the inhibitory effects of osa are not specific to the tumorigenesis phenotype, but extend to the inhibition of genetic transformation to other stable phenotypes.
Although osa inhibited the genetic transformation of plant cells to express various phenotypes stably, it was possible that the effect of osa was on T-DNA integration and not T-DNA transfer or nuclear targeting. We therefore investigated the effect of osa on the transient transformation of plant cells by using two different approaches. The first approach utilized agroinfection with both components of the two-component geminivirus ToMoV. Although never formally proven, it has been widely assumed that agroinfection of multimers of viral genomes contained within the T-DNA results in the release of viral DNA by homologous recombination within the plant nucleus. Successful agroinfection requires nuclear transport of the T-DNA, but presumably not T-DNA integration into the plant genome. The presence of osa within the inciting A. tumefaciens strains completely inhibited agroinfection, as determined by the formation of ToMoV symptoms, and the specific detection of viral DNA by squash blot and PCR analyses. These results indicate that osa inhibits the genetic transformation of plants by A. tumefaciens at or before the stage of nuclear translocation of the T-DNA. These results were confirmed by analyzing the effect of osa upon the transient expression of GUS activity in infected plant cells. We have previously shown that expression of GUS activity, or the production of gusA mRNA, does not require T-DNA integration into the plant genome (29, 31). The inhibition of expression of transient GUS activity in plant cells infected with A. tumefaciens strains containing osa additionally indicates that osa inhibits the transient genetic transformation of plant cells.
We next wished to determine whether osa inhibited the transfer of T-DNA from A. tumefaciens to plant cells. To do this, we utilized the genetic approach of extracellular complementation first described by Otten et al. (34). Our data indicated that osa does not inhibit T-DNA transfer from the T-DNA donor strain A. tumefaciens At221 to plant cells. However, when osa was present in the VirE2 protein donor strain A. tumefaciens LBA4404, extracellular complementation with A. tumefaciens At221 did not occur. We therefore conclude that the primary effect of osa is to prevent VirE2 protein, and not T-DNA, transfer from the bacterium to the plant cell.
To confirm that osa primarily inhibits VirE2 protein but not T-DNA transfer, we utilized two additional approaches. First, we infected D. stramonium stems with the virE2 mutant strain A. tumefaciens At221. This A. tumefaciens mutant incited small tumors on this highly sensitive plant. A. tumefaciens At221 containing pSa or the osa gene retained the ability to incite small tumors on D. stramonium. This result further indicates that osa does not block T-DNA transfer. Second, we infected leaf discs of wild-type and VirE2-producing transgenic tobacco with A. tumefaciens At221 lacking or containing osa. Leaf discs from wild-type plants did not develop tumors with any virE2 mutant A. tumefaciens strain tested (data not shown). As first shown by Citovsky et al. (5), VirE2-producing transgenic plants developed tumors when inoculated with the virE2 mutant A. tumefaciens At221. The virE2 mutant A. tumefaciens strain containing osa incited tumors on these transgenic leaf discs at a frequency comparable to that incited by the strain lacking osa. These results further indicate that we could not detect an effect, either qualitative or quantitative, of osa on T-DNA transfer to plant cells. Binns et al. (2) showed that the presence of pJW323, a derivative of the IncQ plasmid RSF1010, in A. tumefaciens greatly inhibited the ability of the bacterium to serve as a VirE2 protein donor, but inhibited its ability to transfer T-DNA by only twofold. They also showed that pJW323 inhibited tumorigenesis by A. tumefaciens A348 by approximately 90%, but did not completely suppress oncogenesis by this strain. Our results with oncogenic suppression by osa differ from those of Binns et al. in several ways. First, we consistently saw complete oncogenic suppression of A. tumefaciens A348 by pSa or osa (24a). Second, we could not detect an effect of pSa or osa on the ability of A. tumefaciens to transfer T-DNA to plant cells. Third, the expression of only the osa gene was sufficient to effect oncogenic suppression. We have recently shown that the osa gene, when incorporated into the A. tumefaciens chromosome, could still effect oncogenic suppression (24b). Thus, no conjugal intermediate of pSa was necessary. This differs from the IncQ system, in which the formation of a conjugal intermediate is necessary to cause oncogenic suppression (39). Fourth, unlike the situation with the RSF1010 derivative pJW323 (2, 42), we were not able to overcome the oncogenic suppressive effect of osa by the coordinate overexpression of VirB9, VirB10, and VirB11 proteins. However, our virB expression plasmid (pUCD5542) could not reverse oncogenic suppression of RSF1010, as described by Ward et al. (42). Therefore, the differences between our inability to overcome oncogenic suppression of RSF1010 and the success of Ward et al. may have been more quantitative than qualitative. Overall, however, pSa may be more efficient in competing for the virB-encoded VirE2 transport apparatus than is RSF1010. Recent experiments indicate that pSa and RSF1010 directly compete with each other for the same export apparatus (24b).
Although we have shown that the molecular mechanism of oncogenic suppression by osa involves inhibition of VirE2 protein export from A. tumefaciens, we have not yet determined the details of how this inhibition is effected. The observations that VirE2 is an abundant protein in A. tumefaciens (9) and that Osa is immunologically difficult to detect unless overexpressed (24a) suggest that it is unlikely that Osa interferes with VirE2 export by stoichiometrically binding to VirE2. It is possible that Osa interacts with VirE1 protein that is needed for VirE2 protein export from the bacterium (40). It is also possible that Osa interacts with one of the proteins of the virB-virD4-encoded protein export apparatus.
Finally, our data showing that osa inhibits VirE2 protein but not T-DNA transfer, as well as our data showing that a virE2 mutant strain of A. tumefaciens can still incite small tumors on D. stramonium, lend further evidence to the model of Binns et al. (2) and Sundberg et al. (40) that a T-DNA–VirD2 complex is transported to plant cells separately from VirE2 protein.
FIG. 6.
Tumorigenesis of various A. tumefaciens strains on VirE2-producing transgenic tobacco leaf discs. Leaf discs of sterile virE2 transgenic tobacco plants were infected with various A. tumefaciens strains. After 2 days of cocultivation, the discs were incubated on MS medium containing Timentin and photographed 1 month later. (A) Panels: 1, infection with A. tumefaciens At789 (wild-type pTi); 2, A. tumefaciens At793 (no pTi); 3, A. tumefaciens At985 [At221 (pJB31)]; 4, A. tumefaciens At989 (virE2 mutant); 5, A. tumefaciens At990 (At221 plus osa); 6, A. tumefaciens At991 (At221 plus vector). (B) Panels 1, no inoculation; 2, A. tumefaciens At928 [A208(pBISN1)]; 3, A. tumefaciens At931 [A208(pBISN1 plus osa)]; 4, A. tumefaciens At932 [A208(pBISN1 plus vector)].
ACKNOWLEDGMENTS
We thank Vitaly Citovsky for providing seeds of VirE2-producing transgenic tobacco plants and Robert Gilbertson for providing strains and assistance for agroinfection and assistance in squash blot and PCR analyses.
This work was supported by grants from the U.S. Department of Agriculture (95-37301-2040) to S.B.G. and NIH grant GM45550 from the National Institute of General Medicine to C.I.K.
REFERENCES
- 1.Beaupré C E, Bohne J, Dale E M, Binns A N. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1997;179:78–89. doi: 10.1128/jb.179.1.78-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binns A N, Beaupré C E, Dale E M. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C-Y, Kado C I. Inhibition of Agrobacterium tumefaciens oncogenicity by the osa gene of pSa. J Bacteriol. 1994;176:5697–5703. doi: 10.1128/jb.176.18.5697-5703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chernin L S, Lobanok E V, Fomicheva V V, Kartel N A. Crown gall-suppressive IncW R plasmids cause a decrease in auxin production in Agrobacterium tumefaciens. Mol Gen Genet. 1984;195:195–199. [Google Scholar]
- 5.Citovsky V, Zupan J, Warnick D, Zambryski P. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science. 1992;256:1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- 6.Close S M. Genetic and molecular analysis of plasmid pSa conferred suppression of Agrobacterium tumefaciens oncogenicity, and identification of an independent pSa locus encoding a nuclease. Ph.D. thesis. University of California, Davis; 1991. [Google Scholar]
- 7.Close S M, Kado C I. The osa gene of pSa encodes a 21.1-kilodalton protein that suppresses Agrobacterium tumefaciens oncogenicity. J Bacteriol. 1991;173:5449–5456. doi: 10.1128/jb.173.17.5449-5456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Close S M, Kado C I. A gene near the plasmid pSa origin of replication encodes a nuclease. Mol Microbiol. 1992;6:521–527. doi: 10.1111/j.1365-2958.1992.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 9.Engstrom P, Zambryski P, Van Montagu M, Stachel S. Characterization of Agrobacterium tumefaciens virulence proteins induced by the plant factor acetosyringone. J Mol Biol. 1987;197:635–645. doi: 10.1016/0022-2836(87)90470-0. [DOI] [PubMed] [Google Scholar]
- 10.Farrand S K, Kado C I, Ireland C R. Suppression of tumorigenicity by the IncW plasmid pSa in Agrobacterium tumefaciens. Mol Gen Genet. 1981;181:44–51. [Google Scholar]
- 11.Fong S T, Stanisich V A. Location and characterization of two functions on RP1 that inhibit the fertility of the IncW plasmid R388. J Gen Microbiol. 1989;135:499–502. doi: 10.1099/00221287-135-3-499. [DOI] [PubMed] [Google Scholar]
- 12.Frey J, Bagdasarian M. The molecular biology of IncQ plasmids. In: Thomas C M, editor. Promiscuous plasmids of Gram-negative bacteria. New York, N.Y: Academic Press; 1989. pp. 125–163. [Google Scholar]
- 13.Gallie D R, Novak S, Kado C I. Novel high- and low-copy stable cosmids for use in Agrobacterium and Rhizobium. Plasmid. 1985;14:171–175. doi: 10.1016/0147-619x(85)90078-2. [DOI] [PubMed] [Google Scholar]
- 14.Garfinkel D J, Simpson R B, Ream L W, White F F, Gordon M P, Nester E W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981;27:143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- 15.Gelvin S B. Crown gall disease and hairy root disease: a sledgehammer and a tackhammer. Plant Physiol. 1990;92:281–285. doi: 10.1104/pp.92.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbertson R L, Hidayat S H, Martinez R T, Leong S A, Faria J C, Morales F J, Maxwell D P. Differentiation of bean-infecting geminiviruses by nucleic acid hybridization probes and aspects of bean golden mosaic in Brazil. Plant Dis. 1991;75:336–342. [Google Scholar]
- 17.Gilbertson R L, Hidayat S H, Paplomatas E J, Rojas M R, Hou Y-M, Maxwell D P. Pseudorecombination between infectious cloned DNA components of tomato mottle and bean dwarf mosaic geminiviruses. J Gen Virol. 1993;74:23–31. doi: 10.1099/0022-1317-74-1-23. [DOI] [PubMed] [Google Scholar]
- 18.Gilbertson R L, Rojas M R, Russell D R, Maxwell D P. Use of the asymmetric polymerase chain reaction and DNA sequencing to determine genetic variability of bean golden mosaic geminivirus in the Dominican Republic. J Gen Virol. 1991;72:2843–2848. doi: 10.1099/0022-1317-72-11-2843. [DOI] [PubMed] [Google Scholar]
- 19.Grimsley N, Hohn B, Hohn T, Walden R. “Agroinfection,” an alternative route for viral infection of plants by using the Ti plasmid. Proc Natl Acad Sci USA. 1986;83:3282–3286. doi: 10.1073/pnas.83.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernalsteens J P, Villarroel-Mandiola R, Van Montagu M, Schell J. Transposition of Tn1 to a broad-host-range drug resistance plasmid. In: Bukhari A I, Shapiro J A, Adhya S L, editors. DNA insertion elements, plasmids, and episomes. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1977. pp. 179–183. [Google Scholar]
- 21.Jefferson R A, Kavanagh T A, Bevan M W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kado C I. Agrobacterium-mediated transfer and stable incorporation of foreign genes in plants. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 243–254. [Google Scholar]
- 23.Kado C I, Heskett M G. Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology. 1970;60:969–976. doi: 10.1094/phyto-60-969. [DOI] [PubMed] [Google Scholar]
- 24.Klapwijk P, Van Beelen P, Schilperoort R A. Isolation of a recombination deficient Agrobacterium tumefaciens mutant. Mol Gen Genet. 1979;173:171–175. doi: 10.1007/BF00330307. [DOI] [PubMed] [Google Scholar]
- 24a.Lee, L.-Y. Unpublished observations.
- 24b.Lee, L.-Y., et al. Unpublished data.
- 25.Lessl M, Lanka E. Common mechanisms in bacterial conjugation and Ti-mediated T-DNA transfer to plant cells. Cell. 1994;77:321–324. doi: 10.1016/0092-8674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein C, Draper J. Genetic engineering of plants. In: Glover D M, editor. DNA cloning: a practical approach. Vol. 2. Washington, D.C: IRL Press; 1986. pp. 67–119. [Google Scholar]
- 27.Liu C-N, Li X-Q, Gelvin S B. Multiple copies of virG enhance the transient transformation of celery, carrot, and rice tissues by Agrobacterium tumefaciens. Plant Mol Biol. 1992;20:1071–1087. doi: 10.1007/BF00028894. [DOI] [PubMed] [Google Scholar]
- 28.Loper J E, Kado C I. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J Bacteriol. 1979;139:591–596. doi: 10.1128/jb.139.2.591-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mysore, K. S., B. Bassuner, X.-B. Deng, N. S. Darbinian, A. Motchoulski, W. Ream, and S. B. Gelvin. Role of the Agrobacterium tumefaciens VirD2 protein in T-DNA transfer and integration. Mol. Plant-Microbe Interact. 11:668–683. [DOI] [PubMed]
- 30.Nam J, Matthysse A G, Gelvin S B. Differences in susceptibility of Arabidopsis ecotypes to crown gall disease may result from a deficiency in T-DNA integration. Plant Cell. 1997;9:317–333. doi: 10.1105/tpc.9.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narasimhulu S B, Deng X-B, Sarria R, Gelvin S B. Early transcription of Agrobacterium T-DNA genes in tobacco and maize. Plant Cell. 1996;8:873–886. doi: 10.1105/tpc.8.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni M, Cui D, Einstein J, Narasimhulu S, Vergara C E, Gelvin S B. Strength and tissue specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J. 1995;7:661–676. [Google Scholar]
- 33.Ooms G, Molendijk L, Schilperoort R A. Double infection of tobacco plants by two complementing octopine T-region mutants of Agrobacterium tumefaciens. Plant Mol Biol. 1982;1:217–226. doi: 10.1007/BF00021033. [DOI] [PubMed] [Google Scholar]
- 34.Otten L, De Greve H, Leemans J, Hain R, Hooykaas P, Schell J. Restoration of virulence of vir region mutations of Agrobacterium tumefaciens strain B6S3 by coinfection with normal and mutant Agrobacterium strains. Mol Gen Genet. 1984;195:159–163. [Google Scholar]
- 35.Rogowsky P M, Close T J, Chimera J A, Shaw J J, Kado C I. Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. J Bacteriol. 1987;169:5101–5112. doi: 10.1128/jb.169.11.5101-5112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sciaky D A, Montoya A L, Chilton M-D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978;1:238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- 37.Stachel S E, Zambryski P C. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell. 1986;47:155–157. doi: 10.1016/0092-8674(86)90437-x. [DOI] [PubMed] [Google Scholar]
- 38.Stachel S E, Nester E W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986;5:1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl L E, Jacobs A, Binns A N. The conjugal intermediate of plasmid RSF1010 inhibits Agrobacterium tumefaciens virulence and VirB-dependent export of VirE2. J Bacteriol. 1998;180:3933–3939. doi: 10.1128/jb.180.15.3933-3939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundberg C, Meek L, Carroll K, Das A, Ream W. VirE1 protein mediates export of the single-stranded DNA-binding protein VirE2 from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1996;178:1207–1212. doi: 10.1128/jb.178.4.1207-1212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentine C R I, Kado C I. Molecular genetics of IncW plasmids. In: Thomas C M, editor. Promiscuous plasmids of Gram-negative bacteria. New York, N.Y: Academic Press; 1989. pp. 125–163. [Google Scholar]
- 42.Ward J E, Dale E M, Binns A N. Activity of the Agrobacterium T-DNA transfer machinery is affected by VirB gene products. Proc Natl Acad Sci USA. 1991;88:9350–9354. doi: 10.1073/pnas.88.20.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson B, Currier T C, Gordon M P, Chilton M-D, Nester E W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975;123:255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]