Abstract
This study aimed to quantify the relative biological effectiveness (RBE) for epithermal neutron beam contaminated with fast neutrons in the accelerator-based boron neutron capture therapy (BNCT) system coupled to a solid-state lithium target. The experiments were performed in National Cancer Center Hospital (NCCH), Tokyo, Japan. Neutron irradiation with the system provided by Cancer Intelligence Care Systems (CICS), Inc. was performed. X-ray irradiation, which was assigned as the reference group, was also performed using a medical linear accelerator (LINAC) equipped in NCCH. The four cell lines (SAS, SCCVII, U87-MG and NB1RGB) were utilized to quantify RBE value for the neutron beam. Before both of those irradiations, all cells were collected and dispensed into vials. The doses of 10% cell surviving fraction (SF) (D10) were calculated by LQ model fitting. All cell experiments were conducted in triplicate at least. Because the system provides not only neutrons, but gamma-rays, the contribution from the gamma-rays to the survival fraction were subtracted in this study. D10 value of SAS, SCCVII, U87-MG and NB1RGB for the neutron beam was 4.26, 4.08, 5.81 and 2.72 Gy, respectively, while that acquired by the X-ray irradiation was 6.34, 7.21, 7.12 and 5.49 Gy, respectively. Comparison of both of the D10 values, RBE value of SAS, SCCVII, U87-MG and NB1RGB for the neutron beam was calculated as 1.7, 2.2, 1.3 and 2.5, respectively, and the average RBE value was 1.9. This study investigated RBE of the epithermal neutron beam contaminated with fast neutrons in the accelerator-based BNCT system coupled to a solid-state lithium target.
Keywords: neutron beam, solid-state Li target, relative biological effectiveness (RBE), accelerator-based BNCT system, boron neutron capture therapy (BNCT)
INTRODUCTION
Boron neutron capture therapy (BNCT) has been performed using research nuclear reactors as neutron sources, and the favorable clinical outcome have been reported in many studies [1–10]. However, it is difficult to install a nuclear reactor into a hospital due to many related regulations. From recent research and developments, an accelerator-based neutron source can be acquired with required neutron flux to perform BNCT instead of the nuclear reactor [11–14]. It can facilitate the clinical implementation of BNCT into a hospital. Furthermore, the favorable clinical outcome using the accelerator-based neutron source has been also reported as well as that using the research reactors [15–17].
There are two types of the accelerator-based neutron source implemented into the clinical [11, 12, 15–17]. They are mainly divided by a neutron generation method. One of them utilizes the neutron generation via the reaction of 9Be(p, n)9B. It is utilized for the accelerator-based BNCT system provided by Sumitomo Heavy Industries (SHI), Ltd [18]. The other utilizes the reaction of 7Li(p, n)7Be. It is utilized for the accelerator-based BNCT system provided by Cancer Intelligence Care Systems (CICS), Inc., and its system is installed into the National Cancer Center Hospital (NCCH), Tokyo, Japan [19]. One of the major differences between them is the energy of generated neutrons. The maximum neutron energy of the accelerator-based BNCT system provided by SHI is ~28 MeV while that by CICS is less than 800 keV [12, 18]. Because the energy of generated neutrons is too high to be suitable for BNCT in both systems, the generated neutrons pass through moderators, which are designed in each system, to deliver the suitable neutron to a patient. After them, the neutron are decelerated to around 10 keV in both systems, and its neutron beam is delivered to a patient. Its energy is upper limit of the epithermal neutron [20]. However, the neutrons with an energy ranging from the epithermal neutron to the maximum energy are also contaminated into the neutron beam in each system even if it has passed through the moderators [21, 22]. Previous study suggested that relative biological effectiveness (RBE) value of the neutron, derived from recoil protons induced by the reactions between the neutrons and a human, might depend on emitted neutron energy in the accelerator-based BNCT system [23]. Therefore, this study aimed to quantify RBE value for the epithermal neutron beam contaminated with fast neutrons in the linear accelerator-based BNCT system coupled to a solid-state lithium target (CICS-1).
MATERIALS AND METHODS
This study was performed using the accelerator-based BNCT system (manufactured by CICS) at NCCH, which employs a solid-state Li target [19]. Free beam major parameters in the accelerator-based BNCT system is summarized in Table 1. Those parameters were proposed in IAEA TECDOC-1223 [20]. To quantify RBE values for the epithermal neutron beam contaminated with fast neutrons, the four cell lines human squamous cell carcinoma (SAS), mouse squamous cell carcinoma (SCCVII), human malignant glioma (U87-MG) and as normal human skin fibroblast (NB1RGB) were utilized. SAS and NB1RGB were purchased from JCRB Cell Bank and U87-MG was obtained from ATCC. SCCVII was transferred from Dr. Suzuki in Kyoto University. SAS, SCCVII, U87-MG and NB1RGB were cultured in DMEM/Ham’s F-12 medium (11581–15, Nacalai Tesque), RPMI-1640 medium (30264–56, Nacalai Tesque), MEM medium (21442–25, Nacalai Tesque) and alpha-MEM medium (21445–05, Nacalai Tesque), respectively. All types of medium contained 10% fetal bovine serum (Item No.: 10270106, Lot No.: 42G2097K, Gibco), 1% penicillin and streptomycin (09367–34, Nacalai Tesque). Cell culture was performed in 10 cm culture dishes (TR4002, NIPPON Genetics Co, Ltd) and cultures were grown in the humidified CO2 incubator at 37°C. Cell passaging was performed on culture dishes with less than 90% confluency and the culture dishes that were near 100% confluency were not used in any experiment. When cells were collected from culture dishes, the dishes were washed with Phosphate-buffered saline (PBS) and Accutase (12679–54, Nacalai Tesque). At the irradiation, the collected cells were dispensed into cryovials (89020, TPP) at approximately 1 × 106 cells/ml.
Table 1.
Free beam parameters at the central beam axis in the accelerator-based BNCT system (CICS-1)
| Beam parametera | CICS-1 |
|---|---|
| Epithermal neutron flux [cm−2 s−1] | 7.3 × 108 |
| Fast neutron dose per unit epithermal neutron fluenceb [Gy cm2] | 4.7 × 10−13 |
| Gamma dose per unit epithermal neutron fluenceb [Gy cm2] | 1.6 × 10−12 |
aThe beam parameters were proposed in IAEA-TECDOC-1223 [20].
bThe values are reported doses per unit epithermal neutron fluence.
Neutron irradiation
Using the accelerator-based BNCT system (CICS-1), the neutron irradiations were performed without any phantom to make the number of neutrons to the cells as much as possible. Figure 1 shows the experimental geometry. The cells were placed at the bottom surface of the irradiation port of the accelerator-based BNCT system. In each cell, the neutron doses of 0, 0.5, 1, 2 and 3 Gy (physical dose) would be delivered to evaluate required dose for acquiring the survival fraction of 10%. The gamma-rays are contaminated in the neutron when the neutron irradiation is performed with the accelerator-based BNCT system. The ratio of the contaminated gamma-ray dose to the total delivered dose (G/T ratio) was 0.338 in this experimental geometry.
Fig. 1.

Neutron irradiation geometry. (a): Schematic diagram along the beam axis, (b): Photograph of (a), (c): Schematic diagram perpendicular to the beam axis, and (d): Photograph of (c).
X-ray irradiation
X-ray irradiation, which was assigned as the reference group, was performed using the medical linear accelerator (LINAC) (Clinac iX silhouettes, Varian Medical Systems, Palo Alto, CA, USA). The medical LINAC used in study was calibrated based on TRS-398 [24]. In each cell, the doses of 0, 2, 4, 6 and 8 Gy were delivered with using 10 MV X-ray to evaluate required dose for acquiring the survival fraction of 10%. The cell was placed on a phantom made of tough water which had a uniform water-equivalent material for X-ray (Kyoto Kagaku Co., Ltd, Kyoto, Japan) [25], and the dose rate in each cell was 6 Gy/min.
Colony formation assay
Irradiated cells were diluted and seeded on 6 well plates or 60 mm dishes and cultured for 7–14 days. The plates were fixed by ethanol and stained by crystal violet solution. Plating efficiency (PE) and surviving fraction (SF) were calculated as described previously [26], and those values were summarized in Tables 2 and 3. The doses of 10% cell SF (D10) was calculated by the linear quadratic (LQ) or linear model fitting. All cell experiments were conducted in triplicate at least.
Table 2.
Number of cell seeded and PE after X-ray irradiation
| X-ray irradiation | SAS | SCCVII | U87-MG | NB1RGB | ||||
|---|---|---|---|---|---|---|---|---|
| [Gy] | Number of cell | P.E. [%] | Number of cell | P.E. [%] | Number of cell | P.E. [%] | Number of cell | P.E. [%] |
| 0 | 120 | 67% | 200 | 66% | 120 | 16% | 120 | 26% |
| 2 | 400 | 35% | 200 | 47% | 120 | 12% | 400 | 9.4% |
| 4 | 400 | 18% | 400 | 26% | 200 | 5.5% | 400 | 6.6% |
| 6 | 1000 | 7.5% | 1000 | 12% | 500 | 3.2% | 1000 | 2.4% |
| 8 | 8000 | 2.4% | 1000 | 4.3% | 1000 | 1.5% | 1000 | 1.4% |
Table 3.
Number of cell seeded and PE after neutron irradiation
| Neutron irradiation [Gy] |
SAS | SCCVII | U87-MG | NB1RGB | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cell | P.E. [%] | Number of cell | P.E. [%] | Number of cell | P.E. [%] | Number of cell | P.E. [%] | |
| 0 | 120 | 44% | 120 | 71% | 120 | 14% | 200 | 39% |
| 0.5 | 120 | 35% | 200 | 49% | 120 | 13% | 200 | 25% |
| 1 | 400 | 17% | 400 | 31% | 300 | 6.0% | 400 | 9.1% |
| 2 | 1000 | 6.4% | 400 | 14% | 2500 | 1.5% | 2000 | 0.79% |
| 3 | 1000 | 1.9% | 1000 | 4.7% | 4000 | 0.61% | 4000 | 0.13% |
Calculation of RBE values
Because the accelerator-based BNCT system provided not only neutrons, but also gamma-rays, the contribution from the gamma-rays to the survival fraction were subtracted in this study. The subtraction method was expressed as follows:
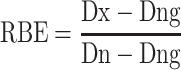 |
(1) |
where Dx is X-ray dose for 10% cell survival, Dn is calculated neutron dose for 10% cell survival and Dng is contaminated gamma-ray dose of Dn. In the equation (1), Dng is subtracted from both Dx and Dn to eliminate the effects to the cell survival fraction from contamination gamma-rays.
The cell survival was calculated by applying the LQ model to calculate the required dose for acquiring the survival fraction of 10%. The LQ model was fitted to each cell survival fraction using a least-square method. In the evaluation of the neutron beam irradiation, only the linear model was applied to the survival fraction after the subtraction method had been applied to eliminate the contribution from the gamma-rays. On the other hand, the LQ model was applied to the survival fraction in the evaluation of the X-ray irradiation. In this case, the LQ model was applied with the restriction that the quadratic term was not lower than zero.
RESULTS
To clarify the level of damage caused by the neutron beam irradiation under free-air condition, cell surviving assay was performed. In the neutron irradiation, median delivered dose rate and median neutron beam fluence rate, which contained epithermal neutron and fast neutron, were 2.45 × 10−2 (range: (2.28–3.20) × 10−2) Gy min−1 and 3.09 × 1010 (range: (2.86–4.02) × 1010) cm−2 min−1, respectively. Median irradiation time (range) for the expected delivered dose of 0.5, 1, 2 and 3 Gy was 30.0 (30.0), 61.0 (60.0–61.7), 123.0 (120.0–125.2) and 185.0 (180.0–193.0) min, respectively. Figure 2 shows the surviving fractions of 4 cell lines with the neutron irradiation and the X-ray irradiation were fitted by linear approximation and LQ model, respectively. The 10% survival doses by the neutron beam irradiation and X-ray irradiation were calculated from each fitted curve as shown in Tables 4 and 5. Contaminated gamma-ray doses in the neutron beam were also calculated using G/T ratio (Table 4). The RBE values of 4 cell lines were calculated, and the values ranged from 1.3 to 2.5 and the average RBE value was 1.9 (Table 6).
Fig. 2.

Surviving fraction curve by X-ray (left side) or neutron irradiation (right side) in (a) SAS, (b) U87-MG, (c) SCCVII and (d) NB1RGB.
Table 4.
Ten percent survival dose obtained from surviving curves of 4 cell lines and each gamma-ray contaminated dose in the epithermal neutron beam contaminated with fast neutrons
| SAS | U87-MG | SCCVII | NB1RGB | |
|---|---|---|---|---|
| 10% survival dose [Gy] | 4.26 | 5.81 | 4.08 | 2.72 |
| Gamma-ray dose in the epithermal neutron beam contaminated with fast neutrons [Gy] | 1.44 | 1.96 | 1.38 | 0.92 |
Table 5.
Ten percent survival dose from surviving curves of 4 cell lines after X-ray irradiation
| SAS | U87-MG | SCCVII | NB1RGB | |
|---|---|---|---|---|
| 10% survival dose [Gy] | 6.34 | 7.12 | 7.21 | 5.49 |
Table 6.
RBE values of the epithermal neutron beam contaminated with fast neutrons in CICS-1
| SAS | U87-MG | SCCVII | NB1RGB | |
|---|---|---|---|---|
| RBE value | 1.7 | 1.3 | 2.2 | 2.5 |
DISCUSSION
This is the first report for quantifying RBE values of the epithermal neutron beam contaminated with fast neutrons in the accelerator-based BNCT system coupled to the solid-state Li target. BNCT had been performed with using the several research reactors, such as KUR, JRR-4, FiR-1, R2–0, MIT-FCB, THOR, HFR-Petten and BMRR. The RBE values of the neutron beam in those reactors range from 3.0 to 3.9 [27–33]. On the other hand, in an accelerator-based BNCT system coupled to Be target (NeuCure, manufactured by SHI), the RBE value of the epithermal neutron beam contaminated with fast neutrons is 2.4 [15], which is a comparable value to that obtained in this study. Because it is known that the RBE value varies with linear energy transfer (LET) of proton particle [34], it was also expected that variations in RBE values due to neutron energy. The neutron energy spectrum in the accelerator-based BNCT system was designed to be lower than that in the research reactor in order to acquire the ideal neutron spectrum for the clinical use of BNCT [27]. According to the previous report, the radiation weighting factor for neutron is slightly increased with increasing the neutron energy (~1 MeV) [35]. Therefore, it is expected that RBE value of the epithermal neutron beam contaminated with fast neutrons in the accelerator-based BNCT systems can be lower than that in the reactor-based BNCT. We expect that new studies on the RBE value will be reported from other accelerator-based BNCT systems in the future, which may reveal the properties in each accelerator-based BNCT system.
It is also not clear about the dose-rate effects by neutron beam irradiation. It was reported that the dose-rate effect of neutron was less than that of gamma-ray irradiation [36]. On the other hands, it has been reported that repair takes longer with low-dose-rate irradiation than with high-dose-rate irradiation in DNA double-strand break repair [37]. In this experiment, maximum irradiation time was 3 hours and the dose-rate was approximately 0.02 Gy/min, while irradiations by NeuCure was much shorter. Therefore, the dose rate effect of neutron beam may also be an important factor in the comparison for deriving the RBE value of the epithermal neutron beam contaminated with fast neutrons.
It is well known that RBE values depend on lineal energy, and the RBE values derived from dose-mean lineal energy have been reported and compared with in vivo and in vitro experiments [38, 39]. According to previous report of the microdosimetric study, the doses for 10% cell survival fraction of HepG2 cells indicate that the RBE values for 6 MV and 10 MV X-rays from a medical LINAC were equivalent to 1.01 and 1.01, respectively, when 60Co γ-ray was used as reference radiation [40]. Furthermore, in HSG cells, the dose-averaged lineal energies of 6 MV X-ray and 60Co γ-ray were comparable (2.26 ± 0.15 keV/μm and 2.27 ± 0.15 keV/μm, respectively). Additionally, the RBE values of 6 MX X-rays could be estimated as 1.04 ± 0.02 when 60Co γ-rays were used as reference radiation [38], and its value was not inconsistent with that reported by Kawahara et al. [40]. Therefore, it was expected that the RBE values obtained in this study were comparable even if 60Co γ-rays were used as the radiation reference.
This study investigated RBE values of the epithermal neutron beam contaminated with fast neutrons in the accelerator-based BNCT system coupled to a solid-state lithium target. The average RBE value of the therapeutic neutron beam, which is decelerated from ~800 keV to around 10 keV, acquired in the four cell lines of SAS, SCCVII, U87-MG and NB1RGB was 1.9 (range: 1.3–2.5). The RBE value in the accelerator-based BNCT was comparable between each system, and tended to be lower than that in the reactor-based BNCT.
ACKNOWLEDGMENTS
We thank Dr. Hitoshi Nakagama, Dr. Yasuaki Arai, Dr. Tomomitsu Hotta, Dr. Takamasa Kayama and the other staff members at the National Cancer Center for supporting our project for the development of an accelerator-based BNCT system.
Contributor Information
Satoshi Nakamura, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Medical Physics Laboratory, Division of Health Science, Graduate School of Medicine, Osaka University, 1-7 Yamadaoka, Suita city, Osaka, 565-0871, Japan.
Shoji Imamichi, Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Central Radioisotope Division, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Department of Molecular and Genomic Biomedicine, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki, 852-8588, Japan.
Kenzi Shimada, Cancer Intelligence Care Systems, Inc. 3-5-7 Ariake, Koto-ku, Tokyo, 135-0063, Japan.
Mihiro Takemori, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Department of Radiological Science, Graduate School of Human Health Sciences, 7-2-10 Higashi-ogu, Arakawa-ku, Tokyo, 116-8551, Japan.
Yui Kanai, Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Central Radioisotope Division, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Laboratory for Zero-Carbon Energy, Institute of Innovative Research, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo, 152-5880, Japan.
Kotaro Iijima, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Takahito Chiba, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Department of Radiological Science, Graduate School of Human Health Sciences, 7-2-10 Higashi-ogu, Arakawa-ku, Tokyo, 116-8551, Japan.
Hiroki Nakayama, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Department of Radiological Science, Graduate School of Human Health Sciences, 7-2-10 Higashi-ogu, Arakawa-ku, Tokyo, 116-8551, Japan.
Tetsu Nakaichi, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Shohei Mikasa, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Yuka Urago, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Department of Radiological Science, Graduate School of Human Health Sciences, 7-2-10 Higashi-ogu, Arakawa-ku, Tokyo, 116-8551, Japan.
Tairo Kashihara, Department of Radiation Oncology, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Kana Takahashi, Department of Radiation Oncology, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Teiji Nishio, Medical Physics Laboratory, Division of Health Science, Graduate School of Medicine, Osaka University, 1-7 Yamadaoka, Suita city, Osaka, 565-0871, Japan.
Hiroyuki Okamoto, Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Jun Itami, Department of Radiation Oncology, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Masamichi Ishiai, Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Central Radioisotope Division, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Minoru Suzuki, Institute for Integrated Radiation and Nuclear Science, Kyoto University, 2 Asashiro-Nishi, Kumatori-cho, Sennan-gun, Osaka, 590-0494, Japan.
Hiroshi Igaki, Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Department of Radiation Oncology, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan.
Mitsuko Masutani, Division of Boron Neutron Capture Therapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Central Radioisotope Division, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan; Department of Molecular and Genomic Biomedicine, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki, 852-8588, Japan.
CONFLICT OF INTEREST
This study was supported by research funds from Cancer Intelligence Care Systems, Inc. (Satoshi Nakamura, Hiroshi Igaki and Mitsuko Masutani). The sponsors had no roles in the design, execution, interpretation, or writing of this study.
FUNDING
This work was supported by a JSPS Grant-in-Aid for Young Scientists (Grant Number 19K17218), and a collaborative research between the National Cancer Center Hospital, Tokyo, Japan, and Cancer Intelligence Care Systems, Inc. This study was partially supported by research funds from Cancer Intelligence Care Systems, Inc. (S.N., H.I., M.M.).
DATA AVAILABILITY
The data underlying this article is available in the article, presented in table format throughout.
REFERENCES
- 1. Kato I, Ono K, Sakurai Y et al. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot 2004;61:1069–73. [DOI] [PubMed] [Google Scholar]
- 2. Nakai K, Yamamoto T, Aiyama H et al. Boron neutron capture therapy combined with fractionated photon irradiation for glioblastoma: a recursive partitioning analysis of BNCT patients. Appl Radiat Isot 2011;69:1790–2. [DOI] [PubMed] [Google Scholar]
- 3. Aihara T, Morita N, Kamitani N et al. BNCT for advanced or recurrent head and neck cancer. Appl Radiat Isot 2014;88:12–5. [DOI] [PubMed] [Google Scholar]
- 4. Futamura G, Kawabata K, Siba H et al. A case of radiation-induced osteosarcoma treated effectively by boron neutron capture therapy. Radiat Oncol 2014;9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sweet WH, Javid M. The possible use of slow neutrons plus boron10 in therapy of intracranial tumors. Trans Am Neurol Assoc 1951;56:60–3. [PubMed] [Google Scholar]
- 6. Farr LE, Sweet WH, Robertson JS et al. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. Am J Roentgenol Radium Therapy, Nucl Med 1954;71:279–93. [PubMed] [Google Scholar]
- 7. Hatanaka H, Sano K. A revised boron-neutron capture therapy for malignant brain tumors. I. Experience on terminally ill patients after Co-60 radiotherapy. Z Neurol 1973;204:309–32. [DOI] [PubMed] [Google Scholar]
- 8. Mishima Y, Honda C, Ichihashi M et al. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet 1989;334:388–9. [DOI] [PubMed] [Google Scholar]
- 9. Finkel GC, Poletti CE, Fairchild RG et al. Distribution of 10B after infusion of Na210B12H11SH into a patient with malignant astrocytoma: implications for boron neutron capture therapy. Neurosurgery 1989;24:6–11. [DOI] [PubMed] [Google Scholar]
- 10. Wang LW, Chen YW, Ho CY et al. Fractionated boron neutron capture therapy in locally recurrent head and neck cancer: a prospective phase I/II trial. Int J Radiat Oncol Biol Phys 2016;95:396–403. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka H, Sakurai Y, Suzuki M et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl Radiat Isot 2011;69:1642–5. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura S, Igaki H, Ito M et al. Neutron flux evaluation model provided in the accelerator-based boron neutron capture therapy system employing a solid-state li target. Sci Rep 2021;11:8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumada H, Matsumura A, Sakurai H et al. Project for the development of the LINAC based NCT facility in University of Tsukuba. Appl Radiat Isot 2014;88:211–5. [DOI] [PubMed] [Google Scholar]
- 14. Watanabe K, Yoshihashi S, Ishikawa A et al. First experimental verification of the neutron field of Nagoya University accelerator-driven neutron source for boron neutron capture therapy. Appl Radiat Isot 2021;168:109553. [DOI] [PubMed] [Google Scholar]
- 15. Hirose K, Konno A, Hiratsuka J et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): an open-label phase II trial. Radiother Oncol 2021;155:182–7. [DOI] [PubMed] [Google Scholar]
- 16. Igaki H, Murakami N, Nakamura S et al. Scalp angiosarcoma treated with linear accelerator-based boron neutron capture therapy: a report of two patients. Clin Transl Radiat Oncol 2022;33:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawabata S, Suzuki M, Hirose K et al. Accelerator-based BNCT for patients with recurrent glioblastoma: a multicenter phase II study. Neurooncol Adv 2021;3:vdab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka H, Takata T, Watanabe T et al. Characteristic evaluation of the thermal neutron irradiation field using 30 MeV cyclotron accelerator-based for basic research on neutron capture therapy. Nucl Instrum Meth Phys Res A 2020;983:164533. [Google Scholar]
- 19. Nakamura S, Igaki H, Ito M et al. Characterization of the relationship between neutron production and thermal load on a target material in an accelerator-based boron neutron capture therapy system employing a solid-state Li target. PLoS One 2019;14:e0225587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burian J, Marek M, Retaj J et al. Current Status of Neutron Capture Therapy (IAEA-TECDOC-1223). Vienna, Austria: International Atomic Energy Agency, 2001. [Google Scholar]
- 21. Tanaka H, Sakurai Y, Suzuki M et al. Characteristics comparison between a cyclotron-based neutron source and KUR-HWNIF for boron neutron capture therapy. Nucl Instrum Meth Phys Res B 2009;267:1970–7. [Google Scholar]
- 22. Nakamura S, Igaki H, Okamoto H et al. Dependence of neutrons generated by 7Li(p,n) reaction on Li thickness under free-air condition in accelerator-based boron neutron capture therapy system employing solid-state Li target. Phys Med 2019;58:121–30. [DOI] [PubMed] [Google Scholar]
- 23. Hopewell JW, Morris GM, Schwint A et al. The radiobiological principles of boron neutron capture therapy: a critical review. Appl Radiat Isot 2011;69:1756–9. [DOI] [PubMed] [Google Scholar]
- 24. Andreo P, Burns DT, Hohlfeld K et al. Absorbed Dose Determination in External Beam Radiotherapy: An International Code of Practice for Dosimetry Based on Standards of Absorbed Dose to Water. IAEA Technical Reports Series No. 398. Vienna: International Atomic Energy Agency, 2000. [Google Scholar]
- 25. Okamoto H, Minemura T, Nakamura M et al. Establishment of postal audit system in intensity-modulated radiotherapy by radiophotoluminescent glass dosimeter and a radiochromic film. Phys Med 2018;48:119–26. [DOI] [PubMed] [Google Scholar]
- 26. Miki S, Imamichi S, Fujimori H et al. Concomitant administration of radiation with eribulin improves the survival of mice harboring intracerebral glioblastoma. Cancer Sci 2018;109:2275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki M, Tanaka H, Sakurai Y et al. Impact of accelerator-based boron neutron capture therapy (AB-BNCT) on the treatment of multiple liver tumors and malignant pleural mesothelioma. Radiother Oncol 2009;92:89–95. [DOI] [PubMed] [Google Scholar]
- 28. Kawabata S, Miyatake S, Kuroiwa T et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res 2009;50:51–60. [DOI] [PubMed] [Google Scholar]
- 29. Seppälä T, Auterinen I, Aschan C et al. Dose planning with comparison to in vivo dosimetry for epithermal neutron irradiation of the dog brain. Med Phys 2002;29:2629–40. [DOI] [PubMed] [Google Scholar]
- 30. Capala J, Stenstam BH, Sköld K et al. Boron neutron capture therapy for glioblastoma multiforme: clinical studies in Sweden. J Neuro-Oncol 2003;62:135–44. [DOI] [PubMed] [Google Scholar]
- 31. Coderre JA, Hopewell JW, Turcotte JC et al. Tolerance of normal human brain to boron neutron capture therapy. Appl Radiat Isot 2004;61:1083–7. [DOI] [PubMed] [Google Scholar]
- 32. Lee JC, Chuang KS, Hsueh Liu YW et al. A comparison of dose distributions in gross tumor volume between boron neutron capture therapy alone and combined boron neutron capture therapy plus intensity modulation radiation therapy for head and neck cancer. PLoS One 2019;14:e0210626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rassow J, Stecher-Rasmussen F, Voorbraak W et al. Comparison of quality assurance for performance and safety characteristics of the facility for boron neutron capture therapy in Petten/NL with medical electron accelerators. Radiother Oncol 2001;59:99–108. [DOI] [PubMed] [Google Scholar]
- 34. Giovannini G, Böhlen T, Cabal G et al. Variable RBE in proton therapy: comparison of different model predictions and their influence on clinical-like scenarios. Radiother Oncol 2016;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Commission on Radiological Protection (ICRP). The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37, 2007. [DOI] [PubMed]
- 36. Kinashi Y, Okumura K, Kubota Y et al. Dose-rate effect was observed in T95G glioma cells following BNCT. Appl Radiat Isot 2014;88:81–5. [DOI] [PubMed] [Google Scholar]
- 37. Nair S, Engelbrecht M, Miles X et al. The impact of dose rate on DNA double-strand break formation and repair in human lymphocytes exposed to fast neutron irradiation. Int J Mol Sci 2019;20:5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okamoto H, Kohno T, Kanai T et al. Microdosimetric study on influence of low energy photons on relative biological effectiveness under therapeutic conditions using 6 MV LINAC. Med Phys 2011;38:4714–22. [DOI] [PubMed] [Google Scholar]
- 39. Sato T, Matsuya Y, Hamada N. Microdosimetric modeling of relative biological effectiveness for skin reactions: possible linkage between in vitro and in vivo data. Int J Radiat Oncol Biol Phys 2022;114:153–62. [DOI] [PubMed] [Google Scholar]
- 40. Kawahara D, Nakano H, Ozawa S et al. Relative biological effectiveness study of Lipiodol based on microdosimetric-kinetic model. Phys Med 2018;46:89–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article is available in the article, presented in table format throughout.


