Abstract
Background
Assisted reproductive technology (ART) has brought good news to infertile patients, but how to improve the pregnancy outcome of poor ovarian response (POR) patients is still a serious challenge and the scientific evidence of some adjuvant therapies remains controversial.
Aim
Based on previous evidence, the purpose of this systematic review and network meta-analysis was to evaluate the effects of DHEA, CoQ10, GH and TEAS on pregnancy outcomes in POR patients undergoing in vitro fertilization and embryo transplantation (IVF-ET). In addition, we aimed to determine the current optimal adjuvant treatment strategies for POR.
Methods
PubMed, Embase, The Cochrane Library and four databases in China (CNKI, Wanfang, VIP, SinoMed) were systematically searched up to July 30, 2022, with no restrictions on language. We included randomized controlled trials (RCTs) of adjuvant treatment strategies (DHEA, CoQ10, GH and TEAS) before IVF-ET to improve pregnancy outcomes in POR patients, while the control group received a controlled ovarian stimulation (COS) regimen only. This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The surface under the cumulative ranking curve (SUCRA) was used to provide a pooled measure of cumulative ranking for each outcome.
Results
Sixteen RCTs (2323 women) with POR defined using the Bologna criteria were included in the network meta-analysis. Compared with the control group, CoQ10 (OR 2.22, 95% CI: 1.05 to 4.71) and DHEA (OR 1.92, 95% CI: 1.16 to 3.16) had obvious advantages in improving the clinical pregnancy rate. CoQ10 was the best in improving the live birth rate (OR 2.36, 95% CI: 1.07 to 5.38). DHEA increased the embryo implantation rate (OR 2.80, 95%CI: 1.41 to 5.57) and the high-quality embryo rate (OR 2.01, 95% CI: 1.07 to 3.78) and number of oocytes retrieved (WMD 1.63, 95% CI: 0.34 to 2.92) showed a greater advantage, with GH in second place. Several adjuvant treatment strategies had no significant effect on reducing the cycle canceling rate compared with the control group. TEAS was the least effective of the four adjuvant treatments in most pooled results, but the overall effect appeared to be better than that of the control group.
Conclusion
Compared with COS regimen, the adjuvant use of CoQ10, DHEA and GH before IVF may have a better clinical effect on the pregnancy outcome of POR patients. TEAS needs careful consideration in improving the clinical pregnancy rate. Future large-scale RCTs with direct comparisons are needed to validate or update this conclusion.
Systematic review registration
PROSPERO CRD42022304723
Supplementary Information
The online version contains supplementary material available at 10.1186/s12958-023-01119-0.
Keywords: Poor ovarian response, IVF-ET, Assisted reproductive technology, NMA, Systematic review
Introduction
Infertility is a common reproductive disease. Although a large number of in vitro Fertilization and Embryo Transplantation (IVF-ET) technologies have been carried out worldwide, the clinical pregnancy rate is only 30–40% [1–3]. During controlled ovarian ovarian stimulation (COS), the incidence of poor ovarian response (POR) is approximately 9–24% [4–6] and the proportion continues to increase [7, 8]. Studies have shown that the cumulative pregnancy rate of POR patients in repeated IVF-ET cycles is only 10–20% [9], which seriously affects the quality of life of patients and requires a large amount of medical resources.
POR is a pathological condition in which the ovary shows a low response to exogenous gonadotropin stimulation, mainly manifested by a decrease in the number and quality of mature oocytes obtained and a decrease in the number of transferable embryos. There are more than 40 criteria used to define POR [10], but no consensus has been reached. In 2011, the European Society of Human Reproduction and Embryology (ESHRE) proposed the Bologna standard [11]. In most subsequent studies, Bologna criteria have been widely used to define POR. For decades, modern medicine has adopted a variety of interventions to improve the outcome of IVF in POR patients, but the results are not ideal [12]. For example, increased gonadotropin use did not result in a higher pregnancy rate [13]. GnRH analogues did not significantly differ between the number of oocytes obtained and the clinical pregnancy rate [14]. Patients with POR did not benefit substantially from natural cycle IVF and the cumulative live birth rate per patient did not exceed 8% [15]. Therefore, some drug intervention before COS has become an important way to enhance the effect of exogenous gonadotrophins and improve the number and quality of oocytes. The commonly used adjuvant interventions include recombinant LH, letrozole, dehydroepiandrosterone, testosterone, growth hormone, clomiphene, estradiol, hCG, clomiphene and aspirin, among others [8].
Previous studies have directly compared the efficacy and safety of adjuvant treatment strategies for POR [7, 14, 16, 17]. However, traditional meta-analysis can only compare direct evidence and cannot determine the most effective treatment measures for patients with POR. Network meta-analysis (NMA) enables the comparison of direct and indirect evidence for multiple treatment measures in a statistical model [18, 19]. In 2020, an NMA involving 19 RCTs and 2677 POR patients assessed the impact of multiple adjuvant treatment strategies on pregnancy rates in POR patients undergoing IVF. The results showed that COS regimens with adjuvant DHEA, CoQ10 and GH showed better clinical outcomes in achieving pregnancy and required a lower dose of gonadotropin for ovulation [20]. Here, we propose another adjunctive treatment measure, transcutaneous electrical acupoint stimulation (TEAS). TEAS, which uses low-frequency pulsed current to generate electrical stimulation through electrodes attached to acupoints, is a noninvasive, painless and safe treatment technique compared with traditional acupuncture [21, 22] and is commonly used to improve IVF-ET pregnancy outcomes.
At present, RCTs continue to increase and the scientific evidence supporting these adjuvant treatments remains controversial and needs to be updated. Therefore, this study selected four adjuvant treatment strategies: DHEA, CoQ10, GH and TEAS for NMA; DHEA, CoQ10 and GH are based on previous evidence [20]. We tried to analyze direct and indirect evidence through NMA to determine the best adjuvant treatment strategy for current POR patients and provide evidence-based support.
Methods
Inclusion criteria
Study design and participants
The protocol for this manuscript has been registered in advance on the PROSPERO platform (CRD42019147503). We will report the literature in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [23]. In order to obtain data directly, only published randomized controlled trials with full text available were included in this study. Subjects were POR patients undergoing IVF-ET and POR was strictly defined using the Bologna criteria. Bologna criteria: (a) advanced maternal age (≥ 40 years) or any other risk factor for POR; (b) a previous POR (≤ 3 oocytes with a conventional stimulation protocol); and (c) an abnormal ovarian reserve test (i.e. AFC < 5–7 follicles or AMH < 0.5–1.1 ng/ml). POR can be diagnosed if at least two of the three characteristics above are met. If POR occurs after two cycles of maximum ovarian stimulation regimen, POR can also be diagnosed directly.
Interventions
DHEA, CoQ10, GH and TEAS were used as adjuvant treatment therapies in the experimental group, all of which could be combined with the COS regimen. The drug dose, drug type, acupoint selection, frequency, waveform and cycle of each RCT are not limited. The control group only received the COS regimen. Comparisons of different doses, points or durations of the same treatment, or the use of two or more adjuvant treatments in the same group were excluded.
Outcomes
The primary outcome was clinical pregnancy rate; secondary outcomes included: (1) embryo implantation rate; (2) high-quality embryo rate; (3) cycle canceling rate; (4) live birth rate; and (5) number of oocytes retrieved. Eligible RCTs included at least the primary outcome clinical pregnancy rate, otherwise they were excluded. Clinical pregnancy was defined as the presence of a gestational sac or fetal heartbeat using ultrasound [24].
Search strategy
PubMed, Embase, The Cochrane Library and four databases in China (CNKI, Wanfang, VIP and SinoMed) were systematically searched up to July 30, 2022, with no language restrictions. Both MeSH terms and text terms were used in the literature search to identify potential RCTs and the search strategy was adjusted according to different databases (Supplementary Table S1). In addition, we performed a manual search of relevant references to identify additional eligible studies.
Study selection and data extraction
Two reviewers independently searched and screened the literature according to the search strategy. Before the data were retrieved, the disputed RCT was discussed by the reproductive experts to determine whether it would eventually be incorporated in the analysis. Two reviewers then independently extracted the basic characteristics of each RCT according to a standardized form, including first author, publication year, language, sample size, age, diagnostic criteria, intervention details and outcomes. All of the above differences were discussed and a consensus was reached by two people. The differences were resolved by the third reviewer.
Quality assessment of risk of bias
Two reviewers used the Cochrane handbook to evaluate each risk of bias (RoB). Assessments included random sequence generation, allocation concealment, blinding of participants and staff, blinding of outcome assessments, incomplete outcome data, selective reporting and other biases. Each area was rated as low risk, high risk, or unclear. Any discrepancies were determined by a third reviewer.
Data synthesis and statistical analysis
NMA analysis was performed using Stata15.1, where the odds ratio (OR) and 95% confidence interval (95% CI) were used for dichotomous data and weighted mean difference (WMD) and 95% CI were used for continuous data. The network graph is used to show the results of direct comparisons of different interventions between studies. The size of nodes in the network graph is the sample size of the intervention and the thickness of lines between nodes represents the number of RCTs directly compared between the two interventions. Then, global and local inconsistency were evaluated (node-splitting method). If P > 0.05, this indicated that there was no significant difference in the estimated effect size between direct and indirect comparisons and the consistency model was used. We used the surface under the cumulative ranking curve (SUCRA) to provide aggregated measures of cumulative ranking for each outcomes, with the results presented as percentages. For direct data, pairwise meta-analysis was performed using a random-effects model and the OR value was converted to number of treatments required (NNT) to account for clinical pregnancy rate, embryo implantation rate, high-quality embryo rate, cycle canceling rate and live birth rate. Finally, we used adjusted comparison funnel plots to assess the impact of small studies.
Results
Study selection and characteristics
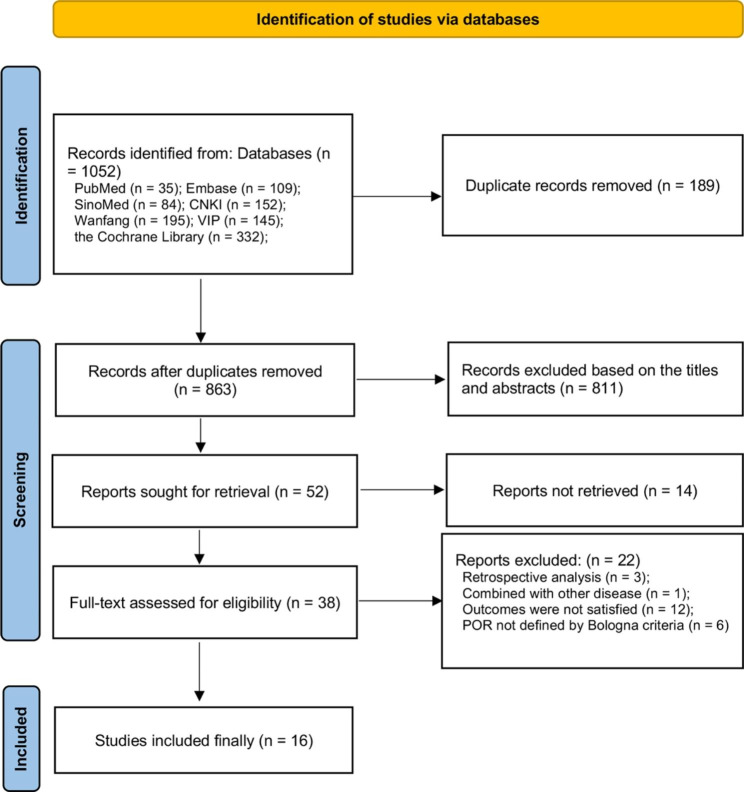
A total of 1052 publications were retrieved from 7 databases, with 189 duplicates excluded as well as 811 papers according to the title and abstract. After further reading the full text, 16 RCTs [25–40] were finally included in this study (see Fig. 1 for the detailed flow chart). Supplementary Table S2 shows the exclusion list and reasons. All 16 RCTs were single-center studies, including 11 in China, 3 in Egypt and the remaining two in Iran and South Korea. Sample sizes for individual RCTs ranged from 38 to 821 and all studies used Bologna diagnostic criteria to define POR. The 16 RCTs included 2323 participants with POR undergoing IVF who were randomly assigned to receive four different adjuvant therapies, including DHEA, GH, CoQ10 and TEAS. The control group was the COS regimen and the specific characteristics of the study are shown in Table 1.
Fig. 1.
The screening flow chart
Table 1.
Characteristics of included studies
| Included studies | Country | Sample size (I/C) | Duration of infertility [y, mean (SD)] (I/C) | BMI [kg/m2] (I/C) | Age [y, mean (SD)] (I/C) | Diagnostic criteria | Intervention (dose/duration) | Control | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Hu R, 2018 | China | 41/37 | 5(4.60)/4.67(3.84) | 21.67(2.81)/22.45(3.12) | 37.33 (3.07)/36.67 (3.86) | Bologna | DHEA (25 mg, tid/3 m) | FSH 300 ~ 450 IU/d, on day 2 ~ 3 of the menstrual cycle; HCG 6000 ~ 10,000 IU | ①②③④ |
| Li XL, 2018 | China | 32/40 | 4.18(2.95)/4.66(2.93) | 22.70(2.86)/21.94(2.79) | 33.13 (4.70)/32.85 (5.31) | Bologna | GH (4 IU, qd/ day 2–3 of the menstrual cycle t to the day of HCG) | one week after ovulation, triptorelin, 0.1 mg/d and changed to 0.5 mg/d after 1–2 weeks; HCG 10,000 IU | ①②③⑥⑤ |
| Liao CR, 2017 | China | 56/47 | 7.25(3.85)/8.29(3.42) | —— | 37.90 (3.08)/ 38.05 (2.35) | Bologna | DHEA (25 mg, tid/3 m) | Gonal-F, 150 ~ 225 U/d, on day 3 of the menstrual cycle; Cetrorelix, 0.25 mg/d; HCG 10,000 IU | ①④⑥ |
| Song H, 2015 | China | 56/56 | 5.85(2.12)/6.28(1.83) | —— | 37.14 (5.21)/36.86 (5.72) | Bologna | DHEA (25 mg, tid/3 m) | HCG 6000 ~ 10000U | ①②③④⑥ |
| Tang Y, 2013 | China | 21/17 | 9.6(1.1)/7.6(0.8) | —— | 34.5 (0.7)/32.8 (1.2) | Bologna | GH (4 IU, qd/ day 2–3 of the menstrual cycle t to the day of HCG) | triptorelin, 0.05 mg/d, 1 ~ 2 day after ovulation; Gonal-F, 300IU/d, 2 ~ 3 day after ovulation; HCG 5000 ~ 10,000 IU | ① |
| Wu XY, 2018 | China | 48/48 | 3.5(1.50)/3.7(1.10) | 24.1(2.60)/23.7(1.80) | 36.4 (3.70)/37.3 (2.90) | Bologna | GH (4.5 IU, qd/ day 2–3 of the menstrual cycle t to the day of HCG) | Gn, 225 ~ 375 IU/d; GnRH-A, 0.25 mg/d, on day 2 ~ 3 of the menstrual cycle; HCG, 250 mg | ①②⑥ |
| Xu Y, 2018 | China | 76/93 | 3(1.54)/2.67(0.76) | 21.85(2.51)/22.24(3.07) | 32.50 (3.30)/31.92 (3.68) | Bologna | CoQ10(200 mg, tid/ 60 d) | Gonal-F and HMG, both 225 IU/d, on day 2 of the menstrual cycle; Cetrorelix 250 μg/d; Ovidrele 250 μg | ①⑤⑥ |
| Safdarian L, 2019 | Iran | 34/26 | 3.71(1.63)/3.16(1.62) | 26.43(3.24)/26.63(3.07) | 33.80 (4.66)/33.91 (4.49) | Bologna | GH (2.5 mg, qd/ day 8 of the menstrual cycle t to the day of HCG) | Gonal-F, 300 ~ 450 IU/d, on day 3 of the menstrual cycle; Cetrotide, 0.25 mg/d; HCG 10,000 IU | ①⑥⑤ |
| Gong Y, 2020 | China | 52/53 | 4.57(3.14)/4.39(3.22) | 22.09(1.73)/22.62(2.73) | 38.41 (2.91)/38.20 (2.79) | Bologna | GH (4 IU, qd/ day 2 of the menstrual cycle t to the day of HCG) | Gonal-F/on day 2 of the menstrual cycle; Ganirelix; HCG | ①②③④⑥ |
| Mi H, 2014 | China | 32/32 | 4.31 (0.60)/ 4.75(0.58) | 23.11 (0.73)/ 24.31(0.53) | 38 (0.92)/ 37.8 (0.55) | Bologna | TEAS (2 Hz, 20–25 mA, 30 min, qd/ 3 m) | Estradiol valerate tablets/2 mg po qd, 21 days, on the fifth day of menstruation; Dydrogesterone Tablets/10 mg po bid, 5 days, 3 periods | ①②③④⑥ |
| Lian F, 2017 | China | 46/46 | 4.03(1.62)/4.28(1.57) | —— | 40.18 (2.82)/ 40.06 (2.62) | Bologna | TEAS (2 Hz, 20–25 mA, 30 min, qd/ 1 m) | HMG, 150 ~ 300 IU/d, on day 2 ~ 4 of menstruation; Cetrorelix Acetate, 0.125 ~ 0.25 mg/d; HCG 5000 ~ 10,000 IU | ①③⑥ |
| Bassiouny YA, 2016 | Egypt | 68/73 | 6.44(3.62)/6.75(3.75) | 23.71(5.06)/22.67(3.71) | 35.79 (5.56)/35.53 (5.98) | Bologna | GH (7.5 IU, qd/ day 6 of hMG stimulation until the day of hCG triggering) | HMG, 300–450 IU/d, from the second day of the cycle; Cetrotide, 0.25 mg/d; HCG 10,000 IU | ①②⑤ |
| Wang Z, 2021 | China | 410/411 | 3.33(3.72)/3.17(3.72) | 23.83(3.16)/24.01(3.07) | 39.00 (4.64)/39.53 (4.39) | Bologna | DHEA (25 mg, tid/4–12 w) | Triptorelin, 0.05 ~ 0.1 mg/on day 2 ~ 3 of menstruation; Menotropins, 150 ~ 225 IU/d; HCG 4000 ~ 10,000 IU | ①⑤ |
| Kotb MMM, 2016 | Egypt | 70/70 | 7.9(2.5)/7.6(2.6) | 25.6(3.4)/25.1(3.4) | 40.05 (3.1)/39.7 (0.5) | Bologna | DHEA (25 mg, tid/3 m) | HMG, 300 ~ 450 IU/d, on the second day of menstruation; Cetrotide 0.25 mg/d; HCG 10,000 IU | ①④ |
| Choe SA, 2018 | South Korea | 62/65 | 3.85(2.69)/4.49(3.57) | 21.2(2.5)/21.1(2.4) | 39.8 (3.6)/39.4 (4.1) | Bologna | GH (20 mg/ three times, mid-luteal, late luteal, and menstrual cycle day 2) | Gonal-F, 225 ~ 375 IU/from menstrual day 3; Cetrotide, 0.25 mg/d; HCG | ①③⑥ |
| Mohammad EH, 2021 | Egypt | 78/78 | 6.62(2.13)/6.35(2.01) | 24.39(1.52)/25.06(3.47) | 34.27 (2.41)/34.74 (1.98) | Bologna | GH (4 IU,qd/ from the second day of the cycle and stopped one day before ovum pickup) | Cetrorelix, 0.25 mg/d, started on day 6 of COH; HCG 10,000 IU | ①② |
I, intervention group; C, control group; w, week; m, month; qd, once a day; tid, hree times a day; DHEA, dehydroepiondrosterone; GH, Growth hormone. FSH, follicle stimulating hormone; HCG, human chorionic gonadotropin; COH, controlled ovarian hyperstimulation; HMG, human menopausal gonadotropin
①: Clinical pregnancy rate; ②: Embryo implantation rate; ③: High-quality embryo rate; ④: Cycle canceling rate; ⑤: Live birth rate; ⑥: Number of oocytes retrieved
Risk of bias
The risk of bias of 16 RCTs was assessed in Supplementary Figure S1. In terms of random sequence generation, 13 RCTs (81.2%) had a low risk of bias. Six RCTs (37.5%) had a low risk of bias in terms of allocation concealment. However, only 4 RCTs (25%) had a low risk of bias on the two assessments associated with blinding. All studies were low risk in terms of data integrity and selective reporting.
Network meta-analysis results
Primary outcomes: clinical pregnancy rate
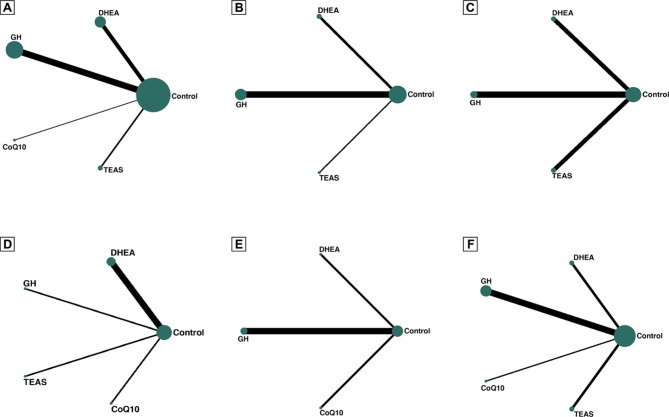
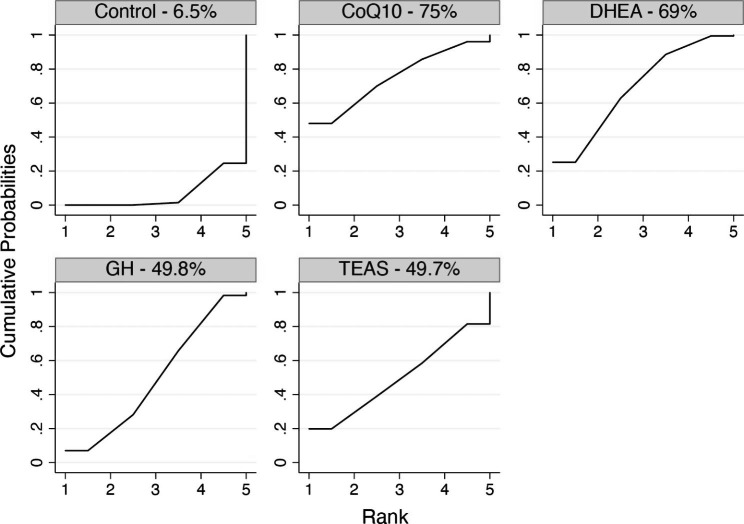
A total of 16 RCTs (2323 participations) reported clinical pregnancy rates. The network plot is shown in Fig. 2A. Due to the lack of inconsistent resources, we used a consistent model. Compared with the control group, CoQ10 (OR 2.22, 95%CI: 1.05 to 4.71) and DHEA (OR 1.92, 95%CI: 1.16 to 3.16) showed obvious advantages in improving the clinical pregnancy rate. The results of network meta-analysis are shown in Table 2. The SUCRA values of CoQ10, DHEA, GH, TEAS and the control were 75%, 69%, 49.8%, 49.7% and 6.5%, respectively (Fig. 3and Supplementary Table S3). The pairwise meta-analysis results conducted with direct data were basically consistent with the above results. Detailed results and NNTs are provided in Supplementary Table S4. The results of the adjusted comparison funnel plot showed that the small-sample study had no effect on the clinical pregnancy rate (Supplementary Figure S2).
Fig. 2.
The network plot (A Clinical pregnancy rates; B Embryo implantation rate; C High-quality embryo rate; D Cycle canceling rate; E Live birth rate; F Number of oocytes retrieved)
Table 2.
Odds ratio (OR) with 95% confidence interval on Clinical pregnancy rate
| CoQ10 | ||||
| 1.16 (0.41,3.26) | DHEA | |||
| 1.42 (0.52,3.89) | 1.23 (0.64,2.37) | GH | ||
| 1.43 (0.37,5.45) | 1.23 (0.41,3.71) | 1.00 (0.34,2.95) | TEAS | |
| 2.22 (1.05,4.71) | 1.92 (1.16,3.16) | 1.56 (0.99,2.58) | 1.56 (0.58,4.18) | Control |
Fig. 3.
SUCRA analysis: MeanRank figure (Clinical pregnancy rate)
Secondary outcomes: embryo implantation rate
Eight RCTs (773 participations) reported on the embryo implantation rate; the network plot is shown in Fig. 2B. Due to the lack of inconsistent resources, we used a consistent model. The results of network meta-analysis are shown in Supplementary Table S5. Compared with the control group, DHEA (OR 2.80, 95%CI: 1.41 to 5.57) and GH (OR 1.60, 95%CI: 1.09 to 2.36) can better improve the embryo implantation rate.The SUCRA values of DHEA, GH, TEAS and control were 87.1%, 57.1%, 53.8% and 8%, respectively (Supplementary Figure S3 and Supplementary Table S6). The paired meta-analysis also showed the same results (Supplementary Table S4).
Secondary outcomes: high-quality embryo rate
A total of 7 RCTs involving 650 participations were included in the network meta-analysis of high-quality embryo rate (see Fig. 2C for the network plot). Due to a lack of inconsistent resources, we used a consistent model. A total of three auxiliary measures were compared with the control group. The results of network meta-analysis showed that DHEA (OR 2.01, 95%CI: 1.07 to 3.78) was associated with a higher rate of high-quality embryos (Supplementary Table S7). The SUCRA values of DHEA, GH, TEAS and the control were 88.1%, 60.1%, 53.1% and 16%, respectively (Supplementary Figure S4 and Supplementary Table S8). The results of pairwise meta-analysis based on direct data were basically consistent with the results of mesh meta-analysis (Supplementary Table S4).
Secondary outcomes: cycle canceling rate
Seven RCTs (771 participations) explored the effect of adjuvant therapy on cycle canceling rate. See Fig. 2D for the network plot. Due to a lack of inconsistent resources, we used a consistent model. Compared with the control group, the four auxiliary measures of DHEA, GH, CoQ10 and TEAS had no obvious advantage in reducing the cycle canceling rate (Supplementary Table S9). The SUCRA values of GH, CoQ10, DHEA, TEAS and control were 81.0%, 69.9%, 49.5%, 30.9% and 18.8%, respectively (Supplementary Figure S5 and Supplementary Table S10). Finally, the paired meta-analysis also showed the same results (Supplementary Table S4).
Secondary outcomes: live birth rate
A total of 5 RCTs (1263 participations) were involved in the live birth rate, as shown in Fig. 2E for the network plot. Due to the lack of inconsistent resources, we used a consistent model. CoQ10 (OR 2.36, 95%CI: 1.07 to 5.38) can effectively improve the live birth rate (Supplementary Table S11). The SUCRA values of CoQ10, GH, control and DHEA were 89.9%, 63.6%, 23.8% and 22.7%, respectively (Supplementary Figure S6 and Supplementary Table S12). The paired meta-analysis results were basically consistent with the above results (Supplementary Table S4).
Secondary outcomes: number of oocytes retrieved
Regarding the effect of the four adjunctive interventions on the number of oocytes retrieved, a total of 10 RCTs involved 949 participants (see Fig. 2F for the network plot). Due to the lack of resources for discordant, we therefore used a concordant model. Compared with the control group, the results of network meta-analysis showed that DHEA (WMD 1.63, 95%CI: 0.34 to 2.92), GH (WMD 1.50, 95%CI: 0.61 to 2.39), CoQ10 (WMD 1.34, 95%CI 0.64 to 1.99), TEAS (WMD 1.04, 95%CI: 0.24 to 3.02) four auxiliary measures could increase the number of oocytes retrieved (Supplementary Table S13). DHEA, GH, CoQ10, TEAS and the Control of SUCRA values were 72.2%, 67.6%, 59%, 47.5% and 3.7% (Supplementary Figure S7 and Supplementary Table S14). Finally, we conducted a pairwise meta-analysis based on direct data; the results were the same as those of mesh analysis, as shown in Supplementary Table S4.
Discussion
Discussion of the main results
To improve IVF pregnancy outcomes in POR patients and determine the best adjuvant treatment strategy, we sought to update the clinical evidence. In this study, we conducted indirect and direct comparisons of CoQ10, DHEA, GH, TEAS and conventional COS regimens, evaluating several clinical outcomes of most concern in the field of reproductive medicine. We found that: [1] CoQ10 was significantly better than DHEA, GH, TEAS and control in improving clinical pregnancy rates and live birth rates; [2] DHEA showed greater advantages in improving the embryo implantation rate, high-quality embryo rate and the number of oocytes retrieved; [3] these adjuvant treatment measures have no significant effect on reducing the cycle canceling rate; and [4] in most pooled results, TEAS had the worst efficacy of the four adjuvant treatments, but the overall effect seemed to be better than that of the control group.
Limited by the Bologna criteria, only one RCT with CoQ10 as adjuvant therapy was included in this study [31]. Analysis of the data obtained revealed that CoQ10 treatment had the highest live birth rate (89.9%), followed by clinical pregnancy rate (75%). CoQ10, as an antioxidant, has been used to improve infertility outcomes, which is associated with increased clinical pregnancy rate (CPR), although the quality of previous evidence remains low [41]. Consistent with our results, a systematic review showed that CoQ10 supplementation significantly increased the clinical pregnancy rate in women with POR and PCOS [42]. In addition, our findings show that CoQ10 was the only statistically significant intervention of the four adjuvant therapies to improve the live birth rate. Despite the limited clinical evidence, CoQ10 has promising applications in adjuvant therapy strategies for POR patients. Of course, these results need to be confirmed by further well-designed prospective RCTs with a large number of participants.
The current meta-analysis included five RCTs with DHEA for POR. Adjuvant treatment with DHEA has a significant impact on various pregnancy outcomes. Our results showed that DHEA produced better clinical outcomes in terms of improving the embryo implantation rate, the high-quality embryo rate and the number of oocytes retrieved. This study suggests that POR patients undergoing IVF improved the conditions of early pregnancy after taking DHEA, which seems to imply an indirect increase in the clinical pregnancy rate. In some direct comparative evidence, DHEA supplementation had a positive effect on women with reduced ovarian reserve (DOR) or POR undergoing IVF/ICSI [43–46], which can improve the ovarian environment for follicle maturation [17]. Compared with placebo or untreated women, the use of DHEA improved the live-birth rate and the ongoing pregnancy rate increased by 3–14% [47]; its mechanism was through the effect on granular cell and ovarian matrix expression of androgen receptor and it also increased the quantity of follicular cavity and AMH level, thereby increasing ovarian reserve [48]. In contrast, several studies have shown that DHEA use is not associated with higher clinical pregnancy rates [49–52], although some scholars suggest that large-scale confirmatory studies are needed to prove the efficacy of DHEA before recommending its routine use [53, 54]. While we support the view of Gleicher and Barad [55]; However, in the case of insufficient available evidence, whether it is possible to supplement the use of DHEA to enhance the effect of exogenous gonadotropins in suitable POR patients to improve ovarian reserve and potential pregnancy outcomes. Further evidence is necessary.
GH has been widely used to treat infertility, especially for patients with POR and the rationale is based on animal and human data. GH may increase the production of insulin-like growth factor 1 (IGF-1) in the ovary. IGF-1 is believed to play an important role in regulating ovarian function [56, 57], stimulating follicle development [58], improving oocyte quality [59] and promoting estrogen production and oocyte maturation [57]. The results of multiple meta-analyses confirmed the beneficial effect of GH on clinical outcomes, including increasing the number of oocytes retrieved, the number of MII oocytes and the number of transferable embryos, thus improving the clinical pregnancy rate and live birth rate. At the same time, the cycle canceling rate and gonadotropin dose of POR patients were decreased [7, 14, 60, 61]. Eight RCTs were evaluated, with GH second only to DHEA in terms of improving the number of oocytes retrieved and the high-quality embryo rate, but there was no significant benefit in improving the clinical pregnancy rate and live birth rate. The Cochrane systematic review noted that because the doses and regimens of GH in the trials were variable, the effects on pregnancy outcomes were uncertain and results needed to be interpreted with caution [62, 63]. However, prospective, large-scale clinical studies may also have different diagnostic criteria and COS protocols, which could increase the risk of bias and imprecision. Therefore, further studies are needed to fully determine the role of GH as an adjunctive treatment for IVF.
TEAS is a non-invasive and painless hybrid therapy that combines transcutaneous electrical nerve stimulation with traditional Chinese medicine acupuncture [21]. TEAS has recently formed a consensus group in reproductive medicine [64]. Multiple RCTs showed that TEAS significantly increased the embryo implantation rate, clinical pregnancy rate and live birth rate [65, 66], improved the basic endocrine level and endometrial receptivity of patients and increased the number of embryos and high-quality embryos [67, 68]. However, in the current meta-analysis, TEAS did not show a better effect in improving pregnancy outcomes in POR patients and did not differ significantly compared with controls. However, the SUCRA value showed that the overall efficacy of combined TEAS in the conventional regimen was better than that of the conventional regimen. Inconsistencies with clinical findings may involve various variable parameters of TEAS, such as frequency, acupoint selection, treatment cycle and even operator level, which may be important factors affecting the clinical effect. Therefore, the potential value of TEAS still needs to be further validated.
Strengths and limitations of the study
Our systematic review and network meta-analysis strictly used the Bologna criteria to define POR, which minimized the risk of heterogeneity and bias. Second, we used network meta-analysis to rank multiple treatment measures in a statistical model [18, 19] and to cover several important clinical outcomes including: (1) embryo implantation rate; (2) high-quality embryo rate; (3) cycle canceling rate; (4) live birth rate; and (5) number of oocytes retrieved. These results can provide a reference for the selection of clinical adjunct regimens for POR.
There are some limitations to our study. There has been no consensus on the definition of POR; after the ESHRE organization proposed the Bologna criteria in 2011, some experts pointed out that the detailed definition of some risk factors had not been solved and the population was very heterogeneous [69]. In 2016, the POSEIDON team proposed a new stratification method based on the number and quality of oocytes, called the POSEIDON criterion [70], but carrying out large RCTs with this criterion can be difficult. First of all, it must be said that our strict use of Bologna criteria to define patients with POR limited the inclusion of some RCTs; only 16 RCTs were included in the network meta-analysis, indirect evidence and the overall risk of bias in the included studies is not optimistic, which may have affected our judgment of the overall quality. Second, there was a gap in the number of RCTs of the four adjuvant therapies, including GH (8 RCTs), DHEA (5 RCTs), TEAS (2 RCTs) and CoQ10 (1 RCT). Due to the limited number of studies, we could not conduct a detailed subgroup analysis according to factors such as adjuvant therapy and exogenous gonadotropin use, initiation time and treatment cycle to reduce heterogeneity. Third, we did not perform an analysis of adverse events due to insufficient primary data; no study provided long-term follow-up data, such as infant growth and development, due to the high time and economic costs involved. Finally, most of the included studies were from Asia or the Middle East, whether population differences had an impact on the outcomes remains to be improved in subsequent studies. Based on current clinical evidence, the clinical efficacy of TEAS remains controversial, but this non-invasive nerve stimulation technique developed from acupoints may provide some new ideas for the treatment of infertility. It may be helpful to reassess the effectiveness of TEAS by summarizing a protocol for prescribing acupoints or standardizing the formation of surface stimulation points.
Conclusion
According to the current evidence, CoQ10, DHEA and GH adjuvant therapy before IVF may have a positive effect on pregnancy outcome in POR patients compared with the conventional COS regimen. TEAS was the worst at improving clinical pregnancy rates, even though it was a noninvasive ex vivo intervention. Infertility patients form a large population worldwide and future large-scale RCTs with direct comparisons are needed to validate or update this conclusion.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material
Supplementary Table S1: Search strategy
Supplementary Table S2: Exclusion list
Supplementary Table S3: SUCRA analysis: MeanRank table (Clinical pregnancy rate)
Supplementary Table S4: Pairwise meta-analysis results for direct comparisons of outcomes
Supplementary Table S5: Odds ratio (OR) with 95% confidence interval on Embryo implantation rate
Supplementary Table S6: SUCRA analysis: MeanRank table (Embryo implantation rate)
Supplementary Table S7: Odds ratio (OR) with 95% confidence interval on High-quality embryo rate)
Supplementary Table S8: SUCRA analysis: MeanRank table (High-quality embryo rate)
Supplementary Table S9: Odds ratio (OR) with 95% confidence interval on Cycle canceling rate
Supplementary Table S10: SUCRA analysis: MeanRank table (Cycle canceling rate)
Supplementary Table S11: Odds ratio (OR) with 95% confidence interval on Live birth rate
Supplementary Table S12: SUCRA analysis: MeanRank table (Live birth rate)
Supplementary Table S14: SUCRA analysis: MeanRank table (Number of oocytes retrieved)
Supplementary Figure S1: Risk of bias assessment results
Supplementary Figure S3: SUCRA analysis: MeanRank figure (Embryo implantation rate)
Supplementary Figure S4: SUCRA analysis: MeanRank figure (High-quality embryo rate)
Supplementary Figure S5: SUCRA analysis: MeanRank figure (Cycle canceling rate)
Supplementary Figure S6: SUCRA analysis: MeanRank figure (Live birth rate)
Supplementary Figure S7: SUCRA analysis: MeanRank figure (Number of oocytes retrieved)
Authors’ contributions
FZ and SY: study conception and design. DC: administrative support. SY, BY and TW: collection and assembly of data. SL, XF and FZ: data analysis and interpretation. FZ, SL, and SY: manuscript writing. All authors: final approval of manuscript. All authors contributed to the article and approved the submitted version.
Funding
No Funding.
Data Availability
Data can be obtained from authors on reasonable request.
Declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simonstein F, Mashiach-Eizenberg M, Revel A, Younis JS. Assisted reproduction policies in Israel: a retrospective analysis of in vitro fertilization-embryo transfer. Fertil Steril. 2014;102(5):1301–6. doi: 10.1016/j.fertnstert.2014.07.740. [DOI] [PubMed] [Google Scholar]
- 2.Kupka MS, D’Hooghe T, Ferraretti AP, de Mouzon J, Erb K, Castilla JA, et al. Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Hum Reprod. 2016;31(2):233–48. doi: 10.1093/humrep/dev319. [DOI] [PubMed] [Google Scholar]
- 3.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from european registries by ESHRE: the european IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Hum Reprod. 2018;33(9):1586–601. doi: 10.1093/humrep/dey242. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Rafael Z, Bider D, Dan U, Zolti M, Levran D, Mashiach S. Combined gonadotropin releasing hormone agonist/human menopausal gonadotropin therapy (GnRH-a/hMG) in normal, high, and poor responders to hMG. J In Vitro Fert Embryo Transf. 1991;8(1):33–6. doi: 10.1007/bf01131588. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins JM, Davies DW, Devonport H, Anthony FW, Gadd SC, Watson RH, et al. Comparison of ‘poor’ responders with ‘good’ responders using a standard buserelin/human menopausal gonadotrophin regime for in-vitro fertilization. Hum Reprod. 1991;6(7):918–21. doi: 10.1093/oxfordjournals.humrep.a137459. [DOI] [PubMed] [Google Scholar]
- 6.Surrey ES, Schoolcraft WB. Evaluating strategies for improving ovarian response of the poor responder undergoing assisted reproductive techniques. Fertil Steril. 2000;73(4):667–76. doi: 10.1016/s0015-0282(99)00630-5. [DOI] [PubMed] [Google Scholar]
- 7.Kolibianakis EM, Venetis CA, Diedrich K, Tarlatzis BC, Griesinger G. Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in-vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2009;15(6):613–22. doi: 10.1093/humupd/dmp026. [DOI] [PubMed] [Google Scholar]
- 8.Papathanasiou A, Searle BJ, King NM, Bhattacharya S. Trends in ‘poor responder’ research: lessons learned from RCTs in assisted conception. Hum Reprod Update. 2016;22(3):306–19. doi: 10.1093/humupd/dmw001. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Li X, Yang X, Cai S, Lu G, Lin G, et al. Cumulative live birth rates in low prognosis patients according to the POSEIDON Criteria: an analysis of 26,697 cycles of in vitro Fertilization/Intracytoplasmic sperm injection. Front Endocrinol (Lausanne) 2019;10:642. doi: 10.3389/fendo.2019.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. 2011;96(5):1058–61. doi: 10.1016/j.fertnstert.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–24. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 12.Ubaldi F, Vaiarelli A, D’Anna R, Rienzi L. Management of poor responders in IVF: is there anything new? Biomed Res Int. 2014;2014:352098. doi: 10.1155/2014/352098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkkanoglu M, Ozgur K. What is the optimum maximal gonadotropin dosage used in microdose flare-up cycles in poor responders? Fertil Steril. 2010;94(2):662–5. doi: 10.1016/j.fertnstert.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril. 2009;91(3):749–66. doi: 10.1016/j.fertnstert.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 15.Polyzos NP, Blockeel C, Verpoest W, De Vos M, Stoop D, Vloeberghs V, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod. 2012;27(12):3481–6. doi: 10.1093/humrep/des318. [DOI] [PubMed] [Google Scholar]
- 16.Sunkara SK, Pundir J, Khalaf Y. Effect of androgen supplementation or modulation on ovarian stimulation outcome in poor responders: a meta-analysis. Reprod Biomed Online. 2011;22(6):545–55. doi: 10.1016/j.rbmo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol. 2011;9:67. 10.1186/1477-7827-9-67 [DOI] [PMC free article] [PubMed]
- 18.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 19.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang C, Shu J, Guo J, Chang HM, Leung PCK, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum Reprod Update. 2020;26(2):247–63. doi: 10.1093/humupd/dmz046. [DOI] [PubMed] [Google Scholar]
- 21.Hsu YC, Liang IT, Huang SY, Wang HS, Soong YK, Chang CL. Transcutaneous electrical acupoint stimulation (TEAS) treatment improves pregnancy rate and implantation rate in patients with implantation failure. Taiwan J Obstet Gynecol. 2017;56(5):672–6. doi: 10.1016/j.tjog.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Szmit M, Agrawal S, Goździk W et al. Transcutaneous Electrical Acupoint Stimulation Reduces Postoperative Analgesic Requirement in Patients Undergoing Inguinal Hernia Repair: A Randomized, Placebo-Controlled Study. J Clin Med. 2021;10(1). 10.3390/jcm10010146 [DOI] [PMC free article] [PubMed]
- 23.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legro RS, Wu X, Barnhart KT, Farquhar C, Fauser BC, Mol B. Improving the reporting of clinical trials of infertility treatments (IMPRINT): modifying the CONSORT statement†‡. Hum Reprod. 2014;29(10):2075–82. doi: 10.1093/humrep/deu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong, Hu, Brand New Qu Effect of dehydroepiandrosterone on ovarian responsiveness and in vitro fertilization-embryo transfer outcomes in patients with low ovarian responsiveness. Int J Reproductive Health/Family Plann. 2018;37(6):458–62. doi: 10.3969/j.issn.1674-1889.2018.06.004. [DOI] [Google Scholar]
- 26.Li X. Effect of growth hormone on embryo quality and pregnancy outcome in patients with low ovarian response with IVF-ET. Yangzhou University. 2018, MA thesis. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201901&filename=1018089597.nh
- 27.Cai-rong Liao PI, Jie ZHU, Jing-xiang LI, Xiao-qing Effect of dehydroepiandrosterone combined with antagonist regimen in vitro fertilization-embryo transfer. Chin J Eugenics and Genetics. 2017;25(2):110–1. [Google Scholar]
- 28.Hui Song Wu, Yan TIAN, Guo-hua LI, Wei YS, Yan-hong HUANG. Effect of dehydroepiandrosterone pretreatment on in vitro fertilization-embryo transfer outcomes in patients with low ovarian response. J Reprod Med. 2015;24(08):622–5. [Google Scholar]
- 29.Tang Y, Guangxiu ZHongLu, Fei G. Application of growth hormone in patients with low ovarian response. Chin J Mod Med. 2013;23(15):49–53. [Google Scholar]
- 30.Wu X-Y, Zhan-Hong TAN, Bei-mei LIU, Chen Ru-jia, Lin Xiao-ping, Jian-Feng XIAO. Effect of antagonist regimen plus GH on endometrial receptivity and pregnancy outcome in POR patients. Chinese Journal of Sex Science (2018) 27(8):79–83. (in Chinese) 10.3969/j.issn.1672-1993.2018.08.024
- 31.Xu Y, Nisenblat V, Lu C, Li R, Qiao J, Zhen X, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol. 2018;16(1):29. doi: 10.1186/s12958-018-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safdarian L, Aghahosseini M, Alyasin A, Samaei Nouroozi A, Rashidi S, Shabani Nashtaei M, et al. Growth hormone (GH) improvement of ovarian responses and pregnancy outcome in poor ovarian responders: a randomized study. Asian Pac J Cancer Prev. 2019;20(7):2033–7. doi: 10.31557/APJCP.2019.20.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong Y, Zhang K, Xiong D, Wei J, Tan H, Qin S. Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: a randomized controlled trial. Reprod Biol Endocrinol. 2020;18(1):91. doi: 10.1186/s12958-020-00648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mi H. Clinical study on the effect of transcutaneous electrical acupoint stimulation on pregnancy outcome of IVF-ET in infertile patients with low ovarian response. Shandong University of Traditional Chinese Medicine. 2018, MA thesis. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201501&filename=1015506882.nh
- 35.Lian F. Clinical study of percutaneous electrical acupoint stimulation combined with luminal physiotherapy in the treatment of renal deficiency type ovarian hyporesponsiveness. Chin J Integr Traditional Western Med. 2017;37(5):522–5. doi: 10.7661/j.cjim.20170315.032. [DOI] [Google Scholar]
- 36.Bassiouny YA, Dakhly DMR, Bayoumi YA, Hashish NM. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil Steril. 2016;105(3):697–702. doi: 10.1016/j.fertnstert.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Yang A, Bao H, Wang A, Deng X, Xue D, et al. Effect of dehydroepiandrosterone administration before in vitro fertilization on the live birth rate in poor ovarian responders according to the Bologna criteria: a randomised controlled trial. BJOG. 2022;129(7):1030–8. doi: 10.1111/1471-0528.17045. [DOI] [PubMed] [Google Scholar]
- 38.Kotb MM, Hassan AM, AwadAllah AM. Does dehydroepiandrosterone improve pregnancy rate in women undergoing IVF/ICSI with expected poor ovarian response according to the Bologna criteria? A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2016;200:1110.1016/j.ejogrb.2016.02.009 [DOI] [PubMed]
- 39.Choe SA, Kim MJ, Lee HJ, Kim J, Chang EM, Kim JW, et al. Increased proportion of mature oocytes with sustained-release growth hormone treatment in poor responders: a prospective randomized controlled study. Arch Gynecol Obstet. 2018;297(3):791–6. doi: 10.1007/s00404-017-4613-4. [DOI] [PubMed] [Google Scholar]
- 40.Mohammad EH, Abou El Serour AG, Mohamed EAH, Abbasy AH, Zaatar M, Rageh KA, et al. Efficacy of growth hormone supplementation with ultrashort GnRH antagonist in IVF/ICSI for poor responders; randomized controlled trial. Taiwan J Obstet Gynecol. 2021;60(1):51–5. doi: 10.1016/j.tjog.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. 2017;7(7):Cd007807. 10.1002/14651858.CD007807.pub3 [DOI] [PMC free article] [PubMed]
- 42.Florou P, Anagnostis P, Theocharis P, Chourdakis M, Goulis DG. Does coenzyme Q(10) supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J Assist Reprod Genet. 2020;37(10):2377–87. doi: 10.1007/s10815-020-01906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarze JE, Canales J, Crosby J, Ortega-Hrepich C, Villa S, Pommer R. DHEA use to improve likelihood of IVF/ICSI success in patients with diminished ovarian reserve: a systematic review and meta-analysis. JBRA Assist Reprod. 2018;22(4):369–74. doi: 10.5935/1518-0557.20180046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Yuan H, Chen Y, Wu H, Wu H, Li L. A meta-analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2015;131(3):240–5. doi: 10.1016/j.ijgo.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Niu W, Wang Y, Xu J, Bao X, Wang L, et al. Dehydroepiandrosterone treatment in women with poor ovarian response undergoing IVF or ICSI: a systematic review and meta-analysis. J Assist Reprod Genet. 2016;33(8):981–91. doi: 10.1007/s10815-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L, Hu C, Liu Q, Li Y. The Effect of Dehydroepiandrosterone (DHEA) supplementation on IVF or ICSI: a Meta-analysis of Randomized controlled trials. Geburtshilfe Frauenheilkd. 2019;79(7):705–12. doi: 10.1055/a-0882-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagels HE, Rishworth JR, Siristatidis CS, Kroon B. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst Rev. 2015;11:Cd009749. 10.1002/14651858.CD009749.pub2 [DOI] [PMC free article] [PubMed]
- 48.Fouany MR, Sharara FI. Is there a role for DHEA supplementation in women with diminished ovarian reserve? J Assist Reprod Genet. 2013;30(9):1239–44. doi: 10.1007/s10815-013-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narkwichean A, Maalouf W, Campbell BK, Jayaprakasan K. Efficacy of dehydroepiandrosterone to improve ovarian response in women with diminished ovarian reserve: a meta-analysis. Reprod Biol Endocrinol. 2013;11:44. doi: 10.1186/1477-7827-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin JC, Fan L, Qin AP. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: evidence from a meta-analysis. J Gynecol Obstet Hum Reprod. 2017;46(1):1–7. doi: 10.1016/j.jgyn.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Richardson A, Jayaprakasan K. The Use of Androgen Priming in Women with Reduced Ovarian Reserve Undergoing Assisted Reproductive Technology. Semin Reprod Med. 2021;39(5–06):207–19. 10.1055/s-0041-1735646 [DOI] [PubMed]
- 52.Bosdou JK, Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Zepiridis L, et al. The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(2):127–45. doi: 10.1093/humupd/dmr051. [DOI] [PubMed] [Google Scholar]
- 53.Sunkara SK, Coomarasamy A, Arlt W, Bhattacharya S. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod. 2012;27(3):637–40. doi: 10.1093/humrep/der464. [DOI] [PubMed] [Google Scholar]
- 54.Yakin K, Urman B. DHEA as a miracle drug in the treatment of poor responders; hype or hope? Hum Reprod. 2011;26(8):1941–4. doi: 10.1093/humrep/der150. [DOI] [PubMed] [Google Scholar]
- 55.Gleicher N, Barad DH. Misplaced obsession with prospectively randomized studies. Reprod Biomed Online. 2010;21(4):440–3. 10.1016/j.rbmo.2010.06.042 [DOI] [PubMed]
- 56.Adashi EY, Resnick CE, D’Ercole AJ, Svoboda ME, Van Wyk JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985;6(3):400–20. doi: 10.1210/edrv-6-3-400. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura Y, Ando M, Nagamatsu S, Iwashita M, Adachi T, Sueoka K, et al. Effects of insulin-like growth factor-I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biol Reprod. 1996;55(1):152–60. doi: 10.1095/biolreprod55.1.152. [DOI] [PubMed] [Google Scholar]
- 58.Adams NR, Briegel JR. Multiple effects of an additional growth hormone gene in adult sheep. J Anim Sci. 2005;83(8):1868–74. 10.2527/2005.8381868x [DOI] [PubMed]
- 59.Weall BM, Al-Samerria S, Conceicao J, Yovich JL, Almahbobi G. A direct action for GH in improvement of oocyte quality in poor-responder patients. Reproduction. 2015;149(2):147–54. doi: 10.1530/rep-14-0494. [DOI] [PubMed] [Google Scholar]
- 60.Cozzolino M, Cecchino GN, Troiano G, Romanelli C. Growth hormone cotreatment for poor responders undergoing in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2020;114(1):97–109. doi: 10.1016/j.fertnstert.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Yang P, Wu R, Zhang H. The effect of growth hormone supplementation in poor ovarian responders undergoing IVF or ICSI: a meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. 2020;18(1):76. doi: 10.1186/s12958-020-00632-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duffy JM, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database Syst Rev. 2010;2010(1):Cd000099. 10.1002/14651858.CD000099.pub3 [DOI] [PMC free article] [PubMed]
- 63.Sood A, Mohiyiddeen G, Ahmad G, Fitzgerald C, Watson A, Mohiyiddeen L. Growth hormone for in vitro fertilisation (IVF). Cochrane Database Syst Rev. 2021;11(11):Cd000099. 10.1002/14651858.CD000099.pub4 [DOI] [PMC free article] [PubMed]
- 64.Qu F, Li R, Sun W, Lin G, Zhang R, Yang J, et al. Use of electroacupuncture and transcutaneous electrical acupoint stimulation in reproductive medicine: a group consensus. J Zhejiang Univ Sci B. 2017;18(3):186–93. doi: 10.1631/jzus.B1600437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang R, Feng XJ, Guan Q, Cui W, Zheng Y, Sun W, et al. Increase of success rate for women undergoing embryo transfer by transcutaneous electrical acupoint stimulation: a prospective randomized placebo-controlled study. Fertil Steril. 2011;96(4):912–6. doi: 10.1016/j.fertnstert.2011.07.1093. [DOI] [PubMed] [Google Scholar]
- 66.Shuai Z, Li X, Tang X, Lian F, Sun Z. Transcutaneous electrical acupuncture point stimulation improves pregnancy outcomes in patients with recurrent implantation failure undergoing in vitro fertilisation and embryo transfer: a prospective, randomised trial. Acupunct Med. 2019;37(1):33–9. doi: 10.1136/acupmed-2017-011483. [DOI] [PubMed] [Google Scholar]
- 67.Zheng Y, Feng X, Mi H, Yao Y, Zhao Y, Li J, et al. Effects of transcutaneous electrical acupoint stimulation on ovarian reserve of patients with diminished ovarian reserve in in vitro fertilization and embryo transfer cycles. J Obstet Gynaecol Res. 2015;41(12):1905–11. doi: 10.1111/jog.12810. [DOI] [PubMed] [Google Scholar]
- 68.Shuai Z, Lian F, Li P, Yang W. Effect of transcutaneous electrical acupuncture point stimulation on endometrial receptivity in women undergoing frozen-thawed embryo transfer: a single-blind prospective randomised controlled trial. Acupunct Med. 2015;33(1):9–15. doi: 10.1136/acupmed-2014-010572. [DOI] [PubMed] [Google Scholar]
- 69.Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod. 2014;29(9):1842–5. doi: 10.1093/humrep/deu139. [DOI] [PubMed] [Google Scholar]
- 70.Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology’ and its proposed marker of successful outcome. F1000Research. 2016;5:2911. 10.12688/f1000research.10382.1 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Table S1: Search strategy
Supplementary Table S2: Exclusion list
Supplementary Table S3: SUCRA analysis: MeanRank table (Clinical pregnancy rate)
Supplementary Table S4: Pairwise meta-analysis results for direct comparisons of outcomes
Supplementary Table S5: Odds ratio (OR) with 95% confidence interval on Embryo implantation rate
Supplementary Table S6: SUCRA analysis: MeanRank table (Embryo implantation rate)
Supplementary Table S7: Odds ratio (OR) with 95% confidence interval on High-quality embryo rate)
Supplementary Table S8: SUCRA analysis: MeanRank table (High-quality embryo rate)
Supplementary Table S9: Odds ratio (OR) with 95% confidence interval on Cycle canceling rate
Supplementary Table S10: SUCRA analysis: MeanRank table (Cycle canceling rate)
Supplementary Table S11: Odds ratio (OR) with 95% confidence interval on Live birth rate
Supplementary Table S12: SUCRA analysis: MeanRank table (Live birth rate)
Supplementary Table S14: SUCRA analysis: MeanRank table (Number of oocytes retrieved)
Supplementary Figure S1: Risk of bias assessment results
Supplementary Figure S3: SUCRA analysis: MeanRank figure (Embryo implantation rate)
Supplementary Figure S4: SUCRA analysis: MeanRank figure (High-quality embryo rate)
Supplementary Figure S5: SUCRA analysis: MeanRank figure (Cycle canceling rate)
Supplementary Figure S6: SUCRA analysis: MeanRank figure (Live birth rate)
Supplementary Figure S7: SUCRA analysis: MeanRank figure (Number of oocytes retrieved)
Data Availability Statement
Data can be obtained from authors on reasonable request.