Abstract
Purpose
Analyzing the prognostic value of Epstein-Barr virus (EBV) DNA load and platelet-to-lymphocyte ratio (PLR) in non-metastatic nasopharyngeal carcinoma (NPC) patients, thereby developing a reliable and effective marker.
Methods
We compared survival rates among different groups using the Kaplan-Meier method and the Log-rank test. The factors affecting the prognosis of NPC patients were determined using univariate and multivariate cox regression analysis. Receiver operating characteristic (ROC) curves were used to identify the cutoff-value and discriminant performance of the model.
Results
The ROC curve indicated a cut-off value of 775 copies/ml for EBV DNA and 203.3 for PLR. Kaplan-Meier and Log-rank tests showed that 3-year overall survival (OS), local recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) of NPC patients in high risk group (HRG) were significantly poorer than those in medium risk group (MRG) and low risk group (LRG). The 3-year OS of NPC patients was significantly correlated with age, N stage and EBV DNA-PLR. The 3-year LRFS were significantly correlated with sex, N stage, histology type, and EBV DNA-PLR. The 3-year DMFS were correlated with histology type. The ROC curve showed that area under the curve (AUC) values of EBV DNA-PLR of 3-year OS, LRFS and DMFS in NPC were higher than those of PLR and EBV DNA.
Conclusion
EBV DNA-PLR is an independent risk factor for the prognosis of NPC. Compared with PLR or EBV DNA alone, the combination of EBV DNA and PLR may be more accurate in predicting the prognosis of NPC patients.
Keywords: Nasopharyngeal carcinoma, Prognosis, Platelet-to-lymphocyte ratio, Epstein-Barr virus DNA
Introduction
In southern China and Southeast Asia, nasopharyngeal carcinoma (NPC) is a common malignant tumor of the head and neck. It has obvious geographical distribution characteristics. Most of its histology types are non-keratinizing carcinoma. NPC lacks specific symptoms in the early stage, and most of them are diagnosed in the late stage. While the pathogenesis of NPC remains unclear, most studies have demonstrated that genetic susceptibility, eating habits, and Epstein-Barr virus (EBV) infection are risk factors [1]. Because the location of NPC is deep and special, cervical lymph node metastasis usually occurs, the effect of operation is poor and difficult, and NPC usually has high radiosensitivity, so radiotherapy is the main treatment of it. In addition, the combination of radiotherapy and chemotherapy is more effective in the treatment of advanced NPC [2]. In the present, the TNM staging system proposed by the International Union Against Cancer/American Joint Committee on Cancer is mainly used to guide clinical management and prognosis of NPC [3, 4]. However, it has been found that this staging system could not always accurately predict the prognosis of patients with NPC. Although patients at the same stage and receiving the same treatment, more than 20% of the patients have poor efficacy, which is due to the defect of the prognosis evaluation of the TNM staging system, which is difficult to reflect the biological behavior and immune heterogeneity of tumors [5–8]. Therefore, in addition to improving the treatment of NPC, looking for reliable and economical prognostic indicators to evaluate the prognosis more accurately is also a necessary condition for us to determine the malignant degree of NPC and optimize the treatment.
Many blood markers have been considered prognostic markers for NPC patients in recent years, including Epstein-Barr virus DNA (EBV DNA) [9], hemoglobin [10], albumin [11], C-reactive protein (CRP) [12] and lactate dehydrogenase (LDH) [13]. There is evidence that EBV DNA can be measured before treatment in most NPC patients, so plasma free EBV DNA load is the most important biomarker to reflect the tumor load of NPC [1, 14]. Due to the different sensitivity of each research center to EBV DNA, there is no unified conclusion on the cut-off value of high or low expression of EBV DNA, and it seems difficult to evaluate the biological characteristics and heterogeneity of NPC patients only on the basis of TNM stage or EBV DNA. Complete blood count is one of the most common laboratory testing methods. Many observational and experimental studies have confirmed that abnormal complete blood count is related to the occurrence and development of tumors. Anemia is closely linked to the prognosis of colorectal cancer and endometrial cancer [15, 16]. Tumor development is influenced by the immune status of the body as well [17]. Lymphocytes are the most important effector cells in tumor immune response. Different types of tumors are significantly associated with inflammatory markers such as neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratios (PLR) [18, 19]. Numerous studies have shown that PLR plays a key role in NPC prognosis in recent years. Therefore, our study combined pre-treatment EBV DNA load with pre-treatment PLR to explore its correlation with general clinical features, survival and prognosis of NPC patients, and to analyze its discriminant performance for prognosis of NPC patients.
Methods
Clinical subjects
One hundred and ninety-eight NPC patients treated in the Radiotherapy Department of the First affiliated Hospital of Guangxi Medical University from November 2017 to August 2019 were retrospectively analyzed for this study. Inclusion criteria for our study were as follows: (1) NPC patients was diagnosed by histology; (2) there was no distant metastasis before or during treatment; (3) had not received any antineoplastic therapy in the past; (4) denied the history of other malignant tumors; (5) physical status score 0–1 for Eastern Cooperative Oncology Group; (6) received radiotherapy or concurrent chemoradiothrapy with / without induction or adjuvant chemotherapy, and completed the entire treatment. (7) clinical data, examination data and follow-up data were available. The exclusion criteria were: (1) distant metastasis was found before or during treatment; (2) serious complications; (3) pregnant or lactating women; (4) previous or simultaneous occurrence of other malignant tumors; (5) unable to complete the treatment. This study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. And this was a retrospective study, so the informed consent was waived by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. Participant information is confidential.
Clinical data collection
The data of the following variables were recorded by consulting medical records: sex, age, histology, TNM stage, smoking history, family history, treatment, complete blood count results and EBV DNA copy number. In the study, PLR was used, and the PLR is calculated as the platelet count per 109/L divided by the lymphocyte count per 109/L.
Therapeutic schedule
In accordance with National Comprehensive Cancer Network guidelines, a standardized treatment plan was established according to the TNM stage of the patients. Stage I patients received radical radiotherapy. Stage II patients received radiotherapy or concurrent chemoradiothrapy combined with platinum drugs. Stage III-IVa patients received concurrent with induction chemotherapy or adjuvant chemotherapy. The patients were all treated with intensity modulated radiotherapy (IMRT). The radiotherapy target areas of NPC include gross tumor volume of nasopharynx (GTVnx), metastatic cervical lymph node volume (GTVnd), surrounding subclinical area (clinical target volume 1, CTV1) and cervical lymphatic drainage area (CTV2) need prophylactic irradiation. Regarding the total prescribed dose, GTVnx was 68 ~ 76 Gy / 30 ~ 33 f, GTVnd was 66 ~ 70 Gy / 30 ~ 33 f, CTV1 was 60 ~ 64 Gy / 30 ~ 33 f;CTV2 was 50 ~ 54 Gy / 30 ~ 33 f. The fractional dose was 2.00 ~ 2.33 Gy/f. Induction or adjuvant chemotherapy included GP regimen, TPF regimen, PF regimen and TP regimen. The GP regimen included gemcitabine doses of 1000 mg/m2 on days 1 and 8 and cisplatin doses of 80 mg/m2 on day 1. The TPF regimen consists of docetaxel 60 mg/m2 on day 1, cisplatin 60 mg/m2 on day 1, and continuous intravenous drips of 5-fluorouracil 600 mg/m2 from day 1 to day 5. The PF regimen consisted of 80 mg/m2 of cisplatin on day 1, 800 ~ 1000 mg/m2 of 5-fluorouracil, continuous intravenous drip from day 1 to day 5. In the TP regimen, docetaxel was administered at 75 mg/m2 on day 1 and cisplatin was administered at 75 mg/m2 on day 1. A total of 2–3 cycles of each regimen were performed every 21 days. Concurrent chemotherapy regimen was mainly cisplatin (80 ~ 100 mg/m2, every 21 days for a total of 2–3 cycles; 30 ~ 40 mg/m2, every 7 days for a total of 5–6 cycles). In the case of patients who were not suitable for cisplatin, other platinum drugs were used instead.
Endpoint and follow-up
Overall survival (OS) was our primary endpoint, followed by distant metastasis-free survival (DMFS) and local recurrence-free survival (LRFS). Generally, the OS is the duration of treatment between the onset of treatment and the death due to any cause. In this study, LRFS was calculated from the beginning of treatment to the first occurrence of local recurrence. The DMFS is calculated from the start of treatment until distant metastases are detected. Patients were followed up every 3 months for the first 2 years after treatment, every 6 months for 3-5-year after treatment, or until death. In follow-up, a magnetic resonance imaging scan of the nasopharynx and neck should be performed, as well as a computed tomography scan of the chest and abdomen. To confirm local recurrence or distant metastasis, fine needle puncture or pathological tissue biopsy should be performed if necessary. Patients who were lost to follow-up or were still alive without distant metastasis or locoregional recurrence at the end of the trial had their data censored at the date of last follow-up.
Statistical analysis
SPSS version 26.0 (IBM Corporation, Armonk, NY, USA) and MedCalc 20.1 (MedCalc Software Ltd, Ostend, Belgium) were used for all statistical analyses. The general characteristics of patients were compared with frequency and descriptive statistics. A chi-square test or Fisher’s exact test was used to compare the characteristics of patients in different groups. To determine the cut-off value of the research indicators based on 3-year OS, Youden index of receiver operating characteristic (ROC) curves were used. Then, the research indicators were divided into high or low according to the cut-off value. To plot survival curves and compare survival among groups, Kaplan-Meier and Log rank tests were used. The factors with P < 0.2 were selected for multivariate analysis based on univariate cox regression analysis. The multivariate cox regression analysis showed independent risk factors for NPC with P < 0.05. A ROC curve was performed to assess whether NPC prognosis could be accurately predicted by EBV DNA combined with PLR. The area under the curve (AUC) > 0.6 is considered to be a predictive value. Delong test was used to compare the classification efficiency of these ROC curves. P < 0.05 indicated a important statistical significance.
Results
EBV DNA-PLR combined score
According to the ROC curve, the cut-off values of EBV DNA and PLR were 775 (copies/ml) and 203.3, respectively. EBV DNA < 775 was considered as low, EBV DNA > 775 was considered as high, and PLR < 203.3 was regarded as low, PLR > 203.3 was regarded as high. To explore the prognostic value of EBV DNA and PLR, we established the EBV DNA-PLR combined score, which is a new prognostic factor based on EBV DNA. Based on the score, patients are divided into three groups: low risk group (LRG), medium risk group (MRG), and high risk group (HRG). The EBV DNA-PLR scoring criteria and grouping were as follows: high EBV DNA and high PLR as high risk group with 2 score, high EBV DNA and low PLR or low EBV DNA and high PLR as medium risk group with 1 score, and low EBV DNA and low PLR as low risk group with 0 score.
Patient characteristics
The median age of 198 NPC patients in our study was 46 years (range 16–73 years), including 140 (70.7%) males and 58 (29.3%) females. In terms of TNM staging, there were 55(27.8%) in stage T1 ~ T2, 74 (37.4%) in stage T3, 69 (34.8%) in stage T4, 87 (43.9%) in stage N0 ~ N1, 76 (38.4%) in stage N2, 35 (17.7%) in stage N3, 111 (56.1%) in stage I ~ III, 87 (43.9%) in stage IVa. In the light of histology, a total of 187 (94.4%) were undifferentiated non-keratinizing carcinomas (WHO histology type III) and 11 (5.6%) were differentiated non-keratinizing carcinomas (WHO histology type II). Among these NPC patients, 17 (8.6%) patients received radiotherapy alone, 93 (47.0%) patients received concurrent chemoradiothrapy, and 88 (44.4%) patients received concurrent chemoradiothrapy plus induction chemotherapy or adjuvant chemotherapy (Table 1).
Table 1.
NPC patients’ characteristics divided by EBV DNA-PLR combined score
| Characteristics | Total(%) | LRG(%) | MRG(%) | HRG(%) | p-Value |
|---|---|---|---|---|---|
| Sex | 0.483a | ||||
| Male | 140(70.7) | 66(75.0) | 57(67.9) | 17(65.4) | |
| Female | 58(29.3) | 22(25.0) | 27(32.1) | 9(34.6) | |
| Age(years) | |||||
| < 46 | 89(44.9) | 49(55.7) | 35(41.7) | 5(19.2) | 0.003 a |
| ≥ 46 | 109(55.1) | 39(44.3) | 49(58.3) | 21(80.8) | |
| T stage | 0.006 a | ||||
| T1-T2 | 55(27.8) | 34(38.6) | 18(21.4) | 3(11.5) | |
| T3 | 74(37.4) | 31(35.2) | 35(41.7) | 8(30.8) | |
| T4 | 69(34.8) | 23(26.1) | 31(36.9) | 15(57.7) | |
| N stage | 0.001 a | ||||
| N0-N1 | 87(43.9) | 50(56.8) | 30(35.7) | 7(26.9) | |
| N2 | 76(38.4) | 31(35.2) | 31(36.9) | 14(53.8) | |
| N3 | 35(17.7) | 7(8.0) | 23(27.4) | 5(19.2) | |
| Overall stage | 0.003 a | ||||
| I-III | 111(56.1) | 60(68.2) | 42(50.0) | 9(34.6) | |
| IVa | 87(43.9) | 28(31.8) | 42(50.0) | 17(65.4) | |
| Histology type | 0.909b | ||||
| II | 11(5.6) | 6(6.8) | 4(4.8) | 1(3.8) | |
| III | 187(94.4) | 82(93.2) | 80(95.2) | 25(96.2) | |
| Smoking | 0.343a | ||||
| No | 122(61.6) | 57(64.8) | 47(56.0) | 18(69.2) | |
| Yes | 76(38.4) | 31(35.2) | 37(44.0) | 8(30.8) | |
| Family history | 0.246a | ||||
| No | 182(91.9) | 79(89.8) | 77(91.7) | 26(100.0) | |
| Yes | 16(8.1) | 9(10.2) | 7(8.3) | 0(0.0) | |
| pre-EBV DNA | 0.001 a | ||||
| Negative (< 400) | 96(48.5) | 70(79.5) | 26(31.0) | 0(0.0) | |
| Positive (> 400) | 102(51.5) | 18(20.5) | 58(69.0) | 26(100.0) | |
| Treatment | 0.597a | ||||
| IMRT | 17(8.6) | 10(11.4) | 4(4.8) | 3(11.5) | |
| CCRT | 93(47.0) | 39(44.3) | 42(50.0) | 12(46.2) | |
| CCRT + IC/AC | 88(44.4) | 39(44.3) | 38(45.2) | 11(42.3) |
Notes: Bold indicates a significant difference among groups with p < 0.05
aChi-square test
bFisher’s exact test
Abbreviations: IMRT, intensity-modulated radiotherapy; CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy. LRG, low risk group; MRG, medium risk group; HRG, high risk group
Survival of different groups in EBV DNA-PLR
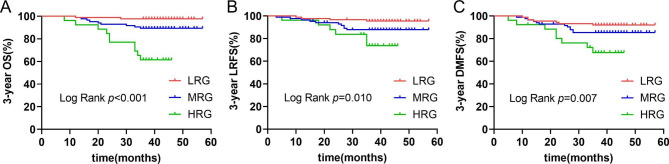
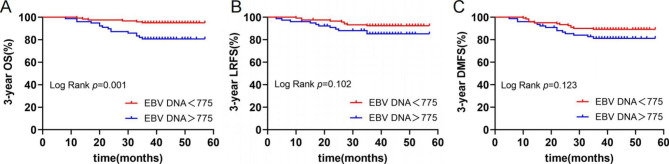
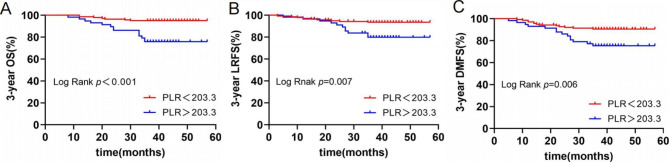
The final follow-up was on August 30, 2022, the median follow-up time was 41 months (range 8–57 months). Nineteen of the 198 NPC patients died, 20 had local recurrences, and 27 had distant metastases. The 3-year OS, LRFS and DMFS were 90.4%, 89.9% and 86.4%, respectively. Two patients died, four had local recurrences, and seven had distant metastases in LRG (0 score). Three-year OS, LRFS, and DMFS were respectively 97.7%, 95.5%, and 92.0%. Nine patients died, 10 had local recurrences, and 12 had distant metastases in MRG (1 score). Three-year OS, LRFS and DMFS were 89.3%, 88.1% and 85.7%, respectively. Ten patients died, 6 had local recurrences, and 8 had distant metastases in HRG (2 score). Three-year OS, LRFS and DMFS were 61.5%, 76.9% and 69.2%, respectively. Kaplan-Meier and log-rank tests showed that the 3-year OS, LRFS, and DMFS of NPC patients in HRG were significantly worse than those in MRG and LRG (Fig. 1). The survival curves of patients in three groups are presented in Fig. 1. The survival curves of patients in different EBV DNA (< 775 vs.> 775) and PLR levels (< 203.3 vs.> 203.3) are presented in Figs. 2 and 3, respectively.
Fig. 1.
Kaplan-Meier survival curves of 3-year OS (A), LRFS (B) and DMFS (C) in LRG, MRG and HRG.
Abbreviations: OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; LRG, low risk group; MRG, medium risk group; HRG, high risk group
Fig. 2.
Kaplan-Meier survival curves of 3-year OS (A), LRFS (B) and DMFS (C) in different EBV DNA levels(< 775 vs.> 775)
Abbreviations: OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival
Fig. 3.
Kaplan-Meier survival curves of 3-year OS (A), LRFS (B) and DMFS (C) in different PLR levels(< 203.3 vs.> 203.3)
Abbreviations: OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival
Univariate and multivariate analysis of prognostic factors for NPC
As shown in Table 2, according to univariate cox regression analysis, a number of variables contributed to 3-year OS, including age, EBV DNA-PLR and clinical stage. Variables associated with 3-year LRFS, including sex, age, N stage, histological type, and EBV DNA-PLR. Age, N stage, histological type, and EBV DNA-PLR are variables associated with 3-year DMFS.
Table 2.
Univariate analysis of prognostic factors in NPC patients
| Characteristics | 3-year OS | 3-year LRFS | 3-year DMFS | |||||
|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | p-Value | HR(95%CI) | p-Value | HR(95%CI) | p-Value | |||
| Sex | ||||||||
| Male | Reference | Reference | Reference | |||||
| Female | 0.944(0.366–2.434) | 0.906 | 2.502(1.041–6.011) | 0.040 | 0.998(0.437–2.280) | 0.997 | ||
| Age(years) | ||||||||
| < 46 | Reference | Reference | Reference | |||||
| ≥ 46 | 5.221(1.538–17.726) | 0.008 | 2.583(0.938–7.108) | 0.066 | 2.023(0.777–5.266) | 0.149 | ||
| T stage | ||||||||
| T1-2 | Reference | Reference | Reference | |||||
| T3 | 1.145(0.323–4.058) | 0.834 | 1.398(0.464–4.171) | 0.548 | 1.406(0.502–3.802) | 0.502 | ||
| T4 | 2.354(0.750–7.394) | 0.143 | 1.009(0.308–3.307) | 0.988 | 1.447(0.526–3.983) | 0.472 | ||
| N stage | ||||||||
| N0-N1 | Reference | Reference | Reference | |||||
| N2 | 2.298(0.698–7.632) | 0.174 | 1.890(0.618–5.777) | 0.264 | 1.284(0.522–3.160) | 0.586 | ||
| N3 | 6.365(1.959–20.678) | 0.002 | 4.048(1.284–12.765) | 0.017 | 2.548(0.982–6.610) | 0.054 | ||
| Histology type | ||||||||
| II | Reference | Reference | Reference | |||||
| III | 1.183(0.159–8.813) | 0.870 | 0.319(0.094–1.091) | 0.069 | 0.306(0.106–0.885) | 0.029 | ||
| Smoking | ||||||||
| No | Reference | Reference | Reference | |||||
| Yes | 1.528(0.649–3.597) | 0.332 | 0.875(0.349–2.194) | 0.776 | 1.356(0.635–2.897) | 0.432 | ||
| Family history | ||||||||
| No | Reference | Reference | Reference | |||||
| Yes | 0.043(0.000-37.654) | 0.363 | 0.043(0.000-41.142) | 0.370 | 0.043(0.000-15.312) | 0.294 | ||
| Treatment | ||||||||
| IMRT | Reference | Reference | Reference | |||||
| CCRT | 1.823(0.233–14.242) | 0.567 | 2.418(0.316–18.486) | 0.395 | 2.826(0.373–21.394) | 0.314 | ||
| CCRT + IC/AC | 1.943(0.249–15.177) | 0.527 | 1.152(0.139–9.570) | 0.896 | 2.152(0.278–16.670) | 0.463 | ||
| EBV DNA-PLR | ||||||||
| LRG (0 point) | Reference | Reference | Reference | |||||
| MRG (1 point) | 4.924(1.064–22.792) | 0.041 | 2.792(0.876–8.904) | 0.083 | 1.885(0.742–4.789) | 0.183 | ||
| HRG (2 point) | 19.867(4.349–90.752) | < 0.001 | 5.985(1.687–21.230) | 0.006 | 4.503(1.632–12.429) | 0.004 | ||
Notes: Bold indicates statistically significant with p < 0.2
Abbreviations: IMRT, intensity-modulated radiotherapy; CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy; LRG, low risk group; MRG, medium risk group; HRG, high risk group. EBV, Epstein-Barr virus; PLR, platelet-to-lymphocyte ratio; OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; HR, hazard ratio; CI, confidence interval
Then, the above variables were introduced in the multivariate cox regression analysis. As shown in Table 3, significant associations were found between 3-year OS and age (P = 0.048), N stage (P = 0.011) and EBV DNA-PLR (P = 0.002). There were also significant correlations between 3-year LRFS and sex (P = 0.048), N stage (P = 0.043), histological (P = 0.009), and EBV DNA-PLR (P = 0.045). Three-year DMFS was significantly correlated with histological (P = 0.010) and EBV DNA-PLR (P = 0.010).
Table 3.
Multivariable analysis of prognostic factors in NPC patients
| Characteristics | 3-year OS | 3-year LRFS | 3-year DMFS | |||||
|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | p-Value | HR(95%CI) | p-Value | HR(95%CI) | p-Value | |||
| Sex | ||||||||
| Male | Reference | |||||||
| Female | 2.449(1.007–5.955) | 0.048 | ||||||
| Age(years) | ||||||||
| < 46 | Reference | Reference | Reference | |||||
| ≥ 46 | 3.681(1.030-13.158) | 0.045 | 2.442(0.833–7.161) | 0.104 | 1.222(0.534–2.796) | 0.634 | ||
| T stage | ||||||||
| T1-2 | Reference | |||||||
| T3 | 0.497(0.134–1.841) | 0.295 | ||||||
| T4 | 0.741(0.205–2.488) | 0.597 | ||||||
| N stage | ||||||||
| N0-N1 | Reference | Reference | Reference | |||||
| N2 | 1.544(0.457–5.209) | 0.484 | 1.331(0.425–4.164) | 0.624 | 1.059(0.423–2.648) | 0.903 | ||
| N3 | 4.900(1.431–16.786) | 0.011 | 3.431(1.038–11.336) | 0.043 | 2.259(0.833–6.125) | 0.109 | ||
| Histology type | ||||||||
| II | Reference | Reference | ||||||
| III | 0.177(0.048–0.652) | 0.009 | 1.222(0.534–2.796) | 0.010 | ||||
| EBV DNA-PLR | ||||||||
| LRG (0 point) | Reference | Reference | Reference | |||||
| MRG (1 point) | 3.317(0.694–15.868) | 0.133 | 1.831(0.547–6.128) | 0.327 | 1.579(0.594–4.198) | 0.360 | ||
| HRG (2 point) | 11.897(2.441–57.977) | 0.002 | 3.856(1.033–14.395) | 0.045 | 4.135(1.401–12.201) | 0.010 | ||
Notes: Bold indicates statistically significant with p < 0.05
Abbreviations: LRG, low risk group; MRG, medium risk group; HRG, high risk group. EBV, Epstein-Barr virus; PLR, platelet-to-lymphocyte ratio; OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; HR, hazard ratio; CI, confidence interval
Discriminant performance of EBV DNA-PLR to the prognosis
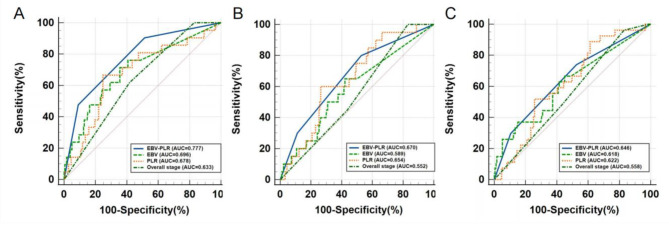
Through the analysis of ROC curve, it was concluded that the AUC values of EBV DNA-PLR of 3-year OS, LRFS and DMFS in NPC were higher than those of PLR, EBV DNA and overall stage. There was significant difference between EBV DNA-PLR and overall stage in AUC values of 3-year OS (P = 0.035) (Table 4; Fig. 4).
Table 4.
Comparison of ROC curves of EBV DNA-PLR, PLR, EBV DNA and Overall stage
| Prognostic factors | 3-year OS | 3-year LRFS | 3-year DMFS | |||
|---|---|---|---|---|---|---|
| AUC | p-Value | AUC | p-Value | AUC | p-Value | |
| EBV DNA-PLR | 0.777 | N/A | 0.670 | N/A | 0.646 | N/A |
| PLR | 0.678 | 0.051c | 0.654 | 0.750c | 0.622 | 0.608c |
| EBV DNA | 0.696 | 0.112d | 0.589 | 0.082d | 0.618 | 0.573d |
| Overall stage | 0.633 | 0.035 e | 0.552 | 0.067e | 0.558 | 0.156e |
Notes: Bold indicates statistically significant with p < 0.05
cComparison between EBV DNA-PLR and PLR.
dComparison between EBV DNA-PLR and EBV DNA.
eComparison between EBV DNA-PLR and Overall stage
Abbreviations: EBV, Epstein-Barr virus; PLR, Platelet lymphocyte ratio; OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; ROC, receiver operating characteristic; AUC, area under the curve
Fig. 4.
ROC curves for EBV DNA-PLR, PLR, EBV DNA and Overall stage of 3-year OS (A), LRFS (B), DMFS (C)
Abbreviations: EBV, Epstein-Barr virus; PLR, platelet-to-lymphocyte ratio; OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; ROC, receiver operating characteristic
Discussion
Since the introduction of intensity modulated radiation therapy (IMRT) and immunotherapy, the local control rate of NPC patients has increased considerably. Five-year overall survival rate of NPC patients has reached over 80% [1, 20]. In spite of this, local recurrence and distant metastasis still remain the main causes of failure of treatment [21, 22]. At present, the prognosis of NPC patients still depends on TNM staging system [3]. Nevertheless, although patients have the same clinical stage and receive the same treatment, there are still different treatment effects, which may be due to tumor heterogeneity, immune and inflammatory responses. In fact, tumor immune response plays a major role in the occurrence and progress of various solid malignant tumors [23]. There is also increasing evidence that the inflammatory response plays a key role in tumor development and shows independent prognostic value, such as NLR [18], PLR [19], CRP [12], LDH [13], etc. Inflammatory and immune responses are associated with all stages of tumorigenesis and progression, including initiation, promotion, and metastasis [24].
Tumor growth requires a rich blood supply, and platelets can promote angiogenesis and release growth factors [25]. Tumor cells mediate platelet aggregation [26]. Platelet aggregation around tumor cells protects it from natural killer cell killing [27]. It could be activated by transforming growth factor-β signal transduction pathway regulates the process of tumor micrometastasis and promotes tumor cell exosmosis [28]. Lymphocytes are the most important effector cells in tumor immunity. PLR, as an indicator of the combination of platelet and lymphocyte counts, can reflect the pro-tumor state, inflammatory response and anti-tumor immune state in the body. A meta-analysis has shown that PLR is a risk factor for poor prognosis of various malignant tumors [19]. It has been demonstrated that PLR predicts OS, PFS, LRFS, and DMFS among patients with NPC by Chen et al. and Peng et al [29, 30].
Now many studies have confirmed that plasma EBV DNA load is the most important biomarker reflecting the tumor burden of NPC [14, 31]. However, the sensitivity of detecting EBV DNA load varies among research centers, which may be attributed to different detection reagents or methods. Therefore, there is no generally accepted and uniform EBV DNA cut-off value, and accurate risk stratification cannot be performed based on this indicator. In this research center, when the EBV DNA load is less than 400 copies/ml, it cannot be detected, and we will record the undetectable EBV DNA as 0 copies/ml.
Relying solely on TNM stage and EBV DNA or PLR to evaluate the prognosis is one-sided, which may ignore the effects of tumor load, heterogeneity, immune and inflammatory response on the development of NPC. Therefore, based on the current research results that both EBV DNA and PLR have an impact on the prognosis of NPC, our study combined these two hematological parameters and established EBV DNA-PLR combined score, and divided the NPC patients into groups according to different scores, and to investigate the correlation between hematological parameters and clinical characteristics, survival and prognosis of patients with NPC. In the comparison of patients’ baseline characteristics, it was found that there were significant differences in age, T stage, N stage and overall stage among different groups of NPC patients. In survival analysis, patients in HRG showed significant differences compared with MRG and LRG, NPC patients with HRG had poorer 3-year OS, LRFS and DMFS. Sex, age, EBV DNA-PLR, T stage, N stage, and histology type were included in multivariate analysis based on univariate analysis. Since Overall stage was determined by T stage and N stage, we did not include it in univariate and multivariate analysis. The results of multivariate analysis showed that the EBV DNA-PLR was an independent prognostic factor for NPC patients. There were significant differences in 3-year OS (HR: 11.897, 95% CI: 2.441–57.977), LRFS (HR: 3.856, 95% CI: 1.033–14.395), and DMFS (HR: 4.135, 95% CI: 1.401–12.201) between HRG and LRG. However, MRG versus LRG, which is, high EBV DNA or high PLR alone did not show a significant difference in prognosis. This result seems to be inconsistent with previous studies, which may be due to the small sample size of the present study compared to previous studies, and it may be also because the development of NPC is a long process, and the follow-up time of this study is insufficient. Other prognostic factors include sex, age, N stage and histology type. In the analysis of ROC curve, the AUC value of EBV DNA-PLR was higher than that of EBV DNA and PLR on 3-year OS, LRFS and DMFS, which showed some advantages in predicting prognosis of NPC patients.
There are some other limitations in this study, such as the EBV DNA-PLR shown in the results is an independent risk factor for 3-year OS (HR: 11.897, 95% CI: 2.441–57.977) in NPC patients, but the 95% confidence interval of HR is wide. And there is not any statistically significant difference in the ROC curve of EBV DNA-PLR, EBV DNA and PLR. This may be related to the insufficient number of final events due to the small sample size. In addition, this is a retrospective study with a relatively short follow-up period and a lack of 5-year survival results, which may affect the correct prediction of long-term outcomes. Then, the treatment of all eligible patients varied according to the choice of doctors in charge, and there are differences in chemotherapy regimens, chemotherapy cycles and drug doses, which may affect the results of the study. In the future, we will need to expand the sample size, further control other factors that may affect the results, and explore a more complete scoring system to better guide clinical diagnosis and treatment.
Conclusion
In summary, the EBV DNA-PLR combined score could be used as an individualized clinical assessment tool to more accurately and easily predict the prognosis of patients with non-metastatic NPC.
Acknowledgements
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HD, ZH, DY and ZL. The first draft of the manuscript was written by HD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 71964003, 71964003, 81760542, 82160467), the Natural Science Foundation of Guangxi Zhuang Autonomous Region (No.2JJA141048), the Research Foundation of the Science and Technology Department of Guangxi Province, China (grant No.2GXNSFAA380252, 2018AB61001 and 2019GXNSFAA185040), the Research Foundation of the Health Department of Guangxi Province, China (No.S2018087), Guangxi Medical University Training Program for Distinguished Young Scholars (2017), Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University (2016).
Data Availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Abbreviations
EBV, Epstein-Barr virus; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; LDH, lactate dehydrogenase; NPC, nasopharyngeal carcinoma; OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; HRG, high risk group; MRG, medium risk group; LRG,low risk group; ROC, receiver operating characteristic; AUC, area under the curve; IMRT, intensity-modulated radiotherapy; CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy; HR, hazard ratio; CI, confidence interval.
Ethics approval and consent to participate.
This study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. And this was a retrospective study, so the informed consent was waived by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. Participant information is confidential.
Consent for publication.
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huan Dong and Zichong Huang contributed equally to this work.
Contributor Information
Yutao Qin, Email: qyt2011@163.com.
Min Kang, Email: kangmin@gxmu.edu.cn.
References
- 1.Chen YP, Chan A, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20(8):2038–44. doi: 10.1200/JCO.2002.08.149. [DOI] [PubMed] [Google Scholar]
- 3.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 4.Tang LL, Chen YP, Mao YP, Wang ZX, Guo R, Chen L, et al. Validation of the 8th Edition of the UICC/AJCC staging system for nasopharyngeal carcinoma from endemic areas in the intensity-modulated Radiotherapy Era. J Natl Compr Canc Netw. 2017;15(7):913–9. doi: 10.6004/jnccn.2017.0121. [DOI] [PubMed] [Google Scholar]
- 5.OuYang PY, Su Z, Ma XH, Mao YP, Liu MZ, Xie FY. Comparison of TNM staging systems for nasopharyngeal carcinoma, and proposal of a new staging system. Br J Cancer. 2013;109(12):2987–97. doi: 10.1038/bjc.2013.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.OuYang PY, Xiao Y, You KY, Zhang LN, Lan XW, Zhang XM, et al. Validation and comparison of the 7th and 8th edition of AJCC staging systems for non-metastatic nasopharyngeal carcinoma, and proposed staging systems from Hong Kong, Guangzhou, and Guangxi. Oral Oncol. 2017;72:65–72. doi: 10.1016/j.oraloncology.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Yang XL, Wang Y, Liang SB, He SS, Chen DM, Chen HY, et al. Comparison of the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma: analysis of 1317 patients treated with intensity-modulated radiotherapy at two centers. BMC Cancer. 2018;18(1):606. doi: 10.1186/s12885-018-4419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li QJ, Mao YP, Guo R, Huang CL, Fang XL, Ma J, et al. A Nomogram based on serum biomarkers and clinical characteristics to predict survival in patients with non-metastatic nasopharyngeal carcinoma. Front Oncol. 2020;10:594363. doi: 10.3389/fonc.2020.594363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang SB, Zhang N, Chen DM, Yang XL, Chen BH, Zhao H, et al. Prognostic value of gross tumor regression and plasma Epstein Barr Virus DNA levels at the end of intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma. Radiother Oncol. 2019;132:223–9. doi: 10.1016/j.radonc.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Gao J, Tao YL, Li G, Yi W, Xia YF. Involvement of difference in decrease of hemoglobin level in poor prognosis of stage I and II nasopharyngeal carcinoma: implication in outcome of radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(4):1471–8. doi: 10.1016/j.ijrobp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Wang K, Liang Z, Guo S, Zhang P, Xu Y, et al. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: a meta-analysis and systematic review. Clin Otolaryngol. 2020;45(2):167–76. doi: 10.1111/coa.13454. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Zhou Y, Yuan Y, Zhang Q, He S, Chen Y, et al. Effect of CRP and kinetics of CRP in prognosis of nasopharyngeal carcinoma. Front Oncol. 2019;9:89. doi: 10.3389/fonc.2019.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou GQ, Tang LL, Mao YP, Chen L, Li WF, Sun Y, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 2012;82(3):e359–65. doi: 10.1016/j.ijrobp.2011.06.1967. [DOI] [PubMed] [Google Scholar]
- 14.Lee HF, Kwong LW, Leung TW, Choi CW, Lee WM. The addition of pretreatment plasma Epstein-Barr virus DNA into the 8th edition of nasopharyngeal cancer TNM stage classification. Int J Cancer. 2018;144(7). 10.1002/ijc.31856. [DOI] [PubMed]
- 15.Gvirtzman R, Livovsky DM, Tahover E, Goldin E, Koslowsky B. Anemia can predict the prognosis of colorectal cancer in the pre-operative stage: a retrospective analysis. World J Surg Oncol. 2021;19(1):341. doi: 10.1186/s12957-021-02452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Zaid A, Alomar O, Abuzaid M, Baradwan S, Salem H, Al-Badawi IA. Preoperative anemia predicts poor prognosis in patients with endometrial cancer: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;258:382–90. doi: 10.1016/j.ejogrb.2021.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Song MM, Zhang X, Ding JS, Ruan GT, Zhang XW, et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle. 2021;12(6):1466–76. doi: 10.1002/jcsm.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Zhou P, Liu Y, Wei H, Yang X, Chen T, et al. Platelet-to-lymphocyte ratio in advanced Cancer: review and meta-analysis. Clin Chim Acta. 2018;483:48–56. doi: 10.1016/j.cca.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–55. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Wu F, Wang R, Lu H, Wei B, Feng G, Li G, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112(1):106–11. doi: 10.1016/j.radonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy–revisited. Nat Rev Drug Discov. 2011;10(8):591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 24.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 26.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelets effects on tumor growth. Semin Oncol. 2014;41(3):359–69. doi: 10.1053/j.seminoncol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100(5):859–65. doi: 10.1111/j.1349-7006.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Sun J, Hu D, Zhang J, Xu Y, Feng H, et al. Predictive value of pretreatment lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio in the survival of nasopharyngeal carcinoma patients. Cancer Manag Res. 2021;13:8767–79. doi: 10.2147/CMAR.S338394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram based on Lactate dehydrogenase-to-albumin ratio (LAR) and platelet-to-lymphocyte ratio (PLR) for Predicting Survival in Nasopharyngeal Carcinoma. J Inflamm Res. 2021;14:4019–33. doi: 10.2147/JIR.S322475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan K, Woo J, King A, Zee B, Lam W, Chan SL, et al. Analysis of plasma Epstein-Barr Virus DNA to screen for nasopharyngeal Cancer. N Engl J Med. 2017;377(6):513–22. doi: 10.1056/NEJMoa1701717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.