Abstract
Background
Prehabilitation aims at enhancing patients’ functional capacity and overall health status to enable them to withstand a forthcoming stressor like surgery. Our aim was to synthesise the evidence on the cost-effectiveness of prehabilitation for patients awaiting elective surgery compared with usual preoperative care.
Methods
We searched PubMed, Embase, the CRD database, ClinicalTrials.gov, the WHO ICTRP and the dissertation databases OADT and DART. Studies comparing prehabilitation for patients with elective surgery to usual preoperative care were included if they reported cost outcomes. All types of economic evaluations (EEs) were included. The primary outcome of the review was cost-effectiveness based on cost–utility analyses (CUAs).
The risk of bias of trial-based EEs was assessed with the Cochrane risk of bias 2 tool and the ROBINS-I tool and the credibility of model-based EEs with the ISPOR checklist. Methodological quality of full EEs was assessed using the CHEC checklist. The EEs’ results were synthesised narratively using vote counting based on direction of effect.
Results
We included 45 unique studies: 25 completed EEs and 20 ongoing studies. Of the completed EEs, 22 were trial-based and three model-based, corresponding to four CUAs, three cost-effectiveness analyses, two cost–benefit analyses, 12 cost–consequence analyses and four cost-minimization analyses. Three of the four trial-based CUAs (75%) found prehabilitation cost-effective, i.e. more effective and/or less costly than usual care. Overall, 16/25 (64.0%) EEs found prehabilitation cost-effective. When excluding studies of insufficient credibility/critical risk of bias, this number reduced to 14/23 (60.9%). In 8/25 (32.0%), cost-effectiveness was unclear, e.g. because prehabilitation was more effective and more costly, and in one EE prehabilitation was not cost-effective.
Conclusions
We found some evidence that prehabilitation for patients awaiting elective surgery is cost-effective compared to usual preoperative care. However, we suspect a relevant risk of publication bias, and most EEs were of high risk of bias and/or low methodological quality. Furthermore, there was relevant heterogeneity depending on the population, intervention and methods. Future EEs should be performed over a longer time horizon and apply a more comprehensive perspective.
Trial registration
PROSPERO CRD42020182813.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02977-6.
Keywords: Prehabilitation, Cost-effectiveness, Health economics, Systematic review, Evidence synthesis
Background
Rationale
Prehabilitation is still a relatively new care concept. It aims at enhancing patients’ functional capacity and overall health status through behaviour change [1] to enable them to withstand a forthcoming stressor [2]. In the surgical context, prehabilitation complements the concept of ‘enhanced recovery after surgery’ (ERAS) and aims to improve surgical outcomes and lower post-operative complication rates [3]. Prehabilitation programmes are delivered preoperatively by a multidisciplinary team and in various settings (e.g. inpatient, outpatient, or at home). Typical modalities include exercise training, promotion of physical activity, nutritional optimisation and psychological support [4], which are provided in addition to elements of ERAS, such as medical optimisation and alcohol or smoking cessation [5].
The potential of prehabilitation is widely recognised. Nevertheless, prehabilitation has not yet been widely adopted by health care systems. Current evidence is still somewhat limited, though much research is still underway to determine the optimal programme types and delivery modalities for different patient populations. Most research activity seems to be in the field of cancer surgery, for example, in an overview of 55 systematic reviews on preoperative prehabilitation, 23 reviews specifically focused on cancer [6]. A likely explanation for this phenomenon is that there is already a large body of evidence demonstrating the positive effects of physical activity on the physical and psychological outcomes of cancer patients [7]. In addition, little is known about the cost-effectiveness of prehabilitation, which is critical for policy-makers considering the implementation of such programmes. By definition, prehabilitation is an approach to reduce healthcare costs [4] and a comprehensive analysis of the value of prehabilitation should incorporate cost outcomes [8].
The aforementioned overview identified only one systematic review on costs [6], but this review focused on nutritional support rather than full prehabilitation programmes [9]. Other reviews that addressed health economic outcomes focused on specific populations [10] or were not systematic reviews [11]. One large systematic review including 178 randomised controlled trials (RCTs) showed that prehabilitation may reduce postoperative length of stay and complications [12], both of which would translate into a cost reduction. However, to our best knowledge, there is currently no comprehensive systematic review on the cost-effectiveness of prehabilitation prior to elective surgery.
Aim and objectives
The aim of this systematic review was to synthesise the evidence on the cost-effectiveness of prehabilitation programmes for patients awaiting elective surgery compared with usual preoperative care to inform decisions about the implementation of prehabilitation programmes and to guide the design of future rigorous economic evaluations of prehabilitation programmes. More specifically, our objectives were to (1) identify all eligible economic evaluations (EEs), (2) assess their validity and (3) systematically present their characteristics, methods and findings.
Methods
We followed general methodological guidance on systematic reviews of interventions [13] as well as guidance specific to systematic reviews of EEs [14–16]. Reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [17, 18] and guidance for systematic reviews without meta-analysis [19]. All raw data collected as part of the review are deposited in the Open Science Framework (OSF) [20].
Registration and protocol
The systematic review was prospectively registered in PROSPERO (CRD42020182813) and we published a protocol [21]. Important protocol changes are reported in Additional file 1: Appendix 1.
Eligibility criteria
The study in- and exclusion criteria are displayed in Table 1 (from the protocol with additional specifications) [21].
Table 1.
Review in- and exclusion criteria
| PICOS | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Patients from any country undergoing elective surgery | Patients undergoing emergency surgery or non-surgical treatments (e.g. chemotherapy) |
| Intervention | A preoperative prehabilitation programme (any setting), defined as a (set of) intervention(s) aimed at optimising functioning and reducing disability in individuals awaiting surgery. The intervention(s) had to include at least one component of physio- or occupational therapy and at least one in-person meeting between the patient(s) and health care professional(s). The ‘dose’, i.e. the programme’s duration (overall and per session) and frequency, had to be sufficiently longa to have an effect if the patients fully adhered to it | Purely medical/nutritional interventions, an intervention combined with additional postoperative rehabilitation, cognitive behaviour therapy or health counselling/education alone, purely web/app-based prehabilitation programmes |
| Control | Usual preoperative care as defined by the study authors, i.e. the routine care that patients with a given condition receive in the respective hospital (extended only by the baseline measurements performed as part of the trial) | Another prehabilitation intervention; no comparator |
| Outcome | Clinical effectiveness and costs, any timeframe for follow-up | Clinical effectiveness only |
| Study type | Full (i.e. cost–benefit, cost-effectiveness and cost–utility analyses) or partial economic evaluations (i.e. cost-minimization analysis), trial-basedb or model-based economic evaluations regardless of their statusc, cost perspective, publication year, language and type (i.e. full article, conference abstract) | Systematic reviews, simple, non-comparative cost analyses (i.e. studies that only calculated the costs of the intervention), commentaries/letters, animal studies |
aAs judged by a physiotherapist (JK), based on current evidence on exercise efficacy and duration
b We included trial-based economic evaluations based on randomised controlled trials as well as non-randomised studies of interventions, as we expected that the latter would provide valuable additional evidence, e.g. from a real-world setting. If a group in a multi-arm study did not meet the inclusion criteria, we included the study but not the group
c We included ongoing studies, i.e. protocols and registration records, as we were interested in their methods
Information sources
We searched PubMed, Embase and the Centre for Reviews and Dissemination (CRD) Database on 31/08/2021, which are the most efficient combination of bibliographic databases for systematic reviews of EEs [22]. Furthermore, we searched OADT.org and the DART-Europe E-theses Portal for grey literature and ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for unpublished and ongoing studies on October 30, 2021. A weekly email alert was created for the search in PubMed (monitored until August 23, 2022). Additionally, we screened the reference lists of included EEs and relevant systematic reviews as well as articles citing the included EEs obtained through Web of Science and Google Scholar. We also contacted the corresponding authors of all included EEs about further relevant EEs.
Search strategy
The database search strategies consisted of search terms, relating to the population (e.g. ‘preoperative’), the intervention (e.g. ‘exercise’) and study type, i.e. terms to search for economic evaluations (e.g. ‘cost’). Full search strategies for all sources can be found in Additional file 1: Appendix 2.
Selection process
Records retrieved from databases were deduplicated, screened and managed using EndNote 20 (Clarivate Analytics, Philadelphia (PA), USA). After deduplication, a randomly selected 10% sample of all unique records was screened against the eligibility criteria by two reviewers (TR, HE) independently based on their titles and abstracts. Disagreement was resolved by consensus. As agreement was above 80%, the remaining 90% were screened by one reviewer (TR). We retrieved the full-text articles for all potentially eligible studies as well as for relevant systematic reviews, so that their references could be screened. Each full-text article was screened for eligibility by two reviewers independently who noted reasons for exclusion. Disagreements were resolved by consensus and by consulting a third reviewer (WQ). Last, all study reports were mapped to unique studies as the unit of interest. No automation tools were used in the process.
Data collection process
Data were extracted into a standardised excel sheet that was piloted by one reviewer (TR). Two reviewers (TR, HE) independently extracted the data of a randomly selected 20% sample of the included completed EEs for calibration. Disagreement was resolved by consensus. As there were no systematic discrepancies, the remaining records were extracted by one reviewer (TR). All outcome data was verified by a second person (JS). We used all documents relevant to the included EEs for data extraction and contacted the study authors via email in case of missing or unclear data. Uncertainties about the methods were only inquired for completed EEs. A reminder email was sent after 2 weeks.
Data items
A list of all data items and detailed descriptions can be found in Additional file 1: Appendix 3. For ongoing studies, we only extracted the study characteristics and, if published as a protocol, the EE methods. For completed EEs, we also extracted post-operative results data (per group and as the difference between groups) on clinical effectiveness and costs. Costs were reported with their original year and currency as well as converted to 2020 EUR. For conversion, we used the ‘Cochrane Campbell Economic Methods Group and the Evidence for Policy and Practice Information and Coordinating Centre Cost Converter’ (version 1.6) [23]. We only extracted unadjusted data for the last available follow-up point based on intention-to-treat analyses.
Risk of bias and methodological quality assessment
The risk of bias of trial-based EEs was assessed on outcome-level using the Cochrane risk of bias tool 2 (RoB 2) [24] for EEs based on RCTs and the ROBINS-I tool [25] for EEs based on non-randomised studies of interventions (NRSI). Among other domains, both tools address the risk of reporting bias. Risk of bias figures were created for each outcome domain separately using the robvis application [26]. Methodological quality of trial-based full EEs was assessed using the Consensus on Health Economic Criteria (CHEC) checklist [27]. Model-based EEs were assessed for credibility using the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) checklist [28]. Assessments were performed by two reviewers (TR, HE) independently in a random 20% sample of the included EEs and continued by one reviewer (TR) as agreement was above 80%.
Effect measures
The review’s primary outcome was the cost-effectiveness from cost–utility analyses (CUAs) based on direction of effect (i.e. reduced costs and/or additional quality-adjusted life year gained). Secondary outcomes were the cost-effectiveness from cost-effectiveness analyses (CEAs), cost–benefit analyses (CBAs), cost-minimisation analyses (CMAs) and cost–consequence analyses (CCAs) based on direction of effect. We calculated effect measures when not reported using risk differences for dichotomous outcomes and mean differences or differences in medians for continuous outcomes. Confidence intervals were extracted when reported. All calculated values are marked as such. All outcomes were reported in disaggregated form in natural units and combined outcome measures, e.g. incremental cost-effectiveness ratios (ICERs), where possible.
Synthesis methods
We were unable to perform a meta-analysis because the only EEs that were sufficiently homogenous had an unquantifiable overlap in patient populations [29–34] or missed crucial information for data transformation [32, 33]. Therefore, structured narrative synthesis in the form of vote counting based on direction of effects was performed [35]. EEs were grouped by design (model-based vs. trials-based) [16] and analysis type (CUA vs. CEA, CBA, CCA, CMA) to reflect the prioritisation of outcomes.
Results were presented graphically in form of a hierarchical permutation matrix [36]. There were ten possible outcomes for incremental costs (which could be higher, lower or same) and effectiveness (which could be better, poorer, same or inconsistent) corresponding to five result categories: cost-effective, neutral, not cost-effective, unclear; incremental analysis required, and unclear; individual decision required). No formal sensitivity analysis was performed but we discussed the influence of excluding EEs that were of critical risk of bias or insufficient credibility. Descriptive post-hoc subgroup analyses were performed to explore heterogeneity in the EEs’ results arising from differences in populations, interventions, methods, funding source and conflict of interest.
Assessment of publication bias
To address publication bias, we searched comprehensively for ongoing studies and grey literature and followed up on their status by searching for related publications and contacting the named investigators. In addition, we discussed how the effectiveness results from the included EEs compare to those of clinical effectiveness studies on prehabilitation using an overview of 55 systematic reviews and meta-analyses of RCTs by McIsaac et al. 2022 [6]. Our hypothesis was that the EEs would appear more beneficial if there truly was a publication bias.
Results
Study selection
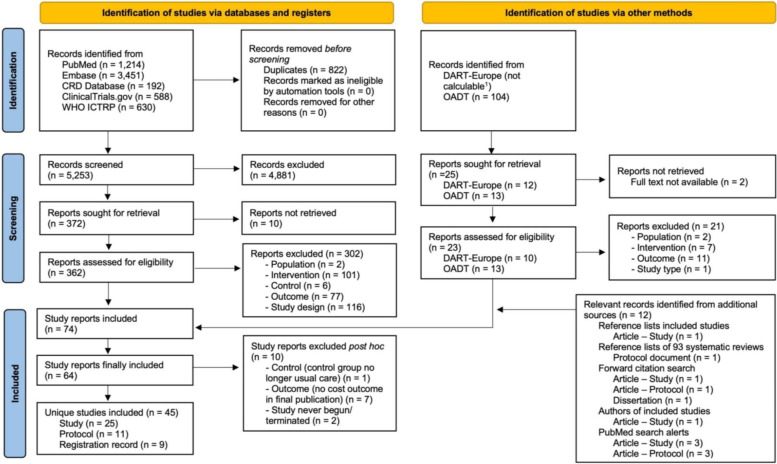
The study selection process is presented in Fig. 1. In total, 45 unique studies were included: 25 completed EEs [29–34, 37–55] and 20 ongoing studies, of which 11 were published as protocol articles [56–66] and nine as registration records [67–75]. Two completed EEs were only published as conference abstracts [53, 54] and two as dissertations [37, 49]. A total of 54 email enquiries were sent to the study authors, of which 23 were answered (response rate 42.6%). A list of all articles excluded after full-text screening can be found in Additional file 1: Appendix 4, with an additional explanation for close misses and articles excluded post hoc [76–85].
Fig. 1.
PRISMA 2020 flow diagram of the search and screening process
Characteristics of economic evaluations
The characteristics of the 25 completed EEs are displayed in Table 2. In summary, there were 22 trial-based EEs (13 RCTs and 9 NRSI), and three model-based EEs (2 decision trees and 1 financial projection) corresponding to four CUAs, three CEAs, two CBAs, 12 CCAs and four CMAs. Nine EEs were performed from a mix of a payer and provider perspective, three EEs each from a payer or provider perspective, and one EE from a patient perspective. The perspective remained unclear in the remaining nine EEs.
Table 2.
Characteristics of the completed economic evaluations
| Study ID, main referencea | Type and design of analysis | Perspective | Location (city/ cities; country) | Enrolment periodb | Inclusion criteria (disease(s); type(s) of surgery; criteria for increased perioperative risk) | Population demographics | Number of patients randomised (total (IG vs. CG)) |
|---|---|---|---|---|---|---|---|
| AlShewaier 2016 [37] | CUA; trial-based (RCT) | Unclear | Riyadh; Saudi Arabia | 07/2014–01/2015 | Isolated ACL injury; ACL reconstruction |
Female: 0%, male: 100% Median age: 27 years |
84 (39 vs. 45) |
| Barberan-Garcia 2019 [38] | CCA; trial-based (RCT) | Mix of payer/provider perspective | Barcelona; Spain | 02/2013–06/2016 | Not specified; major digestive surgery; age > 70 years and/or ASA score ≥ III |
Female: 25%, male: 75% Mean age: 71 years |
125 (62 vs. 63) |
| Beaupre 2004 [39] | CMA; trial-based (RCT) | Payer perspective | Edmonton; Canada | Not reported | Non-inflammatory arthritis; primary TKA |
Female: 55%, male: 45% Mean age: 67 years |
131 (65 vs. 66) |
| Chen 2022 [40] | CBA; model-based (projection) | Provider perspective | Toronto; Canada | Not applicable (model) | Not specified; major elective intra-cavity surgery; higher-than-average risk, limited physiologic reserve, frailty, deconditioned patient, other indication for prehabilitation with explanation | Not reported | 480 (240 vs. 240) |
| Dholakia 2021 [41] | CEA; model-based (decision tree) | Payer perspective | Not reported; USA | Not applicable (model) | Epithelial ovarian cancer; non-emergent primary debulking surgery; frailty |
Female: 100%, male: 0% Age not reported |
8830 (4415 vs. 4415) |
| Englesbe 2017 [29] | CMA; trial-based (NRSI) | Mix of payer/provider perspective | Ann Arbor; USA |
IG: 06/2014–12/2015c CG: 07/2006–06/2011 |
Not specified; major inpatient abdominal and thoracic operative care |
Female: 50%, male: 50% Mean age: 60 years |
364 (182 vs. 182) |
| Fernandes 2017 [42] | CUA; trial-based (RCT) | Mix of payer/ provider/patient perspective | Svendborg; Denmark | 01/2010–03/2011 | Symptomatic osteoarthritis; TKA, THA |
Female: 56%, male: 44% Mean age: 67 years |
165 (84 vs. 81) |
| Gao 2015 [43] | CCA; trial-based (NRSI) | Unclear | Chengdu; China | 11/2008–06/2011 | Lung cancer; lobectomy; > 800 pack-years, quitted smoking < 2 weeks ago, bronchial hyperresponsiveness, impaired lung function |
Female: 59%, male: 41% Mean age: 66 years |
142 (71 vs. 71) |
| Gränicher 2020 [44] | CCA; trial-based (RCT) | Payer perspective | Zürich; Switzerland | 07/2016–03/2017 | Not specified; TKA |
Female: 40%, male: 60% Mean age: 67 years |
20 (10 vs. 10) |
| Howard 2019 [30] | CCA; trial-based (NRSI) | Mix of payer/ provider perspective | Ann Arbor; USA | 01/2012–12/2017c | Not specified; major abdominal surgery |
Female: 49%, male: 51% Mean age: 59 years |
116 (76 vs. 40) |
| Huang 2012 [45] | CCA; trial-based (RCT) | Provider perspective | Changhua; Taiwan | 01/2008–12/2010 | Advanced osteoarthritis; primary TKA |
Female: 72%, male: 28% Mean age: 70 years |
243 (126 vs. 117) |
| Koh 2021 [46] | CCA; trial-based (NRSI) | Patient perspective | Singapore; Singapore |
IG: 02/2017–03/2020 CG: 04/2016–09/2018 |
Colorectal cancer; major colectomy; age ≥ 70 years |
Female: 44%, male: 56% Median age: 78 years |
81 (58 vs. 23) |
| Lai 2017 [32] | CCA; trial-based (RCT) | Unclear; assumed provider perspective | Chengdu; China | 01/2015–12/2015d | Non-small cell lung cancer; lung cancer surgery; > 20 pack-years, age > 75 years, BMI > 30, impaired predicted lung function or COPD |
Female: 42%, male: 58% Mean age: 64 years |
101 (51 vs. 50) |
| Lai 2019 [33] | CCA; trial-based (RCT) | Unclear; assumed provider perspective | Chengdu; China | 01/2018-not reported | Non-small cell lung cancer; lobectomy |
Female: 51%, male: 49% Mean age: 64 years |
68 (34 vs. 34) |
| McGregor 2004 [47] | CEA; trial-based (RCT) | Mix of payer/provider perspective | London; UK | Not reported | Not specified; THA |
Female: 71%, male: 29% Mean age: 72 years |
39 (19 vs. 20) |
| Mouch 2019 [31] | CMA; trial-based (NRSI) | Mix of payer/provider perspective | Multiple cities in Michigan; USA | 01/2014–12/2017c | Not specified; various types of surgery; high risk for complications (according to surgeon) |
Female: 53%, male: 47% Median age: 70 years |
1569 (523 vs. 1046) |
| Nguyen 2022 [48] | CUA; trial-based (RCT) | Mix of payer/provider perspective | Paris, Clermont-Ferrand; France | 10/2012- not reported | Knee osteoarthritis; TKA |
Female: 68%, male: 32% Mean age: 69 years |
262 (131 vs. 131) |
| Pham 2016 [49] | CMA; trial-based (RCT) | Unclear; assumed provider perspective | Sudbury; Canada | Not reported | Osteoarthritis; TKA, THA; BMI ≥ 30 |
Female: 69%, male: 31% Mean age: 64 years |
50 (29 vs. 21) |
| Ploussard 2020 [50] | CCA; trial-based (NRSI) | Mix of payer/provider perspective | Quint-Fonsegrives; France |
IG: 01/2018–12/2019 CG: 01/2016–12/2017 |
Not specified; robot-assisted radical prostatectomy |
Female: 0%, male: 100% Mean age: 66 years |
350 (194 vs. 156) |
| Risco 2022 [51] | CCA; trial-based (NRSI) | Provider perspective | Barcelona, Spain | 06/2017–12/2019 | Not specified; major digestive, cardiac, thoracic, gynaecologic or urologic surgeries; age > 70 years and/or ASA score ≥ III and/or severe deconditioning |
Female: 69%, male: 31% Median age 71 years |
656 (328 vs. 328) |
| Tew 2017 [52] | CEA; trial-based (RCT) | Mix of payer/provider perspective | Middlesbrough, Sheffield, York; UK | 09/2013–07/2015 | Abdominal aortic aneurysms; abdominal aortic aneurysm repair |
Female: 6%, male: 94% Mean age: 75 years |
53 (27 vs. 26) |
| Tveter 2020 [53] | CUA; trial-based (RCT) | Unclear; assumed mix of provider, payer and patient perspective | Trondheim, Bergen, Haugesund; Norway | 04/2013–06/2015 | Carpometacarpal joint osteoarthritis; thumb carpometacarpal joint surgery |
Female: 79%, male: 21% Mean age: 63 years |
180 (90 vs. 90) |
| Van Wijk 2020 [54] | CBA; model-based (decision tree) | Not reported | Netherlands (nationwide) | Not applicable (model) | Not specified; pancreatic surgery; low physical fitness, impaired nutritional status, the presence of iron deficiency anaemia, frailty, and/or intoxications | Not reported | Not reported |
| Wang 2020 [55] | CCA; trial-based (NRSI) | Unclear; assumed provider perspective | Singapore; Singapore | 02/2016–10/2017 | Hepatocellular carcinoma, colorectal liver metastases; liver resection |
Female: 26%, male: 74% Median age: 67 years |
104 (70 vs. 34) |
| Zhou 2017 [34] | CCA; trial-based (NRSI) | Unclear; assumed patient perspective | Chengdu; China | 03/2014–06/2015d | Primary non-small cell lung cancer; lobectomy; ≥ 20 pack-years, BMI ≥ 28, impaired lung function, COPD/asthma/airway hyper reactivity |
Female: 56%, male: 44% Mean age: 59 years |
939 (197 vs. 742) |
ACL anterior cruciate ligament, ASA American Society of Anesthesiologists, BMI body mass index, CBA cost–benefit analysis, CCA cost–consequence analysis, CEA cost-effectiveness analysis, CG control group, CMA cost-minimisation analysis, COPD chronic obstructive pulmonary disease, CUA cost–utility analysis, IG intervention group, NRSI non-randomised study of interventions, RCT randomised controlled trial, THA total hip arthroplasty, TJA total joint arthroplasty, TKA total knee arthroplasty, UK United Kingdom, USA United States of America
a Further references relating to the included economic evaluations are cited in Additional file 1: Appendix 7
b Extracted from article or registration record (in that order)
c Overlapping population (Michigan, United States)
d Overlapping population (Chengdu, China)
Most EEs were published in the last 10 years and came from Europe (10 EEs), Asia (8 EEs) or North America (7 EEs). The EEs covered a wide range of diseases and surgery types that can be broadly categorised as orthopaedic surgery (9 EEs), cancer surgery (8 EEs), mixed major surgery (6 EEs) and other (2 EEs). In 11 EEs, patients had an increased perioperative risk (e.g. old age or frailty). Sample size ranged from 20 to 8830 patients (median 137). The median proportion of women across the EEs was 53%, and the mean or median age ranged from 59 to 78 years, with one outlier (median age 27 years).
Characteristics and methods of the 20 ongoing EEs are reported in Additional file 1: Appendix 5. All are trial-based EEs, with the majority based on RCTs (18 EEs). There were five CUAs, six CEAs, three EEs using both CUA and CEA, and six EEs with unclear analysis type. In addition to the above continents, two ongoing EEs were from Australia and one from South America. The disease and surgery types were similar to the completed EEs (9 EEs on cancer, 8 EEs on major mixed or major other surgeries and 3 EEs on orthopaedics), though there were slightly more EEs from the field of cardiology and focusing on patients with an increased perioperative risk, and less EEs from the field of orthopaedics.
Information on the completed and ongoing EEs’ funding and conflict of interest can be found in Additional file 1: Appendix 6. Nine EEs did not report any information, one received parts of its funding from a commercial funder [57], one from a private donor [46] and in one, it was unclear [68]. Two EEs declared a relevant conflict of interest [29, 42], as authors were related to companies contracted to organise the prehabilitation.
Methods of economic evaluations
Detailed information on the methods can be found study-by-study in Additional file 1: Appendix 7 (completed EEs) and Additional file 1: Appendix 8 (ongoing EEs published as protocols). Most completed EEs used a time horizon for effects and costs of 1 month or less (range: 2 weeks to 24 months), with various EEs following patients until discharge and using the costs of hospital stay. No EE discounted effects or costs. Using bootstrapped precision measures (e.g. 95% confidence intervals) was the most common method for calculating uncertainty around the point estimates. Three EEs applied willingness-to-pay thresholds. In summary, with two exceptions [40, 42], few EEs applied comprehensive economic evaluation methods.
Description of prehabilitation programmes
Characteristics of the completed EEs’ prehabilitation programmes can be found in Table 3. Briefly, in most EEs, the prehabilitation programme was multimodal. All 25 programmes included an exercise element, though the type of training and use of unsupervised sessions varied. Additionally, many included an element of counselling or education (13 EEs) or an element addressing the patients’ nutritional status (11 EEs). The programmes involved various groups of health care professionals, the most common group being physiotherapists (14 EEs). Most programmes were performed in an outpatient (11 EEs) or home setting (8 EEs). The programmes’ overall duration ranged from 3 days to 3 months, with most programmes lasting between 2 and 4 weeks. The frequency of supervised sessions ranged from daily to once per week, with session durations being individual or ranging from 30 to 70 min. Where intensity was reported, we mostly classified it as high, e.g. an 80% of peak work rate for endurance training. Many programmes were not evidence-based. They costed between 100 and 1000 EUR (2020) per patient. Characteristics of the programmes evaluated in the ongoing studies can be found in Additional file 1: Appendix 9.
Table 3.
Characteristics of the prehabilitation programmes evaluated in the completed economic evaluations
| Study ID, main reference | Type and modalities | Involved health care professionals | Setting | Overall duration, frequency and duration per sessiona | Intensity of exercise trainingb | Evidence-based programme | Programme costs in EUR (2020) |
|---|---|---|---|---|---|---|---|
| AlShewaier 2016 [37] | Unimodal: exercise (resistance, proprioception and balance) | Physiotherapists | Outpatient—hospital | 4 weeks, 3x/week, for 45 min | High | Yes | 838 |
| Barberan-Garcia 2019 [38] | Multimodal: counselling/education, exercise (endurance), promotion of physical activity | Specialised physiotherapist | Outpatient—hospital | Individual, but min 4 weeks, 1–3x/week, individual session duration | High | Yes | 457 |
| Beaupre 2004 [39] | Multimodal: counselling/education, exercise (resistance) | Physiotherapists | Outpatient—community | 4 weeks, 3x/week, individual and progressing session duration | High | No | 225 |
| Chen 2022 [40] | Multimodal: exercise (endurance, resistance, disease-specific), nutrition (counselling, supplements), psychosocial (stress management), smoking cessation | Kinesiologist or certified exercise physiologist, dietitian, psychologist | Outpatient—hospital (FBP group), home (HBP group) | Individual; min 2 weeks, 2x/week (FBP), 3–5x/week (HBP), for 60 min | Moderate | Yes | 798 |
| Dholakia 2021 [41] | Various (model): smoking cessation, stabilising diseases, nutrition (counselling, supplements), exercise (endurance, resistance, inspiratory muscles), psychosocial (stress management), counselling/education, other (social/financial support) | Variable (model) | Variable (model) | Variable (model) | Not applicable | No | Variable (model) |
| Englesbe 2017 [29] | Multimodal: promotion of physical activity, exercise (inspiratory muscles), nutrition (counselling), psychosocial (stress management), planning of care, smoking cessation | Not reported | Home | Individual; min 2 weeks, 3-7x/week, individual session duration | Not reported | No | 80 |
| Fernandes 2017 [42] | Multimodal: exercise (resistance, proprioception and balance) | Physiotherapists | Outpatient—hospital | Not reported but ‘An attendance of 12 sessions or more was considered good compliance’, implying ≥ 6 weeks, 2x/week, for 60 min | Not reported | No | 351 (824 when including patient expenses) |
| Gao 2015 [43] | Multimodal: exercise (inspiratory muscles, endurance) | Professional therapists | Inpatient | 3–7 days, 2x/day, for 50–60 min | Not reported | No | 246 |
| Gränicher 2020 [44] | Multimodal: exercise (endurance, stretching and flexibility, resistance; individual exercises when indicated), counselling/education | Physiotherapists | Outpatient—hospital | 3–4 weeks, 1.25–3x/week, individual session duration | Low to moderate | No | 283 |
| Howard 2019 [30] | Multimodal: promotion of physical activity, exercise (inspiratory muscles), nutrition (counselling), psychosocial (stress management), planning of care, smoking cessation | Not reported | Home | Individual; min 2 weeks, 3–7x/week, individual session duration | Not reported | No | 80 |
| Huang 2012 [45] | Multimodal: exercise (resistance), counselling/education | Physiotherapists | Home | 4 weeks, 7x/week, for 40 min (first session); remaining sessions individual | Not reported | No | 20 |
| Koh 2021 [46] | Multimodal: nutrition (supplements), exercise (resistance), counselling/education, drug evaluation, stabilising diseases | Dieticians, physiotherapists | Outpatient—community, hospitalc | 3 weeks, frequency not reported, individual session duration | Not reported | No | Not calculable |
| Lai 2017 [32] | Multimodal: exercise (inspiratory muscles, endurance) | Inpatient | 1 week, 3 + 2 + 1/day, for 45–60 min plus breathing exercises | Not reported | No | Not calculable | |
| Lai 2019 [33] | Multimodal: exercise (inspiratory muscles, endurance) | Specialised nurses, physical therapists | Inpatient | 1 week, 3 + 1/day, for 30 min plus breathing exercises | Not reported | No | Not calculable |
| McGregor 2004 [47] | Unimodal: counselling/education, exercise (not specified) | Not reported | Home | 1–3 weeksc, unclear frequency, individual session duration | Not reported | No | 22 |
| Mouch 2019 [31] | Multimodal: promotion of physical activity, exercise (inspiratory muscles), nutrition (counselling), psychosocial (stress management), planning of care, smoking cessation | Not reported | Home | Individual; min 2 weeks, 3-7x/week, individual session duration | Not reported | No | 54 |
| Nguyen 2022 [48] | Multimodal: counselling/education, nutrition (counselling), psychosocial (stress management, anxiety reduction), exercise (resistance, stretching and flexibility, endurance, proprioception and balance) | Physiotherapist, instructor in physical activity, social worker, dietician, psychologist, occupational therapist | Outpatient—hospital, home | 8 weeks, 2x/week, for 60 min | Low | Yes | 60 |
| Pham 2016 [49] | Multimodal: exercise (stretching and flexibility, resistance, endurance, proprioception and balance), counselling/education | Kinesiologist and/or Human Kinetics graduate student | Outpatient—community | 12 weeks, 3x/week, for 40–60 min | Moderate to high | Yes | 103 |
| Ploussard 2020 [50] | Multimodal: counselling/education, planning of care, exercise (disease-specific), promotion of physical activity, stabilising diseases, psychological (other), nutrition (counselling, supplements) | Urology nurse, physiotherapist, nurse anaesthetist, oncology nurse specialist, cardiologist (if needed), pneumologist (if needed), psychologist, dietician, urologist | Home | 2 weeksc, 2–3x/day, for 1 full day, then individual session duration | Not reported | No | 231 |
| Risco 2022 [51] | Multimodal: counselling/education, promotion of physical activity, exercise (endurance, resistance), nutrition (counselling, supplements), psychosocial (anxiety reduction, stress management, other) | Anaesthesiologists, physiotherapists, dietitians, psychologists and nurses | Outpatient—hospital | Min 4 weeks, 2-3x/week, for 47 min (endurance) and individual session duration (strength, physical activity) | High (endurance), moderate (strength) | Yes | 445 |
| Tew 2017 [52] | Unimodal: exercise (endurance) | Research nurse, physiotherapist | Outpatient—hospital | 4 weeks, 3x/week, for 45 min (after first 3 sessions option to do it in 37 min) | High | Yes | 1341 |
| Tveter 2020 [53] | Multimodal: counselling/education, other (assistive devices, orthoses), exercise (stretching and flexibility, resistance) | Occupational therapist | Home | 12 weeks, 3x/week, individual session duration | Moderate to high | Yes | Not reported |
| Van Wijk 2020 [54] | Various (model): not reported, but assumedly: exercisec, promotion of physical activity, nutrition, stabilizing diseases, alcohol cessation, smoking cessation | Variable (model) | Not reported | Variable (model) | Not reported | No information | 1446 |
| Wang 2020 [55] | Multimodal: exercise (inspiratory muscles), nutrition (counselling, supplements), counselling/education, planning of care, other (financial/social support) | Physiotherapist, dietician, case manager | Not reported | 2–4 weeks, 5x/week, for 30 min plus breathing exercises | Not reported | No | Not reported |
| Zhou 2017 [34] | Multimodal: exercise (inspiratory muscles, endurance) | Lung cancer nurse specialists, physiotherapists | Inpatient | 1 week, 2 + 3 + 1x/day, for 65–70 min | Not reported | No | Not calculable |
FBP facility-based prehabilitation, HBP home-based prehabilitation
a Referring to exercise element if multiple modalities
b As judged by an experienced physiotherapist
c Information obtained through author contact
Risk of bias and methodological quality
The results of the assessment of risk of bias and methodological quality of the included studies can be found in Additional file 1: Appendix 10. The majority of RCT-based EEs were judged to be of high risk of bias with the RoB 2 tool. Only one RCT had a moderate risk of bias in all domains [42], and none had a low risk of bias. The main reason for high risk of bias was the absence of a prospective study protocol/registration record. All NRSI-based EE had at least a high, one even a critical risk of bias [34], the main reason being that most EEs did not adequately control for confounding when selecting or analysing patients.
The methodological quality of full trial-based EEs as judged with the CHEC-checklist ranged from 8 to 15 fulfilled items (of 18 to 19 applicable items) and thus can be considered moderate to low. The credibility of model-based EEs as judged with the ISPOR checklist was acceptable in one EE [40], insufficient in another EE [41] and could not be determined due to lack of information in one EE published as a conference abstract [54].
Results of individual economic evaluations
Table 4 provides an overview of the results of the completed EEs. Smaller values represent a higher benefit unless indicated otherwise. Morbidity refers to the rate of postoperative complications unless indicated otherwise. Detailed cost results can be found in Additional file 1: Appendix 11 including quantities of resource use, unit costs, total costs, incremental cost-effectiveness ratio (ICER) in the original currency and year, and the study authors’ conclusion. Furthermore, adherence and safety/feasibility outcomes can be found in Additional file 1: Appendix 12. Two EEs had adherence rates of less than 35% [48, 51] and in three EEs, drop-out and/or adverse event rates were notably higher in the prehabilitation groups [47, 48, 53].
Table 4.
Results of completed economic evaluations
| Study ID, main reference | Clinical effectiveness (IG vs. CG) | Total costs in EUR (2020) (IG vs. CG) | Cost-effectiveness based on direction of effectsa | Risk of bias/quality |
|---|---|---|---|---|
| Results from model-based economic evaluations | ||||
| Results from CEAs | ||||
| Dholakia 2021 [41] | Mortality: 397/4415 (9.0%) vs. 441/4415 (10.0%); RDb -1.0% | Mean 59,849 vs. 65,304; MDb -5455 | Cost-effective | ISPOR-Q: Insufficiently credible |
| Results from CBAs | ||||
| Chen 2022 [40] | Morbidity: 24/240 (10.0%) vs. 16/240 (6.7%); RDb -3.3% | Meanb 3292 vs. 3742; MDb -450 | Cost-effective; total cost–benefit: 108,022 EUR (2020) | ISPOR-Q: Sufficiently credible |
| Van Wijk 2020 [54] | Morbidity: not reported | 28,001 vs. 30,242; difference -2,241c | Unclear (effectiveness not reported); return of investment: 1.55 | ISPOR-Q: Insufficient information |
| Results from trial-based economic evaluations | ||||
| Results from CUAs | ||||
| AlShewaier 2016 [37] | QALYsd: Median 0.679 (IQR 0.10) vs. 0.573 (0.05); Difference in mediansb 0.106 | Median 20,790 vs. 19,952; difference in mediansb 838 | Unclear; incremental analysis required: ICER: 7906 EUR (2020) per QALY gained, but no WTP reported |
RoB 2: High CHEC: 13/19 items |
| Fernandes 2017 [42] | QALYsd: Mean 0.66 ± BS SE 0.04 vs. 0.61 ± 0.04e; MD 0.04 (95% CI 0.01 to 0.07) | Mean 17,432 ± BS SE 1265 vs. 17,574 ± 1480; MD -142 (95% CI -3952 to 3668) (331b when including patient expenses) | Cost-effective (unclear when including patient expenses); Probability of CEA at a WTP of 40,000 EUR: 84% (approx. 79% when including patient expenses) |
RoB 2: Some concerns CHEC: 16/19 items |
| Nguyen 2022 | QALYsd: Mean 0.7 ± SD 0.3 vs. 0.6 ± 0.3; MDb 0.1 | Mean 15,071 ± SD 7014 vs. 15,472 ± 6309; MD -401 | Cost-effective |
RoB: Some concerns (QALY), high (costs) CHEC: 9/19 items |
| Tveter 2020 [53] | QALYsd: Not reported per group; difference 0.07c | Not reported per group; difference -508c | Cost-effective |
RoB 2: Some concerns (QALY), high (costs) CHEC: 11/19 items |
| Results from CEAs | ||||
| McGregor 2004 [47] | HrQoL: EQ-5D-3L VAS (0–100%)d: mean 75.80 ± SD 14.86 vs. 72.15 ± 22.20f; MDb 3.65%, EQ-5D-3L utilitiesd: mean 0.72 ± SD 0.13 vs. 0.60 ± SD 0.31f; MDb 0.12 | Mean 4148 vs. 5005; MDb -856 | Cost-effective |
RoB 2: High CHEC: 8/19 items |
| Tew 2017 [52] | HrQoL: EQ-5D-5L utilitiesd: mean 0.837 vs. 0.760; MD 0.077 (95% CI 0.005 to 0.148) | BS mean 14,269 ± SD 3542 vs. 13,688 ± 3542; BS MD 582 (95% CI -1588 to 2848) | Unclear; incremental analysis required, but ICER not reported |
RoB 2: High CHEC: 14/19 items |
| Results from CCAs | ||||
| Barberan-Garcia 2019 [38] |
HrQoL: SF-36 PCSd: mean 47 ± SD 7 vs. 44 ± 8; MDb 3 SF-36 MCS scored: mean 51 ± SD 9 vs. 50 ± 9; MDb 1 Morbidity: 19/62 (30.6%) vs. 39/63 (61.9%); RDb -31.3% Mortality: 1/62 (1.6%) vs. 4/63 (6.3%); RDb -4.7% PROMs: YPAS scored: mean 46 ± SD 13 vs. 39 ± 15; MDb 7 HAD total score: mean 6 ± SD 5 vs. 8 ± 6; MDb -2 |
Mean 5428 (range 1792 to 30,532) vs. 6416 (1587 to 34,521); BS MD -955 (95% CI -3109 to 1033) | Cost-effective |
RoB 2: Some concerns (morbidity, mortality), high (PROMs, costs) CHEC: not applicable |
| Gao 2015 [43] | Morbidity: 12/71 (16.9%) vs. 59/71 (83.3%); RDb -66.4% | Mean 9728 ± SD 1130 vs. 8955 ± 888; MDb 773 | Unclear; incremental analysis required, but ICER not applicable for CCA |
ROBINS-I: Serious CHEC: not applicable |
| Gränicher 2020 [44] |
PROMs: Lysholm Scored: mean 87.1 ± SD 9.0 vs. 69.1 ± 14.9; MDb 18 Lysholm Score pain itemd: mean 25.0 ± SD 0.0 vs. 16.5 ± 9.1; MDb 8.5 Tegner Activity Scaled: mean 3.8 ± SD 0.8 vs. 2.5 ± 0.9; MDb 1.3 Physical function: Stair climbing test, time in seconds: mean 12.58 ± SD 4.64 vs. 13.59 ± 5.30; MDb -1.01 Knee ROM (degrees)d: mean 100.5 ± SD 18.7 vs. 103.5 13.7; MDb -3 |
Mean 3187 vs. 4052; MDb -865 | Unclear (inconsistent effectiveness); individual decision required |
RoB 2: Some concerns (PROMs, physical function), high (costs) CHEC: not applicable |
| Howard 2019 [30] |
Morbidity: 12/40 (30.0%) vs. 29/75 (38.7%); RDb -8.7% Mortality: 1/40 (2.5%) vs. 1/75 (1.3%); RDb 1.2% |
Mean 58,300 ± SD 42,590 vs. 75,248 ± 77,516; MDb (incorporating prehabilitation costs) -16,870 | Unclear (inconsistent effectiveness); individual decision required |
ROBINS-I: Serious CHEC: not applicable |
| Huang 2012 [45] |
Morbidity: Infection rate: 2/126 (1.6%) vs. 1/117 (0.9%); RDb 0.7% Rate of deep vein thrombosis: 5/126 (4.0%) vs. 3/117 (2.6%); RDb 1.4% PROMs: Pain (VAS): mean 2.4 ± SD 0.7 vs. 2.5 ± 0.6; MDb -0.1 Physical function: Knee ROM (degrees)d: mean 76 ± SD 22 vs. 74 ± 20; MDb 2 Ambulation statusd: 108/126 (85.7%) vs. 95/117 (81.2%); RDb 4.5% |
Mean 6726 ± SD 283 vs. 6841 ± SD 241; MDb (incorporating prehabilitation costs) -95 | Unclear (inconsistent effectiveness); individual decision required |
RoB 2: High CHEC: not applicable |
| Koh 2021 [46, 86] |
Morbidity: 24/58 (41.4%) vs. 11/23 (47.8%); RDb -6.4% Mortalityg: 0/58 (0%) vs. 0/23 (0%); RDb 0% |
Not reported per group; MD -2584 | Cost-effective |
ROBINS-I: Serious CHEC: not applicable |
| Lai 2017 [32] | Morbidity: 5/51 (9.8%) vs. 14/50 (28.0%); RDb -18.2% | Mean 7677 ± SD 1374 vs. 8608 ± 2482; MDb -931 | Cost-effective |
RoB 2: High CHEC: not applicable |
| Lai 2019 [33] | Morbidity: 4/34 (11.8%) vs. 12/34 (35.3%); RDb -23.5% | Median 10,456 (IQR 9683 to 11,339) vs. 11,285 (10,544 to 13,340); Difference in mediansb -830 | Cost-effective |
RoB 2: High CHEC: not applicable |
| Ploussard 2020 [50] | Mortality: 0/194 (0%) vs. 1/156 (0.6%); RDb -0.6% | Mean 2904 vs. 3282; MDb -379 | Cost-effective |
ROBINS-I: Serious CHEC: not applicable |
| Risco 2022 [51] | Morbidity: Comprehensive complications index: mean 15.1 ± SD 17.1 vs. 16.6 ± 16.9; MDb: -1.5 | Mean 7288 vs. 7142; MD 145 | Unclear; incremental analysis required, but ICER not applicable for CCA |
ROBINS-I: Serious CHEC: not applicable |
| Wang 2020 [55] |
Morbidity: 21/70 (30.0%) vs. 18/34 (52.9%); RD -22.9% Mortality: 1/70 (1.4%) vs. 1/34 (2.9%); RDb -1.5% PROMs (in subsample of n = 33 vs. n = 24): FACT-Hep scored: median 152 (range 102 to 179) vs. 148 (66 to 175); differences in mediansb 4 |
Median 6138 (IQR 4590 to 8833) vs. 7349 (5328 to 11,026); difference in medians -1210 | Cost-effective |
ROBINS-I: Serious CHEC: not applicable |
| Zhou 2017 [34] | Morbidity: 36/197 (18.3%) vs. 194/742 (26.1%); RDb: -7.8% | Not calculable in EUR (2020) as original currency not reported; original values: mean 7131.8 ± SD 2316.6 vs. 77,266.4 ± 1615.0; MDb -134.60 | Cost-effective |
ROBINS-I: Critical CHEC: not applicable |
| Results from CMAs | ||||
| Beaupre 2004 [39] | Not applicable | Mean 1285 ± SD 1196 vs. 1283 ± 1329; MD 2 | Not cost-effective |
RoB 2: High CHEC: not applicable |
| Englesbe 2017 [29] | Not applicable |
Provider perspective: median 16,900 (IQR 10,162 to 30,365) vs. 23,091 (14,993 to 39,017); difference in mediansb -6191 Payer perspective: median 19,216 (IQR 12,122 to 33,840) vs. 24,519 (17,057 to 37,243); difference in mediansb -5303 |
Cost-effective |
ROBINS-I: Serious CHEC: not applicable |
| Mouch 2019 [31] | Not applicable | Mean 24,435 ± SD 20,024 vs. 26,903 ± 24,935; MDb -2468 | Cost-effective |
ROBINS-I: Moderate CHEC: not applicable |
| Pham 2016 [49] | Not applicable | Reported only for a subset of patients (5/29 vs. 11/21): mean 5081 ± SD 298 vs. 5152 ± 656; MDb -71 | Cost-effective |
RoB 2: High CHEC: not applicable |
BS bootstrapped, CBA cost–benefit analysis, CCA cost–consequence analysis, CEA cost-effectiveness analysis, CG control group, CHEC Consensus on Health Economic Criteria, CMA cost-minimisation analysis, CUA cost–utility analysis, EQ-5D-3L EuroQoL 5 dimensions 3 levels, EQ-5D-5L EuroQoL 5 dimensions 5 levels, FACT-Hep Functional Assessment of Cancer Therapy—Hepatobiliary, HAD Hospital Anxiety and Depression Scale, IG intervention group, ICER incremental cost-effectiveness ratio, ISPOR-Q International Society for Pharmacoeconomics and Outcomes Research Questionnaire, IQR interquartile range, MD mean difference, PROMs patient-reported outcome measures, QALY quality-adjusted life-year, HrQoL health-related quality of life, RD relative difference, RoB 2 revised Cochrane risk-of-bias tool for randomised trials, ROBINS-I tool for assessing risk of bias in non-randomised studies of interventions, ROM range of motion, SD standard deviation, SF-36 Short Form 36, VAS visual analogue scale, YPAS Yale Physical Activity Scale
a ‘Cost-effective’ if better effectiveness and same/lower costs, or same effectiveness and lower costs; ‘unclear’ if better effectiveness and higher costs, or same effectiveness and same costs, or inconsistent/poorer effectiveness and lower costs; ‘not cost-effective’ if same effectiveness and higher costs, or poorer effectiveness and same/higher costs
b Calculated by review authors
c Measure of central tendency (mean, median) not reported
d Higher values indicating higher benefit
e When missing values were imputed by linear trend at point and adjusted for baseline EQ-5D-3 L scores
f Values were obtained through author contact
g Survival was also calculated but not mentioned in the methods as an outcome
Results of synthesis
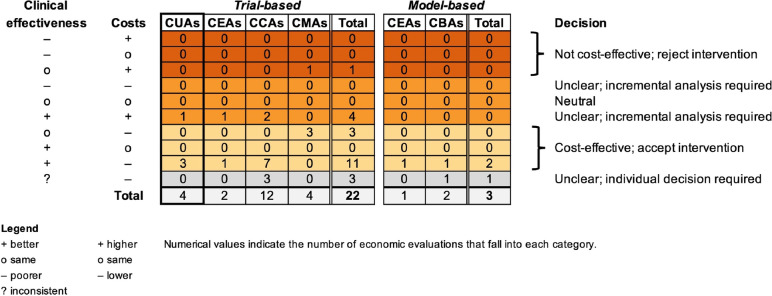
Four trial-based EEs [37, 42, 48, 53] reported data on the primary outcome, i.e. cost-effectiveness based on CUA (Fig. 2, thick bordered column). Based on direction of effects, three CUAs (75%) fell into the cost-effective category, and one fell into the category ‘unclear; incremental analysis required’. The ICER of the latter study was 7906 EUR (2020) per quality-adjusted life year (QALY) gained, which is likely acceptable under common willingness-to-pay (WTP) thresholds [37].
Fig. 2.
Hierarchical permutation matrix presenting the results vote counting based on direction of effects
Three model-based and 18 trial-based EEs reported on the secondary outcomes (Fig. 2), i.e. cost-effectiveness based on other types of EEs, respectively. Based on direction of effects, two model-based EEs fell into the cost-effective category [40, 41], but one was judged insufficiently credible [41]. The remaining model-based EE fell into the category ‘unclear; individual decision required’ [54]. Of the trial-based EEs, 11 fell into the cost-effective category [29, 31–34, 38, 46, 47, 49, 50, 55], one of which was judged to be of critical risk of bias [34], three into the category ‘unclear; incremental analysis required’ [43, 51, 52], three into the category ‘unclear; individual decision required’ [30, 44, 45], and one, a CMA with a difference in total costs of + 2 EUR (2020), into the not cost-effective category [39].
Overall, 16/25 (64.0%) EEs found prehabilitation cost-effective based on direction of effects, (14/23; 60.9% when excluding the EEs of insufficient credibility/critical risk of bias [34, 41]), in 8/25 (32.0%) it was unclear, and one EE (4.0%) found prehabilitation not cost-effective [39]. Descriptive post hoc subgroup analyses revealed heterogeneity in the cost-effectiveness results depending on the population, intervention and methods, but not on conflict of interest and funding source (Additional file 1: Appendix 13). Briefly, cost-effectiveness was more frequently observed in EEs of cancer patients, patients with a high perioperative risk, multimodal programmes, home-based or inpatient prehabilitation, shorter programmes, low-cost programmes and EEs taking a mix of payer/provider perspective.
Publication bias
There was a relevant risk of publication bias regarding the included completed EEs. Firstly, the review had initially included 74 study reports belonging to 54 unique studies. However, ten reports referring to nine unique studies were excluded post hoc [76–85] (see Additional file 1: Appendix 4), of which four protocols [77–80], one registration record [85] and a conference abstract [81] referred to studies that no longer reported on costs in the study publication [87–95]. The authors of two studies confirmed that no economic evaluation was performed [78, 80]. The remaining authors did not respond.
In comparison to the results of the overview by McIsaac et al. 2022 [6], the included EEs on cancer surgery showed more beneficial results regarding morbidity [32–34, 43, 46, 55] and mortality [41, 50, 55], and the included EEs on orthopaedic surgery showed more beneficial results on health-related quality of life (HrQoL) [37, 42, 47, 48, 53]. Apart from that, results were comparable but, overall, the included EEs’ results appear more beneficial suggesting a risk of publication bias.
Discussion
This is the first comprehensive systematic review on the cost-effectiveness of prehabilitation prior to elective surgery including 25 completed and 20 ongoing EEs. Using vote-counting based on direction of effects, the majority of completed EEs found prehabilitation cost-effective, including three CUAs, and only one EE favoured usual care. However, most EEs were of high risk of bias and/or low methodological quality, and we identified a relevant risk of publication bias. Furthermore, the included EEs were heterogeneous in their population, intervention and methods. Therefore, our results should be interpreted with caution. An update of this review might lead to more definite evidence, as it should include at least eight more completed CUAs [58, 59, 62–66].
Cost-effectiveness depended on the population and intervention, with certain groups (e.g. cancer- or high-risk patients) and programmes (e.g. shorter, home-based prehabilitation) resulting more frequently in benefit. Among the included EEs, there was a high variability in populations, whose underlying diseases and surgeries differed in concept (e.g. restoration in orthopaedic surgery and cure in cancer surgery). It is possible that for orthopaedic patients, the restoring character of the surgery might be the crucial element in the recovery of both groups, although the modalities of prehabilitation may also serve as a conservative therapy option for certain orthopaedic patients, delaying or even eliminating the need for surgery [49, 53]. Of course, for other patient groups, cure through prehabilitation is not possible, e.g. for cancer patients whose disease cannot be improved in itself by prehabilitation. Lastly, it might be more (cost-)effective to focus on patients with low functional capacity [96] who are at high-risk for adverse perioperative outcomes because of factors such as old age, relevant co-morbidities [4] and frailty [97], as these patients much room for preoperative improvement.
Our review also showed great variability in the programme modalities, ‘dose’ (i.e. frequency, intensity and duration) and delivery settings. As a result, the programme costs ranged from below 100 EUR (2020) per patient in six (mainly home-based) EEs [29–31, 45, 47, 48] to above 1000 EUR (2020) in two EEs [52, 54]. Although prehabilitation is usually defined as a multi-modal approach, it is not yet clear what intervention designs are most effective and whether they in fact need to be multimodal [6]. For example, in certain indications, a unimodal intervention, such as preoperative breathing exercises, would likely be less costly and hence could turn out to be more cost-effective.
The dose–response relationship of prehabilitation programmes is a crucial aspect for programme effectiveness and depends largely on the length of the preoperative period available for prehabilitation. This again depends on the underlying diseases and how fast these are progressing, i.e. patients with slowly progressing diseases, such as osteoarthritis, can generally wait longer than those with more rapidly progressing diseases, such as most cancer types, who should often be operated within a few weeks following diagnosis [98]. However, cancer patients undergoing neoadjuvant treatment before surgery may be ideal candidates for prehabilitation [99, 100]. Similarly, the waiting period for patients on organ transplant lists may present a window of opportunity to implement a prehabilitation programme [101], and waiting lists in general may aid the early identification of eligible patients [102].
The dose of prehabilitation is also determined by the intensity of individual sessions which must be sufficiently high to have an effect while being tolerable for the target population [103]. Although there were few adverse events directly related to prehabilitation, some EEs reported that patients from the intervention group dropped out due to high-intensity [32–34]. Programmes must be designed in a way to facilitate high adherence rates and thus cost-effectiveness [104]. For instance, offering home-based options may reduce issues regarding transportation, which was found to be a central barrier to adherence to prehabilitation [105]. Though not considered specifically in this review, telemedicine is likely to play an important role in the provision of prehabilitation as well.
Limitations
Some limitations on review and study level apply. First, we could not perform a meta-analysis but had to resort to narrative synthesis in the form of vote counting based on direction of effects. This synthesis method does not provide any information on the magnitude of effects, nor does it account for the EEs’ sample sizes [106]. Second, the review’s broad inclusion criteria led to a large number of included articles that we coined ‘EEs’ for the purpose of the review. However, most of them were trial reports including cost outcomes which understood themselves as pilot and/or feasibility trials and thus did not apply comprehensive EE methods. Third, as there currently is no universally recognised definition of prehabilitation [6] nor common concepts, procedures or measurements [4], the definition of the prehabilitation elements varied between the EEs. The definition of usual care also varied across EEs. For instance, advice on physical activity and smoking cessation were included as standard care in some EEs [38, 50, 51], while those aspects were part of prehabilitation in other EEs [29–31, 40, 41, 54]. Lastly, characteristics of health systems, such as the type of financing (public vs. private) and organisation of care (centralised vs. decentralised), play a crucial role in programme delivery and cost justification. As we did not formally assess the generalisability and transferability of our results to different health systems, we recommend policy-makers interested in implementing prehabilitation to conduct a health technology assessment (HTA) for their government.
Limitations on the study level included the high risk of bias and low methodological quality of the included EEs. However, the exclusion of the two EEs judged to be insufficiently credible/of critical risk of bias only had a small effect on the results. Furthermore, there was a high risk of publication bias associated with trial-based EEs. Trial-based EEs are by nature prone to a specific form of publication bias, namely conduct bias [107], meaning they are not published because they were never performed in the first place, e.g. when the underlying trial was ‘inconclusive’ or had negative results. Although an intervention that is less effective but cheaper than the control may still be cost-effective, it is generally not acceptable from an ethical and quality of care perspective to replace usual care with a less-effective intervention.
Implications for practice and policy
Owing to the limitations described above, our results should be interpreted with caution. As many EEs were based on prospective trials, decision-makers must also consider the possibility that there was a motivational bias among the participants and that the cost-effectiveness of prehabilitation may be lower under ‘real world’ circumstances. Before implementing prehabilitation into routine care, decision-makers should assess potential barriers and facilitators [108, 109], which may differ between health systems and stakeholders, or even individuals. For example, qualitative studies found that group prehabilitation was perceived both as a barrier and facilitator [110, 111]. In their framework for prehabilitation services, Bates et al. 2020 list several considerations for the implementation of prehabilitation, including to involve patients when designing the prehabilitation programme [112].
Finally, decision-makers must determine which patient population(s) should receive prehabilitation and establish screening pathways, accessibility to the programme and strategies to ensure sustainability [113]. This involves performing a budget impact analysis, including the one-time investments into infrastructure (e.g. prehabilitation centres) as well as the running costs for the provision of prehabilitation and maintenance of the infrastructure. Although many EEs found that prehabilitation paid off during the index hospitalisation, the pervasive shortage of health care professionals [114] may hinder implementation of prehabilitation.
Implications for future research
First, future research should address the knowledge gaps discussed above, i.e. which populations benefit most and what the optimal prehabilitation programme for those populations is. If a broadly defined population is included in a clinical trial, it is recommended to consider pre-specified subgroup analyses for economic evaluation [115]. To ensure added value, new clinical research should consider the existing evidence [116] as well as involve patients and stakeholders in all phases of research [117], e.g. when designing the prehabilitation programme [118]. Ideally, these efforts would result in a clinical practice guideline for prehabilitation, the first step of which was taken by Tew et al. 2018 with a guideline on preoperative exercise training in patients awaiting major noncardiac surgery [119].
Second, future research should address the shortcomings of existing EEs. Common issues included inadequate reporting, short time horizons, and the use of limited perspectives. Reporting guidelines are intended to support authors and increase the accuracy and transparency of reporting, but they are frequently used inappropriately, including those for EEs [120]. In our review, reporting guidelines for EEs seemed to have been under-used, as none of the full EEs published as full-text articles after 2013 [37, 40–42, 48, 52] reported following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [121], which is applicable to both trial- and model-based EEs. A possible reason is that only two trial-based EEs were published as separate full-text articles [38, 42]. Hence, we recommend that authors publish full EEs as separate articles and follow the latest version of the CHEERS checklist [122].
Many EEs had a short time horizon of 1 month or less. However, as argued by Grocott and Ludbrook 2019, ‘it is plausible that improved fitness arising from prehabilitation might have a further lingering positive impact on the need for later care’ [123]. Such an impact can only be detected using a longer time horizon but, in our review, only five EEs [39, 41, 42, 48, 53] had a time horizon of 12 months or more. To determine an adequately long-time horizon, we recommend authors to consult guidelines from their national HTA institutes and by the ISPOR [115]. On a closely-related matter, many EEs applied limited perspectives, such as the provider perspective, with the hospital being the provider, and therefore did not consider post-discharge or out-of-hospital resource use. None of the included EEs applied a full societal perspective including costs from other sectors, e.g. productivity loss. When this is not feasible, we suggest that authors adopt a comprehensive health sector perspective including all relevant payers and providers. For example, EEs may consider improved access for other patients through freed-up capacity, e.g. due to earlier discharge of prehabilitated patients [124]. In summary, future EEs should be performed over a longer time horizon and apply a more comprehensive perspective.
Update of the review
We plan to update the review upon publication of our own economic evaluation [59] in 2025/26 by re-running the search strategies modified only by adding the MeSH/Emtree term ‘Preoperative Exercise’.
Conclusions
We found some evidence that prehabilitation for patients awaiting elective surgery is cost-effective compared to usual preoperative care. Cost-effectiveness based on direction of effect was more frequently observed for cancer patients, patients with a high perioperative risk and for low-cost (shorter or home-based) programmes. However, the results should be interpreted with caution as most EEs were of high risk of bias and/or low methodological quality, and we suspect a relevant risk of publication bias. Future research should address clinical knowledge gaps surrounding prehabilitation, e.g. which populations benefit most, as well as the shortcomings of existing EEs, e.g. by adopting a societal perspective.
Supplementary Information
Additional file 1: App1. Important changes made to the protocol. App2. Search strategies. App3. Data items. App4. List of excluded studies. App5. Characteristics of ongoing economic evaluations. App6. Funding and competing interest of included economic evaluations. App7. Methods of completed economic evaluations. App8. Methods of ongoing economic evaluations with a published protocol. App9. Description of prehabilitation programmes in ongoing studies. App10. Risk of bias and methodological quality of included economic evaluations. App11. Detailed costs results of included economic evaluations. App12. Results of adherence and safety outcomes. App13. Results of descriptive post-hoc subgroup analyses to explore heterogeneity in cost-effectiveness results.
Acknowledgements
Not applicable.
Abbreviations
- CBA
Cost-benefit analysis
- CCA
Cost-consequence analysis
- CEA
Cost-effectiveness analysis
- CHEC
Consensus on Health Economic Criteria
- CHEERS
Consolidated Health Economic Evaluation Reporting Standards
- CMA
Cost-minimisation analysis
- CRD
Centre for Reviews and Dissemination
- CUA
Cost-utility analysis
- EE
Economic evaluation
- ERAS
Enhanced recovery after surgery
- HrQoL
Health-related quality of life
- HTA
Health technology assessment
- ICER
Incremental cost-effectiveness ratio
- ICTRP
International Clinical Trials Registry Platform
- ISPOR
International Society for Pharmacoeconomics and Outcomes Research
- NRSI
Non-randomised study of an intervention
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
Quality-adjusted life year
- RCT
Randomised controlled trial
- ROB
Risk of bias
- ROBINS-I
Risk of bias in non-randomised studies of interventions
- WHO
World Health Organization
Authors’ contributions
TR, HE and JS collected and analysed the data. All authors interpreted the data. JK performed content assessments of the prehabilitation programmes. TM provided specific methodological expertise regarding systematic reviews on economic evaluations. WQ supervised the review process. TR was a major contributor in writing the manuscript draft. All authors edited the manuscript draft and have read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The systematic review is part of a larger project which is supported by the Innovation Fund coordinated by the Innovation Committee of the Federal Joint Committee in Germany (Innovationsausschuss beim Gemeinsamen Bundesausschuss (G-BA)), grant number 01NVF18024. The funders had no role in planning or conduct of the review nor in the decision to submit the results for publication.
Availability of data and materials
All raw data collected as part of the review are deposited in the Open Science Framework (OSF) [20].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
TR, HE, JS, JK and WQ are involved in one of the included ongoing economic evaluations [59]. TM declares to have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grimmett C, Bradbury K, Dalton SO, Fecher-Jones I, Hoedjes M, Varkonyi-Sepp J, et al. The role of behavioral science in personalized multimodal prehabilitation in cancer. Front Psychol. 2021;12:634223. doi: 10.3389/fpsyg.2021.634223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin. 2015;33(1):17–33. doi: 10.1016/j.anclin.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Gillis C, Ljungqvist O, Carli F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br J Anaesth. 2022;128(3):434–448. doi: 10.1016/j.bja.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Gurlit S, Gogol M. Prehabilitation is better than cure. Curr Opin Anaesthesiol. 2019;32(1):108–115. doi: 10.1097/ACO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 5.Scheede-Bergdahl C, Minnella EM, Carli F. Multi-modal prehabilitation: addressing the why, when, what, how, who and where next? Anaesthesia. 2019;74(Suppl 1):20–26. doi: 10.1111/anae.14505. [DOI] [PubMed] [Google Scholar]
- 6.McIsaac DI, Gill M, Boland L, Hutton B, Branje K, Shaw J, et al. Prehabilitation in adult patients undergoing surgery: an umbrella review of systematic reviews. Br J Anaesth. 2022;128(2):244–257. doi: 10.1016/j.bja.2021.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Christensen JF, Simonsen C, Hojman P. Exercise training in cancer control and treatment. Compr Physiol. 2018;9(1):165–205. [DOI] [PubMed]
- 8.Ng P, Lee JKD, Tan KY. Finding value with prehabilitation in older persons receiving surgery. Curr Opin Support Palliat Care. 2022;16(1):19–24. doi: 10.1097/SPC.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 9.Zhong JX, Kang K, Shu XL. Effect of nutritional support on clinical outcomes in perioperative malnourished patients: a meta-analysis. Asia Pac J Clin Nutr. 2015;24(3):367–378. doi: 10.6133/apjcn.2015.24.3.20. [DOI] [PubMed] [Google Scholar]
- 10.Gometz A, Maislen D, Youtz C, Kary E, Gometz EL, Sobotka S, et al. The effectiveness of prehabilitation (prehab) in both functional and economic outcomes following spinal surgery: a systematic review. Cureus. 2018;10(5):e2675. doi: 10.7759/cureus.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coderre D, Brahmbhatt P, Hunter TL, Baima J. Cancer prehabilitation in practice: the current evidence. Curr Oncol Rep. 2022;24(11):1569–1577. doi: 10.1007/s11912-022-01304-1. [DOI] [PubMed] [Google Scholar]
- 12.Perry R, Herbert G, Atkinson C, England C, Northstone K, Baos S, et al. Pre-admission interventions (prehabilitation) to improve outcome after major elective surgery: a systematic review and meta-analysis. BMJ Open. 2021;11(9):e050806. doi: 10.1136/bmjopen-2021-050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
- 14.van Mastrigt GAPG, Hiligsmann M, Arts JJC, Broos PH, Kleijnen J, Evers SMAA, et al. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: a five-step approach (part 1/3) Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):689–704. doi: 10.1080/14737167.2016.1246960. [DOI] [PubMed] [Google Scholar]
- 15.Wijnen B, Van Mastrigt G, Redekop WK, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3) Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):723–732. doi: 10.1080/14737167.2016.1246961. [DOI] [PubMed] [Google Scholar]
- 16.Mandrik O, Severens JL, Bardach A, Ghabri S, Hamel C, Mathes T, et al. Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ispor good practices task force report. Value Health. 2021;24(4):463–472. doi: 10.1016/j.jval.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rombey T, Eckhardt H, Kiselev J, Silzle J, Mathes T, Quentin W. Cost-effectiveness of prehabilitation prior to elective surgery: A systematic review of economic evaluations. OSF. 2023. 10.17605/OSF.IO/W3B4Y. [DOI] [PMC free article] [PubMed]
- 21.Rombey T, Eckhardt H, Quentin W. Cost-effectiveness of prehabilitation prior to elective surgery compared to usual preoperative care: protocol for a systematic review of economic evaluations. BMJ Open. 2020;10(12):e040262. doi: 10.1136/bmjopen-2020-040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arber M, Glanville J, Isojarvi J, Baragula E, Edwards M, Shaw A, et al. Which databases should be used to identify studies for systematic reviews of economic evaluations? Int J Technol Assess Health Care. 2018;34(6):547–554. doi: 10.1017/S0266462318000636. [DOI] [PubMed] [Google Scholar]
- 23.Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy. 2010;6(1):51–59. doi: 10.1332/174426410X482999. [DOI] [Google Scholar]
- 24.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:I4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 27.Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care. 2005;21(2):240–245. doi: 10.1017/S0266462305050324. [DOI] [PubMed] [Google Scholar]
- 28.Jaime Caro J, Eddy DM, Kan H, Kaltz C, Patel B, Eldessouki R, et al. Questionnaire to assess relevance and credibility of modeling studies for informing health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health. 2014;17(2):174–182. doi: 10.1016/j.jval.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Englesbe MJ, Grenda DR, Sullivan JA, Derstine BA, Kenney BN, Sheetz KH, et al. The Michigan Surgical Home and Optimization Program is a scalable model to improve care and reduce costs. Surgery. 2017;161(6):1659–1666. doi: 10.1016/j.surg.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Howard R, Yin YS, McCandless L, Wang S, Englesbe M, Machado-Aranda D. Taking control of your surgery: impact of a prehabilitation program on major abdominal surgery. J Am Coll Surg. 2019;228(1):72–80. doi: 10.1016/j.jamcollsurg.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouch CA, Kenney BC, Lorch S, Montgomery JR, Gonzalez-Walker M, Bishop K, et al. Statewide prehabilitation program and episode payment in medicare beneficiaries. J Am Coll Surg. 2020;230(3):306–13.e6. doi: 10.1016/j.jamcollsurg.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Lai Y, Su J, Qiu P, Wang M, Zhou K, Tang Y, et al. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: a randomized trial. Interact Cardiovasc Thorac Surg. 2017;25(3):476–483. doi: 10.1093/icvts/ivx141. [DOI] [PubMed] [Google Scholar]
- 33.Lai Y, Wang X, Zhou K, Su J, Che G. Impact of one-week preoperative physical training on clinical outcomes of surgical lung cancer patients with limited lung function: a randomized trial. Ann Transl Med. 2019;7(20):544. doi: 10.21037/atm.2019.09.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou K, Su J, Lai Y, Li P, Li S, Che G. Short-term inpatient-based high-intensive pulmonary rehabilitation for lung cancer patients: is it feasible and effective? J Thorac Dis. 2017;9(11):4486–4493. doi: 10.21037/jtd.2017.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor. Cochrane Handbook for Systematic Reviews of Interventions version 63 (updated February 2022): Cochrane; 2022. Available from www.training.cochrane.org/handbook.
- 36.Nixon J, Khan KS, Kleijnen J. Summarising economic evaluations in systematic reviews: a new approach. BMJ. 2001;322(7302):1596–1598. doi: 10.1136/bmj.322.7302.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alshewaier SA. Developing a standardised pre- operative physiotherapy programme to improve the outcomes of patients undergoing anterior cruciate ligament reconstruction in Riyadh (KSA) Manchester: Manchester Metropolitan University; 2016. [Google Scholar]
- 38.Barberan-Garcia A, Ubre M, Pascual-Argente N, Risco R, Faner J, Balust J, et al. Post-discharge impact and cost-consequence analysis of prehabilitation in high-risk patients undergoing major abdominal surgery: secondary results from a randomised controlled trial. Br J Anaesth. 2019;123(4):450–456. doi: 10.1016/j.bja.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Beaupre LA, Lier D, Davies DM, Johnston DB. The effect of a preoperative exercise and education program on functional recovery, health related quality of life, and health service utilization following primary total knee arthroplasty. J Rheumatol. 2004;31(6):1166–1173. [PubMed] [Google Scholar]
- 40.Chen MMZ, Sibley D, Au D, Alibhai SMH, Karkouti K, Randall IM, et al. Is the integration of prehabilitation into routine clinical practice financially viable? A financial projection analysis. Curr Anesthesiol Rep. 2022;12(1):166–176. doi: 10.1007/s40140-021-00506-w. [DOI] [Google Scholar]
- 41.Dholakia J, Cohn DE, Straughn JM, Dilley SE. Prehabilitation for medically frail patients undergoing surgery for epithelial ovarian cancer: a cost-effectiveness analysis. J Gynecol Oncol. 2021;32(6):e92. doi: 10.3802/jgo.2021.32.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes L, Roos EM, Overgaard S, Villadsen A, Søgaard R. Supervised neuromuscular exercise prior to hip and knee replacement: 12-month clinical effect and cost-utility analysis alongside a randomised controlled trial. BMC Musculoskelet Disord. 2017;18(1):5. doi: 10.1186/s12891-016-1369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao K, Yu PM, Su JH, He CQ, Liu LX, Zhou YB, et al. Cardiopulmonary exercise testing screening and pre-operative pulmonary rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: A study for 342 cases. Thorac Cancer. 2015;6(4):443–449. doi: 10.1111/1759-7714.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gränicher P, Stöggl T, Fucentese SF, Adelsberger R, Swanenburg J. Preoperative exercise in patients undergoing total knee arthroplasty: a pilot randomized controlled trial. Arch Physiother. 2020;10:13. doi: 10.1186/s40945-020-00085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang SW, Chen PH, Chou YH. Effects of a preoperative simplified home rehabilitation education program on length of stay of total knee arthroplasty patients. Orthop Traumatol Surg Res. 2012;98(3):259–264. doi: 10.1016/j.otsr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Koh FH, Loh CH, Tan WJ, Ho LML, Yen D, Chua JMW, et al. Structured presurgery prehabilitation for aged patients undergoing elective surgery significantly improves surgical outcomes and reduces cost: a nonrandomized sequential comparative prospective cohort study. Nutr Clin Pract. 2021;37(3):1–9. [DOI] [PMC free article] [PubMed]
- 47.McGregor AH, Rylands H, Owen A, Doré CJ, Hughes SP. Does preoperative hip rehabilitation advice improve recovery and patient satisfaction? J Arthroplasty. 2004;19(4):464–468. doi: 10.1016/j.arth.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen C, Boutron I, Roren A, Anract P, Beaudreuil J, Biau D, et al. Effect of prehabilitation before total knee replacement for knee osteoarthritis on functional outcomes: a randomized clinical trial. JAMA Netw Open. 2022;5(3):e221462. doi: 10.1001/jamanetworkopen.2022.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pham JM. Community-based prehabilitation program: a pilot study exploring the impact of exercise and education programs on functional mobility pre-surgery and on length of stay post-total joint arthroplasty. Sudbury: Laurentian University; 2016. [Google Scholar]
- 50.Ploussard G, Almeras C, Beauval JB, Gautier JR, Garnault V, Frémont N, et al. A combination of enhanced recovery after surgery and prehabilitation pathways improves perioperative outcomes and costs for robotic radical prostatectomy. Cancer. 2020;126(18):4148–4155. doi: 10.1002/cncr.33061. [DOI] [PubMed] [Google Scholar]
- 51.Risco R, González-Colom R, Montané-Muntané M, Cano I, Vela E, Sebio R, et al. Actionable factors fostering health value generation and scalability of prehabilitation: a prospective cohort study. Ann Surg. 2022;278(2):e217–e225. doi: 10.1097/SLA.0000000000005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tew GA, Batterham AM, Colling K, Gray J, Kerr K, Kothmann E, et al. Randomized feasibility trial of high-intensity interval training before elective abdominal aortic aneurysm repair. Br J Surg. 2017;104(13):1791–1801. doi: 10.1002/bjs.10669. [DOI] [PubMed] [Google Scholar]
- 53.Tveter A, Kleven L, Østerås N, Nossum R, Eide R, Klokkeide Å, et al. Cost-utility analysis of multimodal occupational therapy in patients with thumb base osteoarthritis. Osteoarthritis Cartilage. 2020;28:S439. doi: 10.1016/j.joca.2020.02.683. [DOI] [Google Scholar]
- 54.van Wijk L, Bos J, Klaase JM. Prehabilitation worth it? A theoretical algorithmic for the cost-effectiveness of a multimodal prehabilitation program for complex abdominal surgery. HPB. 2020;22(Supplement 1):S64. doi: 10.1016/j.hpb.2020.04.525. [DOI] [Google Scholar]
- 55.Wang B, Shelat VG, Chow JJL, Huey TCW, Low JK, Woon WWL, et al. Prehabilitation program improves outcomes of patients undergoing elective liver resection. J Surg Res. 2020;251:119–125. doi: 10.1016/j.jss.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Barberan-Garcia A, Navarro-Ripoll R, Sánchez-Lorente D, Moisés-Lafuente J, Boada M, Messaggi-Sartor M, et al. Cost-effectiveness of a technology-supported multimodal prehabilitation program in moderate-to-high risk patients undergoing lung cancer resection: randomized controlled trial protocol. BMC Health Serv Res. 2020;20(1):207. doi: 10.1186/s12913-020-05078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coca-Martinez M, Lopez-Hernandez A, Montane-Muntane M, Arguis MJ, Gimeno-Santos E, Navarro-Ripoll R, et al. Multimodal prehabilitation as strategy for reduction of postoperative complications after cardiac surgery: a randomised controlled trial protocol. BMJ Open. 2020;10(12):e039885. doi: 10.1136/bmjopen-2020-039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pufulete M, Levett D, Rogers C, Grocott M, Reeves B, McConnell A, et al. Effectiveness and cost-effectiveness of INSPIRatory musclE training (IMT) for reducing postoperative pulmonary complications (PPC): a sham-controlled randomised controlled trial (RCT) (INSPIRE) (version Aug 13, 2020). Bristol: University of Bristol; 2020. Available from: https://fundingawards.nihr.ac.uk/award/16/140/07.
- 59.Schaller SJ, Kiselev J, Loidl V, Quentin W, Schmidt K, Mörgeli R, et al. Prehabilitation of elderly frail or pre-frail patients prior to elective surgery (PRAEP-GO): study protocol for a randomized, controlled, outcome assessor-blinded trial. Trials. 2022;23(1):468. doi: 10.1186/s13063-022-06401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheill G, Guinan E, O'Neill L, Normand C, Doyle SL, Moore S, et al. Preoperative exercise to improve fitness in patients undergoing complex surgery for cancer of the lung or oesophagus (PRE-HIIT): protocol for a randomized controlled trial. BMC Cancer. 2020;20(1):321. doi: 10.1186/s12885-020-06795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamp N, McCann M, Maiorana A, Ngui A, Quested E, Ntomanis N, et al. M22 cardiac prehabilitation: a home-based program. Heart Lung Circ. 2021;30:S10. [Google Scholar]
- 62.Steffens D, Young J, Riedel B, Morton R, Denehy L, Heriot A, et al. PRehabIlitatiOn with pReoperatIve exercise and educaTion for patients undergoing major abdominal cancer surgerY: protocol for a multicentre randomised controlled TRIAL (PRIORITY TRIAL) BMC Cancer. 2022;22(1):443. doi: 10.1186/s12885-022-09492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svinøy OE, Bergland A, Risberg MA, Pripp AH, Hilde G. Better before-better after: efficacy of prehabilitation for older patients with osteoarthritis awaiting total hip replacement-a study protocol for a randomised controlled trial in South-Eastern Norway. BMJ Open. 2019;9(12):e031626. doi: 10.1136/bmjopen-2019-031626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98. doi: 10.1186/s12885-018-5232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.West M, Bates A, Grimmett C, Allen C, Green R, Hawkins L, et al. The Wessex Fit-4-Cancer Surgery Trial (WesFit): a protocol for a factorial-design, pragmatic randomised-controlled trial investigating the effects of a multi-modal prehabilitation programme in patients undergoing elective major intra-cavity cancer surgery. F1000Res. 2021;10:952. [DOI] [PMC free article] [PubMed]
- 66.Yau DKW, Wong MKH, Wong WT, Gin T, Underwood MJ, Joynt GM, et al. PREhabilitation for improving QUality of recovery after ELective cardiac surgery (PREQUEL) study: protocol of a randomised controlled trial. BMJ Open. 2019;9(5):e027974. doi: 10.1136/bmjopen-2018-027974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diaz-Feijoo B. SOPHIE Trial: Surgery in ovarian cancer with prehabilitation in ERAS (NCT04862325). Bethesda: NIH U.S. National Library of Medicine; 2021. Available from: https://ClinicalTrials.gov/show/NCT04862325.
- 68.Hu L. The clinical study of prehabilitation to improve the prognosis of frail patients undergoing gastretomy for adenocarcinoma: a single-center randomized controlled study (ChiCTR2100042131). Chengdu: Chinese Clinical Trial Registry; 2021. Available from: http://www.chictr.org.cn/showproj.aspx?proj=66739.
- 69.Lönnroos E. Interprofessional Preoperative Geriatric Assessment for Older Arthroplasty Patients With Multimorbidity (NCT04001699). Bethesda: NIH U.S. National Library of Medicine; 2019. Available from: https://ClinicalTrials.gov/show/NCT04001699.
- 70.Molenaar C. Multimodal prehabilitation in NSCLC patients undergoing surgery (NL8080). Amsterdam: Nederlands Trialregister; 2019. Available from: https://trialregister.nl/trial/8080.
- 71.Ottawa Hospital Research Institute. The PREPARE trial: exercise before surgery to improve recovery in older people with frailty (NCT04221295). Bethesda: NIH U.S. National Library of Medicine; 2020. Available from: https://ClinicalTrials.gov/show/NCT04221295.
- 72.Santa Mina D. Prehab for Surgery (NCT04155346) 2019. Available from: https://ClinicalTrials.gov/show/NCT04155346.
- 73.Strijker D. Multimodal intensive prehabilitation in high impact surgery to reduce postoperative complications (NL8699). Amsterdam: Nederlands Trialregister; 2020. Available from: https://trialregister.nl/trial/8699.
- 74.Uchoa Rezende M. PARQVE prior to total knee replacement (NCT04017858). Bethesda: NIH U.S. National Library of Medicine; 2019. Available from: https://ClinicalTrials.gov/show/NCT04017858.
- 75.University Hospital Grenoble. Patient empowerment for major surgery preparation @ home (NCT04190719). Bethesda: NIH U.S. National Library of Medicine; 2019. Available from: https://ClinicalTrials.gov/show/NCT04190719.
- 76.McIsaac DI, Saunders C, Hladkowicz E, Bryson GL, Forster AJ, Gagne S, et al. PREHAB study: a protocol for a prospective randomised clinical trial of exercise therapy for people living with frailty having cancer surgery. BMJ Open. 2018;8(6):e022057. doi: 10.1136/bmjopen-2018-022057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berkel AEM, Bongers BC, van Kamp MS, Kotte H, Weltevreden P, de Jongh FHC, et al. The effects of prehabilitation versus usual care to reduce postoperative complications in high-risk patients with colorectal cancer or dysplasia scheduled for elective colorectal resection: study protocol of a randomized controlled trial. BMC Gastroenterol. 2018;18(1):29. doi: 10.1186/s12876-018-0754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blasco JM, Igual-Camacho C, Roig-Casasús S. In-home versus hospital preoperative balance and proprioceptive training in patients undergoing TKR; rationale, design, and method of a randomized controlled trial. BMC Musculoskelet Disord. 2017;18(1):518. doi: 10.1186/s12891-017-1887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janssen TL, Mosk CA, van Hoof-de LC, Wielders D, Seerden TCJ, Steyerberg EW, et al. A multicomponent prehabilitation pathway to reduce the incidence of delirium in elderly patients in need of major abdominal surgery: study protocol for a before-and-after study. BMC Geriatr. 2019;19(1):87. doi: 10.1186/s12877-019-1101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merki-Künzli C, Kerstan-Huber M, Switalla D, Gisi D, Raptis DA, Greco N, et al. Assessing the value of prehabilitation in patients undergoing colorectal surgery according to the enhanced recovery after surgery (ERAS) pathway for the improvement of postoperative outcomes: protocol for a randomized controlled trial. JMIR Res Protoc. 2017;6(10):e199. doi: 10.2196/resprot.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halliday L, Doganay E, Lada H, Wynter-Blyth V, Hanna G, Moorthy K. Prehabilitation in oesophago-gastric cancer: the impact on post-operative outcomes. Br J Surg. 2019;106(Supplement 5):79. [Google Scholar]
- 82.McIsaac DI, Hladkowicz E, Bryson GL, Forster AJ, Gagne S, Huang A, et al. Home-based prehabilitation with exercise to improve postoperative recovery for older adults with frailty having cancer surgery: the PREHAB randomised clinical trial. Br J Anaesth. 2022;129(1):41–48. doi: 10.1016/j.bja.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Radboud University Medical Center. Incremental cost-utility study on prehabilitation among older patients with colorectal cancer undergoing surgery (PreColo CU) (NCT04097795). Bethesda: NIH U.S. National Library of Medicine; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT04097795.
- 84.Manchester University NHS Foundation Trust. Surviving aneurysm surgery: a pilot study on exercise training in abdominal aortic aneurysm patients (SAS) (NCT01805973) Bethesda: NIH U.S. National Library of Medicine; 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT01805973.
- 85.Liang MK. Modifying risk in ventral hernia patients (NCT02365194). Bethesda: NIH U.S. National Library of Medicine; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT02365194.
- 86.Koh FH, Tan WJ, Ho L, Yen F, G K, Sivarajah S, et al. QS342: A structured pre-surgery prehabilitation for elderly patients undergoing elective surgery significantly improves surgical outcomes and reduces cost. Dis Colon Rectum. 2020;63(6):e125.
- 87.Berkel AEM, Bongers BC, Kotte H, Weltevreden P, de Jongh FHC, Eijsvogel MMM, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275(2):e299–e306. doi: 10.1097/SLA.0000000000004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blasco JM, Acosta-Ballester Y, Martínez-Garrido I, García-Molina P, Igual-Camacho C, Roig-Casasús S. The effects of preoperative balance training on balance and functional outcome after total knee replacement: a randomized controlled trial. Clin Rehabil. 2020;34(2):182–193. doi: 10.1177/0269215519880936. [DOI] [PubMed] [Google Scholar]
- 89.Janssen TL, Steyerberg EW, Langenberg JCM, de Lepper CCHAvH, Wielders D, Seerden TCJ, et al. Multimodal prehabilitation to reduce the incidence of delirium and other adverse events in elderly patients undergoing elective major abdominal surgery: an uncontrolled before-and-after study. PLoS One. 2019;14(6):e0218152. doi: 10.1371/journal.pone.0218152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janssen TL, Steyerberg EW, van Hoof-de LC, Seerden TCJ, de Lange DC, Wijsman JH, et al. Long-term outcomes of major abdominal surgery and postoperative delirium after multimodal prehabilitation of older patients. Surg Today. 2020;50(11):1461–1470. doi: 10.1007/s00595-020-02044-0. [DOI] [PubMed] [Google Scholar]
- 91.Gloor S, Misirlic M, Frei-Lanter C, Herzog P, Müller P, Schäfli-Thurnherr J, et al. Prehabilitation in patients undergoing colorectal surgery fails to confer reduction in overall morbidity: results of a single-center, blinded, randomized controlled trial. Langenbecks Arch Surg. 2022;407(3):897–907. doi: 10.1007/s00423-022-02449-0. [DOI] [PubMed] [Google Scholar]
- 92.Taha A, Taha-Mehlitz S, Staartjes VE, Lunger F, Gloor S, Unger I, et al. Association of a prehabilitation program with anxiety and depression before colorectal surgery: a post hoc analysis of the pERACS randomized controlled trial. Langenbecks Arch Surg. 2021;406(5):1553–1561. doi: 10.1007/s00423-021-02158-0. [DOI] [PubMed] [Google Scholar]
- 93.Liang MK, Bernardi K, Holihan JL, Cherla DV, Escamilla R, Lew DF, et al. Modifying risks in ventral hernia patients with prehabilitation: a randomized controlled trial. Ann Surg. 2018;268(4):674–680. doi: 10.1097/SLA.0000000000002961. [DOI] [PubMed] [Google Scholar]
- 94.Bernardi K, Olavarria OA, Dhanani NH, Lyons N, Holihan JL, Cherla DV, et al. Two-year outcomes of prehabilitation among obese patients with ventral hernias: a randomized controlled trial ( NCT02365194) Ann Surg. 2022;275(2):288–294. doi: 10.1097/SLA.0000000000004486. [DOI] [PubMed] [Google Scholar]
- 95.Halliday LJ, Doganay E, Wynter-Blyth V, Osborn H, Buckley J, Moorthy K. Adherence to pre-operative exercise and the response to prehabilitation in oesophageal cancer patients. J Gastrointest Surg. 2021;25(4):890–899. doi: 10.1007/s11605-020-04561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minnella EM, Awasthi R, Gillis C, Fiore JF, Jr, Liberman AS, Charlebois P, et al. Patients with poor baseline walking capacity are most likely to improve their functional status with multimodal prehabilitation. Surgery. 2016;160(4):1070–1079. doi: 10.1016/j.surg.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 97.Carli F, Baldini G. From preoperative assessment to preoperative optimization of frail older patiens. Eur J Surg Oncol. 2021;47(3 Pt A):519–23. doi: 10.1016/j.ejso.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 98.Kucejko RJ, Holleran TJ, Stein DE, Poggio JL. How soon should patients with colon cancer undergo definitive resection? Dis Colon Rectum. 2020;63(2):172–182. doi: 10.1097/DCR.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 99.Schmid S, Minnella EM, Pilon Y, Rokah M, Rayes R, Najmeh S, et al. Neoadjuvant prehabilitation therapy for locally advanced non-small-cell lung cancer: optimizing outcomes throughout the trajectory of care. Clin Lung Cancer. 2022;23(7):593–599. doi: 10.1016/j.cllc.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Trestini I, Cintoni M, Rinninella E, Grassi F, Paiella S, Salvia R, et al. Neoadjuvant treatment: a window of opportunity for nutritional prehabilitation in patients with pancreatic ductal adenocarcinoma. World J Gastrointest Surg. 2021;13(9):885–903. doi: 10.4240/wjgs.v13.i9.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gimeno-Santos E, Coca-Martinez M, Arguis MJ, Navarro R, Lopez-Hernandez A, Castel MA, et al. Multimodal prehabilitation as a promising strategy for preventing physical deconditioning on the heart transplant waiting list. Eur J Prev Cardiol. 2020;27(19):2367–2370. doi: 10.1177/2047487319889709. [DOI] [PubMed] [Google Scholar]
- 102.Levy N, Selwyn DA, Lobo DN. Turning 'waiting lists' for elective surgery into 'preparation lists'. Br J Anaesth. 2021;126(1):1–5. doi: 10.1016/j.bja.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 103.Zhu H, Moffa Z, Wang X, Abdullah S, Julaiti J, Carroll J. Understanding challenges in prehabilitation for patients with multiple chronic conditions. New York: Association for Computing Machinery; 2018. p. 138–47.
- 104.Brown O, Kenney B, Derstine B, Grenda D, Sullivan J, Palazzolo W, et al. Patient engagement drives the positive impact of prehabilitation. Mich J Med. 2018;3(1):21–28.
- 105.Ferreira V, Agnihotram RV, Bergdahl A, van Rooijen SJ, Awasthi R, Carli F, et al. Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer. 2018;26(8):2717–2723. doi: 10.1007/s00520-018-4109-1. [DOI] [PubMed] [Google Scholar]
- 106.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Meta-analysis methods based on direction and p-values. Introduction to meta‐analysis. 2009. p. 325–30.
- 107.Shemilt I AP, Graybill E, Craig D, Henderson C, Drummond M, Wilson ECF, Robalino S, Vale L; on behalf of the Campbell and Cochrane Economics Methods Group. . Chapter 20: Economic evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
- 108.Heil TC, Driessen EJM, Argillander TE, Melis RJF, Maas H, Olde Rikkert MGM, et al. Implementation of prehabilitation in colorectal cancer surgery: qualitative research on how to strengthen facilitators and overcome barriers. Support Care Cancer. 2022;30(9):7373–7386. doi: 10.1007/s00520-022-07144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Provan D, McLean G, Moug SJ, Phillips I, Anderson AS. Prehabilitation services for people diagnosed with cancer in Scotland - Current practice, barriers and challenges to implementation. Surgeon. 2022;20(5):284–290. doi: 10.1016/j.surge.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 110.Agasi-Idenburg CS, Zuilen MK-v, Westerman MJ, Punt CJA, Aaronson NK, Stuiver MM. “I am busy surviving” - Views about physical exercise in older adults scheduled for colorectal cancer surgery. J Geriatr Oncol. 2020;11(3):444–50. [DOI] [PubMed]
- 111.Alsaif H, Goodwin PC, Callaghan MJ, Sudell L, O'Neill TW, Yeowell G. Patient and healthcare provider experience and perceptions of a preoperative rehabilitation class for lumbar discectomy: a qualitative study. Musculoskelet Sci Pract. 2023;64:102740. doi: 10.1016/j.msksp.2023.102740. [DOI] [PubMed] [Google Scholar]
- 112.Bates A, West MA, Jack S. Framework for prehabilitation services. Br J Surg. 2020;107(2):e11–e14. doi: 10.1002/bjs.11426. [DOI] [PubMed] [Google Scholar]
- 113.Davis JF, van Rooijen SJ, Grimmett C, West MA, Campbell AM, Awasthi R, et al. From theory to practice: an international approach to establishing prehabilitation programmes. Curr Anesthesiol Rep. 2022;12(1):129–137. doi: 10.1007/s40140-022-00516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.World health statistics 2022: monitoring health for the SDGs, sustainable development goals. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2022.
- 115.Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–172. doi: 10.1016/j.jval.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 116.Robinson KA, Brunnhuber K, Ciliska D, Juhl CB, Christensen R, Lund H. Evidence-Based Research Series-Paper 1: what evidence-based research is and why is it important? J Clin Epidemiol. 2021;129:151–157. doi: 10.1016/j.jclinepi.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 117.Sheridan S, Schrandt S, Forsythe L, Hilliard TS, Paez KA. The PCORI Engagement Rubric: promising practices for partnering in research. Ann Fam Med. 2017;15(2):165–170. doi: 10.1370/afm.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Francis-Coad JED, Bulsara CE, Barrett-Lennard A, Owen K, Fletcher D, Wood F, Hill A. Partnering with patients to design a prehabilitation program for optimizing the patient experience through general surgery. Patient Exp J. 2021;8(1):135–147. doi: 10.35680/2372-0247.1544. [DOI] [Google Scholar]
- 119.Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery. Anaesthesia. 2018;73(6):750–768. doi: 10.1111/anae.14177. [DOI] [PubMed] [Google Scholar]
- 120.Caulley L, Catalá-López F, Whelan J, Khoury M, Ferraro J, Cheng W, et al. Reporting guidelines of health research studies are frequently used inappropriately. J Clin Epidemiol. 2020;122:87–94. doi: 10.1016/j.jclinepi.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 121.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 122.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi: 10.1016/j.jval.2021.11.1351. [DOI] [PubMed] [Google Scholar]
- 123.Grocott MPW, Ludbrook GL. Economic evaluation of prehabilitation: a true return on investment? Br J Anaesth. 2019;123(6):710–712. doi: 10.1016/j.bja.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 124.Dangor S, Jayaraman-Pillay P, Maddocks S, Chetty V. Pre-operative physiotherapy following unilateral ankle fractures at a tertiary hospital in South Africa: Perceptions of patients and nurses. S Afr J Physiother. 2021;77(1):a1501. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: App1. Important changes made to the protocol. App2. Search strategies. App3. Data items. App4. List of excluded studies. App5. Characteristics of ongoing economic evaluations. App6. Funding and competing interest of included economic evaluations. App7. Methods of completed economic evaluations. App8. Methods of ongoing economic evaluations with a published protocol. App9. Description of prehabilitation programmes in ongoing studies. App10. Risk of bias and methodological quality of included economic evaluations. App11. Detailed costs results of included economic evaluations. App12. Results of adherence and safety outcomes. App13. Results of descriptive post-hoc subgroup analyses to explore heterogeneity in cost-effectiveness results.
Data Availability Statement
All raw data collected as part of the review are deposited in the Open Science Framework (OSF) [20].