Abstract
Background
Studies on the relationship between diet and colorectal cancer (CRC) risk using single food or nutrient approach are widely conducted as opposed to dietary pattern approach. Therefore, this study aimed to determine the major dietary patterns and their association with CRC risk among Malaysians.
Methods
Patients aged between 18 and 80 years old from two teaching hospitals in Peninsular Malaysia were recruited through purposive sampling. Socio-demographic information and anthropometry data were assessed before the colonoscopy procedure, and dietary intake was also recorded using a validated semi-quantitative food frequency questionnaire (FFQ). Cases were those patients having histopathologically proven CRC, while controls were those without.
Results
Four major dietary patterns were identified: the allergenic diet, plant-based diet, processed diet, and energy-dense diet pattern. After adjusting for potential covariates, the processed diet pattern was consistently associated with CRC (OR = 3.45; 95% CI = 1.25–9.52; P = 0.017) while the plant-based diet, energy-dense diet, and allergenic diet were not associated with CRC risk.
Conclusions
The processed diet pattern attributed to a diet high in confectionaries and fast foods was associated with an increased risk of CRC in the Malaysian population. In order to give prevention measures through lifestyle change, more research could be done on the effect of food patterns on faecal microbiota associated with CRC.
Keywords: Colorectal cancer, Dietary patterns, Processed diet, Western diet, Prudent diet
Background
Colorectal cancer (CRC) has a five-year prevalence rate of about 5.3 million people worldwide. With estimates of over 1.9 million and 935,000 fatalities, respectively, CRC is the second most prevalent cause of cancer death and the fourth most common malignancy in terms of diagnoses [1]. A study in European countries showed that the incidence of CRC in Austria, Germany, and the Czech Republic reduced over time [2]. For Denmark, Slovenia, and the Netherlands, the age-standardised incidence rates primarily showed an increment but then subsequently reduced, while the rate is increased for Bulgaria, Norway, Estonia, and Ukraine countries. The age-standardised incidence rates of CRC, on the other hand, were constant and barely varied from 17.03 to 20.01 per 100,000 in northern Malaysia, whereas the age-standardized mortality rates dropped from 12.73 per 100,000 in 2008 to 2.99 per 100,000 in 2017 [3].
There are three main risk factors for CRC, including family and personal medical history, lifestyle and others [4]. Family and personal medical history included family history and genetics, inflammatory bowel disease, colon polyps, diabetes mellitus, and cholecystectomy [4]. As for lifestyle factors, some of these included dietary patterns, overweight and obesity, physical inactivity, smoking, and alcohol intake, while others address gut microbiota, age, gender, race, and socioeconomic status. [4]. Dietary intake has become one of the important factors contributing to CRC development. Populations consuming high intakes of red meat [5], sugar [6], and white bread [7] may be at increased risk of the disease.
Several studies had been conducted to assess the relationship between diet and CRC risk [5, 7, 8]; however, those studies used a single food or nutrient approach rather than a dietary pattern approach. Dietary pattern is more preferred because it involves quantification, variation, or the combination of different foods and beverages in the diet and their habitual consumption frequency [9]. In addition, dietary pattern analysis also is a crucial new technique that may help to explain the relationship [10]. The application of this dietary pattern to non-western population is limited, due to lack of studies in Asian populations evaluating the association between dietary pattern and CRC risk [6, 11–15]. To the best of our knowledge, there is no study on dietary patterns for the determination of CRC risk in Malaysia. Therefore, the aim of this study was to determine the major dietary patterns and their association with CRC risk among the Malaysian population.
Methods
Study design and study population
This hospital-based case–control study was conducted between July 2020 and January 2022 in two teaching hospitals, namely Hospital Universiti Sains Malaysia (HUSM) and Hospital Canselor Tuanku Muhriz Universiti Kebangsaan Malaysia (HCTM UKM). HUSM is located in Kota Bharu (northeastern region of Peninsular Malaysia) with a population density of 122 people per square kilometer, while HCTM UKM is located in Kuala Lumpur (Klang Valley of Peninsular Malaysia) with an 8,045-population density per square kilometer. These tertiary teaching hospitals represented urban (HCTM UKM) and suburban (HUSM) areas, respectively [16]. Around 126 patients (30 cases and 96 controls) were enrolled from HUSM and 138 patients (49 cases and 89 controls) from HCTM UKM.
Sample size
The sample size in this study was computed using Power and Sample software (PS software) version 3.0 by William D. Dupont and Walton D. Plummer (1997–2009). The study from Safari et al. (2013) was adopted as it has the most similar criteria to this study [12]. A total of 78 cases and 156 controls (plus an additional 30% of non-respondents) were needed for the investigation, assuming the expected proportions in case and control were 0.606 and 0.387, 80% study power, alpha 0.05, and a participation ratio of 1:2.
Patient selection
The study protocol was reviewed and approved by the Human Research Ethics Committee of Universiti Sains Malaysia (USM/JEPeM/19060354) and the Universiti Kebangsaan Malaysia Medical and Research Ethics Committee (UKMREC; FF-2020–005). All patients who attended the colonoscopy procedure were approached in a sequential manner, and informed consent was obtained from those who agreed to participate. Inclusion criteria were Malaysian, not being on any special diet that could influence their weight status, have no previous abdominal surgeries associated with bowel obstruction, and females must not be pregnant or breastfeeding. Aged 18 to 80 were selected as the cancer incidence increased after the age of 30 years while the CRC incidence increased after the age of 60 years [17].
After completing the colonoscopy procedure and obtaining the results of histopathology, patients were assigned to the case and control groups. Thus, cases were endoscopically and histopathologically confirmed CRCs, diagnosed no more than one year before study enrolment, and had no previous cancer diagnosis at other anatomic sites. They also did not have severe mental disorders, including major depression, schizophrenia, and anxiety, or physical disabilities affecting independent self-care and diet. Meanwhile, controls were patients with histological tubular adenoma and normal or benign findings such as diverticulum, colitis, appendicitis, and a rectal ulcer.
Data collection
Patients would complete a face-to-face interview in the Malay language during their hospital visit using the same structured questionnaire at both study sites. It consisted of socio-demographics data (age, gender, ethnicity, marital status), smoking status, supplement intake, employment status, household income, educational level, medical and family history of CRC, anthropometric parameters, and dietary intake.
Anthropometric measurements
Anthropometric measurements were taken in accordance with standard procedure [18]. During the measuring process, two researchers were incorporated as measurers and recorders. Only one measurer was involved in the measuring process to minimize inter-measurer bias. The average values were finalized from the duplication measurements taken.
Height was measured using a stadiometer (Seca, 217, Hamburg, Germany) with the respondent standing straight and barefoot, and the reading was measured to the nearest 0.5 cm. Then, weight and body composition were measured using the body composition analyzer [19] which produced estimated values of the measurements taken using the dual-energy X-ray absorptiometry method (DEXA) and bioelectrical impedance analysis (BIA). The obtained height and weight were used to calculate BMI by using the formula: BMI = weight (kg)/height (m2). The BMI cut-off point was classified according to the criteria defined by the World Health Organization (WHO) [20].
A measuring tape was used to measure the waist circumference (WC) and hip circumference (HC) (Seca, 201, Hamburg, Germany). Respondent stood straight with arms hanging by the sides. The tape was positioned at the midpoint between the inferior margin of the last rib and the iliac crest, and then passed firmly around the waist, not compressing the skin. The measurement for WC was taken at the end of expiration when the respondent breathed normally, to the nearest 0.1 cm. HC was measured by adjusting the tape level to the adjudged level of the greatest posterior protuberance of the buttocks. The measurement was taken to the nearest 0.1 cm after it was perpendicular to the trunk's long axis.
Dietary Intake Questionnaire Via FFQ
The validated semi-quantitative food frequency questionnaire (FFQ) consisted of 142 food items from 16 food groups. It is basically a modification of food list related to CRC from the original National Health and Morbidity Survey (NHMS) 2014 questionnaire [21]. The validity and reproducibility were tested, and the FFQ was good for estimating absolute nutrient and food group intakes. The 16 food groups included cereal products, meats, fish, and seafood, eggs, vegetables, legumes, bread spreads, fruits, confectionaries, fast foods, non-sugary drinks, sugar-sweetened drinks, alcoholic drinks, condiments, and dairy products.
The diet assessed was for a period of one year prior to the colonoscopy procedure, which will capture the dietary pattern of cases before cancer diagnosis and the habitual dietary pattern of the controls. During data collection, patients were required to report the frequency of foods and drinks consumed and the amount intake on a daily, weekly, monthly, and yearly/never basis through a face-to-face interview. Photographs of household measurements, including glass, cup, tablespoon, teaspoon, and scoop were provided to aid respondents in estimating the portion sizes of the foods that they consumed.
To obtain the energy and nutrient values, the daily intake of each food item was calculated using the frequency of intake per day x total number of servings x weight of food in one serving. All the dietary information from the semi-quantitative FFQ was analyzed using Nutritionist Pro™ Diet Analysis Software, version 7.8.0 (Axxya Systems, version 2020, Redmond, USA), and the database selected was Nutrient Composition of Malaysian Foods. Recipes that were not available in the reference list were added to the database, where the portion sizes were calculated based on standard recipe sizes (total serving and per serving size). The Atlas of Food Exchanges and Portion Sizes, the Nutrient Composition of Malaysian Foods, the Malaysian Food Album [22], and the Malaysian Food Composition Database (MyFCD) [23] were used to determine the weight of foods or ingredients used in the recipes. The nutritional content of the food product was obtained from its packaging or MyFCD and was inserted into the database.
However, under-reported and over-reported dietary intake were eliminated using the Goldberg cut-off based on confidence limit (CL) of physical activity level (PAL) and the ratio of energy intake (EI) and basal metabolic rate (BMR) [24].
Exploratory factor analysis
All data analysis was performed using IBM SPSS Statistics, Version 26.0 (Chicago, IL, USA), and statistics were considered significant with a p-value < 0.05. Firstly, based on the functions of food and its nutritional properties, the 142 food items in the FFQ would be classified into the predetermined 16 food groups. Then, using exploratory factor analysis (EFA), the identified dietary factors were rotated using an orthogonal varimax rotation approach for the maximum amount of variance explained and the uncorrelated components for improved interpretability. The following criteria were used to determine which factors should be kept: factor interpretability, factor eigenvalues larger than 1.15, scree plot break point, and proportion of variance explained [25]. Food groups with absolute rotation factor loadings equal to or higher than 0.30 were used to designate dietary patterns. This highlights the significance of factor loading when calculating factor scores that correspond to each pattern individually. Furthermore, each patient will receive a factor score determined by a loading matrix for each dietary pattern (factor) by indicating how well their diet matched that pattern. In other words, a person with a higher factor score adhered to that pattern more strongly.
Statistical analyses
To determine the association between dietary pattern and CRC, unconditional logistic models were initially used, and only age and total energy consumption were accounted for in Model 1. Scores of dietary pattern were divided into tertiles with the lowest tertiles serving as the reference group [26]. Additional confounding factors were adjusted using the multiple logistic regression analyses, including age, total daily calorie intake, gender, BMI, marital status, level of education, and history of CRC in the family (referred to as Model 2). These factors were chosen based on the findings of the systematic review and meta-analysis [27]. Risks were reported as odds ratios (OR) and 95 percent confidence intervals (CI). All data analysis was performed using IBM SPSS Statistics, Version 26.0 (Chicago, IL, USA), and statistics were considered significant with a p-value < 0.05.
Results
Recruitment and dietary assessment finalization
Initially, 355 eligible patients were recruited, but 28 did not complete the nutritional assessment (n = 20 for anthropometry and n = 8 for FFQ), 8 withdrew from the study, and 3 were diagnosed with other cancers (brain, urinary bladder, and small intestine). Out of the remaining 316 patients, 52 did not report a plausible intake which consisted of 14 patients underreported and 38 patients overreported their dietary intake. Hence, the final total of patients was 264 (79 cases and 185 controls) (Fig. 1).
Fig. 1.
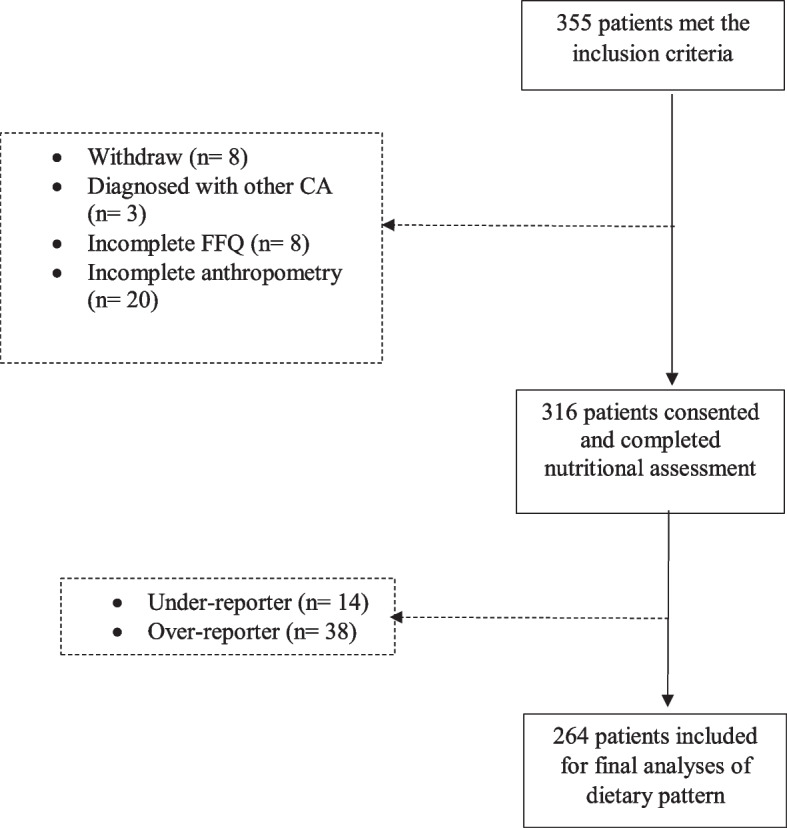
Flow chart indicating screening, recruitment, and dietary assessment finalization. CA, cancer; FFQ, food frequency questionnaire
Baseline characteristics of patients
Patient baseline characteristics are shown in Table 1. Cases were significantly older than controls (61.0 years ± 11.8 vs. 50.8 years ± 15.4) (p < 0.001) and were mostly Malay as compared to controls of Chinese and Indian ethnicity.
Table 1.
Baseline characteristics of the study patients (n = 264)
| Variables | Cases, n (%) | Controls, n (%) | p-value |
|---|---|---|---|
| Age (years)a | 61.0 (11.8) | 50.8 (15.4) | < 0.001 |
| Gender | 0.955 | ||
| Male | 43 (54.4) | 100 (54.1) | |
| Female | 36 (45.6) | 85 (45.9) | |
| Ethnicity | 0.003 | ||
| Malay | 63 (79.7) | 122 (65.9) | |
| Chinese | 16 (20.3) | 40 (21.6) | |
| Indian | 0 (0) | 23 (12.4) | |
| Study site | 0.042 | ||
| Urban area | 49 (62.0) | 89 (48.4) | |
| Suburban area | 30 (38.0) | 96 (51.6) | |
| Marital status | 0.016 | ||
| Single | 7 (8.9) | 37 (20.0) | |
| Married | 49 (62.0) | 117 (63.2) | |
| Divorced/ widowed | 23 (29.1) | 31 (16.8) | |
| Education | 0.116 | ||
| Primary | 13 (16.5) | 20 (10.8) | |
| Secondary/ college | 39 (49.4) | 73 (39.5) | |
| Higher institution | 27 (34.2) | 92 (49.7) | |
| Family history of CRCb | 0.018 | ||
| First degree relative | 23 (29.5) | 30 (16.5) | |
| Second degree relative | 10 (12.8) | 15 (8.2) | |
| Distant relative | 3 (3.8) | 3 (1.6) | |
| No family history CRC | 42 (53.8) | 134 (73.6) | |
| Occupation | 0.074 | ||
| Government/ semi-government worker | 20 (32.7) | 66 (35.9) | |
| Private sector worker | 11 (13.9) | 35 (19.0) | |
| Self-employed/ Own business | 8 (10.1) | 24 (13.0) | |
| Housewife | 14 (17.7) | 16 (8.7) | |
| Unemployed | 4 (5.1) | 11 (6.0) | |
| Pensioner | 22 (27.8) | 32 (17.4) | |
| Monthly household income (MYR)c | 0.128 | ||
| < 3860 | 50 (63.3) | 99 (53.8) | |
| 3861–8319 | 27 (34.2) | 69 (37.5) | |
| ≥ 8320 | 2 (2.5) | 16 (8.7) | |
| Smoking status | 0.606 | ||
| Active smoker | 15 (19.0) | 34 (20.5) | |
| Former smoker | 17 (21.5) | 44 (26.5) | |
| Nonsmoker | 47 (59.5) | 88 (53.0) | |
| Vitamin/ mineral supplement intake | ≤ 0.001 | ||
| Yes | 46 (59.0) | 42 (23.1) | |
| No | 32 (41.0) | 140 (76.9) | |
| BMI | 0.004 | ||
| Underweight | 49 (62.0) | 78 (42.2) | |
| Normal | 4 (5.1) | 5 (2.7) | |
| Overweight/Obese | 26 (32.9) | 102 (55.1) | |
| WC (cm), malea | 89.4 (16.1) | 97.1 (13.1) | 0.004 |
| WC (cm), femalea | 85.4 (13.8) | 90.2 (15.6) | 0.115 |
| HC (cm), malea | 96.8 (5.4) | 101.7 (7) | ≤ 0.001 |
| HC (cm), femalea | 101.3 (13.6) | 103.9 (10.7) | 0.267 |
| WHR (cm), malea | 0.9 (0.2) | 1.0 (0.1) | 0.245 |
| WHR (cm), femalea | 0.8 (0.1) | 0.9 (0.1) | 0.182 |
| Body fat %, malea | 24.5 (6.7) | 26.7 (6.3) | 0.069 |
| Body fat %, femalea | 31.8 (7.5) | 34.5 (8.4) | 0.096 |
| Energy intake (kcal)a | 1881.4 (396.9) | 1946.0 (362.3) | 0.199 |
p-value was calculated using the Chi-Square test
aData presented in mean (SD) and p-value was calculated by the independent T-test
bSample size was not always n = 264 due to missing values
cBased on the cut-off of Eleventh Malaysia Plan (2015)
In addition, vs. controls, cases were more likely to be from an urban area (p = 0.042), divorced or widowed (p = 0.016) and have a positive family history of CRC from the first-, second-, and distant relatives (p = 0.018). The proportions of BMI categories (p = 0.004), WC and HC in males (p = 0.004 and p ≤ 0.001, respectively) were significantly different between cases and controls. Case group significantly had lean BMI (underweight 62.0% and normal weight 5.1%) and lower WC (89.4 cm ± 16.1) and HC (96.8 cm ± 5.4) among males as compared to control group.
Identification of dietary patterns based on EFA
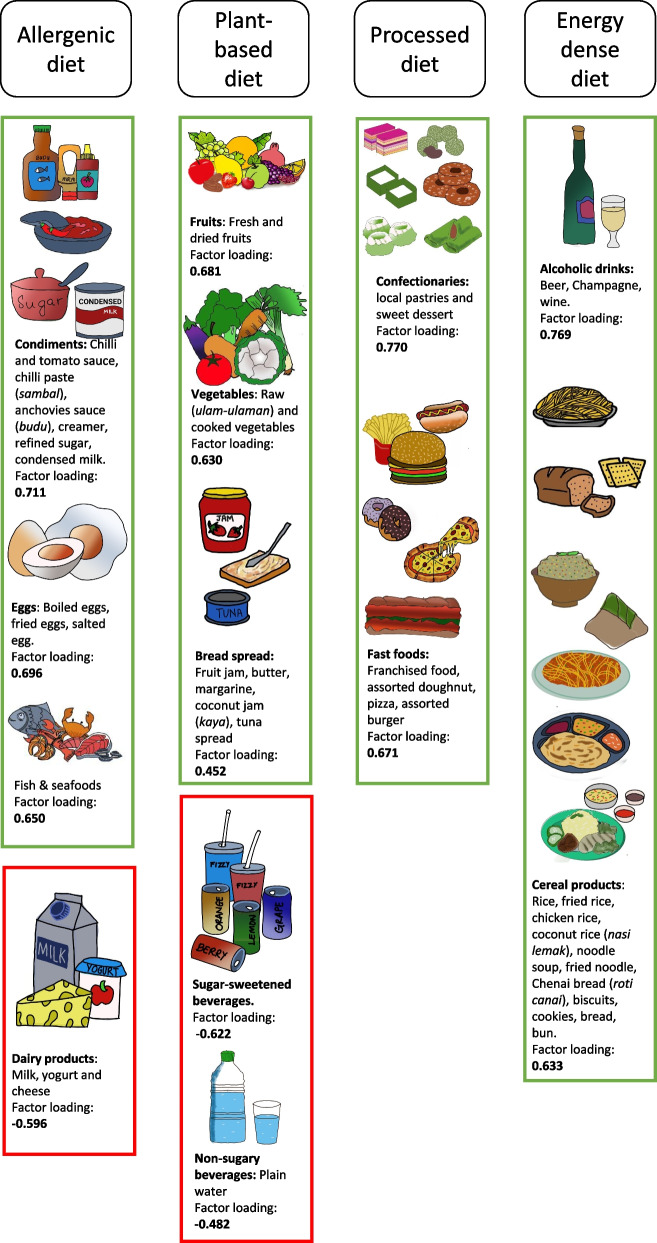
Four major dietary patterns were identified from EFA and factor labeling as shown in Fig. 2. A predefined food group was considered loaded on a specific pattern when its absolute factor loading was ≥ 0.3. The first was the allergenic diet pattern, characterized by high loadings for condiments, eggs, fish and seafood, and dairy products. The second was the plant-based diet pattern, loaded heavily on fresh fruits, dried fruits, raw vegetables, cooked vegetables, and bread spread. Next, the third was the processed diet pattern, which comprised of confectionaries and fast foods. The fourth was an energy-dense diet pattern with high loadings of alcoholic drinks and cereals.
Fig. 2.
Food groups within four dietary patterns with positive and negative loadings. Absolute values less than 0.2 were not listed for simplicity, and those above 0.3 were presented to visually emphasize the strength of association. The green box indicates positive factor loading, and the red box indicates negative factor loading
Association of dietary patterns with CRC
Table 2 reveals the odds ratios and their 95% confidence intervals for CRC by the tertiles of factor scores. After adjusting for potential covariates, the processed diet pattern was found to be associated with CRC (OR = 3.45; 95% CI = 1.25–9.52; P = 0.017). However, when adjusting with only age and total energy intake as confounders, the allergenic diet pattern and the processed diet pattern were found to be associated with CRC (OR = 2.34; 95% CI = 1.12–4.91; P = 0.024; OR = 2.68; 95% CI = 1.06–6.77; P = 0.037). The plant-based diet and energy-dense diets were not significantly associated with CRC (p > 0.05).
Table 2.
Odds ratios and 95% CI of colorectal cancer according to the four major dietary patterns
| Dietary pattern | Tertiles | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| OR | 95% CI | OR | 95% CI | ||
| Allergenic diet | |||||
| dModel 1 | 1.00 | 1.12 | 0.52, 2.41 | 2.34 | 1.12, 4.91 |
| eModel 2 | 1.00 | 0.64 | 0.27, 1.52 | 1.34 | 0.61, 3.14 |
| Plant-based diet | |||||
| dModel 1 | 1.00 | 1.60 | 0.83, 3.09 | 0.68 | 0.32, 1.46 |
| eModel 2 | 1.00 | 1.43 | 0.69, 2.97 | 0.45 | 0.19, 1.04 |
| Processed-diet | |||||
| dModel 1 | 1.00 | 1.43 | 0.68, 2.99 | 2.68 | 1.06, 6.77 |
| eModel 2 | 1.00 | 1.27 | 0.57, 2.84 | 3.45 | 1.25, 9.52 |
| Energy-dense diet | |||||
| dModel 1 | 1.00 | 0.68 | 0.33, 1.38 | 0.45 | 0.19, 1.05 |
| eModel 2 | 1.00 | 0.54 | 0.25, 1.15 | 0.53 | 0.21, 1.31 |
dAdjusted for age and total energy intake, eAdjusted for: age, total energy intake, gender, body mass index, marital status, education, and family history of CRC; significant associations are in bold
Discussion
The current findings provided the first evidence of a link between higher factor loading, which reflected dietary patterns with greater potential food groups, and an elevated risk of CRC in a multi-ethnic Malaysian population. The findings of this population, whose dietary choices differ markedly from those of the more well-studied populations in Western countries, reaffirm that the use of the EFA as a tool for relating the dietary pattern to CRC in a wide range of populations. This study’s findings added to the corroboration that some dietary patterns are incorporated with the CRC studies in United States [25, 28–35], European [36–39], and Asian populations [14, 40–43]. Thus, four dietary patterns were identified from 16 food groups, including the allergenic diet, plant-based diet, processed diet, and energy dense diet. Then, the processed diet was found to be associated with CRC in the Malaysian population.
Predominantly, previous reported studies associated with CRCs have classified the dietary pattern into two groups: the western/unhealthy pattern and the prudent/healthy pattern [29, 30, 35, 44–48]. This current study found that processed diet being associated with CRCs would be considered the western/unhealthy pattern, and the food groups loaded in both patterns shared similarities [38]. The processed diet pattern included confectionaries and fast foods, may have contributed to the patterns known as light meals, Japanese meals, animal food patterns, and high-dairy, high-fruit and vegetable, high-starch and low-alcohol diets (DFSA) [27]. A multicase-control study from 11 Spanish provinces had primarily several studies to assess associations of dietary pattern with gastric, breast and prostate cancer. Another study was reconstructed to observe the association between dietary patterns and CRC. Results produced were consistent with the primary previous study which showed that the western dietary pattern had putative risk for breast and gastric cancer [49]. In addition, western dietary patterns are associated with an increased risk of CRC, particularly distal colon and rectal tumors [35].
It is evident from this study that the processed diet consisted primarily of fast food (particularly processed meat and franchised meals) and confectionaries (mainly refined carbohydrates and sugars from local pastries and sweet desserts). A population-based case–control study in Ontario, Canada, found that westernized dietary patterns were associated with a statistically significant increased risk of early onset CRC. Their western-like dietary pattern of derivation consists of red meat, processed meat, sugary drinks, sugary desserts, fast food, and processed snacks [50]. Meanwhile, higher consumption of ultra-processed foods (meat, poultry, and seafood-based ready-to-eat products) among men and ready-to-eat/heat mixed dishes among women was associated with increased risk of colorectal cancer in three prospective US cohort studies [51]. A systematic review proved that a raised of CRC development from six cohort studies with adherence to western dietary pattern which comprised of refined grain and red and processed meat [52].
A meta-analysis with twenty-eight studies reported an increase in CRC risk (relative risk (RR) = 1.25; 95% CI = 1.11–1.40; P < 0.001) when compared to the western vs. prudent dietary patterns. This confirmed that a diet rich in meats (red and processed), refined grains and sugar-rich food is linked with an increased risk of CRC [27]. This result was also supported by the previous review that found the harms of a diet high in meats, refined grains, and added sugar [53]. Moreover, a recent systematic review of meta-analyses on whole grain and refined grain concluded refined grains was related to gastric and colon cancer [54]. However, most studies covered in that meta-analyses had no exact definition of refined grains. This led to poor outcomes because refined grains were commonly referred to both staple refined grain and indulgent refined grain foods [54]. Bread, cereal, pasta, and rice are examples of staple refined grains, as are cakes, cookies, pastries, sweets or grain-based desserts which are indulgent refined grains [55]. It is necessary to interpret results from the perspective of how refined grains have been defined [55].
A case–control study conducted in Iran showed that sweets and desserts (including pastry), combined with red meat, soft drinks, and high-fat dairies, had higher factor loading in the western dietary pattern and was associated with CRC [56]. This is in line with the current study that showed fast foods, local pastries, and sweet desserts were associated with CRC. An Iranian pattern was significantly associated with an increased odds of CRC which derived from processed meat (sausages, hamburger and salami) and sweets and dessert (chocolate, dry sweet, cookies, cakes, pastry, jam, honey, halvah) [57]. In addition, an Iran case–control study found significant associations were reported between different types of dietary carbohydrates: maltose (OR = 9.03, CI 95%: 3.93–20.78, P < 0.001), fructose (OR = 1.31, CI 95%: 1.19–1.43, P < 0.001), galactose (OR = 1.31, CI 95%: 1.07–1.6, P = 0.008), sucrose (OR = 1.19, CI 95%: 1.12. − 1.25, P < 0.001), glucose (OR = 1.06, CI 95%: 1.01–1.11, P = 0.009), sugar (OR = 1.02, CI 95%: 1.01–1.03, P < 0.001), lactose (OR = 1.009, CI 95%: 1.01–1.18, P = 0.02) and carbohydrate (OR = 1.009, CI 95%: 1.003–1.01, P = 0.002) [58]. However, a prospective Japanese cohort study (Asian population) among middle-aged adults showed insignificant association of all sugar types with CRC risk except a positive association of total sugar intake in women with rectal cancer specifically [59].
Mechanisms of these diets in the causation of CRCs have been studied widely. Various molecules are involved in the causation, including heme iron, N-nitroso-compound (NOC), heterocyclic amines (HCAs), and polycyclic aromatic hydrocarbons (PAHs) [60]. Various theories have also been determined on the potential carcinogenicity of red and processed meat. The probable involvement of PAHs and HACs in the carcinogenesis brought on by DNA mutation is described via a proposed mechanism. Another theory, heme produces cytotoxic and genotoxic aldehydes that leads to the development of cancer by causing lipid peroxidation and the subsequent creation of NOCs [61]. Sugar consumption was linked to inflammation or angiogenesis, as demonstrated in the recent global ColoCare Study [62]. In addition, raised plasma insulin levels and insulin-like growth factor-1, both known to promote tumor growth [27, 63].
This study has several strengths. First, this study represents the Malaysian population with different socio-economic background because the recruitment was conducted from two different geographical location. Each site was west and east Peninsular Malaysia and at the same time, covering urban and suburban regions. Second, the dietary questionnaire used in the current study was specifically modified and validated for this study [21]. Third, patients with dietary reporting bias have been omitted using the Goldberg equation. Fourth, instead of a single nutrient or food approach, EFA was used to derive new and non-correlated variables in order to explain the variations in dietary habits. This allowed the researcher to obtain a more comprehensive and accurate picture of dietary exposures in a population. Finally, when exploring the relationships between dietary patterns and CRC, a multivariate logistic regression model that controlled for a wide range of potential confounding factors was fitted.
There are several noteworthy limitations. First, the purposive sampling can be prone to difficulty in defending the population representativeness, but this has been lessened by recruiting patients from different study sites. Second dietary patterns we observed may not be comparable with other studies due to variances in dietary patterns based on ethnicity, culture, religion, geography, and other socioeconomic determinants. Third, one year of dietary recall might cause memory bias, and overreporting is common when using the FFQ. Thus, this limitation has been minimized by excluding implausible dietary reporters.
Conclusion
The present study has identified four dietary patterns from 16 food groups in the Malaysian diet, including an allergenic diet, a plant-based diet, a processed diet, and an energy-dense diet. Only the processed diet has been found to be associated with CRCs in the Malaysian population. The association between dietary patterns and fecal microbiota could be further investigated to address lifestyle modification for CRC prevention.
Acknowledgements
The authors would like to thank all participants for their valuable input, nurses and doctors for their assistance during the study.
Abbreviations
- BMI
Body mass index
- BMR
Basal metabolic rate
- CL
Confidence limit
- CRC
Colerectal cancer
- DEXA
Dual-energy X-ray absorptiometry method
- DFSA
High-dairy, high-fruit and vegetable, high-starch, low-alcohol diets
- EFA
Exploratory factor analysis
- EI
Energy intake
- CRC
Colorectal cancer
- FFQ
Food frequency questionnaire
- HC
Hip circumference
- HCA
Heterocyclic amines
- HCTM UKM
Hospital Canselor Tuanku Muhriz Universiti Kebangsaan Malaysia
- HUSM
Hospital Universiti Sains Malaysia
- MyFCD
Malaysian Food Composition Database
- NHMS
National and Health Morbidity Survey
- NOC
N-nitroso-compound
- OR
Odds ratio
- PAH
Polycyclic aromatic hydrocarbons
- PAL
Physical activity level
- PS
Power and sample size
- RR
Relative risk
- US
United States
- WC
Waist circumference
- WHO
World Health Organization
Authors’ contributions
HJJM, MRS designed and HJJM, MRS, YYL and RARA supervised the study. AAAR, LSA, and NHS helped gather and LSA conducted the literature review. AAAR analyzes and interprets the data and AAAR and LSA wrote the original draft of the manuscript. All authors corrected and approved the final manuscript.
Funding
The study is funded by the Long-Term Research Grant Scheme (LRGS)-Malaysia Research University Network (MRUN) (203/PPSK/6720021, LRGS/MRUN/FI/02/2018/01, and LR001-2019).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All patients gave written informed consent to be included in the study. The study was conducted in compliance with the Declaration of Helsinki. Ethics approval was granted by the Human Research Ethics Committee of Universiti Sains Malaysia (USM/JEPeM/19060354) and the Universiti Kebangsaan Malaysia Medical and Research Ethics Committee (UKMREC; FF-2020–005).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International agency for research on cancer. Cancer fact sheets. 2020. https://gco.iarc.fr/today/fact-sheets-cancers. Accessed 24 Nov 2022.
- 2.Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–1013. doi: 10.1016/S1470-2045(21)00199-6. [DOI] [PubMed] [Google Scholar]
- 3.Ismail I, Chan HK, Aiman SS, Radzi AHM. A 10-year registry-based incidence, mortality, and survival analysis of colorectal cancer in Northern Malaysia. J Cancer Res Ther. 2022;18(4):931–8. [DOI] [PubMed]
- 4.Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel) 2021;13(9):2025. doi: 10.3390/cancers13092025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takachi R, Tsubono Y, Baba K, Inoue M, Sasazuki S, Iwasaki M, et al. Red meat intake may increase the risk of colon cancer in Japanese, a population with relatively low red meat consumption. Asia Pac J Clin Nutr. 2011;20(4):603–612. [PubMed] [Google Scholar]
- 6.Nayak SP, Sasi MP, Sreejayan MP, Mandal S. A case-control study of roles of diet in colorectal carcinoma in a South Indian Population. Asian Pac J Cancer Prev. 2009;10(4):565–568. [PubMed] [Google Scholar]
- 7.Abu Mweis SS, Tayyem RF, Shehadah I, Bawadi HA, Agraib LM, Bani-Hani KE, et al. Food groups and the risk of colorectal cancer: results from a Jordanian case–control study. Eur J cancer Prev. 2015;24(4):313–320. doi: 10.1097/CEJ.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 8.DellaValle CT, Xiao Q, Yang G, Shu X-O, Aschebrook-Kilfoy B, Zheng W, et al. Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women’s Health Study. Int J cancer. 2014;134(12):2917–2926. doi: 10.1002/ijc.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Villegas A, Martínez-Lapiscina EH. A healthy diet for your heart and your brain. The prevention of cardiovascular disease through the Mediterranean Diet. 2018.
- 10.Labeda I, Syarifuddin E, Uwuratuw J, Pattelongi I, Faruk M. analysis of pik3ca expression to clinicopathology features of colorectal cancer in makassar, Indonesia. Int J Surg Med. 2020;1. Available from: https://www.ejmanager.com/fulltextpdf.php?mno=65553.
- 11.Butler LM, Wang R, Koh WP, Yu MC. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br J Cancer. 2008;99(9):1511–1516. doi: 10.1038/sj.bjc.6604678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safari A, Shariff ZM, Kandiah M, Rashidkhani B, Fereidooni F. Dietary patterns and risk of colorectal cancer in Tehran Province: a case–control study. BMC Public Health. 2013;13(1):1–9. [DOI] [PMC free article] [PubMed]
- 13.Wang Z, Uchida K, Ohnaka K, Morita M, Toyomura K, Kono S, et al. Sugars, sucrose and colorectal cancer risk: the Fukuoka colorectal cancer study. Scand J Gastroenterol. 2014;49(5):581–588. doi: 10.3109/00365521.2013.822091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park Y, Lee J, Oh JH, Shin A, Kim J. Dietary patterns and colorectal cancer risk in a Korean population: A case-control study. Medicine. 2016;95(25):e3759. [DOI] [PMC free article] [PubMed]
- 15.Zheng X, Hur J, Nguyen LH, Liu J, Song M, Wu K, et al. Comprehensive assessment of diet quality and risk of precursors of early-onset colorectal cancer. JNCI J Natl Cancer Inst. 2021;113(5):543–552. doi: 10.1093/jnci/djaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Statistics Malaysia. Current Population Estimates. Prime Minister’s Department of Malaysia. 2022. p. 140. Available from: https://newss.statistics.gov.my/newss-portalx/ep/epFreeDownloadContentSearch.seam?cid=57206. Cited 2022 Jan 17.
- 17.National Cancer Institute. Malaysian National Cancer Registry Report (2012 - 2016). 2019th ed. Malaysia Ministry of Health; 2019. Available from: https://nci.moh.gov.my/index.php/ms/pengumuman/340-national-cancer-registry-report
- 18.National Health and Nutrition Examination Survey (NHANES). Anthropometry Procedure Manual. Ministry of Health of Malaysia. 2013. Available from: https://www.cdc.gov/nchs/nhanes/nhanes2013-2014/manuals13_14.htm. Cited 2023 Feb 27.
- 19.Tanita. Body composition analyzer SC-330 instruction manual. Tokyo: TANITA Corporation; 2008.
- 20.WHO Consultation on Obesity & World Health Organization. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 1999 & 2000. [PubMed]
- 21.Abd Rashid AA, Ashari LS, Shafiee NH, Ali RAR, Yeh LY, Shahril MR, et al. Validity and Reproducibility of Malaysian Food Frequency Questionnaire for Dietary Intake Related to Colorectal Cancer. J Gizi dan Pangan. 2022;17(2):77–86. doi: 10.25182/jgp.2022.17.2.77-86. [DOI] [Google Scholar]
- 22.IPH. Album Makanan Malaysia. Kuala Lumpur: Institute for Public Health, Ministry of Health Malaysia; 2011.
- 23.MYFCD. Welcome to Malaysian Food Composition Database (MyFCD). 2020. Available from: https://myfcd.moh.gov.my. Cited 2022 Nov 2.
- 24.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes. 2000;24(9):1119–30. doi: 10.1038/sj.ijo.0801376. [DOI] [PubMed] [Google Scholar]
- 25.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Zhu Y, Wang PP, Roebothan B, Zhao J, Zhao J, et al. Reported intake of selected micronutrients and risk of colorectal cancer: results from a large population-based case–control study in Newfoundland, Labrador and Ontario. Canada Anticancer Res. 2012;32(2):687–696. [PubMed] [Google Scholar]
- 27.Garcia-Larsen V, Morton V, Norat T, Moreira A, Potts JF, Reeves T, et al. Dietary patterns derived from principal component analysis (PCA) and risk of colorectal cancer: a systematic review and meta-analysis. Vol. 73, European journal of clinical nutrition. 2019. p. 366–86. [DOI] [PubMed]
- 28.Randall E, Marshall JR, Brasure J, Graham S. Dietary patterns and colon cancer in western New York. Nutr Cancer. 1992;18(3):265–76. [DOI] [PubMed]
- 29.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma K-N. Eating patterns and risk of colon cancer. Am J Epidemiol. 1998;148(1):4–16. doi: 10.1093/aje/148.1.4-a. [DOI] [PubMed] [Google Scholar]
- 30.Fung T, Hu FB, Fuchs C, Giovannucci E, Hunter DJ, Stampfer MJ, et al. Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med. 2003;163(3):309–314. doi: 10.1001/archinte.163.3.309. [DOI] [PubMed] [Google Scholar]
- 31.Flood A, Rastogi T, Wirfält E, Mitrou PN, Reedy J, Subar AF, et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr. 2008;88(1):176–184. doi: 10.1093/ajcn/88.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satia JA, Tseng M, Galanko JA, Martin C, Sandler RS. Dietary patterns and colon cancer risk in Whites and African Americans in the North Carolina Colon Cancer Study. Nutr Cancer. 2009;61(2):179–193. doi: 10.1080/01635580802419806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, et al. Dietary patterns, food groups, and rectal cancer risk in Whites and African-Americans. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1552–1561. doi: 10.1158/1055-9965.EPI-08-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller PE, Lazarus P, Lesko SM, Muscat JE, Harper G, Cross AJ, et al. Diet index-based and empirically derived dietary patterns are associated with colorectal cancer risk. J Nutr. 2010;140(7):1267–1273. doi: 10.3945/jn.110.121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR, et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology. 2017;152(8):1944–1953. doi: 10.1053/j.gastro.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terry P, Hu FB, Hansen H, Wolk A. Prospective study of major dietary patterns and colorectal cancer risk in women. Am J Epidemiol. 2001;154(12):1143–1149. doi: 10.1093/aje/154.12.1143. [DOI] [PubMed] [Google Scholar]
- 37.Kesse E, Clavel-Chapelon F, Boutron-Ruault M-C. Dietary patterns and risk of colorectal tumors: a cohort of French women of the National Education System (E3N) Am J Epidemiol. 2006;164(11):1085–1093. doi: 10.1093/aje/kwj324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Wu H, Wang PP, Savas S, Woodrow J, Wish T, et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. 2013;3(2):e002270. doi: 10.1136/bmjopen-2012-002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Wang PP, Woodrow J, Zhu Y, Roebothan B, Mclaughlin JR, et al. Dietary patterns and colorectal cancer: results from a Canadian population-based study. Nutr J. 2015;14:8. [DOI] [PMC free article] [PubMed]
- 40.Kim MK , Sasaki S, Otani T, Tsugane S, Group JPHCPS Dietary patterns and subsequent colorectal cancer risk by subsite: a prospective cohort study. Int J cancer. 2005;115(5):790–8. doi: 10.1002/ijc.20943. [DOI] [PubMed] [Google Scholar]
- 41.Kurotani K, Budhathoki S, Joshi AM, Yin G, Toyomura K, Kono S, et al. Dietary patterns and colorectal cancer in a Japanese population: the Fukuoka Colorectal Cancer Study. Br J Nutr. 2010;104(11):1703–1711. doi: 10.1017/S0007114510002606. [DOI] [PubMed] [Google Scholar]
- 42.Kumagai Y, Chou W-T, Tomata Y, Sugawara Y, Kakizaki M, Nishino Y, et al. Dietary patterns and colorectal cancer risk in Japan: the Ohsaki Cohort Study. Cancer Causes Control. 2014;25:727–736. doi: 10.1007/s10552-014-0375-5. [DOI] [PubMed] [Google Scholar]
- 43.Shin S, Saito E, Sawada N, Ishihara J, Takachi R, Nanri A, et al. Dietary patterns and colorectal cancer risk in middle-aged adults: A large population-based prospective cohort study. Clin Nutr. 2018;37(3):1019–1026. doi: 10.1016/j.clnu.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Wu K, Hu FB, Fuchs C, Rimm EB, Willett WC, Giovannucci E. Dietary patterns and risk of colon cancer and adenoma in a cohort of men (United States) Cancer Causes Control. 2004;15:853–862. doi: 10.1007/s10552-004-1809-2. [DOI] [PubMed] [Google Scholar]
- 45.De Stefani E, Deneo-Pellegrini H, Ronco AL, Correa P, Boffetta P, Aune D, et al. Dietary patterns and risk of colorectal cancer: a factor analysis in Uruguay. Asian Pac J Cancer Prev. 2011;12(3):753–759. [PubMed] [Google Scholar]
- 46.Fung TT, Kashambwa R, Sato K, Chiuve SE, Fuchs CS, Wu K, et al. Post diagnosis diet quality and colorectal cancer survival in women. PLoS ONE. 2014;9(12):e115377. doi: 10.1371/journal.pone.0115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geijsen AJMR, Kok DE, van Zutphen M, Keski-Rahkonen P, Achaintre D, Gicquiau A, et al. Diet quality indices and dietary patterns are associated with plasma metabolites in colorectal cancer patients. Eur J Nutr. 2021;60(6):3171–84. [DOI] [PMC free article] [PubMed]
- 48.Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer. 2020;20(2):125–39. [DOI] [PubMed]
- 49.Castelló A, Amiano P, Fernández de Larrea N, Martín V, Alonso MH, Castaño-Vinyals G, et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr. 2019;58:1495–505. doi: 10.1007/s00394-018-1674-5. [DOI] [PubMed] [Google Scholar]
- 50.Chang VC, Cotterchio M, De P, Tinmouth J. Risk factors for early-onset colorectal cancer: a population-based case–control study in Ontario. CA Cancer J Clin. 2021;32(10):1063–1083. doi: 10.1007/s10552-021-01456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Du M, Wang K, Khandpur N, Rossato SL, Drouin-Chartier JP, et al. Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohort studies. bmj. 2022;378(e068921):1. [DOI] [PMC free article] [PubMed]
- 52.Yusof AS, Isa ZM, Shah SA. Dietary patterns and risk of colorectal cancer: a systematic review of cohort studies (2000–2011) Asian Pacific J Cancer Prev. 2012;13(9):4713–4717. doi: 10.7314/APJCP.2012.13.9.4713. [DOI] [PubMed] [Google Scholar]
- 53.Fung TT, Brown LS. Dietary patterns and the risk of colorectal cancer. Curr Nutr Rep. 2013;2:48–55. doi: 10.1007/s13668-012-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaesser GA. Whole grains, refined grains, and cancer risk: A systematic review of meta-analyses of observational studies. Nutrients. 2020;12(12):3756. doi: 10.3390/nu12123756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaesser GA. Perspective: refined grains and health: genuine risk, or guilt by association? Adv Nutr. 2019;10(3):361–371. doi: 10.1093/advances/nmy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bahrami A, Houshyari M, Jafari S, Rafiei P, Mazandaranian M, Hekmatdoost A, et al. Dietary patterns and the risk of colorectal cancer and adenoma: a case control study in Iran. Gastroenterol Hepatol Bed Bench. 2019;12(3). [PMC free article] [PubMed]
- 57.Azizi H, Asadollahi K, Esmaeili ED, Mirzapoor M. Iranian dietary patterns and risk of colorectal cancer. Heal Promot Perspect. 2015;5(1):72. doi: 10.15171/hpp.2015.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonoush M, Fathi S, Hassanpour Ardekanizadeh N, Khalatbari Mohseni G, Majidi N, Keshavarz SA, et al. The Association Between Different Types of Dietary Carbohydrates and Colorectal Cancer: A Case-Control Study. Front Nutr. 2022;9:898337. doi: 10.3389/fnut.2022.898337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanehara R, Katagiri R, Goto A, Yamaji T, Sawada N, Iwasaki M, et al. Sugar intake and colorectal cancer risk: A prospective Japanese cohort study. Cancer Sci. 2023;114(6):2584. doi: 10.1111/cas.15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouvard V, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 61.Cascella M, Bimonte S, Barbieri A, Del Vecchio V, Caliendo D, Schiavone V, et al. Dissecting the mechanisms and molecules underlying the potential carcinogenicity of red and processed meat in colorectal cancer (CRC): an overview on the current state of knowledge. Infect Agent Cancer. 2018;13. [DOI] [PMC free article] [PubMed]
- 62.Stewart KL, Gigic B, Himbert C, Warby CA, Ose J, Lin T, et al. Association of Sugar Intake with Inflammation- and Angiogenesis-Related Biomarkers in Newly Diagnosed Colorectal Cancer Patients. Nutr Cancer. 2022;74(5):1636–1643. doi: 10.1080/01635581.2021.1957133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stefani C, Miricescu D, Stanescu-Spinu II, Nica RI, Greabu M, Totan AR, et al. Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: where are we now? Int J Mol Sci. 2021;22(19):10260. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.