Abstract

An increasing number of publications over the past ten years have focused on the development of chitosan-based cross-linked scaffolds to regenerate bone tissue. The design of biomaterials for bone tissue engineering applications relies heavily on the ideals set forth by a polytherapy approach called the “Diamond Concept”. This methodology takes into consideration the mechanical environment, scaffold properties, osteogenic and angiogenic potential of cells, and benefits of osteoinductive mediator encapsulation. The following review presents a comprehensive summarization of recent trends in chitosan-based cross-linked scaffold development within the scope of the Diamond Concept, particularly for nonload-bearing bone repair. A standardized methodology for material characterization, along with assessment of in vitro and in vivo potential for bone regeneration, is presented based on approaches in the literature, and future directions of the field are discussed.
Keywords: Chitosan-Based Scaffolds, Bone Tissue Engineering, Diamond Concept, Cross-linking, Osteogenic Cells, Growth Factors
1. Introduction
Advancement of the bone tissue engineering field in recent years has opened new potentials for the design of alternative treatments of various orthopedic conditions. Naturally, bone tissue relies on its self-healing capabilities through direct or indirect healing, but this can be hindered following severe traumas or other underlying conditions.1−3 Current conventional bone graft treatments, while advantageous in some respects, present many limitations including invasiveness, donor site morbidity, limited graft volume, and long hospitalization times.4−10
Interest has since shifted to the design and implementation of tissue engineered substitutes. These designs focus on targeting the regeneration of native tissue both at the structural and functional level using a complex polytherapy approach called the “Diamond Concept”.9,11−13 This design framework established by Giannoudis et al. provides a guideline for the minimal requirements needed for efficient bone healing: osteoconductive scaffolds, osteogenic and angiogenic cells, osteoinductive mediators, and a suitable mechanical environment9−12,14−16 (Figure 1A).
Figure 1.
Comprehensive overview of the literature review presented in this manuscript. (A) “Diamond Concept” for Bone Regeneration, which encompasses an osteoconductive scaffold, osteogenic cells, osteoinductive mediators, and vascularization in a suitable mechanical environment. This builds the foundation for the organization of the review. (B) Increasing number of publications within the chitosan-based cross-linked scaffold/hydrogel domain for bone tissue engineering applications in the last 20 years. (C) The review’s novelty within the combined domain of chitosan based cross-linked hydrogel/scaffolds for bone tissue engineering applications implementing the Diamond Concept.
Each component within this approach plays a critical role in the overall ability of the biological substitute for bone healing and regeneration.12,15,17−21 The biomaterial scaffold acts as a 3D microenvironment for cells by providing structural support thereby necessitating similarities in mechanical properties between a chosen biomaterial and native tissue environment. In terms of bone regeneration, the scaffold needs to have a sufficiently high mechanical strength,22−24 in addition to a biodegradation rate similar to that of new bone tissue formation.12,25−30 The architecture of the scaffold should be porous to allow for cellular migration and vascular network infiltration.17,31
Incorporation of cells is also necessary since cells are responsible for the secretion of the extracellular matrix along with different growth factors and cytokines involved in tissue healing.32,33 For bone applications, this aspect often encompasses osteoblast lineage cells such as preosteoblasts since they are already lineage committed and require less effort in differentiation processes.33,34 More recent work has also focused on the usage of mesenchymal stem cells with subsequent differentiation once encapsulated in the scaffold.33,35 Finally, osteoinductive mediators such as bone morphogenetic proteins 2, 4, and 7 (BMP2, 4, 7) are a crucial consideration as their incorporation aids in developing the scaffold into an optimal environment for cell growth, differentiation, and new tissue formation.20,36−42 While the three previously discussed components make up the tissue engineering triad for the regeneration of many tissues, vascularization and mechanical environment are specific considerations for bone applications.43 These are added due to the complex nature of bone tissue thereby necessitating a more complex design approach.
Polymers represent the largest class of biomaterials investigated for various biomedical and tissue engineering purposes. These chains of repeating monomers can be sourced naturally such as chitosan, alginate, and collagen or fabricated synthetically like poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), and poly(methyl methacrylate) (PMMA), among many others.44−47 As evidenced throughout the literature, chitosan is among the most widely investigated polymers for the design of ideal scaffolds for bone tissue engineering.44−46,48 Its usage is often compounded with a physical or chemical cross-linker, which serves to improve material properties such as compressive strength, elastic modulus, degradation rate, structural stability, and architecture.44,48−51 The implementation of cross-linking for chitosan-based scaffolds in bone applications has continued to become more popular in literature studies, thus requiring a more thorough investigation of its demonstrated benefits (Figure 1B).
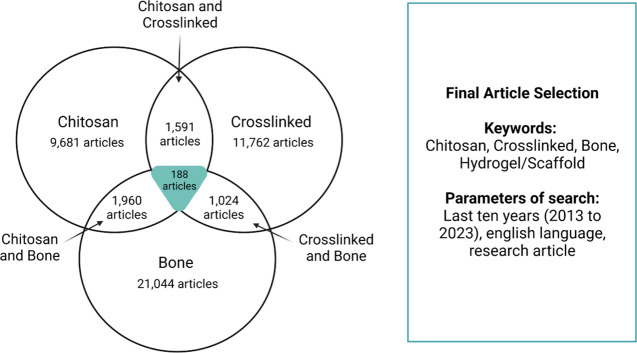
In the scope of this review, we aim to provide in-depth comparisons of material and osteogenic properties for chitosan-based cross-linked scaffolds within the ideals of the Diamond Concept for bone tissue engineering of nonload bearing tissues. Each subcategory of the Diamond Concept will be discussed according to the most recent advances in that component. This literature review is to our knowledge the first of its kind to review the design of chitosan based cross-linked scaffolds for bone in this manner (Figure 1C). To accomplish this, a literature search was conducted using the SCOPUS database with keywords: chitosan, cross-linked, and bone, and resulting articles were further screened for availability and intended application (Figure 2).
Figure 2.
Results of literature search conducted for this manuscript. The search was performed using the keywords: Chitosan, Crosslinked (or Cross-linked), Bone, and Scaffold or Hydrogels. These results were also limited to publications from the last ten years and written in the English language. From this search, 188 articles were found to be suitable and screened further to examine the specific target of the study.
Although the need for assessment of scaffold material properties and bone regenerative capabilities is evident in many studies found in the literature, no clear set standardized methodology has been presented in the literature for bone applications. Thus, our additional goal of this review is to highlight the most popular methods of experimental testing to drive the construction of a more standardized methodology in the development of biomaterial substitutes for bone.
2. Chitosan as a Biomaterial
Among the various polymers available for scaffold fabrication, chitosan remains one of the most attractive biomaterials in the field due to various desirable inherent properties such as excellent biocompatibility and biodegradability, antibacterial properties, as well as its mucoadhesive nature and versatility for further chemical and physical modification.46,52,53 Chitosan is derived from chitin through the deacetylation of N-acetylglucosamine residues into N-glucosamines following treatment with an alkaline compound. Similarities in structure between the attained chitosan and glycosaminoglycans found in the extracellular matrix provide chitosan biomaterials with the ability to interact well with cells.54 Numerous studies have demonstrated the material’s potential for bone applications since it can support the proliferation and adhesion of osteoblastic lineage cells as well as mesenchymal stem cells.22,54 Further to this benefit, the wide abundance of chitin in nature designates chitosan as an accessible and cost-effective biomaterial for use in many different applications including both tissue engineering and pharmaceuticals.53
The physicochemical and biological properties of chitosan are largely influenced by its degree of deacetylation, which is defined by the percentage of N-glucosamine residues formed.55,56 Degree of deacetylation (DD) of commercially available chitosan can vary from low (<50%) to full deacetylation (100%), but many studies have shown that a higher DD is favorable in bone tissue regeneration.57 These newly formed amine groups allow for chitosan to become soluble under dilute acidic conditions, which yields the advantage of fabricating minimally invasive, injectable chitosan-based scaffolds. Use of chitosan with a higher DD often correlates with higher solubility and offers more protonated sites that can be exploited to modify the chitosan chains or complex them with other biomaterials, lipids, proteins, and genetic material for a wide range of therapeutic applications.57 These chemical modifications of chitosan solutions such as quaternized, carboxyalkyl, and thiolated chitosan have more recently emerged as additional options for base materials in designed bone regenerative scaffolds since they aid in attaining more optimal material properties.54
3. Cross-linking for Chitosan Scaffolds
Although chitosan possesses many beneficial properties for consideration as a biomaterial substitute, previous literature has shown insufficiencies in certain parameters of the material needed to achieve optimal bone healing.58−60 For instance, chitosan lacks the necessary mechanical strength for bone related applications and exhibits issues with stability and control of biodegradation rates.44,48−50,61,62 To overcome these limitations, the addition of small molecule cross-linkers is explored to improve chitosan’s capability for healing.58 Particularly, in recent years, cross-linking of chitosan has become a popular trend allowing for not only the development of chitosan scaffolds with optimal properties but also the development of composite scaffolds through cross-linking with other materials (Table 1).
Table 1. Common Cross-linkers for Chitosan-Based Scaffolds in the Literature.
| Scaffolds | Method of Cross-linking | Cross-linkers Used | Influence of Cross-linking on Mechanical/Rheological Properties |
|---|---|---|---|
| Chitosan | Chemical cross-link – Covalent bonding | EDC/NHS64,65 | Increased Young’s modulus for chitosan scaffolds with vanillin (and bioglass) compared to chitosan scaffold.79 Increased resistance to compression for glutaraldehyde cross-linked scaffolds compared to uncross-linked scaffolds, concentration dependent increase in strength for the cross-linker.81 No significant difference in Young’s modulus with different genipin concentrations (0.25 and 0.5 M final concentration) under compression.500 Increased stiffness for cross-linked scaffold with genipin compared to chitosan scaffold73 |
| Genipin66−78 | |||
| Vanillin500,79 | |||
| Glutaraldehyde80−83 | |||
| Schiff base reaction84 | |||
| Hexamethylene-1,6-diaminocarboxysulfonate (HDACS)74 | |||
| Citric acid85 | |||
| Physical cross-link – Other bonding | Purines – Guanosine Diphosphate4,51,86−89 | Increased compressive modulus with increasing concentrations of TPP in scaffolds.93 Increased compressive strength with TPP cross-linking compared to uncross-linked scaffolds.94 Increased stiffness for cross-linked scaffold with pectin compared to chitosan scaffold73 | |
| β-Glycerophosphate84,90−92 | |||
| Tripoly phosphate (TPP)93−98 | |||
| Copper99 | |||
| Pectin73 | |||
| Chitosan-composite | Chemical cross-link – Covalent bonding | EDC/NHS100−102 | Increased elastic and loss Modulus with increasing DF-P1000 cross-linker concentration (Schiff Base and EDC dual cross-linking).100 Increased modulus and compressive strength with cross-linking using glutaraldehyde (increasing concentrations and soaking time).108 Increase in compressive strength for cross-linked scaffolds with glutaraldehyde alone or in combination with calcium cations. (110) Increased compression modulus with genipin cross-linking (also with graphene oxide addition).120 Increase in compressive strength with glyoxal cross-linking compared to uncross-linked scaffolds.106 Increased compressive strength with increasing concentration of inorganic phase (GPTMS)131 |
| Schiff base reaction100,103−105 | |||
| Glyoxal101,106,107 | |||
| Glutaraldehyde108−116 | |||
| Genipin112,117−129 | |||
| N′-Methylene bis(acrylamide)130 | |||
| 3-Glycidoxypropyl trimethoxysilane (GPTMS)131 | |||
| 2-Hydroxyethyl methacrylate (HEMA)111 | |||
| Hexamethylene diisocyanate (HDI)132 | |||
| Physical cross-link – Other bonding | Tripoly phosphate (TPP)101,133−135 | Decreasing compressive strength with lemon grass oil addition due to decreased H-bonding potential of HPMC.136 Increase in compressive strength with addition of calcium cations as cross-linker compared to uncross-linked scaffolds.110 Increased elastic modulus (G′) in scaffold with Mg2+ions and BMP due to increased cross-linking density.130 Increased compressive strength for DHT treated scaffolds compared to IR treated, glutaraldehyde, and HEMA cross-linked111 | |
| Hydroxypropyl methyl cellulose (HPMC)136,137 | |||
| Calcium cations110,113,138 | |||
| Mg2+ ions130 | |||
| Dehydrothermal (DHT) Treatment111 | |||
| Irradiation treatment111 | |||
| Cu2+ ions139 |
Various studies in the literature have examined the use of cross-linkers including genipin, glutaraldehyde, and sodium tripolyphosphate as means to ensure stability within the polymer network.48−51,63 The incorporation of these cross-linkers relies on physical or chemical means through bonding to the polymer’s functional groups either by covalent bonding or supramolecular interactions.58,60 With respect to chitosan, this bonding often implements its amine groups, which can form a bond to hydroxyl groups.46
As discussed previously, this cross-linking can be further improved in chitosan through modifications of the polymer backbone by methacrylate or substitution of carboxymethyl derivatives.130,140,141 While physical methods of cross-linking including UV or gamma irradiation treatments tend to allow for a reversible process in certain pH and temperature environments, the use of chemical treatments for cross-linking is more commonly found in regenerative medicine applications.59,62,63,142 Treatment with chemical reagents has been shown to result in a higher degree of cross-linking and formation of a permanent network rather than a reversible one.60,62
In the selection of a cross-linker for chitosan, many factors need to be considered including the intended use of the scaffold and the properties of the cross-linking agent itself. For tissue engineering applications, the cross-linking agent must not induce an immunogenic effect and should not affect cell and material interactions.58−60 Within the chemical domain, glutaraldehyde and genipin are the most used cross-linkers for chitosan-based scaffold applications since their beneficial abilities have been well-documented in the literature.63 However, the toxicity of the functional aldehyde group of glutaraldehyde has resulted in its limited usage in commercial products.58,60 Conversely, genipin has been shown to be less cytotoxic, as it stems from natural roots.58,60,63
3.1. Methodology for Material Property Studies of Chitosan-Based Scaffolds
Addition of a cross-linking agent to scaffolds is expected to have significant effects with regards to material properties including degradation rate, structural architecture, and mechanical properties. This notion thus requires further testing of non-cross-linked and cross-linked materials for comparison to attain a more thorough understanding of its effects (Table 2, Figure 3). Within recent literature, 91% of material property studies are shown to include some form of evaluation of structural architecture, 71% show an examination of mechanical properties, and 54% look at biodegradation mechanisms.
Table 2. Common Methodologies for Material Property Testing in Chitosan Cross-linked Scaffolds for Bone (N = 100).
| Material Properties | Incorporation in Property Studies (%) | Methodology | Method Usage in Specific Property (%) | Information Obtained |
|---|---|---|---|---|
| Mechanical properties | 71% | Compression testing(64−70,72,73,75,76,79,81,91,93−95,97,98,101−103,105−111,114−117,120,129,131−137,140,141,143−160) | 87.3% | Compressive strength, Young’s modulus |
| Dynamic mechanical analyzer83,130,138,161−163 | 8.4% | Creep and recovery properties | ||
| Tensile testing130,164−166 | 5.6% | Tensile strength, Tensile strain | ||
| Nanoindentation testing65,85,119 | 4.2% | Nanomechanical properties | ||
| Atomic force microscopy79 | 1.4% | Young’s modulus of hydrated samples | ||
| Hardness testing97 | 1.4% | Hardness of material | ||
| Flexural strength testing83 | 1.4% | Flexural strength | ||
| Rheological properties | 28% | Frequency sweep(66,69,86,89,92,100,103,104,112,123,130,134,144,147,149,158,167,168) | 64.3% | Storage modulus (G′) and Loss modulus (G′′) with angular frequency |
| Strain sweep92,104,105,118,121,123,125,126,128,168 | 35.7% | Storage modulus (G′) and loss modulus (G′′) with respect to strain | ||
| Time sweep92,100,112,130,158,162 | 21.4% | Determination of gelation | ||
| Structural architecture properties | 91% | Scanning electron microscopy(4,64−68,70−73,75,79−81,83,85,86,89−95,98,99,101,102,104−112,114−121,123,124,126,128−141,145−171) | 96.7% | Morphology and architecture of the scaffold, Pore size measurements with ImageJ |
| Liquid Displacement69,73,79,83,94,97,102,105,107,109−111,114−116,132,133,136,138,141,148−150,152−154 | 28.6% | Porosity and density measurement | ||
| MicroCT imaging68,87,89,95,120,124 | 7.7% | Porosity and interconnectivity | ||
| Mercury porosimeter65,70,75,126,129,158 | 6.6% | Porosity measurement | ||
| Physisorption analyzer117 | 1.1% | Measurement of specific surface area and pore structure | ||
| Atomic force microscopy65 | 1.1% | Surface structure | ||
| Stereomicroscope154 | 1.1% | Surface structure | ||
| Pycnometer93 | 1.1% | Bulk volume of scaffold to find porosity | ||
| Biodegradation properties | 54% | In solution (PBS, SBF, or water) with additives (lysozymes, muramidase, collagenase I, etc.)(64,69,70,72,73,75,85,89,105,111,119,121,124−126,129,130,136,138,140,141,149,155,158,159) | 46.3% | Percentage of degradation, Weight loss over time |
| In PBS(72,79,94,101,103,104,106,108−110,112,114,115,125,130−132,134,135,137,151,152,155,164,169) | 46.3% | Weight remaining ratio, Weight loss percentage | ||
| In simulated body fluid95,116,133,145 | 7.4% | Degradation weight | ||
| In culture media93,150 | 3.7% | Degradation rate | ||
| In water157 | 1.8% | Degradation percentage |
Figure 3.
Material properties to consider following cross-linking of chitosan scaffolds. Each property is presented with common methodology for testing found in the literature.
With respect to mechanical assessment, the most reported technique (87.3%) in the literature is found to be compression testing due to its ability to closely mimic the experience of bone in vivo. This is expected since it is thought to more accurately represent the material’s mechanical behavior when placed in the body.22 However, in addition to compressive modulus and strength, other parameters such as Young’s modulus, toughness, fatigue strength, and shear modulus of materials have also been reported in the literature but to a lesser extent.9,12,30,172 For derivation of these parameters, other methodologies are utilized including a mechanical tester for tensile properties, atomic force microscopy or nanoindentation for nanoindentation and stiffness, and a dynamic mechanical analyzer to measure the response of force versus displacement.
Further mechanical scaffold characterization has focused on examining the material’s viscoelastic properties under deformation using rheology. For chitosan-based scaffolds, literature studies often include either one or a combination of frequency,86,103,123,134,147,149,167 strain,98,100,112,130,151 or time sweep98,104,105,121,126,128,168 tests, which all provide complementary information about the material’s mechanical properties. However, recent literature has shown a popularity in using frequency sweeps especially since they can provide information such as the elastic and loss modulus with respect to angular frequency. These particular sweeps are performed 64.3% of the time in recent studies, whereas strain sweeps and time sweeps are performed 35.7% and 21.4% of the time, respectively.
With respect to assessment of scaffold morphology and architecture changes, scanning electron microscopy (SEM) is considered as the most popular method with a percentage of usage around 96.4% in recent publications of the field. This technique is so popular as it allows researchers to determine the pore size, interconnectivity, and distribution as well as the distribution of any other added materials within the scaffold. Furthermore, researchers can also use this technique to observe and image the surface morphology of the designed material. Other techniques including liquid displacement (28.6%), Micro CT (7.7%), or porosimeter usage (6.6%) have also been explored in the literature to a lesser extent, either alone or in conjunction with SEM, to determine the percentage of porosity and density. Assessment of biodegradation rate in the literature generally encompasses weight measurements at multiple time points after incubation of scaffolds in either PBS alone, with enzyme solutions, or in media. In the scope of chitosan-based scaffolds, the effect of cross-linker incorporation is beneficial to optimize and increase native material properties for bone related application.
3.2. Influence of Chitosan Cross-linking on Mechanical Properties
The protectoral function of bones in the body necessitates the consideration of mechanical properties of the material in the design of biological substitutes, that will provide sufficient mechanical support and integrity throughout the entire repair process.22,23 This explains why the mechanical environment is considered as one of the essential branches of the diamond concept on its own. Insufficiencies in mechanical strength have been demonstrated to be detrimental to the functionality of encapsulated cells rendering healing unsuccessful.22,173 For instance, variations in stiffness result in the differentiation of cells into other lineages, since cells can sense mechanical changes to their microenvironment through mechanosensation and respond accordingly.22,173 Increased mechanical properties and stiffness of chitosan-based materials with cross-linking can favor the differentiation of mesenchymal stem cells into the osteogenic lineage compared to others since osteogenic cells favor stiffer substrates.24
Design criteria often suggest that a material’s mechanical properties should be similar to those of the native bone tissue, which tend to vary based on the type of bone.22,23 The compressive strength and Young’s modulus of healthy human cortical bone has been illustrated in the literature to be between 130 to 200 MPa and 7 to 30 GPa, respectively, whereas trabecular bone values are reported to be between 0.1 to 16 MPa and 0.05 to 0.5 GPa.174 Mechanical properties of a material tend to vary in response to a variety of factors. Changes in structural items such as the incorporation of other polymeric components,64,70,105,117,154,155 cross-linkers,69,75,81,93,94,97,101,106,110,111,117,131,135,152 and additives64,66,69,81,94,103,105,106,115,120,121,131−133,136,138,143,148,151 can have dramatic effects on the mechanical properties of the chitosan-based biomaterial substitute.175 However, comparison between studies is often difficult since chitosan can be used with different modifications, molecular weights, and degree of deacetylations.22
The incorporation of a cross-linker as previously mentioned is used with the intention of improving the mechanical properties of chitosan scaffolds. Improved compressive strengths and young’s modulus values compared to uncross linked scaffolds have regularly been demonstrated in the literature.94,97 A study by Pinto et al. showed that the addition of glutaraldehyde in increasing concentrations to a chitosan scaffold, produced increasing Young’s modulus values from 0.27 MPa with 0% to 1.7 MPa in 10% resulting in a more rigid and stable structure.81 Since many different cross-linking methods exist, some authors have chosen to compare the effects of multiple cross-linkers on the mechanical properties to determine which methodology is most efficient. Karakecili et al. evaluated different cross-linkers including EDAC/NHS, tripolyphosphate, and glyoxal in chitosan and collagen type I composite scaffolds, with results indicating a significant effect of cross-linking method on mechanical strength.101 The glyoxal cross-linking showed the highest percentage of increase for the compression modulus, whereas EDAC/NHS was found to have the lowest values of the three cross-linkers. Interestingly, significant differences between cross-linkers are not always observed within the literature. A study comparing tripolyphosphate and genipin cross-linkers in a chitosan and nano beta-tricalcium phosphate scaffold suggested a bimodal trend in concentrations with no significant differences between the cross-linkers.75 Demonstration of both these cross-linker modality effects indicates the necessity of significant testing for optimal composition when designing scaffolds prior to exploration of in vitro and in vivo osteogenic potential of materials.
In addition to the influence of cross-linking, some studies of composite chitosan cross-linked scaffolds have also examined the effect of different polymeric ratios within the scaffold. Nair et al. tested for compressive strength differences between chitosan and collagen scaffolds at varying ratios (75/25, 50/50, and 25/75) before and after cross-linking with glyoxal (Figure 4A,B). Results demonstrated that as the concentration of chitosan decreased, the compressive strength of the material also decreased (with cross-linking: CH–CO 75/25:19.3 kPa to CH–CO 25/75:9.3 kPa).106 This is promising as it suggests the possible benefit of chitosan for mechanical properties compared to collagen. Another study was also able to support this possible benefit, but this time examining changes in concentration of N,O-carboxymethyl chitosan with aldehyde containing hyaluronate acid in a scaffold cross-linked through Schiff base reactions.105 Increasing the concentration of N,O-carboxymethyl chitosan in the scaffold resulted in a significant increase in compressive modulus from 4.35 kPa with 4% to 20.99 kPa in 8%.
Figure 4.
Influence of scaffold design changes on the mechanical properties. (A) Compressive strength measurements of chitosan and collagen composite scaffolds at different ratios (75:25, 50:50, 25:75) prior to cross-linking. (B) Measurements of the same composite scaffolds after cross-linking with glyoxal. Both A and B were reproduced with permission from ref (106). Copyright 2021 Royal Society of Chemistry and the Centre National de la Recherche Scientifique. (C) Elastic modulus and compressive strength of gelatin chitosan composite scaffolds with increasing concentrations of bioglass (0 to 30%). C was reproduced and adapted under open access conditions (Creative Commons Attribution License) from ref (115). Copyright 2016 Kanchan Maji et al. and Hindawi.
As the addition of compounds and mediators have been widely studied to improve the material and osteogenic properties of scaffolds, the influence of these additions on mechanical properties must also be considered. Some additives are included for the purpose of improving mechanical properties while others are not expected to have a significant effect. One of the main methods for improving the mechanical strength is the incorporation of hydroxyapatite or the induction of scaffold mineralization . A study by Lu et al. examined compressive stress values of mineralized and nonmineralized N,O-carboxymethyl chitosan scaffolds with cross-linking through amidation reactions using EDC/NHS.141 The mineralization of the scaffold presented a marked increase in ultimate compressive stress from 19.5 to 91.6 kPa. This effect was expected, as n-HA is known for its reinforcing ability. Fucoidan was also examined in this study as a potential drug delivery vehicle, but authors suggest that its addition could improve the ability of the scaffold to mineralize as well. Fucoidan incorporated scaffolds showed increased nonmineralized and mineralized values of compressive stress compared to pure N,O-carboxymethyl chitosan scaffolds alone. Another study demonstrated that through the addition of increasing concentrations of bioactive glass (0, 10, 20, 30 wt %) in chitosan and gelatin scaffolds, the compressive strength and elastic modulus were able to increase (Figure 4C).115 However, not all additives have been shown to have a beneficial effect. An example of this is the addition of lemongrass oil, which was found to produce a decrease in compressive strength with increasing concentrations.136 This undesirable effect was explained to be due to a disruption in cross-linking of hydroxypropyl methyl cellulose and chitosan with this addition.
These results therefore suggest extensive testing for additive effects in scaffold design studies. It is important to note that an intimate relationship exists between mechanical properties and multiple other material parameters, including the structural architecture. Incorporation of higher porosity and larger pores, which are often necessary for cellular functionality and vascular network infiltration, often are a result of decreased cross-linking and thus the materials present decreased mechanical strength.173 Therefore, a balance must be struck between the architectural design and the corresponding scaffold mechanical properties.
3.3. Influence of Chitosan Cross-linking on Rheological Properties
Strain sweep tests are often conducted as a preliminary step to frequency sweeps to determine in what amplitude range the material exhibits viscoelastic properties by applying a strain signal with increasing amplitudes. The frequency sweep test is often performed over a range of frequencies (0.01 to 100 Hz) with a constant strain to attain important parameter values including the shear elastic modulus (G′), loss/viscous modulus (G′′) and loss tangent (tan(δ)) values. Together, these values give further insight to the stability of cross-linked scaffolds. A study by Dessi et al. used frequency sweep testing to demonstrate the deformation behavior of Beta tricalcium phosphate (1 and 1.5%) and chitosan cross-linked composite scaffolds.98 Since the G′ elastic modulus was higher than the loss modulus G′′ throughout the frequency range, authors were able to conclude that the behavior was primarily elastic indicating a stable structure. Interestingly, no significant difference was observed due to concentration changes, but this conclusion should be further examined through comparison to a scaffold without beta tricalcium phosphate (β-TCP). While the loss tangent values were below one for this study, confirming the materials viscoelasticity, the elastic modulus values (at 1 Hz: G′ of 55 Pa for β-TCP 1% and 75 Pa for β-TCP 1.5%) seem relatively low compared to the use of other cross-linkers and additives reported in the literature. Another study examining scaffolds of chitosan-cysteine conjugates with difunctionalized PEG cross-linkers demonstrated a range of elastic modulus values between 962.5 to 1561.8 Pa with increasing weight to volume percentages of chitosan-cysteine solutions from 1.5 to 2.5% (Figure 5A).100 Results presented in this study suggest a beneficial effect of increasing concentrations of chitosan-cysteine solutions as well as an increased concentration of difunctionalized PEG cross-linkers (0.3 to 0.6 w/v %). The large difference (one to 2 orders of magnitude) between G′ and G′′ was observed as well, indicating again the stability of the scaffold. The null effect of pyrophosphatase addition to a chitosan-based guanosine diphosphate cross-linked scaffold on its rheological properties has recently been demonstrated using this methodology.86
Figure 5.
Rheological property evaluation. (A) Effect of increasing chitosan-cysteine (CH–CY) solutions (1.5 to 2.5%) and difunctionalized PEG cross-linkers (0.3 to 0.6 w/v %) on G′ and G′′ values. Reproduced and adapted under open access conditions (Creative Commons Attribution License) from ref (100). Copyright 2022 Qing Min et al. and MDPI. (B) Percentages of published articles for inclusion of mechanical properties, rheological properties, both, and none.
Time sweeps can also be beneficial in providing knowledge about the elastic modulus as a function of time. This particular technique has been well implemented in the literature as means to determine gelation time measurements. For example, An et al. were able to tune the gelation time of a methyl acrylate chitosan and aldehyde containing oxidized hyaluronate acid using this technique.104 The gelation time was determined as the time point where G′ and G′′ crossed over, as an indication of the transition from a liquid to a solid (more elastic) scaffold. Other authors have used this method to establish the elastic modulus changes throughout different time periods after cross-linking.128 From reviewing the literature for chitosan-based cross-linked scaffolds in the last ten years, it is evident that rheological properties of materials have been less explored compared to mechanical and compressive studies (Figure 5B). The viscoelasticity of a material is however an important consideration as it also affects the osteogenic differentiation of cells and their spreading behavior.24 Rapid relaxation of the scaffold following an applied stress encourages differentiation of the mesenchymal stem cells.24 Therefore, although it is more common to find studies that examine both recently, this combination of studies should be a set standard in implementation of Diamond Concept in bone repair, as it allows for a more concrete understanding of the material and possible interactions with body environment and cells.
3.4. Influence of Chitosan Cross-linking on Structural Architecture
The microarchitecture of scaffolds plays a vital role in supporting various functions. Careful consideration is needed when developing scaffolds to achieve the optimal structural architecture, depending on the intended therapeutic application. Bone is a highly porous structure; as such, porosity is an important parameter of a scaffold to better mimic the native tissue.176 An interconnected porous microstructure would favor higher cell infiltration and activity, vascularization, proper exchange of nutrients, and diffusion of therapeutic agents.17,31
In general, chitosan-based scaffolds demonstrate excellent porosity, with a commonly reported range of 80–90%.69,110 Moreover, pore diameter sizes tend to often be >100 μm, which is reported to be optimal for efficacious tissue infiltration and ingrowth.116 However, cross-linking in efforts to enhance the mechanical properties might be detrimental to the desired scaffold architecture, and thus, it is important to determine a right balance between polymer and cross-linker concentrations.
Genipin is among the most commonly used cross-linkers when fabricating chitosan-based scaffolds. While increasing the concentration of this cross-linker can slow down the degradation rate of the scaffold as a consequence of higher cross-linking, Dimida et al. observed no significant change neither in the structural architecture of the formed scaffolds nor in their mechanical properties (Figure 6A).72 Similarly, increasing concentrations of glutaraldehyde, another popular chitosan cross-linker, do not show a great effect on the general microarchitecture but did aid in enhancing the mechanical behavior of the scaffold. On the other hand, the compressive modulus of sodium tripolyphosphate cross-linked chitosan scaffold showed significant increase with higher cross-linking concentrations, but the porosity decreased to as low as 17.2%.93 Such results might suggest glutaraldehyde as an optimal cross-linker to combine improved mechanical strengths with maintained porous microstructure. However, increasing the concentrations of glutaraldehyde can risk an increase in cytotoxic effects. Therefore, more focus should be placed on finding a suitable cross-linker that supports all necessary properties relevant to the Diamond Concept.
Figure 6.
Characterization of structural architecture for various modifications of chitosan scaffold. (A) SEM images of chitosan scaffolds with increasing concentrations of genipin (left −3.75%, right −7.5%). Reproduced and adapted under open access conditions (Creative Commons Attribution License) from ref (72). Copyright 2017 Simona Dimida et al. and Hindawi. (B) Total porosity measurements (T.Po), structure thickness (St.Th), and specific surface (Sp.S) for chitosan scaffolds with increasing graphene oxide (GO) percentages (0, 0.5, 3%). Reproduced and adapted under open access conditions (Creative Commons Attribution License) from ref (177). Copyright 2019 Sorina Dinescu et al. and MDPI. (C) Percentage of porosity for sodium alginate and chitosan composite scaffolds with and without the addition of collagen and graphene oxide using liquid displacement method. Reproduced and adapted with permission from ref (138). Copyright 2018 American Chemical Society.
To complement cross-linking with the enhancement of mechanical and osteogenic properties of a scaffold, the addition of apatites and other ceramics is commonly employed. Hydroxyapatite (HA) is one such frequent additive to chitosan-based cross-linked scaffolds.71,88,95,166 As a major constituent of natural bone, HA not only increases the mechanical strength of scaffolds but also their biocompatibility and bioactivity.101
This, however, can often be accompanied by changes to the overall porosity or pore size.65,152 Compared to the control scaffold with only tripolyphosphate (TPP) cross-linking, Shemshad et al. observed a decrease in pore size following incorporation of nanohydroxyapatite along with bioactive silicate diopside. Nonetheless, the size remained within the range of 40 to 250 μm often recommended for bone tissue engineering applications. The incorporation of these factors did also yield a rougher pore morphology, which can be advantageous for better cell and tissue adhesion.94 β-TCP is another ceramic that can be added to confer to scaffolds similar properties as HA does. However, Jahan et al. observed that β-TCP had a larger particle size compared to HA, resulting in more occluded scaffold pores, thereby decreasing their size.51
To enhance the overall morphology of chitosan-based scaffolds, the addition of graphene oxide (GO) as an additive was attempted. This carbon-based material can aid in cross-linking different polymer chains together or polymers and other biomolecules, as well as increasing the mechanical properties of a scaffold and its biocompatibility, osteogenic properties, and in some instances, the total porosity of chitosan scaffolds.138 Dinescu et al. reported enhanced porosity with the addition of 3% wt. GO in a freeze-dried chitosan scaffold. They also observed that the addition of GO favored the formation of larger pores (Figure 6B).177 In other instances where chemical cross-linking was completed, the addition of GO slightly decreased the pore size of an alginate–chitosan–collagen composite scaffold chemically cross-linked with Ca2+ (Figure 6C).138 Nonetheless, addition of GO commonly yields a more homogeneous architecture with more defined pores, thick pore walls, and rougher morphology all which beneficially influence cell attachment and osteogenic differentiation and improve the overall efficacy of the scaffold for bone tissue engineering.80,120,178
3.5. Influence of Chitosan Cross-linking on Degradation Rate
Since one of the expectations of bone healing in defects is the regeneration of bone tissue over the course of time, the biodegradability of the material is an important consideration of scaffold design. When the scaffold is placed in vivo, it is expected that its degradation rate will match that of the native bone tissue ingrowth thus allowing the gradual transfer of mechanical load.12,25−30 The scaffold’s role is to act as a temporary bridge between both ends of the defect helping cells, as well as acting as a platform to create an optimal microenvironment.25 Degradation rates that are too fast or too slow result in inadequate healing and lead to insufficiencies in mechanical support throughout the healing process.27
Due to the necessity for control, assessment of this property has become a standard practice in material design and characterization, including the design of chitosan-based scaffolds.28 Within the body, degradation of polymers typically occurs through a chain scission process of the polymer induced by enzymes such as lysozymes, which results in the hydrolysis of chitosan and the cleavage of its β-(1–4)-glycosidic bonds.26,51 A study by Kim et al. demonstrated this concept through the incorporation of varying concentrations of lysozymes in a methacrylated glycol chitosan hydrogel.140 Increases in lysozyme concentration from 0.1 to 10 mg/mL resulted in accelerated degradation rates of the hydrogel with a final degradation of around 30% left after 14 days when a lysozyme concentration of 10 mg/mL was used.
Work within the literature on chitosan cross-linked scaffolds has often embraced the lysozyme technique to study in vitro degradation behavior.69,70,72,73,129,138,140 Formation of a composite chitosan and polycaprolactone (PCL) scaffold with genipin cross-linking showed decreased degradation rates compared to chitosan alone cross-linked control groups (Figure 7A).119 The dependence of the chitosan amount on the degradation rate was not surprising since the lysozyme is expected to target the chitosan specifically. Another study by Rahman et al. further demonstrated chemical cross-linking’s influence on biodegradation rates and stability using a chitosan/hydroxyapatite/collagen composite scaffold.111 This scaffold was cross-linked with either glutaraldehyde (GTA), dehydrothermal treatment (DTH), irradiation (IR) or 2-hydroxyethyl methacrylate (HEMA), resulting in Ha·Col1·Cs-GTA, Ha·Col1·Cs-IR, Ha·Col1·Cs-DTH, and Ha·Col1·Cs-HEMA composites. Results indicated that the glutaraldehyde cross-linked scaffold exhibited a 16% degradation on day 21, whereas the scaffold without the cross-linker was 55% degraded at this same time point (Figure 7B). Interestingly, this improvement in degradation rate using cross-linkers was not shown when adding a fibrin coating to a chitosan scaffold.70
Figure 7.
Degradation profiles of composite chitosan scaffolds in lysozyme. (A) Chitosan (CS) and polycaprolactone (PCL) scaffolds with different concentrations of polymers (100:0, 20:80, 40:60, 0:100) cross-linked with genipin. Reproduced and adapted with permission from ref (119). Copyright 2022 Elsevier. (B) Chitosan/hydroxyapatite/collagen composite (Ha-Col1-CS) scaffolds before and after cross-linking with different methodologies: (1) DHT – dehydro-thermal treatment, (2) IR – irradiation, (3) GTA – glutaraldehyde, and (4) HEMA – 2-hydroxyethyl methacrylate. Reproduced and adapted under open access conditions (Creative Commons Attribution License) from ref (111). Copyright 2019 Md. Shaifur Rahman et al. and Springer.
Since composite scaffolds are often formed with chitosan and other polymers such as collagen, the biodegradation of scaffolds in collagenase solution is also sometimes assessed in the literature.107,121,125,126 Grabska-Zielinska et al. examined the degradation profile of a chitosan and fish gelatin scaffold with genipin cross-linking and the benefits of graphene oxide incorporation. Interestingly, when cultured in collagenase 2 solution, the scaffold without graphene oxide mostly degraded (72%) within the first 24 h. However, the addition of graphene oxide in doses above 1 wt % was able to withstand some of this degradation showing percentage values around 44% to 52% depending on concentration.107 Similarly, the addition of amino group functionalized silica particles with alendronate served to slow down the degradation process of chitosan scaffolds with collagen and hyaluronic acid.121 However, the beneficial effects of biodegradation mediators should be further explored for better understanding of mediator-mediated biodegradation.
In addition to the importance of the biodegradation profile itself, the byproducts of this degradation must also be considered in aspects of design. These should be nontoxic and nonteratogenic and do not elicit any undesirable immunogenic response when degradation occurs.12,20,25,27,29,179 Often, byproducts can be beneficial in the development of an adequate microenvironment for healing and can improve bioactivity within the defect site.25,51
4. Cell Encapsulation in Chitosan Cross-linked Scaffolds
In addition to modifications of the scaffold material itself, the Diamond Concept also stresses the necessity of an encapsulated viable cell population. Incorporation of cells is important in successful bone regenerative therapies since bone cells such as osteoblasts, osteoclasts, and osteocytes are responsible for the production, remodeling, and maintenance of bone.180 Previous studies with empty scaffolds have shown almost no cellular ingrowth from the native tissue, which negatively affects the regenerative ability.181 With relevant cell encapsulation, both osteogenic differentiation of the cells and angiogenesis are encouraged when present at the target site, thereby resulting in the secretion of varying growth factors, matrix proteins and cytokines such as vascular endothelial growth factor and interleukin 6.32,33,179,182 Culmination of these secretion activities with successful cellular differentiation can improve the healing outcomes.
4.1. Methodology for Cellular In Vitro Studies in Chitosan Scaffolds
The first step in designing an encapsulated cell-based substitute is to examine the material’s ability to support cellular activity. While cross-linking of chitosan-based scaffolds can result in improved mechanical properties which is beneficial in supporting loading, it can be accompanied by a decrease in porosity and pore size. Since the functionality of cells once encapsulated is known to be heavily dependent on their access to nutrients and oxygen, such significant changes to the internal structure with cross-linking can lead to drastic changes in cellular viability and activity.
Another important consideration is the effect of the method of cross-linking itself on cellular viability and functionality. Some cross-linking techniques, like physical cross-linking, can have low cytotoxicity to cells however, they might require harsh conditions such as changes in temperature or pH and do not always provide the optimal material properties necessary for bone applications.58,183 On the other hand, chemical cross-linking might be more beneficial with respect to material properties due to stronger bonding but can have higher costs and higher risk of cytotoxicity, especially with cross-linkers such as glutaraldehyde.58 Genipin cross-linking, which is commonly used as a cross-linker for chitosan-based materials, is known to be biocompatible and less cytotoxic than other chemical cross-linkers.58
With all the considerations of cross-linker effects on cellular activity, this addition necessitates a thorough evaluation of cellular functionality in chitosan-based scaffolds. For bone applications, the in vitro assessment of cellular activity is thought to be further divided into three subgroups, namely biocompatibility, osteogenic differentiation, and mineralization (Figure 8).
Figure 8.
Common methodologies for in vitro studies using cell cultures in the literature. These steps include testing for biocompatibility, osteogenic differentiation, and biomineralization.
The initial aspect of this often encompasses a quantification of cellular proliferation and metabolic activity using commercial assays such as MTT/XTT/MTS (41.9%), alamarBlue (21.5%), or Cell Counting Kits (18.3%). While these listed tend to be the most popular in terms of quantitative assays, many other techniques encompassing a visual assessment component have also been demonstrated in the literature. For example, around 33.3% of recent articles have examined cellular distribution and morphology through staining using antibodies for phalloidin and nucleic acid. Another common staining found in the literature is Live/Dead or calcein based assays, which stain live and dead cells different colors making it easy to quantify cytotoxicity and overall percentage of viability.
Furthermore, in many studies SEM is used to visually determine cell adhesion properties on the surface of the scaffold. All these methods for biocompatibility provide researchers with a more thorough understanding of whether their material can support generic cellular activities without significant adverse effects. However, not all these methods will provide the same information and therefore it is crucial to understand the differences between testing methods. Table 3 is presented to help differentiate these differences and serve as a resource for experiment planning in future studies.
Table 3. Common Methodologies for In Vitro Testing in Chitosan Cross-linked Scaffolds for Bone (N = 94).
| In Vitro Experiments | Incorporation in In Vitro Studies (%) | Information Obtained | Methodology | Usage in Specific In Vitro (%) |
|---|---|---|---|---|
| Cellular Biocompatibility | 98.9% | Cellular Metabolic Activity, Growth and Proliferation | MTT/XTT/MTS Assay(69,70,72,75,79,90,93−95,98,105,106,108,110,115,116,123,124,128−132,135,137,138,141,148−150,152−155,157−159,170,171) | 41.9% |
| IF Staining – DAPI with or without Phalloidin65,66,70,74−77,79,81,85−89,98,104,106,112,119,123,129,130,137,139,144,151,152,161,166,168,169 | 33.3% | |||
| Alamar Blue Assay72,76,81,86,87,89,92,107,118,121,125−127,134,136,140,143,144,152,164,170 | 21.5% | |||
| Cellular Counting Kits64,65,71,74,100,103,104,114,117,119,133,139,147,151,163,166,169 | 18.3% | |||
| PrestoBlue Kit101,109,112 | 3.2% | |||
| EdU and BrdU Staining161,184 | 2.2% | |||
| Resazurin Assay68,102 | 2.2% | |||
| IF Staining – Ki6765,119 | 2.2% | |||
| Cellular Cytotoxicity and Counting of Viable Cell Numbers | Live Dead Assay/Calcein AM Staining(64−66,75,87,89,95,100,104,105,117,123,124,137,138,140,147,150,159,160,163,167−169) | 25.8% | ||
| Lactic Dehydrogenase Assay124,156,169 | 3.2% | |||
| Trypan Blue Staining93,165 | 2.2% | |||
| FDA Staining71,93 | 2.2% | |||
| Acridine orange/Ethidium bromide staining151 | 1.1% | |||
| Flow Cytometry – Annexin V,64 Propidium Iodide64 | 1.1% | |||
| DNA Content | PicoGreen total DNA Assay(4,70,73,75,101,123,162,170) | 8.6% | ||
| Cellular Biocompatibility, Morphology and Adhesion | Scanning Electron Microscopy(64−66,70,73−75,77,81,85,87,88,94,98,101,103,108,115,117,121,125−127,131,132,137,139,150,152,157,166,169,170) | 35.5% | ||
| Light Microscopy90,157,165,171 | 5.4% | |||
| HE Staining73 | 1.1% | |||
| Osteogenic Differentiation | 63.8% | Osteoblast Differentiation Specific Protein Secretion | Alkaline Phosphatase Testing(4,64−66,69−71,73,75−77,79,81,85,86,88,89,93,94,98,100,101,108,109,114,118,119,121,123,125−127,130,131,133,135,137,139−141,144,151,155−157,159,161,163,169,170) | 84.7% |
| ELISA - Osteocalcin,71,85,163 Type 1 Collagen,100 Osteopontin163 | 5.1% | |||
| Sirius Red Dye Assay112/SirCol Collagen Assay73 | 3.4% | |||
| Osteoblast Differentiation Related Activity Stainings | IHC, ICC, IF Staining for different markers – RUNX2,64,115,120OPN,120BSP,64OCN,86,115,184OSX88 | 11.9% | ||
| Gene Expression of Osteoblast Related Genes | qPCR – ALP,64,65,70,74,81,89,112,129,130,144,147,166,169BSP,64,65,70,79,129COL1A1,64,70,81,101,119,123,127,130,139,140,144,151,157,169OCN,64,65,77,79,81,101,108,119,123,127,130,139,140,144,157RUNX2,64,77,79,81,89,101,108,112,120,127,139,140,144,147,151,157,162,169OPN,65,77,81,101,119,120,123,129,130BMPs,81,151ON,70OSX65 | 44.1% | ||
| Protein Expression of Osteoblast Related Genes | Western Blot – Col1A1,64,119,130RUNX2,64,130ALP,130OPN,64,65,119,130BSP,64,65OSX,64OCN,64,65,70,119ECM Proteins,119B-Catenin,130Smad1,130p-smad1/5/8,130cell growth factors119 | 6.8% | ||
| Mineralization | 36.2% | Quantification of Mineral and Calcium Deposition | Alizarin Red Staining(4,64−66,69,70,86,98,101,104,106,111,114,119,120,123,130,138,140,141,143,147,151,155,156,159,161) | 45.8% |
| Von Kossa Staining73,88,106,144,184 | 8.5% | |||
| Total Calcium Kit70,75,79,156 | 6.8% | |||
| O-Cresol phthalein complexone method73,109 | 3.4% |
In addition to simply testing biocompatibility, the ability of a material to support osteogenic differentiation is another important consideration to address since the target application in many of these studies is bone regeneration. Around 63.8% of relevant literature have included this aspect into their In vitro studies, with the most common technique for studying this being the detection of an early osteogenic marker, alkaline phosphatase (84.7%). Bone ALP is known to regulate the mineralization process since it provides a phosphate reservoir to the matrix through hydrolysis of the mineralization inhibitor, pyrophosphate. Thus, it is arguably a necessary methodology with regards to claiming successful differentiation potential. This assay is sometimes complemented with other techniques such as qPCR, which serves to assess gene expression of other early and late-stage markers in a comprehensive manner.
With respect to mineralization of the material, this is often the least considered and evaluated aspect of in vitro studies, present in only around 36.2% of publications. The most common techniques for this involve mineral staining using either Alizarin Red or Von Kossa but a few studies have also shown promising results using quantitative calcium assays.
4.2. Cell Sources for Bone Tissue Engineering in Chitosan Scaffolds
A variety of factors need to be considered in the decision of cell source including aspects such as the ease of availability for harvesting, low morbidity of donor site, high efficiency of isolation, adequate cell proliferative ability, and sufficient osteogenic differentiation potential. While many different options for cell sources are currently being explored in chitosan-based cross-linked scaffolds for bone tissue engineering, the focus remains on finding a reliable cell source that will meet many of the criteria listed above. Currently, these consist of either osteogenic cells that are already lineage committed or stem cells, which allow for control of differentiation (Table 4).
Table 4. Common Cell Lineages and Lines Presented in the Literature for Chitosan-Based Scaffolds.
| Category | Cells in Literature | Scaffold |
|---|---|---|
| Lineage committed | MC3T3 preosteoblast cells (murine) | Chitosan4,79,87,88,137 |
| Chitosan/gelatin101,109,112,120,124 | ||
| Chitosan/collagen135 | ||
| Chitosan/collagen/alginate138 | ||
| Chitosan-cysteine conjugate100 | ||
| Methacrylic anhydride modified chitosan169 | ||
| Methacrylic anhydride modified chitosan/poly(acrylamide)130 | ||
| Carboxymethyl chitosan/alginate139 | ||
| Chitosan/hyaluronic acid129 | ||
| 7F2 osteoblast cells (murine) | Chitosan73 | |
| Carboxymethyl chitosan/fucoidan141 | ||
| MG-63 osteoblast like cells from osteosarcoma (murine) | Chitosan81,93,94,98 | |
| Chitosan/collagen/hyaluronic acid118,121,125,126 | ||
| Chitosan/gelatin143 | ||
| Chitosan/polyvinyl alcohol131 | ||
| Chitosan/polycaprolactone170 | ||
| Stem cells | Mesenchymal stem cells (MSCs) – attained from multiple sources | Chitosan66,75−77,85,86,156 |
| Chitosan/gelatin115,133,147 | ||
| Chitosan/agarose/gelatin114 | ||
| Chitosan/collagen111,127 | ||
| Thiolated chitosan/gelatin151 | ||
| Carboxymethyl chitosan64,65,71 | ||
| Chitosan/gelatin108 | ||
| Methylacrylylated chitosan/hyaluronic acid104 | ||
| Chitosan/polycaprolactone119 | ||
| Fatty acid modified chitosan/decellularized bone ECM123 | ||
| Methacrylated glycol chitosan140 | ||
| Chitosan/fibrin70 | ||
| Others | C2C12 myoblasts (murine) | Chitosan/alginate161 |
| Chitosan/lactide155 |
4.2.1. Osteogenic Lineage Committed Cells in Chitosan Scaffolds
The first category often discussed when addressing this challenge is osteogenic cells such as osteo-progenitor cells, osteoblasts, and osteocytes.30,33 Although these cells are still derived from mesenchymal cells, their previous commitment to the osteogenic lineage allows them to begin secreting bone matrix components almost immediately upon implantation.33,34 Thus, osteogenic markers such as type 1 collagen and alkaline phosphatase are expected to be present sooner into the regenerative process, therefore improving the rate of bone healing.33 In recent years, the MC3T3 preosteoblastic cell line has become a standard model regarding lineage committed cells for applications in bone tissue engineering.185,186 In many studies with chitosan-based cross-linked scaffolds, these cells have thus been included for evaluation of osteogenic differentiation and mineralization potential in the materials. For example, in a genipin cross-linked chitosan-gelatin scaffold with GO incorporated, Selaru et al. were able to use MC3T3 cells to demonstrate enhanced osteogenic differentiation and mineral deposition compared to scaffolds without GO.120 Similarly, Karakeçili et al. observed enhanced MC3T3 mineralization aided by the addition of n-HA.101 While these committed lineage cells provide many benefits in the healing cascade, they are often time-consuming with regard to the culturing process and their potential for expansion is limited in vitro.30 Furthermore, collection and harvesting processes for primary cells are invasive by nature, therefore encouraging scientists to commonly look at alternatives for cell types such as the incorporation of stem cells.33
Another common category of cells for various in vitro osteogenic models includes osteosarcomas such as MG-63 and Saos-2. These cell lines are derived from bone tumors and can display similar properties as osteogenic cells like alkaline phosphatase (ALP) production and mineral deposition.187 Osteosarcomas have been widely used to investigate the osteogenic suitability of a various range of chitosan-based cross-linked scaffolds.92,95,128,132,152,154,164 In the study by Shemshad et al., osteogenic differentiation of MG-63 cells seeded on a TPP cross-linked chitosan scaffold with addition of hydroxyapatite and bioactive silicate diopside is reported.94 By adding the osteogenic factor strontium to their scaffold, Rodríguez-Méndez et al. observed increased ALP activity of MG-63 cells, whereas Pati et al. demonstrated the benefits of cross-linking with an increase in mineralization (Figure 9).98,170 However, while osteosarcomas can be useful in providing valuable information regarding biocompatibility and osteogenic potential in vitro, their clinical translatability is hindered by their tumoral nature. Therefore, it can be argued that these cells are not ideal within the scope of the Diamond Concept and an alternative source should be considered or tested simultaneously with these cells.
Figure 9.
Assessment of (A) osteoblastic differentiation (ALP activity) and (B) mineralization potential (Alizarin Red Staining) from MG-63 cells over 21 days for chitosan scaffolds with and without tripolyphosphate cross-linker. Reproduced and adapted with permission from ref (98). Copyright 2012 Wiley Periodicals, Inc.
4.2.2. Stem Cells in Chitosan-Based Scaffolds
Stem cells present many advantages for use, since they have self-renewal capabilities and can easily be induced to differentiate into various lineages.179,188,189 Although this broad categorization encompasses several different types, adult mesenchymal stem cells (MSCs) are most extensively used in the literature for bone regeneration application as they are not associated with ethical or safety constraints.33,35 Adult MSCs can be isolated from tissues such as bone marrow and adipose, which are vascularized.30,179,182 They possess beneficial properties including multilineage potential for differentiation (adipogenic, chondrogenic, and osteogenic), high proliferative abilities, and immunomodulatory capabilities.179,180,182 Bone marrow derived stem cells are the frequent cell choice for bone tissue engineering applications and treatment of bone disease since they have demonstrated the highest potential for osteogenic differentiation and bone repair.41,181,182 These cells are harvested during bone marrow biopsies, often from the iliac crest, sternum, or proximal tibia, which all are rich in marrow and are induced for differentiation through exposure of cells to a high phosphate environment.181,182,190
Although bone marrow derived stem cells are commonly employed and have been extensively characterized, they only compose around 0.001 to 0.1% of cells present in the marrow, which make their isolation in large quantities very difficult.181 The cell number for isolation is dependent on various parameters including the donor’s age and associated donor site morbidity and their limited quantity is a significant disadvantage to their use.188
An alternative cell source for osteogenic applications includes adipose derived stem cells (ADSCs). These cells are attained from adipose tissue through minimally invasive surgical procedures such as liposuction.182,188,190 They can be retrieved in high abundance from harvesting, and their differentiation from fibroblast-like morphology makes them very interesting for rapid formation of connective tissue.42,181,182,188,191,192 However, their osteogenic ability has been questioned since some studies have shown lower capability for differentiation compared to bone marrow derived stem cells.33,188 Others have demonstrated that their differentiation potential is adequate and can be enhanced in this cell type through culture with osteogenic medium.181
5. Osteoinductive Mediators’ Encapsulation in Cross-linked Chitosan Scaffolds
The third major aspect in the design of biological substitutes for bone tissue regeneration involves the incorporation of bioactive molecules, drugs, and growth factors with osteoinductive properties. These components are responsible for influencing cellular functionality including the attraction of cells to the injured site, and stimulation of cells to undergo osteogenic differentiation in turn resulting in bone regeneration.193−196 While in cell cultures growth factors can be added to media, it is often more effective to encapsulate them in the scaffold or conjugate them to the polymer backbone through chemical modification.194,197 Within the scaffold, factors can be locally delivered to the injury site in a controlled release manner, allowing for the creation of a similar microenvironment to the natural healing cascade.193,198,199
Many studies have been conducted within the literature examining the application of different osteoinductive mediators with respect to cross-linked chitosan scaffolds including growth factors, mediators, enzymes, drugs, compounds, and nanoparticles (Figure 10).
Figure 10.
Common osteoinductive mediators in the literature for chitosan-based cross-linked scaffolds.
5.1. Encapsulation of Growth Factors in Cross-linked Chitosan Scaffolds
Traditional growth factors, such as BMPs, have great potential in bone regeneration applications since they are known to have a physiological role in the fracture healing cascade.199 BMPs residing within the transforming growth factor-beta (TGF-β) family are one of the most popular osteogenic growth factors currently. These factors encourage bone formation through downstream events that occur due to receptor binding.193−195,199 BMPs 2 and 7 specifically are known to be responsible for bone regeneration and have been demonstrated in the literature for controlled delivery both alone and in conjunction with other mediators from chitosan-based scaffolds.64,77,114,129,130,161,169,193−195 A study by Nath et al. demonstrated the osteogenic effects of BMP2 incorporation into a chitosan and hyaluronic acid scaffold cross-linked with 3 mg of genipin. Results showed that MC3T3 preosteoblast cells in the scaffolds coencapsulated with BMP2 exhibited higher expression levels of early differentiation markers ALP and osteopontin (OPN) at day 7 compared to control groups, as well as significantly higher late-stage expression of OPN at day 28. While this study showed the benefits of coencapsulation, the osteogenic effect of sustained release of BMP2 was not considered for cells outside the scaffold.129 This missing aspect was however explored in the scope of a chitosan-agarose-gelatin scaffold with chitosan oligosaccharide/heparin nanoparticles. Through indirect culture of MSCs, Wang et al. demonstrated that the loading of BMP2 within the nanoparticles resulted in a sustained release over time leading to increased ALP activity and osteogenic induction throughout 21 days. These BMP2 loaded nanoparticles in the chitosan-agarose-gelatin scaffold showed significantly higher ALP levels compared to the nanoparticles or scaffold alone and demonstrated similar levels to the group with BMP2 alone at early time points. At later stages, this BMP2 loaded test group also exhibited more positive alizarin red calcium staining compared to the BMP2 alone control group.114 Interestingly, while BMPs are present in a variety of studies in the literature for chitosan-based scaffolds due to their demonstrated beneficial effects, other alternatives are considered due to their high cost and possible side effects.
5.2. Encapsulation of Bioactive Compounds in Cross-linked Chitosan Scaffolds
In addition to traditional growth factors, studies within the bone tissue engineering domain heavily rely on the incorporation of osteoinductive mediators such as bioceramics, namely HA51,65,69,71,76,81,83,88,93,94,101,103,111,136,141,145,146,152,164−166 and β-TCP.70,75,92,145 Both ceramics contain calcium and phosphate, which make up the main components of mineralized bone.9,200−203 Many studies have included HA within their designed chitosan-based scaffolds due to its biocompatibility with cells and its known bioactivity both in vitro and in vivo.204 Recent work has explored the combination of HA with other compounds for added promotion of osteogenic activities. Lu et al. incorporated n-HA with fucoidan into a hydroxypropyl chitosan scaffold cross-linked with genipin.69 While the incorporation of n-HA alone in these scaffolds resulted in a marked concentration-dependent increase in ALP activity for 7F2 osteoblasts compared to controls, the combination of n-HA with fucoidan produced an even higher ALP activity. The influence of HA incorporation either alone or in combination with fucoidan also showed increased calcium deposition in alizarin red staining, signifying its benefits in mineralization induction. Combination of HA and β-TCP in varying ratios has also been tested in chitosan-based scaffolds68 ,81 ,95 ,133 since this biphasic calcium phosphate composite is expected to have increased osteoconductivity to HA alone.
More recently, studies have focused on this criterial element by incorporating enzymes, natural compounds, and drugs in their chitosan-based scaffolds. A few studies used pyrophosphatase in combination with the purine cross-linker guanosine diphosphate to promote mineralization.4,86,88 Nayef et al. have shown that due to the enzymatic cleavage of the purine during the degradation process, a large quantity of pyrophosphate is found in the culture media.4 Incorporation of pyrophosphatase served to cleave observed pyrophosphates into phosphate ions allowing for an increased ratio of phosphate to pyrophosphate, thus producing mineralization at levels similar to those treated with direct BMP7 injection in MC3T3 cell cultures.4 Additional studies have expanded the pyrophosphatase encapsulation in conjunction with adipose derived stem cells86 in the chitosan scaffold and reported on its beneficial effect in vivo using mouse tibial defect models.88
The loading of bisphosphonate drugs such as Alendronate have also been explored for promoting osteogenic activity in chitosan cross-linked scaffolds.119,121 Alendronate has been shown to increase BMP2 expression, thereby stimulating osteogenic differentiation and mineralization. A recent study by Shi et al. examined these possible beneficial effects in a chitosan and polycaprolactone scaffold cross-linked with genipin.119 Through seeding of ectomesenchyme cells, they demonstrated increased osteogenic induction (ALP activity) and calcium deposition with Alendronate compared to the control scaffolds. Additionally, results showed an increased level of BMP2 proteins within this test group, which could suggest Alendronate as a possible lower-cost alternative to BMP2 growth factor. Since bisphosphonate drugs are often more commonly associated with their roles in osteoclast activity inhibition, their usage proves beneficial in diseases such as osteoporosis or Paget’s disease where additional bone turnover is observed.
Natural medicinal compounds derived from herbs and minerals have also been considered as an alternate option for promotion of osteogenic differentiation to mitigate high costs and long-lasting side effects.83,100,148 Two flavonoid compounds, Quercetin and Icariin, have been explored recently with encapsulation into chitosan-based cross-linked scaffolds as possible agents for promotion of bone regeneration and formation. Both compounds exhibit similar effects in bone metabolism to estrogen, thus inhibiting osteoclast differentiation and promoting osteoblast formation. A study by Min et al. showed a synergistic osteogenic effect of amino-functionalized mesoporous bioglass nanoparticles loaded with Quercetin in a chitosan cysteine conjugate composite (CH–CY) with difunctionalized PEG cross-linkers.100 Hydrogels with loaded nanoparticles exhibited increased ALP activity and Collagen I content at 14 days compared to CH–CY gels alone. The authors suggested that the synergistic effect was a result of both Si and Ca ions released due to nanoparticle degradation as well as quercetin release. While these encouraging results of natural compounds are outlined clearly in the literature, their tested incorporation in the scaffolds serves to design a drug delivery system whereby drug release can be carefully controlled. Often, loading of these compounds relies on the usage of nano or microparticles within the scaffold as can be seen in the previous example.
5.3. Inorganic Mediators Encapsulation in Micro- and Nanoparticles
Nanoparticle encapsulation in chitosan-based cross-linked scaffolds has incorporated the usage of mostly inorganic materials including silica,90,105,118,121,122,125,127 copper,139 zinc oxide,137,148 strontium,170 and titanium oxide.128 Silica is one of the components that make up bioactive glass, thus making its incorporation in studies popular. Unlike the previous compounds and additives, silica has been shown to promote apatite formation in cultures of simulated body fluid as well as osteogenic differentiation. Filipowska et al. demonstrates both effects using hBMSCs in a chitosan and collagen composite scaffold.127 In groups with silica particles, the mRNA expression levels of osteogenic related genes (RUNX2, COL-1, and osteocalcin) were significantly higher than the control group at day 14. Interestingly, results demonstrated a dependency of the mRNA expression on the size of the particles at this early stage where the smaller particles exhibited higher levels. In addition, SEM micrographs of both silica particle groups showed aggregation on the surface of the cell as a sign of mineralization and apatite formation compared to the smooth surface found in the control collagen and chitosan scaffold. EDS analysis for calcium and phosphorus on these surfaces illustrated silica’s effect on calcium and phosphate production. These two test groups had Ca/P ratios around 1.65, which is similar to that of native crystalline hydroxyapatite. Other inorganic materials listed above have also been tested within these scaffolds, however to a much lesser extent. Copper is one of these less explored elements but has been shown to promote bone formation in the literature. A study by Lu et al. demonstrated that the incorporation of copper nanoparticles in a carboxymethyl chitosan and alginate scaffold resulted in increased ALP activity at day 7, and increased COL-1 and osteocalcin mRNA expression at day 14.139 Interestingly, the copper nanoparticles were used in place of Cu2+ ions in this study to slow down the cross-linking process of the carboxymethyl chitosan and alginate scaffold. It has been shown that the Cu2+ ion alone can serve as a cross-linker, thus allowing the scaffold to cross-link slowly as ions are released.
5.4. Investigation of Release Kinetics of Encapsulated Compounds
Since one of the main challenges in the design of biological substitutes with mediators, growth factors, or drugs is the achievement of a sustained release profile, kinetic release studies are often conducted in vitro as part of scaffold characterization.22,205−207 To perform these tests, scaffolds are loaded with a model component and its concentration in the supernatant is measured over a certain amount of time (Figure 11, Table 5). The most common method to assess the release kinetics includes UV–vis spectrophotometry and absorbance readings, with half of the relevant articles having reported on this methodology. Other techniques like enzyme linked immunosorbent assays, HPLC, or protein assays can also be used. Ideally, the release of growth factors and mediators for osteogenic promotion needs to be sustained throughout the healing process. Thus, research incorporating compounds such as BMPs, bisphosphonates, or inorganic materials in chitosan scaffolds often tests this kinetics throughout a designated period. Some studies have examined a short duration of time up to 2 weeks,64,91,135,151,155 while others have examined release past 4 or 5 weeks4,96,130 enabling a more comprehensive understanding of the mediator’s possible effect over time.
Figure 11.
General mechanisms that mediate the release of factors from scaffolds and common methodologies used to assess this release.
Table 5. Common Methodologies for Encapsulation Efficiency and Release Kinetic Studies in Chitosan Cross-linked Scaffolds for Bone (N = 31).
| Growth Factor Properties | Incorporation in Growth Factor Studies (%) | Information Obtained | Methodology | Usage in Specific Property Category testing (%) |
|---|---|---|---|---|
| Encapsulation efficiency | 45.2% | Drug loading rate encapsulation efficiency | UV–vis/ spectrophotometer–absorbance readings(67,83,100,103,121) | 35.7% |
| ELISA4,96,114,167 | 28.6% | |||
| HPLC119 | 7.1% | |||
| Weight measurements of drug105 | 7.1% | |||
| Drug specific testing | BCA protein assay–used commonly for BSA loading(91,129) | 14.3% | ||
| Toluidine Blue staining (presence of heparin)(96,146) | 14.3% | |||
| Release kinetics | 96.7% | Release rate of drug percentage of drug released | UV–vis/ spectrophotometer–absorbance readings(65,68,73,83,87,100,103,105,119,121,132,147,153,157,161,169) | 50% |
| ELISA4,64,77,96,114,129,167 | 26.7% | |||
| Inductively coupled plasma mass spectrometry130,139 | 6.7% | |||
| Aluminum trichloride method158 | 3.3% | |||
| HPLC135 | 3.3% | |||
| Drug specific testing | BCA protein assay–used commonly for BSA loading(91,155,162) | 10% | ||
| Toluidine Blue Assay (concentration of Heparin)146 | 3.3% |
Osteogenic mediators and factors can be added to chitosan scaffolds mainly through covalent or noncovalent binding.208,209 The latter is more prominent in bone-oriented applications and involves the adsorption of the factors onto the scaffolds or their entrapment within the scaffold mesh.22,210 Electrostatic interactions, hydrophobic interactions, hydrogen bonding, or van der Waals forces play a major role in immobilizing factors noncovalently onto the scaffolds, and disruption of these interactions can influence the release kinetics.208,209 Generally, different mechanisms mediate the release of factors from scaffolds including desorption and diffusion of factors outside of the scaffold, swelling of the scaffold, or degradation of the scaffold’s structure, and they have been previously reviewed in a publication by Herdiana et al.211 In addition to the type of factor added, the release mechanism also depends on how the factor interacts with and is bound to the scaffold as well as the properties of the scaffold itself. Thus, changes in the composition of the scaffold can allow for the rate of release to be tuned and controlled more precisely.210
As discussed in the previous sections, cross-linking of chitosan greatly influences the material properties of the formed scaffold, which in turn can affect the release of factors depending on the cross-linker used and its concentration (Table 6). Therefore, it is necessary to take that into consideration when developing cross-linked chitosan scaffolds in order to yield optimal kinetics. As an example, the addition of a model drug, Pentoxifylline, was examined in multiple chitosan scaffold formulations with either genipin (CG) or pectin cross-linkers (CP).73 Results indicated a significant difference in release kinetics among all tested groups with the genipin cross-linker showing the most promise with a lower cumulative release percentage after 6 h (Figure 12A). However, the high percentage values at 6 h (around 80% in CG scaffolds) seems to indicate an initial burst release, which has been demonstrated in other studies in the literature.77,83,87,93,105,114,121,129,155,167 However, it would be interesting in this scenario to determine whether the kinetics becomes more gradual at later points. A study by Ji et al. has indicated that while this burst trend is often observed, it is usually accompanied by two additional stages: a line and slow stage. This was evident in the results of their study, where the release of BMP2 from composite chitosan scaffolds with mineralized COL-I and poly lactic-co-glycolic acid (plga) microspheres was high at day 3 (162.8 pg rhbmp2 per mg) but gradually reached 361.06 pg at the 28th day of culture (Figure 12B).135 This notion of stages for release profiles was also found in other studies where an initial burst release occurred.87
Table 6. Common Osteogenic Mediators Examined in the Literature for Release Kinetics from Chitosan-Based Scaffolds.
| Mediator in Literature | Scaffold Tested | Cross-linking Method | Observed Outcomes |
|---|---|---|---|
| BMP-2 | Chitosan/alginate | EDC/NHS | Lower cross-linking levels showed higher initial burst release, as well as absolute amount of BMP-2 released over 1 month161 |
| Chitosan/hyaluronic acid | Genipin | Initial burst release was observed that became more gradual and sustained after 3 days and lasted over 4 weeks129 | |
| Chitosan/collagen | Sodium tripolyphosphate (STPP) | Initial burst release followed by a linear stage for 3 days after which the kinetics reached a slow-release stage135 | |
| Chitosan | Sodium tripolyphosphate (STPP) | No initial burst release was observed. Release was sustained over 45 days, after which 90% of BMP-2 was released96 | |
| Chitosan/agarose/gelatin | Glutaraldehyde | Zero-order-like release sustained over 15 days114 | |
| Alendronate | Chitosan/polycaprolactone | Genipin | Addition of polycaprolactone to the scaffold demonstrated a more sustained release of alendronate compared to the control which had faster release. More than 90% of the drug was released from either scaffold after 7 days119 |
| Icariin | Chitosan | Glutaraldehyde | 50% of icariin was released after 8 h. By the 24 h mark, 73% of loaded icariin was released, after which the rate of release became slow and sustained83 |
| Ascorbic acid | Chitosan | Guanosine diphosphate | A burst release was observed in the first 5 h, in which 50% of ascorbic acid was released. Afterward, the release became more gradual up to 6 days87 |
| Gentamicin | Chitosan | Β-Glycerophosphate | 100% of the drug was released in 4 h153 |
| Levofloxacin | Chitosan/biphasic calcium phosphates | Genipin | Initial fast release that begins to plateau after 10 min. This burst release is more pronounced with a higher Ca/P ratio. More than half the drug is released after 30 min, regardless of Ca/P ratio68 |
Figure 12.
Cumulative release profiles of different encapsulants from scaffolds. (A) Release percentage of pentoxifylline at the 6 h time point for chitosan scaffolds cross-linked with genipin, pectin, or freeze-dried. Reproduced and adapted with permission from ref (73). Copyright 2015 IOP Publishing. (B) Cumulative release of rhBMP2 from PLGA microspheres, which will be placed in composite chitosan scaffolds with mineralized collagen I. Reproduced and adapted with permission from ref (135). Copyright 2017 Wiley Periodicals, Inc. (C) Release patterns of rhBMP-2 and vancomycin (VAN) from methacrylated chitosan photo-cross-linked scaffolds with or without PLGA microspheres. Reproduced and adapted with permission from ref (169). Copyright 2021 Elsevier. (D) Cumulative release percentage of tetracycline hydrochloride from carboxymethyl chitosan/oxidized gellan gum gels with hydroxyapatite (HAp/gel) and gels with magnetic hydroxyapatite/gelatin microspheres (MHGMs). Reproduced and adapted with permission from ref (103). Copyright 2022 Elsevier.
In other instances, authors have focused their drug delivery studies to the encapsulation of antibacterial or anti-inflammatory agents rather than those to promote bone healing.65,68,103,169 Incorporation of these drugs, while not directly influencing osteogenic differentiation and mineralization, still can have a beneficial effect in these applications since inflammation can hinder successful bone regeneration. Since fracture healing starts with a preliminary inflammatory phase, it could potentially be more useful to deliver these drugs faster such as in the Pentoxifylline study mentioned above. Song et al. also showed early burst release patterns of an antibacterial agent, Vancomycin, with more than 80% released in the first 2 days in a chitosan hydrogel with a coencapsulation of PLGA microparticles loaded with rhBMP2 (Figure 12C).169 While this quick release was expected based on other studies, authors suggested that this rate needs to be further tuned to match that of the antibacterial stage.
Loading of drugs within nano or micro spheres or particles prior to encapsulation into a chitosan scaffold could serve to delay the release of drugs or compounds and encourage a more gradual release. The control of release for antibacterial agents, silver sulfadiazine and tetracycline hydrochloride, has recently been explored using magnetic hydroxyapatite and gelatin microspheres cross-linked with glutaraldehyde.103 Chen et al. demonstrated that due to the solubility of tetracycline hydrochloride in water, the release rate tends to be accelerated by diffusion in the aqueous environment (Figure 12D). While release kinetics from the microspheres and carboxymethyl chitosan and oxidized gellan gum gel alone resulted in a burst release, the incorporation of the nanoparticles within the gel was shown to provide a more sustained release rate. At the 21-day time point, cumulative release was 16.1%, which was decreased compared to the gel alone. Similarly, Dorati et al. observed complete release of gentamicin from a β-glycerophosphate cross-linked chitosan scaffold within only 4 h, which extended to 80 days following the loading of the drugs within PLGA–PEG microparticles.153
6. Vascularization in Bone Tissue Engineering
The fifth element of the diamond concept for proper bone healing is the development of an adequate vascular network throughout.42 Osteogenesis and angiogenesis are closely associated processes, sharing some essential mediators. Skeletal vasculature plays a significant role in the process of bone development (endochondral and intramembranous ossification), regeneration, and remodeling.212,213 As known, bone is highly vascular tissue and the presence of a vascular network aids in providing oxygen and nutrients to cells, calcium and phosphate for mineralization as well as removing waste products from the site.212,214−216 Formation of these vascular networks within biomaterial substitutes has been one of the main challenges in the bone tissue engineering domain and is often overlooked in literature studies. Lack of sufficient vascularization in tested scaffolds has been shown to result in cell hypoxia and death since nutrients and oxygen can only travel around 150 μm from surrounding networks.215,217−219
Vasculogenesis and angiogenesis are thought to be the main mechanisms for vascular network formation in bone. While the vasculogenesis mechanism relies on progenitor cells forming the vasculature without an existing network, angiogenesis builds off pre-existing networks surrounding the defect site.212,220 Assessment of vascular formation within the scaffold in vitro and in vivo relies on measurement of vascular markers.221 These can include vascular endothelial growth factor, von Willebrand factor, vascular endothelial cadherin, and the platelet endothelial cell adhesion molecule among others.221
Recent studies of chitosan-based scaffolds have incorporated a chicken chorioallantois membrane (CAM) assay to examine the induction of angiogenesis. Through this method, Zhang et al. were able to demonstrate that the addition of sphingosine 1-phosphate (S1P) loaded silica nanoparticles (MSNs) to N,O-carboxymethyl chitosan (NOCC) and aldehyde hyaluronic acid (aHa) nanocomposite hydrogels resulted in the formation of a dense capillary network compared to control scaffolds (Figure 13A,B).105 The difference between these groups was shown to be significant with respect to the area of vessels counted from 30.03% in S1P loaded hydrogels to 16.26% in control. This significant effect of S1P addition was also confirmed in vivo subcutaneously in mice (Figure 13C,D). Another chitosan-based composite scaffold was assessed for vascularization potential in vivo as well, but this time using a critical size defect model in the femoral head of a rabbit.133 This study only examined one configuration of their scaffold (2:1:3 mass ratio of chitosan, gelatin, and biphasic calcium phosphate nanoparticles), but hematoxylin and eosin (HE) staining results demonstrated improved vascularity over the course of time from one to three months. No clear pattern emerges from the literature regarding which encapsulants significantly improve vascularization of the scaffold, but some publications have proposed the incorporation of a coculture system to overcome this aspect.87,215,220,222 This involves the coencapsulation of an osteoblast precursor in direct or indirect contact with endothelial cells, allowing for the regeneration of a tissue more similar to the native environment.223 However, the specifics of a coculture platform for healing, while important, are considered to be outside of the scope of this review.
Figure 13.
Effect of sphingosine 1-phosphate (S1P) loaded silica nanoparticles (MSNs) addition to N,O-carboxymethyl chitosan (NOCC) and aldehyde hyaluronic acid (aHa) nanocomposite hydrogels on capillary network formation in vitro and in vivo. (A, B) Results of CAM assay for examination of angiogenesis and quantification of vascular area. (C, D) HE staining images at the 2-week time point after implantation into a mouse. Arrows present the blood vessels. D presents the counted number of vessels shown. Reproduced and adapted with permission from ref (105). Copyright 2022 Royal Society of Chemistry.
7. Pre-clinical Implementation of Diamond Concept Using Chitosan-Based Cross-linked Scaffolds
While in vitro experiments are a necessary step in the assessment of osteogenic differentiation and regeneration potential for designed substitutes, the clinical translation of in vitro findings require preclinical in vivo validations. Often, the in vitro models are not convoluted enough to accurately represent the complexity of the in vivo healing environment.224 As such, incorporating animal studies is an essential part of biological substitute development and evaluation in the framework of the Diamond Concept.225 Within the literature, this has included studies of fracture healing models on many mammals, both large and small, with implantation or injection of chitosan-based cross-linked scaffolds (Table 7).225,226
Table 7. Cross-linked Chitosan-Based Scaffold Formulations That Demonstrated Beneficial Bone Regeneration In Vivo.
| Species | Defect Model | Scaffold | Additions Tested |
|---|---|---|---|
| Rats | Cranial/mandible defect model | Chitosan80,99 | Carbon nanotubes and BMP-2,64, Alendronate,119 Graphene oxide and titanium oxide,80 Bisphosphonates and BMP-2,130 Magnesium ions and polyhedral oligomeric silsesquioxane (POSS) nanoparticles,151 Graphene oxide and nanohydroxyapatite,65 Copper99 |
| Carboxymethyl chitosan64,65 | |||
| Chitosan/polycaprolactone119 | |||
| Methacrylic modified chitosan130 | |||
| Chitosan/collagen72 | |||
| Thiolated chitosan/gelatin151 | |||
| Subcutaneous insertion | Chitosan96 | Silicon-substituted calcium phosphate, rhBMP-2 and heparin96 | |
| Mice | Cranial/mandible defect model | Chitosan76 | Lysozyme,140 Hydroxyapatite76 |
| Methacrylated glycol chitosan140 | |||
| Phenol conjugated glycol167 | |||
| Tibial model | Chitosan88 | Hydroxyapatite and pyrophosphatase88 | |
| Subcutaneous insertion | Chitosan79,120 | Graphene oxide,120,124 Bioglass nanoparticles and BMP-2,79 Hydroxyapatite -decorated silica beads with alendronate,121 BMP-2161 | |
| Chitosan/collagen/hyaluronic acid121 | |||
| Chitosan/gelatin124 | |||
| Chitosan/alginate161 | |||
| Rabbits | Cranial/mandible defect model | Chitosan/collagen111 | Hydroxyapatite111 |
| Femoral model | Chitosan/gelatin133 | Biphasic calcium phosphate nanoparticles133 | |
| Tibial model | Chitosan85 | Citric acid85 | |
| Oleyol chitosan/decellularized bone matrix123 |
As large animal models, rabbits are used in multiple studies for chitosan-based cross-linked scaffolds’ evaluation in vivo.85,111,123,133 These studies have included examinations of both the cross-linking mechanisms and the necessity for cell encapsulation for in vivo studies. Datta et al. examined the influence of human amnion derived MSC encapsulation using a combinatorial genipin cross-linked fatty acid modified chitosan and decellularized bone ECM hydrogel in a rabbit tibial defect model.123 Results demonstrated the positive effect of cellular addition, as cell encapsulated scaffolds produced highly mineralized tissue densities compared to cell free groups. In addition, staining showed that this incorporation of cells resulted in similar morphological features to that of the native bone tissue. This reinforces the notion of osteogenic cells as a crucial subcategory of the Diamond Concept. Another study using a similar rabbit tibial defect model provided a thorough understanding of cross-linking’s role in bone formation. By comparing two types of chitosan-based fibers treated with either a citric acid bath for cross-linking or a dual cross-linking using a citric acid bath and NHS/EDC, the authors were able to demonstrate that the dual cross-linked chitosan fibers showed more collagen and mineral deposition.85 This finding indicated better osteogenic activity and bone regeneration with higher amounts of cross-linking. In addition to tibial defects, other studies in rabbits with chitosan scaffolds have used mandible defects111 and femoral head133 defects models, but these models tend to be less common in both large and small animals.
Use of small animal models such as rat and mouse, is significantly more common than large animal studies since these animals are low cost and can be kept in large quantities, allowing for more groups to be tested and more statistically relevant information to be obtained on differences.225,226 In addition, many of the commercially available monoclonal antibodies used to conduct a more comprehensive evaluation of healing are targeted for rat and mouse.226 Within these rat and mouse models, fracture healing is completed within 4 to 6 weeks, which is faster than the timeline for healing in large animal models.225,226 Assessment of healing in these models is conducted at an early phase (1–2 weeks) and a late phase (3–5 weeks in mice, 4–6 weeks in rats).226
Among these murine models, researchers tend to examine their material’s effect on osteogenic differentiation and bone healing through usage of cranial defects64,76,80,82,99,119,130,135,140,151,167 or subcutaneous incorporation.65,79,96,120,121,139,161 However, a few studies have instead chosen to use tibial88 or femoral defect112 models to better understand the fate of their scaffold for a load bearing bone application. Often, incorporation of chitosan-based scaffolds in these cases is done in combination with a fixator or mechanical stabilizer to bear the load while the bone is healing. Jahan et al. demonstrated the potential of a chitosan-based GDP cross-linked scaffold with hydroxyapatite and pyrophosphatase incorporation in vivo using a rod fixated tibia fracture model of mice (Figure 14A).88 However, although tibial fracture models are well-established in murine models, often, femur fracture models are more beneficial since they provide more consistent results with respect to biomechanical evaluation.225
Figure 14.
In vivo studies using (A–C) MicroCT and (D) HE staining. (A) Images of mouse tibial fractures treated with nothing (SHAM) or chitosan scaffolds with hydroxyapatite (HA) and pyrophosphatase (P). Reproduced and adapted under open access conditions (Creative Commons Attribution License) from ref (88). Copyright 2020 Kaushar Jahan et al. and Springer Nature. (B, C) Imaging and quantitative analysis of a rat cranial defect model at 12 weeks postsurgery treated with control, chitosan and PCL composite scaffolds, and composite scaffolds with Alendronate. (C) Quantification of the mature bone area, bone volume/tissue volume, and bone mineral density for these samples. Both B and C were reproduced and adapted with permission from ref (119). Copyright 2022 Elsevier. (D) Staining for new bone formation in defects treated with nothing (top), chitosan scaffold (middle), or a chitosan scaffold loaded with copper (bottom). Within this figure, the new bone (NB) and old bone (OB) are distinguished. Reproduced and adapted with permission from ref (99). Copyright 2014 Wiley Periodicals, Inc.
7.1. Methodology of In Vivo Bone Healing Assessment in Chitosan-Based Scaffolds
Assessment of the scaffold’s capability for healing in vivo commonly involves three different components: bone imaging, histological staining and quantitative assays225,226 (Figure 15). Table 8 serves to demonstrate the available techniques for each of these components, as well as the percentage of use within recent work on chitosan-based scaffolds for bone.
Figure 15.
Common methodologies for in vivo studies using animal models in the literature. These are divided into 3 components: imaging studies, histological staining, and quantitative assays.
Table 8. Common Methodologies for In Vivo Testing in Chitosan Cross-linked Scaffolds for Bone (N = 33).
| In Vivo Experiments | Incorporation in In Vivo Studies (%) | Methodology | Usage in Specific In Vivo Testing (%) |
|---|---|---|---|
| Imaging techniques | 57.6% | MicroCT and CT imaging(64,65,76,79,88,99,119,123,130,135,139,140,161,163,184) | 78.9% |
| X-ray imaging111,167 | 10.5% | ||
| Radiography82,135 | 10.5% | ||
| Scanning electron microscopy80 | 5.3% | ||
| Stereoscopic imaging80 | 5.3% | ||
| Staining techniques | 87.9% | HE staining(76,79,80,82,85,96,99,105,111,112,119−121,123,130,133,135,139,140,151,158,163,168,170,184) | 86.2% |
| Masson’s trichrome staining76,80,85,112,119,121,123,130,133,139,140,151,163 | 44.8% | ||
| IHC/IF osteocalcin (OCN)119,130,151,163 | 13.8% | ||
| Alizarin Red staining64,96,120,121 | 13.8% | ||
| Von Kossa staining65,85,88 | 10.4% | ||
| CD31 Staining105,123,151 | 10.4% | ||
| Toluidine Blue/Alcian Blue staining88,123 | 10.4% | ||
| Gomori’s Trichrome staining120,124 | 6.9% | ||
| IHC/IF RUNX2120,151 | 6.9% | ||
| IHC/IF Collagen 1151,163 | 6.9% | ||
| IHC/IF osteopontin (OPN)65,120 | 6.9% | ||
| ALP staining163,167 | 6.9% | ||
| Giemsa staining139 | 3.4% | ||
| Von Willebrand factor staining105 | 3.4% | ||
| Alpha smooth muscle actin staining151 | 3.4% | ||
| Calcein staining64 | 3.4% | ||
| TRAP staining167 | 3.4% | ||
| Tetracycline staining64 | 3.4% | ||
| IHC/IF bone sialo protein (BSP)65 | 3.4% | ||
| Assays and quantitative assessments | 9.1% | Quantification of inflammatory cytokines(121,168) | 66.7% |
| ALP quantification in serum120 | 33.3% | ||
| qPCR for gene expression120 | 33.3% |
Scanning techniques are implemented around 57.6% of the time in the literature and are known to aid researchers in fully assessing newly formed bone tissue prior to sectioning. Micro-CT imaging has been shown to be the most popular of the available imaging techniques since it allows for the quantification different parameter values including bone volume to tissue volume or trabecular spacing, among others. These scans are typically performed ex vivo to encourage the examination of newly formed tissue in a higher resolution but the technique could also be adapted for monitoring in vivo throughout the healing process.225,227 Other scanning techniques have also been found in recent literature including X-ray imaging (10.5%) or SEM (5.3%); however, these are significantly less common compared to MicroCT imaging (78.9%).
Histomorphometry of samples by different staining methods is another technique that can be applied to acquire specific desired information on the role of the scaffold on the bone healing process.225 For example, Georgopoulou et al. used HE and Masson Trichrome stainings to assess the ability of a glutaraldehyde cross-linked chitosan and gelatin scaffold to support bone regeneration and formation of the extracellular matrix.228 HE staining is one of the primary methods for assessing bone healing in vivo, with a reported usage of 86.2% in publications with some sort of staining technique. While used for histology of many different tissues, the bone structure in HE-stained images tends to have a dark pink color, whereas connective tissue will be a noticeably lighter shade of pink. Use of other stains such as Masson Trichrome and Movat Pentachrome have become a more set standard in the literature recently since they allow for a more in-depth observation of the callus tissue composition with only one required staining.229,230 Other less common staining options include Von Kossa/Van Gieson which aims to look at mineralization and Alcian Blue to examine cartilage.
Quantification of different parameters using assays does not appear to be an increasingly common trend with respect to in vivo studies as it is with in vitro studies (9.1%). A few studies have used ELISA or kits to measure the production of inflammatory cytokines, which could in future be an important test to further understanding the full biocompatibility of the designed material.
7.2. Influence of Chitosan Cross-linking on In Vivo Studies
The combination of these techniques provides a more accurate representation of the full healing process. Within the literature in the past ten years, very few studies have examined the effect of scaffold formulation, such as polymer addition or cross-linker concentration geared toward improving the material properties, in the in vivo osteogenic potential experiments. Often, the number of tested groups are minimized based on prior investigation of material properties and in vitro cell experiments to reduce the cost for animal studies. This means that only the optimal groups, per se, are considered for in vivo experiments, to determine what encapsulants, or factors play a promotional osteogenic role.
Incorporation of the bisphosphonate, Alendronate, into a Chitosan/polylactic acid scaffold was demonstrated to support bony bridging within the cranial defect model compared to the scaffold alone in micro-CT images (Figure 15B,C).119 This beneficial trend in growth factor addition was also evident with the incorporation of BMP2 in carboxymethyl chitosan scaffolds with carbon nanotubes. While the initial intention of this in vivo study was to assess specifically at the carbon nanotube effect, the addition of BMP2 resulted in a nearly fully covered defect at week 8, whereas the carbon nanotubes alone in the scaffold had significantly reduced bone tissue production.64 The influence of inorganic materials as mediators was also established in a rat critical size defect model using a chitosan scaffold with copper cross-linking.99 Results showed improved mineralized tissue formation in groups with copper compared to controls and chitosan alone (Figure 15D). This supportive effect was also confirmed using HE staining. Generally, results presented here illustrate the necessity of osteogenic compounds in the designed scaffold and encourage the development of additional and alternative growth factors for these applications.
8. Future Perspectives and Conclusion
This review serves to provide a thorough comparison of modifications made to cross-linked chitosan-based scaffolds with respect to the Diamond Concept including changes of cross-linkers, cell types and growth factors. Based on the research results in the development of these scaffolds within the last ten years, it is evident that the synergistic combination of all aspects of the Diamond Concept are necessary to achieving successful bone healing. However, consideration for all these aspects in literature studies of cross-linked chitosan materials remains lacking hence why no clinically relevant chitosan scaffold-based solution has been achieved. The base polymer chitosan, often found in many different variations commercially, should be the preliminary consideration in the design process with emphasis placed on chitosan solutions with high DD and molecular weight (MW). Chitosan has been demonstrated in the literature to have sufficient biocompatibility with cells and presents many beneficial properties with regards to osteogenic activities.54 The large DD allows for the prominence of positive charges in the amine groups throughout the structure thus improving the interaction of the material with the cells.22,54 Both chitosan parameters have shown an inverse correlation with degradation thus necessitating precise control of these to tune the rate of scaffold degradation as new bone formation occurs.22,231 With respect to the use of chemically modified chitosan derivatives for bone specific applications, no clear trend has emerged thus far and further analysis on this should be conducted.
Studies have adequately demonstrated the importance of cross-linking, with many publications using genipin or glutaraldehyde, yet no clear cross-linking agent has emerged as optimal with respect to material properties. Future studies should therefore focus on eliciting a clear trend between different cross-linkers. Concerns within the literature have been raised regarding the usage of synthetic chemical cross-linkers such as glutaraldehyde with respect to cytotoxicity. Thus, a balance should be struck between benefits to material properties and effects on cellular functionality. Cross-linking alone however has not been shown to be sufficient for bone healing and the encapsulation of bioceramics such as hydroxyapatite can be argued as an integral component to meet necessary mechanical and osteogenic properties for bone engineering applications.
There has been no set standardized methodology regarding the assessment of material properties for chitosan-based cross-linked materials, with many studies using varying techniques that may not necessarily yield the same information. This, in turn, makes comparisons between studies significantly more challenging. Future work in this domain should investigate the material properties of the designed scaffold in the following presented manner. First, mechanical properties should be assessed using compression testing since it resembles the experience of the scaffold in the native tissue environment.22 This method should be supplemented with rheological testing as viscoelasticity plays an important role in promoting differentiation of encapsulated cells.24 Second, the structural architecture examination should be completed scanning electron microscopy for surface morphology and complemented with micro-CT for internal structure parameters. While liquid displacement is more popular for porosity assessment, it can be argued that, if not inhibited by cost and availability, micro-CT provides a more comprehensive evaluation of this internal architecture. Finally, biodegradation rates of the chitosan-based scaffolds should be assessed through incubations in solutions of PBS or water with enzymes. This method more accurately serves to simulate the natural body conditions experienced by implanted scaffolds. Although these degradation patterns have been extensively characterized within the literature for various scaffold fabrications in different buffers such as PBS, water, simulated body fluid,75,95,116,133,145 or culture media71,150 no clear standard has been established with regard to the appropriate degradation timeline needed for striking a balance with the rate of new bone tissue formation. A more precise timeline for material degradation should be explored further in future work to allow for the design of optimal chitosan-based biomaterials.
With respect to cellular encapsulation, research should focus on using more clinically relevant cell lines such as stem cells. The last 10 years of chitosan-based scaffold studies demonstrate good advancement in this regard. Assignment of a standardized methodology for cellular biocompatibility assessment is challenging since many techniques have been proven effective for attaining similar information regarding metabolic activity, cytotoxicity, and adhesion. However, the addition of a cross-linker to scaffolds makes the necessity for this assessment evident in all future work. Techniques for studying the potential for cellular differentiation and mineralization should include an evaluation of both early and late stage markers and would benefit greatly from gene expression studies on top of secretion and staining techniques.
Few studies of cellular activity in vitro have sought to regenerate the complex aspects of the bone structure such as vascularization. This aspect is known to be important for successful bone tissue engineering since it serves to provide oxygen and nutrient to cells within the injured sites, but its success in vitro and in vivo remains a challenge in terms of achievement.217,232 The coculture of osteoblastic lineage cells with endothelial cells is a seemingly promising method for this endeavor. Future studies on chitosan-based bone tissue engineering scaffolds can benefit from a comprehensive evaluation of all aspects of the Diamond Concept including vascularization instead of only focusing on osteogenic differentiation and mineralization.
While research examining the combination of the different aspects of the Diamond Concept is prominent in vitro, its application has been lacking in vivo. As previously discussed, in vivo studies in the literature incorporate 3 main components including imaging, staining, and quantitative assays. Micro-CT imaging of the regenerated bone should become the gold standard for imaging methodologies since it provides an abundance of qualitative and quantitative information and can be nondestructive. This property is beneficial as it allows for scanning of the same bones at multiple time points without the addition of more groups, thereby following the reduction principle of animal work (the 3Rs). Imaging of the samples should, in turn, be sequentially followed by sectioning and staining to assess specific aspects of healing. Recent studies have demonstrated the clear benefit of both HE and Masson’s Trichrome since they stain multiple tissue types with only one necessary staining. Thus, the implementation of these stains in animal samples should become standard practice but could be complemented with additional ones if a particular aspect of healing is of specific interest.
Studies exploring the use of quantitative assays within in vivo studies has been sufficiently lacking in recent publications but are recommended to be added in the future. Incorporation of assays and tests such as qPCR for osteogenic gene expression or serum secretion levels of markers such as ALP could be beneficial in conjunction with imaging and staining in attaining a more complete profile of in vivo healing. In addition to this, future studies of chitosan cross-linked scaffolds should also focus on exploring more comprehensively their capabilities in vivo for aiding in bone formation with biomechanical testing including torsional and 3-point bending. This determination of the newly formed bone’s mechanical properties can help to design a detailed therapeutic option for future clinical testing and usage.
Acknowledgments
The authors acknowledge the financial support of CIHR and NSERC via the Collaborative Health Research Program, Canada Research Chair – Tier 1 in Regenerative Medicine and Nanomedicine, as well as FRQ-S funding. All schematic figures were created using the Biorender software under paid subscription, and graphs were created using Graphpad Prism.
The authors declare no competing financial interest.
References
- Marsell R.; Einhorn T. A. The biology of fracture healing. Injury 2011, 42 (6), 551–555. 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L.; Recknagel S.; Ignatius A. Fracture healing under healthy and inflammatory conditions. Nature Reviews Rheumatology 2012, 8 (3), 133–143. 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- Pereira H. F.; Cengiz I. F.; Silva F. S.; Reis R. L.; Oliveira J. M. Scaffolds and coatings for bone regeneration. J. Mater. Sci.: Mater. Med. 2020, 31 (3), 1–16. 10.1007/s10856-020-06364-y. [DOI] [PubMed] [Google Scholar]
- Nayef L.; Mekhail M.; Benameur L.; Rendon J. S.; Hamdy R.; Tabrizian M. A combinatorial approach towards achieving an injectable, self-contained, phosphate-releasing scaffold for promoting biomineralization in critical size bone defects. Acta biomaterialia 2016, 29, 389–397. 10.1016/j.actbio.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Lutolf M.; Hubbell J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature biotechnology 2005, 23 (1), 47 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Schemitsch E. H. Size matters: defining critical in bone defect size!. Journal of orthopaedic trauma 2017, 31, S20–S22. 10.1097/BOT.0000000000000978. [DOI] [PubMed] [Google Scholar]
- Petite H.; Viateau V.; Bensaid W.; Meunier A.; de Pollak C.; Bourguignon M.; Oudina K.; Sedel L.; Guillemin G. Tissue-engineered bone regeneration. Nature biotechnology 2000, 18 (9), 959 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- Mauffrey C.; Barlow B. T.; Smith W. Management of segmental bone defects. JAAOS-Journal of the American Academy of Orthopaedic Surgeons 2015, 23 (3), 143–153. 10.5435/JAAOS-D-14-00018. [DOI] [PubMed] [Google Scholar]
- Jahan K.; Tabrizian M. Composite biopolymers for bone regeneration enhancement in bony defects. Biomaterials science 2016, 4 (1), 25–39. 10.1039/C5BM00163C. [DOI] [PubMed] [Google Scholar]
- Roddy E.; DeBaun M. R.; Daoud-Gray A.; Yang Y. P.; Gardner M. J. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. European Journal of Orthopaedic Surgery & Traumatology 2018, 28 (3), 351–362. 10.1007/s00590-017-2063-0. [DOI] [PubMed] [Google Scholar]
- Andrzejowski P.; Giannoudis P. V. The ‘diamond concept’for long bone non-union management. Journal of Orthopaedics and Traumatology 2019, 20 (1), 21 10.1186/s10195-019-0528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorozhkin S. V. Calcium Orthophosphate (capo4) Scaffolds For Bone Tissue Engineering Applications. Journal of Biotechnology and Biomedical Science 2018, 1 (3), 25 10.14302/issn.2576-6694.jbbs-18-2143. [DOI] [Google Scholar]
- Lobb D. C.; DeGeorge B. R. Jr; Chhabra A. B. Bone graft substitutes: current concepts and future expectations. Journal of hand surgery 2019, 44 (6), 497–505.e2. 10.1016/j.jhsa.2018.10.032. [DOI] [PubMed] [Google Scholar]
- Black C. R.; Goriainov V.; Gibbs D.; Kanczler J.; Tare R. S.; Oreffo R. O. Bone tissue engineering. Current molecular biology reports 2015, 1 (3), 132–140. 10.1007/s40610-015-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter F.Chapter 1 - What is Tissue Engineering? In Tissue Engineering Made Easy; Akter F., Ed.; Academic Press, 2016; pp 1–2. [Google Scholar]
- Vacanti J. P.; Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. lancet 1999, 354, S32–S34. 10.1016/S0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- O’Brien F. J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14 (3), 88–95. 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- Carletti E.; Motta A.; Migliaresi C.. Scaffolds for tissue engineering and 3D cell culture. In 3D cell culture; Springer, 2011; pp 17–39. [DOI] [PubMed] [Google Scholar]
- Liu X.; Ma P. X. Polymeric scaffolds for bone tissue engineering. Annals of biomedical engineering 2004, 32 (3), 477–486. 10.1023/B:ABME.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- Wiraja C.; Chong M. S.; Liao Y.; Chew S. W.; Xu C.. Multi-Functional Biomaterials for Bone Tissue Engineering. In Smart Materials for Tissue Engineering; Royal Society of Chemistry: London, UK, 2017; pp 169–193. [Google Scholar]
- Eltom A.; Zhong G.; Muhammad A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Advances in Materials Science and Engineering 2019, 2019, 3429527 10.1155/2019/3429527. [DOI] [Google Scholar]
- Levengood S. K. L.; Zhang M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2 (21), 3161–3184. 10.1039/c4tb00027g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S.; Roy M.; Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30 (10), 546–554. 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining K. H.; Mooney D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18 (12), 728–742. 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.; Ma J.-X.; Xu L.; Gu X.-S.; Ma X.-L. Biodegradable materials for bone defect repair. Military Medical Research 2020, 7 (1), 1–25. 10.1186/s40779-020-00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Zhou L.; Zhang W. Control of scaffold degradation in tissue engineering: a review. Tissue Engineering Part B: Reviews 2014, 20 (5), 492–502. 10.1089/ten.teb.2013.0452. [DOI] [PubMed] [Google Scholar]
- Collins M. N.; Ren G.; Young K.; Pina S.; Reis R. L.; Oliveira J. M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31 (21), 2010609 10.1002/adfm.202010609. [DOI] [Google Scholar]
- Zhu Y.; Goh C.; Shrestha A. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci. 2021, 21 (4), 2000365 10.1002/mabi.202000365. [DOI] [PubMed] [Google Scholar]
- Abbasi N.; Hamlet S.; Love R. M.; Nguyen N.-T. Porous scaffolds for bone regeneration. Journal of Science: Advanced Materials and Devices 2020, 5 (1), 1–9. 10.1016/j.jsamd.2020.01.007. [DOI] [Google Scholar]
- Salgado A. J.; Coutinho O. P.; Reis R. L. Bone tissue engineering: state of the art and future trends. Macromol. Biosci. 2004, 4 (8), 743–765. 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- Afewerki S.; Sheikhi A.; Kannan S.; Ahadian S.; Khademhosseini A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics. Bioengineering & translational medicine 2019, 4 (1), 96–115. 10.1002/btm2.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery L. D.; Bishop A. E.. Introduction to Tissue Engineering. In Biomaterials, Artificial Organs and Tissue Engineering; Hench L. L., Jones J. R., Eds.; Woodhead Publishing Series in Biomaterials, 2005; pp 193–200. [Google Scholar]
- Kargozar S.; Mozafari M.; Hamzehlou S.; Brouki Milan P.; Kim H.-W.; Baino F. Bone tissue engineering using human cells: a comprehensive review on recent trends, current prospects, and recommendations. Applied Sciences 2019, 9 (1), 174 10.3390/app9010174. [DOI] [Google Scholar]
- Florencio-Silva R.; Sasso G. R. d. S.; Sasso-Cerri E.; Simões M. J.; Cerri P. S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed. research international 2015, 2015, 421746 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoichan A.; Baudequin T.; Al-Jallad H.; Tabrizian M. Encapsulation and differentiation of adipose-derived mesenchymal stem cells in a biomimetic purine cross-linked chitosan sponge. J. Biomed. Mater. Res., Part A 2022, 110 (3), 585–594. 10.1002/jbm.a.37311. [DOI] [PubMed] [Google Scholar]
- Roberts T. T.; Rosenbaum A. J. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8 (4), 114–124. 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya U. R.; Mishra S.; Bano S.. Application of Bone Substitutes and Its Future Prospective in Regenerative Medicine. In Biomaterial-supported Tissue Reconstruction or Regeneration; IntechOpen, 2019; p 85092. [Google Scholar]
- Khan W. S.; Rayan F.; Dhinsa B. S.; Marsh D. An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: how far are we?. Stem cells international 2012, 2012, 236231 10.1155/2012/236231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrektsson T.; Johansson C. Osteoinduction, osteoconduction and osseointegration. European spine journal 2001, 10 (2), S96–S101. 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg T.; Singh O.; Arora S.; Murthy R. Scaffold: a novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2012, 29 (1), 1–63. 10.1615/CritRevTherDrugCarrierSyst.v29.i1.10. [DOI] [PubMed] [Google Scholar]
- Mishra R.; Bishop T.; Valerio I. L.; Fisher J. P.; Dean D. The potential impact of bone tissue engineering in the clinic. Regenerative medicine 2016, 11 (6), 571–587. 10.2217/rme-2016-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R.; Huang Y.; Ma C.; Wu C.; Tian W. Current advances for bone regeneration based on tissue engineering strategies. Frontiers of medicine 2019, 13 (2), 160–188. 10.1007/s11684-018-0629-9. [DOI] [PubMed] [Google Scholar]
- Willie B. M.; Petersen A.; Schmidt-Bleek K.; Cipitria A.; Mehta M.; Strube P.; Lienau J.; Wildemann B.; Fratzl P.; Duda G. Designing biomimetic scaffolds for bone regeneration: why aim for a copy of mature tissue properties if nature uses a different approach?. Soft Matter 2010, 6 (20), 4976–4987. 10.1039/c0sm00262c. [DOI] [Google Scholar]
- Hayashi Y.; Yamada S.; Guchi K. Y.; Koyama Z.; Ikeda T.. Chitosan and fish collagen as biomaterials for regenerative medicine. In Advances in food and nutrition research; Elsevier, 2012; Vol. 65, pp 107–120. [DOI] [PubMed] [Google Scholar]
- Jiang T.; James R.; Kumbar S. G.; Laurencin C. T.. Chitosan as a biomaterial: structure, properties, and applications in tissue engineering and drug delivery. In Natural and synthetic biomedical polymers; Elsevier, 2014; pp 91–113. [Google Scholar]
- Ahmed S.; Ali A.; Sheikh J.; et al. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. 10.1016/j.ijbiomac.2018.04.176. [DOI] [PubMed] [Google Scholar]
- Ibrahim H.; El-Zairy E.. Chitosan as a biomaterial—structure, properties, and electrospun nanofibers. In Concepts, Compounds, and the Alternatives of Antibacterials; IntechOpen, 2015; p 61300. [Google Scholar]
- Dutta P.; Rinki K.; Dutta J.. Chitosan: A promising biomaterial for tissue engineering scaffolds. In Chitosan for biomaterials II; Springer, 2011; pp 45–79. [Google Scholar]
- Benameur L.; Baudequin T.; Mekhail M.; Tabrizian M. The bioconjugation mechanism of purine cross-linkers affects microstructure and cell response to ultra rapidly gelling purine–chitosan sponges. J. Mater. Chem. B 2018, 6 (4), 602–613. 10.1039/C7TB02968C. [DOI] [PubMed] [Google Scholar]
- Mekhail M.; Jahan K.; Tabrizian M. Genipin-crosslinked chitosan/poly-l-lysine gels promote fibroblast adhesion and proliferation. Carbohydr. Polym. 2014, 108, 91–98. 10.1016/j.carbpol.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Jahan K.; Mekhail M.; Tabrizian M. One-step fabrication of apatite-chitosan scaffold as a potential injectable construct for bone tissue engineering. Carbohydr. Polym. 2019, 203, 60–70. 10.1016/j.carbpol.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Croisier F.; Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49 (4), 780–792. 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- Ahsan S. M.; Thomas M.; Reddy K. K.; Sooraparaju S. G.; Asthana A.; Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- LogithKumar R.; KeshavNarayan A.; Dhivya S.; Chawla A.; Saravanan S.; Selvamurugan N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. 10.1016/j.carbpol.2016.05.049. [DOI] [PubMed] [Google Scholar]
- Dash M.; Chiellini F.; Ottenbrite R. M.; Chiellini E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36 (8), 981–1014. 10.1016/j.progpolymsci.2011.02.001. [DOI] [Google Scholar]
- Pérez-Álvarez L.; Ruiz-Rubio L.; Vilas-Vilela J. L. Determining the Deacetylation Degree of Chitosan: Opportunities To Learn Instrumental Techniques. J. Chem. Educ. 2018, 95 (6), 1022–1028. 10.1021/acs.jchemed.7b00902. [DOI] [Google Scholar]
- Lv S. H.7 - High-performance superplasticizer based on chitosan. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal F., Ivanov V., Karak N., Jonkers H., Eds.; Woodhead Publishing, 2016; pp 131–150. [Google Scholar]
- Oryan A.; Kamali A.; Moshiri A.; Baharvand H.; Daemi H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. 10.1016/j.ijbiomac.2017.08.184. [DOI] [PubMed] [Google Scholar]
- Acevedo C. A.; Olguín Y.; Briceño M.; Forero J. C.; Osses N.; Díaz-Calderón P.; Jaques A.; Ortiz R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Materials Science and Engineering: C 2019, 99, 875–886. 10.1016/j.msec.2019.01.135. [DOI] [PubMed] [Google Scholar]
- Krishnakumar G. S.; Sampath S.; Muthusamy S.; John M. A. Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: A systematic review. Materials Science and Engineering: C 2019, 96, 941–954. 10.1016/j.msec.2018.11.081. [DOI] [PubMed] [Google Scholar]
- Li Z.; Ramay H. R.; Hauch K. D.; Xiao D.; Zhang M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26 (18), 3919–3928. 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Calori I. R.; Braga G.; de Jesus P. d. C. C.; Bi H.; Tedesco A. C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621 10.1016/j.eurpolymj.2020.109621. [DOI] [Google Scholar]
- Fang Y.; Zhang T.; Song Y.; Sun W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomedical Materials 2020, 15 (4), 045003 10.1088/1748-605X/ab452d. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Zhang L.; Ji Y.; Deng H.; Long M.; Ge S.; Su Y.; Chan S. Y.; Loh X. J.; Zhuang A.; Ruan J. A non-invasive smart scaffold for bone repair and monitoring. Bioactive Materials 2023, 19, 499–510. 10.1016/j.bioactmat.2022.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Xiao C.; Huang Y.; Chen M.; Wei W.; Yang X.; Zhou H.; Bi X.; Lu L.; Ruan J.; Fan X. Enhanced bioactivity and osteoinductivity of carboxymethyl chitosan/nanohydroxyapatite/graphene oxide nanocomposites. RSC Adv. 2018, 8 (32), 17860–17877. 10.1039/C8RA00383A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.; Liu K.; Chen J.; Li Y.; Liu M.; Lu L.; Zhou C.; Luo B. Dual-Cross-linked Liquid Crystal Hydrogels with Controllable Viscoelasticity for Regulating Cell Behaviors. ACS Appl. Mater. Interfaces 2022, 14, 21966–21977. 10.1021/acsami.2c02689. [DOI] [PubMed] [Google Scholar]
- Vukajlovic D.; Bretcanu O.; Novakovic K. Fabrication and characterization of two types of bone composites made of chitosan-genipin hydrogel and Bioglass 45S5. Open Ceramics 2021, 8, 100174 10.1016/j.oceram.2021.100174. [DOI] [Google Scholar]
- Marques C. F.; Olhero S. M.; Torres P. M. C.; Abrantes J. C. C.; Fateixa S.; Nogueira H. I. S.; Ribeiro I. A. C.; Bettencourt A.; Sousa A.; Granja P. L.; Ferreira J. M. F. Novel sintering-free scaffolds obtained by additive manufacturing for concurrent bone regeneration and drug delivery: Proof of concept. Materials Science and Engineering C 2019, 94, 426–436. 10.1016/j.msec.2018.09.050. [DOI] [PubMed] [Google Scholar]
- Lu H. T.; Lu T. W.; Chen C. H.; Mi F. L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. 10.1016/j.ijbiomac.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Siddiqui N.; Pramanik K. Improvement of cellular responses of genipin cross-linked chitosan/nano β-TCP composite scaffolds by surface modification with fibrin. Biomedical Physics and Engineering Express 2018, 4 (4), 045034 10.1088/2057-1976/aacad1. [DOI] [Google Scholar]
- Zhang N.; Yao R.; Guo J.; He J.; Meng G.; Wu F. Modulation of osteogenic and haemostatic activities by tuning cationicity of genipin-crosslinked chitosan hydrogels. Colloids Surf., B 2018, 166, 29–36. 10.1016/j.colsurfb.2018.02.056. [DOI] [PubMed] [Google Scholar]
- Dimida S.; Barca A.; Cancelli N.; De Benedictis V.; Raucci M. G.; Demitri C. Effects of genipin concentration on cross-linked chitosan scaffolds for bone tissue engineering: Structural characterization and evidence of biocompatibility features. International Journal of Polymer Science 2017, 2017, 8410750. 10.1155/2017/8410750. [DOI] [Google Scholar]
- Dimida S.; Barca A.; Cancelli N.; De Benedictis V.; Raucci M. G.; Demitri C. Effects of genipin concentration on cross-linked chitosan scaffolds for bone tissue engineering: Structural characterization and evidence of biocompatibility features. International Journal of Polymer Science 2017, 2017, 8410750 10.1155/2017/8410750. [DOI] [Google Scholar]
- Liu I. H.; Chang S. H.; Lin H. Y. Chitosan-based hydrogel tissue scaffolds made by 3D plotting promotes osteoblast proliferation and mineralization. Biomedical Materials (Bristol) 2015, 10 (3), 035004 10.1088/1748-6041/10/3/035004. [DOI] [PubMed] [Google Scholar]
- Beringer L. T.; Kiechel M. A.; Komiya Y.; Donius A. E.; Habas R.; Wegst U. G. K.; Schauer C. L. Osteoblast biocompatibility of novel chitosan crosslinker, hexamethylene-1,6-diaminocarboxysulfonate. Journal of Biomedical Materials Research - Part A 2015, 103 (9), 3026–3033. 10.1002/jbm.a.35438. [DOI] [PubMed] [Google Scholar]
- Siddiqui N.; Pramanik K.; Jabbari E. Osteogenic differentiation of human mesenchymal stem cells in freeze-gelled chitosan/nano β-tricalcium phosphate porous scaffolds crosslinked with genipin. Materials Science and Engineering C 2015, 54, 76–83. 10.1016/j.msec.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohbergh M. E.; Katsman A.; Mondrinos M. J.; Stabler C. T.; Hankenson K. D.; Oristaglio J. T.; Lelkes P. I. Osseointegrative properties of electrospun hydroxyapatite-containing nanofibrous chitosan scaffolds. Tissue Engineering - Part A 2015, 21 (5–6), 970–981. 10.1089/ten.tea.2013.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Qiu J.; Zheng L.; Ren N.; Li J.; Liu H.; Miao J. Sustained delivery of BMP-2 enhanced osteoblastic differentiation of BMSCs based on surface hydroxyapatite nanostructure in chitosan-HAp scaffold. Journal of Biomaterials Science, Polymer Edition 2014, 25 (16), 1813–1827. 10.1080/09205063.2014.951244. [DOI] [PubMed] [Google Scholar]
- Su W. T.; Wang Y. T.; Chou C. M. Optimal fluid flow enhanced mineralization of MG-63 cells in porous chitosan scaffold. Journal of the Taiwan Institute of Chemical Engineers 2014, 45 (4), 1111–1118. 10.1016/j.jtice.2013.10.016. [DOI] [Google Scholar]
- Hu J.; Wang Z.; Miszuk J. M.; Zhu M.; Lansakara T. I.; Tivanski A. V.; Banas J. A.; Sun H. Vanillin-bioglass cross-linked 3D porous chitosan scaffolds with strong osteopromotive and antibacterial abilities for bone tissue engineering. Carbohydr. Polym. 2021, 271, 118440 10.1016/j.carbpol.2021.118440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Llano C. H.; Solano M. A.; Grande-Tovar C. D. Nanocomposites of chitosan/graphene oxide/titanium dioxide nanoparticles/blackberry waste extract as potential bone substitutes. Polymers 2021, 13 (22), 3877 10.3390/polym13223877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R. V.; Gomes P. S.; Fernandes M. H.; Costa M. E. V.; Almeida M. M. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Materials Science and Engineering C 2020, 109, 110557 10.1016/j.msec.2019.110557. [DOI] [PubMed] [Google Scholar]
- Chen C. K.; Chang N. J.; Wu Y. T.; Fu E.; Shen E. C.; Feng C. W.; Wen Z. H. Bone formation using cross-linked chitosan scaffolds in rat calvarial defects. Implant Dentistry 2018, 27 (1), 15–21. 10.1097/ID.0000000000000670. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liu T.; Zheng J.; Xu X. Glutaraldehyde-crosslinked chitosan/hydroxyapatite bone repair scaffold and its application as drug carrier for icariin. J. Appl. Polym. Sci. 2013, 130 (3), 1539–1547. 10.1002/app.39339. [DOI] [Google Scholar]
- Zeng J.; Mamitimin M.; Song Y.; Sun W.; Wu Z.; Qi X. Chairside administrated antibacterial hydrogels containing berberine as dental temporary stopping for alveolar ridge preservation. Eur. Polym. J. 2021, 160, 110808 10.1016/j.eurpolymj.2021.110808. [DOI] [Google Scholar]
- Ghosh P.; Rameshbabu A. P.; Das D.; Francis N. K.; Pawar H. S.; Subramanian B.; Pal S.; Dhara S. Covalent cross-links in polyampholytic chitosan fibers enhances bone regeneration in a rabbit model. Colloids Surf., B 2015, 125, 160–169. 10.1016/j.colsurfb.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Karoichan A.; Baudequin T.; Al-Jallad H.; Tabrizian M. Encapsulation and differentiation of adipose-derived mesenchymal stem cells in a biomimetic purine cross-linked chitosan sponge. Journal of Biomedical Materials Research - Part A 2022, 110 (3), 585–594. 10.1002/jbm.a.37311. [DOI] [PubMed] [Google Scholar]
- Baudequin T.; Agnes C.; Tabrizian M. A core-shell guanosine diphosphate crosslinked chitosan scaffold as a potential co-encapsulation platform. Carbohydr. Polym. 2021, 256, 117499 10.1016/j.carbpol.2020.117499. [DOI] [PubMed] [Google Scholar]
- Jahan K.; Manickam G.; Tabrizian M.; Murshed M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci. Rep. 2020, 10, 11603 10.1038/s41598-020-67886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnes C. J.; Murshed M.; Takada A.; Willie B. M.; Tabrizian M. A 6-bromoindirubin-3′-oxime incorporated chitosan-based hydrogel scaffold for potential osteogenic differentiation: Investigation of material properties in vitro. Int. J. Biol. Macromol. 2023, 227, 71–82. 10.1016/j.ijbiomac.2022.12.130. [DOI] [PubMed] [Google Scholar]
- Lewandowska-ŁAńcucka J.; Fiejdasz S.; Rodzik L.; Kozieł M.; Nowakowska M. Bioactive hydrogel-nanosilica hybrid materials: A potential injectable scaffold for bone tissue engineering. Biomedical Materials (Bristol) 2015, 10 (1), 015020 10.1088/1748-6041/10/1/015020. [DOI] [PubMed] [Google Scholar]
- Yasmeen S.; Lo M. K.; Bajracharya S.; Roldo M. Injectable scaffolds for bone regeneration. Langmuir 2014, 30 (43), 12977–12985. 10.1021/la503057w. [DOI] [PubMed] [Google Scholar]
- Dessì M.; Borzacchiello A.; Mohamed T. H. A.; Abdel-Fattah W. I.; Ambrosio L. Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. Journal of Biomedical Materials Research - Part A 2013, 101 (10), 2984–2993. 10.1002/jbm.a.34592. [DOI] [PubMed] [Google Scholar]
- Goh C. Y.; Lim S. S.; Tshai K. Y.; El Azab A. W. Z. Z.; Loh H. S. Fabrication and in vitro biocompatibility of sodium tripolyphosphate-crosslinked chitosan–hydroxyapatite scaffolds for bone regeneration. J. Mater. Sci. 2019, 54 (4), 3403–3420. 10.1007/s10853-018-3087-5. [DOI] [Google Scholar]
- Shemshad S.; Kamali S.; Khavandi A.; Azari S. Synthesis, characterization and in-vitro behavior of natural chitosan-hydroxyapatite-diopside nanocomposite scaffold for bone tissue engineering. International Journal of Polymeric Materials and Polymeric Biomaterials 2019, 68 (9), 516–526. 10.1080/00914037.2018.1466138. [DOI] [Google Scholar]
- Shavandi A.; Bekhit A. E. D. A.; Sun Z.; Ali M. A. Bio-scaffolds produced from irradiated squid pen and crab chitosan with hydroxyapatite/β-tricalcium phosphate for bone-tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1446–1456. 10.1016/j.ijbiomac.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Thanyaphoo S.; Kaewsrichan J. A new biocompatible delivery scaffold containing heparin and bone morphogenetic protein 2. Acta Pharmaceutica 2016, 66 (3), 373–385. 10.1515/acph-2016-0026. [DOI] [PubMed] [Google Scholar]
- Aryaei A.; Liu J.; Jayatissa A. H.; Champa Jayasuriya A. Cross-linked chitosan improves the mechanical properties of calcium phosphate-chitosan cement. Materials Science and Engineering C 2015, 54, 14–19. 10.1016/j.msec.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati F.; Kalita H.; Adhikari B.; Dhara S. Osteoblastic cellular responses on ionically crosslinked chitosan-tripolyphosphate fibrous 3-D mesh scaffolds. Journal of Biomedical Materials Research - Part A 2013, 101A (9), 2526–2537. 10.1002/jbm.a.34559. [DOI] [PubMed] [Google Scholar]
- D’Mello S.; Elangovan S.; Hong L.; Ross R. D.; Sumner D. R.; Salem A. K. Incorporation of copper into chitosan scaffolds promotes bone regeneration in rat calvarial defects. Journal of Biomedical Materials Research - Part B Applied Biomaterials 2015, 103 (5), 1044–1049. 10.1002/jbm.b.33290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Q.; Tan R.; Zhang Y.; Wang C.; Wan Y.; Li J. Multi-Crosslinked Strong and Elastic Bioglass/Chitosan-Cysteine Hydrogels with Controlled Quercetin Delivery for Bone Tissue Engineering. Pharmaceutics 2022, 14 (10), 2048 10.3390/pharmaceutics14102048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakeçili A.; Korpayev S.; Orhan K. Optimizing Chitosan/Collagen Type I/Nanohydroxyapatite Cross-linked Porous Scaffolds for Bone Tissue Engineering. Appl. Biochem. Biotechnol. 2022, 194 (9), 3843–3859. 10.1007/s12010-022-03962-0. [DOI] [PubMed] [Google Scholar]
- Grabska-Zielińska S.; Sionkowska A.; Carvalho A.; Monteiro F. J. Biomaterials with potential use in bone tissue regeneration-collagen/chitosan/silk fibroin scaffolds cross-linked by EDC/NHS. Materials 2021, 14 (5), 1–21. 10.3390/ma14051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Tan H.; Xu W.; Wang Z.; Zhang J.; Li S.; Zhou T.; li J.; Niu X. A self-healing, magnetic and injectable biopolymer hydrogel generated by dual cross-linking for drug delivery and bone repair. Acta Biomaterialia 2022, 153, 159–177. 10.1016/j.actbio.2022.09.036. [DOI] [PubMed] [Google Scholar]
- An P.; Wei H.; Zhang Y.; Zhou Y.; Zhang H.; Li W.; Niu B.; Chen J. A mechanically adaptive “all-sugar” hydrogel for cell-laden injection. Eur. Polym. J. 2022, 174, 111328 10.1016/j.eurpolymj.2022.111328. [DOI] [Google Scholar]
- Zhang Q.; Pei Q.; Yang J.; Guo S.; Yang A.; Qian Y.; Li C.; Feng Q.; Lv H.; Zhou X.; He C. Vascularized nanocomposite hydrogel mechanically reinforced by polyelectrolyte-modified nanoparticles. J. Mater. Chem. B 2022, 10 (28), 5439–5453. 10.1039/D2TB00735E. [DOI] [PubMed] [Google Scholar]
- Nair P. R.; Sreeja S.; Sailaja G. S. Early biomineralizing chitosan-collagen hybrid scaffold withCissus quadrangularisextract for regenerative bone tissue engineering. New J. Chem. 2021, 45 (42), 19733–19745. 10.1039/D1NJ03687D. [DOI] [Google Scholar]
- Grabska-Zielińska S.; Sionkowska A.; Coelho C. C.; Monteiro F. J. Silk fibroin/collagen/chitosan scaffolds cross-linked by a glyoxal solution as biomaterials toward bone tissue regeneration. Materials 2020, 13 (15), 1–20. 10.3390/ma13153433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banafati Zadeh F.; Zamanian A. Glutaraldehyde: Introducing Optimum Condition for Cross-linking the Chitosan/Gelatin Scaffolds for Bone Tissue Engineering. International Journal of Engineering, Transactions A: Basics 2022, 35 (10), 1967–1980. 10.5829/IJE.2022.35.10A.15. [DOI] [Google Scholar]
- Loukelis K.; Papadogianni D.; Chatzinikolaidou M. Kappa-carrageenan/chitosan/gelatin scaffolds enriched with potassium chloride for bone tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1720–1730. 10.1016/j.ijbiomac.2022.04.129. [DOI] [PubMed] [Google Scholar]
- Doustdar F.; Olad A.; Ghorbani M. Effect of glutaraldehyde and calcium chloride as different crosslinking agents on the characteristics of chitosan/cellulose nanocrystals scaffold. Int. J. Biol. Macromol. 2022, 208, 912–924. 10.1016/j.ijbiomac.2022.03.193. [DOI] [PubMed] [Google Scholar]
- Rahman M. S.; Rana M. M.; Spitzhorn L. S.; Akhtar N.; Hasan M. Z.; Choudhury N.; Fehm T.; Czernuszka J. T.; Adjaye J.; Asaduzzaman S. M. Fabrication of biocompatible porous scaffolds based on hydroxyapatite/collagen/chitosan composite for restoration of defected maxillofacial mandible bone. Progress in Biomaterials 2019, 8 (3), 137–154. 10.1007/s40204-019-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulou A.; Papadogiannis F.; Batsali A.; Marakis J.; Alpantaki K.; Eliopoulos A. G.; Pontikoglou C.; Chatzinikolaidou M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci.: Mater. Med. 2018, 29 (5), 59 10.1007/s10856-018-6064-2. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Fang H.; Zhang K.; Yin J. Homologous Sodium Alginate/Chitosan-Based Scaffolds, but Contrasting Effect on Stem Cell Shape and Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10 (8), 6930–6941. 10.1021/acsami.7b18859. [DOI] [PubMed] [Google Scholar]
- Wang B.; Guo Y.; Chen X.; Zeng C.; Hu Q.; Yin W.; Li W.; Xie H.; Zhang B.; Huang X.; Yu F. Nanoparticle-modified chitosan-agarose-gelatin scaffold for sustained release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. 10.2147/IJN.S180859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji K.; Dasgupta S.; Pramanik K.; Bissoyi A. Preparation and Evaluation of Gelatin-Chitosan-Nanobioglass 3D Porous Scaffold for Bone Tissue Engineering. International Journal of Biomaterials 2016, 2016, 9825659 10.1155/2016/9825659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva A. R. P.; Macedo T. L.; Coletta D. J.; Feldman S.; Pereira M. M. Synthesis, characterization and cytotoxicity of Chitosan/Polyvinyl alcohol/bioactive glass hybrid scaffolds obtained by Lyophilization. Revista Materia 2016, 21 (4), 964–973. 10.1590/s1517-707620160004.0089. [DOI] [Google Scholar]
- Zhang S.; Zhao G.; Ma W.; Song Y.; Huang C.; Xie C.; Chen K.; Li X. The root-like chitosan nanofiber porous scaffold cross-linked by genipin with type I collagen and its osteoblast compatibility. Carbohydr. Polym. 2022, 285, 119255 10.1016/j.carbpol.2022.119255. [DOI] [PubMed] [Google Scholar]
- Krajcer A.; Klara J.; Horak W.; Lewandowska-Łańcucka J. Bioactive injectable composites based on insulin-functionalized silica particles reinforced polymeric hydrogels for potential applications in bone tissue engineering. Journal of Materials Science and Technology 2022, 105, 153–163. 10.1016/j.jmst.2021.08.003. [DOI] [Google Scholar]
- Shi W.; Zhang X.; Bian L.; Dai Y.; Wang Z.; Zhou Y.; Yu S.; Zhang Z.; Zhao P.; Tang H.; Wang Q.; Lu X. Alendronate crosslinked chitosan/polycaprolactone scaffold for bone defects repairing. Int. J. Biol. Macromol. 2022, 204, 441–456. 10.1016/j.ijbiomac.2022.02.007. [DOI] [PubMed] [Google Scholar]
- S̨elaru A.; Herman H.; Vlăsceanu G. M.; Dinescu S.; Gharbia S.; Baltă C.; Roşu M.; Mihali C. V.; Ionită M.; Serafim A.; Iovu H.; Hermenean A.; Costache M. Graphene–oxide porous biopolymer hybrids enhance in vitro osteogenic differentiation and promote ectopic osteogenesis in vivo. International Journal of Molecular Sciences 2022, 23 (1), 491 10.3390/ijms23010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilarska A.; Hinz A.; Bzowska M.; Dyduch G.; Kamiński K.; Nowakowska M.; Lewandowska-ŁAńcucka J. Addressing the Osteoporosis Problem-Multifunctional Injectable Hybrid Materials for Controlling Local Bone Tissue Remodeling. ACS Appl. Mater. Interfaces 2021, 13 (42), 49762–49779. 10.1021/acsami.1c17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Yu X.; Tian D.; Yu A.; Wan Y. Thermo-responsive chitosan/silk fibroin/amino-functionalized mesoporous silica hydrogels with strong and elastic characteristics for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1746–1758. 10.1016/j.ijbiomac.2021.05.166. [DOI] [PubMed] [Google Scholar]
- Datta S.; Rameshbabu A. P.; Bankoti K.; Roy M.; Gupta C.; Jana S.; Das A. K.; Sen R.; Dhara S. Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration. Materials Science and Engineering C 2021, 119, 111604 10.1016/j.msec.2020.111604. [DOI] [PubMed] [Google Scholar]
- Vlasceanu G. M.; S̨elaru A.; Dinescu S.; Balta C.; Herman H.; Gharbia S.; Hermenean A.; Ionita M.; Costache M. Comprehensive appraisal of graphene–oxide ratio in porous biopolymer hybrids targeting bone-tissue regeneration. Nanomaterials 2020, 10 (8), 1–19. 10.3390/nano10081444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowska-Łańcucka J.; Gilarska A.; Buła A.; Horak W.; Łatkiewicz A.; Nowakowska M. Genipin crosslinked bioactive collagen/chitosan/hyaluronic acid injectable hydrogels structurally amended via covalent attachment of surface-modified silica particles. Int. J. Biol. Macromol. 2019, 136, 1196–1208. 10.1016/j.ijbiomac.2019.06.184. [DOI] [PubMed] [Google Scholar]
- Gilarska A.; Lewandowska-Łańcucka J.; Guzdek-Zając K.; Karewicz A.; Horak W.; Lach R.; Wójcik K.; Nowakowska M. Bioactive yet antimicrobial structurally stable collagen/chitosan/lysine functionalized hyaluronic acid – based injectable hydrogels for potential bone tissue engineering applications. Int. J. Biol. Macromol. 2020, 155, 938–950. 10.1016/j.ijbiomac.2019.11.052. [DOI] [PubMed] [Google Scholar]
- Filipowska J.; Lewandowska-Łańcucka J.; Gilarska A.; Niedźwiedzki Ł.; Nowakowska M. In vitro osteogenic potential of collagen/chitosan-based hydrogels-silica particles hybrids in human bone marrow-derived mesenchymal stromal cell cultures. Int. J. Biol. Macromol. 2018, 113, 692–700. 10.1016/j.ijbiomac.2018.02.161. [DOI] [PubMed] [Google Scholar]
- Zazakowny K.; Lewandowska-Łańcucka J.; Mastalska-Popławska J.; Kamiński K.; Kusior A.; Radecka M.; Nowakowska M. Biopolymeric hydrogels – nanostructured TiO2 hybrid materials as potential injectable scaffolds for bone regeneration. Colloids Surf., B 2016, 148, 607–614. 10.1016/j.colsurfb.2016.09.031. [DOI] [PubMed] [Google Scholar]
- Nath S. D.; Abueva C.; Kim B.; Lee B. T. Chitosan-hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr. Polym. 2015, 115, 207–214. 10.1016/j.carbpol.2014.08.077. [DOI] [PubMed] [Google Scholar]
- Xiong A.; He Y.; Gao L.; Li G.; Liu S.; Weng J.; Wang D.; Zeng H. The fabrication of a highly efficient hydrogel based on a functionalized double network loaded with magnesium ion and BMP2 for bone defect synergistic treatment. Materials Science and Engineering C 2021, 128, 112347 10.1016/j.msec.2021.112347. [DOI] [PubMed] [Google Scholar]
- Ghorbani F.; Pourhaghgouy M.; Mohammadi-hafshehjani T.; Zamanian A. Effect of Silane-Coupling Modification on the Performance of chitosan-poly vinyl Alcohol-Hybrid Scaffolds in Bone Tissue Engineering. Silicon 2020, 12 (12), 3015–3026. 10.1007/s12633-020-00397-2. [DOI] [Google Scholar]
- Shokri S.; Movahedi B.; Rafieinia M.; Salehi H. A new approach to fabrication of Cs/BG/CNT nanocomposite scaffold towards bone tissue engineering and evaluation of its properties. Appl. Surf. Sci. 2015, 357, 1758–1764. 10.1016/j.apsusc.2015.10.048. [DOI] [Google Scholar]
- Nie L.; Wu Q.; Long H.; Hu K.; Li P.; Wang C.; Sun M.; Dong J.; Wei X.; Suo J.; Hua D.; Liu S.; Yuan H.; Yang S. Development of chitosan/gelatin hydrogels incorporation of biphasic calcium phosphate nanoparticles for bone tissue engineering. Journal of Biomaterials Science, Polymer Edition 2019, 30 (17), 1636–1657. 10.1080/09205063.2019.1654210. [DOI] [PubMed] [Google Scholar]
- Kosowska K.; Domalik-Pyzik P.; Krok-Borkowicz M.; Chłopek J. Polylactide/hydroxyapatite nonwovens incorporated into chitosan/graphene materials hydrogels to form novel hierarchical scaffolds. International Journal of Molecular Sciences 2020, 21 (7), 2330 10.3390/ijms21072330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y.; Wang M.; Liu W.; Chen C.; Cui W.; Sun T.; Feng Q.; Guo X. Chitosan/nHAC/PLGA microsphere vehicle for sustained release of rhBMP-2 and its derived synthetic oligopeptide for bone regeneration. Journal of Biomedical Materials Research - Part A 2017, 105 (6), 1593–1606. 10.1002/jbm.a.35962. [DOI] [PubMed] [Google Scholar]
- Ali H. U.; Iqbal D. N.; Iqbal M.; Ezzine S.; Arshad A.; Zeeshan R.; Chaudhry A. A.; Alshawwa S. Z.; Nazir A.; Khan A. F. HPMC crosslinked chitosan/hydroxyapatite scaffolds containing Lemongrass oil for potential bone tissue engineering applications. Arabian Journal of Chemistry 2022, 15 (7), 103850 10.1016/j.arabjc.2022.103850. [DOI] [Google Scholar]
- Zeeshan R.; Mutahir Z.; Iqbal H.; Ali M.; Iqbal F.; Ijaz K.; Sharif F.; Shah A. T.; Chaudhry A. A.; Yar M.; Luan S.; Khan A. F.; Ihtesham ur R. Hydroxypropylmethyl cellulose (HPMC) crosslinked chitosan (CH) based scaffolds containing bioactive glass (BG) and zinc oxide (ZnO) for alveolar bone repair. Carbohydr. Polym. 2018, 193, 9–18. 10.1016/j.carbpol.2018.03.046. [DOI] [PubMed] [Google Scholar]
- Kolanthai E.; Sindu P. A.; Khajuria D. K.; Veerla S. C.; Kuppuswamy D.; Catalani L. H.; Mahapatra D. R. Graphene Oxide - A Tool for the Preparation of Chemically Crosslinking Free Alginate-Chitosan-Collagen Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2018, 10 (15), 12441–12452. 10.1021/acsami.8b00699. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Li L.; Zhu Y.; Wang X.; Li M.; Lin Z.; Hu X.; Zhang Y.; Yin Q.; Xia H.; Mao C. Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl. Mater. Interfaces 2018, 10 (1), 127–138. 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Cui Z. K.; Koo B.; Zheng J.; Aghaloo T.; Lee M. Chitosan-Lysozyme Conjugates for Enzyme-Triggered Hydrogel Degradation in Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10 (48), 41138–41145. 10.1021/acsami.8b15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. T.; Lu T. W.; Chen C. H.; Lu K. Y.; Mi F. L. Development of nanocomposite scaffolds based on biomineralization of N,O-carboxymethyl chitosan/fucoidan conjugates for bone tissue engineering. Int. J. Biol. Macromol. 2018, 120, 2335–2345. 10.1016/j.ijbiomac.2018.08.179. [DOI] [PubMed] [Google Scholar]
- Pita-López M. L.; Fletes-Vargas G.; Espinosa-Andrews H.; Rodriguez-Rodriguez R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176 10.1016/j.eurpolymj.2020.110176. [DOI] [Google Scholar]
- Tejo-Otero A.; Ritchie A. C. Biological and mechanical evaluation of mineralized-hydrogel scaffolds for tissue engineering applications. Journal of Biomaterials Applications 2021, 36 (3), 460–473. 10.1177/0885328221995425. [DOI] [PubMed] [Google Scholar]
- Pizzolitto C.; Scognamiglio F.; Sacco P.; Lipari S.; Romano M.; Donati I.; Marsich E. Immediate stress dissipation in dual cross-link hydrogels controls osteogenic commitment of mesenchymal stem cells. Carbohydr. Polym. 2023, 302, 120369 10.1016/j.carbpol.2022.120369. [DOI] [PubMed] [Google Scholar]
- Azaman F. A.; Zhou K.; Blanes-Martínez M. D. M.; Brennan Fournet M.; Devine D. M. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels 2022, 8 (11), 696 10.3390/gels8110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak F. Z.; Yar M.; Rehman I. U. Hydroxyapatite-Integrated, Heparin-and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. International Journal of Molecular Sciences 2022, 23 (10), 5370 10.3390/ijms23105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.; Chen R.; Wang W.; Liu Y.; Shi C.; Tang S.; Tang G. Fabrication of Naturally Derived Double-Network Hydrogels With a Sustained Aspirin Release System for Facilitating Bone Regeneration. Frontiers in Chemistry 2022, 10, 874985 10.3389/fchem.2022.874985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer S.; Athipornchai A.; Pabunrueang P.; Trakulsujaritchok T. Development of mangiferin loaded chitosan-silica hybrid scaffolds: Physicochemical and bioactivity characterization. Carbohydr. Polym. 2021, 261, 117905 10.1016/j.carbpol.2021.117905. [DOI] [PubMed] [Google Scholar]
- Li Y.; Sun S.; Gao P.; Zhang M.; Fan C.; Lu Q.; Li C.; Chen C.; Lin B.; Jiang Y. A tough chitosan-alginate porous hydrogel prepared by simple foaming method. J. Solid State Chem. 2021, 294, 121797 10.1016/j.jssc.2020.121797. [DOI] [Google Scholar]
- Suneetha M.; Rao K. M.; Han S. S. Mechanically improved porous hydrogels with polysaccharides via polyelectrolyte complexation for bone tissue engineering. Int. J. Biol. Macromol. 2020, 144, 160–169. 10.1016/j.ijbiomac.2019.12.096. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Huang P.; Jiang G.; Zhang M.; Yu F.; Dong X.; Wang L.; Chen Y.; Zhang W.; Qi Y.; Li W.; Zeng H. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Materials Science and Engineering C 2021, 121, 111868 10.1016/j.msec.2021.111868. [DOI] [PubMed] [Google Scholar]
- Patil T.; Saha S.; Biswas A. Preparation and Characterization of HAp Coated Chitosan-Alginate PEC Porous Scaffold for Bone Tissue Engineering. Macromol. Symp. 2017, 376 (1), 1600205 10.1002/masy.201600205. [DOI] [Google Scholar]
- Dorati R.; De Trizio A.; Genta I.; Merelli A.; Modena T.; Conti B. Formulation and in vitro characterization of a composite biodegradable scaffold as antibiotic delivery system and regenerative device for bone. Journal of Drug Delivery Science and Technology 2016, 35, 124–133. 10.1016/j.jddst.2016.04.008. [DOI] [Google Scholar]
- Lou C. W.; Wen S. P.; Lin J. H. Chitosan/gelatin porous bone scaffolds made by crosslinking treatment and freeze-drying technology: Effects of crosslinking durations on the porous structure, compressive strength, and in vitro cytotoxicity. J. Appl. Polym. Sci. 2015, 132 (17), 41851 10.1002/app.41851. [DOI] [Google Scholar]
- Kim S.; Kang Y.; Mercado-Pagán A. E.; Maloney W. J.; Yang Y. In vitro evaluation of photo-crosslinkable chitosan-lactide hydrogels for bone tissue engineering. Journal of Biomedical Materials Research - Part B Applied Biomaterials 2014, 102 (7), 1393–1406. 10.1002/jbm.b.33118. [DOI] [PubMed] [Google Scholar]
- Tsai W. B.; Chen Y. R.; Liu H. L. RGD-conjugated crosslinked chitosan scaffolds for culture and osteogenic differentiation of mesenchymal stem cells. Journal of the Taiwan Institute of Chemical Engineers 2013, 44 (1), 1–7. 10.1016/j.jtice.2012.09.003. [DOI] [Google Scholar]
- Fattahi R.; Soleimani M.; Khani M.-M.; Rasouli M.; Hosseinzadeh S. A three-dimensional structure with osteoconductive function made of O-carboxymethyl chitosan using aspirin as a cross-linker. International Journal of Polymeric Materials and Polymeric Biomaterials 2023, 1–17. 10.1080/00914037.2022.2155156. [DOI] [Google Scholar]
- Arpornmaeklong P.; Jaiman N.; Apinyauppatham K.; Fuongfuchat A.; Boonyuen S. Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels. Polymers 2023, 15 (2), 416 10.3390/polym15020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S.; Srivastava P.; Mishra A. Generation of bioactive porous chitosan/gelatin based scaffold modified with tri-calcium phosphate/nano-bioglass for bone tissue engineering applications. Journal of Porous Materials 2022, 1–15. 10.1007/s10934-022-01406-y. [DOI] [Google Scholar]
- Uswatta S. P.; Okeke I. U.; Jayasuriya A. C. Injectable porous nano-hydroxyapatite/chitosan/tripolyphosphate scaffolds with improved compressive strength for bone regeneration. Materials Science and Engineering: C 2016, 69, 505–512. 10.1016/j.msec.2016.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridade S. G.; Monge C.; Almodóvar J.; Guillot R.; Lavaud J.; Josserand V.; Coll J. L.; Mano J. F.; Picart C. Myoconductive and osteoinductive free-standing polysaccharide membranes. Acta Biomaterialia 2015, 15, 139–149. 10.1016/j.actbio.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond M. J.; Krebs M. D. Tunable chitosan-calcium phosphate composites as cell-instructive dental pulp capping agents. Journal of Biomaterials Science, Polymer Edition 2021, 32 (11), 1450–1465. 10.1080/09205063.2021.1925390. [DOI] [PubMed] [Google Scholar]
- Bi S.; Wang P.; Hu S.; Li S.; Pang J.; Zhou Z.; Sun G.; Huang L.; Cheng X.; Xing S.; et al. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019, 224, 115176 10.1016/j.carbpol.2019.115176. [DOI] [PubMed] [Google Scholar]
- Kosowska K.; Domalik-Pyzik P.; Krok-Borkowicz M.; Chłopek J. Synthesis and characterization of chitosan/reduced graphene oxide hybrid composites. Materials 2019, 12 (13), 2077 10.3390/ma12132077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Castillo S.; Bernal-Ballen A.; Bernal-López C.; Segura-Puello H.; Nieto-Mosquera D.; Villamil-Ballesteros A.; Muñoz-Forero D.; Munster L. Synthesis and characterization of poly(vinyl alcohol)-chitosan-hydroxyapatite scaffolds: a promising alternative for bone tissue regeneration. Molecules 2018, 23 (10), 2414 10.3390/molecules23102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechel M. A.; Beringer L. T.; Donius A. E.; Komiya Y.; Habas R.; Wegst U. G. K.; Schauer C. L. Osteoblast biocompatibility of premineralized, hexamethylene-1,6-diaminocarboxysulfonate crosslinked chitosan fibers. Journal of Biomedical Materials Research - Part A 2015, 103 (10), 3201–3211. 10.1002/jbm.a.35451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil S. V.; Brittain S. B.; Kan H. M.; Drissi H.; Rowe D. W.; Nair L. S. Evaluation of enzymatically crosslinked injectable glycol chitosan hydrogel. J. Mater. Chem. B 2015, 3 (27), 5511–5522. 10.1039/C5TB00663E. [DOI] [PubMed] [Google Scholar]
- Maturavongsadit P.; Paravyan G.; Shrivastava R.; Benhabbour S. R. Thermo-/pH-responsive chitosan-cellulose nanocrystals based hydrogel with tunable mechanical properties for tissue regeneration applications. Materialia 2020, 12, 100681 10.1016/j.mtla.2020.100681. [DOI] [Google Scholar]
- Song W.; Xiao Y. Sequential drug delivery of vancomycin and rhBMP-2 via pore-closed PLGA microparticles embedded photo-crosslinked chitosan hydrogel for enhanced osteointegration. Int. J. Biol. Macromol. 2021, 182, 612–625. 10.1016/j.ijbiomac.2021.03.181. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Méndez I.; Fernández-Gutiérrez M.; Rodríguez-Navarrete A.; Rosales-Ibáñez R.; Benito-Garzón L.; Vázquez-Lasa B.; Román J. S. Bioactive Sr(II)/chitosan/poly(ε-caprolactone) scaffolds for craniofacial tissue regeneration. In vitro and in vivo behavior. Polymers 2018, 10 (3), 279 10.3390/polym10030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S.; Maria K. H.; Ishtiaque M. S.; Nahar A.; Das H.; HOQUE S. Evaluation of a novel nanocrystalline hydroxyapatite powder and a solid hydroxyapatite/Chitosan-Gelatin bioceramic for scaffold preparation used as a bone substitute material. Turkish Journal of Chemistry 2020, 44 (4), 884–900. 10.3906/kim-1912-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadh S.; Wong R. C. W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Science International 2018, 15 (2), 48–55. 10.1016/S1348-8643(18)30005-3. [DOI] [Google Scholar]
- Babis G. C.; Soucacos P. N. Bone scaffolds: the role of mechanical stability and instrumentation. Injury 2005, 36 (4), S38–S44. 10.1016/j.injury.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Gerhardt L.-C.; Boccaccini A. R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3 (7), 3867–3910. 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L.; Wu Q.; Long H.; Hu K.; Li P.; Wang C.; Sun M.; Dong J.; Wei X.; Suo J.; et al. Development of chitosan/gelatin hydrogels incorporation of biphasic calcium phosphate nanoparticles for bone tissue engineering. Journal of Biomaterials Science, Polymer Edition 2019, 30 (17), 1636–1657. 10.1080/09205063.2019.1654210. [DOI] [PubMed] [Google Scholar]
- Chen H.; Han Q.; Wang C.; Liu Y.; Chen B.; Wang J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinescu S.; Ionita M.; Ignat S.-R.; Costache M.; Hermenean A. Graphene oxide enhances chitosan-based 3D scaffold properties for bone tissue engineering. International journal of molecular sciences 2019, 20 (20), 5077 10.3390/ijms20205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermenean A.; Codreanu A.; Herman H.; Balta C.; Rosu M.; Mihali C. V.; Ivan A.; Dinescu S.; Ionita M.; Costache M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7 (1), 16641 10.1038/s41598-017-16599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseti L.; Parisi V.; Petretta M.; Cavallo C.; Desando G.; Bartolotti I.; Grigolo B. Scaffolds for bone tissue engineering: state of the art and new perspectives. Materials Science and Engineering: C 2017, 78, 1246–1262. 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Manzini B. M.; Machado L. M. R.; Noritomi P. Y.; da Silva J. V. L. Advances in Bone tissue engineering: A fundamental review. Journal of Biosciences 2021, 46 (1), 1–18. 10.1007/s12038-020-00122-6. [DOI] [PubMed] [Google Scholar]
- Szpalski C.; Barbaro M.; Sagebin F.; Warren S. M. Bone tissue engineering: current strategies and techniques—part II: cell types. Tissue Engineering Part B: Reviews 2012, 18 (4), 258–269. 10.1089/ten.teb.2011.0440. [DOI] [PubMed] [Google Scholar]
- Mende W.; Götzl R.; Kubo Y.; Pufe T.; Ruhl T.; Beier J. P. The role of adipose stem cells in bone regeneration and bone tissue engineering. Cells 2021, 10 (5), 975 10.3390/cells10050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Zhuang S. Chitosan-based materials: Preparation, modification and application. Journal of Cleaner Production 2022, 355, 131825 10.1016/j.jclepro.2022.131825. [DOI] [Google Scholar]
- Maglione M.; Spano S.; Ruaro M. E.; Salvador E.; Zanconati F.; Tromba G.; Turco G. In vivo evaluation of chitosan-glycerol gel scaffolds seeded with stem cells for full-thickness mandibular bone regeneration. Journal of Oral Science 2017, 59 (2), 225–232. 10.2334/josnusd.16-0235. [DOI] [PubMed] [Google Scholar]
- Allen N. B.; Abar B.; Johnson L.; Burbano J.; Danilkowicz R. M.; Adams S. B. 3D-bioprinted GelMA-gelatin-hydroxyapatite osteoblast-laden composite hydrogels for bone tissue engineering. Bioprinting 2022, 26, e00196 10.1016/j.bprint.2022.e00196. [DOI] [Google Scholar]
- Wang D.; Liao X.; Qin X.; Shi W.; Zhou B. A novel chimeric peptide binds MC3T3-E1 cells to titanium and enhances their proliferation and differentiation. Molecular Medicine Reports 2013, 7 (5), 1437–1441. 10.3892/mmr.2013.1352. [DOI] [PubMed] [Google Scholar]
- Pautke C.; Schieker M.; Tischer T.; Kolk A.; Neth P.; Mutschler W.; Milz S. Characterization of Osteosarcoma Cell Lines MG-63, Saos-2 and U-2 OS in Comparison to Human Osteoblasts. Anticancer Res. 2004, 24 (6), 3743. [PubMed] [Google Scholar]
- Yousefi A.-M.; James P. F.; Akbarzadeh R.; Subramanian A.; Flavin C.; Oudadesse H. Prospect of stem cells in bone tissue engineering: a review. Stem cells international 2016, 2016, 6180487 10.1155/2016/6180487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevlin R.; Walmsley G.; Marecic O.; Hu M. S.; Wan D.; Longaker M. Stem and progenitor cells: advancing bone tissue engineering. Drug delivery and translational research 2016, 6 (2), 159–173. 10.1007/s13346-015-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C. L. M.; Teo E. Y.; Chong M. S.; Liu Y.; Choolani M.; Chan J. K.. Advances in bone tissue engineering. In Regenerative medicine and tissue engineering; IntechOpen, 2013; p 55916. [Google Scholar]
- Orciani M.; Fini M.; Di Primio R.; Mattioli-Belmonte M. Biofabrication and bone tissue regeneration: cell source, approaches, and challenges. Frontiers in Bioengineering and Biotechnology 2017, 5, 17 10.3389/fbioe.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaei S.; Keshavarz G.; Bozorgi A.; Nazari H.; Khazaei M. Adipose tissue-derived stem cells: A comparative review on isolation, culture, and differentiation methods. Cell and tissue banking 2022, 23 (1), 1–16. 10.1007/s10561-021-09905-z. [DOI] [PubMed] [Google Scholar]
- Toosi S.; Behravan J. Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. Biofactors 2020, 46 (3), 326–340. 10.1002/biof.1598. [DOI] [PubMed] [Google Scholar]
- Kneser U.; Schaefer D.; Munder B.; Klemt C.; Andree C.; Stark G. Tissue engineering of bone. Minimally Invasive Therapy & Allied Technologies 2002, 11 (3), 107–116. 10.1080/136457002320174177. [DOI] [PubMed] [Google Scholar]
- Kneser U.; Schaefer D. J.; Polykandriotis E.; Horch R. E. Tissue engineering of bone: the reconstructive surgeon’s point of view. Journal of cellular and molecular medicine 2006, 10 (1), 7–19. 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari B.; Davaran S.; Aghanejad A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. 10.1016/j.ijbiomac.2021.02.052. [DOI] [PubMed] [Google Scholar]
- Howard D.; Buttery L. D.; Shakesheff K. M.; Roberts S. J. Tissue engineering: strategies, stem cells and scaffolds. Journal of anatomy 2008, 213 (1), 66–72. 10.1111/j.1469-7580.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A.; Byambaa B.; Morshed M.; Cheikh M. I.; Shakoor R. A.; Mustafy T.; Marei H. E. Advances in osteobiologic materials for bone substitutes. Journal of Tissue Engineering and Regenerative Medicine 2018, 12 (6), 1448–1468. 10.1002/term.2677. [DOI] [PubMed] [Google Scholar]
- Oliveira É. R.; Nie L.; Podstawczyk D.; Allahbakhsh A.; Ratnayake J.; Brasil D. L.; Shavandi A. Advances in growth factor delivery for bone tissue engineering. International Journal of Molecular Sciences 2021, 22 (2), 903 10.3390/ijms22020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ana I. D.Bone Substituting Materials in Dental Implantology. In Bone Management in Dental Implantology; Springer, 2019; pp 121–141. [Google Scholar]
- Baino F.; Novajra G.; Vitale-Brovarone C. Bioceramics and scaffolds: a winning combination for tissue engineering. Frontiers in bioengineering and biotechnology 2015, 3, 202 10.3389/fbioe.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber-Samandari S.; Saber-Samandari S.; Ghonjizade-Samani F.; Aghazadeh J.; Sadeghi A. Bioactivity evaluation of novel nanocomposite scaffolds for bone tissue engineering: The impact of hydroxyapatite. Ceram. Int. 2016, 42 (9), 11055–11062. 10.1016/j.ceramint.2016.04.002. [DOI] [Google Scholar]
- Saber-Samandari S.; Saber-Samandari S.; Kiyazar S.; Aghazadeh J.; Sadeghi A. In vitro evaluation for apatite-forming ability of cellulose-based nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 86, 434–442. 10.1016/j.ijbiomac.2016.01.102. [DOI] [PubMed] [Google Scholar]
- Hajiali H.; Ouyang L.; Llopis-Hernandez V.; Dobre O.; Rose F. R. Review of emerging nanotechnology in bone regeneration: progress, challenges, and perspectives. Nanoscale 2021, 13 (23), 10266–10280. 10.1039/D1NR01371H. [DOI] [PubMed] [Google Scholar]
- Bharathi R.; Ganesh S. S.; Harini G.; Vatsala K.; Anushikaa R.; Aravind S.; Abinaya S.; Selvamurugan N. Chitosan-based scaffolds as drug delivery systems in bone tissue engineering. Int. J. Biol. Macromol. 2022, 222, 132–153. 10.1016/j.ijbiomac.2022.09.058. [DOI] [PubMed] [Google Scholar]
- Salave S.; Rana D.; Sharma A.; Bharathi K.; Gupta R.; Khode S.; Benival D.; Kommineni N. Polysaccharide Based Implantable Drug Delivery: Development Strategies, Regulatory Requirements, and Future Perspectives. Polysaccharides 2022, 3 (3), 625–654. 10.3390/polysaccharides3030037. [DOI] [Google Scholar]
- Turnbull G.; Clarke J.; Picard F.; Riches P.; Jia L.; Han F.; Li B.; Shu W. 3D bioactive composite scaffolds for bone tissue engineering. Bioactive materials 2018, 3 (3), 278–314. 10.1016/j.bioactmat.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J.; Krebsbach P. H. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Advanced drug delivery reviews 2012, 64 (12), 1239–1256. 10.1016/j.addr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Ochoa D.; Robles-Ovalle P.; Mayolo-Deloisa K.; Brunck M. E. Immobilization of growth factors for cell therapy manufacturing. Frontiers in bioengineering and biotechnology 2020, 8, 00620 10.3389/fbioe.2020.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood K. A.; Bock N.; Dargaville T. R.; Ann Woodruff M. Scaffolds for growth factor delivery as applied to bone tissue engineering. International Journal of Polymer Science 2012, 2012, 174942 10.1155/2012/174942. [DOI] [Google Scholar]
- Herdiana Y.; Wathoni N.; Shamsuddin S.; Muchtaridi M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674 10.1016/j.heliyon.2021.e08674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowska J.; Tomaszewski K. A.; Niedźwiedzki Ł.; Walocha J. A.; Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20 (3), 291–302. 10.1007/s10456-017-9541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B.; Wang W.; Li Q.; Wang Z.; Yan B.; Zhang Z.; Wang L.; Huang M.; Jia C.; Lu J.; et al. Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat. Commun. 2016, 7 (1), 1–13. 10.1038/ncomms13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. A.; Davaine J.-M.; Layrolle P. Pre-vascularization of bone tissue-engineered constructs. Stem cell research & therapy 2013, 4 (4), 96 10.1186/scrt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; van den Beucken J. J.; Yang F.; Both S. K.; Cui F.-Z.; Pan J.; Jansen J. A. Coculture of osteoblasts and endothelial cells: optimization of culture medium and cell ratio. Tissue Engineering Part C: Methods 2011, 17 (3), 349–357. 10.1089/ten.tec.2010.0215. [DOI] [PubMed] [Google Scholar]
- Ghiasi M. S.; Chen J.; Vaziri A.; Rodriguez E. K.; Nazarian A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone reports 2017, 6, 87–100. 10.1016/j.bonr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Pagán Á. E.; Stahl A. M.; Shanjani Y.; Yang Y. Vascularization in bone tissue engineering constructs. Annals of biomedical engineering 2015, 43 (3), 718–729. 10.1007/s10439-015-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. E.; Dohle E.; Kirkpatrick C. J. Improving vascularization of engineered bone through the generation of pro-angiogenic effects in co-culture systems. Advanced drug delivery reviews 2015, 94, 116–125. 10.1016/j.addr.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Jin G.-Z.; Kim H.-W. Co-culture of human dental pulp stem cells and endothelial cells using porous biopolymer microcarriers: a feasibility study for bone tissue engineering. Tissue engineering and regenerative medicine 2017, 14 (4), 393–401. 10.1007/s13770-017-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T.; Zhang T.; Lin Y.; Cai X. Vascularization in craniofacial bone tissue engineering. Journal of dental research 2018, 97 (9), 969–976. 10.1177/0022034518767120. [DOI] [PubMed] [Google Scholar]
- Lafage-Proust M.-H.; Roche B.; Langer M.; Cleret D.; Bossche A. V.; Olivier T.; Vico L. Assessment of bone vascularization and its role in bone remodeling. BoneKEy reports 2015, 4, 662 10.1038/bonekey.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. I.; Unger R. E.; Sousa R. A.; Reis R. L.; Kirkpatrick C. J. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone–starch scaffold and the in vitro development of vascularization. Biomaterials 2009, 30 (26), 4407–4415. 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Yang C.; Han B.; Cao C.; Yang D.; Qu X.; Wang X. An injectable double-network hydrogel for the co-culture of vascular endothelial cells and bone marrow mesenchymal stem cells for simultaneously enhancing vascularization and osteogenesis. J. Mater. Chem. B 2018, 6 (47), 7811–7821. 10.1039/C8TB02244E. [DOI] [PubMed] [Google Scholar]
- Rouwkema J.; Gibbs S.; Lutolf M. P.; Martin I.; Vunjak-Novakovic G.; Malda J. In vitro platforms for tissue engineering: implications for basic research and clinical translation. Journal of tissue engineering and regenerative medicine 2011, 5 (8), e164–e167. 10.1002/term.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein J.; Garcia P.; Histing T.; Kristen A.; Scheuer C.; Menger M.; Pohlemann T. Advances in the establishment of defined mouse models for the study of fracture healing and bone regeneration. Journal of orthopaedic trauma 2009, 23, S31–S38. 10.1097/BOT.0b013e31819f27e5. [DOI] [PubMed] [Google Scholar]
- Histing T.; Garcia P.; Holstein J.; Klein M.; Matthys R.; Nuetzi R.; Steck R.; Laschke M.; Wehner T.; Bindl R.; et al. Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone 2011, 49 (4), 591–599. 10.1016/j.bone.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Jahan K.; Manickam G.; Tabrizian M.; Murshed M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci. Rep. 2020, 10 (1), 1–17. 10.1038/s41598-020-67886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulou A.; Papadogiannis F.; Batsali A.; Marakis J.; Alpantaki K.; Eliopoulos A. G.; Pontikoglou C.; Chatzinikolaidou M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci.: Mater. Med. 2018, 29 (5), 1–13. 10.1007/s10856-018-6064-2. [DOI] [PubMed] [Google Scholar]
- Kruck B.; Zimmermann E. A.; Damerow S.; Figge C.; Julien C.; Wulsten D.; Thiele T.; Martin M.; Hamdy R.; Reumann M. K.; et al. Sclerostin neutralizing antibody treatment enhances bone formation but does not rescue mechanically induced delayed healing. Journal of Bone and Mineral Research 2018, 33 (9), 1686–1697. 10.1002/jbmr.3454. [DOI] [PubMed] [Google Scholar]
- Luca L.; Rougemont A. L.; Walpoth B. H.; Boure L.; Tami A.; Anderson J. M.; Jordan O.; Gurny R. Injectable rhBMP-2-loaded chitosan hydrogel composite: Osteoinduction at ectopic site and in segmental long bone defect. J. Biomed. Mater. Res., Part A 2011, 96 (1), 66–74. 10.1002/jbm.a.32957. [DOI] [PubMed] [Google Scholar]
- Saravanan S.; Leena R.; Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. 10.1016/j.ijbiomac.2016.01.112. [DOI] [PubMed] [Google Scholar]
- Rouwkema J.; Rivron N. C.; van Blitterswijk C. A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26 (8), 434–441. 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]