Abstract
One type of firefighting foam, referred to as aqueous filmforming foams (AFFF), is known to contain per- and polyfluoroalkyl substances (PFAS). The concerns raised with PFAS, and their potential environmental and health impacts, have led to a surge in research on fluorine-free alternatives both in the United States and globally. Particularly, in January 2023, a new military specification (MIL-PRF-32725) for fluorine-free foam was released in accordance with Congressional requirements for the U.S. Department of Defense. This paper provides a critical analysis of the present state of the various fluorine-free options that have been developed to date. A nuanced perspective of the challenges and opportunities of more sustainable replacements is explored by examining the performance, cost, and regulatory considerations associated with these fluorine-free alternatives. Ultimately, this evaluation shows that the transition to fluorine-free replacements is likely to be complex and multifaceted, requiring careful consideration of the trade-offs involved. Yet, the ongoing work will provide valuable insights for future research on alternatives to AFFF and enhancing the safety and sustainability of fire suppression systems.
Keywords: aqueous film-forming foams, per- and polyfluoroalkyl substances, fluorine-free foam, firefighting foam
Graphical Abstract

INTRODUCTION: FIVE DECADES WITH AFFF
All aqueous film forming foams (AFFF) containing PFAS were developed in the 1970s for Class B firefighting operations to combat flammable liquid fires like petroleum-based fuel. The key discovery was that fluorinated surfactants enable much improved fire extinguishment capability because PFAS are dissolved by fuel and have a lower surface energy than fuels.1 For approximately 50 years, AFFF have been used by the military, civilian airports, industry, and fire departments to combat flammable liquid fires. PFAS-containing formulations have also been used in manufacturing firefighters’ personal protective equipment.2 PFAS exposures to firefighters using AFFF were first captured when blood samples from firefighters at the World Trade Center disaster were tested, and PFAS concentrations were approximately 2-fold higher than the general population.3 Originally, it was thought that the pervasiveness of these chemicals was limited to long-chain PFAS. As a result, in 2006, multiple firefighting foam manufacturers committed to move from long-chain PFAS to short-chain PFAS by 2015.4 This turned out to be a regrettable substitution since additional research showed the short-chain fluorinated replacements to have higher mobility that causes a wider spread in the environment and are harder to remove from water than long-chain PFAS.5 As a result of growing awareness of the health hazards6–8 and environmental contamination9–12 of PFAS, the U.S. EPA proposed, as appropriate under the PFAS Strategic Roadmap, best management practices to address PFAS-containing AFFF for stormwater permits.13,14 Therefore, the manufacturers and users of AFFF have been under increasing pressure to transition to PFAS-free replacements (Figure 1).15–18
Figure 1.

Timeline of some milestones related to PFAS use in AFFF from production to phase out.
U.S. Updates.
Efforts to develop fluorine-free foams (F3) or PFAS-free foams (PFF) started in the early 2000s; However, they have gained more attention recently after the growing concern with PFAS.19 The transition entailed the U.S. Department of Defense (DoD) to revise the military specification (MilSpec; MIL-PRF-24385F),20 which required fluorocarbon surfactants and includes fire suppression performance criteria and physical properties that have not yet been matched by PFAS-free commercial foams. In the 2020 National Defense Authorization Act (NDAA 2020), the U.S. Congress directed the DoD to phase out use of AFFF on military installations by October 2024 (with extensions until 2026).21 The fluorine-free foam MilSpec (MIL-PRF-32725) for land-based and freshwater applications was published in January 202322 and will be followed by updates to the Qualified Product List with tested and certified F3 and a cease in the DoD purchases of AFFF for shore-based uses by October 2023. F3 is required by the new 2023 MilSpec to contain a maximum of 1 ppb PFAS. The Federal Aviation Administration (FAA) no longer requires the use of fluorinated surfactants according to the FAA Reauthorization Act of 2018—followed up by a statement in October 2021—but still requires that the performance standards are met.23,24 The FAA will adopt the F3MilSpec and follow the DoD transition.24 Led by the state of Washington in 2018, 22 states as of 2022 have regulated AFFF use and sales, with exemptions such as military, airport, and chemical plants. Many other states have proposed legislation or have set up discharge notifications or buyback programs.19 In 2022, the National Fire Protection Association (NFPA) published a roadmap report to assist fire departments and industrial facilities.25 Some petroleum industry and fire departments, like the San Francisco Fire Department,26 are making the transition early. This transition is likely to happen quickly once adopted by the DoD and FAA. However, product selection will be specific to the user, application, and distribution system as no commercial foam is a complete drop-in replacement for AFFF.
International Updates.
A global trend toward transition from PFAS has been in progress for over a decade. In Europe, the European Union (EU) has taken a leading role in phasing out PFAS. The European Chemical Agency (ECHA) has classified several PFAS as substances of very high concern and has implemented measures to restrict the use of these substances, including the use of certain PFAS in AFFF.27 Several countries have also implemented their own measures to phase out PFAS. Sweden, for instance, has implemented a national action plan to phase out PFAS, including the use of these substances in foams. Denmark has also developed similar measures, including a ban on the use of some PFAS in foams for firefighting and other applications.28 Canada has enforced a ban on the use of certain PFAS in food packaging and foams, while it has also applied a phaseout plan for the use of these substances in other applications.19 Australia uses an alternative to AFFF at all of its 27 major airports, and in 2018, the state of South Australia issued the first governmental ban of AFFF following a phaseout period that ended in 2020.29 In addition to country-specific efforts to phase out the use of PFAS, there have been international efforts to address the use of these substances. The Stockholm Convention on Persistent Organic Pollutants, for example, is an international treaty that aims to eliminate or restrict the use of persistent organic pollutants, including PFOS, PFOA, and their salts. The treaty has been ratified by 180 countries, including many countries that have implemented their own measures to phase out the use of PFAS. The petroleum and chemical manufacturing industries have already seen some transition by some companies and industrial facilities (e.g., BP, ExxonMobil, Statoil, BASF, AkzoNobel, Pfizer, and Lilly).30 At the end of 2022, 3M, which is a major PFAS manufacturer, announced that the company will stop making PFAS by the end of 2025.31 While progress has been made in some countries, there is still more work to be done to fully phase out the use of PFAS and transition to safer PFAS-free alternatives.
FIRE SUPPRESSION PERFORMANCE OF COMMERCIAL F3
PFAS-containing AFFF extinguish a fire by confining the fuel vapors below a layer of fluorinated surfactant film and a blanket of bubbles. F3 do not form a continuous film on the fuel surface and rely mainly on the bubble blanket to contain the fuel vapors. As a result, capabilities of F3 have a greater dependence on foam quality (i.e., aspiration and expansion ratio).25 Firefighting foams are approved using a range of application-specific standards that contain various test parameters and performance requirements. For instance, many commercially available F3 already meet some U.S. and international standards such as Underwriters Laboratories (UL), European Standards (EN), and International Civil Aviation Organization (ICAO) standards. However, none have yet been qualified for the U.S. DoD performance requirements defined in Mil-PRF-24385F. Most of the ongoing activities by the U.S. DoD on F3 formulation development and small-scale performance testing are performed under the Strategic Environmental Research and Development Program (SERDP), with large-scale testing, demonstration, and validation being performed by the Environmental Security Technology Certification Program (ESTCP), the U.S. Air Force, and the U.S. Naval Research Laboratory.32–34
Recently, a multiphase validation study of the leading F3 for DoD land-based applications was conducted under two SERDP Programs (WP21–3461 and WP21–3465) against representative scale Aircraft Rescue and Firefighting (ARFF) type scenarios.32–34 These fire scenarios included both three-dimensional running fuel fires (with a growing spill fire component) and large uncontained fuel spill fires. Approximately 150 validation tests and demonstrations were conducted during the four phases of this study (i.e., 107 hose line tests and 43 turret tests), and it was concluded that the leading F3 typically took about 1.5–2 times longer than AFFF to extinguish the fires in most scenarios. Specifically, the spill fire extinguishment times for AFFF were between 30 and 45 s (i.e., 30 s using the hose line and 45 s using the turret) while the F3 extinguishment times were between 45 and 60 s for the hose line and 60–90 s using the turret. The running fuel fires were typically extinguished using AFFF in less than 60 s and in about 90–120 s using the F3. The tested F3 all demonstrated similar extinguishment capabilities and times.
The burnback capabilities of the F3 were also assessed during the spill fire scenarios. Immediately after the spill fires were extinguished, a small hole was created in the foam blanket and ignited using a propane brush burner torch. The burnback time was defined as the time from ignition until the fire had grown to 100 ft.2 In general, all the foams tested during this program demonstrated good burnback capabilities against the F-24 spill fire scenarios. The burnback time for AFFF was about 3 min when discharged through the standard nozzle and about 4 min when discharged through the foam tube(s). The addition of the foam tube typically increased the burnback times by about a minute for almost all the foams. When comparing the F3 to AFFF, the burnback times were typically about 30 s shorter (i.e., the fire burned back slightly faster) for the Newtonian F3 but were slightly better (i.e., longer burnback times) for the non-Newtonian F3.
It should be noted that the previous capabilities discussion was for land-based scenarios, where the concentrate is diluted with freshwater. This is distinct from applications where seawater is used because foam properties will differ when using saltwater compared to using freshwater. In addition, proportioning devices are used to mix the foam concentrate with water in a specific ratio, to create an optimal foam solution for extinguishing fires. However, many new F3 have higher viscosities with different surface tensions than AFFF which may make them incompatible with existing AFFF proportioning devices. This can lead to reduced foam aspiration, which refers to the ability of the foam to be mixed with water and sprayed out of the discharge device. Thus, legacy discharge devices may also be an issue due to the potential need for better foam aspiration. Fire performance approval tests (bench- and large-scale pool fires) remain the best way to test new F3 products. Since the firefighting capabilities of current F3 are much more dependent on the application conditions compared to AFFF, product selection, hardware selection, and firefighter training are increasingly important for effective use of these foams. SERDP has increased investments to address needs associated with firefighting tools, equipment, training, and improving benchscale tests to approximate larger fire events.
The petroleum industry has also been evaluating performance led by the international consortium LASTFIRE (for example, the 2018 Dallas Fort Worth Fire Research and Training Center workshop).25 A list of commercially available products was compiled in 2019 by the Interstate Chemicals Clearinghouse (IC2) and the New York State Pollution Prevention Institute; the list included almost 100 alternative foams.35 Overall, the market is rapidly changing. Next generation products with higher performance are expected, and new standards and specs are being written with this in mind.
PFAS REPLACEMENTS FOR FIREFIGHTING FOAM APPLICATIONS
Although PFAS are the main source of effectiveness for AFFF, these foam formulations contain less than 2% of PFAS and about 5%–10% hydrocarbon surfactants.36,37 Common solvents with surfactant properties include diethylene glycol butyl ether (DGBE).38 Other additives include polymers, stabilizers and preservatives, salts, corrosion inhibitors, chelating agents, and biocides.38 Unsurprisingly, there are many similarities between the basic composition of AFFF and the first generation of current commercial F3. However, research and attention have been primarily focused on the characterization of the PFAS fraction of AFFF, and the same practice is being done when dealing with F3. As a consequence, little is known about the other surfactants and even less about organic corrosion inhibitors (e.g., benzotriazoles).39 Thus, further research is needed on other classes of hydrocarbon surfactants found in AFFF, including alkyl amidobetaines, alkyl glucosides, alkyl sulfates, alkyl ether sulfates, alkyl coco amidoglycinates, alkyl amino dipropionates, octylphenol polyethoxylates, sulfobetaines, olefin sulfonates, and linear alkyl benzenesulfonates (Figure 2).37
Figure 2.

Examples for surfactants most often used in firefighting foams. R designates C8–C14 alkyl chains.40
The synthesis of novel halogen-free surfactants or additives with the potential to directly replace PFAS is an area of active research (Figure 3). The desire for a drop-in replacement for AFFF means that it is likely that next generation foams will include unknown active ingredients and flame-retardant additives that can mimic the ability of the PFAS tail group to be both hydrophobic and oleophobic. Currently, nitrogen, phosphorus, and silicon-containing surfactants and polymers are being tested.41 Also under consideration are formulations with various compositions that employ different mechanisms for fire suppression, e.g., hollow glass microspheres,42 ionic liquids,19 and gel foams.43 It is important to highlight that our analysis here is based on current compositions of replacements.44 However, many chemical development efforts are expected in the following 10–20 years, and next generation foams might differ more from AFFF and warrant careful attention to standard safety testin protocols.
Figure 3.
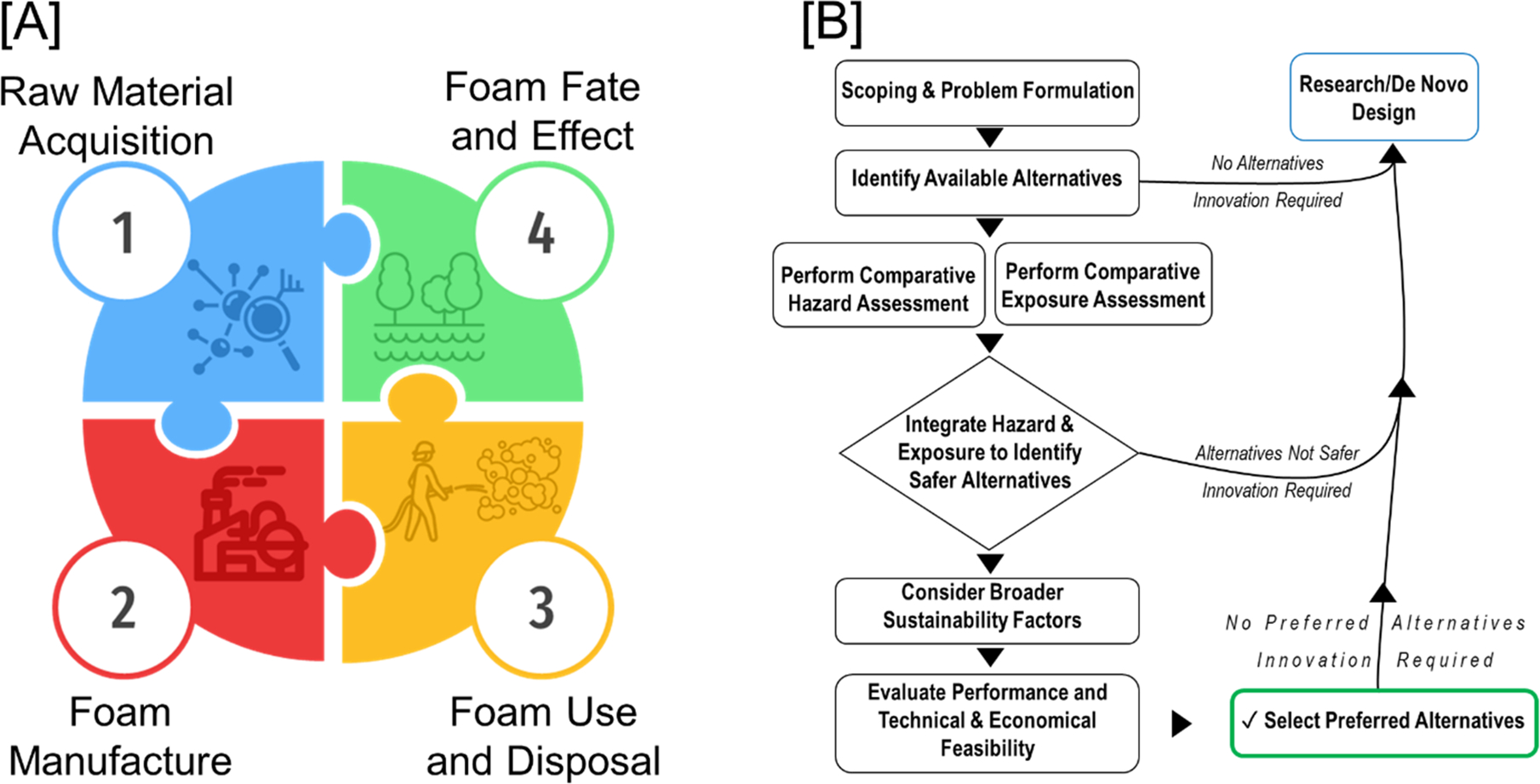
(A) Fire-fighting foam product flow. (B) Generic alternatives assessment framework by OECD Guidance.
The 2019 IC2 report35 compiled safety data sheets for F3 disclosing both specific chemicals and surfactant classes like alkyl sulfates or the more general “anionic surfactants”. This suggests that the current market trend is F3 foams with bulk compositions (surfactants, solvents, water, and additives) similar to AFFF with some overlapping nonfluorinated surfactants. However, no alternative chemicals evaluated have shown better performance than PFAS-containing AFFF.46 Therefore, the new alternative active ingredients are being used in higher concentrations in F3 than their PFAS counterparts in AFFF.47 Therefore, this suggests that the relative health impacts of PFAS, F3, and other surfactants will remain an important area of continued research.
AVOIDING REGRETTABLE SUBSTITUTIONS
Current Efforts.
It is imperative to transition away from AFFF as the treatment and remediation of PFAS is challenging and expensive. As defined by the National Research Council,48 a regrettable substitution occurs when a chemical is replaced with a similar, but new or untested, alternative without thoroughly researching its potential toxic effects and impacts, often leading to similar issues and harm.48 Historically, response to environmental pollution has only occurred after the event or discovery of the problem. However, the longevity of PFAS emphasizes the urgent need for a prevention-focused approach to pollution and the elimination of replacement chemicals that may pose threats to the environment. For example, while hydrocarbon surfactants are more biodegradable than PFAS under aerobic conditions, this may not always be the case in certain environments such as groundwater. As regulatory and societal pressure drives the transition away from PFAS, it presents an opportunity to proactively assess the health and environmental impacts of potential replacements and inform the selection of products. In this regard, the new F3MilSpec (MIL-PRF-32725) set tight limits of PFAS occurrence in new formulations and requires performing biodegradation and certain toxicity tests. Formulations that fail to meet the criteria established in the F3MilSpec will be disapproved for use, and individual F3 batches that fail to conform with the requirements will be rejected.
Among the independent verifications of F3’s environmental sustainability, GreenScreen Certified is a means of certifying PFAS-free products, including F3 foams.49 As of February 2023, 35 commercially available Class B foams have received GreenScreen certification.50 In order to attain this designation, foam manufacturers must divulge all ingredients, which are then examined for toxicity through a database. The platinum, gold, and silver levels of certification are based on aquatic toxicity, and all currently certified foams are at the silver level. Restricted substances include alkylphenols and alkylphenol ethoxylates, cyclic volatile methyl siloxanes, organohalogens, and specified chemicals on the Zero Discharge of Hazardous Chemicals Manufacturing Restricted Substances List. Analysis of total organic fluorine is performed using combustion ion chromatography (CIC), and the level must be less than 1 ppm to be qualified by GreenScreen. However, this limit is 3 orders of magnitude higher than the limit of 1 ppb set by the new F3MilSpec (MIL-PRF-32725). The F3MilSpec paves the road toward developing long-term, sustainable, and economically feasible solutions. Relying solely on GreenScreen certification has some limitations, such as toxicity being based on individual compounds, the potential for incomplete toxicity data for next-generation F3 foams with novel surfactants, a focus on acute toxicity rather than chronic toxicity, and an inability to evaluate the fate and impact of discharge into water sources.
Design for the Environment (DfE).
DfE51 is a non-regulatory U.S. EPA initiative that began in the 1990s, which provides transparent publicly available criteria for comparing chemical alternatives based on several human health, ecotoxicity, and fate endpoints. Frameworks such as Green-Screen build upon the DfE approach. Conducting alternative assessments using tools such as GreenScreen usually requires substantial time and resources.52 In 2019, Vegosen and Martin53 developed the Hazard Comparison Dashboard (HCD), to rapidly compare chemical alternatives. HCD attempts to assign ordinal scores (i.e., low, medium, high, or very high) for each of the DfE human health, ecotoxicity, and fate categories. The scores are not aggregated into a single score so that users can decide which categories are most relevant to their alternatives assessment. For example, if there is potential for release into natural bodies of water, the scores for acute and chronic aquatic ecotoxicities are especially relevant.
The database within HCD contains over 990,000 score records compiled from publicly available online sources, including hazardous chemical lists, the Globally Harmonized System (GHS) hazard codes (H-codes) or hazard categories from government health agencies, experimental quantitative toxicity values, and predicted values obtained using quantitative structure activity relationship (QSAR) models. QSAR model predictions were obtained using the U.S. EPA’s Toxicity Estimation Software Tool (T.E.S.T.). If a chemical has multiple score records for a given hazard category, the final score is assigned from the most hazardous score from the most authoritative source. The authority levels in the database, in decreasing order of authority, are authoritative, screening, and predictive. As an example, representative HCD results for known components of AFFF and some F3 are presented in Figure 4. The weight percentage of each component should be also considered to facilitate a fair comparison of alternatives (i.e., sometimes a component is more hazardous but requires a much lower concentration to be effective).
Figure 4.
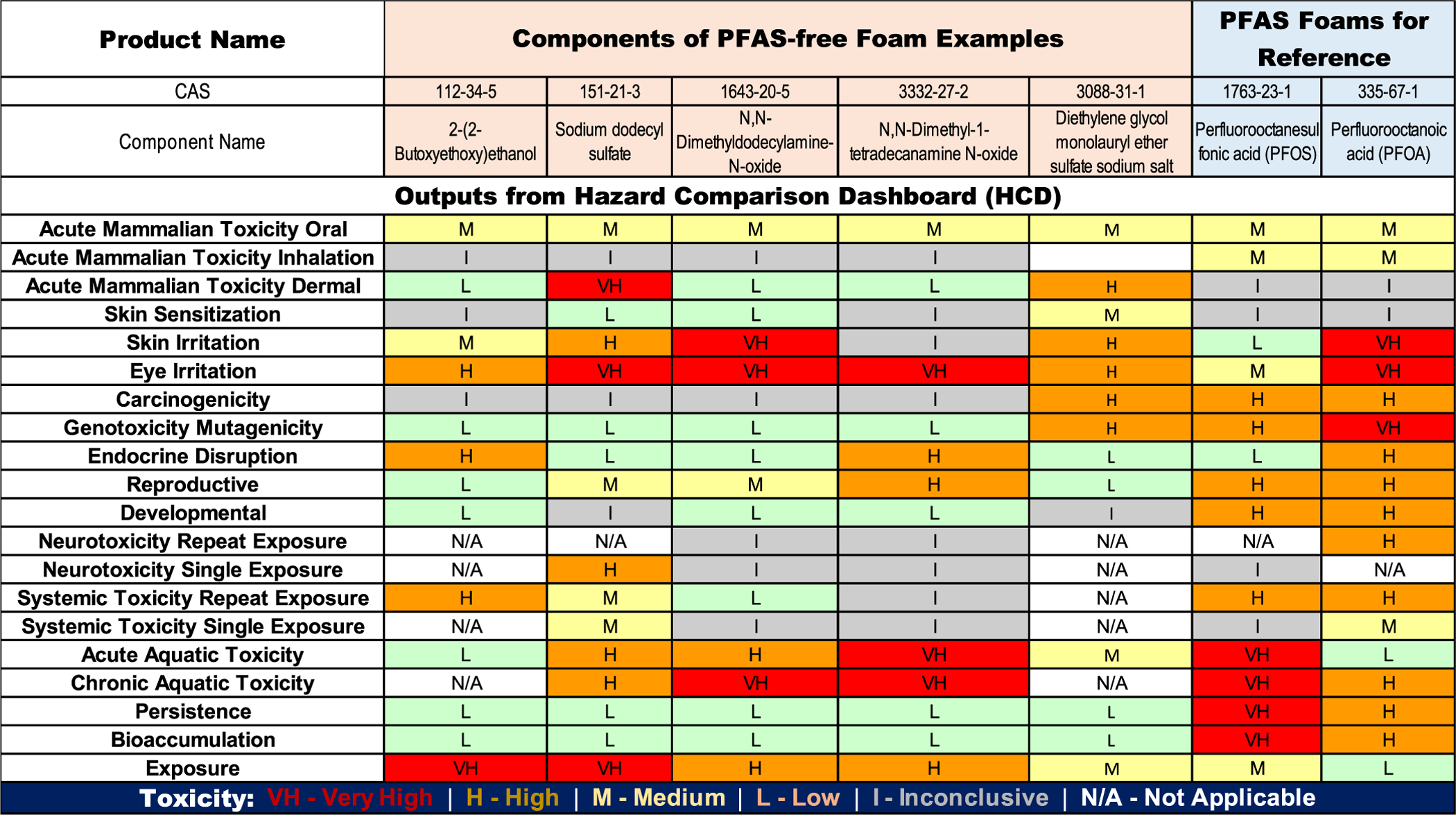
Example for the output from the Hazard Comparison Dashboard (HCD) for chemicals disclosed in the safety data sheets (SDS) of some F3s.
As the PFAS components in AFFF are proprietary, HCD results are provided for the most extensively studied PFAS, perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). PFOS and PFOA have received high or very high scores in the hazard categories pertinent to chronic exposure. This is of particular significance because PFAS compounds are highly persistent (scores of high or very high). The other components in AFFF vary in their hazard levels in terms of their HCD scores when compared to PFOA/PFOS. For the example non-PFAS foam alternatives, the surfactants used have lower scores than PFOS and PFOA for most of the categories relevant to chronic exposure. Additionally, these chemicals are not persistent (the persistence score is low) and thus should pose a lower risk to human health due to low chronic exposure (risk is the product of hazard and exposure). It is worth noting that the HCD exposure scores were provided from the SEEM exposure model, which calculates median population intake rates, which are not relevant to the exposure from firefighting foams.54 While GreenScreen and DfE are highly valuable screening tools, more comprehensive and experimental evaluations on the actual foams are needed to fully understand the potential human health and environmental impacts from non-PFAS alternatives.
ENVIRONMENTAL IMPACTS OF F3: CURRENT RESEARCH
Research into the environmental impacts of F3 is a relatively new field, with the first peer-reviewed papers on the topic published only in the past couple of years. At present, the available ecotoxicological data on foam formulations are limited to studies on acute toxicity in 14 freshwater and marine aquatic species,55 acute toxicity in one terrestrial invertebrate (Caenorhabditis elegans),56 acute oral toxicity in one avian species (northern bobwhite quail),57 phytotoxicity in one plant species (Brassica rapa),58 and aerobic biodegradation.59 This limited body of research suggests that further investigation is needed to fully understand the environmental impacts of these foams, particularly when compared to the long-term effects of AFFF.
Analysis of F3 and F3-Impacted Sites.
Some studies on PFAS-containing AFFF have identified the presence of hydrocarbon surfactants through nontargeted analysis and suspect screening.60–63 These surfactants are a component class shared by AFFF and F3. During human health impact and bioaccumulation studies of AFFF, Yang et al.64 and Li et al.65 found evidence of both hydrocarbon surfactants and PFAS. Garcia et al.62 identified eight classes of hydrocarbon surfactants in AFFF-impacted groundwater, suggesting that these substances can persist in anaerobic conditions. Rana et al.66 used nontargeted analysis to identify PFAS in surface water runoff after F3 were used to extinguish a chemical warehouse fire in Australia but did not focus on or identify nonfluorinated surfactants. These findings highlight the need for further research on ensuring the availability of analytical methods and standards for hydrocarbon surfactants in AFFF and F3.
Toxicology.
In some cases, research has found that F3 can demonstrate equal or greater acute toxicity than C6 AFFF, particularly for aquatic species, which are often more sensitive than terrestrial species and mammals.67 Some studies have also focused on the sublethal growth and reproductive impacts of these foams on worms68 and plants.45 However, it is important to distinguish between toxicity tests on a foam, formulation, or product and toxicological assessments based on individual components or constituents. This latter type of assessment relies on chemical and compositional information disclosed by the manufacturer in a safety data sheet (SDS) or extensive analysis to characterize unknown components.58,59,61,69 In general, more is known about the acute toxicity of individual components in F3, but there is limited information available on their chronic, reproductive, and developmental toxicity (Figure 4).70
Fate and Biodegradation.
Gharehveran et al.59 conducted research on the fate and biodegradation of F3 and found that they are mostly readily biodegradable. However, they did not examine the biodegradation products of these foams. Wu et al.58 discovered the presence of metabolites in plants exposed to F3 foams, but it is unclear whether these metabolites were formed in the soil or in the plants themselves. Yao et al.71 also found nonfluorinated byproducts resulting from the pyrolysis of AFFF, and Etz et al.72 conducted modeling on the byproducts that are formed during the thermal degradation of trimethylsiloxane surfactants. Overall, there is limited information available on the degradation products of F3, and further research is needed to fully understand their fates and impacts on the environment. Ecotoxicological impacts of F3 are directly tied to their potential ability to be more biodegradable and less persistent than PFAS.
Unanswered Questions.
There are several knowledge gaps in the current ecotoxicological understanding of F3, including their chronic toxicity and the effects of low exposures associated with diluted environmental releases, particularly the fraction of nonfluorinated surfactants that serve as PFAS replacements in the first generation of foams (Figure 4). While this information may be available for certain known individual components, it does not necessarily translate to their mixtures in the foam products as a whole and their diluted applications. Research on the reproductive and developmental toxicity, behavioral impacts, and gene expression or molecular toxicity of new or unknown ingredients of F3 will craft a better understanding of potential ecotoxicological impacts, especially as compared to AFFF. The current body of knowledge is also restricted to studies under limited types of ecosystems and climatic zones. Similarly, little is known about the environmental persistence and mobility of F3 in groundwater and surface water. As the use of F3 is expected to increase in the future, likely leading to an increase in releases into the environment, more research is needed on the fate and transport of F3 and hydrocarbon surfactants, as well as their impact on the remediation and treatment of AFFF-contaminated sites.73–75 While there is some research on hydrocarbon surfactants in wastewater treatment,76,77 more information is also needed on their fates during disinfection and in drinking water, including the formation of disinfection byproducts. It is likely that investigating these gaps is important to further strengthen our understanding about potential impacts on the environment and developing effective management strategies for F3.
OUTLOOK AND RESEARCH NEEDS
The ubiquity of PFAS in various environmental media is indicative of the far-reaching ramifications of poorly understood chemicals. These consequences manifest in environmental and human health issues associated with the chemical family. These issues necessitate site remediation and removal from AFFF-related equipment to mitigate future environmental and human health impacts. Additionally, there is a need to evaluate and test potential substitutes to prevent a regrettable substitution. Consequently, the evaluation of PFAS-free alternatives to AFFF will need to consider multiple factors across the lifecycle of the replacement chemical.
Given that F3 are currently unable to achieve the firefighting efficacy of AFFF, further research on the mechanisms of fire suppression provided by PFAS in AFFF is necessary, and alternative chemicals with similar capabilities need to be identified. It is possible, and maybe likely, that some proposed alternatives may have similar environmental impacts as PFAS. In a broader sense, the relationship between chemical structure and purpose in the final product needs to be better understood.
Furthermore, many chemicals undergo degradation in the environment due to microbial and chemical action, resulting in degradation products that may have similar or greater environmental impacts. There is a need to develop data, methods, and tools to assist in reliably identifying and predicting the long-term potential environmental and human health effects of chemicals, including breakdown products. For instance, development efforts of F3 may benefit from considering the concepts of PMT (persistent, mobile, and toxic) and vPvM (very persistent and very mobile) substances to have alternative chemicals or materials that are safer for human health and the environment.
Economic considerations also play a role in alternative selection. For instance, the need for a drop-in replacement for AFFF is largely driven by the cost of replacing physical firefighting equipment compared to removing AFFF from them. In order to reuse existing equipment, it must be effectively cleaned, and the cost of cleaning will be influenced by disposal costs of effluent cleaning fluid. Furthermore, techniques for cleaning firefighting equipment need to be developed, allowing for a full economic comparison of equipment cleaning versus replacement. If a drop-in replacement is not economically feasible due to cleaning costs, then a broader range of alternative foam technologies may be considered.
This work outlines some of the considerations involved in replacing AFFF. Once performance characteristics and potential environmental impacts are established, leading candidate foams can be chosen from the various alternatives proposed. Even after significant effort has been made to characterize the performance and environmental impacts of the alternatives, information on the fire suppression performance and environmental implications of the candidate replacements will be based on limited data and may be incomplete. As a result, the selection of next-generation foams will be challenging, with different stakeholders choosing products based on their specific needs. Trade-offs between firefighting performance and environmental and health impacts will need to be made.
The replacement of AFFF has been shown to be a multifaceted task requiring expertise from various scientific disciplines. The timelines and resources for replacing AFFF were provided by the U.S. Congress in 2020. These resources have enabled extensive study of both the firefighting performance and environmental impacts of a number of potential replacements. Despite these resources and partly due to the short time frame, the performances of the replacements currently do not match those of AFFF, and limited environmental information has been generated. However, recent research suggests that current F3 exhibit greater biodegradability and lower potential for environmental persistence than PFAS. Nevertheless, the understanding gained from this work will be invaluable in guiding future research on foam replacements and improving the safety and sustainability of fire suppression systems.
ACKNOWLEDGMENTS
Gerard G. Back acts as subject matter expert (SME) on fire suppression and fire suppression systems for the Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP) and is funded under SERDP WP21–3465 and ESTCP WP20–5373. This document has been subjected to the U.S. Environmental Protection Agency’s review and has been approved for publication. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the Agency. Any mention of trade names, products, or services does not imply an endorsement by the Agency. The Agency does not endorse any commercial products, services, or enterprises.
Biographies

Dr. Mohamed (Moha) Ateia Ibrahim is an environmental engineer and a group leader with the U.S. EPA’s Center for Environmental Solutions and Emergency Response (CESER). Moha combines his expertise in environmental chemistry and materials chemistry to develop and evaluate innovative water treatment technologies to remove and/or degrade emerging contaminants, such as PFAS and microplastics. In parallel, Moha has initiated and is currently leading a project to evaluate the environmental impacts of PFAS replacement chemicals and formulations in firefighting foams and consumer products. He is a member of the Weapons Systems and Platforms Technical Committee of the SERDP-ESTCP. Moha is also an adjunct assistant professor at the Chemical and Biomolecular Engineering Department, Rice University.

Jean Van Buren researches PFAS and emerging contaminants in environmental media at the U.S. EPA Office of Research and Development’s Center for Environmental Solutions and Emergency Response, with a focus on nontargeted analysis, analytical method development, and contaminant treatment and fate. She received a Ph.D. in chemistry from the University of California at Berkeley where she studied oxidative water treatment of organic contaminants, after which she focused on potable water reuse as a University of Southern California postdoc and then PFAS as an ORISE postdoc before joining the U.S. EPA in 2022.

Dr. William Barrett’s research and development activities involve the use of information, software, and networking to reduce the environmental footprint of manufacturing processes. He has developed chemical process simulation-based tools for the evaluation of environmental impacts related to chemical manufacturing processes. He is currently a member of the Weapons Systems and Platforms Technical Committee of the joint EPA, DoD, and DOE Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESCTP), helping to reduce the environmental impacts of building and maintaining DoD assets.

Dr. Todd Martin has over 15 years of experience in computational toxicology. He is the lead developer of T.E.S.T. (Toxicity Estimation Software Tool) which allows users to easily estimate several toxicity and physical property endpoints from molecular structures. He has authored over 20 papers in the areas of computational toxicology, pollution prevention, and alternative assessments.

Jerry Back is a senior fire protection engineer with Jensen Hughes out of Baltimore, Maryland. He has over 38 years of experience covering the spectrum of fire protection topics/issues. Over the course of his career, he has published over 100 papers on fire protection-related topics and has been responsible for project management and administration (planning, execution, and analysis) of fire protection research, development, testing, and evaluation programs. He has a bachelor of science in mechanical engineering and a master of science in fire protection engineering, both from the University of Maryland. He is either the principal or alternate on every NFPA foam and aviation committee (12 total).
Footnotes
The authors declare no competing financial interest.
Contributor Information
Mohamed Ateia, Center for Environmental Solutions & Emergency Response, U.S. Environmental Protection Agency, Cincinnati, Ohio 45204, United States; Department of Chemical and Biomolecular Engineering, Rice University, Houston, Texas 77005, United States.
Jean Van Buren, Center for Environmental Solutions & Emergency Response, U.S. Environmental Protection Agency, Cincinnati, Ohio 45204, United States.
William Barrett, Center for Environmental Solutions & Emergency Response, U.S. Environmental Protection Agency, Cincinnati, Ohio 45204, United States.
Todd Martin, Center for Computational Toxicology and Exposure, U.S. Environmental Protection Agency, Cincinnati, Ohio 45204, United States.
Gerard G. Back, Jensen Hughes, Inc., Halethorpe, Maryland 21227, United States
REFERENCES
- (1).Tuve R; Jablonski E, Method of extinguishing liquid hydrocarbon fires. U.S. Patent US3258423A, 1966. [Google Scholar]
- (2).Gore RW Process for producing porous products. U.S. Patent US3953566A, 1976. [Google Scholar]
- (3).Tao L; Kannan K; Aldous KM; Mauer MP; Eadon GA Biomonitoring of perfluorochemicals in plasma of New York State personnel responding to the World Trade Center disaster. Environ. Sci. Technol. 2008, 42 (9), 3472–3478. [DOI] [PubMed] [Google Scholar]
- (4).Brendel S; Fetter E; Staude C; Vierke L; Biegel-Engler A Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH. Environmental Sciences Europe 2018, 30 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Heidari H; Abbas T; Ok YS; Tsang DC; Bhatnagar A; Khan E GenX is not always a better fluorinated organic compound than PFOA: A critical review on aqueous phase treatability by adsorption and its associated cost. Water Res. 2021, 205, 117683. [DOI] [PubMed] [Google Scholar]
- (6).Li W; Hu Y; Bischel HN In-vitro and in-silico assessment of per-and polyfluoroalkyl substances (PFAS) in aqueous film-forming foam (AFFF) binding to human serum albumin. Toxics 2021, 9 (3), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gilliland FD; Mandel JS Mortality among employees of a perfluorooctanoic acid production plant. Journal of Occupational Medicine 1993, 35, 950–954. [DOI] [PubMed] [Google Scholar]
- (8).Olson CT; Andersen ME The acute toxicity of perfluorooctanoic and perfluorodecanoic acids in male rats and effects on tissue fatty acids. Toxicology and applied pharmacology 1983, 70 (3), 362–372. [DOI] [PubMed] [Google Scholar]
- (9).Anderson RH; Thompson T; Stroo HF; Leeson A US Department of Defense-funded Fate and transport research on perand polyfluoroalkyl substances at aqueous film-forming foamimpacted sites. Environ. Toxicol. Chem. 2021, 40 (1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Moody CA; Field JA Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 2000, 34 (18), 3864–3870. [Google Scholar]
- (11).Anderson RH; Long GC; Porter RC; Anderson JK Occurrence of select perfluoroalkyl substances at US Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685. [DOI] [PubMed] [Google Scholar]
- (12).Giesy JP; Kannan K Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35 (7), 1339–1342. [DOI] [PubMed] [Google Scholar]
- (13).PFAS Strategic Roadmap: EPA’s Commitments to Action 2021–2024. U.S. Environmental Protection Agency, 2021. https://www.epa.gov/pfas/pfas-strategic-roadmap-epas-commitments-action-2021-2024 (accessed 2023-04-07).
- (14).Memorandum, Addressing PFAS Discharges in NPDES Permits and Through the Pretreatment Program and Monitoring Programs. U.S. Environmental Protection Agency. https://www.epa.gov/system/files/documents/2022-12/NPDES_PFAS_State%20Memo_December_2022.pdf (accessed 2023-04-07).
- (15).Hagenaars A; Meyer I; Herzke D; Pardo B; Martinez P; Pabon M; De Coen W; Knapen D The search for alternative aqueous film forming foams (AFFF) with a low environmental impact: physiological and transcriptomic effects of two Forafac® fluorosurfactants in turbot. Aquatic Toxicology 2011, 104 (3–4), 168–176. [DOI] [PubMed] [Google Scholar]
- (16).Sheinson RS; Williams BA; Green C; Fleming JW; Anleitner R; Ayers S; Maranghides A; Barylski D The future of aqueous film forming foam (AFFF): performance parameters and requirements. Naval Research Laboratory, 2002. https://www.nist.gov/system/files/documents/el/fire_research/R0201327.pdf (accessed 2023-04-07). [Google Scholar]
- (17).Minutes of Fluorochemical Study Committee Meeting. 3M. https://www.ag.state.mn.us/Office/Cases/3M/docs/PTX/PTX1279.pdf (accessed 2023-04-07). [Google Scholar]
- (18).AF awards replacement firefighting foam contract. U.S. Air Force. https://www.af.mil/News/Article-Display/Article/915057/afawards-replacement-firefighting-foam-contract/ (accessed 2023-04-07).
- (19).Ross I; Storch P; Schaefer T; Ramsden N Fluorine Free Foams: Transitioning Guide. In Forever Chemicals; CRC Press: 2021; pp 23–50. [Google Scholar]
- (20).McDonald R; Lumley A; Johnson G; Broxson S; Luckarift H; Bridgett A Evaluation of the Chemical and Physical Properties of Commercial Fluorine Free Foams, 2022. Arctos Technology Solutions. https://apps.dtic.mil/sti/citations/AD1172169 (accessed 2023-04-07). [Google Scholar]
- (21).Jacobson R; Ferraro MF Environmental Deconfliction 2020: The National Defense Authorization Act for FY 2020. Envtl. L. Rep. 2020, 50, 10983. [Google Scholar]
- (22).MIL-PRF-XX727, Fire Extinguishing Agent, Fluorine-Free Foam (F3) Liquid Concentrate, For Land-Based, Fresh Water Applications. U.S. DoD. https://sam.gov/opp/43aafb84be8e495da7edb458d456e554/view (accessed 2023-04-07). [Google Scholar]
- (23).Andrews DQ; Hayes J; Stoiber T; Brewer B; Campbell C; Naidenko OV Identification of point source dischargers of perand polyfluoroalkyl substances in the United States. AWWA Water Science 2021, 3 (5), No. e1252. [Google Scholar]
- (24).Elwell DK FAA Reauthorization Act of 2018 (2018 Act or Act), 2018. U.S. Department of Transportation. https://www.transportation.gov/testimony/implementation-faa-reauthorization-act-2018#_msoanchor_2 (accessed 2023-04-07).
- (25).Grant C; Hawthorne E; Back J In Firefighting Foams: Transition Roadmap for the Fire Service; 2022 NFPA Conference & Expo, 2022; NFPA, 2022. [Google Scholar]
- (26).San Francisco Fire Department Health Safety and Wellness Report. SFFD. https://sf-fire.org/media/1821/download?inline (accessed 2023-04-07). [Google Scholar]
- (27).Cousins IT; DeWitt JC; Gluge J; Goldenman G; Herzke D; Lohmann R; Miller M; Ng CA; Scheringer M; Vierke L; Wang Z Strategies for grouping per-and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environmental Science: Processes & Impacts 2020, 22 (7), 1444–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Glüge J; Scheringer M; Cousins IT; DeWitt JC; Goldenman G; Herzke D; Lohmann R; Ng CA; Trier X; Wang Z An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environmental Science: Processes & Impacts 2020, 22 (12), 2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bluteau T; Cornelsen M; Day G; Holmes N; Klein R; Olsen K; McDowall J; Stewart R; Tisbury M; Webb S In Global PFAS problem: Fluorine-free alternatives as solutions firefighting foams and other sources—Going fluorine-free; White Paper prepared for IPEN by members of the IPEN Expert Panel and associates, Meeting of the Stockholm Convention Conference of the Parties (COP9), Geneva, Switzerland, 2019. https://ipen.org/sites/default/files/documents/the_global_pfas_problem-v1_5_final_18_april.pdf (accessed 2023-04-07). [Google Scholar]
- (30).Allcorn M; Bluteau T; Corfield J; Day G; Cornelsen M; Holmes N; Klein R; McDowall J; Olsen K; Ramsden N Fluorine-free firefighting foams (3F)-Viable alternatives to fluorinated aqueous film-forming foams (AFFF). Independent Expert Panel Convened by IPEN. Saatavissa. https://ipen.org/documents/fluorine-free-firefighting-foams (accessed 2023-04-07). [Google Scholar]
- (31).3M to Exit PFAS Manufacturing by the End of 2025. 3M. https://news.3m.com/2022-12-20-3M-to-Exit-PFAS-Manufacturing-by-the-End-of-2025). (accessed 2023-04-07). [Google Scholar]
- (32).Developing New Replacements. SERDP-ESTCP. https://www.serdp-estcp.org/focusareas/c49b7f51-cd5c-428e-b733-9231179b44ac/developing-new-replacements (accessed 2023-04-07).
- (33).Field Demonstrations. SERDP-ESTCP. https://www.serdpestcp.org/focusareas/93321f8e-f3cb-4c84-9478-37341a0e4abc/field-demonstrations (accessed 2023-04-07).
- (34).Management of PFAS in the Environment. SERDP-ESTCP. https://www.serdp-estcp.org/focusareas/9a62d079-00d0-4482-a2a2-d2d8157abec9/management-of-pfas-in-the-environment (accessed 2023-04-07).
- (35).Per- and Polyfluorinated Substances in Firefighting Foam, 2018. IC2, Rochester Institute of Technology, New York State Pollution Prevention Institute. https://cswab.org/wp-content/uploads/2019/03/PFAS-in-Firefighting-Foam-New-York-State-Pollution-Prevention-Institute-Dec-2018.pdf (accessed 2023-04-07). [Google Scholar]
- (36).Ruyle BJ; Pickard HM; LeBlanc DR; Tokranov AK; Thackray CP; Hu XC; Vecitis CD; Sunderland EM Isolating the AFFF signature in coastal watersheds using oxidizable PFAS precursors and unexplained organofluorine. Environ. Sci. Technol. 2021, 55 (6), 3686–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).García RA; Chiaia-Hernández AC; Lara-Martin PA; Loos M; Hollender J; Oetjen K; Higgins CP; Field JA Suspect screening of hydrocarbon surfactants in AFFFs and AFFF-contaminated groundwater by high-resolution mass spectrometry. Environ. Sci. Technol. 2019, 53 (14), 8068–8077. [DOI] [PubMed] [Google Scholar]
- (38).Field J; Sedlak D; Alvarez-Cohen L Characterization of the Fate and Biotransformation of Fluorochemicals in AFFF-Contaminated Groundwater at Fire/Crash Testing Military Sites, 2017. Oregon State University Corvallis United States. https://apps.dtic.mil/sti/citations/AD1037940 (accessed 2023-04-07). [Google Scholar]
- (39).Titaley IA; Khattak J; Dong J; Olivares CI; DiGuiseppi B; Lutes CC; Field JA Neutral Per-and Polyfluoroalkyl Substances, Butyl Carbitol, and Organic Corrosion Inhibitors in Aqueous Film-Forming Foams: Implications for Vapor Intrusion and the Environment. Environ. Sci. Technol. 2022, 56 (15), 10785–10797. [DOI] [PubMed] [Google Scholar]
- (40).Pabon M; Corpart J Fluorinated surfactants: synthesis, properties, effluent treatment. J. Fluorine Chem. 2002, 114 (2), 149–156. [Google Scholar]
- (41).Sheng Y; Xue M; Ma L; Zhao Y; Wang Q; Liu X Environmentally Friendly Firefighting Foams Used to Fight Flammable Liquid Fire. Fire Technol. 2021, 57, 2079–2096. [Google Scholar]
- (42).Li R; Fan G; Wang P; Ouyang X; Ma N; Wei H Effects of silane coupling agent modifications of hollow glass microspheres on syntactic foams with epoxy matrix. Polymers and Polymer Composites 2021, 29, S1191–S1203. [Google Scholar]
- (43).Sheng Y; Peng Y; Zhang S; Guo Y; Ma L; Wang Q; Zhang H Study on Thermal Stability of Gel Foam Co-Stabilized by Hydrophilic Silica Nanoparticles and Surfactants. Gels 2022, 8 (2), 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Dubocq F; Wang T; Yeung LW; Sjöberg V; Kärrman A Characterization of the chemical contents of fluorinated and fluorine-free firefighting foams using a novel workflow combining nontarget screening and total fluorine analysis. Environ. Sci. Technol. 2020, 54 (1), 245–254. [DOI] [PubMed] [Google Scholar]
- (45).Wu X; Nguyen H; Kim D; Peng H Chronic toxicity of PFAS-free AFFF alternatives in terrestrial plant Brassica rapa. Science of The Total Environment 2022, 850, 158100. [DOI] [PubMed] [Google Scholar]
- (46).Banerjee A; Liu Y Essential factor of perfluoroalkyl surfactants contributing to efficacy in firefighting foams. Langmuir 2021, 37 (30), 8937–8944. [DOI] [PubMed] [Google Scholar]
- (47).Yu X; Jiang N; Miao X; Li F; Wang J; Zong R; Lu S Comparative studies on foam stability, oil-film interaction and fire extinguishing performance for fluorine-free and fluorinated foams. Process Safety and Environmental Protection 2020, 133, 201–215. [Google Scholar]
- (48).A framework to guide selection of chemical alternatives, 2014. NRC, National Research Council. https://nap.nationalacademies.org/catalog/18872/a-framework-to-guide-selection-of-chemical-alternatives (accessed 2023-04-07). [PubMed] [Google Scholar]
- (49).Standard for Firefighting Foam. GreenScreen. https://www.greenscreenchemicals.org/images/ee_images/uploads/resources/GSCFirefightingFoamStandardV2.0_20200909_pdf (accessed 2023-04-07). [Google Scholar]
- (50).GreenScreen, GreenScreen Certified Products - Firefighting Foam. https://www.greenscreenchemicals.org/certified/products/category/firefighting (accessed 2023-04-07).
- (51).Design for the Environment Program Alternatives Assessment Criteria for Hazard Evaluation, Version 2.0, 2011. U.S. EPA. https://www.epa.gov/sites/default/files/2014-01/documents/aa_criteria_v2.pdf (accessed 2023-04-07). [Google Scholar]
- (52).Wehage K; Chenhansa P; Schoenung JM An open framework for automated chemical hazard assessment based on GreenScreen for Safer Chemicals: A proof of concept. Integr. Environ. Assess. Manag. 2017, 13 (1), 167–176. [DOI] [PubMed] [Google Scholar]
- (53).Vegosen L; Martin TM An automated framework for compiling and integrating chemical hazard data. Clean technologies and environmental policy 2020, 22 (2), 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Ring CL; Arnot JA; Bennett DH; Egeghy PP; Fantke P; Huang L; Isaacs KK; Jolliet O; Phillips KA; Price PS; Shin H-M; Westgate JN; Setzer RW; Wambaugh JF Consensus modeling of median chemical intake for the US population based on predictions of exposure pathways. Environ. Sci. Technol. 2019, 53 (2), 719–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Jones DK; Quinlin KA; Wigren MA; Choi YJ; Sepulveda MS; Lee LS; Haskins DL; Lotufo GR; Kennedy A; May L; Harmon A; Biber T; Melby N; Chanov MK; Hudson ML; Key PB; Chung KW; Moore DW; Suski JG; Wirth EF; Hoverman JT Acute Toxicity of Eight Aqueous Film-Forming Foams to 14 Aquatic Species. Environ. Sci. Technol. 2022, 56 (10), 6078–6090. [DOI] [PubMed] [Google Scholar]
- (56).Yu X; Ceja-Navarro JA; Wu X Sublethal Toxicity of Fluorine-Free Firefighting Foams in Soil Invertebrate Caenorhabditis elegans. Environmental Science & Technology Letters 2022, 9 (6), 561–566. [Google Scholar]
- (57).Hossain F; Dennis NM; Subbiah S; Karnjanapiboonwong A; Guelfo JL; Suski J; Anderson TA Acute Oral Toxicity of Nonfluorinated Fire-Fighting Foams to Northern Bobwhite Quail (Colinus virginianus). Environ. Toxicol. Chem. 2022, 41 (8), 2003–2007. [DOI] [PubMed] [Google Scholar]
- (58).Wu X; Nguyen H; Kim D; Peng H Chronic toxicity of PFAS-free AFFF alternatives in terrestrial plant Brassica rapa. Sci. Total Environ. 2022, 850, 158100. [DOI] [PubMed] [Google Scholar]
- (59).Gharehveran MM; Walus AM; Anderson TA; Subbiah S; Guelfo J; Frigon M; Longwell A; Suski JG Per- and polyfluoroalkyl substances (PFAS)-free aqueous film forming foam formulations: Chemical composition and biodegradation in an aerobic environment. Journal of Environmental Chemical Engineering 2022, 10 (6), 108953. [Google Scholar]
- (60).Titaley IA; Khattak J; Dong J; Olivares CI; DiGuiseppi B; Lutes CC; Field JA Neutral Per- and Polyfluoroalkyl Substances, Butyl Carbitol, and Organic Corrosion Inhibitors in Aqueous Film-Forming Foams: Implications for Vapor Intrusion and the Environment. Environ. Sci. Technol. 2022, 56 (15), 10785–10797. [DOI] [PubMed] [Google Scholar]
- (61).Dubocq F; Wang T; Yeung LWY; Sjoberg V; Karrman A Characterization of the Chemical Contents of Fluorinated and Fluorine-Free Firefighting Foams Using a Novel Workflow Combining Nontarget Screening and Total Fluorine Analysis. Environ. Sci. Technol. 2020, 54 (1), 245–254. [DOI] [PubMed] [Google Scholar]
- (62).Garcia RA; Chiaia-Hernandez AC; Lara-Martin PA; Loos M; Hollender J; Oetjen K; Higgins CP; Field JA Suspect Screening of Hydrocarbon Surfactants in AFFFs and AFFF-Contaminated Groundwater by High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2019, 53 (14), 8068–8077. [DOI] [PubMed] [Google Scholar]
- (63).Annunziato KM; Doherty J; Lee J; Clark JM; Liang W; Clark CW; Nguyen M; Roy MA; Timme-Laragy AR Chemical characterization of a legacy aqueous film-forming foam sample and developmental toxicity in zebrafish (Danio rerio). Environ. Health Perspect. 2020, 128 (9), 097006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Yang D; Han J; Hall DR; Sun J; Fu J; Kutarna S; Houck KA; LaLone CA; Doering JA; Ng CA; Peng H Nontarget Screening of Per- and Polyfluoroalkyl Substances Binding to Human Liver Fatty Acid Binding Protein. Environ. Sci. Technol. 2020, 54 (9), 5676–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Li W; Hu Y; Bischel HN In-Vitro and In-Silico Assessment of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous Film-Forming Foam (AFFF) Binding to Human Serum Albumin. Toxics 2021, 9 (3), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Rana S; Marchiandi J; Partington JM; Szabo D; Heffernan AL; Symons RK; Xie S; Clarke BO Identification of novel polyfluoroalkyl substances in surface water runoff from a chemical stockpile fire. Environ. Pollut. 2022, 313, 120055. [DOI] [PubMed] [Google Scholar]
- (67).Jones DK; Quinlin KA; Wigren MA; Choi YJ; Sepulveda MS; Lee LS; Haskins DL; Lotufo GR; Kennedy A; May L; Harmon A; Biber T; Melby N; Chanov MK; Hudson ML; Key PB; Chung KW; Moore DW; Suski JG; Wirth EF; Hoverman JT Acute Toxicity of Eight Aqueous Film-Forming Foams to 14 Aquatic Species. Environ. Sci. Technol. 2022, 56 (10), 6078–6090. [DOI] [PubMed] [Google Scholar]
- (68).Yu X; Ceja-Navarro JA; Wu X Sublethal Toxicity of Fluorine-Free Firefighting Foams in Soil Invertebrate Caenorhabditis elegans. Environmental Science & Technology Letters 2022, 9 (6), 561–566. [Google Scholar]
- (69).Hinnant KM; Willey JL Perfluorinated Alkyl Substance (PFAS) Analyte Testing and Additional Analytical Evaluation of Relevant Firefighting Foam Formulations and Samples, 2022. Naval Research Lab. https://apps.dtic.mil/sti/citations/AD1181396 (accessed 2023-04-07). [Google Scholar]
- (70).Holden L; East A; Narizzano A Toxicology Assessment for Strategic Environmental Research and Development Program: Perand Polyfluoroalkyl Substances - Free Aqueous Film Forming Foams. Army Public Health Center. https://apps.dtic.mil/sti/citations/AD1175175 (accessed 2023-04-07). [Google Scholar]
- (71).Yao B; Sun R; Alinezhad A; Kubatova A; Simcik MF; Guan X; Xiao F The first quantitative investigation of compounds generated from PFAS, PFAS-containing aqueous film-forming foams and commercial fluorosurfactants in pyrolytic processes. J. Hazard Mater. 2022, 436, 129313. [DOI] [PubMed] [Google Scholar]
- (72).Etz BD; Mifkovic M; Vyas S; Shukla MK High-temperature decomposition chemistry of trimethylsiloxane surfactants, a potential Fluorine-Free replacement for fire suppression. Chemosphere 2022, 308, 136351. [DOI] [PubMed] [Google Scholar]
- (73).Zhang Q; Wu X; Lyu X; Gao B; Wu J; Sun Y Effects of anionic hydrocarbon surfactant on the transport of perfluorooctanoic acid (PFOA) in natural soils. Environmental Science and Pollution Research 2022, 29, 24672. [DOI] [PubMed] [Google Scholar]
- (74).Ji Y; Yan N; Brusseau ML; Guo B; Zheng X; Dai M; Liu H; Li X Impact of a hydrocarbon surfactant on the retention and transport of perfluorooctanoic acid in saturated and unsaturated porous media. Environ. Sci. Technol. 2021, 55 (15), 10480–10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Brusseau ML; Van Glubt S The influence of surfactant and solution composition on PFAS adsorption at fluid-fluid interfaces. Water research 2019, 161, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Lara-Martin PA; Gonzalez-Mazo E; Brownawell BJ Multiresidue method for the analysis of synthetic surfactants and their degradation metabolites in aquatic systems by liquid chromatography-time-of-flight-mass spectrometry. J. Chromatogr A 2011, 1218 (30), 4799–807. [DOI] [PubMed] [Google Scholar]
- (77).Cowan-Ellsberry C; Belanger S; Dorn P; Dyer S; McAvoy D; Sanderson H; Versteeg D; Ferrer D; Stanton K Environmental Safety of the Use of Major Surfactant Classes in North America. Crit Rev. Environ. Sci. Technol. 2014, 44 (17), 1893–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


