Abstract
The complex structure and function of a plant microbiome is driven by many variables, including the environment, microbe-microbe interactions, and host factors. Likewise, resident microbiota may influence many host phenotypes. Gnotobiotic growth systems and controlled environments empower researchers to isolate these variables, and standardized methods equip a global research community to harmonize protocols, replicate experiments, and collaborate broadly. We developed two easily-constructed peat-based gnotobiotic growth platforms – the FlowPot system and the GnotoPot system. Sterile peat is amenable to colonization by microbiota and supports growth of the model plant Arabidopsis thaliana in the presence or absence of microorganisms. The FlowPot system uniquely allows one to flush substrate with water, nutrients, and/or suspensions of microbiota via an irrigation port, and a mesh retainer allows for the inversion of plants for dip or vacuum infiltration protocols. The irrigation port also facilitates passive drainage, preventing root anoxia. In contrast, the GnotoPot system utilizes a compressed peat pellet, widely used in the horticultural industry. GnotoPot construction has fewer steps and requires less user handling, thereby reducing the risk of contamination. Both protocols take up to four days to complete with four to five hours of hands-on time, including substrate and seed sterilization. In this protocol, we provide detailed assembly and inoculation procedures for the two systems. Both systems are modular, do not require a sterile growth chamber, and cost less than $2 (USD) per vessel.
Keywords: Microbiome, gnotobiotic, phytobiome, germ-free, microbiota, axenic, holoxenic, Arabidopsis, dysbiosis, host-microbiota interactions
EDITORIAL SUMMARY
This protocol describes two peat-based plant growth systems for microbiome research in Arabidopsis. While both systems support microbe-free plants and input microbiota control, GnotoPots have advantages for throughput and FlowPots for versatility.
TWEET
A new protocol describing two peat-based plant gnotobiotic systems for microbiome research in Arabidopsis. #FlowPot #GnotoPot
COVER TEASER
Gnotobiotic systems for plant microbiome research
Introduction
Multicellular organisms are in constant contact with diverse microbial communities, which reside in and on their body parts. These microbes, collectively called microbiota, play crucial roles in their host health and disease1-3 . Plant microbiota is essential for maintaining plant health and promoting crop productivity, thus supporting human life and health. Much progress has been made to elucidate plant-microbiome interactions in open field or greenhouse conditions, but there are limitations to resolve the cause and effect of plant-microbiome interactions under such conditions4,5. In particular, soils vary tremendously in their biochemical composition, microbial composition, and geochemical and physical attributes. Likewise, the air to which plants are exposed in open systems in different locations fluctuates in microbial composition and load. These variations are not amenable for a universally obtainable substrate for a global research community to replicate experiments easily. A deeper understanding of plant-microbiome interactions, especially the cause and effect relationship, is paramount to the use of microbiome for sustainable agriculture. This is a research priority for the plant microbiome community to develop standardized methods to elucidate the rules of microbiome assembly, functional plant-microbiome interactions, and isolate experimental variables to refine the understanding plant genotype-by-environment-by-microbiome-by-management interactions4,5. Development of a set of gnotobiotic plant growth systems that can be widely adopted by the research community could facilitate the advancement of global plant microbiome studies. In previous studies, nutrient agar and calcine clay have been used in enclosed systems to investigate plant-microbiome interactions6-10 (Table 1). However, these systems have limitations, as they do not contain organic matters and/or soil-like structure. Accordingly, development of plant gnotobiotic growth systems based on substrates that contain organic matter, such as peat-based potting mixtures, could more closely approximate a natural or agriculturally-relevant state.
Table 1.
Advantages and disadvantages of substrates used in gnotobiotic systems
| Growth system | Advantage | Disadvantage |
|---|---|---|
| Nutrient agar |
|
|
| Hydroponic |
|
|
| Mineral substrates (calcined clay, sand, quartz, vermiculite) |
|
|
| Sterilized Soil |
|
|
| Peat (FlowPot and GnotoPot) |
|
|
Careful consideration must be taken when designing an axenic growth system to minimize artifacts and sample variability, but not at the expense of versatility. Environmental factors are the major drivers of differential taxonomic composition and diversity among host-associated microbiota11-13. Thus, abiotic factors must be controlled to ensure reproducibility of observed host phenotypes and community dynamics. For example, in the model plant Arabidopsis, humidity greatly influences microbiota composition and the plant’s ability to defend against foliar pathogens14. Other ecological drivers of soil microbiota composition are associated with physical and chemical attributes of the soil, including, but not limited to: pH, porosity and its gaseous composition, water retention, organic carbon, inorganic nitrogen and orthophosphate (Pi) availability, organic matter content, and cation exchange capacity15,16. When conducting microbiota colonization experiments to compare gnotobiotic plants colonized with a known microbial community to axenic (germ-free) plants, it is essential to consider edaphic factors of the substrate as well as the growth environment, particularly if the experiment is intended to recapitulate the diverse microbial communities found in organic matter-rich soils4,5.
Here, we present two axenic growth systems with peat-based substrate for plant microbiota research. These procedures allow for the growth of Arabidopsis in axenic (no viable microorganisms detected), gnotobiotic (inoculated with a defined community of bacteria), and holoxenic (inoculated with undefined microbiota extracted directly from a natural environment) conditions. Procedure 1 and Procedure 2 describe how to prepare and assemble the FlowPot and the GnotoPot systems, respectively. The FlowPot system has sterile peat substrate contained within a chamber. An irrigation port allows for precise control of watering and inoculation with suspensions of microbiota, nutrients and other inputs. Furthermore, FlowPots can be inverted while retaining substrate for dipping or vacuum-infiltration experiments. On the other hand, the GnotoPot system uses sterile peat wafers that can also be saturated with the same inputs as described for FlowPots. Their ease of GnotoPot construction may be conducive to experiments with higher throughput requirements.… We have recently used both systems in plant microbiota colonization studies for elucidating the significance of inter-kingdom microbial interaction in plant survival3 and establishing a causal relationship for phyllosphere microbial communities in promoting microbiota dysbiosis in plant leaves2. While the FlowPot and GnotoPot systems were designed specifically for Arabidopsis growth, either system can likely be adapted to support the growth of other plants.
Development of the approaches
For the FlowPot system, we developed a protocol in which various substrates, including soil or peat mixes, could be used to support plant growth in a contained environment. Axenic plant growth requires that the substrate be sterilized, which in the case of peat, can be accomplished by three autoclave cycles. Upon final assembly of the FlowPots, two successive aseptic flushing steps with water and with a nutrient solution will support plant growth2,3. The Luer lock irrigation port at the base of the FlowPot supports subsequent irrigation, and the mesh substrate retainer allows researchers to invert the FlowPot for versatile microbial inoculation methods, including dipping and vacuum infiltration of leaves.
The GnotoPot system relies on using commercially available Jiffy-7® peat pellet substrate (manufactured by Jiffy Products, Norway, https://www.jiffygroup.com/). Since its introduction to market in 1967, the Jiffy-7® pellet which is prepared from Sphagnum peat moss and coir fibers, has been used extensively by hobbyists and professional growers for starting a wide variety of crops and ornamentals. We found that a hydrated Jiffy-7® pellet could be effectively sterilized by two autoclave cycles and that supplemental nutrients at the initial hydration step and final preparation step are sufficient to ensure healthy plant growth2. Assembly of the GnotoPot system involves minimal handling steps, yet supports axenic plant growth in organic matter-rich plant growth substrate.
Overview of the procedures
Both procedures described here are based on predominantly peat-based substrates and utilize similar conditions for plant growth inside the same gas permeable Microbox tissue culture containers (SacO2, Belgium), however, the growth apparatus construction makes each procedure unique in terms of its potential applications and versatility.
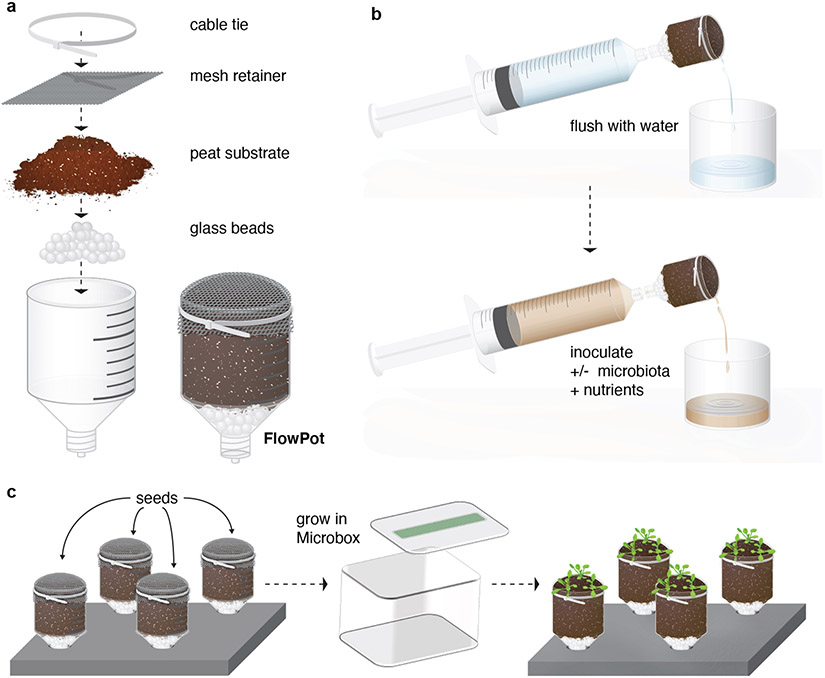
For the FlowPot system (Procedure 1), each FlowPot (the gnotobiotic pot holding the substrate) is assembled using inexpensive and routinely available labware. In short, reusable components of the FlowPot system are constructed from truncated syringes (which form individual FlowPots bases) and modified pipette tip inserts (Steps 1-4). Soil or peat substrates are sterilized by autoclaving (Steps 5-7), then added to FlowPots bases, covered with a mesh retainer, and secured with a cable tie (Steps 8-10) (Fig. 1a). The FlowPot system features an inoculation port on each vessel (Fig. 1a) that enables substrate rinsing to remove soluble byproducts of soil sterilization, provides drainage, and accommodates homogenous inoculation with microbiota and/or nutrients. Assembled FlowPots are then autoclaved once more and aseptically irrigated from the inoculation port with nutrients and any desired input microbial suspensions (Steps 11-14)(Fig. 1b). Subsequently each FlowPot is placed into a sterile Microbox supported by a stand, and microbiota-free Arabidopsis seeds are sown on each FlowPot (Steps 15-16). The tissue culture boxes containing FlowPots are placed in a plant growth chamber with desired lighting and temperature conditions to support plant growth (Steps 17-18).
Fig. 1 ∣. Schematic illustration of the FlowPot system.
Each FlowPot is prepared by (a) adding glass beads to the Luer end of a truncated syringe, followed by the addition of twice-autoclaved peat, covered with a mesh retainer, and then secured with a cable tie. Assembled FlowPots are then autoclaved, (b) aseptically irrigated with sterile Milli-Q water, and inoculated with nutrients and any desired input microbiota. (c) FlowPots are then aseptically placed into Microboxes on stands and microbe-free Arabidopsis seeds are sown onto each FlowPot. The Microboxes containing FlowPots are placed in a growth chamber with desired lighting and temperature conditions for plant growth.
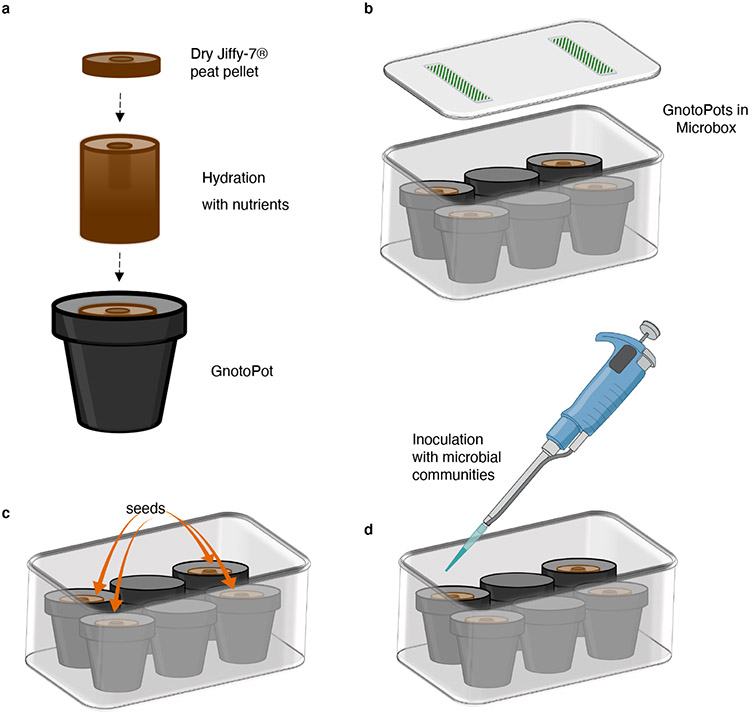
For the GnotoPot system (Procedure 2), the assembly of each unit begins with an initial hydration step of a compressed Jiffy-7® pellet. Here, a dry pellet is placed inside a small polypropylene pot and hydrated with a nutrient solution (Steps 1-2)(Fig. 2a). Next, GnotoPots are transferred to the Microboxes and secured in place with empty plastic pots (Steps 3-7)(Fig. 2b). Then, Microboxes containing GnotoPots are placed inside an autoclavable plastic bag, looselysealed, and autoclaved (Steps 8-12). At the final preparation steps (Fig. 2c,d), Arabidopsis seeds are sown aseptically on GnotoPots, desired input microbiota communities are inoculated and the Microboxes are placed inside a tissue culture growth chamber with desired lighting and temperature conditions to support plant growth (Steps 22-26).
Fig. 2 ∣. Schematic illustration of the GnotoPot system.
Soaking the Jiffy-7® peat pellet with nutrient solution results in expansion of the dry discs and formation of the GnotoPot (a). GnotoPots are placed inside the Microboxes flanking two empty pots (b). After the autoclaving cycles the GnotoPots are rehydrated with nutrient solution and seeds are sown aseptically (c). Lastly, desired input microbiota is inoculated using a 1 mL pipette (d), the lids are snapped closed and Microboxes are transferred to growth chambers.
Features and applications of peat-based gnotobiotic systems
The gnotobiotic plant growth procedures presented here enable researchers to perform in-depth studies on the phenotype of axenic plants as well as plants colonized by soil-derived microbiota under highly controlled environmental parameters (Fig. 3 and Fig.4). These setups are effective platforms to study plant-microbiota interactions in situ, including microbial competitions within a synthetic root microbiota3 and determining the causal relationships contributing to leaf microbiota dysbiosis using microbiota transplantation experiments2. Colonization of plants by synthetic or complex microbial communities can be performed at the seed stage or later during plant development via soil drench or other foliar applications.
Fig. 3 ∣. Arabidopsis thaliana grown in FlowPots with axenic or holoxenic substrate.
Growth phenotype of Arabidopsis plants grown in FlowPots, 4 weeks post germination. Holoxenic substrate was inoculated with a soil slurry, and axenic substrate was inoculated with a heat-killed version of the same soil slurry. Rosette images are representative of at least three replicated experiments.
Fig. 4 ∣. Arabidopsis thaliana grown in GnotoPots with axenic or holoxenic substrate.
Growth phenotype of Arabidopsis plants grown in GnotoPots at 6.5 weeks post germination. Holoxenic substrate was inoculated with a soil slurry, axenic substrate was inoculated with a heat-killed version of the same soil slurry. Rosette images are representative of at least three replicated experiments.
The versatility of the FlowPot system facilitates the use of different soil and soil-like substrates for microbiota colonization studies. Additionally, using the mesh retainer allows FlowPots to be inverted for a variety of downstream applications, including dip inoculation or vacuum infiltration of aerial plant tissues with bacterial suspensions and other solutions.
Although the GnotoPot system is limited to the peat pellet substrate, the widespread availability of Jiffy-7® peat pellets make the GnotoPot system suitable for comparison of Arabidopsis microbiota colonization experiments across different labs. Furthermore, the larger size of the peat pellet substrate (relative to the FlowPots constructed in Procedure 1) supports plant growth for a longer period of time, making the GnotoPots system suitable for microbiota studies in fully expanded leaves during later plant developmental stages (Fig. 4). Lastly, the simplicity and minimal steps in the assembly of the GnotoPot procedure makes it feasible for an individual with minimal training to assemble more than 48 GnotoPots in 2 hours.
While the gnotobiotic systems described here have been designed for Arabidopsis thaliana root and phyllosphere microbiota research, we anticipate potential applications of peat-based gnotobiotic systems beyond Arabidopsis for growth of other model and non-model plant species under gnotobiotic conditions with minor modifications. However, this application will be limited to seedling and early vegetative growth stages of plants and will require the use of a larger gas permeable container (available from SacO2, Belgium) to accommodate larger plant species.
Comparison with other growth methodology for plant microbiome research
Axenic Arabidopsis growth can be accomplished with routine tissue culture methodology on a phytonutrient agar substrate (or similar) contained within a light- and gas-permeable container (see Table 1). However, routine tissue culture systems do not provide a soil-simulating growth substrate for microbial colonization. Furthermore, agar-based systems are notorious for non-uniform nutrient and O2 delivery over time17. Hydroponic and aeroponic systems can alleviate issues with nutrient uniformity and O2-delivery by agitation and media replenishment, but such systems still do not provide a soil-simulating growth substrate for microbial colonization18. Furthermore, it can be challenging to maintain axenic conditions or prevent cross-contamination in common-reservoir hydroponic systems.
Non-soil substrates, such as sand, quartz, vermiculite, and calcined clay, are frequently used in gnotobiotic systems6,7,19-21. These substrates are porous, thus providing varied surfaces for microbial colonization and root penetration. However, one major limitation of non-soil gnotobiotic systems is the lack of organic carbon typical of soil, which is beneficial to plant and microbial survival and/or growth. Furthermore, batch-to-batch variation of ceramic substrates can result in a wide range of labile ions20. Calcined clay, for example, has sorptive properties that can reduce labile concentrations of P, Fe, Cu and Zn, and desorptive properties that can cause excess labile B, Mg, Ca, S, K, and Mn, leading to potential toxicity of the latter22. While thorough washing or soaking of the non-soil substrate can reduce the initial excess of labile ions, flow and drainage are important to reduce significant changes in chemistry over time. Nevertheless, compared to peat-based gnotobiotic systems described here, non-soil substrates, such as phytonutrient agar and calcined clay, may be suitable to mimic defined mineral nutrient deficiencies and other specific applications, thus highlighting the importance of multiple standardized gnotobiotic system methodologies.
Natural and agricultural soils have been used as substrates in axenic systems, but often present challenges, such as contamination or hindered plant growth due to suboptimal sterilization procedures. Numerous sterilization methods have been used with soil, including: autoclaving, dry heat, irradiation, microwave, fumigation by gaseous chemicals, and saturation with various sterilants23. In our hands, soil sterilization methods need to be carefully optimized for plant growth due to potential phytotoxic effects of chemical residue and unintended artifacts of the sterilization process. Autoclaving soil has been shown to increase levels of water-soluble carbon, some ions and reduce pH24, leading to potential nutritional imbalance, but not significantly alter ion exchange capacity. Gamma-irradiation has been reported to minimally disrupt the physical attributes of soils but can result in the generation of reactive oxygen species, capable of depolymerizing the C-C bond of polysaccharides25. Both autoclaving and gamma irradiation can result in changes of the physical structure of the soil, exposing more surface area and thus altering sorptive properties. However, complete sterilization of some soils can be achieved with minimal chemical alterations by autoclaving a thin layer of soil for three short (<45 min) autoclave cycles with 18-24 hour intervals26-28.Subsequent flushing of sterile soil substrate can increase plant productivity, presumably by rinsing away soluble phytotoxic byproducts. However, in our hands, gnotobiotic systems based on heat-sterilized soils often do not provide a conducive environment for growing healthy Arabidopsis plants.
MATERIALS FOR PROCEDURE 1
Biological materials
Arabidopsis seeds (from ABRC - Arabidopsis Biological Research Center; https://abrc.osu.edu/). See Box 2 for seed preparation, sterilization, and stratification and Box 3 for plant growth conditions.
Sample containing input microbiota (e.g., from soil, see Box 1)
Box 2 ∣. Seed preparation, sterilization, and stratification ⚫ Timing 3 d.
Arabidopsis seed sterilization can be performed via a variety of methods29. Here we describe vapor phase sterilization as it allows high-throughput processing of multiple aliquots of different genotypes at once and can be performed ahead of time. To promote uniform germination and plant growth, seeds are first selected based on size using a sieve. Additionally, proper seed storage and cold stratification help ensure higher germination rates. As FlowPot and GnotoPot systems can be used to study plant interactions with vertically transmitted endophytes, specialized steps, such as antibiotic or fungicide treatment before seed harvest in the prior generation insect-free growth chambers, will need to be developed.
Reagents:
Arabidopsis seeds (from ABRC - Arabidopsis Biological Research Center; https://abrc.osu.edu/)
-
Bleach (common household bleach, 5.25% sodium hypochlorite (wt/vol); e.g. Clorox)
! CAUTION Bleach is corrosive. Use protective equipment.
! CRITICAL Use freshly opened bleach.
-
Hydrochloric acid (HCl, 37% (vol/vol); Sigma-Adrich, cat. no. 320331)
! CAUTION HCl is corrosive. Use protective equipment.
Equipment:
Metal Sieve, US Standard 60 mesh (250 μm) (Fisher Scientific, cat. no. AA41200ON)
Metal Sieve, US Standard 50 mesh (300 μm) (Fisher Scientific, cat. no. AA39985ON)
Microcentrifuge tubes (USA Scientific, cat. no. 1415-2500)
Polypropylene storage box (USA Scientific, cat. no. 2310-5848)
Erlenmeyer flask (Corning, cat. no. 4980-500)
Glass pipette and bulb (Fisher Scientific, cat. nos. 13-678-20 and 03-448-25, respectively)
Glass desiccator (Corning, cat. no. 3081-250)
Vacuum grease (Dow Corning, cat. no. 1597418)
Chemical fume hood
Biosafety cabinet or laminar flow hood (e.g., Logic+ Class II A2 Biological Safety Cabinet, Labconco, cat. no. 302611100; or Console Horizontal Airflow Workstation, Nuaire, cat. no. NU-301-530)
Auto-desiccator cabinet (Bel-Art, cat. no. F42074-0116)
Seed preparation ⚫ TIMING 5 min
-
Pass dried Arabidopsis seeds harvested from healthy plants through two metal sieves with sieve mesh size 50 placed on top of sieve mesh size 60. Only collect the seeds in between two sieves. This will result in selection of seeds with sizes between 250 μm and 300 μm and reduce variation in germination rates.
PAUSE POINT Seeds can be stored under dry, cool conditions for at least one year prior to further processing.
Seed sterilization ⚫ TIMING 6-8 h
-
2
Aliquot approximately 50-250 seeds into a labeled 1.5 mL microcentrifuge tube. Do not close the lid. Repeat for the desired number of aliquots.
CRITICAL STEP Chlorine gas generated in subsequent steps will react with some commonly used inks and may interfere with sample labeling. Use chemical-resistant inks.
-
3
Place open microcentrifuge tubes in a plastic microcentrifuge storage box, but do not close the box lid. Place the open microcentrifuge storage box with open microcentrifuge tubes containing seeds and an Erlenmeyer flask containing 100 mL undiluted bleach in a glass desiccator located in a chemical fume hood.
-
4
Carefully add 1-2 mL of concentrated HCl using a glass pipette to the Erlenmeyer flask containing bleach and immediately place the lid on the glass desiccator, ensuring a proper seal. Sterilize seeds for 6-8 hrs30.
! CAUTION Chlorine gas is toxic to humans! Use proper safety precautions.
! CRITICAL STEP Vacuum grease can help ensure a sufficient seal is made.
-
5
After sterilization, allow seed aliquots to off-gas residual chlorine gas before closing the lids on individual seed aliquots. Close the storage box lid and store the entire box containing seed aliquots at 4°C. For long-term cold storage, store seeds in the dark under low humidity. We found seeds stored in an auto-desiccator cabinet were sterile and viable after more than one year in storage.
! CAUTION Chlorine gas is caustic! Use proper safety precautions.
! CRITICAL STEP Residual chlorine gas can be removed by cracking the lid to the desiccator for several minutes and moving the seeds to a laminar flow hood. It is important to maintain sterile technique upon sterilization.
! CRITICAL STEP Checking for effective decontamination of an aliquot of seeds is crucial for maintaining axenic growth conditions (see Box 4).
! CRITICAL STEP Storing seeds in the dark at low humidity is important to maintain high germination rates.
PAUSE POINT Aliquots of seed can be sterilized in bulk and stored under appropriate conditions for future use.
Seed stratification ⚫ TIMING 2 d
-
6
Prior to an experiment, allow seeds to imbibe during a 48-hour stratification period in sterile Milli-Q water at 4°C in the dark prior to sowing. This helps promote uniform germination.
? TROUBLESHOOTING
Box 3 ∣. Plant growth conditions.
A plant tissue culture growth chamber (Percival) was used for growing plants in gnotobiotic setups. We routinely use the following conditions for Arabidopsis plant growth: 22 °C with 12h day/12h night photoperiod cycle at ~90-100 μE m−2 s−1. The light intensity and temperatures on the tissue culture chamber were adjusted based on measurement done using probes placed inside the Microboxes to attain the expected growth parameters. We recommend rotating gnotobiotic boxes in a growth chamber every 2-3 days to ensure uniform plant growth. Since Microboxes are engineered to have a high water retention capacity inside the containers make sure to adapt Microbox-grown plants to desired relative humidity at the time of performing experiments, if relevant.
Box 1 ∣. Preparation of the soil extract source microbiota.
This box provides information on how to collect and store soil for extraction of complex microbial communities. Soil collection is done when the soil has not recently experienced extreme conditions. Procurement of a source microbiota can be performed in advance of the experiment and modified depending on the input community characteristics and experimental parameters of your choosing.
Equipment:
Whirl-Pak sterile sampling bags (Nasco, cat. no. B01065WA)
3 mm galvanized steel screen
Collection method
Collect and store soil using the following steps:
Remove topsoil (typically 10-15 cm) including any vegetation. At the sites of our soil collection, this helps to avoid variability of surface debris and organic matter. However, less topsoil may be removed if soil surfaces are less variable in debris and organic matter. Then collect more than 5 cm deep soil and transfer to the lab.
Let the soil sit for 1 week at room temperature (22-25 °C) with ~50% relative humidity.
Next, sift through a 3 mm galvanized steel screen to remove large debris.
Aliquot soil in 100 g increments and store at 4°C in Whirl-Pak bags.
Reagents
Sterile Milli-Q water (reverse osmosis filtered, or an equivalent quality water)
-
Multi-Terge™ detergent (EMD Millipore, cat. no. 65068); diluted to 2% (v/v)
! CAUTION Multi-Terge detergent may be corrosive to metals and cause skin irritation. Use personal protective equipment as described by the manufacturer.
-
Spor-Klenz™ disinfectant (Steris, USA, cat. no. 652026); diluted to 3% (v/v)
! CAUTION Spor-Klenz is a strong oxidizer and corrosive. Use personal protective equipment as described by the manufacturer.
Linsmaier & Skoog (LS) medium buffered with 2-(N-morpholino) ethanesulfonic acid (MES) to pH 5.7 (Caisson Labs, cat. no. LSP03)
-
Ethanol, 100% or 95% (v/v) (Fisher Scientific, cat. no. 04-355-451)
! CAUTION Avoid ignition sources and ensure proper ventilation when working with fire and flammable solvents such as ethanol.…
Equipment
Luer lock PP syringes, 50 mL (Jensen Global, cat. no. JG50CC-LL)
Female Luer x female Luer adapter, nylon (autoclaved prior to use; Cole-Parmer, cat. no. EW-45502-22)
Mesh fiberglass “Phiferglass”, 18 X 14 standard charcoal mesh (Phifer Incorporated, cat. no. 3003906)
Soda-glass beads, 3 mm (Sigma-Aldrich, cat. no. Z265926)
Microbox container (SacO2, cat. no., TP1600+TPD1200; #40 green filter, autoclavable)
Filament tape model 893, 18 mm (Scotch Company)
- Redi-Earth plug and seedling mix (Sun Gro Horticulture, Canada). Contains fine Canadian sphagnum peat moss, vermiculite, dolomitic limestone, and a wetting agent
- ! CRITICAL: this can be substituted with alternative substrates, but plant performance may vary.
Medium vermiculite, horticultural grade
Polypropylene trays (United Scientific Supplies, cat. no. 81701)
Sterilization wrap (Medline, cat. no. GEM1124S)
Cable ties, 22 mm (TENAX Corporation, Baltimore, USA, cat. no. 120094)
Sun bags (Sigma-Aldrich, cat. no. B7026)
Cell strainer, 70 μm (Celltreat Scientific, cat. no. 229483)
Drill bit, 8.8 mm (e.g., Chicago-Latrobe, cat no. 47329)
Blocks of polypropylene, 12 cm X 8 cm x 1 cm (United States Plastic Corp, cat. no. 42605); alternatively, use Rainin RT-L1000 or similar tip box inserts
General equipment
Biosafety cabinet or laminar flow hood (e.g., Logic+ Class II A2 Biological Safety Cabinet, Labconco, cat. no. 302611100; or Console Horizontal Airflow Workstation, Nuaire, cat. no. NU-301-530)
Test tube clamp or clamp modified hemostat (e.g., Stoddard Clamp, United Scientific Supplies, cat. no. TTCL03)
Pipet and 1 mL filter tips (e.g., classic PR-1000 pipette and 1 mL RT-LTS filter tips, Rainin, cat. nos. 17008653 and 30389214)
Funnel, 150 mm (Fisher Scientific, cat. no. 10-500-3)
Glass Erlenmeyer flasks, 2 L (Corning, cat. no. 4980-2L)
Sterile glass media bottles with screw cap, 2 L (Corning, cat. no. 1395-2L)
Sterile graduated cylinders, 500 mL (Thermo Scientific, cat. no. 36620500)
Bunsen burner (e.g., Humboldt Manufacturing Company, cat. no. H5870)
Test tube racks (Thermo Scientific, cat. no. 59700020)
Miter saw (e.g., Ryobi, cat. no. DC970K-2)
Drill (e.g., 18-Volt Compact Drill/Driver, Dewalt, cat. no. DC970K-2)
Growth chamber with desired lighting (e.g., Percival cat. no. CU36L5)
Reagent Setup
Multi-Terge detergent
Dilute Multi-Terge concentrate to 2% (v/v) in water. Diluted detergent can be stored at room temperature (22-25°C) for several weeks.
Spor-Klenz disinfectant
Dilute Spor-Klenz concentrate to 3% (v/v) in water. Prepare fresh solution daily.
LS nutrient solution
Prepare LS solutions by dissolving the LS powder in water at 4.73g/L for a 1X solution. Autoclave the solution for 45 min. After autoclaving, cool down the media bottles to room temperature (22-25°C) then tighten the lid. Prepared LS nutrient solution can be stored at room temperature for at least three months. A 1X concentrate of buffered LS from Caisson Labs contains: NH4NO3 (1650 mg/L), H3BO3 (6.2 mg/L), CaCl2 (332.2 mg/L), CoCl2 . 6H2O (0.025 mg/L), CuSO4 . 5H2O (0.025 mg/L), EDTA disodium dihydrate (37.26 mg/L), MES (200 mg/L), MgSO4 (180.7 mg/L), MnSO4 . H2O (16.9 mg/L), Na2MoO4 . 2H2O (0.25 mg/L), Myo-Inositol (100 mg/L), KHCO3 (98 mg/L), KI (0.83 mg/L), KNO3 (1900 mg/L), KH2PO4 (170 mg/L), Thiamine hydrochloride (0.4 mg/L), ZnSO4 . 7H2O (8.6 mg/L).
MATERIALS FOR PROCEDURE 2
Biological materials
Arabidopsis seeds (from ABRC - Arabidopsis Biological Research Center; https://abrc.osu.edu/). See Box 2 for seed preparation, sterilization, and stratification and Box 3 for plant growth conditions.
Sample containing input microbiota (e.g., from soil, see Box 1)
Reagents
Linsmaier & Skoog (LS) medium buffered with 2-(N-morpholino) ethanesulfonic acid (MES) to pH 5.7 (Caisson Labs, cat. no. LSP03).
Sterile Milli-Q water (reverse osmosis filtered, or an equivalent quality water)
-
Spor-Klenz™ disinfectant (Steris, USA, cat. no. 652026); dilute to 3% (v/v)
! CAUTION Spor-Klenz is a strong oxidizer and corrosive. Use personal protective equipment as described by the manufacturer.
Equipment
Biosafety cabinet or laminar flow hood (e.g., Logic+ Class II A2 Biological Safety Cabinet, Labconco, cat. no. 302611100; or Console Horizontal Airflow Workstation, Nuaire, cat. no. NU-301-530)
Standard Sterile Petri plates, 100 x 15 mm (VWR, cat. no. 25384-302)
Jiffy-7® peat pellet, 36mm (Amazon, cat. no. B01LWMB93K)
Small (2 inch) polypropylene nursery pots (Amazon, cat no. B00LH1NMV0)
Microbox container (SacO2, cat. no., TP1600+TPD1200; #40 green filter, autoclavable)
Sun bags (Sigma-Aldrich, cat. no. B7026)
25 mL disposable serological pipets (Genesee Scientific, cat. no.12-106 )
Electronic pipette controller (Scilogex, cat. no. 740200029999)
Cell strainer, 70 μm (Celltreat Scientific, cat. no. 229483)
Sterilization wrap (Medline, cat. no. GEM1124S)
Pipet with 1 mL and 20 uL filter tips (e.g., classic PR-1000 pipette with 1 mL and 20 uL RT-LTS filter tips, Rainin, cat. nos. 17008653, 30389214, and 30389296, respectively
Growth chamber with desired lighting (e.g., Percival cat. no. CU36L5)
Reagent Setup
LS nutrient solutions
Prepare LS solutions by dissolving the LS powder in water at 4.73g/L or 2.37g/L for 1X or 1/2X solution, respectively. Autoclave the solution for 45 min. After autoclaving, cool down the media bottles to room temperature (22-25°C) then tighten the lid. Prepared LS nutrient solution can be stored at room temperature for at least three months. A 1X concentrate of buffered LS from Caisson Labs contains: NH4NO3 (1650 mg/L), H3BO3 (6.2 mg/L), CaCl2 (332.2 mg/L), CoCl2 . 6H2O (0.025 mg/L), CuSO4 . 5H2O (0.025 mg/L), EDTA disodium dihydrate (37.26 mg/L), MES (200 mg/L), MgSO4 (180.7 mg/L), MnSO4 . H2O (16.9 mg/L), Na2MoO4 . 2H2O (0.25 mg/L), Myo-Inositol (100 mg/L), KHCO3 (98 mg/L), KI (0.83 mg/L), KNO3 (1900 mg/L), KH2PO4 (170 mg/L), Thiamine hydrochloride (0.4 mg/L), ZnSO4 . 7H2O (8.6 mg/L).
Spor-Klenz solution
Dilute Spor-Klenz concentrate to 3% (v/v) in water. Prepare fresh solution daily.
PROCEDURE 1. THE FLOWPOT SYSTEM
Construction of FlowPots ⚫ TIMING ~1.5 h
! CRITICAL: In this procedure, a FlowPot is described as an individual growth vessel that contains peat substrate, and the final assembled setup consists of four assembled FlowPots within a Microbox. FlowPots are reusable. Steps 1-4 of the procedure only need to be performed for initial construction.
-
For each individual FlowPot, remove the piston from a 50 mL polypropylene (PP) Luer taper syringe. Using a miter saw with a fine-tooth blade, cut the syringe at the “20 mL” mark, retaining only the portion with the Luer connector. Mount the blade on the miter saw backwards for a smoother cut, and sand if needed. Remove any residual shards with a vacuum and a moist cloth. Soak the syringe tops for 20 minutes in 2% (v/v) Multi-Terge ionic detergent, and subsequently rinse the syringe top in Milli-Q water to remove all traces of the detergent. Autoclave prior to FlowPot construction.
! CRITICAL STEP Avoid syringes that have silicon oil or other lubricants within the barrel or wash thoroughly prior to initial use.
! CAUTION Use proper eye protection and keep hands out of the path of the blade when cutting plastic.
Cut 5 x 5 cm squares of mesh fiberglass. Autoclave prior to FlowPot construction.
Rinse 3 mm soda-glass beads 6 times with Milli-Q water. Dry and autoclave prior to FlowPot construction.
-
To construct a FlowPot stand, drill four holes in a 12 x 8 x 1 cm block of autoclave-compatible plastic (polypropylene or polycarbonate; e.g. disposable inserts from Rainin RT-L1000 or other pipette tip boxes) using an 8.8 mm drill bit. Orient the holes so they are evenly distributed with adequate spacing from stand edge so that the FlowPots do not exceed the stand boundaries.
! CAUTION Use proper eye protection and keep hands out of the path of blades and drill bits when cutting plastic and drilling inserts.
Sterilization of the substrate ⚫ TIMING ~3 d
-
5
Blend a 1:1 (vol:vol) ratio of peat potting mix and medium vermiculite (substrate). Moisten with Milli-Q water to achieve moisture content of approximately 60% moisture content. Evenly distribute the substrate on clean polypropylene laboratory trays at a depth of approximately 2 cm. Cover the surface of each tray with sterilization wrap in such a way that liquid will not collect on top during autoclaving and flow onto the substrate. Autoclave for 30 minutes on liquid cycle (121°C, 18 PSI, slow exhaust with forced liquid cooling) and bring to room temperature (22-25°C) immediately after autoclaving.
! CRITICAL STEP Do not let materials sit in the autoclave after cycling because this may cause the substrate to dry out, resulting in increased hydrophobicity and suboptimal plant growth.
-
6
Homogenize substrate in a sterile container and subsequently distribute on polypropylene laboratory trays. Let sit covered with sterilization wrap at room temperature for 24-48 hours.
! CRITICAL STEP The rest between autoclave cycles provides an opportunity for dormant microbial spores to germinate, which can then be killed during the second autoclave cycle.
-
7
Autoclave the substrate a second time (to kill any spores) for 30 minutes on liquid cycle (121°C, 18 PSI, slow exhaust with forced liquid cooling). Pre-clean the surface of a laminar flow hood using Spor-Klenz. Immediately after autoclaving, place the autoclaved trays of substrate in the pre-cleaned laminar flow hood and bring to room temperature. Once at room temperature, aseptically homogenize the substrate in the sterile laminar flow hood. Cover the trays of substrate with sterilization wrap. Leave covered at room temperature for 24-48 hours.
! CRITICAL STEP Depending on the moisture content of your substrate, relative humidity, and the calibration of your autoclave, autoclave parameters may need to be optimized to ensure sterility whilst preserving the integrity of the substrate.
! CAUTION Spor-Klenz is caustic and an eye/skin irritant. Use personal protective equipment as described by the manufacturer.
Assembly of FlowPots ⚫ TIMING ~2 h
-
8
Aseptically place 10 sterile glass beads (from Step 3) into each of autoclaved syringe tops (from Step 1). To stabilize FlowPots during assembly, use a sterile test tube rack (from Step 4). Gently fill each syringe top with the twice-autoclaved substrate mixture (from Step 7) until slightly heaping (~0.5 cm). Cover barrel end of the syringe top with the square mesh (from Step 2) and secure with a cable tie. Trim the excess edges of the square mesh.
! CRITICAL STEP Do not overpack the substrate. Compaction can lead to suboptimal plant growth. Within an experiment, it is critical to maintain the same relative compaction for all FlowPots.
! CRITICAL STEP: We recommend using a cable tie gun: Thomas & Betts Ty-Rap Tool (http://www.cableorganizer.com/thomas-betts/ty-rap-tool.html).
-
9
Once the test tube rack is full, place the test tube rack full of assembled FlowPots in a Sun bag and loosely close the end with autoclave tape such that the risk of contamination is minimized when the bag is removed from the autoclave, yet steam may still permeate the bag during sterilization. Autoclave for 30 minutes on liquid cycle (121°C, 18 PSI, slow exhaust with forced liquid cooling). Immediately after autoclaving, seal the opening of the Sun bag and move to a sterile hood.
! CRITICAL STEP Alternative autoclave-safe bags can be used instead of Sun bags. FlowPots can also be autoclaved directly in Microboxes as well, as long as care is taken to ensure steam can penetrate assembled FlowPots during autoclaving.
PAUSE POINT Sterile FlowPots inside sealed Sun bags can be stored for several days.
-
10
Center and fasten the drilled FlowPot stand to the inside bottom of a Microbox tissue culture vessel using filament tape. Autoclave constructed boxes and lids according to the manufacturer’s instructions prior to use. Immediately after autoclaving, aseptically move to a sterile hood and place four assembled, autoclaved FlowPots into each Microbox.
! CRITICAL STEP We routinely use 18 mm filament tape model 893 (Scotch, USA), but alternative tapes are suitable.
PAUSE POINT Upon cooling, autoclaved Microboxes containing sterile, assembled FlowPots can be snapped closed and stored for several weeks.
? TROUBLESHOOTING
FlowPot irrigation and inoculation ⚫ TIMING ~2 h
-
11
Add 950 mL of sterile distilled H2O and 50 g of sieved soil (see Box 1) to a sterile 2-L Erlenmeyer flask. Agitate soil slurry on a rotary shaker for 20 minutes at room temperature at 100-200 rpm, and subsequently let settle for 5 minutes. Filter the supernatant through a 40 μm cell strainer into a sterile 2-L Nalgene media bottle.
! CRITICAL STEP Allowing the slurry to settle increases reproducibility of colonization31 and reduces filter clogging.
-
12
Divide the soil slurry into two. Prepare the holoxenic inoculum by directly mixing the strained soil slurry with equal parts 1x LS media. To prepare a sterile mock inoculum, autoclave the remaining portion of the strained soil slurry for 45 minutes (121°C, 18 PSI, slow exhaust with forced liquid cooling), then mix with equal parts 1x LS media in a sterile laminar flow hood, bringing the final concentration of LS to 1/2x.
! CRITICAL STEP: The amount of inoculum needed for each condition will be determined by the number of FlowPots being prepared.
-
13
In a sterile hood, attach a sterile female Luer x female Luer adapter to a sterile 50 mL syringe. Using a flame-sterilized test tube clamp, grasp each FlowPot (from Step 10) and invert over a sterile funnel placed atop a waste flask. While inverted, use the sterile 50 mL syringe with attached adapter to aseptically infiltrate each FlowPot with 50 mL of sterile H2O. Apply even pressure during the infiltration. After water infiltration, place the FlowPot back into its Microbox or on a sterile test tube rack. To reduce the risk of contamination, we recommend ethanol-flaming the test tube clamps between each FlowPot infiltration. The preparation of axenic FlowPots should be performed separately from those that are holoxenic and in a Biosafety cabinet or laminar flow hood.
! CAUTION Avoid ignition sources and ensure proper ventilation when working with fire and flammable solvents such as ethanol.
! CRITICAL STEP Occasionally, the glass beads become oriented in a way that the infiltration port is obscured. In this case, a sterile syringe needle may be inserted into the infiltration port to clear the blockage.
! CRITICAL STEP An alternative to the test tube clamp holder is a modified hemostat with semicircular stainless steel bands to grip the FlowPots.
? TROUBLESHOOTING
-
14
Let water-infiltrated FlowPots sit for 30 minutes, then infiltrate the FlowPots with 50 mL of a desired input community mixture (from Step 12). Evenly mix the input community prior to infiltration. Because of the small size of seeds to be placed on the surface of a prepared pot (see step 15), colonization of germinating plants is not expected to need inoculation of the entire system. For some other applications where a total saturation of the systems becomes necessary (e.g., study of microbial activities in different locations within a pot), an investigation of the evenness of inoculation within a pot will need to be conducted.
-
15
Place irrigation port of inoculated FlowPots in the drilled holes of the FlowPots stand within the sterile Microboxes. We recommend 4 FlowPots per Microbox for plants to receive even light coverage.
Sowing seeds ⚫ TIMING ~0.5 h
Plant growth ⚫ TIMING Up to 4.5 weeks
-
17
Place Microboxes with planted FlowPots in the plant tissue culture growth chamber (see Box 3).
! CRITICAL STEP After sowing, make sure the Microbox lids are completely sealed to maintain consistent humidity and sterility.
-
18
Aseptically thin boxes to 3 plants per pot using flamed forceps 7-10 days after germination.
! CRITICAL STEP Check sterility of plants and substrate during plant growth by using information from Box 4.
! CRITICAL STEP The syringe barrels can be reused after the experiment is completed to construct new FlowPots. In order to do so, discard contents, rinse thoroughly, and autoclave before storage or new FlowPot construction. …
? TROUBLESHOOTING
Box 4 ∣. Assessment of the sterility of gnotobiotic systems.
This box presents culture-based methods for testing the sterility of the gnotobiotic systems. To ensure axenic conditions are maintained throughout plant growth, it is important to check the sterility both before the experiment and after plant growth. For plants grown in the FlowPot system we test 7-10 days old seedlings at the time of thinning (Procedure1, Step 20) and again at the time of using plants for planned experiments. For plants grown in the GnotoPot system we check sterility of axenic plants and peat substrate at the time of using plants for planned experiments.
Reagents
R2A agar medium (DIFCO, cat. no. 218263)
Potato dextrose agar (PDA) (BD Difco, BD 213400)
Standard sterile Petri plates, 100 x 15 mm (VWR, cat. no. 25384-302)
Sterile tweezers or disposable inoculation loops (Fisher Scientific, cat no. 16-100-110 or 08-757-133, respectively)
REAGENT SETUP
R2A
Dissolve 18.2 g of powder in 1 L of water. Mix thoroughly. Autoclave at 121°C for 20 minutes on liquid cycle. Cool the medium to ~65°C and pour it into petri dishes in a sterile hood. Once solidified, the plates can be stored at 4°C for at least three months. R2A media from Difco contains: yeast extract (0.5 g/L), proteose peptone No.3 (0.5 g/L), casamino acids (0.5 g/L), dextrose (0.5 g/L), soluble starch (0.5 g/L), sodium pyruvate (0.3 g/L), dipotassium phosphate (0.3 g/L), magnesium sulfate (0.05 g/L), agar (15 g/L).
PDA
Dissolve 39 g of powder tin 1 L of water. Mix thoroughly. Autoclave at 121°C for 30 min on liquid cycle. Cool the medium to ~65°C and pour it into petri dishes in a sterile hood. Once solidified, the plates can be stored at 4°C for at least three months. PDA from Difco contains: potato starch (4 g/L), dextrose (20 g/L), agar (15 g/L).
Testing for culturable microbial contamination of seeds ⚫ Timing 2-7 days
-
Check for seed-borne contaminants and germination efficiency by incubating an aliquot of sterilized seeds on R2A agar at 22°C for at least one week.
? TROUBLESHOOTING
Testing for culturable microbial contamination of plants and substrate ⚫ Timing 2-7 days
-
2
To test for bacterial contamination after plant growth, transfer plant material or small amounts of peat substrate to R2A agar plates. Spread peat material evenly. Incubate for at least one week at 22°C looking for any possible bacterial contamination.
-
3
Use the same approach and plate on PDA to test for fungal contamination. Incubate for at least one week at 22°C looking for any possible fungal contamination.
? TROUBLESHOOTING
PROCEDURE 2. THE GNOTOPOT SYSTEM
Preparation of the GnotoPot system (day 1) ⚫ TIMING ~45 min
! CRITICAL In this procedure, a hydrated Jiffy-7® peat pellet inside a plastic pot is referred to as a GnotoPot, and the final assembled setup consists of four GnotoPots within a Microbox. This procedure is for preparing 48 GnotoPots for within 12 Microboxes.
Place 48 plastic nursery pots inside a large autoclave bin and add one dry compressed Jiffy-7® disc per pot.
-
Add 3 L of freshly prepared ½ X LS nutrient solution to completely hydrate the pellets. Wait for 30-40 min. The pellets will expand to seven times the original height of the dry discs.
? TROUBLESHOOTING
While pots are being thoroughly soaked in nutrient solution add a small piece of labelling tape to 12 Microbox containers on the side with an opening crack for labelling each box at the time of the experiment.
Transfer two empty plastic pots to the center of each Microbox container.
Transfer four fully hydrated GnotoPots to each Microbox flanking the central empty pots (Fig. 2b).
Add 50 mL of the excess nutrient solution from hydration autoclave bin to each box.
Loosely place the Microbox container lid on top of each box. Do not snap close the lids.
Transfer two assembled Microboxes with loose lids into an autoclavable bag (Sun bag) and close the bag with a piece of labelling tape after folding the opening of the bag inside and then down.
-
Place assembled bags into an autoclave bin, cover with sterilization wrap and secure the wrap with binder clips.
! CRITICAL STEP Make sure that the final assembled setup does not go through any strong mechanical disturbances since the pots will tip over and would not be usable after the autoclave cycles.
Sterilization of the GnotoPot system (day 1 and day 3) ⚫ TIMING ~3 days
-
10
Autoclave the assembled setup from Step 9 for 45 min using a liquid cycle at 121°C (18 PSI). After the autoclave cycle is done allow the chamber to cool down to 50°C. Then store the assembled setup at room temperature (22-25°C).
-
11
After two nights (36 h to 48 h) repeat the autoclave cycle as Step 10 and cool down at room temperature.
! CRITICAL STEP The rest between autoclave cycles provides an opportunity for dormant microbial spores to germinate, which can then be killed during the second autoclave cycle.
-
12
After 3-4 h or sufficient cool down remove the sterilization wrap and snap close the lids while inside the Sun Bag. Store the Microboxes containing GnotoPots while inside Sun Bags at room temperature for at least 1 day before sowing seeds (Step 23).
PAUSE POINT At this stage, Microboxes containing GnotoPots inside the Sun bags could be stored away for future use up to at least two months without any contamination issues.
! CRITICAL STEP If the external surfaces of the Microbox lids have significant condensation after 1 day, allow more time for drying before use.
Preparation of the complex holoxenic community (day 4) ⚫ TIMING ~3 hours
-
13
Use the soil material prepared based on information in Box 1. To extract soil microbial communities, transfer 10 g of the soil aliquot to an autoclaved 1L Erlenmeyer flask and add 200 mL of autoclaved Milli-Q water.
-
14
Place the flask in a shaker for 20 min at 100-200 rpm at room temperature.
-
15
Store the flasks on the bench for 5 min allowing for separation of large soil particles from soil slurry.
-
16
Filter the soil slurry through a cell strainer and split into two 100 mL aliquots inside 1 L round media storage bottles.
-
17
Add 100 mL of autoclaved 1X LS to 100 mL of soil slurry for viable microbial community inoculation.
-
18
Autoclave the second half of soil slurry for 45 min for heat-killed community control. After cooling down to room temperature, place the heat-killed community inside a biosafety cabinet and add 100 mL of autoclaved 1X LS solution.
Transferring Microboxes containing GnotoPots to a biosafety cabinet (day 4) ⚫ TIMING ~30 min
-
19
To surface sterilize the interior environment of the biosafety cabinet use the germicidal UV lamp for 10 min. After lifting up the sash spray and wipe out all the working areas with freshly prepared Spor-Klenz solution.
! CAUTION Spor-Klenz is caustic and an eye/skin irritant. Use personal protective equipment as described by the manufacturer.
! CRITICAL STEP To ensure that the GnotoPots remain axenic during the handling steps, thorough aseptic practice is recommended.
-
20
Spray all the items to be used inside the biosafety cabinet with freshly prepared Spor-Klenz solution and store inside the biosafety cabinet.
-
21
Remove the Microboxes containing GnotoPots from Sun Bags and move the bags out of the biosafety cabinet.
! CRITICAL STEP in cases that the external surfaces of the Microbox lids are still wet at this point, allow the boxes to dry out further inside the biosafety cabinet before handling.
Seed sowing on GnotoPots (day 4) ⚫ TIMING ~45 min
-
22
Open each Microbox and add 15 mL of 1X LS solution to individual GnotoPots by top irrigation of the pots to restore the Jiffy-7® pellet water content to full saturation. At this step label each Microbox with appropriate information for different plant genotypes and treatments.
-
23
Using a P20 pipette transfer 1 or 2 seeds (see Box 2) per pot to the edge of the central divot of the GnotoPots.
! CRITICAL STEP To achieve a uniform plant growth in this procedure only one seed per pot is being used, therefore it is important to make sure that the seeds are of high quality with high germination rates following the steps described earlier (see Box 2).
-
24
For axenic plants with no further treatments snap close boxes and move them out of the biosafety cabinet.
Inoculation with microbial communities (day 4) ⚫ TIMING ~15 min
-
25
To inoculate with live complex microbial communities or synthetic bacterial communities2 gradually irrigate the top section of pots with 1 mL of the microbial community solution (from Step 17) in a dropwise manner covering the entire top surface. For axenic plants treated with heat-killed community as a control uniformly add 1 mL of autoclaved and cooled down solution from Step 22 to the top section of GnotoPots. Then snap close the lids and move boxes out of the biosafety cabinet.
Plant growth ⚫ TIMING Up to 6.5 weeks
Sampling GnotoPots for contamination tests ⚫ Timing ~30 min for six Microboxes containing GnotoPots
-
27
Test for sterility before using germ-free plant material for experiments by sampling the peat pellet from each GnotoPot for microbial contamination. Spray germ-free boxes with Spor-Klenz and store under the biosafety cabinet for 10 min. Then open up the boxes and, using a sterile loop, scoop a small amount of peat pellet from any part of the GnotoPots including the central hollow core and process samples using instructions from Box 4. Alternatively, test standing liquid inside Microboxes for contamination.
! CAUTION Spor-Klenz is caustic and an eye/skin irritant. Use personal protective equipment as described by the manufacturer.
! CRITICAL STEP Make sure that sampling is done without damaging the plant material or contaminating the system. Use this sample in the next step.
Troubleshooting
The FlowPot system requires more steps to construct and implement than the GnotoPot system. It is important to minimize the variability associated with construction of FlowPots (Procedure 1 Steps 1-4), and several attempts may be required to optimize Procedure 1. Table 2 summarizes several troubleshooting suggestions to help facilitate the optimization process. Table 1 also contains several troubleshooting tips for the GnotoPot system as well.
Table 2.
Troubleshooting table.
| Procedure | Step | Problem | Possible reason | Solution |
|---|---|---|---|---|
| Box 2 | 6 | Poor germination rate | Sterilization is too harsh. | Reduce exposure to chlorine gas or reduce the amount of HCl added to the Erlenmeyer flask. Verify germination rate on 1/2X LS plates without sucrose. |
| Seeds storage suboptimal. | Ensure seeds are stored in the dark with low humidity. Verify germination rate on 1/2X LS plates without sucrose. | |||
| Box 4 | 1 | Seeds are contaminated | Seeds were not properly decontaminated. | Use fresh bleach during sterilization and ensure adequate sterilization time. Alternatively, increase the exposed surface area of seed aliquots by reducing the number of seeds in each tube. It may be necessary to empirically determine the duration of sterilization based on the volume of seeds in individual aliquots. Alternatively, surface sterilize seeds with Ethanol solution (50% Ethanol (v/v) +0.01% Triton X-100) for 5 min, followed by fresh bleach solution (10% commercial bleach+ 0.01% Triton X-100) for 5 min and wash 4 times with sterile Milli-Q water. |
| 3 | Plants or substrate are contaminated | Substrate was not properly sterilized. | Ensure sufficient time between autoclave cycles (24-48 h) during substrate sterilization. The rest provides an opportunity for dormant spores to germinate, which can then be killed during the second autoclave cycle. | |
| Tissue culture box not sealed. | Check filters and seals of Microbox. Discard if damaged. | |||
| Water or media was contaminated during irrigation or inoculation. | Ensure clean, undamaged glassware and proper sterile technique are being used. | |||
| 1 - FlowPot | 12 | Microbox becomes deformed after autoclaving. | Lid was sealed on the Microbox while autoclaving. | Do not seal lids while autoclaving and follow manufacturer’s instructions for Microbox sterilization. |
| 15 | Liquid is unable to be passed through the FlowPot. | Glass beads or debris are obstructing the irrigation port. | Insert a sterile needle into the bottom of the FlowPot to clear the irrigation port. | |
| 15 | The mesh retainer is dislodged from the FlowPot. | Glass beads or debris are partially obstructing the irrigation port, resulting in increased backpressure. | Insert a sterile needle into the bottom of the FlowPot to clear the irrigation port. | |
| Substrate is packed too tightly into the FlowPot, resulting in increased back pressure. | Assemble FlowPots using less substrate and do not pack. | |||
| The nylon cable tie relaxed during autoclaving. | Do not fully tighten cable ties until after autoclaving or use a cable tie gun. Alternatively, change the material of the cable ties and avoid nylon. | |||
| 20 | Plants appear chlorotic | Recalcitrant byproducts of sterilization may be deleterious to plant growth. | In our experience, using 50-60 °C water to flush the substrate improves performance if plants appear chlorotic. | |
| 20 | Excess condensation builds up on the walls of the Microbox during plant growth. | Too much moisture is contained within the system. | The depth filter of the described Microbox is hydrophobic, so water is generally retained within a sealed box for quite some time. Decrease the amount of FlowPots per box to reduce excess moisture. We recommend no more than four FlowPots per Microbox. | |
| Temperature fluctuations within the Microbox are increasing the rate of evaporation from FlowPots. | Maintain constant temperatures within the Microbox. Adjusting day/night temperatures can help account for the radiant energy absorbed by the Microboxes from the light source. Alternatively, we found that placing growth chamber temperature probes into sealed Microboxes with humidity levels corresponding to those of boxes growing plants helped mitigate temperature fluctuations. Additionally, insulating Microboxes from metal surfaces by placing them on matte black ceramic tiles may also help reduce thermal fluctuations and the build up of condensation. | |||
| 20 | Plants are water stressed. | Substrate is drying out. | Aseptically apply ~8 mL sterile water with a needle and syringe to the center of each FlowPot, avoiding damage to plant roots. We have found that watering plants after sowing is generally unnecessary and that the substrate sustains plant growth for at least 8 weeks without intervention. In our experience, the drying of substrate is usually due to temperature fluctuations increasing the rate of evaporation. The water is usually retained within the system on the bottom or sides of the Microbox as the filter is hydrophobic. | |
| 20 | Plants are nutrient stressed. | Nutrients are depleted from the substrate. | Apply ~8 mL 1/2X LS media with a needle and syringe to the center of each FlowPot as needed. We have found that supplementing FlowPots with nutrients after sowing is generally unnecessary and that the substrate sustains plant growth for at least 8 weeks without intervention. | |
| 20 | Plants fail to germinate. | Seeds sterilized for too long. | Decrease sterilization time. It may be necessary to empirically determine the duration of sterilization based on the volume of seeds in individual aliquots. We found that 6 h sterilization time had no adverse effect on germination rate. Alternatively, liquid bleach can be used to sterilize seeds. | |
| 2 - GnotoPot | 2 | Occasionally, the dry pellets do not fully expand. | Variance in manufacturing. | Visually inspect the dry pellets before placing inside the 2 inch pots to make sure that the mesh covering peat is not ruptured. Also after full expansion make sure that the central part of the Jiffy-7® is accessible for placing the seed at the time of seed sowing. If the central hole is not accessible use a pair of tweezers to manually open up the mesh. |
| 26 | Too much algal growth inside the box. | Excess of microbial community inoculum from soil. | Use a smaller amount of the soil for preparing the soil tea. Also make sure that the soil inoculum is only added to the central area of peat pellet | |
| 26 | The peat pellet looks dry after 6 weeks. | Excessive water loss | Although the GnotoPots perform very well in retaining the moisture, depending on the changes in the seasonal ambient humidity levels in different geographical locations some pots might have a drier peat pellet later in the growth phase. To hydrate these pots simply raise one side of the Microbox to about 10 degree angle to move the sitting solution to one side of the box and wait for 1-2 minutes. Perform this step for all the relevant controls as well. |
Timing
Any required soil or seed preparation should be performed prior to the start of the experiment.
Procedure 1 for preparation of 12 FlowPots
Pre-experiment preparation:
Steps 1-4, construction of 12 FlowPots (3 FlowPot boxes): ~1.5 h
Days 1-4:
Steps 5-7, sterilization of the substrate: ~3 d
Steps 8-10, assembly of FlowPots: ~2 h
Steps 11-15, FlowPot irrigation and inoculation: ~2 hSteps 16, sowing seeds: ~0.5 h
Procedure 2 for preparation of 48 GnotoPots
Days 1-4:
Steps 1-12, preparation of the 48 GnotoPots (within 12 Microboxes) and two autoclave cycles: 3 d
Steps 13-18, preparation of the complex holoxenic community: ~3 h
Step 19-25, soil slurry and heat killed community preparation, and seeds sowing in GnotoPot: ~4 hours
Anticipated Results
We have shown that the FlowPot and the GnotoPot systems can be used to study microbiota dynamics in planta2,3 under a controlled environment using plants grown in organic matter-based peat substrates that more closely approximates a natural state as compared to other existing methods (Fig. 3 and Fig. 4). We expect that both systems will enable researchers to investigate a variety of questions related to the plant-microbiota interactions, ranging from community assembly to epigenetic impacts on the host, which will undoubtedly provide new insights in plant and microbial sciences. Specific scientific objectives may necessitate modification of either the FlowPot or GnotoPot systems, however, the procedures described here provide a strong foundation for future research.
The FlowPot and GnotoPot systems can be adapted to suit many research applications, including microbiota colonization studies as well as studies to determine the phenotypic impact different microbiotas may have on the host plant. A fundamental attribute of both systems is the ability to support the robust growth of axenic Arabidopsis plants. To demonstrate this feature, we performed Procedure 1 and Procedure 2 to produce axenic and holoxenic Arabidopsis plants in the FlowPot system (Fig. 3) and the GnotoPot system (Fig. 4). Briefly, FlowPots and GnotoPots were constructed according to the procedures described in this Protocol, and subsequently treated with a soil slurry or mock-treated with the heat-killed soil slurry. The soil slurry was generated from soil we collected from an agricultural field located at Michigan State University (East Lansing, MI, USA) (Supplementary Table 1). Arabidopsis seeds were sown, and plants were grown for 4 weeks (Fig. 3) or 6.5 weeks (Fig. 4) under the recommended growth chamber conditions. To prevent overcrowding, each FlowPot and GnotoPot was aseptically thinned to a single plant using sterile forceps. The axenic plants were verified to be axenic using the procedure described in this Protocol, and representative images of mature rosettes were photographed (Fig.3 and Fig. 4). Plant samples from both systems could be subsequently used for various plant phenotyping experiments and microbial community studies to determine the functional impact of microbiota on the host, microbiome composition, and to identify microbial strains that most successfully colonize host plant tissues.
One of the fundamental questions regarding host-microbiota interactions across different domains of life is how a host shapes its microbiome, and ultimately, how does this promote health and disease. The FlowPot and GnotoPot systems provide researchers with tools to address such questions. For example, Xin et al. (2017) noticed that two Arabidopsis polymutants deficient in aspects of pattern-triggered immunity and defense-associated vesicle trafficking (min7/bak1/bkk1/cerk1 and min7/fls2/efr/cerk1) (referred to mbbc and mfec, respectively) developed spontaneous disease symptoms in the absence of pathogen inoculation under high humidity when grown in growth chambers with conventional potting mix substrate, which contains natural microbiota. To determine whether the phenotype was microbiome-dependent, the GnotoPot system was deployed by Chen et al. (2020), who performed an experiment to study leaf phenotypes in wildtype vs mfec Arabidopsis in the presence or absence of microbiota. Only holoxenic mfec had clearly visible chlorosis, demonstrating the microbiota-dependent nature of the phenotype. We repeated this experiment using the mbbc polymutant and observed a similar result (Fig. 5). Collectively, the GnotoPot system enabled experiments to demonstrate a causal role of microbiota in the observed spontaneous leaf damage associated with dysbiosis. We anticipate the use of the FlowPot and GnotoPot systems will enable researchers to broadly address questions about various microbiota functions.
Fig. 5 ∣. Application for microbiota function studies in planta.
Six-week old wildtype (Col-0) and mbbc plants were subjected to high humidity (95% relative humidity) for 11 days. A chlorosis phenotype is observed in holoxenic mbbc plants but not axenic mbbc plants, indicating that chlorosis is dependent on exposure to a microbial community. Holoxenic substrate was inoculated with a soil slurry, axenic substrate was inoculated with a heat-killed version of the same soil slurry.
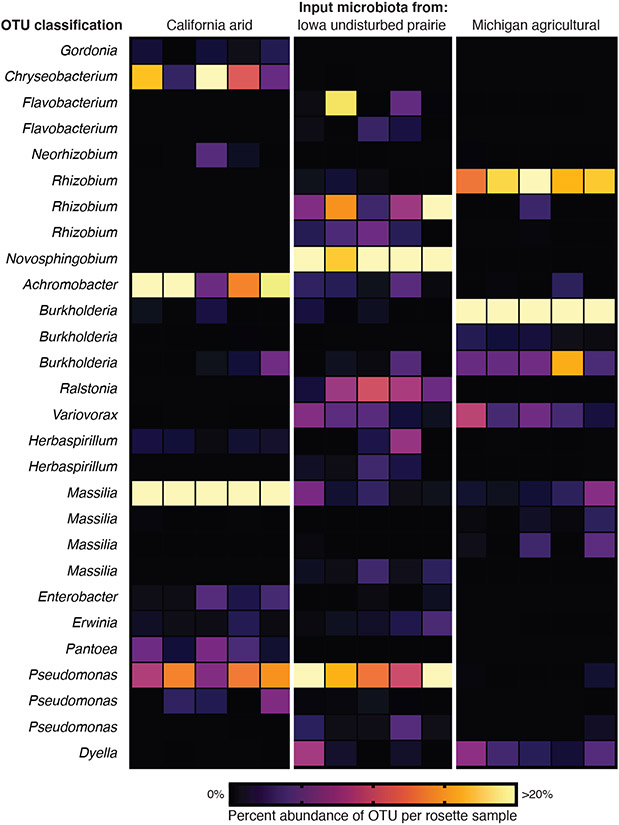
The FlowPot and GnotoPot systems can also be used to determine the relative success of different microorganisms for their ability to colonize and persist in host tissues. Critically, MicroBoxes allow isolation of samples/treatments within a common growth chamber, preventing microbiota cross-contamination and thus allowing researchers to use multiple input microbial communities in a single experiment with limited space. Both defined microbiotas (for example, synthetic communities) and undefined natural microbiotas (for example, a soil slurry) can be used as input microbiotas in either system. The FlowPot system homogeneously saturates entire pots with input microbial suspensions (Fig. 1), while the GnotoPot system allows experimenters to apply microbial suspensions to the surface of Jiffy-7 pellets (Fig. 2). Here, we provide an example of a colonization workflow (Fig. 6). Using three distinct soils as input microbiotas (Supplementary Table 1), we inoculated FlowPots, collected whole Arabidopsis rosettes after four weeks of growth, and surveyed the bacterial community composition of rosette tissue (Supplementary Methods) to identify the most abundant bacteria present. The 16S rRNA gene operational taxonomic units (OTUs) that account for >2% of total reads in any given sample are displayed as a heatmap (Fig. 6), and closest-match OTU classifications at the genus level were also determined for this example application (Supplementary Table 2). Applications similar to this community profiling experiment have broad utility, not limited to 16S rRNA gene community profiling. For example, Duran et al. (2018) successfully used this approach to characterize fungal and oomycete communities associated with plant tissues.
Fig. 6 ∣. Application for microbial community studies.
FlowPots were inoculated with soil slurries from three distinct environments (arid, undisturbed prairie, agricultural) (Supplementary Table 1) with 20 replicate FlowPots per treatment contained in 5 Microboxes (4 per box). Arabidopsis seeds (wildtype Col-0) were sowed, and total rosettes were collected at 4 weeks. Each sample consists of bulk DNA extracted from 4 pooled rosettes from the same Microbox. The heatmap represents the relative abundance of Operational Taxonomic Units (OTUs) for all OTUs that accounted for >2% of total reads in any given sample. Genus-level classifications are given for each OTU (Supplementary Table 2). See Supplementary Methods for DNA extraction and microbial community profiling.
With all animal and plant gnotobiotic systems there is always the challenge of keeping the enclosed system devoid of any unwanted microbial colonizers. The protocols described here are designed to effectively eliminate substrate- and air-borne microorganisms without using antibiotic treatments and exclude microbes from the gnotobiotic systems prior to the start of gnotobiotic experiments in a cost-effective way. By using best practices of sterile techniques along with recommended troubleshooting steps included in this paper, we expect that the FlowPot and the GnotoPot systems could be successfully adapted by the plant-microbiota research community.
Supplementary Material
Supplementary Table 1. Geographic locations of soils used in this study
Supplementary Table 2. OTU information for the example community profiling workflow used to generate the heatmap in Fig. 6
Acknowledgements
We would like to thank Caleigh Griffin, Alec Bonifer, Alan Mundakkal, Franchesca Dion, Trevor Ulrich, Jennifer Martz, and Tim Johnson for assistance with FlowPot assembly and workflow optimization, Dr. M. Amine Hassani and Dr. Stephane Hacquard for critical reading and helpful comments on this manuscript, and Dr. Brian Kvitko and Dr. JP Jerome for their contributions to FlowPot growth system development. Figure 2 was partially created with Biorender.com. This project was supported by funding from Gordon and Betty Moore Foundation (GBMF3037), National Institutes of Health (GM109928), and Plant Resilience Institute, Michigan State University.
Related links
Key references using this protocol:
- Durán P. et al. Cell 175, 973–983 (2018). 10.1016/j.cell.2018.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. et al. Nature 580, 653–657 (2020). 10.1038/s41586-020-2185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Competing interests
The authors declare no competing interests.
Data availability
All data are presented in this paper and available from the authors without restrictions. Information about the input soil microbial communities is available in Supplementary Table 1. Raw source 16S rRNA gene sequences from this project (for Fig. 6 and Supplementary Table 2) are available in the Sequence Read Archive database under BioProject PRJNA689857, accession numbers SAMN17220890 to SAMN17220933.
References
- 1.Round JL & Mazmanian SK The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol 9, 313–323 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T. et al. A plant genetic network for preventing dysbiosis in the phyllosphere. Nature 580, 653–657 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durán P. et al. Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival. Cell 175, 973–983.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busby PE et al. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 15, e2001793 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y-X, Qin Y & Bai Y Reductionist synthetic community approaches in root microbiome research. Current Opinion in Microbiology vol. 49 97–102 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Bai Y. et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528, 364–369 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Lebeis SL et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 349, 860–864 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Zamioudis C. et al. Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB 72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. The Plant Journal vol. 84 309–322 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkel OM et al. The effects of soil phosphorus content on plant microbiota are driven by the plant phosphate starvation response. PLoS Biol. 17, e3000534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innerebner G, Knief C & Vorholt JA Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol 77, 3202–3210 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiffer JA et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. U. S. A 110, 6548–6553 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spor A, Koren O & Ley R Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol 9, 279–290 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Adair KL & Douglas AE Making a microbiome: the many determinants of host-associated microbial community composition. Curr. Opin. Microbiol 35, 23–29 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Xin X-F et al. Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539, 524–529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrew DR et al. Abiotic factors shape microbial diversity in Sonoran Desert soils. Appl. Environ. Microbiol 78, 7527–7537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fierer N & Jackson RB The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. U. S. A 103, 626–631 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunning T & Cahill DM A Soil-free Plant Growth System to Facilitate Analysis of Plant Pathogen Interactions in Roots. Journal of Phytopathology 157, 497–501 (2009). [Google Scholar]
- 18.Jackson MB et al. Ventilation in plant tissue cultures and effects of poor aeration on ethylene and carbon dioxide accumulation, oxygen depletion and explant development. Ann. Bot 67, (1991). [Google Scholar]
- 19.Kloepper JW, Schroth - Phytopathology MN & 1981. Plant growth-promoting rhizobacteria and plant growth under gnotobiotic conditions. apsnet.org (1981). [Google Scholar]
- 20.Henry A. et al. An axenic plant culture system for optimal growth in long-term studies. J. Environ. Qual 35, 590–598 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Carlström CI et al. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nature Ecology & Evolution vol. 3 1445–1454 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams C, Jacobson A & Bugbee B Ceramic Aggregate Sorption and Desorption Chemistry: Implications for Use as a Component of Soilless Media. J. Plant Nutr 37, 1345–1357 (2014). [Google Scholar]
- 23.Trevors JT Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 26, 53–59 (1996). [Google Scholar]
- 24.Shaw LJ, Beaton Y, Glover LA, Killham K & Meharg AA Re-inoculation of autoclaved soil as a non-sterile treatment for xenobiotic sorption and biodegradation studies. Appl. Soil Ecol 11, 217–226 (1999). [Google Scholar]
- 25.Bank TL et al. Effects of gamma-sterilization on the physico-chemical properties of natural sediments. Chem. Geol 251, 1–7 (2008). [Google Scholar]
- 26.Berns AE et al. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur. J. Soil Sci 59, 540–550 (2008). [Google Scholar]
- 27.Wolf DC, Dao TH, Scott HD & Lavy TL Influence of Sterilization Methods on Selected Soil Microbiological, Physical, and Chemical Properties. J. Environ. Qual 18, 39–44 (1989). [Google Scholar]
- 28.Lotrario JB et al. Effects of sterilization methods on the physical characteristics of soil: implications for sorption isotherm analyses. Bull. Environ. Contam. Toxicol 54, 668–675 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Lindsey BE 3rd, Rivero L, Calhoun CS, Grotewold E & Brkljacic J Standardized Method for High-throughput Sterilization of Arabidopsis Seeds. J. Vis. Exp (2017) doi: 10.3791/56587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clough SJ & Bent AF transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Seedorf H. et al. Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159, 253–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Geographic locations of soils used in this study
Supplementary Table 2. OTU information for the example community profiling workflow used to generate the heatmap in Fig. 6
Data Availability Statement
All data are presented in this paper and available from the authors without restrictions. Information about the input soil microbial communities is available in Supplementary Table 1. Raw source 16S rRNA gene sequences from this project (for Fig. 6 and Supplementary Table 2) are available in the Sequence Read Archive database under BioProject PRJNA689857, accession numbers SAMN17220890 to SAMN17220933.